Abstract
Neuropeptide Y receptors belong to the G-protein-coupled receptor superfamily and mediate a wide variety of physiological functions, including blood pressure regulation, hormone release, appetite control, seizure propensity, cognition, and emotion. The recent description of a new neuropeptide Y receptor, Y5, expressed in hypothalamic nuclei in rat brain, raised the possibility that Y5 was the receptor mediating the feeding and appetite-related functions of neuropeptide Y. This was supported by subsequent data showing a downregulation of this “feeding” receptor in the brain of the obese Zucker rat (Widdowson, 1997). We have performed a detailed analysis of Y5 expression in rat brain using in situ hybridization histochemistry with digoxygenin-labeled riboprobes and compared this to expression of Y5 in human brain regions. mRNA for the human Y5 receptor was highly expressed in human hypothalamic and thalamic nuclei. In particular, the arcuate and paraventricular nuclei of the hypothalamus, midline thalamic nuclei, and amygdala showed very high levels of expression with high levels in hippocampus. The striking conservation of expression of the rat and human Y5 receptors in relevant hypothalamic and other nuclei implies sharing of a major neuroendocrine functional role by this receptor.
Keywords: NPY, Y5, in situ hybridization, appetite, hypothalamus, paraventricular, arcuate, thalamus
Neuropeptide Y (NPY) is the most abundant neuropeptide in the mammalian CNS (Heilig and Widerlöv, 1990) and mediates diverse physiological responses, including alterations in blood pressure, hormone release, induction of anxiolysis, enhancement of memory retention, and alterations in circadian rhythms (Wahlestedt and Reis, 1993; Heilig and Widerlöv, 1995; Wettstein et al., 1995). NPY also has a well established role in the regulation of appetite and feeding (Zimanyi et al., 1998), and evidence from the NPY-deficient mouse has implicated NPY with a major protective role in kainic acid-induced seizures (Baraban et al., 1997).
The richest source of NPYergic neurons is found in the arcuate nucleus of hypothalamus, which sends dense projections to the paraventricular nucleus (PVN) (Stanley et al., 1993). This pathway appears to be the crucial NPY system acting on feeding as intracerebral injection of NPY into PVN results in increased food intake (Levine and Morley, 1984;Stanley and Leibowitz, 1984) and, in rats, chronic administration of NPY produces a prolonged effect on food intake with resultant obesity (Zarjevski et al., 1993). Leptin administration reduces NPY mRNA in the arcuate and PVN (Schwartz et al., 1996; Wang et al., 1997), and fasting increases NPY secretion from PVN (Kalra et al., 1991). Thus, NPYergic arcuate-PVN neurons form part of a homeostatic loop regulating body fat mass in which leptin acts as a signal of energy excess or deficiency (Flier, 1998).
The NPY family of peptides mediates the specificity of the actions of NPY through a family of G-protein-coupled receptors (Dumont et al., 1993; Balasubramaniam, 1997; Blomqvist and Herzog, 1997). Five different subtypes, Y1, Y2, Y4, Y5, and y6, have been cloned from several species (Blomqvist and Herzog, 1997). Both binding studies and in situ hybridization histochemistry (ISH) have been used to examine receptor distribution. ISH has shown that several of the subtypes are relatively abundant in rat brain: Y1 (Mikkelsen and Larsen, 1992; Larsen et al., 1993b), Y2 (Gustafson et al., 1997), and the newest subtype, Y5 (Gerald et al., 1996). Gerald et al. (1996) found Y5 present at significant levels in the PVN and arcuate nucleus, thalamus, and amygdala; a distribution suggesting Y5 might mediate appetite regulation at a hypothalamic level. In contrast,Dumont et al. (1998), using autoradiographic binding techniques to deduce the distribution of the “Y5 receptor-like” subtype in rat brain, found little Y5 in hypothalamus. This discrepancy clearly highlights the need for high-resolution localization studies to delineate the relative functional contribution of Y5 and the other Y receptor subtypes in CNS.
The human Y5 gene has been cloned and characterized by a number of groups, including our own (Hu et al., 1996; Herzog et al., 1997;Borowsky et al., 1998). To delineate further the role of this receptor in mediating the function of NPY in CNS, particularly related to appetite regulation, we examined expression of Y5 by high-resolution, nonradioactive ISH in human brain and compared this to a detailed analysis of Y5 in rat brain.
MATERIALS AND METHODS
Preparation of riboprobes for in situhybridization histochemistry. The entire coding region of the human Y5 cDNA isolated from human hypothalamic cDNA libraries (Clontech, Palo Alto, CA) was subcloned into pcDNA3 vector (Invitrogen, San Diego, CA). A 1199 bp EcoRI–BglII cDNA fragment (coding region 3–1196) of the rat Y5 receptor isolated from a rat hypothalamic library (Stratagene, La Jolla, CA) was subcloned into pBluescript (Stratagene). Sense and antisense RNA probes incorporating digoxigenin were generated according to the manufacturer's instructions using the DIG RNA labeling kit (SP6/T7/T3; Boehringer Mannheim, Mannheim, Germany) to produce complementary RNA (cRNA) and mRNA transcripts (for details of riboprobe preparation, see Holtke et al., 1990; Parker and Herzog, 1998). Transcripts of 616 bp cRNA and 465 bp mRNA were produced from the rat Y5 construct after linearization with NcoI, and human cRNA (265 bp) and mRNA (463 bp) riboprobes were synthesized after digestion with BglII orNcoI, respectively. The digoxigenin-labeled riboprobes were purified by successive extractions in phenol–chloroform followed by centrifugation through a spin column prepared with Sephadex G25 material (Amersham Pharmacia Biotech).
Collection of rat tissues. Adult male Wistar rats (200–250 gm) were maintained on a 12 hr light/dark cycle with access to food and water ad libitum. Animals were anesthetized with sodium pentobarbital (70 mg/kg, i.p) and transcardially perfused with 20 ml of PBS followed by 200 ml of ice-cold 4% paraformaldehyde (PFA) in PBS. All efforts were made to minimize animal suffering, and animal numbers were kept to a minimum. Experimental work using animals was done in accordance with the guidelines outlined in the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (1990). Dissected tissues were post-fixed for 2 hr in fresh 4% PFA, dehydrated through a series of alcohol using an automated Leica tissue processor, then orientated and embedded in paraffin wax. Six micrometer serial sections were collected onto gelatin–chrome alum-subbed slides and stored at 4°C until used.
Collection of human tissues. Postmortem brain tissue was obtained from two patients (17-yr-old male, 20 hr postmortem delay; 69-yr-old male, 16 hr postmortem delay) without neurological disease as part of routine autopsies. Tissue blocks from a variety of brain regions were dissected and fixed by immersion in formalin for 36 hr and then paraffin-embedded as detailed above. Six micrometer serial sections were collected onto gelatin–chrome alum-subbed slides and stored at 4°C until required. The data presented here were all obtained from the 17-yr-old male patient, however, we found no significant difference in the pattern of hybridization signal between the two individuals.
