Abstract
Glial cell line-derived neurotrophic factor (GDNF) is a potent neurotrophic factor for several populations of CNS and peripheral neurons. Synthesis and storage of GDNF by the neuron-like adrenal medullary cells suggest roles in adrenal functions and/or in the maintenance of spinal cord neurons that innervate the adrenal medulla. We show that unilateral adrenomedullectomy causes degeneration of all sympathetic preganglionic neurons within the intermediolateral column (IML) of spinal cord segments T7–T10 that project to the adrenal medulla. In situ hybridization revealed that IML neurons express the glycosylphosphatidylinositol-linked α receptor 1 and c-Ret receptors, which are essential for GDNF signaling. IML neurons also display immunoreactivity for transforming growth factor-β (TGF-β) receptor II. Administration of GDNF (recombinant human, 1 μg) in Gelfoam implanted into the medullectomized adrenal gland rescued all Fluoro-Gold-labeled preganglionic neurons projecting to the adrenal medulla after four weeks. Cytochrome c applied as a control protein was not effective. The protective effect of GDNF was prevented by co-administration to the Gelfoam of neutralizing antibodies recognizing all three TGF-β isoforms but not GDNF. This suggests that the presence of endogenous TGF-β was essential for permitting a neurotrophic effect of GDNF. Our data indicate that GDNF has a capacity to protect a population of autonomic spinal cord neurons from target-deprived cell death. Furthermore, our results demonstrate for the first time that the previously reported requirement of TGF-β for permitting trophic actions of GDNF in vitro (Krieglstein et al., 1998) also applies to the in vivo situation.
Keywords: preganglionic sympathetic neurons, intermediolateral column, adrenal chromaffin cells, GDNF receptors, TGF-β receptors, spinal cord
Glial cell line-derived neurotrophic factor (GDNF), a member of the superfamily of transforming growth factor-βs (TGF-βs), is a potent neurotrophic molecule for a variety of peripheral and CNS neuron populations. GDNF signals through a receptor complex, which consists of the transmembrane tyrosine kinase receptor c-Ret and a glycosylphosphatidylinositol-linked α receptor (GFRα), of which four isoforms are presently known (Enokido et al., 1998; for review, see Unsicker et al., 1998). The nigrostriatal dopaminergic projection is the only system known to date in which a role for GDNF as a target-derived neurotrophic factor has been convincingly demonstrated (Beck et al., 1995; Kearns and Gash, 1995;Sauer et al., 1995; Tomac et al., 1995a,b; for review, see Olson, 1997). GDNF is synthesized in the striatum and axonally transported by dopaminergic cells in the substantia nigra, which express c-Ret and GFRα. The protective effects of GDNF for target-deprived dopaminergic neurons in animal models of Parkinson’s disease (for review, seeOlson, 1997) has raised hopes that GDNF may have relevance in the treatment of this neurodegenerative disorder.
Several lines of evidence suggest that GDNF and its receptors may have important roles in the development of the peripheral autonomic nervous system. Mice deficient for GDNF or c-Ret (Schuchardt et al., 1994;Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996) lack large portions of the enteric nervous system and display significant neuron losses in the superior cervical ganglion of the paravertebral sympathetic chain. Alterations in the enteric nervous system of GDNF mutant mice are consistent with synthesis of GDNF in smooth muscle layers of the gastrointestinal tract, i.e., in the target areas of enteric neurons (Suvanto et al., 1996; Bar et al., 1997). We have previously shown that GDNF is stored in and released from another major target tissue of autonomic neurons, the adrenal medulla (Krieglstein et al., 1996, 1998). The neuron-like chromaffin cells of the adrenal medulla receive a dense cholinergic projection from neurons, whose cell bodies are located in the intermediolateral column (IML) of the spinal cord (Schramm et al., 1975). Degeneration and death of these neurons after selective destruction of their target in the adult rat are well documented (Schober et al., 1998a). In the present study we have addressed the question of whether GDNF may protect these IML neurons after ablation of their target. We show that IML neurons express the GDNF receptors c-Ret and GFRα-1 and are fully protected by GDNF administered to the medullectomized adrenal gland. To understand mechanisms underlying the neurotrophic effect of GDNF in vivo, we asked whether TGF-β, which, like GDNF, is stored in adrenal medullary chromaffin cells (Krieglstein and Unsicker, 1995;Krieglstein et al., 1998), was required for permitting GDNF to act as a neurotrophic factor (Krieglstein et al., 1998). We show now for the first time that the previously documented requirement of TGF-β in neurotrophic actions of GDNF in vitro also applies to its neurotrophic potential in vivo.
A preliminary account of our study has been presented in abstract form (Schober et al., 1998b).
MATERIALS AND METHODS
Animals. Thirty-five Hanover–Wistar male rats (250 gm) were used. They were kept under standard laboratory conditions with food and water ad libitum and a 12 hr light/dark cycle. Rats were killed according to S1 method of humane killing (Animal Scientific Procedures, 1986, United Kingdom).
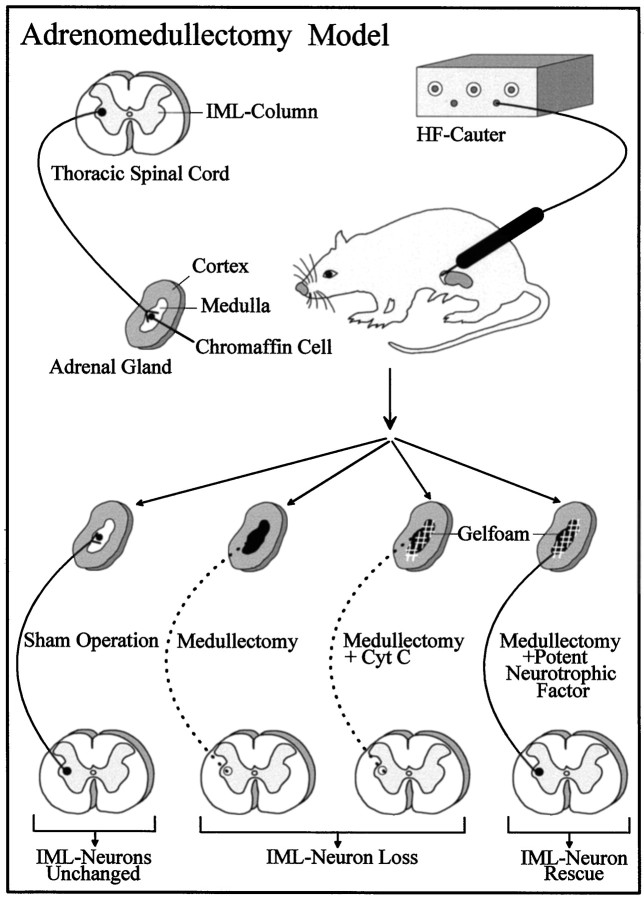
Adrenomedullectomy (compare Fig. 1). Rats were deeply anesthetized by an intraperitoneal injection of 5% chloral hydrate (1 ml/100 gm of body weight). The left adrenal gland was exposed retroperitoneally and medullectomized by electrocauterization using a fine-needle electrode (diameter, 0.5 mm) that was connected to a radiotome (Martin ME 80). The free tip of the isolated electrode was inserted carefully through the cortex into the adrenal medulla. The coagulation was performed by a brief pulse. To test the completeness of the chromaffin tissue destruction, histological control examinations were performed. Thereafter, a small piece (1 mm3) of Gelfoam (Spongostan; Ferrosan, Soeburg, Denmark) soaked with GDNF (recombinant human, lot 065641; IC Chemikalien) or cytochromec (Cyt c, 1 μg/Gelfoam; Serva, Heidelberg, Germany) was implanted into the wound cavity and covered by a small drop of tissue glue (Roth GmbH). In an additional series of experiments GDNF (1 μg) and a neutralizing pan-TGF-β antibody (5 μg/Gelfoam; Genzyme, Boston, MA), which recognizes all three TGF-β isoforms (cf.Krieglstein et al., 1998), were co-administered in Gelfoam to the medullectomized adrenal gland of six male rats. Controls were performed by substituting of the pan-TGF-β antibody by a horse anti-mouse IgG (5 μg/Gelfoam; Dako, Glostrup, Denmark), which was co-implanted with GDNF (1 μg) into adrenomedullectomized rats (n = 6).
