Abstract
This study examined the contribution of frequency-dependent short-term depression of PSP amplitude to low-pass temporal filtering in the weakly electric fish Eigenmannia. Behavioral and neurophysiological methods were used. Decelerations of the electric organ discharge frequency were measured in response to continuous and discontinuous electrosensory stimuli. Decelerations were strongest (median = 4.7 Hz; range, 3.5–5.9 Hz) at continuous beat rates of ∼5 Hz and weakest (median = 0.4 Hz; range, 0.0–0.8 Hz) at beat rates of 30 Hz. Gating 20 or 30 Hz stimuli at a rate of 5 Hz, however, elicited decelerations that were sixfold greater than that of continuous stimuli at these beat rates (median = 2.6 Hz; range, 2.0–4.7 Hz for 30 Hz). These results support the hypothesis that short-term processes enhance low-pass filtering by reducing responses to fast beat rates. This hypothesis was tested by recording intracellularly the responses of 33 midbrain neurons to continuous and discontinuous stimuli. Results indicate that short-term depression of PSP amplitude primarily accounts for the steady-state low-pass filtering of these neurons beyond that contributed by their passive and active membrane properties. Previous results demonstrate that passive properties can contribute up to 7 dB of low-pass filtering; PSP depression can add up to an additional 12.5 dB (median = 4.5). PSP depression increased in magnitude with stimulus frequency and showed a prominent short-term component (t1 = 66 msec at 30 Hz). Initial PSP amplitude recovered fully after a gap of 150 msec for most neurons. Remarkably, recovery of PSP amplitude could be produced by inserting a brief low–temporal frequency component in the stimulus.
Keywords: whole-cell patch, sensory processing, adaptation, torus semicircularis, jamming avoidance response, synaptic depression, plasticity
A fundamental function of sensory systems is to extract biologically relevant information. Although in many systems there is a good understanding of the stimulus selectivity of sensory neurons in particular central regions, there is comparatively little known about the mechanisms that are responsible for generating these filtering properties.
The electrosensory system of the weakly electric fishEigenmannia is well suited for investigating how central filters are generated. Behavioral and neurophysiological studies have clearly identified the filtering and computational processes that underlie electrosensory behaviors. One particularly well studied behavior is the jamming avoidance response (JAR), which persists in neurophysiological preparations. In the JAR, Eigenmanniaadjusts the frequency of its electric organ discharges (EODs) to avoid detrimental interference from EODs of neighboring fish. When two fish of similar EOD frequencies approach, the combination of their EODs produce amplitude and phase modulations that can interfere with both animals’ ability to electrolocate (Matsubara and Heiligenberg, 1978). Modulations (“beat rates”) of 3–8 Hz are most detrimental to electrolocation and elicit the largest JARs (Bullock et al., 1972;Heiligenberg et al., 1978; Partridge et al., 1981; Bastian and Yuthas, 1984), whereas beat rates of >20 Hz do not impair electrolocation. During a JAR, the fish with the lower initial EOD frequency lowers its frequency while the other fish raises its frequency, thereby increasing the beat rate to values that have little effect on electrolocation.
These behavioral observations indicate that Eigenmanniaselectively extracts patterns of afferent activity that reflect slow modulations of signal amplitude. Selectivity for beat rates of 3–8 Hz emerges at the midbrain (see Fig. 1B), where most neurons respond poorly to rates of 20 Hz or more (Partridge et al., 1981). The rejection of fast temporal frequency information in the torus is also found in the phylogenetically older ampullary electrosensory system (Fortune and Rose, 1997a). The mechanisms that underlie the low-pass temporal-filtering properties of toral neurons are incompletely understood.
Fig. 1.
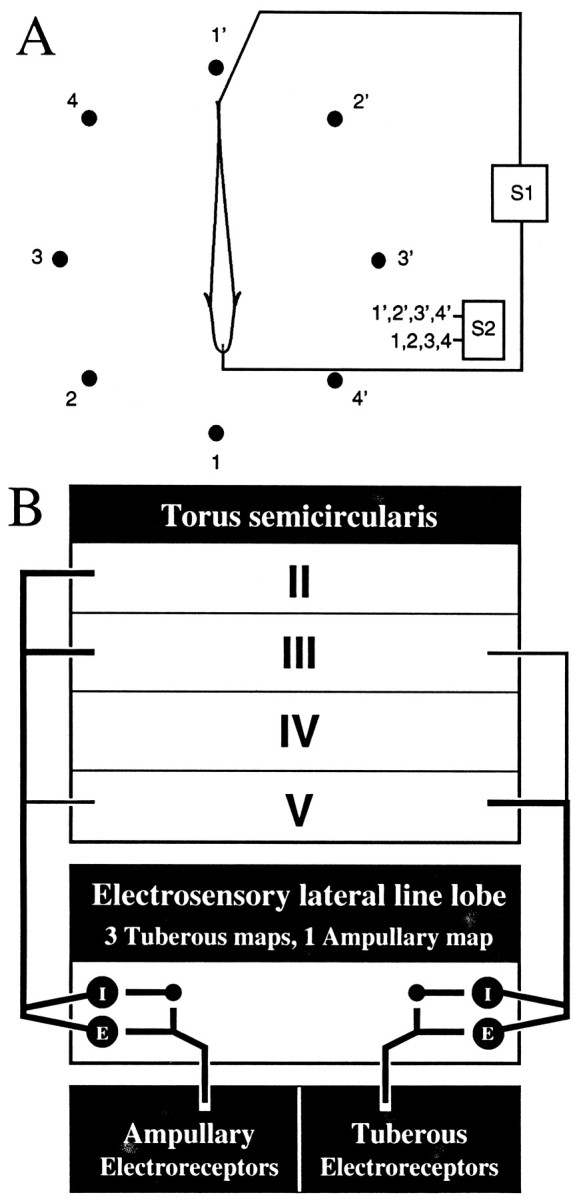
Schematic diagrams of the stimulation apparatus and ascending electrosensory pathways. A, A sinusoidal mimic of the subject’s EOD (S1) is presented through electrodes placed at the tail and in the mouth. Jamming and other signals (S2) can be presented through pairs of carbon electrodes surrounding the fish and numbered 1–4 and 1′–4′. S2 signals also can be electronically added to the S1 signal. B, Ampullary and tuberous electrosensory systems have parallel projections. Ampullary and P-type tuberous afferents project into the electrosensory lateral line lobe, forming synapses on basilar pyramidal neurons and granule neurons (small dots). Granule neurons in turn have inhibitory synapses on nonbasilar pyramidal neurons. Basilar and nonbasilar pyramidal neurons respond ∼180° out-of-phase with respect to the stimulus; they are known as E and I units, respectively. In the tuberous system, E units respond to rises in stimulus amplitude, and I units respond to decreases. Pyramidal neurons project into various laminae in the dorsal torus semicircularis. Recordings were made in layers 2–5.
Both passive and active membrane properties contribute to the low-pass temporal-filtering characteristics of toral neurons (Fortune and Rose, 1997b). Rarely, however, do passive and active membrane properties account entirely for the filtering characteristics of toral neurons to sensory stimuli. For these neurons, PSP amplitude declined by as much as 20 dB as either the beat rate or the sinusoidal frequency of the stimulus was varied from 2 to 30 Hz, even when the role of voltage-dependent conductances was minimized.
In addition to active and passive membrane properties, frequency- and time-dependent processes could, theoretically, contribute to the temporal-filtering properties of neurons; synaptic depression (Zucker, 1989) is one mechanism for achieving such attenuation. At stimulus onset, PSP amplitude would be a function of a neuron’s passive and active membrane properties. As the high-frequency stimulus is maintained, however, PSP amplitude might be attenuated until a steady state is reached. This hypothesis was examined using both behavioral and neurophysiological techniques. Results indicate that frequency-dependent depression of PSP amplitude (“PSP depression”) enhances low-pass temporal filtering.
MATERIALS AND METHODS
Experimental procedures were similar to those described previously (Heiligenberg and Rose, 1985; Rose and Call, 1993; Fortune and Rose, 1997a,b). Fish, ∼1-year-old, of the genusEigenmannia were used. Animal husbandry, anesthesia, and surgical procedures were performed under the guidelines established by the Society for Neuroscience. For experiments, a fish’s EOD was measured and then attenuated (∼1000 fold) by intramuscular injection of Flaxedil (4 μg/gm of fish). Additional injections of Flaxedil were made during the experiment as necessary to maintain the attenuation of the EOD. The fish’s EOD was replaced by a sinusoidal mimic (S1) that was delivered through electrodes placed at the tail and in the mouth. The amplitude and frequency of the S1 were adjusted to approximate the fish’s EOD before the injection of Flaxedil. Additional electrosensory stimuli were delivered through an array of carbon electrodes that surrounded the fish (Fig.1A). In five fish, behavioral experiments (see below) were performed under these conditions before surgery.