In situ hybridization histochemistry. Sections were dewaxed, washed in PBS, and pretreated for 20 (rat tissue) or 30 min (human tissue) at 37°C with 5 μg/ml proteinase K (Boehringer Mannheim) in 50 mm Tris and 5 mm EDTA, pH 7.5. Sections were washed three times with 0.1 m glycine in PBS for 2 min then once in PBS. Sense and antisense digoxigenin-labeled riboprobes were diluted in hybridization buffer (2× SSPE, 50% formamide, 5% dextran sulfate, 1× Denhardt's reagent, 100 μg/ml tRNA type X-SA), and amounts equivalent to ∼200 ng/ml were added to each tissue section. The sections were hybridized at 42°C (rat) or 50°C (human) for 16 hr in a humidified environment using a Hybaid OmniSlide Thermal Cycler (Hybaid). After hybridization, sections were washed at room temperature (RT) in 2× SSC for 10 min, then 0.2× SSC and 0.1× SSC for 30 min each at 55 and 60°C, respectively. The sections were then treated with 20 μg/μl ribonuclease A (Sigma, St. Louis, MO) in 10 mm Tris, 15 mm NaCl, pH 7.5, for 15 min at RT, washed in 2× SSC for 5 min at RT then in 0.2× SSC at 37°C for 30 min. The tissues were then processed for immunological detection according to the manufacturer's instructions (Boehringer Mannheim) using an alkaline phosphatase-conjugated anti-digoxigenin antiserum diluted 1:500. The labeled probes were visualized using nitroblue tetrazolium and bromochloroindoyl phosphate (with 1 mm levamisole) as substrates for 16 hr in the dark. Sections were washed for 10 min in 10 mm Tris-HCl, 1 mm EDTA, pH 8.0, mounted and photographed using a ZeissAxiophot photomicroscope with Nomarski optics and Kodak TMAX 100 film.
Analysis of the distribution of Y5 mRNA in human and rat brain. A detailed comparative study was made of the distribution of Y5 mRNA in human and rat brain tissues using ISH techniques and digoxigenin-labeled sense and antisense riboprobes. In rat, coronal sections were analyzed and mapped with reference to Paxinos and Watson (1997). A detailed analysis of hybridization was performed using sections obtained from three rats, and a similar pattern of hybridization was found in all animals examined. Each area of brain was hybridized at least three times with the digoxigenin-labeled riboprobes in different experiments. In human, sections from regional brain blocks were cut and stained by hemotoxylin and eosin–luxol fast blue to identify nuclei, and structures of interest and mapped with reference to Mai et al. (1997). Each area of brain was hybridized at least three times with the digoxigenin-labeled riboprobes in different experiments. A sense riboprobe was run to serve as a control on an adjacent section in parallel with the antisense riboprobe. Hybridization intensity in both rat and human tissues was judged by two independent observers and appeared to be dependent on the concentration of probe used, in that increasing amounts of probe led to greater intensity of hybridization signal. However, the relative intensity of hybridization between brain regions and identical nuclei within a section did not differ significantly between experimental runs or with the concentration of probe used and was entirely reproducible from animal to animal. The intensity of hybridization signal was determined by arbitrarily dividing the range of intensity of hybridization signal in brain into high, moderate, and low intensity, reflecting both the number of positively hybridized neuronal cell bodies as well as the amount of reaction product within cells.
RESULTS
Expression of Y5 mRNA in rat brain
In rat brain, the highest levels of Y5 mRNA expression were found in hypothalamic nuclei, thalamic nuclei, piriform cortex, hippocampus, and specific nuclei in the brainstem (Table1). In particular, high levels of Y5 mRNA were found in neurons of the supraoptic (SON), arcuate, suprachiasmatic, and ventromedial hypothalamic nuclei (Fig.1). In the SON most, if not all, neurons showed high levels of hybridization signal (Fig. 1A), and these magnocellular neurons are known to express oxytocin and vasopressin. Neurons in the arcuate nucleus and the median eminence also showed high levels of Y5 mRNA (Fig. 1C). In the arcuate nucleus, neurons were highly labeled in all parts of the nucleus; the ventromedial part, the ventrolateral part where larger neurons are apparent, and the dorsomedial part. However, there were clearly neurons in the ventrolateral part that were negative for hybridization, and some of these are shown in Figure 1C. A few sparse but highly hybridizing neurons were found in the region of the median eminence (Fig. 1C). In the PVN there was distinctive regionalization of expression of Y5 with high levels of hybridization in neurons of the lateral magnocellular division (PaLM) and moderate levels in the ventral parvocellular division (PaV) (Fig.1D–F). The smaller neurons of the medial parvocellular division (PaMP) of the PVN showed only low levels of hybridization or were unlabeled by the Y5 antisense riboprobe. In contrast, periventricular (Pe) neurons, although scattered and small, were moderately labeled. The magnocellular neurons of the PVN are known to express vasopressin and oxytocin and have significant projections to the neural lobe of the pituitary gland. This same region of the PVN would also be designated as the posterior magnocellular group using the terminology of Swanson and Kuypers (1980), in which the PVN is divided into seven subnuclei, including the anterior magnocellular, medial magnocellular, posterior magnocellular, anterior parvocellular, medial parvocellular, dorsal parvocellular, and lateral parvocellular regions. This particular group of neurons has been defined as being vasopressin neuron-enriched compared to magnocellular neurons in the other subdivisions (Fénelon and Herbison, 1996). The smaller, moderately labeled cells in the parvocellular regions of the PVN would include a heterogeneous population of neurons containing angiotensin II, atrial naturetic peptide, bombesin, cholecystokinin, and corticotrophin-releasing hormone, as well as other hormones and peptides.
Table 1.
The expression of rat Y5 mRNA in areas of rat brain and brainstem
| Rat brain regions | Intensity |
|---|---|
| Cortex | |
| Piriform | +++ |
| Entorhinal | ++ |
| Subiculum | + |
| Cingulate | ++ |
| Olfactory tubercle | ++ |
| Olfactory tract | +++ |
| Islands of Calleja | + |
| Nucleus of horizontal diagonal band | + |
| Lateral globus pallidus | ++ |
| Caudate putamen | − |
| Nucleus accumbens | − |
| Lateral septum | − |
| Hypothalamus | |
| Supraoptic n | +++ |
| Paraventricular n | |
| PaAM | +++ |
| PaAP | +/− |
| PaLM | +++ |
| PaV | ++ |
| Pe | ++ |
| PaMP | +/− |
| Dorsomedial n | ++ |
| Ventromedial n | +++ |
| Lateral n | ++ |
| Anterior n | + |
| Arcuate n | +++ |
| Retrochiasmatic n | +++ |
| Suprachiasmatic n | ++ |
| Medial preoptic n | +++ |
| Lateral mammillary n | +++ |
| Tuberomammillary n | ++ |
| Median eminence | +++ |
| Tuber cinereum | ++ |
| Hippocampus | |
| CA1 | + |
| CA2 | ++ |
| CA3 | +++ |
| Dentate gyrus | +++ |
| Amygdala | |
| Anterior amygdala n | − |
| Cortex-amygdala trans zone | +++ |
| Anterior cortical amygdaloid n | +++ |
| Posterior cortical amygdaloid n | ++ |
| Basomedial amygdaloid n | +/− |
| Basolateral amygdaloid n | + |
| Lateral amygdaloid n | + |
| Cerebellum | |
| Cortex | − |
| Purkinje cells | ++ |
| Basket cells | − |
| Granule cells | − |
| Deep cerebellar n | ++ |
| Thalamic nuclei | |
| Paraventricular n | ++ |
| Anterodorsal n | ++ |
| Laterodorsal n | ++ |
| Ventrolatelar n | +++ |
| Ventroposterior n | +++ |
| Intermediodorsal n | ++ |
| Rhomboid n | ++ |
| Reuniens n | ++ |
| Anteroventral n | ++ |
| Mediodorsal n | ++ |
| Reticular n | ++ |
| Medial habenular n | ++ |
| Subthalamic nucleus | +++ |
| Sup and inf colliculi | − |
| Basal n of Meynert | + |
| Substantia nigra | +++ |
| Pontine nuclei | +++ |
| Olivary n | ++ |
| Locus coeruleus | +++ |
| Reticulotegmental n pons | ++ |
| Ventral cochlear n | +++ |
| Motor root of trigeminal n | +++ |
| Red nucleus | +++ |
+ corresponds to low levels of hybridization signal, ++ to moderate levels of signal, and +++ to high levels of signal. − indicates the region was examined and was negative for hybridization.
