Abstract
To understand better how spontaneous motoneuron activity and intramuscular nerve branching influence motoneuron survival, we chronically treated chicken embryos in ovo with eitherd-tubocurarine (dTC) or muscimol during the naturally occurring cell death period, assessing their effects on activity by in ovo motility measurement and muscle nerve recordings from isolated spinal cord preparations. Because muscimol, a GABAA agonist, blocked both spontaneous motoneuron bursting and that elicited by descending input but did not rescue motoneurons, we conclude that spontaneous bursting activity is not required for the process of normal motoneuron cell death. dTC, which rescues motoneurons and blocks neuromuscular transmission, blocked neither spontaneous nor descending input-elicited bursting and early in the cell death period actually increased burst amplitude. These changes in motoneuron activation could alter the uptake of trophic molecules or gene transcription via altered patterns of [Ca2+]i, which in turn could affect motoneuron survival directly or indirectly by altering intramuscular nerve branching. A good correlation was found between nerve branching and motoneuron survival under various experimental conditions: (1) dTC, but not muscimol, greatly increased branching; (2) the removal of PSA from NCAM partially reversed the effects of dTC on both branching and survival, indicating that branching is a critical variable influencing motoneuron survival; (3) muscimol, applied with dTC, prevented the effect of dTC on survival and motoneuron bursting and, to a large extent, its effect on branching. However, the central effects of dTC also appear to be important, because muscimol, which prevented motoneuron activity in the presence ofdTC, also prevented the dTC-induced rescue of motoneurons.
Keywords: motoneuron survival, cell death period, activity blockade, intramuscular nerve branching, muscimol, spontaneous burst activity
Chronic administration ofd-tubocurarine (dTC), an acetylcholine receptor (AChR) antagonist, during development induces neuromuscular activity blockade and rescues motoneurons from naturally occurring cell death (Hamburger, 1975; Oppenheim and Chu-Wang, 1977; Pittman and Oppenheim, 1978). It also produces an increase in intramuscular nerve branching and synapse formation at the onset of the cell death period, leading to the suggestion that enhanced trophic factor uptake at these sites might play a critical role in their enhanced survival (Dahm and Landmesser, 1988, 1991; Oppenheim, 1989; Landmesser, 1992; Calderó et al., 1998). Previous work had shown that both dTC and α-bungarotoxin (αBTX, another AChR antagonist) could block neuromuscular activity by acting not only at the neuromuscular junction (NMJ) but also by acting centrally within the spinal cord (Landmesser and Szente, 1986). High densities of the α7 nicotinic AChR are, in fact, present in the lateral motor column during the motoneuron cell death period (Renshaw et al., 1993; Renshaw and Goldie, 1996). Also supporting a site of action for dTC other than the NMJ was the observation of Hory-Lee and Frank (1995) that subparalytic doses ofdTC rescued motoneurons from cell death.
Thus, it is presently unclear whether dTC rescues motoneurons from cell death by acting at central or peripheral sites or both. The increase in motoneuron survival could result from any combination of the following observed effects of dTC: neuromuscular activity blockade (Oppenheim and Chu-Wang, 1977; Pittman and Oppenheim, 1978; Ding et al., 1983; Dahm and Landmesser, 1988), increases in intramuscular nerve branching and synaptogenesis (Ding et al., 1983; Dahm and Landmesser, 1988, 1991; Oppenheim, 1989;Landmesser, 1992), blockade of peripheral AChRs (postsynaptic AChRs at the NMJ or presynaptic ones on motoneuron axons), or blockade of central AChRs within the spinal cord (Landmesser and Szente, 1986;Renshaw et al., 1993; Renshaw and Goldie, 1996).
To test whether neuromuscular activity blockade per se was the critical feature of the dTC treatments that influenced motoneuron survival, we blocked activity in a manner that avoided any direct effects on nicotinic receptors. In the chick embryonic spinal cord, during the period of motoneuron cell death, GABAergic immunoreactive interneurons are present (Antal et al., 1994) and are an important component of the circuit that drives motoneurons in periodic bursts of electrical activity (Sernagor et al., 1995; Chub and O’Donovan, 1998). We found that application of the GABAA agonist muscimol blocked spontaneous motoneuron bursting in isolated spinal cord preparations and blocked spontaneous motility when it was appliedin ovo. Thus, by acting on the circuitry within the spinal cord, possibly including motoneuron somas, muscimol indirectly blocks neuromuscular activity but does not act on nicotinic receptors at either the NMJ or in the spinal cord. In this study we compare the effects of dTC versus muscimol-induced activity blockade on motoneuron survival and intramuscular nerve branching.
MATERIALS AND METHODS
White Leghorn fertile chick eggs (CWRU Squire Farm) were incubated in a humidified forced-air draft incubator at 38°C and used as described below. An opening was made in stage 23–25 (Hamburger and Hamilton, 1951) eggs, and the square hole subsequently was sealed with paraffin and a sterile coverslip.
Drug treatments. These began at stage (st.) 28–29, with the drug applied daily for dTC (2 mg/d; Sigma, St. Louis, MO) or twice a day for muscimol (0.1 mg/d; Sigma) and diluted in buffered Tyrode’s solution [containing (in mm) 139 NaCl, 3 KCl, 17 NaHCO3, 1 MgCl, and 3 CaCl2]. These concentrations were used because they are just sufficient to block the motility of the hindlimb in ovo. The actual concentration of the drug in the embryo was estimated to be approximately 5 × 10−6m fordTC because this concentration was just sufficient to block transmission at the NMJ in the isolated cord preparation (see Fig.1g). For muscimol, the estimated concentration was approximately 5 × 10−6m because this concentration was also just sufficient to block spontaneous bursting in the isolated cord preparation (see Fig. 1a,b). Endosialidase-N (endo-N) was used and injected as previously described (Landmesser et al., 1990). Briefly, 0.1–1.0 μl of a highly purified solution of endo-N (30,000 U/ml; gift of Dr. Urs Rutishauser, Sloan-Kettering Institute, New York, NY) was injected with a glass micropipette into the dorsoposterior thigh on one side of a st. 26–27 embryo. A tracer dye, trypan blue in Tyrode’s solution (sterile 0.04% trypan blue; Life Technologies, Grand Island, New York), was coinjected to ensure the location of the injection. Controls of dye and buffer injection alone showed no abnormalities when compared with noninjected embryos. Single localized injections of endo-N into the dorsal thigh were shown previously to remove polysialic acid (PSA) from the injected thigh for up to 3 d but not to affect the PSA levels in the contralateral limb or spinal cord (Landmesser et al., 1990).
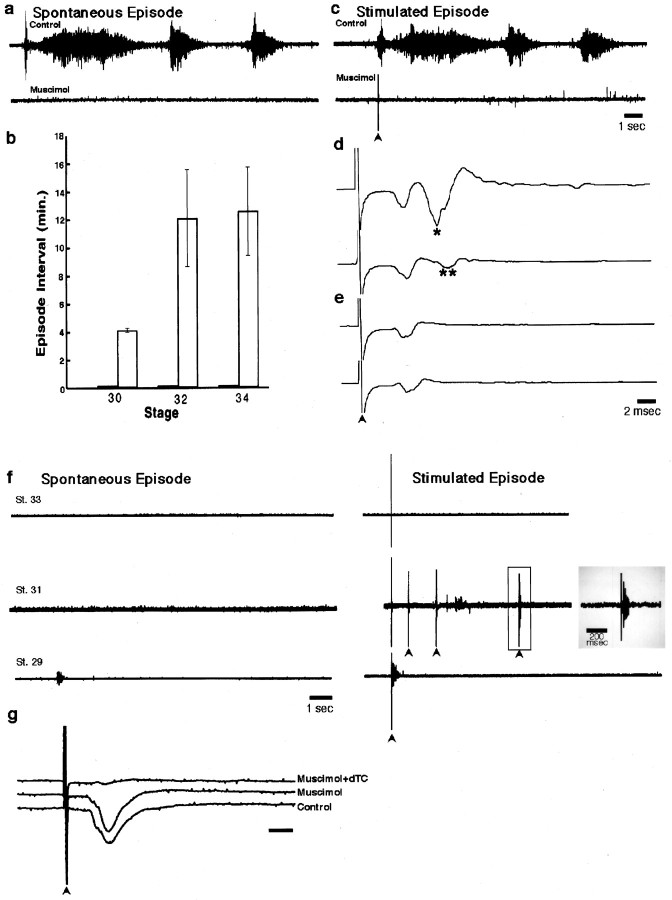
Fig. 1.
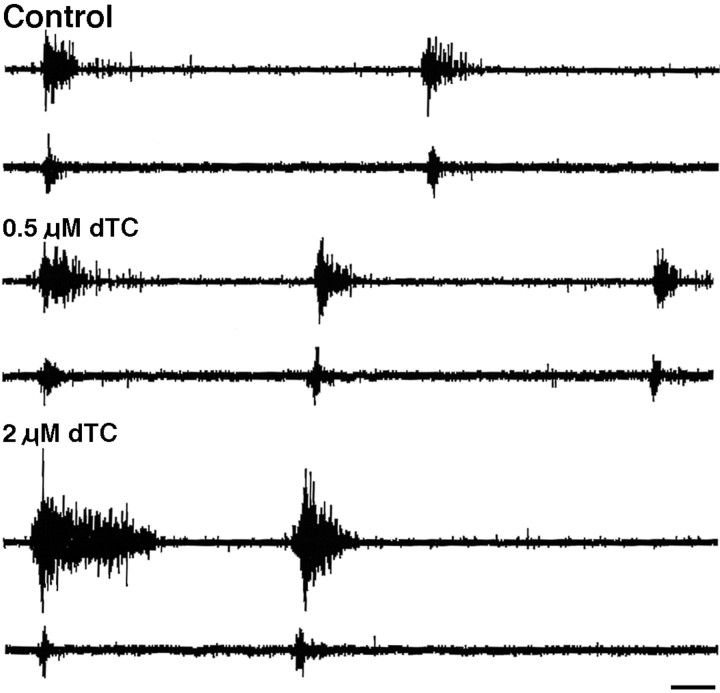
Characterization of the effects of muscimol: the acute (a–e) and chronic (f) effects of muscimol on motoneuron bursting in an isolated cord preparation. a, Neurogram recording from the femorotibialis muscle nerve of an episode of spontaneous bursting from a st. 32 chick embryo under control conditions (top trace) and in the presence of 5 × 10−6m muscimol (bottom trace). Note the absence of any bursts. b, Mean time interval ± SEM between spontaneous bursting episodes under control (open bars) and muscimol treatments (dark bars) at st. 30, 32, and 34 (n = 7, 6, and 11, respectively). c, A single stimulus (arrowhead) to the cervical/thoracic spinal cord of a st. 32 chick embryo elicits a bursting episode before (toptrace), but not after (bottom trace), the application of 5 × 10−6m muscimol. d, In the presence of 5 × 10−6mmuscimol, 1/sec stimulation of the motoneurons directly at LS2 elicits two peaks (top trace; single asterisk denotes second peak); the second peak (two asterisks), but not the first, fatigues with 10 Hz stimulation (bottom trace).e, In the presence of 5 × 10−6m muscimol and low Ca2+ Tyrode’s solution, 1/sec stimulation of the motoneurons directly at LS2 elicits one peak (top trace), which does not fatigue at 10 Hz stimulation (bottom trace); st. 32 chick embryo for a, c, d, and e. Calibration bars: c(also applies to a), 1 sec; d, e, 2 msec. Gain for d, e, and g is 5× that ofa–c and f. f, Effect of chronic muscimol treatment as follows: at st. 33 (top traces), no bursting activity occurred spontaneously nor could it be elicited by stimulation; at st. 31(middle traces), no bursting occurred spontaneously or after single stimuli. However, sometimes with multiple stimuli (arrowheads) a very short burst could be elicited, in this case after the fourth stimulus. This very short burst (enclosed in open gray box) is displayed on an expanded time scale at the end of the trace (shaded gray box). The very brief length of these bursts can be seen by comparing this trace with the control inc. At st. 29 (bottom traces) very brief bursts occurred spontaneously (left) and after stimulation of descending input (right).g, Effect of muscimol on synaptic transmission at the NMJ was assessed by recording the compound action potential elicited in a st. 29 sartorius muscle by stimulation (arrowhead) of the LS1 spinal nerve. Under both control conditions and in the presence of 5 × 10−6m muscimol, the sartorius muscle exhibited a vigorous response. However, the addition of 5 × 10−6 mdTC blocked the postsynaptic muscle response. Calibration bar, 5 msec.