At the conclusion of the experiment, not >4 hr after the first neuron was filled, animals were deeply anesthetized by the flow of 2% (w/v) urethane across the gills. Animals were perfused transcardially with saline–heparin solution followed by 4% (w/v) paraformaldehyde in 0.2m phosphate buffer, pH 7.4. After perfusion, the brain was removed and stored at 4°C overnight in the paraformaldehyde solution. Sections, 100 μm thick, were cut on a vibratome and reacted using an avidin–biotin peroxidase kit (Vector Laboratories, Burlingame, CA). Sections were dehydrated, cleared in xylenes, mounted on slides, and coverslipped.
Behavioral procedures. After injection of Flaxedil, the fish’s EOD was replaced by a sinusoidal mimic (S1) that was delivered through electrodes placed at the tail and in the mouth (Fig.1A). The residual EOD was recorded differentially via a suction electrode fitted to the tail and amplified (model P15D; Grass Instruments). EOD frequency was measured to an accuracy of 0.1 Hz using a window discriminator (SA Instrumentation) and a frequency counter (BK 1822). The fish’s resting EOD frequency, measured in the presence of the S1 alone, was determined before and after each experimental stimulus was presented. EOD frequency was monitored until it reached a stable level, usually >1 min for each stimulus. Water temperature was held at 25°C.
Experimental stimuli were generated by electronically adding a second sinusoidal signal (S2) to the S1. The frequency of the S2 was 1, 2, 5, 10, 20, or 30 Hz above or below the frequency of the S1 (Fig.2A), which produces a signal that “beats” at a rate equal to the frequency difference between the S1 and S2. Two classes of experimental stimuli were used: “continuous” and “discontinuous.” For continuous experimental stimuli, the S2 signal was presented and maintained until the fish had reached a stable EOD frequency. Discontinuous stimuli were generated by gating the S2 signal on and off at a rate of 5 Hz; the frequency of the S2 was 10, 20, or 30 Hz above or below the S1 frequency. The stimulus waveform that results from gating, at a rate of 5 Hz, an S2 that is 20 Hz greater than the S1 frequency is shown in Figure2B. The starting phase of the S2 signals was adjusted such that the amplitude of the combined (S1 + S2) signal started at the amplitude of the S1 alone, thereby minimizing sharp transients in stimulus amplitude.
Fig. 2.
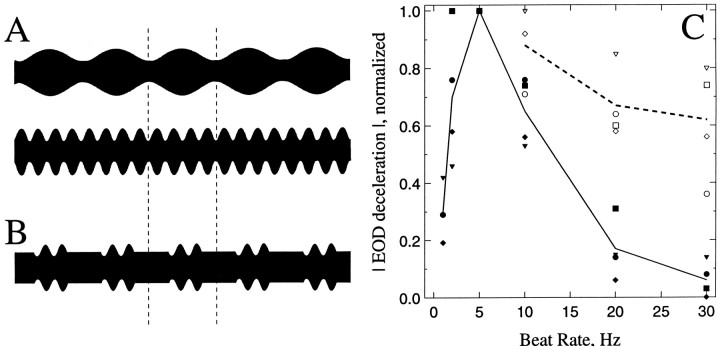
Behavioral evidence of short-term PSP depression.A, B, Oscillograms of continuous electrosensory stimuli with beat rates of 5 Hz (A; top) and 20 Hz (A; bottom) and of a discontinuous stimulus with a beat rate of 20 Hz that is gated at a rate of 5 Hz (B). Responses to the stimulus inB are the open symbolsabove 20 Hz in C. Dashed vertical lines represent the time for a full cycle at 5 Hz, 200 msec. C, Magnitude of EOD decelerations versus the beat rate for continuous (closed symbols) and discontinuous (open symbols) stimuli. The magnitude of responses was normalized for each fish to the maximum deceleration evoked from that fish. Each symbol type–-e.g., triangles,diamonds—represents data from an individual fish. Thesolid line is the mean response across fish to continuous stimuli. The dashed line is the mean for discontinuous stimuli.
The added S1 and S2 signals were delivered through the electrodes in the mouth and at the tail. This stimulus arrangement elicited decelerations of the EOD frequency from its resting level (Takizawa et al., 1999).
Intracellular recording procedures. Whole-cell patch recordings were made as described in detail by Rose and Fortune (1996). Intracellular recordings were made from 33 neurons in the dorsal 5 layers of the torus semicircularis of adult Eigenmannia(Fig. 1B). Patch pipettes were constructed from borosilicate or aluminosilicate capillary glass [1 mm outer diameter and 0.58 mm inner diameter (#5960; A-M Systems); 1 mm outer diameter and 0.75 mm inner diameter (#5810; A-M Systems), respectively] using a Flaming-Brown type puller (model P-97; Sutter Instruments). Electrodes were pulled to resistances between 10 and 25 MΩ. Electrode tips were backfilled with a solution (pH = 7.4; 285 mOsm.) consisting of (values in mm): 100 potassium acetate or potassium gluconate, 2 KCl, 1 MgCl2, 5 EGTA, 10 HEPES, 20 KOH, and 43 biocytin. Biocytin was replaced by mannitol in the solution used to fill pipette shanks. Electrodes were mounted in a Plexiglas holder with a pressure port. This port allowed the application of pressure pulses (40–80 msec; 40 psi) from a Picospritzer (General Valve, Fairfield, NJ) or the manual application of suction or pressure from a 30 cc syringe. The electrode was advanced in 1.5 μm steps (Burleigh 6000 microdrive) through the dorsal 5 layers of the torus. Responses were amplified using an electrometer (model 767; World Precision Instruments, Sarasota, FL) and stored on videotape at 40 kHz with 16-bit resolution (model 3000; Vetter Instruments).
Recordings generally were made at several levels of negative holding current. This procedure permitted PSPs to be observed in the absence of spiking and other fluctuations associated with voltage-dependent conductances; generally less than −0.2 nA was used. As in previous studies (Fortune and Rose, 1997a,b), these holding currents did not, with the exception in some neurons of the all-or-nothing components of EPSPs, affect the temporal-filtering properties of neurons. In addition, in the present study no qualitative differences in short-term PSP depression were observed over this range of holding currents. Resting potentials were between −55 and −75 mV. At the conclusion of each intracellular recording, neurons were filled with biocytin by applying 1–2 nA of positive DC for 1–3 min.
Stimuli for intracellular recordings. The search stimulus was designed to elicit responses from both ampullary and tuberous neurons in the torus. The ampullary component of the search stimulus was a linear frequency sweep (2–30 Hz; 10 sec duration; 1–2 mV/cm at the fish’s head) that was added to the S1 and presented through the electrodes in the mouth and at the tail. The tuberous component was the S1 and a sine wave (S2) 4 Hz higher than the S1 frequency that was delivered concurrently through one pair of the array of carbon electrodes surrounding the fish. The addition of the S2 generated broad-field amplitude and phase modulations at a rate equal to the difference in frequencies of the S1 and S2; the modulation frequency is known as the beat rate.
After a recording was established, the best stimulus (ampullary or tuberous) and stimulus orientation (pairs of carbon electrodes surrounding the fish) were determined. Stimulus orientation was chosen to elicit the strongest and most consistent responses from the neuron. Eighteen neurons were tuberous, and 15 were ampullary. The data here and in previous reports (Fortune and Rose, 1997a,b) indicate that ampullary and tuberous neurons in the torus are indistinguishable on the basis of their temporal-filtering properties, appearance of their PSPs, and anatomy.
Responses were first recorded while the stimulus frequency (ampullary) or beat rate (tuberous) was linearly scanned from ∼2 to 30 Hz. These “sensory scans” were 10 sec in duration. Subsequently “sensory bursts” were delivered; the stimulus frequency (ampullary) or beat rate (tuberous) was held at 5, 10, 20, or 30 Hz. The bursts were 1 sec in duration and presented at an interval of 2 sec. For tuberous stimulation, the S2 was gated on at the zero-crossing of an S1 cycle, and its starting phase was adjusted differentially for E-type and I-type units. For neurons that were excited primarily by amplitude increases (E units), the stimulus burst began with an amplitude decrease; the opposite relation held for I units. This stimulation paradigm was used so that the first PSP elicited by the stimulus burst was in response to the same magnitude of amplitude modulation as subsequent PSPs. For ampullary stimulus bursts, only a single low-frequency signal was presented. The starting phase of the signal was adjusted such that the first quarter cycle of stimulation did not excite the neuron. Rarely neurons were encountered that had both strong E and I components of their responses. These neurons were excluded from the analyses presented in this paper.