Fig. 1.
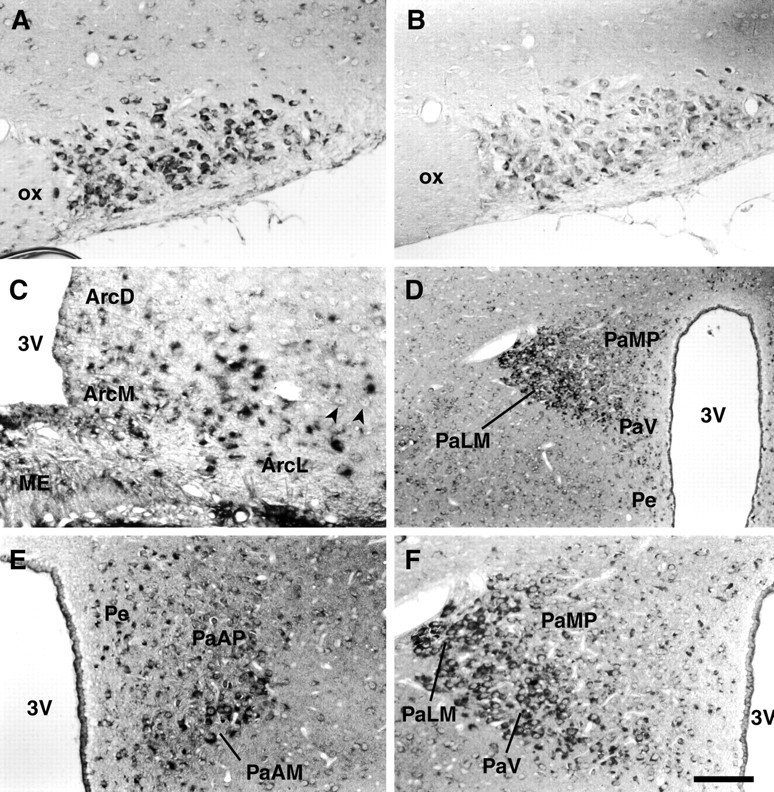
Localization of Y5 mRNA in rat hypothalamus. Representative photomicrographs showing the localization of Y5 mRNA in coronal sections of rat hypothalamus detected byin situ hybridization histochemistry using digoxigenin detection. A, High levels of hybridization signal for Y5 mRNA were found in the large magnocellular neurons of the supraoptic nucleus of the hypothalamus. B, Comparative sections for each brain region examined were hybridized with the sense riboprobe, and in all instances there was no significant signal. This is shown here for the supraoptic nucleus. C, In the arcuate nucleus the most highly hybridizing region was the ventromedial part, but there was also some hybridization in the dorsomedial part and the ventrolateral part, where larger neurons are apparent. There were neurons in the ventrolateral division that were clearly negative for hybridization, and some of these are indicated (arrowheads). D, The PVN showed distinct regionalization of Y5 mRNA expression with the most highly hybridizing neurons localized to the PaLM and less so to the PaV. Only low levels of hybridization were found in the PaMP. In the Pe, scattered positive neurons were present. E, In this view through the parvocellular region of the PVN, the Y5 signal is clearly preferentially expressed by the magnocellular neurons (PaAM). F, A higher powered view of a region of D shows the highly hybridizing neurons selectively situated in the lateral magnocellular region (PaLM) of the PVN with much less hybridization in the PaMP. Scale bar (in F): A,B, 100 μm; C, 70 μm;D, 400 μm; E, F, 200 μm. Arc D, Arcuate nucleus, dorsal part; Arc L, arcuate nucleus, lateral part; Arc M, arcuate nucleus, medial part; ME, median eminence;PaAM, paraventricular nucleus, anterior magnocellular division; PaAP, paraventricular nucleus, anterior parvocellular division; PaLM, paraventricular nucleus, lateral magnocellular division; PaV, paraventricular nucleus, ventral parvocellular division; PaMP, paraventricular nucleus, medial parvocellular division;Pe, periventricular hypothalamic nucleus;3V, third ventricle; ox, optic chiasm.
Other hypothalamic regions that labeled highly but are not illustrated here include the ventromedial nucleus, suprachiasmatic nucleus, and the lateral mammillary nucleus. Sense riboprobes were hybridized on comparative serial sections for each brain region and were negative for hybridization in all cases; a coronal section through the SON hybridized with the sense riboprobe is shown in Figure1B.
High levels of expression of Y5 mRNA were also found in regions of the thalamus, and the anterodorsal thalamic nucleus is shown (Fig.2A). High levels were also found in the basal forebrain, basal ganglia, and the large neurons of the substantia nigra (data not shown). Hybridization was found throughout the dorsal hippocampus with high levels of hybridization in the CA3 region and the dentate gyrus (Fig. 2B,C). Some areas of cortex also showed high levels of hybridization, especially the piriform cortex (Fig. 2D) and to a lesser extent entorhinal and cingulate cortex. In general, other cortical areas were negative for hybridization. The amygdala was moderately labeled, particularly in the central cortical nuclei (Fig.2D). Specific areas of brainstem, such as the pontine nuclei, ventral cochlear nucleus, olivary nucleus, trigeminal nuclei, and locus coeruleus (Fig. 2E) were highly labeled with the Y5 antisense probe, as was the red nucleus (Fig.2F). In the cerebellum, moderate levels of hybridization were found in the Purkinje cells and the deep cerebellar nuclei. Corresponding sections of rat brain from each area were hybridized with a sense probe to the Y5 receptor, and these showed no specific labeling in any region.
Fig. 2.
