Abstract
Diffuse noxious inhibitory controls (DNIC), which involve supraspinal structures and modulate the transmission of nociceptive signals, were investigated at different stages during the development of adjuvant-induced monoarthritis in the rat. After behavioral evaluation, recordings of trigeminal convergent neurons were performed in anesthetized animals with acute (24–48 hr) or chronic (3–4 weeks) monoarthritis of the ankle. Inhibitions of C-fiber-evoked neuronal responses during and after the application of noxious conditioning stimuli to the ankle were measured to evaluate DNIC. The conditioning stimuli consisted of mechanical (maximal flexion and graded pressures) and graded thermal stimuli and were applied alternately to normal and arthritic ankles. Behaviorally, the two groups of animals exhibited a similar increased sensitivity to mechanical stimuli applied to the arthritic joint (i.e., an increased ankle-bend score and a decreased vocalization threshold to pressure stimuli). However, they showed different electrophysiological profiles. In the animals with acute monoarthritis, the DNIC-induced inhibitions produced by mechanical or thermal stimulation of the arthritic joint were significantly increased at all intensities compared with the normal joint. In contrast, in the chronic stage of monoarthritis, the DNIC-induced inhibitions triggered by thermal or pressure stimuli were similar for both ankles, except with the most intense mechanical stimuli. This discrepancy between the behavioral and electrophysiological findings suggests that inputs activated during chronic monoarthritis may fail to recruit DNIC and may thus be functionally different from those activated in the acute stage of inflammation.
Keywords: nociception, pain modulation, chronic inflammation, animal model, diffuse noxious inhibitory controls, descending controls
The output of spinal nociceptive neurons can be modulated by descending, propriospinal, and segmental systems, which may tonically or phasically inhibit or facilitate the spinal transmission of nociceptive signals (for review, seeZieglgänsberger et al., 1986; Willis, 1988; Fields and Basbaum, 1994). Although it is widely accepted that these endogenous modulatory systems affect sensations of pain, the way in which they are brought into play and their consequences on nociceptive behavior have rarely been addressed in the context of tissue injury (Cervero et al., 1991; Morgan et al., 1991; Schaible et al., 1991; Ren and Dubner, 1996).
Diffuse noxious inhibitory controls (DNIC) are supraspinally mediated inhibitory controls triggered by the application of noxious stimuli. In normal healthy rats, spinal and trigeminal convergent neurons are inhibited in an intensity-dependent manner when noxious stimuli are applied to heterotopic areas of the body, i.e., regions remote from the excitatory receptive field of the neuron under study (Le Bars et al., 1979a,b; Dickenson and Le Bars, 1983; Morton et al., 1987; Ness and Gebhart, 1991). In man, heterotopic noxious stimuli inhibit sensations of pain and the spinal nociceptive flexion (RIII) reflex (Willer et al., 1984). In both rats and man, these phenomena are sustained by a loop involving supraspinal structures and endogenous opioidergic systems (Le Bars et al., 1979b, 1981; Cadden et al., 1983;Roby-Brami et al., 1987; Willer et al., 1990; Bouhassira et al., 1993,1995). It has been hypothesized that DNIC may facilitate the integration of pain information by the complementary processes of segmental excitation and diffuse inhibition (Le Bars et al., 1979b).
The aim of this work was to study whether DNIC would be modified during the course of inflammation and to examine how modifications of these descending controls might be related to pain behavior in the context of acute and chronic pain. DNIC have been evaluated previously in rats rendered polyarthritic by intradermal injections of complete Freund adjuvant (CFA) into the tail (Calvino et al., 1987). However, CFA-induced polyarthritis is a severe widespread systemic disease, and it is not possible with this model to compare directly the effects of a stimulus on an inflamed joint and the effects of the same stimulus on a normal contralateral joint. To study the temporal evolution of DNIC during the course of chronic inflammation without the widespread systemic consequences of polyarthritis, we reinvestigated these inhibitory controls in the acute and chronic stages of CFA-induced monoarthritis, a model of circumscribed, persistent inflammatory pain (Grubb et al., 1991; Butler et al., 1992). We compared DNIC-induced inhibitions of convergent trigeminal neurons triggered by graded conditioning stimulation of either the normal or the arthritic ankle in rats with acute or chronic monoarthritis. Our hypothesis was (1) that the sensitization of nociceptive neurons during inflammation would be paralleled by an exacerbation of DNIC and (2) that chronicity would possibly be associated with modifications of this phenomenon as a result of central plastic changes induced by prolonged pain.
MATERIALS AND METHODS
The study was performed on three groups of Sprague Dawley male rats weighing 300–400 gm at the time of the experiments: a control group of healthy rats (n = 12); a group of rats with acute monoarthritis of the ankle (n = 16); and a group with chronic monoarthritis of the ankle (n = 15). Monoarthritis was induced by the intracapsular injection of 50 μl of CFA into the right tibiotarsal joint. This procedure was performed under brief halothane–N2O–O2 anesthesia, as described previously (Butler et al., 1992). The CFA was prepared as follows: 60 mg of dead mycobacterium butyricum (Difco, Detroit, MI) was added to a mixture of paraffin oil (6 ml), 9% NaCl (4 ml), and Tween 80 (1 ml), was mixed thoroughly, and was autoclaved for 30 min at 120°C. The acute monoarthritis group consisted of animals 24–48 hr after the injection; these animals showed acute inflammation of the ankle. The chronic monoarthritis group consisted of animals 3–4 weeks after the injection that showed pronounced swelling of the right ankle. Animals with polyarthritis and animals with no or minimal swelling of the right ankle were discarded.