Fig. 1.
Illustration of the adrenomedullectomy model used for studying the neuroprotective effect of GDNF for target-deprived IML neurons in vivo.
Specificity controls for the pan-TGF-β antibody.Specificity of the pan-TGF-β antibody was tested by dot blot analysis. Two microliters of TGF-β3 (=10, 1, and 0.1 ng/2 μl) and 2 μl of GDNF, FGF-2, BDNF, and CNTF (=100, 30, and 3 ng/2 μl) were loaded onto nitrocellulose membrane. The membrane was blocked with 3% low-fat milk powder and 0.1% BSA in Tris-buffered saline (TBS, pH 7.3) incubated with primary antibody (5 μg/ml pan-anti-TGF-β in 0.1% BSA and TBS) overnight at 4°C followed by peroxidase-conjugated anti-mouse antibody (1:2000 in 0.1% BSA and TBS). Finally, the membrane was developed using the Amersham (Arlington Heights, IL) enhanced chemiluminescence detection system.
Fluoro-Gold labeling and tissue preparation. Intraperitoneal injection of the fluorescent tracer Fluoro-Gold (FG) labels the entire population of sympathetic preganglionic neurons in the adult rat spinal cord (compare Fig. 2; Anderson and Edwards, 1994; Schober et al., 1998a). Twenty-six days after unilateral adrenomedullectomy, rats received an intraperitoneal injection of 400 μl FG (0.2%, Fluorochrome Inc.). Forty-eight hours later animals were reanesthetized and transcardially perfused by 4% paraformaldehyde (PFA). The upper thoracic spinal cord was exposed, and spinal cord segments T7-T10 and the operated side were marked. Adrenal glands were removed for monitoring completeness of the medullectomy. After 12 hr of post-fixation (4% PFA), longitudinal serial sections of the spinal cord were performed on a vibrating blade microtome (VT 1000 E; Leica, Nussloch, Germany), collected free-floating, and mounted on gelatin-coated slides.
Fig. 2.
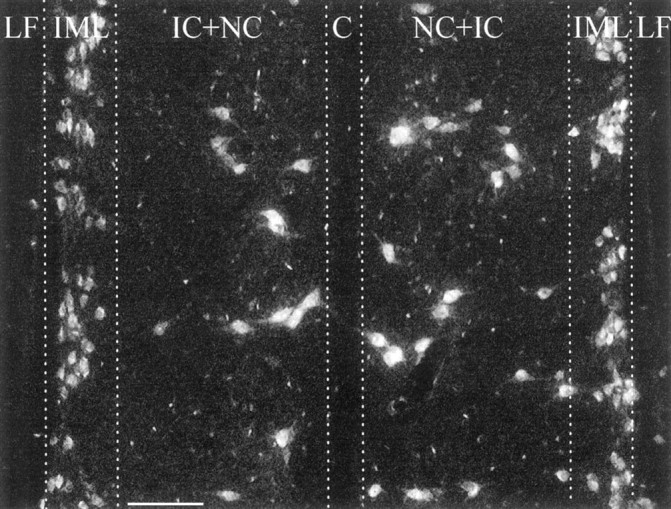
Identification of preganglionic sympathetic neurons in the spinal cord (longitudinal section at level T9-T10). Neurons were labeled by intraperitoneally injected FG.LF, Lateral funciculus; IML, intermediolateral column; IC+NC, intercalated nucleus and central nucleus; C, central canal. Scale bar, 100 μm.
Quantitative analysis. Numbers of FG-labeled preganglionic neurons were determined by cell counts of complete series of horizontal sections through the IML column of the upper thoracic spinal cord (T7-T10). In all animal groups (GDNF, Cyt c, GDNF plus IgG, and GDNF plus anti-pan-TGF-β), counts were performed on the left (operated) and right (control) sides of the spinal cord. Sham-operated and untreated animals were used to determine the left/right ratio of total numbers of FG-labeled IML neurons. Neuron counts on the right side (control side) were set as 100%. Sections were examined by a Zeiss Axiophot fluorescence microscope using a UV filter set (Zeiss; excitation filter, 390–420 nm; barrier filter, 425–450 nm). Only brightly fluorescent IML neurons containing a clearly visible nucleus were counted. Total numbers were corrected for possible double counts of split nuclei according to Abercrombie’s formula (Konigsmark, 1970). Results are given as mean values in percent ± SEM and tested for statistical significance of side differences by Student’s ttest.
