Abstract
The effects of acute nicotine (0.5 mg/kg, s.c.) on dopamine (DA) metabolism and Fos protein expression in striatal and limbic areas of rats on the seventh day of chronic nicotine infusion (4 mg · kg−1 · d−1) and after 24 or 72 hr withdrawal were investigated. In saline-infused rats, acute nicotine elevated striatal and limbic 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) concentrations significantly. During the nicotine infusion, no such increases were seen in the striatum, but limbic HVA was somewhat elevated. After 24 hr withdrawal when no nicotine was found in the plasma, acute nicotine elevated striatal DOPAC and HVA and limbic HVA. However, the limbic DOPAC was unaffected. Acute nicotine increased Fos immunostaining (IS) in the caudate-putamen (CPU), the core of nucleus accumbens (NAcc), the cingulate cortex (Cg), and the central nucleus of amygdala (ACe) significantly. During nicotine infusion the nicotine-induced responses were attenuated in CPU and NAcc, whereas in ACe and Cg Fos immunostaining was increased as in saline-infused rats. After 24 hr withdrawal, acute nicotine did not increase Fos immunostaining in CPU, NAcc, and Cg, but increased it clearly in ACe. After 72 hr withdrawal, nicotine’s effects were restored. Our findings suggest that the nicotinic receptors in the striatal areas are desensitized more easily than those in the limbic areas. Furthermore, the effects of nicotine on various DA metabolites differ. We also found evidence for long-lasting inactivation of nicotinic receptors in vivo regulating limbic dopamine metabolism and Fos expression in striatal and limbic areas. These findings might be important for the protective effects of nicotine in Parkinson’s disease and in its dependence-producing properties.
Keywords: nicotine, constant infusion, striatal dopamine metabolism, limbic dopamine metabolism, Fos protein, desensitization, tolerance
Acute nicotine enhances striatal and limbic dopamine (DA) turnover and metabolism (Nose and Takemoto, 1974;Haikala et al., 1986; Grenhoff and Svensson, 1988). Nicotine increases 3H-DA release in vitro from striatal slices and synaptosomes (Westfall, 1974;Rapier et al., 1988; Grady et al., 1992) and from the nucleus accumbens (NAcc) (Rowell et al., 1987). Nicotine also elevates extracellular DA in the striatum (Imperato et al., 1986) and in the NAcc (Imperato et al., 1986; Benwell and Balfour, 1992). There is evidence that nicotine activates the limbic DA system more easily than the striatal one (Imperato et al., 1986; Grenhoff and Svensson, 1988; Benwell and Balfour, 1997). We recently found that in mice tolerance develops to the nicotine-induced increase of striatal dopamine metabolism at 24 hr after withdrawal from 7 week oral nicotine administration (Pietilä et al., 1996). The effect of chronic nicotine treatment seems to depend on the method of administration. During prolonged constant nicotine infusion the acute nicotine-evoked increase of extracellular DA in the NAcc and dorsal striatum was inhibited, and the elevation of accumbal DA metabolites was attenuated, suggesting desensitization of the receptors mediating nicotine-induced mesolimbic and nigrostriatal DA responses (Benwell et al., 1995; Benwell and Balfour, 1997). On the other hand, Damsma et al. (1989) and Nisell et al. (1996) reported no change in the nicotine-induced increase of DA release in the NAcc of rats when nicotine was given by repeated injections. After intermittent nicotine treatment, even sensitization of the nicotine response has been reported (Benwell and Balfour, 1992;Shoaib et al., 1994; Marshall et al., 1997).
Drug treatments affecting dopaminergic transmission modulate the expression of Fos protein encoded by c-fos proto-oncogene in the striatum (Graybiel et al., 1990; Robertson et al., 1990; Herrera and Robertson, 1996; Moratalla et al., 1996). Acute nicotine elevates Fos expression in various brain regions, including dopaminergic target areas (Ren and Sagar, 1992; Matta et al., 1993; Pang et al., 1993; Kiba and Jayaraman 1994; Panagis et al., 1996; Salminen et al., 1996; Valentine et al., 1996). Fos expression is also elevated in rats trained to self-administer intravenous nicotine (Pagliusi et al., 1996;Merlo Pich et al., 1997). Dopamine D1-receptor mediates nicotine-induced Fos expression in striatum, NAcc, and medial prefrontal cortex because D1-antagonist SCH 23390 completely blocks this effect (Kiba and Jayaraman, 1994; Nisell et al., 1997).
In this study we investigated the effects of acute nicotine on dopamine metabolism in the striatum and limbic forebrain of rats during chronic nicotine infusion and after 24 or 72 hr withdrawal. The estimated dopamine metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) are considered indicators of different aspects of DA dynamics. DOPAC is formed by monoamine oxidase mainly intraneuronally and thus can be used as an indicator of intraneuronal synthesis and metabolism. HVA is formed by both monoamine oxidase and catechol-O-methyltransferase, indicating the sum of DA synthesis, metabolism, and release (Roffler-Tarlov et al., 1971;Westerink and Spaan, 1982). Also we studied the expression of Fos protein in dopaminergic target areas to determine whether the nicotine-induced changes in DA metabolism could be detected in postsynaptic neurons at the level of immediate-early genes.