The time course of recovery from PSP depression was assessed by systematically reducing the interval between the end of one stimulus burst and the beginning of the next burst (“gap duration”). Each stimulus burst was 952 msec in duration except for those with gaps >48 msec; beyond 48 msec, gap duration was increased by decreasing the burst duration. Within each burst the beat rate or sinusoidal frequency was held at 20 Hz. The starting and ending phase of the stimuli was adjusted for each cell such that PSPs were not evoked by the first and last quarter cycles of the stimulus burst.
Finally, whenever possible, the sensory stimulus was removed, and a 0.1 nA peak-to-peak sinusoidal current sweep, 2–30 Hz, was injected into the soma via the recording electrode. Constant-frequency current bursts with a positive-going peak amplitude of 0.1 nA, a duration of 1 sec, and intervals of 1 sec were also used. Burst frequencies were 5, 10, 20, and 30 Hz. Negative holding current was used to hyperpolarize the neuron so that positive-going sinusoidal current injection, 0 to +0.1 nA, produced depolarizations in the neuron that were similar to EPSPs elicited by sensory stimuli.
Analysis of neurophysiological data. As in previous studies (Fortune and Rose, 1997b), the temporal-filtering profiles of neurons were determined by Fourier analysis of segments of the intracellular responses to sensory and current scans. The peak of the power spectrum near to the stimulus frequency was used as a measure of the amplitude of stimulus-related PSPs at that frequency. In repeated measures of PSP amplitude using this methodology, we found that the values varied by less than ± 0.5 dB; each value represents an average of the responses to several stimulus cycles. PSP depression was measured by comparing the PSP amplitude as measured by Fourier analysis of responses to the initial 100 msec segment of a burst with that to a segment of identical duration at the end of the burst. In some cases PSP depression was activated in <50 msec. In those cases the peak-to-peak amplitude (in millivolts) of PSPs was measured at the beginning (Vi) and end (Ve) of the burst. The ratio of these values was taken and expressed in decibels: dB = 20 log (Ve/Vi).
For sinusoidal current injection data, the voltage drop attributable to the access resistance (electrode and patch resistances) was subtracted from the total voltages recorded. Access resistance was measured as the first exponential component in the voltage response to square-wave current injection. This value was subtracted from the individual voltage values for particular stimulation rates. Decibel values were computed using the corrected amplitudes.
RESULTS
Behavior
To assess the potential role of short-term depression in temporal filtering, we compared EOD deceleration responses (Takizawa et al., 1999) of five fish with continuous and discontinuous electrosensory stimuli. Discontinuous stimuli were designed to reduce or eliminate the contribution of short-term processes that require up to 100 msec for activation.
Continuous beat rates of 5 Hz (Fig. 2A) elicited the greatest (up to 6 Hz) decelerations in EOD frequency (Fig.2C, solid line, closed symbols). Responses to beat rates of 20 Hz and above were ∼1/10 the maximum magnitude. These data are similar to those obtained in a previous report (Takizawa et al., 1999).
Subsequently, stimuli of 10, 20, and 30 Hz beat rate were presented in a discontinuous pattern, 100 msec bursts alternating with 100 msec gaps (Fig. 2B). The effects of this stimulus regimen resembled a continuous 5 Hz beat rate stimulus in that, at least for E-type tuberous electrosensory neurons (those responding to amplitude increases), activity should be restricted to the 100 msec segments in which the amplitude of the signal was modulated. Discontinuous stimuli elicited responses that were up to sixfold stronger than the continuous stimuli at high beat rates (Fig. 2C, dashed line, open symbols). These responses were, however, only 60–70% the amplitude of those to the continuous 5 Hz beat stimuli. These data suggest that short-term processes (e.g., short-term depression) contributed to the generation of low-pass filtering in this behavior.
Intracellular physiology
Strong low- and bandpass temporal-filtering, neural correlates of the behavioral responses to continuous stimuli are well developed at the level of the torus. This conclusion is supported by data from previous studies in which the stimulus beat rate was scanned linearly from 2 to 30 Hz over 10 sec. The hypothesis that short-term processes contribute to low-pass temporal-filtering properties of neurons was tested by recording the responses of 33 low- and bandpass toral neurons to continuous and discontinuous sensory stimuli.
Neurons were divided into two groups based on a combination of physiological and anatomical properties (Fortune and Rose, 1997b). The first group included 26 neurons that had voltage responses to injection of sinusoidal current that declined by at least 2.6 dB over the range 2–30 Hz and/or had large, complex dendritic arbors with many spines (“spiny neurons”). The second group included 7 neurons that had voltage responses to injection of sinusoidal current that declined by <2.0 dB and/or had small, simple dendritic arbors with few or no spines (“aspiny neurons”).
The temporal-filtering properties of these neurons were first assessed by recording responses to continuous stimuli in which the beat rate or frequency of the stimulus was swept linearly from 2 to 30 Hz over 10 sec (sensory scans). For this stimulus regimen, neurons showed low-pass filtering ranging from approximately a 2 dB reduction in PSP amplitude to almost 20 dB (median = 10.5 dB). Only aspiny neurons had low-pass filtering of <5 dB. Both aspiny and spiny neurons were found that showed low-pass filtering in the range of 5 to ∼9 dB. Only spiny neurons, however, showed low-pass filtering of >9 dB.
Evidence of short-term PSP depression
The role of short-term depression of PSP amplitude in low-pass and bandpass temporal filtering was assessed by comparison of PSP amplitude at the beginnings and ends of sensory bursts. In these stimuli, the beat rate or frequency was held constant at 5, 10, 20, or 30 Hz for 1 sec and repeated at a rate of 0.5 Hz.
PSP depression, a form of short-term plasticity, is defined here as a reduction in the amplitude of PSPs over time to a stimulus of constant frequency or beat rate, e.g., adaptation. Alternatively, neurons could produce constant-amplitude PSPs over time or could show an increase in PSP amplitude (facilitation). Approximately 60% of low- and bandpass neurons showed >3 dB short-term depression of PSP amplitude. This PSP depression was frequency dependent, increasing in magnitude with stimulation frequency.
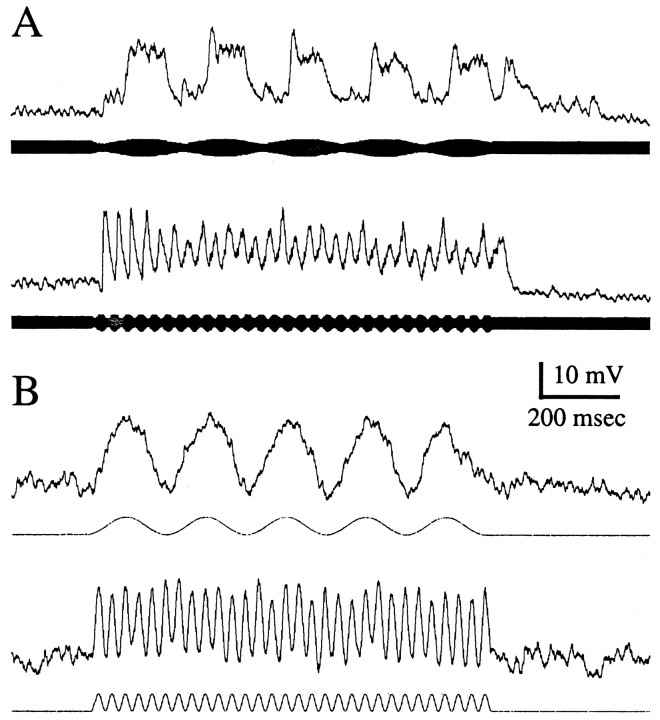
Figures 3 and4 show primary data from aspiny and spiny neurons, respectively. Figures 3A and 4Ashow examples of neurons with little or no evidence of PSP depression, and Figures 3B and 4B show examples with strong PSP depression. In the case of aspiny neurons, which are characterized by having little passive low-pass filtering, PSP depression primarily accounted for their low-pass filtering to sensory stimuli. Aspiny neurons without appreciable PSP depression had weak (<5 dB) low-pass filtering over 2–30 Hz. The bandpass neuron shown in Figure 3A exhibited ∼2 dB of passive low-pass filtering and 2 dB of PSP depression. These contributions add to match the 4 dB of filtering observed in response to the continuous sensory scan. The lack of response to the lowest frequency beat rates is at present unexplained but is likely to result from filtering properties of their afferents from the electrosensory lateral line lobe (ELL) (see Shumway, 1989). The large PSP after the high-frequency stimulus in Figure3A is also currently unexplained; the presence of such PSPs is not correlated with the strength of PSP depression.