The motility of the embryo after target innervation was observed as described previously (Dahm and Landmesser, 1988) by counting whole hindlimb movements (kicks) for a 3 min observation period, using a dissecting microscope focused through the hole in the egg onto the hindlimb. For dTC drug treatments the hindlimb movement blockade was observed as reported previously (Oppenheim, 1975; Dahm and Landmesser, 1988). For muscimol drug treatments, careful observations were made to characterize the precise motions of the hindlimb in both acute and chronic treatments and in both the isolated cord preparation and in ovo. The total number of embryos used included the following: for dTC treatments, n = 108 (mortality rate = 65%); for muscimol, n = 119 (mortality rate = 18%); for endo-N + dTC,n = 108 (mortality rate = 82%); for endo-N,n = 28 (mortality rate = 25%); for muscimol +dTC, n = 20 (mortality rate = 30%). The mortality rate with muscimol-induced chronic activity blockade was consistently much lower than the relatively high rate that we and others have found with dTC. The reasons for this are unclear but might arise because dTC would act at multiple sites, including autonomic ganglia. In the present study it appeared that muscimol reduced the mortality rate caused by dTC. However, the dTC-induced mortality rate varies enough from study to study that such a conclusion is not warranted at this time. Embryos were decapitated at the appropriate stage, placed in an oxygenated Tyrode’s bath, and processed as described below for either whole mounts or for sectioning.
Muscle whole mounts. These were done as described previously (Dahm and Landmesser, 1988). Briefly, the iliofibularis (IFIB) muscle was exposed by the removal of all other posterior thigh muscles and connective tissue; it was fixed in ice-cold acetone for 5 min, washed [wash = three times for 5 min in PBS (1× PBS)], incubated with the C2 monoclonal antibody (against chick neurofilament) for 1–3 hr, and then washed again. Next, the muscle was fixed further in 3.7% formaldehyde, diluted in 1× PBS for 30 min, and then washed in PBS for three times at 15 min each. Secondary antibody incubation was with goat anti-mouse IgG-FITC (Sigma) overnight at 4°C. After a wash, the IFIB was dissected completely from the hindlimb and mounted in 50% glycerol/PBS with 0.03 mg/ml p-phenylenediamine (Sigma). The immunofluorescence of the labeled intramuscular nerve branching was viewed and photographed on a Nikon Microphot microscope with an FITC cube at a total magnification of 100×. To reconstruct the entire IFIB muscle, we made photographic montages from the individual pictures. Each montage of a muscle was photographed, and from that negative a print of the muscle was made. Then the print was scanned, using the Duophot scanner combined with Fotolook/Adobe Photoshop/Corel Draw for final figure labeling and scale bar placement.
Motoneuron quantification. This was done as previously described (Tanaka and Landmesser, 1986). St. 36 spinal cords were dissected and fixed in Bouin’s solution, dehydrated in ethanol and xylene, and paraffin-embedded. Serial 14 μm sections were cut throughout the lumbosacral cord and then were stained with hematoxylin and eosin-orange G. Serial reconstruction of the spinal cord was done by noting the presence of the DRG and the lateral motor column (LMC) enlargement, which together allow for the identification of the lumbar (L1–L8) spinal cord. Criteria for counting cells as motoneurons included the size of the cell and its location in the LMC and the presence of a nucleus. Cell counts were made of every fifth section in both the left and right LMCs (for endo-N-treated embryos only the injected side was counted). The total cell number per LMC side was obtained by adding together all of the counted cells in each left or right LMC and multiplying the total number of cells by five. Averages of left and right LMC were made, and statistics were generated from these numbers. No corrections were made for double counting on the basis of the criteria stated in Abercrombie (1946), because only relative and not absolute values were needed for comparisons. Nuclear diameters were measured in each drug treatment, and no significant difference in nuclear size was found for any treatment when it was compared with control. Thus, the numbers used in the graphs that are presented reflect raw, uncorrected (Abercrombie, 1946) counts showing the average cell count in a LMC in any one treatment.
Physiological characterization of the effects of dTC and muscimol. The electrophysiology was done as described previously (Landmesser and O’Donovan, 1984; Landmesser and Szente, 1986; Rafuse et al., 1996). Control embryos were decapitated and immediately placed in chilled and oxygenated Tyrode’s, and eviscerated; a ventral laminectomy was performed. Dissection was done to expose the appropriate hindlimb muscle as well as the muscle nerves. The isolated cord–hindlimb preparation was placed in 30°C Tyrode’s to equilibrate for several hours until spontaneous bursting of regular intervals began. Either electromyogram (EMG) or neurogram recordings were done by placing fine tip suction electrodes (flame-pulled polyethylene tubing, PE-190; Clay Adams) onto the muscle or muscle nerve, respectively, and applying suction to develop a tight seal. Such recordings were usually stable over many hours because the tapered flexible tubing moved with any contraction-generated movement, and therefore there was no damage to the nerve or muscle. The absolute amplitude of these extracellular recordings, which generally ranged between 0.2 and 0.5 mV, will vary with the tightness of the seal and thus is not a meaningful parameter. However, once a stable recording is obtained, the relative amplitude of the response before and after drug treatment provides a good estimate of the number of axons being activated. In all cases in which we observed a drug to increase or decrease the amplitude of the EMGs or neurograms, the amplitude returned to pre-drug levels on washout of the drug. Direct activation of a motoneuron pool by cord stimulation resulted in compound action potentials caused by the synchronous activation of many axons; these compound action potentials were generally in the range of 5–20 mV in amplitude. The signal was amplified, filtered at 10 Hz low-pass and 30 kHz high-pass (Grass P15 AC preamplifier; Quincy, MA), displayed on an oscilloscope (Tektronix R5030; Beaverton, CO) and Gould chart recorder (Gould, Cleveland, OH), and simultaneously recorded on a Vetter VCR (model 500H; Rebersburg, PA). Stimulation of the exposed thoracic or lumbar spinal cord was delivered by a standard stimulator (Grass S88), which was isolated from ground with a photoelectric stimulus isolation unit (Grass PSIU6B). Subsequent analysis was done by digitizing the recorded segment of interest, using MaCADIOS ADPO (GW Instruments, Somerville, MA) and subsequently by using SuperScope II Software (GW Instruments) for analysis and figure generation, except for Figure1g, which used DigiData 1200 Series Interface (Axon Instruments, Foster City, CA) and Axoscope 7 Software (Axon Instruments) for digitization and analysis and Microcal Origin 5 (Northampton, MA) for figure generation. To remove background noise, we low-pass HAM-filtered Figure 1, a, c, and f, at 20% frequency cutoff at 200 Hz (low-pass filtering passes low frequencies and attenuates the high frequencies). HAM uses a hamming window, which implements a rough low-pass filter; its transfer function follows a sin(f)/f shape with a stopband 43 dB down from its pass band. The drugs muscimol anddTC used in the isolated cord preparation bath were obtained from Sigma.
RESULTS
Characterization of muscimol-induced blockade of activity
During the naturally occurring cell death period (st. 30–36) a number of important developmental events occur (for review, seeHamburger, 1977; Oppenheim, 1991). First, just before the onset of motoneuron cell death at st. 30 (Hamburger, 1975), the motoneuron axons reach their target muscles in the hindlimb (Dahm and Landmesser, 1988). By st. 31, after the nerve has grown further into the muscle, pronounced visible movements of the hindlimb can be observed (Hamburger and Balaban, 1963; Hamburger et al., 1965; Hamburger, 1975; Bekoff et al., 1975) and are produced by bursts of spontaneous motoneuron activity. The latter can be quantified by using electrodes to record from muscles or from muscle nerves (Bekoff et al., 1975). Thus, at this early stage in the cell death period, neuromuscular synapses are present (Dahm and Landmesser, 1991) and functional (Landmesser, 1978b;O’Donovan and Landmesser, 1987). Previous studies showed that thein ovo application of dTC, a nicotinic acetylcholine receptor antagonist, blocked or greatly reduced the frequency of hindlimb movements (Pittman and Oppenheim, 1978; Dahm and Landmesser, 1988). However, these observations did not reveal what effects dTC may have had on the spontaneous bursting of motoneurons during the cell death period. To determine this, as well as to explore the importance of central versus peripheral activity blockade, we treated embryos either with dTC or with muscimol, a GABAA receptor agonist, and characterized the effects of these drugs on motoneuron activity and motoneuron survival. In these experiments we sought to understand howdTC was influencing increased motoneuron survival.
Before we used muscimol to block neuromuscular activity, it was necessary to ascertain optimal blocking concentrations and to characterize further its mode of action. To do this, we used an isolated spinal cord–hindlimb preparation (Landmesser and O’Donovan, 1984; Chub and O’Donovan, 1998) and recorded from either a hindlimb muscle (EMG) or a muscle nerve (neurogram). The isolated spinal cord preparation undergoes episodes of spontaneous bursting activity, with the number and length of the bursts within each episode varying with developmental stage (Fig. 1a;entire top trace = an episode). The interval between each of these spontaneous bursting episodes increases with age. As shown in Figure 1b, the mean interval between episodes increased from 4.15 ± 0.13 min (mean ± SD) at st. 30 to 12.12 ± 3.45 min (mean ± SD) at st. 32 and 12.6 ± 3.14 min (mean ± SD) at st. 34. An episode of bursting activity also can be triggered by a single stimulus to cervical or thoracic spinal cord, which activates descending input to the lumbar motoneurons (Fig. 1c, top trace). Such triggered episodes are identical in all respects to those occurring spontaneously.
By directly stimulating spinal nerves and recording EMGs, we found that muscimol (up to 1 × 10−5m) did not block neuromuscular transmission (Fig.1g). As shown in this st. 32 example, the compound action potential recorded from the muscle was not altered by the application of 5 × 10−6mmuscimol, but it was blocked by 5 × 10−6mdTC, as expected. However, by recording directly from muscle nerves, we found that the bath application of 5 × 10−6m muscimol blocked spontaneous motoneuron bursting activity for up to 3 hr (the longest time period tested) at all stages of the naturally occurring cell death period (Fig. 1a, lower trace, b). Because this was the minimum dose that blocked activity in the isolated spinal cord preparation, we assume that a similar concentration was achieved when muscimol applied in ovo blocked hindlimb movement and that this concentration also would block the spontaneous bursting of the motoneurons in ovo.
Because descending input from the brainstem can drive motoneuron bursting in the isolated cord preparation (Sholomenko and O’Donovan, 1995) and presumably could be active in ovo, we determined whether muscimol also could block such elicited bursting. As shown in the example of a st. 32 embryo (Fig. 1c, lower trace), 5 × 10−6mmuscimol also prevented descending input from activating motoneurons throughout the cell death period.
