Abstract
The Caenorhabditis elegans ASH sensory neurons mediate responses to nose touch, hyperosmolarity, and volatile repellent chemicals. We show here that distinct signaling pathways mediate the responses to touch and hyperosmolarity. ASH neurons distinguish between these stimuli because habituation to nose touch has no effect on the response to high osmolarity or volatile chemicals (1-octanol). Mutations in osm-10 eliminate the response to hyperosmolarity but have no effect on responses to nose touch or to volatile repellents. OSM-10 is a novel cytosolic protein expressed in ASH and three other classes of sensory neurons. Mutations in two other osmosensory-defective genes, eos-1 andeos-2, interact genetically with osm-10. Our analysis suggests that nose touch sensitivity and osmosensation occur via distinct signaling pathways in ASH and that OSM-10 is required for osmosensory signaling.
Keywords: Caenorhabditis elegans, osmosensation, mechanosensation, neurodegeneration, OSM-10, eos-1, eos-2, degenerin
A class of polymodal sensory neurons has been described in the nematode Caenorhabditis elegans. The bilateral ASH sensory neurons mediate the responses to nose touch, hyperosmolarity, and volatile repellents; each stimulus evokes backward locomotion (Bargmann et al., 1990; Kaplan and Horvitz, 1993; Troemel et al., 1995). ASH neurons provide direct synaptic input to command interneurons that drive locomotion (White et al., 1986). An AMPA-type glutamate receptor, GLR-1, is expressed in ASH synaptic targets and is required for ASH-mediated touch sensitivity but not for osmotic or volatile sensitivities mediated by ASH (Hart et al., 1990; Maricq et al., 1995). These results suggest that the ASH-to-interneuron synaptic signals evoked by touch differ from those signals evoked by osmotic and volatile repellents. However, it is unclear whether common or distinct pathways mediate sensory transduction by the different ASH sensory stimuli.
Touch and hyperosmolarity are both thought to be detected by mechanically gated ion channels. The C. elegans degenerin proteins have been proposed to act as mechanosensory receptors. The degenerins MEC-4 and MEC-10, distantly related to epithelial sodium channels, are required for the response to body touch mediated by the “touch neurons” (ALM, PLM, AVM, and PVM). Dominant mec-4and mec-10 mutations cause neurodegeneration, hence the name degenerins. The putative mechanoreceptor complexes are proposed to contain combinations of degenerin subunits and proteins that tether these complexes to cytoskeletal and extracellular matrix proteins (Driscoll and Kaplan, 1997). Although these complexes are an attractive model for the mechanoreceptor of the touch cells, it is unclear whether other mechanosensory neurons like ASH use a similar receptor complex. An alternative candidate receptor is OSM-9, a C. eleganshomolog of the capsaicin receptor (Colbert et al., 1997). The vertebrate capsaicin receptor encodes an ion channel gated by either capsaicin or high temperature (Caterina et al., 1997). However,osm-9 mutants are defective in response to all stimuli detected by ASH (Colbert et al., 1997). No homologs of other receptors implicated in osmosensation or mechanosensation in either yeast orEscherichia coli have been identified in C. elegans.
Here we characterize further the mechanisms underlying ASH sensory transduction and modality coding. We show that ASH neurons distinguish between touch and other ASH stimuli and that ASH and the microtubule touch cells use distinct mechanoreceptors, and we characterize a modality-specific gene, osm-10 (osmosensory defective). A mutation in osm-10 disrupts ASH-mediated osmosensation but has no effect on other ASH-mediated responses. Taken together, our results suggest that the ASH responses to touch and hyperosmolarity are mediated by distinct signaling pathways and identify a novel intracellular protein that is required for osmosensation.
MATERIALS AND METHODS
Behavioral assays
Nose touch, osmotic avoidance, and volatile repellent assays were performed as described previously (Culotti and Russell, 1978;Kaplan and Horvitz, 1993; Hart et al., 1995). Behaviors were quantitated as follows: nose touch avoidance (Not) was quantitated as the percentage of trials in which animals responded to touch with an eyelash by stopping forward movement or reversing, osmotic avoidance (Osm) was quantitated as the percentage of animals that escape a ring of 8 m glycerol in <8 min, and volatile avoidance (Sos) was quantitated as the average time to initiate backward movement in response to an eyelash dipped in 1-octanol. The morphology of ASH neurons was examined by dye filling using DiD, DiI, or DiO (Molecular Probes, Eugene, OR). DiD facilitates unambiguous identification of green fluorescent protein (GFP)-expressing cells, even at low levels of GFP expression (P. W. Faber, J. Alter, M. MacDonald, and A. C. Hart, unpublished observations). To generate transheterozygotes unambiguously for behavioral assays, we used recessive genetic markers (e.g., dpy-17) or osm-10:: GFPreporter constructs to identify cross progeny. Strains used includeddpy-17 (e164) osm-10 (n1602); eos-1(nu288) rtEx61(pKP#58); eos-1(nu288) lin-15(n765);nDf16/dpy-17(e164) unc-32(e189); and individual mutant strains listed in Tables 1-6. unc-8(n491n1193); deg-1(u506u550) (HA27) was constructed fromdpy20(e1282ts)IV;deg-1(u506u550)X (HA4) andunc-8(n491n1193). HA27 construction was confirmed by sequencing allele-specific polymorphisms. Animals were raised at 25° unless otherwise indicated. Direct injection of pKP#58 intoeos-1 created rtEx61; strains were maintained by selecting for GFP expression each generation on a GFP dissection microscope (Leica/Kramer Scientific, Burlington, MA).
