Abstract
Approximately 28% of dorsal horn neurons (DHNs) in lamina V of the rat spinal cord generate voltage-dependent plateau potentials underlying accelerating discharges and prolonged afterdischarges in response to steady current pulses or stimulation of nociceptive primary afferent fibers. Using intracellular recordings in a transverse slice preparation of the cervical spinal cord, we have analyzed the ionic mechanisms involved in the generation and maintenance of plateau potentials in lamina V DHNs. Both the accelerating discharges and afterdischarges were reversibly blocked by Mn2+ and enhanced when Ca2+ was substituted with Ba2+. The underlying tetrodotoxin-resistant regenerative depolarization was sensitive to dihydropyridines, being blocked by nifedipine and enhanced by Bay K 8644. Substitution of extracellular Na+ withN-methyl-d-glucamine or choline strongly decreased the duration of the plateau potential. Loading the neurons with the calcium chelator BAPTA did not change the initial response but clearly decreased the maximum firing frequency and the duration of the afterdischarge. A similar effect was obtained with flufenamate, a specific blocker of the calcium-activated nonspecific cation current (ICAN). We conclude that the plateau potential of deep DHNs is supported by both Ca2+ influx through intermediate-threshold voltage-gated calcium channels of the L-type and by subsequent activation of a can current. Ca2+ influx during the plateau is potentially of importance for pain integration and the associated sensitization in spinal cord.
Keywords: dorsal horn neurons, plateau potentials, bistability, afterdischarge, nociceptive integration, dihydropyridine-sensitive intermediate voltage-activated Ca2+ current, CAN current, slice–intracellular technique
One correlate of central sensitization to pain is an increased background activity of spinal nociceptive neurons and the production of long-lasting afterdischarges.In vivo, afterdischarges are generated by deep dorsal horn neurons (DHNs) in response to nociceptive primary afferent inputs (Woolf and King, 1987; De Koninck and Henry, 1991). Their expression in response to peripheral stimulation is exaggerated in experimental models of persistent pain (Palecek et al., 1992; Laird and Bennett, 1993; Sotgiu et al., 1995; Grubb et al., 1996) and has been related to intense and prolonged behavioral responses to noxious stimuli (Laird and Bennett, 1993; Asada et al., 1996). These prolonged afterdischarges, apparently determinants for the perception of pain, are mediated in part by long-lasting excitatory synaptic potentials elicited in DHNs via the activation of neurokinin or amino acid receptors (Urban and Randic, 1984; Yoshimura and Jessell, 1990; De Koninck and Henry, 1991; Gerber et al., 1991; Nagy et al., 1993;Yoshimura et al., 1993).
In addition to synaptic components, however, intrinsic regenerative membrane properties of DHNs contribute significantly to the long-lasting nociceptive responses (Morisset and Nagy, 1996, 1998;Russo and Hounsgaard, 1996). We reported previously that ∼28% of deep dorsal horn neurons in the rat spinal cord exhibited, in vitro, voltage-dependent plateau potentials positively modulated by the activation of metabotropic glutamate receptors (Morisset and Nagy, 1998). Expression of these regenerative depolarizations can profoundly alter the output properties of deep DHNs in response to sensory inputs. Nociceptive primary afferent stimulation elicited intense and prolonged responses in plateau-generating DHNs, whereas brief bursts of spikes were evoked in the absence of regenerative potential. Because plateau potentials had slow activation kinetics and were voltage-dependent, plateau-generating neurons presented nonlinear input–output relationships in both the amplitude and time domains. Together, these results suggested that the ability of deep DHNs to generate plateau potentials might be crucial for the perception of pain in vivo. They also suggested that limiting the expression of regenerative membrane properties of DHNs is potentially of clinical interest as an alternative way of controlling pain-related central hyperexcitability. A prerequisite, however, is a reasonable knowledge of the ionic basis for these properties. Plateau potentials of deep DHNs were shown to depend on calcium (Morisset and Nagy, 1996), but the underlying conductances of the plateau were not precisely known in the rat.
Using a slice preparation from the cervical region of the rat spinal cord, we have analyzed in the present paper the membrane conductances involved in the generation and the maintenance of plateau potentials in lamina V DHNs. We show that the plateau potential is carried by both Ca2+ influx through voltage-gated calcium channels (VGCC) of the L-type and subsequent activation of a calcium-activated nonspecific cation current.
MATERIALS AND METHODS
The methods were described previously (Morisset and Nagy, 1996,1998). Wistar rats of both sexes, aged from 17 to 26 d, were anesthetized with ether and decapitated. The excised cervical spinal cord was sliced transversally (400 μm sections) in the region C6–C8 using a vibratome (Campden Instruments Ltd, Leics, UK). The slices were transferred into the recording chamber on a layer of optical paper (interface-type chamber) on which they were perfused from below at a rate of 0.5 ml/mn with a Krebs’ solution containing (in mm): 124.0 NaCl, 2.4 KCl, 2.4 CaCl2, 1.3 Mg SO4, 1.2 KH2PO4, 26.0 NaHCO3, 1.25 HEPES, and 10.0 glucose. The solution, maintained at a temperature of 30°C, was oxygenated with 95% O2–5% CO2, pH 7.4. Recording began after 2 hr of equilibration.
Either a sharp electrode filled with 1% biocytin (Sigma, St. Quentin Fallavier, France) in 1 m K-acetate (tip resistance of 160–200 MΩ) or a patch electrode (tip resistance of 9–12 MΩ) was placed under visual control into the deep dorsal horn (lamina V of Rexed). The internal solution of patch pipettes had the following composition (in mm): 120.0 K-gluconate, 20.0 KCl, 0.1 CaCl2, 1.3 Mg Cl2, 1.0 EGTA, 10.0 HEPES, 0.1 GTP, 0.2 cAMP, 0.1 leupeptin, 3.0 Na2-ATP, and 77.0d-Mannitol, pH 7.3 (308 mOsm; 8 mOsm hyperosmotic to extracellular Krebs’ solution). Signals were recorded with an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA) and displayed on an oscilloscope (DSO 630; Gould, Ilford, Essex, UK) and a chart recorder (TA11; Gould). Acquisition and analysis were conducted with a Digidata 1200 system and the pClamp 6 software (Axon Instruments) connected to a 486 IBM-compatible computer. Current injection was controlled by the Digidata 1200 system or by a stimulator (Master 8; AMPI, Jerusalem, Israel). Current was injected into the neurons through the same electrode via a bridge circuit in the amplifier. Bridge balance was monitored throughout experiments. Subsequent data analysis was performed with the pClamp 6 software (Axon Instruments), Excel 5.0 (Microsoft, Seattle, WA) and SigmaPlot 4.16 (SPSS Inc., Chicago, IL). During electrophysiological recordings with sharp electrodes, neurons were filled with biocytin for subsequent morphological characterization. The morphological characteristics and the types of sensory input integrated by the plateau-generating deep dorsal horn cells have been presented previously (Morisset and Nagy, 1998). Mean resting membrane potential and neuronal input resistance were calculated for a subset of the recorded neurons (resting membrane potential, −57.7 ± 1.2 mV; input resistance, 98.7 ± 6.5 MΩ; mean ± SD; n = 30).