GABAergic interneurons, acting primarily via GABAA receptors, have been shown to be an important component of the network of neurons within the lumbar cord that drives the motoneurons in spontaneous bursts (Chub and O’Donovan, 1998). It also is known that GABA can be excitatory as well as inhibitory at early stages of development (Wu et al., 1992; Owens et al., 1996; Rohrbough and Spitzer, 1996). Although muscimol clearly blocked spontaneous bursting and that elicited by descending input, we wanted to determine whether stimulating more locally within the lumbar spinal cord could still activate motoneurons either directly or indirectly. Thus, while recording from the femorotibialis muscle nerve in the presence of 5 × 10−6m muscimol (Fig. 1d,e, upper traces), we found that stimulation at LS2, the region containing the femorotibialis motoneurons (Landmesser, 1978a,b), elicited a compound action potential with one or two peaks [second peak marked byasterisk(s)]. The first, shorter latency peak was attributable to direct activation of the motoneurons, because it persisted at 10 Hz stimulation (Fig. 1d, lower trace), a frequency that blocks synaptic transmission at this stage of development, and in the presence of low Ca2+ and high Mg2+ (Fig. 1e). The second, longer latency peak (marked with two asterisks) appears to be attributable to synaptic activation because it was blocked by 10 Hz stimulation (Fig. 1d, lower trace) and by low Ca2+ Tyrode’s (0.2 mm Ca2+ and 7 mm Mg2+; Fig.1e).
Thus, although muscimol prevents the spontaneous bursting of motoneurons and the ability of such bursting to be elicited by descending input, motoneurons in the presence of muscimol still could be activated both directly and synaptically. These data, taken together, suggest that muscimol is acting centrally to block the network of local interneurons that drive motoneuron bursting (Sernagor et al., 1995; Chub and O’Donovan, 1998) and the ability of descending input to activate this network. Furthermore, motoneurons do not appear to be extremely depolarized or hyperpolarized, because they still can be activated synaptically or directly.
Having shown that muscimol can block activity in the isolated spinal cord preparation, we proceeded to test its effects in ovo. Earlier studies had shown that GABA reduced, whereas the GABAA antagonist bicuculline increased, spontaneous motility in ovo (Reitzel et al., 1979). Because we had ascertained that muscimol does not block the NMJ (Fig.1g), any effects it has on motility should be attributable to alterations in motoneuron bursting episodes. In control embryos, hindlimb movements occur in episodes in which a series of strong and sustained kicks moves the limb over a considerable angle. We found that 0.1 mg/d per egg was the minimal dose needed to block such movements over a 24 hr period. Such movements were reduced in number by 88% with respect to controls (for example at st. 34, controls exhibited a mean of 24 kicks per 3 min period vs only 3 kicks in muscimol-treated embryos). This level of reduction in movement is similar to that previously reported for dTC, which reduced movements by 82% from control levels (Pittman and Oppenheim, 1979). However, some brief low-amplitude movements persisted during chronic muscimol treatment, and we felt that it was important to determine whether these movements were produced by bursts of motoneuron activity or whether they simply represented the fibrillatory movements that occur when muscle is chronically paralyzed.
Because it is impossible to make in ovo muscle nerve recordings at these stages, we addressed this question by treating embryos chronically in ovo with muscimol, and then we dissected these embryos in the continuous presence of 5 × 10−6m muscimol. Such chronically treated embryos did exhibit small, uncoordinated movements of different parts of the body, including the hindlimbs. However, these were not associated with motoneuron bursts as recorded from muscle nerves. Thus, many of the small movements observed in muscimol-treated embryos in ovo appear to be muscle-generated fibrillations and probably do not reflect motoneuron activity. By recording over periods of several hours we found that spontaneous bursting episodes were blocked in these embryos at stages encompassing most of the cell death period. As examples, in one st. 33 embryo and one st. 31 embryo (one midway through and one just after the onset of the cell death period, respectively) no spontaneous bursts occurred during the several hours of the recording period. However, in a st. 29 embryo (just before the cell death period), very short spontaneous bursts occurred with an irregular frequency of 10.7 ± 6.04 min (mean ± SD; n = 8) in 5 × 10−6m muscimol, although these were abolished when the muscimol concentration was increased to 1 × 10−5m. Because we cannot be certain of the in ovo muscimol concentrations, we cannot exclude the possibility that such brief bursts occurred in ovo at this young stage. However, as shown in Figure 1f, such episodes were extremely brief, one burst of ∼100–200 msec, when compared with control-bursting episodes of similar stages (three bursts each of 2 sec; Fig. 1a). Thus, the in ovo muscimol treatments greatly reduced the overall amount of spontaneous bursting activity that motoneurons were exposed to when compared with controls or with dTC-treated embryos, as described later.
The ability of descending input to generate bursting episodes also was greatly curtailed in these chronically treated embryos. At st. 33, no bursts could be elicited by stimulating descending input at cervical or rostrothoracic cord levels. At st. 31, as well, most stimuli failed to evoke bursts, although occasionally a very short single burst could be elicited, especially after repeated stimuli (see Fig. 1f, open gray box; solid gray box shows the burst elicited by the fourth stimulus on an expanded time scale). At st. 29, however, stimulation of the rostral cord, even in the higher dose (1 × 10−5m) of muscimol, was able to evoke single very brief bursts similar to those occurring spontaneously. Thus, if descending input is periodically active at this stage in ovo, it could elicit bursts, but, once again, the overall bursting activity during the cell death period should be greatly reduced.
Effect of muscimol treatment on motoneuron survival
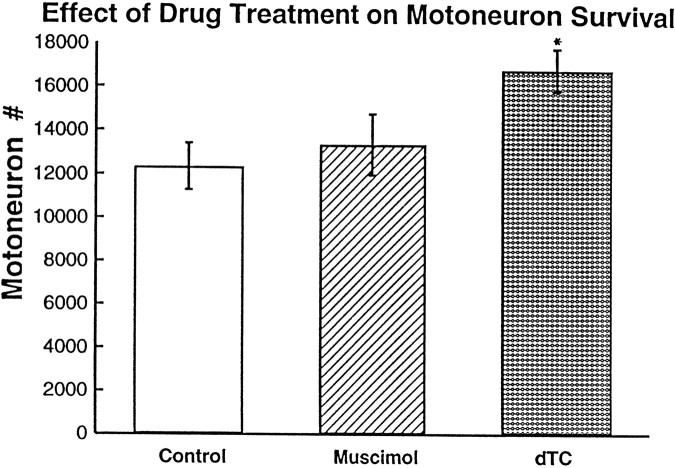
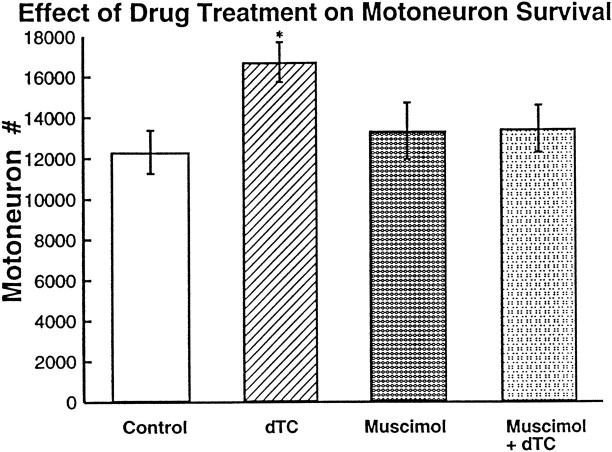
Pittman and Oppenheim (1979) had shown that the blockade of neuromuscular activity by dTC during the cell death period resulted in an increase in motoneuron survival. We duplicated these results, finding that dTC increased cell survival 36% over controls (Fig. 2). However, blocking activity with muscimol during the same period did not increase motoneuron survival; motoneuron number after muscimol-induced activity blockade was not significantly different from control but was significantly less than the motoneuron number in thedTC-treated embryos. This difference between muscimol anddTC treatments is interesting because, although both drug treatments block neuromuscular activity, motoneuron survival was increased only in the dTC treatment. Thus, although the blockade of muscle activation and contraction occurred in muscimol-treated embryos, this inactivity itself was not sufficient to alter cell survival. This suggests that there must be an effect of thedTC treatment, in addition to neuromuscular activity blockade, that affects cell survival.
Fig. 2.
The number of motoneurons (on one side of the lumbar lateral motor column) surviving at the end of the naturally occurring cell death period after muscimol ordTC-induced activity blockade. The number of motoneurons is significantly different from control after dTC treatment (*by t test: p = 3.6 × 10−5 for one-tailed and p = 7.2 × 10−5 for two-tailed;n = 3 for control and dTC). The number of motoneurons after muscimol treatment is significantly fewer than that after dTC treatment (t test:p = 2 × 10−4 for one-tailed and p = 4 × 10−4 for two-tailed; n = 6 for muscimol). Error bars represent SD.
Effect of muscimol-induced activity blockade on intramuscular nerve branching
Neuromuscular activity blockade induced by dTC during the naturally occurring cell death period also results in an increase in intramuscular nerve branching. To compare the effects ofdTC and muscimol on intramuscular nerve branching, we chose the iliofibularis (IFIB) muscle, in which the branching was quantified previously (Dahm and Landmesser, 1988). The IFIB has discrete regions that contain fast and slow myotubes (Fredette and Landmesser, 1991), which are innervated by distinct classes of motoneurons (Rafuse et al., 1996; Milner et al., 1998). The motoneuron axons that innervate the slow region have a characteristic collateral pattern of branching (shown in the st. 34 control muscle, Fig.3a, solid bracket; magnified in 3e, g, i, and k), with the main nerve trunk growing parallel to the myotubes and small side branches exiting this main nerve trunk at right angles to both the myotubes and the main nerve trunk. The fast region (Fig. 3a, dashed bracket; magnified in 3f, h, j, and l) has a characteristic reductive pattern of branching, with the main trunk growing perpendicular to the myotubes and successively splitting into smaller branches (Dahm and Landmesser, 1988; Landmesser et al., 1990). Confirming previous observations (Dahm and Landmesser, 1988), we found that dTC (Fig. 3d) treatment during the cell death period resulted in a profound alteration in the intramuscular nerve branching. Nerve trunks in both the fast (compare the fast magnified regions in Fig. 3f,l) and the slow regions (Fig. 3e,l) were much more defasciculated than in the controls. In addition, the fast region was more highly branched (Fig.3l), and many more side branches (arrows) emerged per unit length from the slow nerve trunks (compare the picture of the entire muscle, Fig. 3a,d; compare the slow magnified regions, Fig. 3e,k).
Fig. 3.
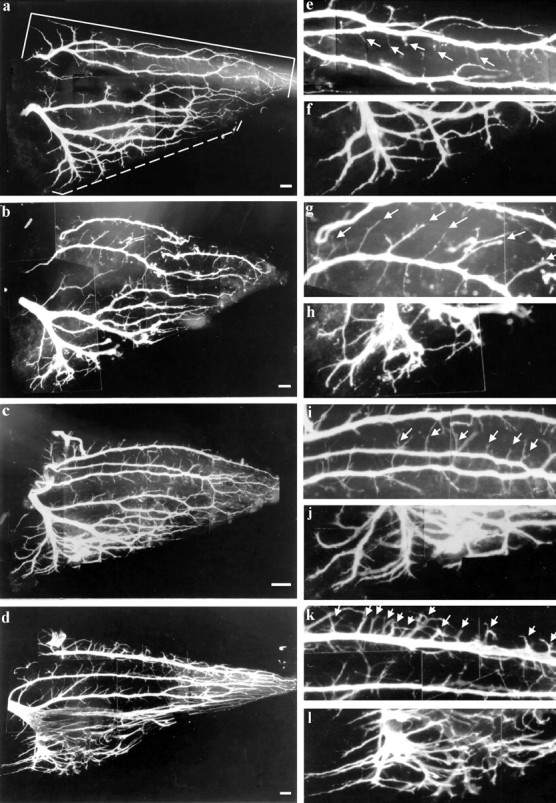
Whole mounts of a st. 34 chick IFIB muscle showing the intramuscular nerve branching pattern visualized by immunostaining for neurofilament, using the antibody C2. Left panelsshow whole muscle; right panels show portions of the slow (top) and the fast (bottom) regions of the muscle at higher magnification. a, Control; note the labeled fast (dashed bracket) and slow (solidbracket) regions.b, Muscimol-treated embryo resembles control but has longer side branches in the slow region. c, Another muscimol-treated embryo showing some increase in branching in both the fast and the slow region, but not as much as that shown ind for dTC. d, dTC-treated embryo; note the high frequency of side branches (arrows) in the slow region and the extensive defasciculation and branching in the fast region. e, f, Magnification of the control (shown in a); note the length and frequency of the side branches (arrows) in the slow region (e) and the extent of branching in the fast region (f). g, h, Magnification of the muscimol (shown in b) slow region (g); note the low frequency but longer length of the side branches (arrows) and the fast region (h) that shows some defasciculation. Magnification of the muscimol-treated muscle (shown inc) slow region (i) and fast region (j); note the longer length and moderate increase in the frequency of side branches (arrows). k, l, Magnification of the dTC-treated muscle (shown in d) in the slow region (k) and the fast region (l); note the greatly increased frequency of the side branches (arrows in k); also note the extensive degree of defasciculation and branching in the fast region (l), which is considerably more than in muscimol-treated muscles (compare l withj, h, and f for higher magnification view or compare d witha–c). Photographed at 100× magnification. Scale bars: a–c, 10 μm; d, 15 μm. e–l are a 2.75× magnification of each respective figure to theleft.