RNA preparation and RT-PCR. RT-PCR was applied to investigate expression of GDNF in the adult rat adrenal medulla. As a positive control the B49 cell line was used. Adult female Sprague Dawley rats were obtained from Charles River Laboratories (Sulzfeld, Germany). Adrenal glands were quickly removed, cleaned, snap frozen in isopentane, and collected on dry ice. Adrenal glands were then sliced with a razor blade, and adrenal medullas were microdissected with stainless steel punching needles. Cultures of B49 cell lines were washed twice in sterile PBS (Life Technologies, Gaithersburg, MD), scraped off from culture dishes, and snap frozen. Dissected tissues and harvested cells were homogenized with a Bandelin Sonopuls HD 2070 microsonicator. Total RNA was then isolated by an RNeasy Mini kit (Qiagen) according to the manufacturer‘s instructions. Total cellular RNA was treated with RQ1 DNase (Promega, Madison, WI) to exclude amplification of contaminating genomic DNA in subsequent manipulations and quantified spectrophotometrically. First-strand cDNA was reverse-transcribed from 1 μg of total RNA using random hexamer primers in a 20 μl reaction volume. Reactions consisted of 1 μg of total RNA and final concentrations of 1× first-strand buffer (5× first strand buffer, in mm: 250 Tris-HCl, pH 8.3, 375 KCl, and 15 MgCl2; Life Technologies), 10 mm dithiothreitol, a 1 mm concentration of each dNTP (Pharmacia, Piscataway, NJ), 25 ng/μl random hexamer primers (Boehringer Mannheim, Mannheim, Germany), 1 U/μl RNase inhibitor (MBI Fermentas), and 20 U/μl Moloney murine leukemia virus reverse transcriptase (Life Technologies). The reaction mixture was incubated at 37°C for 60 min. After reverse transcription, 3.5 μl of the cDNA samples were subjected to PCR amplification using specific primers described by Springer et al. (1994). Reactions were performed in a Perkin-Elmer (Norwalk, CT) GeneAmp 9600 thermal cycler PCR system in 0.2 ml thin-walled reaction tubes using the “hot-start” method. Reagents were assembled in a final volume of 100 μl, and final concentrations of reagents were as follows: 3.5 μl of first-strand cDNA, 1 μm forward primer, 1 μm reverse primer, 1× PCR buffer (10× PCR buffer, in mm: 200 Tris-HCl, pH 8.4, and 500 KCl; Life Technologies), 2.5 mmMgCl2, 0.1 mm dNTPs, and RNase-free water to 100 μl. Samples were initially denatured at 94°C for 4 min, and 2.5 U of recombinant Taq DNA polymerase (Life Technologies) were then added. Thermocycling parameters were then 30 sec denaturation at 94°C, 45 sec annealing at 65°C, and 1 min extension at 72°C repeated for 35 cycles with a final extension step at 72°C for 5 min. Eight microliters of PCR reactions were analyzed by electrophoresing 8 μl aliquots in a 2% agarose gel (Life Technologies) in 1× TAE buffer (in m: 0.04 Tris-acetate and 0.001 EDTA). After soaking gels in 0.5 μg/ml ethidium bromide solution in 1× TAE for 20 min, reaction products were visualized by UV transilluminator (Renner GmbH). The bands were of the expected size (633 and 555 bp). Images were captured by a computer-assisted gel documentation system (Intas). The identity of the amplified products was further checked with appropriate restriction enzymes.
Immunocytochemistry. Perfused adrenal glands and spinal cords were cryoprotected overnight (30% sucrose) and cut on a cryostat (10 μm). Sections were then mounted on gelatin-coated slides, dried at room temperature, for 30 min, and placed in 0.1 mphosphate buffer (PB), pH 7.4. Nonspecific binding sites were blocked by preincubation with 5% normal goat serum containing 0.1% Triton X-100 diluted in PB for 1 hr at room temperature. Cryosections of adrenal glands were immunostained as follows: (1) incubation with GDNF polyclonal antibody (D-20, sc-328; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:200 in PB for 24 hr at room temperature; (2) incubation with biotinylated anti-rabbit IgG (diluted 1:200 in PB; Vector Laboratories, Burlingame, CA); and (3) incubation with Cy3-conjugated streptavidin (indocarbocyanine; Jackson ImmunoResearch, West Grove, PA) diluted 1:4000. Controls were performed by (1) preabsorbing the antibody to a 20-fold molar excess of the antigen, (2) using the corresponding normal serum, (3) omitting the respective antiserum. For the demonstration of the TGF-βII receptor (TβRII) immunoreactivity, paraffin sections (4 μm) from adult rat spinal cord were cut and mounted onto glycerol–gelatin-coated slides. Sections were deparaffinated, rehydrated, and incubated with polyclonal rabbit anti-TβR-II antibody (1:200, sc-400; Santa Cruz) for 48 hr at 4°C, followed by incubation with a Cy3-conjugated anti-rabbit IgG (1:200; Dianova, Hamburg, Germany) for 1 hr at room temperature. Finally, sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 1:1000; Boehringer Mannheim), rinsed three times in PB, dried, and mounted with Kaiser’s glycerol gelatin.
In situ hybridization of GFRα-1, GFRα-2, and c-Ret receptor mRNAs. In situ hybridizations (ISHs) were performed on paraffin sections of the adrenal gland and on longitudinal vibratome sections of the thoracic spinal cord, essentially as described byArumäe et al. (1993). Few modifications were applied. The digestion time with proteinase K was prolonged to 30 min, and the prehybridization using the hybridization mixture was performed at 52°C for 2 hr before hybridization. All cRNA probes used were synthesized from linearized plasmids and labeled by35S-UTP: (1) rat GFRα-1, nucleotides 294–1039 (Suvanto et al., 1997); (2) rat GFRα-2, full-length cDNA (Luukko et al., 1997); and (3) mouse c-Ret, nucleotides 2534–3217, covering the tyrosine kinase domain (Reeben et al., 1998). After hybridization, all sections were dipped and exposed for 2–3 weeks, counterstained with hematoxylin, air-dried and embedded in DePex (Serva, Heidelberg, Germany). Generally, no hybridization signal was detected with the probes on sense orientation.
Retrograde axonal transport of GDNF. Left adrenal glands of 12 anesthetized adult female Sprague Dawley rats were exposed for microinjection using a 10 μl Hamilton syringe. One group (n = 8) received intramedullary injections of125I-GDNF (human recombinant, 100 μCi/μg) in 2 μl/adrenal (50 ng/μl; injection rate, 1 μl/min). A control group (n = 4) received the same volume (2 μl) consisting of125I-GDNF and a 20-fold excess of cold GDNF. After 18 hr (n = 4) or 24 hr (n = 8), respectively, animals were killed by decapitation, spinal cord segments T7-T10 were frozen on dry ice, and horizontal serial cryostat sections (14 μm) were collected on coated slides, processed for emulsion autoradiography (cf. Grothe and Unsicker, 1992), and counterstained with cresyl violet.
