Abstract
Activation of ATP/P2Y purinergic receptors stimulates proliferation of astrocytes, but the mitogenic signaling pathway linked to these G-protein-coupled receptors is unknown. We have investigated the role of extracellular signal-regulated protein kinase (ERK) in P2Y receptor-stimulated mitogenic signaling as well as the pathway that couples P2Y receptors to ERK. Downregulation of protein kinase C (PKC) in primary cultures of rat cerebral cortical astrocytes greatly reduced the ability of extracellular ATP to stimulate ERK. Because occupancy of P2Y receptors also leads to inositol phosphate formation, calcium mobilization, and PKC activation, we explored the possibility that signaling from P2Y receptors to ERK is mediated by a phosphatidylinositol-specific phospholipase C (PI-PLC)/calcium pathway. However, neither inhibition of PI-PLC nor chelation of calcium significantly reduced ATP-stimulated ERK activity. Moreover, a preferential inhibitor of calcium-dependent PKC isoforms, Gö6976, was significantly less effective in blocking ATP-stimulated ERK activity than GF102903X, an inhibitor of both calcium-dependent and -independent PKC isoforms. Furthermore, ATP stimulated a rapid translocation of PKCδ, a calcium-independent PKC isoform, but not PKCγ, a calcium-dependent PKC isoform. ATP also stimulated a rapid increase in choline, and inhibition of phosphatidylcholine hydrolysis blocked ATP-evoked ERK activation. These results indicate that P2Y receptors in astrocytes are coupled independently to PI-PLC/calcium and ERK pathways and suggest that signaling from P2Y receptors to ERK involves a calcium-independent PKC isoform and hydrolysis of phosphatidylcholine by phospholipase D. In addition, we found that inhibition of ERK activation blocked extracellular ATP-stimulated DNA synthesis, thereby indicating that the ERK pathway mediates mitogenic signaling by P2Y receptors.
Keywords: purinergic receptors, MAP kinase, protein kinase C, proliferation, phospholipases, astrocytes
Emerging evidence points to a crucial role for extracellular signal-regulated protein kinases (ERK1 and ERK2) in regulating cellular proliferation and differentiation (for review, see Marshall, 1995; Neary, 1997). ERKs are part of a family of serine/threonine protein kinases known as mitogen-activated protein kinases (MAPKs) (Seger and Krebs, 1995). These enzymes are components of a signaling cascade consisting of at least three cytoplasmic protein kinases that are activated sequentially. ERKs are activated by phosphorylation on tyrosine and threonine residues by a dual-specificity protein kinase termed MAPK/ERK kinase (MEK). MEK in turn is activated by phosphorylation on serine/threonine residues, which can be catalyzed either by the Raf family of protein kinases, the protooncogene product Mos, or MEK kinases (Avruch et al., 1994). The ERK/MAPK cascade is stimulated shortly after extracellular signals bind to cell surface receptor tyrosine kinases or heterotrimeric G-protein-coupled receptors (GPCRs). These receptors are linked to the ERK/MAPK cascade by a sequence of protein–protein interactions and by upstream protein kinases such as protein kinase C (PKC). The activated ERK can then translocate to the nucleus where it can activate or induce transcription factors such as Elk-1 and c-Fos (Karin, 1995). In this manner, ERKs provide a link between cytoplasmic and nuclear signaling, ultimately leading to changes in gene expression involved in proliferation and differentiation.
Recent studies on the role of extracellular ATP in the brain have demonstrated that in addition to serving as an excitatory neurotransmitter (Edwards et al., 1992; Harms et al., 1992; Shen and North, 1993), ATP can also exert mitogenic and morphogenic activity on glial and neuronal cells (Neary et al., 1996). For example, treatment of astrocytes with ATP leads to increases in DNA synthesis (Rathbone et al., 1992; Abbracchio et al., 1994; Neary et al., 1994b), in process formation (Neary et al., 1994b) and elongation (Abbracchio et al., 1994, 1995), and in the content of glial fibrillary acidic protein (Neary et al., 1994b), an astrocyte-specific marker that is upregulated after brain injury (Eng, 1988). These trophic actions of ATP may be important in development and synaptogenesis as well as in tissue injury and repair, but the signaling mechanisms underlying the trophic activity of ATP are unknown.
The biological actions of extracellular ATP are mediated by cell surface receptors designated as P2 purinoceptors (Burnstock, 1978). This general class of receptors has been categorized into two major types: ligand-gated ionotropic receptors (P2X) and G-protein-coupled metabotropic receptors (P2Y) (Abbracchio and Burnstock, 1994). P2Y receptors are expressed in astrocytes, and these receptors are coupled to phosphatidylinositol-specific phospholipase C (PI-PLC), leading to inositol phosphate formation and calcium mobilization (Pearce et al., 1989; Neary et al., 1991; Kastritsis et al., 1992; Salter and Hicks, 1994, 1995; King et al., 1996; Centemeri et al., 1997). After activation of the PI-PLC/calcium pathway, PKC is then activated.
We have shown that P2Y receptors in astrocytes are also linked to the ERK/MAPK cascade (Neary and Zhu, 1994; King et al., 1996). However, the signaling elements that couple the P2Y receptors to the ERK/MAPK cascade have not been defined. Preliminary evidence indicates that signaling from P2Y receptors to ERK in astrocytes is dependent on PKC (Neary, 1996). Because P2Y receptor signaling can involve PKC in both PI-PLC and ERK pathways, we have investigated the hypothesis that PI-PLC/calcium are upstream of ERK. We report here for the first time that P2Y receptors are coupled independently to PI-PLC and ERK pathways and that the latter pathway involves hydrolysis of phosphatidylcholine (PC) and a calcium-independent isoform of PKC. We also provide evidence that the ERK pathway mediates ATP-induced mitogenic signaling in astrocytes.
MATERIALS AND METHODS
Cell culture and treatment. Primary astrocytes were obtained from neonatal rat (Fischer) cerebral cortices as described previously (Neary et al., 1994a). Cells were seeded at densities of 150,000 cells/well in 24-well plates (for DNA synthesis studies) or at 300,000 cells/35 mm or 600,000 cells/60 mm plates (for ERK studies); cells were not replated before use. At least 99% of the cell population were astrocytes, as determined by staining with cell-specific markers (Neary et al., 1994a). Experiments were conducted with 3- to 6-week-old cultures. Before treatment with nucleotides or other agents, cells that had been maintained in DMEM containing 10% horse serum were shifted to the quiescent phase by incubation in DMEM containing 0.5% horse serum for 48–72 hr. Stock solutions of nucleotides and drugs were divided into single-use aliquots and stored at −80°C, except for D609 (Research Biochemicals International, Natick, MA), which was prepared fresh for each experiment.
DNA synthesis.3H-thymidine incorporation was measured as described previously (Neary et al., 1994a) using confluent cells in the stationary phase of growth. In brief, nucleotides or PD 098059 (Research Biochemicals International) or both at the indicated final concentrations were added to the media in the wells in triplicate or quadruplicate. PD 098059 was added 60 min before addition of nucleotides. As a positive control, some wells were treated with 10% horse serum, whereas untreated cultures were used for negative controls. After 18 hr, 3H-thymidine (0.5 μCi/ml culture media; 83 Ci/mmol; ICN Biochemicals, Costa Mesa, CA) was added to the media in the wells for an additional 4 hr. Cells were then rinsed with Dulbecco’s PBS (D-PBS), incubated in 10% trichloroacetic acid for 30 min on ice, rinsed again with D-PBS, and lysed in 1% SDS, 0.3 N NaOH. Aliquots were neutralized and counted by liquid scintillation spectrometry.