Interestingly, activity blockade induced by muscimol did not produce the same effect on intramuscular nerve branching as that induced bydTC. With respect to the amount and pattern of branching, the muscimol-treated muscles were more similar to the control than todTC-treated muscles. As illustrated in the two examples shown in Figure 3, b and c, muscimol treatment did not produce the extensive defasciculation that dTC did, especially in the fast region (compare Fig. 3h,j, withl). Although the overall branching pattern more closely resembled the controls, there clearly was some increase in the number of side branches in the slow region and probably an increase in side branch length (Fig. 3g,i). However, branching was much less extensive than in the dTC-treated muscle (Fig.3d).
Within the muscimol-treated embryos there was a range in the effect of muscimol-induced activity blockade on branching; some, as in the example in Figure 3b, were quite similar to controls, whereas others (Fig. 3c) were somewhat more branched. Increasing the daily dose of the muscimol fourfold did not result in an increase in branching (data not shown), indicating that the range in response was probably not attributable to the in ovomuscimol concentrations being near a threshold for response. To see whether the embryos with more extensive intramuscular nerve branching (i.e., Fig. 3c,i) had higher levels of motoneuron survival, we made motoneuron counts from three such st. 36 embryos. No correlation was found (t test: one tail, p = 0.066; two tail, p = 0.131).
Although the lack of a robust effect of muscimol on intramuscular nerve branching is consistent with the intramuscular nerve branching/trophic factor access hypothesis (Dahm and Landmesser, 1988, 1991; Oppenheim 1989; Landmesser, 1992), some increase in branching, especially in the slow region, did occur after muscimol treatment, with no correlated increase in motoneuron survival. Others have shown that subparalytic doses of dTC can rescue motoneurons from cell death with only moderate increases in intramuscular branching (Hory-Lee and Frank, 1995). This suggests that there is some unique aspect of thedTC-induced increase in branching that results in motoneuron survival (i.e., such as the extensive defasciculation observed in both fast and slow regions with dTC, but not with muscimol). This possibility could be evaluated once the downstream consequences ofdTC treatment that actually rescue motoneurons (such as enhanced trophic factor uptake) are identified. Alternatively,dTC could be affecting survival by an additional mechanism. If the critical effect of dTC in promoting survival is via increased branching, then preventing this increase in the continued presence of dTC should negate the effect of dTC on survival and provide support for the access hypothesis. We tested this as described below.
Effects of combined treatment with dTC and endo-N on intramuscular nerve branching
The large increase in intramuscular nerve branching afterdTC treatments was shown to be caused at least in part by an upregulation of PSA, a large carbohydrate added to neural cell adhesion molecule (NCAM) (Landmesser et al., 1990). NCAM, containing high levels of PSA, can act in a de-adhesive manner (Acheson et al., 1991), and the highly sialylated form of NCAM therefore can promote the defasciculation of axons within the muscle, resulting in increased intramuscular nerve branching (Landmesser et al., 1990). This increase in branching was hypothesized to facilitate trophic factor uptake, supporting increased motoneuron survival (Dahm and Landmesser, 1988). Therefore, the next series of experiments was designed to test whether the increased intramuscular branching produced by dTC was a critical variable in motoneuron survival. This was achieved by preventing the dTC-induced increase in branching by the simultaneous application of dTC and endo-N. Endo-N, which removes PSA from NCAM, previously has been shown to reduce the amount of branching produced by dTC (Landmesser et al., 1990; Tang and Landmesser, 1993).
It also was reported that chronic endo-N did not alter embryonic motility nor prevent the ability of descending input to elicit a normal bursting episode when it was acutely applied to an isolated cord preparation (Tang and Landmesser, 1993). However, that study did not record spontaneous bursting episodes from embryos chronically treated with endo-N.
Therefore, we first confirmed that chronic endo-N treatment did not alter spontaneous motility of the hindlimb, an indirect measure of motoneuron bursting. We counted the number of hindlimb movements per 3 min at st. 29, 30–31, 32–33, and 34 and found that, although there is a steep relationship between age and the total movements, at no stage were the counts for the endo-N-treated embryos statistically different from age-matched controls. For example, at st. 32–33 the endo-N-treated embryos exhibited 18.9 ± 3.7 versus 19.1 ± 3.6 kicks (mean ± SD) for the controls.
In one case we made an isolated cord preparation from a st. 29 embryo that had been chronically treated with endo-N for 24 hr. As shown by the recording from the femorotibialis and sartorius nerves, such embryos exhibited spontaneous episodes with the normal number and pattern of bursts (see Fig. 5a; compare with control st. 29 embryo in Fig. 6c). In this endo-N-treated embryo the mean interepisode interval of 7.32 ± 0.8 min (mean ± SD) differed slightly from that of a control embryo at this stage, 5.6 ± 2.1 min (mean ± SD), but such variation is within that expected because of the steep relationship between developmental stage and motility.
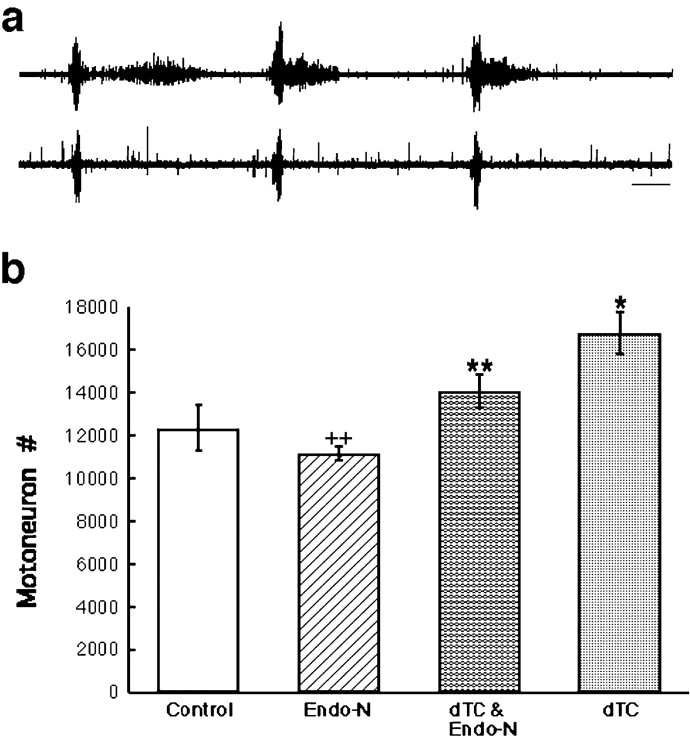
Fig. 5.
a, Neurogram recording from a chronically endo-N-treated embryo showing a spontaneous bursting episode. Top trace, femorotibialis. Bottom trace, sartorius. Calibration bar, 1 sec. b,Motoneuron counts from the lumbar lateral motor column at st. 36 after treatment with endo-N, dTC, or both together.dTC (*) is significantly different from control (see Fig. 2 legend). dTC plus endo-N (**) is significantly different from dTC treatment alone by two-tailedt test, p = 8.62 × 10−3 (one tail) and p = 0.0172 (two tail) but also significantly different from control by two-tailedt test, p = 0.0291 (one tail) andp = 0.0583 (two tail). Endo-N treatment alone (++) is not significantly different from control by two-tailed test, p = 0.0309 (one tail) andp = 0.0618 (two tail), but it is significantly different from the combined dTC plus endo-N by two-tailed t test, p = 0.0182 (one tail) and p = 0.0364 (two tail).
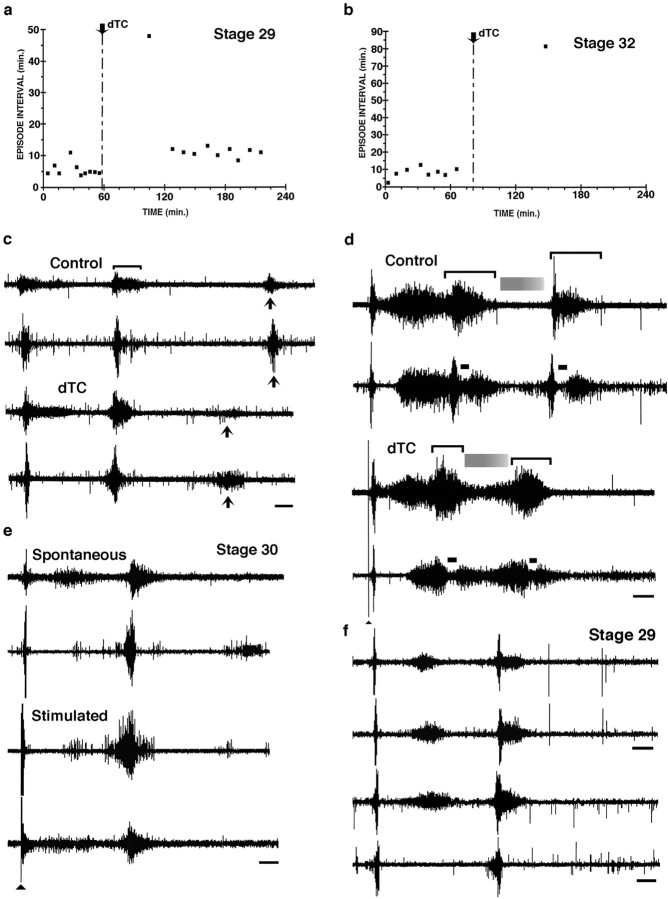
Fig. 6.