RESULTS
GDNF rescues preganglionic neurons after unilateral adrenomedullectomy
We have previously shown that unilateral adrenomedullectomy in adult rats causes the loss of all IML neurons in spinal cord segments T7-T10 that project to the adrenal medulla (cf. Schober et al., 1998a). If GDNF had a role in maintaining IML neurons, supplementation of GDNF to the medullectomized adrenal gland should protect these neurons from death induced by ablation of their GDNF-providing target cells. Animals were unilaterally adrenomedullectomized by electrocauterization and received a Gelfoam implant into the organ cavity (compare Fig. 1). Gelfoams were soaked with GDNF (1 μg) or the nontrophic control protein cytochromec. Twenty-six days after surgery animals were intraperitoneally injected with FG to label all viable IML neurons (compare Fig. 2). Forty-eight hours later animals were killed and perfused, and adrenal glands and spinal cords were processed for histology. In previous experiments we had established (Schober et al., 1998a) that IML neurons labeled by intraperitoneal injection of FG included the subset of neurons that project to the adrenal medulla and can be retrogradely labeled with fast blue. Counts of IML neurons containing FG revealed a 24% loss on the operated compared with the nonoperated side in animals that had been treated with cytochrome c containing Gelfoam implants (Fig. 3A). From previous studies (Blottner et al., 1989a,b; Schober et al., 1998a) we know that this 24% loss reflects the loss of all IML neurons that project to the adrenal medulla. Animals that had received GDNF-containing Gelfoam implants did not show a significant decrease in IML neuron numbers on the lesioned compared with the unlesioned side (Fig. 3B). Thus, implantation of GDNF-containing Gelfoams into the medullectomized adrenal gland prevents death of IML neurons induced by target deprivation.
Fig. 3.
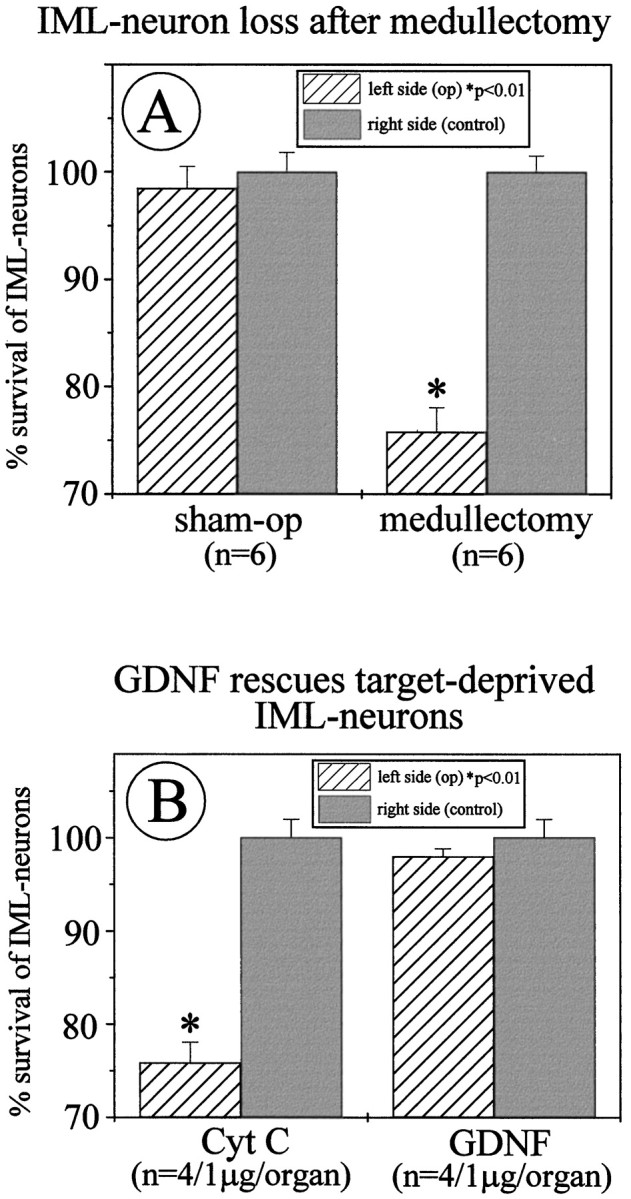
A, Quantitative determination of IML neuron losses after unilateral adrenomedullectomy. Twenty-four percent of FG-labeled IML neurons within spinal cord segments T7-T10 have disappeared. These neurons constitute the full set of IML neurons that project to the adrenal medulla. B, Administration of GDNF (1 μg) in Gelfoam implants to the medullectomized adrenal gland rescues all target-deprived IML neurons after 4 weeks compared with cytochrome c treatments.
GDNF and GDNF receptors in the adult rat adrenal gland and spinal cord
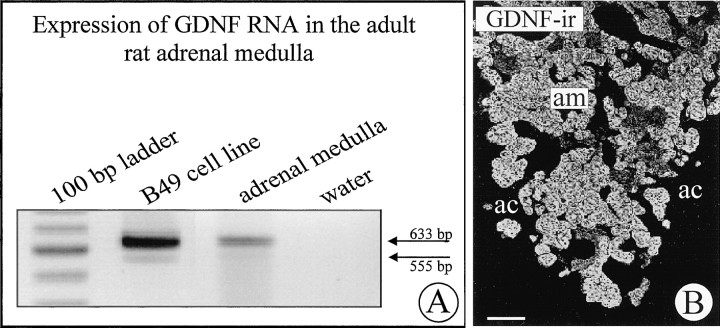
To investigate whether neurotrophic actions of GDNF on IML neurons may reflect a physiological role of GDNF beyond its documented pharmacological effect, we studied expression of GDNF and its receptors in the adult rat adrenal gland and IML of the spinal cord. Figure4A reveals the presence of GDNF mRNA in micropunches of tissue from the adult rat adrenal medulla. Figure 4B shows that GDNF immunoreactivity can be localized to the chromaffin cells of adult rat adrenal medulla. ISH revealed that adrenal medullary cells did not express the GDNF receptors c-Ret and GFRα-1 (results not shown). However, as shown in Figure 5, neurons in the IML of the spinal cord clearly expressed both c-Ret and GFRα-1 mRNA, suggesting that this neuron population and its axons that project to the adrenal medulla are targets for GDNF.
Fig. 4.
A, Expression of GDNF mRNA in the adult rat adrenal medulla and in the B49 cell line as revealed by RT-PCR. B, GDNF-ir in chromaffin cells of the adult rat adrenal medulla (am). The adrenal cortex is devoid of detectable levels of GDNF-ir. Scale bar, 100 μm.
Fig. 5.