MATERIALS AND METHODS
Male Wistar rats (body weight 200–300 gm at the beginning of experiments) bred locally in the Laboratory Animal Center, University of Helsinki, were divided randomly into nicotine-receiving and control animals. The experimental animals were maintained in accordance with internationally accepted principles, and the experimental setup was approved by the Committee for Animal Experiments of the Faculty of Science of the University of Helsinki. The rats had free access to food and water and were housed singly after surgery. The lights were on from 6 A.M. until 6 P.M., and the ambient temperature was kept at 20–22°C. Osmotic minipumps (Alzet 2001) containing saline or nicotine hydrogen tartrate (Sigma, St.Louis, MO) solutions were implanted subcutaneously under halothane anesthesia. Nicotine was infused at a dose of 4 mg · kg−1 · d−1 for 7 d. The dose refers to the base. Withdrawal was induced by removing the minipumps surgically on the seventh day of chronic treatment.
The rats received 0.9% NaCl solution (saline, s.c.) or acute nicotine (0.5 mg/kg s.c.) 1 hr before decapitation on the seventh day with the minipumps still in place and after 24 or 72 hr withdrawal. For injections (0.1 ml/100 gm) (−)-nicotine base (Fluka, Buchs, Switzerland) was diluted with 0.9% NaCl solution, and the final solution was adjusted to 7.0–7.4 pH with 0.05 m HCl in 0.9% NaCl solution. After decapitation the striatum and the limbic forebrain (containing inter alia the olfactory tubercles, medial part of the nucleus accumbens, the central nucleus of amygdala, and part of the paleocortex) were dissected and collected on dry ice within 5 min. Tissues were weighed and stored at −80°C until assayed. When estimating dopamine and its metabolites, nicotine and cotinine concentrations, and performing Fos immunohistochemistry, all four treatment groups (saline infusion + acute saline, saline infusion + acute nicotine, nicotine infusion + acute saline, nicotine infusion + acute nicotine) were analyzed simultaneously. However, the 7 d, 24 hr, and 72 hr withdrawal experiments were performed and analyzed separately.
Measurement of dopamine and its metabolites. The striatal and limbic concentrations of DA and its metabolites, DOPAC and HVA, were measured by HPLC with electrochemical detection after Sephadex G-10 gel chromatographic cleanup of samples. Tissue samples were homogenized in 1 ml 0.2 m HClO4, and the homogenates were adjusted to 2.4 pH by KOH/HCOOH buffer. After centrifugation (15 min, 28,000 × g, 4°C), 1 ml of supernatant was put on an acidic (0.01 m HCl) Sephadex G-10 column (bed height 8 mm) prepared in a long Pasteur pipette. After the supernatants had passed through the columns, the compounds were eluted, and a 200 μl portion of each collected fraction was injected through a high-pressure injection valve in an HPLC column. The C-18 reverse-phase (Spherisorb octadecylsilane 2 μm) HPLC column (25 cm, 4.6 mm inner diameter) was connected to the electrochemical detector (Coulochem II, ESA). The assay is described in detail by Haikala (1987).
Measurement of nicotine and cotinine. The plasma concentrations of nicotine and cotinine were measured on the seventh day of chronic nicotine infusion and after 24 hr withdrawal using gas chromatography–mass spectrometry. The trunk blood of rats was collected by decapitation 60 min after a challenge injection of 0.5 mg/kg nicotine or saline. Blood samples were treated and measured according to Leikola-Pelho et al. (1990).
Fos immunohistochemistry. The nicotine- or saline-infused rats received saline or nicotine (0.5 mg/kg, s.c.) 1 hr before the pentobarbital (Orion Pharma, Espoo, Finland) anesthesia (100 mg/kg, i.p.) and intracardial perfusion on the seventh day with the minipumps still in place and after 24 or 72 hr withdrawal. Rats were perfused with 0.9% PBS followed by 4% paraformaldehyde in 0.1 msodium phosphate buffer, pH 7.4. The brains were post-fixed with the same fixative for 4 hr at room temperature after perfusion. The brains were immersed in a 20% sucrose solution at 4°C until used. The sections (40 μm) were cut on a cryostat. The sections were first incubated in 2% normal rabbit serum (NRS) (Vector Laboratories, Burlingame, CA) in PBS + 0.5% Tween 20 + 0.2% NRS for 60 min to block nonspecific staining. The sections were then incubated in primary fos antibody (OA-11–824, Genosys Biotechnologies, Cambridge, UK) diluted 1:1000 (experiments during chronic nicotine) or 1:2000 (withdrawal experiments) in PBS (in 0.5% Tween 20 + 4% NRS) for 72 hr at 4°C. The antibody used was sheep polyclonal antibody to fos oncoproteins to a synthetic peptide Met-Phe-Ser-Gly-Phe-Asn-Ala-Asp-Tyr-Glu-Ala-Ser-Ser-Ser-Arg-Cys, selected from a conserved region of mouse and human c-fos(van Straaten et al., 1983). The sections were processed with the avidin–biotin method (Vectastain Kit, Vector Laboratories) with diaminobenzidine (Sigma) as the chromagen. The sections were then mounted on gelatin/chrome alum-coated slides, air-dried, dehydrated through graded ethanols to xylene, and coverslipped with DePex (BDH Laboratory Supplies, Poole, England). Controls for the immunostaining, which included omission of either primary or secondary antibody, demonstrated no Fos immunostaining. The atlas of Paxinos and Watson (1986) was used to identify the brain areas. Figure1 shows the areas in which Fos-positive nuclei were counted. The Fos-positive nuclei were counted with a 10× objective with the assistance of a LEICA QWin image analysis system on selected brain areas within a rectangular area of 480 × 360 μm. A group mean (±SEM) was determined from the counts of four to seven rats in each treatment group.
Fig. 1.