Recording from muscle nerves in isolated spinal cord preparations treated acutely (a–d) and chronically (e, f) with dTC.a, Scatter plot showing the intervals between spontaneous bursting episodes in a st. 29 embryo that, under control conditions, burst every 5.6 ± 2.1 min (mean ± SD;n = 10). The addition of dTC transiently blocked bursting, which then resumed at a lower frequency; the time between episodes was 14.4 ± 1.29 min (mean ± SD;n = 9). b, Scatter plot showing the interval between spontaneous bursting episodes in a st. 32 embryo; under control conditions an episode occurred every 8.2 ± 3.03 min (mean ± SD; n = 8), whereas after the addition of dTC only a single episode occurred after 82 min and none occurred with an additional 50 min. c, In the same embryo as in a, the entire bursting episode before dTC is shown (top pair oftraces; femorotibialis and sartorius, respectively); a single burst is indicated by a bracket. Thebottom pair of traces shows the burst structure of spontaneous episodes after the recovery of bursting in the continued presence of 5 × 10−6mdTC. Note the changes in burst structure: increased amplitude of the first two bursts and the decrease in the third burst and its time of appearance (compare solid arrows).d, The structure and length of the episode (the same embryo as in b) are altered from control (top pair of traces; femorotibialis and sartorius, respectively) after the addition of 5 × 10−6mdTC (bottom pair of traces; femorotibialis and sartorius, respectively; see Results for more details). Brackets indicate the two bursts occurring in each episode; shaded grayrectanglesindicate a quiescent period between bursts, and the solid bar indicates an inhibitory period that occurs in sartorius at the onset of each burst. Solid arrowhead indicates a stimulus artifact for lower two traces only.e, In a chronically treated dTC embryo in the continuous presence of 5 × 10−6mdTC, both spontaneous and stimulated bursting episodes occur. Femorotibialis (top) and sartorius (bottom) are shown in each pair of traces.f, Neurogram recordings from embryos treated with endo-N plus dTC. The top pair oftraces is from a st. 29 embryo chronically treated withdTC and recorded in the continued presence of 5 × 10−6mdTC. The burst structure of the femorotibialis from the limb injected with endo-N at the onset of the chronic dTC treatment (lower trace) is essentially the same as that recorded from the femorotibialis in the noninjected hindlimb. The lower pair of traces is from an embryo that was chronically injected with endo-N alone. Both traces from the endo-N-injected limb (top trace, femorotibialis;bottom trace, sartorius) when 5 × 10−6mdTC was acutely added to the bath show that the burst structure is similar to the embryo chronically treated with dTC (top pair of traces) and that this differed from the burst structure before dTC treatment (as shown in Fig.5a). Thus acute dTC treatments produce the same effects as chronic dTC treatments, and this is not affected by the presence or absence of endo-N. Calibration bars:c–f, 1 sec.
Endo-N, applied during the in ovo dTC treatment (st. 29–36), removed PSA as ascertained by immunostaining at multiple time points during the cell death period (data not shown). In most of the embryos that were observed from st. 28 to 36, immunofluorescent labeling for PSA was absent, whereas simultaneous immunofluorescence for the cell adhesion molecule L1 was positive in all sections (n = 11). Only one of these embryos, at st. 34, had some positive staining for PSA. Thus, removal of PSA from the injected thigh was achieved during the critical time period when most motoneurons are committing to live or die.
The dual treatment with endo-N and dTC resulted in less extensive branching than dTC treatment alone. Figure4a shows the control IFIB branching pattern in a st. 34 embryo. Treatment with endo-N alone reduced branching in both the fast and slow regions (Fig.4b). dTC treatment alone (Fig. 4d) produced the large increase in branching that was described previously. However, the addition of endo-N to dTC (Fig. 4c) resulted in a clear decrease in branching in both the fast (comparedashed bracket area, Fig. 4c,d) and slow regions when compared with dTC treatment alone. Thus, endo-N was able to partially prevent the dTC-induced increase in branching, even under the conditions of activity blockade. However, such dually treated muscles still had more nerve branching in both the fast and the slow regions than did the control muscles (compare Fig.4c with a). Figure 4 shows examples of whole mounts from st. 34 embryos near the middle of the cell death period, and these are representative of others done at earlier and later stages.
Fig. 4.
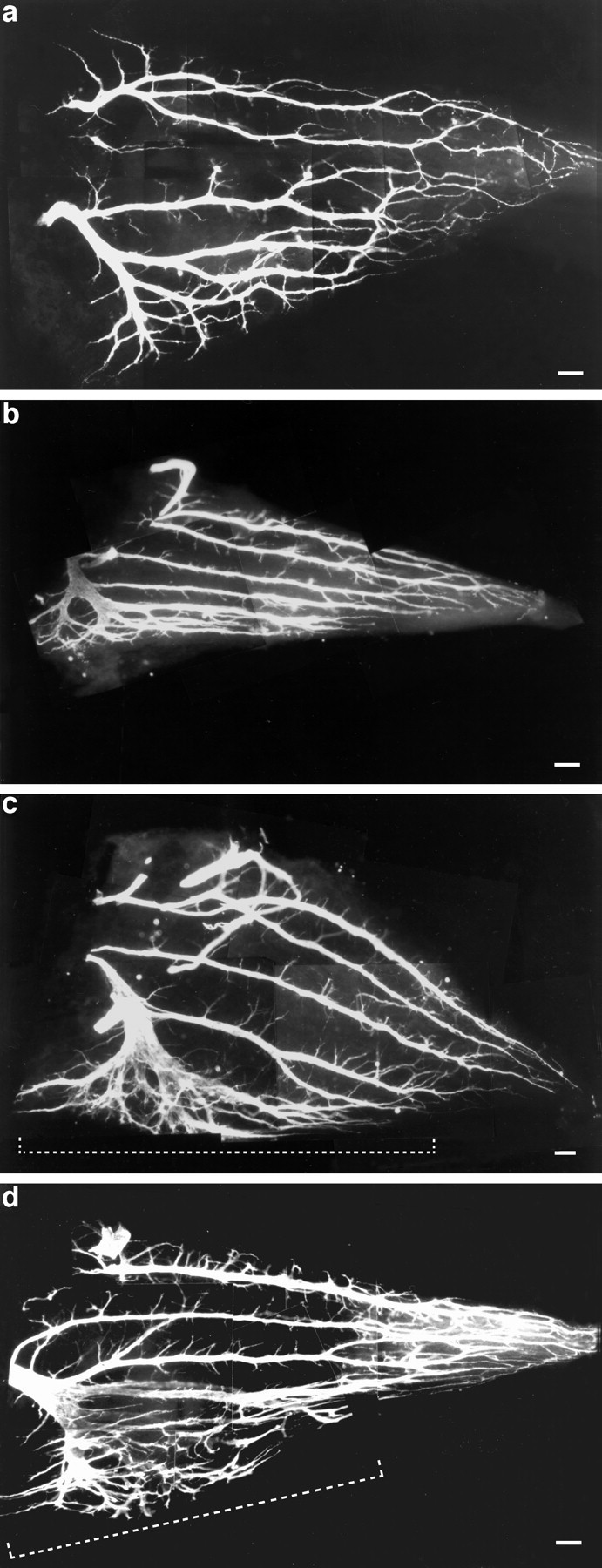
Whole mounts of st. 34 IFIB muscles showing the intramuscular nerve branching pattern, using C2 immunoreactivity.a, Control. b, Endo-N treatment, showing very fasciculated branches; although this muscle appeared slightly smaller than the control, this is within the range found in the controls. c, Endo-N plus dTC; note the decrease in defasciculation and branching in the fast region (bracket) and a decrease in branching in the slow region when compared with dTC treatment alone.d, dTC treatment alone (photographed at 100× magnification). Dashed brackets indicate the fast region. Scale bars: a, b, d, 10 μm; c, 5 μm.
The effect of combined dTC and endo-N treatment on motoneuron survival
Motoneuron counts at st. 36, the end of the cell death period, after the dual dTC and endo-N treatment are shown in Figure5b. These results show that the increased motoneuron survival produced by dTC was reduced significantly by simultaneous treatment with endo-N. As shown previously (Tang and Landmesser, 1993), endo-N alone was able to reduce survival significantly when compared with controls, although the effect in the present experiments was modest (i.e., a 10% reduction). As shown in Figure 5b for the dual treatment, althoughdTC was present and able to act at both peripheral and central sites, the presence of endo-N was able to reduce motoneuron survival toward control levels.
Thus, the combined treatment of dTC and endo-N reduced both intramuscular nerve branching and motoneuron survival to a value in between the control and the dTC-treated embryos. This is consistent with the idea that the degree of intramuscular branching can affect motoneuron survival, perhaps via trophic uptake at the NMJ (Dahm and Landmesser, 1988; DiStefano et al., 1992). However, these data do not exclude the possibility that dTC also could affect motoneuron survival by acting directly on motoneurons. Thus, we determined whether dTC had any effects on the spontaneous bursting episodes at different stages during the cell death period.
Effects of dTC on motoneuron bursting activity
Previous work, characterizing the effects of dTC at the end of the naturally occurring cell death period (st. 36), had shown that dTC, either applied chronically from st. 30 in ovo or applied acutely to the isolated spinal cord preparation, could block the patterned bursting activity evoked by stimulation of the cervical spinal cord (Landmesser and Szente, 1986). The blocking effect of chronically applied dTC was reversed by washing for 1–6 hr, indicating that the cord circuitry responsible for generating bursting activity was not damaged by the chronic in ovo dTC treatment. Although dTC blocked stimulated bursting activity at st. 36, it was not known how dTC would affect stimulated or spontaneous bursting activity at earlier stages, especially during this period of naturally occurring cell death. Thus, we first characterized the effects of dTC applied acutely to the isolated cord preparation on both stimulated and spontaneous bursting activity at earlier time points.
At st. 29, just before the onset of the cell death period,dTC transiently suppressed spontaneous bursting, as illustrated in Figure 6a, in which the interval between episodes of spontaneous bursting is plotted as a function of time. Before dTC application, the mean interval between episodes was 5.6 ± 2.1 min (mean ± SD;n = 10). The application of 5 × 10−6mdTC suppressed bursting for 48 min. However, bursting episodes then resumed, but at a lower frequency (the interval between episodes being 14.4 ± 1.3 min; mean ± SD; n= 9). This recovery of spontaneous bursting in the continued presence of drug appears to reflect plasticity in the early cord circuits that are capable of using several transmitter systems to drive spontaneous bursting. Because the details of this phenomenon are not germane to the present paper, they will not be considered further, but they have been characterized elsewhere (Chub and O’Donovan, 1998; Milner and Landmesser, 1999).
In addition to affecting the frequency of spontaneous bursting episodes, dTC also altered the structure of the bursts (a single burst is bracketed in Fig. 6c, upper trace) within an episode. This effect, as well as the effects on spontaneous bursting, were highly dependent on developmental stage. For example, at st. 28–28.5, 5 × 10−7mdTC increased the number of bursts per episode and increased the amplitude and length of the individual bursts, especially for extensors such as the femorotibialis (see, for example, Fig. 7). By st. 29 (Fig. 6c), this enhancement was less pronounced; for example, the last burst in the three burst sequence, which is characteristic of st. 29, usually did not occur or was greatly diminished (Fig. 6c, arrows) in the presence of 5 × 10−6mdTC. However, the earlier bursts were still enhanced in amplitude.
Fig. 7.
Dose-dependent alterations in a spontaneous bursting episode produced by dTC. In an isolated cord preparation of a st. 28 embryo, 5 × 10−7mdTC, a dose that does not block the NMJ, induces the appearance of an extra third burst. dTC (2 × 10−6m) alters the burst structure and increases burst length and amplitude, especially in the femorotibialis (top trace in each pair) and sartorius (bottom trace of each pair) as compared with control. At 5 × 10−7mdTC, the third burst no longer occurs, but the first bursts are increased in amplitude. Calibration bar (for all six traces), 1 sec.
Somewhat later during the cell death period, st. 32, the same concentration of dTC (5 × 10−6m) had a much greater effect on suppressing spontaneous bursting (Fig.6b). At this stage the interval between bursting episodes in controls had increased to 8.2 ± 3.03 min (mean ± SD;n = 8). After the application of dTC, only one spontaneous burst occurred in 1.5 hr. However, even in the presence of dTC, a bursting episode could be elicited reliably by single stimuli to the brachial or thoracic cord to activate descending input (Fig. 6d, lower two traces; arrowheadindicates stimulus). In this embryo, two bursts (indicated bybrackets) were elicited per episode, and the characteristic differences between the femorotibialis (top trace) and the sartorius (bottom trace) had begun to emerge (O’Donovan and Landmesser, 1987). For example, the sartorius burst exhibits an inhibitory period (solid bar) at the onset of each burst, whereas the femorotibialis does not. Although these characteristic differences were still observed in the presence of dTC (Fig.6d, bottom pair of traces), the overall length of the bursting episode was shortened, as if the episode had been compressed in time. Additionally, the femorotibialis did not become quiescent between bursts (gray shaded bar), as in controls, and the sartorius also exhibited higher activation between bursts.