ERK activity. After treatment with nucleotides or other agents for the times and concentrations indicated, cells were rinsed quickly in ice-cold D-PBS and lysed in a buffer containing 20 mm Tris, pH 7.0, 0.27 m sucrose, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 1 mm dithiothreitol (DTT), 1 mm sodium orthovanadate, 10 mm β-glycerophosphate, 5 mmsodium pyrophosphate, and 1% Triton X-100. In some cases, cells were lysed in a buffer containing 20 mm Tris, pH 7.5, 100 mm NaCl, 50 mm NaF, 2 mm EGTA, 50 mm β-glycerophosphate, 1 mm sodium orthovanadate, 100 μg/ml 4-(2-aminoethyl) benzenesulfonylfluoride (AEBSF; Calbiochem, La Jolla, CA), 0.3 U/ml aprotinin, and 1% Triton X-100. The lysates were centrifuged in a microfuge for 5 min at 4°C. ERK activity was measured as described previously (Neary and Zhu, 1994). In brief, aliquots (20 μl containing 10–20 μg protein) of the supernatants were assayed at 30°C for 20 min in a final reaction solution containing 10 μm ATP (0.2 μCi [γ32P]ATP; 3000 Ci/mmol; New England Nuclear), 10 mm MgCl2, 1 μm okadaic acid, and 0.33 mg/ml bovine brain myelin basic protein (MBP) in a final volume of 40 μl. Under these conditions, the reaction is linear with respect to time and enzyme concentration. Reactions were stopped by pipetting 20 μl aliquots onto 1 × 2 cm strips of phosphocellulose paper (Sevetson et al., 1993) and immediately placing the strips in 75 mm phosphoric acid. Strips were washed for a minimum of 2 hr and rinsed three times for 5 min each in 75 mm phosphoric acid and once in ethanol. Strips were dried and transferred to scintillation vials, and radioactivity was assessed by liquid scintillation counting. ERK activity was expressed as picomoles of 32P transferred per minute per milligram of protein. Protein concentrations were determined by the modified Lowry procedure as described (Peterson, 1983), with bovine serum albumin as standard. The validity of the phosphocellulose strip assay under these conditions was confirmed by comparing the data with phosphorylation of MBP obtained by SDS-PAGE of the samples followed by autoradiography and densitometry (Bio-Rad GS-670 imaging densitometer; Bio-Rad, Hercules, CA); a correlation coefficient of 0.935 was obtained (Neary and Zhu, 1994). Results obtained by this procedure were consistent with those obtained by immunocomplex kinase assays after immunoprecipitation with anti-ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA) as described previously (Neary and Zhu, 1994) or by determination of phosphorylated ERK1/2 as described below.
Anion exchange chromatography. The procedure of Ahn et al. (1990) was used with some modifications. After treatment of cultures with ATP (six 60 mm plates per group), plates were rinsed quickly in ice-cold PBS. Cells were scraped and homogenized in buffer A (50 mm β-glycerophosphate, pH 7.3, 0.1 mm sodium orthovanadate, 1.5 mm EGTA, 1 mm DTT), and the homogenate was centrifuged at 13,000 × g for 10 min at 4°C. Supernatants were stored at −70°C. Before chromatography, supernatants were thawed and centrifuged for 30 min at 23,000 ×g, 4°C. Supernatants containing equivalent amounts of protein were loaded onto a 1 ml Hi-Trap Q (Amersham Pharmacia Biotech) column at a flow rate of 0.5 ml/min. Fractions (1 ml) were collected with a Gradifrac protein purification system (Amersham Pharmacia Biotech), and the column was washed with 8 ml buffer A. Elution was conducted with a 60 ml, linear NaCl gradient (0–400 mmNaCl in buffer A). Fractions (20 μl) were assayed for ERK activity as described above using MBP as substrate. In addition, ERK1/2 was detected in column fractions and cellular extracts by immunoblotting as described below.
Membrane preparation. After quiescent cultures were treated with ATP or 12-O-tetradecanoylphorbol-13-acetate (TPA) for the time periods indicated in the text, cells were quickly rinsed twice with ice-cold PBS and collected in 200 μl (per 35 mm plate; four plates/group) of a buffer containing 50 mm Tris, pH 7.7, 2 mm EGTA, 5 mm DTT, 100 μg/ml AEBSF, and 10 μg/ml leupeptin. Cells were homogenized (20 strokes) using a Teflon–glass homogenizer, and homogenates were centrifuged at 100,00 × g for 60 min, 4°C, in a Beckman TL-100 centrifuge. Pellets were suspended in 800 μl of the homogenizing buffer containing 1% Triton X-100. Suspensions were incubated on ice for 30 min and sonicated for 5–10 sec (Ultrasonic 40W, Heat Systems, Farmingdale, NY). Protein concentrations were determined by the modified Lowry procedure (Peterson, 1983). Fractions were diluted in 2× Laemmli SDS sample buffer (Laemmli, 1970) and heated in a boiling water bath for 5 min.
Immunoblotting. Samples containing equal amounts of protein were subjected to SDS-PAGE (Laemmli, 1970) using 11% acrylamide and transferred to nitrocellulose filters with a Genie electrophoretic blotter (Idea Scientific, Minneapolis, MN) for 1 hr at 12 V in a transfer buffer containing 25 mm Tris, 192 mmglycine, and 20% methanol. Filters were incubated with a blocking solution containing 20 mm Tris, pH 7.7, 137 mmNaCl, 0.1% Tween 20 (TTBS), and 5% nonfat dry milk for 1 hr at room temperature, rinsed in TTBS, and then incubated for 1 hr at room temperature with specific antibodies diluted in TTBS [anti-phospho-ERK1/2 (1/20,000; Promega, Madison, WI), anti-ERK1/2 (1/500; Santa Cruz Biotechnology), anti-PKCδ (1/200; Transduction Laboratories, Lexington, KY), or anti-PKCγ (1/5000; Transduction Laboratories)]. After three rinses in TTBS, filters were incubated for 1 hr at room temperature with peroxidase-conjugated anti-rabbit or anti-mouse IgG diluted in TTBS (1/20,000 or 1/10,000, respectively; Amersham Life Sciences, Arlington Heights, IL). Filters were washed three times in TTBS, and proteins were detected by enhanced chemiluminescence (Amersham Life Sciences).
Inositol phosphate formation. Inositol phosphates were measured by a previously described procedure that involves prelabeling cells overnight with myo-[2-3H(N)]inositol (12.3Ci/mmol; New England Nuclear), stimulating with ATP as described in the text, applying aqueous extracts to Dowex AG 1-X8 columns, and eluting inositol phosphates with 1 m ammonium formate/0.1m formic acid (Bender et al., 1993). The 3H recovered in inositol phosphates was then standardized to an incorporation of 105 cpm in the lipid fraction.
PC hydrolysis. Cells were incubated with3H-choline, and water-soluble3H-choline-labeled metabolites were separated and quantitated as described (Vance et al., 1980) with some modifications. In brief, cultures grown on 35 mm dishes were incubated in DMEM/0.5% horse serum containing 2.5 μCi [methyl-3H]-choline (NEN; 60–90 Ci/mmol)/ml for 48 hr followed by a 24 hr incubation in DMEM/0.5% horse serum. After treatment with ATP for the times indicated, the media was quickly removed, and 2.1 ml of extraction solution (methanol/10 mm glycine, pH 3.0; 5:2, v/v) was added immediately. Dishes were scraped, and the extract was transferred to 15 ml centrifuge tubes. Chloroform (0.8 ml) was added, tubes were vortexed, 0.8 ml water and 0.8 ml chloroform were added, tubes were vortexed again, and phases were separated by centrifugation at 660 × g for 5 min. The lipid phase was removed, and the aqueous phase was re-extracted with 0.8 ml chloroform, followed by vortexing and centrifugation as described above. Aqueous phases were combined, and carrier choline, phosphocholine, cytidine 5′-diphospho-choline, and glycerophosphocholine (50 μg each) were added to a 0.5 ml aliquot that was concentrated to 25 μl by vacuum centrifugation. Samples were applied to a silica gel thin layer plate that was developed in 0.5% NaCl, ethanol, methanol, NH4OH (5:3:2:0.5; v/v). The plate was dried at room temperature, and spots were detected by iodine vapor, scraped into scintillation vials containing 0.75 ml 0.1N NaOH, and incubated overnight at room temperature. Acetic acid (75 μl of 1.5N) was added to each vial, and radioactivity was determined by scintillation spectrometry. The 3H recovered in the choline-containing fractions was then standardized to an incorporation of 105 cpm in the aqueous phase.
Statistical analyses. All experiments were conducted a minimum of three times, each time with cultures from different seedings. Data were analyzed by Student’s t tests for two groups or ANOVA followed by post hoc comparisons for multiple groups with an Instat software package (GraphPad Software).
