Abstract
The expression and coupling of endothelin (ET) receptors were studied in rat pituitary somatotrophs. These cells exhibited periods of spontaneous action potential firing that generated high-amplitude fluctuations in cytosolic calcium concentration ([Ca2+]i). The message and the specific binding sites for ETA, but not ETB, receptors were found in mixed pituitary cells and in highly purified somatotrophs. The activation of these receptors by ET-1 led to an increase in inositol 1,4,5-trisphosphate production and the associated rise in [Ca2+]i and growth hormone (GH) secretion. The Ca2+-mobilizing action of ET-1 lasted for 2–3 min and was followed by an inhibition of action potential-driven Ca2+ influx and GH secretion to below the basal levels. As in somatostatin-treated cells, the ET-1-induced inhibition of spontaneous electrical activity and Ca2+ influx was accompanied by the inhibition of adenylyl cyclase and by the stimulation of inward rectifier potassium current. In contrast to somatostatin, ET-1 did not inhibit voltage-gated Ca2+ channels. During prolonged agonist stimulation a gradual recovery of Ca2+influx and GH secretion occurred. In somatotrophs treated with pertussis toxin overnight, the ET-1-induced Ca2+-mobilizing phase was preserved, but it was followed immediately by facilitated Ca2+influx and GH secretion. Both somatostatin- and ET-1-induced inhibitions of adenylyl cyclase activity were abolished in pertussis toxin-treated cells. These results indicate that the transient cross-coupling of Ca2+-mobilizing ETAreceptors to the Gi/Go pathway in somatotrophs provides an effective mechanism to change the rhythm of [Ca2+]i signaling and GH secretion during continuous agonist stimulation.
Keywords: somatotrophs, growth hormone, calcium, endothelin, somatostatin, electrical activity
Action potential (AP)-driven Ca2+ influx through voltage-gated calcium channels (VGCCs) is operative in various endocrine and neuroendocrine cells, including pituitary somatotrophs. Because many different ionic channels act in concert to control AP firing, this pathway is referred to as the membrane potential (Vm)-dependent pathway for Ca2+ signaling (Stojilkovic, 1998). Several of the ionic channels contributing to theVm pathway in somatotrophs have been identified. These include VGCCs, voltage-gated sodium channels, pacemaker and ATP-gated cationic channels, and several types of potassium channels, including inwardly rectifying potassium channels (Kir) (Lewis et al., 1988; Sims et al., 1991; Brake et al., 1994; Koshimizu et al., 1998). Although resting somatotrophs have a fluctuatingVm, Kwiecien and colleagues (1997)have found that these pacemaker fluctuations were unable to initiate spontaneous APs in a majority of cultured somatotrophs. Consequently, they suggested that somatotrophs behave as “conditional pacemakers,” because the activation of adenylyl cyclase-coupled receptors is required for AP generation. However, others have observed spontaneous APs (Sims et al., 1991) and extracellular Ca2+-dependent and dihydropyridine-sensitive oscillations in intracellular Ca2+ concentration ([Ca2+]i transients) (Holl et al., 1988; Cuttler et al., 1992; Naumov et al., 1994).
The Vm pathway in somatotrophs is controlled by two hypothalamic neuropeptides, growth hormone-releasing hormone (GHRH) and somatostatin. GHRH activates its seven membrane domain receptors that are coupled positively to adenylyl cyclase. The increase in cAMP stimulates tetrodotoxin-insensitive pacemaker current to depolarize the membrane and initiate L-type Ca2+ channel-driven AP firing and the associated [Ca2+]i transients (Lussier et al., 1991a; Kwiecien et al., 1997). In contrast, somatostatin receptors are coupled negatively to adenylyl cyclase (Reisine and Bell, 1995), and their activation hyperpolarizes the cells to abolish GHRH-induced APs and [Ca2+]i transients (Mollard et al., 1988; Lussier et al., 1991c; Sims et al., 1991). A reduction in the amplitude of voltage-gated Ca2+ currents and the facilitation ofKir account for this inhibition (Yamashita et al., 1986; Sims et al., 1991).
Excitable cells also rely on the release of intracellularly stored Ca2+ by the activation of Ca2+-mobilizing receptors in a manner that is well characterized in nonexcitable cells. The specificity in the operation of Ca2+-mobilizing receptors in excitable cells is in the need for the synchronization of voltage-gated Ca2+ influx with Ca2+ mobilization (Stojilkovic, 1998). For example, in pituitary lactotrophs and GT1 neurons the activation of thyrotropin-releasing hormone (TRH) and gonadotropin-releasing hormone (GnRH) receptors, respectively, generates biphasic changes inVm and [Ca2+]i. A transient spike increase in [Ca2+]i caused by Ca2+ mobilization is associated with the activation of Ca2+-controlled K+ channels, which induces membrane hyperpolarization. This is followed by a sustained membrane depolarization, accompanied with enhanced Ca2+ influx (Sankaranarayanan and Simasko, 1996; Van Goor et al., 1999). In pituitary somatotrophs, however, the expression and operation of a Ca2+-mobilizing pathway have not been observed. In this study we demonstrate the presence of phospholipase C-coupled endothelinA (ETA) receptors in pituitary somatotrophs and characterize their effects on [Ca2+]i and theVm pathway. In addition to being coupled to Ca2+ mobilization, they are cross-coupled to pertussis toxin-sensitive G-proteins, leading to a unique pattern of Ca2+ signaling and hormone secretion.
MATERIALS AND METHODS
Cell cultures and hormone secretion. Anterior pituitary glands from adult female Sprague Dawley rats obtained from Charles River (Wilmington, MA) were dispersed into single cells by a trypsin/DNase (Sigma, St. Louis, MO) treatment procedure. All experiments were performed in either mixed pituitary cell populations or purified somatotroph populations. Purification was done by a two-stage Percoll discontinuous density gradient centrifugation (Lussier et al., 1991b). For cell column perifusion, 12 × 106 mixed cells were incubated with preswollen Cytodex-1 beads (Pharmacia, Piscataway, NJ) in 60 mm culture dishes for 2 d. Then the cells were loaded into temperature-controlled chambers; they were perifused at 37°C with Hanks’ medium 199 containing 20 mm HEPES and 0.1% BSA for 60 min at a flow rate of 0.8 ml/min for 2 hr, after which a stable basal growth hormone (GH) secretion rate was established. During the test period 1 min fractions were collected, and the perifusate subsequently was stored at −20°C. For static culture secretion, 0.25 × 106 cells/well were sited in 24-well plates. After 2 d the incubation medium was replaced with Hanks’ medium 199 containing selected concentrations of agonists and was incubated for 3 hr at 37°C. GH release was determined by radioimmunoassay, using the reagents and standards provided by the National Pituitary Agency (Torrance, CA).
cAMP and inositol 1,4,5-triphosphate (InsP3) measurements. Radioimmunoassay of cAMP was performed as previously described (Baukal et al., 1994), using a specific cAMP antiserum at a titer of 1:5000. This antibody showed no cross-reaction with cGMP, 2′3′-cAMP, or isobutylmethylxanthine (IBMX), the latter being used to downregulate the phosphodiesterase activity. For inositol phosphate measurements, on the second day of cell culture in four-well plates the medium was changed to inositol-free medium 199 with Hanks’ salt solution containing 5 μCi ofmyo[3H]inositol, NaHCO3 (1.4 gm/l), and 0.1% BSA. After 24 hr of incubation the cells were washed three times with inositol-free medium 199 containing 25 mm HEPES, pH 7.4, and 0.1% bovine serum albumin and were treated with 100 nmET-1 or somatostatin. The radioactivity incorporated into the individual or total inositol phosphates was determined as previously described (Stojilkovic et al., 1994).
Single-cell calcium measurements. Cells were plated on coverslips coated with poly-l-lysine and cultured in medium 199 containing Earle’s salts, sodium bicarbonate, horse serum, and antibiotics for 24–48 hr. For [Ca2+]imeasurements the cells were incubated for 60 min at 37°C with 2 μm fura-2 AM in phenol red-free medium 199 containing Hanks’ salts, 20 mm sodium bicarbonate, and 20 mm HEPES. Coverslips with cells were washed with phenol red-free physiological buffer and mounted on the stage of an Axiovert 135 microscope (Carl Zeiss, Oberkochen, Germany) attached to an Attofluor Digital Fluorescence Microscopy System (Atto Instruments, Rockville, MD). Cells were examined under a 40× oil immersion objective during exposure to alternating 340 and 380 nm light beams; the intensity of light emission at 505 nm was measured. [Ca2+]i is shown as the ratio of intensities measured at 340 and 380 nm [F340/F380].
Measurement of ionic currents. Ionic currents were measured by using perforated-patch or regular whole-cell voltage-clamp recording techniques as previously described (Van Goor et al., 1999). Briefly, voltage-clamp recordings were performed at room temperature with an Axopatch 200 B patch-clamp amplifier (Axon Instruments, Foster City, CA) and were low-pass-filtered at 2 kHz. Patch-clamp pipettes were fabricated from borosilicate glass (type 7740; World Precision Instruments, Sarasota, FL), using a horizontal micropipette puller (Model P-87; Sutter Instrument, Novato, CA), and were heat-polished to a final tip resistance of 2–4 MΩ. Before seal formation the liquid junction potentials were canceled. When necessary, series resistance compensation was optimized, and all Ca2+ current records were corrected for a linear leakage and capacitance via a P/-N procedure. Pulse generation, data acquisition, and analysis were done with a PC equipped with a Digidata 1200 A/D interface in conjunction with pCLAMP 7 (Axon Instruments). For the recording ofKir, patch pipette tips were immersed briefly in a solution containing (in mm): 70 KCl, 70 K-aspartate, 1 MgCl2, and 10 HEPES (pH-adjusted to 7.2 with KOH) and then back-filled with the same solution containing amphotericin B (240 μg/ml). The bath contained a high extracellular K+ solution containing (in mm): 120 NaCl, 20 KCl, 1.26 CaCl2, 2 MgCl2, 0.7 MgSO4, 10 HEPES, and 10 glucose, pH-adjusted to 7.4 with NaOH. For the recording of isolated Ca2+ currents, the bath contained (in mm): 100 teramethylammonium (TMA)-Cl, 20 TEA, 2.6 or 10 CaCl2, 1 MgCl2, 1 μm TTX, and 10 HEPES, pH-adjusted to 7.4 with TMA-OH; the pipette solution contained (in mm): 120 CsCl, 20 TEA-Cl, 4 MgCl2, 10 EGTA, 9 glucose, 20 HEPES, 0.3 Tris-GTP, 4 Mg-ATP, 14 CrPO4, and 50 U/ml creatine phosphokinase, pH-adjusted to 7.2 with Tris base.
