Abstract
Calcium-dependent activator protein for secretion (CAPS) is a neural/endocrine cell-specific protein that has been shown to function at the Ca2+-dependent triggering step of dense-core vesicle (DCV) exocytosis in permeabilized PC12 cells. To evaluate the function of CAPS under physiological conditions, we introduced affinity-purified anti-CAPS IgGs into calf adrenal chromaffin (AC) cells via a patch pipette and tested the kinetics of catecholamine secretion using both amperometric and membrane capacitance techniques. The antibodies reacted with a single major ∼145 kDa protein in AC cells based on immunoblot analysis. AC cells stimulated with sequential trains of action potentials at 7 Hz resulted in successive secretory episodes of equivalent magnitude. When either of two different anti-CAPS IgGs or their Fab fragments were present, a rapid and progressive inhibition of catecholamine release ensued to a maximum of >80%. The effect was specific because preabsorption of IgGs with the respective antigens ablated the inhibitory effect, and the IgGs had no effect on Ca currents. CAPS immunoneutralization not only reduced the number of amperometric spikes but markedly altered the kinetic characteristics of the residual events. The remaining spikes were much smaller (by 85%) and broader (by ∼3.5-fold) than those in control cells, suggesting that CAPS plays a role in determining release of vesicle contents via the fusion pore. Anti-CAPS IgGs also slowed the rate of the initial exocytotic capacitance burst, representing the docked-and-primed vesicle pool, by ∼90% but had no effect on the kinetics of rapid endocytosis. These results suggest that CAPS is a key component regulating the fusion of DCVs to the plasma membrane, and possibly fusion pore dilation, in catecholamine secretion from AC cells.
Keywords: dense-core vesicles, exocytosis, CAPS, adrenal chromaffin cells, fusion pore, amperometric recording, capacitance measurements, catecholamine release
Much has been learned in recent years about the molecular basis of exocytosis from small synaptic vesicles (SSVs) in the nervous system. Several important components of the SSV and presynaptic membrane have been cloned, and interactions between them have been studied in some detail (for review, see Sudhof, 1995). Considerably less is known about exocytosis from dense-core vesicles (DCVs) that store biogenic amines as well as neuropeptides. Significant differences may exist in the characteristics of secretion between these different types of vesicles (Martin, 1997). For example, although SSVs are released largely from well-defined active zones, DCVs frequently appear to have a more diffuse pattern of release. Perhaps as a result, secretion from DCVs is often, but not always, slower than that from SSVs. Despite these kinetic differences, there are substantial similarities between several components of the core secretory machinery associated with SSVs and DCVs. For example, the proteins synaptotagmin, synaptobrevin (VAMP), and rab 3a are all present on both types of vesicle. In addition, secretion in either system is sensitive to clostridial protease toxins that selectively cleave various protein components of the secretory apparatus. These findings suggest that regulated SSV and DCV exocytosis largely involves a common set of proteins and mechanisms (for review, see Martin, 1997).
One approach to distinguishing between SSV and DCV secretion at the molecular level is to identify proteins that may be critical to one type of vesicle exocytosis but not the other. Calcium-dependent activator protein for secretion (CAPS) is a recently discovered protein that has been suggested to be specific to DCV secretion (Walent et al., 1992; Ann et al., 1997; Berwin et al., 1998; Tandon et al., 1998). Its initial purification was based on the ability to reconstitute catecholamine secretion from permeabilized PC12 cells (Walent et al., 1992). Both permeabilized PC12 and adrenal chromaffin (AC) cells secrete catecholamines by exocytosis when both ATP and Ca2+ are exogenously supplied. The process can be divided into two stages: an ATP-dependent “priming” step that can take place in the absence of Ca2+, and a Ca2+-dependent “triggering” step for which ATP is not required (Bittner and Holz, 1992; Hay and Martin, 1992). Extensive washing reduces secretion from permeabilized cells, which can then be recovered by addition of exogenous concentrated rat brain cytosol (Hay and Martin, 1992). Fractionation of the cytosol led to the identification of three proteins that could reconstitute, in a purified form, both the priming and triggering steps of secretion. Two of the required proteins for the ATP-dependent priming step were identified as components involved in phosphoinositide phosphorylation (Hay and Martin, 1993), whereas the ∼145 kDa CAPS was found to act at the triggering (Ca2+-dependent) step (Walent et al., 1992). Subsequent cloning of CAPS revealed that it is homologous to the Caenorhabditis elegans gene unc-31 (Ann et al., 1997). Loss-of-function mutations in this gene result in pleiotropic nervous system abnormalities, suggesting that CAPS plays a fundamental role in neurosecretion in these invertebrates (Avery et al., 1993). Antibodies to CAPS are able to inhibit secretion in permeabilized PC12 cells (Walent et al., 1992; Ann et al., 1997) and selectively target catecholamine secretion in perforated rat brain synaptosomes (Tandon et al., 1998). Anti-CAPS IgG blocks norepinephrine secretion from PC12 cells and semi-intact synaptosomes but fails to antagonize glutamate secretion in the latter preparation. These findings indicate that CAPS plays a dedicated role in DCV but not SSV exocytosis.
Although studies on permeabilized preparations strongly suggest that CAPS is required for DCV exocytosis, they lack the kinetic resolution that can be obtained with electrophysiological analysis of intact cells. To further our understanding of the role of CAPS in secretion, we turned to patch-clamped calf AC cells. Secretion from these cells have been well characterized and can be recorded with millisecond resolution using either capacitance or amperometric techniques (Neher and Marty, 1982; Wightman et al., 1991; Chow et al., 1992; Artalejo et al., 1994; Elhamdani et al., 1998). We previously showed by using capacitance measurements that catecholamine secretion in these cells is preferentially coupled to a particular type of L-type Ca channel termed the facilitation Ca channel (Artalejo et al., 1994). More recent experiments using the high-resolution amperometric technique revealed that catecholamine secretion consists of two kinetic components (Elhamdani et al., 1998). Surprisingly, secretion elicited by activation of facilitation Ca channels is remarkably rapid (delay of ∼3 msec after the depolarization; termed “strongly coupled” secretion), approaching the speeds characteristic of synaptic transmission (Elhamdani et al., 1998). We attributed strongly coupled secretion to colocalization of facilitation Ca channels with DCV release sites. Slower secretion (delay of >25 msec or weakly coupled) is also observed, probably attributable to Ca channels that are not colocalized with the release apparatus (Klingauf and Neher, 1997;Elhamdani et al., 1998). Capacitance recordings also reveal multiple phases of secretion with an initial “exocytotic burst,” manifest as a very high initial rate of secretion, being caused by an already docked-and-primed “release-ready” pool of DCVs preceding a slower phase that may reflect secretion of newly recruited vesicles (Parsons et al., 1995). This kinetic diversity, along with the opportunity to analyze other parameters revealed by electrochemical analysis, such as the shape of unitary amperometric spikes, permits us in the present study to examine the effects of intracellular antagonism of CAPS on several aspects of catecholamine secretion in detail. The results suggest that CAPS plays a role at the very final step in DCV exocytosis where fusion of the vesicle with the membrane surface takes place.
MATERIALS AND METHODS
Cell culture
Bovine calf (average age 10–12 weeks) chromaffin cells were prepared by digestion of adrenal glands obtained from local slaughterhouses. Cells were purified and cultured using previously described methods (Artalejo et al., 1991). Cells plated at a density of 3 × 105 cells on collagen-coated 35-mm-diameter dishes were used in all studies, within 1 week of plating.