RESULTS
Signaling from ATP/P2Y receptors to ERK in astrocytes is dependent on PKC
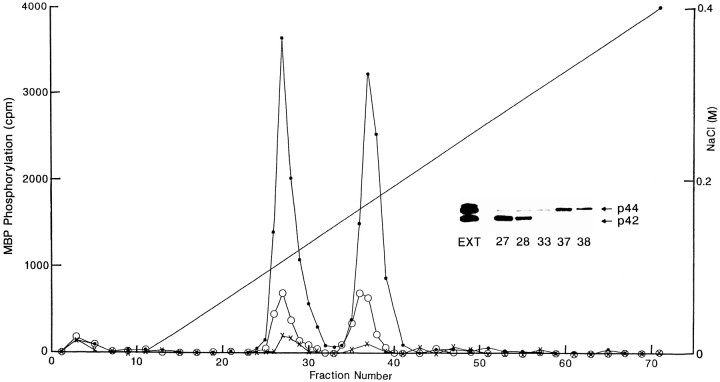
P2Y receptors are members of the heterotrimeric GPCR superfamily (Abbracchio and Burnstock, 1994). Signaling from GPCRs to the ERK/MAPK cascade can proceed by several distinct pathways, some of which involve PKC (van Biesen et al., 1996b; Neary, 1997). To determine whether signaling from astrocytic P2Y receptors to ERK is dependent on PKC, primary cultures of rat cortical astrocytes were treated overnight with phorbol ester (TPA; 100 nm) to downregulate PKC; such chronic treatment with TPA reduces PKC activity in astrocytes by at least 90% (Neary et al., 1988). After addition of extracellular ATP, cellular homogenates were then prepared and fractionated by anion exchange chromatography under conditions that separate p42 and p44 isoforms of ERK (Ahn et al., 1990). After stimulation of astrocytes with ATP (100 μm, 15 min), two peaks of ERK activity were eluted at ∼110 and 175 mm NaCl (Fig.1). Downregulation of PKC by chronic phorbol ester treatment reduced the ability of extracellular ATP to stimulate the two regions of ERK activity by ∼85% (Fig. 1). To confirm the presence of ERK in these two regions of activity, immunoblots of peak fractions and of a fraction between the peaks were probed with an antibody that recognizes both ERK1 and ERK2. As shown in the inset of Figure 1, the first peak contained ERK2 and the second peak contained ERK1. In separate experiments, astrocytes were treated acutely with phorbol ester (100 nm, 5 min); ERK activity was stimulated 2.95 ± 0.13-fold, thereby indicating that PKC can activate ERK in astrocytes.
Fig. 1.
Signaling from P2Y receptors to ERK is dependent on PKC. Primary rat astrocyte cultures were treated with 100 μm ATP for 15 min (●–●), with 100 nm TPA for 24 hr before application of 100 μm ATP for 15 min (○–○), or were untreated (×–×). Homogenates were centrifuged, and supernatants containing equivalent amounts of protein were applied to a 1 ml Hi-Trap Q anion exchange column as described in Materials and Methods. Fractions (1 ml) were collected at a flow rate of 0.5 ml/min, and proteins were eluted with a linear, 60 ml NaCl gradient (0–400 mm). Two peaks of ERK activity eluted at 110 and 175 mm NaCl. Inset, Immunoblot of column fractions from the peak ERK regions and a fraction between the peaks. The indicated column fractions and the cellular extract (EXT) from the ATP-treated, normal cells were subjected to SDS-PAGE, and immunoblots were probed with anti-ERK1/2. The arrows indicate the two ERK isoforms p42 and p44, before and after fractionation by anion exchange chromatography. Similar results were obtained by chromatography on a 1 ml Resource Q (Amersham Pharmacia Biotech) column.
Inhibition of PI-PLC or chelation of calcium does not block ATP-stimulated ERK activation
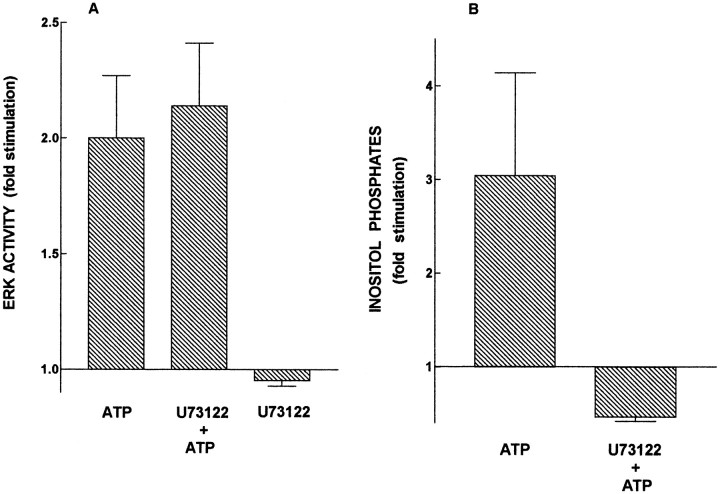
In astrocytes as in other cells, activation of P2Y receptors leads to mobilization of intracellular calcium via the PI-PLC pathway; the increased calcium, together with the PI-PLC-catalyzed increase in diacylglycerol, can then activate PKC. The finding that signaling from P2Y receptors to ERK is dependent on PKC raised the possibility that PI-PLC, calcium, PKC, and ERK are part of the same signal transduction pathway. To determine whether PI-PLC and calcium are upstream of ERK, several approaches were used. To block signaling from P2Y receptors to ERK via PI-PLC, we used an inhibitor of PI-PLC, the steroidalamine U73122 (Bleasdale et al., 1990; Salter and Hicks, 1995). Cultures were treated with U73122 (10 μm) for 30 min before application of extracellular ATP, after which ERK activity and inositol phosphate formation were measured. As shown in Figure2A, U73122 did not diminish the ability of ATP to activate ERK. However, U73122 was effective in blocking ATP-evoked inositol phosphate formation (Fig.2B). These findings suggest that activation of ERK by stimulation of P2Y receptors can proceed independently of the PI-PLC pathway.
Fig. 2.
Inhibition of PI-specific PLC does not block ATP-stimulated ERK activity. In A, primary rat astrocyte cultures were treated with 10 μm U73122 for 30 min before addition of ATP (100 μm, 15 min). ERK activity data (mean ± SEM) were obtained from three experiments, each conducted with duplicate culture plates. ERK activity in untreated cultures was 43.5 ± 8.2 pmol phosphate transferred per minute per milligram of protein. In B, primary rat astrocyte cultures were treated with 10 μm U73122 for 30 min before addition of ATP (500 μm, 30 min), and inositol phosphates were extracted and measured as described in Materials and Methods.
The ability of extracellular ATP to stimulate ERK independently of PI-PLC suggests that calcium mobilization is not required for activation of ERK by P2Y receptors. To test this, we used BAPTA-AM to chelate intracellular calcium. Treatment of cells with 30 μm BAPTA-AM for 30 min before application of ATP did not significantly reduce ERK activation (fold-stimulation: ATP, 2.64 ± 0.05; BAPTA + ATP, 2.68 ± 0.12; p > 0.05). As another approach, EGTA was used to minimize entry of extracellular calcium across the plasma membrane, an event that can occur in response to depletion of intracellular stores after application of ATP. However, treatment with 3 mm EGTA for 5 min, a condition known to block carbachol- and bradykinin-evoked activation of ERK (Lev et al., 1995), did not significantly reduce ATP-stimulated ERK activity (fold-stimulation: ATP, 2.57 ± 0.25; EGTA + ATP, 2.47 ± 0.20; p > 0.05).
A calcium-independent PKC isoform mediates signaling from P2Y receptors to ERK
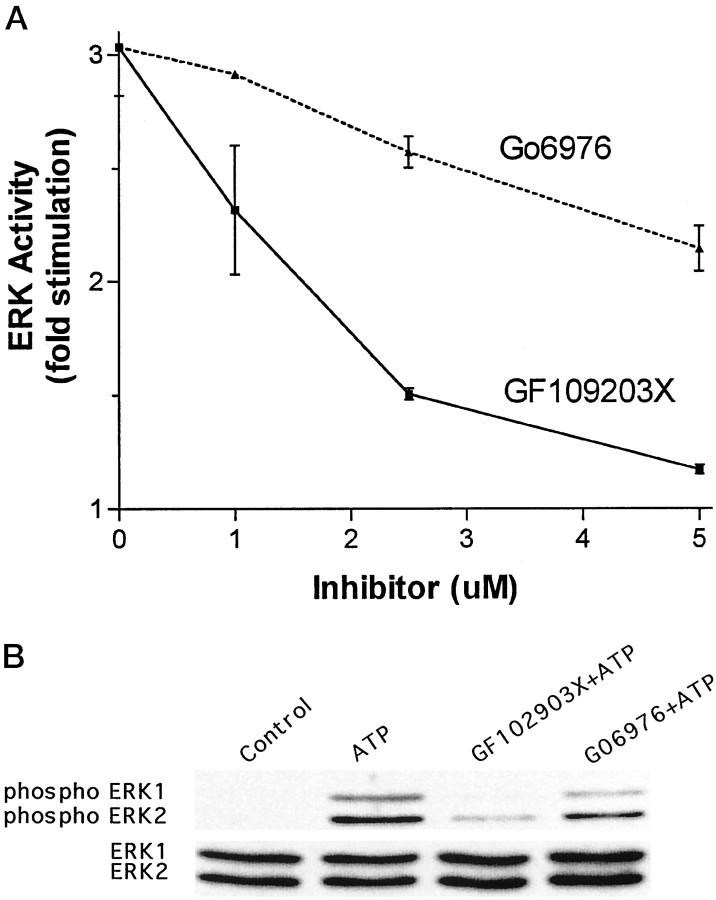
Because P2Y receptor signaling to ERK is independent of PI-PLC and calcium, but dependent on PKC, we reasoned that a calcium-independent isoform of PKC may be involved in the P2Y/ERK pathway. If this is the case, a preferential inhibitor of calcium-dependent PKCs, Gö 6976 (Martiny-Baron et al., 1993), should have less of an effect on the stimulation of ERK by agonists of P2Y receptors than GF102903X, an inhibitor of both calcium-dependent and independent PKCs. To test this, cultures were treated with varying concentrations (1, 2.5, or 5 μm) of Gö 6976 or GF102903X for 20 min before addition of ATP (100 μm; 5 min). As shown in Figure3A, Gö 6976 was less effective than GF102903X in inhibiting ATP stimulation of ERK activity. The greater degree of inhibition observed with GF109203X was significantly different from that observed with Gö 6976 for each of the concentrations used (p < 0.01). To confirm this observation, we tested the effect of these inhibitors on ATP-evoked ERK phosphorylation, which was measured by immunoblot analysis with an antibody that recognizes dually phosphorylated ERK1 and ERK2 (Thr183, Tyr185). As shown in Figure 3B, phosphorylation of ERK1 and ERK2 was only slightly reduced by Gö 6976, whereas GF102903X almost completely blocked ATP-evoked phosphorylation. These experiments support the concept that P2Y receptors are coupled to ERK by a calcium-independent isoform of PKC.