Simultaneous measurements of [Ca2+]i andVm. Purified pituitary somatotrophs were incubated for 60 min at 37°C in phenol red-free medium 199 containing Hanks’ salts, 20 mm sodium bicarbonate, 20 mm HEPES, and 2 μm indo-1 AM (Molecular Probes, Eugene, OR). The coverslips with cells were washed twice with a recording solution containing (in mm): 120 NaCl, 4.7 KCl, 1.26 CaCl2, 2 MgCl2, 0.7 MgSO4, 10 HEPES, and 10 glucose (pH-adjusted to 7.4 with NaOH) and were mounted on the stage of an inverted epifluorescence microscope (Nikon, Tokyo, Japan). A photon counter system (Nikon) was used to measure simultaneously the intensity of light emitted at 405 and 480 nm after excitation at 340 nm, with correction for background intensity at each emission wavelength. Perforated-patch, current-clamp recordings were performed with an Axopatch 200 B patch-clamp amplifier (Axon Instruments) as previously described (Van Goor et al., 1999). Patch pipette tips (3–5 MΩ) were immersed briefly in a solution containing (in mm): 70 KCl, 70 K-aspartate, 1 MgCl2, and 10 HEPES (pH-adjusted to 7.2 with KOH) and then back-filled with the same solution containing amphotericin B (240 μg/ml). Before seal formation the liquid junction potentials were canceled. The data were digitized at 4 kHz, using a PC equipped with the pCLAMP 7 software package in conjunction with a Digidata 1200 A/D converter (Axon Instruments). All reportedVm values were corrected for a liquid junction potential error of 10 mV (Barry, 1994). The bath contained <500 μl of saline and was perfused continuously at a rate of 2 ml/min with a gravity-driven superfusion system. A solid Ag/AgCl reference electrode was connected to the bath via a 3 m KCl agar bridge.
RNA isolation and RT-PCR. Total RNA was extracted from GH3, primary pituitary, purified somatotrophs, and A10 cells with Trizol Reagent (Life Technologies, Gaithersburg, MD) and quantified spectrophotometrically; its purity was determined by the A260/A280 ratio. RNA samples were subjected to RT-PCR to determine whether these cells contained ET receptor mRNA. To eliminate residual genomic DNA, we treated the RNA samples with DNase I. Total RNA (5 μg) from each sample with or without DNase treatment was reverse-transcribed into cDNA in a 20 μl reaction mixture containing oligo-dT18 primer that used SuperScript II reverse transcriptase (Life Technologies) according to the supplier’s instructions. An aliquot of 0.5 μl of the RT reaction was amplified for 30 cycles with a PCR reagent system (Life Technologies) in a final volume of 25 μl containing 1.5 mmMgCl2, 0.2 μm of each primer, and 0.2 mm of each dNTP. PCR conditions for each cycle included the following: denaturation at 94°C for 1 min, annealing at 55°C for 30 sec, and extension at 72°C for 1 min. The PCR products were analyzed by agarose gel (1.5%) electrophoresis and visualized with ethidium bromide. ETA-specific primers corresponded to the rat endothelin type A receptor sequence (Lin et al., 1991): nucleotides 873–894 for the sense primer (5′-ATTGCCCTCAGCGAACACCT-3′) and 981–1000 for the antisense primer (5′-CATAGACGGTTTTCTTCAAA-3′). ETB-specific primers corresponded to the rat endothelin type B receptor sequence (Sakurai et al., 1990): nucleotides 680–700 for the sense primer (5′-TCTCTGTGGTTCTGGCTGTC-3′) and 1004–1024 for the antisense primer (5′-TGCTGAGGTGAAGGGGAAGC-3′). The same volume of samples used for ET receptor message analysis also was subjected to PCR reaction, using GAPDH-specific primers; sequences for sense and antisense primers were 5′-GGCATCCTGGGCTACACTG-3′ and 5′-TGAGGTCCACCACCCTGTT-3′, respectively. The authenticity of the PCR-amplified products for ETA and ETB receptors was confirmed further by Southern blot analysis. The samples run on the gels were transferred onto nylon membranes (Schleicher & Schuell, Keene, NH) and probed with radiolabeled ETA- and ETB-specific sequences (internal to the PCR primers): 5′-GCCCTGTGCTGGTTCCCTCTTCACTTAAGCCG-3′ (nucleotides 946–977) and 5′-TGGTGGCTGTTCAGTTTCTACTTCTGCTTGCC-3′ (nucleotides 820–851), respectively. Probes were labeled with the 5′-end labeling kit (Amersham, Arlington Heights, IL), and membranes were hybridized at 40°C for 20 hr, using Hybrisol solution (Oncor, Gaithersburg, MD) containing 30% formamide. The membranes were washed under stringent conditions and visualized by radioautography. A10 cells and rat ETA cDNA (kindly provided by M. Yanagisawa, University of Texas Southwestern Medical Center, Dallas, TX) were used as a positive control for the ETA receptor. Human ETB receptor sequence (Sakamoto et al., 1991) was used as a positive control for the ETB receptor. Reactions without an RNA sample or RT were run also and served as negative controls.
RESULTS
Ca2+ signaling in unstimulated somatotrophs
Both identified somatotrophs from mixed populations of anterior pituitary cells and highly purified somatotrophs were used for single-cell [Ca2+]imeasurements. In mixed populations of anterior pituitary cells, ∼50% of cells are somatotrophs, and their identification in our experiments was based on the actions of somatostatin (typical for somatotrophs and some lactotrophs), TRH (typical for lactotrophs and thyrotrophs), and GnRH (typical for gonadotrophs). In purified cultures, ∼94% of cells were somatotrophs (Lussier et al., 1991b). The procedure used for the purification of cells separates a subfraction of high-density cells, which previously have been shown to respond to GHRH by the facilitation of Ca2+ influx in an adenylyl cyclase-dependent manner (Lussier et al., 1991a,b; Ramirez et al., 1998). Purified somatotrophs were used to evaluate the dependence of spontaneous and agonist-induced Ca2+signaling on surrounding cell composition, i.e., to eliminate potential paracrine effects. In addition, purified somatotrophs provide an internal control for experiments with somatotrophs identified in mixed populations of cells.
Approximately 50% of identified somatotrophs from mixed populations of cells (155 of 312 cells) were quiescent (Fig.1A), whereas the residual cells exhibited spontaneous fluctuations in [Ca2+]i. The patterns of these [Ca2+]i transients were variable. In general, they could be characterized as discrete baseline-like [Ca2+]i transients (Fig. 1B) or prolonged bursting-like periods (Fig.1C). In a fraction of somatotrophs (34 of 155), transitions from active to quiescent status and from quiescent to active status were observed also (Fig. 1D). Other populations of anterior pituitary cells also exhibited spontaneous activity, but the amplitudes of their [Ca2+]i transients were ∼20–40% of that commonly observed in somatotrophs (data not shown). Finally, in cultures of highly purified somatotrophs some cells were quiescent (8 of 19 cells; Fig. 1E,tracing a), and others were spontaneously active (Fig. 1E, tracings b,c). The pattern of spontaneous [Ca2+]i transients in these cells was highly comparable to that observed in identified somatotrophs from mixed populations of anterior pituitary cells. Thus, both quiescence and spontaneous activities are functional stages of somatotrophs. Because the spontaneous activity is not affected by the removal of other subpopulations of anterior pituitary cells, it is likely that the generation of [Ca2+]i transients is an intrinsic characteristic of somatotrophs.
Fig. 1.
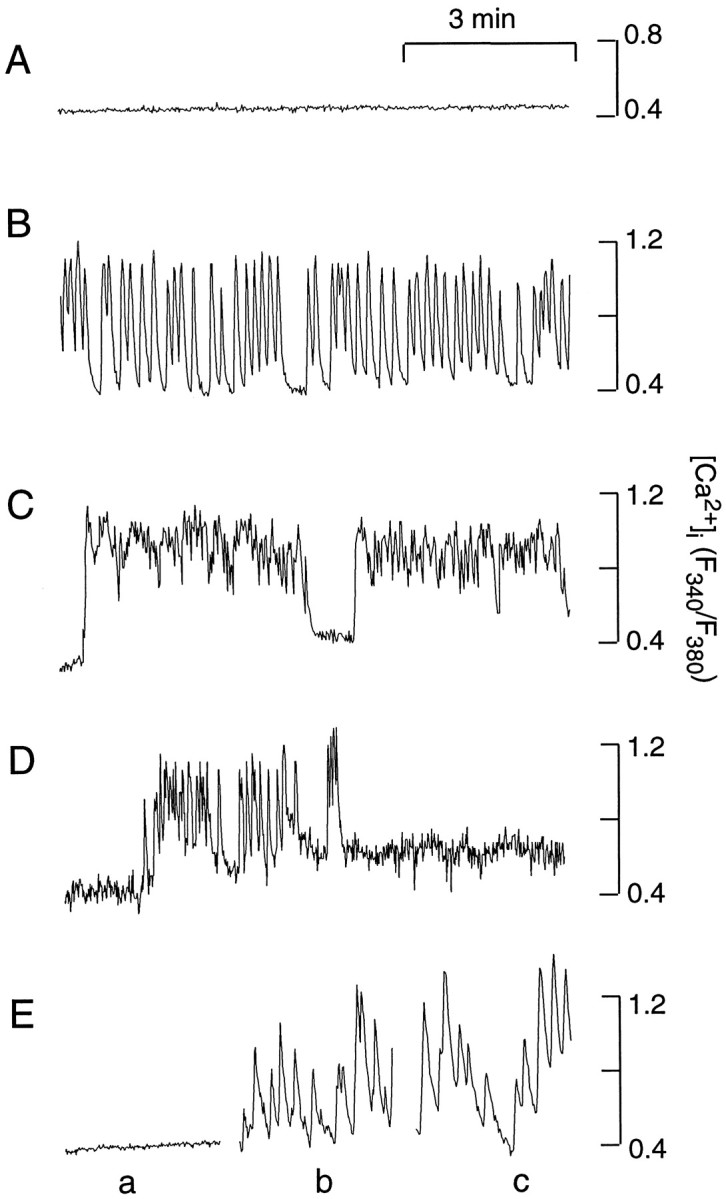
Basal [Ca2+]i in cultured somatotrophs. The tracings shown are from four representative somatotrophs in mixed populations of cells (A–D) and three somatotrophs from purified cultures (E, tracingsa–c). In this and the following figures, fura 2-AM was used for [Ca2+]i recordings, if not otherwise specified. Recordings were done at room temperature.