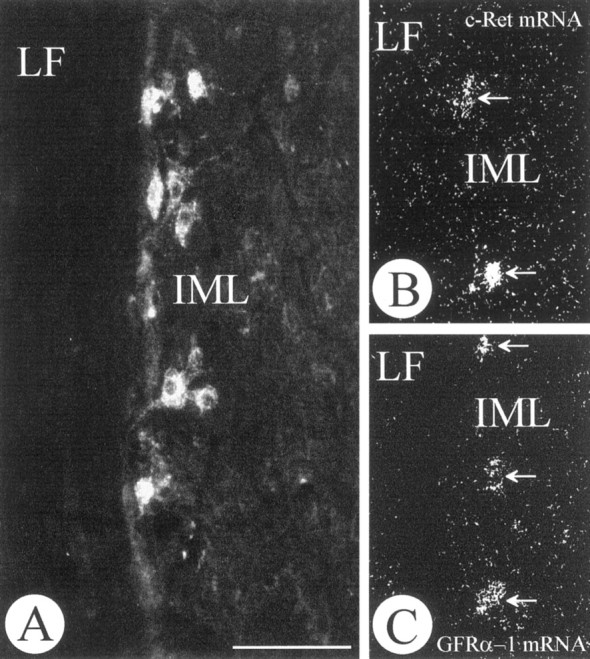
Longitudinal section of the rat thoracic spinal cord at the level T9-T10. IML neurons were identified by retrograde tracing with FG from the adrenal medulla (A). ISH reveals that IML neurons (white arrows) express the GDNF receptors c-Ret (B) and GFRα-1.LF, Lateral funciculus; IML, intermediolateral column. Scale bar, 100 μm.
Retrograde transport of GDNF from the adrenal gland to the spinal cord was not detectable
To address molecular mechanisms underlying the neuroprotective effect of GDNF, we next investigated whether its administration to the intact and medullectomized adrenal gland was followed by axonal transport to the spinal cord. Autoradiography was performed on longitudinal sections of the spinal cord (T7-T10) of eight animals each after 18 and 24 hr, respectively, after injection of iodinated GDNF into unlesioned or medullectomized adrenal glands. In a separate series of experiments iodinated GDNF was unilaterally injected into the striatum of adult rats (Tomac et al., 1995b). Although transport of GDNF occurred in the nigrostriatal dopaminergic system (Tomac et al., 1995b), we did not find evidence for transport of GDNF from the adrenal medulla to the spinal cord. Moreover, intra-adrenal implants of GDNF (10 μg) did not result in an immunocytochemically detectable signal in the spinal cord after 36 hr (results not shown).
GDNF requires TGF-β to exert its neurotrophic potential on IML spinal cord neurons
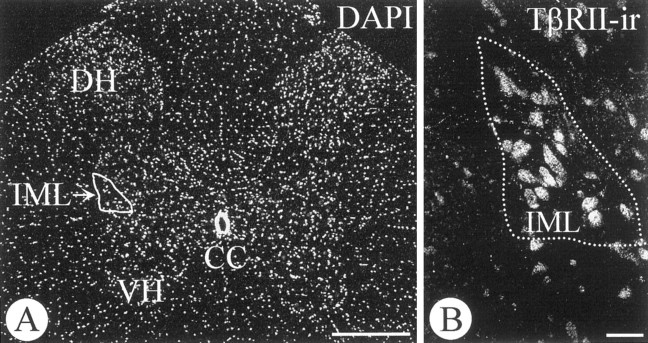
In previous studies we have documented (Krieglstein et al., 1998) that GDNF requires TGF-β for exerting its full neurotrophic potential on peripheral and CNS neurons in vitro. TGF-β is expressed by both adrenal medullary (Krieglstein and Unsicker, 1995; Blottner et al., 1996) and cortical cells (Thompson et al., 1989). To investigate putative implications of TGF-β in the neurotrophic action of GDNF on IML neurons after adrenomedullectomy, a neutralizing pan-TGF-β antibody (5 μg) recognizing all three mammalian TGF-β isoforms (-β1, -β2, and -β3) was co-administered together with GDNF (1 μg) in Gelfoam implants to the medullectomized adrenal gland. As shown in Figure 6A, the pan-TGF-β antibody fully prevented the protective effect of GDNF on IML spinal cord neurons. Figure 6B provides evidence that the antibody to TGF-β specifically recognized TGF-β, but not GDNF, FGF-2, BDNF, or CNTF. Finally, Figure7 documents the presence of the TβRII-ir on IML neurons, indicating that TGF-β can affect this neuron population. Together these data suggest, in extension of our previous in vitro demonstration (Krieglstein et al., 1998), that GDNF also requires TGF-β for exerting its trophic effect in anin vivo model of neuron death caused by target deprivation.
Fig. 6.
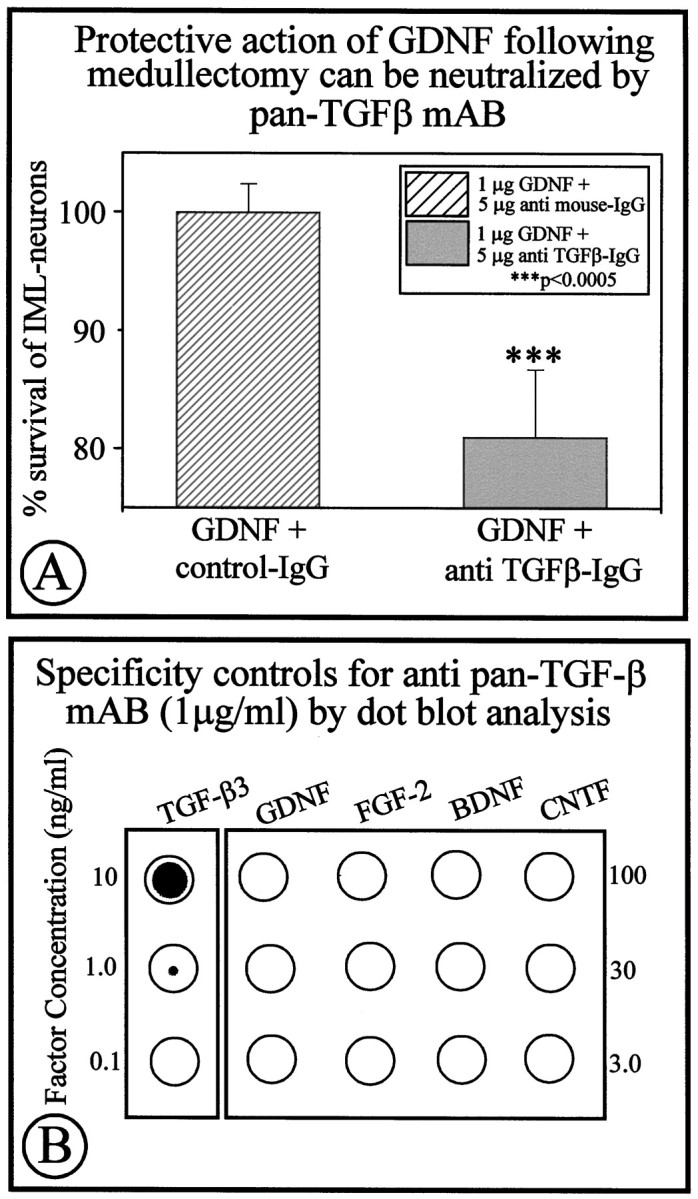
A, Co-application of a neutralizing anti-pan-TGF-β antibody significantly reduces the neuroprotective effect of GDNF after target deprivation of IML neurons. Co-application of a control IgG did not affect the protective effect of GDNF.B, Specificity controls showing that the anti-pan-TGF-β antibody recognizes TGF-β3 (as well as TGF-β1 and -β2; data not shown) without recognizing GDNF, FGF-2, BDNF, and CNTF.