Electrophysiology
Conventional patch-clamp current and capacitance recording. Our patch-clamp techniques have been published previously (Artalejo et al., 1995); an Axopatch 200 B (Axon Instruments, Foster City, CA) was used as the patch-clamp amplifier throughout these experiments. Capacitance was measured by a computer program using a phase-tracking technique. A standard protocol of ten 50 msec depolarizations from a holding potential of −90 mV to +10 mV, each pulse preceded by a prepulse to +120 mV to recruit facilitation Ca channels, was used to evoke secretion. After the secretory phase, rapid endocytosis (RE) manifests as a decrease in capacitance; both the rate and extent of RE were measured, as well as the rate and extent of exocytosis and the magnitude of Ca current. All experiments were performed at room temperature (24°C). The standard patch pipette solution contained (in mm): Cs-glutamate 110, Cs-EGTA 0.1, HEPES 40, MgCl2 5, ATP 2, GTP 0.35, pH 7.2, with CsOH (nucleotides and various anti-CAPS IgGs as indicated in the figure legends were added fresh to the stock salt solution just before the experiment). The external solution consisted of (in mm): CaCl2 2, TEA-Cl 150, HEPES 10, glucose 10, MgCl2 1, and 1 μm tetrodotoxin, pH 7.3.
Current-clamp recording. To evoke APs, cells maintained at a Vm of −70 to −90 mV, through application of a holding current of −1 to −4 pA, were stimulated by depolarizing currents (+10 to +20 pA) of 20 msec (Elhamdani et al., 1998). An eight-pole Bessel filter was set to a corner frequency of 2 kHz for Vm recording, then sampled at 5 kHz. The pipette solution contained (in mm): K-glutamate 100, K-EGTA 0.1, NaCl 12, HEPES 30, MgCl2 5, ATP 2, GTP 0.35, pH 7.2, with KOH. The external solution consisted of (in mm): NaCl 140, glucose 10, HEPES 10, MgCl2 1, KCl 5.5, CaCl2 2, pH 7.3, with NaOH.
Carbon-fiber amperometry for catecholamine detection
Highly sensitive low-noise polypropylene-insulated carbon-fiber electrodes were used for electrochemical monitoring of quantal release of catecholamines from single AC cells as described previously (Wightman et al., 1991; Chow et al., 1992; Elhamdani et al., 1998). Briefly, a 7-μm-diameter carbon fiber (Amoco Performance Products, Greenville, SC) was inserted into a polypropylene pipette (Continental Lab Products). The tip was then heated and pulled with a homemade CFE puller. The ProCFE was prepared for recording by inserting it into a micromanipulator and cutting the tip with a microscissor. To obtain electrical contact between the carbon fiber and the Ag wire input to the head stage of the amplifier, the shank of the ProCFE was back-filled with mercury. Each ProCFE was then used in a maximum of one to three cells to ensure the highest possible sensitivity. The tip of the electrode was closely apposed to the cell surface to minimize the diffusion distance from release sites. The amperometric current (Iamp), generated by oxidation of catecholamines at the exposed tip of the CFE, was measured using an Axopatch 200A amplifier, operated in the voltage-clamp mode at a holding potential of +780 mV. Amperometric signals were low-pass-filtered at 1 kHz, then sampled at 2 kHz by an Axobasic system. The data were collected and then analyzed by computer using IGOR software (WaveMetrics, Lake Oswego, OR). Latencies (defined as the time from the peak of the AP to the beginning of the current spike) were analyzed using latency histograms (Elhamdani et al., 1998). The beginning of the current spike was located where the leading edge of the transient (which includes the “foot” signal when present) exceeded the baseline current by two times the SD of the baseline noise level. Measurements are given as means ± SEM.
Preparation of anti-CAPS antibodies. Antisera (referred to as bCAPS and FP5 antibody, respectively) were generated by immunization of rabbits with recombinant CAPS proteins: a full-length protein (bCAPS) produced by baculovirus-encoded expression in insect cells (Ann et al., 1997) or a truncated protein (FP5 corresponding to amino acids 264–364) produced as a glutathioneS-transferase fusion protein in E. coli. IgGs for patch-clamp studies were purified by chromatography on protein A-Sepharose. IgG fractions depleted for CAPS-neutralizing antibodies were prepared by affinity chromatography on the FP5 protein coupled to Sepharose 4B by CNBr activation.
Analysis of CAPS expression in chromaffin cells.Extracts of calf AC cells, PC12 cells, and rat1a fibroblasts were prepared by sonication in a lysis buffer containing (in mm): 50 Tris-HCl, pH 7.4, 2 EDTA, 1 EGTA, 5 2-mercaptoethanol, 0.5 PMSF, and 0.01 leupeptin. After sonication, aliquots were taken for protein assay, and the extracts were immediately solubilized in SDS sample buffer. Samples (100 μg) were separated by SDS-7.5% PAGE, and proteins were transferred to nitrocellulose and subjected to immunoblotting procedures as described previously (Artalejo et al., 1995). AC cells for immunocytochemical analysis were also prepared as before (Artalejo et al., 1995). After incubation in primary IgG (1 μg/ml), coverslips were washed and incubated for 30 min in biotin-coupled goat anti-rabbit IgG (0.2 μg/ml) followed by 10 min in Cy3-coupled streptavidin (0.1 mg/ml). Fluorescence was viewed in a Noran Odyssey confocal microscope.
RESULTS
CAPS is an abundant protein in calf AC cells
Previous studies showed that CAPS expression was restricted to neural and secretory endocrine tissues in the rat and is also present in adult bovine AC cells (Ann et al., 1997). To verify that the calf AC cells used in the present study also contain CAPS and that the antibodies used are specific, we performed immunoanalysis of these cells. As shown in Figure1A, two anti-CAPS IgGs used in the present study react with a major ∼145 kDa protein in immunoblots of total calf AC cell protein; rat PC12 cells were also positive but rat fibroblasts were negative, in line with previous studies showing a limited tissue distribution for this protein (Ann et al., 1997). Immunoreactivity was completely blocked by preabsorption of IgGs with the respective antigens (data not shown). The same antibodies stained calf AC cells in situ (Fig. 1B), confirming that CAPS is an abundant protein in these cells.
Fig. 1.