Effects of endothelin and somatostatin on [Ca2+]i in somatotrophs
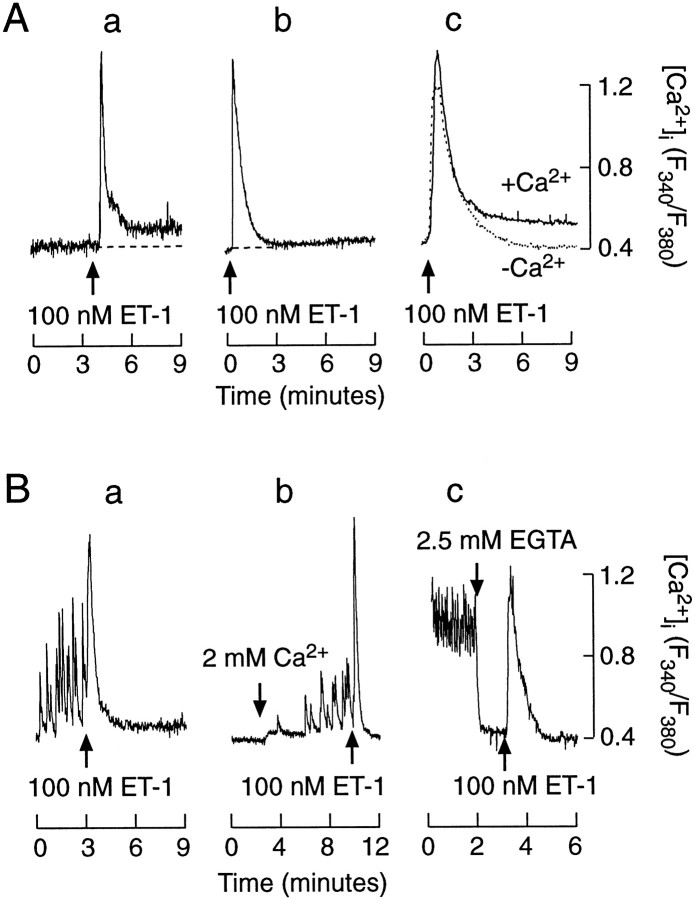
The actions of ET-1 and somatostatin on Ca2+ signaling were studied in both quiescent and spontaneously active cells. In a majority of quiescent cells bathed in Ca2+-containing medium (117 of 133), 100 nm ET-1 induced a biphasic change in [Ca2+]i, composed of an early spike phase and a sustained low-amplitude plateau phase (Fig.2A, tracing a; Table 1). In the residual cells the plateau phase was not observed. Like the cells bathed in Ca2+-containing medium, a majority of somatotrophs bathed in Ca2+-deficient medium (183 of 207 cells) responded to 100 nmET-1. Depletion of extracellular Ca2+ did not affect the ET-1-induced spike phase, whereas the plateau response was abolished. Figure 2A, tracing b, illustrates an example of an ET-1-induced [Ca2+]i profile in a cell bathed in Ca2+-deficient medium, and tracings shown in tracing c are the mean values (n = 15 per group) from ET-1-stimulated cells in Ca2+-containing and -deficient medium. These results indicate that ET-1 induces a rapid Ca2+ mobilization from intracellular stores, followed by a sustained Ca2+influx of a limiting capacity. In this respect, the action of ET-1 in quiescent somatotrophs does not differ from those observed in nonexcitable cells (Stojilkovic, 1998).
Fig. 2.
Endothelin-induced [Ca2+]i response in identified somatotrophs from mixed populations of anterior pituitary cells.A, Agonist-induced [Ca2+]i signals in quiescent somatotrophs. a, Biphasic [Ca2+]i response in cells bathed in Ca2+-containing medium—single-cell recording.b, Monophasic [Ca2+]iresponse in cells bathed in Ca2+-deficient medium—single-cell recording (free [Ca2+]i was ∼10 μm).c, Mean values (n = 15 for each group) of [Ca2+]i profiles of ET-1-stimulated cells bathed in Ca2+-containing (+Ca2+) and Ca2+-deficient medium (−Ca2+). B, Effects of ET-1 on [Ca2+]i in spontaneously active cells.a, ET-1-induced spike [Ca2+]i response and inhibition of spontaneous [Ca2+]i transients.b, c, Extracellular Ca2+ dependence of spontaneous [Ca2+]i transients and independence of ET-1-induced spike [Ca2+]i response. Cells initially were bathed in Ca2+-deficient medium (tracingb) or 2 mmCa2+ (tracingc). In this and the following figures thearrows indicate the moment of drug applications. The drugs were added in 1 ml volumes and were present throughout the recording. The concentrations indicated above thearrows are final.
Table 1.
Concentration-dependent effects of ET-1 on facilitation of Ca2+ release and inhibition of Ca2+ influx in identified somatotrophs from mixed populations of anterior pituitary cells
| ET-1 (in nm) | Spontaneously active cell | Quiescent cells | |||||
|---|---|---|---|---|---|---|---|
| Mobilization and influx (%) | Influx only (%) | No effect (%) | n | Mobilization (%) | No effect (%) | n | |
| 100 | 75 | 20 | 5 | 147 | 88 | 12 | 151 |
| 10 | 43 | 38 | 19 | 74 | 58 | 42 | 59 |
| 1 | 10 | 70 | 20 | 20 | 42 | 58 | 12 |
The effects of ET-1 on [Ca2+]i in spontaneously active somatotrophs were more complex than those observed in quiescent somatotrophs. The addition of 100 nm ET-1 was associated in a majority of spontaneously active cells with a transient spike increase in [Ca2+]i, which usually had a higher amplitude and a longer duration than that of the spontaneously generated [Ca2+]i transients (Fig. 2B, tracings a–c; Table1). The amplitudes and duration of ET-1-induced spikes in the active cells were highly comparable to those observed in quiescent cells (Fig.2A). After the spike phase, 100 nm ET-1 abolished [Ca2+]i transients in all responding cells. In spontaneously active cells the addition of EGTA also abolished spontaneous [Ca2+]itransients, demonstrating their dependence on Ca2+ influx (Fig. 2B,tracing c). In accord with this, the addition of 2 mm Ca2+ in cells bathed in Ca2+-deficient medium generated spontaneous [Ca2+]i transients (Fig. 2B, tracing b). These results indicate that ET-1 has a dual action on calcium signaling in somatotrophs; it stimulates the Ca2+mobilization pathway independently of extracellular Ca2+ concentration and inhibits Ca2+ influx in spontaneously active cells.
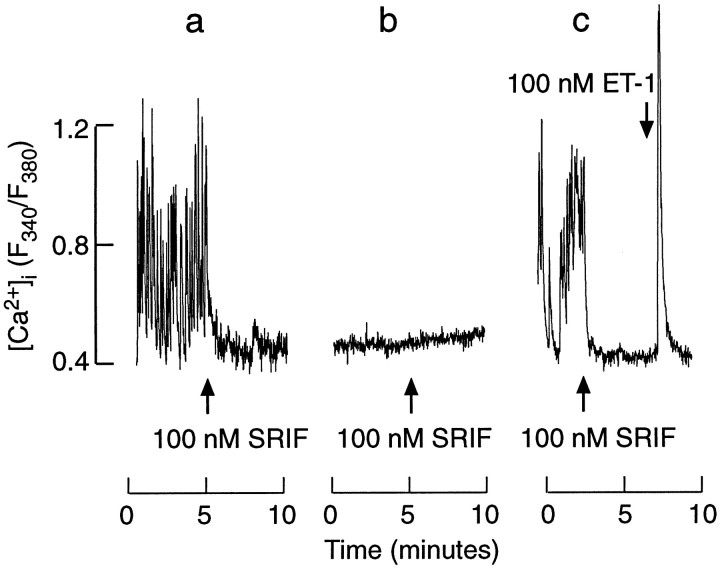
The inhibitory action of ET-1 on Ca2+influx in somatotrophs resembled the effects of somatostatin, an agonist for these cells. Somatostatin abolished the [Ca2+]i transients in 40 of 43 spontaneously active cells that were studied (Fig.3, tracing a). In contrast to ET-1, somatostatin-induced inhibition was never preceded by a spike increase in [Ca2+]i. Also, in 15 of 16 quiescent cells somatostatin did not affect [Ca2+]i (Fig. 3,tracing b). The addition of 100 nm ET-1 in the presence of somatostatin induced a monophasic rise in [Ca2+]i in spontaneously active cells (Fig. 3, tracing c). These results suggest that ET-1 and somatostatin may act via similar pathways to inhibit spontaneous and extracellular calcium-dependent [Ca2+]itransients. Furthermore, ET-1, but not somatostatin, also can activate the Ca2+ mobilization pathway.
Fig. 3.
Somatostatin-controlled [Ca2+]i signals in identified somatotrophs from mixed populations of anterior pituitary cells.a, c, Somatostatin-induced inhibition of spontaneous [Ca2+]i transients. b, Ineffectiveness of somatostatin on [Ca2+]i in quiescent cells.c, Monophasic [Ca2+]iresponse to ET-1 in cells bathed in the presence of somatostatin.
However, not all of the spontaneously active somatotrophs responded to ET-1 with both Ca2+ mobilization and the inhibition of Ca2+ influx. Approximately 75% of the cells stimulated with 100 nm responded with Ca2+ mobilization followed by the inhibition of Ca2+ influx, whereas 20% of cells responded only with the inhibition of Ca2+ influx (n = 147). As shown in Table 1, the percentage of spontaneously active cells responding only with the inhibition of Ca2+ influx increased with decreasing ET-1 concentration. Furthermore, the percentage of quiescent cells responding with Ca2+ mobilization decreased with a decrease in ET-1 concentrations (Table 1). These results indicate the different sensitivity of ET receptors in controlling Ca2+ mobilization and Ca2+ influx-dependent pathways.
