Abstract
We previously showed that the associative enhancement ofAplysia siphon sensorimotor synapses in a cellular analog of classical conditioning is disrupted by infusing the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N-N′,N′-tetraacetic acid into the postsynaptic motor neuron before training or by training in the presence of the NMDA receptor antagonistdl-2-amino-5-phosphonovalerate (APV). Our earlier experiments with APV used a nondifferential training protocol, in which different preparations were used for associative and nonassociative training. In the present experiments we extended our investigation of the role of NMDA receptor type potentiation in learning inAplysia to differential conditioning. A cellular analog of differential conditioning was performed with a reduced preparation that consisted of the CNS plus two pedal nerves. A siphon motor neuron and two siphon sensory neurons, both of which were presynaptically connected to the motor neuron, were impaled with sharp microelectrodes. One sensorimotor synapse received paired stimulation with a conditioned stimulus (brief activation of a single sensory neuron) and an unconditioned stimulus (pedal nerve shock), whereas the other sensorimotor synapse received unpaired stimulation. Training in normal artificial seawater (ASW) resulted in significant differential enhancement of synapses that received the paired stimulation. Training in APV blocked this differential synaptic enhancement. A comparison of the present data with the data from earlier experiments that used nondifferential training is consistent with the possibility that differential training comprises competition between the presynaptic sensory neurons. Synaptic competition may contribute significantly to the associative effect of paired stimulation in the differential training paradigm.
Keywords: Aplysia californica, classical conditioning, learning and memory, long-term potentiation (LTP), NMDA, synaptic plasticity, synaptic competition
The siphon-withdrawal reflex of the marine snail Aplysia can express a form of classical conditioning. The conditioned stimulus (CS) used for this form of associative learning is weak stimulation of the siphon; the unconditioned stimulus (US) is strong electrical shock of the tail (Carew et al., 1981). The original demonstration of classical conditioning of the withdrawal reflex used a nondifferential training protocol, in which separate groups of animals received either associative training—paired presentation of the CS and US—or nonassociative training—presentation of the US alone or unpaired presentation of the CS and US. A more rigorous demonstration of the dependence of classical conditioning of the withdrawal reflex on the paired presentation of the CS and US was provided by the report of differential classical conditioning by Carew et al. (1983). In their study each animal received two conditioned stimuli, which consisted of weak stimulation of two separate sites on the skin of the animal. The delivery of one of the stimuli (CS+) was paired with the US, whereas the delivery of the other stimulus (CS−) was explicitly unpaired with the US. After differential training, animals exhibited a longer withdrawal response to the CS+ than to the CS−. An advantage of differential conditioning is that each animal serves as its own control. This not only permits a more convincing separation of the associative and nonassociative effects of training but also provides an advantageous experimental paradigm for a cellular analysis of classical conditioning in Aplysia. In 1983 two groups, using somewhat different experimental paradigms, demonstrated a cellular analog of differential conditioning in Aplysia(Hawkins et al., 1983; Walters and Byrne, 1983). In this cellular analog of differential conditioning, synapses made by two different sensory neurons, each of which was connected to same motor neuron, were differentially trained. Activation of the CS+sensory neuron was paired with the US (tail or tail nerve shock), whereas activation of the CS− sensory neuron was unpaired with the US. Both Hawkins et al. (1983) and Walters and Byrne (1983) found that the synapses made by the CS+sensory neurons were significantly more enhanced after training than were the synapses made by the CS− sensory neurons. Based on these results, the two groups jointly proposed a cellular mechanism to explain classical conditioning in Aplysia. This mechanism, known as “activity-dependent presynaptic facilitation (ADPF)” is hypothesized to be an elaboration of the mechanism of presynaptic facilitation of sensorimotor synapses (Byrne and Kandel, 1996). As originally conceived, ADPF is a non-Hebbian mechanism, involving strictly presynaptic processes.
Evidence that classical conditioning in Aplysia might be mediated by a different mechanism came from the discovery by Lin and Glanzman (1994a,b, 1997) that sensorimotor synapses possess the capacity for Hebbian long-term potentiation (LTP). This discovery led to the hypothesis that classical conditioning of the withdrawal reflex is mediated, in part, by Hebbian potentiation (Glanzman, 1994; Lin and Glanzman, 1994b; Glanzman, 1995). In support of this hypothesis we previously showed that the cellular analog of nondifferential classical conditioning of the withdrawal reflex is blocked by infusing the rapid Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N-N′,N′-tetraacetic acid into the postsynaptic motor neuron (Murphy and Glanzman, 1996) and by the NMDA receptor antagonist dl-2-amino-5-phosphonovalerate (APV) (Murphy and Glanzman, 1997). In the present experiments we tested whether the cellular analog of differential conditioning (Hawkins et al., 1983; Walters and Byrne, 1983), like the cellular analog of nondifferential conditioning, depends on activation of NMDA-type receptors (Collingridge et al., 1983; Dale and Kandel, 1993).
MATERIALS AND METHODS
Preparation. A reduced preparation was used (Hawkins et al., 1983; Murphy and Glanzman, 1996, 1997). Aplysia californica (100–200 gm, obtained from local suppliers) were anesthetized by injecting isotonic MgCl2 (equal to approximately one-half body weight) into the hemocoel. The CNS, except for the buccal ganglia, was removed from the animal, together with two posterior pedal nerves (P9) that innervate the animal's tail. The tail, together with the rest of the animal's body, was discarded. After the abdominal ganglion was lightly fixed with glutaraldehyde [0.5% in 50% MgCl2 and 50% normal artificial seawater (ASW; 460 mm CaCl2, 11 mmCaCl2, 10 mm KCl, 55 mmMgCl2, and 10 mm HEPES buffer, pH 7.6)], the preparation was pinned to the Sylgard-lined bottom of a recording chamber containing 50% MgCl2 and 50% normal ASW (Fig. 1A). The abdominal ganglion was then partially desheathed, and the two P9 nerves were drawn into suction electrodes. Afterward the MgCl2-containing solution was washed out by perfusing the recording chamber with normal ASW for at least 30 min before the start of the experiment.