When needed, the following drugs were added to the normal Krebs’ solution and continuously superfused on the preparation: 1S,3R-1-amino-1,3-cyclopentanedicarboxylic acid (1S,3R-ACPD), (±)-2-amino-5-phosphonopentanoic acid (AP-5), apamin, bicuculline, and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were from Research Biochemicals (Natick, MA); BAPTA-AM was from Calbiochem (La Jolla, CA); cAMP, Na2-ATP, Bay-K 8644, choline chloride, EGTA, flufenamic acid (FFA), GTP, HEPES, leupeptin, nifedipine,N-methyl-d-glucamine (NMDG), strychnine, and tetrodotoxin (TTX) were from Sigma. Nifedipine and FFA were freshly dissolved in dimethylsulfoxide (DMSO) for each experiment. Care was taken to protect nifedipine from light. DMSO had no effects per se at the concentration used (0.1 and 0.2%, respectively). Low-sodium saline consisted of normal perfusion medium in which 124.0 mm NaCl was replaced with 124.0 mm NMDG or 124.0 mm choline chloride. Mn2+ was added to a modified perfusion medium containing (in mm): 124.0 NaCl, 3.6 KCl, 2.4 CaCl2, 1.3 Mg Cl2, 26.0 NaHCO3, 1.25 HEPES, and 10.0 glucose. Ba2+ saline consisted of the same modified medium in which 2.4 CaCl2 was substituted with equimolar BaCl2. When needed in the presence of a drug, bias current was injected to keep same holding potential or to reach same initial firing frequency as in control. Data presented in all the figures were obtained in the presence of a mixture of 50 μm AP-5, 20 μm CNQX, 20 μm bicuculline, and 50 μm strychnine to block NMDA receptors and most of the fast excitatory and inhibitory synaptic transmission. In Figures2 and 4-6, plateau potentials were induced in the presence of the metabotropic glutamate receptor agonist 1S,3R-ACPD (Morisset and Nagy, 1996,1998). Recordings were obtained with patch pipettes (whole-cell configuration) in Figure 6 and with sharp electrodes in all other figures.
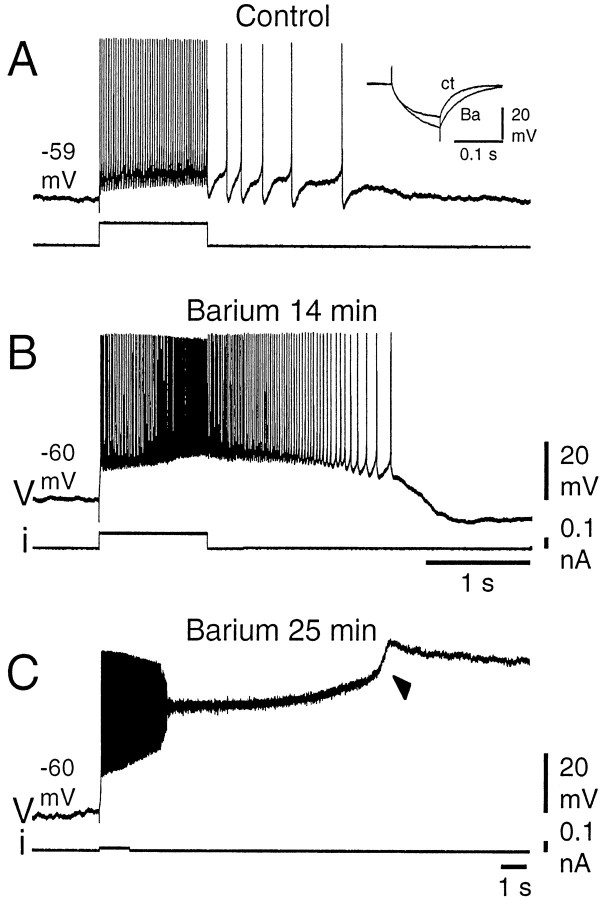
Fig. 2.
Plateau-potential amplification in the presence of barium. A, In control conditions, a DHN responded to a 1 sec depolarizing current pulse with a discharge of moderate acceleration, followed by a weak afterdischarge. B, After 14 min in the barium solution (Ca2+substituted with equimolar Ba2+), the latter were substantially increased. Barium also caused an increase in the neuron input resistance (A, inset).C, After a more prolonged exposure to the barium solution (25 min), the amplitude of the plateau potential was enhanced, leading to inactivation of action potentials, and a long-lasting afterdepolarization was produced. Note after 4.5 sec a further depolarizing step in the plateau potential (arrowhead). Recordings were made in the presence of the mGluR agonist 1S,3R-ACPD (25 μm).
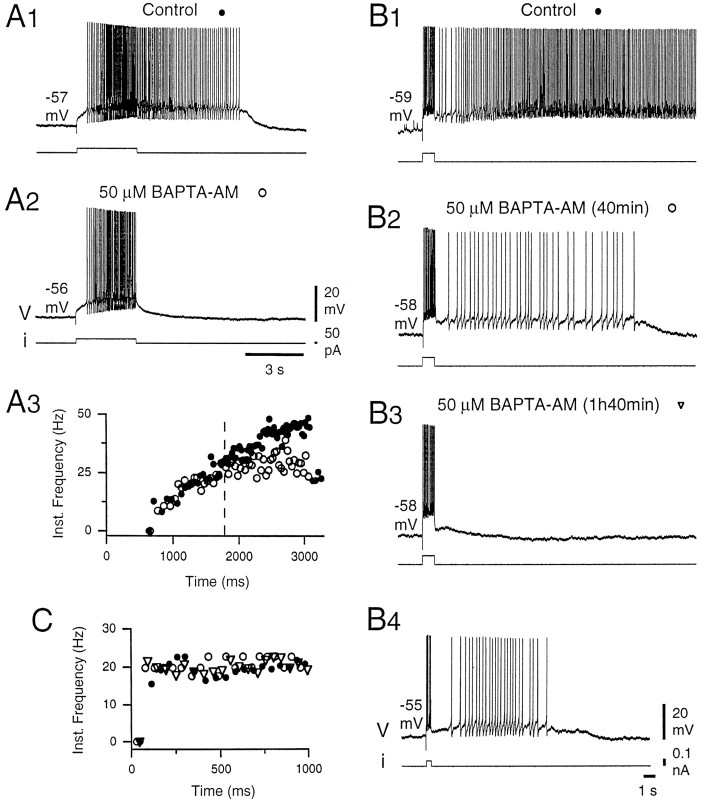
Fig. 4.