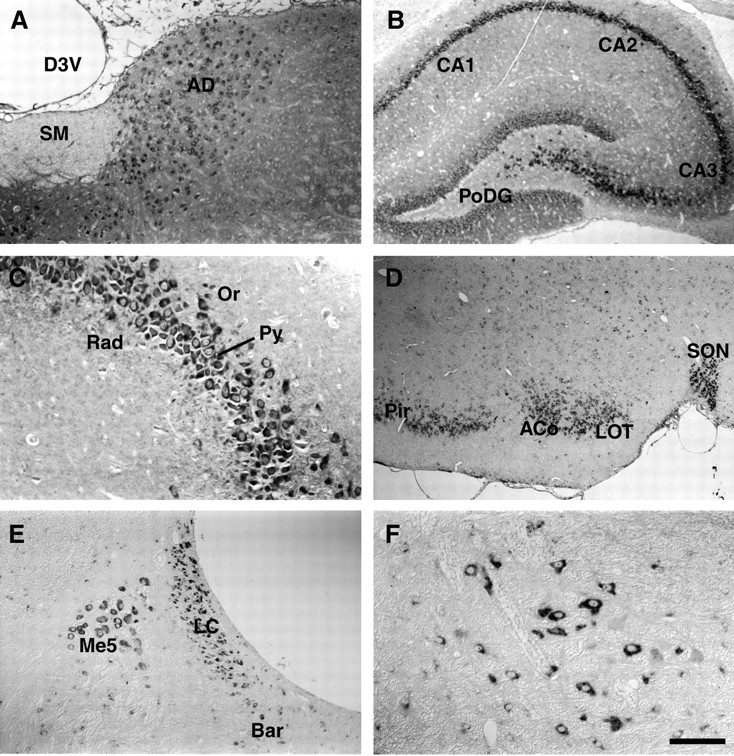
Localization of Y5 mRNA in rat brain and brainstem regions. Representative photomicrographs showing the localization of Y5 mRNA in coronal sections of rat brain and brainstem regions detected by in situ hybridization histochemistry. A, The anterodorsal thalamic nucleus was uniformly and moderately labeled. B, A low-powered view through the dorsal hippocampus shows highly hybridizing pyramidal neurons of CA3, especially near the dentate gyrus. There was also highly significant label in dentate gyrus and CA2 in lesser amounts.C, A high-powered view through the CA3 region of hippocampus. D, A low-powered view shows high levels of hybridization in neurons of the piriform cortex, anterior cortical amygdala, lateral olfactory tract, and supraoptic nucleus.E, In the brainstem, many nuclear groups had high levels of hybridization, including the locus coeruleus and mesencephalic nucleus shown here, and F, the red nucleus. Scale bar (in F): A, E, 200 μm; B, 300 μm; C, F, 100 μm; D, 385 μm. ACo, Anterior cortical amygdala nucleus; AD, anterodorsal thalamic nucleus; Bar, Barrington's nucleus; CA1, field CA1 of the hippocampus; CA2, field CA2 of the hippocampus; CA3, field CA3 of the hippocampus;D3V, dorsal third ventricle; LC, locus coeruleus; LOT, nucleus of the lateral olfactory tract;Me5, mesencephalic nucleus; Or, oriens layer of the hippocampus; Pir, piriform cortex;PoDG, polymorphic layer of dentate gyrus;Py, pyramidal cell layer of the hippocampus;Rad, stratum radiatum of the hippocampus;SM, stria medullaris of the thalamus;SON, supraoptic nucleus.
These results are presented in schematic form in Figure3, illustrating six representative coronal rat brain sections hybridized with the Y5 antisense probe. This detailed distribution of the Y5 receptor mRNA is generally consistent with the briefer initial report by Gerald et al. (1996) using a radioactive method of detection in which the most intense hybridization was reported in the dentate gyrus and CA3 region of hippocampus and cingulate cortex. Gerald et al. (1996) also found abundant signal in most hypothalamic nuclei including the PVN, lateral hypothalamus, and SON as well as the arcuate and central amygdaloid nuclei. Our results are in agreement with this earlier report, but provide a significantly more extensive analysis at high resolution at an individual cellular level, as well as concentrating on regionalization of localization in hypothalamic nuclei.
Fig. 3.
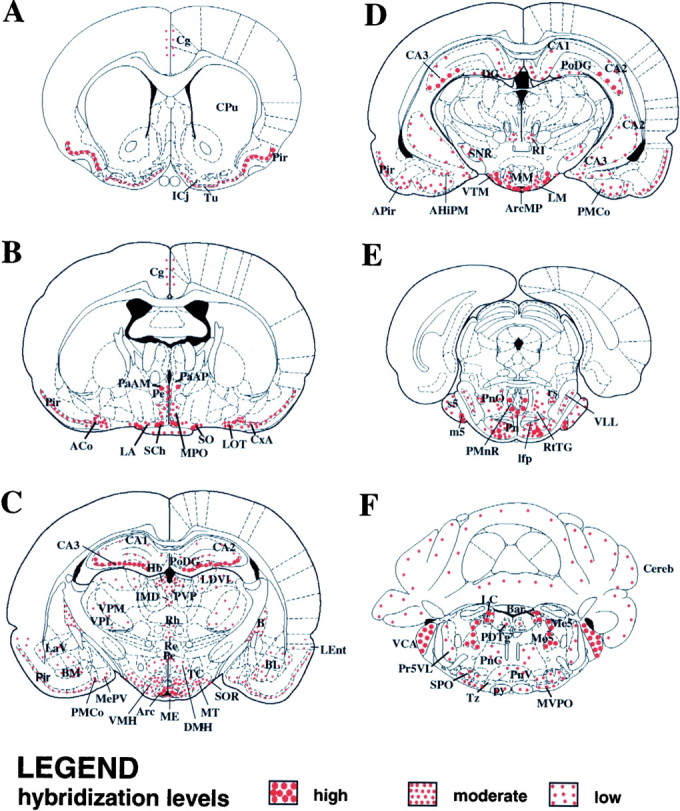
Detailed Y5 mRNA localization in rat brain. Schematic representation of intensity and localization of Y5 mRNA expression determined by in situ hybridization in six representative coronal sections through the rat brain. Coronal sections corresponding to bregma 1.00 mm, −0.92 mm, −3.14 mm, −4.52 mm, −7.8 mm, and −9.80 mm (panels A–F, respectively) were examined for the pattern and intensity of in situhybridization signal, and the results are indicated inred. Hybridization intensity was judged by the density of positive neuronal cell bodies as well as intensity of reaction product and was graded high, moderate, or low. This is indicated by the size and density of dots shown in the legend. This schematic was generated using Paxinos and Watson (1997). AHiPM, Amygdalohippocampal area, posteromedial part; APir, amygdalopiriform transition zone; Arc, arcuate nucleus;ArcMP, arcuate nucleus, medial posterior part;B, basal nucleus of Meynert; BL, basolateral amygdaloid nucleus; BM, basomedial amygdaloid nucleus; Cereb, cerebellum;Cg, cingulate cortex; CPu, caudate putamen; CxA, cortex-amygdala transition zone;DG, dentate gyrus; DMH, dorsomedial hypothalamic nucleus; Hb, habenular nucleus;ICj, islands of Calleja; Ifp, longitudinal fascic pons; IMD, intermediodorsal thalamic nucleus; LA, lateroanterior hypothalamic nucleus;LaV, lateral amgydaloid nucleus, ventral part;LDVL, laterodorsal thalamic nucleus, ventrolateral part;LEnt, lateral entorhinal cortex; LM, lateral mammillary nucleus; m5, motor root of the trigeminal nerve; ME, median eminence;MePV, medial amygdaloid nucleus, posteroventral part;MM, mammillary nucleus; Mo5, motor trigeminal nucleus; MPO, medial preoptic nucleus; MTu, medial tuberal nucleus;MVPO, medioventral periolivary nucleus;PDTg, posterodorsal tegmental nucleus;PMCo, posteromedial cortical amygdaloid nucleus;PMnR, paramedian raphe nucleus; Pn, pontine nucleus; PnC, pontine reticular nucleus, caudal part; PnO, pontine reticular nucleus;PnV, pontine reticular nucleus, ventral part;Pr5VL, principal sensory trigeminal nucleus;PVP, paraventricular thalamic nucleus, posterior part;py, pyramidal tract; Re, reuniens thalamic nucleus; Rh, rhomboid thalamic nucleus;rs, rubrospinal tract; RtTG, reticulotegmental nucleus of the pons; s5, sensory root of the trigeminal nerve; SCh, suprachiasmatic nucleus;SNR, substantia nigra; SO, supraoptic nucleus; SOR, supraoptic nucleus, retrochiasmatic part;SPO, superior paraolivary nucleus; TC, tuber cinereum; Tu, olfactory tubercle;Tz, trapezoid body; VCA, ventral cochlear nucleus; VLL, ventral nucleus of the lateral lemniscus;VMH, ventromedial hypothalamic nucleus;VP, ventral pallidum; VPL, ventral posterolateral thalamic nucleus; VTM, ventral tuberomammillary nucleus. All other abbreviations are as indicated in previous figures.