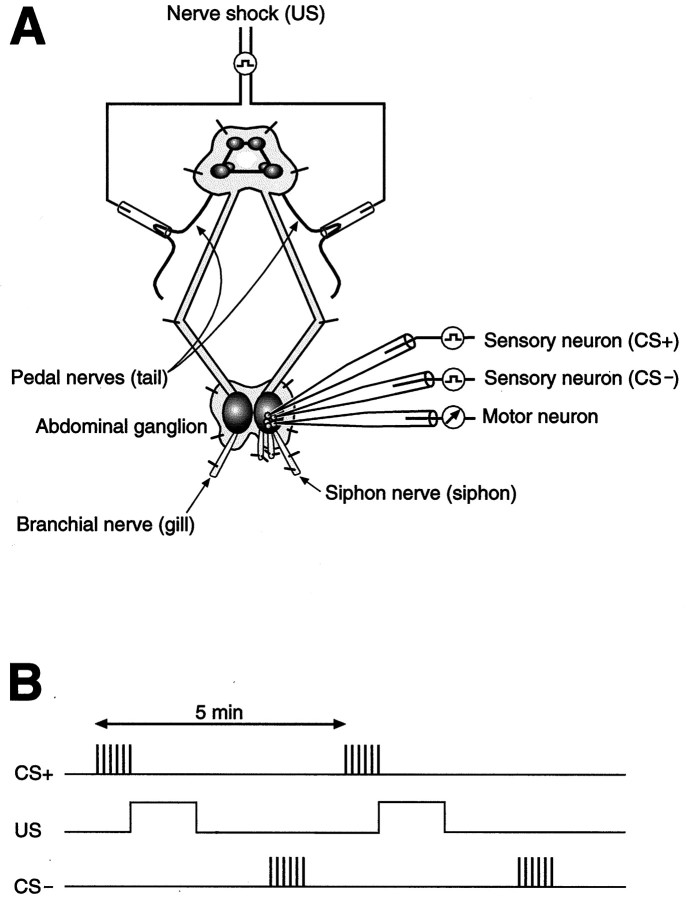
Fig. 1.
Experimental preparation and pattern of stimulation used for the cellular analog of differential classical conditioning. A, Illustration of the reduced preparation (see Methods and Materials for details). Note that the abdominal ganglion is shown artificially enlarged relative to the other ganglia. Sharp microelectrode recordings were made from a single small siphon (LFS) motor neuron and two siphon sensory (LE) neurons, both of which were presynaptically connected to the motor neuron. B, Differential conditioning was performed by pairing activation of one of the sensory neurons, the CS+ sensory neuron, with the US (tail nerve shock), whereas the activation of the other sensory neuron, the CS− sensory neuron, was specifically unpaired with the US. The US was delivered once per 5 min; the CS− stimulation occurred 2.5 min after the US. All stimuli were presented five times during training (see Fig. 2).
Electrophysiology. A single small siphon (LFS) motor neuron (Frost et al., 1988; Hickie and Walters, 1995) and two siphon sensory (LE) neurons (Byrne et al., 1974), each of which was presynaptically connected to the motor neuron, were impaled with sharp micropipettes (20–30 MΩ); the micropipettes were filled with a solution of 2 m K-acetate, 0.5 m KCl, and 10 mm HEPES, pH 7.2. Stimulation and recording were performed using standard methods, and the electrophysiological data were digitized with a Maclab 4e system (ADInstruments, Castle Hill, Australia). Before the start of an experiment the motor neuron was hyperpolarized to 50 mV below its resting potential by passing negative current through the bridge circuit of the amplifier. This hyperpolarizing current was maintained throughout the experiment to prevent both spontaneous firing by the motor neuron and evoked firing in response to test stimulation of the sensory neurons. Action potentials were elicited in LE neurons by brief (20 msec) injections of positive current.
Experimental protocol. The differential training protocol was similar to that of Hawkins et al. (1983). The CS was brief activation of the sensory neuron (12 action potentials at 25 Hz) (Figs.1B, 2). The level of current used to stimulate the sensory neuron during the CS was twice the threshold level for eliciting an action potential. The specific levels of current used for the CS were 0.4–1.8 nA. The US was a 1 sec shock (3 msec pulses at 25 Hz) delivered to the P9 nerves via the suction electrodes. The intensity of the US was six times the threshold level for evoking an EPSP in the motor neuron. This shock intensity was 7–9 V, measured from the front panel of the stimulator (S88; Grass Medical Instruments, Quincy MA). During training only the activity of one sensory neuron—the CS+ sensory neuron—was paired with the US. Activity in the other sensory neuron (the CS− sensory neuron) was specifically unpaired with the US. Assignment of a sensory neuron to the CS+ or CS− condition was based on the size of its EPSP evoked on pretest 2; the assignment was made to minimize the difference between the CS+ and CS− groups (and between the CS+/APV and CS−/APV groups) with respect to the mean group value of the pretest 2 EPSP. In each experiment the US was presented five times once per 5 min. Sensorimotor synapses in the CS+ condition received five paired presentations of the CS and US. During each bout of paired (CS+) stimulation, the onset of the CS preceded that of the US by 500 msec. The unpaired CS (CS−) occurred 2.5 min after the US. Before training there were four pretests with a 15 min interval between the tests. During each pretest the two sensory neurons were each fired one time, and the resulting EPSPs were recorded from the motor neuron. Training was begun 5 min after the fourth pretest. Two post-tests were performed on each synapse, one at 15 min and the other at 60 min, after the delivery of the final CS in each experimental condition. For example, the 15 min post-test for the CS− synapse occurred 15 min after the last unpaired CS and 2.5 min after the 15 min post-test for the CS+ synapse (Fig. 2). One of the pretests (pretest 2) and both of the post-tests were performed in the presence of a modified ASW that contained elevated levels of Mg2+and Ca2+. This so-called “2:1” ASW (NaCl, 368 mm; CaCl2, 13.8 mm; KCl, 8 mm; MgCl2, 101 mm; MgSO4, 20 mm; and HEPES buffer, 10 mm, pH 7.6) reduces the interneuronal contribution to the sensorimotor EPSP (Trudeau and Castellucci, 1992). The 2:1 ASW was perfused into the recording chamber after pretest 1 and was washed out immediately after pretest 2. The 2:1 ASW was reintroduced after the final bout of paired stimulation and remained in the recording chamber for both post-tests. The training was performed with normal ASW in the recording chamber. To test the potential contribution of NMDA-type receptors to associative plasticity, in some experiments APV (Sigma, St. Louis, MO) was dissolved in normal ASW (100 μm) and perfused into the recording chamber immediately after the pretest 2; it was washed out immediately after the fifth delivery of the CS−.
Fig. 2.
