Abstract
The movements that define behavior are controlled by motoneuron output, which depends on the excitability of motoneurons and the synaptic inputs they receive. Modulation of motoneuron excitability takes place over many time scales. To determine whether motoneuron excitability is specifically modulated during the active versus the quiescent phase of rhythmic behavior, we compared the input–output properties of phrenic motoneurons (PMNs) during inspiratory and expiratory phases of respiration.
In neonatal rat brainstem–spinal cord preparations that generate rhythmic respiratory motor outflow, we blocked excitatory inspiratory synaptic drive to PMNs and then examined their phase-dependent responses to superthreshold current pulses. Pulses during inspiration elicited fewer action potentials compared with identical pulses during expiration. This reduced excitability arose from an inspiratory-phase inhibitory input that hyperpolarized PMNs in the absence of excitatory inspiratory inputs. Local application of bicuculline blocked this inhibition as well as the difference between inspiratory and expiratory firing. Correspondingly, bicuculline locally applied to the midcervical spinal cord enhanced fourth cervical nerve (C4) inspiratory burst amplitude. Strychnine had no effect on C4 output. Nicotinic receptor antagonists neither potentiated C4 output nor blocked its potentiation by bicuculline, further indicating that the inhibition is not from recurrent inhibitory pathways. We conclude that it is bulbospinal in origin.
These data demonstrate that rapid changes in motoneuron excitability occur during behavior and suggest that integration of overlapping, opposing synaptic inputs to motoneurons is important in controlling motor outflow. Modulation of phasic inhibition may represent a means for regulating the transfer function of PMNs to suit behavioral demands.
Keywords: phrenic motoneuron, brainstem, spinal cord, respiration, GABA, neonatal rat
Motoneurons shape motor patterns by transforming inputs into appropriate outputs controlling muscle contraction. This transformation of input into output, excitability, is determined by an interaction between synaptic inputs and the expression and modulation of intrinsic voltage- and ligand-gated conductances (Hultborn and Kiehn, 1992). Excitability is not static. It changes over multiple time scales (Feldman et al., 1990), such as during development (Berger et al., 1996) and in transitions between sleep states (Soja et al., 1991; Kubin et al., 1993). Because the output of multiple motoneuron pools must be coordinated to optimize movements for various behaviors, motoneuron properties may also change over faster time scales to achieve changes in excitability specific to a given behavior (Dickinson, 1995; Krawitz et al., 1997) or to a particular phase of behavior (Brownstone et al., 1992).
Various mechanisms could underlie such rapid changes in motoneuron excitability. (1) Coactivation of neuromodulatory pathways to motoneurons during behaviors is suggested by reductions in action potential threshold during fictive locomotion (Krawitz et al., 1997) and by monoamine-induced plateau potentials (Hounsgaard et al., 1988;Hounsgaard and Kiehn, 1989; Hultborn and Kiehn, 1992). (2) The strength of recurrent inhibitory pathways can be modulated (Hultborn et al., 1979; Hilaire et al., 1986). (3) The balance of concurrent excitatory and inhibitory synaptic inputs may be adjusted. Indirect evidence for such opposing inputs to hypoglossal motoneurons during inspiration (Withington-Wray et al., 1988; Woch and Kubin, 1995), and evidence for GABAergic gain modulation of respiratory premotoneurons (McCrimmon et al., 1997), led us to test whether concurrent inhibition and excitation underlie phase-specific modulation of motoneuron excitability.
Determining whether motoneuron excitability is differentially modulated between phases of a behavior requires preparations exhibiting behavior. Moreover, investigating motoneuron excitability during the active phase of behavior presents a challenge, because changes in membrane potential and input resistance produced by excitatory synaptic drive can mask underlying modulatory effects. For example, increased excitability of lumbar motoneurons during the active versus quiescent phase of the fictive locomotor cycle (Brownstone et al., 1992) suggests that activation of central rhythmic behavioral circuits changes the input–output function of motoneurons. However, interpretation of these results is confounded by the presence of ongoing rhythmic locomotor drive potentials and the associated changes in membrane conductance. In most rhythmic behaviors, block of these postsynaptic drive potentials to motoneurons will disrupt generation of behavioral rhythm, due to proximity of the motoneurons to the rhythm-generating circuitry (Robertson and Stein, 1988; Brownstone et al., 1992).
To address these problems and test for rapid, phase-specific modulation of motoneuron excitability, we used a neonatal rat brainstem–spinal cord preparation that generates rhythmic inspiratory drive to motoneurons (Smith and Feldman, 1987). This preparation was chosen because phrenic motoneurons (PMNs), which drive the diaphragm, are spatially segregated from the rhythm-generating networks that produce their primary behavioral (inspiratory) input, thereby facilitating pharmacological manipulation of fast excitatory inputs at the motoneuron level without disruption of respiratory rhythm. In addition,in vitro conditions allow complete block of excitatory postsynaptic rhythmic drive currents.
MATERIALS AND METHODS
Brainstem–spinal cord preparations. Experiments were performed on brainstem–spinal cord preparations (n = 74) from neonatal Wistar rats ranging in age from 0 to 3 d postnatal (P0–P3). Preparations were isolated using methods previously described for Sprague Dawley rats (Smith and Feldman, 1987; Dong and Feldman, 1995). Briefly, an animal was anesthetized with diethyl ether and decerebrated, then the brainstem–spinal cord isolated in a dissection chamber containing artificial CSF (aCSF) containing (in mm): 128 NaCl, 3 KCl, 1.5 CaCl2, 1 MgSO4, 21 NaHCO3, 0.5 NaH2PO4, 30 d-glucose, pH 7.4, at 20–22°C, oxygenated with 95%O2/5%CO2. Preparations extended from the midpontine level rostrally to the seventh cervical segment caudally.
After isolation, the brainstem–spinal cord was pinned, ventral surface up, on Sylgard resin in a recording chamber (2 ml volume) perfused with oxygenated aCSF at a flow rate of 2–2.5 ml/min. The pia mater was then removed from the ventral surface of the spinal cord just lateral to midline at the level of the C4 nerve root to allow introduction of whole-cell recording electrodes into the PMN column and facilitate drug diffusion. Bath temperature was gradually increased to 26–27°C before recording.
Extracellular recording of whole-nerve inspiratory activity.Inspiratory motoneuron activity was recorded from the severed ends of C1 or C4 cervical roots using suction electrodes (80–100 μm internal diameter). In experiments requiring block of glutamatergic drive to individual PMNs, C4 nerve output was also abolished. Thus C1 nerve output was recorded as an index of respiratory cycle timing. In experiments designed to determine the effect of drugs on PMN population output, we recorded from the C4 nerve. Nerve recordings were amplified (50,000 times), bandpass-filtered (0.1–3 kHz), full wave-rectified, and integrated using a leaky integrator (τ = 50 msec).
Whole-cell recording. Intracellular recordings from PMNs (n = 54) were made using “blind” whole-cell patch-clamp recording techniques (Blanton et al., 1989). Patch electrodes (resistance 4.0–5.5 MΩ; 1.5–2 μm tip size) were pulled on a horizontal puller (Sutter Model P-97) from 1.2 mm outer diameter, filamented borosilicate glass (Clark/WPI) and filled with solution containing (in mm): K+-gluconate 125, NaCl 5, CaCl2 1, HEPES 10, BAPTA 10, ATP (Mg2+ salt) 2. Intracellular solution pH was adjusted to 7.2–7.3 with 5 m KOH. Signals were amplified and filtered with a patch-clamp amplifier (5 kHz Bessel filter, Axopatch 1D; Axon Instruments, Foster City, CA).
Series resistance and whole-cell capacitance were estimated under voltage-clamp conditions by using short voltage pulses (100 Hz, −10 mV, 3.0 msec). Series resistance ranged from 10 to 32 MΩ, (mean, 17 ± 6 MΩ) and was monitored throughout experiments. Data were discarded if series resistance changed between control and test conditions. Voltage–current (V–I) relationships were obtained in current clamp by applying a series of current steps (500 msec, +50 to −300 pA). In voltage clamp, current–voltage (I–V) relationships were obtained by applying a series of voltage steps (+24 to −36 mV from resting membrane potential). Cell input resistance (RN) was calculated from the slope or the inverse of the slope of a least squares regression line fitted to V–I or I–Vcurves, respectively. Rheobase was estimated by increasing the amplitude of depolarizing square-wave current steps (400–600 msec duration) until a single spike was elicited.
Neurons included in the database satisfied criteria described previously for PMNs (Liu et al., 1990; Dong and Feldman, 1995). Briefly, they had resting membrane potentials of −60 mV or more hyperpolarized, received rhythmic synaptic drive currents/potentials in phase with inspiratory burst activity on C1 ventral nerve roots, produced action potentials that overshot 0 mV, and were located at intermediate laterality 110–260 μm deep from the ventral surface at the level of C4.
Pharmacological substances and application. Drugs that were used included bicuculline methbromide [Research Biochemicals (RBI), Natick, MA; 0.1–1.0 mm], 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX) (RBI; 0.25–0.5 mm), GABA (Sigma, St. Louis, MO; 1 mm), hexamethonium bromide (Serva Feinbiochemica, Heidelberg, Germany; 0.1–0.5 mm), 3-[2-methylphenoxy]-1,2-propanediol (mephenesin) (Sigma; 1 mm), mecamylamine hydrochloride (RBI; 50 μm), (+)-MK-801 hydrogen maleate (MK801) (RBI; 0.5–1.0 mm), 5-aminomethyl-3-hydroxyisoxazole (muscimol) (Sigma; 10–100 mm), 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium (NBQX) (RBI; 0.375–0.5 mm), strychnine hydrochloride (Sigma; 1 mm), and tetrodotoxin (TTX) (RBI; 0.5–1.0 μm). All drugs were made up in aCSF and stored as frozen aliquots, with the exception of hexamethonium, which was made fresh before each use.
Drugs were either applied locally over the ventral surface of the C4 spinal cord over the region containing the PMN pool, or added to the bath. Local application of drugs from triple-barreled pipettes (6 μm per barrel outside diameter at the tip) was via timed pressure injection controlled by solenoid valves.
Drugs were bath-applied when multisegmental action of a given drug was required (e.g., when we wanted to block recurrent inhibition throughout the cervical cord rather than at C4 alone). The recording chamber was divided into two compartments (split-bath configuration) by construction of a petroleum jelly barrier at the spinomedullary junction. This allowed selective application of drugs to the spinal cord aCSF without affecting processes in the brainstem where respiratory rhythm originates. A minimum of 10 min was allowed for drug equilibration. The split-bath configuration involved analysis of C4 population output only, because the petroleum jelly used for the partition interferes with formation of GΩ seals required for whole-cell recording. The integrity of the partition was tested at the start and the end of each experiment by removing all solution from one compartment and verifying that no solution leaked through.