Chelating intracellular calcium decreased the late phase of plateau potentials. A, In control conditions, the neuron responded to a 3 sec pulse of depolarizing current with an accelerating discharge, followed by an afterdischarge (A1, A3, filled circles). Application of the calcium chelator BAPTA-AM (50 μm) did not change the initial response of the neuron (A3, open circles) but interrupted the acceleration of discharge after 1.5 sec (A3,dashed line) and abolished the afterdischarge (A2). A3, Instantaneous frequency plot calculated as in Figure 1 for the discharges recorded inA1 and A2. B, In control conditions, a long-lasting and intense plateau potential was triggered by a short current pulse (1 sec) in another DHN (B1). Note the slow increase in firing frequency during the afterdischarge. In the presence of BAPTA-AM, the duration and intensity of the afterdischarge gradually decreased (B2, 40 min), until it disappeared (B3, 1 hr 40 min). Conversely, the firing frequency during the 1 sec stimulation did not change (C, instantaneous frequency plot calculated forB1–B3). Under the same conditions, a weak afterdischarge could be triggered by a shorter pulse of current (B4). All the recordings were obtained in the presence of the mGluR agonist 1S,3R-ACPD (25 μm).A, B, Different neurons.
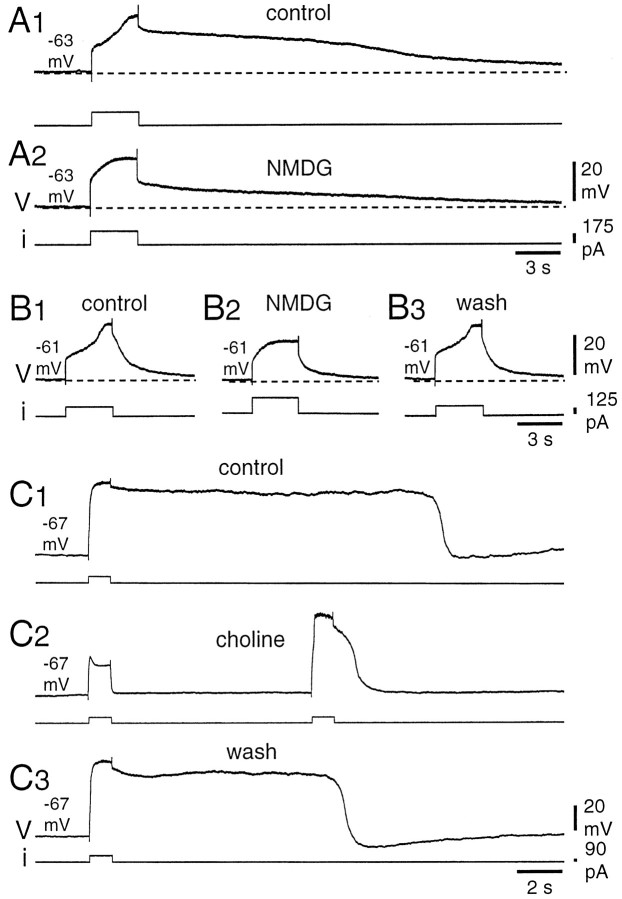
Fig. 5.
Sodium substitution decreased the amplitude and duration of TTX-resistant plateau potentials. All the recordings were obtained in the presence of 1 μm TTX and 50 μm 1S,3R-ACPD.A, When most of the Na+ was replaced by NMDG in the perfusion medium, the amplitude of both the late depolarizing phase during the stimulation and the afterdepolarization were reduced (A2) compared with control conditions (A1). In neurons producing short plateaus (B1), the NMDG medium reduced the late phase of the regenerative depolarization (B2). The reduction was reversible (B3). C, Similar reversible effects were obtained when most of the Na+ was replaced by choline in the bath. In the choline medium, note the difference between a subthreshold (C2, first stimulation) and a suprathreshold response (second stimulation).
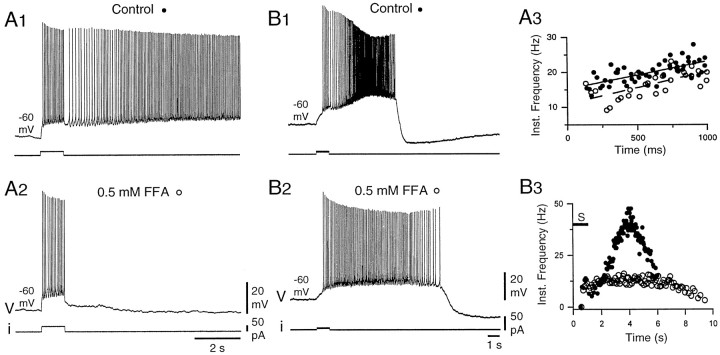
Fig. 6.
The ICANblocker FFA blocked the late phase of plateau potentials.A, In control conditions, a full plateau potential supported a prolonged afterdischarge. Again, note the progressive firing acceleration after termination of the 1 sec stimulation. The afterdischarge was suppressed in the presence of 0.5 mm FFA (A2), although the firing pattern during the stimulation was not affected (A3). A3, The instantaneous frequency plot was calculated as in Figure 1 for two discharges in control conditions (filled circles) and for two discharges in the presence of FFA (open circles). Lines are linear regressions through the data points (solid line, control; dashed line, FFA) (correlation coefficients, 0.68 in both cases; see Results). B, In another DHN for which the plateau quickly repolarized (B1), application of FFA strongly reduced the maximum firing frequency and prolonged the duration of the afterdischarge (B2, B3,open circles) (see Results). B3, The instantaneous frequency plots were calculated for the discharges inB1 and B2 (S, 1 sec depolarizing pulse). All recordings were obtained in the presence of 25 μm 1S,3R-ACPD.
RESULTS
Nifedipine-sensitive Ca2+ component of the plateau potential
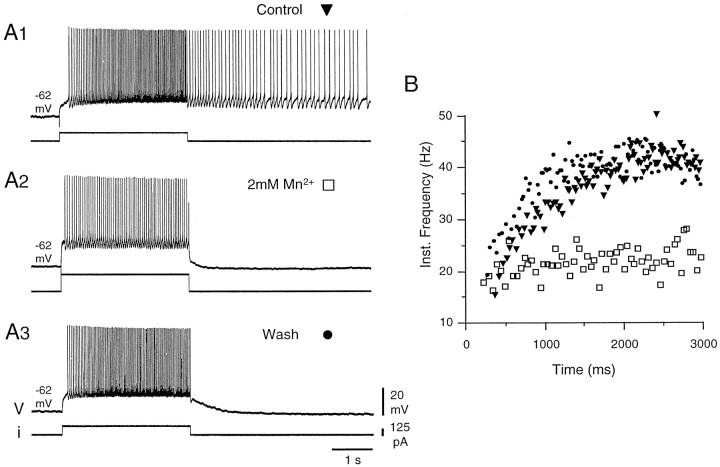
In plateau-generating DHNs, both the acceleration of firing during the injection of a pulse of depolarizing current (Fig.1A1,B,filled triangles) and the afterdischarge were suppressed in the presence of 2 mmMn2+, which is known to block voltage-activated calcium currents (Fig.1A2,B, open squares) (n = 3). Note that the intensity of the current pulse was adjusted to elicit the same initial firing frequency as in the control (mean firing frequency of 18.6 and 19.3 Hz, respectively, during the first 100 msec of the discharge). Nevertheless, the subsequent evolution of the discharges was radically different (mean firing frequency during the last 100 msec of the discharge of 40.9 Hz in control and 20.6 Hz in the presence of Mn2+). The block by Mn2+ was reversible (Fig.1A3, 1B, filled circles). Conversely, plateau potentials were enhanced when Ca2+ was substituted with Ba2+ in the bathing medium (four of four DHNs). Barium is a better charge carrier through VGCCs, does not activate Ca2+-dependent K+ conductances, and subsequently blocks most of the potassium channels from the inner face of the membrane. The effects of the substitution are illustrated in Figure2 for a neuron that expressed moderate acceleration of firing and reduced afterdischarge in response to a pulse of depolarizing current in control conditions (Fig.2A). After 14 min in the presence of barium (Fig.2B), the firing frequency during and after the stimulation was much higher (118.6 ± 9.7 Hz; n = 3 stimulations during the last 100 msec of the stimulation in Ba2+; 46.7 ± 1.4 Hz,n = 3 in control), and the afterdischarge was longer. A longer exposure to the Ba2+ solution further increased the amplitude and duration of the plateau potentials, leading to spike inactivation (Fig. 2C). These enhanced plateau potentials obtained in the presence of Ba2+ could still be repolarized by the injection of hyperpolarizing current pulses (data not shown).
