Abstract
Recent evidence has shown that brain-derived neurotrophic factor (BDNF) is involved in hippocampal long-term potentiation (LTP). Because the reagents used in acute experiments react not only with BDNF but also with neurotrophin-4/5 (NT4/5) and neurotrophin-3 (NT3), we examined the involvement of these neurotrophins in LTP using two highly specific, function-blocking monoclonal antibodies against BDNF and NT3, as well as a TrkB-IgG fusion protein. Our results show that NT3 antibodies did not have any effects on LTP. However, both TrkB-IgG fusion proteins and BDNF antibody similarly reduced LTP, suggesting that only BDNF but no other ligands of the TrkB-receptor are likely to be involved in LTP induction. The reduction in LTP depended on the inducing stimuli and was only observed with theta-burst stimulation (TBS) but not with tetanic stimulation. We further observed that LTP was only reduced if BDNF was blocked before and during TBS stimulation, and BDNF antibodies did not affect early or late stages of LTP if they were applied 10, 30, or 60 min after TBS stimulation. These results point toward a specific and unique role of endogenous BDNF but not of other neurotrophins in the process of TBS-induced hippocampal LTP. Additionally, they suggest that endogenous BDNF is required for a limited time period only shortly before or around LTP induction but not during the whole process of LTP.
Keywords: BDNF, NT3, NT4/5, monoclonal antibodies, TrkB-IgG fusion protein, LTP
Neurotrophins are proteins that control the survival and differentiation of many types of neurons during development (Davies, 1994; Lewin and Barde, 1996). In addition, there is increasing evidence indicating that neurotrophins are also involved in activity-dependent neuronal plasticity in the developing CNS (for review, see Lo, 1995; Thoenen, 1995; Berninger and Poo, 1996; Bonhoeffer, 1996). Compared with NGF, brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT3), mRNAs are highly expressed in the hippocampus and neocortex (Wetmore et al., 1989;Ernfors et al., 1990; Hofer et al., 1990; Maisonpierre et al., 1990), and the expression of BDNF is tightly regulated by neuronal activity (Zafra et al., 1990, 1991; Castrén et al., 1992; Knipper et al., 1994; for review, see Lindholm et al., 1994). Interestingly, tetanization, a stimulation paradigm used to induce long-term potentiation (LTP) in the hippocampus, can induce both BDNF and NT3 mRNA expression in different hippocampal areas (Patterson et al., 1992;Dragunow et al., 1993). Also, the release of neurotrophins is dependent on neuronal activity (Blöchl et al., 1995; Blöchl and Thoenen, 1995; Griesbeck et al., 1995). Using BDNF and NT3, Lohof et al. (1993) were the first to show a direct influence of exogenous neurotrophins on synaptic transmission in neuromuscular synapses in developing Xenopus. Later, several reports demonstrated that acute application of exogenous BDNF, neurotrophin-4/5 (NT4/5), or NT3 can alter or potentiate synaptic transmission in rat hippocampal cultures and slices (Lessmann et al., 1994; Kang and Schuman, 1995;Levine et al., 1995). Although these experiments indirectly suggested that neurotrophins can participate in synaptic plasticity, work with mice carrying a null mutation in the BDNF gene showed that the lack of endogenous BDNF leads to drastically impaired LTP (Korte et al., 1995;Patterson et al., 1996) and to a limited capability of these animals to perform certain learning tasks (Linnarsson et al., 1997). Importantly, it was also shown that reexpression of the BDNF gene (Korte et al., 1996) or treatment of slices with recombinant BDNF (Patterson et al., 1996) were both able to restore LTP in slices of these mutant mice within <14 hr, making unspecific developmental deficits unlikely as an explanation for impaired LTP.
An additional approach to determine the involvement of endogenous neurotrophins in LTP is to block their function acutely in slices from wild-type animals. Two recent studies used a TrkB-IgG fusion protein (FP) and antibodies (Abs) against the TrkB receptor to block the ligands or the function of the TrkB receptor. This led to impaired LTP in slices from rat hippocampus (Figurov et al., 1996; Kang et al., 1997). Because both BDNF and NT4/5 can interact with the TrkB receptor, these experiments still leave the issue unresolved as to which of the two particular ligands actually contribute to LTP. The situation is further complicated by the fact that TrkB FPs are not selective for BDNF and NT4/5 but also bind NT3 (Shelton et al., 1995). The availability of specific, function-blocking monoclonal antibodies against BDNF and NT3 allowed us to acutely and selectively interfere with these neurotrophins and to determine their function in hippocampal LTP. We compared their effects on LTP with those of TrkB-IgG FPs and assessed the time period relative to the induction of LTP during which neurotrophins need to be available.
MATERIALS AND METHODS
Slice preparation. Hippocampal transversal slices (400-μm-thick) were prepared from male wild-type mice of SV129 strain (4–8 weeks old) using conventional techniques (Korte et al., 1995) and maintained under standard conditions [medium contained (in mm): 124 NaCl, 3 KCl, 1.25 KH2PO4, 2 Mg2SO4, 26 NaHCO3, 2.5 CaCl2, and 10 glucose; at room temperature) and gassed with 95% O2 and 5% CO2. Slices were allowed to recover in an incubation chamber for at least 1.5 hr at room temperature before they were transferred to the perfusion chamber and used for the electrophysiological experiments.
Perfusion of slices with antibodies. The following antibodies were used for LTP experiments: (1) a TrkB receptor body, which is a fusion protein between the extracellular domain of the chick TrkB receptor and the Fc part of a human IgG antibody (Dechant et al., 1993); (2) a mouse monoclonal antibody (MAB) (clone #21, IgG2B) raised against BDNF, characterized by its function blocking action with the same specificity as MAB clone #9 described by Kolbeck et al. (1999); and (3) a mouse monoclonal function-blocking NT3 antibody (IgG1) (Gaese et al., 1994). Antibody solutions were freshly prepared in perfusate artificial CSF (ACSF) from frozen antibody aliquots. The final concentration used for TrkB-IgG fusion protein, anti-BDNF, or anti-NT3 antibody was 4 μg/ml. In the course of this study, we realized that it is of paramount importance to counteract adhesion of the antibodies to surfaces of beakers, tubing, etc. Therefore, tubing and beakers were siliconized for 1 hr and dried afterward. Before the actual experiments, siliconized tubing was washed extensively with fresh ACSF for 30 min. Additionally, BSA (0.1 mg/ml) was added to the antibody, as well as to the control solutions. To test for the presence and stability of the antibodies in the perfusion solution, the level of antibodies was determined from probes taken at different time points during perfusion using a specific enzyme immunoassay (ELISA). All experiments were performed in a blind way; the experimenter performing the electrophysiological recordings did not know what type of solution was added to the perfusion medium. A closed-loop perfusion system was used for all experiments with a total volume of 30 ml of perfusion medium. During preincubation of slices with either antibody or control solution, perfusion rates were alternated between fast rate at 40 ml/min, which potentially facilitates diffusion of exogenously applied substances into slices (Kang et al., 1996), and slow rate at 1 ml/min to prevent possible damage to the slices. For electrophysiological recordings, perfusion rate was kept constant at 1 ml/min during all experiments. After each recording, tubings and the recording chamber were extensively washed with fresh ACSF for 5–10 min.
