Abstract
Neurons and glia of the cerebral cortex are thought to arise from a common, multipotent progenitor cell that is instructed toward alternate fates by extracellular cues. How do these cells behave when confronted with conflicting cues? We show here that nestin-positive neuroepithelial (NE) cells from embryonic day 14 rat cortex coexpress surface receptor proteins for ciliary neurotrophic factor (CNTF) and platelet-derived growth factor (PDGF). Both sets of these receptor proteins are functional in NE cells, as shown by ligand-dependent activation of downstream signal-generating proteins. Transient (30′) exposure to CNTF instructs NE cells toward an astrocyte fate. Brief exposure to PDGF initiates neuronal differentiation. However, when challenged with conflicting cues, PDGF is dominant to CNTF. Moreover, CNTF-treated NE cells can be “redirected” by a subsequent exposure to PDGF to form neurons instead of astrocytes, whereas the converse is not true. The asymmetric relationship between CNTF and PDGF indicates that these two growth factors act on a common progenitor cell that has, at a minimum, two fates available to it rather than separate populations of precommitted neuroblasts and astroblasts. This bipotent progenitor cell processes conflicting cues for neurons and glia in a hierarchical manner.
Keywords: neurons, astrocytes, CNTF, PDGF, cortical development, neuroepithelial cells, signal transduction
Histogenetic studies of the rat brain have contributed greatly to our current understanding of mammalian cerebral cortical development (Bayer and Altman, 1991). During embryonic days 13 and 14 (E13–E14) of a 21 d gestational cycle, neuroepithelial (NE) cells expressing the intermediate filament nestin (Hockfield and McKay, 1985) undergo cell division within the ventricular zone of the developing brain. At E16, the subventricular germinal zone develops, and postmitotic neurons start to appear in both the ventricular and subventricular areas. These neurons migrate along radial glia cells to their eventual destinations within the deeper cortical layers from E17 to E21. At E19, the mostly depleted ventricular zone stops producing neurons, whereas the subventricular zone continues to generate neurons and now also glial cells. The subventricular zone neurons migrate to form the superficial layers of cortex from E21 to postnatal day 3. From E21 on, the subventricular zone is thought to produce only glial cells.
The cell of origin for both neurons and glia is thought to be a common, multipotent progenitor cell. Transplantation studies show that the fate choices of this progenitor cell can be dictated by extracellular cues in the local environment (Brustle et al., 1998; Flax et al., 1998). These fate choices are obviously mutually exclusive. A fully differentiated cell can be a neuron, an astrocyte, or an oligodendrocyte, but not a combination of the two or three lineages. Given the unlikelihood that the extracellular signals controlling fate choice can be strictly compartmentalized in space and time within the developing embryo, a question arises. How do multipotent neural progenitor cells process conflicting cues?
This question can be addressed in vitro using undifferentiated, nestin-positive NE cells excised from the ventricular zone of E14 rats. These NE cell cultures give rise to neurons, astrocytes, or oligodendrocytes in response to specific growth factors. Platelet-derived growth factor (PDGF) for example initiates neuronal differentiation, whereas (under varying conditions of cell culture) bone morphogenic proteins, basic fibroblast growth factor (bFGF), or ciliary neurotrophic factor (CNTF) induce astrocyte development, and thyroid hormone stimulates oligodendrocyte development (Gross et al., 1996; Johe et al., 1996; Bonni et al., 1997; Williams et al., 1997). In principle, NE cell cultures can be composed of unipotent progenitor cells that each generate a single cell type, bipotent progenitor cells that generate restricted pairs of cell types, and/or multipotent progenitor cells that give rise to all three cell types. In the experiments summarized here, we show that NE cell cultures taken from the ventricular zone of E14 rats contain an abundant population of cells that are, at a minimum, developmentally bipotent. These bipotent NE cells process conflicting cues to form neurons and astrocytes in a hierarchical manner. The data provide a possible explanation for why neurons appear before astrocytes in the developing mammalian brain.
MATERIALS AND METHODS
Cell cultures. The cerebral cortices of Wistar Furth (Harlan, Madison, WI) rat E14 embryos were dissected in serum-free medium (DMEM supplemented with glucose, transferrin, insulin, selenium, progesterone, thyroxine, tri-iodothyronine, putrescine, and bovine serum albumin) (Williams et al., 1991; Williams and Price, 1995) and dissociated into a single cell suspension by trituration through 21 and 23 gauge hypodermic needles. The cells were centrifuged and resuspended in serum-free medium before plating on poly-d-lysine-coated 12 mm glass coverslips (Fisher Scientific, Pittsburgh, PA) placed in 24-well tissue plates (Falcon, Lincoln Park, NJ). The initial plating densities were 2 × 105 cells per coverslip in a volume of 50 μl. After allowing 30 min for the cells to settle and attach to the coverslips, 0.45 ml of additional serum-free medium was added to each well. For clonal analysis experiments, cultures were infected at this time with the BAG retroviral vector that encodes the enzyme β-galactosidase (β-gal) (Price et al., 1987). The following day, cultures were stimulated with CNTF (Upstate Biotechnologies, Lake Placid, NY), PDGF (Upstate Biotechnologies), or solvent controls as indicated, washed three times with serum-free medium, and maintained in serum-free medium. For experiments in which the cells were stimulated simultaneously, CNTF (30 ng/ml) and PDGF B-B (The homedimeric B-B isoform of PDGF) (30 ng/ml) were present for 4 hr and then washed out three times with serum-free medium. In the sequential stimulation experiments, cultures were exposed to the first growth factor for 4 hr, washed three times with serum-free medium, exposed to the second growth factor for 4 hr, again washed three times with serum-free medium, and then maintained in serum-free medium. Thereafter, all cultures were fed every 2 d with serum-free medium, and cell staining and immunoblotting analyses were performed at the indicated times. In some experiments, NE cell cultures were first exposed to 15 μg/ml 5,6-dichloro-β-d-ribofuranosyl-benzimidazole (DRB) (Sigma, St. Louis, MO), a reversible inhibitor of RNA synthesis alone for 1 hr before stimulation with CNTF (30 ng/ml) or control solvent for 4 hr in the continued presence of DRB. The DRB with or without CNTF was removed by washing three times with serum-free medium after the 4 hr growth factor stimulation period.