Expression of Y5 mRNA in human brain
Using ISH we examined expression of human Y5 mRNA in the major nuclei of the human hypothalamus and thalamus as well as other brain regions, and these results are tabulated in Table2. Of the regions examined, the highest cellular levels of Y5 mRNA were found in neurons of the amygdala, thalamic nuclei, and the substantia nigra. In the human hypothalamus, moderate to high levels of Y5 mRNA were found in the supraoptic, paraventricular, lateral, and posterior nuclear regions, with moderate levels of hybridization in the arcuate nucleus, mammillary body, and intercalatus nucleus (Fig.4A–C). The large magnocellular neurons of the SON are illustrated in Figure4A. Figure 4B shows a coronal section through the human PVN demonstrating hybridization signal in the typical magnocellular neurons of the PVN with distinctive peripheral Nissl substance. In addition, medium and small neurons in the field are also labeled. There are also clearly nonhybridizing neurons visible in this field of view. Hence, the highest levels of hybridization were in the magnocellular neurons of the PVN but smaller neurons, consistent with parvocellular neurons, also hybridized. Neurons in thalamic areas adjacent to the hypothalamus showed particularly high levels of hybridization (Fig. 4D,E). Cells of the anterior lobe of pituitary gland were examined and were negative for hybridization (Fig. 4F).
Table 2.
The expression of human Y5 mRNA in areas of human brain and brainstem
| Human brain regions | Intensity |
|---|---|
| Cortex | |
| Parietal | − |
| Occipital | − |
| Subiculum | + |
| Hypothalamus | |
| Supraoptic n | ++/+++ |
| Paraventricular n | ++/+++ |
| Dorsomedial n | ++ |
| Ventromedial n | ++ |
| Lateral hypothalamic n | ++/+++ |
| Posterior hypothalamic n | ++/+++ |
| Arcuate n | ++ |
| Mammillary body | ++ |
| Lat mamm/intercalatus n | ++ |
| Hippocampus | |
| CA1 | + |
| CA2 | ++ |
| CA3 | ++ |
| CA4 | ++ |
| Dentate gyrus | ++ |
| Amygdala | +++ |
| Thalamus | |
| Lateral geniculate n | ++/+++ |
| Ventrolateral n | +++ |
| Ventroanterior n | +++ |
| Substantia nigra | +++ |
| Red nucleus | ++ |
| Horiz limb diag band | ++ |
| Pituitary | − |
| Cerebellum | |
| Purkinje cells | − |
| Granular layer | − |
| Deep cerebellar nuclei | ++ |
| Brainstem nuclei | |
| Dorsal n of vagus | ++ |
| Nucleus of Roller | ++ |
| Inferior olivary n | ++ |
+ corresponds to low levels of hybridization signal, ++ moderate levels of signal and +++ high levels of signal. − indicates the region was examined and was negative for hybridization.
Fig. 4.
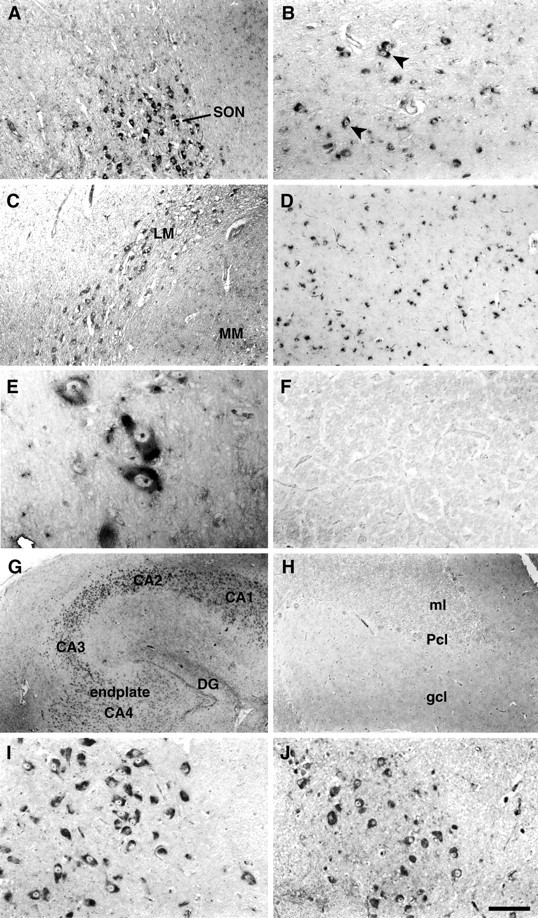
Localization of Y5 mRNA in human brain regions. Representative photomicrographs showing the localization of Y5 mRNA in coronal (A–C, G–I) and horizontal (D–F) sections of human brain and in an axial section through brainstem (J) detected by in situ hybridization histochemistry using an antisense riboprobe and digoxigenin detection. A, The large magnocellular neurons of the supraoptic nucleus showed moderate to high levels of hybridization. B, The magnocellular neurons of the paraventricular nucleus are characterized by their peripheral cresenteric Nissl substance and showed moderate to high levels of labeling (arrowheads). In addition, medium- and small-sized neurons in the nucleus also hybridized.C, The lateral mammillary/intercalatus nucleus and mammillary nucleus hybridized moderately. D, High levels of hybridization were found throughout the ventrolateral thalamic nucleus. E, A high-powered view through the ventrolateral thalamus shows highly hybridizing magnocellular neurons.F, A representative section through the anterior lobe of pituitary gland showing no significant signal with the antisense probe.G, In the hippocampus, moderate hybridization is illustrated in neurons in CA4, CA3, and the dentate gyrus as well as CA2. H, The cerebellar hemispheres were completely negative for hybridization with the antisense probe. I,The large, distinctive pyramidal cells of the substantia nigra showed high levels of hybridization. J, Many brainstem nuclei hybridized positively, and here the inferior olivary nucleus is illustrated. Scale bar (in J): A,C, D, H, 200 μm;B, 50 μm; E, 32 μm; F,I, J, 100 μm; G, 400 μm. endplate CA4, Field CA4 of the hippocampus;gcl, granular cell layer; ml, molecular layer; Pcl, Purkinje cell layer. All other abbreviations are as indicated in previous figures.