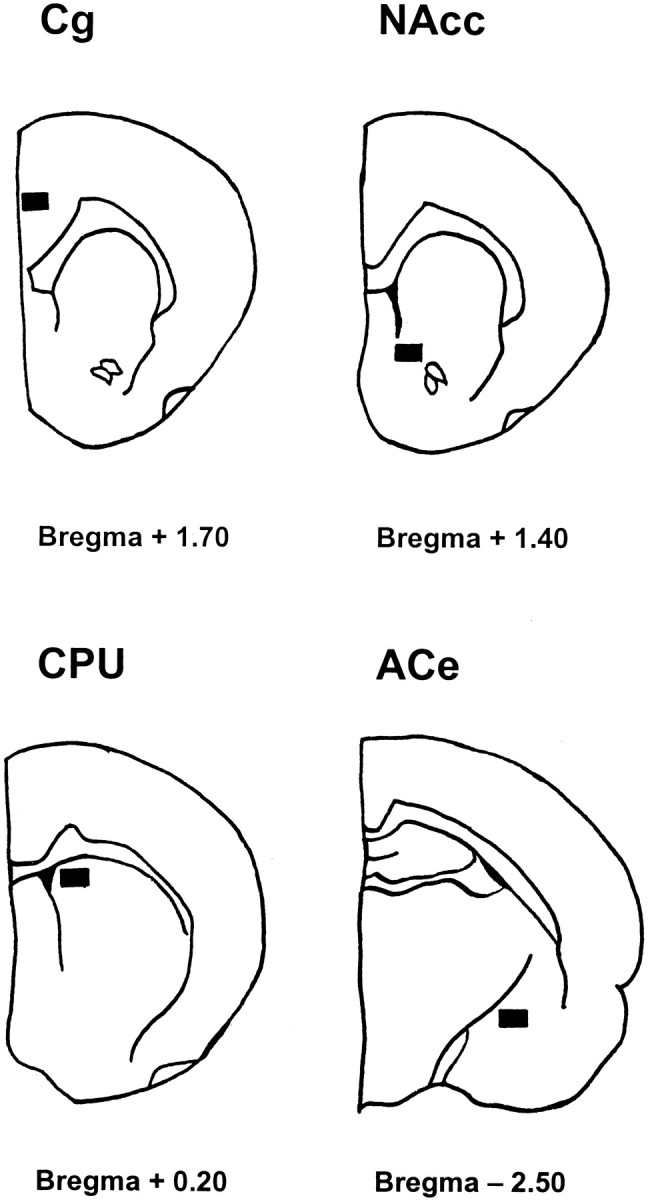
Schematic drawings indicating areas [according toPaxinos and Watson (1986)] in which Fos-positive nuclei were counted.Cg, Cingulate cortex; NAcc, core of nucleus accumbens; CPU, caudate-putamen;ACe, central nucleus of amygdala.
Statistics. Because the metabolite results consisted of several experiments, the statistical analyses were performed by three-way ANOVA (experiment × chronic treatment × acute challenge). Because no significant interactions between the experiment and other factors were observed, the randomized block two-way ANOVA was performed using experiments as blocks. If there were significant chronic × acute nicotine interactions (p< 0.1), the analysis was continued by comparing appropriate cell means with linear contrasts. The Fos immunostaining data were analyzed by Kruskal–Wallis nonparametric ANOVA followed by the Mann–WhitneyU test. The data concerning plasma nicotine and cotinine concentrations were analyzed by Student’s t test.
RESULTS
Plasma nicotine and cotinine concentrations
As shown in Table 1, the plasma nicotine and cotinine concentrations were clearly elevated on the seventh day of chronic infusion, but at 24 hr after removal of the minipumps no nicotine and only traces of cotinine were found in the plasma. Acutely administered 0.5 mg/kg nicotine elevated the plasma nicotine and cotinine concentrations in the withdrawn rats to the same degree as in the control rats, but did not clearly increase the nicotine or the cotinine concentrations in the plasma of rats with the minipumps still in place.
Table 1.
Plasma nicotine and cotinine concentrations in rats infused chronically with nicotine for 7 d at 60 min after acute saline or nicotine
| Acute treatment | Control rats | Nicotine-infused rats | ||
|---|---|---|---|---|
| Nicotine (ng/ml) | Cotinine (ng/ml) | Nicotine (ng/ml) | Cotinine (ng/ml) | |
| On the seventh day | ||||
| Saline | 0 | 0 | 76 ± 7 | 858 ± 142 |
| Nicotine | 80 ± 22 | 103 ± 25 | 114 ± 24 | 639 ± 106 |
| After 24 hr withdrawal | ||||
| Saline | 0 | 0 | 0 | 22 ± 12 |
| Nicotine | 78 ± 6 | 119 ± 15 | 79 ± 6 | 149 ± 15 |
Results are the means ± SEM of seven to nine observations. Rats were infused with saline or nicotine (4 mg · kg−1 · d−1) via subcutaneously implanted osmotic minipumps for 7 d. Some of the rats received either saline (s.c.) or nicotine (0.5 mg/kg, s.c.) on the seventh day of chronic treatment with the minipumps still in place, and the rest of rats were given saline or nicotine acutely 24 hr after the removal of the minipumps.
Striatal dopamine metabolism
As shown in Figure 2, in saline-infused control rats acute nicotine (0.5 mg/kg, s.c., 60 min) elevated DOPAC and HVA concentrations significantly in the striatum (DOPAC by 24–40%, HVA by 20–46%, respectively). No such increases were seen when acute nicotine was given on the seventh day of chronic nicotine infusion. Neither chronic nicotine infusion nor nicotine withdrawal (24 and 72 hr) altered DOPAC and HVA concentrations. At 24 and 72 hr after removal of the minipumps, acute nicotine elevated DOPAC (24 hr, 16%; 72 hr, 22%) and HVA (24 hr, 33%; 72 hr, 22%) in the nicotine-infused rats to almost the same extent as in the saline-infused rats. Striatal DA concentrations were not altered by any treatment.