Behavioral testing. On the day of the experiments, animals were weighed, the circumference of each ankle was measured, and the sensitivity to mechanical stimulation of each ankle was assessed. For this purpose, the animals were gently restrained in a soft towel, allowing access to both hindlimbs while at the same time restricting movement. Vocalization to flexion and extension of the ankle within its limits of movement were recorded; for each paw, two manipulations were made in each direction, and the total number of vocalizations was recorded. Forceps incorporating strain gauges, which were connected to an amplifier (Hottinger Baldwin Messtechnik, Darmstadt, Germany), were used to evaluate the vocalization threshold to pressure. An increasing pressure was delivered dorsoventrally to the ankle through the forceps, and the vocalization threshold was measured for each paw by averaging three successive values obtained at 1 min intervals. All observations were performed by one of the authors to minimize inter-observer differences. The tester could not be blind to the experimental group because rats with acute and chronic monoarthritis could be easily distinguished (a certain degree of cutaneous desquamation was characteristic of the chronic stage).
Animal preparation for electrophysiological experiments.Immediately after the behavioral testing, the animals were deeply anesthetized (2% halothane in a 2:1 mixture of NO2 and O2). A tracheal cannula was inserted, and the jugular vein was cannulated. The rats were artificially ventilated at a rate of 50 strokes per minute, with the volume of ventilation adjusted to maintain a normal acid/base equilibrium; this was assessed with a capnometer, which also measured O2, NO2, and halothane levels throughout the experiment. Heart rate was monitored continuously, and core temperature was maintained at 37 ± 0.5°C by means of a homeothermic blanket system. The animals were mounted in a stereotaxic frame, with the head fixed in a ventroflexed position. The caudal medulla was then exposed by removing the overlying musculature, atlanto-occipital membrane, and dura mater. After surgery, the administration of NO2 was stopped, and the level of halothane was reduced to 1.2%. This anesthetic regimen allows a very stable level of anesthesia, under which neither motor nor cardiovascular reactions are observed during the application of strong stimuli.
Recording of trigeminal neurons. Extracellular recordings from convergent neurons in nucleus caudalis were made with the use of steel microelectrodes (9–12 MΩ; Frederick Haer & Co. Inc., Bowdoinham, ME). The electrode was inserted on the left side, 1.5–2 mm caudal to the obex and 1.5–2.5 mm lateral to the midline. The signal was led to a differential alternating current preamplifier, from where it was displayed on an oscilloscope and gated so that only single-unit activity was recorded. Responses to electrical stimulation of the receptive field were quantified by a computerized data-acquisition system (Notocord Inc., Croissy, France) and stored for further analysis. Neurons were classified as convergent (i.e., wide dynamic range) on the basis of their responses to both mechanical and transcutaneous electrical stimulation of their receptive field. The poststimulus histograms were analyzed to distinguish responses attributable to A- and C-fiber inputs by their latencies. Once a cell had been identified, the extent of its excitatory receptive field was determined. Graded transcutaneous electrical stimuli (single square wave pulses of 2 msec duration) were applied to the center of the excitatory receptive field to determine the electrical threshold of the C-fiber input. Only cells that presented no serious alterations in spike amplitude or waveform during the complete experimental procedure were considered. Only one or two neurons per animal were characterized.
Assessment of DNIC. The test stimuli consisted of sequences of repetitive electrical stimulation (two times the C-fiber threshold, 0.66 Hz) of the excitatory receptive fields of the trigeminal neurons. The heterotopic conditioning stimuli consisted of mechanical or thermal stimulation of the ankle. Only the C-fiber-evoked responses were considered. To obtain a steady discharge, the first 30 responses of the neuron were not considered. The poststimulus histogram built from responses 31–45 was used as a control for the sequence. The conditioning stimulus was applied for 37 sec from test stimuli 46–70, and the poststimulus histogram built from responses 56–70 was used to assess the effects of the conditioning stimulation. The DNIC-induced inhibition triggered by each heterotopic stimulus was expressed as a percentage of decrease in the number of spikes with reference to the control poststimulus histogram. The poststimulus histograms built from responses 71–85 and 86–100 allowed postconditioning effects to be monitored over two successive 22 sec periods after the cessation of the conditioning stimulus. Sequences were performed at 5 min intervals.
Three types of conditioning stimuli were used: flexion of the ankle within its range of movements; graded pressure applied ventrodorsally to the ankle with a constant intensity (4, 8, or 16 N/cm2); and immersion of the hindpaw up to the ankle in a 44, 46, or 48°C water bath. In the animals with acute monoarthritis, complete flexion of the arthritic ankle could be performed easily. Flexion of an ankle with chronic monoarthritis was generally limited by ankylosis and required more force to overcome the stiffness of the joint. Each ankle was stimulated alternately, starting with the weakest and ending with the most intense stimulus. Consecutive stimuli to either ankle were therefore separated by 10 min intervals.
Statistical analyses. Results are presented as mean ± SEM. DNIC-induced inhibitions triggered by stimulation of the normal and arthritic paws were compared within each group and between groups with the use of ANOVA and post hoc Fisher’s least significant difference test. p < 0.05 was regarded as significant.