Fig. 1.
Plateau potentials were sensitive to blockers of voltage-gated calcium channels. In control conditions, a DHN responded to a 3 sec pulse of depolarizing current with an accelerating discharge and a prolonged afterdischarge (A1, B, filled triangles). In the presence of 2 mm Mn2+, the discharge was tonic during the stimulation, and no afterdischarge was observed (A2, B, open squares). This effect was reversible (A3,B, filled circles). B, Instantaneous frequency plot calculated for the discharges recorded inA during the 3 sec of the stimulation and expressed against time. Note the same initial firing frequency in all three cases. For that, the intensity of the current pulse was adjusted inA2. In this and the following figures, most of the excitatory and inhibitory synaptic transmission was blocked by a mixture of 20 μm CNQX, 50 μm AP-5, 20 μm bicuculline, and 50 μm strychnine.
As shown previously (Morisset and Nagy, 1996), the Ca2+-dependent bistability was also maintained under 1 μm tetrodotoxin (n = 24), further indicating that the ability to produce plateau potentials is an endogenous membrane property of the deep DHNs. Under these conditions, a slow-rising plateau potential still developed during injection of square pulse of depolarizing current and was followed by a long-lasting afterdepolarization (Figs.3A1,control) that could be interrupted by a brief pulse of hyperpolarizing current (data not shown).
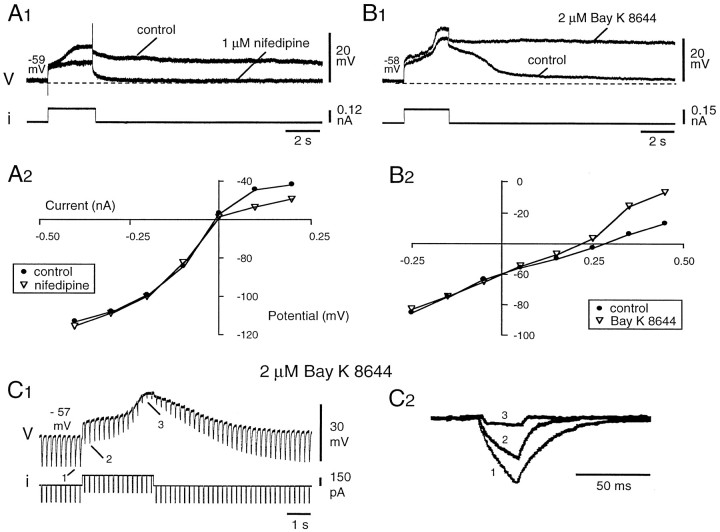
Fig. 3.
TTX-resistant plateau potentials were sensitive to dihydropyridines. A1, The plateau potential was blocked in the presence of 1 μm nifedipine (traces in control andnifedipine superimposed). A2, TheI–V plot obtained from another neuron (potential measured at the end of 800 msec hyperpolarizing and depolarizing current pulses injected from a holding potential of −56 mV) shows that the nifedipine-sensitive component of the plateau potential activated at approximately −55 mV. B1, The plateau potential was increased in both amplitude and duration in the presence of 2 μm Bay K 8644. B2, The I–Vplot obtained from the same neuron again shows that the threshold of the Bay K 8644-sensitive component was approximately −55 mV.C1, The neuron input resistance decreased during the development of the plateau potential and progressively recovered during the repolarization. C2, Voltage deflections in response to 25 msec hyperpolarizing pulses shown at larger time and amplitude scales before (1) and during (2,3) the depolarizing phase of the plateau (same records as in C1). All the recordings were obtained in the presence of 1 μm TTX. B, C, Same neuron. A1, A2, B, Different neurons.
TTX-resistant plateau potentials were also highly sensitive to dihydropyridines, which are known to modulate negatively (nifedipine) and positively (S-Bay K 8644) L-type calcium currents (Fox et al., 1987b). They were almost completely blocked by bath-applied nifedipine (1 μm, n = 2 of 2; 10 μm, n = 9 of 9) (Fig.3A1). Conversely, both the amplitude of the plateau during the stimulation and the duration of the afterdepolarization were enhanced by 2 μmS-Bay K 8644 (Fig.3B1) (n = 3 of 3). TheI–V plots obtained at the end of 3 sec depolarizing current pulses of increasing intensities in the presence of nifedipine (10 μm) (Fig. 3A2) orS-Bay K 8644 (Fig. 3B2) begin to differentiate from the control I–V plots at approximately −55 mV. The threshold for the dihydropyridine-sensitive current was therefore in agreement with the plateau-potential threshold measured in normal saline (−53.9 ± 1.3 mV; n = 6). Both the regenerative depolarization during the stimulation and the afterdepolarization were correlated with a strong decrease in membrane resistance (Fig. 3C), whereas the slow repolarizing phase after the plateau corresponded to a progressive recovery of the control value. The preceding results demonstrate that bistability in DHNs is supported by a nifedipine-sensitive Ca2+-dependent plateau potential. Accordingly, in the absence of TTX, both the acceleration of firing during injection of a depolarizing current pulse and the afterdischarge were suppressed in the presence of nifedipine (n = 3 of 3; data not shown). Together, our results indicate that L-type calcium channels are involved in the endogenous plateau properties of deep DHNs underlying accelerating discharges and prolonged afterdischarges.
Ca2+-dependent depolarizing component of the plateau potential
Because Ca2+ entry through VGCCs could subsequently activate calcium-dependent depolarizing conductances, such as a calcium-activated nonspecific cationic conductance (ICAN) (Swandulla and Lux, 1985; Partridge et al., 1994), we addressed the question of whether the calcium was in itself a charge carrier responsible for the plateau potential or whether the generation and maintenance of regenerative depolarizations were caused, in part or totally, by Ca2+-dependent conductances.