Table 1.
mec genes are not required for ASH function
| Genotype | Osm (% defective) | Sos (reversal time in sec) | Not (% responses) |
|---|---|---|---|
| N2 (wild type) | 8 ± 2 | 1.8 ± 0.6 | 86 ± 2 |
| eat-4(ky5)III | 77 ± 4 | 13.8 ± 0.8 | 4 ± 3 |
| osm-3(p802) | 88 ± 6 | 0.4 ± 0.7 | 24 ± 5 |
| mec-4(u45) | 10 ± 6 | 1.4 ± 0.2 | 92 ± 4 |
| mec-10(e1515) | 15 ± 12 | 1.8 ± 0.3 | 83 ± 10 |
| mec-2(e75) | 13 ± 10 | 2.7 ± 0.6 | 70 ± 6 |
| mec-5(e1340) | 8 ± 6 | 3.1 ± 0.8 | 82 ± 7 |
| mec-6(u686) | 4 ± 3 | 1.3 ± 0.3 | 86 ± 5 |
| mec-9(e1494) | 3 ± 3 | 1.3 ± 0.2 | 88 ± 4 |
| unc-8(n491n1193) | 0 ± 0 | 2.0 ± 0.3 | 86 ± 6 |
ASH-mediated sensory responses to high osmolarity (Osm), volatile repellents (Sos), and nose touch (Not) were compared. For each determination, means ± SE are reported; osmotic avoidance (Osm) was analyzed in at least six animals and three trials, avoidance of 1-octanol (Sos) was determined in at least three animals and usually a total of 10 trials, and nose touch avoidance (Not) was determined in at least three animals with 10 trials per animal.
Table 2.
Gain-of-function mutations in the deg-1 gene cause ASH degeneration
| Genotype | Dye filling (% defective) |
|---|---|
| N2 (wild type) | 0 (n = 30) |
| deg-1(u38) | 27 (n = 15) |
| deg-1(u38); mec-6 | 0 (n = 37) |
| deg-1(u506) | 73 (n = 15) |
| deg-1(u506u550) | 0 (n = 39) |
| unc-8(n491) | 0 (n = 10) |
| unc-8(n491n1193) | 0 (n = 24) |
ASH degeneration was assessed by dye filling with DiO. The number of neurons scored (n) is indicated. Swollen, degenerating ASH neurons were detected in dye-filling defective strains.
Table 3.
degenerin genes are not required for the response mediated by ASH
| Genotype | Osm (% defective) | Sos (reversal time in sec) | Not (% responses) |
|---|---|---|---|
| unc-8(n491n1193) | 0 ± 0 | 2.0 ± 0.3 | 86 ± 6 |
| deg-1(u506)X | – | – | 14 ± 4 |
| deg-1(u506u550)X | 12 ± 6 | 2.1 ± 0.3 | 92 ± 5 |
| deg-1(u506u550); unc-8 (n491n1193) | 3 ± 1 | 2.2 ± 0.5 | 84 ± 3 |
| deg-1(u38) | – | – | 32 ± 9 |
| +/+ | – | – | 76 ± 9 |
unc-8 and deg-1 mutations were characterized and analyzed as described in Table 1. +/+, Control animals; –, not determined.
Table 4.
Nose touch habituation does not alter responses to other ASH stimuli
| Assays on 10 wild-type animals | Naive | After Not habituation | After recovery |
|---|---|---|---|
| Not (% responses) | 87 ± 2 | 45 ± 20 | 79 ± 11 |
| Sos (reversal time in sec) | 1.1 ± 2.5 | 1.0 ± 1.7 | – |
| Osm (% defective) | 0 | 0 | – |
Animals were habituated by 40 rapid nose touch trials; the ability to respond to a single Osm or Sos trial is reported as the percentage responding or the average time to reverse, respectively. Analysis was as described in Table 1.
Table 5.
osm-10, eos-1, and eos-2 are specifically required for osmotic avoidance
| Genotype | Osm (% defective) | Sos (reversal time in sec) | Not (% responses) |
|---|---|---|---|
| N2 (wild type) | 5 ± 3 | 1.9 ± 0.1 | 87 ± 2 |
| osm-10(n1602)III | 91 ± 4 | 1.5 ± 0.5 | 82 ± 13 |
| osm-10(nr2076)III | 83 ± 5 | 1.6 ± 0.5 | 94 ± 4 |
| eat-4(ky5)III | 77 ± 2 | 13.8 ± 0.8 | 4 ± 3 |
| eos-1(nu288)IV | 28 ± 8 | 2.8 ± 0.5 | 94 ± 3 |
| eos-2(nu268)III | 35 ± 11 | 1.8 ± 0.2 | 78 ± 4 |
| nDf16/osm-10(n1602) | 100 ± 0 | 2.5 ± 0.8 | 75 ± 15 |
| osm-10(n1602)/+; eos-1 (nu288)/+ | 41 ± 11 | 2.0 ± 0.2 | 85 ± 15 |
| eos-2(nu268)/osm-10 (n1602) | 69 ± 12 | 1.7 ± 0.3 | 86 ± 3 |
| eos-2(nu268)/+; eos-1 (nu288)/+ | 53 ± 15 | 1.7 ± 0.3 | 94 ± 6 |
| nDf16/+; eos-1 (nu288)/+ | 63 ± 13 | 2.8 ± 0.4 | 85 ± 15 |
| nDf16/+ | 29 ± 4 | 2.6 ± 0.3 | 94 ± 4 |
| osm-10(n1602)/+ | 0 ± 0 | 1.3 ± 0.2 | 86 ± 5 |
| eos-1(nu288)/+ | 5 ± 3 | 1.7 ± 0.1 | 92 ± 4 |
| eos-2(nu268)/+ | 16 ± 9 | 1.7 ± 0.3 | 86 ± 3 |
Animals were characterized and analyzed as described in Table1.