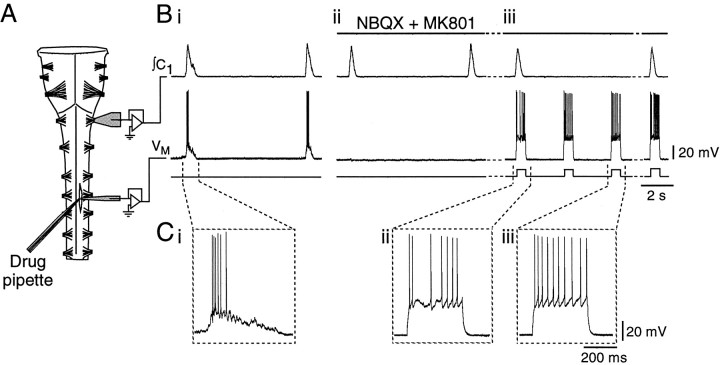
Neuronal properties during inspiratory and expiratory periods: repetitive firing protocol. The paradigm used to compare repetitive firing elicited during inspiration with firing elicited between inspiratory bursts, which we refer to as expiration, is illustrated in Figure 1. First, C1 ventral root population inspiratory activity and whole-cell recording of PMN inspiratory synaptic inputs were established (see Fig. 1Bi). After inspiratory synaptic inputs were characterized under voltage- and current-clamp conditions, they were blocked via continuous local application of a glutamate receptor antagonist mixture over the C4 PMN pool until no depolarization of PMNs was observed concurrent with inspiratory bursts on the C1 nerve (see Fig. 1Bii). The antagonist mixture comprised 0.25–0.5 mm CNQX and 0.5 mm MK801, or 0.375 mm NBQX and 0.5 mm MK801, or 0.5 mm CNQX, 0.375 mmNBQX, and 0.5 mm MK801. C1 nerve inspiratory activity remained unblocked by the glutamate antagonist cocktail because of its spatial separation from the site of drug injection and therefore was used as an index of cycle phase. The rising phase of C1-integrated inspiratory activity elicited a TTL pulse from a window discriminator that initiated an eight-cycle series of injected current pulse triplets. The first pulse was delivered during inspiration; the second and third pulses of equal magnitude were delivered during the expiratory period (between inspiratory bursts) at 2–4 sec intervals (see Fig. 1Biii). The third pulse was applied to control for the possibility that accommodative properties may have produced different PMN responses to the second of a pair of identical current pulses independent of any phasic alteration of neuron properties. Temporal characteristics of the square-wave pulses were controlled with a Master-8 Stimulator (A.M.P.I., Jerusalem, Israel) and Axodata software. Duration of the pulses was varied from 400 to 600 msec to match the duration of the inspiratory phase of the preparation. Injected current amplitude incremented or decremented with each respiratory cycle (i.e., after one inspiratory and two expiratory pulses), with a total of eight equal steps chosen empirically to produce a range of firing frequencies comparable between PMNs.
Fig. 1.
Experimental set-up and protocol for comparison of PMN firing behavior during inspiratory and expiratory phases.A, Schematic of the rhythmically active brainstem–spinal cord preparation showing the configuration of the suction electrode for recording first cervical nerve (C1) activity, the whole-cell recording electrode at the level of the fourth cervical nerve for recording PMN activity, and the triple-barrel drug ejection pipette. B, Rectified, integrated recording of C1 output (∫C1, top trace) showing population inspiratory activity and whole-cell current-clamp record of a PMN (VM, middle trace) (i) under control conditions with inspiratory synaptic potential present, (ii) after complete blockade of the PMN excitatory inspiratory input with NBQX and MK801, and (iii) during the repetitive firing protocol in the continued presence of NBQX and MK801. After block of excitatory inspiratory drive to the PMN (Bii), population inspiratory activity on ∫C1 was used to trigger injection of square-wave current pulses during inspiration (Biii). Responses of PMNs to current pulses injected during inspiratory and expiratory periods were then compared. C, Expanded versions of voltage traces (VM) inB, showing the firing responses of the PMN to (i) endogenous inspiratory synaptic input, (ii) a 380 pA pulse delivered during inspiration, and (iii) the same amplitude current pulse delivered during expiration.
Block of primary excitatory inspiratory synaptic drive was essential for these experiments. (1) It prevented changes in motoneuron input conductance associated with activation of the ligand-gated glutamate receptor channels that mediate inspiratory drive (Liu et al., 1990); (2) it removed rapid membrane potential fluctuations associated with inspiratory synaptic drive (see Fig. 1Bi), facilitating analysis of repetitive firing behavior; and most importantly, (3) it ensured that inspiratory-phase responses were not evoked from more depolarized membrane potentials than expiratory-phase responses. If excitatory synaptic inputs were not blocked, inspiratory-phase responses would be from the summed action of synaptic and injected currents, whereas expiratory-phase responses would represent responses to injected current only. Thus PMNs were not subjected to the repetitive firing protocol if inspiratory-phase depolarizations were still observed.
Tests for phase-dependent changes inRN. PMN RN during inspiratory and expiratory periods was compared, after block of glutamatergic inputs, from the slope of least squares regression line fitted through voltage–current relationships generated by plotting the steady-state voltage responses to incrementing or decrementing current pulses (50 pA steps; −200 to +50 pA) delivered during the inspiratory and expiratory periods. As above, the rising phase of C1 inspiratory activity was used as the index of inspiratory onset.
Data acquisition and analysis. Signals were displayed on line on a chart recorder and oscilloscope and were recorded on videotape via pulse code modulation (Vetter 402 or 3000A; A. R. Vetter, Rebersberg, PA) (sampled at 10–40 kHz/channel) for storage and off-line analysis. Selected portions of data were digitized at 1–20 kHz using AxoData software and a National Instruments NBMIO-16 A/D board and stored on computer for subsequent analysis. Peaks of EPSPs and IPSPs and of integrated nerve activity were calculated using the peak detection analysis in AxoGraph software (version 3.0). The effects of bath-applied and locally applied drugs on frequency and peak amplitude of C4 inspiratory bursts were assessed using a custom-written LabVIEW acquisition and analysis program.
PMNs exhibited a wide range of rheobase,RN, and maximum firing frequency values. Thus, the absolute magnitude of the eight current pulses injected to examine repetitive firing varied between PMNs. Therefore to pool data on firing frequency versus injected current from different PMNs for statistical analysis, firing frequency was plotted against the current step number rather than absolute current. Each of the eight steps produced a similar increment in PMN output relative to maximum firing for that cell.
Data are reported as mean ± SE. Means of data on C4 burst amplitude and PMN subthreshold properties were compared using a two-tailed Student’s paired t test. An arc sine transform was performed to normalize all percentage data before statistical comparison. Comparison of PMN firing behavior during the inspiratory and expiratory phase was made with Statistical Analysis Software using an 8 × 3 or 3 × 3 univariate one-way ANOVA for unbalanced data sets. Linear contrast coefficients were used to partition the sum of squares to determine whether firing was significantly different between inspiration and expiration or between the two expiratory pulses. Values of p < 0.05 were assumed to be significant.
RESULTS
Behavior of brainstem–spinal cord preparations isolated from P0–P3 Wistar rats was indistinguishable from that of similar preparations isolated from Sprague Dawley rats [Smith and Feldman (1987); Lin et al. (1990); Dong and Feldman (1995); also see Connelly et al. (1992)]. Preparations produced spontaneous inspiratory-related bursts of activity (5–12/min) on ventral cervical nerve roots. Burst envelopes were rapidly incrementing and slowly decrementing (Fig.1).
PMN properties were also similar to those described previously (Liu et al., 1990; Dong and Feldman, 1995). PMNs received rhythmic synaptic input (200–1500 pA) in phase with C1 inspiratory nerve activity, had resting membrane potentials of −64 ± 1 mV,RN of 79 ± 5 MΩ in control solution (n = 41), and fired repetitively in response to square-wave depolarizing current pulses (rheobase, 370 ± 210 pA).
Repetitive firing is reduced during inspiration versus expiration
To facilitate comparison of repetitive firing behavior of PMNs during inspiration and expiration, excitatory synaptic inputs mediating the inspiratory drive were blocked with local application of NBQX or CNQX + MK801 or both. Drugs were applied continuously to ensure continued block of excitatory synaptic drive until protocols were completed. Although 95% of the inspiratory synaptic inputs to PMNs can be blocked by 5–50 μm CNQX (Liu et al., 1990), complete block of excitatory input required up to 500 μm CNQX or NBQX. MK-801 was included in the antagonist solution to ensure that all ionotropic glutamatergic receptors were blocked (Greer et al., 1991). Complete block of excitatory drive was determined by the absence of inspiratory-phase depolarizing inputs to the recorded PMN. Although recent observations suggest a possible metabotropic glutamate receptor contribution to the inspiratory drive to PMNs (Dong and Feldman, 1996), our ability to completely block inspiratory-phase depolarization in 32 of 37 PMNs suggests that a metabotropic component is not present in all cells, or that, where present, it was blocked by the high concentrations of antagonists. Complete block typically took 5–15 min. The antagonist mixture was associated with a 22 ± 4% increase inRN (to 97 ± 6 MΩ; n = 26).
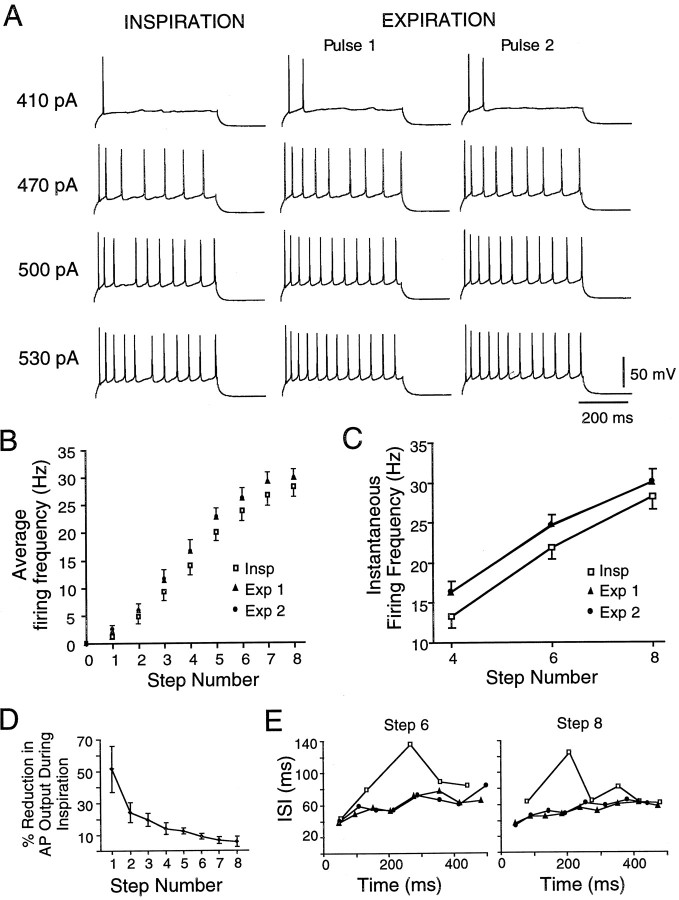
The repetitive firing protocol was completed in 20 of the PMNs in which all inspiratory-phase depolarization was blocked. Visual inspection of the responses revealed a clear reduction in inspiratory-phase firing in 14 PMNs, as shown for one PMN in Figure2A. In the remaining six PMNs, differences between inspiratory and expiratory firing responses were not obvious. We compared firing frequencies between inspiration and expiration on data pooled from all 20 PMNs and on data from the 14 cells showing obvious reductions in inspiratory-phase firing. Inspiratory-phase firing was significantly lower than expiratory firing regardless of whether 20 or 14 cells were used for analysis (see below). Because the 14 PMNs with obvious phase differences in firing may represent a subpopulation of PMNs that receive inspiratory modulation, we report only the quantitative results from analysis of those cells. Firing frequencies were calculated in two ways. (1) To quantify the overall response to a current pulse, the number of action potentials produced was divided by pulse duration. This gave a value for average firing frequency that enabled comparison of firing between inspiration and expiration at current levels that did not produce enough action potentials to calculate a representative instantaneous firing frequency. (2) Instantaneous firing frequency, the inverse of the interspike interval duration, was also averaged at the fourth, sixth, and eighth current steps to ensure that average and instantaneous firing frequencies responded similarly to inspiratory and expiratory input.