Fig. 3.
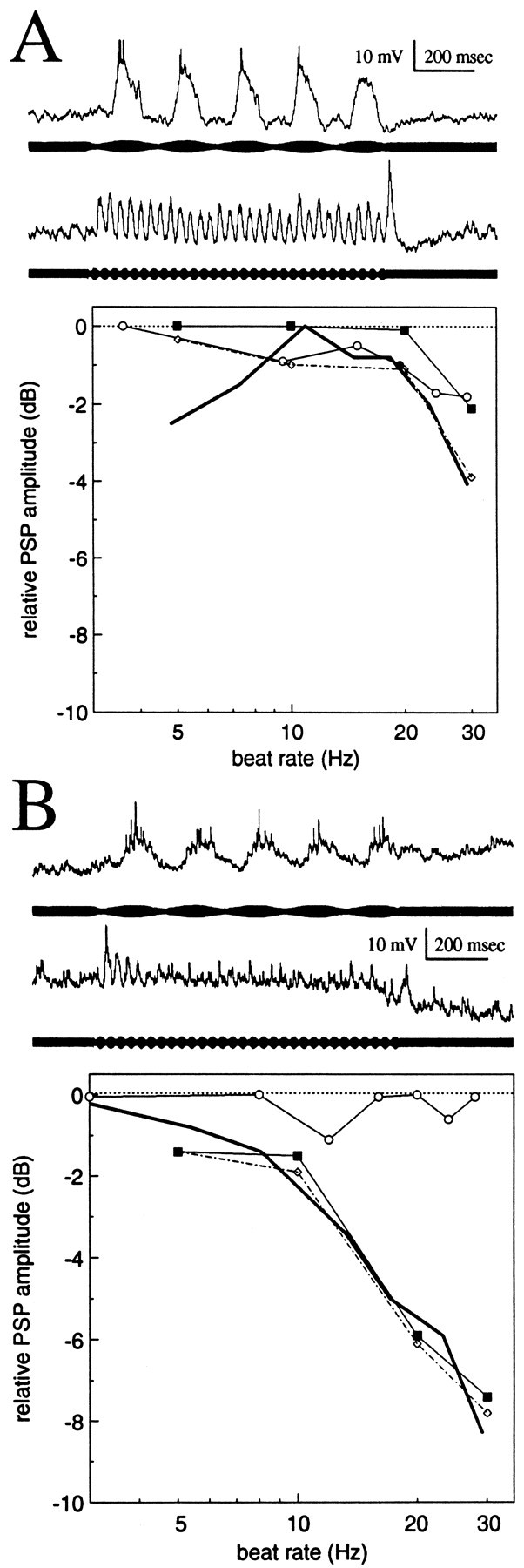
Responses of aspiny neurons to stimulus bursts.A, Neuron with little or no PSP depression.B, Neuron with strong PSP depression.Traces are responses to sensory bursts of 5 Hz (top trace) and 30 Hz (bottom trace) beat rates. The relative amplitudes of PSPs (in decibels) are plotted versus stimulation rate. Responses are to sensory scans (beat rate swept linearly from 2 to 30 Hz; thick lines), sinusoidal current injection (frequency swept linearly from 2 to 30 Hz;open circles), and stimulus bursts (closed squares). Values, in decibels, are normalized to the maximum PSP amplitude for each stimulus condition. For bursts, values are the differences in the magnitude of initial PSPs and last PSPs of each burst. These values therefore are a measurement of PSP depression. The dotted line with open diamondsindicates the combined effects of passive electrical filtering (as determined by injection of sinusoidal current) and PSP depression.
Fig. 4.
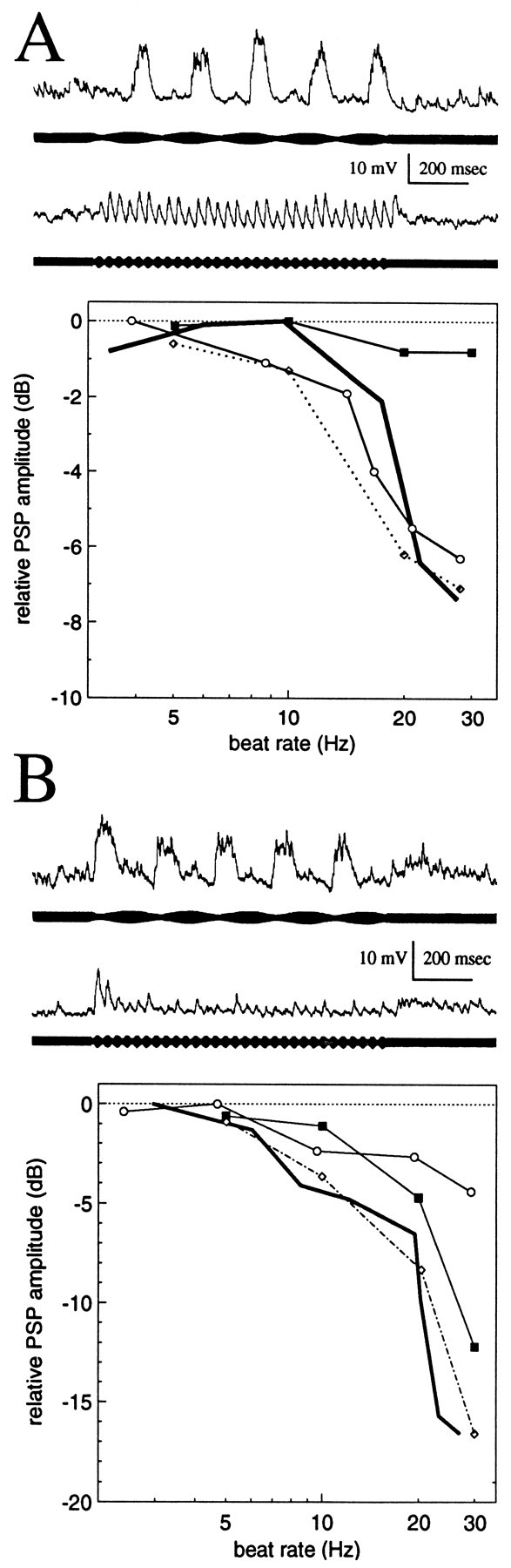
Responses of spiny neurons to sensory bursts.A, Neuron with little or no PSP depression.B, Neuron with strong PSP depression. Figure components are described in Figure 3.
Data from an aspiny neuron that exhibited strong PSP depression is shown in Figure 3B. The amplitude of the initial PSP to a high-frequency stimulus was almost equal in amplitude to PSPs elicited by low-frequency stimuli. Within five beat cycles, <150 msec, the amplitude of PSPs declined by >7 dB. In aspiny neurons the magnitude of PSP depression was up to 7.5 dB (Fig.5, closed circles).
Fig. 5.
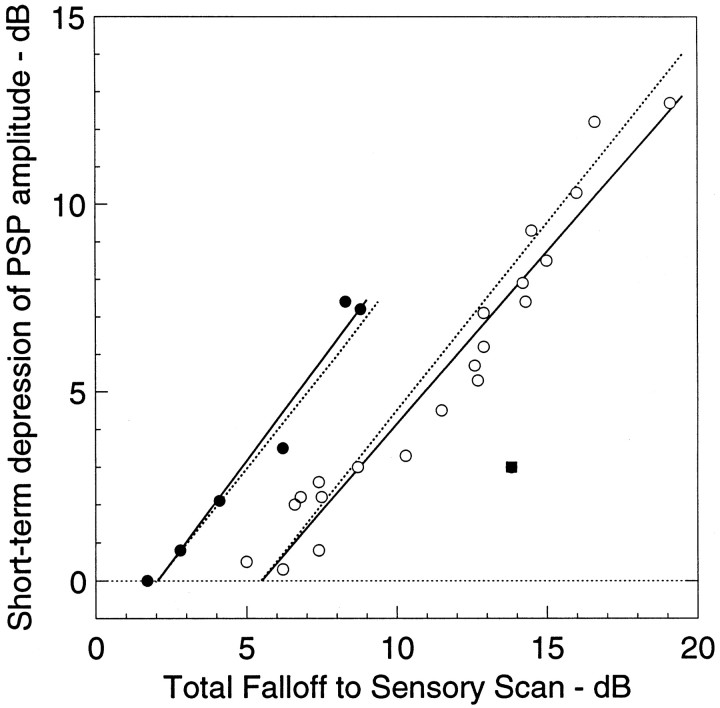
Relation between magnitudes of low-pass filtering (measured from responses to sensory scans) and PSP depression (measured from responses to sensory bursts). Solid lines are linear regressions for data from aspiny (closed circles) and spiny (open circles) neurons.Dotted lines represent the hypothesis that all low-pass filtering beyond that attributable to membrane properties of the neuron is caused by PSP depression. Theclosed square is a datum that was omitted from the regression analysis.