Electrophysiology. Recordings were performed at 32°C. Slices were transferred from the incubation chamber to the recording chamber. In the pretreatment LTP experiments, they were perfused for 1 hr in either control or antibody-containing medium before electrophysiological recordings were started. In the other experiments in which antibody or control aliquots were added at different time points after theta-burst stimulation (TBS), the control or anti-BDNF antibody solutions were washed into the recording chamber at 10, 30, or 60 min after TBS, respectively. Control and antibody experiments were performed at the same experimental day. Field EPSPs (fEPSPs) of CA1 apical dendrites were recorded extracellularly with a glass electrode filled with 3 m NaCI solution (resistance of 4–10 MΩ) at a depth of ∼120 μm below the slice surface. Two stimulating electrodes (monopolar, coated tungsten) were placed on either side of the recording electrode (within 200–400 μm). Stimuli were delivered alternating between these two independent Schaffer collateral–CA1 pathways, one serving as test and the other as control pathway, at a frequency of 0.1 Hz to each pathway. Stimulation in each pathway was set to elicit a fEPSP with an amplitude of ∼40–50% of maximum. LTP was induced in the testing pathway by either tetanus consisting of 3 × 30 pulses (100 Hz, 5 sec intertrain interval) or theta-burst stimulation of three trains (10 sec intertrain interval), each consisting of 15 [200 msec interstimulus interval (ISI)] × 4 pulses (100 Hz).
Data analysis. Data were collected with a program written in LABVIEW (National Instruments, Austin, TX) and stored onto computer. The initial slope and amplitude of fEPSPs evoked by stimulation of the Schaffer collateral afferents was observed online but later reanalyzed off-line. Responses in each experiment were normalized to baseline, which was defined as the mean value obtained in 100 responses before tetanus or TBS, and shown as mean ± SEM. Experiments were excluded from the experimental data set if (1) the baseline of testing pathway was unstable (variability more than ±20%), or (2) the control pathway or the testing pathway were unstable or showed an apparent drift (variability more than ±20%). The “identity” of all slices was not revealed to the experimenter performing the electrophysiological recordings before the analysis was completed. As statistical test, the unpaired one-tailed Student’s t test was used. A one-tailed test was chosen because the effect of the antibody and fusion protein treatments, if any, should be directional i.e., with smaller LTP in the experimental group than the respective controls. Significance levels are indicated in text. p> 0.05 were considered as not significant.
RESULTS
Effects of TrkB-IgG fusion protein and BDNF antibodies on basal synaptic transmission
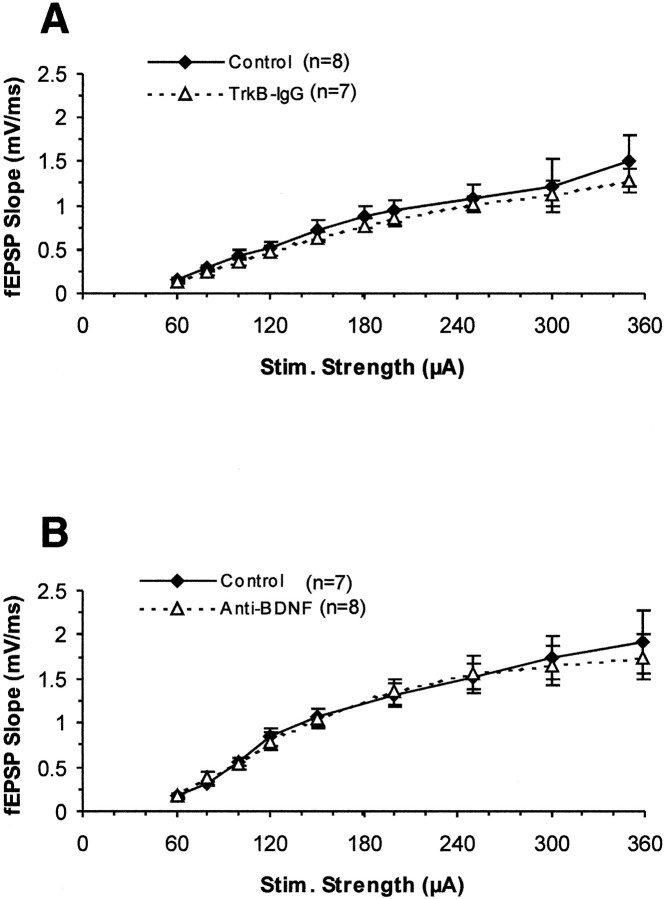
We first tested whether the basal synaptic transmission at the Schaffer collateral–CA1 synapses was affected by pretreatment of the slices with TrkB-IgG FP or anti-BDNF antibody. Basal synaptic transmission was assessed in two ways. First, we measured the input–output curves of synaptic strength by plotting the slope of the fEPSPs versus the stimulus strength in control and antibody–fusion protein-treated slices. As shown in Figure1A, the size of field EPSP slope in TrkB-IgG FP-treated slices was not significantly lower than in the controls for all the stimuli used. Also, incubation of slices with anti-BDNF monoclonal antibody for 1 hr did not show any effect on the input–output curves (Fig. 1B). We also compared the maximal response that we could elicit in control and antibody treated slices. The maximal slope of field EPSPs under treatments with both antibodies was also not significantly reduced from control slices (data not shown). The data above suggest that there is no deficit in basal synaptic transmission after acutely blocking endogenous BDNF with BDNF antibodies or other TrkB ligands with a TrkB-IgG fusion protein in hippocampal slices.
Fig. 1.
Basal synaptic transmission at the Schaffer collateral–CA1 pathway of hippocampal slices is not affected by treatment with TrkB-IgG fusion protein or BDNF antibody. The slope of field EPSPs was plotted against stimulus strength (in microamperes).A, TrkB-IgG fusion protein (triangles,dotted line; n = 7 slices, 4 mice) and controls (diamonds, solid line;n = 8 slices, 4 mice) (p> 0.2). B, BDNF antibody (triangles,dotted line; n = 8 slices, 4 mice) and controls (diamonds, solid line;n = 7 slices, 4 mice) (p> 0.3).