Immunofluorescence. To determine the presence of growth factor receptors on NE cells, cells plated on glass coverslips as described above were fixed with 4% paraformaldehyde and incubated with primary antibodies (Abs) against nestin (Developmental Studies Hybridoma Bank, Iowa City, IA) (Hockfield and McKay, 1985), the CNTF receptor (CNTF-R) (Santa Cruz Biotechnology, Santa Cruz, CA), and the PDGF receptor (PDGF-R) (Santa Cruz Biotechnology). After washing, cells were incubated with species and isotype-specific aminomethylcoumarin acetate (AMCA)-, indocarbocyanine (Cy3)-, or cyanine (Cy2)-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) and viewed using a Nikon (Tokyo, Japan) Optophot-2 microscope. Control coverslips were also fixed with 4% paraformaldehyde but incubated only with the species and isotype-specific Cy3- or Cy2-conjugated secondary antibodies. When viewed using a Nikon Optophot-2 microscope, there was no cell-associated fluorescence. To demonstrate the signaling ability of these growth factor receptors, NE cells were stimulated for 10 min with either CNTF or PDGF before fixation with 4% paraformaldehyde. They were then stained with primary antibodies against nestin and either phosphorylated signal transducer and activator of transcription (STAT 3) (gift of D. Frank, Dana-Farber Cancer Institute, Boston, MA) or phosphorylated mitogen-activated protein (MAP) kinase (Promega, Madison, WI), respectively. After washing, cells were incubated with the secondary antibodies noted above. Determination of cell phenotypes was performed as described previously (Williams et al., 1991; Williams and Price, 1995). Neurons were identified using the monoclonal antibody (mAb) TuJ1 (Geisert and Frankfurter, 1989) and confirmed by staining with the monoclonal antibodies anti-neurofilament 68, 160, or 200 kDa (Sigma), MAP2 (Sigma), or anti-neuron synaptosomal-associated protein (SNAP-25) (specific for a 25 kDa neuron synaptosomal-associated protein) (Transduction Laboratories, Lexington, KY). Astrocytes were identified using anti-GFAP monoclonal antibodies (Sigma) (Bignami et al., 1972), and oligodendrocytes were identified using the O4 antibody (Boehringer Mannheim, Indianapolis, IN) (Sommer and Schachner, 1981). NE cells were identified as cells that stained with an anti-nestin antibody but not with antibodies that recognize differentiated cell types. Determination of clones was performed using rabbit anti-β-galactosidase serum (Williams et al., 1991), and NE clones are defined as clones in which all lacZ-positive cells are also positive for nestin and negative for all other markers. Likewise, astrocyte and neuronal clones are defined as clones in which all lacZ-positive cells are also positive for GFAP or TuJ1, respectively, and negative for all other markers. In some experiments, total cells were scored rather than β-gal-positive clones. When scoring total cells, the percentage of GFAP-positive astrocytes or TuJ1-positive neurons was determined by counting five fields per coverslip under a fluorescent microscope using a 63× objective. For both clonal analysis and total cell analysis, the significance of the increase in astrocytes or neurons observed was calculated using the Bonferroni–Dunn procedure.
Immunoblots. E14 cell cultures were stimulated with CNTF and lysed at the specified time intervals with NP-40. For determination of GFAP expression, lysates were size fractionated on an SDS-polyacrylamide gel, transferred to Immobilon-P membranes (Millipore, Bedford, MA), and immunoblotted with anti-GFAP monoclonal antibodies. For determination of signal transduction molecule activation, lysates were immune precipitated with an anti-phosphotyrosine mAb (4G10) (Upstate Biotechnologies), size fractionated on an SDS-polyacrylamide gel, transferred to Immobilon-P membranes and immunoblotted with anti-gp130 (Upstate Biotechnologies), anti-STAT 1 (gift of D. Frank), or anti-STAT 3 Abs. All antibody signals were visualized with ECL (Amersham, Arlington Heights, IL) and/or quantitated with Attophos (JBL Scientific, Inc., San Luis Obispo, CA) using a fluorimager apparatus (Molecular Dynamics, Sunnyvale, CA). In some experiments, NE cell cultures were also stimulated with CNTF in the absence or presence of DRB and similarly processed as described.