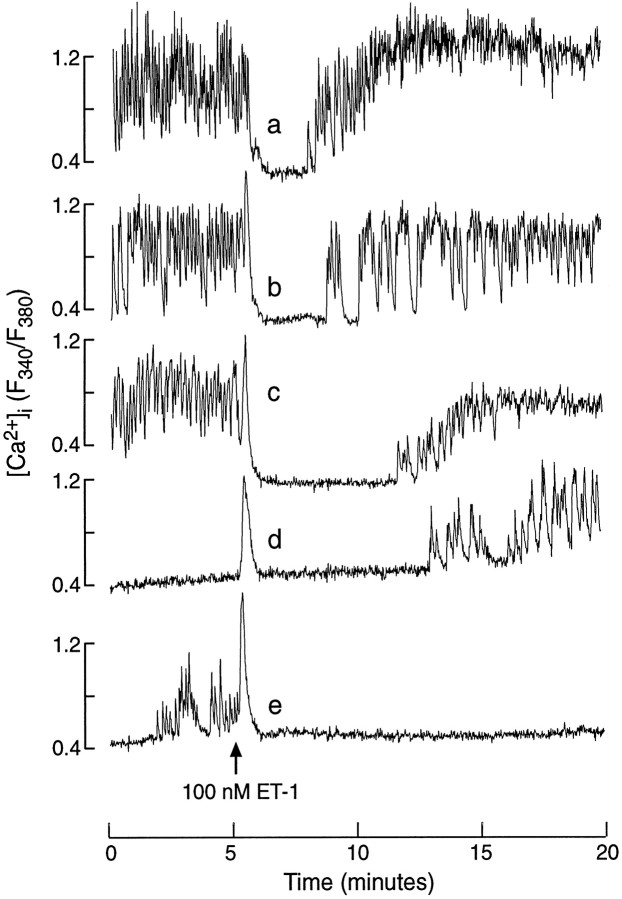
Although ET-1 was more effective in inhibiting spontaneous [Ca2+]i transients than in activating the Ca2+ mobilization pathway, such inhibition was temporal. In general, the recovery of calcium spiking occurred more rapidly in cells when the spike rise in [Ca2+]i was not triggered (Fig. 4, tracing a). The recovery also was observed in spontaneously active cells in which the Ca2+ mobilization phase was triggered by ET-1 (Fig. 4, tracings b, c). In quiescent somatotrophs a prolonged exposure to ET-1 frequently was associated with the initiation of [Ca2+]i transients (Fig. 4, tracing d). In some cells the recovery of [Ca2+]itransients did not occur during the recording (Fig. 4,tracing e). In cultures stimulated with 100 nm ET-1 for 15 min, 11 of 27 spontaneously active cells showed the recovery of Ca2+ spiking, and 12 of 24 quiescent cells showed the initiation of Ca2+ spiking.
Fig. 4.
Patterns of [Ca2+]i signaling during the prolonged stimulation with ET-1. a–c, Recovery of [Ca2+]i transients in cells stimulated with 100 nm ET-1. Note the lack of a spike [Ca2+]i response in a.d, Initiation of [Ca2+]i transients in quiescent cells during the prolonged ET-1 stimulation. e, The lack of recovery of [Ca2+]i transients in a spontaneously active cell during a 15 min exposure to 100 nm ET-1.
GH secretion in unstimulated and agonist-stimulated cells
In parallel to Ca2+ signaling in single cells, ET-1 induced a bidirectional change in GH release in perifused pituitary cells that was composed of a transient spike-like stimulation and a sustained inhibition. A rapid (within 20 sec) substitution of ET-1 with somatostatin was followed by a further reduction in the rate of GH secretion (Fig.5A). When somatostatin was applied initially, an immediate reduction in GH secretion was observed. After a substitution of somatostatin with ET-1, the secretion resumed a typical bidirectional pattern composed of an early spike response and a sustained inhibition. Again, the somatostatin-induced inhibition of GH secretion was greater than that induced by ET-1 (Fig.5B).
Fig. 5.
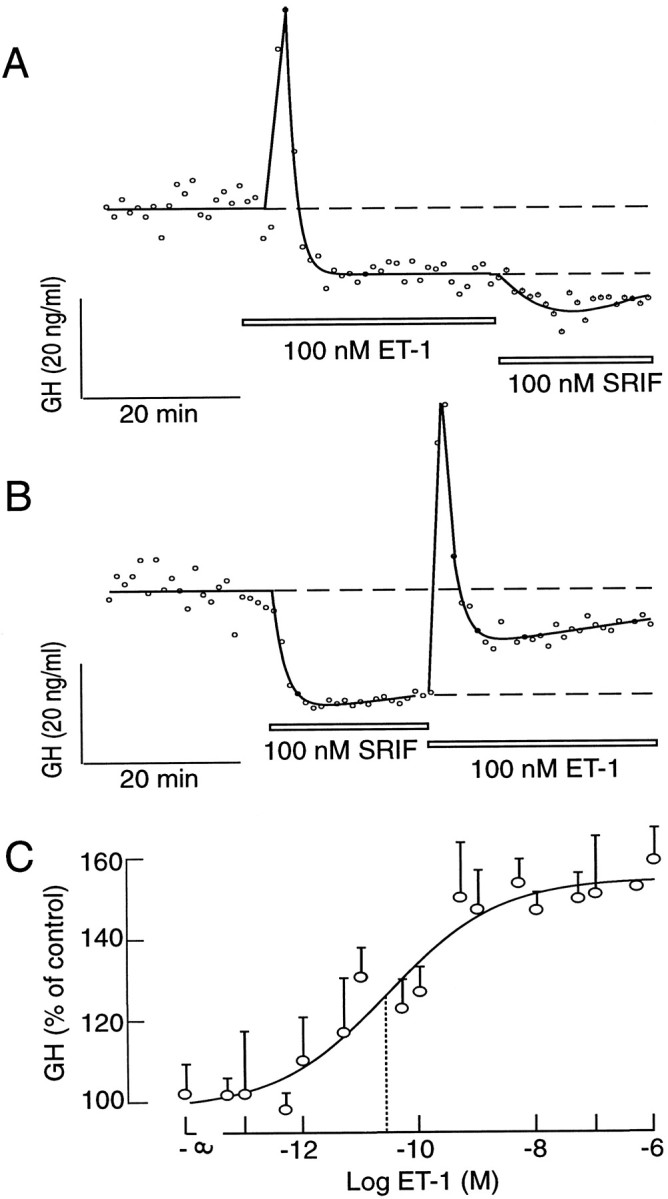
Endothelin-induced GH secretion in perifused pituitary cells. A, B, Comparison of the effects of ET-1 and somatostatin on GH secretion. Cells were perifused at a flow rate of 0.8 ml/min, and samples were collected every minute. Thetracings shown are representative from three experiments. C, Concentration dependence of ET-1 on GH secretion in static cultures of pituitary cells (0.25 × 106 cells/well). Cultures were stimulated with ET-1 for 3 hr. The results shown are the mean ± SEM from sextuplicate incubation in one from three independent experiments. Thedotted lines indicate the EC50 for ET-1, derived from the fitted logistic curve (Kaleida Graph program).
Because of the transient nature of the ET-1-induced inhibition of [Ca2+]i spiking (see Fig. 4), we speculated that prolonged receptor activation would have an overall stimulatory effect on GH secretion. To test this hypothesis, we stimulated the cells in static culture for 3 hr with increasing concentrations of ET-1, and we collected the medium at the end of the stimulation for GH analysis. As shown in Figure5C, ET-1 increased GH secretion in a concentration-dependent manner, with an EC50 of ∼25 pm, which was comparable to the IC50 observed in binding studies (see below). These results indicate that basal GH secretion is high and can be modulated by ET and somatostatin. Because the activation of both receptors leads to the abolition of [Ca2+]itransients, it is reasonable to postulate that basal GH secretion is controlled by spontaneous [Ca2+]itransients.
Characterization of ET receptor subtypes expressed in somatotrophs
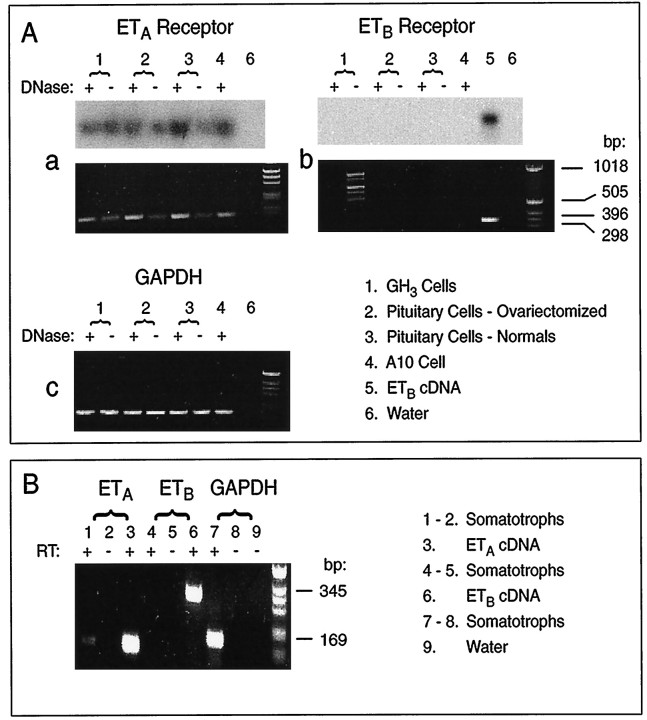
The findings that ET-1 induces Ca2+mobilization and mimics the inhibitory action of somatostatin on Ca2+ influx are consistent with the operation of both ETA and ETB receptors in somatotrophs, the first being facilitatory and the second inhibitory. However, RT-PCR analysis that used ETA- and ETB-specific primers revealed that pituitary cells and GH3-immortalized cells express only the message for ETA receptors. The message was identified in anterior pituitary tissue from normal and ovariectomized rats and GH3-immortalized lacto-somatotrophs (Fig.6A, panelsa, b), as well as in highly purified somatotrophs (Fig. 6B). The ETB message was observed only in control samples (Fig. 6A,panel b). Southern blot analysis also indicated the expression of ETA, but not ETB, receptors in anterior pituitary cells (Fig.6A, top panels).
Fig. 6.
Detection of endothelin receptor transcripts in a variety of rat pituitary cells. A, RNA samples isolated from GH3 cells, primary pituitary cells, and A10 cells (controls) were subjected to RT-PCR, using ETA and ETB receptors or GAPDH-specific primers as described under Materials and Methods. Ethidium bromide-stained agarose gels ran with the PCR-amplified products. Southern blots of the same gels are shown in the top panels. A 1 kb DNA ladder was run to provide size markers. The expected size bands, based on the primers that were used, were 127 bp for ETA, 345 bp for ETB, and 169 bp for GAPDH. B, Purified somatotroph fractions were used for RT-PCR analysis of ET receptor expression. A cDNA synthesis reaction was performed with and without reverse transcriptase (RT). The PCR primers that were used are the same as above.