Effects of TrkB-IgG fusion proteins and BDNF antibodies on short-term plasticity
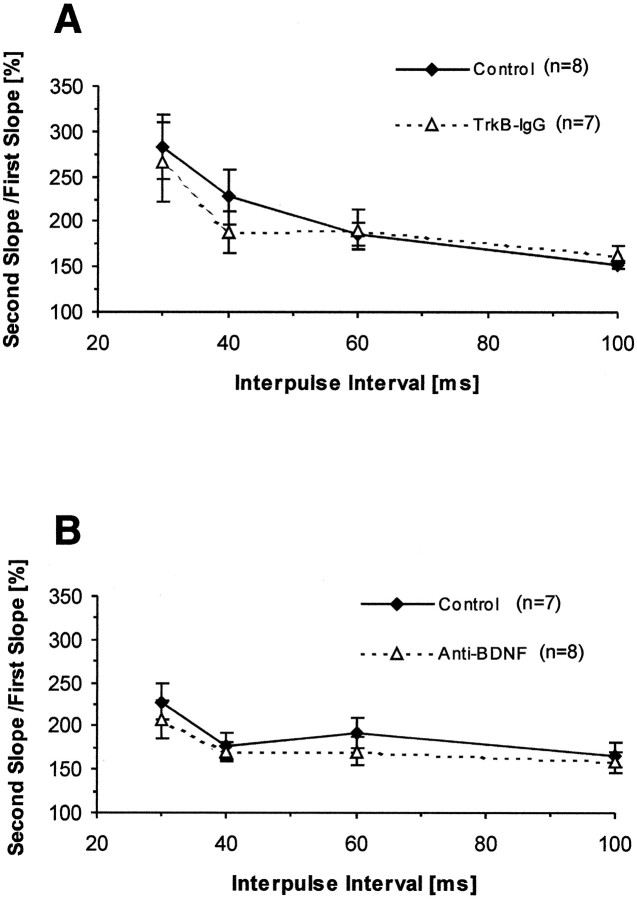
We next examined the presynaptic function in antibody-treated slices checking two forms of short-term synaptic plasticity in the Schaffer collateral–CA1 pathway of hippocampal slices: paired-pulse facilitation (PPF) and post-tetanic potentiation (PTP). First, we measured PPF in control and antibody-treated slices. PPF is a form of presynaptic short-term plasticity in which the response of a neuron to the second of two consecutive stimuli with a given ISI to the first one is increased (Katz and Miledi, 1968;Zucker, 1989). One hour after perfusion of the slices with control or antibody solutions, we tested PPF by using ISIs of 30, 40, 60, and 100 msec in control and antibody-treated slices. We found that PPF was reduced in neither TrkB-IgG fusion protein-treated slices compared with control slices ( p > 0.2 for all ISIs; one-tailedt test) (Fig.2A) nor in BDNF antibody-treated slices (p > 0.2 for all ISIs; one-tailed t test) (Fig. 2B). No deficits in PPF suggest that presynaptic forms of short-term plasticity stay normal after blocking endogenous BDNF or other TrkB ligands in antibody-treated slices. These results are consistent with our earlier findings in BDNF mutant mice (Korte et al., 1995). We also measured PTP, another form of short-term plasticity induced by tetanic stimulation, by averaging the EPSPs during the first minute after tetanic stimulation (average of six EPSPs) and calculating the ratio between this and the baseline value. We found that the size of PTP immediately after tetanic stimulation was not significantly reduced in TrkB receptor body-treated slices (TrkB-IgG, 301 ± 43%;n = 6; control, 281 ± 20%; n = 6; p > 0.3; one-tailed t test) (Fig.3A), as well as BDNF antibody-treated slices compared with their controls (BDNF Ab, 324 ± 27%; n = 7; control, 264 ± 25%;n = 7; p > 0.08; one-tailedt test) (Fig.4A). Together, the above results indicate that neither TrkB-IgG FP nor BDNF antibody alter short-term plasticity in hippocampal slices, thus suggesting that presynaptic neurotransmitter release is not disturbed by blocking ligands of TrkB receptor.
Fig. 2.
Short-term plasticity measured by PPF remains normal and unaffected at the Schaffer collateral–CA1 pathway of hippocampal slices after treatment with TrkB-IgG fusion protein or BDNF antibody. Plot depicting the percent ratio of responses to the second pulse relative to the first one. Pairs of pulses were delivered at interpulse intervals of 30, 40, 60, and 100 msec. A, TrkB-IgG fusion protein-treated slices (triangles,dotted line; n = 7 slices, 4 mice) versus control slices (diamonds, solid line; n = 8 slices, 4 mice). B, BDNF antibody-treated slices (triangles, dotted line; n = 8 slices, 4 mice) versus control slices (diamonds, solid line;n = 7 slices, 4 mice).
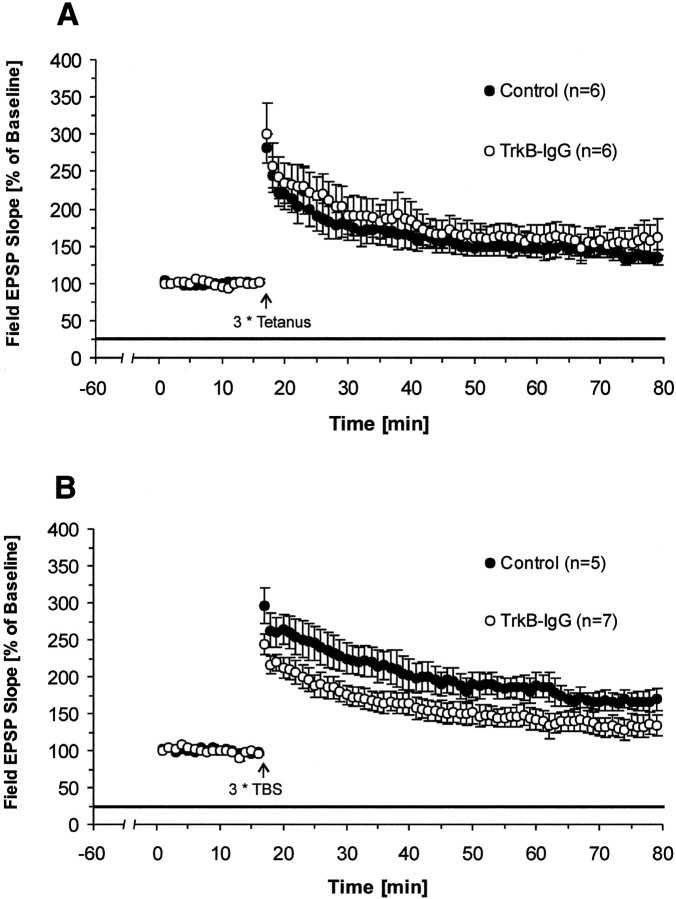
Fig. 3.
