Abstract
Synapses are critical sites of information transfer in the nervous system, and it is important that their functionality be maintained under stressful conditions to prevent communication breakdown. Here we show that synaptic transmission at the Drosophila larval neuromuscular junction is protected by prior exposure to heat shock that strongly induces expression of heat shock proteins, in particular hsp70. Using a macropatch electrode to record synaptic activity at individual, visualized boutons, we found that prior heat shock sustains synaptic performance at high test temperatures through pre- and postsynaptic alterations. After heat shock, nerve impulses release more quantal units at high temperatures and exhibit fewer failures of release (presynaptic modification), whereas the amplitude of quantal currents remains more constant than does that in nonheat-shocked controls (postsynaptic modification). The time course of these physiological changes is similar to that of elevated hsp70. Thus, stress-induced neuroprotective mechanisms maintain function at synapses by modifying their properties.
Keywords: Drosophila, heat shock proteins, quanta, thermal stress, presynaptic, postsynaptic, neuromuscular
Prior exposure to high temperatures (heat shock) induces expression of heat shock proteins (hsps) and protects tissues and organisms from injury and death because of subsequent exposure to normally lethal temperatures and other forms of stress (Morimoto et al., 1994). Nervous tissue is no exception to this general rule, and it is well established that the heat shock response can protect nervous systems against subsequent insults (Mailhos et al., 1993; Mayer and Brown, 1994; Fink et al., 1997; Yenari et al., 1998). Protective effects of a prior heat shock treatment have been noted in neural cells grown in tissue culture (Lowenstein et al., 1991; Rordorf et al., 1991). Other studies have reported protective effects of brief heat shock in the intact nervous system. For example, previous heat shock at 42°C protects rat embryos from developmental neural defects that are normally caused by heat shock at 43°C (Walsh et al., 1987,1989). Prior heat shock also protects retinal photoreceptors from degeneration induced by bright light (Barbe et al., 1988); the time course of hsp70 induction parallels the time course of the protective effect (Tytell et al., 1994).
An intriguing question is whether prior heat shock protects critical neural processes such as synaptic function from subsequent stress. The neurophysiological consequences of heat shock have not been thoroughly investigated, so it is not known how neuronal communication might be modified and protected against stressful conditions. UsingDrosophila, an organism that lends itself to genetic and molecular manipulation, we have investigated whether heat shock, sufficient to induce robust expression of heat shock proteins, affects temperature sensitivity of synaptic transmission at the larval neuromuscular junction.
The heat shock response and subsequent thermotolerance were first described in Drosophila (Ritossa, 1962), and it has been amply demonstrated that these phenomena are truly adaptive in promoting survival and consequent reproductive success in this organism’s ecological niche of necrotic fruit on summer days (Feder et al., 1996;Feder and Krebs, 1997, 1998). In Drosophila, synthesis of most cellular proteins is down-regulated during thermal stress, but the predominant heat shock protein hsp70 is rapidly induced and plays a major role in protective mechanisms (Parsell and Lindquist, 1993; Feder et al., 1996). There is evidence in the locust that heat shock protects the neural circuitry controlling flight rhythm and protects synaptic delay at one of the synapses in this circuitry (Dawson-Scully and Robertson, 1998), suggesting that synapses may be affected by prior heat shock. To ascertain whether neuroprotective effects of heat shock influence presynaptic and postsynaptic events of synaptic transmission, we studied synaptic transmission and hsp70 inDrosophila.
The Drosophila larval neuromuscular junction has become one of the premier preparations for investigations of synaptic transmission (Restifo and White, 1990; Keshishian et al., 1996). Application of the macropatch recording technique (Dudel, 1981) at individual, visualized boutons allows inferences to be made about pre- or postsynaptic changes that alter synaptic strength or reliability (Mallart et al., 1991;Cooper et al., 1995; Stewart et al., 1996). Here, we show that prior heat shock increases the percentage of neuromuscular junctions functional at higher test temperatures and sustains a higher quantal content of transmission, indicating presynaptic effects. Quantal size increases with temperature in control preparations but remains more constant in heat-shocked preparations, indicating postsynaptic stabilization. Thus, heat shock has both pre- and postsynaptic protective and stabilizing effects on synaptic transmission, paralleling a robust induction of hsp70.
MATERIALS AND METHODS
Animals. Drosophila melanogaster wandering third-instar larvae of the Canton-S strain reared on cornmeal medium at 25°C (60–70% relative humidity) were used (Atwood et al., 1993). Physiological comparisons were made between control larvae and heat-shocked larvae. The latter, in standard Petri dishes containing filter paper moistened with phosphate-buffered solution and taped shut to preserve high humidity, were exposed to an elevated temperature of 36°C for 1 hr. Larvae were then allowed to recover at 25°C for 1/2 hr before experimentation.
Western blot analysis of heat shock proteins. Control and heat-shocked third-instar larvae at the appropriate time points were collected, quick frozen on dry ice, and stored at −70°C. Groups of two to three larvae were homogenized in 100 μl of 0.32 msucrose in 1.5 ml microfuge tubes with 20 passes with a fitted Teflon pestle. Protein concentrations were determined using the Bio-Rad (Hercules, CA) protein assay. Aliquots of 50 μg of protein were solubilized by boiling for 5 min with an equal volume of dissociation buffer (8 m urea, 2% SDS, 2% β-mercaptoethanol, and 20% glycerol). PAGE was performed in the presence of SDS on 10% gels with a 5% stacking gel using the discontinuous buffer system of Laemmli (1970). The proteins were transferred onto nitrocellulose membranes for 16–18 hr in a solution of 50 mm boric acid, 4 mm β-mercaptoethanol, and 2 mm EDTA, at 400 nA. Blots were stained with Ponceau S to check for equal loading of protein in all lanes.