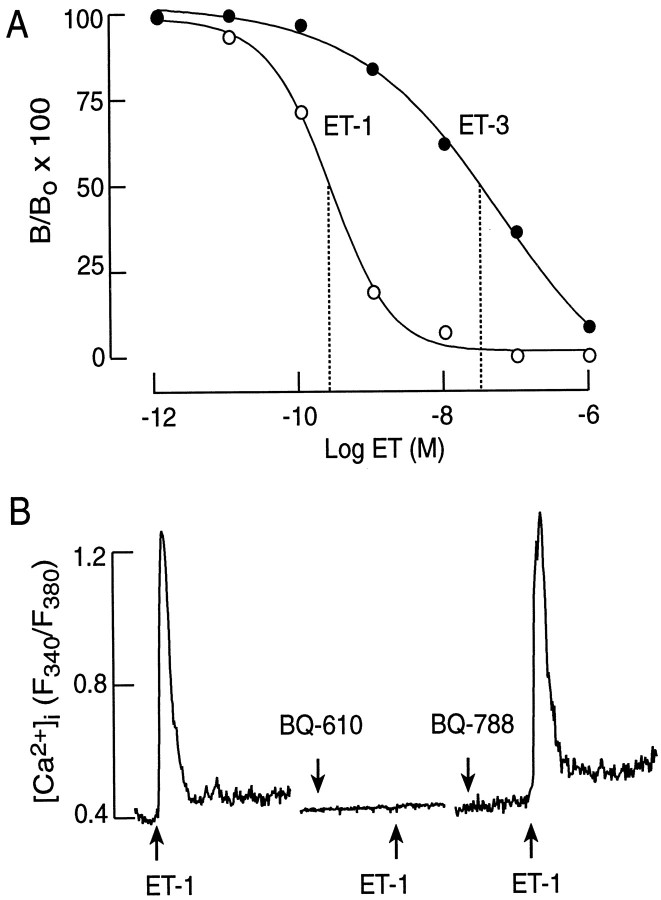
Displacement studies with 125I–ET-1 and unlabeled ET-1 and ET-3 confirmed the expression of ETA receptors, but not ETBreceptors, in pituitary cells. As shown in Figure7A, ET-1 was more potent than ET-3 in ligand displacement experiments. Scatchard analysis of these data revealed the expression of a single class of ETA receptors with aKD of 35 pm. Moreover, the ET-1-induced rise in [Ca2+]i was prevented by the addition 1 μm BQ-610, a specific antagonist for ETA receptors (n = 26), but not by 1 μmBQ-788, a specific antagonist for ETB receptors (n = 10; Fig. 7B). Thus, ET-1-induced stimulation of Ca2+ mobilization, the bidirectional changes in the pacemaker activity, and the accompanied changes in the rhythm of GH secretion were mediated by a single class of typical ETA receptors.
Fig. 7.
Characterization of ET receptor subtypes in pituitary cells. A, Displacement of125I–ET-1 by unlabeled ET-1 and ET-3. Thedottedlines indicate the IC50 values for these two ligands, derived from fitted logistic curves. The results shown are the means from a triplicate incubation. B, Effects of BQ-610 (1 μm) and BQ-788 (1 μm), the ETA and ETB receptor antagonists, respectively, on the ET-1-induced (10 nm) [Ca2+]i response in silent somatotrophs.
Coupling of ETA receptors to intracellular messengers
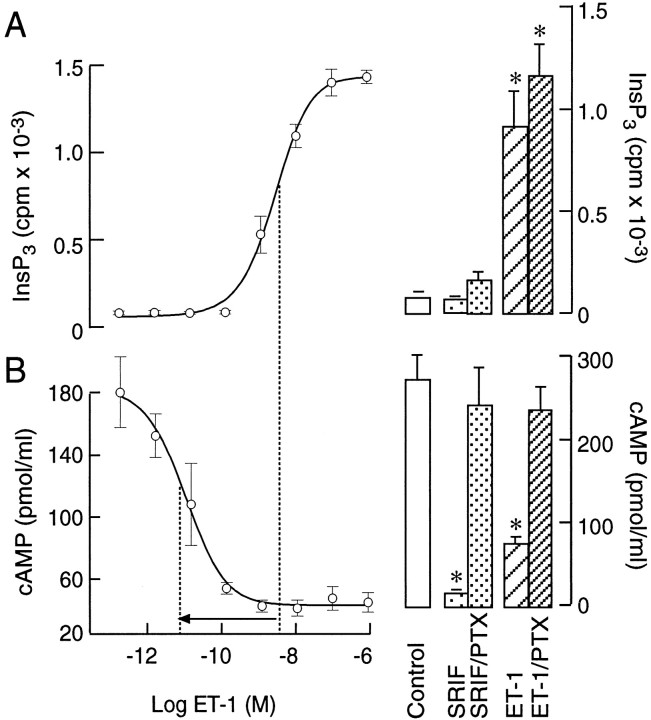
Consistent with the coupling of ETAreceptors to phospholipase C, ET-1 induced a concentration-dependent increase in InsP3 production (Fig.8A, left panel). Endothelin-1 also mimicked the inhibitory action of somatostatin on cAMP accumulation in IBMX-treated cells (Fig. 8B, left panel). In parallel to a different sensitivity of ETA receptor coupling to Ca2+ mobilization and Ca2+ influx pathways (Table 1), there was an obvious difference in the EC50 values for ET-1-induced InsP3 production and the inhibition of cAMP production (illustrated by an arrow in Fig. 8). Endothelin-1 and somatostatin-induced inhibition of cAMP accumulation was reduced in pertussis toxin-treated cells (250 ng/ml for 16 hr; Fig.8B, right panel). In contrast, pertussis toxin treatment did not affect ET-1-induced InsP3production (Fig. 8A, right panel). These results indicate that both ETA and somatostatin receptors in pituitary cells are coupled negatively to adenylyl cyclase through the Gi/Go-dependent signaling pathway. In addition, ETA receptors, but not somatostatin receptors, are coupled to the phospholipase C pathway in a pertussis toxin-insensitive manner.
Fig. 8.
Coupling of ETA receptors to phospholipase C and adenylyl cyclase pathways. A,Left, Concentration dependence of ET-1 on InsP3 production. A, Right, The lack of effects of pertussis toxin (PTX) on somatostatin (100 nm) and ET-1-induced (100 nm) InsP3 production. B, Left, Concentration dependence of ET-1 on cAMP production. B, Right, Effects of pertussis toxin on somatostatin (100 nm) and ET-1-induced (100 nm) cAMP production. Cells were exposed to 250 ng/ml PTX overnight. The results shown are the mean ± SEM from sextuplicate incubation. The dotted lines indicate the EC50 and IC50 for the ET-1-induced increase in InsP3 and the decrease in cAMP productions, respectively, derived from fitted logistic curves.
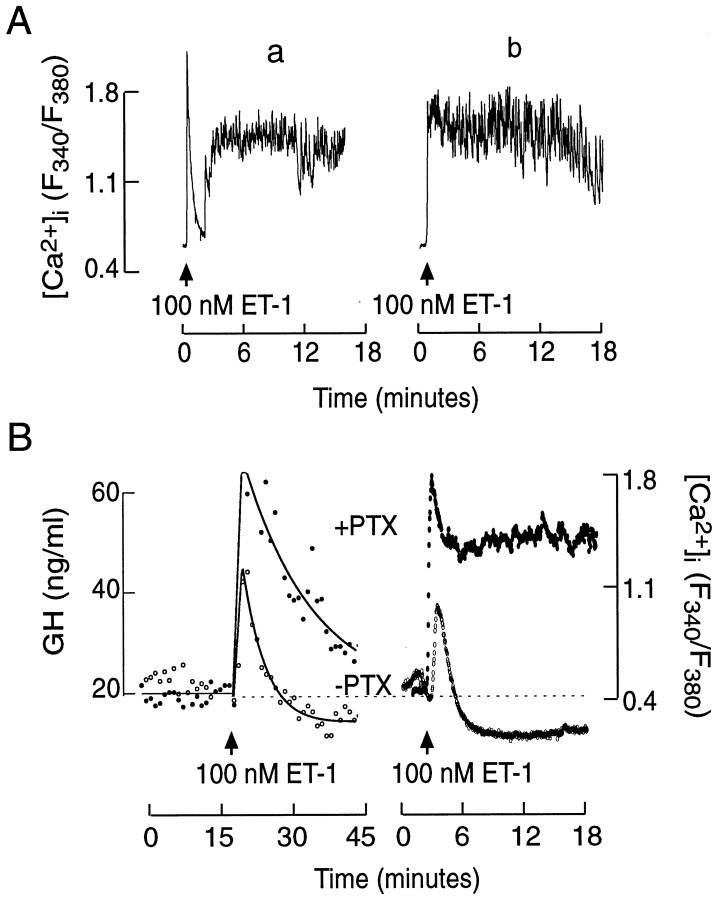
To examine the impact of the cross-coupling of ETA receptors to the Gi/Go signaling pathway, we studied the ET-1-induced Ca2+ signals in pertussis toxin-treated cells. Consistent with the lack of effects of pertussis toxin on InsP3 production, the ET-1-induced spike [Ca2+]i response was preserved in such treated cells (Fig.9A). In contrast to controls, however, it was followed by a sustained elevation in [Ca2+]i (see Fig.2B vs 9A). In 110 of 162 cells the initial Ca2+-mobilizing phase was clearly separated from the sustained spiking (Fig. 9A,tracing a). In the residual cells no obvious separation of two phases was observed (Fig. 9A,tracing b). Changes in the pattern of Ca2+ signaling induced by pertussis toxin also altered the ET-induced GH secretion in perifused pituitary cells. As shown in Figure 9B, left panel, ET-1-induced GH secretion was enhanced as compared with untreated cells. The secretory profile was also comparable to the average values of ET-1-induced [Ca2+]i in controls and pertussis toxin-treated cells (Fig. 9B, right panel).
Fig. 9.
ET-1-induced calcium signaling and GH secretion in pertussis toxin-treated pituitary cells. A, Two different patterns of ET-1-induced Ca2+ signals, labeled as a and b. B, The profiles of ET-1-induced GH secretion and [Ca2+]i in control and PTX-treated pituitary cells. Calcium tracings are the mean values from 25 individual recordings.
To identify the pathways responsible for the initial and sustained elevation in [Ca2+]i, we stimulated cells with ET-1 in Ca2+-deficient medium, and 1.2 mm Ca2+ was added 5 min later. Consistent with the findings shown in Figure 2A, the addition of Ca2+ to pertussis toxin-untreated cultures induced a minor increase in [Ca2+]i (Fig.10A;n = 8). In a majority of pertussis toxin-treated cells stimulated with ET-1 (12 of 19 cells), however, the addition of Ca2+ was associated with a major elevation in [Ca2+]i (Fig.10B). Furthermore, in pertussis toxin-treated cells bathed in normal Ca2+-containing medium, the addition of nifedipine, an L-type Ca2+channel blocker, abolished the sustained [Ca2+]i spiking (n = 15; Fig. 10C). These results indicate that enhanced voltage-gated Ca2+ influx through L-type channels accounts for the sustained rise in [Ca2+]i in pertussis toxin-treated ET-stimulated cells.