RESULTS
Behavioral testing
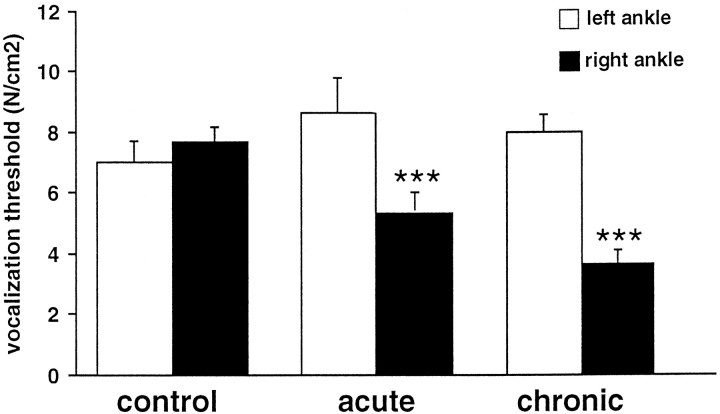
The circumference of the arthritic (right) ankle was increased to a very similar extent in the acute and chronic monoarthritis groups [40 ± 1 and 41 ± 2 mm compared with 27.0 ± 0.2 and 27.0 ± 0.3 mm for the normal (left) ankle, respectively]. The number of vocalizations to flexion and extension of the arthritic ankle within its range of movements was maximal (i.e., four of four) for all selected animals with acute or chronic monoarthritis. No animal with monoarthritis (either acute or chronic) vocalized when its normal ankle was extended or flexed. This was also true for the control animals. The vocalization threshold to pressure on the normal ankle was not significantly different between the three groups, and, as would be expected, there was no left–right difference in the controls (Fig.1). In contrast, the vocalization threshold to pressure of the right ankle was significantly different across the three groups (F(2,33) = 10.03;p < 0.001). Compared with control rats, the vocalization threshold to pressure of the arthritic ankle was significantly lower in both the acute (p < 0.05) and the chronic (p < 0.0001) monoarthritis groups (Fig. 1). This threshold was significantly lower in the chronic than in the acute monoarthritis group (p < 0.05). However, the left–right difference in threshold was not significantly different between these two groups.
Fig. 1.
Vocalization thresholds to pressure on the ankle observed in the different groups of animals. control, Control normal rats; acute, rats with acute monoarthritis (24–48 hr); chronic, rats with chronic monoarthritis (3–4 weeks). ***p < 0.001 for comparison between right and left sides.
Characteristics of the trigeminal neurons
Sixty-two convergent neurons were recorded in the left trigeminal nucleus caudalis (16, 24, and 22 in the control, acute, and chronic monoarthritis groups, respectively). The electrophysiological characteristics of these neurons were very similar in the three groups: all exhibited a small receptive field located unilaterally on the lips and/or the muzzle; spontaneous activity was very low (mean value less than one spike per second) in all three groups; the threshold for triggering C-fiber-evoked responses was similar in all three groups (6.6 ± 0.9, 5.7 ± 0.6, and 6.0 ± 0.9 mA in the control, acute, and chronic monoarthritis groups, respectively); and the responses to C-fiber activation during the control sequences were similar in the three groups (6.6 ± 1.0, 7.4 ± 0.9, and 7.5 ± 1.3 spikes per stimulus in the control, acute, and chronic monoarthritis groups, respectively).
DNIC triggered by flexion of the ankle
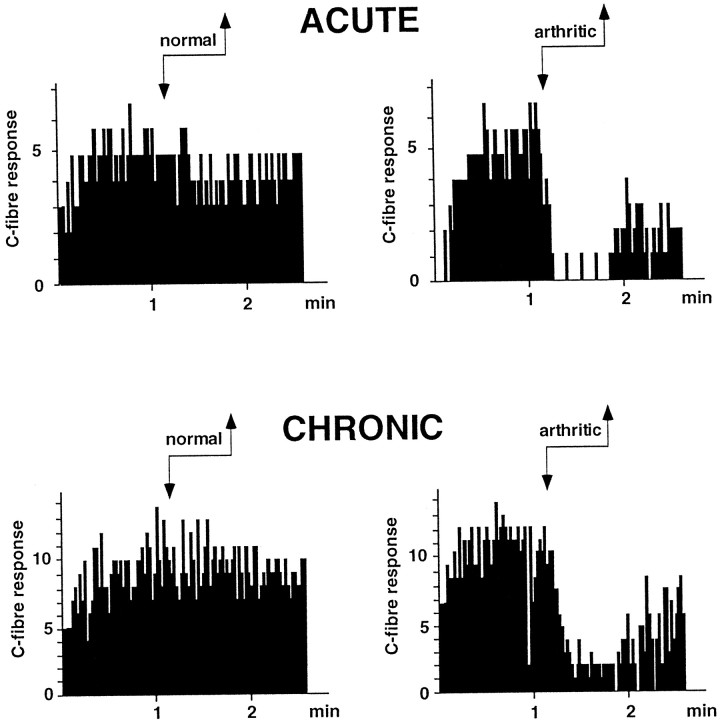
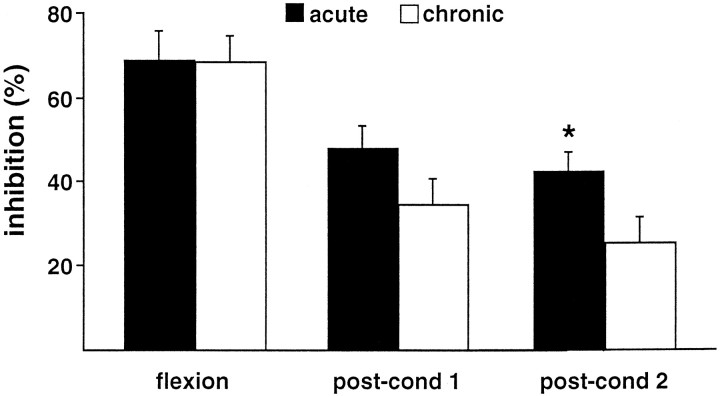
In the three groups, flexion of the normal ankle triggered virtually no inhibition of trigeminal neuronal activity. In contrast, flexion of the arthritic ankle in animals with either acute or chronic monoarthritis triggered a potent inhibition (Fig. 2). These inhibitions were similar in the acute and chronic monoarthritis groups (69 ± 7 and 68 ± 6%, respectively; p < 0.001 in both cases compared with the normal ankle). Postconditioning effects, however, were significantly more pronounced and prolonged in the acute than in the chronic monoarthritis group (p < 0.05) (Fig. 3).