Fig. 2.
PMN excitability is reduced in inspiration relative to expiration. A, Responses of one PMN to four levels of current steps presented during inspiration and at two times during expiration after block of excitatory inspiratory synaptic input. Responses to pulses presented during the inspiratory phase are shown in the left-hand traces(INSPIRATION); responses to pulses presented during the expiratory phase are shown in the middle(EXPIRATION, Pulse 1) and right-hand traces (EXPIRATION, Pulse 2). Pulse amplitude is indicated to the left of each triplet. B, Plot of total pulse firing frequency versus current step number calculated from responses to pulses presented during inspiration (Insp) and at two times during expiration (Exp 1, Exp 2) (n = 14). C, Plot of instantaneous firing frequency versus current step number calculated from responses to the fourth, sixth, and eighth current steps during inspiration (Insp) and at two times during expiration (Exp 1, Exp 2) (n = 14).D, Plot of percentage reduction in inspiratory-phase action potential (AP) output (number of APs relative to expiratory phase discharge) as a function of current step number.E, Plots of interspike interval (ISI) duration versus interval number for repetitive firing in one PMN. Firing was elicited by the sixth (left-hand trace) and eighth (right-hand trace) current steps delivered during inspiration (■) and by the same current levels at two times during the expiratory phase (Exp 1, ▴; Exp 2, ●). Compare with Figure 4C.
The relationship of firing frequency to injected current step was shifted to the right during inspiration (i.e., more current was needed to produce a given firing frequency). Thus the overall mean spike frequency during inspiration (15.4 ± 1.1 Hz) was significantly lower than during expiration (17.4 ± 1.1 Hz; n = 14; p < 0.001) (Fig. 2B). Similarly, instantaneous firing frequency was significantly lower during inspiration than during expiration. At the fourth, sixth, and eighth steps, average instantaneous firing frequencies during inspiration were 13 ± 1, 22 ± 1, and 28 ± 2 Hz, respectively, versus 16 ± 1, 25 ± 1, and 30 ± 2 Hz during expiration (p < 0.001) (Fig. 2C). Firing frequency responses to pulses delivered early and late in expiration were not different at any level of current (p > 0.5) regardless of whether average or instantaneous firing frequencies were compared (Fig. 2B,C). The reduction in firing was manifest as one or two fewer action potentials per 400–600 msec current pulse, independent of current level. Thus, when expressed in terms of relative change in action potential output, the reduction in inspiratory versus expiratory discharge was greater at the lower firing levels (Fig. 2D). Relative to the expiratory phase, inspiratory firing was reduced by 49 ± 15% and 7 ± 3% for steps 1 and 8, respectively (n = 14).
Plots of interspike interval duration against time, calculated from the firing responses to square-wave pulses, revealed that interspike interval durations increased during inspiration relative to expiration (Fig. 2E), indicating that excitability is reduced during the inspiratory phase.
The reduction in inspiratory firing is caused by phasic inspiratory inhibition
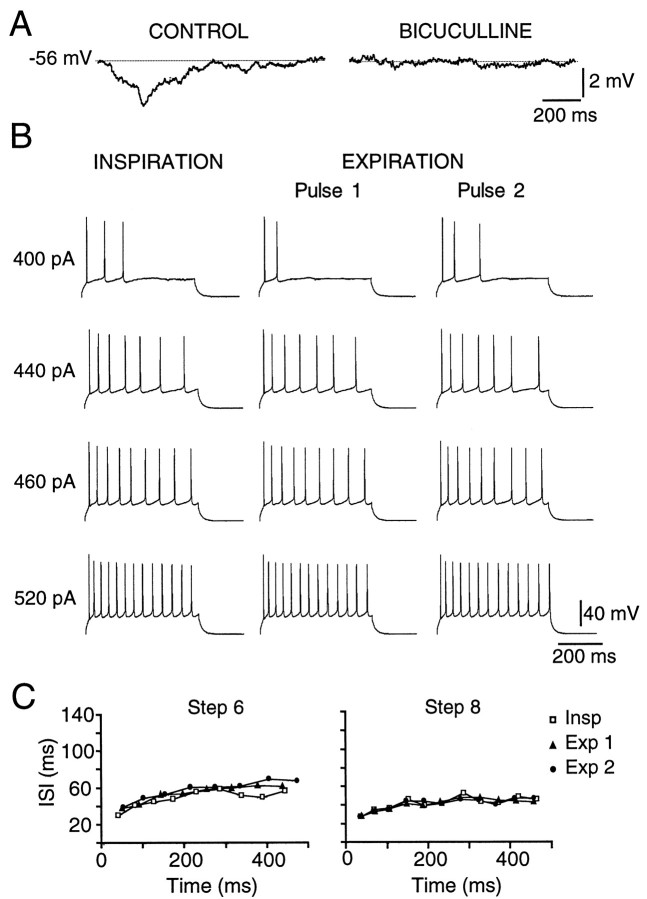
The increase in duration of interspike intervals during evoked repetitive firing in the inspiratory phase indicated that PMN excitability was decreased during inspiration. Examination of membrane potential during the inspiratory phase (after block of excitatory synaptic input) revealed that in 16 of the 32 PMNs in which the inspiratory-phase depolarization was completely blocked, a small hyperpolarizing input arrived coincident with inspiratory bursts on the C1 nerve. Ten of the PMNs exhibiting this hyperpolarization were among those subjected to the repetitive firing protocol. Of these 10 PMNs, nine showed a clear reduction in inspiratory firing. Failure to observe a hyperpolarization in ∼50% of the PMNs may reflect that not all PMNs are phasically inhibited or that where unobserved, the hyperpolarization was masked by incompletely blocked excitatory drive. The latter possibility was supported by the finding that blocking the hyperpolarization with bicuculline (100–200 μm) in 10 cells revealed a small (<2 mV) remaining inspiratory-phase depolarization in four of them (data not shown).
An example of the inspiratory hyperpolarization in one PMN in current clamp is shown in Figure 3A. Hyperpolarizations had an average duration of 580 ± 97 msec at a membrane potential of −55 mV. Peak magnitude was dependent on membrane potential, increasing from −0.7 ± 0.1 mV at resting membrane potential (−62 mV), to −1.1 ± 0.4 mV and −1.5 ± 0.5 mV at −55 and −50 mV, respectively (n = 4). The hyperpolarization reversed between −65 and −75 mV (Fig.3A).
Fig. 3.
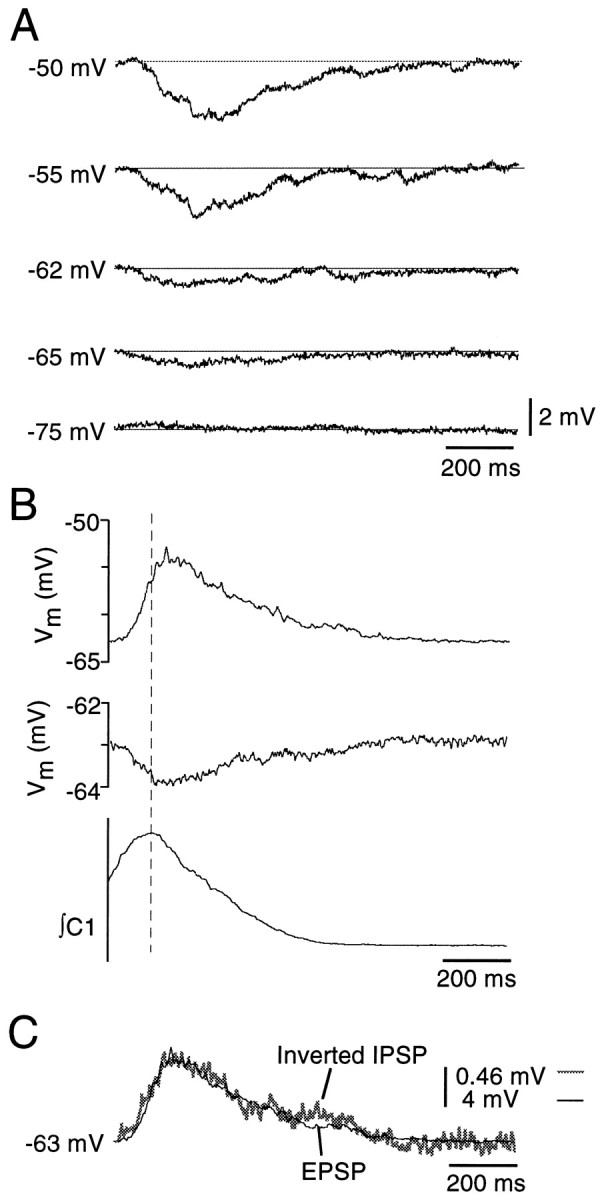
PMNs receive hyperpolarizing input during inspiration. A, Current-clamp recordings from one PMN illustrating the magnitude of the inspiratory hyperpolarization at five different membrane potentials (shown at left of traces). Traces represent averages of five inspiratory cycles. B, Time course of the excitatory (top trace) and inhibitory inspiratory potentials (middle trace) relative to the inspiratory burst recorded from C1 (bottom trace). Traces represent averages of 10 inspiratory cycles. C, The high degree of overlap between these inspiratory excitatory and inhibitory synaptic inputs is demonstrated by inverting and amplifying (8.7 times) the inhibitory input (gray trace) and superimposing it on the excitatory input (thin black trace).
Durations of the inhibitory (580 ± 97 msec) and excitatory (690 ± 66 msec) inputs to PMNs were similar (n = 6), and a rapidly incrementing, slowly decrementing envelope was common to both. The temporal relationship between excitatory and inhibitory inspiratory synaptic inputs could not be established directly because the inhibitory input was only visible during block of the excitatory input. Instead, the timing of the peak of the excitation, measured before application of glutamate antagonists, and the peak of the inspiratory inhibition, measured after glutamate receptor block, were compared relative to the peak of the C1 ventral root inspiratory burst. Peaks of the inspiratory synaptic excitation and inhibition were thus determined to be nearly coincident, arriving 10 ± 10 msec and 34 ± 22 msec (n = 5), respectively, after the peak of the C1 nerve inspiratory burst (Fig. 3B).