Fig. 2.
The effect of an acute nicotine challenge (0.5 mg/kg, s.c., 60 min) on the striatal dopamine (DA), dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) concentrations in rats on the seventh day of chronic nicotine infusion (4 mg · kg−1 · d−1) with the minipumps still in place, and at 24 or 72 hr after removal of the minipumps. Given are the mean striatal concentrations of DA and the metabolites (columns) ± SEM (vertical bars) of 7–14 observations. *p < 0.05, **p < 0.01, ***p < 0.001 as compared with the control rats given acute saline subcutaneously;oop < 0.01, ooop < 0.001 as compared with the control rats given acute nicotine; ♦p < 0.05, ♦♦p < 0.01 as compared with chronic nicotine rats given saline acutely. White columns, Sham-operated control rats + acute saline; dotted columns, control rats + acute nicotine; checkered columns, chronic nicotine + acute saline; black columns, chronic nicotine + acute nicotine.
Limbic dopamine metabolism
Figure 3 shows that in saline-infused control rats acute nicotine (0.5 mg/kg, s.c., 60 min) elevated DOPAC and HVA concentrations in the limbic areas (DOPAC by 49–70% and HVA by 66–86%). Neither chronic nicotine nor nicotine withdrawal altered the limbic DOPAC concentration, whereas HVA concentration was significantly elevated in rats with the minipumps still in place but not in rats from which the nicotine-infusing minipumps had been removed. Acute nicotine did not elevate the limbic DOPAC concentration on the seventh day of chronic infusion or at 24 hr after its withdrawal, but after 72 hr withdrawal acute nicotine elevated DOPAC by 82%. On the seventh day of chronic nicotine infusion, acute nicotine somewhat increased the limbic HVA concentration, although significantly less than in the control rats. After 24 and 72 hr withdrawal, the nicotine-induced elevations of HVA in the nicotine-infused rats were similar to those of the controls (24 hr, 73%; 72 hr, 55%). Limbic DA was not altered by any of the treatments.
Fig. 3.
The effect of an acute nicotine challenge (0.5 mg/kg, s.c., 60 min) on the limbic dopamine (DA), dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) concentrations in rats on the seventh day of chronic nicotine infusion (4 mg · kg−1 · d−1) with the minipumps still in place and at 24 and 72 hr after removal of the minipumps. Given are the mean limbic concentrations of DA and the metabolites (columns) ± SEM (vertical bars) of 7–14 observations. **p < 0.01, ***p < 0.001 as compared with the control rats given acute saline subcutaneously; op< 0.05 as compared with the control rats given acute nicotine;♦♦♦p < 0.001 as compared with chronic nicotine rats given saline acutely. White columns, Sham-operated control rats + acute saline;dotted columns, control rats + acute nicotine;checkered columns, chronic nicotine + acute saline;black columns, chronic nicotine + acute nicotine.
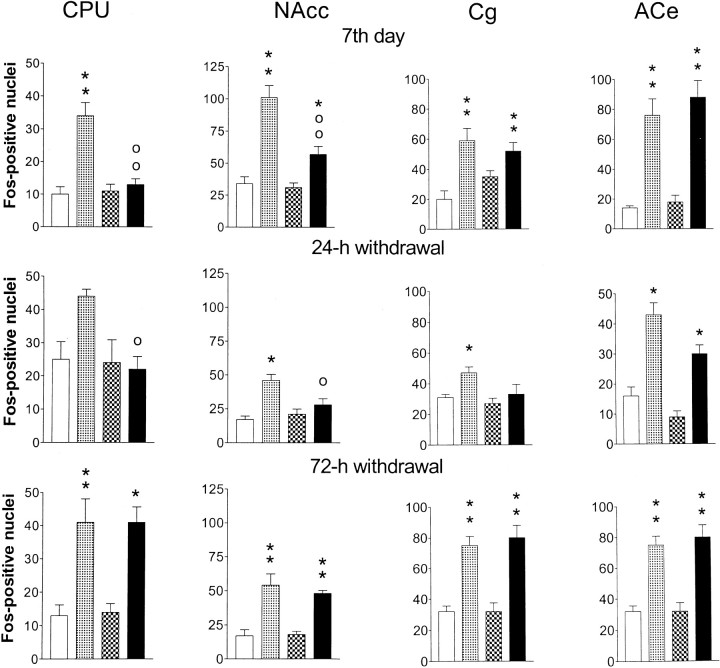
Fos immunohistochemistry
Figure 4 shows that in saline-infused control rats acute nicotine (0.5 mg/kg, s.c., 60 min) increased the number of Fos-positive nuclei in the dorsomedial CPU, in the NAcc, in the Cg, and in the ACe. The increase of Fos expression by acute nicotine occurred mainly in the core part of the NAcc, whereas no change in the number of Fos-positive nuclei was observed in the shell (data not shown). Therefore, Fos-positive nuclei were counted only in the area shown in Figure 1. On the seventh day of chronic nicotine infusion or 24 or 72 hr after removal of the minipumps, the numbers of Fos-positive nuclei in the areas studied were similar in nicotine-infused and saline-infused control rats. Thus, chronic nicotine infusion or its withdrawal did not alter the Fos expression.
Fig. 4.