Fig. 2.
Individual examples of DNIC-induced inhibitions triggered by flexion of the ankle in a rat with acute monoarthritis and in a rat with chronic monoarthritis. Each histogram corresponds to a sequence of 100 stimuli during which C-fiber-evoked responses of a trigeminal convergent neuron were recorded before, during (arrows), and after flexion of either the normal or the arthritic ankle. Ordinate, Number of C-fiber-evoked spikes; abscissa, time.
Fig. 3.
Comparison of DNIC-induced inhibitions triggered by flexion of the arthritic ankle in animals with acute (black columns) or chronic (white columns) monoarthritis. The percentage of inhibition of the C-fiber responses of trigeminal neurons triggered by flexion of the arthritic ankle are represented for each group during flexion and in two successive 22 sec (postconditioning) periods after the cessation of flexion (post-cond 1, post-cond 2). The same maneuver applied to the normal ankle of arthritic or normal rats triggered virtually no inhibition (data not shown). *p < 0.05 for comparison between acute and chronic monoarthritis groups.
DNIC triggered by graded pressure on the ankle
Inhibitions triggered by graded pressure on the normal ankle were very similar in the three groups. In the control rats, inhibitions triggered by pressure of either the right or the left ankle were very similar at all intensities of conditioning stimuli (Fig.4A). In all cases, inhibitions triggered by pressure of the ankle increased with the intensity of conditioning stimulation. In the acute monoarthritis group, the DNIC-induced inhibitions were more pronounced at all intensities when pressure was applied to the arthritic ankle (F(1,94) = 18.05; p < 0.001) (Fig. 4B). In animals with acute monoarthritis, postconditioning effects were also significantly more pronounced when pressure was applied to the arthritic ankle (first postconditioning period, F(1,94) = 14.41; p < 0.001; second postconditioning period, F(1,94) = 9.9; p < 0.01). In contrast, the animals with chronic monoarthritis did not exhibit significant left–right differences in the inhibitions induced by pressure (F(1,100) = 1.39; NS). In this group, only the most intense pressure (16 N/cm2) triggered significantly greater inhibitions when applied to the arthritic ankle (Fig. 4C).
Fig. 4.
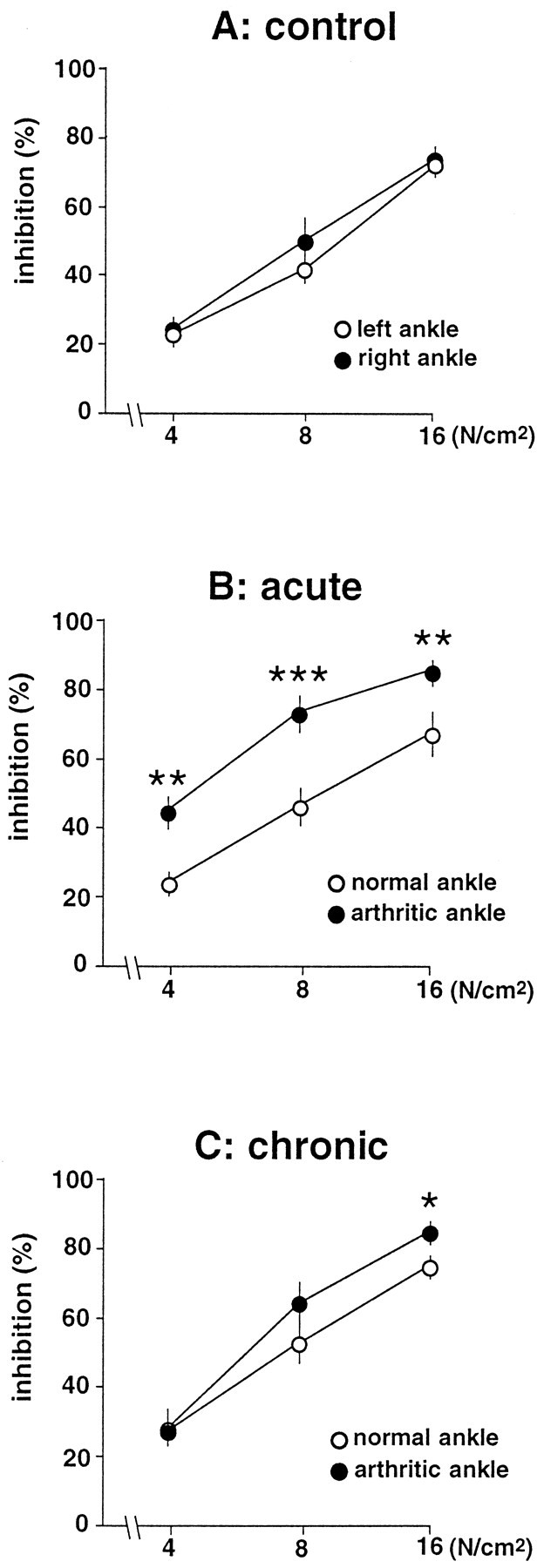
Percentage of inhibition (ordinate) of C-fiber responses of trigeminal neurons induced by graded pressure (abscissa in logarithmic scale) applied to each ankle in control animals (A) and in rats with acute (B) or chronic (C) monoarthritis. *p < 0.05; **p< 0.01; ***p < 0.001 for comparison between right and left sides.
