Abstract
The electrophysiological and neurochemical characteristics of the nondopaminergic nigrostriatal (NO-DA) cells and their functional response to the degeneration of dopaminergic nigrostriatal (DA) cells were studied. Three different criteria were used to identify NO-DA cells: (1) antidromic response to striatal stimulation with an electrophysiological behavior (firing rate, interspike interval variability, and conduction velocity) different from that of DA cells; (2) retrograde labeling after striatal injection of HRP but showing immunonegativity for DA cell markers (tyrosine hydroxylase, calretinin, calbindin-D28k, and cholecystokinin); and (3) resistance to neurotoxic effect of 6-hydroxydomine (6-OHDA). Our results showed that under normal conditions, 5–8% of nigrostriatal neurons are immunoreactive for GABA, glutamic acid decarboxylase, and parvalbumin, markers of GABAergic neurons, a percentage that reached 81–84% after 6-OHDA injection. Electrophysiologically, NO-DA cells showed a behavior similar to that found in other nigral GABAergic (nigrothalamic) cells. In addition, the 6-OHDA degeneration of DA cells induced a modification of their electrophysiological pattern similar to that found in GABAergic nigrothalamic neurons. Taken together, the present data indicate the existence of a small GABAergic nigrostriatal pathway and suggest their involvement in the pathophysiology of Parkinson’s disease.
Keywords: nigrostriatal pathway, dopaminergic cells, GABAergic cells, GABA, glutamic acid decarboxylase, parvalbumin, calretinin, calbindin-D28k, cholecystokinin, Parkinson’s disease
The substantia nigra (SN) is a major output center of the basal ganglia (DeLong, 1990; Gerfen et al., 1990;Graybiel, 1990; Obeso et al., 1997), participating in their sensory-motor functions (DeLong et al., 1983; Hikosaka and Wurtz, 1983a–c; Schwarz et al., 1984; Schultz, 1986). This, together with its direct implication on the physiopathology of Parkinson’s disease (Albin et al., 1989; DeLong, 1990; Graybiel, 1990; Obeso et al., 1997;Viteck et al., 1997), has increased the interest in studying this mesencephalic center. The SN is composed primarily of two projection neurons. Dopaminergic (DA) cells, located in the pars compacta (SNc) and pars reticulata (SNr) and projecting to the striatum, and GABA cells, located in the pars reticulata and projecting to the thalamus, superior colliculus, and pedunculopontine nucleus (Dahlström and Fuxe 1964; Kilpatrick et al., 1980; Childs and Gale, 1983; Chiodo, 1988; Hedreen and DeLong, 1991; Parent and Hazrati, 1995; Hazrati, 1995a,b). Some preliminary evidence suggests that, besides the dopaminergic nigrostriatal system, there are nondopaminergic nigral cells projecting to the striatum. Thus, not all SN cells projecting to the striatum show catecholamine fluorescence (van der Kooy et al., 1981) or tyrosine hydroxylase immunoreactivity (Swanson, 1982; Gerfen et al., 1987). Early electrophysiological studies classified nigrostriatal cells as belonging to slow conduction velocity (0.5 m/sec) pars compacta neurons and higher conduction velocity (1.7 m/sec) pars reticulata neurons (Deniau et al., 1978; Guyenet and Aghajanian, 1978). The slow conduction velocity neurons were subsequently identified as dopaminergic (Grace and Bunney, 1980, 1983a). Most studies have been focused on this neuron type (Grace and Bunney, 1980,1983a–c, 1984a,b, 1985a,b; DeLong et al., 1983; Schwarz et al., 1984;Schultz, 1986; Chiodo, 1988; Schultz and Romo, 1990), because they are probably conditioned by their direct involvement in various neurological disorders, including Parkinson’s disease (Ehringer and Hornykiewicz, 1960), whereas the higher conduction velocity neurons have remained practically unexplored.
The main aim of this study was the electrophysiological and neurochemical characterization of nondopaminergic nigroestriatal (NO-DA) cells. Before analyzing their electrophysiological behavior, SN cells were classified as nigrostriatal or nigrothalamic according to their antidromic response (Guyenet and Aghajanian, 1978; Grace and Bunney, 1980, 1983a–c; Ruffieux and Schultz, 1980; Sanderson et al., 1986; Chiodo, 1988; MacLeod et al., 1990; Castellano and Rodríguez, 1991; Castellano et al., 1993). In the neurochemical study, nigrostriatal neurons were first identified by the retrograde transport of horseradish peroxidase (HRP) from the striatum. Then they were classified as DA or GABA cells using different neurochemical markers. For DA cells, besides tyrosine hydroxylase (TH, the rate-limiting enzyme in the DA synthesis), we used the peptide cholecystokinin (CCK; Hökfelt et al., 1980, 1985; Chiodo, 1988;Freeman and Chiodo, 1988) and the calcium-binding proteins calretinin (CR) and calbindin (CB; Goodman et al., 1979; Rogers, 1987; Yamada et al., 1990; Heizmann and Hunziker, 1991; Iacopino et al., 1994; Liang et al., 1996), three substances contained in DA neurons (Résibois and Rogers, 1992; Liang et al., 1996; McRitchie et al., 1996). For GABA cells, besides GABA and glutamic acid decarboxylase (GAD, the synthesizing enzyme for GABA), we used parvalbumin (PV), a calcium-binding protein that, in the SN, is expressed by and restricted to GABAergic neurons (Reiner and Anderson, 1993; Rajakumar et al., 1994).
In addition, taking into account experimental (Arbuthnott, 1974;Ruffieux and Schultz, 1980; Sanderson et al., 1986; Mitchell el al., 1989; DeLong, 1990; MacLeod et al., 1990; Robledo and Féger, 1992; Herrero et al., 1993; Burbaud et al., 1995) and clinical (Bernheimer et al., 1973; Condé, 1992) data suggesting that some Parkinsonian motor disturbances, acquired after the destruction of DA cells, are induced by a functional modification in SN GABAergic cells projecting to the thalamus, we have also studied the effect of experimentally produced degeneration of DA cells on the electrophysiological behavior of NO-DA cells.
MATERIALS AND METHODS
Animals. Experiments were performed on male Sprague Dawley rats (300–350 gm; Panlab, Barcelona, Spain). Animals were housed at 22°C, two per cage, under normal laboratory conditions on a standard 12 hr light/dark schedule (with lights on from 3 A.M. to 3 P.M.) and with free access to food and water. Experimental protocols were in accordance with the European Community Council Directive of November 24, 1986 (86/609/EEC) regarding the care and use of animals for experimental procedures. Adequate measures were also taken to minimize pain and discomfort.
A group of animals were lesioned using a procedure that induces a marked and selective destruction of DA neurons (Hökfelt and Ungerstedt, 1973; Castro et al., 1985). Under anesthesia (80 mg/kg ketamine plus 12 mg/kg xylazine, i.p.), rats received a double unilateral injection of 6-hydroxydopamine hydrochloride (6-OHDA; Sigma, St. Louis, MO; 8 μg in 4 μl of a 0.9% saline solution with 0.3 μg/μl ascorbic acid/injection; 1 μl/min) or vehicle in the medial forebrain bundle (Castro et al., 1985; Burunat et al., 1988;Barroso and Rodríguez, 1996), according to the stereotaxic coordinates of Paxinos and Watson (1988): 4.0 mm anterior to λ, 1.6 mm to the right of the midline, and 8 mm below the dura (first injection); and 4.6 mm anterior to λ, 1.4 mm to the right of the midline, and 8 mm below the dura (second injection). The behavioral evaluation of lesion degree (Castro et al., 1985) was performed 2–3 weeks later. Animals showing more than eight turns per minute in response to apomorphine hydrochloride (1 mg/kg, i.p.; Sigma) were included in the study.
Electrophysiological studies. Unlesioned, sham-lesioned, and 6-OHDA lesioned (4–6 weeks after lesion) rats were anesthetized with chloral hydrate (400 mg/kg, i.p.), and the extracellular activity of SN neurons was monitored according to the procedure of Castellano and Rodríguez (1991). Recordings (2.8–3.4 mm anterior to λ, 1.8–2.4 mm lateral to the midline, and 7–8.5 mm below the cortical surface) were obtained from rats kept between 36.5 and 37.0°C by using electrodes (glass tubing filled with 2 m NaCl containing 2% Pontamine Sky Blue; BDH Chemicals, Poole, England) with a 6–9 mΩ impedance (at 1000 Hz). The signal was amplified (3000×) and filtered (200–5000 Hz) in a high input impedance differential amplifier (CAN96T; Telcan, Tenerife, Spain).