In the human hippocampus, moderate levels of Y5 mRNA were found in all neurons of the CA2, CA3, and CA4 regions and dentate gyrus with lower levels in CA1 (Fig. 4G). The cerebellar hemispheres were negative (Fig. 4H), except for distinctive labeling in large neurons of the deep cerebellar nuclei. Very high levels of hybridization were also found in the distinctive neurons of the substantia nigra (Fig. 4I). Brainstem was examined in the region of the inferior olives and showed moderate labeling in nuclei, including the dorsal nucleus of the vagus, the nucleus of Roller, and the inferior olivary nucleus (Fig. 4J). The human frontal and occipital cortical regions examined were negative for hybridization, however, neurons in the subiculum showed low levels of labeling. The amygdaloid nuclei had highly hybridizing neurons, particularly in the central region. Corresponding sections for all regions were hybridized with the Y5 sense riboprobe and showed no significant labeling.
In summary, in the human brain Y5 mRNA was highly expressed in a quite novel expression pattern: highest in hypothalamic and thalamic nuclei, amygdala, and substantia nigra as well as in specific brainstem nuclei. There was moderate expression in the hippocampus and relatively little expression in the cerebral cortex. In contrast to our findings in the rat cerebellum, no significant Y5 mRNA hybridization signal was detected in human Purkinje cells.
DISCUSSION
Using ISH with a digoxigenin method of detection, we have examined Y5 mRNA expression in human CNS and compared this to CNS expression of Y5 in rat. Our most important finding is almost complete conservation of Y5 mRNA expression in major hypothalamic nuclei between man and rat. In particular, we focused on examination of the arcuate and PVN in hypothalamus, nuclei known to play a pivotal role in feeding and appetite that are densely innervated by NPYergic neurons (Jhanwar-Uniyal et al., 1993; Stanley et al., 1993). In these regions, major nuclear groups showed moderate to high levels of Y5 mRNA expression in both species. This crucial finding suggests an important conserved role for this receptor in mediating the function of NPY in both species and makes it more likely that current physiological studies of Y5 undertaken in rat and mouse will have direct relevance to human physiology and pharmacology.
The high levels of expression of rat Y5 in both the arcuate nucleus and PVN suggest that Y5 mediates some of the action of NPY at these sites. To date, Y1 immunoreactivity (Zhang et al., 1994; Fuxe et al., 1997), and Y2 (Gustafson et al., 1997) and Y5 (Gerald et al., 1996) mRNAs have been localized to cell bodies in the arcuate nucleus, suggesting a complex interaction of NPY and its receptors here within individual neurons. In the arcuate, Y2 mRNA is predominantly located rostrally (Gustafson et al., 1997), whereas we found high levels of Y5 in ventromedial arcuate, an area containing high levels of NPY and leptin receptors (Hakansson et al., 1996; Mercer et al., 1996; Schwartz et al., 1996) and also believed to lack a blood–brain barrier (Shaver et al., 1992). Leptin may activate receptors through the peripheral circulation at this site, explaining its pivotal role in the regulation of feeding and other metabolic responses.
Studies have shown that the PVN has moderate to high levels of expression of Y1 and Y5 mRNA (Larsen et al., 1993b; Gerald et al., 1996) with Y2 present in only a few scattered neurons (Gustafson et al., 1997). Our high-resolution study defined clear regionalization of expression of Y5 mRNA in rat PVN with predominant expression in the lateral magnocellular division, particularly PaLM, the division containing vasopressin rather than oxytocin-expressing neurons and having major projections to arcuate, median eminence, and posterior pituitary. Hence, Y5 possesses the anatomical substrate to be a major receptor in the complex NPYergic loop existing between the median eminence, arcuate, and PVN.
Regionalized expression of Y5 in neurons of the lateral magnocellular PVN is interesting because NPY is not normally detectable in these neurons. It is feasible that Y5 may be functioning postsynaptically, binding NPY released from neurons projecting from the arcuate nucleus. NPY immunoreactivity and mRNA levels are increased in the magnocellular neurons of the PVN, however, in normal rats under hyperosmotic stimulation (Larsen et al., 1993a) and in the obese Zucker rat, which demonstrates hydro-osmotic abnormalities in addition to obesity (Fetissov and Nicolaidis, 1998). Although extrapolation from the Zucker rat model of obesity to the normal physiological state should be undertaken with caution, such observations suggest that NPY could be involved in the central regulation of water intake as well as appetite and feeding.
Our results should be viewed in light of two recent studies showing a lack of correlation between hypothalamic Y5 mRNA levels and receptor binding (Dumont et al., 1998; Statnick et al., 1998). Using competitive RT-PCR techniques, high levels of Y5 were found in human hypothalamic nuclei, however, radioligand binding studies could not detect Y5-like binding sites in homogenates of human hypothalamus (Statnick et al., 1998). This latter finding is consistent with the finding of low Y5-like binding in rat hypothalamic nuclei (Dumont et al., 1998). These authors acknowledged the discrepancy between their data and ISH reported by Gerald et al. (1996) and considered it could be attributable to: (1) Y5 mRNA being translated at low efficiency into protein; (2) detection of the binding signal requiring a higher resolution technique; (3) the very high levels of NPY in hypothalamus saturating available receptors, and (4) the translated protein being localized distantly on nerve terminals. Our ISH data in rat brain concurs well with the report by Gerald et al. (1996), confirming highly significant levels of Y5 in hypothalamic nuclei. Hence, it is likely that at least one of these four factors is contributing to explain the binding studies. Immunohistochemical studies with Y5 are needed to reveal the exact sites of Y5 protein expression, i.e., is it present on the cell bodies of PVN neurons or on their rather distant projections, or both? Such studies will ultimately elucidate the sites of action of NPY on Y5 receptors.
The conservation of expression of Y5 between human and rat in areas apart from the hypothalamus, including the hippocampus, dentate gyrus, and amygdala, may implicate the receptor as mediating memory and learning functions, perhaps specifically related to feeding behavior. In the rat brain, we identified hybridizing nuclear regions in the amygdala with highest levels in the central nucleus of the amygdala. This finding is important because the amygdala has been implicated with both an inhibitory and excitatory role in the control of food intake (Zimanyi et al., 1998). Other Y receptor subtypes have also been identified in the amygdala, Y1, and Y2 at relatively high levels (Widdowson, 1993; Gustafson et al., 1997).
Previously reported ISH studies of Y receptors in human used autoradiographic ISH techniques: Y1 (Jacques et al., 1996; Caberlotto et al., 1997), Y2 (Caberlotto et al., 1998), and Y5 (Jacques et al., 1998). Our findings are in almost complete agreement with the recent study in human in finding high levels of Y5 in major hypothalamic areas, the arcuate, and dentate gyrus and adds significantly to this data in providing detailed localization of Y5 within a number of nuclei, including the arcuate and PVN. In human, Y1 and Y2 mRNA are also highly expressed in the dentate gyrus with moderate expression in hypothalamic regions and amygdala. We also found high levels of Y5 mRNA in some midline thalamic areas, in contrast to Jacques et al. (1998)who reported low levels. Other Y receptors, Y1 and Y2, show little or no appreciable mRNA in any thalamic regions examined. Although both Y1 and Y2 mRNA are widely distributed in parts of the human cerebral cortex, it appears that cortical Y5 mRNA expression is limited, based on our data and the data of Jacques et al. (1998).