Fig. 7.
Transverse section through the adult rat spinal cord (T9-T10) showing DAPI staining for nuclei (A) and TβR-II-ir IML neurons (B). VH, Ventral horn;DH, dorsal horn; IML, intermediolateral column; CC, central canal. Scale bars: A, 1 mm; B, 50 μm.
DISCUSSION
The present data identify GDNF as an important protective neurotrophic factor for a population of preganglionic autonomic neurons in the IML of the spinal cord. These neurons are important for controlling peripheral cardiovascular and metabolic functions as well as secretion of biogenic amine and neuropeptide hormones from intra- and extra-adrenal chromaffin tissue (Blaschko et al., 1975; Winkler, 1993). Autonomic efferent neurons in the IML have a common origin with motoneurons in the ventral horn of the spinal cord and share with them responsiveness to various neurotrophic and growth factors (Blottner et al., 1989a,b, 1996; Blottner and Unsicker, 1990;Oppenheim, 1996; Sendtner et al., 1996; Sendtner, 1997; Schober et al., 1998a). The adrenal medulla and its chromaffin cells represent a prominent target tissue for IML neurons, and the population of IML neurons that projects to the adrenal medulla has been precisely mapped by retrograde tracing studies (Schramm et al., 1975; Schober et al., 1998a). The respective cell bodies are mainly located within spinal cord segments T7 and T10, where they constitute one-fourth (24%) of the total population of IML neurons (Blottner et al., 1996; Schober et al., 1998a). After destruction of the adrenal medulla, exactly this 24% population undergoes degeneration and death (Blottner and Baumgarten, 1992; Schober et al., 1998a), suggesting that these neurons crucially depend on factors provided by their target.
IML neurons resemble motoneurons: multiple neurotrophic factors can protect them against target deprivation
Several candidate factors have been identified that have a capacity to prevent cell death of IML neurons after target ablation. These include FGF-2 (Blottner et al., 1989b), CNTF (Blottner et al., 1989a), and neurotrophin-4 (NT-4) (Schober et al., 1998a). NT-4 and FGF-2 are of particular interest, because NT-4- as well as FGF-2-deficient mice show cell losses in the IML (Dono et al., 1998;Schober et al., 1998a). The trophic effects of these factors on IML neurons are consistent with expression and presence of respective receptors, including FGF receptor 1 (Blottner et al., 1997), CNTF receptor-α (MacLennan et al., 1996), and trkB (Schober et al., 1998a). The present study has added expression of the c-Ret and GFRα-1 receptors by IML neurons to this list, thereby implying that GDNF may be a relevant molecule in the regulation IML neuron survival and differentiation. Thus, IML neurons resemble motoneurons in the ventral horn in that their survival after target deprivation can be supported by a large number of neurotrophic factors (for review, seeOppenheim, 1996).
GDNF receptors are expressed by IML neurons
GDNF is a member of a subfamily of TGF-βs and most closely related to neurturin (NTN) and persephin (for review, see Unsicker et al., 1998). In contrast to all other members of the TGF-β superfamily, GDNF, NTN, and probably also persephin signal through a heteromeric receptor complex of a tyrosine kinase receptor, c-Ret, and a GFRα. Whether GDNF and its congeners may also signal in the absence of c-Ret, and whether c-Ret-related tyrosine kinases with a capacity to serve GDNF, NTN, and persephin signaling exist are unknown. Given the presence in and release of GDNF from adrenal chromaffin cells (Krieglstein et al., 1996, 1998), it was important to identify putative target cells for the chromaffin cell-derived GDNF. In the absence of detectable levels of c-Ret and GFRα-1 mRNA within the adrenal gland and co-expression of c-Ret and GFRα-1 by IML neurons, we hypothesized that this population of cells were likely candidates for GDNF provided by adrenal chromaffin cells.
GDNF requires TGF-β to accomplish its protective effect on target-deprived IML neurons
The present data are consistent with a role of GDNF in protecting a population of autonomic spinal cord neurons, which express the receptor complex for GDNF, from death induced by target deprivation. The issue of underlying mechanisms has been addressed in this study in two directions: (1) retrograde axonal transport of GDNF and (2) co-factors permitting the neurotrophic effect of GDNF. Surprisingly, we could not detect labeled GDNF in IML neurons of the spinal cord after its administration to the adrenal gland, although the protocol used was identical to that used in previous transport studies with GDNF in the dopaminergic nigrostriatal system (Tomac et al., 1995b). Because axons of IML neurons are probably the only target structure for GDNF within the adrenal gland, GDNF is likely to address IML neurons directly rather than triggering secretion of other neurotrophic molecules from adrenal cells. Absence of retrograde axonal transport would then indicate that GDNF might use other mechanisms for transferring information from the axon terminals to the perikarya of IML neurons. In the case of FGF-2 it has been shown that activation and subsequent retrograde transport of a set of G-proteins rather than retrograde transport of FGF-2 itself constitutes the mechanism, by which the FGF-2 signal is transmitted from the axon terminal to the neuronal cell body (Hendry et al., 1995a,b).