Spiny neurons showed 5–20 dB low-pass filtering. Spiny neurons with relatively weak low-pass filtering (5–9 dB) generally responded to sensory bursts with PSPs that diminished little over time; i.e., PSP amplitude was relatively constant throughout the duration of both low- and high-frequency bursts (Fig. 4A). Nonetheless, there was an appreciable decrease in PSP amplitude as stimulus frequency was raised from 5 to 30 Hz. Injection of sinusoidal current revealed that the passive electrical properties of these neurons accounted for up to ∼7 dB of this low-pass filtering (Fig. 4A).
In contrast, for spiny neurons with strong low-pass filtering, PSP amplitude decreased dramatically from the beginning to the end of sensory bursts when the beat rate was 20 Hz or more (Fig.4B). For this case, PSP amplitude fell to steady-state values within ∼200 msec; the fluctuations of PSP amplitude throughout the remainder of the stimulus may be a feature resulting from the mechanisms underlying short-term PSP depression (see below).
The total magnitude of low-pass filtering can be seen by comparison of the amplitudes of the last few PSPs of the high- and low-frequency responses shown in Figure 4B. The passive filtering properties of this neuron, as determined by sinusoidal current injection, accounted for only ∼4 dB of the low-pass sensory filtering. The effect of passive filtering is evident in the amplitudes of the first PSPs of both traces in Figure4B; the initial response to the low beat rate stimulus was almost 4 dB greater than the initial response to the high beat rate stimulus. For a beat rate of 30 Hz, the magnitude of short-term PSP depression was ∼12 dB and can be seen by comparing the amplitude of the initial PSPs with that of the last PSPs (Fig.4B).
Thus, the passive electrical properties of spiny neurons accounted for a maximum of ∼7 dB of their low-pass filtering over the range 2–30 Hz. The additional low-pass filtering appeared to result from frequency-dependent short-term depression of PSP amplitude (Fig.4B). In spiny neurons short-term PSP depression had magnitudes of up to 12.5 dB (range, 0–12.5 dB; median = 4.5 dB) (Fig. 5, open circles).
Contribution of short-term depression of PSP amplitude to low- and bandpass filtering
The intracellular data shown above suggest that, when the role of active membrane properties was minimized by passing hyperpolarizing current, low-pass filtering of sensory information resulted from a combination of the passive electrical properties of neurons and the frequency-dependent short-term depression of PSP amplitude. The hypothesis that PSP depression accounts for all low-pass filtering of sensory information beyond that contributed by the neuron’s passive and active membrane properties was evaluated.
The passive electrical low-pass filtering of spiny and aspiny neurons is significantly different (Fortune and Rose, 1997b). Most aspiny neurons show 1–2 dB of passive low-pass filtering, and most spiny neurons have 5–6 dB, excluding the contribution of active membrane properties (Fortune and Rose, 1997b). If all additional low-pass filtering is a result of short-term PSP depression, the relation between total low-pass filtering and the magnitude of short-term PSP depression should have a slope of 1 with an intercept between 1 and 2 dB for aspiny and between 5 and 6 dB for spiny neurons.
Linear regressions of aspiny and spiny neurons recorded in this study (Fig. 5) have slopes near 1 (aspiny, slope = 1.07;r = 0.98; n = 6; spiny, slope = 0.92; r = 0.97; n = 21). A slope of 1 indicates that for every additional decibel of filtering beyond the contribution of passive filtering there is 1 dB of filtering caused by short-term PSP depression. These correlations did not result from systematic differences in PSP amplitude. There was no correlation of PSP amplitude to the magnitude of PSP depression across neurons. In this analysis one datum was excluded (Fig. 5, closed square); this neuron showed strong low-pass filtering but little PSP depression. It is likely that the afferents of this neuron were low-pass I-type neurons from the centromedial map of the ELL (Shumway, 1989).
Time course of short-term depression of PSP amplitude
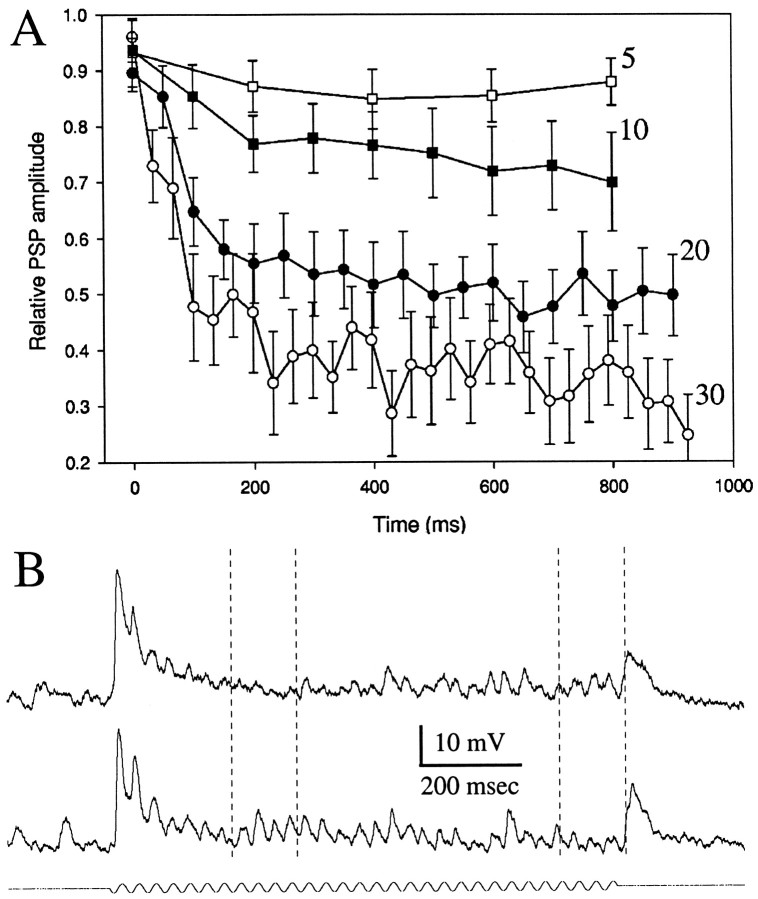
The mean time course and frequency dependence of PSP depression (Fig. 6A) were analyzed for 10 neurons. Sensory stimuli were either beating or sinusoidal signals, and frequencies of 5 Hz (open squares), 10 Hz (closed squares), 20 Hz (closed circles), and 30 Hz (open circles) were used. For each neuron, PSP amplitude was normalized with respect to the largest PSP. PSP depression was greatest at stimulation frequencies of 30 Hz and minimal at 5 Hz. For frequencies of 20 and 30 Hz, these data were well fit (R2= 0.94 and 0.91, respectively) by double-exponential functions having first-order time constants of 111 msec (20 Hz) and 66 msec (30 Hz) and second-order time constants of 23 sec (20 Hz) and 3.1 sec (30 Hz). Thus, stimulation at 30 Hz elicited greater and faster PSP depression than did stimulation at 20 Hz, even though initial PSPs were generally smaller for 30 Hz stimulation.
Fig. 6.
A, Time course and frequency dependence of PSP depression. PSPs were elicited by delivering sensory stimuli 1 sec in duration at 2 sec interstimulus intervals. Frequencies or beat rates of stimulation were 5 Hz (open squares), 10 Hz (closed squares), 20 Hz (closed circles), or 30 Hz (open circles). For each neuron, PSP amplitudes were normalized with respect to the largest (mean) PSP recorded for each stimulus condition. The time (x-axis) of occurrence of the first PSP in response to the stimulus was designated as the 0 point. Means and SEs are plotted. B, Intracellular recordings from an ampullary neuron. Top andbottom traces are responses to 30 Hz sinusoidal signals of ∼1 and 0.5 mV/cm, respectively.