Effects of blocking all endogenous TrkB ligands on LTP. Pretreatment with TrkB-IgG fusion protein for 1 hr reduces LTP in a stimulus-dependent manner; only LTP induced by TBS at the Schaffer collateral–CA1 synapses of hippocampal slices is affected but not LTP induced by tetanic stimulation. Field EPSP slopes were plotted as percent of baseline (mean ± SEM). Straight black line represents the presence of TrkB-IgG fusion protein.First data point after LTP induction represents PTP.A, Tetanus-induced LTP and the effects of TrkB-IgG fusion protein (TrkB-IgG, n = 6 slices, 4 mice; control, n = 6 slices, 4 mice). B, TBS-induced LTP and the effects of TrkB-IgG fusion protein (TrkB-IgG,n = 7 slices, 4 mice; control,n = 5 slices, 4 mice).
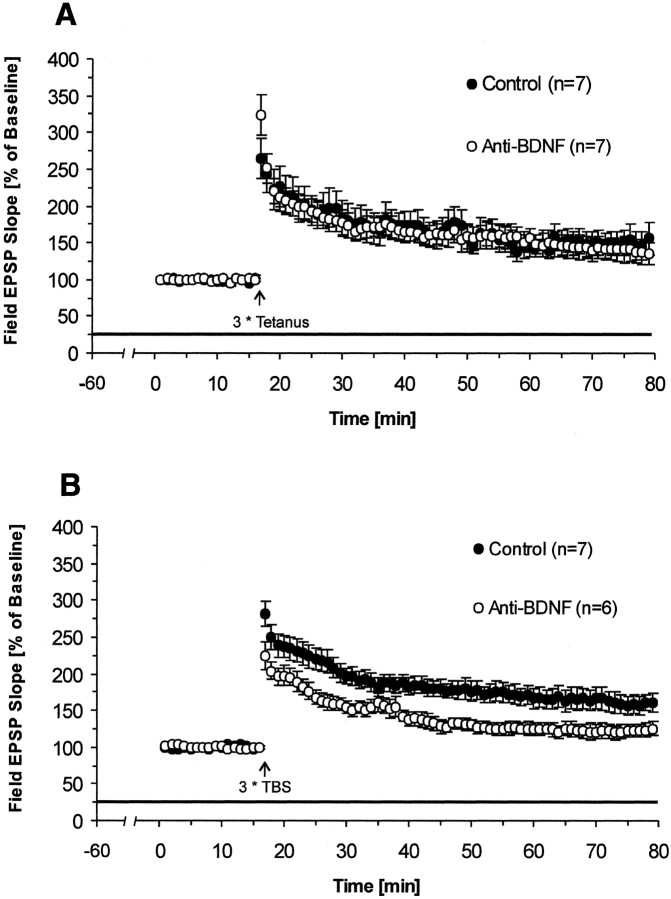
Fig. 4.
Effect of selectively blocking endogenous BDNF on LTP. Pretreatment with BDNF antibody for 1 hr significantly reduces LTP induced by TBS but not by tetanic stimulation. A, Effects of BDNF antibody on tetanus-induced LTP (BDNF Ab,n = 7 slices, 4 mice; control,n = 7 slices, 4 mice). B, Effects of BDNF antibody on TBS-induced LTP (BDNF Ab, n = 6 slices, 4 mice; control, n = 7 slices, 4 mice).
TrkB-IgG fusion protein attenuates LTP induced by TBS but not by tetanus
At present, two kinds of LTP-inducing stimuli, tetanus and TBS, are widely used to induce LTP in hippocampal slices. Both protocols were applied in our experiments in combination with the blockade of neurotrophins. First, we checked the effect of TrkB-IgG FP on LTP induced by tetanus. After the slice was superfused by medium containing either 4 μg/ml FP or the control solution for 1 hr, three trains of 100 Hz tetani were delivered to the Schaffer collateral–CA1 synapses to induce LTP. We found that LTP measured 1 hr after tetanus was not reduced by the TrkB-IgG FP compared with control slices (average slope of fEPSPs to baseline 55–60 min after tetanus: TrkB-IgG, 157 ± 24%; n = 6; control, 135 ± 10%; n = 6; p > 0.2; one-tailedt test) (Fig. 3A). TBS constitutes another important stimulus protocol to induce LTP. Compared with tetanus, TBS is a weak stimulus, and it is designed to mimic the theta rhythm in the hippocampus. We therefore also used this stimulus paradigm to induce LTP. In contrast to the tetanus-induced LTP, we found a significant reduction in LTP 1 hr after TBS in TrkB-IgG fusion protein-treated slices compared with their controls (average slope of fEPSPs to baseline 55–60 min after TBS: TrkB-IgG, 132 ± 14%;n = 7; control, 167 ± 12%; n = 5; p < 0.04; one-tailed t test) (Fig.3B). Interestingly, PTP was also significantly lower in the treatment group, similar to the results of Figurov et al. (1996), who used a similar stimulation paradigm to test the effects of TrkB-IgG fusion proteins. Synaptic transmission in the control pathway remained unaffected (average slope of fEPSPs to baseline in the nontetanized pathway 1 hr after TBS: TrkB-IgG, 93 ± 5%; n = 7; control, 93 ± 3.0%; n = 5; p> 0.45; one-tailed t test). This confirms previous reports (Figurov et al., 1996; Kang et al., 1997) suggesting that ligands of TrkB receptor might have direct effects on TBS-induced LTP.
BDNF antibody reduces LTP induced by TBS but not by tetanus
The experiments above showed that blocking ligands of TrkB receptor reduced TBS-induced LTP. However, it still remains unclear which of the endogenous ligands of the TrkB receptor (BDNF, NT4/5, or both) participate in the process of LTP. Therefore, in the following experiments, we also used a specific monoclonal antibody against BDNF to determine whether blocking endogenous BDNF alone will affect hippocampal LTP in the same way. As before, we first checked the effect of BDNF antibody on tetanus-induced LTP and found that, in the presence of 4 μg/ml BDNF antibody, LTP was again not significantly reduced (average slope of fEPSPs to baseline 55–60 min after tetanus: BDNF Ab, 138 ± 16%; n = 7; control, 151 ± 18%;n = 7; p > 0.3; one-tailedt test) (Fig. 4A). Slices pretreated with 4 μg/ml BDNF antibody for 1 hr and stimulated with TBS, however, showed significant reduction in LTP (average slope of fEPSPs to baseline 55–60 min after TBS: BDNF Ab, 122 ± 7%;n = 6; control, 157 ± 12%; n = 7; p < 0.018; one-tailed t test) (Fig.4B), whereas basal synaptic transmission in the control pathway remained unaffected (average slope of fEPSPs to baseline in the nontetanized pathway 55–60 min after TBS: BDNF Ab, 85 ± 1%; n = 4; control, 90 ± 4%;n = 4; p > 0.2; one-tailedt test). The difference between the two groups became apparent immediately after TBS. Thus, PTP after TBS stimulation was reduced by application of BDNF antibodies in contrast to PTP after tetanic stimulation. Together, these results show that hippocampal LTP is significantly reduced by the BDNF-antibody and to a comparable degree as seen with TrkB-IgG FP.