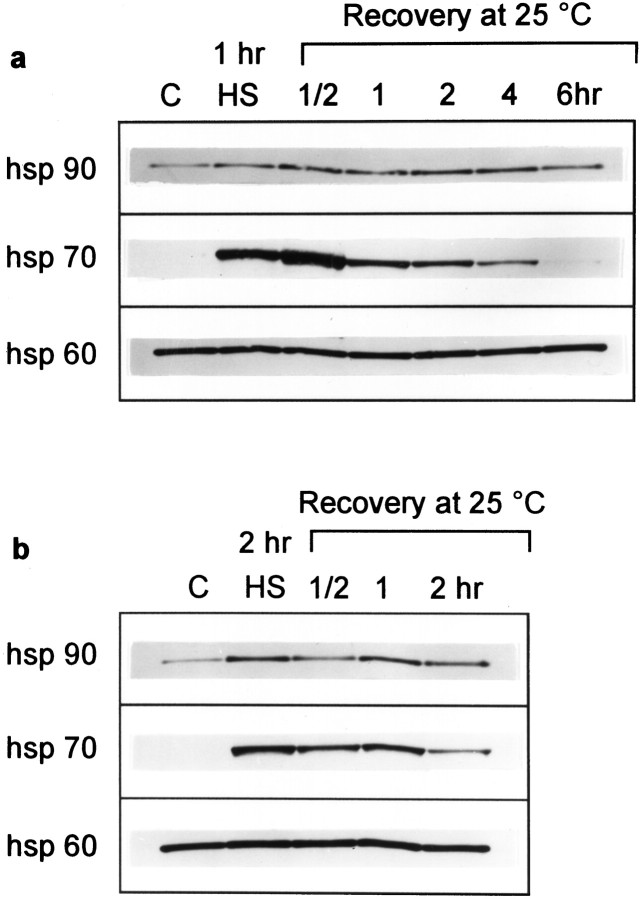
For Western analysis of hsp70 protein, the blots were washed for 10 min in 0.1 m PBS, pH 7.4, blocked for 2 hr in 5% milk powder and PBS, and then incubated overnight in primary antibody diluted 1:10,000 (Drosophila hsp70-specific monoclonal antibody 7FB; gift from Dr. S. Lindquist). Blots were then washed three times for 10 min each in PBS plus 0.1% Tween 20, incubated for 1 hr in secondary antibody diluted 1:20,000 (rat IgG adsorbed with human IgG), and then washed three times for 5 min each in PBS plus 0.3% Tween 20, followed by three times for 5 min each in PBS plus 0.1% Tween 20. Immunoreactive bands were visualized by use of enhanced chemiluminescence (ECL) Western blotting detection reagents (RPN 2106; Amersham, Arlington Heights, IL). Blots were stripped of the hsp70 signal by washing three times for 15 min each with 100 mmsodium citrate, pH 3.5, and were reprobed to detect hsp90 (also known as Drosophila hsp83) and subsequently hsp60. Blots were first washed four times for 5 min each in Tris-buffered saline with Tween (TBST; 0.25 m NaCl, 0.05% Tween 20, and 10 mm Tris, pH 7.5), blocked for 2 hr in 5% milk powder and TBST, and then incubated overnight in primary antibody diluted 1:5000 (monoclonal 29A anti-hsp90 antibody; gift from Dr. A. C. Wikstrom;Akner et al., 1992). Blots were then washed four times for 10 min each in TBST plus 1% BSA, incubated for 2 hr in secondary antibody diluted 1:5000 (anti-mouse IgG), washed six times for 5 min each in TBST plus 1% BSA, and processed for ECL analysis as described above. Blots were then stripped and reprobed with monoclonal anti-hsp60 antibody diluted 1:20,000 (gift from Dr. R. Gupta) using the method described for the hsp90 antibody. Western blot data (see Fig. 1) representative of four sets of Drosophila larvae are shown.
Fig. 1.
Transient expression of hsp70 inDrosophila larvae after heat shock. Whole wandering third-instar larvae were exposed to either a 1 or 2 hr heat shock at 36°C (a, b, respectively) and then placed at a recovery temperature of 25°C for the indicated number of hours. Whole-body lysates of the larvae were analyzed by Western blotting to detect hsp90, hsp70, and hsp60 (50 μg of protein loaded per lane). The recovery interval at 25°C following heat shock is indicated in hours. C, Control larvae raised at 25°C; HS, larvae exposed to heat shock at 36°C for either 1 or 2 hr.
Physiological measurements. Larvae were dissected to remove internal organs and to expose the nervous system and body-wall muscles, as in previous work (Atwood et al., 1993; Stewart et al., 1994). Electrophysiological recordings were made from muscle 6 of segment 3. The standard hemolymph-like solution (HL3) was used, with the following ionic composition (in mm): Na+, 70.0; K+, 5.0; Ca2+, 1.0; Mg2+, 20.0; NaHCO3, 10.0; Trehalose, 5.0; sucrose, 115.0; andN,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid, 5.0 (Stewart et al., 1994).
Experiments were conducted over a range of test temperatures (22, 27, 31, and 35°C). The temperature of the preparation was changed by superfusing it with solution passed over a heating coil before the solution entered the preparation dish. Once at the desired temperature, the preparation was equilibrated for 4 min before measurements were taken.
The preparation was viewed with a 40× water immersion lens (numerical aperture, 0.55) and Nomarski optics. Live nerve terminals were viewed through a low-light intensity television camera (Panasonic WV-BP310, mounted on the microscope) and displayed on the monitor screen of a computer (Apple Power Macintosh 7500/100) using the built-in frame grabber.
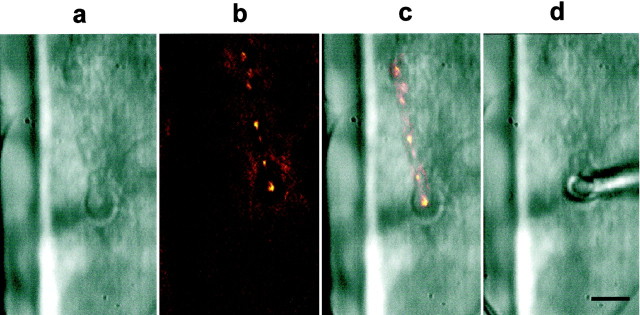
Individual boutons of type Ib (Atwood et al., 1993) were selected visually for focal macropatch recordings of synaptic currents. To facilitate identification of boutons, we incubated the preparation for 45 sec in the mitochondrial dye 3,3′-diethyloxadicarbocyanine iodide (DiOC2(5)), at a concentration of 0.3 μm in HL3 saline (Lavidis and Bennett, 1992). The preparation was then thoroughly rinsed with HL3 saline and viewed under epifluorescence. Live images from the low-light intensity television camera were viewed to locate suitable regions where the boutons were well separated and distinguishable. Four images of that region were then averaged with NIH Image software (NIH, Bethesda, MD) to improve the quality of the final image (see Fig. 2). The same field was again viewed under Nomarski optics; identifying landmarks and the position of the boutons were sketched onto an overhead transparency film attached to the computer monitor screen to aid in electrode placement and repositioning.
Fig. 2.
Macropatch recordings from individual, visualized synaptic boutons of the larval neuromuscular junction. Placement of the focal macropatch electrode over individual Ib boutons at the larval neuromuscular junction was under visual control to record synaptic currents generated at that site. a, Strings of Ib and Is boutons can be seen innervating the surface of muscle 6 under Nomarski optics. b, Under DiOC2(5) fluorescence, the same string of Ib boutons can be seen clearly, and the surrounding subsynaptic reticulum (SSR) does not fluoresce; however, fluorescence appears where the SSR meets the muscle. c, Overlay of the fluorescence image on the Nomarski image is shown.d, The focal macropatch electrode is gently placed over the chosen Ib bouton. The bouton can be seen through the lumen of the electrode. At this concentration of DiOC2(5) and exposure to illumination for fluorescence, synaptic transmission was unaffected compared with that in controls, and electron microscopy revealed no obvious damage to synaptic structure (S. Karunanithi, L. Marin, and H. L. Atwood, unpublished observations). Scale bar, 10 μm.