Table 6.
Role of OSM-10-expressing cells in osmosensation
| Genotype/Cells ablated | Osm (min to escape) |
|---|---|
| Wild type (N2 andnuIs11) | 11.0 ± 0.4 (n = 15) |
| Mock ablation | 11.7 ± 0.5 (n = 26) |
| osm-10(n1602) | 2.8 ± 0.5 (n = 8) |
| osm-3(p802) | 1.7 ± 0.3 (n = 12) |
| ASH−, ASI−, PHA−, PHB− | 2.8 ± 0.5 (n = 9) |
| ASH− | 1.8 ± 0.4 (n = 17) |
| ASI−, PHA−, PHB− | 12.0 ± 1.1 (n = 5) |
Indicated neurons were killed in young larvae with a laser microbeam as described (Avery and Horvitz, 1989). The average time required to cross a 1 cm diameter, 8 m-glycerol osmotic barrier was determined three or four times for each animal 3 d after the operation or determined once for specified genotypes. To facilitate cell identifications and to monitor laser killing, we usednuIs11 [osm-10:: gfp] transgenic animals for most of the laser experiments. Additional laser ablations in wild-type animals yielded similar results. These data were pooled for this table. Cell killing was confirmed by dye filling and/or by GFP staining.
Positional cloning of osm-10
Mapping data. n1602 was mapped by recombination with MT5427 (sma-3 6 mec-14 4osm-10 7 ncl-1 9 unc-36) and MT4837 (sma-3 8 osm-10 5 lin-39) to an interval between mec-14 and lin-39 on chromosome III by the use of the Osm assay format described below. Cosmids from the region were obtained from the C. elegansgenome-sequencing project. Twenty or more transgenic F3 generation KP497 animals [osm-10 (n1602)III; lin-15 (n765) X] were tested for osmotic avoidance for each cosmid or plasmid to assess rescue activity. pHA#51 contains a 3.2 kbPstI/XhoI fragment that corresponds to 12848–16050 of cosmid T20H4 and contains the 3′ one-half of T20H4.2 and all of T20H4.1 and all of osm-10 except for part of the 3′-untranslated region.
Sequencing. The products of three independent PCR reactions from osm-10(n1602) or eos-2(nu268) genomic DNA were sequenced to identify allele-specific polymorphisms. No mutations were identified in osm-10 exons in eos-2(nu268). The genomic structure of osm-10 predicted by GENEFINDER and the C. elegans genome-sequencing project was confirmed by reverse transcription (RT)–PCR from wild-type animals using Superscript Reverse Transcriptase (Life Technologies, Gaithersburg, MD) and the Marathon cDNA Race Kit (Clontech, Palo Alto, CA). The translation initiation site was confirmed by sequencing an RT–PCR product containing a stop codon, in frame, 10 codons upstream of the predicted initiator methionine codon.
osm-10(nr2076). The osm-10 deletion allele was generated using a PCR-based strategy by the Nemapharm division of Axys Pharmaceuticals (South San Francisco, CA) (Liu et al., unpublished observations).
osm-10 expression
Antisera. The second exon of osm-10 was amplified by PCR and inserted into pET21b (Novagen, Madison, WI) for bacterial expression with a polyHIS tag. Affinity-purified sera from three immunized rabbits detected OSM-10 with varying specificity in whole mounts fixed with Bowin’s (Nonet et al., 1997) and on Western blots. Confocal resolution was obtained with optical Z-series sectioning and Openlab deconvolution image processing (Improvision, Coventry, England). Western blots were blocked with 5% dry milk and 1% E. coli acetone powder in TBS plus Tween and were probed with a 1:1000 dilution of affinity-purified (M. Koelle, personal communication) anti-OSM-10 antisera. HRP-coupled goat anti-rabbit antiserum (Amersham, Arlington Heights, IL) and ECL (Dupont NEN, Boston, MA) were used to detect protein.
GFP constructs. In pKP#58 the GFP gene was amplified from pPD95.67 and inserted in frame into the NruI site [nucleotide (nt) 14155] in the osm-10 rescue construct pKP#51 (see above). Additional GFP rescue constructs with different insertion sites were generated; all resulted in the same expression pattern in vivo. Coinjection of pKP#58 and pJM#24 intolin-15(n765) and subsequent gamma irradiation resulted in the insertion of nuIs11 into the X chromosome.nuIs11 was backcrossed four times before laser ablation and the other experiments reported in this manuscript.
Heterologous expression constructs. OSM-10 protein was expressed using promoters from the srb-6 andsre-1 genes using pHA#2 and pHA#1, respectively (Troemel et al., 1995). srb-6 is expressed in ADL, ADF, ASH, PHA, PHB, and the vulval region; sre-1 is expressed in ADL and ASJ(faint). PCR-amplified osm-10 genomic DNA (T20H4, nt 13738–15801) replaced GFP in pTU#62 betweenSmaI/EcoRI sites to create pKP#82.SphI/SmaI sites in srb-6 andsre-1 constructs (Troemel et al., 1995) were used to subclone the srx promoter into pKP#82. Transgenic strain construction was described in the previous section;lin-15(n765) animals were injected.
Isolation of eos-2(nu268) and eos-1(nu288)
Hermaphrodite N2 animals were mutagenized with ethylmethanesulfonate; 11,220 haploid genomes were screened, and 50 mutants that were defective in detecting high osmolarity but had normal dye filling of the amphid neurons were isolated, includingeos-2(nu268) and eos-1(nu288).