Although these analyses clearly show that PSP depression has a strong short-term component, they do not adequately represent the “fine structure” of the time course of depression. In general, the pattern of frequency-dependent short-term depression of PSP amplitude generally resembled that of a damped oscillation. This feature was prominent in stimuli with large amplitudes (compare top andbottom traces in Fig. 6B). For the larger stimulus amplitude (Fig. 6B, top trace), PSP amplitude was largest at the beginning of sensory bursts and declined to a minimum of 1–2 mV within ∼250 msec and then rebounded to 2–3 mV over the next 250 msec. This waxing and waning of PSP amplitude continued as long as the stimulus was maintained. At the lower stimulus amplitude (Fig. 6B, bottom trace), the magnitude of the initial decline in PSP amplitude was reduced. Further analysis of the time course of recovery from depression will be studied in future experiments in which direct stimulation of the afferents to toral neurons permits analysis with regard to the roles of specific mechanisms for synaptic plasticity.
Time course of recovery from short-term depression of PSP amplitude
The time course of recovery from PSP depression was studied for six neurons. Segments of responses from a tuberous neuron and an ampullary neuron are shown in Figure 7,A and B, respectively. For the tuberous neuron, initial PSP amplitude recovered fully after a gap equal to or greater than ∼28 msec. For a gap of 16 msec, the amplitude of the initial PSP was 81% of control values, and the second PSP was smaller than those during the last 100 msec of the burst. As the gap was increased from 16 to 48 msec, the amplitude of the second PSP increased threefold, whereas the amplitude of the initial PSP was relatively constant. This neuron had the fastest recovery that was observed. The time course of this fluctuation in PSP amplitude, however, is a function of the duration of the gap. For the ampullary neuron, the recovery of full amplitude was not complete for gap durations of <48–100 msec. Although the time course of PSP depression was similar for these two neurons when the gap was 48 msec, it differed for the shorter gaps; for gaps of 8–20 msec, the second PSP of this ampullary neuron was actually slightly larger than the first.
Fig. 7.
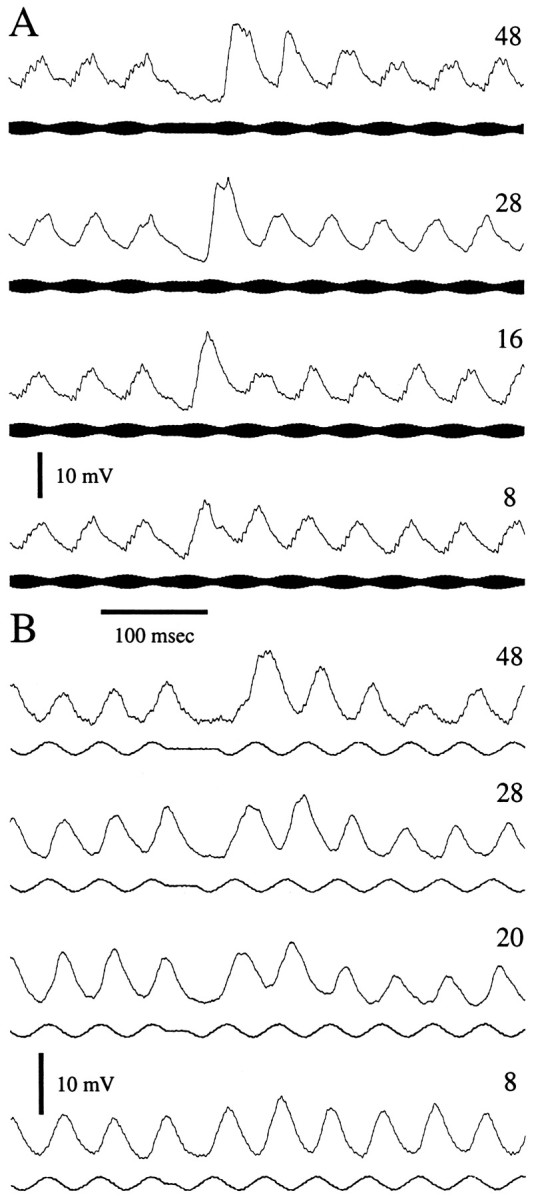
Effects of stimulus gaps on recovery from PSP depression. Averaged segments of recordings from a tuberous (A) and an ampullary (B) neuron. Stimuli were continuous except for gaps, 8–48 msec in duration, in which stimulus amplitude was held constant (tuberous) or set to 0 (ampullary) at ∼1 sec intervals. Stimulus beat rate or frequency was 20 Hz. Neurons were current clamped at −0.1 nA.Traces are the averaged responses to 4–8 repetitions of the stimulus.
All six neurons studied showed full recovery of initial PSP amplitude for gaps >100–150 msec (median = 96 msec; range, 46–150 msec) (Fig. 8). However, as shown above, the time course of PSP depression appeared to be a complex function of the gap interval.
Fig. 8.
Time course of recovery from PSP depression. Recovery values represent the ratio of the amplitude of the first PSP after a stimulus gap of 8–200 msec to that of the first PSP after a stimulus gap of 1 sec. Data are from six neurons. Open circles are measurements from the neurons shown in Figure 7A. Open squaresare from Figure 7B.
Other experiments revealed that recovery from PSP depression was not simply a function of gap duration. Interestingly, when the phase of the stimulus was adjusted such that the gap was an excitatory stimulus, a PSP was produced, and the depression process was reversed (Fig.9). In the case shown in Figure9A, gaps of 8–48 msec were inserted in the rising phase of stimulus amplitude. Because this was primarily an E-type neuron (excited by amplitude rises), a PSP was elicited during the gap. Remarkably, for excitatory gaps of 16 msec or greater, the next rise in stimulus amplitude, at the beginning of the next burst, evoked a PSP that was of the same amplitude as PSPs at the onset of a stimulus burst after a 1 sec gap. This “resetting” of the depression process occurred despite the fact that the PSP peaks were ≤50 msec apart throughout the stimulation and gap periods.
Fig. 9.
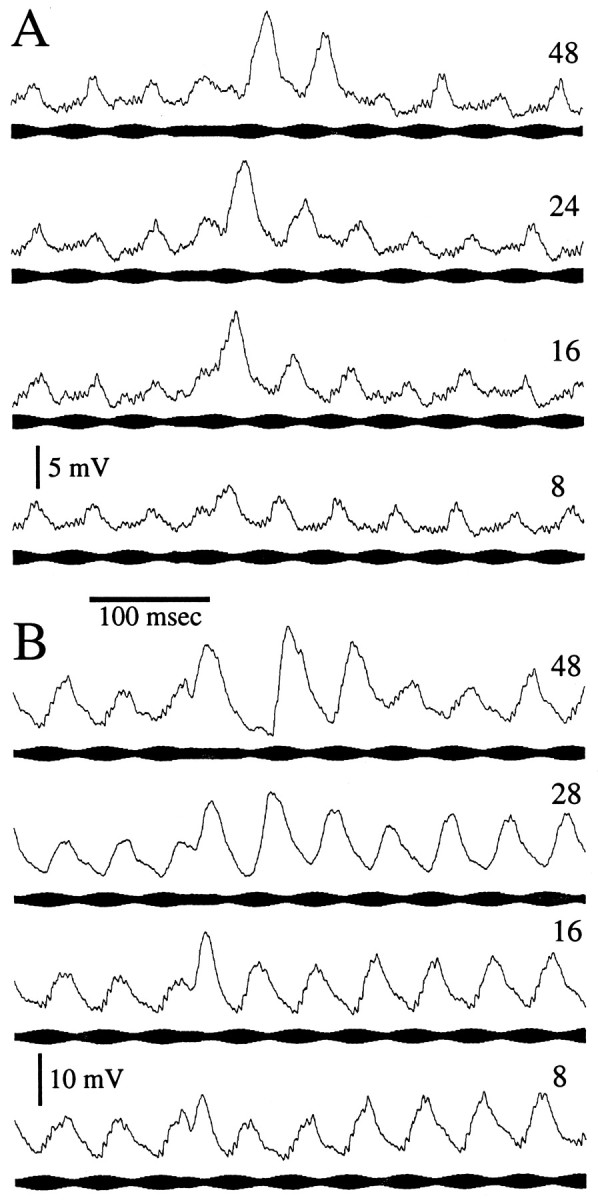
Resetting of PSP depression by a low–temporal frequency excitatory stimulus. A, Responses of an E-type toral neuron to 20 Hz beat rate stimuli in which 8–48 msec constant-amplitude segments were embedded. Holding current was −0.3 nA. B, Responses of an I-type toral neuron to 20 Hz beat rate stimuli in which similar duration constant-amplitude segments were embedded. Holding current was −0.1 nA.