Fig. 3.
Effects of inhibitors of calcium-independent and -dependent PKC isoforms on signaling from P2Y receptors to ERK. InA, primary rat astrocyte cultures were treated with ATP (100 μm, 5 min) or with Gö 6976 (1–5 μm, 20 min), which preferentially inhibits calcium-dependent PKC isoforms, or GF109203X (1–5 μm, 20 min), an inhibitor of both calcium-dependent and -independent PKC isoforms, before addition of ATP (100 μm, 5 min). ERK activity data were obtained from a minimum of three experiments, each conducted with duplicate culture plates. In B, primary rat astrocyte cultures were treated with ATP (100 μm, 5 min) or with Gö 6976 (2.5 μm) or GF109203X (2.5 μm) for 20 min before addition of ATP (100 μm, 5 min), cells were lysed, and lysates containing equivalent amounts of protein were subjected to SDS-PAGE. Immunoblots were probed with antibodies that recognize phosphorylated ERK1/ERK2 (top panel) or ERK1/ERK2 (bottom panel).
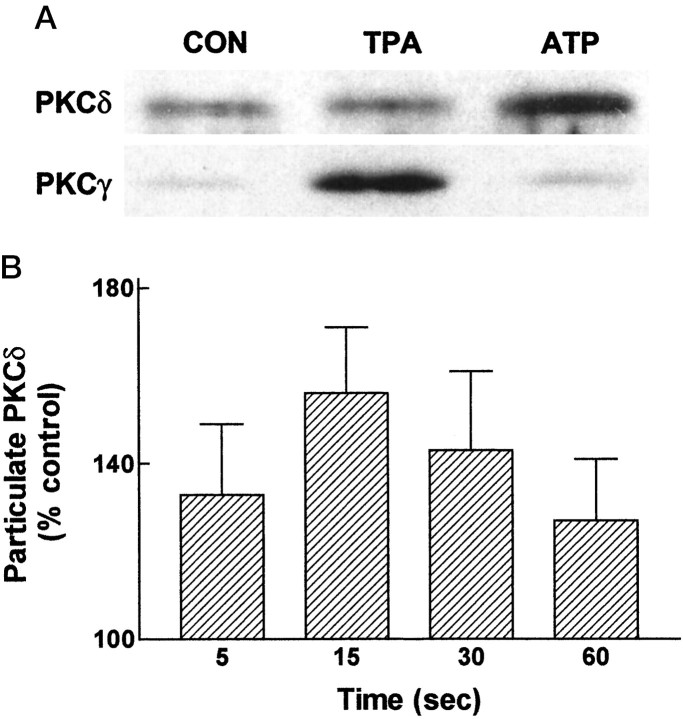
To determine whether activation of P2Y receptors leads to translocation of a calcium-independent PKC isoform, membrane fractions from untreated and ATP-treated astrocytes were immunoblotted and probed with antibodies raised against PKCδ, a calcium-independent PKC isoform present in astrocytes (Gott et al., 1994). For comparison, translocation of PKCγ, a calcium-dependent PKC isoform, was also examined. As shown in Figure4A, ATP stimulated a rapid translocation (15 sec) of PKCδ, but not PKCγ. By contrast, PKCγ was translocated by a phorbol ester TPA (100 nm, 5 min) (Fig. 4A). Time course studies revealed that ATP-evoked translocation of PKCδ was observed at 5 sec, reached a peak at 15 sec, and declined from 30 to 60 sec (Fig.4B). This rapid translocation of PKCδ can be compared with time course studies of ERK activation, which showed that ATP-stimulated ERK activity was detected at 1.5 min (Neary and Zhu, 1994), thereby indicating that PKCδ translocation occurs before ERK activation.
Fig. 4.
Extracellular ATP stimulates translocation of a calcium-independent PKC isoform. In A, primary rat astrocyte cultures were treated with or without (CON) 100 μm ATP for 15 sec, or with 100 nm TPA for 5 min, and particulate fractions were prepared as described in Materials and Methods. Equal amounts of protein (3.9 μg) were subjected to SDS-PAGE and analyzed by immunoblotting with monoclonal antibodies raised against PKCδ or PKCγ. In B, astrocytes were treated with 100 μm ATP for the times indicated, and particulate fractions were obtained. Equal amounts of protein within each experiment (ranging from 4 to 5 μg) were analyzed by immunoblotting with a monoclonal antibody raised against PKCδ. Values given are mean ± SEM; densitometric analysis (Bio-Rad GS-670) was conducted on a minimum of four to six independent experiments, each representing different culture seedings.
Signaling from P2Y receptors to ERK is dependent on hydrolysis of phosphatidylcholine
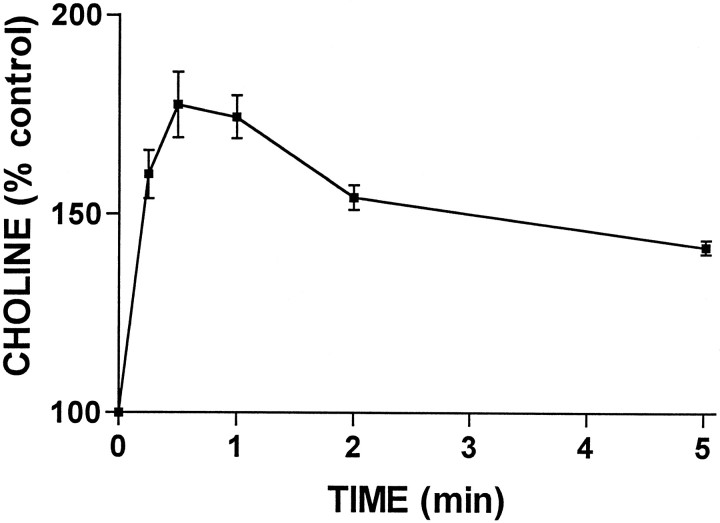
If P2Y receptors signal to ERK by a pathway that involves a calcium-independent PKC and is independent of PI hydrolysis, we reasoned that the diacylglycerol needed to activate PKC may come from the hydrolysis of PC. To test this, time course studies were conducted to examine the ability of ATP to stimulate PC hydrolysis. We found that treatment of cultures with ATP led to the production of choline, which was observed at 15 sec and peaked at 30 to 60 sec (Fig.5). This finding, together with a lack of increase in phosphocholine (data not shown), suggests that astrocytic P2Y receptors are coupled to phospholipase D (PLD) rather than PC-specific PLC. The time course of choline formation is consistent with the rapid translocation of PKCδ and subsequent activation of ERK.
Fig. 5.
Extracellular ATP rapidly stimulates choline formation. Primary rat astrocyte cultures were incubated with3H-choline (2.5 μCi/ml) for 48 hr followed by 24 hr in choline-free culture medium before treatment with ATP (100 μm) for the indicated times. Choline formation was measured as described in Materials and Methods. Similar results were obtained in two additional experiments.