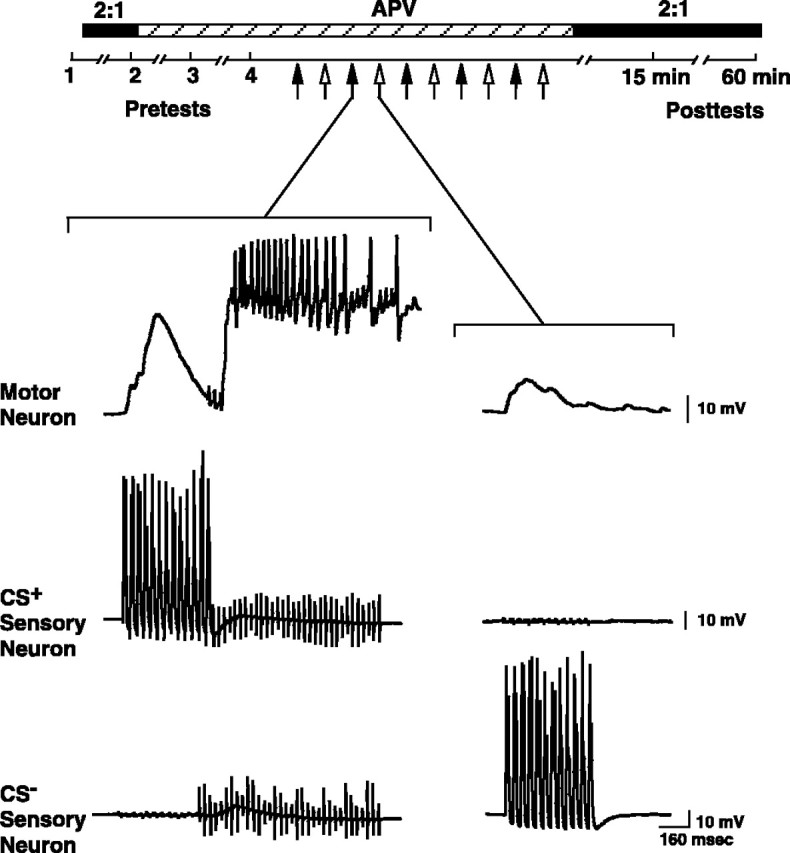
Experimental protocol used for differential conditioning. Four pretests were performed on each of the two sensorimotor synapses at an intertest interval of 15 min. The first was performed in normal ASW. After the first pretest, 2:1 ASW (see Materials and Methods), indicated by the black bars above the time line, was perfused into the recording chamber. The second pretest was then performed in 2:1 ASW. After the second pretest the 2:1 ASW was replaced with normal ASW, and the third and fourth pretests were performed. Training began 5 min after pretest 4 and was performed either in normal ASW or in ASW that contained 100 μm APV (indicated by the hatched bar above thetime line). Each of the stimuli (CS+, US, and CS−) was presented 5 times. The filled arrows indicate paired presentations of the CS+and US; the open arrows indicate presentations of the CS−. The paired stimuli were delivered at a rate of one per 5 min, and the unpaired stimuli were separated from the paired stimuli by 2.5 min. Immediately after training 2:1 ASW was again perfused into the recording chamber, and two posttests were performed; the post-tests occurred 15 and 60 min after the final delivery of the CS for each synapse (see Materials and Methods). Representative traces are from the motor neuron and the two presynaptic sensory neurons recorded during the sequential presentation of the stimuli (CS+, US and CS−) in an experiment in which APV was present during training. Notice that the US produced strongdepolarization and firing of the motor neuron.
Statistics. The summary statistics are presented as means ± SEM. In the figures and text n refers to the number of preparations. A nonparametric paired t test (Wilcoxon signed rank test) was used to assess the statistical significance of differences between two conditions within the same preparations. Intragroup comparisons (between pre- and post-tests) were also made with Wilcoxon signed rank tests. Planned comparisons between two experimental groups were made with unpaired nonparametric Mann–Whitney tests, and planned multiple comparisons were made with a nonparametric ANOVA (Kruskal–Wallis). All reported levels of statistical significance represent two-tailed values.
RESULTS
Effect of APV on differential conditioning
There were no differences among the mean raw EPSP amplitudes recorded on pretest 2 in the four different experimental groups (CS+ EPSP = 5.0 ± 0.6 mV; CS− EPSP = 6.3 ± 1.6 mV; CS+/APV EPSP = 5.0 ± 1.5 mV; CS−/APV EPSP = 5.4 ± 1.3 mV; Kruskal–Wallis = 2.6; p > 0.05). Synapses that received paired stimulation (CS+ group; Fig.3) showed significant enhancement for both the 15 min post-test (normalized EPSP = 194 ± 17%) and 60 min post-test (normalized EPSP = 163 ± 8%), based on the comparison with the pretest 2 EPSP (p < 0.01 for each comparison). Unpaired presentations of the CS and US (CS− group; Fig. 3) did not produce a significant enhancement of the EPSP for either the 15 min post-test (normalized EPSP = 127 ± 16%) or 60 min post-test (normalized EPSP = 80 ± 9%), compared with the pretest 2 EPSP (p > 0.05 for both comparisons). The training protocol produced significant differential enhancement of synapses in the CS+ group, as indicated by the significant difference between the CS+ EPSP and CS− EPSP on both post-tests (p < 0.01 for each comparison).
Fig. 3.
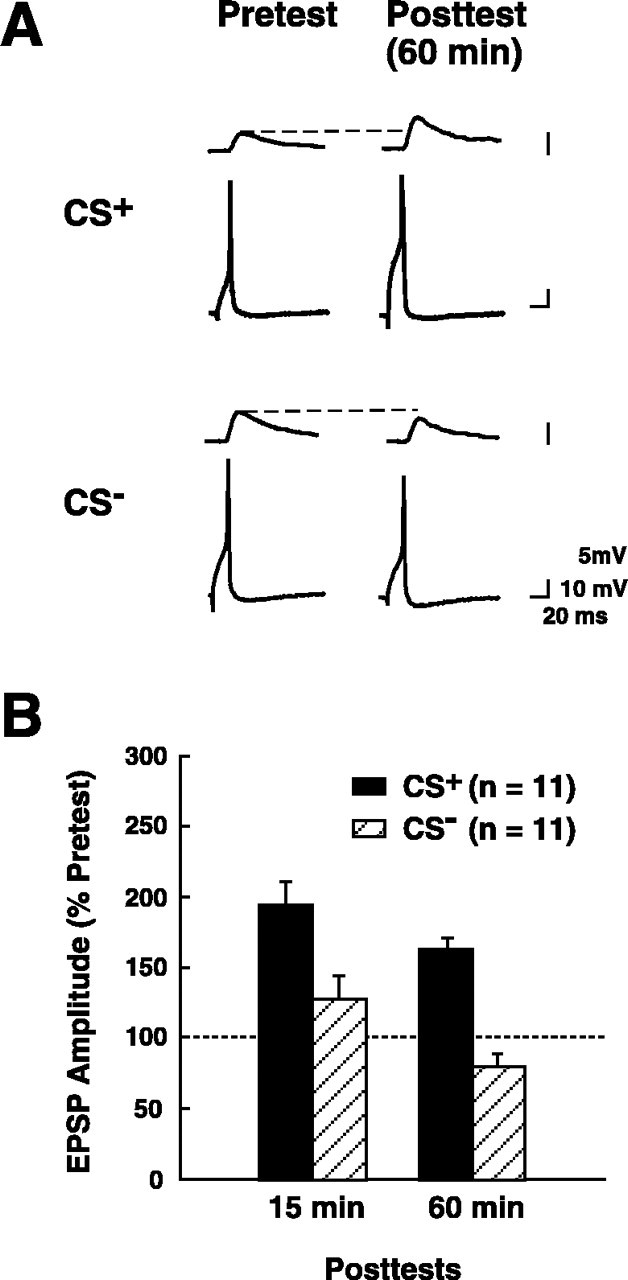
Training in normal ASW produces differential enhancement of the synapse made by the CS+ sensory neuron. A, Representative records of the responses of the two presynaptic sensory neurons (bottom traces) and the postsynaptic motor neuron (top traces) during the second pretest and the 60 min post-test. Both tests were performed in 2:1 ASW (see Materials and Methods). B, Group data for experiments in which training was performed in normal ASW. The amplitude of the EPSP on each of the posttests was normalized to the amplitude of the EPSP on pretest 2 (see Fig. 2). The data are presented as means ± SEM. The EPSP in synapses that received paired CS–US stimulation (the CS+ group) was significantly enhanced compared with the normalized pretest EPSP (indicated by the dashed line). Furthermore, the CS+ EPSP was significantly more enhanced after training than was the EPSP in synapses that received unpaired training (the CS− group; see Results).