Not only was its time course similar: the inspiratory inhibition corresponded closely in shape to the inverted excitatory drive. This is shown for one PMN in Figure 3C where the inhibitory potential envelope averaged from 10 inspiratory cycles was inverted, scaled (in this case amplified 8.7 times), and superimposed on the averaged excitatory inspiratory input (before it was blocked) from the same cell. The close correspondence of the relationships between inhibitory and excitatory synaptic inputs (Fig. 3C) is consistent with the possibility that inhibitory and excitatory drives are proportional.
To test whether the inspiratory hyperpolarization was mediated via GABA receptors, we examined the effects of locally applied bicuculline (GABAA receptor antagonist; 100–200 μm) on the inspiratory-related hyperpolarization. Bicuculline blocked the hyperpolarization in all PMNs tested (n = 11) (Fig.4A). It also eliminated the difference between inspiratory and expiratory firing (Fig.4B) (n = 3) and blocked the differences between inspiratory and expiratory firing behavior evident in the plots of interspike interval versus time (Fig. 4C). These data support our hypothesis that the inspiratory-phase hyperpolarization was responsible for reduced firing during the inspiratory relative to the expiratory phase.
Fig. 4.
The hyperpolarizing inspiratory inhibition of PMNs and the reduction in PMN firing during inspiration are blocked by bicuculline. A, The same cell as in Figure3A at −56 mV. The membrane potential of a PMN during the inspiratory phase, before (CONTROL) and after local application of 200 μm bicuculline (BICUCULLINE). Traces are averages of eight inspiratory cycles. B, Bicuculline (200 μm) blocked the reduction in spike output during inspiration relative to expiration (same cell as in Fig. 2A). C, Bicuculline also blocked the increases in inspiratory interspike interval (same cell and current steps as in Fig.2E).
GABA receptor activation inhibits PMN excitability
We tested the effects of GABA and muscimol (GABAAreceptor agonist) on PMN properties and repetitive firing behavior to determine whether their actions supported our hypothesis that the reductions in inspiratory firing were caused by endogenous activation of GABAA receptors.
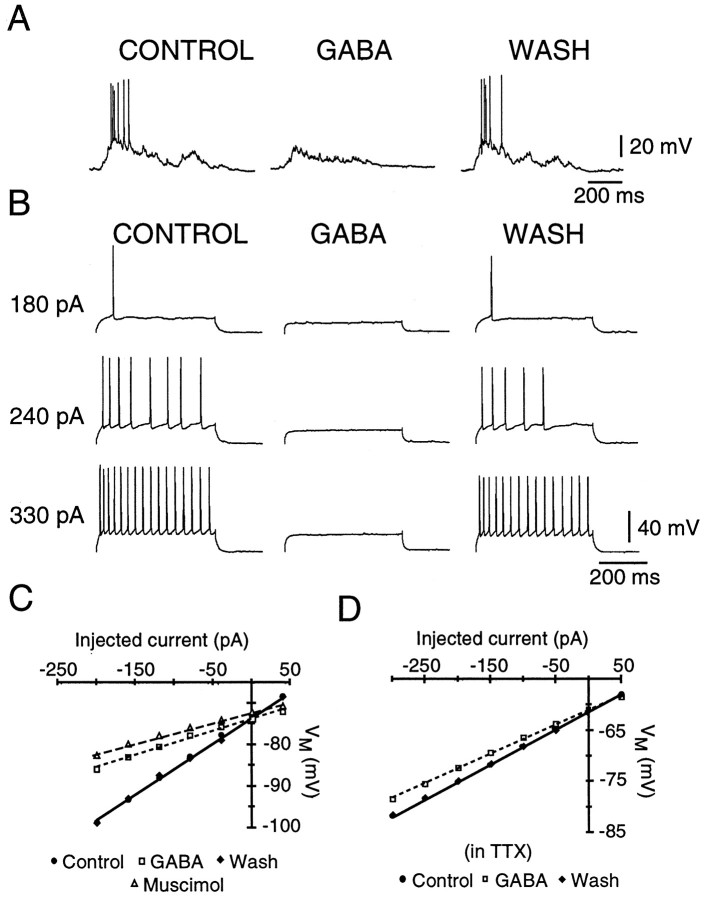
GABA (1 mm, applied locally) rapidly (within one inspiratory cycle) and reversibly reduced the number of spikes produced during the inspiratory phase (Fig.5A), completely blocking inspiratory-phase firing in six of six cells. Similarly, GABA greatly reduced repetitive firing elicited by current injection (n = 6) (Fig. 5B). This was not due to block of cells’ ability to fire action potentials, because higher amplitude current pulses still elicited repetitive firing (data not shown). The associated increase in rheobase did not appear to be attributable to a change in spike threshold but to a reduction in cellRN by GABA to 33 ± 8% of control (n = 10) (Fig. 5C,D). The GABA-induced decrease in RN persisted in TTX (n = 3) (Fig. 5D), suggesting that it is mediated at least in part by postsynaptic receptors. The effects of GABA were mimicked by the GABAA receptor agonist muscimol (0.01–1.0 mm), which similarly decreasedRN to 48 ± 12% of control (n = 4) (Fig. 5C). No consistent effects on membrane potential were observed in response to GABA or muscimol at resting membrane potential (−63 ± 5 mV).
Fig. 5.
GABA reduces PMN excitability. A, Current-clamp recording from a PMN before (CONTROL), during (GABA), and after (WASH) local application of GABA (1 mm) over the C4 phrenic motoneuron pool. During GABA application, endogenous excitatory synaptic drive did not elicit action potentials. B, The same PMN as in A, showing that injected current pulses that elicited firing in control did not bring the cell to threshold during GABA application. C, Plot of membrane potential versus injected current for one PMN, showing a decrease in slope of theV–I relationship in the presence of GABA (1 mm, ■) or muscimol (0.01 mm, ▵) relative to control (●), indicating a reduction in cellRN. D, Application of GABA (1 mm) also reduced RN after block of synaptic transmission with 0.8 μm TTX (different cell than in C).
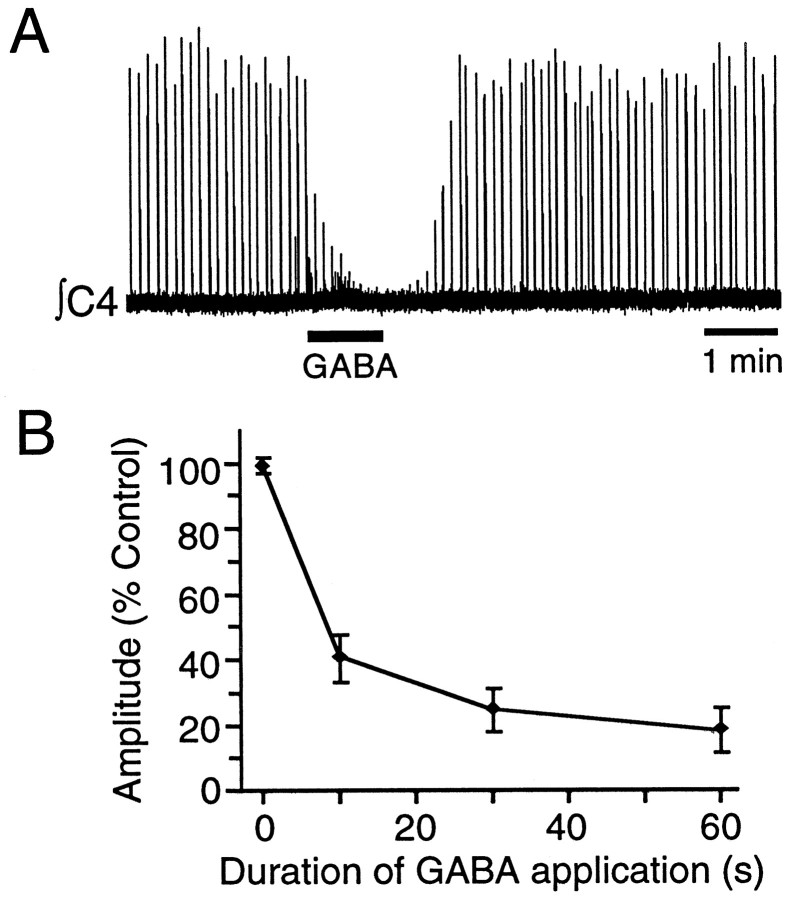
In keeping with its observed effects on PMN activity, local application of 1 mm GABA over the C4 spinal cord for 10, 30, and 60 sec significantly reduced C4 nerve burst amplitude to 40 ± 7, 25 ± 7, and 21 ± 7% of control (Fig.6) (n = 5).
Fig. 6.
A, Sixty second application of GABA (1 mm) over the PMN pool nearly abolished ∫C4 population inspiratory activity in a P1 rat. B, Pooled data showing the dose-dependent reduction in ∫C4 burst amplitude (expressed as percentage of control amplitude) produced by 10, 30, and 60 sec applications of 1 mm GABA over the C4 motoneuron pool (n = 5; mean ± SE).
Bicuculline potentiates C4 inspiratory activity
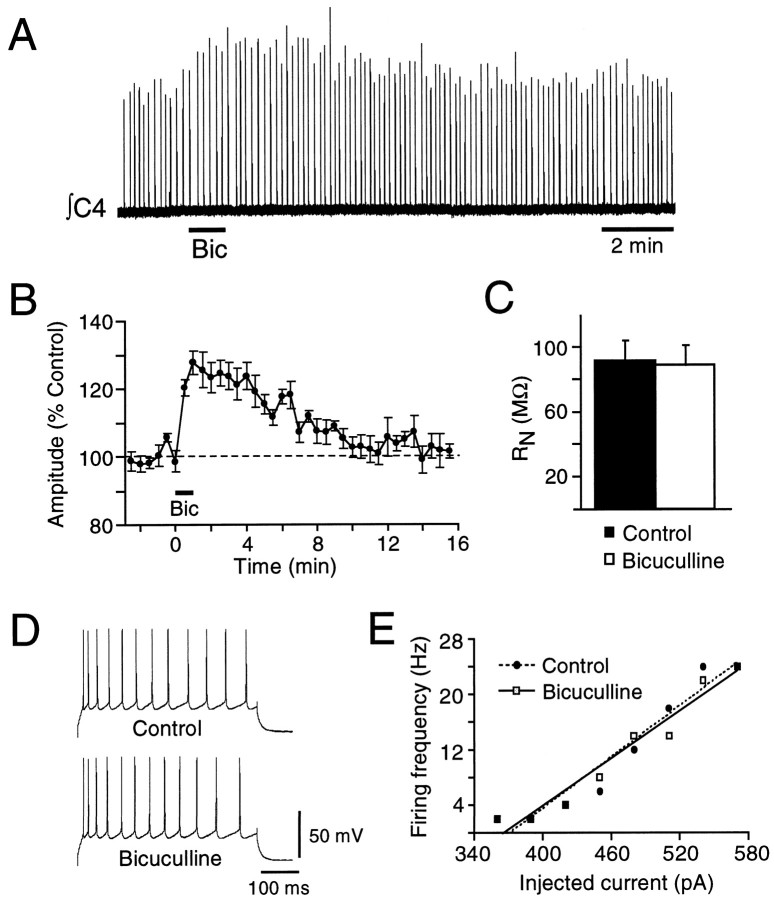
To assess the impact of the bicuculline-sensitive inspiratory inhibition of PMNs on inspiratory input to the diaphragm, we measured changes in C4 nerve burst activity after locally applying bicuculline (1 mm) over the C4 PMN pool. Bicuculline (1 mm; 30–60 sec) significantly increased C4 nerve burst amplitude by 33 ± 9% (Fig. 6) (n = 14; p < 0.05). The bicuculline-induced potentiation peaked at 1–2 min and returned to control levels 10–15 min after application.