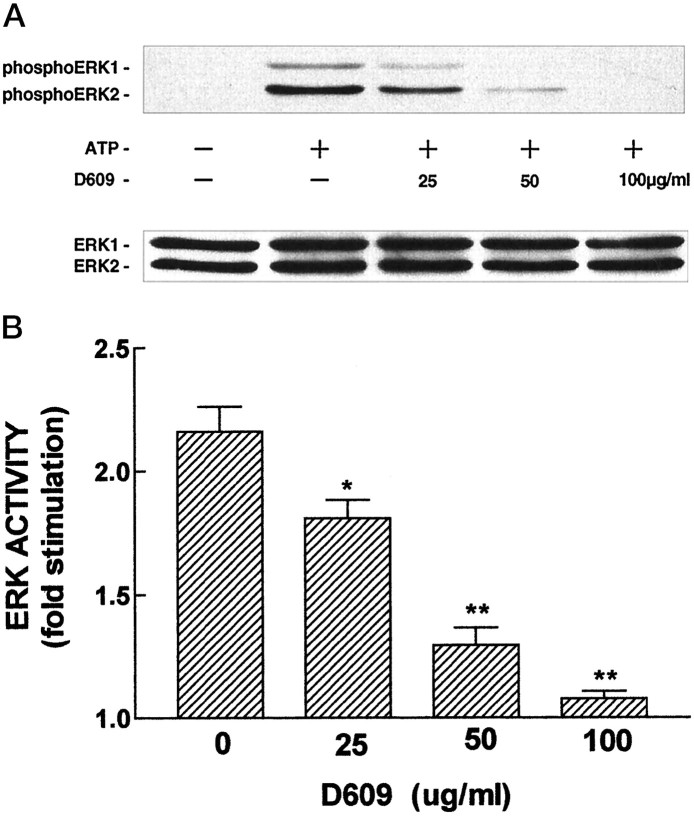
To determine whether PC hydrolysis is upstream of ERK activation, we used tricyclodecan-9-yl-xanthogenate (D609), which inhibits the hydrolysis of PC but not PI (Muller-Decker, 1989; Schutze et al., 1992;Cai et al., 1993; Inui et al., 1994; Kiss and Tomono, 1995; van Dijk et al., 1997). Cultures were treated with D609 for 60 min before stimulation by ATP (100 μm, 5 min). We found that D609 inhibited ATP-stimulated phosphorylation of ERK1 and ERK2 (Fig.6A) and ERK activity (Fig. 6B) in a dose-dependent manner. At 50 μg/ml D609, a concentration frequently used to inhibit PC hydrolysis and diacyglycerol formation in intact cells (Muller-Decker, 1989; Schutze et al., 1992; Inui et al., 1994; Kiss and Tomono, 1995), ATP-stimulated ERK activity was reduced by ∼75%, thereby indicating that signaling from P2Y receptors to ERK involves PC hydrolysis.
Fig. 6.
Inhibition of PC hydrolysis decreases the ability of ATP to stimulate ERK activity. Primary rat astrocytes were treated without or with the indicated concentrations of D609 for 60 min before addition of ATP (100 μm) for 5 min. In A, phosphorylated ERK1/ERK2 (top panel) and ERK1/ERK2 (bottom panel) were detected by immunoblotting as described in Materials and Methods. InB, ERK activity data (mean ± SEM) were obtained from three independent experiments, each representing different seedings and conducted with duplicate culture plates. “0” indicates cultures treated with ATP only. ERK activity in untreated cultures was 43.3 ± 4.9 pmol phosphate transferred per minute per milligram of protein. **p < 0.001; *p < 0.05 for comparisons of ATP versus D609 + ATP.
Signaling from P2Y receptors to ERK via pertussis toxin-sensitive and -insensitive G-proteins
The type(s) of G-proteins involved in the P2Y/ERK pathway was studied by using pertussis toxin; this agent inactivates α subunits of the Gi and Go families but does not affect Gs or Gq subunits (Yahame and Fung, 1993). Cultures were treated with 100 ng/ml pertussis toxin overnight (18–20 hr) before addition of ATP (100 μm) for 5 min. We found that pertussis toxin diminished the ability of ATP to stimulate ERK activity by 68 ± 3% (mean ± SEM; p < 0.0001; n = 8). These results suggest that coupling of P2Y receptors in astrocytes to the ERK/MAPK cascade is mainly via pertussis toxin-sensitive G-proteins.
Role of the ERK/MAPK cascade in mediating mitogenic signaling by P2Y receptors
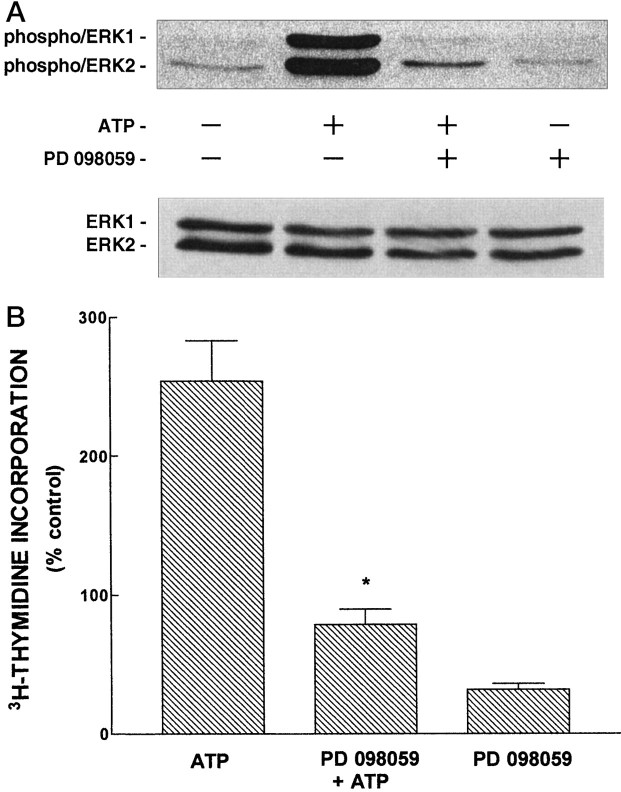
To determine whether the ERK/MAPK cascade is involved in extracellular ATP-induced mitogenesis, we used PD 098059 [2-(2′amino-3′methoxypheny)-oxanaphthalen-4-one], a selective inhibitor of MEK1 that was shown to inhibit growth factor-induced DNA synthesis without altering cell viability (Dudley et al., 1995). First, we examined the ability of PD 098059 to inhibit activation of ERK by extracellular ATP. Primary cultures of astrocytes were treated with PD 098059 (50 μm) for 30 min before addition of ATP (100 μm, 5 min), cellular lysates were subjected to SDS-PAGE, and phosphorylated ERK1 and ERK2 were detected by immunoblot analysis. As shown in Figure 7A, PD 098059 greatly reduced extracellular ATP-evoked phosphorylation of ERK1 and ERK2. ERK activity assays confirmed this finding (data not shown). We then investigated the ability of PD 098059 to inhibit ATP-induced mitogenic signaling. Primary cultures of astrocytes were treated with PD 098059 (50 μm) for 30 min before the addition of ATP (100 μm), and its effect on ATP-induced DNA synthesis was measured. As shown in Figure 7B, PD 098059 blocked the ability of ATP to stimulate DNA synthesis. Additional experiments demonstrated that 50 μm PD 098059 did not affect cellular viability as assessed by measuring protein synthesis (3H-leucine incorporation; data not shown). These results demonstrate that the ERK/MAPK cascade mediates mitogenic signaling by P2Y receptors.
Fig. 7.
Extracellular ATP-evoked mitogenic signaling is mediated by the ERK/MAPK cascade. In A, primary rat astrocyte cultures were treated with ATP (100 μm, 5 min), or with PD 098059 (50 μm) for 30 min before addition of ATP (100 μm, 5 min), cells were lysed, and lysates containing equivalent amounts of protein were subjected to SDS-PAGE. Immunoblots were probed with antibodies that recognize phosphorylated ERK1/ERK2 (top panel) or ERK1/ERK2 (bottom panel). In B, primary rat astrocyte cultures were treated with ATP (100 μm), or with PD 098059 (50 μm) for 30 min before addition of ATP, and DNA synthesis was determined as described in Materials and Methods. Values are given as the mean ± SE of the mean and were obtained from three independent experiments, each conducted in quadruplicate and with different seedings. 3H-thymidine incorporation in untreated cultures was 1055 ± 358 cpm per culture well. *p < 0.001 for comparison of ATP versus PD 098059 + ATP.
DISCUSSION
The main findings of the studies presented here are that (1) signaling from P2Y receptors to ERK is independent of the PI-PLC/calcium pathway and involves a calcium-independent PKC isoform as well as PC hydrolysis, which may be catalyzed by PLD, and (2) the ERK/MAPK cascade mediates mitogenic signaling by P2Y receptors.