The recordings displayed in Figure 9B show a similar resetting of PSP amplitude for an I unit. In this case, a decrease in stimulus amplitude was the effective stimulus, and the PSP during the gap was broadened on its leading edge. In contrast to that in Figure9A, the PSP elicited by the gap was, for gaps of 16 msec or greater, of nearly full (nondepressed) amplitude. For gaps of 48 msec or greater, the subsequent PSP was at full amplitude. For gap durations of 16 and 28 msec, successive PSP peaks were not separated by >51 msec throughout the stimuli. Also, throughout these stimulation periods the membrane potential only briefly (<10 msec) remained near that seen when the gaps were ineffective stimuli. When the opposite phase gap configuration was used, such that gaps were inserted into the falling (in this case ineffective) phase of stimulus amplitude (Fig.7A), full recovery of PSP amplitude occurred only when ∼70 msec separated the last PSP in response to a burst from the first PSP in the next burst.
Finally, we determined whether postsynaptic fluctuations in the membrane potential were sufficient to induce depression. Voltage fluctuations were produced by injecting constant-frequency bursts of sinusoidal current (0.1 nA peak-to-peak; 1 sec duration). The temporal composition of these bursts was identical to that of sensory bursts. Injection of sinusoidal current bursts never elicited short-term depression even in neurons that showed strong PSP depression to sensory stimuli (Fig. 10). If depression resulted from a time-dependent increase in conductance, the amplitude of these fluctuations and the hyperpolarization induced by the −0.1 nA holding current should decrease over time. These data show that PSP depression does not simply result from postsynaptic conductance changes in response to high-frequency fluctuations in the membrane potential.
Fig. 10.
Current injection alone does not elicit PSP depression. A, Evidence of PSP depression in this neuron. The bottom trace shows 6 dB of depression to 30 Hz beat rate sensory stimuli. B,Responses to positive-going 0.1 nA sinusoidal current injection. Note that the amplitude of these voltage fluctuations did not change throughout the current injection stimuli. Holding current was −0.1 nA.
DISCUSSION
We examined the role that frequency-dependent short-term depression of PSP amplitude plays in behaviorally relevant temporal filtering. The principle findings were that (1) fast beat rates were more effective in eliciting decelerations of the fish’s EOD when presented discontinuously (at a rate of 5 Hz) versus continuously and (2) PSP depression primarily accounts for the low-pass temporal-filtering characteristics of toral neurons beyond that attributable to their passive and active membrane properties.
Behavioral considerations
Mechanisms for generating low-pass temporal filtering are only adaptive if they do not preclude detection of slow changes in signal amplitude that are concurrent with fast temporal fluctuations. An important question, therefore, is whether processes for PSP depression impede responses to slow changes in signal amplitude that are concurrent with or directly follow fast AMs.
Rose et al. (1994) showed that superimposing fast modulations onto slow modulations did not attenuate the slow PSPs of low-pass toral neurons. Because PSP depression was not measured in that study, the findings suggest, but do not prove, that PSP depression does not preclude response to low–temporal frequency information.
Remarkably, the present study shows that even a brief low–temporal frequency component, e.g., maintaining constant signal amplitude (peak-to-peak) for as little as 16 msec, could evoke large PSPs and partially reset the sensitivity of the cell to fast fluctuations of signal amplitude. In these cases the strong response that occurred at or followed the low–temporal frequency component could not be accounted for simply by recovery time; PSP depression could be reset even when the interval between successive PSP peaks was ∼50 msec or less.
With these findings in mind, PSP depression can be viewed from a “learning perspective.” By sampling several beat cycles, the system evaluates the temporal frequency of the stimulus and, over time, attenuates its responses if the temporal frequency is above a particular value. This PSP depression process, a potential substrate of habituation, can then be reversed either by a sufficient gap or by a low–temporal frequency (sensitizing) component in the stimulus. This process may permit fish, therefore, to habituate to the rapid fluctuations in signal amplitude, such as those resulting from jamming, while preserving its capacity to respond to slow modulations, as are experienced during electrolocation of objects. This process may also allow for the detection of the low-frequency components resulting from “chirps,” brief (up to ∼100 msec) interruptions in a fish’s EOD pattern, that are involved in social communication. Importantly, this neural dishabituation does not occur in response to any change in the temporal pattern of the stimulus. For example, PSP depression develops while the stimulus beat rate was changed slowly from 2 to 30 Hz.
PSP depression may also contribute to the determination of direction of relative movement of images over the sensory array. In the electrosensory system, as in mammalian visual systems, selectivity for the direction of stimulus movement (Reid at al., 1991; Jagadeesh et al., 1993) is seen at sites, or postsynaptic to sites, in the respective pathways in which low-pass filtering and short-term, frequency-dependent depression/adaptation are also found [electrosensory system (Bastian, 1982; Heiligenberg and Rose, 1987); visual system (Orban et al., 1985)]. In visual cortex, short-term synaptic depression, apparently because of presynaptic processes, underlies their adaptation properties (Varela et al., 1997). These findings have led investigators to postulate that adaptation (short-term synaptic depression) might be a mechanism for generating shifts in the timing of peak responses resulting from stimulation of particular sites on the body surface. These temporal shifts could be used in directional selectivity (Jagadeesh et al., 1993; Chance et al., 1998). This hypothesis could be tested in the electrosensory system by determining whether neurons that show strong PSP depression respond in a directionally selective manner to the motion of electric images.
Finally, measurements in this study of the “AM deceleration response” (Takizawa et al., 1999) for continuous 20 Hz beat stimuli versus 20 Hz beat stimuli that were gated at 5 Hz provide strong evidence that frequency-dependent short-term depression of PSP amplitude is an important component of the AM filter. The stimulus that was gated on and off at a rate of 5 Hz elicited responses that were approximately sixfold stronger than those for the continuous stimulus. This result suggests that PSP depression greatly enhanced the rejection of sustained high–temporal frequency signals. For 5 Hz–gated stimuli, the magnitude of deceleration responses declined by ∼30% as the beat rate was changed from 10 to 30 Hz. This finding probably reflects the role of passive filtering properties of toral neurons (Fortune and Rose 1997b). Amplification of PSP amplitude, attributable to active membrane properties, at low beat rates (5 Hz) may account for the slightly larger deceleration response at 5 Hz, relative to that of the 10 Hz stimulus that was gated at a rate of 5 Hz.
Evidence of gain control
The detailed time course of PSP depression suggests that it might be appropriately viewed as a gain-control process. For fast temporal frequencies, in many cases, PSP amplitude was largest for the first few beat cycles, quickly decreased to its lowest values within the next 100 msec, and then increased to near steady-state values. That is, during the initial segment of the burst stimulus, the short-term depression process overshot its intended set point. With lower stimulus amplitudes or depths of modulation, the degree of overshooting the set point was less. This gain-control hypothesis should be explored further in future studies by testing over a wider range of stimulus amplitudes or modulation depths and durations.
Gain control has been demonstrated in the responses of ELL pyramidal neurons to stepwise increases or decreases in stimulus amplitude and is mediated by the negative feedback projection from the N. praeeminentialis to the ELL (Bastian, 1986a,b). This descending control system, however, appears to reduce the responses of ELL pyramidal cells to very slow beat rates but does not give rise to low-pass temporal filtering; ELL cells showing strong gain control nevertheless respond well to fast beat rates. This conclusion is also supported by the minimal low-pass filtering exhibited by most ELL neurons for beat rates up to ∼20 Hz (Bastian, 1981; Shumway, 1989). It appears likely, therefore, that the frequency-dependent PSP depression demonstrated in the present study occurs in the torus.