To identify nigrostriatal and nigrothalamic cells (Castellano et al., 1993; Rodríguez and Barroso, 1996), stimulating electrodes were placed in the head of the caudate nucleus (0.0 mm anterior to bregma, 3.2 mm to the right of the midline, and 5 mm ventral to the brain surface) and in the ventral thalamus (2.5 mm posterior to bregma, 1.5 mm to the right of the midline, and 6.5–7 mm ventral to the brain surface). The collision test was applied to determine the antidromic activation from these nuclei, using a Grass (West Warwick, RI) S-8800 and a bipolar stimulating electrode made with stainless steel rods (250 μm diameter; A-M Systems, Everet, WA). Two rods were electroetched with a 60 Hz alternating current [1:1 (v/v) 96% sulfuric acid/85% phosphoric acid] and insulated with Sylgar 184 (Dow Corning, Wiesbaden, Germany), and their conductive tips (0.5 mm) were placed 500 μm apart. Neurons were considered antidromically activated (0.5 mA/0.3 msec square pulse) when they showed (1) an antidromic spike per stimulus and a fixed latency from the stimulus artifact (<0.05 msec) during high-rate stimulation (50 Hz) and (2) a collision between the spontaneously occurring action potentials and the stimulation-elicited spikes. DA neurons were identified by a 2–5 msec triphasic extracellular wave form (with a positive first phase), often displaying a prominent notch (initial segment–somatodendritic break) in the initial positive rising, a spontaneous activity <10 spikes/sec, and a long-latency antidromic response (conduction velocity of ∼0.5 m/sec) to striatal stimulation, mostly consisting of the initial segment component of the action potential. GABAergic nigrothalamic neurons were identified by their biphasic extracellular wave form (often showing a duration of <1 msec) and a short-latency antidromic response (a conduction velocity of >1 m/sec) evoked from the ventromedial nucleus of the thalamus.
The basal activity of neurons was recorded 10 min after their antidromic identification. Extracellular signals were simultaneously digitized on a Pentium-based computer by using a 16 bit analog-to-digital converter (LTI-C30; Madrid, Spain), recorded on magnetic tape for off-line analysis, and displayed on a storage oscilloscope. Only recordings with single-unit activity were used. Action potentials were considered as displayed by the same neuron when the spike shape, analyzed with both hardware (SD1 spike discriminator; Tucker–Davis Technologies, Gainesville, Florida) and software (hybrid multilayer artificial neural network; Garcia et al., 1998) procedures, did not change during the recording session. The firing rate (number of spikes per second) and variation coefficient of interspike intervals (VC; SD of interspike intervals/mean of interspike intervals) were computed for a recording time of at least 4 min.
At the end of each experiment, the recording sites were marked by iontophoretic injection of Pontamine Sky Blue (−20 μA, 30 min), and the stimulus sites were marked by means of an iron deposited by passing a current of 10 μA for 30 sec. Rats were perfused with 0.9% saline and 1% potassium ferricyanide in 10% formaldehyde. Brains were post-fixed in the same solution for 6–10 hr and cryoprotected overnight in 30% sucrose at 4°C. Serial sections (50 μm thick) were obtained with a freezing microtome and stained with formal thionine.
Morphological studies. These studies were performed by combining retrograde transport of HRP and immunocytochemistry for different markers of nigral neurons. Under anesthesia (80 mg/kg ketamine and 12 mg/kg xylazine, i.p.) and aseptic conditions, rats received a single injection of 0.2–0.4 μl of 40% HRP (Boehringer Mannheim, Mannheim, Germany) in the striatum 42–68 hr before killing. Given that under normal conditions the soma concentrations of some neuropeptides (i.e., CCK) and enzymes (i.e., GAD) are so low that they are difficult to detect with immunocytochemistry, a group of rats were injected in the lateral ventricle (ipsilateral to the HRP injection) with 10 μl of colchicine (10 mg/ml, Sigma) dissolved in saline 24 hr before killing. This is a successful procedure to block the axonal transport, increasing the immunoreactivity in somata (Fallon et al., 1983; Seroogy et al., 1989; Campbell et al., 1991). Rats were heavily anesthetized and transcardially perfused with 0.9% saline and a fixative solution of 1% paraformaldehyde and 1.25% glutaraldehyde in 0.1 m phosphate buffer, pH 7.4. Brains were removed and stored in the same fixative at 4°C for 4–12 hr. Midbrains were initially obtained by using a brain blocker (Activational System, Warren, MI) and then cut at 30 μm with a vibratome. Sections were collected in parallel series, treated for inhibition of endogenous peroxidase with 3% H2O2, and processed for double labeling. First, they were processed for histochemical localization of HRP by using 3,3′-diaminobenzidine (DAB, Sigma) intensified by cobalt chloride (Sigma; Adams, 1977) or nickel amonium sulfate (Serva, Heidelberg, Germany; Adams, 1981) as chromogen, which produces a black granular reaction product, and thereafter for immunocytochemical detection of distinct markers (one series per marker) using nonintensified DAB, which produces a brown diffuse reaction product.
As primary antibodies we used a mouse anti-TH monoclonal antibody (1:8000, Sigma), a mouse anti-GABA monoclonal antibody (1:12,000; Matute and Streit, 1986), a rabbit anti-GAD polyclonal antibody (1:2000; Chemicon, Temecula, CA), a rabbit anti-CCK-8 polyclonal antibody (1:12000, Sigma), a mouse anti-CB monoclonal antibody (1:1000, Sigma), a rabbit anti-CR polyclonal antibody (1:5000, Chemicon), and a mouse anti-PV monoclonal antibody (1:6000, Sigma). After HRP histochemistry, sections were washed in 0.1 m PBS, pH 7.4, and immersed for 60 min in the preincubation solution (PIS): 3% normal goat serum (NGS; Vector Laboratories, Burlingame, CA) for GAD, CCK, and CR, or 3% normal horse serum (NHS, Vector Laboratories) for TH, GABA, CB, and PV; and 0.5% BSA (Serva) and 0.1% Triton X-100 (TX-100, Sigma) in PBS. Sections were then incubated overnight in the primary antibody dissolved in PIS. In GAD and GABA immunocytochemistry TX-100 was omitted. Sections were washed several times in PBS and incubated for 60 min in 1:200 biotinylated goat anti-rabbit antiserum (for GAD, CCK, and CR; Vector Laboratories) or 1:200 biotinylated horse anti-mouse antiserum (for TH, GABA, CB, and PV; Vector Laboratories) and either 1:200 NGS or 1:200 NHS in PBS. Immunoreactions were visualized by incubation for 2 hr in ExtrAvidin (1:5000, Sigma) in PBS and for 10 min in 0.005% DAB (Sigma) and 0.001% H2O2 in cacodylate buffer. After several rinses in PBS, sections were mounted on gelatinized slides, dehydrated, cleared in xylene, and coverslipped with DPX (BDH). Control experiments to demonstrate the specificity of the immunolabeling were performed by removing the primary or secondary antibodies, resulting in negative staining.
The percentage of nigrostriatal neurons that were immunostained for the different markers was computed by counting the number of double-labeled neurons in a total of 900 HRP-positive cells per series in each unlesioned rat [700 in SN, 160 in ventral tegmental area (VTA), and 40 in retrorubral field (RRF)] and 70 HRP-positive cells per series in each lesioned rat (all then in SN). In each series, HRP-positive neurons were randomly elected in 10 sections separated by a distance of 210 μm from each other and taken from rostral SN to caudal RRF. The quantitative study was performed with the aid of a Magiscan image analysis system using the Genias program (Joyce-Loebl) and dividing the SN, VTA, and RRF into fields of 400 × 400 μm at a magnification of 200×. Taking into account that antisera only penetrate 8–10 μm from the surface, whereas HRP-positive neurons are evident at all depths of the section, we have only counted those single- and double-labeled neurons exposed on the surface of sections, excluding diffusely HRP-labeled cells.
Statistical comparisons. The statistical analyses were performed using a one-way ANOVA followed by appropriate post hoc tests (Statistica; Statsoft, Tulsa, OK). In all cases, a level of p < 0.05 was considered critical for assigning statistical significance.
Experiment 1: electrophysiological identification of nondopaminergic nigrostriatal cells. The extracellular electrophysiological activity of 374 nigral cells was recorded in 33 unlesioned rats. Electrical stimulation of the head of the caudate nucleus and ventral thalamus was used for identifying the target of recorded neurons. Multiunitary recordings were excluded, and only cells showing antidromic response to striatal (n = 142) or thalamic (n = 62) stimulation were finally included.