In a previous study we have identified TGF-β as an essential component in GDNF-mediated neurotrophic actions in vitro(Krieglstein et al., 1998). The present data take this observation to the in vivo level, showing for the first time that neutralization of endogenous TGF-β using antibodies that recognize all three TGF-β isoforms abolishes a neuroprotective effect of GDNFin vivo. Localization of both GDNF and TGF-β receptors in IML neurons is consistent with a synergistic action of GDNF and TGF-β on IML neurons. Sources for TGF-β within the medullectomized adrenal gland are likely to include cortical cells (Thompson et al., 1989) and macrophages (Assoian et al., 1987), which have both been identified as sites of storage and synthesis of TGF-β. Our previous in vitro studies have suggested that GDNF and TGF-β may synergize, at least on two distinct levels, the recruitment and/or stabilization of the GFRα and phosphatidylinositol-3 kinase (Krieglstein et al., 1998). Although mice deficient for each of the three TGF-β isoforms have been generated (Shull et al., 1992; Kulkarni et al., 1993; Kaartinen et al., 1995; Proetzel et al., 1995; Sanford et al., 1997), complete elimination of TGF-β from an animal would require a triple knock-out plus prevention of TGF-β1 transfer from mothers to offspring through milk. Thus, future studies using conditional gene knock-outs for the TGF-β receptors will hopefully help further underscore the fundamental significance of TGF-β in the signaling of GDNF and, most likely, other neurotrophic factors (cf. Krieglstein and Unsicker, 1996; Krieglstein et al., 1998).
Footnotes
This work was supported by European Union BioMedII Grant BMH4-97-2157 and Deutsche Forschungsgemeinschaft Grant SFB 317/D4. We thank Johan Widenfalk and Lars Olson (Karolinska Institute Stockholm, Sweden) for conducting the retrograde transport studies.
Correspondence should be addressed to Dr. Klaus Unsicker, Neuroanatomy, The University of Heidelberg, Im Neuenheimer Feld 307, D-69120 Heidelberg, Germany.
REFERENCES
- 1.Anderson CR, Edwards SL. Intraperitoneal injections of Fluoro-Gold reliably labels all sympathetic preganglionic neurons in the rat. J Neurosci Methods. 1994;53:137–141. doi: 10.1016/0165-0270(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 2.Arumäe U, Pirvola U, Palgi J, Kiema T-R, Palm K, Moshnyakov M, Ylikoski J, Saarma M. Neurotrophins and their receptors in rat peripheral trigeminal system during maxillary nerve growth. J Cell Biol. 1993;122:1053–1065. doi: 10.1083/jcb.122.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assoian RK, Fleurdelys BE, Stevenson HC, Madtes DK, Raines EW, Ross R, Sporn MB. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar KJ, Facer P, Williams NS, Tam PK, Anand P. Glial-derived neurotrophic factor in human adult and fetal intestine and in Hirschsprung’s disease. Gastroenterology. 1997;112:1381–1385. doi: 10.1016/s0016-5085(97)70154-9. [DOI] [PubMed] [Google Scholar]
- 5.Beck KD, Valverde J, Alexi T, Paulsen K, Moffat B, Vandlen RA, Rosenthal RA, Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- 6.Blaschko H, Sayers G, Smith DA, editors. Handbook of physiology, Sec 7, Endocrinology, Vol VI, Adrenal gland. American Physiological Society; Washington, DC: 1975. [Google Scholar]
- 7.Blottner D, Baumgarten HG. Basic fibroblast growth factor prevents neuronal death and atrophy of retrogradely labeled preganglionic neurons in vivo. Exp Neurol. 1992;118:35–46. doi: 10.1016/0014-4886(92)90020-q. [DOI] [PubMed] [Google Scholar]
- 8.Blottner D, Unsicker K. Maintenance of intermediolateral spinal cord neurons by fibroblast growth factor administered to the medullectomized rat adrenal gland: dependence on intact organ innervation and cellular organization of implants. Eur J Neurosci. 1990;2:378–382. doi: 10.1111/j.1460-9568.1990.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 9.Blottner D, Brüggemann W, Unsicker K. Ciliary neurotrophic factor supports target-deprived preganglionic sympathetic spinal cord neurons. Neurosci Lett. 1989a;105:316–320. doi: 10.1016/0304-3940(89)90640-x. [DOI] [PubMed] [Google Scholar]
- 10.Blottner D, Westermann R, Grothe C, Böhlen K, Unsicker K. Basic fibroblast growth factor in the adrenal gland. Eur J Neurosci. 1989b;1:471–478. doi: 10.1111/j.1460-9568.1989.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 11.Blottner D, Wolf N, Lachmund A, Flanders KC, Unsicker K. TGF-β rescues target deprived preganglionic sympathetic neurons in the spinal cord. Eur J Neurosci. 1996;8:202–210. doi: 10.1111/j.1460-9568.1996.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 12.Blottner D, Stapf C, Meisinger C, Grothe C. Localization, different expression, and retrograde axonal transport suggest physiological role of FGF-2 in spinal autonomic neurons of the rat. Eur J Neurosci. 1997;9:368–377. doi: 10.1111/j.1460-9568.1997.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 13.Dono R, Texido G, Dussel R, Ehmke H, Zeller R. Impaired cerebral cortex development and blood pressure regulation in FGF-2-deficient mice. EMBO J. 1998;17:4213–4225. doi: 10.1093/emboj/17.15.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enokido Y, De Sauvage F, Hongo JA, Ninkina N, Rosenthal A, Buchman VL, Davies AM. GFRα-4 and the tyrosine kinase Ret form a functional receptor complex for persephin. Curr Biol. 1998;8:1019–1022. doi: 10.1016/s0960-9822(07)00422-8. [DOI] [PubMed] [Google Scholar]
- 15.Grothe C, Unsicker K. Basic fibroblast growth factor in the hypoglossal system: specific retrograde transport, trophic, and lesion-related response. J Neurosci Res. 1992;32:317–328. doi: 10.1002/jnr.490320304. [DOI] [PubMed] [Google Scholar]
- 16.Hendry IA, Johanson SO, Heydon K. Retrograde axonal transport of the alpha subunit of the GTP-binding protein Gz to the nucleus of sensory neurons. Brain Res. 1995a;700:157–163. doi: 10.1016/0006-8993(95)00945-m. [DOI] [PubMed] [Google Scholar]
- 17.Hendry IA, Johanson SO, Heydon K. Developmental signalling. Clin Exp Pharmacol Physiol. 1995b;22:563–568. doi: 10.1111/j.1440-1681.1995.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 19.Kearns CM, Gash DM. GDNF protects nigral dopaminergic neurons against 6-hydroxydopamine in vivo. Brain Res. 1995;672:104–111. doi: 10.1016/0006-8993(94)01366-p. [DOI] [PubMed] [Google Scholar]
- 20.Konigsmark BW. Methods for counting neurons. In: Nauta WJH, Ebesson SOE, editors. Contemporary research methods in neuroanatomy. Springer; New York: 1970. pp. 315–340. [Google Scholar]