DNIC triggered by thermal stimulation of the hindpaws
Inhibitions triggered by immersion of the normal hindpaw in hot water (44–48°C) did not differ significantly between the three groups. In the control rats, the inhibitions were virtually superimposable for the right and left hindpaws (Fig.5A). Significant left–right differences in the DNIC-induced inhibitions were found only in the acute monoarthritis group (F(1,62) = 8.04;p < 0.01) (Fig. 5B). Compared with the normal paw, the inhibitions seen during immersion of the arthritic paw were significantly greater at 46 and 48°C. Postconditioning effects were also significantly more pronounced on the arthritic side at these temperatures (F(1,62) = 13.69; p< 0.001 for the first postconditioning period). In contrast, animals with chronic monoarthritis did not show any significant left–right differences in DNIC-induced inhibitions during and after thermal stimulation of the hindpaws (Fig. 5C).
Fig. 5.
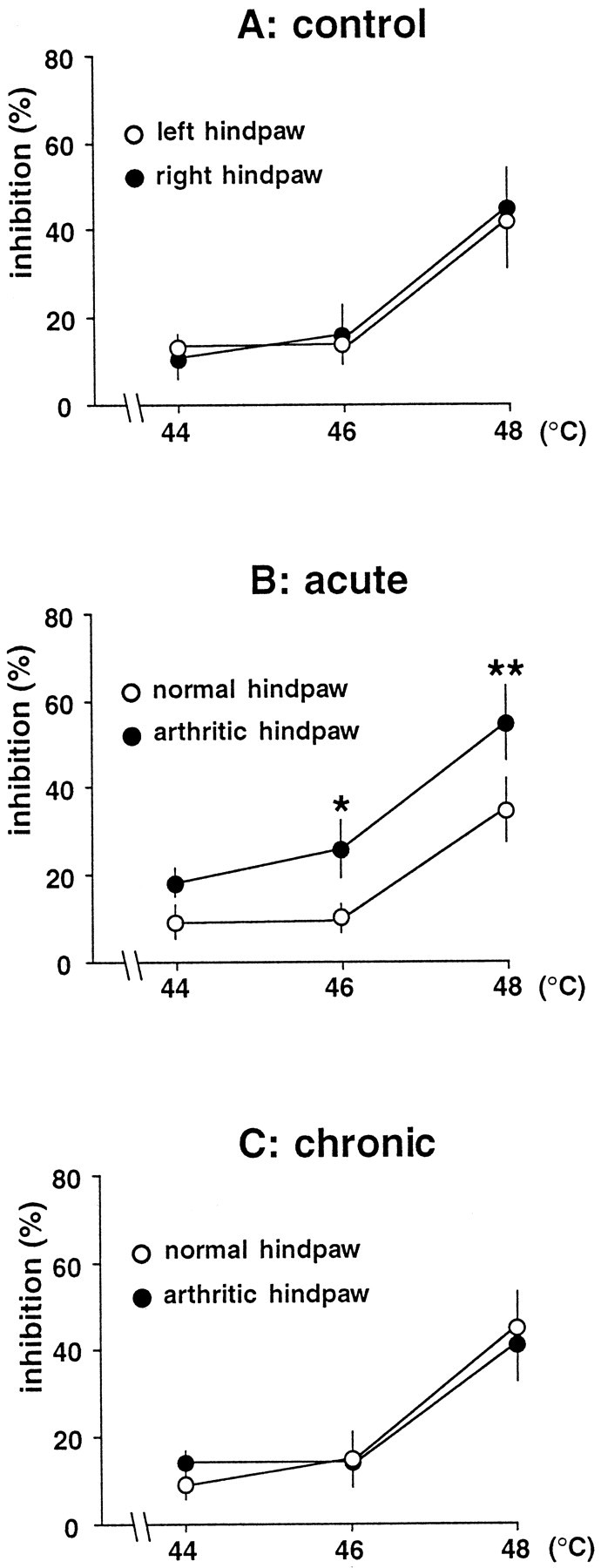
Percentage of inhibition (ordinate) of C-fiber responses of trigeminal neurons induced by immersion of each hindpaw in hot water in control animals (A) and in rats with acute (B) or chronic (C) monoarthritis. *p < 0.05; **p < 0.01 for comparison between right and left sides.
DISCUSSION
DNIC-induced inhibitions of convergent trigeminal neurons triggered by graded conditioning stimulation of either a normal or an arthritic ankle were compared in rats with acute or chronic monoarthritis. Behaviorally, the two groups of rats exhibited a similar increased sensitivity to mechanical stimuli applied to the arthritic joint but presented different electrophysiological profiles. In the acute stage of inflammation, DNIC-induced inhibitions elicited by stimulation of the arthritic joint were significantly increased, regardless of the conditioning stimulus. This exacerbation of DNIC fitted well with the behavioral changes. In contrast, in the chronic form of the disease, there was a discrepancy between the electrophysiological and behavioral results. Indeed, compared with the acute monoarthritis group, animals with chronic monoarthritis showed a relative decrease in the DNIC-induced inhibitions triggered by stimulation of the arthritic joint.