Experiment 2: morphological and neurochemical identification of nondopaminergic nigrostriatal cells. To identify cells projecting to the striatum, 12 rats were injected in the caudate nucleus with HRP. Two days later, 6 of them were injected in the lateral ventricle with colchicine. After 2 d in the case of the colchicine-untreated rats and 3 d in the case of colchicine-treated rats, they were heavily anesthetized, perfused, and processed for the histochemical and immunocytochemical studies.
Experiment 3: effect of dopaminergic nigrostriatal cells degeneration on nondopaminergic nigrostriatal neurons. Forty-four rats were used to evaluate the effects of DA cell degeneration on nondopaminergic nigrostriatal and nigrothalamic neurons. Two to 3 weeks after 6-OHDA (n = 22) or vehicle (n = 22) injections, rats were behaviorally tested to determine the lesion degree. Twenty to 40 d later they were anesthetized for the electrophysiological (16 sham and 16 6-OHDA-injected rats) or neurochemical (6 sham and 6 6-OHDA-injected rats) studies, following the same protocols used in experiments 1 and 2, but in this experiment colchicine was not used.
RESULTS
Experiment 1: electrophysiological identification of nondopaminergic nigrostriatal cells
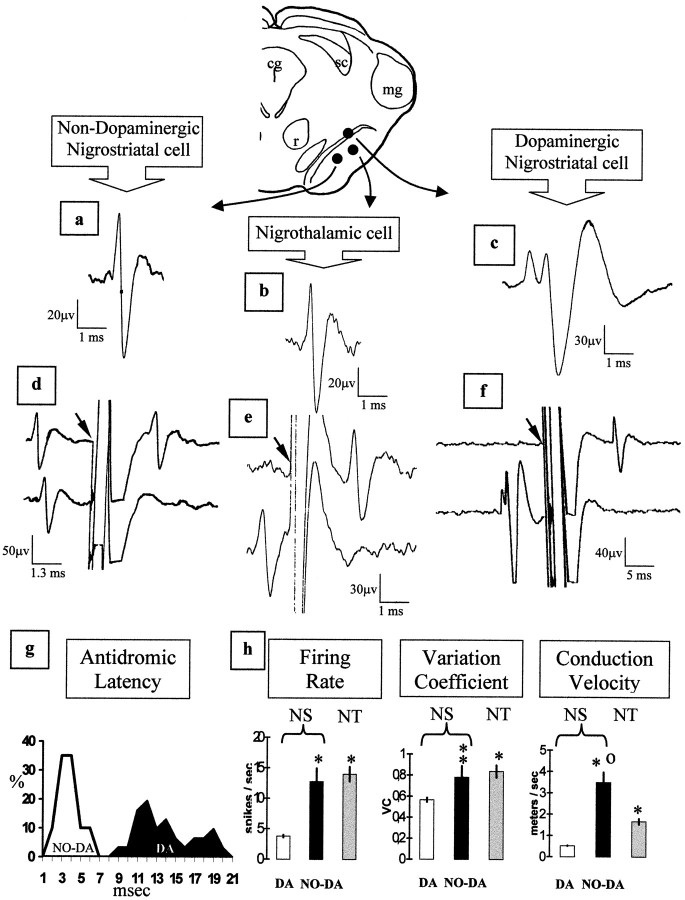
During this experiment 123 DA cells, 19 NO-DA cells, and 62 nigrothalamic cells were recorded. DA cells showed the characteristic triphasic action potential with the first phase being positive (often displaying a notch on the initial rising) and a duration of 2–6 msec (Fig. 1c). The DA cells showed a stable antidromic latency (oscillations <0.05 msec) and were identified as nigrostriatal neurons by the collision test (Fig.1f). The conduction velocity was 0.52 ± 0.023 m/sec (mean ± SE; Fig. 1h) with an antidromic latency of 7–20 msec (Fig. 1g). NO-DA cells showed a biphasic short-duration (often <1 msec) spike with a positive first phase (Fig.1a) and an antidromic response to striatal stimulation with a positive collision test (Fig. 1d). The conduction velocity was 3.48 ± 1.80 m/sec (Fig. 1h) with an antidromic latency of 0.5–5 msec (Fig. 1g). Nigrothalamic neurons displayed a characteristic biphasic action potential with a positive first phase and a duration of <1 msec (data not shown). The thalamic stimulation induced a stable antidromic response with a positive collision test (Fig. 1e) and a conduction velocity of 1.65 ± 1.03 m/sec (Fig. 1h). Thus, the conduction velocity was different in the three cell groups (ANOVA,F = 42.19; p < 0.0001), with the nigrothalamic neurons showing values lower than those of NO-DA cells (p < 0.0001) and higher than those of DA neurons (p < 0.0001).
Fig. 1.
Electrophysiological identification of NO-DA, DA, and nigrothalamic cells. An example of the spike shape (a–c) and collision test (d–f) in NO-DA, nigrothalamic, and DA cells, respectively, is shown. The antidromic response consisted of the full spike in NO-DA cells (d) and nigrothalamic cells (e) and the initial segment in DA cells (f). Because the antidromic stimulation of DA cells often displays the initial segment but not the somatodendritic component of the spike (spike dissociation), the antidromic spike shown at the top of f is clearly different (somatodendritic component virtually absent) from the spontaneous spike showed at the bottom off. g, Antidromic latency histogram of nigrostriatal cells. Values are expressed as a percentage of the total number of NO-DA (n = 19) or DA (n = 123) recorded neurons. h, Basal activity and conduction velocity of DA and NO-DA nigrostriatal (NS) and nigrothalamic (NT) cells. *p < 0.0001 versus NS DA cells; **p < 0.01 versus NS DA cells. °p < 0.0001 versus NT cells.Arrows in c and d indicate the initial part of the striatal stimulus artifact. Values represent the mean ± SE.
Statistical differences were also found for firing rate and VC (Fig.1h). Both NO-DA (p < 0.0001) and nigrothalamic (p < 0.0001) cells showed a firing rate higher than that found in DA cells (ANOVA,F = 67.42; p < 0.0001), with no difference between NO-DA and nigrothalamic neurons (p = 0.43). VC also showed higher values in NO-DA (p < 0.01) and nigrothalamic (p < 0.0001) cells than in DA cells (ANOVA,F, = 15.34; p < 0.0001), with no statistical difference between NO-DA and nigrothalamic cells (p = 0.51). Whereas a group of DA and nigrothalamic neurons showed a bursting activity, most NO-DA cells presented a regular tonic discharge, and only a few of them displayed irregular discharges.
Experiment 2: morphological and neurochemical identification of nondopaminergic nigrostriatal cells
Although as expected, after HRP injection in the striatum, most HRP-positive neurons in the basal midbrain were immunopositive for TH, a significant number of them were TH-negative (15.3%). They were preferentially located in the SNr (Figs.2, 3), in a lower proportion in VTA, and only a few of them in RRF (Fig. 3).
Fig. 2.

HRP-TH double labeling after HRP injection in the striatum of unlesioned rats. a, Rostrolateral region of the SN; b, boxed area ina; c, caudomedial region of the SN.Arrows in b and c indicate single HRP-stained neurons in focus with double-labeled neurons (arrowheads). Scale bars: a, 200 μm;b, c, 50 μm.
Fig. 3.
Mesencephalic distribution of cells projecting to the striatum according to their tyrosine hydroxylase immunoreactivity (n = 10,800 HRP cells). TH(+), Tyrosine hydroxylase-immunopositive cells; TH (−), tyrosine hydroxylase-immunonegative cells.
As mentioned above, to identify these nigrostriatal neurons neurochemically, alternate sections were immunostained with different nigral neuron markers. In this experiment, we focused on GABAergic neuron markers, using GAD, GABA, and PV immunocytochemistry in both colchicine-treated and untreated rats.
Double-labeled neurons were found in both colchine-treated and untreated rats (Fig. 4). The number of the GAD–HRP-positive cells (Fig. 4a–c) was slightly higher in the colchicine-treated rats, in which they reached levels of 5.2% of the total number of HRP-positive cells. In untreated rats, this proportion was 3.8%. The number of GABA–HRP-positive neurons (Fig.4d,e) was 1.5% of the total number of HRP neurons. PV–HRP-positive neurons (Fig. 4f) displayed the largest proportion of double-labeled neurons (7.7%). The percentage of GABA–HRP- and PV–HRP-labeled cells did not change after colchicine injection.
Fig. 4.