These results show that dTC has strong effects on the cord circuitry responsible for spontaneous bursting, affecting both the circuit responsible for the timing of spontaneous episodes as well as that which regulates the burst structure. On the one hand,dTC appears to result in a stronger activation of the motoneuron pool during each episode, especially for the extensors such as the femorotibialis. Enhanced central activation of motoneurons in the presence of dTC also may explain the result of Hory-Lee and Frank (1995), who reported that subparalytic doses ofdTC could both increase intramuscular nerve branching and promote motoneuron survival. As shown in Figure7, at st. 28 just as motoneurons begin to grow into their target muscle, 5 × 10−7mdTC, a dose that does not block the neuromuscular junction and that also has no effect on the frequency of motoneuron bursting episodes, elicits an extra burst in each episode without affecting the burst structure. At a higher dose of 2 × 10−6mdTC, both the amplitude and length of the burst is affected, which is even more extreme at 1 × 10−5mdTC (latter not shown).
On the other hand, dTC reduced, to a varying extent depending on the developmental stage, the frequency of spontaneous bursting episodes. However, at no stage did dTC prevent the ability of descending input to evoke a robust bursting episode. Thus, activation of bursting in ovo either by descending input or by sensory input appears likely. To understand better how cord circuits would be affected by chronic dTC application, we treated several embryos in ovo for 1–2 d and then made isolated cord preparations in the continued presence of 5 × 10−6mdTC.
As shown by the example in Figure 6e, the st. 30 embryo, chronically treated with dTC, produced spontaneous bursting episodes consisting of well formed high-amplitude bursts, similar to what would have occurred in a control embryo. As in the acutely treateddTC embryos, however, the frequency of bursting episodes was reduced and more irregular in timing, with a mean inter-episode interval of 17.4 ± 11.3 min (mean ± SD; n = 7) as compared with 4.2 ± 0.13 min (mean ± SD;n = 7) for the control. Similar bursting episodes also could be evoked by stimulation of the descending input (see Fig.6e, bottom pair of traces). Thus, in summary, although we cannot be certain of the precise frequency with which bursting occurs in the dTC-treated embryos in ovo, the overall effect is clearly opposite from muscimol, which blocks or greatly attenuates bursting under all circumstances.
As described above, we found that an injection of endo-N to remove PSA from the limb of an embryo during chronic dTC treatment partially reversed the effect of dTC on both motoneuron survival and intramuscular nerve branching. This might have occurred because endo-N was able to prevent the dTC-induced increased expression of PSA, which has been postulated to promote increased branching (Landmesser et al., 1990). The reduced branching in turn might have contributed to reduced motoneuron survival. However, it was necessary to determine whether endo-N also might be acting by altering in some way the effect of dTC on spontaneous motoneuron activity. Although endo-N did not reverse the dTC-induced motility blockade and thus peripheral NMJ blockade (present study), no studies have tested its effect on motoneuron activity in embryos treated chronically with dTC.
To assay this, we chronically treated embryos with dTC and removed PSA from only one limb as we had done earlier. We then dissected such embryos in the continued presence of 5 × 10−6mdTC and recorded spontaneous bursting activity from both the endo-N-injected side and the contralateral limb (the latter serving as a control).
In a neurogram recording (Fig. 6f) comparing the femorotibialis muscle nerve on the left side (noninjected hindlimb) and the right side (injected hindlimb), we found that both muscles were activated in complete synchrony and that there were no differences in burst structure. The frequency of bursting episodes in this dually treated embryo (5.3 ± 7.8 min; mean ± SD; n= 9) was irregular, because we observed intervals ranging from 1.4 to 26 min. Such irregular bursting was common after chronic dTC treatment, and on washout of dTC the interval from this embryo became faster and more regular (4.9 ± 0.6 min; mean ± SD; n = 16), similar to that of the control embryo shown in Figure 6a (5.6 ± 2.1 min; mean ± SD;n = 10). We also observed the effects of acutedTC application on a st. 29 chronically treated endo-N embryo (Fig. 6f, lower pair of traces; femorotibialis and sartorius muscle nerve recordings, respectively). Although this embryo exhibited a burst structure and interepisode interval (see Fig. 5a and accompanying text) similar to the control, on the addition of dTC (5 × 10−6m) the structure changed (Fig. 6f, lower pair of traces) to that seen previously in the presence of dTC (compare with Fig. 6c,e). Overall then, in ovo injections of endo-N do not alter bursting from control, nor do they alter the effects on bursting produced by either the acute or chronic application of dTC.
Effect of combined muscimol and dTC treatment
To test whether the different effects the two drugs had on motoneuron survival might be explained by their different effects on motoneuron bursting activity (i.e., dTC possibly enhancing and muscimol blocking spontaneous activity), we first determined the effect of combined muscimol and dTC drug treatments on electrical activity. In the isolated spinal cord preparation we found that, in the combined presence of 5 × 10−6m muscimol anddTC, no spontaneous bursts occurred, nor could they be elicited by stimulation of the rostral cord at st. 29 or 32 (data not shown). Thus, although dTC was present and potentially acting at both peripheral and central sites, the simultaneous presence of muscimol prevented motoneuron bursting activity. We then determined how chronic application of the two drugs in ovo affected motoneuron survival.
The results of the combined dTC and muscimol treatment during the cell death period on motoneuron survival are shown in Figure8. Interestingly, the total lumbar motoneuron cell counts in this combined treatment were very similar to muscimol treatment alone; neither was significantly different from control. These results sharply contrast with the dTC treatment alone, in which there was a 36% increase in motoneuron survival over control. Thus, it appears that the effects of muscimol dominate over the influence of dTC on affecting cell survival.
Fig. 8.
Effect of combined muscimol and dTC treatment during the cell death period on motoneuron survival as shown from counts of the LMC at the end of the naturally occurring period of cell death (st. 36). The number of motoneurons in the dual treatment is not significantly different from those of control but is significantly different from those of dTC treatment alone (byt test: p = 8.7 × 10−5 for one-tailed and p = 1.7 × 10−4 for two tailed;n = 3 for each drug treatment except muscimol alone, which is n = 6).
Additionally, we assessed the effect of the combined muscimol anddTC treatment on intramuscular nerve branching patterns. The branching pattern in the combined treatment clearly differed from that of dTC alone; specifically, the fast region was not so highly defasciculated and branched (Fig.9b), nor was there a large increase in side branch number in the slow region. However, there did appear to be some increase in branching over that of muscimol alone.
Fig. 9.
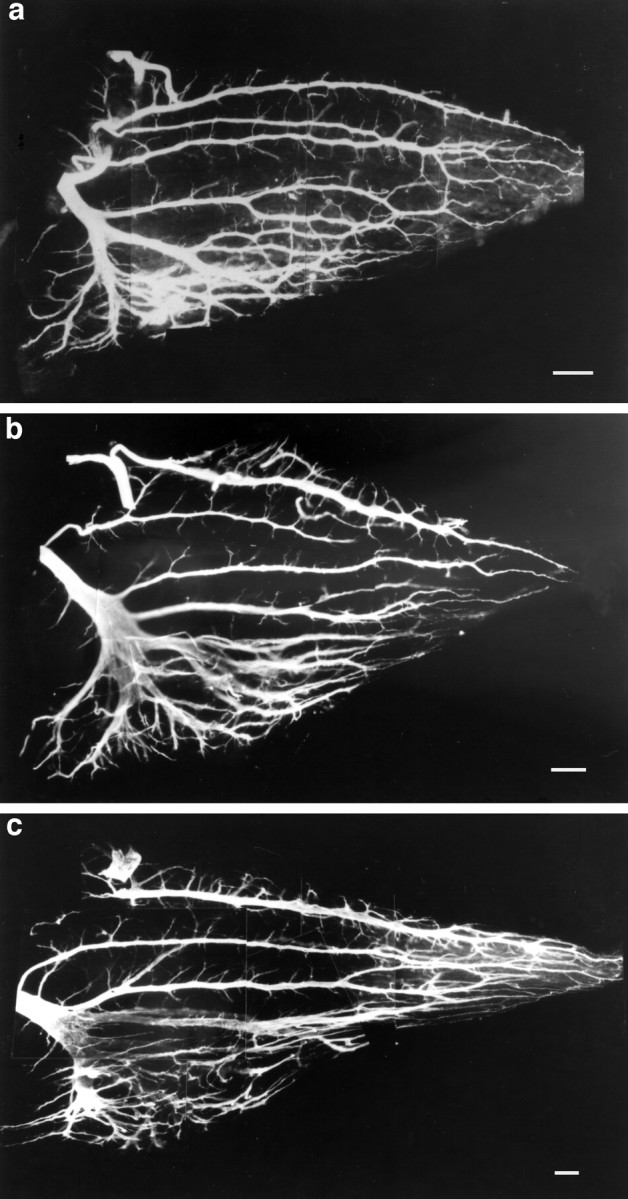
Effect of combined muscimol and dTC treatment during the cell death period on the intramuscular nerve branching pattern seen in st. 34 chick IFIB muscle. a, Muscimol treatment alone. b, Combined muscimol anddTC treatment. c, dTC treatment alone. Although there was some increase in branching in the combined treatment, when compared with muscimol alone it was considerably less than that produced by dTC treatment alone. Scale bars: a, b, 15 μm; c, 10 μm.
In summary, the effects of the combined treatment on the spontaneous bursting activity and motoneuron cell survival are similar to the effect of muscimol alone. Although dTC was present, it was not able to prevent the blockade of spontaneous bursting caused by muscimol; in turn, we did not observe the large increase in survival or in intramuscular nerve branching that dTC treatment alone elicits.
DISCUSSION
The present studies were designed to (1) explore why in vivo blockade of neuromuscular activity with dTC rescues motoneurons from naturally occurring cell death as shown earlier (Pittman and Oppenheim, 1978), and (2) to test further the hypothesis (Dahm and Landmesser, 1988, 1991; Oppenheim, 1989) that thedTC-induced increase in intramuscular nerve branching was critical to this rescue. We found that, although the GABAA agonist muscimol indirectly blocked neuromuscular activity by suppressing the spontaneous bursting of motoneurons centrally, it did not rescue motoneurons from cell death. Thus, consistent with earlier observations (Oppenheim et al., 1989), we conclude that preventing the activation of the NMJ and any downstream consequences alone are insufficient to rescue motoneurons.
A novel finding was that, early in the motoneuron cell death period,dTC not only blocked the neuromuscular junction but also had strong central effects on motoneuron bursting activity, which were the opposite of muscimol. We propose that the different central effects of muscimol and dTC, by their direct actions on the activity of cord circuits and motoneurons and/or by indirectly affecting intramuscular nerve branching, may explain their differential effects on motoneuron survival. Additionally, because muscimol had no effect on naturally occurring motoneuron death and because it did block both spontaneous motoneuron activity and the ability of descending input to evoke such activity, we conclude that motoneuron activity, per se, and any downstream consequences are not essential for the normal process of motoneuron cell death. This is a novel finding, because none of the studies assaying motility has assessed motoneuron activity directly.