DNIC in the acute stage of monoarthritis
In animals with acute monoarthritis, gentle flexion of the inflamed ankle triggered a potent and prolonged inhibition of the C-fiber-evoked responses of trigeminal convergent neurons. This result is in agreement with the fact that innocuous movements become nociceptive when the ankle is inflamed. DNIC-induced inhibitions triggered by pressure on the arthritic ankle were also clearly increased at all intensities compared with the normal ankle. The stimulus–response curve shifted to the left but remained parallel to the corresponding curve for the normal ankle, suggesting that the encoding properties of DNIC were not impaired. The increase in DNIC-induced inhibitions during mechanical stimulation of the arthritic ankle may have been caused by an increase in the afferent inputs during acute monoarthritis. In the normal rat, DNIC depend on the activation of thin myelinated (Aδ) and unmyelinated (C) fibers (Bouhassira et al., 1987), and the majority of these afferents have been shown to develop a long-lasting sensitization to mechanical stimuli after the onset of joint inflammation (Coggeshall et al., 1983;Schaible and Schmidt, 1985, 1988; Grigg et al., 1986; for review, see Schaible and Grubb, 1993). Changes in the discharge properties of spinal cord neurons have also been demonstrated during acute monoarthritis in studies performed in rats (Grubb et al., 1993), cats (Schaible et al., 1987; Neugebauer and Schaible, 1990), and monkeys (Dougherty et al., 1992). Grubb et al. (1993), whose work is the most relevant to our study, showed that 48 hr after the induction of monoarthritis by the injection of CFA into an ankle, superficial or deep dorsal horn neurons with joint inputs exhibited lower mechanical thresholds compared with those seen in normal rats.
The increase in DNIC-induced inhibitions during thermal stimulation of the inflamed paw fits well with the increase in heat sensitivity observed on the inflamed hindpaw in the first few days after CFA injections (Iadarola et al., 1988; Ren and Dubner, 1996; Jasmin et al., 1998). This suggests that cutaneous afferents and nociceptive neurons with cutaneous inputs may be sensitized to heat during the acute stage of monoarthritis.
Evolution of DNIC from acute to chronic monoarthritis
In agreement with the behavioral data, animals with chronic monoarthritis showed a significant increase in DNIC-induced inhibitions during flexion of the arthritic ankle compared with the normal ankle. However, although the conditioning stimulation might have been stronger in animals with chronic arthritis because of the force required to overcome the joint stiffness, the DNIC-induced inhibitions during flexion of the inflamed ankle were similar in the two groups, and yet the postconditioning effects were significantly less pronounced in the animals with chronic monoarthritis. In addition, although these animals showed a clear decrease in their vocalization thresholds to pressure on the arthritic side, DNIC-induced inhibitions during pressure on the inflamed ankle were similar to those elicited from the normal ankle, except with the most intense pressure (i.e., approximately four and a half times the vocalization threshold). This discrepancy between behavioral and electrophysiological profiles contrasts with the data obtained in animals with acute monoarthritis. It is also in contrast with the results obtained in rats with CFA-induced polyarthritis in which light pressure applied to the inflamed areas was shown to trigger pronounced inhibition of trigeminal convergent neurons (Calvino et al., 1987). However, the increase in DNIC-induced inhibitions in these animals may be partly related to systemic metabolic (Godefroy et al., 1987) or neurological (Reiber et al., 1984) disorders induced by the disease. The differences in the results obtained with the two models might also be explained by the fact that the affected joints may be more severely injured in polyarthritic than in chronic monoarthritic rats.
In contrast with the acute monoarthritis group, no left–right difference in DNIC-induced inhibitions was found during thermal stimulation of the hindpaws in the chronic stage of inflammation. This is in accordance with recent data concerning the evolution of the sensitivity to heat during the course of CFA-induced inflammation of the hindpaw in the rat (Jasmin et al., 1998). The complete regression of heat hyperalgesia in the chronic stage of CFA-induced inflammation suggests that skin nociceptors may be sensitized only for a short period after the induction of monoarthritis, after which nociceptors in the joint and surrounding deep tissues become preferentially involved. This hypothesis is further supported by the results of two electrophysiological studies showing that the threshold to noxious heat is not decreased but increased in parabrachial neurons and in ventrobasal thalamic neurons, in the chronic stage of adjuvant polyarthritis, compared with normal animals (Gautron and Guilbaud, 1982; Matsumoto et al., 1996).
How can we explain the decrease in pressure-induced inhibitions in the chronic stage of monoarthritis? One simple explanation is that the afferent input induced by pressure might have decreased in the chronic stage. We found that the vocalization threshold to pressure was still significantly decreased in animals with chronic monoarthritis, but this result could be confounded by paw movement. However, electrophysiological experiments have shown that joint afferents and spinal cord neurons with joint inputs display a similar degree of sensitization during acute and chronic stages of CFA-induced monoarthritis (Birrell et al., 1990; Grubb et al., 1991, 1993; McQueen et al., 1991; Schaible and Schmidt, 1996). Thus, the relative decrease in the DNIC-induced inhibitions that was observed during pressure of the chronically inflamed ankle may not simply be caused by a reduction in the afferent input to the spinal cord. Moreover, because this relative decrease was specific to the inflamed ankle, it cannot be explained by a global decrease of DNIC. Rather, our data suggest that inputs activated during chronic monoarthritis may fail to recruit DNIC. This functional difference between acute and chronic monoarthritis may be attributable to the fact that the populations of activated neurons receiving inputs from the inflamed ankle might not be the same during the acute and chronic stages of monoarthritis. Alternatively, an inhibitory modulation of these activated neurons may have developed during the course of monoarthritis and interfered with the spino–bulbo–trigeminal loop subserving DNIC. In any case, the present results suggest that chronic inflammatory processes induce a reorganization of the spinal transmission of nociceptive signals, which modifies the recruitment of DNIC. Furthermore, the dissociation between DNIC and nociceptive behavior in animals with chronic monoarthritis suggests that, in the context of chronic pain, mechanisms that are independent of DNIC may play a key role in the enhancement of pain perception.