Osm assay. Approximately 20 μl of 8 m glycerol (dyed with bromphenol blue) is distributed in a 1.5 cm ring on an agar plate. The ring is allowed to dry for 2–3 min. Up to 300 well-fed adult worms are washed three times with S Basal and placed in the center of the ring. Excess S Basal is blotted off, and the percentage of animals escaping after 10 min is reported.
Mapping. eos-2(nu268) or eos-1(nu288)was mapped with DA438 [bli-4(e397)I; rol-6(e187)II; daf-2(e1368) vab-7(e1562)III; unc-31(e928)IV; dpy-11(e224)V; lon-2(e678)X]. Five of 17 eos-2(nu268) animals were heterozygous forvab-7. eos-1(nu288) linkage to IV was confirmed with the dominant markers unc-8(n491dm) dpy-13(e184sd) IV. Fourteen of 16 F2 animals that did not carry unc-8 dpy-13were homozygous for eos-1(nu288).
RESULTS
Relationship between mechanoreceptors in ASH and microtubule touch cells
Both touch and increased osmolarity are thought to be detected by mechanically gated ion channels. The genes mec-2,mec-4, mec-5, mec-6, mec-9, and mec-10 encode components of a putative mechanoreceptor complex that mediates touch sensitivity by the touch cells in C. elegans (Driscoll and Kaplan, 1997). These genes are not required for ASH-mediated behavioral responses (Table1). The degenerins DEG-1 and UNC-8 were also considered likely candidates for ASH mechanoreceptors (Driscoll and Chalfie, 1991; Huang and Chalfie, 1994; Driscoll and Kaplan, 1997) because unc-8 is expressed in ASH (Tavernarakis et al., 1997) and gain-of-function alleles deg-1(u38) anddeg-1(u506) cause ASH neurons to undergo neurodegeneration (Table 2). The deg-1(u38)mutation caused temperature-sensitive dominant nose touch insensitivity, whereas deg-1 null alleles do not perturb the nose touch response (Table 3) (Garcia-Anoveros et al., 1995). Double mutants containing null alleles for both unc-8 and deg-1 are normal for all ASH-mediated responses (Table 3), suggesting that the mechanically gated ion channels used by ASH for nose touch and osmotic detection may be distinct from those used by the microtubule touch cells.
Animals distinguish between the ASH sensory stimuli
Although they mediate responses to three sensory stimuli, it is possible that ASH neurons cannot distinguish between the different stimuli detected. If this were the case, one would predict that any treatment that causes a decrement in one response would also produce a defect in the other responses. We found that repeated nose touch stimuli reduced responsiveness to further nose touch stimulation; however, these habituated animals remained fully sensitive to hyperosmolarity or volatile repellent chemicals (Table4). Similar experiments habituating the responses to hyperosmolarity or to volatile repellents were not possible because chronic exposure to these stimuli is lethal. However, a short exposure to 1-octanol partially habituates response without deleterious effects; control animals reverse in 1.4 ± 0.3 sec, but after two 15 sec exposures to 1-octanol, the average reversal time is 4.9 ± 0.5 sec (n = 11 animals). This brief habituation to 1-octanol neither increased nor decreased sensitivity to nose touch (72 ± 6% response vs 80 ± 15% response in control animals). These results suggest that the ASH neurons distinguish nose touch from hyperosmolarity and the volatile repellent 1-octanol.
osm-10, a modality-specific gene required for osmosensation
To identify genes involved in ASH sensory transduction, we sought mutations that selectively impair ASH-mediated osmosensation but not other ASH-mediated behaviors. One previously identified mutation,osm-10(n1602), fit our criteria. As previously shown,osm-10(n1602) mutants failed to avoid either 8 mglycerol or 4 m fructose (Table5) (Bargmann et al., 1990), indicating that they are defective for osmosensation. However, we found thatosm-10(n1602) animals responded normally to nose touch and volatile repellents, indicating that the mutant ASH neurons retained some sensory functions (Table 5). By contrast, other mutations that cause generalized defects in ASH function or structure (e.g.,osm-3 and eat-4) impaired multiple ASH-mediated sensory behaviors (Table 1). The n1602 mutation is likely to cause a severe defect in osm-10 function because the behavior of osm-10(n1602) homozygotes was indistinguishable from that of osm-10(n1602)/nDf16heterozygotes (Table 5).
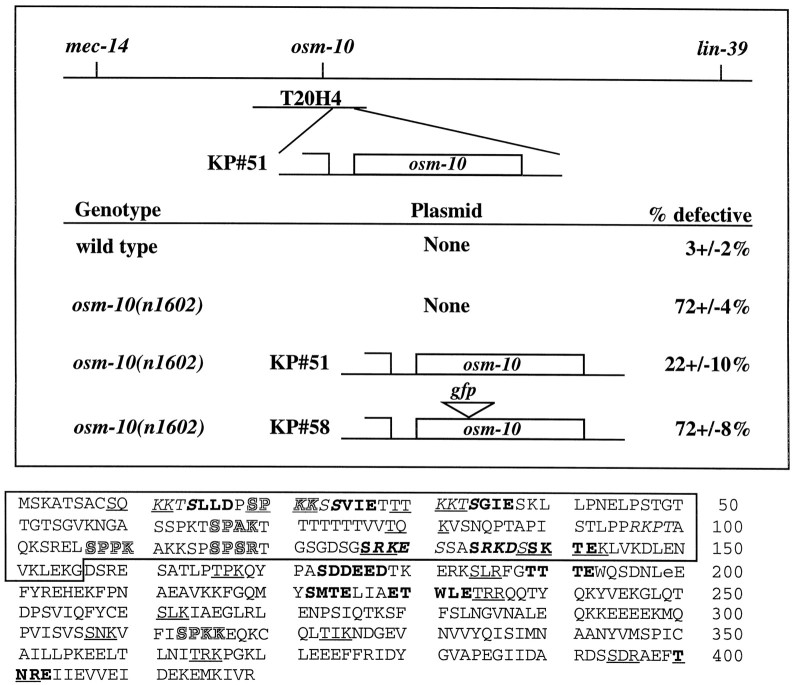
We positionally cloned osm-10. We mappedosm-10(n1602) to a small interval on chromosome III betweenmec-14 and lin-39. A single cosmid clone from this region, T20H4, and plasmid subclones derived from it were able to rescue the osm-10 defect in transgenic animals (Fig.1, top). In this manner, theosm-10–rescuing activity was mapped to a single gene predicted by the C. elegans genome-sequencing project (T20H4.1), and the n1602 mutation was shown to correspond to a missense mutation (E199K) in one of the predicted exons. We concluded that the osm-10 gene corresponds to T20H4.1.