In a first series of experiments, we have examined the effect of loading plateau-generating neurons with a calcium chelator that prevents the activation of Ca2+-dependent conductances. Figure 4 shows that applying in the bath 50 μm of the membrane-permeable calcium chelator BAPTA-AM clearly decreased the expression of plateau potentials. A DHN that produced an accelerating discharge and an afterdischarge in response to a 3 sec depolarizing current pulse in control conditions (Fig.4A1) became unable to generate the afterdischarge, regardless of the membrane potential in the presence of BAPTA-AM (Fig.4A2). Interestingly, in the presence of the calcium chelator, the discharge was still accelerating during the stimulation. However, analysis of the instantaneous firing frequency (Fig.4A3, open circles) shows that, although initially similar to the control (Fig. 4A3,filled circles), the firing acceleration stopped after 1.5 sec (Fig. 4A3, dashed line), and the mean firing frequency during the last 100 msec of the stimulation was lower (28.0 Hz) than in control (44.8 Hz). Therefore, in the presence of BAPTA-AM, a regenerative depolarization was still observed, but a delayed depolarizing component of the response disappeared, preventing the afterdischarge. The fact that different components participate in the initial acceleration of firing and in the afterdischarge appears more clearly in Figure 4, B and C, in which another DHN was stimulated with shorter current pulses. In the control conditions (Fig. 4B1), the neuron produced an afterdischarge over >30 sec. After the end of the stimulus, the firing frequency raised gradually to peak after 10 sec at 14.4 Hz (mean frequency over 0.5 sec) yielding to a mean frequency of 10.8 Hz during the first 20 sec of the afterdischarge. After 40 min in the presence of 50 μm BAPTA-AM (Fig. 4B2), the afterdischarge produced in response to the same stimulation lasted for 14.9 sec with a much lower firing frequency (2.5 Hz). After 1 hr 40 min in the presence of the chelator, no afterdischarge was generated, regardless of the membrane potential (Fig. 4B3). Interestingly, in the three situations, the same firing pattern was produced during the stimulation (mildly accelerating discharge of ∼20 Hz) (Fig. 4C). Moreover, in the same conditions as in Figure4B3 (1 hr 40 min in BAPTA-AM), a shorter stimulation (0.35 sec) (Fig. 4B4) was able to elicit an afterdischarge. Most probably, during the brief stimulation, the cumulative spike-associated afterhyperpolarizing potential did not develop enough to counteract the calcium current-mediated depolarization. The afterdischarge, however, was shorter than 8 sec with a mean firing frequency of 3.4 Hz. Similar results were obtained in three of three neurons. Together, they indicate that the plateau potential in deep DHNs is supported by both an L-type Ca2+ current and a Ca2+-activated depolarizing current and that the calcium current by itself can only sustain a mild and relatively short afterdischarge, if any.
In a second series of experiments, we investigated further the type of calcium-dependent current involved by testing the possible implication of a calcium-activated nonspecific cationic current. For that purpose, plateau potentials were tested in mediums in which Na+ was substituted with NMDG or choline. The former substance is known to have very low permeability through thecan channels (Bal and McCormick, 1993; Wilson et al., 1996). The permeability of choline varies depending on the neuronal type (Partridge et al., 1994; Rekling and Feldman, 1997). These substitutions were performed in the presence of 1 μm TTX. Figure 5A2 shows that, when Na+was substituted with NMDG, the peak depolarization during the current injection, as well as the afterdepolarization after the stimulation, were clearly reduced in amplitude compared with the control (Fig.5A1). Figure 5B illustrates a similar effect for another type of plateau-generating DHN, producing only very short afterdepolarization in control. Again, the peak depolarization during the stimulation was reduced in the NMDG medium (Fig. 5B2), the reduction being reversible (Fig. 5B3). The effects of NMDG substitution were obtained in three of three neurons. Similar reduction of the plateau potential was also obtained in three of three other DHNs when Na+ was substituted with choline. In the example of Figure 5C, in control conditions (Fig. 5C1), the duration of the plateau potential was variable but always longer than 10 sec (n = 6 stimulations), whereas in the choline medium, it fell to 3.5 ± 0.2 sec (n = 6) when elicited from the same holding potential (Fig. 5C2). Again, the effect was reversible (Fig. 5C3). Note in Figure 5C2 the difference between a subthreshold (left) and a suprathreshold (right) stimulation. The residual regenerative depolarization may be attributable to either partial permeability of both NMDG and choline through can channels or calcium current through the L-type VGCC.
Participation of a can current in the plateau potential was further investigated by using FFA, a specific antagonist ofICAN (Shaw et al., 1995). Figure6 shows that the late phase of the plateau potential was strongly reduced in the presence of 0.5 mm FFA. In the example of Figure6A, the neuron responded to a 1 sec depolarizing current pulse with an afterdischarge longer than 20 sec (Fig.6A1). In the presence of FFA, no afterdischarge was obtained (Fig. 6A2), regardless of the holding potential or the stimulus intensity. Again, however, there was no clear difference in the type of firing pattern during the stimulation compared with control. As indicated by the slope of the regression lines in Figure 6A3, during the first second of firing, the acceleration was 8.0 Hz/sec in control and 9.4 Hz/sec in the presence of FFA (linear regression coefficients, 0.68 in both cases). The early and late phases of the plateau have, therefore, the same different sensitivity to FFA as to BAPTA (Fig.4A,B). In the case of the neuron in Figure 6B, the stimulus triggered a short plateau that lasted only for 5.5 sec and led to an intense firing during the afterdischarge (Fig. 6B1, 6B3,peak at 47.6 Hz, filled circles). In response to the same stimulation in the presence of 0.5 mmFFA (Fig. 6B2), the maximum firing frequency during the afterdischarge was much lower (16.1 Hz) (Fig.6B3, open circles), although the mean firing frequency during the first second of the discharge was similar to the control (9.6 and 9.9 Hz, respectively). Unexpectedly, the afterdischarge was prolonged in the presence of FFA. This was most probably caused by a weaker afterhyperpolarizing potential associated with the much lower depolarization during the plateau potential. Strong reduction of the plateau potential was obtained in four of four plateau-generating neurons.
In summary, our results demonstrate that the plateau potential in deep DHNs is supported by both an L-type Ca2+current and a calcium-activated nonspecific cationic current. The former is the major depolarizing component during the initial phase of the plateau, and the latter is necessary for the expression of long-lasting afterdischarges.
DISCUSSION
Expression of voltage-dependent plateau potentials by dorsal horn neurons in response to the stimulation of primary afferent fibers is of particular importance for the processing of nociceptive information in the spinal cord (Morisset and Nagy, 1996, 1998; Russo and Hounsgaard, 1996). In the present study, we analyzed the ionic basis for the intrinsic regenerative properties of the rat DHNs.