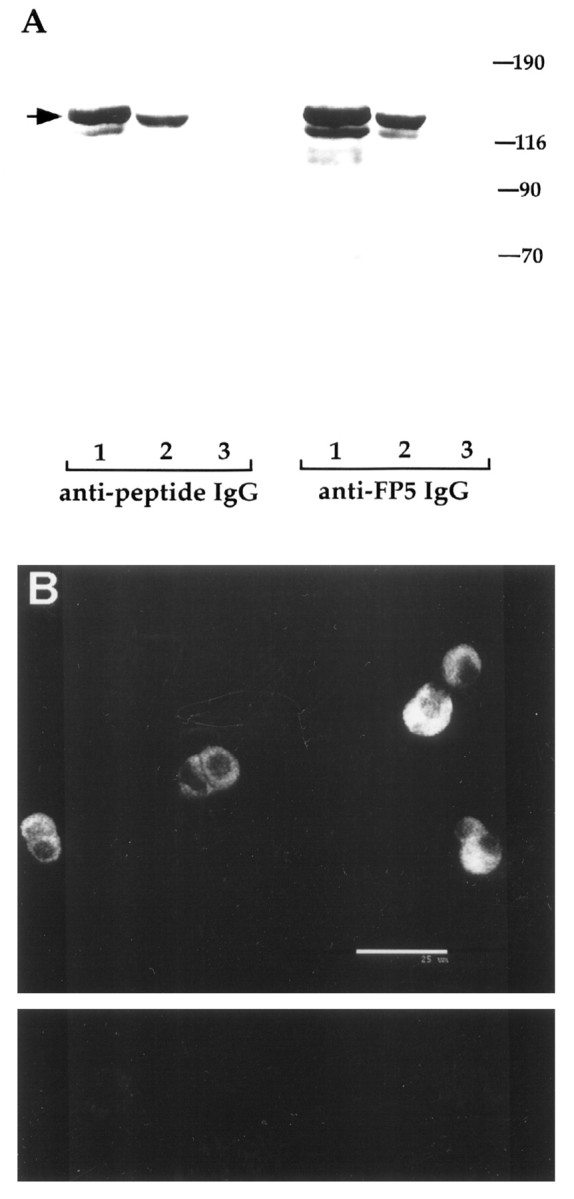
CAPS is an abundant protein in calf AC cells.A, Immunoblots showing the reactivity of the two CAPS IgGs used in the present study with calf AC cell (lanes 1), PC12 cell (lanes 2), and rat 1a fibroblast (lanes 3) cell extracts. Each lane contains 100 μg of total cell protein. Both anti-CAPS-FP5 and anti-CAPS peptide IgGs were used at 1 μg/ml. Detection was with peroxidase-coupled secondary antibodies and ECL. Note the presence of the major ∼145 kDa species in lanes 1 and 2 but its absence inlanes 3. B, Immunofluorescence of anti-CAPS-FP5 IgG with calf AC cells. Fixed calf AC cells on coverslips were incubated with anti-CAPS IgG (1 μg/ml), then with biotin-labeled goat anti-rabbit secondary antibodies followed by streptavidin-Cy3 label. The cells were visualized in a Noran Odyssey confocal microscope of which a typical section is shown (top panel). Scale bar, 25 μm. Only faint background signal was observed when staining was performed with preabsorbed antibody (lower panel).
Anti-CAPS IgGs progressively and specifically inhibit catecholamine secretion
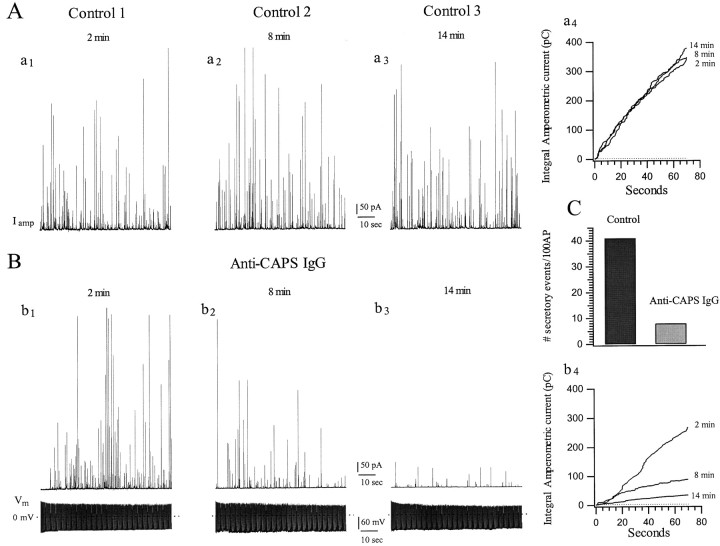
To ascertain the effects of CAPS antagonism on secretion, we introduced two anti-CAPS IgG preparations or their controls into calf AC cells via the patch pipette and recorded secretion using an extracellular carbon-fiber electrode. As described previously (Elhamdani et al., 1998), when such cells are stimulated using sequential trains of action potentials delivered at a frequency of 7 Hz, rates of secretion as quantitated by cumulative amperometric current are similar in successive periods (Fig.2A). Introduction of anti-CAPS IgG (anti-bCAPS raised against recombinant CAPS generated in insect cells; 1.5 mg/ml) resulted in a progressive decline in the rate and magnitude of secretion from these cells (Fig.2B). After 8 min the cumulative amperometric spike amplitude was reduced by ∼67%, and by 14 min the reduction had increased to 86%, presumably reflecting the time course of IgG diffusion into the cell from the pipette, and did not decline further. Similar results were obtained when the antibody was allowed to diffuse into a resting cell for 14 min before any stimulation; when these cells were then stimulated at 7 Hz, secretion was still inhibited by 84.5 ± 6.8% (n = 9 cells) compared with control cells with no antibody. When stimulation was performed at a lower frequency (1 Hz), >95% inhibition of secretion was observed in anti-CAPS IgG-treated cells after 14 min (data not shown).
Fig. 2.
Anti-CAPS IgGs progressively inhibit catecholamine secretion from calf AC cells. The effect of intracellular anti-CAPS IgG (bCAPS antibody raised against recombinant CAPS generated in insect cells; 1.5 mg/ml) on secretion was tested with amperometry. A calf AC cell was stimulated by three successive trains of 490 APs at 7 Hz (bottom traces); 5–6 min separated each train. Amperometric spikes were recorded with an extracellular carbon fiber electrode (top traces) as described in Materials and Methods. A, Under control conditions (either no IgG or preimmune IgG in the pipette), secretion in the third round (14 min) was not significantly different from the first (2 min) or second (8 min) rounds, indicating that depletion of releasable vesicles did not occur (a1–a3). Graph onright (a4) represents cumulative amperometric spike current as a function of time during the three recording periods. B, Cell loaded with anti-CAPS IgG: quantal release during the first series of APs (2 min,b1) was not significantly different from control, but thereafter (b2, b3) a progressive inhibition of secretion ensued, such that the total quantal release during the third round (14 min) was reduced by 86%, as determined by reduction in cumulative amperometric spike current (b4), compared with secretion in the first round.C, Anti-CAPS IgG reduces the total number of amperometric events by 80%.
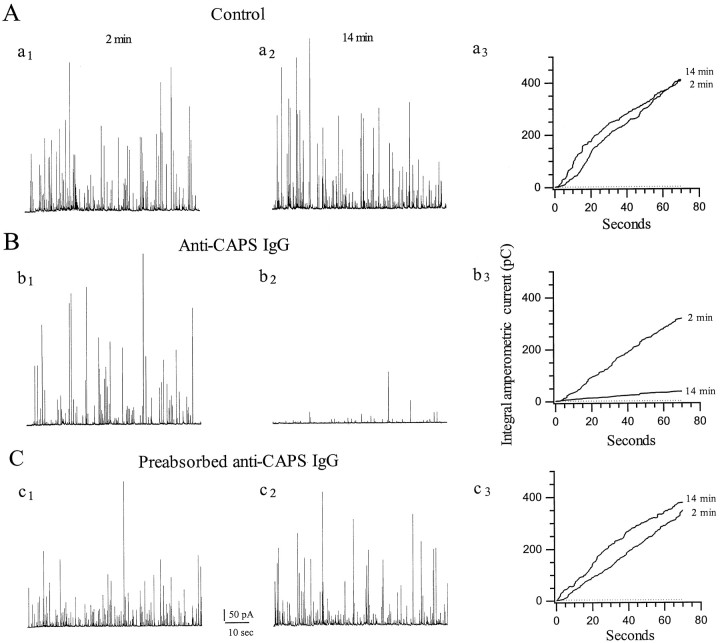
To test for the specificity of this effect, we used a second anti-CAPS IgG (anti-FP5 antibody raised against a CAPS fusion protein) and compared the response of the IgG alone with that of IgG that had been preabsorbed with an excess of the antigen (Fig.3). The anti-FP5 antibody (1.5 mg/ml) also reduced secretion by ∼80% after 14 min when stimulation was conducted at 7 Hz (Fig. 3B), whereas preabsorbed (Fig.3C) or preimmune (Table 1) IgGs were without discernible effect. None of the IgG preparations influenced the magnitude of the Ca current, indicating that the reduction in secretion was not caused by alteration in the amount of Ca2+ entering the cell during stimulation. As a further negative control for the FP5 antibody, we used “immune” serum that was depleted of neutralizing antibody by passage through an affinity column of the antigen; such antibodies were also ineffective in blocking secretion. These results are summarized in Table 1.