The effect of an acute nicotine challenge (0.5 mg/kg, s.c., 60 min) on Fos immunostaining in rat brain striatal and limbic areas on the seventh day of chronic nicotine infusion (4 mg · kg−1 · d−1) with the minipumps still in place and at 24 and 72 hr after removal of the minipumps. Given are the mean numbers of Fos-positive nuclei (columns) ± SEM (vertical bars) of four to seven observations. *p < 0.05, **p < 0.01 as compared with control rats given acute saline subcutaneously; op < 0.05,oop < 0.01 as compared with the control rats given acute nicotine. White columns, Control rats + acute saline; dotted columns, control rats + acute nicotine; checkered columns, chronic nicotine + acute saline; black columns, chronic nicotine + acute nicotine. CPU, Dorsomedial caudate-putamen;NAcc, core of nucleus accumbens; Cg, cingulate cortex; ACe, central nucleus of amygdala.
On the seventh day of the chronic nicotine infusion, the effect of acute nicotine on the number of Fos-positive nuclei varied. Thus, in the CPU of rats infused chronically with nicotine, acute nicotine had no effect, and in the NAcc there was a slight increase of Fos-IS, which was significantly less, however, than in saline-infused control rats. In the ACe and Cg, acute nicotine increased Fos-IS in nicotine-infused rats to the same extent as it did in saline-infused rats. No increases of Fos-IS were seen in the CPU, in the NAcc, or in the Cg when acute nicotine was given to rats withdrawn for 24 hr from 7 d nicotine infusion. However, in the ACe of these nicotine-withdrawn rats acute nicotine increased the Fos-IS in the same way as in the control rats. At 72 hr after removal of the nicotine-releasing minipumps, acute nicotine elevated Fos-IS in all four brain areas studied.
DISCUSSION
On the seventh day of constant nicotine infusion, we found profound attenuation of nicotine’s effects on striatal and limbic DA metabolism. After 24 hr withdrawal, nicotine’s effects on DA metabolism were fully recovered, except that on limbic DOPAC, which was still attenuated. The postsynaptic effects of nicotine in major dopaminergic target areas as estimated by Fos expression were attenuated on the seventh day of nicotine infusion, and acute nicotine did not activate Fos expression in these areas at 24 hr after nicotine withdrawal. Nicotine’s effects that were studied were restored after 72 hr withdrawal.
In contrast to the saline-infused rats, acute nicotine treatment induced no changes in the striatal DA metabolism in rats on the seventh day of continuous nicotine infusion. Also, the nicotine-induced elevations of the limbic DOPAC and HVA were reduced when acute nicotine was given during nicotine treatment. These findings agree with microdialysis studies (Benwell et al., 1994, 1995; Benwell and Balfour, 1997) where the effects of acute nicotine challenge on extracellular concentration of DA in the dorsal striatum and on those of DA, DOPAC, and HVA in the NAcc were attenuated during constant nicotine infusion as compared with controls. This phenomenon could be caused by the continuous presence of an agonist, in this case nicotine, desensitizing the nicotinic acetylcholine receptors (nAChRs) regulating the dopaminergic neurons. We found that the attenuation of nicotine’s effect on DA metabolism occurred with prolonged exposure to nicotine concentrations approximately similar to those found in the plasma of heavy smokers (Table 1). Furthermore, such plasma concentrations indicate high cerebral nicotine concentrations in rats (Deutsch et al., 1992; Rowell and Li, 1997). Lippiello et al. (1995) observed that nicotine tends to stabilize nAChRs in the high-affinity conformation that is related to the process of functional desensitization. Thus, our experiments indicate that nAChRs involved in the control of striatal and limbic dopamine turnover were desensitized during constant nicotine infusion.
After 24 and 72 hr withdrawal from nicotine infusion, acute nicotine elevated striatal DOPAC and HVA to the same extent in nicotine-infused and saline-infused rats. The loss of action of the nAChRs regulating striatal DA metabolism during chronic nicotine infusion thus is a reversible phenomenon that is recovered within 24 hr of withdrawal. Acute nicotine also elevated the limbic HVA concentrations similarly in control and nicotine-withdrawn rats. However, the nicotine-induced elevation of limbic DOPAC was still abolished after 24 hr withdrawal but not after 72 hr withdrawal. Thus, nAChRs mediating nicotine-induced elevation of limbic DOPAC were still inactivated after 24 hr withdrawal, when no nicotine and only traces of cotinine were detected in the plasma. The lack of response of limbic DOPAC to acute nicotine could be attributed to long-lasting inactivation of nAChR function, which in in vitro experiments could be distinguished from reversible receptor desensitization and is caused by the prolonged pretreatment or high concentrations of nicotine (Rowell and Duggan, 1998). We could not demonstrate any nicotine-induced sensitization in either striatal or limbic DA metabolism when nicotine was withdrawn for 24 or 72 hr. This agrees with previous findings when nicotine was given by constant infusion (Benwell et al.,1995; Marshall et al., 1997).
In agreement with the study of Grenhoff and Svensson (1988), acute nicotine elevated limbic dopamine metabolites somewhat more than the striatal ones in this study. Furthermore, we now found differences in the responses of striatal and limbic DA metabolites to chronic infusion as well as to acute nicotine administration in nicotine-infused rats. Our findings suggest that at least some of the limbic nAChRs mediating nicotine’s effects on dopamine turnover are less easily desensitized than the striatal ones. This finding agrees with the suggestion ofHenningfield et al. (1996) based on in vitro experiments (Rapier et al., 1988; Brodie, 1991; Grady et al., 1994) that the somatodendritic nAChRs located on mesolimbic DA neurons appear to desensitize much less readily than nAChRs located on the terminals of the nigrostriatal pathway. In vitro the subunit composition of nAChRs determines the degree to which receptors are desensitized by various concentrations of nicotine (Fenster et al., 1997). We have previously suggested that nAChRs involved in the regulation of the intraneuronal DA metabolism are different from those in impulse-mediated DA release at least in their response to chronic nicotine administration in mice (Leikola-Pelho et al., 1990). The differences we now found in the effect of nicotine on limbic DOPAC and HVA in rats give further support to this suggestion. Thus, there may be variations in the functional states or in the subunit compositions of nAChRs mediating nicotine’s various effects on dopaminergic transmission or differences in receptor distribution in the limbic and striatal areas and their input areas.