Paired stimulation in the presence of APV did not produce significant enhancement of the EPSP (CS+/APV group; Fig.4). Neither the mean normalized EPSP for the 15 min post-test (101 ± 12%) nor that for the 60 min post-test (105 ± 10%) was significantly different from the pretest 2 EPSP (p > 0.7 for each comparison). Furthermore, the presence of APV during the training significantly blocked differential synaptic enhancement. The mean EPSP in the CS+/APV group was not significantly different from the mean EPSP in the CS−/APV group for either the 15 min post-test (CS−/APV EPSP = 131 ± 17%) or the 60 min post-test (CS−/APV EPSP = 129 ± 14%) (p > 0.05 for each comparison). Unpaired stimulation with the CS and US in the presence of APV produced a small increase in mean EPSP amplitude on the post-tests compared with the pretest 2 EPSP. This increase was statistically significant for the 15 min post-test (p < 0.05), although not quite for the 60 min post-test (p > 0.07). Therefore, in contrast to its disruption of associative synaptic enhancement, APV did not reduce the effect of unpaired stimulation on the EPSP. The mean EPSP in the CS−/APV group was not significantly different from the mean EPSP amplitude in the CS− group trained without APV (Fig. 3) for the 15 min posttest (p> 0.05) and was actually significantly greater for the 60 min post-test (p < 0.01).
Fig. 4.
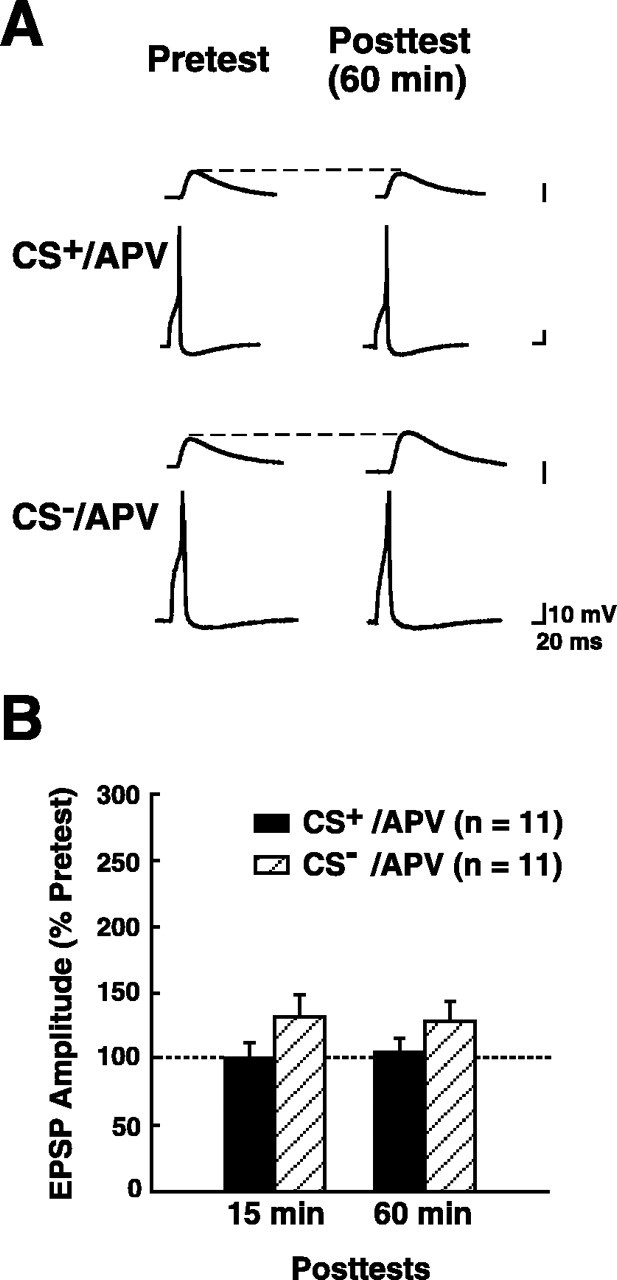
Training in APV blocks differential synaptic enhancement. A, Representative records of the responses of the two presynaptic sensory neurons (bottom traces) and the postsynaptic motor neuron (top traces) during the second pretest and the 60 min post-test. Both tests were performed in 2:1 ASW (see Materials and Methods). B, Group data for experiments in which training was performed in ASW containing APV (100 μm). The amplitude of the EPSP on each of the post-tests was normalized to the amplitude of the EPSP on pretest 2 (see Fig. 2). The data are presented as means ± SEM. The EPSP in synapses that received paired stimulation in APV (the CS+/APV group) did not exhibit significant enhancement compared with the normalized pretest EPSP (indicated by the dashed line) on either post-test. Furthermore, the CS+/APV EPSP was not significantly different from the CS−/APV EPSP on either post-test.
Effect of APV on the excitatory drive of the motor neuron during tail nerve shock
A potential explanation for the disruption of associative synaptic plasticity by APV is that the drug might have reduced the efficacy of excitatory or modulatory interneurons that are activated by tail shock (Frost and Kandel, 1995). The effect of the excitatory interneurons is to strongly depolarize the siphon motor neurons during the US. A decrease in postsynaptic depolarization during the US would be expected to interfere with Hebbian potentiation of the sensorimotor synapses. To assess the possibility that APV might have interfered with US-induced excitatory drive on the motor neuron during paired training, we quantified the amount of postsynaptic depolarization produced by the US in our experiments. This was done by mathematically integrating the area under the postsynaptic membrane during the US (Fig. 5A, shaded area). This integration was made for each bout of paired stimulation for both the CS+ and CS+/APV groups. (The integration was performed off-line with software options provided by our data acquisition system. See Materials and Methods.) The presence of APV during training did not decrease the amount of depolarization produced during the US, as indicated by the results from integrating the area under the postsynaptic membrane potential (p > 0.05; Fig. 5B). As a second method of quantifying the US-induced drive on the motor neuron in our experiments, we also counted the number of action potentials (spikes) produced in the motor neuron during the US. The drug did not decrease the number of postsynaptic spikes during the US (p > 0.05; Fig. 5B). Therefore, the block of associative plasticity produced by APV cannot be attributed to a decrease in the activity, or efficacy, of excitatory interneurons during training.
Fig. 5.