RESULTS
Coordinate expression of functional receptors for CNTF and PDGF in cultured NE cells
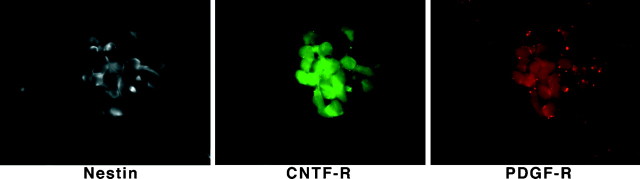
Cells isolated from the developing cerebral cortices of E14 rats were plated and cultured in serum-free medium overnight. Immunofluorescence staining (Fig. 1) shows that >75% of the total cells in these cultures express the intermediate filament nestin but are negative for markers found on differentiated cell types and are thus identifiable as NE cells. More than 90% of the NE cells express surface receptors for both CNTF and PDGF (Fig. 1). These CNTF and PDGF receptors are uniformly ligand-responsive and functional. This can be shown by exposing the cells to CNTF or PDGF and monitoring the activation of downstream signal-generating proteins with phosphopeptide-directed antibodies. CNTF is thought to induce cellular responses primarily (though not exclusively) through activation of JAK–STAT signal transduction pathways (Bonni et al., 1997). Conversely, PDGF signals primarily (though again, not exclusively) via the Ras–Raf–MAP kinase pathway (Schlessinger and Ullrich, 1992). Accordingly, we stimulated NE cells with either CNTF or PDGF for 10 min and then conducted immunofluorescent staining with antibodies directed against the phosphorylated, activated forms of either STAT 3 or MAP kinase, respectively. As shown in Figure 2, both CNTF and PDGF activate downstream signaling proteins in the majority of NE cells. Collectively, the images in Figures 1 and 2 show that functional receptors for CNTF and PDGF are coordinately expressed in cultured NE cells.
Fig. 1.
Neuroepithelial cells express both CNTF and PDGF receptors. One day after plating on glass coverslips, E14 cultures were fixed with 4% paraformaldehyde and then immunostained with antibodies to nestin, CNTF receptor, or PDGF receptor. Secondary antibodies conjugated to AMCA, Cy2, and Cy3, respectively, were used to visualize the target molecules.
Fig. 2.
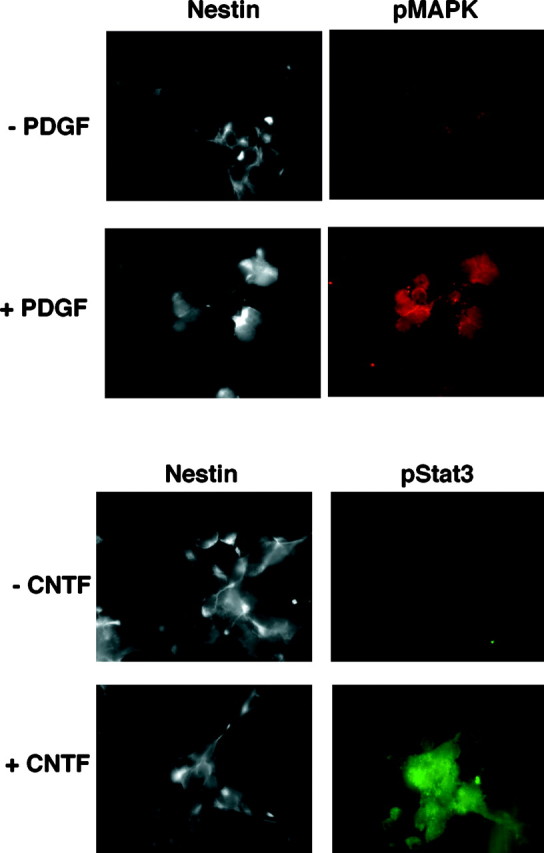
CNTF-R and PDGF-R expressed on NE cells are functional. E14 cells plated on glass coverslips were stimulated with CNTF (30 ng/ml) or PDGF (30 ng/ml) and stained with antibodies directed against nestin and either activated STAT-3 or MAP kinase, respectively. Secondary antibodies conjugated to AMCA, Cy2, and Cy3, respectively, were used to visualize the target molecules.
Transient activation of CNTF receptors initiates astrocyte differentiation in NE cell cultures
In previous studies, we reported that even transient (30′) exposures to PDGF can induce neuronal differentiation in E14 NE cell cultures. Although outward signs of neuronal development do not appear until several days after PDGF treatment, the sustained developmental response does not reflect persistently activated PDGF receptors. This is demonstrated by the ability to uncouple receptor activation from neuronal development by a reversible inhibitor of RNA (Williams et al., 1997). These central features of the response to PDGF are seen also in the astrocytic differentiation of NE cells in response to CNTF. In accordance with previous studies using bFGF-expanded NE cell cultures (Johe et al., 1996), nanomolar concentrations of CNTF can promote astrocyte differentiation in primary, nonexpanded cell populations as well (Fig. 3). Outward signs of astrocyte formation are not detectable until 3–4 d after exposure to CNTF (Fig.4), but CNTF stimulation periods as brief as 30 min are sufficient to initiate this response (Fig.5).