Like nicotine’s effects on striatal DA metabolism, Fos expression in response to acute nicotine was attenuated in the dorsomedial CPU and in the core of the NAcc on the seventh day of nicotine infusion. Moreover, in agreement with earlier experiments (Nisell et al., 1997), acute nicotine at the dose of 0.5 mg/kg did not significantly increase Fos expression in the shell of the NAcc. The core of the NAcc projects to the dorsolateral part of the ventral pallidum, which in turn projects to the subthalamic nucleus and substantia nigra (Heimer et al., 1991;Meredith et al., 1992) comprising a striatal sector that takes part predominantly in motor functions (Deutch and Cameron, 1992). The attenuation of nicotine-induced Fos expression in these striatal regions may be attributable to the same kind of desensitization phenomenon as discussed above regarding striatal dopamine during the chronic nicotine infusion. However, at this time point, in the ACe and Cg, acute nicotine increased the Fos immunostaining in both the nicotine-infused and saline-infused rats. ACe forms part of the mesolimbic DA system; it is a major target of the DA pathway originating largely in the A10 and A9 (Loughlin and Fallon, 1983; Kilts et al., 1988) and also from there arises input to substantia nigra (Price and Amaral, 1981; Gonzales and Chesselet 1990). Cg is one of the terminal regions of the mesocorticolimbic DA pathway (Zilles and Wree, 1995). Thus, like nicotine’s effects on limbic DA metabolism, its Fos expression-increasing effects in the limbic brain areas seem to be less easily attenuated during chronic nicotine treatment than its effects on the striatal Fos. Thus, nAChRs mediating nicotine’s postsynaptic effects in the striatal areas may desensitize more easily than the ones in the limbic areas. The observed differences in the striatal and limbic areas between nicotine’s effects on both DA metabolites and Fos expression thus suggest that nAChRs in these brain areas might differ.
After 24 hr withdrawal, acute nicotine did not increase the Fos immunostaining in CPU, nor did it alter the Fos immunostaining in NAcc or Cg at this time point. The withdrawal itself (24 or 72 hr) did not change the Fos expression. Similar to acute nicotine’s effects on DA metabolites, its effects on Fos expression were fully restored within 72 hr after withdrawal of nicotine infusion. The inability of nicotine to induce Fos expression in CPU and NAcc after 24 hr withdrawal could be caused by the prolonged inactivation of nAChRs, as discussed above (Rowell and Duggan, 1998). It should be noted that on the seventh day of treatment, acute nicotine did not increase Fos immunostaining in CPU and did so only to a small degree in NAcc. Furthermore, the inability of nicotine to induce Fos expression in NAcc agrees with the finding that acute nicotine did not elevate limbic DOPAC in rats withdrawn for 24 hr. However, the inability of acute nicotine to induce Fos expression in CPU does not correlate with our findings on DA metabolites. Also the inability of nicotine to increase Fos immunostaining in Cg after the 24 hr withdrawal is somewhat puzzling and may be attributed to unspecific response. Dopaminergic pathways are not necessarily the only ones involved in mediating nicotine’s effects on Fos protein expression during withdrawal. Cortical and thalamic glutamatergic inputs to the striatum may modulate the dopaminergic activation of postsynaptic intracellular mechanisms that lead to changes in Fos expression (Harlan and Garcia, 1998).
In conclusion, we observed differences in the effects of nicotine on dopamine systems as well as on Fos expression in striatal and limbic brain areas. The nAChRs in the striatal areas seem to be desensitized more easily than those in the limbic areas during prolonged nicotine infusion. We also found evidence for a long-lasting inactivation of nAChRs mediating nicotine’s effects on limbic dopamine metabolism and on Fos expression in striatal and limbic areas in vivo. The variations in responses to nicotine are interesting when studying the protective effect of nicotine in Parkinson’s disease as well as in its reinforcing and dependence-producing properties.
Footnotes
This work was supported by grants from the University of Helsinki, the Research Council for Health of the Academy of Finland, and the Finnish Cultural Foundation. We thank Marjo Vaha for skillful assistance
Correspondence should be addressed to Outi Salminen, Division of Pharmacology and Toxicology, P.O. Box 56 (Viikinkaari 5), University of Helsinki, FIN-00014 Helsinki, Finland.