The focal macropatch electrodes (tip diameter, ∼5 μm) were manufactured as described previously (Stewart et al., 1994) and filled with HL3 solution. The diameters of the tip openings were selected to enclose the chosen bouton, minimizing direct pressure on it (see Fig.2). Signals from the focal macropatch electrode were amplified using the Axoclamp-2A amplifier (Axon Instruments, Foster City, CA) under bridge mode. The signals, which indicate the time course and relative amplitude of current flow into the postsynaptic membrane, were recorded as the extracellular voltage drop at the tip of the macropatch electrode. Seal resistance was monitored and remained constant during each experiment. The MacLab/4S data acquisition system (AD Instruments) was used to record the electrical signals with the same computer used simultaneously for visualization. Data points were sampled at 40 kHz; 300 excitatory junctional extracellular currents (EJCs; at 1 Hz stimulation) and 25–50 miniature EJCs (mEJCs) were collected at each test temperature for every experiment.
Data measurement and evaluation. The MacLab data files were converted to Igor Pro files for analysis with subroutines written for the Igor Pro 3 software analysis package (Wavemetrics) using standard methods described previously (Sayer et al., 1989; Karunanithi et al., 1995). Data for statistical comparisons were tested for normality and equal variance, and appropriate parametric and nonparametric tests were applied using commercial software (Sigmastat; Jandel Scientific, Corte Madera, CA). Significance was assessed at p < 0.05.
RESULTS
Transient expression of hsp70 protein in Drosophilalarvae following heat shock
The induction profile of hsp70 protein was determined in whole wandering third-instar Drosophila larvae exposed to a 36°C heat shock for either 1 or 2 hr (Fig.1a,b, respectively). Whole-body lysates of control larvae raised at 25°C showed no detectable hsp70 in the absence of heat stress (Fig. 1,lane C). After a 1 hr heat shock treatment at 36°C, a robust induction of hsp70 was apparent (Fig. 1a, lane HS). Larvae were then placed at a recovery temperature of 25°C, and a further increase in hsp70 was noted at the 1/2 hr recovery point (Fig. 1a). Thereafter hsp70 rapidly declined and was barely detectable by the 6 hr recovery time at 25°C. A more prolonged induction was observed for hsp90, but it was not as robust as that observed for hsp70. No augmentation of the hsp70 induction inlane HS was noted when the 36°C heat shock period was extended from 1 to 2 hr (Fig. 1b).
In contrast to stress-inducible hsp70, constitutive expression of hsp60 was detected in the nonheat shock control larvae (Fig. 1, lane C), and the level of this mitochondrial hsp did not change in response to the heat shock treatments. The constant hsp60 signal in all lanes of the Western blots shown in Figure 1 serves as a control to verify equal loading of larval protein at the various time points.
Figure 1 indicates that maximal induction of hsp70 protein was apparent in the third-instar larvae at the 1/2 hr recovery time point following the 1 hr heat shock at 36°C. This observation provided the rationale for selecting this recovery time for the subsequent neurophysiological experiments that explored whether previous heat shock protects synaptic function from subsequent stress. In addition, physiological observations were made at the 6 hr time point, when hsp70 returns to near-control levels, to determine whether physiological changes are correlated with the amount of hsp70.
Success rate of synaptic transmission at individual boutons following heat shock
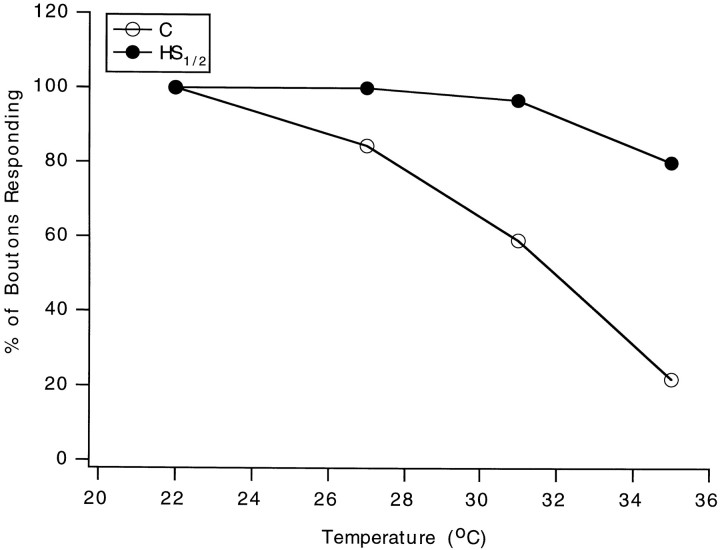
Heat shock has been reported to protect cells from subsequent stress (Parsell and Lindquist, 1993; Mayer and Brown, 1994; Morimoto et al., 1994; Feder et al., 1996). Whether this includes protection of synaptic function was assessed by determining the overall success rate of synaptic transmission at individual boutons at high test temperatures in heat-shocked and control neuromuscular junctions. The focal macropatch electrode was used to record both spontaneous and evoked postsynaptic events resulting from released transmitter at individual, visualized Ib boutons of the larval neuromuscular junction (Fig. 2). The overall success of synaptic transmission, represented as the percentage of boutons giving at least some responses (evoked or spontaneous events), declined much more at higher temperatures in control preparations (Fig.3). In the control group, 84% of the preparations survived to 27°C, 59% to 31°C, and 22% to 35°C, whereas in the heat shock group, 100% survived to 27°C, 97% to 31°C, and 80% to 35°C. The improvement in the number of preparations surviving in the latter group at the higher test temperatures reveals that prior heat shock affords neuroprotection to synapses and ensures more stable synaptic performance under subsequent heat stress. The effects of heat shock on pre- and postsynaptic parameters were assessed next.
Fig. 3.
The success of synaptic transmission at individual boutons is maintained at the higher test temperatures in preparations derived from heat-shocked larvae. The results were obtained from 29 control and 30 heat-shocked neuromuscular junctions. C, Control (open circles);HS1/2, heat shock followed by a 1/2 hr recovery (filled circles).
Spontaneous quantal events at individual boutons
In the absence of nerve stimulation, the macropatch electrode samples the spontaneous postsynaptic response to a single quantum of transmitter contained in a synaptic vesicle. This is termed an mEJC. The amplitude of an mEJC (quantal size) is determined by the number of open postsynaptic channels, the channel conductance, and the open times of these channels.