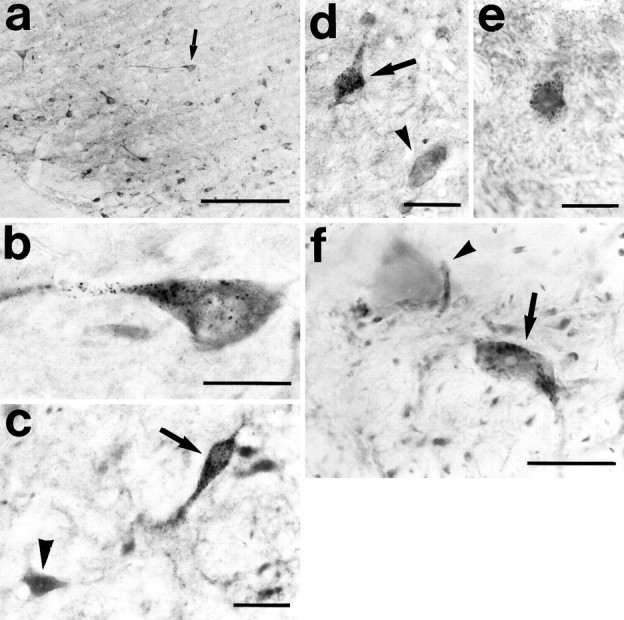
Double-labeled neurons for HRP and GAD (a–c), HRP and GABA (d, e), and HRP and PV (f) in the SNr (a, b, d–f) and VTA (c) of unlesioned rats. The arrow in a indicates the neuron shown in b. In c, d, andf, arrows indicate double-labeled neurons; arrowheads indicate single immunostained neurons. Scale bars: a, 200 μm; b, f, 20 μm; c–e, 30 μm.
The distribution pattern of double-labeled neurons was very similar to that of single HRP-stained neurons in material processed for TH with the largest number of GABA-projecting (77.8%), GAD-projecting (74.3%), and PV-projecting (80.2%) neurons localized in the SNr (Fig.4a,b,d,e,f), only a few of them in VTA (Fig.4c), and none in the SNc and RRF. Morphologically, they constitute a polymorphic population of elongated and round cells, with their largest diameter ranging between 15 and 23 μm. Although the aim of this study was not to investigate the topography of this projection, we observed that the localization of double-labeled cells in the SNr varied depending on the injection site in striatum. As shown in Figure5, neurons projecting to the medial part of striatum localize in medial regions of the SNr, and those projecting to the lateral part of striatum localize in the lateral part of the SNr. In the rostrocaudal axis, this topographic relation was not as evident as in the mediolateral one, because after injection in any place in the striatum, double-labeled cells were present at any rostrocaudal level of the SNr. However, after rostral injections, the largest number of double-labeled cells was in the rostral half of the SNr, and after caudal injections, they localize perferentially in caudal regions of the SNr.
Fig. 5.
Distribution of GAD-IR nigrostriatal neurons.a, Example of HRP injection in the striatum.b, Schematic drawing showing the localization of the HRP injection in some representative cases. c, Distribution of GAD-HRP neurons in the SN in two cases. Solid circlesindicate the localization of double-labeled neurons after injection in the lateral half of striatum (shown in a, black area in b). Open circles indicate the localization of double-labeled neurons after injection in the medial half of striatum (shadowed area inb). Each circle corresponds to three neurons. Modified drawing from Paxinos and Watson (1988). The distance of each section to the interaural line is shown in millimeters.ac, Anterior commissure; cc, corpus callosum; f, fornix; GP, globus pallidus;ic, internal capsule; lv, lateral ventricle; MT, medial terminal nucleus of the accessory optical tract; S, septum; SNl, substantia nigra pars lateralis; St, striatum. Scale bar, 1 mm.
Experiment 3: effect of dopaminergic nigrostriatal cells degeneration on nondopaminergic nigrostriatal neurons
The injection of 6-OHDA into the very center of the medial forebrain bundle led to the degeneration of practically all nigral DA-neurons ipsilateral to injection (Figs.6a,b, 8), affecting their different neurochemical subpopulations in the same way, as demonstrated by CCK (see Fig. 8), CR (Figs. 6e,f, 8) and CB (Figs.6g,h, 8) immunocytochemistry. By comparing the number of double labeled neurons in sham and lesioned rats (Fig.7), we found that after lesion, the percentage of HRP cells showing immunoreactivity for TH decreased from 82.3 to 1.2%, for CCK from 61.4 to 0.9%, for CB from 21.1 to 1.6%, and for CR from 86.1 to 1.6%.
Fig. 6.

TH (a–d), CR (e, f), and CB (g, h) immunocytochemistry after 6-OHDA injection in the right medial forebrain bundle and HRP injection in the ipsilateral striatum.a, e, g, Lesioned side; b, f, h, unlesioned side. c, d, Boxed areas ina; arrows indicate single HRP-stained neurons. Scale bars: a, b, e–h, 150 μm; c, d, 35 μm.
Fig. 8.
Percentage of nigrostriatal cells showing TH, CCK, CB, CR, GABA, GAD, and PV immunoreactivity in sham (n = 4200 HRP cells per series) and 6-OHDA-injected (n = 420 HRP cells per series) rats.
Fig. 7.

Double-labeled neurons for HRP and GAD (a), HRP and GABA (b), and HRP and PV (c, d) in SNr of 6-OHDA lesioned rats.d, Boxed area in c;arrows in b and d indicate double-labeled neurons; arrowhead in dindicates a single PV-immunostained neuron. Scale bars: a, b, 20 μm; c, 200 μm; d, 30 μm.
Despite the massive degeneration of DA cells, a number of retrogradely labeled neurons survived the neurotoxic effect of 6-OHDA (Fig.6a,c,d), most of them showing immunoreactivity for different GABA cell markers (Figs. 7, 8). Thus, when compared with the sham group, the percentage of HRP cells showing immunoreactivity for GABA (Fig. 7b) increased from 1.2 to 84.3%, those showing immunoreactivity for GAD (Fig. 7a) increased from 4.9 to 81.3%, and those showing immunoreactivity for PV (Fig. 7c,d) increased from 8.3 to 83.0%.
The electrophysiological study showed a functional modification of NO-DA and nigrothalamic cells after the 6-OHDA degeneration of DA neurons (Fig. 9). Although no statistical differences were found in the firing rate (p = 0.64) and conduction velocity (p = 0.45), NO-DA cells showed a higher VC in the 6-OHDA lesioned than in the sham-lesioned rats (p < 0.05). This effect was similar to that found in the nigrothalamic GABAergic neurons, with no modification in the firing rate (p = 0.45) and conduction velocity (p = 0.22) but with an increase in the VC (p < 0.05).
Fig. 9.
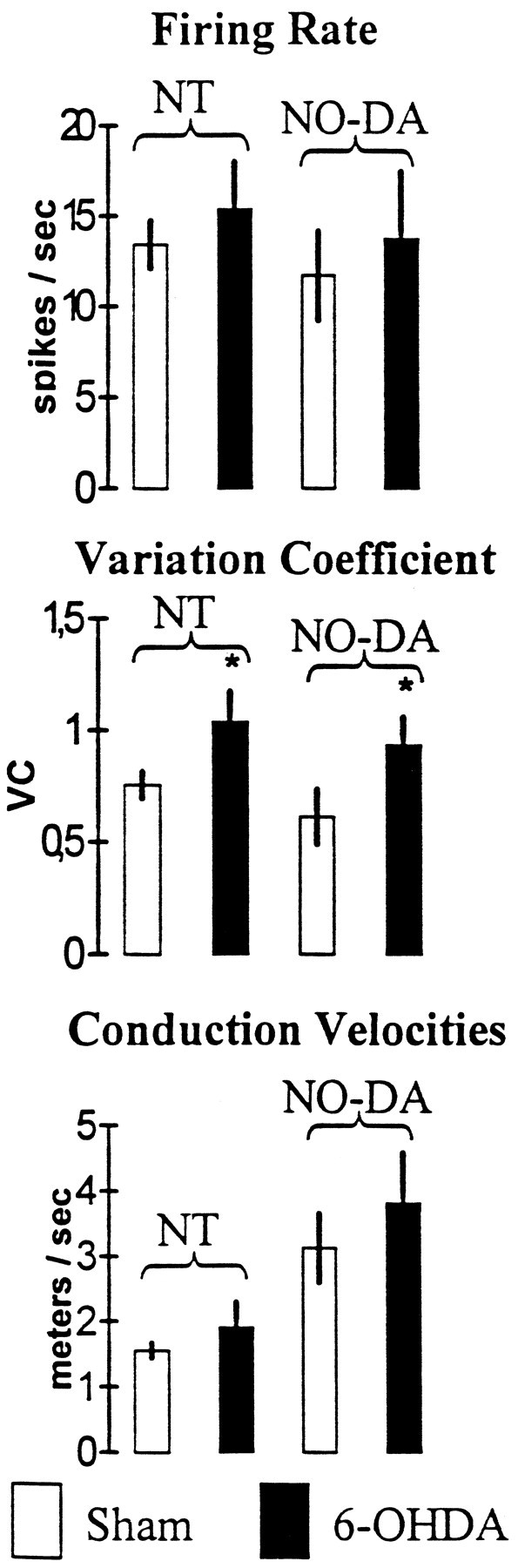
Functional effect of DA cell degeneration on NO-DA and nigrothalamic (NT) cells. *p < 0.05 versus sham-lesioned rats. Values represent the mean ± SE.