A bicuculline-induced potentiation of C4 burst amplitude could result from block of tonic rather than inspiratory-phase GABA-mediated inhibition of PMN activity. We therefore examined the effects of bicuculline on PMN membrane properties and repetitive firing behavior during expiration. Consistent with a phase-dependent (inspiratory) inhibition, bicuculline (0.2 or 1.0 mm) did not increaseRN during the expiration (n = 9) (Fig. 7C), nor did it alter the relationship between injected current and firing frequency (n = 4) (Fig. 7D,E). Establishing that the potentiation of C4 burst amplitude by bicuculline results from block of an inspiratory-phase inhibition enabled us to later use bicuculline-induced potentiation as an indirect measure of the magnitude of the inspiratory inhibition (Figs. 7, 9, 10).
Fig. 7.
Bicuculline increases C4 inspiratory output without changing RN or firing responsiveness of PMNs during expiration. A, Rectified, integrated C4 nerve recording from a P1 rat showing increased burst amplitude in response to a 60 sec application of bicuculline (1 mm) over the PMN pool (bar under trace). B, Time course of the effects of bicuculline on C4 inspiratory burst amplitude expressed as a percentage of control (n = 5; mean ± SE). C, Bicuculline (0.2–1.0 mm) had no significant effect onRN of PMNs measured during expiration (n = 10). D, Current-clamp recording from a PMN showing repetitive firing during expiration elicited by current steps injected under control conditions and during local application of bicuculline (1 mm) over the C4 motoneuron pool. E, Plot of firing frequency versus injected current during expiration for a PMN in control (●) and bicuculline (1 mm, ■).
Fig. 9.
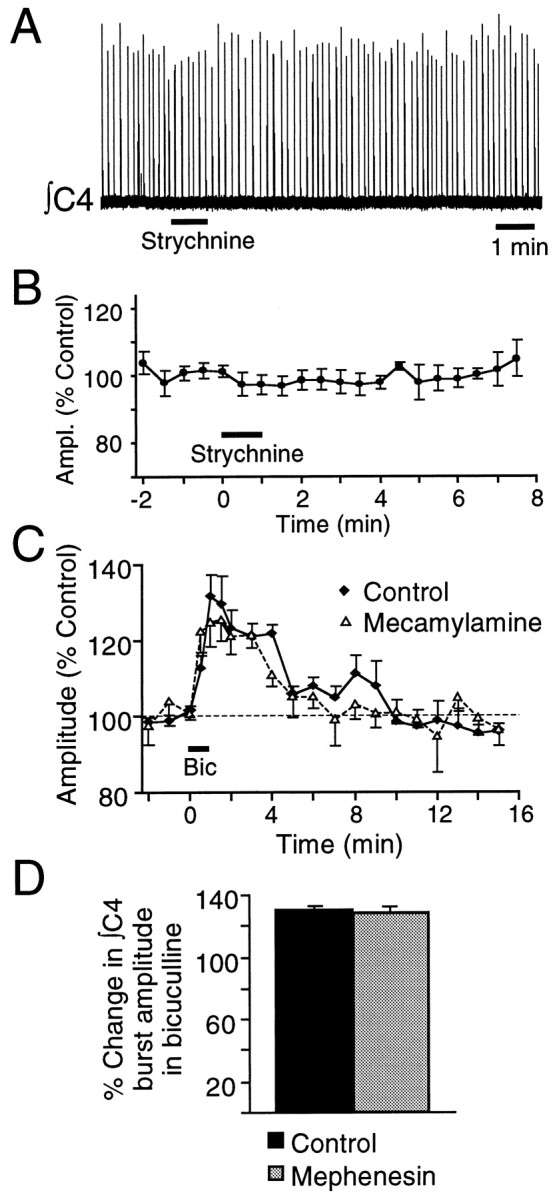
Renshaw cells do not mediate the bicuculline-sensitive inspiratory inhibition. A, Rectified, integrated record of C4 inspiratory activity from a P1 rat brainstem–spinal cord showing no change in nerve burst amplitude after a 60 sec application of 1 mm strychnine (bar under trace) to the C4 PMN pool. B, Pooled data confirm that strychnine applied to the C4 PMN pool (60 sec starting att = 0, indicated by bar) had no effect on C4 inspiratory output (n = 6; mean ± SE). C, Addition of mecamylamine (50 μm) to the medium perfusing the spinal cord (split-bath configuration) did not block the potentiation of ∫C4 inspiratory burst amplitude induced by local application of bicuculline (1 mm, 60 sec) (n = 4; error bars indicate SE). D, Potentiation of C4 burst amplitude induced by local application of bicuculline (60 sec, 1 mm) over the PMN pool (Control) was not affected by addition of mephenesin (1 mm) to the spinal cord bath (n = 5; error bars indicate SE).
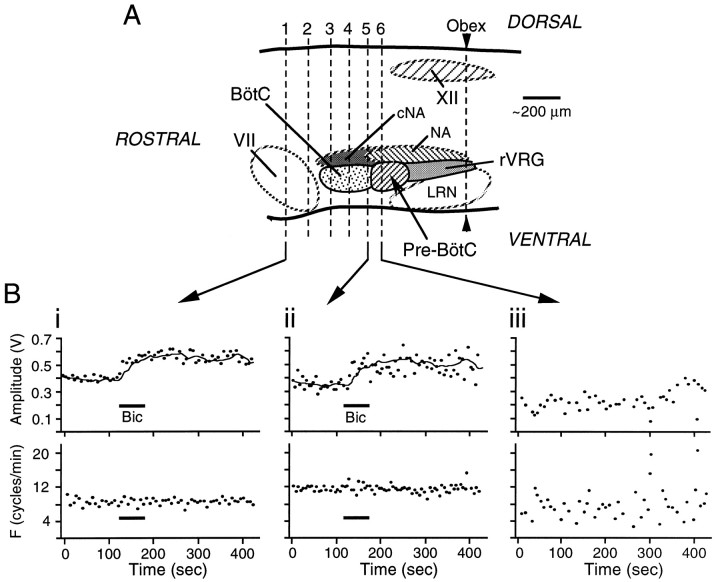
Fig. 10.
The inspiratory-related inhibition does not arise from structures rostral to the pre-Bötzinger Complex.A, Sagittal view of the brainstem showing levels of transection (dashed lines labeled 1through 6) taken serially, in relation to organization of ventrolateral brainstem respiratory nuclei.B, Time course of the effects on ∫C4 inspiratory burst amplitude (in volts) and burst frequency (F) of locally applying bicuculline (60 sec, 1 mm) over the C4 PMN pool after transection of the brainstem at levels 1(Bi) and 5 (Bii). Individual data points represent amplitude and frequency of single inspiratory cycles. The line in the top panel ofBi represents the moving average of the C4 burst amplitude response to bicuculline applied after transection at level1. This moving average is included in Biito illustrate that the effects of bicuculline were unaffected with progressively more caudal transections up to level 5. Removal of the rostral component of the pre-Bötzinger Complex with transection at level 6 disrupted both respiratory rhythm and C4 burst amplitude (Biii), precluding further analysis of responses to bicuculline. BötC, Bötzinger Complex; VII, facial nucleus;XII, hypoglossal nucleus; cNA, compact division of nucleus ambiguus; LRN, lateral reticular nucleus; NA, nucleus ambiguus;Pre-BötC, pre-Bötzinger Complex;rVRG, rostral–ventral respiratory group).
GABA-mediated gain modulation of PMN excitability
The shapes of IPSPs and EPSPs appear similar (Fig. 3). This raises the possibility that the GABA-mediated inhibition provides a means for gain control over PMN output. Gain control is defined as a process whereby the output discharge frequency of a neuron,Fo(t), is the product of its discharge frequency in the absence of modulation,Fi(t), and a modulation coefficient (1 − α) (McCrimmon et al., 1997). If endogenous GABAergic inhibition decreases gain of the PMN to inspiratory inputs, it would reduce the peak output of the PMN, reduce the slope of the relation between Fo(t) andFi(t) below identity, and cause minimal change in burst duration, such thatFo(t) = (1 − α)Fi(t). Blocking this inhibition would have the opposite effect.
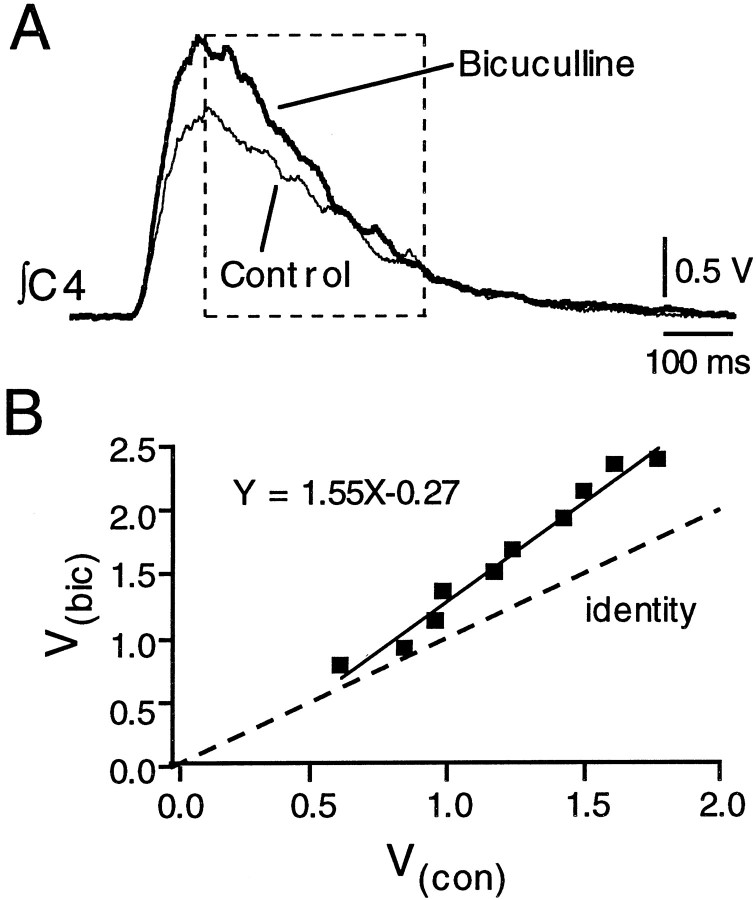
To test for gain modulation, we examined the effect of bicuculline applied over the PMN pool on C4 nerve inspiratory bursts. We examined C4 output rather than individual PMN output because (1) C4 output provides a reliable estimate of PMN activity, as evident in the close correspondence between the shape of the inspiratory synaptic drive potential and C4 burst envelope (Fig. 8), and (2) firing of individual PMNs during inspiration was often low, making it difficult to establish a reliable relationship between discharge Fo(t) andFi(t). Thus, we compared voltage of the integrated C4 nerve recording during bicuculline (V(bic)) versus control (V(con)). Voltage was measured at 25 msec intervals during the decrementing phase of the cycle (Fig.8A, boxed region). Figure 8A shows the voltage profile of the integrated C4 nerve burst (averaged from 10 cycles) for one preparation before and after bicuculline application. Bicuculline increased the peak C4 burst amplitude (also apparent in Fig. 7). Linear regression indicated that the slope of the relationship between V(bic) and V(con)was greater than unity (control) (Fig. 8B). The slope and intercept were 1.47 ± 0.10 and 0.20 ± 0.04, respectively (n = 3). Burst duration was not significantly affected by bicuculline (673 ± 72 msec in control; 714 ± 113 msec in bicuculline). These data suggest that phasic GABAergic input modulates PMN gain during inspiration. Although this may appear inconsistent with the lack of a change in slope in the relationship between firing and injected current step in Figure2B, a change in slope would not be expected in that relationship because the square-wave pulses used to elicit the firing did not match the waveform of the inspiratory inhibition. The proposed gain control arises from the proportionality of the excitatory and inhibitory inputs.