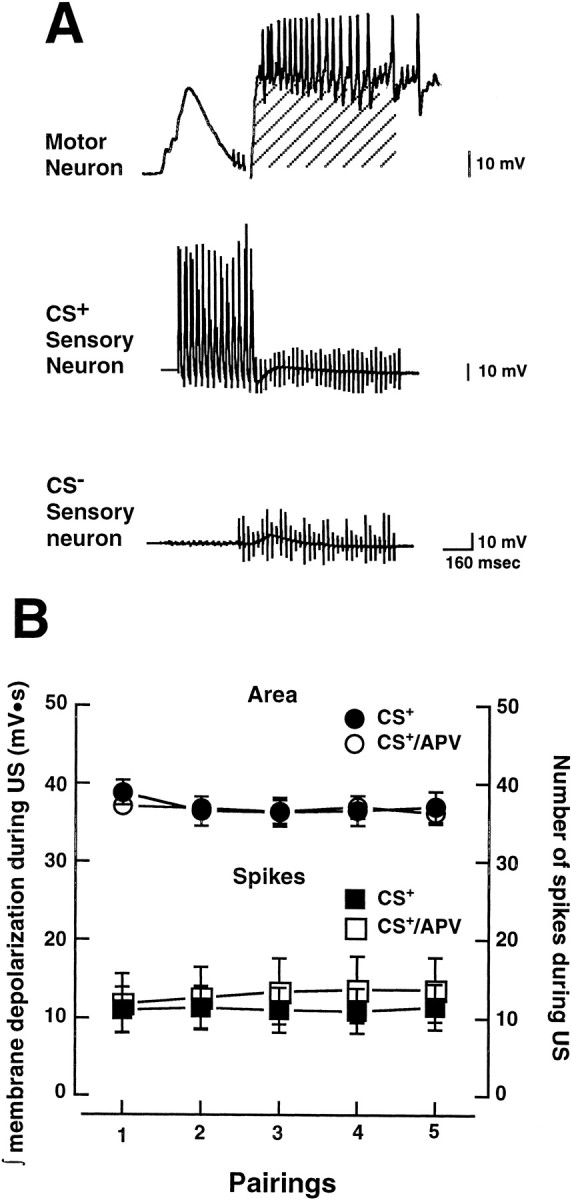
APV does not alter the effect of the US on the postsynaptic motor neuron. A, Examples of the responses of a motor neuron and the two presynaptic sensory neurons during a bout of paired stimulation (CS+ and US). The excitatory drive on the motor neuron produced by the US (tail nerve shock) was approximated by mathematically integrating the area under the postsynaptic membrane potential during the 500 msec of the US (shaded area). B, Group data (means ± SEM) for the experiments performed in normal ASW and the experiments performed in ASW containing APV. There were no significant differences between the two groups in the amount of postsynaptic depolarization or in the number of postsynaptic action potentials (spikes) produced by the US.
These data, together our previous demonstration that APV does not alter the nonassociative enhancement of the sensorimotor EPSP resulting from unpaired stimulation (Murphy and Glanzman, 1997), permit us to rule out a significant effect of the drug on the actions of excitatory and facilitatory interneurons. We cannot, however, completely exclude an effect of APV on interneuronal pathways. Notice that sensorimotor EPSPs were relatively more enhanced on the 60 min posttest after unpaired training in APV than after unpaired training in normal ASW (compare Figs. 3B, 4B). This effect may be attributable to a disruptive effect of APV on the influence of presynaptic inhibitory interneurons activated by the US (Mackey et al., 1987; Marcus et al., 1988).
Possible recruitment of synaptic competition during differential conditioning
Our present results differed in one major respect from the results of our previous conditioning experiments, which used a nondifferential training protocol wherein preparations received only paired or unpaired stimulation (Murphy and Glanzman, 1997). In the earlier nondifferential experiments we observed significant synaptic enhancement 15 min after training in preparations that received unpaired training in normal ASW (Murphy and Glanzman, 1997, their Fig. 2D; the CS− EPSP was significantly greater than its pretest value in both the 15 and 60 min post-tests). But we did not observe significant synaptic enhancement attributable to unpaired training in the present experiments for the 15 min post-test (Fig. 3; the CS− EPSP in the 15 min post-test was not significantly different from its pretest value). Figure6 shows the post-test data from Murphy and Glanzman (1997), together with the post-test data from the present study, for those experiments in which training was performed in normal ASW. The data from the two studies have been replotted as the mean difference in millivolts between the EPSPs for pretest 2 and the post-tests. The data are plotted in this manner to permit examination of the absolute results from the two independent studies. In the study by Murphy and Glanzman (1997) the mean synaptic enhancement in the CS+ group (2.5 ± 0.8 mV) was not significantly different from that in the CS− group (1.5 ± 0.7 mV) for the 15 min post-test (Fig. 6A;p > 0.05, Mann–Whitney test). However, the difference between the two groups was significant for the 60 min post-test. Paired training produced a mean synaptic enhancement of 2.7 ± 1.2 mV (CS+ group), whereas unpaired training produced almost no enhancement (0.1 ± 0.6 mV, (CS−group; p < 0.05, Mann–Whitney test). In contrast to the nondifferential training protocol, the differential training protocol used in the present experiment resulted in significant differences between the CS+ and CS− groups for both posttests (Fig.6B). The mean synaptic enhancement for the 15 min post-test was 4.4 ± 1.0 mV in the CS+ group but only 0.1 ± 1.2 mV in the CS− group (p < 0.01, Mann–Whitney test). The mean synaptic enhancement for the 60 min post-test was 3.0 ± 0.5 mV in the CS+ group; in the CS− group there was a synaptic decrease of 1.7 ± 0.9 mV (p < 0.0001, Mann–Whitney test).
Fig. 6.
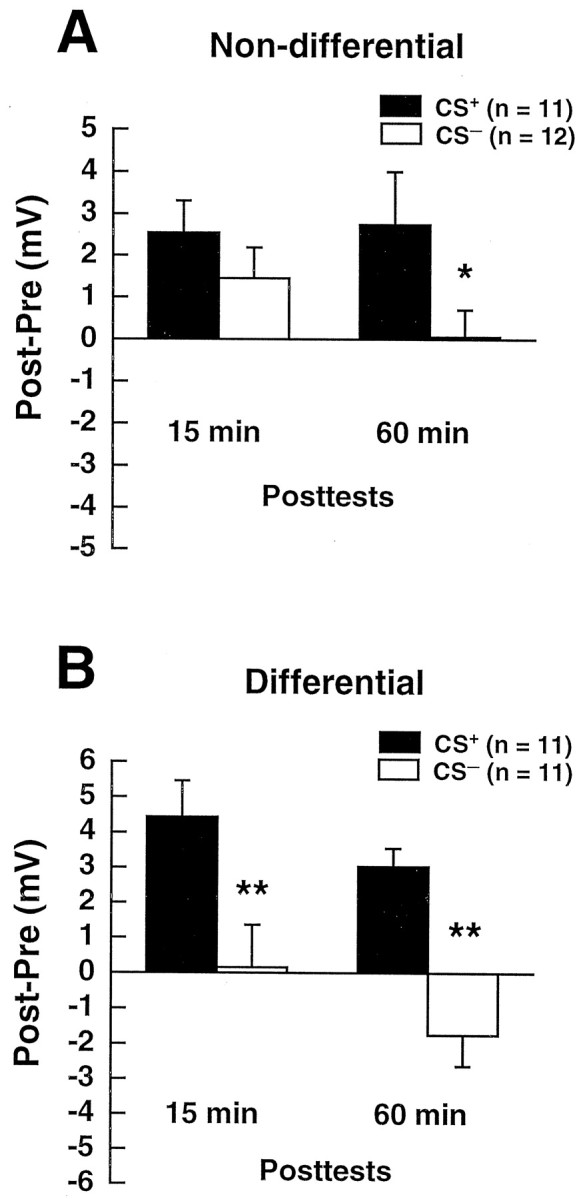
Comparison of the synaptic effects of nondifferential and differential training. A, Replotted data from Murphy and Glanzman (1997). The data are expressed as the mean ± SEM difference (in millivolts) between the non-normalized EPSP on each of the post-tests and the non-normalized EPSP on pretest 2 (see Fig. 2). Paired and unpaired training in these experiments was performed on different preparations. B, Data from the present experiments, in which paired and unpaired training was performed on the same preparations. The data are presented as difference scores as in A. Statistical comparisons between groups (CS+ vs CS−) inA and B were made using Wilcoxon signed rank tests. Asterisks indicate statistical significance for the between-group comparison; *p < 0.05; **p < 0.01.