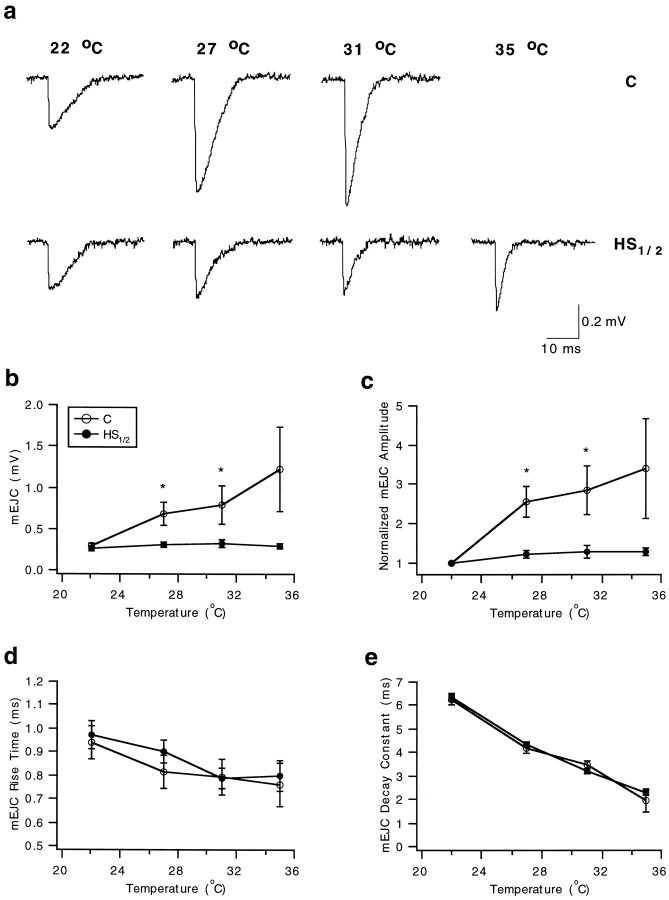
mEJCs appeared at a low frequency (generally 0.1 Hz or less) at the initial test temperature (22°C). As the test temperature increased, several changes were consistently observed (Fig.4a). In particular, the time course of the response shortened, and the amplitude increased relative to that at 22°C.
Fig. 4.
Postsynaptic stabilization of synaptic performance following heat shock at all test temperatures as revealed by the constant amplitude of mEJCs. mEJC parameters were assessed in control (C, open circles) and heat-shocked (HS1/2, 1/2 hr recovery,filled circles) experiments at the four test temperatures. Significant differences between the two groups at each test temperature assessed using a t test are denoted with an asterisk. a, Exemplarytraces of mEJCs recorded from individual Ib boutons in control (top) and heat-shocked (bottom) preparations at the four test temperatures. Each record represents an average of three individual events selected close to the mean amplitude. The time course of the unitary events is shortened at the higher test temperature, and the change in amplitude is more pronounced in the control recordings. b, mEJC amplitude. c, Normalized mEJC amplitude. Amplitudes at 27, 31, and 35°C were normalized to that at 22°C in each experiment. Note the larger variance in the control samples.d, mEJC rise times (time to peak). e, mEJC decay time constant obtained by fitting a single exponential to the decay phase of the mEJC. No marked differences in temporal parameters were evident between C andHS1/2 recordings. Error bars represent SEs.
Preparations subjected to prior heat shock differed from controls in several respects. First, the quantal size increased strikingly in controls, whereas in heat-shocked preparations, the amplitude remained constant over the entire temperature range (Fig.4a,b). There was no significant difference in quantal size between the control (0.30 ± 0.03 mV;n = 22) and heat shock group (0.27 ± 0.02 mV;n = 20) at 22°C (t test, p= 0.582). Thus, the effects of heat shock were not evident at room temperature. To make comparisons between the two groups, we normalized events recorded at the higher test temperatures to those recorded at 22°C in each preparation (Fig. 4c). Significant effects of heat shock on relative quantal size were observed; in heat-shocked boutons, quantal size was more resistant to change with increases in temperature. Also, some of the control boutons failed to produce spontaneous quantal events at the highest test temperatures (Fig.4a), whereas almost all of the heat-shocked boutons continued to generate quantal events. Using a two-way repeated measures ANOVA test, we confirmed that there were significant overall differences (p = 0.0029) between the control and heat shock groups. At 27 and 31°C (Fig. 4c), control mEJCs (n = 19) were significantly larger than those of the heat shock group (n = 20) by 101.7% (ttest, p = 0.004) and 119.3% (t test,p = 0.030), respectively. Pairwise comparisons could not be made at 35°C because only a few preparations continued to respond in the control group (n = 4) compared with the heat shock group (n = 16). Multiple pairwise comparisons (using the Student–Newman–Keuls method) also revealed that amplitudes at 27, 31, and 35°C were significantly larger than that at 22°C for the control group but not for the heat shock group (Fig. 4b). At each test temperature, the standard error (SE) of mEJC amplitude is dramatically reduced in the heat shock group compared with the control group (Fig. 4b,c). The increase in quantal size indicates that there is not a desensitization to transmitter at the higher temperatures in controls; thus, failure to detect quantal events in some of the controls likely represents failure of transmitter to be released rather than failure of postsynaptic response.
The possibility that increased quantal size could arise from a larger amount of transmitter liberated by the synaptic vesicles after exposure to a higher temperature does not apply in several other synapses that have been examined, including frog neuromuscular junctions (Cohen and Van der Kloot, 1983) and mammalian central synapses (Wall and Usowicz, 1998). We examined electron micrographs of Drosophilaneuromuscular junctions fixed after a 1 hr exposure to 37°C and after holding for the same length of time at room temperature to see whether any change in the size of synaptic vesicles occurred in type Ib boutons. Diameters of 50 vesicles measured in each of four boutons after exposure to 37°C and in each of two boutons after exposure to 22°C revealed no difference in vesicle size; mean values were 35.8 and 35.7 nm, respectively. Synaptic vesicle size is thought to be correlated with the amount of contained transmitter and with the amplitude of the quantal event in some synapses (Wilson and Frerking, 1998). Therefore, the results for Drosophila neuromuscular junctions indicate that vesicle size and, by inference, the amount of contained transmitter are not much altered by exposure to a higher temperature.
For both control and heat-shocked boutons, mEJCs showed more rapid rise and decay times at the highest test temperatures, but there was no difference between the two groups for these parameters (Fig.4d,e). The two-way repeated measures ANOVA revealed no significant differences for heat shock treatment (p = 0.348), effects of temperature (p = 0.09), and overall group comparison (p = 0.974). Similarly, the time constant of decay, derived by fitting a single exponential to the decay phase of the mEJC, did not differ for the two groups at the four test temperatures (Fig. 4e). The two-way repeated measures ANOVA revealed that, overall, no differences could be discerned between the two groups (p = 0.497). Heat shock does not produce significant differences in the rise time and time constant of decay between the two groups, implying that postsynaptic receptor channel kinetics is not modified by this treatment.