DISCUSSION
In this study we present evidence supporting the existence of GABAergic nigrostriatal cells with electrophysiological and neurochemical characteristics similar to those of GABAergic nigrothalamic cells but different from DA nigrostriatal neurons. After 6-OHDA injection, which produced the disappearance of the DA neurons, these cells remained intact, showing a change in the electrophysiological behavior similar to that found in nigrothalamic cells. Taken together, present findings indicate the existence of a GABAergic nigrostriatal pathway and suggest its involvement in the pathophysiology of Parkinson’s disease.
Data supporting a nondopaminergic nigrostriatal pathway
Our morphological data show a subpopulation of SN neurons retrogradely labeled after HRP injection in the striatum that is nonimmunoreactive for TH, CCK, CR, or CB, proteins expressed by DA cells (Hökfelt et al., 1980, 1985; Résibois and Rogers, 1992; Liang et al., 1996; McRitchie et al., 1996). The fact that these neurons do not degenerate after the injection of 6-OHDA, a neurotoxin selectively taken up by a transporter only contained in DA neurons (Hökfelt and Ungerstedt, 1973; Cohen and Heikkila, 1974;Walkinshaw and Waters, 1994; Ciliax et al., 1995; Betarbet et al., 1997; Miller et al., 1997), also supports the existence of NO-DA cells. Present findings confirm previous morphological studies reporting nigrostriatal cells without catecholamine fluorescence (van der Kooy et al., 1981) or TH immunoreactivity (Swanson, 1982; Gerfen et al., 1987) and provide further morphological evidence showing that they are not immunoreactive for CR, CB, and CCK.
The electrophysiological studies also identified a group of nigrostriatal neurons with a conduction velocity several times higher than that found in the DA cells. Although this cell type was mentioned in the early works of Deniau et al. (1978) and Guyenet and Aghajanian (1978), they were never systematically studied. As shown in Figure1f, these cells displayed a higher basal firing rate and VC than those of DA neurons. In the 6-OHDA lesioned rats, surviving nigral neurons showing an antidromic response to striatal stimulation presented a conduction velocity and basal firing rate similar to those found for NO-DA cells in unlesioned rats. This finding also indicates that 6-OHDA does not affect NO-DA cells.
Data supporting a GABAergic nigrostriatal pathway
Double labeling for HRP and GABA, GAD, or PV showed that 5–8% of nigrostriatal cells are GABAergic. This percentage reached 81–84% of all retrogradely labeled neurons in 6-OHDA lesioned rats. The difference observed in the experiment 2, between the percentage of nigrostriatal neurons showing TH immunonegativity (15%) and those showing GAD, GABA, and PV immunopositivity (5–8%), could suggest that not all NO-DA cells are GABAergic. The fact that after lesion (experiment 3) almost all TH, CCK, CR, and CB nigrostriatal neurons disappeared, whereas >80% of HRP neurons were immunopositive for GAD, GABA, and PV, indicates that if a subpopulation of NO-DA cells is not GABAergic, this is quantitatively negligible. It is possible that, despite our effort counting only those neurons lying in the surface of sections, and because of the limited penetration of antisera (8–10 μm) compared with that of HRP (evident at all depths of the sections), the number of single HRP-stained neurons was overestimated. So, we must be cautious when interpreting our quantitative data.
GABAergic nigrostriatal neurons are restricted to the SNr, and their distribution varies as a function of the injection site in striatum. In the mediolateral axis, they follow a topographic pattern similar to that previously described for the dopaminergic nigrostriatal projection (Fallon and Moore, 1978) and for strionigral descending fibers (Gerfen, 1985). In the rostrocaudal axis, the topography of GABAergic nigrostriatal neurons is also biased according to the injection site, matching that of the dopaminergic ones (Fallon and Moore, 1978) but differing from that of descending strionigral fibers that, independently of the injection site, are distributed homogeneously throughout the rostrocaudal axis of the SNr (Gerfen, 1985). Although our material does not allow us to establish a precise spatial correspondence in the GABAergic nigrostrial pathway, we can suggest that this may be involved in the modular organization of the nigro-strio-nigral loop (Deniau and Chevalier, 1992; Parent and Hazrati, 1995; Deniau and Thierry, 1997).
A further support for the GABAergic nature of NO-DA cells comes from electrophysiological data. The firing rate and VC of NO-DA cells were higher than those of DA cells (Grace and Bunney, 1980, 1983a–c,1984a,b, 1985a,b; DeLong et al., 1983; Schwarz et al., 1984; Schultz, 1986; Chiodo, 1988) but similar to those of other SN GABAergic neurons (Sanderson et al., 1986; Burbaud et al., 1995). Contrary to that reported in DA cells (Grace and Bunney, 1984a,b; Overton and Clark, 1997), NO-DA cells showed no clear evidence of burst discharge. Most of them displayed tonic discharges with different degrees of regularity, a pattern similar to that reported in two subgroups of nigrothalamic neurons: the regular tonic and the irregular tonic discharging groups (Sanderson et al., 1986; Burbaud et al., 1995). On the other hand, the conduction velocity suggests that these GABAergic nigrostriatal cells are probably composed of myelinated fibers of a relatively small diameter (3.48 ± 2.03 m/sec), in contrast to the unmyelinated axon of DA neurons (0.52 ± 0.02 m/sec).
It is known that, besides the dopaminergic nigrostriatal projection, there are DA cells projecting to the striatum from both VTA and RRF (Parent et al., 1983; Charara and Parent, 1984; Lewis and Sesack, 1997). Along with these cells, we found nondopaminergic cells transporting HRP from the striatum to VTA and RRF. Recently, GABAergic cells have been reported in the VTA (Steffensen et al., 1998). Our data show that the striatum is one of the targets for these cells, although the electrophysiological and neurochemical details of this projection require further study.