Generation and maintenance of the plateau potential
Plateau potentials, or more generally regenerative depolarizations outlasting the duration of a stimulus, have been described in a variety of neuronal types in both invertebrates (Golowasch and Marder, 1992;Kiehn and Harris-Warrick, 1992; Zhang and Harris-Warrick, 1995; Zhang et al., 1995; Angstadt and Choo, 1996; Wilson et al., 1996; Mills and Pitman, 1997) and vertebrates (Hounsgaard and Mintz, 1988; Kiehn, 1991;Fraser and MacVicar, 1996; Russo and Hounsgaard, 1996; Overton and Clark, 1997; Rekling and Feldman, 1997; Viana di Prisco et al., 1997;Hsiao et al., 1998; Sandler et al., 1998), and the mechanisms are diverse. The present data show that, in DHNs of the rat, the plateau potential is calcium-dependent, being both resistant to TTX and blocked by Mn2+. In addition, maximum firing frequency and duration of the afterdischarge supported by the plateau are strongly reduced when intracellular Ca2+ concentration is maintained low by the calcium chelator BAPTA, indicating that the plateau potential is supported by both a Ca2+ conductance and a Ca2+-dependent conductance. The calcium chelator was applied in the bath under its membrane-permeable form BAPTA-AM. Therefore, it could have also perturbed a spontaneous release of transmitters in the slice preparation, whereby inducing indirect modifications of the DHNs regenerative properties. However, our experiments conducted in a mixture of synaptic blockers eliminated the possibility of indirect effects via ionotropic neurotransmission. The indirect decrease of plateau properties via modifications in the release of neuromodulators is also unlikely because the initial part of the plateaus was almost unchanged in the presence of the chelator. Moreover, ionic substitutions and application of blockers confirmed the implication of a Ca2+-dependent conductance in the plateau potential of the deep DHNs.
Application of these substances indicated that the calcium-dependent conductance involved in the depolarizing phase of the plateau potential was a Ca2+-activated nonselective cation current (ICAN) (Partridge et al., 1994), which was shown in a variety of neurons to sustain burst discharge, afterdischarge, and plateau potentials, or oscillations (Swandulla and Lux, 1985; Zhang et al., 1995; Fraser and MacVicar, 1996; Wilson et al., 1996; Klink and Alonso, 1997; Viana di Prisco et al., 1997; Beurrier et al., 1999). In the rat DHNs, the amplitude and/or the duration of the plateau potential were strongly reduced when most of extracellular Na+ was replaced by impermeable molecules through the can channels (NMDG or choline), or during superfusion with the specificICAN blocker FFA. FFA, one member of a class of nonsteroidal anti-inflammatory drugs, was shown to affect two calcium-activated conductances in neurons of the snail Helix aspersa (Shaw et al., 1995). It induced a transient increase inICAN and in a calcium-activated chloride current, consecutive to a rise in intracellular calcium concentration; subsequently, it blocked the two calcium-activated conductances. To our knowledge, no calcium-activated chloride current was described in the DHNs, and, in any case, blockade of such a hyperpolarizing conductance by FFA would have prolonged plateau potentials. On the other hand, a CAN current was reported in a proportion of rat DHNs (Murase et al., 1989), and the clear and permanent decrease of the late phase of the plateaus in the presence of FFA, together with the effects of Ca2+chelation and Na+ substitutions, indicates that ICAN is actually involved in this late depolarizing phase of the plateau potentials. Dynamic interactions between ICAN and intermediate-to-high threshold Ca2+ currents were reported in other plateau-producing neurons (Zhang et al., 1995; Fraser and MacVicar, 1996). In the latter cases, blocking theICAN completely eliminated the plateau potentials. When ICAN was blocked in DHNs, however, the reduction only concerned the late phase of the plateau, leaving the early phase unchanged. Moreover, a weak afterdischarge can still show up in the presence of BAPTA. Therefore,ICAN was not the only depolarizing component of the regenerative plateau potential.
Our data indicate that the plateau potential in DHNs of the rat spinal cord was also carried by calcium influx through VGCC, which activated a few millivolt positive to resting membrane potential. This intermediate threshold calcium current was mediated probably by L-type calcium channels, being sensitive to dihydropyridines. Plateau potentials supported by similar calcium currents were described in DHNs of the turtle spinal cord (Russo and Hounsgaard, 1996) and various motoneurons (Hounsgaard and Mintz, 1988; Hsiao et al., 1998). In the latter cases, as well as in the rat DHNs (present paper), the involved L-type calcium current activated at a more negative membrane potential than for typical L-type currents (Fox et al., 1987a; Tsien et al., 1988). Dihydropyridine sensitivity was reported for a number of low- or intermediate-voltage-activated calcium currents, showing little or no inactivation (Marchetti et al., 1995; Avery and Johnston, 1996;Kavalali and Plummer, 1996). In DHNs of the rat spinal cord, voltage-clamp studies described modulation by dihydropyridines of a sustained Ca2+ current for membrane potentials positive to −50 mV (Huang, 1989; Ryu and Randic, 1990). Interestingly, both this intermediate-threshold Ca2+ current and anICAN were reported to be activated or enhanced by substance P in a proportion of the rat DHNs (Murase et al., 1989; Ryu and Randic, 1990), just like the plateau potential (Russo et al., 1997). A possibility remains thatICAN is activated by other sources of calcium. High-voltage-activated calcium currents were described in rat DHNs (Ryu and Randic, 1990), including one of the N-type (Huang, 1989). They appeared, however, insufficient to support plateau potentials, which were readily blocked in the presence of nifedipine. Another potential activator of ICAN is the calcium released from the intracellular store (Zhang et al., 1995). In any case, it would have to be via a Ca2+-induced Ca2+ release (for review, see Simpson et al., 1995) consecutive to the activation of L-type VGCC. These additional possibilities require further studies.
Firing acceleration, maximum firing frequency, and duration of the afterdischarge are rather variable depending on the neuron (Russo and Hounsgaard, 1996; Morisset and Nagy, 1998). This variability is such that it often prevents normalized quantification of the effects of channel blockers (Figs. 5, compare A, B; 6, compare A, B). This indicates that, in the balance of the intrinsic conductances, the relative weight of L-typeICa and ICAN, and as stressed for turtle DHNs (Russo and Hounsgaard, 1996) of the Ca2+-dependent K+ current, is probably set differently in different neurons.
In summary, in DHNs of the rat spinal cord, an L-type calcium current is the principal depolarizing component during the early phase of the plateau potential, ensuring the initial regenerative depolarization and firing acceleration. It subsequently triggers and maintains a CAN current, responsible for the high-frequency firing, and expression of prolonged afterdischarge. Regenerative properties of the rat DHNs, therefore, appear more complex than those described for the same class of spinal neurons and for motoneurons in the turtle in which the plateau potential is supported essentially by a noninactivating L-type current (Hounsgaard and Mintz, 1988; Russo and Hounsgaard, 1996).
Plateau potential-mediated Ca2+ influx and pain processing in spinal cord
As reported previously, plateau-generating cells in the deep dorsal horn are comprised preferentially of wide-dynamic range neurons and, to a smaller extent, of nociceptive specific neurons (Morisset and Nagy, 1998). The regenerative depolarizations associated with plateau potentials of these neurons are functionally important for nociceptive integration in the spinal cord. They introduce nonlinearity in the information processing and enable the production of intense firing and afterdischarges in response to stimulation of nociceptive inputs (Morisset and Nagy, 1996, 1998; Russo and Hounsgaard, 1996). We have shown in the present paper that Ca2+acts as a charge carrier participating in these prolonged depolarizations. However, intracellular calcium may act also as a second messenger directly and by stimulation of other second messenger systems. These effects, including synaptic plasticity and regulation of neuronal gene expression, are potentially of importance in pain processing (for review, see Woolf, 1996). Ca2+ influx during plateau potentials must contribute substantially to elevate the internal calcium concentration.