The frequency of mEJCs increased with temperature in both control and heat shock groups, but statistical significance between the groups was not shown by ANOVA or t tests. At 22°C, mEJCs occurred at a frequency of 0.064 ± 0.012 Hz (± SE; n = 22) in controls and 0.047 ± 0.007 Hz (n = 19) in the heat shock group. At 35°C, frequency increased to 0.233 ± 0.131 Hz (n = 4) in controls and 0.155 ± 0.029 Hz (n = 16) in the heat shock group.
Nerve-evoked responses at individual boutons
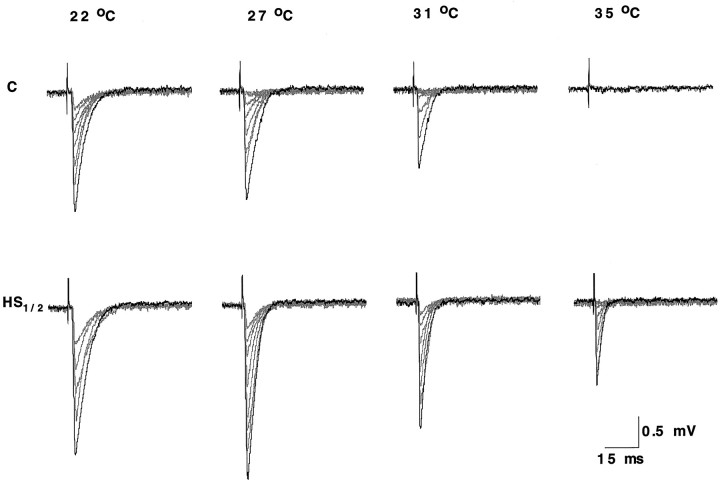
Nerve stimulation causes the release of a variable number of quanta per impulse during a train of stimuli. The resulting postsynaptic response detected by the macropatch electrode is termed an EJC. If quantal units are undetected following a stimulus, this is termed a failure. At 22°C, both control and heat-shocked boutons produced one or more quantal events for each nerve impulse, with no failures (Fig. 5). As the test temperature increased, the mean amplitude of the response declined, and failures became evident, particularly in the control preparations. As shown in Figure 5, transmission failed completely in the control bouton at 35°C but was maintained (with a few failures of transmission) in the heat-shocked bouton.
Fig. 5.
Representative traces of nerve-evoked EJCs recorded from individual Ib boutons in control (C) and heat-shocked (HS1/2, 1/2 hr recovery) preparations at the four test temperatures. In this experiment, there was no response at 35°C for the control preparation.
As the test temperature was increased, the averaged nerve-evoked responses showed a decline in relative amplitude in controls and only at the highest temperatures in heat-shocked boutons. This is the reverse of what was seen for the mEJCs (Fig.6a, C,HS1/2; n = 10 at each test temperature for each category), suggesting that a larger decline in transmitter release overcomes the increase in quantal size. Normalized values were significantly different for control and heat-shocked boutons at two of the higher test temperatures (Fig.6b, C, HS1/2). At 35°C, the difference was not significant, but, as shown in Figure 3, only a few control boutons provided data at this temperature. Measurements of the time course of the evoked responses (data not shown) showed little difference between control and heat-shocked boutons, in keeping with the results for mEJCs.
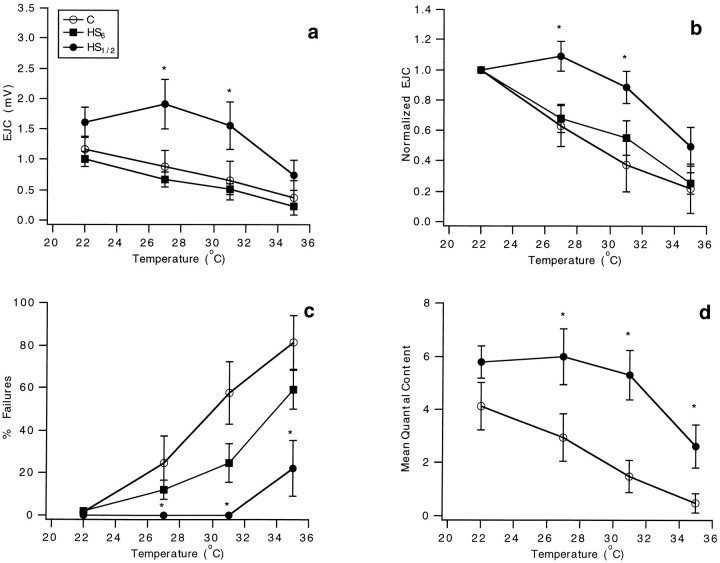
Fig. 6.
Presynaptic performance is stabilized after heat pretreatment, but this neuroprotection diminishes with increased recovery time when hsp70 levels are decreased. EJC parameters were assessed in control (C, open circles) and heat shock (HS1/2, 1/2 hr recovery,filled circles; HS6, 6 hr recovery, filled squares) experiments at the four test temperatures. Significant differences between C andHS1/2 at each test temperature assessed using a t test are denoted with anasterisk. a, EJC amplitude.b, Normalized EJC amplitude. Amplitudes at 27, 31, and 35°C were normalized to that at 22°C in each experiment.c, The percentage of transmission failures.d, The estimated mean quantal content. Error bars represent SEs.
The most striking difference between the responding boutons of the two groups was in the percentage of impulses failing to evoke transmission (Fig. 6c, C,HS1/2). As indicated in Figures 3 and 5, failures of transmission became evident at lower test temperatures in controls and only at 35°C in heat-shocked boutons. Statistical tests (two-way repeated measures ANOVA) confirmed highly significant effects of heat shock treatment (p < 0.0001) and temperature (p < 0.0001), with highly significant overall differences (p = 0.0082) between the control and heat shock groups. The percentage of failures for the heat-shocked boutons at 35°C (22.4 ± 13.0%;n = 10) almost equals that at 27°C (24.6 ± 12.7%; n = 10) for the control boutons; thus, heat shock protects boutons against transmission failure, extending the temperature range for transmission by up to 8°C.
The quantal content of transmission, which is the average number of vesicles liberating transmitter per stimulus, was estimated from the ratio of the mean amplitudes of evoked to spontaneous events (Cooper et al., 1995). Quantal content was significantly greater for heat-shocked preparations at all test temperatures above 22°C (Fig. 6d,C, HS1/2; t test, 27°C, p = 0.043; 31°C, p = 0.039; 35°C, p = 0.034). For the two-way repeated measures ANOVA tests, both heat shock treatment (p < 0.0001) and temperature effects (p = 0.0003) individually have significant effects, but the overall group difference was not significant (p = 0.57). In heat-shocked preparations, the quantal content determined at room temperature was maintained to approximately the same value up to 31°C, but at 35°C there was a decline; in control, the quantal content declined as temperature increased above 22°C. Thus, prior heat shock extends the safety factor for presynaptic performance, as evidenced by the constancy of quantal content values, by 9°C compared with controls. Although there is a decline in quantal content at 35°C following heat shock, the relative quantal size remains close to what is seen at the lowest test temperatures, indicating that heat shock affords greater post- than presynaptic stabilization.