GABAergic nigrostriatal pathway and dopaminergic cell degeneration
In the current model of the organization of basal ganglia, the symptoms of different diseases have been explained on the basis of modifications in the firing rate of specific cell groups (Albin et al., 1989; Alexander and Crutcher, 1990a,b; DeLong, 1990), disturbances that may be attenuated by new neurosurgical therapies that modify the balance between excitatory and inhibitory inputs (Marsden et al., 1997;Obeso et al., 1997; Pollak et al., 1997; Viteck et al., 1997). It has been suggested that a reduction of striatal dopamine leads to activation of the inhibitory GABAergic nigrothalamic input (Bernheimer et al., 1973; Mitchell et al., 1989; DeLong, 1990; MacLeod et al., 1990; Condé, 1992; Herrero et al., 1993; Burbaud et al., 1995), and a subsequent reduction of thalamo-cortical activation (DeLong, 1990; Obeso et al., 1997). The final result could be a cortical inhibition that may be directly involved in different motor disturbances and especially in the hypokinesia often found in Parkinson’s disease. This hypothesis is supported by previous experiments showing that the degeneration of dopaminergic nigrostriatal cells produces an increase of 2-deoxyglucose uptake (Mitchell el al., 1989) and GAD mRNA levels (Herrero et al., 1993; Vila et al., 1996) in SNr cells. However, the overexpression of GAD and GABA does not necessarily imply an increase of GABAergic cell activity or GABA release. Contradictory results have been reported about the functional consequences of the DA cell degeneration on the GABA turnover (Tanaka et al., 1986; Lindgren, 1987; Samuel et al., 1988; Houssain and Weiner, 1995) and firing rate (Arbuthnott, 1974; Ruffieux and Schultz, 1980;Sanderson et al., 1986; MacLeod et al., 1990; Robledo and Féger, 1992; Burbaud et al., 1995) of SNr cells. Our data show that the increase of GAD expression previously reported by others in 6-OHDA lesioned rats is not associated with changes in the firing rate, at least in nigrothalamic and nigrostriatal GABAergic neurons. However, the VC increased in both groups. Despite the fact that we did not block 6-OHDA uptake into noradrenergic fibers, the effect appears to be secondary to the degeneration of DA neurons, because this was similar to that consistently reported in previous studies for nigrothalamic neurons (Ruffieux and Schultz, 1980; Sanderson et al., 1986; MacLeod et al., 1990). It has been suggested that a firing pattern code could be a more important feature than the mean firing rate for explaining the basal ganglia activity (Chesslet and Delfs, 1996; Wichmann and DeLong, 1996; Parent and Ciccheti, 1998). However, the characteristics of this code remain unknown (Eggermont, 1990), and only indirect measurements of the information processing in the basal ganglia can be taken. A suitable measure in this case is the VC, an indicator of the interspike interval variability that is invariant to the firing rate. From this point of view, the modification of VC observed in nigrothalamic and nigrostriatal GABAergic cells after 6-OHDA lesion suggests that DA cells modulate the SNr cell activity in a complex manner and do not induce a simple modification of the firing rate. Previous evidence showed that whereas the GABAergic neurons of SNr process and transmit information concerning different sensory or motor functions (DeLong et al., 1983; Hikosaka and Wurtz, 1983a–c; Schwarz et al., 1984; Joseph and Boussaoud, 1985; Schultz, 1986; Condé, 1992), the DA cells of SNc only have a role in maintaining stable levels of dopamine in the striatum (Gonon and Buda, 1985; Gonon, 1988, 1997; Levey et al., 1993;Overton and Clark, 1997), responding in a similar and stereotyped manner to sensory stimuli and not encoding detailed movement parameters (DeLong and Georgopoulos, 1979; Georgopoulos et al., 1983; Schultz et al., 1983; Schultz and Romo, 1990; Alexander and Crutcher, 1990a,b; Apicella et al., 1992; Schultz, 1998). Despite the fact that DA may not be directly involved in the information processing, its presence is necessary for nigrothalamic cells to analyze and transmit information to the thalamus on its return to the cerebral cortex (Penney and Young, 1983; Alexander at el., 1986; DeLong, 1990; Gerfen et al., 1990;Graybiel, 1990). Present data show a new pathway that can be used to reintroduce the information in the striatum, once it has been processed in the SN. Because the 6-OHDA lesion induces a similar modification in GABAergic cells projecting to the thalamus (a pathway extensively involved in the Parkinsonian symptoms) and striatum, present data suggest that GABAergic nigrostriatal neurons participate in the pathophysiology of this basal ganglia disorder.
Footnotes
This work was supported by Ministerio de Sanidad y Consumo, FIS, Grant 98/1499) and Gobierno Autonomo de Canarias Grant PI1998/008, Spain.
Correspondence should be addressed to Manuel Rodríguez, Department of Physiology, Faculty of Medicine, University of La Laguna, La Laguna, Tenerife, Canary Islands, Spain.
REFERENCES
- 1.Adams JC. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2:141–145. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- 2.Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- 3.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 4.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990a;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990b;68:945–960. doi: 10.1152/jn.1990.64.1.133. [DOI] [PubMed] [Google Scholar]
- 6.Alexander GE, DeLong MP, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurobiol. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 7.Arbuthnott GW. Spontaneous activity of spontaneous units in the striatum after unilateral destruction of the dopamine input. J Physiol (Lond) 1974;239:121–122. [PubMed] [Google Scholar]
- 8.Barroso N, Rodriguez M. Action of β-phenylethylamine and related amines on nigrostriatal dopamine neurotransmission. Eur J Pharmacol. 1996;297:195–203. doi: 10.1016/0014-2999(95)00757-1. [DOI] [PubMed] [Google Scholar]
- 9.Betarbet R, Turner R, Chockkan V, DeLong MR, Allers KA, Walters J, Levey AI, Greenamyre JT. Dopaminergic neurons intrinsic to the primate striatum. J Neurosci. 1997;17:6761–6768. doi: 10.1523/JNEUROSCI.17-17-06761.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbaud P, Gross C, Benazzouz A, Coussemacq M, Bioulac B. Reduction of apomorphine-induced rotational behaviour by subthalamic lesion in 6-OHDA lesioned rats is associated with a normalization of firing rate and discharge pattern of pars reticulata neurons. Exp Brain Res. 1995;105:48–58. doi: 10.1007/BF00242181. [DOI] [PubMed] [Google Scholar]
- 11.Burunat E, Castro R, Diaz-Palarea MD, Rodriguez M. Conditioning of the early behavioral response to apomorphine in the rotational model of Parkinson’s disease. Eur J Pharmacol. 1988;145:323–328. doi: 10.1016/0014-2999(88)90436-0. [DOI] [PubMed] [Google Scholar]
- 12.Campbell KJ, Takada M, Hattori T. Co-localization of tyrosine hydroxylase and glutamate decarboxylase in a subpopulation of single nigrotectal projection neurons. Brain Res. 1991;558:239–244. doi: 10.1016/0006-8993(91)90774-p. [DOI] [PubMed] [Google Scholar]
- 13.Castellano MA, Rodriguez M. Nigrostriatal dopaminergic cell activity is under control by substantia nigra of the contralateral brain side: electrophysiological evidence. Brain Res Bull. 1991;27:213–218. doi: 10.1016/0361-9230(91)90070-z. [DOI] [PubMed] [Google Scholar]
- 14.Castellano MA, Rivero FL, Rodriguez M. Spontaneous firing of nigrostriatal dopaminergic neurons in split-brain rats. Neurosc Lett. 1993;162:1–4. doi: 10.1016/0304-3940(93)90545-v. [DOI] [PubMed] [Google Scholar]
- 15.Castro R, Abreu P, Calzadilla CH, Rodriguez M. Increased or decreased locomotor response in rats following repeated administration of apomorphine depends on dosage intervals. Psychopharmacology. 1985;85:333–339. doi: 10.1007/BF00428198. [DOI] [PubMed] [Google Scholar]
- 16.Charara A, Parent A. Brainstem dopaminergic, cholinergic and serotonergic afferents to the pallidum in the squirrel monkey. Brain Res. 1984;640:155–170. doi: 10.1016/0006-8993(94)91870-8. [DOI] [PubMed] [Google Scholar]
- 17.Chesslet MF, Delfs JM. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996;19:417–422. doi: 10.1016/0166-2236(96)10052-7. [DOI] [PubMed] [Google Scholar]
- 18.Childs J, Gale K. Neurochemical evidence for a nigrotegmental GABAergic projection. Brain Res. 1983;258:109–114. doi: 10.1016/0006-8993(83)91233-7. [DOI] [PubMed] [Google Scholar]