Fig. 3.
Anti-CAPS IgGs specifically inhibit catecholamine secretion. Continuous amperometric recordings, using a stimulation paradigm identical to that in Figure 2, from AC cells in which (A) no protein, (B) anti-CAPS IgG (FP5 antibody raised against a CAPS fusion protein; 1.5 mg/ml), or (C) anti-CAPS IgG (1.5 mg/ml) preabsorbed with an approximate fourfold molar excess of FP5 were included in the patch pipette. Note in A andC that both rounds of exocytosis occur with similar quantal release, whereas in B the second round of release is strongly blocked after the antibody has diffused into the cell (in the first round, secretion is normal because insufficient antibody has diffused into the cell). Similar results were also obtained with anti-bCAPS IgG (Table 1). From n = 46 treated cells, anti-CAPS IgG blocks the total charge of the amperometric spikes by 83.6 ± 4.1%. From n = 7 treated cells, anti-FP5 Fab blocks the total charge of the amperometric spikes by 82.4 ± 5.4%.
Table 1.
Statistical analysis of anti-CAPS IgG effects on catecholamine secretion in calf chromaffin cells
| Condition | τ (msec) | Integral Qamp(pC) | Events/AP |
|---|---|---|---|
| Control (n = 48) | 3.32 ± 0.11 | 346.12 ± 16.5 | 0.384 ± 0.019 |
| FP5 IgG (n = 23) | 53.21 ± 5.3* | 0.075 ± 0.01* | |
| bCAPS IgG (n = 16) | 54 ± 5.5* | 0.073 ± 0.009* | |
| FP5 Fab (n = 7) | 59.1 ± 6.2* | 0.079 ± 0.01* | |
| Preabsorbed IgG (n = 15) | 3.34 ± 0.206 | 336.5 ± 29.3 | 0.372 ± 0.012 |
| Preimmune IgG (n = 12) | 3.22 ± 0.244 | 318.74 ± 16.94 | 0.352 ± 0.01 |
τ is calculated from the individual amperometric delay histograms in each condition. τ is obtained from a monoexponential fit of the strongly coupled events in each histogram. IntegralQamp = ∫ Iamp dt is calculated from the amperometric traces (70 sec) under the different conditions described in the Table. Events/AP represents average quantal release induced by APs at a frequency of 7 Hz, under the different conditions described in the Table. *Values are significantly different from the control, preabsorbed, and preimmune IgGs at p< 0.0001. Measurements are given as means ± SEM.
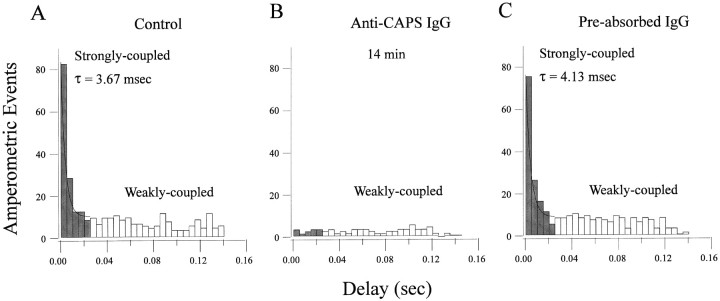
We have shown previously that secretion of catecholamines from calf AC cells occurs in two kinetically distinct phases that we term strongly coupled and weakly coupled (Elhamdani et al., 1998). This distinction is based on the analysis of amperometric latency histograms that depict the temporal relationship between the evoked action potential and the resultant extracellular amperometric spike (Chow et al., 1992). Accordingly, during stimulation at 7 Hz we found that many amperometric events occurred <5 msec after the action potential, forming an early strongly coupled peak in histograms (Fig.4A). This early peak is abolished by the Ca channel antagonist nisoldipine, suggesting that it is caused entirely by the action of facilitation L-type Ca channels in these cells. By contrast, weakly coupled secretion (Fig.4A) occurs with a significant delay (>25 msec) after the action potential and contributes a plateau phase in these histograms that is relatively resistant to nisoldipine (Elhamdani et al., 1998). As shown in Figure 4B, anti-CAPS IgG inhibited both phases of secretion after 14 min of intracellular dialysis. No inhibition of either peak was obtained with either the preabsorbed (Fig. 4C) or preimmune (Table 1) IgGs, indicating that the effect was specific.
Fig. 4.
Anti-CAPS blocks both strongly and weakly coupled secretion. To determine the contribution of CAPS to the different kinetic components of secretion in calf AC cells (Elhamdani et al., 1998), we analyzed latency histograms from amperometric experiments. These histograms reflect the delay in the amperometric spike after the action potential. Two successive stimulation periods of 7 Hz (980 APs) were performed in cells loaded with (A) no addition, (B) anti-CAPS IgG at 14 min, or (C) preabsorbed CAPS antibody. In control cells, or those loaded with preabsorbed antibody, 46% of the events had a latency <25 msec, whereas 54% were >25 msec. In B, the strongly coupled component is reduced by ∼89%, whereas the weakly coupled component is reduced by ∼59%.