Fig. 8.
GABA-mediated gain modulation of PMN inspiratory activity. A, Superimposed voltage profiles of the integrated C4 nerve inspiratory burst envelopes (averaged from 10 cycles) for one preparation before (Control) and after local application of bicuculline (1 mm) over the C4 PMN pool. Bicuculline increased C4 burst amplitude and the slope of dV/dt, but did not change burst duration. B, Measurements of voltage, taken at 25 msec intervals through the decrementing phase (region enclosed in box) of the control and bicuculline C4 burst envelopes shown in Awere used to generate a plot of voltage in bicuculline (V(bic)) versus control (V(con)). Linear regression provided estimates of the slope (1.55) and intercept of this relationship and indicated a 1.55-fold increase in gain of the population PMN inspiratory input–output relationship. The line of identity was produced by plotting V(con) versusV(con).
Source of inspiratory inhibition
The endogenous GABA-mediated inspiratory inhibition to PMNs had two potential sources: (1) recurrent (feedback) inhibition arising from PMN activation of Renshaw cells or (2) concurrent (feedforward) inhibition from the brainstem via either direct monosynaptic inhibition of PMNs from bulbospinal inspiratory inhibitory neurons, or polysynaptic inhibition through activation of GABAergic interneurons in the spinal cord by bulbospinal inspiratory excitatory neurons.
Recurrent Inhibition
Recurrent inhibition sometimes has a GABAergic component (Curtis et al., 1976; Cullheim and Kellerth, 1981; Schneider and Fyffe, 1992); however, there are no reports of recurrent inhibition in which glycine does not play a major role. Thus, if recurrent inhibition were the source of the inspiratory hyperpolarization, block of glycine receptors should potentiate C4 inspiratory burst amplitude. Strychnine (1 mm), a glycinergic antagonist, had no significant effect on C4 output when applied for up to 2 min (n = 6) (Fig.9A,B) over the C4 motoneuron pool at the same site where GABA inhibited (Fig. 6), and bicuculline potentiated (Fig. 7A,B), C4 inspiratory burst amplitude.
Motoneuronal excitation of Renshaw cells, which mediate recurrent inhibition, is largely via nicotinic acetylcholine receptors (nAChRs) (Curtis and Ryall, 1966; Noga et al., 1987). Thus, we used the split-bath configuration to further test for involvement of recurrent inhibitory pathways in producing the inspiratory inhibition. The nAChR antagonists hexamethonium and mecamylamine were applied in the spinal bath to determine whether they would increase C4 inspiratory burst amplitude and occlude the bicuculline-mediated response. Hexamethonium (500 μm) had no effect on C4 burst amplitude, nor did it block potentiation of C4 nerve burst amplitude induced by locally applied bicuculline (1 mm) (n = 2; data not shown). Similarly, C4 burst amplitude was unaffected by application of mecamylamine (50 μm) to the spinal cord bath. The bicuculline-mediated potentiation of C4 burst amplitude was reduced slightly in mecamylamine, from 42 ± 5 to 35 ± 4% (n = 5), but was never abolished (Fig.9C).
Feedforward inhibition from inspiratory-modulated brainstem areas
The minimal change in the C4 inspiratory output after disruption of recurrent inhibitory spinal networks suggested that the inspiratory inhibition originates supraspinally. To test whether the inspiratory inhibition of PMN activity was monosynaptic or polysynaptic (see above), mephenesin was applied to the solution perfusing the spinal cord (split-bath configuration) to selectively block transmission in polysynaptic versus monosynaptic pathways within the spinal cord (Farkas et al., 1989). Mephenesin (1 mm) reduced C4 output by 30–40% but did not alter the bicuculline-mediated increase in C4 burst amplitude (n = 4), which was 28 ± 3% in control and 29 ± 3% in mephenesin (Fig. 9D).
We next tested the hypothesis that the inspiratory inhibition of PMNs originated within the Bötzinger Complex, a region in the rostral medulla that has widespread inhibitory inputs to many brainstem and spinal respiratory neurons (Fedorko and Merrill, 1984; Merrill and Fedorko, 1984). The brainstem–spinal cord preparation was pinned onto a paraffin-coated chuck in a vibratome bath, and serial sections (60–100 μm) were removed in the rostrocaudal direction starting at the caudal pons. The response of C4 inspiratory burst amplitude to local application of bicuculline (1 mm) over C4 was recorded after each section. Sections were fixed in 4% paraformaldehyde in 0.1 m phosphate buffer and stained with cresyl violet to identify structures. In the three preparations tested, complete removal of the Bötzinger Complex up to the rostral border of the pre-Bötzinger Complex had no effect on the bicuculline-mediated potentiation of C4 burst amplitude. Amplitude potentiation was 51% before and 56% after removal of the Bötzinger Complex. Tests of bicuculline responses after removal of more caudal structures were not performed because sectioning of the pre-Bötzinger Complex substantially reduced burst amplitude and disrupted respiratory rhythm (Fig.10Biii) (Smith et al., 1991).
DISCUSSION
We describe an inspiratory-phase inhibitory input to PMNs that (1) reduces inspiratory output of the C4 nerve, (2) reduces PMN excitability during inspiration relative to expiration, (3) is mediated via GABAA receptors, (4) is unlikely to arise from recurrent inhibitory pathways or from descending inputs from the Bötzinger Complex, and (5) is synchronous with, and of similar shape to, the inspiratory excitatory drive to PMNs. We propose that this concurrent inhibition allows for rapid modulation of PMN excitability.
Mechanism of inspiratory inhibition
GABAergic inhibition
Complete block of the inspiratory inhibition by bicuculline in all PMNs tested and reduction of PMN RN and action potential discharge by GABA and muscimol indicate that the inhibition is mediated by GABAA receptors. Potentiation of C4 inspiratory output by bicuculline, but not strychnine, further supports the conclusion that the inhibition is GABA-mediated, with minimal contribution of glycine receptors. The possibility that bicuculline produced its actions through direct effects (i.e., non-receptor-mediated), as reported in cultured mouse neurons (Heyer et al., 1982), is unlikely because bicuculline had no effect on subthreshold properties or firing behavior of PMNs during expiration. Our studies did not establish whether the GABAergic inhibition is presynaptic or postsynaptic; however, GABA receptors are present on PMNs (Zhan et al., 1989), and reduction in PMNRN by GABA [and muscimol (Su and Chai, 1998)] after block of synaptic transmission with TTX is consistent with postsynaptic GABA receptor involvement.
Recurrent inhibition
Innervation of Renshaw cells by PMNs and the demonstration of recurrent inhibition of PMNs (Hilaire et al., 1983, 1986; Lipski et al., 1985) raise the possibility that the inspiratory inhibition arises from a recurrent inhibitory pathway. However, several lines of evidence argue against this. (1) Glycine is an important transmitter in recurrent inhibitory pathways (Curtis et al., 1976; Cullheim and Kellerth, 1981; Schneider and Fyffe, 1992), yet strychnine had no effect on C4 inspiratory output. (2) Motoneurons activate Renshaw cells primarily via nAChRs (Curtis and Ryall, 1966; Noga et al., 1987); however, nAChR antagonists neither potentiated C4 inspiratory burst output nor occluded its potentiation by bicuculline. (3) The inspiratory inhibition was present during prolonged application of glutamate receptor antagonists over the C4 motoneuron pool, which would have greatly reduced the number of PMNs capable of activating recurrent pathways.
Descending inhibition from the brainstem: concurrent inhibition
Given that recurrent inhibition appears minimal, we propose that the inspiratory inhibition arises from inspiratory-modulated neurons within the brainstem.
Direct bulbospinal pathways. Respiratory-modulated bulbospinal inputs to PMNs originate primarily from the Bötzinger Complex, the rostral–ventral respiratory group (rVRG), the dorsal respiratory group (DRG) and the raphe obscuris and pallidus (Fedorko and Merrill, 1984; Ellenberger et al., 1990a,b; Dobbins and Feldman, 1994). The Bötzinger Complex is unlikely to be the source of the inspiratory inhibition. Bötzinger Complex neurons provide expiratory- rather than inspiratory-phase inhibition to PMNs (Merrill and Fedorko, 1984; Milano et al., 1992), and in our study, removal of the Bötzinger Complex by serial sectioning was without effect on the bicuculline-mediated potentiation of C4 inspiratory burst amplitude.
In rat, the predominant direct inspiratory bulbospinal projection to PMNs originates from the rVRG (Ellenberger and Feldman, 1988;Ellenberger et al., 1990b; Saji and Miura, 1990; Dobbins and Feldman, 1994), with minimal contributions from the DRG (Onai et al., 1987;Dobbins and Feldman, 1994). Although data indicate that these projections are primarily excitatory (Ellenberger et al., 1990a; Tian and Duffin, 1996b), they may not be exclusively so. Some inspiratory neurons in the rVRG inhibit other respiratory neurons (Segers et al., 1987; Ezure et al., 1989; Schmid et al., 1996; Ramirez et al., 1997), and a population of inspiratory rVRG neurons may provide inhibitory inputs to PMNs, as suggested by the presence of a small proportion of symmetrical densities at rVRG–PMN synapses (Ellenberger et al., 1990a). Coactivation of bulbospinal inhibitory and excitatory premotoneuron pools would account for the similar time course of inhibitory and excitatory synaptic inputs we observed in PMNs.
Medullary raphe neurons may also provide the inspiratory inhibition of PMNs. Neurons in the raphe obscuris and pallidus project to the region of the phrenic nucleus (Holtman et al., 1984; Onai et al., 1987;Dobbins and Feldman, 1994) and show GAD immunoreactivity (Jones et al., 1991; Holmes et al., 1994) and respiratory-modulated activity (Lindsey et al., 1987; Hosagai et al., 1993; Gilbey et al., 1995).