- 19.Chiodo LA. Dopamine-containing neurons in the mammalian central nervous system: electrophysiology and pharmacology. Neurosci Biobehav Rev. 1988;12:49–91. doi: 10.1016/s0149-7634(88)80073-3. [DOI] [PubMed] [Google Scholar]
- 20.Ciliax BJ, Heilman C, Demchyshysn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen G, Heikkila RE. The generation of hydrogen peroxide, superoxide radical and hydroxyradical by 6-hydroxydopamine, dialuric acid and related cytotoxic agents. J Biol Chem. 1974;249:2447–2452. [PubMed] [Google Scholar]
- 22.Condé H. Organization and physiology of the substantia nigra. Exp Brain Res. 1992;88:233–248. doi: 10.1007/BF02259099. [DOI] [PubMed] [Google Scholar]
- 23.Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. demonstration of monoamines in the central nervous system. Acta Physiol Scand [Suppl] 1964;62:232. [PubMed] [Google Scholar]
- 24.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 25.DeLong MR, Georgopoulos AP. Motor functions of basal ganglia as revealed by studies of single cell activity in the behaving primate. Adv Neurol. 1979;24:131–140. [Google Scholar]
- 26.DeLong MR, Crutcher MD, Georgopoulos AP. Relations between movement and single cell discharge in the substantia nigra of the behaving monkey. J Neurosci. 1983;3:1599–1606. doi: 10.1523/JNEUROSCI.03-08-01599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deniau JM, Chevalier G. The lamellar organization of the rat substantia nigra pars reticulata: distribution of projection neurons. Neuroscience. 1992;46:361–377. doi: 10.1016/0306-4522(92)90058-a. [DOI] [PubMed] [Google Scholar]
- 28.Deniau JM, Thierry . Anatomical segregation of information processing in the rat substantia nigra pars reticulata. In: Obeso JA, Delong MR, Ohye C, Marsden CD, editors. Advances in neurology, Vol 74, The basal ganglia and new surgical approaches for Parkinson’s disease. Lippincott–Raven; Philadelphia: 1997. pp. 83–96. [PubMed] [Google Scholar]
- 29.Deniau JM, Hammond C, Riszk A, Feger J. Electrophysiological properties of identified output neurons of the rat substantia nigra (pars compacta and pars reticulata): evidences for the existence of branched neurons. Exp Brain Res. 1978;32:409–422. doi: 10.1007/BF00238711. [DOI] [PubMed] [Google Scholar]
- 30.Eggermont JJ. The correlative brain. Springer; Berlin: 1990. [Google Scholar]
- 31.Ehringer H, Hornykiewicz O. Verteilung von noradrenalin und dopamin (3-hydroxy-tyramin) im gehirn des menschen und ihr verhalten bei erkrankungen des extrapyramidalen systems. Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- 32.Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- 33.Fallon JH, Hicks BR, Loughlin SE. The origin of cholecystokinin terminals in the basal forebrain of the rat: evidence from immunofluorescence and retrograde tracing. Neurosci Lett. 1983;37:29–35. doi: 10.1016/0304-3940(83)90500-1. [DOI] [PubMed] [Google Scholar]
- 34.Freeman AS, Chiodo LA. Multiple effects of cholecystokinin octapeptide on identified nigrostriatal dopaminergic neurons. Brain Res. 1988;439:266–274. doi: 10.1016/0006-8993(88)91483-7. [DOI] [PubMed] [Google Scholar]
- 35.Garcia P, Paz C, Rodríguez J, Rodríguez M. Unsupervised classification of neural spikes with a hybrid multilayer artificial neural network. J Neurosci Methods. 1998;82:59–73. doi: 10.1016/s0165-0270(98)00035-1. [DOI] [PubMed] [Google Scholar]
- 36.Gerfen CR. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985;236:454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- 37.Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerfen CR, Engber TR, Mahan LC, Suzel Z, Chase TN, Monsma FR, Sibbley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;1990:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 39.Georgopoulos AP, DeLong MR, Crutcher MD. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci. 1983;3:1586–1598. doi: 10.1523/JNEUROSCI.03-08-01586.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- 41.Gonon FG. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonon FG, Buda MJ. Regulation of dopamine release by impulse flow and by autoreceptors as studied by in vivo voltametry in the rat striatum. Neuroscience. 1985;14:765–774. doi: 10.1016/0306-4522(85)90141-1. [DOI] [PubMed] [Google Scholar]
- 43.Goodman M, Perchére JF, Haiech J, Demaille JG. Evolutionary diversification of structure and function in the family of intracellular calcium-binding proteins. J Mol Evol. 1979;13:331–352. doi: 10.1007/BF01731373. [DOI] [PubMed] [Google Scholar]
- 44.Grace AA, Bunney BS. Nigral dopamine neurons: intracellular recording and identification with l-DOPA injection and histofluorescence. Science. 1980;210:645–656. doi: 10.1126/science.7433992. [DOI] [PubMed] [Google Scholar]
- 45.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons-1. Identification and characterization. Neuroscience. 1983a;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 46.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons-2. Action potential generating mechanisms and morphological correlates. Neuroscience. 1983b;10:317–331. doi: 10.1016/0306-4522(83)90136-7. [DOI] [PubMed] [Google Scholar]
- 47.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons-3. Evidence of electrotonic coupling. Neuroscience. 1983c;10:333–348. doi: 10.1016/0306-4522(83)90137-9. [DOI] [PubMed] [Google Scholar]
- 48.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984a;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984b;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985a;333:271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- 51.Grace AA, Bunney BS. Low doses of apomorphine elicit two opposing influences on dopamine cell electrophysiology. Brain Res. 1985b;333:285–298. doi: 10.1016/0006-8993(85)91582-3. [DOI] [PubMed] [Google Scholar]
- 52.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 53.Guyenet PG, Aghajanian GK. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 1978;150:69–84. doi: 10.1016/0006-8993(78)90654-6. [DOI] [PubMed] [Google Scholar]
- 54.Hedreen JC, DeLong MR. Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque. J Comp Neurol. 1991;304:569–595. doi: 10.1002/cne.903040406. [DOI] [PubMed] [Google Scholar]
- 55.Heizmann CW, Hunziker W. Intracellular calcium-binding proteins: more sites than insights. Trends Mol Sci. 1991;16:98–103. doi: 10.1016/0968-0004(91)90041-s. [DOI] [PubMed] [Google Scholar]
- 56.Herrero MT, Ruberg M, Hirsch EC, Movatt A, Obeso JA, Agid Y, Javoy-Agid F. In situ hybridization of GAD mRNA in monkey and human brain: quantification at both regional and cellular levels. Neurosci Lett. 1993;157:57–61. doi: 10.1016/0304-3940(93)90642-x. [DOI] [PubMed] [Google Scholar]
- 57.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983a;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- 58.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. II. Visual responses related to fixation of gaze. J Neurophysiol. 1983b;49:1254–1267. doi: 10.1152/jn.1983.49.5.1254. [DOI] [PubMed] [Google Scholar]
- 59.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983c;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- 60.Houssain MA, Weiner N. Interactions of dopamienrgic and GABAergic neurotransmission: impact of 6-hydroxydopamine lesions into the substantia nigra of rats. J Pharmacol Exp Ther. 1995;275:237–244. [PubMed] [Google Scholar]
- 61.Hökfelt T, Ungerstedt U. Specificity of 6-hydroxydopamine induced degeneration of central monoamine neurones: an electron and fluorescence microscopic study with special reference to intracerebral injection on the nigro-striatal dopamine system. Brain Res. 1973;60:269–297. doi: 10.1016/0006-8993(73)90791-9. [DOI] [PubMed] [Google Scholar]
- 62.Hökfelt T, Rehfeld JF, Skirboll L, Iremark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and cholecystokinin in the mesolimbic neurons. Nature. 1980;285:476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- 63.Hökfelt T, Skirboll L, Everitt B, Meister B, Brownstein M, Jacobs M, Faden S, Kuga S, Goldstein M, Markstein R, Dockray G, Rehfeld J. Distribution of cholecystokinin-like immunoreactivity in the nervous system: co-existence with classical neurotransmitter and other neuropeptides. Ann NY Acad Sci. 1985;448:255–274. doi: 10.1111/j.1749-6632.1985.tb29922.x. [DOI] [PubMed] [Google Scholar]
- 64.Iacopino AM, Quintero EM, Miller EK. Calbindin-D28k: a potential neuroprotective protein. Neurodegeneration. 1994;3:1–20. [Google Scholar]
- 65.Joseph JP, Boussaoud D. Role of the cat substantia nigra pars reticulata in eye and head movements I. Neural activity. Exp Brain Res. 1985;57:286–296. doi: 10.1007/BF00236534. [DOI] [PubMed] [Google Scholar]