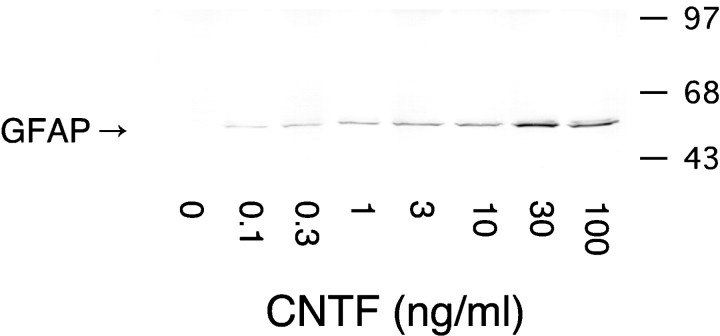
Fig. 3.
CNTF induces the expression of GFAP. A dose–response curve for astrocyte formation was established by exposing NE cultures to increasing concentrations of CNTF for 4 hr. Expression of GFAP in cell cultures was assessed by immunoblotting 5 d later using an anti-GFAP monoclonal antibody.
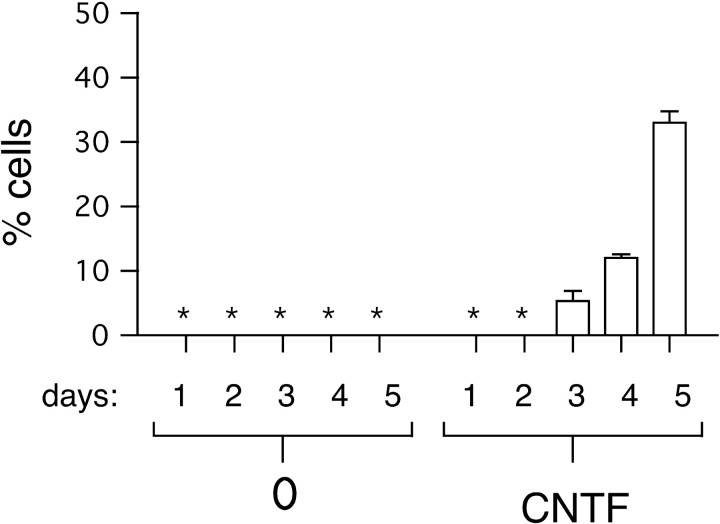
Fig. 4.
CNTF induces the differentiation of astrocytes. A time course for the developmental response to CNTF was determined by exposing NE cells to CNTF (30 ng/ml) for 4 hr. On the following 5 consecutive days, coverslips were stained with an anti-GFAP mAb, and the percentages of GFAP-positive cells were determined. * denotes 0.
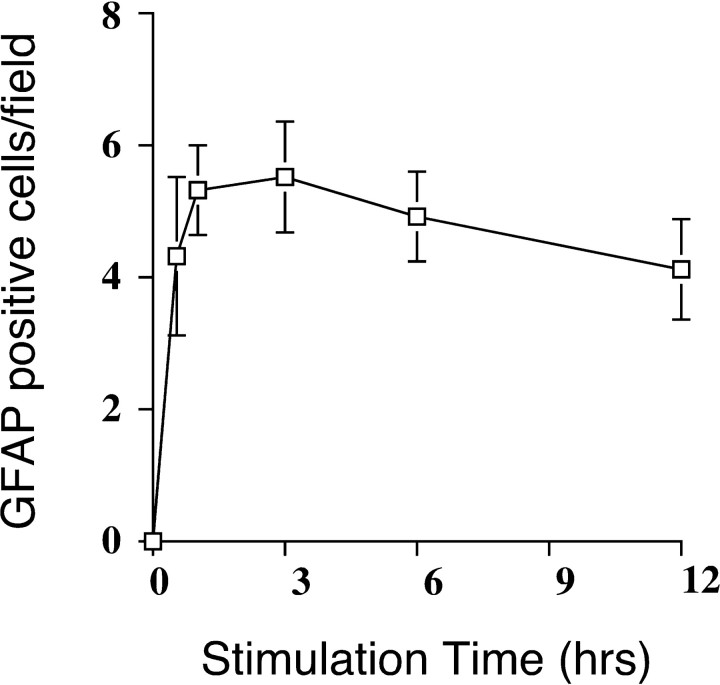
Fig. 5.
Transient exposures of CNTF are sufficient to induce astrocyte differentiation. The minimum time interval needed for NE cells to become instructed by CNTF to form astrocytes was determined by exposing cultures to CNTF (30 ng/ml) for increasing amounts of time. At the indicated times, CNTF was removed, and cultures were washed with serum-free medium. GFAP expression was assessed after 5 d of culture in serum-free medium. The number of GFAP-positive cells per field under a 63× objective as defined by immunostaining is shown.