Footnotes
We thank Dr. S. W. Cadden for advice in the preparation of this manuscript.
Correspondence should be addressed to Didier Bouhassira, Institut National de la Santé et de la Recherche Médicale U-161, 2 Rue d’Alésia, 75014 Paris, France.
REFERENCES
- 1.Birrell GJ, McQueen DS, Iggo A, Grubb BD. The effects of 5-HT on articular sensory receptors in normal and arthritic rats. Br J Pharmacol. 1990;101:715–721. doi: 10.1111/j.1476-5381.1990.tb14146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouhassira D, Le Bars D, Villanueva L. Heterotopic activation of Aδ- and C-fibres triggers inhibition of trigeminal and spinal convergent neurones in the rat. J Physiol (London) 1987;389:301–317. doi: 10.1113/jphysiol.1987.sp016658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouhassira D, Le Bars D, Bolgert F, Laplane D, Willer JC. Diffuse noxious inhibitory controls (DNIC) in man. A neurophysiological investigation of a patient with a form of Brown-Séquard syndrome. Ann Neurol. 1993;34:536–543. doi: 10.1002/ana.410340406. [DOI] [PubMed] [Google Scholar]
- 4.Bouhassira D, Chitour D, Villanueva L, Le Bars D. The spinal transmission of nociceptive information: modulation by the caudal medulla. Neuroscience. 1995;69:931–938. doi: 10.1016/0306-4522(95)00269-o. [DOI] [PubMed] [Google Scholar]
- 5.Butler SH, Godefroy F, Besson JM, Weil-Fugazza J. A limited arthritic model for chronic pain studies in the rat. Pain. 1992;48:73–81. doi: 10.1016/0304-3959(92)90133-V. [DOI] [PubMed] [Google Scholar]
- 6.Cadden SW, Villanueva L, Chitour D, Le Bars D. Depression of activities of dorsal horn convergent neurones by propriospinal mechanisms triggered by noxious inputs; comparison with diffuse noxious inhibitory controls (DNIC). Brain Res. 1983;275:1–11. doi: 10.1016/0006-8993(83)90412-2. [DOI] [PubMed] [Google Scholar]
- 7.Calvino B, Villanueva L, Le Bars D. Dorsal horn (convergent) neurons in the intact anaesthetized arthritic rat. II. Heterotopic inhibitory influences. Pain. 1987;31:359–379. doi: 10.1016/0304-3959(87)90165-5. [DOI] [PubMed] [Google Scholar]
- 8.Cervero F, Schaible HG, Schmidt RF. Tonic descending inhibition of spinal cord neurons driven by joint afferents in normal cats and in cats with an inflamed knee joint. Exp Brain Res. 1991;83:675–678. doi: 10.1007/BF00229846. [DOI] [PubMed] [Google Scholar]
- 9.Coggeshall RE, Hong KAP, Langford LA, Schaible HG, Schmidt RF. Discharge characteristics of fine medial articular afferents at rest and during passive movements of inflamed knee joints. Brain Res. 1983;272:185–188. doi: 10.1016/0006-8993(83)90379-7. [DOI] [PubMed] [Google Scholar]
- 10.Dickenson AH, Le Bars D. Diffuse noxious inhibitory controls (DNIC) involve trigeminothalamic and spinothalamic neurones in the rat. Exp Brain Res. 1983;49:174–180. doi: 10.1007/BF00238577. [DOI] [PubMed] [Google Scholar]
- 11.Dougherty PM, Sluka KA, Sorkin LS, Westlund KN, Willis WD. Neural changes in acute arthritis in monkeys. I. Parallel enhancement of responses of spinothalamic tract neurons to mechanical stimulation and excitatory amino acids. Brain Res Rev. 1992;17:1–13. doi: 10.1016/0165-0173(92)90002-4. [DOI] [PubMed] [Google Scholar]
- 12.Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Wall PD, Melzack R, editors. Textbook of pain. Churchill Livingstone; Edinburgh: 1994. pp. 243–257. [Google Scholar]
- 13.Gautron M, Guilbaud G. Somatic responses of ventrobasal thalamic neurons in polyarthritic rats. Brain Res. 1982;237:459–471. doi: 10.1016/0006-8993(82)90457-7. [DOI] [PubMed] [Google Scholar]
- 14.Godefroy F, Weil-Fugazza J, Besson JM. Complex temporal changes in 5-hydroxytryptamine synthesis in the central nervous system induced by experimental polyarthritis in the rat. Pain. 1987;28:223–238. doi: 10.1016/0304-3959(87)90118-7. [DOI] [PubMed] [Google Scholar]
- 15.Grigg P, Schaible HG, Schmidt RF. Mechanical sensitivity of group III and IV afferents from posterior articular nerve in normal and inflamed cat knee. J Neurophysiol. 1986;55:635–643. doi: 10.1152/jn.1986.55.4.635. [DOI] [PubMed] [Google Scholar]
- 16.Grubb BD, Birrell GJ, McQueen DS, Iggo A. The role of PGE2 in the sensitization of mechanoreceptors in normal and inflamed ankle joints of the rat. Exp Brain Res. 1991;84:383–392. doi: 10.1007/BF00231460. [DOI] [PubMed] [Google Scholar]
- 17.Grubb BD, Stiller RU, Schaible HG. Dynamic changes in the receptive field properties of spinal cord neurons with ankle input in rats with chronic unilateral inflammation in the ankle region. Exp Brain Res. 1993;92:441–452. doi: 10.1007/BF00229032. [DOI] [PubMed] [Google Scholar]