Components of the signaling pathway from P2Y purinergic receptors to the ERK/MAPK cascade in primary cultures of rat cortical astrocytes
The role of PKC in GPCR/ERK signaling pathways has not been completely defined. In some cases, GPCRs can be coupled to ERK by both PKC-dependent and -independent pathways, thereby suggesting multiple signaling pathways from a specific receptor to ERK. For example, signaling from endothelin-1 receptors to ERK was reduced ∼50% by chronic phorbol ester treatment in mesangial cells (Wang et al., 1992), cardiac myocytes (Bogoyevitch et al., 1994), and astrocytes (Cazaubon et al., 1993). The studies presented here indicate that PKC is a crucial element of the pathway linking P2Y receptors to the ERK/MAPK cascade in primary cultures of cortical astrocytes because inhibition or downregulation of PKC reduced the ability of extracellular ATP to stimulate ERK activity by ∼90%. To our knowledge, these studies are the first to document in brain cells that PKC is upstream of ERK in this P2Y receptor signaling pathway.
It is well established that P2Y receptors in astrocytes are also coupled to PI-PLC (Pearce et al., 1989; Neary et al., 1991; Kastritsis et al., 1992; Salter and Hicks, 1994, 1995; King et al., 1996;Centemeri et al., 1997). Because signaling from P2Y receptors to ERK is dependent on PKC, this raised the possibility that P2Y receptors signal to ERK via PI-PLC. However, evidence presented here indicates that this is not the case. Inhibition of PI-PLC blocked the ability of extracellular ATP to stimulate formation of inositol phosphates but did not significantly reduce activation of ERK, thereby indicating that P2Y receptors are coupled independently to PI-PLC and ERK. Moreover, chelation of intracellular or extracellular calcium did not decrease the ability of extracellular ATP to activate ERK, thereby suggesting that calcium-dependent protein kinases such as Pyk2 (Lev et al., 1995) or calcium-dependent isoforms of PKC are not upstream of ERK. Consistent with this was the finding that Gö 6976, which preferentially inhibits calcium-dependent PKC isoforms (Martiny-Baron et al., 1993), was significantly less effective in reducing the ability of extracellular ATP to activate ERK than GF10290X, an inhibitor of both calcium-dependent and -independent PKC isoforms. A previous study using the PKC inhibitor CGP 41251, which displays selectivity for calcium-dependent PKC isoforms, implicated the involvement of a calcium-independent PKC isoform in signaling from P2Y receptors to ERK in renal mesangial cells, but this report did not examine PKC translocation induced by ATP (Huwiler and Pfeilschifter, 1994). Here we have found that a calcium-independent isoform, PKCδ, was rapidly translocated from the cytosol to the membrane on stimulation of P2Y receptors, and this translocation occurred before ERK activation. Although studies with antisense oligonucleotides or dominant negative mutants are needed to further establish the role of PKCδ, our findings in primary cells are in agreement with a recent report using COS cells in which overexpression of a constitutively active mutant of PKCδ was sufficient to activate MEK and ERK (Ueda et al., 1996). Overexpression of another calcium-dependent isoform, PKCε, or a calcium-dependent isoform, PKCα, did not activate MEK or ERK. In addition, PKCδ-evoked activation of MEK and ERK was not blocked by overexpression of an inactive mutant of Ras, thereby indicating that PKCδ can activate the ERK/MAPK cascade in a manner independent of Ras. Previous studies suggest that signaling from P2Y receptors to ERK in astrocytes does not involve Ras (Neary and Zhu, 1994; Neary, 1996).
Because a calcium-independent PKC isoform mediates signaling from P2Y receptors to the ERK/MAPK cascade in primary cultures of rat cortical astrocytes, we investigated the possibility that P2Y receptors are coupled to ERK by phospholipases that are not directly linked to calcium signaling and yet can generate diacylglycerol for PKC activation. PC-PLC and PLD are two such enzymes that have been implicated in mitogenesis (Cai et al., 1993; Boarder, 1994). Hydrolysis of PC by PC-PLC yields diacylglycerol, which can then activate PKC isoforms. Alternatively, PC hydrolysis by PLD generates choline and phosphatidic acid; the latter can be converted to diacylglycerol by phosphatidic acid phosphohydrolase. Because ATP stimulated a rapid formation of choline rather than phosphocholine, PLD rather than PC-PLC may catalyze PC hydrolysis after occupancy of P2Y receptors. Previous studies have reported that P2Y receptors are linked to PLD in astrocytes (Gustavsson et al., 1993) as well as endothelial cells (Martin and Michaelis, 1989; Pirotton et al., 1990; Purkiss and Boarder, 1992), promyelocytic leukemia cells (Xie et al., 1991), renal mesangial cells (Pfeilschifter and Merriweather, 1993), and canine kidney cells (Balboa et al., 1994). However, no information is available on the role of PLD-catalyzed PC hydrolysis in coupling P2Y receptors to the ERK/MAPK cascade.
To determine the involvement of PC hydrolysis in signaling from P2Y receptors to ERK, we used an inhibitor of PC hydrolysis and diacylglycerol production, D609 (Muller-Decker, 1989; Schutze et al., 1992; Cai et al., 1993; Inui et al., 1994; Kiss and Tomono, 1995; van Dijk et al., 1997). Studies with this inhibitor have shown that PC hydrolysis is upstream of the ERK/MAPK cascade in some signaling systems. For example, in NIH 3T3 cells, D609 inhibited EGF-, TPA-, and serum-evoked phosphorylation of Raf (Cai et al., 1993). In Rat-1 fibroblasts, D609 inhibited PDGF-induced activation of ERK, but EGF-induced activation of ERK was unaffected by D609 (van Dijk et al., 1997). Expression of the G-protein-coupled 5-HT1A receptor in Chinese hamster ovary cells promoted activation of ERK2, and this activation was partially inhibited by D609 (Cowen et al., 1996). As reported here, we found that D609 inhibited extracellular ATP stimulation of ERK activity, thereby indicating for the first time that PC hydrolysis plays an important role in coupling P2Y receptors to the ERK/MAPK cascade.
Inhibition of the ATP-evoked activation of ERK/MAPK by pertussis toxin suggests that the response is mediated at least in part by Gα subunits such as Gi or Go, which are sensitive to pertussis toxin. By contrast, previous work has shown that pertussis toxin does not affect ATP-evoked mobilization of intracellular calcium in astrocytes from rat cerebral cortex (Bruner and Murphy, 1993a,b) and spinal cord (Ho et al., 1995; Salter and Hicks, 1995), thereby indicating that signaling from P2Y receptors to PI-PLC involves a G-protein insensitive to pertussis toxin, such as Gq/G11 subclass, which lacks a site for ADP ribosylation. Our finding that the coupling of P2Y receptors to the ERK/MAPK cascade is not dependent on PI-PLC is consistent with these results because if PI-PLC were involved in signaling from P2Y receptors to ERK, pertussis toxin would not be expected to diminish the ability of ATP to activate ERK. Instead, we found that signaling from P2Y receptors to ERK was reduced by 65–70%, thereby suggesting the involvement of a pertussis toxin-sensitive G-protein. Interestingly, pertussis toxin did not inhibit hypo-osmotic activation of ERK in astrocytes; in this case, ERK activation was dependent on calcium but independent of PKC (Schliess et al., 1996). Thus, hypo-osmotic signaling to the ERK/MAPK cascade in astrocytes differs markedly from the P2Y/ERK pathway, because in the latter case our findings indicate that signaling is dependent on PKC, independent of calcium, and partially blocked by pertussis toxin. The pertussis toxin-sensitive protein involved in signaling from P2Y receptors to the ERK/MAPK cascade may be Gi or Go. On the basis of the findings of van Biesen et al. (1996a), we speculate that Gois more likely to be implicated because coupling of GPCRs to the ERK/MAPK cascade by Gi involved a protein tyrosine kinase but not PKC, whereas coupling of GPCRs to the ERK/MAPK cascade via PKC involved a Go protein.
As demonstrated here, signaling from P2Y receptors to the ERK cascade in astrocytes is independent of the PI-PLC/calcium pathway. Activation of signaling pathways by GPCRs may depend on the type of G-protein that links receptors to specific effectors, e.g., P2Y receptors may be coupled to PI-PLC via Gq and to ERK signaling via Go or Gi. Another possibility is that because four subtypes of P2Y receptors, P2Y1, P2Y2, P2Y4, and P2Y6, have been cloned from rat tissues, ERK and PI-PLC pathways could be activated by separate subtypes. The P2Y receptor agonists ATP, UTP, and 2-methylthio-ATP (2-MeSATP) stimulate both calcium mobilization and ERK activation in rat cortical astrocytes (King et al., 1996). This suggests that receptor subtypes such as P2Y1 (2-MeSATP- and ATP-preferring), P2Y2 and P2Y4 (ATP- and UTP-preferring), and P2Y6(UTP-preferring) may be coupled to both pathways. However, further studies are needed to determine the array of P2Y receptor subtypes expressed in astrocytes and their linkage to specific signaling pathways. Such efforts would be aided by the development of highly selective agonists and antagonists to distinguish between endogenous P2Y receptor subtypes.