Propriospinal pathways. Inspiratory phase inhibition with a profile similar to the excitatory input could arise polysynaptically. In cat spinal cord, agonist motoneurons and Ia inhibitory interneurons that inhibit antagonist motoneurons receive parallel descending synaptic drive (Baldissera et al., 1981).
Inspiratory and expiratory interneurons with unknown connectivity are located proximal to the phrenic nucleus in cat (Bellingham and Lipski, 1990; Grelot et al., 1993) and rabbit (Palisses et al., 1989). A group of interneurons 200 μm from PMNs may be interposed between bulbospinal neurons and PMNs (Dobbins and Feldman, 1994). However, a role for either group in mediating the inspiratory inhibition that we observed is doubtful. Their proximity to the phrenic nucleus means that their inspiratory activation would have been greatly reduced, if not blocked, by local application of glutamatergic antagonists.
Contributions from more distant propriospinal inspiratory neurons in the upper cervical cord (Nakazono and Aoki, 1994; Tian and Duffin, 1996a) cannot be ruled out. However, mephenesin, which preferentially blocks polysynaptic versus monosynaptic transmission (Farkas et al., 1989), failed to block the bicuculline-induced potentiation of C4 burst amplitude when applied to the spinal cord bath, arguing against a propriospinal-mediated inhibition of PMN activity.
In summary, we conclude that the inspiratory-phase inhibition of PMNs does not originate from recurrent inhibition at the level of the spinal cord or from descending Bötzinger projections. Its proposed bulbospinal origin remains to be verified.
Functional significance of concurrent inhibitory and excitatory inputs
The physiological relevance of the inspiratory inhibition of PMNs is apparent in that blocking it with bicuculline produces an ∼30% increase in C4 inspiratory output. This inhibition may serve a number of functions, as described below.
Competing synaptic drives in the control of motoneuron excitability
Integration of simultaneous excitatory and inhibitory synaptic inputs is important in shaping neuronal discharge patterns at the interneuronal/premotoneuronal level in various motor systems, including respiration (Ballantyne and Richter, 1984; Schmid et al., 1996; Ramirez et al., 1997). GABA receptor-mediated inhibition of medullary inspiratory neuron discharge during inspiration is well documented (Wang et al., 1982; Paton and Richter, 1995; Schmid et al., 1996;McCrimmon et al., 1997; Ramirez et al., 1997). In contrast, at the level of the motoneuron, it is generally held that excitatory and inhibitory synaptic inputs arrive alternately, not simultaneously, and thus underlie rhythmic alternation between active and quiescent phases in behaviors including scratching, locomotion, and respiration (Perret, 1983; Merrill and Fedorko, 1984; Shefchyk and Jordan, 1985; Robertson and Stein, 1988). Periods of overlap between inhibitory and excitatory synaptic inputs are typically brief and presumed to bring about phase transitions. In inspiratory-modulated hypoglossal motoneurons, however, IPSPs arrive concurrently with excitatory inspiratory drive (Withington-Wray et al., 1988), and RN is reduced during inspiration (Woch and Kubin, 1995). These findings, in conjunction with our observation of simultaneous excitation and inhibition of PMNs during inspiration and observations of large periods of overlap between excitatory and inhibitory inputs in cat motoneurons during fictive locomotion (Perret, 1986) and in turtle motoneurons during some forms of fictive scratch (Robertson and Stein, 1988), suggest that integration of overlapping opposing synaptic inputs at the level of the motoneuron may play multiple roles in controlling motor output. It may control gain, shape discharge pattern, establish recruitment order, or smooth force production and provide greater flexibility of control (Robertson and Stein, 1998; Feldman and Smith, 1995).
Phase-specific control of motoneuron excitability
Evidence for behavioral- or phase-specific modulation of motoneuron properties is limited. Krawitz et al. (1997) showed that firing of lumbar motoneurons is enhanced during all phases of fictive locomotion. They suggest that this may result from a locomotion-related modulation of motoneuron excitability, perhaps via a mechanism similar to that which underlies bistable behavior [i.e., “plateau potentials” (see Hultborn and Kiehn, 1992)]. Brownstone et al. (1992) found that motoneuron excitability increases specifically during the active phase of the locomotor cycle and attributed this to a phase-specific reduction in afterhyperpolarization. In our study, block of rhythmic excitatory drive revealed a GABA-mediated inhibition of PMNs during the inspiratory phase. Relative to other motoneuron systems with partially overlapping inhibitory and excitatory inputs (Perret, 1983; Orsal et al., 1986; Robertson and Stein, 1988), the unique aspect of the inspiratory-phase inhibition of PMNs is that its shape and time course match those of the excitatory input (Fig. 3). It therefore provides a means for inspiratory-specific gain control of PMN output. Gain control via GABAergic inhibition may play a role in controlling the excitability of respiratory premotoneurons (McCrimmon et al., 1997), where it is proposed to contribute to the optimization of respiratory and nonrespiratory behaviors. A similar mechanism may operate at the motoneuron level. Inspiratory inhibition may arise from neurons that provide the interface between respiratory and nonrespiratory behavior (Orem, 1989), and modulation of its amplitude may adjust PMN output from that optimal for respiration to that suited to other behaviors such as apnea, gasping, coughing, vocalization, or defecation (Chang, 1992; Grelot et al., 1992; Gestreau et al., 1996).
Footnotes
This research was supported by grants from the Marsden Fund, the New Zealand Lottery Grants Board, the Health Research Council of New Zealand, and National Institutes of Health (Grant NS-24742). We thank Ms. C. Walsh, Dr. D. Robinson, and Mr. A. Frankcom-Burgess for excellent technical assistance.
Correspondence should be addressed to to Dr. M. A. Parkis, Department of Physiology, Faculty of Medicine and Health Science, University of Auckland, Private Bag 92019, Auckland, New Zealand.
REFERENCES
- 1.Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of physiology, Section I, The nervous system, Vol II, Motor control, Part I. American Physiological Society; Bethesda, MD: 1981. pp. 509–595. [Google Scholar]
- 2.Ballantyne D, Richter DW. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol (Lond) 1984;348:67–87. doi: 10.1113/jphysiol.1984.sp015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res. 1990;533:141–146. doi: 10.1016/0006-8993(90)91807-s. [DOI] [PubMed] [Google Scholar]
- 4.Berger AJ, Bayliss DA, Viana F. Development of hypoglossal motoneurons. J Appl Physiol. 1996;81:1039–1048. doi: 10.1152/jappl.1996.81.3.1039. [DOI] [PubMed] [Google Scholar]
- 5.Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 6.Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk SJ. On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Exp Brain Res. 1992;90:441–455. doi: 10.1007/BF00230927. [DOI] [PubMed] [Google Scholar]
- 7.Chang F-CT. Modification of medullary respiratory-related discharge patterns by behaviors and states of arousal. Brain Res. 1992;571:281–292. doi: 10.1016/0006-8993(92)90666-w. [DOI] [PubMed] [Google Scholar]
- 8.Connelly CA, Otto-Smith MR, Feldman JL. Blockade of NMDA receptor-channels by MK-801 alters breathing in adult rats. Brain Res. 1992;596:99–110. doi: 10.1016/0006-8993(92)91537-o. [DOI] [PubMed] [Google Scholar]
- 9.Cullheim S, Kellerth JO. Two kinds of recurrent inhibition of cat spinal alpha-motoneurones as differentiated pharmacologically. J Physiol (Lond) 1981;312:209–224. doi: 10.1113/jphysiol.1981.sp013624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis DR, Ryall RW. The synaptic excitation of Renshaw cells. Exp Brain Res. 1966;2:81–96. doi: 10.1007/BF00234362. [DOI] [PubMed] [Google Scholar]
- 11.Curtis DR, Game CJ, Lodge D, McCulloch RM. A pharmacological study of Renshaw cell inhibition. J Physiol (Lond) 1976;258:227–242. doi: 10.1113/jphysiol.1976.sp011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson PS. Interaction among neural networks for behavior. Curr Opin Neurobiol. 1995;5:792–798. doi: 10.1016/0959-4388(95)80108-1. [DOI] [PubMed] [Google Scholar]
- 13.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 14.Dong X-W, Feldman JL. Modulation of inspiratory drive to phrenic motoneurons by presynaptic adenosine A1 receptors. J Neurosci. 1995;15:3458–3467. doi: 10.1523/JNEUROSCI.15-05-03458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong X-W, Feldman J. Role and mechanism of endogenously activated mGluR1 in modulating synaptic transmission to phrenic motoneurons. Soc Neurosci Abstr. 1996;22:598. [Google Scholar]
- 16.Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol. 1988;269:47–57. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- 17.Ellenberger HH, Feldman JL, Goshgarian HG. Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. J Comp Neurol. 1990a;302:707–714. doi: 10.1002/cne.903020403. [DOI] [PubMed] [Google Scholar]
- 18.Ellenberger HH, Vera PL, Haselton JR, Haselton CL, Schneiderman N. Brainstem projections to the phrenic nucleus: an anterograde and retrograde HRP study in the rabbit. Brain Res Bull. 1990b;24:163–174. doi: 10.1016/0361-9230(90)90201-a. [DOI] [PubMed] [Google Scholar]
- 19.Ezure K, Manabe M, Otake K. Excitation and inhibition of medullary inspiratory neurons by two types of burst inspiratory neurons in the cat. Neurosci Lett. 1989;104:303–308. doi: 10.1016/0304-3940(89)90593-4. [DOI] [PubMed] [Google Scholar]
- 20.Farkas S, Tarnawa I, Berzsenyi P. Effects of some centrally acting muscle relaxants on spinal root potentials: a comparative study. Neuropharmacology. 1989;28:161–173. doi: 10.1016/0028-3908(89)90053-1. [DOI] [PubMed] [Google Scholar]
- 21.Fedorko L, Merrill EG. Axonal projections from the rostral expiratory neurones of the Botzinger complex to medulla and spinal cord in the cat. J Physiol (Lond) 1984;350:487–496. doi: 10.1113/jphysiol.1984.sp015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman JL, Smith JC. Neural control of respiratory pattern in mammals: an overview. In: Dempsey JA, Pack AI, editors. Regulation of breathing, Vol 79. M. Dekker; New York: 1995. pp. 39–69. [Google Scholar]