Fig. 1.
Positional cloning of osm-10.Top, The osm-10 gene maps betweenmec-14 and lin-39 on chromosome III. The osmotic avoidance defect of osm-10(n1602) was rescued by the cosmid T20H4 and the plasmid pKP#51 that contains theosm-10 gene T20H4.1. See Results for details.Bottom, The sequence of the osm-10 cDNA was determined by RT–PCR, leading to the predicted protein sequence shown. The n1602 mutation corresponds to the missense mutation E199K; E199 is lowercase. Amino acids translated in the predicted osm-10(nr2076) protein are in the box. Potential S/T kinase and Y kinase sites are indicated as follows (number of sites): protein kinase C (17) sites areunderlined; casein kinase II (11) sites are inbold; cAMP- and cGMP-dependent kinase phosphorylation sites (6) are in italics; and cyclin-dependent kinase (5) sites are outlined.
After failing to isolate osm-10 cDNAs from available libraries, we determined the intron/exon structure, the 5′ and 3′ ends of the osm-10 mRNA, by RT–PCR. These experiments confirmed that the predicted OSM-10 protein contains 419 amino acids and is rich in serine and threonine residues (20.8%). Database searches for similar proteins failed to identify any osm-10 homologs. OSM-10 contains 39 potential serine and threonine phosphorylation sites for members of the protein kinase C and the cAMP- and cGMP-dependent, casein, and cyclin-dependent kinase families. The n1602mutation perturbs a potential tyrosine kinase phosphorylation site (Fig. 1, bottom). An additional allele,osm-10(nr2076), was obtained using reverse genetic techniques and removed nucleotides 14324–15446 within T20H4.1. The predicted mutant protein consists of 156 amino acids of OSM-10 before a frame shift and premature termination of translation (after exon 2).osm-10(nr2076) animals are phenotypically indistinguishable from osm-10(n1602) animals.
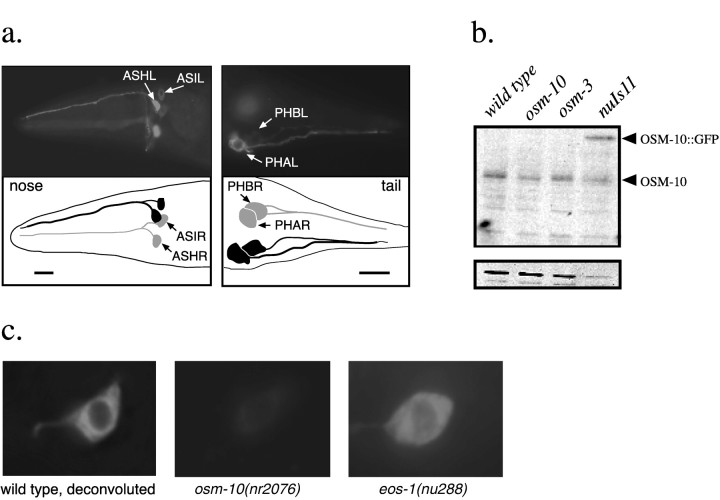
Polyclonal anti-OSM-10 antisera detected a 49 kDa protein on Western blots of wild-type and osm-10(n1602) animals (Fig.2b), which is consistent with the predicted size of the wild-type OSM-10 protein. Subcellular localization of OSM-10 was addressed by expressing GFP reporter constructs and by staining fixed animals with anti-OSM-10 antisera. We detected OSM-10 expression in four classes of chemosensory neurons in all larval and adult animals (ASH, ASI, PHA, and PHB). Expression commences just before hatching. OSM-10 expression was never observed in any other cells (Fig. 2a). The OSM-10 protein was uniformly distributed throughout the cell bodies, sensory processes, and axons of the expressing cells but was excluded from nuclei on the basis of confocal resolution microscopy (data not shown). OSM-10 expression was not altered in osm-10(n1602), osm-9(ky10), orosm-3(p802) animals. OSM-10 expression was reduced to barely detectable levels in osm-10(nr2076) animals. These results demonstrate that OSM-10 is a novel cytoplasmic protein expressed in the osmosensory ASH neurons.