What might account for the discrepancy between our nondifferential and differential data on the 15 min post-test? One possibility, suggested by related experiments on sensorimotor synapses in cell culture (Glanzman et al., 1991; Schacher et al., 1997; Wright et al., 1999), is that the presynaptic sensory neurons compete with each other for synaptic interaction with the motor neuron. According to this idea, the significant effect of paired stimulation at 15 min after training in the differential experiments (Figs. 3, 6B) predominately reflects a competitive interaction between the two presynaptic cells. In the differential experiments both the CS+ and CS− sensory neurons synapsed onto the same target motor neuron. By contrast, only one presynaptic sensory neuron per preparation was studied in the nondifferential experiments; no effect of synaptic competition would therefore be expected in the nondifferential results. Consequently, the significant synaptic enhancement for the 60 min post-test in the CS+ group in the nondifferential experiments is probably attributable to a purely associative mechanism. The lack of a difference between the paired and unpaired results at 15 min after training in the nondifferential experiments suggests that this associative synaptic mechanism has a relatively delayed onset, being absent at 15 min but present at 60 min after training. We believe that the significant difference between the CS+ and CS− groups at 60 min after training in the differential experiments is also attributable to a delayed associative synaptic enhancement. However, it is possible that synaptic competition contributes to the 60 min post-test results in the differential experiments as well (see Discussion).
DISCUSSION
Comparison with earlier results
The present results demonstrate that the strengthening of the monosynaptic sensorimotor synapses that occurs during the cellular analog of differential classical conditioning in Aplysiarequires activation of NMDA, or NMDA-related, receptors (Dale and Kandel, 1993). These results, together with our previous findings (Murphy and Glanzman, 1996, 1997), support the hypothesis that Hebbian potentiation of sensorimotor synapses (Lin and Glanzman, 1994a,b, 1997) plays a role in classical conditioning of Aplysia's siphon-withdrawal reflex. In our previous study examining the potential contribution of NMDA-type LTP of sensorimotor synapses to classical conditioning (Murphy and Glanzman, 1997), we used a nondifferential protocol. Experiments involving paired stimulation (both in normal ASW and in APV) were performed separately from those involving unpaired stimulation using different preparations. It has therefore been suggested that the apparent disruption of associative plasticity by APV we observed in the earlier study might have been caused, instead, by fluctuation in the magnitude of the nonassociative effects of training over time (Hawkins, 1998). The present data, in which associative and nonassociative training were performed within the same preparation at the same time, obviate this interpretation of the effect of APV. Our new data therefore confirm our previous conclusion that APV blocks associative, conditioning-related synaptic enhancement of sensorimotor synapses (Murphy and Glanzman, 1997, 1998).
Potential contribution from synaptic competition in differential conditioning
An unexpected result from our earlier study was that there was no associative effect of paired training in normal ASW on the 15 min post-test (Fig. 6A; also see Murphy and Glanzman, 1997, their Fig. 2D). This result contrasts with those of Hawkins et al. (1983), who found significant associative synaptic enhancement in the CS+ group as early as 5 min after five bouts of paired stimulation. The study by Hawkins et al. (1983), unlike our earlier study, used a differential paradigm. In the present study, which also used a differential paradigm, we observed an associative effect of paired training on the 15 min post-test for the CS+ versus CS− protocol (Figs.3, 6B). This suggests that the early (15 min) associative effect of paired training is peculiar to the differential paradigm. We hypothesize that the differential conditioning paradigm, unlike the nondifferential paradigm, recruits synaptic competition between the CS+ and CS− sensory neurons, and that this competition accounts for the associative effect we observed at 15 min after training in the present study.
Evidence from studies of sensorimotor cocultures supports the idea that the strength of different sensory inputs to a common target motor neuron is regulated by a competitive interaction, during both development and learning-related plasticity. Glanzman and colleagues (1991) found that the mean size of the sensorimotor EPSP of “mature” (5-d-old) sensorimotor cocultures with two presynaptic neurons was half the size of the EPSP of cocultures with one presynaptic neuron. Furthermore, Schacher and colleagues (1997) found that long-term associative enhancement of a connection made by one sensory neuron [attributable to paired stimulation with serotonin (5-HT) and tetanus] interferes with long-term nonassociative enhancement (attributable to 5-HT alone) of the connection made by a second sensory neuron with the same target motor neuron. Schacher et al. (1997) refer to this phenomenon as “pathway-specific facilitation.” These in vitro results parallel the disparity we observed between our nondifferential and differential conditioning experiments with respect to the CS−data for the 15 min post-test (Fig. 6). Interestingly, Schacher et al. (1997) found that both postsynaptic hyperpolarization and APV disrupt pathway-specific facilitation. The results of Schacher et al. (1997)indicate that an associative increase in strength of the connection made by one sensory neuron with a motor neuron comes at the expense of the potential nonassociative enhancement of the connection made by a second sensory neuron with the same motor neuron. The results further indicate that postsynaptic NMDA-type receptors may play a role in the apparent competition among presynaptic inputs.