- 23.Feldman JL, Smith JC, Ellenberger HH, Connelly CA, Liu G, Greer JJ, Lindsay AD, Otto MR. Neurogenesis of respiratory rhythm and pattern: emerging concepts. Am J Physiol. 1990;259:R879–R886. doi: 10.1152/ajpregu.1990.259.5.R879. [DOI] [PubMed] [Google Scholar]
- 24.Gestreau C, Milano S, Bianchi AL, Grelot L. Activity of dorsal respiratory group inspiratory neurons during laryngeal-induced fictive coughing and swallowing in decerebrate cats. Exp Brain Res. 1996;108:247–256. doi: 10.1007/BF00228098. [DOI] [PubMed] [Google Scholar]
- 25.Gilbey MP, Futuro-Neto HA, Zhou SY. Respiratory-related discharge patterns of caudal raphe neurones projecting to the upper thoracic spinal cord in the rat. J Auton Nerv Syst. 1995;50:263–273. doi: 10.1016/0165-1838(94)00097-4. [DOI] [PubMed] [Google Scholar]
- 26.Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol (Lond) 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grelot L, Milano S, Portillo F, Miller AD, Bianchi AL. Membrane potential changes of phrenic motoneurons during fictive vomiting, coughing, and swallowing in the decerebrate cat. J Neurophysiol. 1992;68:2110–2119. doi: 10.1152/jn.1992.68.6.2110. [DOI] [PubMed] [Google Scholar]
- 28.Grelot L, Milano S, Portillo F, Miller AD. Respiratory interneurons of the lower cervical (C4–C5) cord: membrane potential changes during fictive coughing, vomiting, and swallowing in the decerebrate cat. Pflügers Arch. 1993;425:313–320. doi: 10.1007/BF00374181. [DOI] [PubMed] [Google Scholar]
- 29.Heyer EJ, Nowak LM, Macdonald RL. Membrane depolarization and prolongation of calcium-dependent action potentials of mouse neurons in cell culture by two convulsants: bicuculline and penicillin. Brain Res. 1982;232:41–56. doi: 10.1016/0006-8993(82)90609-6. [DOI] [PubMed] [Google Scholar]
- 30.Hilaire G, Khatib M, Monteau R. Spontaneous respiratory activity of phrenic and intercostal Renshaw cells. Neurosci Lett. 1983;43:97–101. doi: 10.1016/0304-3940(83)90135-0. [DOI] [PubMed] [Google Scholar]
- 31.Hilaire G, Khatib M, Monteau R. Central drive on Renshaw cells coupled with phrenic motoneurons. Brain Res. 1986;376:133–139. doi: 10.1016/0006-8993(86)90907-8. [DOI] [PubMed] [Google Scholar]
- 32.Holmes CJ, Mainville LS, Jones BE. Distribution of cholinergic GABAergic and serotonergic neurons in the medial reticular formation and their projections studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1155–1178. doi: 10.1016/0306-4522(94)90351-4. [DOI] [PubMed] [Google Scholar]
- 33.Holtman JR, Jr, Norman WP, Gillis RA. Projections from the raphe nuclei to the phrenic motor nucleus in the cat. Neurosci Lett. 1984;44:105–111. doi: 10.1016/0304-3940(84)90229-5. [DOI] [PubMed] [Google Scholar]
- 34.Hosagai M, Matsuo S, Nakao S. Firing pattern and location of respiratory neurons in cat medullary raphe nuclei. Neurosci Lett. 1993;161:149–152. doi: 10.1016/0304-3940(93)90281-o. [DOI] [PubMed] [Google Scholar]
- 35.Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol (Lond) 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol (Lond) 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hultborn H, Kiehn O. Neuromodulation of vertebrate motor neuron membrane properties. Curr Opin Neurobiol. 1992;2:770–775. doi: 10.1016/0959-4388(92)90132-5. [DOI] [PubMed] [Google Scholar]
- 38.Hultborn H, Lindstrom S, Wigstrom H. On the function of recurrent inhibition in the spinal cord. Exp Brain Res. 1979;37:399–403. doi: 10.1007/BF00237722. [DOI] [PubMed] [Google Scholar]
- 39.Jones BE, Holmes CJ, Rodriguez-Veiga E, Mainville L. GABA-synthesizing neurons in the medulla: their relationship to serotonin-containing and spinally projecting neurons in the rat. J Comp Neurol. 1991;313:349–367. doi: 10.1002/cne.903130210. [DOI] [PubMed] [Google Scholar]
- 40.Krawitz S, Fedirchuk B, Dai Y, Jordan LM, McCrea DA. Locomotion hyperpolarizes the voltage threshold of cat lumbar motoneurones. Soc Neurosci Abstr. 1997;23:759. [Google Scholar]
- 41.Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- 42.Lindsey BG, Segers LS, Shannon R. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. II. Evidence for inhibitory actions of expiratory neurons. J Neurophysiol. 1987;57:1101–1117. doi: 10.1152/jn.1987.57.4.1101. [DOI] [PubMed] [Google Scholar]
- 43.Lipski J, Fyffe RE, Jodkowski J. Recurrent inhibition of cat phrenic motoneurons. J Neurosci. 1985;5:1545–1555. doi: 10.1523/JNEUROSCI.05-06-01545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- 45.McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CF, Stuth EA, Tonkovic-Capin M, Krolo M, Hopp FA. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respir Physiol. 1997;110:161–176. doi: 10.1016/s0034-5687(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 46.Merrill EG, Fedorko L. Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Botzinger neurons. J Neurosci. 1984;4:2350–2353. doi: 10.1523/JNEUROSCI.04-09-02350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milano S, Miller AD, Grelot L. Multi-phase expiratory inhibition of phrenic motoneurons in the decerebrate cat. NeuroReport. 1992;3:307–310. doi: 10.1097/00001756-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Nakazono Y, Aoki M. Excitatory connections between upper cervical inspiratory neurons and phrenic motoneurons in cats. J Appl Physiol. 1994;77:679–683. doi: 10.1152/jappl.1994.77.2.679. [DOI] [PubMed] [Google Scholar]
- 49.Noga BR, Shefchyk SJ, Jamal J, Jordan LM. The role of Renshaw cells in locomotion: antagonism of their excitation from motor axon collaterals with intravenous mecamylamine. Exp Brain Res. 1987;66:99–105. doi: 10.1007/BF00236206. [DOI] [PubMed] [Google Scholar]
- 50.Onai T, Saji M, Miura M. Projections of supraspinal structures to the phrenic motor nucleus in rats studied by a horseradish peroxidase microinjection method. J Auton Nerv Syst. 1987;21:233–239. doi: 10.1016/0165-1838(87)90026-9. [DOI] [PubMed] [Google Scholar]
- 51.Orem J. Behavioral inspiratory inhibition: inactivated and activated respiratory cells. J Neurophysiol. 1989;62:1069–1078. doi: 10.1152/jn.1989.62.5.1069. [DOI] [PubMed] [Google Scholar]
- 52.Orsal D, Perret C, Cabelguen JM. Evidence of rhythmic inhibitory synaptic influences in hindlimb motoneurons during fictive locomotion in the thalamic cat. Exp Brain Res. 1986;64:217–224. doi: 10.1007/BF00238216. [DOI] [PubMed] [Google Scholar]
- 53.Palisses R, Persegol L, Viala D. Evidence for respiratory interneurones in the C3–C5 cervical spinal cord in the decorticate rabbit. Exp Brain Res. 1989;78:624–632. doi: 10.1007/BF00230250. [DOI] [PubMed] [Google Scholar]
- 54.Paton JFR, Richter DW. Role of fast inhibitory synaptic mechanisms in respiratory rhythm generation in the maturing mouse. J Physiol (Lond) 1995;484:505–521. doi: 10.1113/jphysiol.1995.sp020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perret C. Centrally generated pattern of motoneuron activity during locomotion in the cat. Symp Soc Exp Biol. 1983;37:405–422. [PubMed] [Google Scholar]
- 56.Perret C. Synaptic influences contributing to the pattern of limb motoneuron activity during fictive locomotion in the cat. In: Grillner S, Stein PSG, Stuart DG, Forsberg H, Hermann RM, editors. Neurobiology of vertebrate locomotion. Macmillan; Basingstroke, UK: 1986. pp. 173–184. [Google Scholar]
- 57.Ramirez JM, Telgkamp P, Elsen FP, Quellmalz UJ, Richter DW. Respiratory rhythm generation in mammals: synaptic and membrane properties. Respir Physiol. 1997;110:71–85. doi: 10.1016/s0034-5687(97)00074-1. [DOI] [PubMed] [Google Scholar]
- 58.Robertson GA, Stein PS. Synaptic control of hindlimb motoneurones during three forms of the fictive scratch reflex in the turtle. J Physiol (Lond) 1988;404:101–128. doi: 10.1113/jphysiol.1988.sp017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saji M, Miura M. Evidence that glutamate is the transmitter mediating respiratory drive from medullary premotor neurons to phrenic motoneurons: a double labeling study in the rat. Neurosci Lett. 1990;115:177–182. doi: 10.1016/0304-3940(90)90451-e. [DOI] [PubMed] [Google Scholar]
- 60.Schmid K, Foutz AS, Denavit-Saubie M. Inhibitions mediated by glycine and GABAA receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res. 1996;710:150–160. doi: 10.1016/0006-8993(95)01380-6. [DOI] [PubMed] [Google Scholar]
- 61.Schneider SP, Fyffe RE. Involvement of GABA and glycine in recurrent inhibition of spinal motoneurons. J Neurophysiol. 1992;68:397–406. doi: 10.1152/jn.1992.68.2.397. [DOI] [PubMed] [Google Scholar]
- 62.Segers LS, Shannon R, Saporta S, Lindsey BG. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. I. Evidence for excitatory and inhibitory actions of inspiratory neurons. J Neurophysiol. 1987;57:1078–1100. doi: 10.1152/jn.1987.57.4.1078. [DOI] [PubMed] [Google Scholar]
- 63.Shefchyk SJ, Jordan LM. Excitatory and inhibitory postsynaptic potentials in α-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol. 1985;53:1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- 64.Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- 65.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soja PJ, Lopez-Rodriguez F, Morales FR, Chase MH. The postsynaptic inhibitory control of lumbar motoneurons during the atonia of active sleep: effect of strychnine on motoneuron properties. J Neurosci. 1991;11:2804–2811. doi: 10.1523/JNEUROSCI.11-09-02804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su C-K, Chai C-Y. GABAergic inhibition of neonatal phrenic motoneurons. Neurosci Lett. 1998;248:191–194. doi: 10.1016/s0304-3940(98)00361-9. [DOI] [PubMed] [Google Scholar]
- 68.Tian GF, Duffin J. Connections from upper cervical inspiratory neurons to phrenic and intercostal motoneurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1996a;110:196–204. doi: 10.1007/BF00228551. [DOI] [PubMed] [Google Scholar]
- 69.Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1996b;111:178–186. doi: 10.1007/BF00227296. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Boyarsky LL, Frazier DT. The effect of transmitter antagonists on phasic respiratory neurons. J Neurosci Res. 1982;8:657–664. doi: 10.1002/jnr.490080410. [DOI] [PubMed] [Google Scholar]
- 71.Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience. 1988;25:1041–1051. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]
- 72.Woch G, Kubin L. Non-reciprocal control of rhythmic activity in respiratory-modulated XII motoneurons. NeuroReport. 1995;6:2085–2088. doi: 10.1097/00001756-199510010-00031. [DOI] [PubMed] [Google Scholar]
- 73.Zhan WZ, Ellenberger HH, Feldman JL. Monoaminergic and GABAergic terminations in phrenic nucleus of rat identified by immunohistochemical labeling. Neuroscience. 1989;31:105–113. doi: 10.1016/0306-4522(89)90033-x. [DOI] [PubMed] [Google Scholar]