Fig. 2.
a, OSM-10 is expressed inASH, ASI, PHA, andPHB. Expression was monitored by staining fixed animals in whole-mount preparations with a polyclonal rabbit anti-OSM-10 antibody. Cells were identified on the basis of their nuclear positions and axon morphologies (Sulston et al., 1983; White et al., 1986). The OSM-10 protein is diffusely distributed throughout the cell body, sensory processes, cilium, and axons of expressing cells. The cellular and subcellular expression pattern of the OSM-10:: GFP reporter construct nuIs11I is identical. Cells ingray are located on the right side of the animal; cells in black are on the left. Scale bars, 25 μm. b, Polyclonal rabbit anti-OSM-10 antisera recognize a 49 kDa band on Western blots from extracts of wild-type, osm-10(n1602), osm-3(p802), and nuIs11(pKP#58, pJM#24) animals. An additional band at ∼60 kDa corresponding to the OSM-10:: GFP fusion protein is visible in the nuIs11I lane. The same blot probed with anti-tubulin antibodies (N356; Amersham) is shown at thebottom. c, OSM-10 immunoreactivity inASH neurons (visualized as in a) is shown. OSM-10 immunoreactivity is excluded from the nucleus.Left, Deconvoluted images of anASH cell body are shown. Middle,Right, OSM-10 immunoreactivity is severely reduced inosm-10(nr2076) animals but is found at wild-type levels in eos-1(nu288) or eos-2(nu268) (data not shown) animals.
The OSM-10 expression pattern suggested that ASI, PHA, and PHB might also play a role in osmosensation. To test this possibility, we killed ASH, ASI, PHB, and PHA (the primary synaptic input for PHB) with a laser microbeam in various combinations, and the osmotic avoidance behavior of the operated animals was analyzed (Table6). We found that the osmotic avoidance behavior of operated animals lacking ASH neurons (ASH−) was indistinguishable from that of osm-3 mutants, whereas the response of animals lacking ASI, PHA, and PHB was indistinguishable from that of mock-ablated animals. osm-3 mutants have defects in the sensory endings of amphid and phasmid neurons, including ASH, ASI, PHA, and PHB (Perkins et al., 1986). On the basis of these results, we conclude that the ASH neurons play the predominant role in osmotic avoidance in this assay. It remains possible that ASI, PHA, and PHB mediate osmosensory regulation of other behaviors.
The restricted expression of OSM-10, together with the osmotic avoidance defects in osm-10 mutant animals, suggested that OSM-10 expression might be sufficient to confer osmotic sensitivity on other sensory neurons. We tested this possibility by ectopically expressing OSM-10 using the promoter for the putative chemosensory receptor gene sre-1, which is expressed in the sensory neurons ADL and ASJ (Troemel et al., 1995). Like ASH, the synaptic targets of ADL include the interneurons that control locomotion inC. elegans (White et al., 1986). Ectopic expression of OSM-10 in ADL and ASJ did not rescue the osm-10(n1602)mutant phenotype (81 ± 3% defective; n = 6 lines). In contrast, osmosensitivity was partially restored when OSM-10 was expressed in ASH, ADF, ADL, PHA, and PHB using the srb-6promoter (44 ± 9% defective; n = 7 lines).srb-6 is another putative chemosensory receptor gene (Troemel et al., 1995). We conclude that OSM-10 expression is not sufficient to confer osmosensitivity on other sensory neurons and that OSM-10 expression in ASH, PHA, and PHB is sufficient for partial rescue of osm-10.
Genes that interact with osm-10
We examined the role of OSM-10 in ASH-mediated sensory behaviors further by testing for genetic interactions of osm-10(n1602)with mutations in other osmosensory defective genes (J. Kass and J. Kaplan, unpublished observations). Two new genes were found that genetically interact with osm-10 (Table 5). Double heterozygotes [e.g., osm-10(n1602)/+ IIIC; eos-1(nu288)/+ IV or osm-10(n1602)/eos-2(nu268) III] are defective for osmotic avoidance. All of these mutations are recessive as single heterozygotes [e.g., osm-10(n1602)/+,eos-1(nu288)/+, or eos-2(nu268)/+], and all are normal for osmotic avoidance. This type of genetic interaction, termed nonallelic noncomplementation, is often seen with genes involved in a common biochemical pathway. Consistent with this hypothesis,eos-2(nu268)/+; eos-1(nu288)/+ animals are defective in osmotic avoidance. Yet, eos-1 andeos-2 mutant animals are morphologically normal and indistinguishable from wild type for other behaviors examined, including chemotaxis, volatile repellent avoidance, nose touch response, dauer formation, male mating, locomotion, and egg laying. Expression and subcellular localization of OSM-10 is unchanged ineos-1(nu288) animals. Expression of OSM-10 ineos-2(nu268) and interactions of eos mutations with nr2076 are under examination (A. C. Hart, unpublished observations) These results suggest that osm-10,eos-1, and eos-2 are part of a common signal transduction pathway for ASH-mediated osmosensation.
DISCUSSION
We have shown that ASH-mediated osmotic and touch sensitivities are mediated by distinct signaling pathways. First, habituating the nose touch response has no effect on osmotic or 1-octanol avoidance. This result demonstrates that animals distinguish nose touch from other ASH sensory modalities. Second, mutations in osm-10 disrupt ASH-mediated osmosensation but not ASH-mediated touch or volatile sensitivities. The specificity of the sensory defects observed inosm-10(nr2076) clearly demonstrates that these pathways are distinct.
This result is somewhat surprising because the responses to hyperosmolarity and touch are both thought to be mediated by mechanically gated ion channels. Examples include mechanically gated channels in the cochlea, putative cytoskeletal-anchored ion channels inC. elegans, and ubiquitous stretch-activated channels in osmosensation (Oliet and Bourque, 1993, 1996). Hence, a single receptor could have functioned in both sensory modalities analogous to the dual function proposed for the capsaicin receptor in chemical and thermal sensation (Caterina et al., 1997). On the other hand, separate receptors would facilitate differentiation of disparate stimuli. Although touch is a ubiquitous and relatively innocuous stimulus, osmotic shock and volatile chemicals are lethal. Thus, animals may need to distinguish between these stimuli. Stimulus strength may, in part, underlie ASH modality encoding.