CAPS antagonism affects the time course of quantal catecholamine secretion
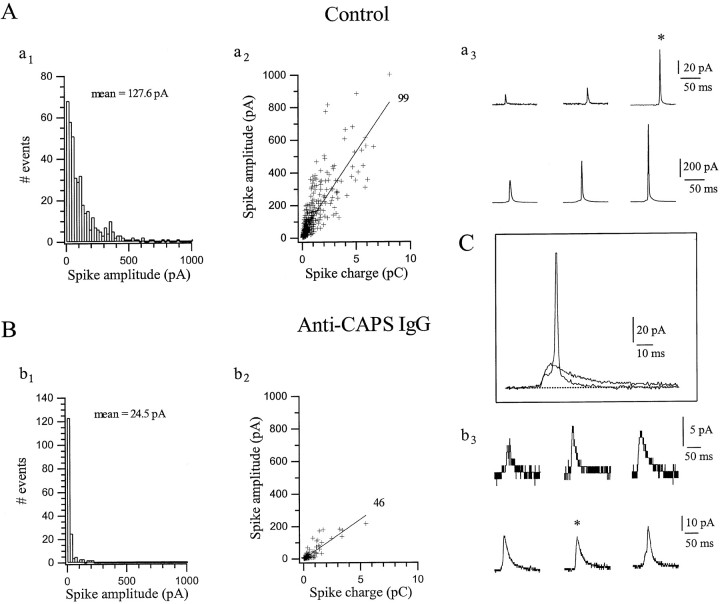
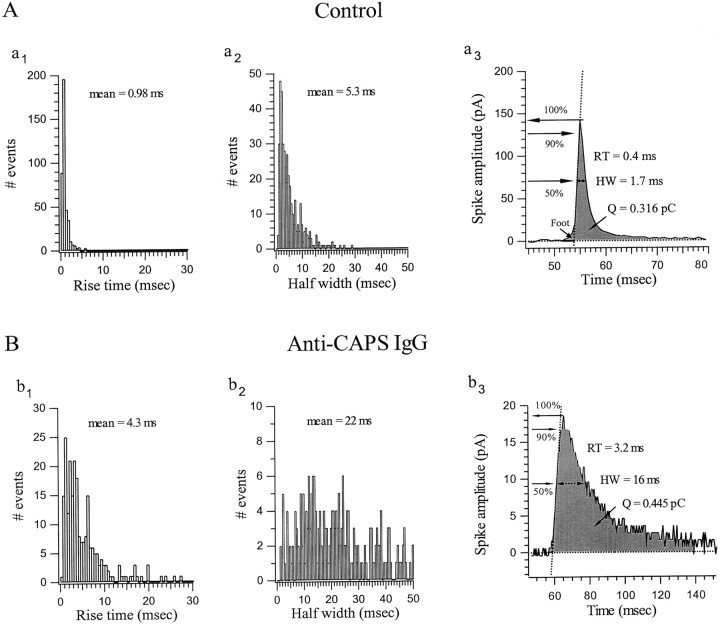
When stimulation was performed at 7 Hz even in the presence of high anti-CAPS IgG concentrations (2 mg/ml), inhibition of secretion was incomplete after 14 min. We investigated the kinetic properties of the residual amperometric spikes to assess whether they differed from those in control cells. Four distinctive parameters were analyzed in the absence and presence of anti-CAPS IgG (Figs.5, 6): maximum spike amplitude, total charge (or quantal content,Q) including the “foot” (Chow et al., 1992) when present, rise time (RT), and half-width (HW). The spike amplitude and spike charge histograms reflect the amount and distribution of catecholamine that is released per quantal event (for review, see Travis and Wightman, 1998). In control cells, amplitude histograms formed a broad distribution with a mean value of 127.6 pA, and only 32.7% of the spikes had an amplitude <40 pA (Fig. 5A, a1, and legend). Amplitude-charge distributions were well-fit with a linear relationship having a slope of 99 pA/pC (Fig. 5A, a2), indicating that spike charge is directly proportional to amplitude, as seen in the representative amperometric spikes in Figure 5A,a3. By contrast, cells loaded with anti-CAPS IgG exhibited only low amplitude spikes, with ∼88% of the events <40 pA (mean 24.5 pA) (Fig. 5B, b1, and legend). The linear regression fit of the spike amplitude versus charge plot gave a slope of only 46 pA/pC. Thus anti-CAPS antibodies reduced spike amplitude to one-sixth the control value but only halved the quantal content.
Fig. 5.
Effect of CAPS antagonism on the kinetics of individual amperometric spikes: amplitude and charge histograms.A, Control cells. a1, Amplitude histogram (bin of 20 pA) for spikes obtained during stimulation of two cells with 980 APs. A broad distribution is apparent with a mean value of 127.6 pA. a2, Distribution of spike amplitude versus charge (Q) fitted by linear regression (Y = A + BX;B = 99 pA/pC). a3, Representative current spike obtained from the two cells analyzed ina1. Their amplitude and charge from leftto right was 13.5 pA/0.042 pC, 21 pA/0.06 pC, 142 pA/0.316 pC, 225 pA/1.31pC, 415 pA/1.52 pC, and 774 pA/2.047 pC. The average values (n = 10 cells) for amplitude, charge, and amplitude/charge were 142.6 ± 25 pA, 1.21 ± 0.17 pC, and 96 ± 5.9 pA/pC, respectively. B, Anti-CAPS IgG-treated cells. Data were collected from two representative cells dialyzed with anti-CAPS IgG (anti-FP5) for 14 min to achieve maximum blockade of secretion and then stimulated with 980 APs. b1, Amplitude histograms show that 88% of the events are <40 pA (average 24.5 pA). Anti-CAPS IgG reduces the amplitude of the spike by approximately sixfold. b2, Distribution of spike amplitude versus charge gives a much reduced slope of 46 pA/pC compared with control. b3, Representative amperometric spikes illustrate their typical reduced magnitude compared with controls (a3). Their amplitude and charge from left to right were 5.5 pA/0.071 pC, 7 pA/0.105 pC, 6.5 pA/0.199 pC, 19.5 pA/0.464 pC, 18.5 pA/0.445 pC, and 23 pA/0.375 pC. Note the different scale betweentop and bottom traces. The average values (n = 15 cells) for amplitude, charge, and amplitude/charge were 23.3 ± 1.4 pA, 0.53 ± 0.04 pC, and 33.1 ± 3.7 pA/pC, respectively. Fromn = 7 cells treated with anti-FP5 Fab, the average values were 31.3 ± 6.4 pA, 0.66 ± 0.13 pC, and 34.3 ± 5.8 pA/pC, respectively. C, Superimposition of two amperometric spikes, from a control cell (note small foot preceding a very fast spike) versus an anti-CAPS IgG-treated cell (sustained flickering foot), to illustrate the kinetic differences.
Fig. 6.
Effect of CAPS antagonism on the kinetics of individual spikes: rise time (RT) and half-width (HW) analyses. Time course of quantal catecholamine release in the absence (A) and presence (B) of anti-CAPS IgG. A,a1, a2, Histograms of the rise time and half-width of spikes obtained in the same control cells as depicted in Figure 5A. The average RT is 0.98 msec, and the HW is 5.3 msec. a3, Representative spike on fast time base, selected from Figure 5A (a3, *) is used to illustrate how the RT and HW are generated. RT is the period from 50 to 90% of the maximum spike amplitude [RT = T(90%) − T(50%)]. HW is the time corresponding to the width of the amperometric spike at 50% of its maximum amplitude. The charge (Q = ∫idt) is the integral of the amperometric current corresponding to the gray area between the baseline (dashed line) and the amperometric spike. From n = 10 control cells, the average values for RT and HW were 1.18 ± 0.08 and 6.43 ± 0.29 msec, respectively. B, Histograms of the RT (b1) and HW (b2) of spikes obtained in the same anti-CAPS IgG-treated cells as depicted in Figure5B. The average value for RT is 4.3 msec and for HW is 22 msec, both approximately four times longer than control.b3, A typical amperometric spike, selected from Figure5B (b3, *) to explain, as in Figure6A, how the analysis has been performed. Fromn = 15 antibody-treated cells, the average values for RT and HW were 4.23 ± 0.39 and 21 ± 1.6 msec, respectively. From n = 7 cells treated with anti-FP5 Fab, the average values were 4.2 ± 0.3 and 22.1 ± 1.5 msec, respectively.
The fact that spike amplitudes were small even for quite large charge events is reflective of the fact that most spikes were broader in anti-CAPS IgG-treated cells, as is readily apparent in the representative examples shown in Figures 5B, b3, and 6C. This was quantitated by measuring the rise time and half-width of spikes in control versus fully anti-CAPS-inhibited cells. In control cells the mean rise time and half-width were 1.18 ± 0.08 msec and 6.43 ± 0.29 msec (n = 10), respectively (Fig. 6A, a1, a2). These values are quite comparable with those found previously in adult bovine AC cells stimulated with high [K+] (Pihel et al., 1996). Anti-CAPS slowed the rise time and prolonged the half-width to values of 4.3 and 22 msec (n = 15), respectively (Fig.6B, b1, b2). In summary, these kinetic results indicate that the residual quantal events seen in CAPS-inhibited cells had different characteristics than those of control cells. Such kinetic alterations in spike morphology suggest that CAPS plays a role at the final stage of Ca2+-triggering secretion, possibly at the level of the fusion process itself (see Discussion).