Fig. 10.
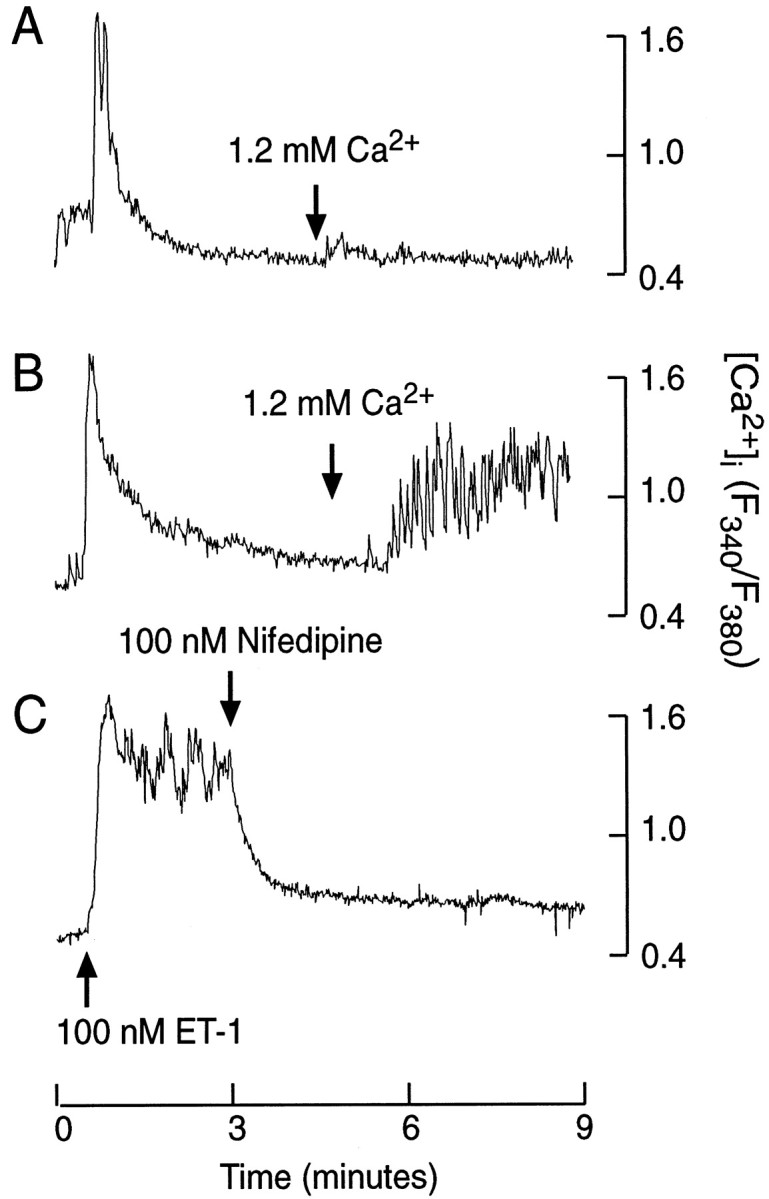
Characterization of sustained Ca2+ influx in ET-1-stimulated somatotrophs.A, B, The effects of extracellular Ca2+, added during sustained ET-1 stimulation, on [Ca2+]i in control (A) and pertussis toxin-treated (B) cells. C, Nifedipine sensitivity of ET-1-induced plateau [Ca2+]i transients in pertussis toxin-treated somatotrophs.
Modulation of plasma membrane channels by ET-1
Simultaneous measurements of Vmand [Ca2+]i in purified somatotrophs revealed the existence of burstingVm oscillations that fire 2–10 APs, giving rise to transient increases in [Ca2+]i (Fig.11). The addition of 100 nm somatostatin hyperpolarized the membrane, leading to the abolition of AP firing and a decrease in [Ca2+]i (Fig.11A). ET-1 also induced a cessation of electrical activity because of a transient hyperpolarization, but it was less effective than somatostatin (panels A vsB, top tracings). Inhibition of AP firing also led to a continuous decrease in [Ca2+]i, but it was preceded by a spike rise in [Ca2+]i (Fig.11B, bottom tracing). In pertussis toxin-treated cells, ET-1-induced Ca2+ mobilization was associated with the sustained depolarization of cells and increased firing frequency (Fig. 11C).
Fig. 11.
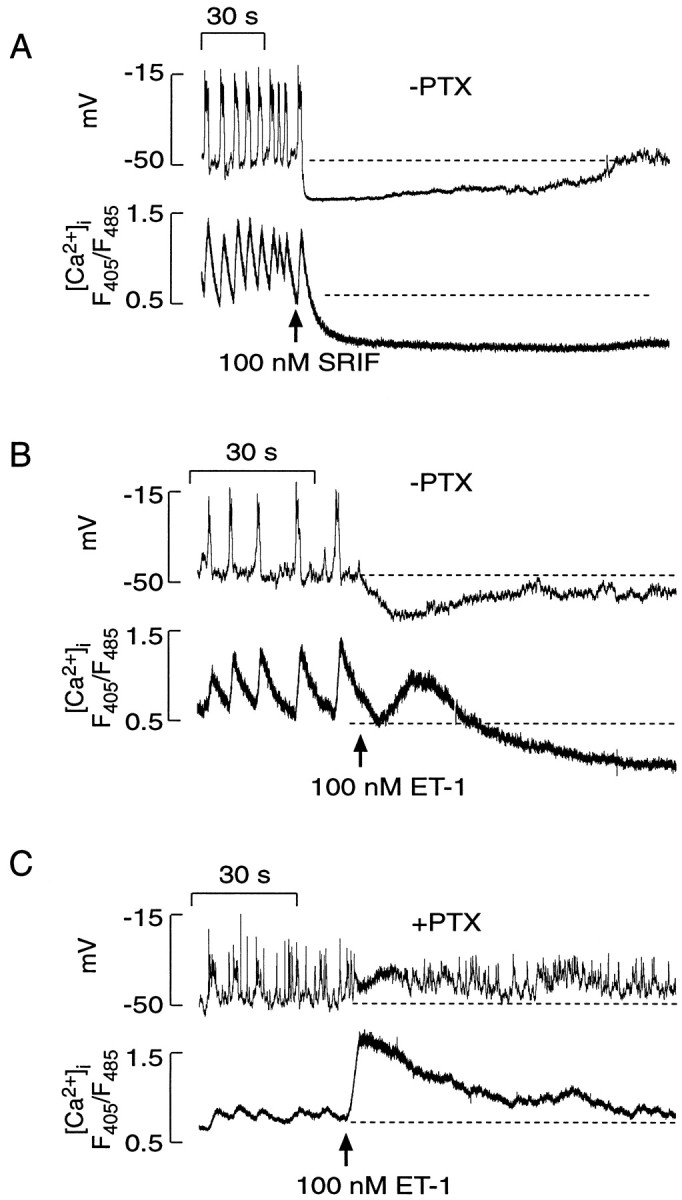
Simultaneous measurements of electrical activity (Vm) and [Ca2+]i transients in purified somatotrophs. A, Effects of somatostatin on electrical activity and [Ca2+]i.B, Effects of ET-1 on Vm and [Ca2+]i. C, Effects of PTX treatment on ET-1-induced changes in Vmand [Ca2+]i. The dotted lines illustrate the baseline potential and basal [Ca2+]i in spontaneously active cells. In these experiments indo-1 was used as a calcium dye, andarrows indicate the moment of the delivery of drugs via a perfusion system.
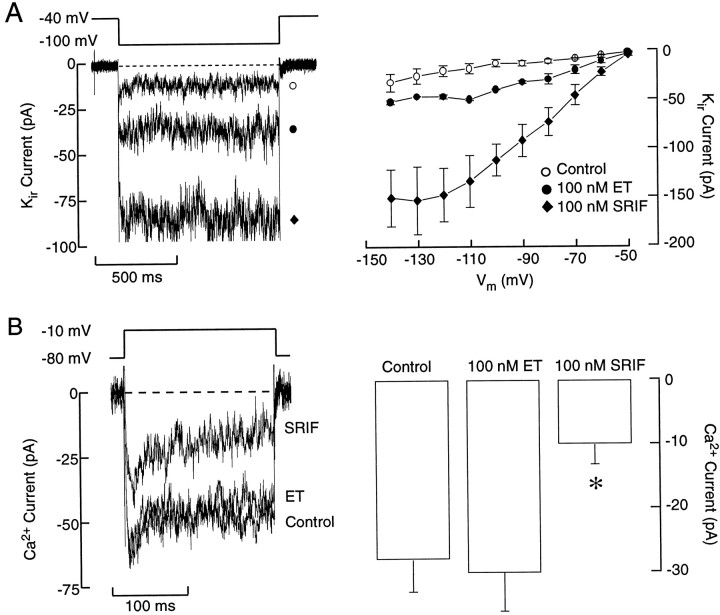
Both somatostatin and ET-1 increased the magnitude of theKir current in purified somatotrophs, which probably accounts for the sustained hyperpolarization of the cells (Fig. 12A). Consistent with the difference in the level of hyperpolarization (see Fig. 11), the amplitude of the Kircurrent was larger in somatostatin-treated cells than in those treated with ET-1 (Fig. 12). Somatostatin also inhibited voltage-gated L-type Ca2+ current (Fig. 12B, left panel), whereas ET-1 did not (right panel). Thus, the more pronounced inhibition of GH secretion by a supramaximal concentration of somatostatin when compared with ET-1-induced inhibition (see Fig. 5) probably results from a dual action of somatostatin receptors onKir channels and VGCCs.
Fig. 12.
Receptor-induced modulation of calcium and inward rectifier potassium currents in purified somatotrophs.A, Effects of ET-1 and somatostatin on inward rectifier potassium current (Kir), recorded in perforated patch-clamp conditions, with 20 mmof extracellular potassium. B, Effects of ET-1 and somatostatin on voltage-gated calcium currents. The whole-cell recording conditions were used for these measurements (see Materials and Methods for the solutions that were used). Data shown onright panels are derived from six independent experiments.
DISCUSSION
Here we show that cultured somatotrophs exhibit periods of spontaneous electrical activity associated with the generation of high-amplitude [Ca2+]itransients, which are critical in the control of basal GH secretion. These cells also express phospholipase C-coupled ETA receptors, and their activation leads to an increase in InsP3 production and the release of intracellular Ca2+ in both spontaneously active and quiescent cells. In spontaneously active cells the Ca2+ mobilization phase is followed by bidirectional modulation of theVm-dependent pathway for Ca2+ signaling. Initially, Ca2+ influx is inhibited, but it is recovered during prolonged agonist stimulation. In quiescent cells the Ca2+-mobilizing action of ET-1 frequently is associated with a delayed activation of Ca2+ influx. In parallel to changes in [Ca2+]i, GH secretion is stimulated during the early Ca2+ mobilization phase of ET-1 action and bidirectionally controlled during sustained agonist stimulation. The cross-coupling of ETA receptors to pertussis toxin-sensitive pathway accounts for the downregulation of adenylyl cyclase activity and the stimulation ofKir, leading to a transient inhibition of Ca2+ influx and GH secretion.