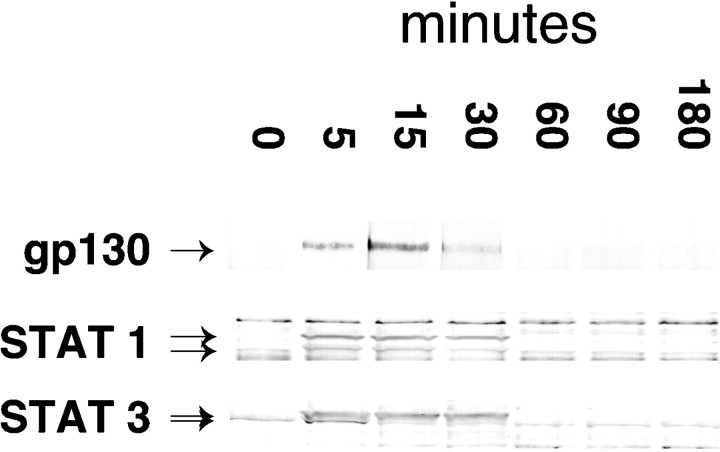
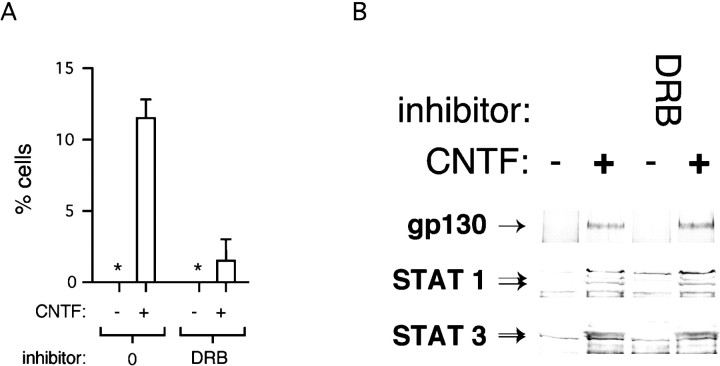
As with PDGF, the long-term developmental response to a brief pulse of CNTF cannot reflect persistently activated downstream signaling molecules. Activation of the CNTF receptor subunit gp130 and downstream signal generating proteins STAT 1 and STAT 3 is relatively transient, even in the continued presence of CNTF (Fig.6). Moreover, receptor activation and signal transduction can be uncoupled from the developmental response by a reversible inhibitor of RNA synthesis, DRB. Cells treated with DRB before, and during the time of, CNTF stimulation fail to develop into astrocytes, even when cultures are maintained for up to 2 weeks (Fig.7A). Immunoblot studies show that DRB has no effect on the activation of gp130, STAT 1, and STAT 3 by CNTF (Fig. 7B), nor does DRB act as an irreversible poison of astrocyte formation. Cells treated with DRB, but washed before subsequent stimulation with CNTF alone, develop into astrocytes as expected (data not shown). This is in keeping with previously published results on the effects of DRB. When used at a concentration of 15 μg/ml as in these experiments, DRB inhibits total RNA synthesis by 66%. Total reversal of its effects occurs within 15 min of its withdrawal (Smith and Stiles, 1981). The simplest interpretation of the data in Figures 3-7 is that short exposures to CNTF commit NE cells to differentiate into astrocytes as a result of downstream signaling events that require new RNA synthesis.
Fig. 6.
CNTF activates gp130 and STATs in NE cells. NP-40 lysates of CNTF (30 ng/ml) stimulated NE cell cultures were made at the times indicated. The lysates were then immune precipitated with an anti-phosphotyrosine mAb (4G10), size fractionated on an SDS-polyacrylamide gel, and immunoblotted with anti-gp130, anti-STAT 1, and anti-STAT 3 mAbs.
Fig. 7.
Differentiation of astrocytes is transcription-dependent. A, NE cell cultures were stimulated with CNTF (30 ng/ml) for 30 min in the absence or presence of DRB, a reversible inhibitor of RNA synthesis, as described previously (Williams et al., 1997). After 4 d incubation in factor-free medium, cells were assessed for GFAP expression using mAb. The percentage of GFAP-positive cells per culture is shown. * denotes 0. B, As controls for the experiment inA, NE cell cultures were stimulated with CNTF (30 ng/ml) for 15 min in the absence or presence of DRB. Cell lysates were then processed and immunoblotted with anti-gp130, anti-STAT 1, and anti-STAT 3 mAbs.
The PDGF cue for neuronal differentiation is dominant to the CNTF cue for astrocyte differentiation
As shown in Figures 1 and 2, CNTF and PDGF receptors are coordinately expressed and functional on virtually all NE cells. We therefore examined the effects of simultaneous and sequential growth factor stimulation on single NE cells using a retroviral vector that encodes the enzyme β-gal. Infection of cells with a limiting titer of retroviral particles allows for the subsequent identification of cell populations derived from an individual clone. As shown in Table1 (columns 1–6), the total number of clones in control, or growth factor-treated, cultures is essentially constant. Thus the factors do not affect the viability of the cells in our clonal analysis. The results of exposure to CNTF or PDGF singly are shown in Table 1 (columns 2 and 3). Relative to untreated cultures (column 1), transient exposure to CNTF converts ∼70% of the NE clones into colonies that contain astrocytes (p= 0.001). Transient exposure to PDGF converts ∼80% of the NE clones into colonies that contain neurons (p = 0.005).
Table 1.