Anti-NT3 antibody does not block LTP induced by either tetanus or TBS
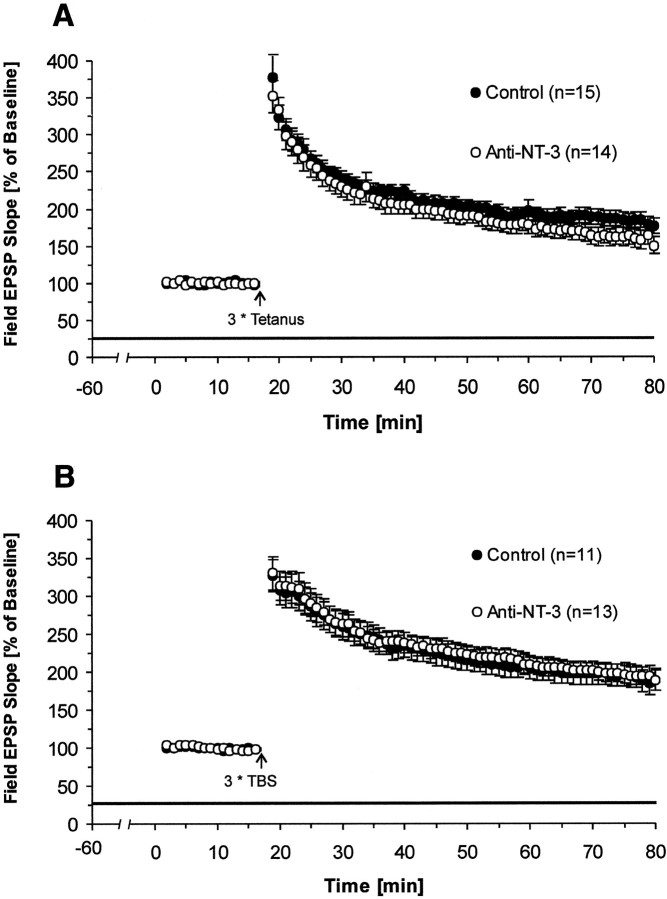
Previous studies that applied neurotrophins exogenously to the medium have suggested a direct role for NT3 in the process of LTP. We therefore asked the question whether blocking of endogenous NT3 by a specific function-blocking monoclonal antibody alters the induction or expression of hippocampal LTP. First, we checked the antibody effect on basal synaptic transmission and short-term plasticity. We found that neither basal synaptic transmission nor short-term synaptic plasticity in hippocampal slices were affected (data not shown). Second, we pretreated slices with antibodies for 1 hr and recorded LTP in control and NT3 antibody-treated slices. We also did not find any significant reduction effect in NT3 antibody-treated slices on either tetanus-induced LTP (average slope of fEPSPs to baseline 55–60 min after tetanus: NT3 Ab, 160 ± 12%; n = 14; control, 182 ± 10%; n = 15; p > 0.09; one-tailed t test) (Fig.5A) or TBS-induced LTP (average slope of fEPSPs to baseline 55–60 min after TBS: NT3 Ab, 190 ± 11%; n = 13; control, 194 ± 16%;n = 11; p > 0.2; one-tailedt test) (Fig. 5B). These results indicate that endogenous NT3 seems not to be directly involved in hippocampal LTP.
Fig. 5.
Effect of blocking endogenous NT3 on LTP. NT3 antibody does not affect LTP induced by either tetanus or TBS. Pretreatment time for slices was again 1 hr before recordings were started. A, Effects of NT3 antibody on tetanus-induced LTP (NT3 Ab, n = 14 slices, 10 mice; control,n = 15 slices, 9 mice). B, Effects of NT3 antibody on TBS-induced LTP (NT3 Ab, n = 13 slices, 10 mice; control, n = 11 slices, 8 mice).
BDNF antibody does not significantly reduce LTP when applied after TBS
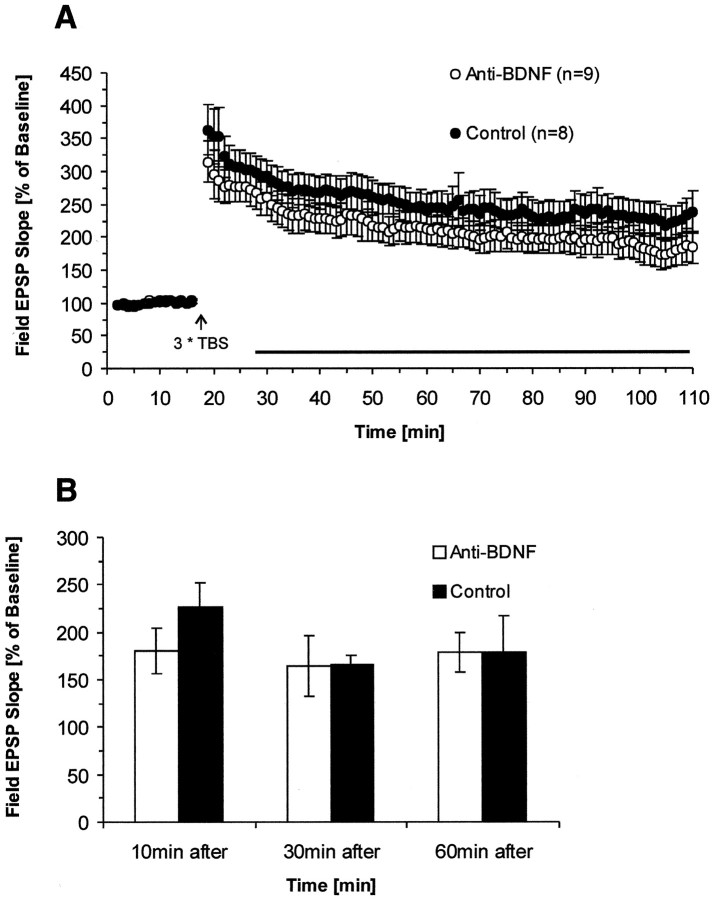
Because the data thus far indicate that endogenous BDNF but not NT-4/5 or NT3 directly participates in TBS-induced LTP, we were then interested in determining the time during which BDNF needs to be present. Is endogenous BDNF required during a longer time period before and after LTP induction, arguing for a more permissive role, or might it be only required during the time period of LTP induction, in line with an instructive role of the protein? To address this question, we washed the BDNF antibody into the recording chamber at different time points (10, 30, and 60 min after delivery of TBS), and LTP was recorded for at least 1 hr after antibody infusion. In these experiments, we only used slices with a strong initial PPF (>200% at 30 msec ISI), because these slices expressed reliably more stable and higher levels of LTP, important for long-term recordings. When the BDNF antibody was applied 10 min after TBS, we found that early phase LTP in BDNF antibody-treated slices was reduced; however, this reduction was not significant (average slope of fEPSPs to baseline 90 min after TBS: BDNF Ab, 177 ± 23%; n = 9; control, 223 ± 24%;n = 8; p > 0.1; one-tailedt test; control pathway: BDNF Ab, 84 ± 5%n = 6; control, 97 ± 8%; n = 5;p > 0.1) (Fig.6A). Although the greater variability of the data in this experiment require a cautious interpretation and conclusion about the absence of a significant reduction effect, it is important to note that wash-in of the antibodies does not cause any increase in the difference between the two groups. Second, the smaller LTP present in the antibody group already exists from the very beginning after TBS, that is, at a time before the antibodies were washed in. Together, this experiment suggest that, after induction, endogenous BDNF does not seem to be required anymore for the maintenance of early phase LTP. We also tested whether blocking of BDNF had effects on the later periods of LTP. For this, antibodies were also applied 30 or 60 min after TBS, and long-term recordings (>3 hr) were made. There was no significant reduction of long-lasting LTP in antibody-treated slices (30 min after treatment–average slope of fEPSPs to baseline 180 min after TBS: BDNF Ab, 164 ± 32%; n = 6; control, 167 ± 9%; n = 4; p > 0.5; one-tailed t test; 60 min after treatment–average slope of fEPSPs to baseline 180 min after TBS: BDNF Ab, 179 ± 21%;n = 5; control, 180 ± 38%; n = 5; p > 0.5; one-tailed t test) (Fig.6B). These results strongly suggest that, after the delivery of TBS, endogenous BDNF is not required, for neither early nor medium-to-late phase LTP.
Fig. 6.