The hypothesis that synaptic competition is recruited during differential conditioning in Aplysia is supported by a negative correlation we observed in our data between a large initial size of the non-normalized CS+ EPSP relative to that of the non-normalized CS− EPSP [pretest 2 CS+ (mV) − pretest 2 CS−(mV)], and the absolute amount of differential conditioning produced by the training [(Δ CS+ EPSP amplitude) − (Δ CS− EPSP amplitude), where Δ CS× EPSP = posttest CS×EPSP − pretest 2 CS× EPSP; Murphy and Glanzman, unpublished data]. We have considered and rejected several alternative hypotheses that might explain this negative correlation. For example, one possible explanation for the negative correlation is the existence of a ceiling effect for EPSP size. According to a ceiling effect hypothesis, initially large CS+ EPSPs undergo less positive differential conditioning because of an intrinsic upper limit for sensorimotor EPSP size. But we found no significant correlation, either negative or positive, between the non-normalized size of the pretest 2 CS+ EPSP (in millivolts) and the absolute change in the size of the CS+ because of training [posttest CS+ EPSP (mV) − pretest 2 CS+ EPSP (mV)] for either the 15 or 60 min post-test, contrary to what a ceiling effect hypothesis would predict (G. G. Murphy and D. L. Glanzman, unpublished data).
A mechanism that could account for our conditioning data, as well as for the data from in vitro studies of the sensorimotor synapse (Glanzman et al., 1991; Schacher et al., 1997), is homeostatic regulation of the sizes of multiple sensory inputs to a common motor neuron. According to a current theory (Miller, 1996), the total strength of excitatory inputs to a postsynaptic cell is relatively fixed. Consequently, if some inputs increase in strength, the strength of others must correspondingly decrease. Such a homeostatic mechanism has been referred to as “postsynaptic normalization” (Buonomano and Merzenich, 1998). A model incorporating postsynaptic normalization can explain the pattern of synaptic alterations we observed during the cellular analog of differential classical conditioning. According to this model, conditioning is a zero-sum situation: a large increase in the strength of the synaptic connection made by the sensory neuron that received paired training (CS+ sensory neuron) is compensated for by decreases in some (or possibly all) of the other sensory neurons that synapse with the motor neuron. The algebraic sum of all of these synaptic decreases is approximately equal to the inverse of the training-induced increase in strength of the synapse made by the CS+ sensory neuron. Further experimental work will be required to test the validity of such a model for classical conditioning in Aplysia.
Various cellular mechanisms contributing to classical conditioning in Aplysia
The present data, together with those of our earlier study (Murphy and Glanzman, 1997), suggest that classical conditioning of the siphon-withdrawal reflex comprises at least three different cellular mechanisms. First, there is a sensitization-related, nonassociative component (Carew et al., 1971). This component results, in part, from presynaptic facilitation of sensorimotor synapses (Byrne and Kandel, 1996) attributable to 5-HT released by serotonergic interneurons activated by tail shock (Glanzman et al., 1989; Mackey et al., 1989). The approximate time course of the nonassociative enhancement under the conditions of our experiments is indicated by the CS− data in the nondifferential conditioning experiments (Fig. 6A; Murphy and Glanzman, 1997). The nonassociative component is maximal shortly after training but is still present at 1 hr after training, as indicated by the significant difference in the nondifferential data for the 60 min post-test between the amplitude of the (normalized) CS− EPSP and the amplitude of the EPSP in preparations that received only test stimuli (test-alone preparations; see Murphy and Glanzman, 1997, their Fig. 2D). The second cellular component is a hypothesized competitive mechanism that operates whenever different synapses on the same motor neuron receive different types of plasticity-inducing stimulation. We infer the presence of synaptic competition among sensory neurons from the difference between the nondifferential and differential data with respect to the CS− results at 15 min after training (Fig. 6; also compare the CS− results in Fig. 3B for the 15 min post-test with those of Murphy and Glanzman, 1997, their Fig. 2C). The outcome of the proposed synaptic competition appears to interact with Hebbian, NMDA receptor-dependent potentiation, because it is blocked by the NMDA receptor antagonist APV (Fig. 4;Schacher et al., 1997). Furthermore, the effects of the putative competition between the CS+ and CS− sensory neurons appear to have their onset relatively early, because they are apparent at 15 min after training in the differential conditioning experiments (Figs. 3, 6). The third component of conditioning is a purely associative process, which we believe to be Hebbian potentiation. As indicated by the lack of a significant difference between the CS+ and CS− data on the 15 min post-test in the nondifferential experiments (Fig. 6A, Murphy and Glanzman, 1997, their Fig. 2D), the associative component of conditioning appears to have a delayed onset. It is robust, however, by 60 min after training, as indicated by the significant difference between the CS+ and CS− data in the nondifferential experiments (Fig.6A).
We propose that the specific outcome of behavioral conditioning inAplysia is determined, at least in part, by a parametric interaction among the above three cellular components. For example, the significant difference between the CS+ and CS− groups on the 15 min post-test in the present experiments appears to be attributable predominately to the effect of synaptic competition between the CS+ and CS− sensory neurons. The difference between these two groups on the 60 min post-test, however, appears to be attributable to the associative component and, possibly, the competitive mechanism as well.
At present the nature of the associative cellular mechanism recruited by classical conditioning in Aplysia is controversial. Originally, this associative mechanism was thought to be exclusively presynaptic (Hawkins et al., 1983; Walters and Byrne, 1983; Carew et al., 1984; Abrams, 1985; Buonomano and Byrne, 1990). Given our data (Murphy and Glanzman, 1996, 1997; present study), together with the other data (Schacher et al., 1997; Bao et al., 1998), however, this idea is no longer tenable. One recent proposal is that conditioning-related associative plasticity of sensorimotor synapses is attributable to a combination of presynaptic and postsynaptic coincidence detectors (Bao et al., 1998; Lechner and Byrne, 1998). Alternatively, it is possible that detection of the coincident occurrence of the CS and US occurs postsynaptically, but that presynaptic mechanisms are critically involved in the persistent expression of the synaptic change. Further experiments will be required to delineate the relative roles of presynaptic and postsynaptic mechanisms in the associative strengthening of sensorimotor synapses during classical conditioning in Aplysia.
Footnotes
This work was supported by National Institutes of Health Grant NS29563 and National Science Foundation Grant IBN-9410579 to D.L.G., as well as National Institute of Mental Health Grant F31-MH11136 to G.G.M. We thank Drs. M. Barish, D. Buonomano, M. Fanselow, and W. Wright for helpful comments.
Correspondence should be addressed to Dr. David L. Glanzman, Departments of Physiological Science and Neurobiology, 695 Young Drive South, Room 2506C, Box 951761, University of California, Los Angeles, CA 90095-1761. E-mail: dglanzman@physci.ucla.edu.
Dr. Murphy's present address: Department of Neurobiology, School of Medicine, University of California, Los Angeles, CA 90095-1761.