After 6 hr of recovery from heat shock of the larvae, the values for EJC and normalized EJC were close to control values at the different test temperatures (Fig. 6a,b,HS6; n = 10 at each test temperature). Statistical tests (two-way repeated ANOVA) revealed no difference between control and heat shock, 6 hr recovery data at all test temperatures (p = 0.398), but significant differences between heat shock, 6 hr recovery data and heat shock, 1/2 hr recovery data (p < 0.0001). The percentage of transmission failures at responding boutons was also closer to that of controls (Fig. 6c, C,HS6). Thus, immediately after heat shock, the protective effects were maximal, extending the safety factor of presynaptic performance by an extra 8–9°C. However, after 6 hr, much of the protection is lost, paralleling a decrease in induced hsp70 levels (Fig. 1a).
DISCUSSION
In this report we show for the first time that heat shock alters key synaptic parameters at individual synaptic boutons both pre- and postsynaptically; both quantal content and quantal size become more resistant to the effects of temperature change after prior heat shock, whereas the kinetic properties of neurotransmission are not affected. In addition, heat shock increases the safety factor for synaptic transmission, extending stable presynaptic performance by an extra 9°C and postsynaptic stabilization over the whole range of test temperatures, compared with controls. These neuroprotective effects coincide with robust induction of hsp70, a major heat shock protein inDrosophila. Interestingly, the protective effects diminish with the decline of hsp70 expression. The coincidence of physiological and biochemical changes supports the initial hypothesis that induction of hsp70 (and possibly other hsps) is responsible for the physiological stabilization of synaptic transmission. The hypothesis of hsp involvement in the synaptic neuroprotective effects can be tested further in Drosophila by using mutants and genetically engineered flies that make more or less than the normal amount of heat shock proteins. Previous work has shown that heat shock has protective effects on locomotion and neural function in the locust flight system (Robertson et al., 1996; Dawson-Scully and Robertson, 1998; Gray and Robertson, 1999), but the physiological mechanisms subject to these protective effects have not been defined.
Promoter sequences derived from heat shock genes have been widely used to drive ectopic expression of specific genes of interest inDrosophila and other systems (Brand et al., 1994). Our present results demonstrate that heat shock alone alters key pre- and postsynaptic parameters. Thus, controls should be included in the above-mentioned experimental paradigms to assess the effect of heat shock without the presence of the inducible transgene.
Induction of heat shock proteins
The time and temperature used in the present experiment result in a very rapid induction of hsp70, which then declines in <8 hr. Hsp70 attains its maximum level at 1/2 hr of recovery. InDrosophila, hsp70 is the major stress-inducible heat shock protein that serves as a molecular chaperone (Parsell and Lindquist, 1993; Feder et al., 1996; Feder and Krebs, 1997, 1998). It is known that synthesis of most other proteins is down-regulated inDrosophila by heat shock treatment (Parsell and Lindquist, 1993). Greater heat stresses (at 38°C) lead to a longer-lasting appearance of hsp70, but this is accompanied by some tissue damage (Feder et al., 1996). Thus, the protocol used in the present experiments is more suitable for physiological studies.
Heat shock affords neuroprotection to larval synapses at sublethal temperatures
Heat shock increases thermotolerance and results in the survival of the organism at previously lethal temperatures (Parsell and Lindquist, 1993; Feder et al., 1996; Feder and Krebs, 1997, 1998). The present study shows that what is true for the intact organism is also reflected in the neuromuscular synapse preparation in vitro. Our electrophysiological measurements of synaptic responses recorded from individual boutons using a macropatch electrode showed clearly that responsiveness was retained better at higher test temperatures after heat shock (Fig. 3). We offer an important caveat: neuromuscular function in Drosophila is known to be strongly influenced by the physiological solutions used for isolated preparations (Stewart et al., 1994), and because the intact larvae survive high temperatures better than the isolated preparations, we think that the physiological solution we used is not optimal for studies of the neuromuscular junction at high temperatures. Thus, the physiological “survival” we found is probably less than that in an intact larva. Nevertheless, it shows that heat shock protects synaptic responsiveness.
Alterations of presynaptic performance
Strong effects of previous heat shock on transmitter release were found; at high temperatures, failures of transmission decrease, and quantal content is sustained. These effects could be attributable to the interaction of hsps with presynaptic proteins involved in transmitter release. For example, the cysteine string proteins (csps), which have a J-domain that may interact with hsc70 (Chamberlain and Burgoyne, 1997a,b), are synaptic vesicle proteins involved in the regulation of release by Ca2+ ions (Mastrogiacomo et al., 1994; Ranjan et al., 1998; Umbach et al., 1998). Null mutants for csps show impaired synaptic transmission (Heckmann et al., 1997) with profound temperature sensitivity (Umbach et al., 1994).
Alterations of postsynaptic performance
Because heat shock helps synapses to function at sublethal temperatures, we investigated parameters of the postsynaptic response: amplitude, rise time, and time constant of decay. Interestingly, there was little effect of prior heat shock on the kinetic parameters; but after heat shock, the relative quantal size remained stable as temperature was increased, whereas in control preparations, it increased (Fig. 4). The effect in controls could be attributable to activation of more postsynaptic receptors by released glutamate or to increased conductance of individual receptor-gated channels. Precedents for both effects have been found in other systems; synaptic plasticity in mammalian CNS is known to involve recruitment of AMPA-type and kainate glutamate receptors by phosphorylation (Wang et al., 1991,1993), and activity-dependent modulation of unitary conductance also plays a role in the strengthening of glutamate synapses (Wall and Usowicz, 1998). Functional GABA receptors are rapidly recruited in the mammalian CNS by insulin (Wan et al., 1997). Further experiments at the single-channel level (Heckmann and Dudel, 1995, 1997) would be required to determine which of these mechanisms applies in Drosophilaas temperature is increased.
Another possibility is that the amount of transmitter released per vesicle to generate a quantal response becomes greater in controls at high temperature but not after heat shock. This possibility is unlikely for two reasons. (1) Electron micrographs of synaptic vesicles inDrosophila boutons show that the vesicles do not increase in size after heat treatment, indicating that the quantal unit size is not altered by the occurrence of larger vesicles containing more transmitter. (2) Other workers have shown that a temperature-dependent increase in quantal size at mammalian synapses and at the frog neuromuscular junction is attributable to increased postsynaptic conductance and not to an increase in the number of transmitter molecules in each vesicle (Cohen and Van der Kloot, 1983; Wall and Usowicz, 1998). Thus, the increase in quantal size is most likely a postsynaptic effect, at Drosophila synapses and elsewhere.