CAPS antagonism reduces the exocytotic burst but does not affect the rate of rapid endocytosis
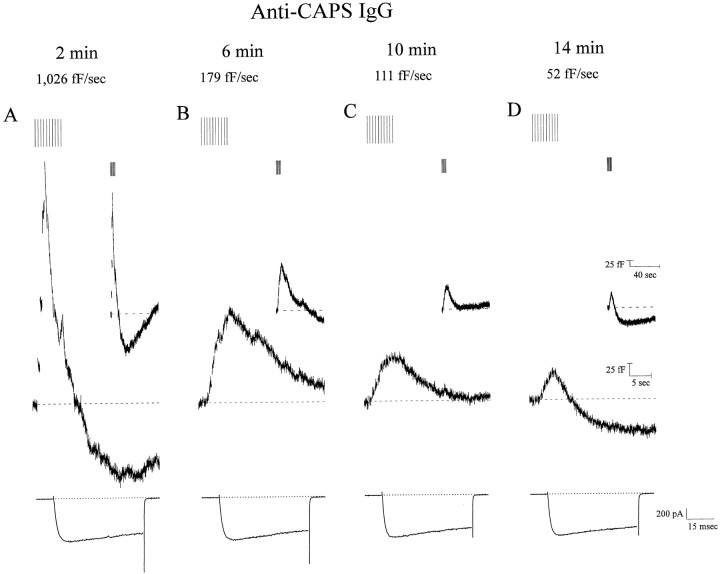
An alternative method of estimating secretion in single AC cells is that of membrane capacitance measurements. It is well established that secretion in AC cells is accompanied by an increase in membrane capacitance, and we have shown that this can be achieved through physiological stimulation of cells with short depolarizing stimuli (Artalejo et al., 1994, 1995). Apart from special circumstances, there is generally good agreement between the results obtained by capacitance and those simultaneously gathered by amperometry (Haller et al., 1998). In AC cells the initial exocytotic capacitance burst represents the release of already docked-and-primed granules because it can be elicited by a rise in Ca2+ in the absence of ATP (Parsons et al., 1995). If CAPS plays a postpriming role in exocytosis during the Ca2+-triggering step, then CAPS antagonism should slow the rate of the initial exocytotic burst. Indeed, introduction of anti-CAPS IgG (anti-FP5) into AC cells progressively slowed the maximal rate of exocytosis from 934.8 ± 29.7 fF/sec to 80.1 ± 4.1 fF/sec (n= 18) after 14 min (Fig. 7). It could be argued that repetitive stimulation in the presence of anti-CAPS IgGs had depleted the release-ready vesicular pool under these conditions and that the subsequent reduction in exocytotic rate might reflect an inhibitory effect of anti-CAPS IgGs on docking and/or priming of new DCVs. To control for this possibility, we introduced anti-CAPS IgGs into calf AC cells and waited 14 min before any stimulation. Under these conditions the initial capacitance burst was still inhibited 91 ± 1.4% (n = 12 cells) compared with control cells, suggesting that CAPS acts at a postpriming step in exocytosis.
Fig. 7.
CAPS plays a central role in exocytosis but is not involved in rapid endocytosis. Time course of CAPS antagonism on membrane capacitance measurements (Cm). Continuous recordings after formation of whole-cell configuration from a cell loaded with anti-CAPS IgG (anti-FP5, 1.5 mg/ml). Secretion was elicited by a train of depolarizations at the times indicated above the capacitance traces. The Ca current elicited by the first test depolarization is plotted below theCm trace. CAPS antagonism inhibited exocytosis without affecting endocytosis. A, Initial rates of exocytosis during the first exocytotic burst were not significantly different from control, reflecting the possibility that insufficient antibody has diffused into the cell at this time. The rate of exocytosis was obtained by measuring the steepest upward slope of theCm. Each panel shows records at a fast time base to reveal in detail the amplitude of the first fourCm jumps and therefore the rate of secretion during the first second of the train (“exocytotic burst”). During the four sequential trains of depolarization, the magnitude and rate of secretion were inhibited by 88 and 95%, respectively. Slower time base records (insets) show that rapid endocytosis is not significantly different from control.
We have demonstrated previously that membrane capacitance reproducibly declines back to baseline when stimulation is terminated in calf AC cells (Artalejo et al., 1995, 1996). We termed this membrane retrieval mechanism RE to distinguish it from other endocytotic processes in the cell (Artalejo et al., 1995). Quantitation of RE kinetics indicated that they remained resistant to CAPS immunoneutralization despite the large reduction in exocytotic rate (Fig. 7). In anti-CAPS IgG-treated cells, a τ value of 10.4 ± 0.9 sec (n = 18) for the fast-2 component of RE [τ of the exponential that fit the decline of Cm on termination of secretion (Artalejo et al., 1995)] was found, not significantly different from that in control cells with a similar magnitude of exocytosis (τ of 10.9 ± 0.6 sec; n= 25). The percentages of membrane retrieval were 101.6 ± 2.7 and 100.9 ± 1.1, respectively, for the same groups of cells. These results suggest that CAPS plays a central role in the last Ca2+-triggering step of exocytosis but is not involved in endocytosis in calf AC cells.
DISCUSSION
The exocytotic machinery is a complex assemblage of proteins whose coordinate function ensures correct targeting and fusion of secretory vesicles with the plasma membrane. A further layer of complexity is introduced in regulated secretion because of the requirement for a triggering step that usually requires a rise in intracellular Ca2+. Several proteins associated with SSVs and/or DCVs exhibit Ca2+ sensitivity, so there may not be a single “Ca2+sensor” for exocytosis but several factors at different steps of secretion requiring the presence of optimal [Ca2+]. Thus, although synaptotagmin I has been suggested as the primary Ca2+sensor for secretion at many nerve terminals (Sudhof, 1995), asynchronous secretion still occurs in the absence of this protein (Geppert et al., 1994). In this study we show that another putative Ca2+-binding protein associated with the secretory apparatus is essential for DCV exocytosis in calf AC cells. Introduction of anti-CAPS antibodies into these cells resulted in a dramatic decline in total catecholamine secretion as determined by both amperometric and capacitance recordings. To our knowledge, this is the first report of antibody effects on secretion visualized with the amperometric technique. The effects of anti-CAPS IgGs were specific and selective because neither Ca currents or APs nor RE, another Ca2+-dependent event in the calf AC cell secretory cycle (Artalejo et al., 1995, 1996), were modified, and preabsorption of anti-CAPS IgGs with their antigens abolished the inhibitory effect. In addition, it is unlikely that the antibodies inhibited secretion merely as a result of cross-linking DCVs because Fab fragments exerted an effect similar to that of intact IgGs. Moreover, we have also shown that introduction of IgGs to DCV surface proteins that are thought not to be involved directly in exocytosis (e.g., cytochrome b561) had no effect on secretion, suggesting that steric hindrance of DCV integration with the secretory machinery cannot explain the inhibitory effects of anti-CAPS IgGs.