REFERENCES
- 1.Abrams TW. Activity-dependent presynaptic facilitation: an associative mechanism in Aplysia. Cell Mol Neurobiol. 1985;5:123–145. doi: 10.1007/BF00711089. [DOI] [PubMed] [Google Scholar]
- 2.Bao JX, Kandel ER, Hawkins RD. Involvement of presynaptic and postsynaptic mechanisms in a cellular analog of classical conditioning at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci. 1998;18:458–466. doi: 10.1523/JNEUROSCI.18-01-00458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buonomano DV, Byrne JH. Long-term synaptic changes produced by a cellular analog of classical conditioning in Aplysia. Science. 1990;249:420–423. doi: 10.1126/science.2165631. [DOI] [PubMed] [Google Scholar]
- 4.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 5.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne J, Castellucci V, Kandel ER. Receptive fields and response properties of mechanoreceptor neurons innervating siphon skin and mantle shelf in Aplysia. J Neurophysiol. 1974;37:1041–1064. doi: 10.1152/jn.1974.37.5.1041. [DOI] [PubMed] [Google Scholar]
- 7.Carew TJ, Castellucci VF, Kandel ER. An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int J Neurosci. 1971;2:79–98. doi: 10.3109/00207457109146995. [DOI] [PubMed] [Google Scholar]
- 8.Carew TJ, Walters ET, Kandel ER. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981;1:1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carew TJ, Hawkins RD, Kandel ER. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983;219:397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- 10.Carew TJ, Hawkins RD, Abrams TW, Kandel ER. A test of Hebb's postulate at identified synapses which mediate classical conditioning in Aplysia. J Neurosci. 1984;4:1217–1224. doi: 10.1523/JNEUROSCI.04-05-01217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol (Lond) 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dale N, Kandel ER. l-Glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proc Natl Acad Sci USA. 1993;90:7163–7167. doi: 10.1073/pnas.90.15.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost WN, Kandel ER. Structure of the network mediating siphon-elicited siphon withdrawal in Aplysia. J Neurophysiol. 1995;73:2413–2427. doi: 10.1152/jn.1995.73.6.2413. [DOI] [PubMed] [Google Scholar]
- 14.Frost WN, Clark GA, Kandel ER. Parallel processing of short-term memory for sensitization in Aplysia. J Neurobiol. 1988;19:297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- 15.Glanzman DL. Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture. J Neurobiol. 1994;25:666–693. doi: 10.1002/neu.480250608. [DOI] [PubMed] [Google Scholar]
- 16.Glanzman DL. The cellular basis of classical conditioning in Aplysia californica—it's less simple than you think. Trends Neurosci. 1995;18:30–36. doi: 10.1016/0166-2236(95)93947-v. [DOI] [PubMed] [Google Scholar]
- 17.Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glanzman DL, Kandel ER, Schacher S. Target-dependent morphological segregation of Aplysia sensory outgrowth in vitro. Neuron. 1991;7:903–913. doi: 10.1016/0896-6273(91)90336-x. [DOI] [PubMed] [Google Scholar]
- 19. Hawkins RD. Learning and the sensorimotor synapse in Aplysia (in Technical Comments) Science 281 1998. 619a Full text at www.sciencemag.org/cgi/content/full/281/5377/619a. [Google Scholar]
- 20.Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983;219:400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- 21.Hickie C, Walters ET. Motor neuronal control of tail-directed and head-directed siphon responses in Aplysia californica. J Neurophysiol. 1995;74:307–321. doi: 10.1152/jn.1995.74.1.307. [DOI] [PubMed] [Google Scholar]
- 22.Lechner HA, Byrne JH. New perspectives on classical conditioning: a synthesis of Hebbian and non-Hebbian mechanisms. Neuron. 1998;20:355–358. doi: 10.1016/s0896-6273(00)80977-0. [DOI] [PubMed] [Google Scholar]
- 23.Lin XY, Glanzman DL. Long-term potentiation of Aplysia sensorimotor synapses in cell culture: regulation by postsynaptic voltage. Proc R Soc Lond B Biol Sci. 1994a;255:113–118. doi: 10.1098/rspb.1994.0016. [DOI] [PubMed] [Google Scholar]
- 24.Lin XY, Glanzman DL. Hebbian induction of long-term potentiation of Aplysia sensorimotor synapses: partial requirement for activation of an NMDA-related receptor. Proc R Soc Lond B Biol Sci. 1994b;255:215–221. doi: 10.1098/rspb.1994.0031. [DOI] [PubMed] [Google Scholar]
- 25.Lin XY, Glanzman DL. Effect of interstimulus interval on pairing-induced LTP of Aplysia sensorimotor synapses in cell culture. J Neurophysiol. 1997;77:667–674. doi: 10.1152/jn.1997.77.2.667. [DOI] [PubMed] [Google Scholar]
- 26.Mackey SL, Glanzman DL, Small SA, Dyke AM, Kandel ER, Hawkins RD. Tail shock produces inhibition as well as sensitization of the siphon-withdrawal reflex of Aplysia: possible behavioral role for presynaptic inhibition mediated by the peptide Phe-Met-Arg-Phe-NH2. Proc Natl Acad Sci USA. 1987;84:8730–8734. doi: 10.1073/pnas.84.23.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackey SL, Kandel ER, Hawkins RD. Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglia of Aplysia produce presynaptic facilitation of siphon sensory neurons. J Neurosci. 1989;9:4227–4235. doi: 10.1523/JNEUROSCI.09-12-04227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcus EA, Nolen TG, Rankin CH, Carew TJ. Behavioral dissociation of dishabituation, sensitization, and inhibition in Aplysia. Science. 1988;241:210–213. doi: 10.1126/science.3388032. [DOI] [PubMed] [Google Scholar]
- 29.Miller KD. Synaptic economics: competition and cooperation in synaptic plasticity. Neuron. 1996;17:371–374. doi: 10.1016/s0896-6273(00)80169-5. [DOI] [PubMed] [Google Scholar]
- 30.Murphy GG, Glanzman DL. Enhancement of sensorimotor connections by conditioning-related stimulation in Aplysia depends upon postsynaptic Ca2+. Proc Natl Acad Sci USA. 1996;93:9931–9936. doi: 10.1073/pnas.93.18.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy GG, Glanzman DL. Mediation of classical conditioning in Aplysia californica by LTP of sensorimotor synapses. Science. 1997;278:467–471. doi: 10.1126/science.278.5337.467. [DOI] [PubMed] [Google Scholar]
- 32. Murphy GG, Glanzman DL. Learning and the sensorimotor synapse in Aplysia (in Technical Comments). Science 281 1998. 619a Full text at www.sciencemag.org/cgi/content/full/281/5377/619a. [Google Scholar]
- 33.Schacher S, Wu F, Sun ZY. Pathway-specific synaptic plasticity: activity-dependent enhancement and suppression of long-term heterosynaptic facilitation at converging inputs on a single target. J Neurosci. 1997;17:597–606. doi: 10.1523/JNEUROSCI.17-02-00597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trudeau LE, Castellucci VF. Contribution of polysynaptic pathways in the mediation and plasticity of Aplysia gill and siphon withdrawal reflex: evidence for differential modulation. J Neurosci. 1992;12:3838–3848. doi: 10.1523/JNEUROSCI.12-10-03838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walters ET, Byrne JH. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983;219:405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- 36.Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimuli. J Neurophysiol. 1983;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- 37.Wright WG, Yong R, Glanzman DL. Synaptic competition at the Aplysia sensorimotor synapse: tetanic stimulation of one presynaptic input depresses a second presynaptic input. Soc Neurosci Abstr. 1999;25:1314. [Google Scholar]