At 35°C, the better stabilization of postsynaptic performance over presynaptic performance indicates that heat pretreatment is relatively more effective on the postsynaptic than on the presynaptic side of the synapse. Postsynaptic localization of hsps has been shown in postsynaptic density fractions isolated from mammalian CNS (Freedman et al., 1981). There are many muscle nuclei in close proximity to the neuromuscular junction; thus, synthesis and transport of hsps to postsynaptic sites could be rapid. Presynaptically, the motor neuron’s nucleus and the cell body are localized in the central ganglion. Thus, transport of synthesized hsps to the presynaptic side of the neuromuscular junction may require longer times. Alternatively, hsps may be derived from glial cells adjacent to the motor axons (Sheller et al., 1998). In either case, more delay in the effects of hsps would likely occur in the presynaptic terminal than postsynaptically.
In conclusion, prior heat shock affords neuroprotection to synapses and stabilizes synaptic performance at sublethal temperatures through both pre- and postsynaptic alterations. Heat shock proteins could contribute to the alterations because they are preferentially synthesized inDrosophila during thermal stress (Parsell and Lindquist, 1993). In particular, expression of hsp70 was maximal when synaptic neuroprotection was observed, and the protective effects declined in parallel with the diminished presence of hsp70. These observations demonstrate that synaptic transmission can be modified in response to stressful conditions, providing subsequent protection. The knowledge that neuroprotective mechanisms alter synaptic properties could be of use in the treatment of injurious conditions such as ischemia and stroke.
Footnotes
The work was supported by grants from the National Sciences and Engineering Research Council of Canada to R.M.R. and H.L.A. and from the Medical Research Council of Canada to I.R.B. Mr. Alan Wong assisted with collection of samples and analysis of the data, Sheila Rush helped with the Western blots, and Marianne Hegström-Wojtowicz assisted with preparation of this manuscript. We thank Dr. Konrad Zinsmaier (University of Pennsylvania) for critical comments on a previous draft of this manuscript.
Correspondence should be addressed to Dr. H. L. Atwood, Department of Physiology, University of Toronto, Toronto, Ontario, Canada M5S 1A8.
REFERENCES
- 1.Akner G, Mossberg K, Sundqvist KG, Gustafsson JA, Wikstrom AC. Evidence for reversible non-microtubule and non-microfilament dependent nuclear translocation of hsp90 after heat shock in human fibroblasts. Eur J Cell Biol. 1992;58:356–364. [PubMed] [Google Scholar]
- 2.Atwood HL, Govind CK, Wu C-F. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. doi: 10.1002/neu.480240803. [DOI] [PubMed] [Google Scholar]
- 3.Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science. 1988;241:1817–1820. doi: 10.1126/science.3175623. [DOI] [PubMed] [Google Scholar]
- 4.Brand AH, Manoukian AS, Perrimon N. Ectopic gene expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain LH, Burgoyne RD. The molecular chaperone function of the secretory vesicle cysteine string proteins. J Biol Chem. 1997a;272:31420–31426. doi: 10.1074/jbc.272.50.31420. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain LH, Burgoyne RD. Activation of the ATPase activity of heat-shock proteins Hsc70/Hsp70 by cysteine-string protein. Biochem J. 1997b;322:853–858. doi: 10.1042/bj3220853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen IS, Van der Kloot W. Effects of low temperature and terminal membrane potential on quantal size at frog neuromuscular junction. J Physiol (Lond) 1983;336:335–344. doi: 10.1113/jphysiol.1983.sp014584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper RL, Stewart BA, Wojtowicz JM, Wang S, Atwood HL. Quantal measurement and analysis methods compared for crayfish and Drosophila neuromuscular junctions, and rat hippocampus. J Neurosci Methods. 1995;61:67–78. doi: 10.1016/0165-0270(95)00024-o. [DOI] [PubMed] [Google Scholar]
- 9.Dawson-Scully K, Robertson RM. Heat shock protects synaptic transmission in flight motor circuitry of locusts. NeuroReport. 1998;9:2589–2593. doi: 10.1097/00001756-199808030-00030. [DOI] [PubMed] [Google Scholar]
- 10.Dudel J. The effect of reduced calcium on quantal unit current and release at the crayfish neuromuscular junction. Pflügers Arch. 1981;391:35–40. doi: 10.1007/BF00580691. [DOI] [PubMed] [Google Scholar]
- 11.Feder ME, Krebs RA. Ecological and evolutionary physiology of heat shock proteins and the stress response in Drosophila: complementary insights from genetic engineering and natural variation. In: Bijlsma R, Loeschoke V, editors. Environmental stress, adaptation and evolution. Birkhäuser; Basel: 1997. pp. 155–173. [DOI] [PubMed] [Google Scholar]
- 12.Feder ME, Krebs RA. Natural and genetic engineering of the heat-shock protein Hsp70 in Drosophila melanogaster: consequences for thermotolerance. Am Zool. 1998;38:503–517. [Google Scholar]
- 13.Feder ME, Cartaño NV, Milos L, Krebs RA, Lindquist SL. Effect of engineering Hsp70 copy number on Hsp70 expression and tolerance of ecologically relevant heat shock in larvae and pupae of Drosophila melanogaster. J Exp Biol. 1996;199:1837–1844. doi: 10.1242/jeb.199.8.1837. [DOI] [PubMed] [Google Scholar]
- 14.Fink SL, Chang LK, Ho DY, Sapolsky RM. Defective herpes simplex virus vectors expressing the rat brain stress-inducible heat shock protein 72 protect cultured neurons from severe heat shock. J Neurochem. 1997;68:961–969. doi: 10.1046/j.1471-4159.1997.68030961.x. [DOI] [PubMed] [Google Scholar]
- 15.Freedman MS, Clark BD, Cruz TF, Gurd JW, Brown IR. Selective effects of LSD and hyperthermia on the synthesis of synaptic proteins and glycoproteins. Brain Res. 1981;207:129–145. doi: 10.1016/0006-8993(81)90683-1. [DOI] [PubMed] [Google Scholar]
- 16.Gray JR, Robertson RM (1999) Effects of heat stress on axonal conduction in the locust flight system. Comp Biochem Physiol [A], in press.