Effect on LTP is dependent on the time when endogenous BDNF is blocked by the antibodies. A, BDNF antibodies do not reduce LTP significantly when applied 10 min after TBS. B, Applications 30 or 60 min after TBS also do not affect LTP and its maintenance. Field EPSP slopes were plotted as percent of baseline. Bars indicate level of LTP at the end of each recording averaged over a 5 min period. Late phase LTP (3 hr after TBS) was measured for 30 and 60 min treatment groups (10 min group: BDNF Ab, n = 9 slices, 7 mice; control,n = 8 slices, 7 mice; 30 min group: BDNF Ab,n = 6 slices, 6 mice; control,n = 4 slices, 4 mice; 60 min group: BDNF Ab,n = 5 slices, 5 mice; control,n = 5 slices, 5 mice).
DISCUSSION
Although several recent studies have focused on the role of BDNF in neurotransmission and LTP within the hippocampus, data on the potential involvement of other neurotrophins are sparse and mainly based on experiments using application of exogenous neurotrophins. With regard to potentiation of synaptic strength in CA1 (the area investigated in our study), BDNF and NT3 seem to be equally effective (Kang and Schuman, 1995). In this study, we specifically addressed the question as to what role the endogenous neurotrophins BDNF, NT4/5, and NT3 play in the process of LTP.
Specificity of neurotrophic action on LTP
Direct evidence for the involvement of endogenous BDNF on LTP has been obtained by experiments using BDNF knock-out mice that show greatly reduced enhancement (Korte et al., 1995). Unlike NT3 −/− animals, BDNF null mutants survive long enough for the kind of experiments to be performed in the postnatal hippocampus. LTP can be “rescued” in BDNF −/− and +/− animals by incubating these slices either in recombinant BDNF (Patterson et al., 1996) or by reintroducing the BDNF gene (Korte et al., 1996). Additionally, two recent studies demonstrate that blocking the endogenous ligands of the TrkB receptor in slices by TrkB-IgG FP also affects LTP (Figurov et al., 1996; Kang et al., 1997). These studies left the question unresolved as to whether the effect was obtained by blockade of endogenous BDNF, by blockade of NT4/5, or both. Although the relative abundance of BDNF could be taken as evidence for a significant physiological role (Ernfors et al., 1990;Hofer et al., 1990; Martinez et al., 1998), it is clear that NT4/5 is also expressed in the hippocampus during all stages of development (Klein et al., 1992; Timmusk et al., 1993). Experiments using a TrkB-IgG FP and a BDNF-specific antibody enabled us to distinguish between the roles of NT4/5 and BDNF in hippocampal LTP. Although TrkB fusion protein bind equally to NT4/5 and BDNF and with slightly lower affinity, even to NT3 (Shelton et al., 1995), the similar reduction in LTP seen in response to the fusion protein and the BDNF-specific antibody argues against a direct and necessary involvement of NT-4/5 in hippocampal LTP.
So far, no clear evidence is available on the role of endogenous NT3 in the process of hippocampal CA1 LTP. Although NT3 and TrkC are strongly expressed early in development, the expression of both is weaker but still present within the adult hippocampus (Ernfors et al., 1990;Maisonpierre et al., 1990; Lamballe et al., 1994; Martinez et al., 1998). Previous studies have shown that exogenous NT3 added to the perfusion medium of slices leads to a fast potentiation of synaptic strength in the CA1 region of hippocampus, similar to the effects obtained with BDNF (Kang and Schuman, 1995), suggesting that NT3 could be potentially as important as BDNF for LTP. The experiments presented here tested the role of endogenous NT3 in LTP by blocking its function through a specific antibody. They show that blocking of NT3 affects neither TBS- nor tetanus-induced LTP. Given that both the NT3 and the BDNF antibodies are IgG molecules, their diffusion properties should be very similar, and both should reach the same sites. Therefore, our results strongly argue against a direct role of endogenous NT3 in the induction of hippocampal CA1 LTP. A similar conclusion was recently drawn by Kokaia et al. (1998) for LTP in the lateral perforant path–granular cell pathway. However, it was based on results using heterozygous +/− NT3 knock-out mice, because of the nonviability of the homozygous −/− mice. Several reports document the fact that the presence of one NT3 allele has readily measurable functional consequences, for example, on the number of sensory neurons or on muscles spindles (Ernfors et al., 1994; Airaksinen and Meyer, 1996; Airaksinen et al., 1996).