- 21.Krieglstein K, Unsicker K. Bovine chromaffin cells release a transforming growth factor-β-like molecule contained within chromaffin granules. J Neurochem. 1995;65:1423–1426. doi: 10.1046/j.1471-4159.1995.65031423.x. [DOI] [PubMed] [Google Scholar]
- 22.Krieglstein K, Unsicker K. Distinct modulatory actions of TGF-β and LIF on neurotrophin-mediated survival of developing sensory neurons. Neurochem Res. 1996;21:843–850. doi: 10.1007/BF02532308. [DOI] [PubMed] [Google Scholar]
- 23.Krieglstein K, Deimling F, Suter-Crazzolara C, Unsicker K. Expression and localization of GDNF in developing and adult chromaffin cells. Cell Tissue Res. 1996;286:263–268. doi: 10.1007/s004410050696. [DOI] [PubMed] [Google Scholar]
- 24.Krieglstein K, Henheik P, Farkas L, Jaszai J, Galter D, Krohn K, Unsicker K. GDNF requires TGF-β for establishing its neurotrophic activity. J Neurosci. 1998;18:9822–9834. doi: 10.1523/JNEUROSCI.18-23-09822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni AB, Huh C-G, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luukko K, Suvanto P, Saarma M, Thesleff I. Expression of GDNF and its receptors in developing tooth is developmentally regulated and suggests multiple roles in innervation and organogenesis. Dev Dyn. 1997;210:463–471. doi: 10.1002/(SICI)1097-0177(199712)210:4<463::AID-AJA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 27.McLennan AJ, Vinson EN, Marks L, McLaurin D, Pfeifer M, Lee N. Immunohistochemical localization of ciliary neurotrophic factor receptor α expression in the rat nervous system. J Neurosci. 1996;15:621–630. doi: 10.1523/JNEUROSCI.16-02-00621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 29.Olson L. The coming age of the GDNF family and its receptors: gene delivery in a rat Parkinson model may have clinical implications. Trends Neurosci. 1997;20:277–279. doi: 10.1016/s0166-2236(97)01098-9. [DOI] [PubMed] [Google Scholar]
- 30.Oppenheim RW. Neurotrophic survival molecules for motoneurons: an embarrassment of riches. Neuron. 1996;17:195–197. doi: 10.1016/s0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 31.Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 32.Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeben M, Laurikainen A, Hiltunen JO, Castren E, Saarma M. The messenger RNAs for both glial cell line-derived neurotrophic factor receptors, c-Ret and GDNFR alpha, are induced in the rat brain in response to kainate-induced excitation. Neuroscience. 1998;83:151–159. doi: 10.1016/s0306-4522(97)00361-8. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez MP, Silos Santigo I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 35.Sanford LP, Ormsby I, Gittenberger de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGF-β2 knockout mice have multiple developmental defects that are non-overlapping with other TGF-β knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauer H, Rosenblad C, Björklund A. Glial cell line-derived neurotrophic factor but not transforming growth factor beta 3 prevents delayed degeneration of nigral dopaminergic neurons following striatal 6-hydroxydopamine lesion. Proc Natl Acad Sci USA. 1995;92:8935–8939. doi: 10.1073/pnas.92.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schober A, Wolf N, Huber K, Hertel R, Krieglstein K, Minichiello L, Kahane N, Widenfalk J, Kalcheim C, Olson L, Klein R, Lewin GR, Unsicker K. TrkB and neurotrophin-4 are important for development and maintenance of sympathetic preganglionic neurons innervating the adrenal medulla. J Neurosci. 1998a;18:7272–7284. doi: 10.1523/JNEUROSCI.18-18-07272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schober A, Hertel R, Arumäe U, Krieglstein K, Unsicker K. GDNF rescues sympathetic preganglionic neurons from target-deprived cell death in vivo. Soc Neurosci Abstr. 1998b;24:296. [Google Scholar]
- 39.Schuchardt A, D’Agati V, Larsson Blomberg L, Constantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 40.Schramm CP, Adair JR, Stribling JM, Gray LP. Preganglionic innervation of the adrenal gland of the rat. Exp Neurol. 1975;49:540–553. doi: 10.1016/0014-4886(75)90107-7. [DOI] [PubMed] [Google Scholar]
- 41.Sendtner M. Gene therapy for motor neuron disease. Nat Med. 1997;3:380–381. doi: 10.1038/nm0497-380. [DOI] [PubMed] [Google Scholar]
- 42.Sendtner M, Holtmann B, Hughes RA. The response of motoneurons to neurotrophins. Neurochem Res. 1996;21:831–841. doi: 10.1007/BF02532307. [DOI] [PubMed] [Google Scholar]
- 43.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Springer JE, Mu X, Bergmann LW, Trojanowski JQ. Expression of GDNF in RNA in rat and human nervous tissue. Exp Neurol. 1994;127:167–170. doi: 10.1006/exnr.1994.1091. [DOI] [PubMed] [Google Scholar]
- 45.Suvanto P, Hiltunen JO, Arumäe U, Moshnyakov M, Sariola H, Sainio K, Saarma M. Localization of glial cell line-derived neurotrophic factor (GDNF) mRNA in embryonic rat by in situ hybridization. Eur J Neurosci. 1996;8:816–822. doi: 10.1111/j.1460-9568.1996.tb01267.x. [DOI] [PubMed] [Google Scholar]
- 46.Suvanto P, Wartiovaara K, Lindahl M, Arumae U, Moshnyakov M, Horelli-Kuitunen N, Airaksinen MS, Palotie A, Sariola H, Saarma M. Cloning, mRNA distribution and chromosomal localization of the gene for glial cell line-derived neurotrophic factor receptor beta, a homologue to GDNFR-alpha. Hum Mol Gen. 1997;6:1267–1273. doi: 10.1093/hmg/6.8.1267. [DOI] [PubMed] [Google Scholar]
- 47.Thompson NL, Flanders KC, Smith M, Ellingsworth LR, Roberts AB, Sporn MB. Expression of transforming growth factor-β1 in specific cells and tissue of adult and neonatal mice. J Cell Biol. 1989;108:661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomac A, Lindquist E, Lin LFH, Ögren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal system by GDNF in vivo. Nature. 1995a;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 49.Tomac A, Widenfalk J, Lin LFH, Kohono T, Ebendal T, Hoffer BJ, Olson L. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci USA. 1995b;92:8274–8278. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unsicker K, Suter-Crazzolara C, Krieglstein K. Neurotrophic roles of GDNF and related receptors. In: Hefti F, editor. Handbook of experimental pharmacology, Vol 137, Neurotrophic factors. Springer; Berlin: 1998. pp. 189–224. [Google Scholar]
- 51.Winkler H. The adrenal chromaffin granule: a model for large dense core vesicles of endocrine and nervous tissue. J Anat. 1993;183:237–252. [PMC free article] [PubMed] [Google Scholar]