- 17.Heckmann M, Dudel J. Recordings of glutamate-gated ion channels in outside-out patches from Drosophila larval muscle. Neurosci Lett. 1995;196:53–56. doi: 10.1016/0304-3940(95)11836-l. [DOI] [PubMed] [Google Scholar]
- 18.Heckmann M, Dudel J. Desensitization and resensitization kinetics of glutamate receptor channels from Drosophila larval muscle. Biophys J. 1997;72:2160–2169. doi: 10.1016/S0006-3495(97)78859-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heckmann M, Adelsberger H, Dudel J. Evoked transmitter release at neuromuscular junctions in wild type and cysteine string protein null mutant larvae of Drosophila. Neurosci Lett. 1997;228:167–170. doi: 10.1016/s0304-3940(97)00390-x. [DOI] [PubMed] [Google Scholar]
- 20.Karunanithi S, Phipps MC, Robinson J, Bennett MR. Statistics of quantal secretion during long trains of sympathetic nerve impulses in mouse vas deferens. J Physiol (Lond) 1995;489:171–181. doi: 10.1113/jphysiol.1995.sp021039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keshishian H, Broadie K, Chiba A, Bate M. The Drosophila neuromuscular junction: a model system for studying synaptic development and function. Annu Rev Neurosci. 1996;19:545–575. doi: 10.1146/annurev.ne.19.030196.002553. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the heat of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lavidis NA, Bennett MR. Probabilistic secretion of quanta from visualized sympathetic nerve varicosities in mouse vas deferens. J Physiol (Lond) 1992;454:9–26. doi: 10.1113/jphysiol.1992.sp019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowenstein DH, Chan PH, Miles MF. The stress protein response in cultured neurons: characterization and evidence for a protective role in excitotoxicity. Neuron. 1991;7:1053–1060. doi: 10.1016/0896-6273(91)90349-5. [DOI] [PubMed] [Google Scholar]
- 25.Mailhos C, Howard MK, Latchman DS. Heat shock protects neuronal cells from programmed cell death by apoptosis. Neuroscience. 1993;55:621–627. doi: 10.1016/0306-4522(93)90428-i. [DOI] [PubMed] [Google Scholar]
- 26.Mallart A, Angaut-Petit D, Bourret-Poulain C, Ferrus A. Nerve terminal excitability and neuromuscular transmission in T(X;Y)V7 and Shaker mutants of Drosophila melanogaster. J Neurogenet. 1991;7:75–84. doi: 10.3109/01677069109066212. [DOI] [PubMed] [Google Scholar]
- 27.Mastrogiacomo A, Parsons SM, Zampighi GA, Jenden DJ, Umbach JA, Gundersen CB. Cysteine string proteins: a potential link between synaptic vesicles and presynaptic Ca2+ channels. Science. 1994;263:981–982. doi: 10.1126/science.7906056. [DOI] [PubMed] [Google Scholar]
- 28.Mayer J, Brown IR. Heat shock proteins in the nervous system. Academic; London: 1994. [Google Scholar]
- 29.Morimoto RI, Tissiers A, Georgopoulos C. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1994. Progress and perspectives on the biology of heat shock proteins and molecular chaperones. [Google Scholar]
- 30.Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 31.Ranjan R, Bronk P, Zinsmaier KE. Cysteine string protein is required for calcium secretion coupling of evoked neurotransmission in Drosophila but not for vesicle recycling. J Neurosci. 1998;18:956–964. doi: 10.1523/JNEUROSCI.18-03-00956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Restifo LL, White K. Molecular and genetic approaches to neurotransmitter and neuromodulator systems in Drosophila. Adv Insect Physiol. 1990;22:115–218. [Google Scholar]
- 33.Ritossa FM. A new puffing pattern induced by a temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- 34.Robertson RM, Xu H, Shoemaker KL, Dawson-Scully K. Exposure to heat shock affects thermosensitivity of the locust flight system. J Neurobiol. 1996;29:367–383. doi: 10.1002/(SICI)1097-4695(199603)29:3<367::AID-NEU8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Rordorf G, Koroshetz WJ, Bonventre JV. Heat shock protects culture neurons from glutamate toxicity. Neuron. 1991;7:1043–1051. doi: 10.1016/0896-6273(91)90348-4. [DOI] [PubMed] [Google Scholar]
- 36.Sayer RJ, Redman SJ, Andersen P. Amplitude fluctuations in small EPSPs recorded from CA1 pyramidal cells in guinea pig hippocampal slice. J Neurosci. 1989;9:840–850. doi: 10.1523/JNEUROSCI.09-03-00840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheller RA, Smyers ME, Grossfeld RM, Ballinger ML, Bittner GD. Heat-shock proteins in axoplasm: high constitutive levels and transfer of inducible isoforms from glia. J Comp Neurol. 1998;396:1–11. doi: 10.1002/(sici)1096-9861(19980622)396:1<1::aid-cne1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Stewart BA, Atwood HL, Renger JJ, Wang J, Wu C-F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol [A] 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- 39.Stewart BA, Schuster CM, Goodman CS, Atwood HL. Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology. J Neurosci. 1996;16:3877–3886. doi: 10.1523/JNEUROSCI.16-12-03877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tytell M, Barbe MF, Brown IR. Induction of heat shock (stress) protein 70 and its mRNA in the normal and light-damaged retina after whole body hyperthermia. J Neurosci Res. 1994;38:19–31. doi: 10.1002/jnr.490380105. [DOI] [PubMed] [Google Scholar]
- 41.Umbach JA, Zinsmaier KE, Eberle KK, Buchner E, Benzer S, Gundersen CB. Presynaptic dysfunction in Drosophila csp mutants. Neuron. 1994;13:899–907. doi: 10.1016/0896-6273(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 42.Umbach JA, Saitoe M, Kidokoro Y, Gundersen CB. Attenuated influx of calcium ions at nerve endings of csp and shibire mutant Drosophila. J Neurosci. 1998;18:3233–3240. doi: 10.1523/JNEUROSCI.18-09-03233.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wall MJ, Usowicz MM. Development of the quantal properties of evoked and spontaneous synaptic currents at a brain synapse. Natu Neurosci. 1998;1:675–682. doi: 10.1038/3677. [DOI] [PubMed] [Google Scholar]
- 44.Walsh DA, Klein NW, Hightower LE, Edwards MJ. Heat shock and thermotolerance during early rat embryo development. Teratology. 1987;36:181–191. doi: 10.1002/tera.1420360205. [DOI] [PubMed] [Google Scholar]
- 45.Walsh DA, Li K, Speirs J, Crowther CE, Edwards MJ. Regulation of the inducible heat shock 71 genes in early neural development of cultured rat embryos. Teratology. 1989;40:321–334. doi: 10.1002/tera.1420400404. [DOI] [PubMed] [Google Scholar]
- 46.Wan Q, Xiong ZG, Man HY, Ackerley CA, Brounton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABAA receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 47.Wang L-Y, Salter MW, MacDonald JF. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991;253:1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- 48.Wang L-Y, Taverna FA, Huang X-P, MacDonald JF, Hampson DR. Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993;259:1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]
- 49.Wilson M, Frerking M. The possible origin of variability in miniature PSC amplitude in cultured amacrine neurons. In: Faber DS, Korn H, Redman SJ, Thompson SM, Altman JS, editors. Central synapses: quantal mechanisms and plasticity. Human Frontier Science Program; Strasbourg, France: 1998. pp. 99–108. [Google Scholar]
- 50.Yenari M, Fink SL, Sun GH, Patel M, Kunis D, Onley D, Sapolsky RM, Steinberg GK. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann Neurol. 1998;44:584–591. doi: 10.1002/ana.410440403. [DOI] [PubMed] [Google Scholar]