One possible outcome of our studies might have been an uncoupling of alterations in intramuscular nerve branching from motoneuron survival, casting doubt on the hypothesis that increased branching and synapse formation were critical to enhanced survival (Dahm and Landmesser, 1988, 1991; Oppenheim, 1989). However, in contrast to this possibility, we found a good correlation between intramuscular nerve branching and survival. Thus, removal of PSA with endo-N partially prevented thedTC-induced increase in both intramuscular nerve branching and motoneuron survival. Similarly, the presence of muscimol prevented the dTC-induced enhancement of motoneuron survival and greatly reduced the dTC-induced increase in branching. Other observations are consistent with this hypothesis: removal of PSA alone reduces both branching and motoneuron survival (Tang and Landmesser, 1993); nicotine and decamethonium produce activity blockade but do not increase motoneuron survival or synapse formation (Oppenheim et al., 1989). Excitation contraction coupling mutants in both the mouse (mdg/mdg; Rieger and Pinçon-Raymond, 1981; Powell et al., 1984; Oppenheim et al., 1986; Houeneou et al., 1990) and the chick (cn/cn; Oppenheim et al., 1997) increase both branching and survival. Even subparalytic doses of dTC that result in increased survival also increase branching (Hory-Lee and Frank, 1995).
Despite such correlation, the effects of dTC on motoneuron survival could result in part from its central effects. Although several previous studies have demonstrated the presence of nicotinic receptors within the spinal cord (Renshaw et al., 1993; Renshaw and Goldie, 1996), our observations are the first to show that blocking nicotinic receptors actually alters the functional activation of motoneurons during the cell death period. Furthermore, the effect of cholinergic antagonists, such as dTC, on motoneuron activity was, in general, opposite to the effects of muscimol, which did not enhance survival. One possibility is that enhanced activation of motoneurons may influence their survival directly, possibly by altering [Ca 2+]i; (Vijayaraghavan et al., 1992; Zhang et al., 1994). This, in turn, could lead to alterations in signaling cascades and gene expression (for review, see Ghosh and Greenberg, 1995; Finkbeiner and Greenberg, 1996;Ginty, 1997; Berridge, 1998) that could interfere directly with the cell death process (for review, see Henderson, 1996). It is interesting then that we observed that subparalytic doses of dTC, which rescues motoneurons (Hory-Lee and Frank, 1995), also enhances motoneuron activity over control levels. Also consistent with this hypothesis is our observations that muscimol, when applied alone, prevented both motoneuron activity and increased motoneuron survival. Additionally, muscimol, which suppressed motoneuron activity in the presence of dTC, also prevented the effects ofdTC on survival. However, opposing such a direct effect ofdTC as the sole explanation for increased motoneuron survival was the finding that PSA removal was able to attenuate significantly the effect of dTC on survival, althoughdTC was present and able to act at both central and peripheral sites. We also demonstrated that endo-N did not produce this effect by acting centrally to alter the effects of dTC on spontaneous motoneuron bursting.
Another possibility is that dTC-induced alterations in motoneuron activation could affect survival by altering motoneuron uptake of trophic molecules, either centrally or peripherally. Although it is known that the periphery can regulate the survival of motoneurons (Hamburger, 1958; Hollyday and Hamburger, 1976; Oppenheim et al., 1978) and in vitro studies have identified candidate trophic molecules (Oppenheim, 1996; Calderó et al., 1998), it is not clear how trophic molecules actually affect motoneuron survivalin vivo or how dTC might influence the process. One possibility is that dTC could result in the enhanced release of trophic molecules centrally or peripherally via activity-dependent mechanisms (Ghosh et al., 1994; Boulanger and Poo, 1999) (for review, see Thoenen, 1995). Second, dTC could cause increased cell surface expression of neurotrophin receptors (depolarization of cultured DRG neurons does lead to such enhanced expression; Meyer-Franke et al., 1998). Third, increases in peripheral branching, as discussed above, could result in increased uptake of trophic factors (Dahm and Landmesser, 1988; DiStefano et al., 1992; Yan et al., 1993) (for review, see Fitzsimonds and Poo, 1998). Although it should be possible to compare Trk receptor expression on motoneurons chronically treated with dTC versus muscimol, existing methodologies do not have sufficient resolution to detect possible alterations in either the release or uptake of neurotrophins.
An important question to resolve is whether dTC is simply acting pharmacologically to short-circuit the normal process of motoneuron cell death, or, alternatively, whether it is altering a variable that is used physiologically to regulate naturally occurring cell death. In this regard, it is useful to compare the current findings with other experimental situations that rescue motoneurons from cell death. In the crooked neck dwarf mutant (cn/cn), chick motoneurons are activated normally, as is neuromuscular transmission, and muscle fibers exhibit action potentials. However, because of an excitation-coupling defect, contraction does not occur (Oppenheim et al., 1997). Why are motoneurons rescued in this situation and in the mdg/mdg mutant mouse (Oppenheim et al., 1986;Houeneou et al., 1990)? One possibility is that Ca2+ released from internal muscle fiber stores after excitation–contraction coupling normally inhibits the production and/or release of sprouting factors or neurotrophins. When this fails to occur in the mutant, motoneuron survival could be enhanced because of the increased supply of trophins. Alternatively, increased release of sprouting factors could enhance access to neurotrophins by increasing branching, which is, in fact, increased in the cn/cn mutant. However, contraction and release of Ca2+ from internal muscle cell stores also would be blocked in the muscimol treatment, which does not result in increased survival.
One way of reconciling these and other observations (Oppenheim et al., 1986; Houeneou et al., 1990, 1991; Oppenheim et al., 1997) is to propose that some event triggered by target inactivity (such as the increased release of sprouting factors/neurotrophins) needs to be coupled to normally active motoneurons. Motoneuron bursting activity might be required either for the uptake of such factors or for appropriately responding to them (see Boulanger and Poo, 1999); thus enhanced survival would occur in cases in which motoneurons were active (dTC) but not when they were inactive (muscimol).
Motoneuron activity also could alter intramuscular nerve branching by regulating the expression of adhesion molecules on nerve or muscle. In both spinal cord (O’Donovan et al., 1994) and retina (Meister et al., 1991; Catsicas et al., 1998), episodes of spontaneous activity result in periodic increases in [Ca2+]i, and the pattern of such oscillations would have differed with the drug treatments we performed. In this regard, it is interesting that the expression of the neural adhesion molecule L1, which can mediate both the growth and the fasciculation of axons (Landmesser et al., 1988;Burden-Gulley et al., 1997), is sensitive to the pattern of electrical activity (Itoh et al., 1995). Polysialylation of NCAM, which has strong effects on intramuscular nerve branching (Landmesser et al., 1990; present results) also is regulated by activity via changes in [Ca2+]i (Rafuse and Landmesser, 1996; Brusés and Rutishauser, 1998). Alternately, exposure to different patterns of electrical activity can affect how axons regulate [Ca2+]i (Lnenicka et al., 1998), and Ca2+ transients can affect directly the growth and/or retraction of neurites (Gomez and Spitzer, 1999). Finally, ACh released by the activity from motoneuron growth cones could bind to axonal nicotinic receptors and affect growth by altering [Ca2+]i or other consequences of AChR activation (see Pugh and Berg, 1994). It will be important now to determine which downstream consequences of motoneuron activity are modulating both intramuscular axonal branching and motoneuron survival.
Footnotes
This work was supported by National Institutes of Health Grant NS19640 from the National Institute of Neurological Diseases and Stroke. We thank Drs. V. Rafuse, S. Banerjee, and L. Milner for helpful input during the course of this research as well as for critical reading of this manuscript. In addition, we thank Dr. Urs Rutishauser for the gift of the endosialidase-N.
Correspondence should be addressed to Dr. Lynn T. Landmesser, Department of Neurosciences, Case Western Reserve University, School of Medicine, Cleveland, OH 44106-4975.
REFERENCES
- 1.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 2.Acheson A, Sunshine JL, Rutishauser U. NCAM polysialic acid can regulate both cell–cell and cell–substrate interactions. J Cell Biol. 1991;114:143–153. doi: 10.1083/jcb.114.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antal M, Berki AC, Horvàth L, O’Donovan MJ. Developmental changes in the distribution of gamma-aminobutyric acid-immunoreactive neurons in the embryonic chick lumbosacral spinal cord. J Comp Neurol. 1994;343:228–236. doi: 10.1002/cne.903430204. [DOI] [PubMed] [Google Scholar]
- 4.Bekoff A, Stein PSG, Hamburger V. Coordinated motor output in the hindlimb of the 7-day chick embryo. Proc Natl Acad Sci USA. 1975;72:1245–1248. doi: 10.1073/pnas.72.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 6.Boulanger L, Poo M. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat Neurosci. 1999;2:346–351. doi: 10.1038/7258. [DOI] [PubMed] [Google Scholar]
- 7.Brusés JL, Rutishauser U. Regulation of neural cell adhesion molecule polysialylation: evidence for nontranscriptional control and sensitivity to an intracellular pool of calcium. J Cell Biol. 1998;140:1177–1186. doi: 10.1083/jcb.140.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burden-Gulley SM, Pendergast M, Lemmon V. The role of cell adhesion molecule L1 in axonal extension, growth cone motility, and signal transduction. Cell Tissue Res. 1997;290:415–422. doi: 10.1007/s004410050948. [DOI] [PubMed] [Google Scholar]
- 9.Calderó J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, Oppenheim RW. Peripheral target regulation of the development and survival of spinal sensory and motoneurons in the chick embryo. J Neurosci. 1998;18:356–370. doi: 10.1523/JNEUROSCI.18-01-00356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catsicas M, Bonness V, Becker D, Mobbs P. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr Biol. 1998;8:283–286. doi: 10.1016/s0960-9822(98)70110-1. [DOI] [PubMed] [Google Scholar]
- 11.Chub N, O’Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. J Neurosci. 1998;18:294–306. doi: 10.1523/JNEUROSCI.18-01-00294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahm LM, Landmesser LT. The regulation of intramuscular nerve branching during normal development and following activity blockade. Dev Biol. 1988;130:621–644. doi: 10.1016/0012-1606(88)90357-0. [DOI] [PubMed] [Google Scholar]
- 13.Dahm LM, Landmesser LT. The regulation of synaptogenesis during normal development and following activity blockade. J Neurosci. 1991;11:238–255. doi: 10.1523/JNEUROSCI.11-01-00238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding R, Jansen KS, Laing NG, Tønnesen H. The innervation of skeletal muscles in chickens curarized during early development. J Neurocytol. 1983;12:887–919. doi: 10.1007/BF01153341. [DOI] [PubMed] [Google Scholar]
- 15.DiStefano P, Friedman B, Radziejewski C, Alexander C, Boland P, Schick C, Lindsay R, Wiegand S. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 16.Finkbeiner S, Greenberg ME. Ca2+-dependent routes to Ras: mechanisms for neuronal survival, differentiation, and plasticity? Neuron. 1996;16:233–236. doi: 10.1016/s0896-6273(00)80040-9. [DOI] [PubMed] [Google Scholar]
- 17.Fitzsimonds RM, Poo MM. Retrograde signaling in the development and modification of synapses. Physiol Rev. 1998;78:143–170. doi: 10.1152/physrev.1998.78.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Fredette BJ, Landmesser LT. Relationship of primary and secondary myogenesis to fiber type development in embryonic chick muscle. Dev Biol. 1991;143:1–18. doi: 10.1016/0012-1606(91)90050-d. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 21.Ginty DD. Calcium regulation of gene expression: isn’t that spatial? Neuron. 1997;18:183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 22.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–354. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 23.Hamburger V. Regression versus peripheral control of differentiation in motor hypoplasia. Am J Anat. 1958;102:365–409. doi: 10.1002/aja.1001020303. [DOI] [PubMed] [Google Scholar]