Average ± SEM number of clones per coverslip
| Clone type | Control | PDGF | CNTF | CNTF + PDGF | CNTF → PDGF | PDGF → CNTF |
|---|---|---|---|---|---|---|
| Neuron | 2.9 ± 0.9 | 6.6 ± 1.7 | 2.7 ± 0.2 | 4.0 ± 0.0 | 5.1 ± 0.8 | 5.4 ± 1.3 |
| Astrocyte | 0 | 0.1 ± 0.1 | 2.6 ± 0.4 | 1.1 ± 0.7 | 0.3 ± 0.3 | 0.3 ± 0.4 |
| NE | 5.0 ± 2.0 | 0.8 ± 0.6 | 1.4 ± 0.3 | 0.6 ± 0.1 | 0.5 ± 0.3 | 0.4 ± 0.4 |
| Mixed | 0 | 0.4 ± 0.4 | 1.9 ± 0.8 | 1.1 ± 0.7 | 1.0 ± 0.8 | 0.5 ± 10 |
| Total β-gal+ clones per coverslip | 7.9 ± 1.1 | 7.9 ± 1.9 | 8.6 ± 0.5 | 6.8 ± 0.1 | 6.9 ± 1.5 | 6.6 ± 2.0 |
Clonal analysis of CNTF and PDGF stimulated cells. Single NE cells respond asymmetrically to CNTF and PDGF. E14 NE cultures were infected with the β-gal retrovirus as described previously (Williams et al., 1997) and then treated with PDGF BB, CNTF, CNTF and PDGF simultaneously, CNTF followed by PDGF, or PDGF followed by CNTF. In all cases (single, simultaneous, or sequential treatment), growth factors were used at a final concentration of 30 ng/ml for 4 hr. After growth factor(s) treatment, cultures were washed and grown in factor-free media for an additional 5 d. The composition of β-gal-positive clones was then analyzed. Neurons were identified using anti-TuJ1, astrocytes were identified using anti-GFAP, and NE cells were identified as cells labeling with anti-nestin antibody but not with antibodies that recognize differentiated cell types (Williams et al., 1997). Mixed clones refer to clones composed of astrocytes, neurons, and NE cells. Results shown are the mean ± SEM of three separate experiments. Relative to CNTF alone, the decrease in astrocyte clones caused by PDGF added at the same time (CNTF + PDGF), after (CNTF → PDGF), or before (PDGF → CNTF) exposure to CNTF is significant (p = 0.01, 0.05, and 0.005, respectively). Conversely, the increase in neuronal clones under the same conditions is significant (p = 0.01, 0.001, and 0.05, respectively).
Superficially, the data in columns 1–3 would indicate that CNTF and PDGF act on a common progenitor cell that is, at a minimum, developmentally bipotent. The alternative possibility, that CNTF and PDGF induce precommitted astroblasts and neuroblasts to complete their developmental programs, is arithmetically untenable because the overlap of these two cell types would account for greater than 100% of the original starting population. At a minimum, between 20 and 30% of the NE cells would have to be developmentally bipotent to generate the data in columns 1–3 of Table 1. A less likely but conceptually feasible explanation of these results is that CNTF activates precommitted astroblasts and kills precommitted neuroblasts, whereas PDGF does the opposite. To evaluate this possibility, we exposed cells simultaneously and also sequentially to the two growth factors (Table 1, columns 4–6). When cells are exposed simultaneously to CNTF and PDGF for time periods sufficient to commit them to either astrocytes or neurons, mostly neurons are formed. Additionally, cells that have been exposed initially to CNTF can be redirected to form neurons instead of astrocytes if they are exposed subsequently to PDGF. The converse does not hold. The asymmetry of the response to CNTF and PDGF indicates that the clonal analysis data in columns 1–3 of Table 1 cannot reflect offsetting developmental and cytotoxic responses for two different kinds of cells. Assuming that cells infected by the β-gal retrovirus are representative of the population at large, the competition and order-of-addition experiments indicate that at least 20–30%, and likely more, of the NE cells have alternate fates available to them that can be initiated by CNTF or PDGF. Oligodendrocytes as determined by O4 and galC immunostaining are infrequently present in these cultures (data not shown).
DISCUSSION
Intrinsic genetic programs and extracellular growth factors are both necessary for the development of the cerebral cortex. We and others have used culture systems in which embryonic ventricular zone cells are harvested and grown in chemically defined, serum-free media. Through manipulation of both the concentration and timing of growth factor addition and withdrawal, the role of extracellular signals in cortical development can be studied (Gage et al., 1995).
Although the culture systems used by various authors have differed with regard to the species, anatomic location, and developmental age of the harvested tissue, a common theme has been the addition of either bFGF or epidermal growth factor (EGF) to the growth media. These growth factors function as initial mitogenic agents to expand the population of primary cortical progenitor cells (Gensburger et al., 1987; Cattaneo and McKay, 1990; Reynolds et al., 1992; Kilpatrick and Bartlett, 1993;Ghosh and Greenberg, 1995; Gross et al., 1996). The additional effects of bFGF and its interaction with other growth factors have however varied across, and sometimes even within, the studies published to date (Kilpatrick and Bartlett, 1993; Baird, 1994; Temple and Qian, 1995;Vicario-Abejon et al., 1995). EGF has likewise been shown to have pleiotropic effects on developing cells (Ferri and Levitt, 1995; Ferri et al., 1996; Kilpatrick and Bartlett, 1993, 1995). The cultures described here were explicitly propagated in the absence of bFGF and EGF to avoid their potential confounding effects on the two growth factors of interest, CNTF and PDGF. In doing so, we also obtained a starting cell population representative of that found in vivo rather than one enriched for a mitotically active subpopulation of cells.