Additional support for the functional importance of Ca2+-dependent plateau potentials of DHNs in processing of nociceptive information comes from in vivostudies showing that L-type VGCCs are involved in the hyperalgesia and allodynia resulting from various nociceptive stimulations and inflammatory conditions in the rat (Martin et al., 1996; Neugebauer et al., 1996; Sluka, 1997). Interestingly, L-type calcium channels might not mediate electrically evoked synaptic release of transmitters (Holz et al., 1988; Takahashi and Momiyama, 1993), leaving for the plateau-mediated calcium influx a potential role in the Ca2+-mediated DHNs sensitization.
Footnotes
This work was supported by grants from the DRET (95–148), the Conseil Régional d’Aquitaine (950301216), and the Institut UPSA de la Douleur. We are very grateful to Dr. D. Voisin and Dr. S. Oliet for careful reading and critical comments on this manuscript and to Dr. G. Le Masson for helpful discussions.
Correspondence should be addressed to Dr. Frédéric Nagy, Institut National de la Santé et de la Recherche Médicale E.9914, Physiopathologie des Réseaux Neuronaux Médullaires, Institut François Magendie, 1 rue Camille Saint-Saëns, 33077 Bordeaux Cedex, France.
REFERENCES
- 1.Angstadt JD, Choo JJ. Sodium-dependent plateau potentials in cultured retzius cells of the medicinal leech. J Neurophysiol. 1996;76:1491–1502. doi: 10.1152/jn.1996.76.3.1491. [DOI] [PubMed] [Google Scholar]
- 2.Asada H, Yamaguchi Y, Tsunoda S, Fukuda Y. Relation of abnormal burst activity of spinal neurons to the recurrence of autotomy in rats. Neurosci Lett. 1996;213:99–102. doi: 10.1016/0304-3940(96)12844-5. [DOI] [PubMed] [Google Scholar]
- 3.Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci. 1996;16:5567–5582. doi: 10.1523/JNEUROSCI.16-18-05567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bal T, McCormick DA. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol (Lond) 1993;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurons switch from single-spike activity to burst- firing mode. J Neurosci. 1999;19:599–609. doi: 10.1523/JNEUROSCI.19-02-00599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Koninck Y, Henry JL. Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proc Natl Acad Sci USA. 1991;88:11344–11348. doi: 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol (Lond) 1987a;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox AP, Nowycky MC, Tsien RW. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol (Lond) 1987b;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser DD, MacVicar BA. Cholinergic-dependent plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci. 1996;16:4113–4128. doi: 10.1523/JNEUROSCI.16-13-04113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber G, Cerne R, Randic M. Participation of excitatory amino acid receptors in the slow excitatory synaptic transmission in rat spinal dorsal horn. Brain Res. 1991;561:236–251. doi: 10.1016/0006-8993(91)91600-6. [DOI] [PubMed] [Google Scholar]
- 11.Golowasch J, Marder E. Ionic currents of the lateral pyloric neuron of the stomatogastric ganglion of the crab. J Neurophysiol. 1992;67:318–331. doi: 10.1152/jn.1992.67.2.318. [DOI] [PubMed] [Google Scholar]
- 12.Grubb BD, Riley RC, Hope PJ, Pubols L, Duggan AW. The burst-like firing of spinal neurons in rats with peripheral inflammation is reduced by an antagonist of N-methyl-d-aspartate. Neuroscience. 1996;74:1077–1086. doi: 10.1016/0306-4522(96)00272-2. [DOI] [PubMed] [Google Scholar]
- 13.Holz GG, Dunlap K, Kream RM. Characterization of the electrically evoked release of substance P from dorsal root ganglion neurons: methods and dihydropyridine sensitivity. J Neurosci. 1988;8:463–471. doi: 10.1523/JNEUROSCI.08-02-00463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol (Lond) 1988;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao C-F, Del Negro CA, Trueblood PR, Chandler SH. Ionic basis for serotonin-induced bistable membrane properties in guinea pig trigeminal motoneurons. J Neurophysiol. 1998;79:2847–2856. doi: 10.1152/jn.1998.79.6.2847. [DOI] [PubMed] [Google Scholar]
- 16.Huang L-YM. Calcium channels in isolated rat dorsal horn neurones, including labelled spinothalamic and trigeminothalamic cells. J Physiol (Lond) 1989;411:161–177. doi: 10.1113/jphysiol.1989.sp017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavalali ET, Plummer MR. Multiple voltage-dependent mechanisms potentiate calcium channel activity in hippocampal neurons. J Neurosci. 1996;16:1072–1082. doi: 10.1523/JNEUROSCI.16-03-01072.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiehn O. Plateau potentials and active integration in the “final common pathway” for motor behaviour. Trends Neurosci. 1991;14:68–73. doi: 10.1016/0166-2236(91)90023-n. [DOI] [PubMed] [Google Scholar]
- 19.Kiehn O, Harris-Warrick RM. Serotonergic stretch receptors induce plateau properties in a crustacean motor neuron by a dual-conductance mechanism. J Neurophysiol. 1992;68:485–495. doi: 10.1152/jn.1992.68.2.485. [DOI] [PubMed] [Google Scholar]
- 20.Klink R, Alonso A. Ionic mechanisms of muscarinic depolarization in entorhinal cortex layer II neurons. J Neurophysiol. 1997;77:1829–1843. doi: 10.1152/jn.1997.77.4.1829. [DOI] [PubMed] [Google Scholar]
- 21.Laird JMA, Bennett GJ. An electrophysiological study of dorsal horn neurons in the spinal cord of rats with an experimental peripheral neuropathy. J Neurophysiol. 1993;69:2072–2085. doi: 10.1152/jn.1993.69.6.2072. [DOI] [PubMed] [Google Scholar]
- 22.Marchetti C, Amico C, Usai C. Functional characterization of the effect of nimodipine on the calcium current in rat cerebellar granule cells. J Neurophysiol. 1995;73:1169–1180. doi: 10.1152/jn.1995.73.3.1169. [DOI] [PubMed] [Google Scholar]
- 23.Martin MI, Del Val VL, Colado MI, Goicoechea C, Alfaro MJ. Behavioral and analgesic effects induced by administration of nifedipine and nimodipine. Pharmacol Biochem Behav. 1996;55:93–98. doi: 10.1016/0091-3057(95)02289-9. [DOI] [PubMed] [Google Scholar]
- 24.Mills JD, Pitman RM. Electrical properties of a cockroach motor neuron soma depend on different characteristics of individual Ca components. J Neurophysiol. 1997;78:2455–2466. doi: 10.1152/jn.1997.78.5.2455. [DOI] [PubMed] [Google Scholar]