The ERK/MAPK cascade mediates mitogenic signaling by ATP/P2Y receptors in astrocytes
Recent studies have demonstrated the importance of the ERK/MAPK cascade in cellular proliferation and differentiation (for review, seeMarshall, 1995; Seger and Krebs, 1995). Much of the evidence for the critical role of the ERK/MAPK cascade in cell growth has come from experiments in which active or inactive mutants of members of the cascade or upstream signaling elements have been overexpressed in transformed cells. This information can then be used as a basis to investigate the role of the ERK/MAPK cascade in mitogenic signaling from specific receptors in nontransformed, primary cultures as well as the components of the pathway that link the receptors to the cascade. Extracellular ATP acts as a mitogenic signal in primary cultures of cerebral cortical astrocytes from newborn rats (Neary et al., 1994a,b). Because ATP also stimulates ERK activity in these cells (Neary and Zhu, 1994; King et al., 1996), this suggests that ERK is involved in ATP-induced mitogenesis. However, in addition to phosphorylating and activating transcription factors involved in gene expression needed for proliferation and differentiation, ERK has other targets, including proteins in the cytoplasm, membrane, and cytoskeleton; therefore, ERK may have other roles besides regulating cell growth (Seger and Krebs, 1995). For example, by phosphorylating myosin light-chain kinase, ERK can regulate cell motility by a pathway independent of gene transcription (Klemke et al., 1997). Thus, the stimulation of ERK by extracellular ATP does not provide sufficient evidence to conclude that ERK mediates mitogenic signaling by ATP/P2Y receptors in astrocytes. To investigate this question, we used PD 098059, an inhibitor of the ERK activator MEK1 (Dudley et al., 1995). PD 098059 has been shown to block ERK stimulation and to inhibit growth factor-induced proliferation in Swiss 3T3 mouse fibroblasts and rat kidney cells at concentrations that were not cytotoxic. PD 098059 is highly selective for MEK1, as evidenced by its failure to inhibit 18 other serine/threonine protein kinases in vitro and in vivo, including the ERK homolog Jun N-terminal kinase (also known as stress-activated protein kinase) (Alessi et al., 1995). If the ATP-evoked activation of ERK and the ATP-induced mitogenic response are causally related, PD 098059 should block the ability of ATP to stimulate DNA synthesis. Indeed, this was observed (Fig. 7B), thereby indicating the importance of the ERK/MAPK cascade in mediating mitogenic signaling by ATP receptors in astrocytes.
Footnotes
This work was supported by the Department of Veterans Affairs.
Correspondence should be addressed to Dr. J. T. Neary, Research Service 151, Veterans Affairs Medical Center, 1201 NW 16th Street, Miami, FL 33125.
REFERENCES
- 1.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 2.Abbracchio MP, Saffrey MJ, Hopker V, Burnstock G. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience. 1994;59:67–76. doi: 10.1016/0306-4522(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 3.Abbracchio MP, Ceruti S, Langfelder R, Cattabeni F, Saffrey MJ, Burnstock G. Effects of ATP analogues and basic fibroblast growth factor on astroglial cell differentiation in primary cultures of rat striatum. Int J Dev Neurosci. 1995;13:685–693. doi: 10.1016/0736-5748(95)00064-x. [DOI] [PubMed] [Google Scholar]
- 4.Ahn NG, Weie JE, Chan CP, Krebs EG. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990;265:11487–11494. [PubMed] [Google Scholar]
- 5.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 6.Avruch J, Zhang X-F, Kyriakis JM. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 7.Balboa MA, Firestein BL, Godson C, Bell KS, Insel PA. Protein kinase Cα mediates phospholipase D activation by nucleotides and phorbol ester in Madin-Darby canine kidney cells. J Biol Chem. 1994;269:10511–10516. [PubMed] [Google Scholar]
- 8.Bender AS, Neary JT, Norenberg MD. Role of phosphoinositide hydrolysis in astrocyte volume regulation. J Neurochem. 1993;61:1506–1514. doi: 10.1111/j.1471-4159.1993.tb13646.x. [DOI] [PubMed] [Google Scholar]
- 9.Bleasdale JE, Thaker R, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neurotrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 10.Boarder MR. A role for phospholipase D in control of mitogenesis. Trends Pharmacol Sci. 1994;15:57–62. doi: 10.1016/0165-6147(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 11.Bogoyevitch MA, Glennon PE, Andersson MB, Clerk A, Lazou A, Marshall CJ, Parker PJ, Sugden PH. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase cascade in cardiac myocytes. J Biol Chem. 1994;269:1110–1119. [PubMed] [Google Scholar]
- 12.Bruner G, Murphy S. Purinergic P2Y receptors on astrocytes are directly coupled to phospholipase A2. Glia. 1993a;7:219–224. doi: 10.1002/glia.440070305. [DOI] [PubMed] [Google Scholar]
- 13.Bruner G, Murphy S. UTP activates multiple second messenger systems in cultured rat astrocytes. Neurosci Lett. 1993b;162:105–108. doi: 10.1016/0304-3940(93)90571-2. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G. A basis for distinguishing two types of purinergic receptors. In: Bolis L, Straub RW, editors. Cell and membrane receptors for drugs and hormones: a multidisciplinary approach. Raven; New York: 1978. pp. 107–118. [Google Scholar]
- 15.Cai H, Erhardt P, Troppmair J, Diaz-Meco MT, Sithanandam G, Rapp UR, Moscat J, Cooper GM. Hydrolysis of phosphatidylcholine couples Ras to activation of Raf protein kinase during mitogenic signal transduction. Mol Cell Biol. 1993;13:7645–7651. doi: 10.1128/mcb.13.12.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cazaubon S, Parker PJ, Strosberg AD, Couraud P-O. Endothelins stimulate tyrosine phosphorylation and activity of p42/mitogen-activated protein kinase in astrocytes. Biochem J. 1993;293:381–386. doi: 10.1042/bj2930381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centemeri C, Bolego C, Abbracchio MP, Cattabeni F, Puglisi L, Burnstock G, Nicosia S. Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br J Pharmacol. 1997;121:1700–1706. doi: 10.1038/sj.bjp.0701293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowen DS, Sowers RS, Manning DR. Activation of a mitogen-activated protein kinase (ERK2) by the 5-hydroxytryptamine 1A receptor is sensitive not only to inhibitors of phosphatidylinositol 3-kinase but to an inhibitor of phosphatidylcholine hydrolysis. J Biol Chem. 1996;271:22297–22300. doi: 10.1074/jbc.271.37.22297. [DOI] [PubMed] [Google Scholar]
- 19.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 21.Eng LF. Regulation of glial intermediate filaments in astrogliosis. In: Norenberg MD, Hertz L, Schousboe A, editors. The biochemical pathology of astrocytes. Alan R. Liss; New York: 1988. pp. 79–90. [Google Scholar]
- 22.Gott AL, Mallon BS, Paton A, Groome N, Rumsby MG. Rat brain glial cells in primary culture and subculture contain the delta, epsilon,and zeta subspecies of protein kinase C as well as the conventional subspecies. Neurosci Lett. 1994;171:117–120. doi: 10.1016/0304-3940(94)90619-x. [DOI] [PubMed] [Google Scholar]
- 23.Gustavsson L, Lundqvist C, Hansson E. Receptor-mediated phospholipase D activity in primary astroglial cultures. Glia. 1993;8:249–255. doi: 10.1002/glia.440080405. [DOI] [PubMed] [Google Scholar]
- 24.Harms L, Finta EP, Tschopl M, Illes P. Depolarization of rat locus coeruleus neurons by adenosine 5′-triphosphate. Neuroscience. 1992;48:941–952. doi: 10.1016/0306-4522(92)90282-7. [DOI] [PubMed] [Google Scholar]
- 25.Ho C, Hicks J, Salter MW. A novel P2-purinoceptor expressed by a subpopulation of astrocytes from the dorsal spinal cord of the rat. Br J Pharmacol. 1995;116:2909–2918. doi: 10.1111/j.1476-5381.1995.tb15944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huwiler A, Pfeilschifter J. Stimulation by extracellular ATP and UTP of the mitogen-activated protein kinase cascade and proliferation of rat renal mesangial cells. Br J Pharmacol. 1994;113:1455–1463. doi: 10.1111/j.1476-5381.1994.tb17160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inui H, Kitami Y, Tani M, Kondo T, Inagami T. Differences in signal transduction between platelet-derived growth factor (PDGF) α and β receptors in vascular smooth muscle cells: PDGF-BB is a potent mitogen, but PDGF-AA promotes only protein synthesis without activation of DNA synthesis. J Biol Chem. 1994;269:30546–30552. [PubMed] [Google Scholar]