Previous findings about NT3 and synaptic transmission may then have been caused by fast, short-term effects of NT3 through increase in evoked EPSC amplitude or increase in miniature EPSC frequency akin to the effects observed for BDNF of minis, not directly related to LTP (Lohof et al., 1993; Lessman et al., 1994; Levine et al., 1995; Li et al., 1998). Moreover, the increase of synaptic transmission by exogenous NT3 may also have been brought about by the secondary release of BDNF. Neurotrophins can trigger the release of other neurotrophins in dissociated cultures of hippocampal neurons (Canossa et al., 1997) and PC12 cells (Kruttgen et al., 1998). Neurotrophin-induced neurotrophin release could therefore not only be an explanation of the NT3 results, but it could also constitute an important factor during normal LTP induction. Still, the lack of any effects of NT3 blockade on LTP makes it highly unlikely that this neurotrophin is directly involved in LTP or that BDNF-induced release of other members of the neurotrophin family is involved in LTP induction.
Stimulus dependency
Interestingly, blocking of BDNF by antibodies only impaired LTP induced by theta-burst stimulation but not by tetanic stimulation, whereas in BDNF knock-out mice, tetanic stimulation-induced LTP was strongly impaired (Korte et al., 1995, 1996; Patterson et al., 1996). One possible explanation for the failure of BDNF antibodies to block tetanus-induced LTP is that BDNF levels after a tetanus might exceed the buffering capacities of the antibody in the tissue at the critical time point. In fact, a number of reports showing that BDNF is released in an activity-dependent manner (Blöchl and Thoenen 1995;Blöchl et al., 1995; Thoenen, 1995) would predict that BDNF concentrations are considerably higher after a tetanus than after TBS, thereby providing a straightforward explanation for this observation.
A second explanation is that tetanus and TBS activate different signaling pathways that are necessary for LTP induction (Kang et al., 1997). Small differences in stimulation paradigms are known to trigger different signaling pathways leading to potentiation, depotentiation, or depression of synapses (Stäubli and Chun, 1996; Xu et al., 1998). Thus, unlike tetanic stimulation, TBS could act through a pathway that involves immediate BDNF effects. In particular, application of exogenous BDNF can occlude subsequent potentiation induced by TBS but not by tetanus (Kang et al., 1997). However, because tetanic stimulation-induced LTP was so strongly diminished in BDNF knock-out mice, these additional signaling pathway(s) would have to depend on the constitutive presence of BDNF. BDNF could activate gene expression through cAMP-response element binding protein, an important component of LTP in hippocampus (Bourtchuladze et al., 1994; Finkbeiner et al., 1997). Thus, these additional pathways in LTP may be impaired and not be activated by tetanic stimulation in BDNF knock-out mice while they were active when endogenous BNDF was blocked by antibodies only for a short period of time.
Time dependency of BDNF requirement
A major question concerning the role of BDNF in LTP is the time period during which BDNF has to be present to exert its effects. One hypothesis is that BDNF acts as a retrograde messenger that is released during LTP induction from the dendrites of pyramidal neurons and subsequently induces the potentiation of synaptic transmission. Direct evidence proving this hypothesis is still missing. Using the BDNF antibody, we could acutely block endogenous BDNF during different time periods relative to the induction of LTP to determine when endogenous BDNF is needed for induction or expression of LTP. Our results show that the presence of BDNF antibodies in the superfusion solution for 80 min before LTP induction significantly reduced LTP induced by TBS from the beginning, which suggests that BDNF is needed during the induction process or before. Figurov et al. (1996) have recently described for young animals that BDNF may influence LTP by changing the effectiveness of tetanic stimulation or TBS via facilitation of synaptic transmission. This effect also led to enhanced paired-pulse facilitation at short interpulse intervals. In our experiments, blockade of BDNF had no measurable effects on basic synaptic transmission (Figs. 1, 2) or on synaptic depression measured during TBS or tetanus stimulation. BDNF therefore seems to affect LTP induction directly rather than by changing the effectiveness of the LTP-inducing stimulus.
Infusion of the antibody starting at different time points after TBS did not significantly reduce early (90 min after TBS) or late (up to 3 hr after TBS) LTP. Although in the 10 min postapplication experiment the larger variability of the data may have obscured a potential significant reduction in LTP by the antibodies, later stages of LTP (up to 3 hr after TBS) clearly remained unaffected when the antibodies were applied 30 or 60 min after TBS. Endogenous BDNF therefore seems to be only required within a short time period before (80 min) or around the time of induction of TBS-induced LTP. Assuming similar short penetration times of the BDNF antibody, these results seem to be in contrast to the results obtained by Kang et al. (1997). Using TrkB-IgG fusion proteins, they found that the application 30–60 min after LTP could still block late phase of LTP, whereas later application had no effect. Because in these experiments a different tetanic stimulation protocol (1 sec at 100 Hz, three times, 5 min apart) was used to induce late phase LTP, this could indicate that the pattern of stimulation might have relevant consequences on the effect of BDNF on later stages of LTP. Another explanation for the different findings may be differences between the onset of blocking in the two sets of experiments. A delayed onset of blocking in the postapplication experiments with the antibody could exceed a potential critical time window during which BDNF is needed. Unfortunately, the question of when the onset of the critical blocking concentration of the antibodies within tissue is reached cannot conclusively be answered. Thus, postapplication experiments in which the antibodies were applied after LTP induction can only give a rough estimate of the temporal requirements of BDNF for LTP.
In summary, our results provide clear evidence that endogenous BDNF plays a unique and specific role in LTP. We could show that, under the same stimulus conditions and similar blocking antibody concentrations, other neurotrophins are not involved in CA1 LTP. Our study demonstrates that BDNF affects LTP directly and not via secondary effects through induced release of other neurotrophins. Although the exact mechanism of the BDNF action still remains unclear, our results further show that the lack of BDNF for a relatively short period of time, before but not after LTP-induction, is sufficient to block LTP. This seems to point toward the possibility that BDNF is important during LTP induction. However, the need for a constitutive activation of TrkB receptors before LTP induction cannot be excluded and may be very important for the activation of other LTP relevant pathways.
Footnotes
This work was supported in part by the Deutsche Forschungsgemeinschaft (SFB391) and the European Community Biotech Program (T.B.). We thank Volker Staiger for excellent technical assistance, Martin Korte for critical comments on this manuscript, and Ilse Bartke at Roche Diagnostics (Penzberg, Germany) for providing us with the monoclonal neurotrophin antibodies.