Relationship between ASH and microtubule mechanoreceptor neurons
A molecular model for the touch cell mechanoreceptor has been proposed by Chalfie and colleagues (Garcia-Anoveros et al., 1995; Gu et al., 1996). We found that none of the mec genes that constitute this putative mechanoreceptor are required for the ASH-mediated touch of osmotic responses. In addition, the degenerinsdeg-1 and unc-8, proposed to be mechanoreceptors on the basis of their homology to mec genes (Chalfie et al., 1993), were not required for ASH-mediated responses. These results suggest that the mechanoreceptors used by ASH are distinct from those used by the touch cells, although mec or degenerin genes could play redundant roles in ASH. In contrast, the analysis of mammalian osmosensing neurons suggests that amiloride-sensitive stretch-activated channels may be required for detecting changes in osmolarity (Oliet and Bourque, 1993, 1996). It is not surprising that ASH and the microtubule touch cells might use distinct mechanoreceptors, because the sensory endings of these cells are dissimilar ultrastructurally. The ASH sensory endings have ciliary axonemes (Albert et al., 1981; Perkins et al., 1986), whereas the microtubule touch cells have an unusual cytoskeleton containing 15 protofilament microtubules (Chalfie and Thomson, 1979). Our results provide further evidence of the proposal that the mechanoreceptors of ciliated and microtubule-containing mechanosensory neurons are distinct, as suggested previously by others (Kernan and Zuker, 1995).
Role of OSM-10 in osmosensation
The selectivity of the sensory defects in osm-10animals suggests that OSM-10 plays a specific role in transducing osmosensory signals and is not required for general aspects of ASH function or development. The specificity of theosm-10(nr2076) defect suggests that the ASH response to high osmolarity differs from responses to nose touch and volatile repellents and that the latter two do not require OSM-10 activity.
Although all living cells respond to changes in osmolarity, the biochemical mechanisms of osmoregulation are only now being elucidated. Generally, the response to osmotic shock is twofold, with a relatively immediate activation of stretch-modulated ion channels and a slower induction of protein kinase cascades. At present, only one stretch-activated channel has been molecularly defined, the E. coli channel MscL, which may mediate efflux of organic solutes in response to osmotic shock (for review, see Sukharev et al., 1997). In mammalian cells, osmotic shock activates unidentified potassium and chloride channels (Hallows and Knauf, 1994; Strange et al., 1996). In both yeast and mammalian cells, hypertonic shock induces the HOG-1 p38 MAP kinase (Brewster et al., 1993; Galcheva-Gargova et al., 1994). In yeast, the osmosensing MAP kinase cascades are coupled to either of two alternative osmosensory receptors (Sln1 or Sho1) (Maeda et al., 1994,1995). In mammalian cells, swelling-activated channel activity is not blocked by agents that prevent activation of osmosensory MAP kinases, suggesting that these aspects of the osmosensory response are mechanistically distinct (Tilly et al., 1996). No MscL, Sln1, or Sho1 homologs have been found in any organism including C. elegans, although the C. elegans genome sequence is nearly completed. C. elegans MAP kinases and potassium and chloride channels have not been implicated in osmosensation.
On the basis of these precedents, OSM-10 could play various roles in osmosensation. OSM-10 might act as a cytoplasmic regulator of the osmotically activated channels, analogous to pICln, which regulates swelling-activated chloride channels (Krapivinsky et al., 1994). OSM-10 might act as a chaperonin specifically required for the trafficking or localization of osmosensory receptor complexes. Alternatively, OSM-10 might act as a cytoplasmic target of an osmosensory receptor, such as Ypd1, which is phosphorylated by the two-component osmosensor Sln1p in yeast (Maeda et al., 1994; Posas et al., 1996).
Detecting and responding to changes in external osmolarity are critical physiological functions of all living cells. Changes in osmolarity alter cell size and shape-evoking compensatory changes in membrane permeability to ions and organic solutes. Despite the fundamental importance of these osmoregulatory effects, relatively little is known about the molecular mechanisms underlying osmotic regulation of cellular volume or sensory detection of changes in osmotic regulation or detection. Further characterization of the osm-10 andeos genes should provide new insights into the mechanisms underlying osmosensation.
Footnotes
This work was supported by National Institutes of Health Grant NS32196 to J.M.K. and by grants from the Medical and Whitehall foundations to A.C.H. J.M.K. is a Pew Scholar in the Biomedical Sciences. A.C.H. is a Searle Scholar. We thank J. Thomas and R. Horvitz for isolation and initial characterization of osm-10(n1602)and of the role of ASH in osmosensation, the Nemapharm division of Axys Pharmaceuticals for osm-10(nr2076), C. Bargmann for plasmids, C. Korey and R. Moeller for assistance with phenotypic characterization, the C. elegans Genetics Center and M. Chalfie for strains, the C. elegans genome-sequencing project for clones, and members of the J. M. Kaplan, G. Ruvkun, and S. van den Heuvel laboratories for comments and advice.
Correspondence should be addressed to Dr. Anne C. Hart, Department of Pathology, Harvard Medical School, and Massachusetts General Hospital Cancer Center, 149–7202 13th Street, Charlestown, MA 02129.