- 18.Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- 19.Jasmin L, Kohan L, Franssen M, Janni G, Goff JR. The cold plate as a test of nociceptive behaviors: description and application to the study of chronic neuropathic and inflammatory pain models. Pain. 1998;75:367–382. doi: 10.1016/s0304-3959(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 20.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979a;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 21.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979b;6:305–327. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- 22.Le Bars D, Chitour D, Kraus E, Dickenson AH, Besson JM. Effect of naloxone upon diffuse noxious inhibitory controls (DNIC) in the rat. Brain Res. 1981;204:387–402. doi: 10.1016/0006-8993(81)90597-7. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto N, Bester H, Menendez L, Besson JM, Bernard JF. Changes in the responsiveness of parabrachial neurons in the arthritic rat: an electrophysiological study. J Neurophysiol. 1996;76:4113–4126. doi: 10.1152/jn.1996.76.6.4113. [DOI] [PubMed] [Google Scholar]
- 24.McQueen DS, Iggo A, Birrell GJ, Grubb BD. Effects of paracetamol and aspirin on neural activity of joint mechanonociceptors in adjuvant arthritis. Br J Pharmacol. 1991;104:178–182. doi: 10.1111/j.1476-5381.1991.tb12404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan MM, Gold MS, Liebeskind JC, Stein C. Periaqueductal grey stimulation produces a spinally mediated, opioid antinociception for the inflamed hindpaw of the rat. Brain Res. 1991;545:17–23. doi: 10.1016/0006-8993(91)91264-2. [DOI] [PubMed] [Google Scholar]
- 26.Morton CR, Maisch B, Zimmermann M. Diffuse noxious inhibitory controls of lumbar spinal neurons involve a supraspinal loop in the cat. Brain Res. 1987;410:347–352. doi: 10.1016/0006-8993(87)90336-2. [DOI] [PubMed] [Google Scholar]
- 27.Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. II. Noxious visceral stimuli inhibit cutaneous nociceptive neurones and reflexes. J Neurophysiol. 1991;66:29–39. doi: 10.1152/jn.1991.66.1.29. [DOI] [PubMed] [Google Scholar]
- 28.Neugebauer V, Schaible HG. Evidence for a central component in the sensitization of spinal neurons with joint input during development of acute arthritis in cat’s knee. J Neurophysiol. 1990;64:299–311. doi: 10.1152/jn.1990.64.1.299. [DOI] [PubMed] [Google Scholar]
- 29.Reiber H, Suckling AJ, Rumsby MG. The effect of Freund’s adjuvant on blood-cerebrospinal fluid barrier permeability. J Neurol Sci. 1984;63:55–61. doi: 10.1016/0022-510x(84)90108-4. [DOI] [PubMed] [Google Scholar]
- 30.Ren K, Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. J Neurophysiol. 1996;76:3025–3037. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]
- 31.Roby-Brami A, Bussel B, Willer JC, Le Bars D. An electrophysiological investigation into the pain-relieving effects of heterotopic nociceptive stimuli: possible involvement of a supraspinal loop. Brain. 1987;110:1497–1508. doi: 10.1093/brain/110.6.1497. [DOI] [PubMed] [Google Scholar]
- 32.Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55:5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- 33.Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol. 1985;54:1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 34.Schaible HG, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988;60:2180–2195. doi: 10.1152/jn.1988.60.6.2180. [DOI] [PubMed] [Google Scholar]
- 35.Schaible HG, Schmidt RF. Neurophysiology of chronic inflammatory pain: electrophysiological recordings from spinal cord neurons in rats with prolonged acute and chronic unilateral inflammation at the ankle. Prog Brain Res. 1996;110:167–176. doi: 10.1016/s0079-6123(08)62573-x. [DOI] [PubMed] [Google Scholar]
- 36.Schaible HG, Schmidt RF, Willis WD. Enhancement of the responses of ascending tract cells in the cat spinal cord by acute inflammation of the knee joint. Exp Brain Res. 1987;66:489–499. doi: 10.1007/BF00270681. [DOI] [PubMed] [Google Scholar]
- 37.Schaible HG, Neugebauer V, Cervero F, Schmidt RF. Changes in tonic descending inhibition of spinal neurons with articular input during the development of acute arthritis in the cat. J Neurophysiol. 1991;66:1021–1032. doi: 10.1152/jn.1991.66.3.1021. [DOI] [PubMed] [Google Scholar]
- 38.Willer JC, Roby-Brami A, Le Bars D. Psychophysical and electrophysiological approaches to the pain relieving effect of heterotopic nociceptive stimuli. Brain. 1984;107:1095–1112. doi: 10.1093/brain/107.4.1095. [DOI] [PubMed] [Google Scholar]
- 39.Willer JC, De Broucker T, Le Bars D. Diffuse noxious inhibitory controls (DNIC) in man: involvement of an opioidergic link. Eur J Pharmacol. 1990;182:347–355. doi: 10.1016/0014-2999(90)90293-f. [DOI] [PubMed] [Google Scholar]
- 40.Willis WD. Anatomy and physiology of descending control of nociceptive responses of dorsal horn neurons: comprehensive review. Prog Brain Res. 1988;77:1–29. doi: 10.1016/s0079-6123(08)62776-4. [DOI] [PubMed] [Google Scholar]
- 41.Zieglgänsberger W, Bloom FE, Geiger SR. Central control of nociception. In: Mountcastle VB, editor. Handbook of physiology: the nervous system IV. Williams and Wilkins; Baltimore: 1986. pp. 581–645. [Google Scholar]