G. C.’s present address: Center for Neuroscience, The University of Edinburgh, Edinburgh EH8 9LE, UK.
Correspondence should be addressed to Dr. Tobias Bonhoeffer, Max-Planck-Institut für Neurobiologie, Am Klopferspitz 18 A, 82152 München-Martinsried, Germany.
REFERENCES
- 1.Airaksinen MS, Meyer M. Most classes of dorsal root ganglion neurons are severely depleted but not absent in mice lacking neurotrophin-3. Neuroscience. 1996;73:907–911. doi: 10.1016/0306-4522(96)00203-5. [DOI] [PubMed] [Google Scholar]
- 2.Airaksinen MS, Koltzenburg M, Lewin GR, Masu Y, Helbig C, Wolf E, Brem G, Toyka KV, Thoenen H, Meyer M. Specific subtypes of cutaneous mechanoreceptors require neurotrophin-3 following peripheral target innervation. Neuron. 1996;16:287–295. doi: 10.1016/s0896-6273(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 3.Berninger B, Poo M-M. Fast action of neurotrophic factors. Curr Opin Neurobiol. 1996;6:324–330. doi: 10.1016/s0959-4388(96)80115-2. [DOI] [PubMed] [Google Scholar]
- 4.Blöchl A, Thoenen H. Characterization of nerve growth factor (NGF) release from hippocampal neurons: evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 5.Blöchl A, Berninger B, Berzaghi M, Castrén E, Lindholm D, Thoenen H. Neurotrophins as mediators of neuronal plasticity. In: Ibanez CF, editor. Life and death in the nervous system. Elsevier; Oxford: 1995. pp. 260–272. [Google Scholar]
- 6.Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 7.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 8.Canossa M, Griesbeck O, Berninger B, Campana G, Kolbeck R, Thoenen H. Neurotrophin release by neurotrophins; implications for activity dependent neuronal plasticity. Proc Natl Acad Sci USA. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castrén E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in the visual cortex. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies AM. The role of neurotrophins in the developing nervous system. J Neurobiol. 1994;25:1334–1349. doi: 10.1002/neu.480251103. [DOI] [PubMed] [Google Scholar]
- 11.Dechant G, Biffo S, Okazawa H, Kolbeck R, Pottgiesser J, Barde Y-A. Expression and binding characteristics of the BDNF receptor chick trkB. Development. 1993;119:545–558. doi: 10.1242/dev.119.2.545. [DOI] [PubMed] [Google Scholar]
- 12.Dragunow M, Beilharz E, Mason B, Lawlor P, Abraham W, Gluckman P. Brain-derived neurotrophic factor expression after long-term potentiation. Neurosci Lett. 1993;160:232–236. doi: 10.1016/0304-3940(93)90420-p. [DOI] [PubMed] [Google Scholar]
- 13.Ernfors P, Wetmore C, Olsom L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the NGF family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 14.Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 15.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 16.Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 17.Gaese F, Kolbeck R, Barde Y-A. Sensory ganglia require neurotrophin-3 early in development. Development. 1994;120:1613–1619. doi: 10.1242/dev.120.6.1613. [DOI] [PubMed] [Google Scholar]
- 18.Griesbeck O, Blöchl A, Carnahan JF, Nawa H, Thoenen H. Characterization of brain-derived neurotrophic factor (BDNF) secretion from hippocampal neurons. Soc Neurosci Abstr. 1995;21:417. [Google Scholar]
- 19.Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 21.Kang H, Jia LZ, Suh K-Y, Tang L, Schuman EM. Determinants of BDNF-induced hippocampal plasticity: role of the trkB receptor and the kinetics of neurotrophin delivery. Learn Mem. 1996;3:188–196. doi: 10.1101/lm.3.2-3.188. [DOI] [PubMed] [Google Scholar]
- 22.Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for trkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 23.Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol (Lond) 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein R, Lamballe F, Bryant S, Barbacid M. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992;8:947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- 25.Knipper M, Berzaghi M, Blöchl A, Breer H, Thoenen H, Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994;6:668–671. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 26.Kokaia M, Asztely F, Olofsdotter K, Balet Sindreu C, Kullmann DM, Olle Lindvall Endogenous neurotrophin-3 regulates short-term plasticity at lateral perforant path–granule cell synapses. J Neurosci. 1998;18:8730–8739. doi: 10.1523/JNEUROSCI.18-21-08730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolbeck R, Bartke I, Eberle W, Barde YA. Brain-derived neurotrophic factor (BDNF) levels in the nervous system of wild-type and neurotrophin gene mutant mice. J Neurochem. 1999;72:1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- 28.Korte M, Carroll P, Wolff E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruttgen A, Moller JC, Heymach JV, Shooter EM. Neurotrophins induce release of neurotrophins by the secretory pathway. Proc Natl Acad Sci USA. 1998;95:9614–9619. doi: 10.1073/pnas.95.16.9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamballe F, Smeyne RJ, Barbacid M. Developmental expression of trkC, the neurotrophin-3 receptor, in the mammalian nervous system. J Neurosci. 1994;14:14–28. doi: 10.1523/JNEUROSCI.14-01-00014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lessmann V, Gottman K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurons. NeuroReport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 33.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewin G, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 35.Li Y-X, Xu Y, Ju D, Lester HA, Davidson N, Schuman EM. Expression of a dominant negative TrkB receptor, T1, reveals a requirement for presynaptic signaling in BDNF-induced synaptic potentiation in cultured hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:10884–10889. doi: 10.1073/pnas.95.18.10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindholm D, Castren E, Berzaghi M, Blöchl A, Thoenen H. Activity-dependent and hormonal regulation of neurotrophin mRNA levels in the brain- implications for neuronal plasticity. J Neurobiol. 1994;25:1362–1372. doi: 10.1002/neu.480251105. [DOI] [PubMed] [Google Scholar]
- 37.Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 38.Lo DC. Neurotrophic factors and synaptic plasticity. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 39.Lohof AM, Ip NY, Poo M-M. Potentiation of developing neuromuscular synapses by neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 40.Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 41.Martinez A, Alcantara S, Borrell V, Delrio JA, Blasi J, Otal R, Campos N, Boronat A, Barbacid M, Silossantiago I, Soriano E. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connection. J Neurosci. 1998;18:7336–7350. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1080–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 43.Patterson SL, Abel T, Deuel TAA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 44.Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stäubli U, Chun D. Factors regulating the reversibility of long-term potentiation. J Neurosci. 1996;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 47.Timmusk T, Belluardo N, Metsis M, Persson H. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. Eur J Neurosci. 1993;5:605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 48.Wetmore C, Ernfors P, Persson H, Olson L. Localization of BDNF mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1989;109:141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- 49.Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394:891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- 50.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zafra F, Castrén E, Thoenen H, Lindholm D. Interplay between glutamate and γ-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci USA. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]