- 24.Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975;160:535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- 25.Hamburger V. The developmental history of the motor neuron. Neurosci Res Program Bull [Suppl] 1977;15:iii–37. doi: 10.1007/978-1-4899-6743-5_5. [DOI] [PubMed] [Google Scholar]
- 26.Hamburger V, Balaban M. Observations and experiments on spontaneous rhythmical behavior in the chick embryo. Dev Biol. 1963;7:533–545. doi: 10.1016/0012-1606(63)90140-4. [DOI] [PubMed] [Google Scholar]
- 27.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–82. [PubMed] [Google Scholar]
- 28.Hamburger V, Balaban M, Oppenheim R, Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool. 1965;159:1–14. doi: 10.1002/jez.1401590102. [DOI] [PubMed] [Google Scholar]
- 29.Henderson CE. Programmed cell death in the developing nervous system. Neuron. 1996;17:579–585. doi: 10.1016/s0896-6273(00)80191-9. [DOI] [PubMed] [Google Scholar]
- 30.Hollyday M, Hamburger V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976;170:311–320. doi: 10.1002/cne.901700304. [DOI] [PubMed] [Google Scholar]
- 31.Hory-Lee F, Frank E. The nicotinic blocking agents d-tubocurare and α-bungarotoxin save motoneurons from naturally occurring death in the absence of neuromuscular blockade. J Neurosci. 1995;15:6453–6460. doi: 10.1523/JNEUROSCI.15-10-06453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houeneou LJ, Pinçon-Raymond M, Garcia L, Harris AJ, Rieger F. Neuromuscular development following tetrodotoxin-induced inactivity in mouse embryos. J Neurobiol. 1990;21:1249–1261. doi: 10.1002/neu.480210809. [DOI] [PubMed] [Google Scholar]
- 33.Houeneou LJ, McManaman JL, Prevette D, Oppenheim RW. Regulation of putative muscle-derived neurotrophic factors by muscle activity and innervation: in vivo and in vitro studies. J Neurosci. 1991;11:2829–2837. doi: 10.1523/JNEUROSCI.11-09-02829.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh K, Stevens B, Schachner M, Fields RD. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–1372. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- 35.Landmesser L. The distribution of motoneurones supplying chick hind limb muscles. J Physiol (Lond) 1978a;284:371–389. doi: 10.1113/jphysiol.1978.sp012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landmesser L. The development of motor projection patterns in the chick hind limb. J Physiol (Lond) 1978b;284:391–414. doi: 10.1113/jphysiol.1978.sp012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landmesser L. The relationship of intramuscular nerve branching and synaptogenesis to motoneuron survival. J Neurobiol. 1992;23:1131–1139. doi: 10.1002/neu.480230906. [DOI] [PubMed] [Google Scholar]
- 38.Landmesser LT, O’Donovan MJ. Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol (Lond) 1984;347:189–204. doi: 10.1113/jphysiol.1984.sp015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landmesser LT, Szente M. Activation patterns of embryonic chick hind limb muscles following blockade of activity and motoneurone cell death. J Physiol (Lond) 1986;380:157–174. doi: 10.1113/jphysiol.1986.sp016278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landmesser L, Dahm L, Schultz K, Rutishauser U. Distinct roles for adhesion molecules during innervation of embryonic chick muscle. Dev Biol. 1988;130:645–670. doi: 10.1016/0012-1606(88)90358-2. [DOI] [PubMed] [Google Scholar]
- 41.Landmesser L, Dahm L, Tang J, Rutishauser U. Polysialic acid as a regulator of intramuscular nerve branching during embryonic development. Neuron. 1990;4:655–667. doi: 10.1016/0896-6273(90)90193-j. [DOI] [PubMed] [Google Scholar]
- 42.Lnenicka GA, Arcaro KF, Calabro JM. Activity-dependent development of calcium regulation in growing motor axons. J Neurosci. 1998;18:4966–4972. doi: 10.1523/JNEUROSCI.18-13-04966.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meister M, Wong ROL, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- 44.Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Jr, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane on CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity prior to target contact. J Neurosci. 1999;19:3007–3022. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milner LD, Rafuse VF, Landmesser LT. Selective fasciculation and divergent pathfinding decisions of embryonic chick motor axons projecting to fast and slow muscle regions. J Neurosci. 1998;18:3297–3313. doi: 10.1523/JNEUROSCI.18-09-03297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Donovan MJ, Landmesser LT. The development of hindlimb motor activity studied in the isolated spinal cord of the chick embryo. J Neurosci. 1987;7:3256–3264. doi: 10.1523/JNEUROSCI.07-10-03256.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Donovan MJ, Ho S, Yee W. Calcium imaging of rhythmic network activity in the developing spinal cord of the chick embryo. J Neurosci. 1994;14:6354–6369. doi: 10.1523/JNEUROSCI.14-11-06354.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oppenheim R. The role of supraspinal input in embryonic motility: a re-examination in the chick. J Comp Neurol. 1975;160:37–50. doi: 10.1002/cne.901600104. [DOI] [PubMed] [Google Scholar]
- 50.Oppenheim RW. The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci. 1989;12:252–255. doi: 10.1016/0166-2236(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 51.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 52.Oppenheim RW. Neurotrophic survival molecules for motoneurons: an embarrassment of riches. Neuron. 1996;17:195–197. doi: 10.1016/s0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 53.Oppenheim RW, Chu-Wang IW. Spontaneous cell death of motoneurons following peripheral innervation in the chick embryo. Brain Res. 1977;125:154–160. doi: 10.1016/0006-8993(77)90367-5. [DOI] [PubMed] [Google Scholar]
- 54.Oppenheim RW, Chu-Wang IW, Maderdrut JL. Cell death of motoneurons in the chick embryo spinal cord. III. The differentiation of motoneurons prior to their induced degeneration following limb-bud removal. J Comp Neurol. 1978;177:87–112. doi: 10.1002/cne.901770107. [DOI] [PubMed] [Google Scholar]
- 55.Oppenheim RW, Houenou L, Pinçon-Raymond M, Powell JA, Rieger F, Standish LJ. The development of motoneurons in the embryonic spinal cord of the mouse mutant, muscular dysgenesis (mdg/mdg): survival, morphology, and biochemical differentiation. Dev Biol. 1986;114:426–436. doi: 10.1016/0012-1606(86)90207-1. [DOI] [PubMed] [Google Scholar]
- 56.Oppenheim RW, Bursztajn S, Prevette D. Cell death of motoneurons in the chick embryo spinal cord. XI. Acetylcholine receptors and synaptogenesis in skeletal muscle following the reduction of motoneuron death by neuromuscular blockade. Development. 1989;107:331–341. doi: 10.1242/dev.107.2.331. [DOI] [PubMed] [Google Scholar]
- 57.Oppenheim RW, Prevette D, Houenou LJ, Pinçon-Raymond M, Dimitriadou V, Donevan A, O’Donovan M, Wenner P, McKemy DD, Allen PD. Neuromuscular development in the avian paralytic mutant crooked neck dwarf (cn/cn): further evidence for the role of neuromuscular activity in motoneuron survival. J Comp Neurol. 1997;381:353–372. doi: 10.1002/(sici)1096-9861(19970512)381:3<353::aid-cne7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 58.Owens DF, Boyce LH, Davis MBE, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pittman RH, Oppenheim RW. Neuromuscular blockade increases motoneurone survival during normal cell death in the chick embryo. Nature. 1978;271:364–366. doi: 10.1038/271364a0. [DOI] [PubMed] [Google Scholar]
- 60.Pittman RH, Oppenheim RW. Cell death in the chick embryo spinal cord. IV. Evidence that a functional neuromuscular interaction is involved in the regulation of naturally occurring cell death and the stabilization of synapses. J Comp Neurol. 1979;187:425–446. doi: 10.1002/cne.901870210. [DOI] [PubMed] [Google Scholar]
- 61.Powell JA, Rieger F, Blondet B, Dreyfus P, Pinçon-Raymond M. Distribution and quantification of ACh receptors and innervation in diaphragm muscle of normal and mdg mouse embryos. Dev Biol. 1984;101:168–180. doi: 10.1016/0012-1606(84)90127-1. [DOI] [PubMed] [Google Scholar]
- 62.Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994;14:889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rafuse VF, Landmesser L. Contractile activity regulates isoform expression and polysialylation of NCAM in cultured myotubes: involvement of Ca2+ and protein kinase C. J Cell Biol. 1996;132:969–983. doi: 10.1083/jcb.132.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rafuse VF, Milner LD, Landmesser L. Selective innervation of fast and slow muscle during early chick neuromuscular development. J Neurosci. 1996;16:6864–6877. doi: 10.1523/JNEUROSCI.16-21-06864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reitzel JL, Maderdrut JL, Oppenheim RW. Behavioral and biochemical analysis of GABA-mediated inhibition in the early chick embryo. Brain Res. 1979;172:487–504. doi: 10.1016/0006-8993(79)90581-x. [DOI] [PubMed] [Google Scholar]
- 66.Renshaw GMC, Goldie R. Neuronal bungarotoxin displaces [125I] α-bungarotoxin binding at the neuromuscular junction as well as to the spinal cord during embryogenesis. Brain Res. 1996;709:316–318. doi: 10.1016/0006-8993(95)01388-1. [DOI] [PubMed] [Google Scholar]
- 67.Renshaw G, Rigby P, Self G, Lamb A, Goldie R. Exogenously administered α-bungarotoxin binds to embryonic chick spinal cord: implications for the toxin-induced arrest of naturally occurring motoneuron death. Neuroscience. 1993;53:1163–1172. doi: 10.1016/0306-4522(93)90498-5. [DOI] [PubMed] [Google Scholar]
- 68.Rieger F, Pinçon-Raymond M. Muscle and nerve in muscular dysgenesis in the mouse at birth: sprouting and multiple innervation. Dev Biol. 1981;87:85–101. doi: 10.1016/0012-1606(81)90063-4. [DOI] [PubMed] [Google Scholar]
- 69.Rohrbough J, Spitzer NC. Regulation of intracellular Cl− levels by Na+-dependent Cl− cotransport distinguishes depolarizing from hyperpolarizing GABAA receptor-mediated responses in spinal neurons. J Neurosci. 1996;16:82–91. doi: 10.1523/JNEUROSCI.16-01-00082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sernagor E, Chub N, Ritter A, O’Donovan MJ. Pharmacological characterization of the rhythmic synaptic drive onto lumbosacral motoneurons in the chick embryo spinal cord. J Neurosci. 1995;15:7452–7464. doi: 10.1523/JNEUROSCI.15-11-07452.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sholomenko GN, O’Donovan MJ. Development and characterization of pathways descending to the spinal cord in embryonic chick. J Neurophysiol. 1995;73:1223–1233. doi: 10.1152/jn.1995.73.3.1223. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka H, Landmesser LT. Cell death of lumbosacral motoneurons in chick, quail, and chick–quail chimera embryos: a test of the quantitative matching hypothesis of neuronal cell death. J Neurosci. 1986;6:2889–2899. doi: 10.1523/JNEUROSCI.06-10-02889.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang J, Landmesser L. Reduction of intramuscular nerve branching and synaptogenesis is correlated with decreased motoneuron survival. J Neurosci. 1993;13:3095–3103. doi: 10.1523/JNEUROSCI.13-07-03095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thoenen H. Neurotrophins and plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 75.Vijayaraghavan S, Pugh PC, Zhang Z, Rathouz MM, Berg DK. Nicotinic receptors that bind α-bungarotoxin on neurons raise intracellular free Ca2+. Neuron. 1992;8:353–362. doi: 10.1016/0896-6273(92)90301-s. [DOI] [PubMed] [Google Scholar]
- 76.Wu W, Ziskind-Conhaim L, Sweet MA. Early development of glycine- and GABA-mediated synapses in rat spinal cord. J Neurosci. 1992;12:3935–3945. doi: 10.1523/JNEUROSCI.12-10-03935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan Q, Elliott JL, Matheson C, Sun J, Zhang L, Mu X, Rex KL, Snider WD. Influences of neurotrophins on mammalian motoneurons in vivo. J Neurobiol. 1993;24:1555–1577. doi: 10.1002/neu.480241202. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Z, Vijayaraghavan S, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin with high affinity function as ligand-gated ion channels. Neuron. 1994;12:167–177. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]