A comparison of Y receptor mRNA expression in rat and human brain shows conservation in most regions of brain but highlights some differences between species. For example, Y1 and Y2 are moderately to highly expressed in the rat thalamus (Larsen et al., 1993b; Gustafson et al., 1997) but show low levels of expression in the human thalamus (Caberlotto et al., 1997, 1998). Our comparison of Y5 expression between rat and human brain showed almost complete conservation in all areas examined. The only significant exception was finding moderate Y5 expression in Purkinje cells in the rat and lack of expression in human. Our study included many areas of the human brain, but it was not exhaustive so further examination may reveal other interspecies differences. An analysis of the overall patterns of distribution of Y1, Y2, and Y5 shows that there are definite differences in expression of the receptor subtypes, suggesting they mediate differential functions of NPY. In a relatively simplistic overview, Y1 predominates in neuronal systems related to motor and limbic function, Y2 to those related to sensory functions, learning, and memory, and Y5 to neuroendocrine, limbic, learning, and memory regions.
Gerald et al. (1996) suggested that the Y5 receptor subtype was the “feeding” receptor based on its CNS distribution and the activation of this receptor by the ligand, which induces feeding in rat models. Such in vivo experiments cannot distinguish conclusively between Y5 activation and that of other receptor subtypes. Recently, Y5 null mice have been shown to feed normally but develop late-onset obesity, however, their response to NPY and related peptides was reduced or absent (Marsh et al., 1998). It is most likely that there are multiple interrelated pathways and feedback loops subserving a pivotal function so crucial to the individual's survival as is appetite and feeding. Nonetheless, our detailed analysis of rat Y5 CNS expression, combined with finding parallel expression in key, central regulatory nuclei in human, would strengthen the putative role of Y5 as a feeding and appetite receptor. Based on its conserved neuroanatomical expression pattern in rat and human, Y5 very likely also plays an important role in memory, antiepileptogenesis, and automatic behaviors, not necessarily restricted to appetite and feeding. Recent studies showing a discrepancy between Y5 levels in CNS, determined by ISH versus receptor binding, highlight the need to distinguish between mRNA levels and the expression of functional protein. Experiments applying double-labeling mRNA techniques to address the question of diversity of NPY receptor subtype expression within individual neuronal populations as well as immunohistochemical localization of receptors will reveal important information about the complexity of interactions of NPY with its receptors.
Footnotes
This work was supported by Bristol Myers-Squibb Pharmaceutical Research Institute, the National Health and Medical Research Council of Australia, and the Garnett Passe and Rodney Williams Memorial Foundation. We thank Francis Pemper, Tanya Wyatt, and Yvonne Hort for their excellent technical assistance and Barbara Depczynski for her helpful input.
Correspondence should be addressed to Dr. A. M. Cunningham, Neurobiology Program, The Garvan Institute of Medical Research, 384 Victoria Street, Darlinghurst, New South Wales 2010, Australia. E-mail:a.cunningham@garvan.unsw.edu.au.
REFERENCES
- 1.Balasubramaniam AA. Neuropeptide Y family of hormones: receptor subtypes and antagonists. Peptides. 1997;18:445–457. doi: 10.1016/s0196-9781(96)00347-6. [DOI] [PubMed] [Google Scholar]
- 2.Baraban SC, Hollopeter G, Erickson JC, Schwartzkroin PA, Palmiter RD. Knock-out mice reveal a critical antiepileptic role for neuropeptide Y. J Neurosci. 1997;17:8927–8936. doi: 10.1523/JNEUROSCI.17-23-08927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomqvist AG, Herzog H. Y-receptor subtypes: How many more? Trends Neurosci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- 4.Borowsky B, Walker MW, Bard J, Weinshank RL, Laz TM, Vaysse P, Branchek TA, Gerald C. Molecular biology and pharmacology of multiple NPY Y5 receptor species homologs. Regul Peptides. 1998;75–76:45–53. doi: 10.1016/s0167-0115(98)00052-4. [DOI] [PubMed] [Google Scholar]
- 5.Caberlotto L, Fuxe K, Sedvall G, Hurd YL. Localisation of neuropeptide Y Y1 mRNA in the human brain: abundant expression in cerebral cortex and striatum. Eur J Neurosci. 1997;9:1212–1225. doi: 10.1111/j.1460-9568.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 6.Caberlotto L, Fuxe K, Rimland JM, Sedvall G, Hurd YL. Regional distribution of neuropeptide Y Y2 receptor messenger RNA in the human post mortem brain. Neuroscience. 1998;86:167–178. doi: 10.1016/s0306-4522(98)00039-6. [DOI] [PubMed] [Google Scholar]
- 7.Dumont Y, Fournier A, St-Pierre S, Quirion R. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J Neurosci. 1993;13:73–86. doi: 10.1523/JNEUROSCI.13-01-00073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumont Y, Fournier A, Quirion R. Expression and characterization of the neuropeptide Y Y5 receptor subtype in the rat brain. J Neurosci. 1998;18:5565–5574. doi: 10.1523/JNEUROSCI.18-15-05565.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fénelon VS, Herbison AE. Plasticity in GABAA receptor subunit mRNA expression by hypothalamic magnocellular neurons in the adult rat. J Neurosci. 1996;16:4872–4880. doi: 10.1523/JNEUROSCI.16-16-04872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fetissov S, Nicolaidis S. Neuropeptide Y in the magnocellular hypothalamic neurons of obese Zucker rats. Neuropeptides. 1998;32:63–66. doi: 10.1016/s0143-4179(98)90018-x. [DOI] [PubMed] [Google Scholar]
- 11.Flier JS. What's in a name? In search of leptin's physiologic role. J Clin Endocrinol Metab. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- 12.Fuxe K, Tinner B, Caberlotto L, Bunnemann B, Agnati LF. NPY Y1 receptor-like immunoreactivity exists in a subpopulation of beta-endorphin immunoreactive nerve cells in the arcuate nucleus: a double immunolabelling analysis in the rat. Neurosci Lett. 1997;225:49–52. doi: 10.1016/s0304-3940(97)00184-5. [DOI] [PubMed] [Google Scholar]
- 13.Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, Vaysse P, Durkin MM, Laz TM, Linemeyer DL, Schaffhauser AO, Whitebread S, Hofbauer KG, Taber RI, Branchek TA, Weinshank RL. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- 14.Gustafson EL, Smith KE, Durkin MM, Walker MW, Gerald C, Weinshank R, Branchek TA. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Mol Brain Res. 1997;46:223–235. doi: 10.1016/s0169-328x(97)00017-x. [DOI] [PubMed] [Google Scholar]
- 15.Hakansson ML, Hulting AL, Meiste B. Expression of leptin receptor mRNA in the hypothalamic arcuate nucleus-relationship with NPY neurones. NeuroReport. 1996;7:3087–3092. doi: 10.1097/00001756-199611250-00059. [DOI] [PubMed] [Google Scholar]