Consideration of both in situ and in vitro data has led to the elaboration of a multistep model for exocytosis (Martin, 1997). In brief, vesicles are first recruited from a tethered pool to an anchored state at the plasma membrane. The subsequent docked stage, defined morphologically in AC cells as that fraction of DCVs in close apposition to the plasma membrane, may correspond biochemically to the formation of the SNARE [soluble N-ethylmaleimide-sensitive fusion (NSF) attachment protein (SNAP) receptor] complex. This is followed by a priming step, which is MgATP dependent and may involve NSF-dependent disassembly of the SNARE complex. Finally, docked-and-primed vesicles require only triggering by elevation of cytoplasmic Ca2+ to fuse with the plasma membrane. Previously, CAPS was found to play a role at the latter Ca2+-dependent triggering step in permeabilized PC12 cells, not at any antecedent step in this sequence (Hay and Martin, 1992; Ann et al., 1997; Tandon et al., 1998). Our results are consistent with a role for CAPS at a Ca2+-dependent triggering step in DCV secretion, rather than at an earlier vesicle recruitment, docking, or priming step, because of the effects of anti-CAPS IgGs on the shape of residual amperometric spikes and the rate of the initial exocytotic burst. If CAPS were playing a role at a step before fusion, such as recruitment or docking, then removing CAPS would be expected to affect only the total number of amperometric spikes observed (i.e., a reduced number of docked and primed vesicles) but not their unitary characteristics. Similarly, in capacitance experiments we would expect a decline in the magnitude of the initial exocytotic burst but not in its rate if CAPS were only involved in a step before Ca2+-dependent triggering and fusion. In fact, we found effects of anti-CAPS IgGs on both the shape of individual amperometric spikes and the rate of the capacitance burst.
Detailed analysis of amperometric recordings revealed that several parameters were altered by CAPS antibodies. It was clear that although anti-CAPS IgGs severely reduced the total number of amperometric spikes, some secretion remained, especially at high-frequency stimulation. Although the spared secretion seen at 7 Hz might suggest the existence of a CAPS-independent population of DCVs, this does not appear to be the case, because stimulation at 1 Hz in the presence of anti-CAPS IgGs did lead to a virtually complete block in secretion. Moreover, the remaining spikes in anti-CAPS-treated cells had substantially lower amplitudes and were much broader than spikes from untreated cells, suggesting that the antibodies did not “select” a minor population of spikes present under normal conditions. Altered spike parameters are thought to reflect the rate of diffusion of catecholamine from the DCV into the extracellular space, rather than any subsequent event (for review, see Travis and Wightman, 1998), although release events occurring far from the electrode tip could result in broad, low-amplitude spikes caused by diffusional effects (Schroeder et al., 1992). This explanation is unlikely to apply in our studies because the amperometric electrode was always close to the cell surface, and broad spikes like those seen in anti-CAPS IgG-treated cells were never seen in control recordings. Instances of spike-broadening of a much smaller magnitude were observed previously in pharmacological experiments on adult bovine AC cells and were attributed to an altered rate of catecholamine dissociation from the vesicular core (Jankowski et al., 1994; Wightman et al., 1995).
In the present case it is difficult to see how a cytoplasmic/peripheral DCV surface protein like CAPS could alter intravesicular chemistry to effect the much larger changes in spike shape seen here. A more attractive explanation is that spike broadening is caused by changes in fusion pore kinetics (Alvarez de Toledo, 1993). Fusion pores have been seen in various cells, including AC cells (Albillos et al., 1997), and exhibit a continuum of conductances (for review, see Monck and Fernandez, 1996). Moreover, fusion pore dilation has been reported to be Ca2+ dependent (Hartmann and Lindau, 1995), implying that a Ca2+ sensor is involved in the process. If CAPS plays a role in governing fusion pore opening, perhaps by stabilizing the DCV membrane interface, then its progressive immunoneutralization may gradually hinder pore dilation, leading to a slower and inefficient exit of catecholamines. Fusion pores can also flicker; thus spike broadening could involve repeated flickering to a constant pore diameter rather than reduction in size of an open pore (Rosenboom and Lindau, 1994). As with a narrower fusion pore diameter, release of DCV contents is thought to be slower through a flickering pore (Alvarez de Toledo et al., 1993). Further studies of fusion pore kinetics in calf AC cells may help to resolve this problem.
Corroborative evidence that CAPS is involved at a late step in DCV exocytosis but is not involved in the vesicle recovery process known as RE became apparent in capacitance measurements. Several studies have shown that secretion from AC cells is characterized by an early fast rise in Cm followed by a slower more sustained phase (Parsons et al., 1995). The early phase is resistant to ATP removal and hence may represent docked and primed DCVs that simply require an elevation in Ca2+ to fuse with the membrane and might correspond to the Ca-dependent but ATP-independent secretion seen in permeabilized cells. In the present experiments, anti-CAPS IgGs dramatically reduced the rate of the initial exocytotic burst, consistent with a role for CAPS at the final Ca2+-triggering step. Because it is difficult to determine in these experiments whether CAPS affects both priming and triggering, we conducted experiments in which no stimulation was conducted during antibody loading in an attempt to preserve the docked and primed vesicle pool. The reduction in exocytotic release rate after 14 min was similar to that seen in cells that underwent previous stimulation, again suggesting that CAPS exerts an effect at the triggering rather than at the vesicle recruitment stage. There is increasing evidence that secretion from AC cell DCVs can occur by a “kiss-and-run” mechanism where full incorporation of the vesicle membrane into the plasma membrane does not take place. After fusion and release of catecholamine, the “empty” vesicle is retracted intact by a dynamin-dependent rapid endocytotic mechanism (Albillos et al., 1997; Artalejo et al., 1998). Within this scenario it is likely that CAPS function is linked to the fusion but not the fission process, because CAPS neutralization had no effect on the kinetics of RE.
The precise mechanism of CAPS action is still unknown. CAPS has several domains with weak homology to other proteins (Ann et al., 1997), but it is unclear which domains of CAPS are essential for its function. CAPS binds Ca2+ with low affinity (Ann et al., 1997), and the protein also binds to PtdIns(4,5)P2, which has been implicated in secretory events in several systems (Loyet et al., 1998). In permeabilized PC12 cells, the production of PtdIns(4,5)P2 seems to be essential at the priming step of exocytosis because two proteins involved at this step are PtdIns4P 5-kinase and PtdIns transfer protein, which together may synthesize vesicular PtdIns(4,5)P2 (Martin, 1997). The conformational change brought about by PtdIns(4,5)P2 binding may permit CAPS to penetrate the lipid bilayer and enable the protein to mediate increased contacts between the fusion partner membranes. Ca2+ appears to switch the phospholipid specificity of CAPS: it binds to PtdIns(4,5)P2 in the absence of Ca2+, but in the range of 10–100 μm Ca2+, it exhibits a preference for phosphatidylcholine/phosphatidylethanolamine-containing liposomes (Loyet et al., 1998). Ca2+influx may trigger the fusion process at least in part by changing the phospholipid-binding properties of CAPS. CAPS has a pleckstrin homology domain that has a preference for binding to PtdIns(4,5)P2, and this may well be the locus of interaction of the protein with the phospholipid bilayer (Loyet et al., 1998). However, it is likely that there are other protein–protein interactions that define the binding specificity of CAPS with some precision. If the CAPS dimer interacts with both the DCV surface and the inner face of the plasmalemma, the association between the two membranes may be stabilized; this may act as an essential prelude to the formation of the fusion pore through which catecholamines escape from the vesicle. A central question that remains is to what extent CAPS represents a crucial divalent cation receptor for secretion from DCVs. Other divalent cation binding proteins such as synaptotagmin are present in chromaffin cell DCVs (Trifaro et al., 1989). The Ca2+-dependent actin-severing protein scinderin is also thought to play an important role in catecholamine secretion (Rodriguez Del Castillo et al., 1990). It may be that, as with SSVs, several Ca2+binding proteins must be activated simultaneously to effect optimal secretion. Further studies similar to those described here will be needed to define the relative importance of these different species in secretion by DCVs from intact AC cells.