Examination of the NE cells with monoclonal antibodies reveals that they all express receptors for PDGF as well as CNTF. To determine whether the receptors are functional, we stimulated cells with either PDGF or CNTF. PDGF stimulation leads to the activation of MAP kinase within the NE cells. CNTF stimulation is not as effective in activating MAP kinase (data not shown) but does induce the rapid and short-lived activation of the signal transduction molecules gp130 and STAT 1 and STAT 3. In contrast, previous studies by Rajan and McKay (1998) have shown that CNTF treatment leads to the prolonged activation of the JAK–STAT pathway (8 d). A possible explanation for the discrepancy is the difference between the two culture systems in use. Although both systems begin with E14 cells, the cells used by Rajan and McKay are first expanded and passaged in bFGF-containing media. The potential modulating role of bFGF in JAK–STAT signaling was not addressed in their paper. The cells in our study are stimulated in the absence of bFGF and on the day after plating.
Through a transcription-dependent mechanism, a brief pulse of CNTF induces the differentiation of NE cells into astrocytes. PDGF stimulation, also through a transcription-dependent mechanism, leads to the induction of neuronal differentiation. When CNTF and PDGF are presented to NE cells simultaneously or in succession, PDGF is dominant to CNTF and can override its effects as well. The target NE cells therefore appear to be, at a minimum, bipotent. Another finding of the clonal analysis experiments is that, although a vast majority of the NE cells are undifferentiated at the time of harvesting (as defined by their expression of nestin), not all of them are necessarily uncommitted. In fact, approximately one-third of the cells appear to be committed to a neuronal fate in the absence of in vitro PDGF stimulation, reflecting perhaps their previous in vivoexposure to PDGF (Williams et al., 1997). The apparent heterogeneity of NE cells, even within the ventricular zone itself, may explain why subtle differences in timing of tissue harvest and culture conditions have led to disparate results by various research groups.
One concern regarding results obtained using relatively specificin vitro conditions is their relevance to in vivoprocesses. The experiments described here demonstrate that PDGF and CNTF can act as growth factors to induce the development of neurons and astrocytes, respectively. This in itself has now been shown by ourselves and others in three different culture systems (Johe et al., 1996; Bonni et al., 1997; Williams et al., 1997), supporting its congruity to in vivo events. An obvious next question is how does the developing cortex reconcile these conflicting cues and form first neurons then glia? Qian et al. (1997) have hypothesized that the default fate of NE cells is neuronal, and the development of glia requires exposure to additional signals. Our results do not support this hypothesis because the formation of a significant number of neurons requires the presence of PDGF, and PDGF itself can override the CNTF-driven development of astrocytes. The readily apparent explanations for the differing results are the use of rat versus murine cells and the exclusion of bFGF from our cultures for the reasons given above. Burrows et al. (1997) have also addressed this question and report that the timing of progenitor cell maturation is regulated by developmental changes in EGF receptor (EGFR) expression levels. Ventricular zone cells initially express low levels of EGFR and respond to EGF by differentiating into neurons. During later stages of development, cells (strictly speaking, now part of the subventricular zone) divide and differentiate into astrocytes in response to EGF (Burrows et al., 1997). Because our cultures do not contain any EGF, it is difficult to compare and contrast our results with theirs. A conclusion that may however be drawn is that there are likely multiple routes to astrocyte differentiation within the CNS (Rajan and McKay, 1998).
The competing and dominant effects of PDGF over CNTF on determining cell fate suggest an alternative explanation for the temporal sequence of brain development in vivo. The intrinsic developmental sequence may instead be hardwired into multipotent neural progenitors at the level of competing growth factor signaling pathways. Induction of astrocyte formation by CNTF is channeled primarily through the JAK–STAT signaling pathway (Bonni et al., 1997), whereas pathways responsible for neuron formation in PDGF-treated cultures have yet to be defined. In general, however, cellular responses to receptor tyrosine kinases such as PDGF draw more heavily on Ras-dependent signaling pathways (Schlessinger and Ullrich, 1992). It is possible that, during development in vivo, STAT-dependent cues for astrocyte formation cannot be acted on until Ras-dependent signals for neuron formation have been removed. Consistent with this, developmental studies of the rat brain have shown decreases in subventricular zone PDGF expression after E19 (Sasahara et al., 1992), the time at which a significant number of cortical astrocytes begin to appear. Ongoing studies are directed at better defining the signal transduction mechanisms regulating the orderly development of the cerebral cortex.
Footnotes
This work was supported by National Institutes of Health Grant PO1 HD24826–10. J.K.P. was supported by a fellowship from the American Brain Tumor Association. B.P.W. received support from the University of London, Central Research Fund, University College London Graduate School, and the Royal Society. We gratefully acknowledge Stephan Muhlebach for technical assistance and Dr. David Frank for the gift of phosphorylation state-specific antibodies to STAT 1 and STAT 3. In compliance with Harvard Medical School Guidelines on possible conflict of interest, we disclose that C.D.S. has a consulting relationship with Upstate Biotechnology and Novartis Pharmaceuticals, Inc.