Earlier studies have indicated that ETs are released by the pituitary and act as autocrine/paracrine hormones in at least two subpopulations of anterior pituitary cells, gonadotrophs and lactotrophs (Samson et al., 1990; Stojilkovic et al., 1990; Burris et al., 1991; Samson and Skala, 1992). Both cell types express functional ET receptors, the activation of which leads to different patterns of Ca2+ signaling and hormone secretion in each cell type (Dymshitz et al., 1992; Samson, 1992; Stojilkovic et al., 1992; Kanyicska and Freeman, 1993; Kanyicska et al., 1995). In gonadotrophs, ETs stimulate oscillatory Ca2+ release and facilitate voltage-gated Ca2+ influx (Stojilkovic et al., 1992). In lactotrophs, on the contrary, ETs stimulate Ca2+ release in a nonoscillatory manner and inhibit voltage-gated Ca2+ influx. This inhibition lasts for several hours and effectively downregulates prolactin secretion (Samson et al., 1990; Lachowicz et al., 1997). The inhibitory effects of ET-1 in somatotrophs resemble those observed in lactotrophs, except that the inhibition is transient rather than long-lasting. A difference in the actions of ETs on Ca2+ influx within the pituitary cells is consistent with the expression of different ET receptor subtypes and/or different cross-coupling of a single class of ET receptors to an additional intracellular signaling pathway in lactotrophs and somatotrophs versus gonadotrophs.
ETA and ETB receptors belong to a subfamily of G-protein-coupled receptors that activate multiple types of G-proteins. It is well established that ETA receptors operate through the Gq/phospholipase C pathway in a variety of tissues, including pituitary cells (Stojilkovic et al., 1994). These receptors frequently are cross-coupled to the Gspathway, as indicated by the ability of ET-1 to increase cAMP formation in rat vascular smooth muscle cells (Eguchi et al., 1993), Chinese hamster ovary cells (Fohr et al., 1989), and bovine tracheal cells (Oda et al., 1992), all expressing ETA receptors. Rat ETB receptors are coupled to the Gq pathway as well, but they also are cross-coupled to the Gi/Gopathway, leading to a decrease in cAMP formation (Fohr et al., 1989;Eguchi et al., 1993; Lin and Chuang, 1993). In this regard, the simultaneous facilitation of Ca2+mobilization and the inhibition of Ca2+influx by ET-1 are more consistent with the expression of ETB receptors in somatotrophs. First, it has been shown previously that cAMP controls a sodium-conducting pacemaker current in these cells (Lussier et al., 1991a; Naumov et al., 1994). Also, the stimulation of adenylyl cyclase by GHRH and several other Gs-coupled receptors in somatotrophs elevates cAMP production, leading to the activation of the depolarizing pacemaker current and the generation of APs (Cuttler et al., 1992;Bluet-Pajot et al., 1998). This, in turn, stimulates voltage-gated Ca2+ influx and GH secretion (Lussier et al., 1991b). Finally, somatostatin inhibits both adenylyl cyclase and electrical activity in somatotrophs (Sims et al., 1991).
However, none of the methods used in our study (RT-PCR analysis, binding studies, or [Ca2+]i recordings in the presence of specific ETA and ETB receptor antagonists) revealed the expression of ETB receptors. All of these analyses were consistent with the operation of a single class of ETA receptors in mixed populations of anterior pituitary cells, immortalized GH3lacto-somatotrophs, and purified somatotrophs. Thus, the ability of ET-1 to mimic the action of somatostatin on electrical activity and Ca2+ signaling is consistent with the negative coupling of ETA receptors to adenylyl cyclase. Contrary to that, it has been reported previously that ETs stimulate cAMP accumulation in pituitary cells (Domae et al., 1994), an action that should mimic the effects of GHRH in these cells. In our experiments, however, ET-1 induced a pronounced and concentration-dependent inhibition in cAMP formation to the levels comparable to those observed in somatostatin-treated cells. Also, in parallel to somatostatin action, ET-1-induced inhibition of cAMP production was abolished by pertussis toxin pretreatment, demonstrating that rat pituitary ETA receptors are cross-coupled to Gi/Goproteins. The finding that ET-1 inhibits adenylyl cyclase in our experiments also indicates that this enzyme is active in resting cells and produces a significant amount of cAMP. Because the pacemaker activity in somatotrophs and immortalized pituitary cells is controlled by cAMP/protein kinase A-dependent channels (Bluet-Pajot et al., 1998), this may provide an explanation for the occurrence of spontaneous firing of APs in somatotrophs.
The cross-coupling of ETA receptors to the Gi/Go pathway is not unique for somatotrophs; ETA receptors also are coupled negatively to the adenylyl cyclase-signaling pathway in guinea pig myocytes, an action that accounts for cardiac inhibition mediated by the hyperpolarization of cells (Ono et al., 1994). Furthermore, the stimulation of Ca2+-mobilizing ETA receptors leads to the inhibition of protein kinase A-dependent chloride conductance in these cells (James et al., 1994). These observations are consistent with a view that the α-subunit of these proteins inhibits adenylyl cyclase, and the β/γ dimer stimulates Kir channels (Wickman and Clapham, 1995). Somatotrophs also express G-protein-regulated Kirchannels, the activation of which by somatostatin hyperpolarizes the cell membrane (Yamashita et al., 1987; Yatani et al., 1987; Mollard et al., 1988; Sims et al., 1991). As shown here, ET-1 activates these channels like somatostatin, but it is not as effective.
In addition to the stimulation of Kirchannels, somatostatin inhibits L-type calcium channels (Lewis et al., 1986; Kleuss, 1995; Tallent et al., 1996). This provides a dual negative control of voltage-gated Ca2+influx, by hyperpolarization of cells and by reduction in the capacity of L-type channels to conduct Ca2+. In contrast to somatostatin receptors, the activation of ETA receptors does not affect L-type channels. At the present time the biochemical reason for the difference in the coupling of two receptors, both operating through the pertussis toxin-sensitive pathway, is not clear. The physiological significance of that, however, has been established. ET-1-induced inhibition of Ca2+ influx and GH secretion is transient and partial, as compared with that induced by somatostatin.
In conclusion, somatotrophs express a single class of ETA receptors. Their activation leads to a typical sequence of intracellular events controlled by a Ca2+-mobilizing receptor. These receptors also mimic the action of pertussis toxin-sensitive somatostatin receptors in their coupling to adenylyl cyclase andKir channels. ETA receptors are less effective than somatostatin receptors in the inhibition of voltage-gated Ca2+ influx and GH secretion. The coupling of ETA receptors to pertussis toxin-sensitive and -insensitive G-proteins provides an effective mechanism to shift from aVm-dependent pathway for Ca2+ signaling to a Ca2+ mobilization pathway. This, in turns, changes the rhythm of GH secretion.
Footnotes
We thank Dr. Albert F. Parlow for help in establishing radioimmunoassay for GH and Drs. Lazar Krsmanovic, Lixin Zheng, and Agnieszka Lachowicz for help in InsP3 and cAMP measurements.
Correspondence should be addressed to Dr. Stanko Stojilkovic, Section on Cellular Signaling, Endocrinology and Reproduction Research Branch, National Institute of Child Health and Human Development, Building 49, Room 6A-36, 49 Convent Drive, Bethesda, MD 20892-4510.