REFERENCES
- 1.Benwell MEM, Balfour DJK. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benwell MEM, Balfour DJK. Regional variation in the effects of nicotine on catecholamine overflow in rat brain. Eur J Pharmacol. 1997;325:13–20. doi: 10.1016/s0014-2999(97)00101-5. [DOI] [PubMed] [Google Scholar]
- 3.Benwell MEM, Balfour DJK, Khadra LF. Studies on the influence of nicotine infusions on mesolimbic dopamine and locomotor responses to nicotine. Clin Invest. 1994;72:233–239. doi: 10.1007/BF00189320. [DOI] [PubMed] [Google Scholar]
- 4.Benwell MEM, Balfour DJK, Birrell CE. Desensitization of the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. Br J Pharmacol. 1995;114:454–460. doi: 10.1111/j.1476-5381.1995.tb13248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodie M. Low concentrations of nicotine increase the firing rate of neurons of the rat ventral tegmental area in vitro. In: Adlkofer F, Thurau K, editors. Effects of nicotine on biological systems. Birkhäuser; Basel: 1991. pp. 373–377. [Google Scholar]
- 6.Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 1989;168:363–368. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- 7.Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46:49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch J, Hegedus L, Greig NH, Rapoport SI, Soncrant TT. Electron-impact and chemical ionization detection of nicotine and cotinine by gas chromatography-mass spectrometry in rat plasma and brain. J Chromatogr. 1992;579:93–98. doi: 10.1016/0378-4347(92)80366-x. [DOI] [PubMed] [Google Scholar]
- 9.Fenster CP, Rains MF, Noerager B, Quick MW, Leister RAJ. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzales C, Chesselet MF. Amygdalonigral pathway: an anterograde study in the rat with Phaseolus vulgaris leucoagglutinin (PHA-L). J Comp Neurol. 1990;297:182–200. doi: 10.1002/cne.902970203. [DOI] [PubMed] [Google Scholar]
- 11.Grady S, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated 3H-dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992;59:848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- 12.Grady SR, Marks MJ, Collins AC. Desensitization of nicotine-stimulated 3H-dopamine release from mouse striatal synaptosomes. J Neurochem. 1994;62:1390–1398. doi: 10.1046/j.1471-4159.1994.62041390.x. [DOI] [PubMed] [Google Scholar]
- 13.Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grenhoff J, Svensson TH. Selective stimulation of limbic dopamine activity by nicotine. Acta Physiol Scand. 1988;133:595–596. doi: 10.1111/j.1748-1716.1988.tb08450.x. [DOI] [PubMed] [Google Scholar]
- 15.Haikala H. Use of a novel type of rotating disc electrode and a flow cell with laminar flow pattern for the electrochemical detection of biogenic monoamines and their metabolites after Sephadex gel chromatographic purification and high-performance liquid chromatographic isolation from rat brain. J Neurochem. 1987;49:1033–1041. doi: 10.1111/j.1471-4159.1987.tb09991.x. [DOI] [PubMed] [Google Scholar]
- 16.Haikala H, Karmalahti T, Ahtee L. The nicotine-induced changes in striatal dopamine metabolism of rats depend on body temperature. Brain Res. 1986;375:313–319. doi: 10.1016/0006-8993(86)90751-1. [DOI] [PubMed] [Google Scholar]
- 17.Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Mol Neurobiol. 1998;16:221–267. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- 18.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–126. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 19.Henningfield JE, Keenan RM, Clarke PBS. Nicotine. In: Schuster CR, Kuhar MJ, editors. Handbook of experimental pharmacology, Vol 118. Springer-Verlag; Berlin: 1996. pp. 271–314. [Google Scholar]
- 20.Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 21.Imperato A, Mulas A, DiChiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- 22.Kiba H, Jayaraman A. Nicotine induced c-fos expression in the striatum is mediated mostly by dopamine D1-receptor and is dependent on NMDA stimulation. Mol Brain Res. 1994;23:1–13. doi: 10.1016/0169-328x(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 23.Kilts CD, Anderson CM, Ely TD, Mailman RB. The biochemistry and pharmacology of mesoamygdaloid dopamine neurons. Ann NY Acad Sci. 1988;537:173–187. doi: 10.1111/j.1749-6632.1988.tb42105.x. [DOI] [PubMed] [Google Scholar]
- 24.Leikola-Pelho T, Heinämäki J, Laakso I, Ahtee L. Chronic nicotine treatment changes differentially the effects of acute nicotine on the three main dopamine metabolites in mouse striatum. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:400–406. doi: 10.1007/BF00169456. [DOI] [PubMed] [Google Scholar]
- 25.Lippiello PM, Bencherif M, Prince RJ. The role of desensitization in CNS nicotinic receptor function. In: Clarke PBS, Quik M, Adlkofer F, Thurau K, editors. Effects of nicotine on biological systems II. Advances in pharmacological sciences. Birkhäuser; Basel: 1995. pp. 79–85. [Google Scholar]
- 26.Loughlin SE, Fallon JH. Dopaminergic and non-dopaminergic projections to amygdala from substantia nigra and ventral tegmental area. Brain Res. 1983;262:334–338. doi: 10.1016/0006-8993(83)91029-6. [DOI] [PubMed] [Google Scholar]
- 27.Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J Neurochem. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- 28.Matta SG, Foster CA, Sharp BM. Nicotine stimulates the expression of cFos protein in the parvocellular paraventricular nucleus and brainstem catecholaminergic regions. Endocrinology. 1993;132:2149–2156. doi: 10.1210/endo.132.5.8386611. [DOI] [PubMed] [Google Scholar]
- 29.Meredith GE, Agolia R, Arts MPM, Groenewegen HJ, Zahm DS. Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat. Neuroscience. 1992;50:149–162. doi: 10.1016/0306-4522(92)90389-j. [DOI] [PubMed] [Google Scholar]