Footnotes
This work was supported by Public Health Service Grants NIH-DK46928 (C.R.A.), NSF-IBN-9604849 (C.R.A.), DGICYT P.M.95–0035 (C.R.A.), NIH-DK40428 (T.F.J.M.), and NIH-DK25861 (T.F.J.M.). We thank Drs. Clive Palfrey and Rodrigo Andrade for helpful criticism of this manuscript. We also thank Dr. Bruce Porter for the production and purification of the antibodies.
Correspondence should be addressed to Cristina R. Artalejo, Department of Pharmacology, Wayne State University, School of Medicine, 540 E. Canfield Avenue, Detroit, MI 48201.
REFERENCES
- 1.Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez de Toledo G, Fernandez-Chacon R, Fernandez JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 3.Ann K, Kowalchyk JA, Loyet KM, Martin TFJ. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J Biol Chem. 1997;272:19637–19640. doi: 10.1074/jbc.272.32.19637. [DOI] [PubMed] [Google Scholar]
- 4.Artalejo CR, Dahmer MK, Perlman RL, Fox AP. Facilitation of Ca current in bovine chromaffin cells is due to recruitment of a second type of whole-cell current with novel properties. J Physiol (Lond) 1991;432:681–707. doi: 10.1113/jphysiol.1991.sp018406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artalejo CR, Adams ME, Fox AP. Three types of Ca channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- 6.Artalejo CR, Henley J, McNiven M, Palfrey HC. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP and dynamin but not clathrin. Proc Natl Acad Sci USA. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- 8.Artalejo CR, Elhamdani A, Palfrey HC. Dense-core vesicles can kiss-and-run too. Curr Biol. 1998;8:R62–R65. doi: 10.1016/s0960-9822(98)70036-3. [DOI] [PubMed] [Google Scholar]
- 9.Avery L, Bargmann CI, Horwitz HR. The C. elegans unc-31 gene affects multiple nervous system controlled functions. Genetics. 1993;134:455–464. doi: 10.1093/genetics/134.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berwin B, Floor E, Martin TFJ. CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron. 1998;21:137–145. doi: 10.1016/s0896-6273(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 11.Bittner MA, Holz RW. Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J Biol Chem. 1992;267:16219–16225. [PubMed] [Google Scholar]
- 12.Chow R, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 13.Elhamdani A, Zhou Z, Artalejo CR. Timing of dense-core vesicle exocytosis depends on the facilitation of L-type Ca channel in adrenal chromaffin cells. J Neurosci. 1998;18:6230–6240. doi: 10.1523/JNEUROSCI.18-16-06230.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 15.Haller M, Heinemann C, Chow RH, Heidelberger R, Neher E. Comparison of secretory responses as measured by membrane capacitance and by amperometry. Biophys J. 1998;74:2100–2113. doi: 10.1016/S0006-3495(98)77917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann J, Lindau M. A novel Ca2+-dependent step in exocytosis subsequent to vesicle fusion. FEBS Lett. 1995;363:217–220. doi: 10.1016/0014-5793(95)00318-4. [DOI] [PubMed] [Google Scholar]
- 17.Hay JC, Martin TFJ. Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic proteins. J Cell Biol. 1992;119:139–151. doi: 10.1083/jcb.119.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hay JC, Martin TFJ. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- 19.Jankowski JA, Finnegan JM, Wightman RM. Extracellular ionic composition alters kinetics of vesicular release of catecholamines and quantal size during exocytosis at adrenal medullary cells. J Neurochem. 1994;63:1739–1747. doi: 10.1046/j.1471-4159.1994.63051739.x. [DOI] [PubMed] [Google Scholar]
- 20.Klingauf J, Neher E. Modeling buffered Ca2+ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loyet KM, Kowalchyk JA, Chaudhary A, Chen J, Prestwich GD, Martin TFJ. Specific binding of phosphatidylinositol 4,5-bisphosphate to calcium-dependent activator protein for secretion (CAPS), a potential phosphoinositide effector protein for regulated exocytosis. J Biol Chem. 1998;273:8337–8343. doi: 10.1074/jbc.273.14.8337. [DOI] [PubMed] [Google Scholar]
- 22.Martin TFJ. Stages of regulated exocytosis. Trends Cell Biol. 1997;7:271–276. doi: 10.1016/S0962-8924(97)01060-X. [DOI] [PubMed] [Google Scholar]
- 23.Monck JR, Fernandez JM. The fusion pore and mechanisms of biological membrane fusion. Curr Opin Cell Biol. 1996;8:524–533. doi: 10.1016/s0955-0674(96)80031-7. [DOI] [PubMed] [Google Scholar]
- 24.Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons TD, Coorssen JR, Horstmann H, Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 26.Pihel K, Travis ER, Borges R, Wightman RM. Exocytotic release from individual granules exhibits similar properties at mast and chromaffin cells. Biophys J. 1996;71:1633–1640. doi: 10.1016/S0006-3495(96)79368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez Del Castillo A, Lemaire S, Tchakarov L, Jeyapragasan M, Doucet JP, Vitale ML, Trifaro JM. Chromaffin cell scinderin, a novel calcium-dependent actin filament-severing protein. EMBO J. 1990;9:43–52. doi: 10.1002/j.1460-2075.1990.tb08078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenboom H, Lindau M. Exo-endocytosis and closing of the fission pore during endocytosis in single pituitary nerve terminals. Proc Natl Acad Sci USA. 1994;91:5267–5271. doi: 10.1073/pnas.91.12.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroeder TJ, Jankowski JA, Kawagoe KT, Wightman RM, Lefrou C, Amatore C. Analysis of diffusional broadening of vesicular packets of catecholamines released from biological cells during exocytosis. Anal Chem. 1992;64:3077–3083. doi: 10.1021/ac00048a003. [DOI] [PubMed] [Google Scholar]
- 30.Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 31.Tandon A, Bannykh S, Kowalchyk JA, Banerjee A, Martin TFJ, Balch WE. Differential regulation of exocytosis by calcium and CAPS in semi-intact synaptosomes. Neuron. 1998;21:147–154. doi: 10.1016/s0896-6273(00)80522-x. [DOI] [PubMed] [Google Scholar]
- 32.Travis ER, Wightman RM. Spatio-temporal resolution of exocytosis from individual cells. Annu Rev Biomol Struct. 1998;27:77–103. doi: 10.1146/annurev.biophys.27.1.77. [DOI] [PubMed] [Google Scholar]
- 33.Trifaro JM, Fournier S, Novas ML. The p65 protein is a calmodulin-binding protein present in several types of secretory vesicles. Neuroscience. 1989;29:1–8. doi: 10.1016/0306-4522(89)90327-8. [DOI] [PubMed] [Google Scholar]
- 34.Walent JH, Porter BW, Martin TFJ. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- 35.Wightman RM, Jankowsky JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wightman RM, Schroeder TJ, Finnegan JM, Ciolkowski EL, Pihel K. Time course of release of catecholamines from individual vesicles during exocytosis at adrenal medullary cells. Biophys J. 1995;68:383–390. doi: 10.1016/S0006-3495(95)80199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]