REFERENCES
- 1.Albert PS, Brown SJ, Riddle DL. Sensory control of dauer larva formation in C. elegans. J Comp Neurol. 1981;198:435–451. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- 2.Avery L, Horvitz H. Effects of killing identified pharyngeal neurons on feeding behavior in Caenorhabditis elegans. Neuron. 1989;3:473–485. doi: 10.1016/0896-6273(89)90206-7. [DOI] [PubMed] [Google Scholar]
- 3.Bargmann CI, Thomas JH, Horvitz HR. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1990;55:529–538. doi: 10.1101/sqb.1990.055.01.051. [DOI] [PubMed] [Google Scholar]
- 4.Brewster J, de Valoir T, Dwyer N, Winter E, Gustin M. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- 5.Caterina M, Schumacher M, Tominaga M, Rosen T, Levine J, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 6.Chalfie M, Thomson JN. Organization of neuronal microtubules in the nematode C. elegans. J Cell Biol. 1979;82:278–289. doi: 10.1083/jcb.82.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalfie M, Driscoll M, Huang M. Degenerin similarities. Nature. 1993;361:504. doi: 10.1038/361504a0. [DOI] [PubMed] [Google Scholar]
- 8.Colbert H, Smith T, Bargmann C. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culotti JG, Russell RL. Osmotic avoidance defective mutants of the nematode Caenorhabditis elegans. Genetics. 1978;90:243–256. doi: 10.1093/genetics/90.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driscoll M, Chalfie M. The mec-4 gene is a member of a family of C. elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- 11.Driscoll M, Kaplan J. Mechanotransduction. In: Riddle D, Blumenthal T, Meyer B, Priess J, editors. C. elegans II. Cold Spring Harbor; Cold Spring Harbor, NY: 1997. pp. 645–677. [PubMed] [Google Scholar]
- 12.Galcheva-Gargova Z, Derijard B, Wu I, Davis R. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Anoveros J, Ma C, Chalfie M. Regulation of Caenorhabditis elegans degenerin proteins by a putative extracellular domain. Curr Biol. 1995;5:441–448. doi: 10.1016/s0960-9822(95)00085-6. [DOI] [PubMed] [Google Scholar]
- 14.Gu G, Caldwell GA, Chalfie M. Genetic interactions affecting touch sensitivity in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1996;93:6577–6582. doi: 10.1073/pnas.93.13.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallows K, Knauf P. Principles of cell volume regulation. In: Strange K, editor. Cellular and molecular physiology of cell volume regulation. CRC; Boca Raton, FL: 1994. pp. 3–29. [Google Scholar]
- 16.Hart A, Kramer H, Van Vactor D, Paidhunghat M, Zipursky SL. Induction of cell fate in the Drosophila retina: the bride of sevenless protein is predicted to contain a large extracellular domain and seven transmembrane segments. Genes Dev. 1990;4:1835–1847. doi: 10.1101/gad.4.11.1835. [DOI] [PubMed] [Google Scholar]
- 17.Hart A, Sims S, Kaplan J. A synaptic code for sensory modalities revealed by analysis of the C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–85. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- 18.Huang M, Chalfie M. Gene interactions affecting mechanosensory transduction in C. elegans. Nature. 1994;367:467–470. doi: 10.1038/367467a0. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan JM, Horvitz HR. A dual mechanosensory and chemosensory neuron in C. elegans. Proc Natl Acad Sci USA. 1993;90:2227–2231. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernan M, Zuker C. Genetic approaches to mechanosensory transduction. Curr Opin Neurobiol. 1995;5:443–448. doi: 10.1016/0959-4388(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 21.Krapivinsky G, Ackerman M, Gordon E, Krapivinsky L, Clapham D. Molecular characterization of a swelling-induced chloride conductance regulatory protein, pICln. Cell. 1994;76:439–448. doi: 10.1016/0092-8674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 22.Maeda T, Wurgler-Murphy S, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- 23.Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- 24.Maricq AV, Peckol E, Driscoll M, Bargmann C. glr-1, a C. elegans glutamate receptor that mediates mechanosensory signaling. Nature. 1995;378:78–81. doi: 10.1038/378078a0. [DOI] [PubMed] [Google Scholar]
- 25.Nonet M, Staunton J, Kilgard M, Fergestad T, Hartwieg E, Horvitz H, Jorgensen E, Meyer B. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci. 1997;17:8061–8073. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliet S, Bourque C. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993;364:341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- 27.Oliet S, Bourque C. Gadolinium uncouples mechanical detection and osmoreceptor potential in supraoptic neurons. Neuron. 1996;16:175–181. doi: 10.1016/s0896-6273(00)80034-3. [DOI] [PubMed] [Google Scholar]
- 28.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 29.Posas F, Wurgler-Murphy S, Maeda T, Witten E, Thai T, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 30.Strange K, Emma F, Jackson P. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 31.Sukharev S, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu Rev Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- 32.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 33.Tavernarakis N, Shreffler W, Wang S, Driscoll M. unc-8, a DEG/ENaC family member, encodes a subunit of a candidate mechanically gated channel that modulates C. elegans locomotion. Neuron. 1997;18:107–119. doi: 10.1016/s0896-6273(01)80050-7. [DOI] [PubMed] [Google Scholar]
- 34.Tilly B, Gaestel M, Engel K, Edixhoven M, de Jonge H. Hypo-osmotic cell swelling activates the p38 MAP kinase signalling cascade. FEBS Lett. 1996;385:133–136. doi: 10.1016/0014-5793(96)01028-9. [DOI] [PubMed] [Google Scholar]
- 35.Troemel E, Chou H, Dwyer N, Colbert H, Bargmann C. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 36.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of Caenorhabditis elegans. Philos Trans R Soc Lond [Biol] 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]