- 25.Morisset V, Nagy F. Modulation of regenerative membrane properties by stimulation of metabotropic glutamate receptors in rat deep dorsal horn neurons. J Neurophysiol. 1996;76:2794–2798. doi: 10.1152/jn.1996.76.4.2794. [DOI] [PubMed] [Google Scholar]
- 26.Morisset V, Nagy F. Nociceptive integration in the rat spinal cord: role of nonlinear membrane properties of deep dorsal horn neurons. Eur J Neurosci. 1998;10:3642–3652. doi: 10.1046/j.1460-9568.1998.00370.x. [DOI] [PubMed] [Google Scholar]
- 27.Murase K, Ryu PD, Randic M. Tachykinins modulate multiple ionic conductances in voltage-clamped rat spinal dorsal horn neurons. J Neurophysiol. 1989;61:854–865. doi: 10.1152/jn.1989.61.4.854. [DOI] [PubMed] [Google Scholar]
- 28.Nagy I, Maggi CA, Dray A, Woolf CJ, Urban L. The role of neurokinin and N-methyl-d-aspartate receptors in synaptic transmission from capsaicin-sensitive primary afferents in the rat spinal cord in vitro. Neuroscience. 1993;52:1029–1037. doi: 10.1016/0306-4522(93)90549-u. [DOI] [PubMed] [Google Scholar]
- 29.Neugebauer V, Vanegas H, Nebe J, Rümenapp P, Schaible HG. Effects of N- and L-type calcium channel antagonists on the responses of nociceptive spinal cord neurons to mechanical stimulation of the normal and the inflamed knee joint. J Neurophysiol. 1996;76:3740–3749. doi: 10.1152/jn.1996.76.6.3740. [DOI] [PubMed] [Google Scholar]
- 30.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 31.Palecek J, Paleckova V, Dougherty PM, Carlton SM, Willis WD. Responses of spinothalamic tract cells to mechanical and thermal stimulation of skin in rats with an experimental peripheral neuropathy. J Neurophysiol. 1992;67:1562–1573. doi: 10.1152/jn.1992.67.6.1562. [DOI] [PubMed] [Google Scholar]
- 32.Partridge LD, Muller TH, Swandulla D. Calcium-activated non-selective channels in the nervous system. Brain Res Rev. 1994;19:319–325. doi: 10.1016/0165-0173(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 33.Rekling JC, Feldman JL. Calcium-dependent plateau potentials in rostral ambiguous neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1997;78:2483–2492. doi: 10.1152/jn.1997.78.5.2483. [DOI] [PubMed] [Google Scholar]
- 34.Russo RE, Hounsgaard J. Plateau-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. J Physiol (Lond) 1996;493:39–54. doi: 10.1113/jphysiol.1996.sp021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo RE, Nagy F, Hounsgaard J. Modulation of plateau properties in dorsal horn neurones in a slice preparation of the turtle spinal cord. J Physiol (Lond) 1997;499:459–474. doi: 10.1113/jphysiol.1997.sp021941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryu PD, Randic M. Low- and high-voltage-activated calcium currents in rat spinal dorsal horn neurons. J Neurophysiol. 1990;63:273–285. doi: 10.1152/jn.1990.63.2.273. [DOI] [PubMed] [Google Scholar]
- 37.Sandler VM, Puil E, Schwarz DWF. Intrinsic response properties of bursting neurons in the nucleus principalis trigemini of the gerbil. Neuroscience. 1998;83:891–904. doi: 10.1016/s0306-4522(97)00415-6. [DOI] [PubMed] [Google Scholar]
- 38.Shaw T, Lee RJ, Partridge LD. Action of diphenylamine carboxylate derivatives, a family of non-steroidal anti-inflammatory drugs, on [Ca2+]i and Ca2+-activated channels in neurons. Neurosci Lett. 1995;190:121–124. doi: 10.1016/0304-3940(95)11518-2. [DOI] [PubMed] [Google Scholar]
- 39.Simpson PB, Challiss RAJ, Nahorski SR. Neuronal Ca2+ stores: activation and function. Trends Neurosci. 1995;18:299–306. doi: 10.1016/0166-2236(95)93919-o. [DOI] [PubMed] [Google Scholar]
- 40.Sluka KA. Blockade of calcium channels can prevent the onset of secondary hyperalgesia and allodynia induced by intradermal injection of capsaicin in rats. Pain. 1997;71:157–164. doi: 10.1016/s0304-3959(97)03354-x. [DOI] [PubMed] [Google Scholar]
- 41.Sotgiu ML, Biella G, Riva L. Poststimulus afterdischarges of spinal WDR and NS units in rats with chronic nerve constriction. NeuroReport. 1995;6:1021–1024. doi: 10.1097/00001756-199505090-00018. [DOI] [PubMed] [Google Scholar]
- 42.Swandulla D, Lux HD. Activation of a nonspecific cation conductance by intracellular Ca2+ elevation in bursting pacemaker neurons of Helix pomatia. J Neurophysiol. 1985;54:1430–1443. doi: 10.1152/jn.1985.54.6.1430. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- 44.Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 45.Urban L, Randic M. Slow excitatory transmission in rat dorsal horn: possible mediation by peptides. Brain Res. 1984;290:336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]
- 46.Viana di Prisco G, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science. 1997;278:1122–1125. doi: 10.1126/science.278.5340.1122. [DOI] [PubMed] [Google Scholar]
- 47.Wilson GF, Richardson FC, Fisher TE, Olivera BM, Kaczmarek LK. Identification and characterization of a Ca2+-sensitive nonspecific cation channel underlying prolonged repetitive firing in Aplysia neurons. J Neurosci. 1996;16:3661–3671. doi: 10.1523/JNEUROSCI.16-11-03661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66:105–108. [PubMed] [Google Scholar]
- 49.Woolf CJ, King AE. Physiology and morphology of multireceptive neurons with C-afferent fiber inputs in the deep dorsal horn of the rat lumbar spinal cord. J Neurophysiol. 1987;58:460–479. doi: 10.1152/jn.1987.58.3.460. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimura M, Jessell TM. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol (Lond) 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimura M, Shimizu T, Yajiri Y, Inokuchi H, Nishi S. Primary afferent-evoked slow EPSPs and responses to substance P of dorsal horn neurons in the adult rat spinal cord slices. Regul Pept. 1993;46:407–409. doi: 10.1016/0167-0115(93)90102-e. [DOI] [PubMed] [Google Scholar]
- 52.Zhang B, Harris-Warrick RM. Calcium-dependent plateau potentials in a crab stomatogastric ganglion motor neuron. I. Calcium current and its modulation by serotonin. J Neurophysiol. 1995;74:1929–1937. doi: 10.1152/jn.1995.74.5.1929. [DOI] [PubMed] [Google Scholar]
- 53.Zhang B, Wootton JF, Harris-Warrick RM. Calcium-dependent plateau potentials in a crab stomatogastric ganglion motor neuron. II. Calcium-activated slow inward current. J Neurophysiol. 1995;74:1938–1946. doi: 10.1152/jn.1995.74.5.1938. [DOI] [PubMed] [Google Scholar]