- 30.Merlo Pich E, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- 31.Moratalla R, Elibol B, Vallejo M, Graybiel AM. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- 32.Nisell M, Nomikos GG, Hertel P, Panagis G, Svensson TH. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse. 1996;22:369–381. doi: 10.1002/(SICI)1098-2396(199604)22:4<369::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Nisell M, Nomikos GG, Chergui K, Grillner P, Svensson TH. Chronic nicotine enhances basal and nicotine-induced Fos immunoreactivity preferentially in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 1997;17:151–161. doi: 10.1016/S0893-133X(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 34.Nose T, Takemoto H. Effect of oxotremorine on homovanillic acid concentration in the striatum of the rat. Eur J Pharmacol. 1974;25:51–55. doi: 10.1016/0014-2999(74)90093-4. [DOI] [PubMed] [Google Scholar]
- 35.Pagliusi SR, Tessari M, DeVevey S, Chiamulera C, Merlo Pich E. The reinforcing properties of nicotine are associated with a specific patterning of c-fos expression in the rat brain. Eur J Neurosci. 1996;8:2247–2256. doi: 10.1111/j.1460-9568.1996.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 36.Panagis G, Nisell M, Nomikos GG, Chergui K, Svensson TH. Nicotine injections into the ventral tegmental area increase locomotion and Fos-like immunostaining in the nucleus accumbens of the rat. Brain Res. 1996;730:133–142. doi: 10.1016/0006-8993(96)00432-5. [DOI] [PubMed] [Google Scholar]
- 37.Pang Y, Kiba H, Jayaraman A. Acute nicotine injections induce c-fos mostly in non-dopaminergic neurons of the midbrain of the rat. Mol Brain Res. 1993;20:162–170. doi: 10.1016/0169-328x(93)90122-6. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1986. [DOI] [PubMed] [Google Scholar]
- 39.Pietilä K, Salminen O, Leikola-Pelho T, Ahtee L. Tolerance to nicotine’s effects on striatal dopamine metabolism in nicotine-withdrawn mice. Eur J Pharmacol. 1996;318:17–22. doi: 10.1016/s0014-2999(96)00767-4. [DOI] [PubMed] [Google Scholar]
- 40.Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rapier C, Lunt GG, Wonnacott S. Stereo-selective nicotine-induced release of dopamine from striatal synaptosomes: concentration dependence and repetitive stimulation. J Neurochem. 1988;50:1123–1130. doi: 10.1111/j.1471-4159.1988.tb10582.x. [DOI] [PubMed] [Google Scholar]
- 42.Ren T, Sagar SM. Induction of c-fos immunostaining in the rat brain after the systemic administration of nicotine. Brain Res Bull. 1992;29:589–597. doi: 10.1016/0361-9230(92)90127-j. [DOI] [PubMed] [Google Scholar]
- 43.Robertson GS, Vincent SR, Fibinger HC. Striatonigral projection neurons contain D1 dopamine receptor-activated c-fos. Brain Res. 1990;523:288–290. doi: 10.1016/0006-8993(90)91498-6. [DOI] [PubMed] [Google Scholar]
- 44.Roffler-Tarlov S, Sharman D, Tegerdine P. 3,4-Dihydroxy-phenylacetic acid and 4-hydroxy-3-methoxyphenylacetic acid in the mouse striatum: a reflection of intra- and extra-neuronal metabolism of dopamine. Br J Pharmacol. 1971;42:343–351. doi: 10.1111/j.1476-5381.1971.tb07118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowell PP, Duggan DS. Long-lasting inactivation of nicotinic receptor function in vitro by treatment with high concentrations of nicotine. Neuropharmacology. 1998;37:103–111. doi: 10.1016/s0028-3908(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 46.Rowell PP, Li M. Dose-response relationship for nicotine-induced up-regulation of rat brain nicotinic receptors. J Neurochem. 1997;68:1982–1989. doi: 10.1046/j.1471-4159.1997.68051982.x. [DOI] [PubMed] [Google Scholar]
- 47.Rowell PP, Carr LA, Garner AC. Stimulation of 3H-dopamine release by nicotine in rat nucleus accumbens. J Neurochem. 1987;49:1449–1454. doi: 10.1111/j.1471-4159.1987.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 48.Salminen O, Lahtinen S, Ahtee L. Expression of Fos protein in various rat brain areas following acute nicotine and diazepam. Pharmacol Biochem Behav. 1996;54:241–248. doi: 10.1016/0091-3057(95)02132-9. [DOI] [PubMed] [Google Scholar]
- 49.Shoaib M, Benwell MEM, Akbar MT, Stolerman IP, Balfour DJK. Behavioural and neurochemical adaptations to nicotine in rats: influence of NMDA antagonists. Br J Pharmacol. 1994;111:1073–1080. doi: 10.1111/j.1476-5381.1994.tb14854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valentine JD, Matta SG, Sharp BM. Nicotine-induced cFos expression in the hypothalamic paraventricular nucleus is dependent on brainstem effects: correlations with cFos in catecholaminergic and noncatecholaminergic neurons in the nucleus tractus solitarius. Endocrinology. 1996;137:622–630. doi: 10.1210/endo.137.2.8593811. [DOI] [PubMed] [Google Scholar]
- 51.van Straaten F, Muller R, Curran T, Van Beveren C, Verma I. Complete nucleotide sequence of a human c-onc-gene: deduced amino acid sequence of the human c-fos protein. Proc Natl Acad Sci USA. 1983;80:3183–3187. doi: 10.1073/pnas.80.11.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westerink BHC, Spaan SJ. Estimation of the turnover of 3-methoxytyramine in the rat striatum by HPLC with electrochemical detection: implications for the sequence in the cerebral metabolism of dopamine. J Neurochem. 1982;38:342–347. doi: 10.1111/j.1471-4159.1982.tb08634.x. [DOI] [PubMed] [Google Scholar]
- 53.Westfall TC. Effect of nicotine and other drugs on the release of 3H-norepinephrine and 3H-dopamine from rat brain slices. Neuropharmacology. 1974;13:693–700. doi: 10.1016/0028-3908(74)90015-x. [DOI] [PubMed] [Google Scholar]
- 54.Zilles K, Wree A. Cortex: areal and laminar structure. In: Paxinos G, editor. The rat nervous system, Ed 2. Academic; New York: 1995. pp. 651–653. [Google Scholar]