- 16.Heilig M, Widerlöv E. Neuropeptide Y: an overview of central distribution, functional aspects and possible involvement in neuropsychiatric illnesses. Acta Psychiatr Scand. 1990;82:95–114. doi: 10.1111/j.1600-0447.1990.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 17.Heilig M, Widerlöv E. Neurobiology and clinical aspects of neuropeptide Y. Crit Rev Neurobiol. 1995;9:115–136. [PubMed] [Google Scholar]
- 18.Herzog H, Darby K, Ball H, Hort Y, Beck-Sickinger A, Shine J. Overlapping gene structure of the human neuropeptide Y receptor subtypes Y1 and Y5 suggests co-ordinate transcriptional regulation. Genomics. 1997;41:315–319. doi: 10.1006/geno.1997.4684. [DOI] [PubMed] [Google Scholar]
- 19.Holtke H-J, Seibl R, Burg J, Muhlegger K, Kessler C. Non-radioactive labeling and detection of nucleic acids II Optimization of the digoxigenin system. Biol Chem Hoppe-Seyler. 1990;371:929–938. doi: 10.1515/bchm3.1990.371.2.929. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Bloomquist BT, Cornfield LJ, DeCarr LB, Flores-Riveros JR, Friedman L, Jiang P, Lewis-Higgins L, Sadlowski Y, Schaefer J, Velazquez N, McCaleb ML. Identification of a novel hypothalamic neuropeptide Y receptor associated with feeding behaviour. J Biol Chem. 1996;271:26315–26319. [PubMed] [Google Scholar]
- 21.Jacques D, Tong Y, Dumont Y, Shen SH, Quirion R. Expression of the neuropeptide Y Y1 receptor mRNA in the human brain: an in situ hybridization study. NeuroReport. 1996;7:1053–1056. doi: 10.1097/00001756-199604100-00020. [DOI] [PubMed] [Google Scholar]
- 22.Jacques D, Tong Y, Shen SH, Quirion R. Discrete distribution of the neuropeptide Y Y5 receptor gene in the human brain: an in situ hybridization study. Mol Brain Res. 1998;61:100–107. doi: 10.1016/s0169-328x(98)00208-3. [DOI] [PubMed] [Google Scholar]
- 23.Jhanwar-Uniyal M, Beck B, Jhanwar YS, Burlet C, Leibowitz SF. Neuropeptide Y projection from arcuate nucleus to parvocellular division of paraventricular nucleus: specific relation to the ingestion of carbohydrate. Brain Res. 1993;631:97–106. doi: 10.1016/0006-8993(93)91192-u. [DOI] [PubMed] [Google Scholar]
- 24.Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci USA. 1991;88:10931–10935. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen PJ, Mikkelsen JD, Jessop DS, Lightman SL, Chowdrey HS. Neuropeptide Y mRNA and immunoreactivity in hypothalamic neuroendocrine neurons: effects of adrenalectomy and chronic osmotic stimulation. J Neurosci. 1993a;13:1138–1147. doi: 10.1523/JNEUROSCI.13-03-01138.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen PJ, Sheikh SP, Jakobsen CR, Schwartz TW, Mikkelsen JD. Regional distribution of putative NPY Y1 receptors and neurons expressing Y1 mRNA in forebrain areas of the rat central nervous system. Eur J Neurosci. 1993b;5:1622–1637. doi: 10.1111/j.1460-9568.1993.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 27.Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behaviour in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- 28.Mai JK, Assheuer J, Paxinos G. Atlas of the human brain. Academic; London: 1997. [Google Scholar]
- 29.Marsh DJ, Hollopeter G, Kafer KE, Palmiter RD. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med. 1998;4:718–721. doi: 10.1038/nm0698-718. [DOI] [PubMed] [Google Scholar]
- 30.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ, Trayhurn P. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J Neuroendocrinol. 1996;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. [DOI] [PubMed] [Google Scholar]
- 31.Mikkelsen JD, Larsen PJ. A high concentration of NPY (Y1)-receptor mRNA-expressing cells in the rat arcuate nucleus. Neurosci Lett. 1992;148:195–198. doi: 10.1016/0304-3940(92)90837-w. [DOI] [PubMed] [Google Scholar]
- 32.Parker RM, Herzog H. Comparison of Y-receptor subtype expression in the rat hippocampus. Regul Peptides. 1998;75–76:109–115. doi: 10.1016/s0167-0115(98)00059-7. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Compact Ed 3. Academic; New York: 1997. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaver SW, Wall KM, Wainman DS, Gross PM. Regional quantitative permeability of blood-brain barrier lesions in rats with chronic renal hypertension. Brain Res. 1992;579:99–106. doi: 10.1016/0006-8993(92)90747-w. [DOI] [PubMed] [Google Scholar]
- 36.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 37.Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF. The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s). Brain Res. 1993;604:304–317. doi: 10.1016/0006-8993(93)90382-w. [DOI] [PubMed] [Google Scholar]
- 38.Statnick MA, Schober DA, Gackenheimer S, Johnson D, Beavers L, Mayne NG, Burnett JP, Gadski R, Gehlert DR. Characterization of the neuropeptide Y5 receptor in the human hypothalamus: a lack of correlation between Y5 mRNA levels and binding sites. Brain Res. 1998;810:16–26. doi: 10.1016/s0006-8993(98)00855-5. [DOI] [PubMed] [Google Scholar]
- 39.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 40.Wahlestedt C, Reis DJ. Neuropeptide Y-related peptides and their receptors-are the receptors potential therapeutic drug targets? Annu Rev Pharmacol Toxicol. 1993;32:309–352. doi: 10.1146/annurev.pa.33.040193.001521. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Bing C, Al-Barazanji K, Mossakowaska DE, Wang XM, McBay DL, Neville WA, Taddayon M, Pickavance L, Dryden S, Thomas ME, McHale MT, Gloyer IS, Wilson S, Buckingham R, Arch JR, Trayhurn P, Williams G. Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes. 1997;46:335–341. doi: 10.2337/diab.46.3.335. [DOI] [PubMed] [Google Scholar]
- 42.Wettstein JG, Earley B, Junien JL. Central nervous system pharmacology of neuropeptide Y. Pharmacol Ther. 1995;65:397–414. doi: 10.1016/0163-7258(95)98598-k. [DOI] [PubMed] [Google Scholar]
- 43.Widdowson PS. Quantitative receptor autoradiography demonstrates a differential distribution of neuropeptide-Y Y1 and Y2 receptor subtypes in human and rat brain. Brain Res. 1993;631:27–38. doi: 10.1016/0006-8993(93)91182-r. [DOI] [PubMed] [Google Scholar]
- 44.Widdowson PS. Regionally-selective down-regulation of NPY receptor subtypes in the obese Zucker rat. Relationship to the Y5 “feeding” receptor. Brain Res. 1997;758:17–25. doi: 10.1016/s0006-8993(97)00160-1. [DOI] [PubMed] [Google Scholar]
- 45.Zarjevski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, Jeanrenaud B. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology. 1993;133:1753–1758. doi: 10.1210/endo.133.4.8404618. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Bao L, Xu ZQ, Kopp J, Arvidsson U, Elde R, Hokfelt T. Localisation of neuropeptide Y Y1 receptors in the rat nervous system with special reference to somatic receptors on small dorsal root ganglion neurons. Proc Natl Acad Sci USA. 1994;91:11738–11742. doi: 10.1073/pnas.91.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimanyi IA, Fathi Z, Poindexter GS. Central control of feeding behaviour by neuropeptide Y. Curr Pharm Des. 1998;4:349–366. [PubMed] [Google Scholar]