- 28.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 29.Kastritsis CH, Salm AK, McCarthy K. Stimulation of the P2y purinergic receptor on type 1 astroglia results in inositol phosphate formation and calcium mobilization. J Neurochem. 1992;58:1277–1284. doi: 10.1111/j.1471-4159.1992.tb11339.x. [DOI] [PubMed] [Google Scholar]
- 30.King BF, Neary JT, Zhu Q, Wang S, Norenberg MD, Burnstock G. P2 purinoceptors in rat cortical astrocytes: expression, calcium-imaging and signalling studies. Neuroscience. 1996;74:1187–1196. doi: 10.1016/0306-4522(96)00209-6. [DOI] [PubMed] [Google Scholar]
- 31.Kiss Z, Tomono M. Compound D609 inhibits phorbol ester-stimulated phospholipase D activity and phospholipase C-mediated phosphatidylethanolaminehydrolysis. Biochim Biophys Acta. 1995;1259:105–108. doi: 10.1016/0005-2760(95)00148-6. [DOI] [PubMed] [Google Scholar]
- 32.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 35.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 36.Martin TW, Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989;264:8847–8856. [PubMed] [Google Scholar]
- 37.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 38.Muller-Decker K. Interruption of TPA-induced signals by an antiviral and antitumoral xanthate compound: inhibition of a phospholipase C-type reaction. Biochem Biophys Res Commun. 1989;162:198–205. doi: 10.1016/0006-291x(89)91981-5. [DOI] [PubMed] [Google Scholar]
- 39.Neary JT. Trophic actions of extracellular ATP on astrocytes, synergistic interactions with fibroblast growth factors, and underlying signal transduction mechanisms. Ciba Found Symp. 1996;198:130–141. doi: 10.1002/9780470514900.ch7. [DOI] [PubMed] [Google Scholar]
- 40.Neary JT. MAPK cascades in cell growth and death. News Physiol Sci. 1997;12:286–293. [Google Scholar]
- 41.Neary JT, Zhu Q. Signaling by ATP receptors in astrocytes. NeuroReport. 1994;5:1617–1620. doi: 10.1097/00001756-199408150-00019. [DOI] [PubMed] [Google Scholar]
- 42.Neary JT, Norenberg LOB, Norenberg MD. Protein kinase C in primary astrocyte cultures: cytoplasmic localization and translocation by a phorbol ester. J Neurochem. 1988;50:1179–1184. doi: 10.1111/j.1471-4159.1988.tb10590.x. [DOI] [PubMed] [Google Scholar]
- 43.Neary JT, Laskey R, van Breemen C, Blicharska J, Norenberg LOB, Norenberg MD. ATP-evoked calcium signal stimulates protein phosphorylation/dephosphorylationinastrocytes. Brain Res. 1991;566:89–94. doi: 10.1016/0006-8993(91)91684-s. [DOI] [PubMed] [Google Scholar]
- 44.Neary JT, Whittemore SR, Zhu Q, Norenberg MD. Synergistic activation of DNA synthesis in astrocytes by fibroblast growth factor and extracellular ATP. J Neurochem. 1994a;63:490–494. doi: 10.1046/j.1471-4159.1994.63020490.x. [DOI] [PubMed] [Google Scholar]
- 45.Neary JT, Baker L, Jorgensen SL, Norenberg MD. Extracellular ATP induces stellation and increases GFAP content and DNA synthesis in primary astrocyte cultures. Acta Neuropathol. 1994b;87:8–13. doi: 10.1007/BF00386249. [DOI] [PubMed] [Google Scholar]
- 46.Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- 47.Pearce B, Murphy S, Jeremy J, Morrow C, Dandona P. ATP-evoked Ca2+ mobilization and prostanoid release from astrocytes: P2-purinergic receptors linked to phosphoinositide hydrolysis. J Neurochem. 1989;52:971–977. doi: 10.1111/j.1471-4159.1989.tb02549.x. [DOI] [PubMed] [Google Scholar]
- 48.Peterson GL. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- 49.Pfeilschifter J, Merriweather C. Extracellular ATP and UTP activation of phospholipase D is mediated by protein kinase C-ε in rat renal mesangial cells. Br J Pharmacol. 1993;110:847–853. doi: 10.1111/j.1476-5381.1993.tb13890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pirotton S, Robaye B, Lagneau C, Boeynaems J-M. Adenine nucleotides modulate phosphatidylcholine metabolism in aortic endothelial cells. J Cell Physiol. 1990;142:449–457. doi: 10.1002/jcp.1041420303. [DOI] [PubMed] [Google Scholar]
- 51.Purkiss JR, Boarder MR. Stimulation of phosphatidate synthesis in endothelial cells in response to P2-receptor activation. Biochem J. 1992;287:31–36. doi: 10.1042/bj2870031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rathbone MP, Middlemiss PJ, Kim J-L, Gysbers JW, DeForge SP, Smith RW, Hughes DW. Adenosine and its nucleotides stimulate proliferation of chick astrocytes and human astrocytoma cells. Neurosci Res. 1992;13:1–17. doi: 10.1016/0168-0102(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 53.Salter MW, Hicks JL. ATP-evoked increases in intracellular calcium in neurons and glia from the dorsal spinal cord. J Neurosci. 1994;14:1563–1575. doi: 10.1523/JNEUROSCI.14-03-01563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salter MW, Hicks JL. ATP causes release of intracellular Ca2+ via the phospholipase Cβ/IP3 pathway in astrocytes from the dorsal spinal cord. J Neurosci. 1995;15:2961–2967. doi: 10.1523/JNEUROSCI.15-04-02961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schliess F, Sinning R, Fischer R, Schmalenbach C, Haussinger D. Calcium-dependent activation of Erk-1 and Erk-2 after hypo-osmotic astrocyte swelling. Biochem J. 1996;320:167–171. doi: 10.1042/bj3200167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kB by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 57.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 58.Sevetson BR, Kong X, Lawrence JC., Jr Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1993;90:10305–10309. doi: 10.1073/pnas.90.21.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen KZ, North RA. Excitation of rat locus coeruleus neurons by adenosine 5′-triphosphate: ionic mechanism and receptor characterization. J Neurosci. 1993;13:894–899. doi: 10.1523/JNEUROSCI.13-03-00894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueda Y, Hirai S, Osada S, Suzuki A. Protein Kinase C δ activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 61.van Biesen T, Hawes BE, Raymond JR, Luttrell LM, Koch WJ, Lefkowitz RJ. Go-protein alpha-subunits activate mitogen-activated protein kinase via a novel protein kinase C-dependent mechanism. J Biol Chem. 1996a;271:1266–1269. doi: 10.1074/jbc.271.3.1266. [DOI] [PubMed] [Google Scholar]
- 62.van Biesen T, Luttrell LM, Hawes BE, Lefkowitz RJ. Mitogenic signaling via G protein-coupled receptors. Endocr Rev. 1996b;17:698–714. doi: 10.1210/edrv-17-6-698. [DOI] [PubMed] [Google Scholar]
- 63.van Dijk MCM, Muriana FJG, de Widt J, Hilkmann H, van Blitterswijk WJ. Involvement of phosphatidylcholine-specific phospholipase C in platelet-derived growth factor-induced activation of the mitogen-activated protein kinase pathway in rat-1 fibroblasts. J Biol Chem. 1997;272:11011–11016. doi: 10.1074/jbc.272.17.11011. [DOI] [PubMed] [Google Scholar]
- 64.Vance DE, Trip E, Paddon HB. Poliovirus increases phosphatidylcholine biosynthesis in HeLa cells by stimulation of the rate-limiting reaction catalyzed by CTP:phosphocholine cytidylytransferase. J Biol Chem. 1980;255:1064–1069. [PubMed] [Google Scholar]
- 65.Wang Y, Simonson MS, Pouyssegur J, Dunn MJ. Endothelin rapidly stimulates mitogen-activated protein kinase activity in rat mesangial cells. Biochem J. 1992;287:589–594. doi: 10.1042/bj2870589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie M, Jacobs LS, Dubyak GR. Regulation of phospholipase D and primary granule secretion by P2-purinergic- and chemotactic peptide-receptor agonists is induced during granulocytic differentiation of HL-60 cells. J Clin Invest. 1991;88:45–54. doi: 10.1172/JCI115303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yahame HK, Fung BKK. Covalent modifications of G proteins. Annu Rev Pharmacol Toxicol. 1993;33:201–241. doi: 10.1146/annurev.pa.33.040193.001221. [DOI] [PubMed] [Google Scholar]