Drs. Park and Williams contributed equally to this work.
Correspondence should be addressed to Charles D. Stiles, Dana-Farber Cancer Institute, 44 Binney Street, Boston, MA 02115. E-mail:charles_stiles@dfci.harvard.edu.
REFERENCES
- 1.Baird A. Fibroblast growth factors: activities and significance of non-neurotrophin neurotrophic growth factors. Curr Opin Neurobiol. 1994;4:78–86. doi: 10.1016/0959-4388(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 2.Bayer SA, Altman J. Neocortical development. Raven; New York: 1991. [Google Scholar]
- 3.Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 4.Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg MA. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 5.Brustle O, Choudhary K, Karram K, Huttner A, Murray K, Dubois-Dalcq M, McKay RDG. Chimeric brains generated by intraventricular transplantation of fetal human brain cells into embryonic rats. Nat Biotechnol. 1998;16:1040–1044. doi: 10.1038/3481. [DOI] [PubMed] [Google Scholar]
- 6.Burrows RC, Wancio D, Levitt P, Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- 7.Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- 8.Ferri RT, Levitt P. Regulation of regional differences in the differentiation of cerebral cortical neurons by EGF family-matrix interactions. Development. 1995;121:1151–1160. doi: 10.1242/dev.121.4.1151. [DOI] [PubMed] [Google Scholar]
- 9.Ferri RT, Eagleson KL, Levitt P. Environmental signals influence expression of a cortical areal phenotype in vitro independent of effects on progenitor cell proliferation. Dev Biol. 1996;175:184–190. doi: 10.1006/dbio.1996.0106. [DOI] [PubMed] [Google Scholar]
- 10.Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 11.Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 12.Geisert EE, Jr, Frankfurter A. The neuronal response to injury as visualized by immunostaining of class III beta-tubulin in the rat. Neurosci Lett. 1989;102:137–141. doi: 10.1016/0304-3940(89)90068-2. [DOI] [PubMed] [Google Scholar]
- 13.Gensburger C, Labourdette G, Sensenbrenner M. Brain basic fibroblast growth factor stimulates the proliferation of rat neuronal precursor cells in vitro. FEBS Lett. 1987;217:1–5. doi: 10.1016/0014-5793(87)81230-9. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh A, Greenberg ME. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 15.Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 16.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 18.Kilpatrick TJ, Bartlett PF. Cloning and growth of multipotential neural precursors: requirements for proliferation and differentiation. Neuron. 1993;10:255–265. doi: 10.1016/0896-6273(93)90316-j. [DOI] [PubMed] [Google Scholar]
- 19.Kilpatrick TJ, Bartlett PF. Cloned multipotential precursors from the mouse cerebrum require FGF-2, whereas glial restricted precursors are stimulated with either FGF-2 or EGF. J Neurosci. 1995;15:3653–3661. doi: 10.1523/JNEUROSCI.15-05-03653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price J, Turner D, Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci USA. 1987;84:156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian X, Davis AA, Goderie SK, Temple S. FGF2 concentration regulates the generation of neurons and glia from multipotent cortical stem cells. Neuron. 1997;18:81–93. doi: 10.1016/s0896-6273(01)80048-9. [DOI] [PubMed] [Google Scholar]
- 22.Rajan P, McKay RDG. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18:3620–3629. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasahara A, Kott JN, Sasahara M, Raines EW, Ross R, Westrum LE. Platelet-derived growth factor B-chain-like immunoreactivity in the developing and adult rat brain. Dev Brain Res. 1992;68:41–53. doi: 10.1016/0165-3806(92)90246-s. [DOI] [PubMed] [Google Scholar]
- 25.Schlessinger J, Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- 26.Smith JC, Stiles CD. Cytoplasmic transfer of the mitogenic response to platelet-derived growth factor. Proc Natl Acad Sci USA. 1981;78:4363–4367. doi: 10.1073/pnas.78.7.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- 28.Temple S, Qian X. bFGF, neurotrophins, and the control or cortical neurogenesis. Neuron. 1995;15:249–252. doi: 10.1016/0896-6273(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 29.Vicario-Abejon C, Johe KK, Hazel TG, Collazo D, McKay RD. Functions of basic fibroblast growth factor and neurotrophins in the differentiation of hippocampal neurons. Neuron. 1995;15:105–114. doi: 10.1016/0896-6273(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 30.Williams BP, Price J. Evidence for multiple precursor cell types in the embryonic rat cerebral cortex. Neuron. 1995;14:1181–1188. doi: 10.1016/0896-6273(95)90265-1. [DOI] [PubMed] [Google Scholar]
- 31.Williams BP, Read J, Price J. The generation of neurons and oligodendrocytes from a common precursor cell. Neuron. 1991;7:685–693. doi: 10.1016/0896-6273(91)90381-9. [DOI] [PubMed] [Google Scholar]
- 32.Williams BP, Park JK, Alberta JA, Muhlebach SG, Hwang GY, Roberts TM, Stiles CD. A PDGF-regulated immediate early gene response initiates neuronal differentiation in ventricular zone progenitor cells. Neuron. 1997;18:553–562. doi: 10.1016/s0896-6273(00)80297-4. [DOI] [PubMed] [Google Scholar]