- 66.Kilpatrick IC, Starr MS, Fletchar A, James TA, MacLeod NK. Evidence for a GABAergic nigrothalamic pathway in the rat. Exp Brain Res. 1980;40:45–54. doi: 10.1007/BF00236661. [DOI] [PubMed] [Google Scholar]
- 67.Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewis DA, Sesack SR. Dopamine systems in the primate brain. In: Bloom FE, Björklund A, Hökfelt T, editors. Handbook of chemical neuroanatomy, Vol 13, The primate nervous system, Pt I. Elsevier; Amsterdam: 1997. pp. 263–375. [Google Scholar]
- 69.Liang CL, Sinton CM, German DC. Midbrain dopaminergic neurons in the mouse: co-localization with calbindin-D28k and calrretinin. Neuroscience. 1996;75:523–533. doi: 10.1016/0306-4522(96)00228-x. [DOI] [PubMed] [Google Scholar]
- 70.MacLeod NK, Ryman A, Arbuthnott GW. Electrophysiological properties of nigrothalamic neurons after 6-hydroxydopamine lesions in the rat. Neuroscience. 1990;38:447–456. doi: 10.1016/0306-4522(90)90041-2. [DOI] [PubMed] [Google Scholar]
- 71.Marsden CD, Linazasoro G, Obeso JA. An introduction to the new surgery for Parkinson’s disease: past and present problems. In: Obeso JA, DeLong MR, Ohye C, Marsden CD, editors. Advances in neurology, Vol 74, The basal ganglia and new surgical approaches for Parkinson’s disease. Lippincott–Raven; New York: 1997. pp. 143–148. [PubMed] [Google Scholar]
- 72.McRitchie DA, Hardman CD, Halliday GM. Cytoarchitectural distribution of calcium binding proteins in midbrain dopaminergic regions of rats and humans. J Comp Neurol. 1996;364:121–150. doi: 10.1002/(SICI)1096-9861(19960101)364:1<121::AID-CNE11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 73.Miller GW, Staley JK, Heilman CJ, Perez JT, Mash DC, Rye DB, Levey AI. Immunocytochemical analysis of dopamine transporter protein in Parkinson’s disease. Ann Neurol. 1997;41:530–539. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- 74.Obeso JA, Rodríguez MC, DeLong MR. Basal ganglia pathophysiology: a critical review. In: Obeso JA, DeLong MR, Ohye C, Marsden CD, editors. Advances in neurology, Vol 74, The basal ganglia and new surgical approaches for Parkinson’s disease. Lippincott–Raven; New York: 1997. pp. 3–18. [PubMed] [Google Scholar]
- 75.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 76.Parent A, Ciccheti F. The current model of basal ganglia organization under scrutiny. Mov Disord. 1998;13:199–202. doi: 10.1002/mds.870130202. [DOI] [PubMed] [Google Scholar]
- 77.Parent A, Hazrati L-N. Functional anatomy of the basal ganglia. I. The cortico-basall ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 78.Parent A, Mackey A, De Bellefeuille L. The subcortical afferents to caudate nucleus and putamen in primate: a fluorescence retrograde double labeling study. Neuroscience. 1983;10:1137–1150. doi: 10.1016/0306-4522(83)90104-5. [DOI] [PubMed] [Google Scholar]
- 79.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1988. [DOI] [PubMed] [Google Scholar]
- 80.Penney JB, Young AB. Speculation on the functional anatomy of basal ganglia disorders. Annu Rev Neurosci. 1983;6:73–94. doi: 10.1146/annurev.ne.06.030183.000445. [DOI] [PubMed] [Google Scholar]
- 81.Pollak P, Benabid A-L, Limousin P, Benazzouz A. Chronic intracerebral stimulation in Parkinson’s disease. In: Obeso JA, DeLong MR, Ohye C, Marsden CD, editors. Advances in neurology, Vol 74, The basal ganglia and new surgical approaches for Parkinson’s disease. Lippincott–Raven; New York: 1997. pp. 113–120. [PubMed] [Google Scholar]
- 82.Rajakumar N, Elisevich K, Flumerfelt BA. Parvalbumin-containing GABAergic neurons in the basal ganglia output system of the rat. J Comp Neurol. 1994;350:324–336. doi: 10.1002/cne.903500214. [DOI] [PubMed] [Google Scholar]
- 83.Reiner A, Anderson KD. Co-occurrence of gamma-aminobutyric acid, parvalbumin and the neurotensin-related neuropeptide LANT6 in pallidal, nigral and striatal neurons in pigeons and monkeys. Brain Res. 1993;624:317–325. doi: 10.1016/0006-8993(93)90096-6. [DOI] [PubMed] [Google Scholar]
- 84.Résibois A, Rogers JH. Calretinin in the rat brain: an immunohistochemical study. Neuroscience. 1992;46:101–134. doi: 10.1016/0306-4522(92)90012-q. [DOI] [PubMed] [Google Scholar]
- 85.Robledo P, Féger J. Acute monoaminergic depletion in the rat potentiates the excitatory effect of the subthalamic nucleus in the substantia nigra pars reticulata but not in the pallidal complex. J Neural Transm Gen Sect. 1992;86:115–126. doi: 10.1007/BF01250572. [DOI] [PubMed] [Google Scholar]
- 86.Rodríguez M, Barroso N. Beta-phenylethylamine regulation of dopaminergic nigrostriatal cell activity. Brain Res. 1996;703:201–204. doi: 10.1016/0006-8993(95)01098-x. [DOI] [PubMed] [Google Scholar]
- 87.Rogers JH. A gene for a novel calcium-binding protein expressed principally in neurons. J Cell Biol. 1987;105:1343–1353. doi: 10.1083/jcb.105.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruffieux A, Schultz W. Dopaminergic activation of reticulata neurons in the substantia nigra. Nature. 1980;285:240–241. doi: 10.1038/285240a0. [DOI] [PubMed] [Google Scholar]
- 89.Samuel D, Kumar U, Niequllon A. Gamma-aminobutyric acid function in the rat striatum is under the double influence of nigrostriatal dopaminergic and thalamostriatal inputs: two models of regulation? J Neurochem. 1988;51:1704–1710. doi: 10.1111/j.1471-4159.1988.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 90.Sanderson P, Mavoungou R, Elbe-Fessard D. Changes in substantia nigra pars reticulata activity following lesions of the substantia nigra pars compacta. Neurosci Lett. 1986;67:25–30. doi: 10.1016/0304-3940(86)90202-8. [DOI] [PubMed] [Google Scholar]
- 91.Schultz W. Activity of pars reticulata neurons of monkey substantia nigra in relation to motor, sensory, and complex events. J Neurophysiol. 1986;55:660–677. doi: 10.1152/jn.1986.55.4.660. [DOI] [PubMed] [Google Scholar]
- 92.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 93.Schultz W, Romo R. Dopamine neurons of the monkey midbrain: contingencies of responses to stimuli eliciting immediate behavioral reactions. J Neurophysiol. 1990;63:607–624. doi: 10.1152/jn.1990.63.3.607. [DOI] [PubMed] [Google Scholar]
- 94.Schultz W, Ruffieux A, Aebischer P. The activity of pars compacta neurons of the monkey substantia nigra in relation to motor activation. Exp Brain Res. 1983;51:377–387. [Google Scholar]
- 95.Schwarz M, Sontag KH, Wand P. Sensory-motor processing in substantia nigra pars reticulata in conscious cats. J Physiol (Lond) 1984;347:129–147. doi: 10.1113/jphysiol.1984.sp015057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seroogy KB, Dangaran K, Lim S, Haycock JW, Fallon JH. Ventral mesencephalic neurons containing both cholecystokinin- and tyronine hydroxylase-like immunoreactivities project to forebrain regions. J Comp Neurol. 1989;279:397–414. doi: 10.1002/cne.902790306. [DOI] [PubMed] [Google Scholar]
- 97.Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 99.Tanaka Y, Niijima K, Mizuno Y, Yoshida M. Changes in GABA, glutamate, glycine and taurine contents in the striatum after unilateral nigrostriatal lesion in rats. Exp Neurol. 1986;91:259–268. doi: 10.1016/0014-4886(86)90066-x. [DOI] [PubMed] [Google Scholar]
- 100.van der Kooy D, Coscina DV, Hattori T. Is there a non-dopaminergic nigrostriatal pathway? Neuroscience. 1981;6:345–357. doi: 10.1016/0306-4522(81)90128-7. [DOI] [PubMed] [Google Scholar]
- 101.Vila M, Herrero MT, Levy R, Faucheux B, Ruberg M, Guillen J, Luquin MR, Guridi J, Javoy-Agid F, Agid Y, Obeso JA, Hirsch EC. Consequences of nigrostriatal denervation on the gamma-aminobutyric acid neurons of substantia nigra pars reticulata and superior colliculus in parkinsonian syndromes. Neurology. 1996;46:802–809. doi: 10.1212/wnl.46.3.802. [DOI] [PubMed] [Google Scholar]
- 102.Viteck JL, Bakay AE, DeLong MR. Microelectrode-guided pallidotomy for medically intractable Parkinson’s disease. In: Obeso JA, DeLong MR, Ohye C, Marsden CD, editors. Advances in neurology, Vol 74, The basal ganglia and new surgical approaches for Parkinson’s disease. Lippincott–Raven; New York: 1997. pp. 183–198. [PubMed] [Google Scholar]
- 103.Walkinshaw G, Waters CM. Neurotoxin-induced cell death in neuronal PC12 cells is mediated by induction of apoptosis. Neuroscience. 1994;63:975–987. doi: 10.1016/0306-4522(94)90566-5. [DOI] [PubMed] [Google Scholar]
- 104.Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- 105.Yamada T, McGeer PL, Baimbridge KG, McGeer EG. Relative sparing in Parkinson’s disease of substantia nigra dopamine neurons containing calbindin-D28k. Brain Res. 1990;526:303–307. doi: 10.1016/0006-8993(90)91236-a. [DOI] [PubMed] [Google Scholar]