REFERENCES
- 1.Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial, and bilayer measurements. J Neurosci Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 2.Baukal AJ, Hunyady L, Catt KJ, Balla T. Evidence for participation of calcineurin in potentiation of agonist-stimulated cyclic AMP formation by the calcium-mobilizing hormone, angiotensin II. J Biol Chem. 1994;269:24546–24549. [PubMed] [Google Scholar]
- 3.Bluet-Pajot M-T, Epelbaum J, Gourdji D, Hammond C, Kordon C. Hypothalamic and hypophyseal regulation of growth hormone secretion. Cell Mol Neurobiol. 1998;18:101–123. doi: 10.1023/A:1022579327647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 5.Burris TP, Kanyicska B, Freeman ME. Inhibition of prolactin secretion by endothelin-3 is pertussis toxin-sensitive. Eur J Pharmacol. 1991;198:223–225. doi: 10.1016/0014-2999(91)90627-3. [DOI] [PubMed] [Google Scholar]
- 6.Cuttler L, Glaum SR, Collins BA, Miller RJ. Calcium signaling in single growth hormone-releasing factor-responsive pituitary cells. Endocrinology. 1992;130:945–953. doi: 10.1210/endo.130.2.1733736. [DOI] [PubMed] [Google Scholar]
- 7.Domae M, Yamada K, Inoue T, Satoh M, Furukawa T. Endothelins stimulate cyclic AMP accumulation in the isolated rat anterior pituitary gland: possible involvement of ETA receptor activation and prostaglandin E2 production. J Pharmacol Exp Ther. 1994;270:55–60. [PubMed] [Google Scholar]
- 8.Dymshitz J, Laudon M, Ben-Jonathan N. Endothelin-induced biphasic response of lactotrophs cultured under different conditions. Neuroendocrinology. 1992;55:724–729. doi: 10.1159/000126192. [DOI] [PubMed] [Google Scholar]
- 9.Eguchi S, Hirata Y, Imai T, Kanno K, Marumo F. Endothelin receptor subtypes are coupled to adenylate cyclase via different guanyl nucleotide-binding proteins in vasculature. Endocrinology. 1993;132:524–529. doi: 10.1210/endo.132.2.7678793. [DOI] [PubMed] [Google Scholar]
- 10.Fohr KJ, Scott J, Hilger GA, Gratzl M. Characterization of the inositol 1,4,5-trisphosphate-induced calcium release from permeabilized endocrine cells and its inhibition by decavanadate and p-hydroxymercuribenzoate. Biochem J. 1989;262:83–89. doi: 10.1042/bj2620083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holl RW, Thorner MO, Mandell GL, Sullivan JA, Sinha YN, Leong DA. Spontaneous oscillations of intracellular calcium and growth hormone secretion. J Biol Chem. 1988;263:9682–9685. [PubMed] [Google Scholar]
- 12.James AF, Xle L-H, Fujtani Y, Hayashi S, Horle M. Inhibition of the cardiac protein kinase A-dependent chloride conductance by endothelin-1. Nature. 1994;370:297–300. doi: 10.1038/370297a0. [DOI] [PubMed] [Google Scholar]
- 13.Kanyicska B, Freeman ME. Characterization of endothelin receptors in the anterior pituitary gland. Am J Physiol. 1993;265:E601–E608. doi: 10.1152/ajpendo.1993.265.4.E601. [DOI] [PubMed] [Google Scholar]
- 14.Kanyicska B, Livingstone JD, Freeman ME. Long-term exposure to dopamine reverses the inhibitory effect of endothelin-1 on prolactin secretion. Endocrinology. 1995;136:990–994. doi: 10.1210/endo.136.3.7867609. [DOI] [PubMed] [Google Scholar]
- 15.Kleuss C. Somatostatin modulates voltage-dependent Ca2+ channels in GH3 cells via a specific Go splice variant. Ciba Found Symp. 1995;190:171–186. doi: 10.1002/9780470514733.ch11. [DOI] [PubMed] [Google Scholar]
- 16.Koshimizu T, Tomic M, Van Goor F, Stojilkovic SS. Functional role of alternative splicing in pituitary P2X2 receptor-channel activation and desensitization. Mol Endocrinol. 1998;12:901–913. doi: 10.1210/mend.12.7.0129. [DOI] [PubMed] [Google Scholar]
- 17.Kwiecien R, Tseeb V, Kurchikov A, Kordon C, Hammond C. Growth hormone-releasing hormone triggers pacemaker activity and persistent Ca2+ oscillations in rat somatotrophs. J Physiol (Lond) 1997;499:613–623. doi: 10.1113/jphysiol.1997.sp021954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachowicz A, Van Goor F, Katzur AC, Bonhomme G, Stojilkovic SS. Uncoupling of calcium mobilization and entry pathways in endothelin-stimulated pituitary lactotrophs. J Biol Chem. 1997;272:28308–28314. doi: 10.1074/jbc.272.45.28308. [DOI] [PubMed] [Google Scholar]
- 19.Lewis DL, Weight FF, Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci USA. 1986;83:9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis DL, Goodman MB, St. John PA, Barker JL. Calcium currents and fura-2 signals in fluorescence-activated cell sorted lactotrophs and somatotrophs of rat anterior pituitary. Endocrinology. 1988;123:611–621. doi: 10.1210/endo-123-1-611. [DOI] [PubMed] [Google Scholar]
- 21.Lin HY, Kaji EH, Winkel GK, Ives HE, Lodish HF. Cloning and functional expression of a vascular smooth muscle endothelin-1 receptor. Proc Natl Acad Sci USA. 1991;88:3185–3189. doi: 10.1073/pnas.88.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin WW, Chuang DM. Endothelin- and ATP-induced inhibition of adenylyl cyclase activity in C6 glioma cells: role of Gi and calcium. Mol Pharmacol. 1993;44:158–165. [PubMed] [Google Scholar]
- 23.Lussier BT, French MB, Moor BC, Kraicer J. Free intracellular Ca2+ concentration and growth hormone (GH) release from purified rat somatotrophs. III. Mechanism of action of GH-releasing factor and somatostatin. Endocrinology. 1991a;128:592–603. doi: 10.1210/endo-128-1-592. [DOI] [PubMed] [Google Scholar]
- 24.Lussier BT, French MB, Moor BC, Kraicer J. Free intracellular Ca2+ concentration ([Ca2+]i) and growth hormone release from purified rat somatotrophs. I. GH-releasing factor-induced Ca2+ influx raises [Ca2+]i. Endocrinology. 1991b;128:570–582. doi: 10.1210/endo-128-1-570. [DOI] [PubMed] [Google Scholar]
- 25.Lussier BT, Wood DA, French MB, Moor BC, Kraicer J. Free intracellular Ca2+ concentration ([Ca2+]i) and growth hormone release from purified rat somatotrophs. II. Somatostatin lowers [Ca2+]i by inhibiting Ca2+ influx. Endocrinology. 1991c;128:583–591. doi: 10.1210/endo-128-1-583. [DOI] [PubMed] [Google Scholar]
- 26.Mollard P, Vacher P, Dufy B, Barker JL. Somatostatin blocks Ca2+ action potential activity in prolactin-secreting pituitary tumor cells through coordinate actions of K+ and Ca2+ conductances. Endocrinology. 1988;123:721–732. doi: 10.1210/endo-123-2-721. [DOI] [PubMed] [Google Scholar]
- 27.Naumov AP, Herrington J, Hille B. Actions of growth hormone-releasing hormone on rat pituitary cells: intracellular calcium and ionic currents. Pflügers Arch. 1994;427:414–421. doi: 10.1007/BF00374255. [DOI] [PubMed] [Google Scholar]
- 28.Oda K, Fujitani Y, Watakabe T, Inui T, Okada T, Urade Y, Okuda-Ashitaka E, Ito S. Endothelin stimulates both cAMP formation and phosphatidylinositol hydrolysis in cultured embryonic bovine tracheal cells. FEBS Lett. 1992;299:187–191. doi: 10.1016/0014-5793(92)80244-b. [DOI] [PubMed] [Google Scholar]
- 29.Ono K, Tsujimoto G, Sakamoto A, Eto K, Masaki T, Ozaki Y, Satake M. EndothelinA receptor mediates cardiac inhibition by regulating calcium and potassium currents. Nature. 1994;370:301–304. doi: 10.1038/370301a0. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez JL, Torronteras R, Malagon MM, Castano JP, Garcia-Navarro S, Gonzales de Aguilar JL, Martinez-Fuentes AJ, Garcia-Navarro F. Growth hormone-releasing factor mobilizes cytosolic free calcium through different mechanisms in two somatotrope subpopulations from porcine pituitary. Cell Calcium. 1998;23:207–217. doi: 10.1016/s0143-4160(98)90119-1. [DOI] [PubMed] [Google Scholar]
- 31.Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16:427–442. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto A, Yanagisawa M, Sakurai T, Takuwa Y, Yanagisawa H, Masaki T. Cloning and functional expression of human cDNA for the ETB endothelin receptor. Biochem Biophys Res Commun. 1991;178:656–663. doi: 10.1016/0006-291x(91)90158-4. [DOI] [PubMed] [Google Scholar]
- 33.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 34.Samson WK. The endothelinA receptor subtype transduces the effects of the endothelins in the anterior pituitary gland. Biochem Biophys Res Commun. 1992;187:590–595. doi: 10.1016/0006-291x(92)91235-i. [DOI] [PubMed] [Google Scholar]
- 35.Samson WK, Skala KD. Comparison of the pituitary effects of the mammalian endothelins: vasoactive intestinal contractor (endothelinB, rat endothelin-2) is a potent inhibitor of prolactin secretion. Endocrinology. 1992;130:2964–2970. doi: 10.1210/endo.130.5.1533364. [DOI] [PubMed] [Google Scholar]
- 36.Samson WK, Skala KD, Alexander BD, Huang FL. Pituitary site of action of endothelin: selective inhibition of prolactin release in vitro. Biochem Biophys Res Commun. 1990;169:737–743. doi: 10.1016/0006-291x(90)90393-2. [DOI] [PubMed] [Google Scholar]
- 37.Sankaranarayanan S, Simasko SM. Characterization of an M-like current modulated by thyrotropin-releasing hormone in normal rat lactotrophs. J Neurosci. 1996;16:1668–1678. doi: 10.1523/JNEUROSCI.16-05-01668.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sims SM, Lussier BT, Kraicer J. Somatostatin activates an inwardly rectifying K+ conductance in freshly dispersed rat somatotrophs. J Physiol (Lond) 1991;441:615–637. doi: 10.1113/jphysiol.1991.sp018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stojilkovic SS. Handbook of physiology, Vol I, Cellular endocrinology, Sec 7, The endocrine system. Oxford UP; Oxford: 1998. Calcium signaling systems. [Google Scholar]
- 40.Stojilkovic SS, Merelli F, Iida T, Krsmanovic LZ, Catt KJ. Endothelin stimulation of cytosolic calcium and gonadotropin secretion in anterior pituitary cells. Science. 1990;248:1663–1666. doi: 10.1126/science.2163546. [DOI] [PubMed] [Google Scholar]
- 41.Stojilkovic SS, Balla T, Fukuda S, Cesnjaj M, Merelli F, Krsmanovic LZ, Catt KJ. Endothelin ETA receptors mediate the signaling and secretory actions of endothelins in pituitary gonadotrophs. Endocrinology. 1992;130:465–474. doi: 10.1210/endo.130.1.1309344. [DOI] [PubMed] [Google Scholar]
- 42.Stojilkovic SS, Tomic M, Kukuljan M, Catt KJ. Control of calcium spiking frequency in pituitary gonadotrophs by a single-pool cytoplasmic oscillator. Mol Pharmacol. 1994;45:1013–1021. [PubMed] [Google Scholar]
- 43.Tallent M, Liapakis G, O’Carroll A-M, Lolait SF, Dichter M, Reisine T. Somatostatin receptor subtypes SSTR2 and SSTR5 couple negatively to an L-type Ca2+ current in the pituitary cell line AtT-20. Neuroscience. 1996;71:1073–1081. doi: 10.1016/0306-4522(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 44.Van Goor F, Krsmanovic LZ, Catt KJ, Stojilkovic SS. Control of action potential-driven calcium influx in GT1 neurons by the activation status of sodium and calcium channels. Mol Endocrinol. 1999;13:587–603. doi: 10.1210/mend.13.4.0261. [DOI] [PubMed] [Google Scholar]
- 45.Wickman K, Clapham DE. Ion channel regulation by G-proteins. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- 46.Yamashita N, Shibuya N, Ogata E. Hyperpolarization of the membrane potential caused by somatostatin in dissociated human pituitary adenoma cells that secrete growth hormone. Proc Natl Acad Sci USA. 1986;83:6198–6202. doi: 10.1073/pnas.83.16.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashita N, Kojima I, Shibuya N, Ogata E. Pertussis toxin inhibits somatostatin-induced K+ conductance in human pituitary tumor cells. Am J Physiol. 1987;16:E28–E32. doi: 10.1152/ajpendo.1987.253.1.E28. [DOI] [PubMed] [Google Scholar]
- 48.Yatani A, Codina J, Sekura RD, Birnbaumer L, Brown AM. Reconstitution of somatostatin and muscarinic receptor-mediated stimulation of K+ channels by isolated GK protein in clonal rat anterior pituitary cell membranes. Mol Endocrinol. 1987;1:283–289. doi: 10.1210/mend-1-4-283. [DOI] [PubMed] [Google Scholar]