Recovery from PSP depression seems to be a complex process. The gap in stimulation that was required for full recovery of initial PSP amplitude, i.e., amplitude of the first PSP after the gap, was in most cases ∼100–150 msec. With stimulus gaps of this magnitude, however, the amplitude of the second and third PSPs was strongly depressed relative to control values (PSPs of the same rank after a 1 sec gap). This result is consistent with the idea that synaptic efficacy and synaptic reserve are distinct and under separate regulation (Galarreta and Hestrin, 1998). The time course for full recovery of response appears to be related to the duration of the stimulus and was not investigated systematically in the present study. For a stimulus duration of ∼1 sec, a gap of 1 sec was sufficient for full recovery; however additional work is needed to determine the relationships between stimulus pattern, duration, and consequent recovery times.
Mechanism of short-term PSP depression
The frequency-dependent depression of PSP amplitude in the responses of toral neurons could be caused by cellular or network properties or both. For example, frequency-dependent synaptic depression, as has been shown in the mammalian neocortex (Galarreta and Hestrin, 1998), could underlie the rapid decline in PSP amplitude at stimulation frequencies of 20–30 Hz. In cortical slices, frequency-dependent synaptic depression is strongest for excitatory synapses. In the absence of negative current clamp, the stimulus-related depolarizations in the current study, if of sufficient amplitude, triggered spikes and therefore can be considered to be primarily EPSPs. Frequency-dependent synaptic depression is believed to be a presynaptic process (Torii et al., 1997) and therefore should not be influenced or elicited by postsynaptic manipulations.
The finding that high-frequency fluctuations of the membrane potential induced by current injection did not affect changes in the biophysical properties of neurons or PSP depression is consistent with this idea. Also, holding neurons at levels of current clamp of −0.1 to approximately −0.3 nA failed to alter the magnitude of PSP depression. Holding currents of this magnitude translated to ∼10–30 mV of hyperpolarization and significantly influenced PSP amplitude. Presynaptic mechanisms that could underlie frequency-dependent synaptic depression include depletion of readily releasable vesicles or decreased efficacy of release, possibly involving inactivation of voltage-gated calcium currents (Forsythe et al., 1998). Postsynaptic receptor desensitization (Jones and Westbrook, 1996) could also contribute to frequency-dependent depression of PSP amplitude.
Footnotes
This work was supported by National Science Foundation Grants IBN-9421039 and IBN-91156789 and by National Institutes of Health Fellowship 1-F32 NS 09779.
Correspondence should be addressed to Dr. Gary J. Rose, Department of Biology, University of Utah, 257 South 1400 East, Salt Lake City, UT 84112-0840.
REFERENCES
- 1.Bastian J. Electrolocation. II. The effects of moving objects and other electrical stimuli on the activities of two categories of lateral line lobe cells in Apternotus albifrons. J Comp Physiol [A] 1981;144:481–494. [Google Scholar]
- 2.Bastian J. Vision and electroreception: integration of sensory information in the optic tectum of the weakly electric fish Apternotus albifrons. J Comp Physiol [A] 1982;147:287–297. [Google Scholar]
- 3.Bastian J. Gain control in the electrosensory system: a role for the descending projections to the electrosensory lateral line lobe. J Comp Physiol [A] 1986a;158:505–515. doi: 10.1007/BF00603796. [DOI] [PubMed] [Google Scholar]
- 4.Bastian J. Gain control in the electrosensory system mediated by descending inputs to the electrosensory lateral line lobe. J Neurosci. 1986b;6:553–562. doi: 10.1523/JNEUROSCI.06-02-00553.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastian J, Yuthas J. The jamming avoidance response of Eigenmannia: properties of a diencephalic link between sensory processing and motor output. J Comp Physiol [A] 1984;154:895–908. [Google Scholar]
- 6.Bullock TH, Hamstra RH, Scheich H. The jamming avoidance response of high frequency electric fish. 1. General features. J Comp Physiol [A] 1972;77:1–22. [Google Scholar]
- 7.Chance FS, Nelson SB, Abbott LF. Synaptic depression and the temporal response characteristics of V1 cells. J Neurosci. 1998;18:4785–4799. doi: 10.1523/JNEUROSCI.18-12-04785.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- 9.Fortune ES, Rose GJ. Temporal filtering properties of ampullary electrosensory neurons in the torus semicircularis of Eigenmannia: evolutionary and computational implications. Brain Behav Evol. 1997a;49:312–323. doi: 10.1159/000113000. [DOI] [PubMed] [Google Scholar]
- 10.Fortune ES, Rose GJ. Passive and active membrane properties contribute to the temporal filtering properties of midbrain neurons, in vivo. J Neurosci. 1997b;17:3815–3825. doi: 10.1523/JNEUROSCI.17-10-03815.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galarreta M, Hestrin S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat Neurosci. 1998;1:587–594. doi: 10.1038/2822. [DOI] [PubMed] [Google Scholar]
- 12.Heiligenberg W, Rose GJ. Phase and amplitude computations in the midbrain of an electric fish: intracellular studies of neurons participating in the jamming avoidance response of Eigenmannia. J Neurosci. 1985;5:515–531. doi: 10.1523/JNEUROSCI.05-02-00515.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiligenberg W, Rose GJ. The optic tectum of the gymnotiform electric fish Eigenmannia: labelling of physiologically identified cells. Neuroscience. 1987;22:331–340. doi: 10.1016/0306-4522(87)90224-7. [DOI] [PubMed] [Google Scholar]
- 14.Heiligenberg W, Baker C, Matsubara J. The jamming avoidance response in Eigenmannia revisited: the structure of a neural democracy. J Comp Physiol [A] 1978;127:267–286. [Google Scholar]
- 15.Jagadeesh B, Wheat HS, Ferster D. Linearity of summation of synaptic potentials underlying direction selectivity in simple cells of the cat visual cortex. Science. 1993;262:1901–1904. doi: 10.1126/science.8266083. [DOI] [PubMed] [Google Scholar]
- 16.Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- 17.Matsubara J, Heiligenberg W. How well do electric fish electrolocate under jamming? J Comp Physiol [A] 1978;149:339–351. [Google Scholar]
- 18.Partridge BL, Heiligenberg W, Matsubara J. The neural basis for a sensory filter in the jamming avoidance response: no grandmother cells in sight. J Comp Physiol [A] 1981;145:153–168. [Google Scholar]
- 19.Orban GA, Hoffmann KP, Duysens J. Velocity selectivity in the cat visual system. I. Responses of LGN cells to moving bar stimuli: a comparison of areas 17 and 18. J Neurophysiol. 1985;54:1026–1049. doi: 10.1152/jn.1985.54.4.1026. [DOI] [PubMed] [Google Scholar]
- 20.Reid RC, Soodak RE, Shapley RM. Direction selectivity and spatiotemporal structure of receptive fields of simple cells in cat striate cortex. J Neurophysiol. 1991;66:505–529. doi: 10.1152/jn.1991.66.2.505. [DOI] [PubMed] [Google Scholar]
- 21.Rose GJ, Call SJ. Temporal filtering properties of neurons in the midbrain of an electric fish: implications for the function of dendritic spines. J Neurosci. 1993;13:1178–1189. doi: 10.1523/JNEUROSCI.13-03-01178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose GJ, Fortune ES. New techniques for making whole-cell recordings from CNS neurons in vivo. Neurosci Res. 1996;26:89–94. doi: 10.1016/0168-0102(96)01074-7. [DOI] [PubMed] [Google Scholar]
- 23.Rose GJ, Etter N, Alder TB. Responses of electrosensory neurons in the torus semicircularis of Eigenmannia to complex beat stimuli: testing hypotheses of temporal filtering. J Comp Physiol [A] 1994;175:467–474. [Google Scholar]
- 24.Shumway C. Multiple electrosensory maps in the medulla of weakly electric Gymnotiform fish. I. Physiological differences. J Neurosci. 1989;9:4388–4399. doi: 10.1523/JNEUROSCI.09-12-04388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takizawa Y, Rose GJ, Kawasaki M. Resolving competing theories for control of the jamming avoidance response: the role of amplitude modulations in electric organ discharge decelerations. J Exp Biol. 1999;202:1281–1289. doi: 10.1242/jeb.202.10.1377. [DOI] [PubMed] [Google Scholar]
- 26.Torii N, Tsumoto T, Uno L, Astrelin AV, Voronin LL. Quantal analysis suggests presynaptic involvement in expression of neocortical short- and long-term depression. Neuroscience. 1997;79:317–321. doi: 10.1016/s0306-4522(97)00129-2. [DOI] [PubMed] [Google Scholar]
- 27.Varela JA, Sen K, Gibson J, Fost J, Abbott LF, Nelson SB. A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. J Neurosci. 1997;17:7926–7940. doi: 10.1523/JNEUROSCI.17-20-07926.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]