Abstract
Recent work suggests that limiting the activation of the trkB subtype of neurotrophin receptor inhibits epileptogenesis, but whether or where neurotrophin receptor activation occurs during epileptogenesis is unclear. Because the activation of trk receptors involves the phosphorylation of specific tyrosine residues, the availability of antibodies that selectively recognize the phosphorylated form of trk receptors permits a histochemical assessment of trk receptor activation. In this study the anatomy and time course of trk receptor activation during epileptogenesis were assessed with immunohistochemistry, using a phospho-specific trk antibody. In contrast to the low level of phosphotrk immunoreactivity constitutively expressed in the hippocampus of adult rats, a striking induction of phosphotrk immunoreactivity was evident in the distribution of the mossy fibers after partial kindling or kainate-induced seizures. The anatomic distribution, time course, and threshold for seizure-induced phosphotrk immunoreactivity correspond to the demonstrated pattern of regulation of BDNF expression by seizure activity. These results provide immunohistochemical evidence that trk receptors undergo activation during epileptogenesis and suggest that the mossy fiber pathway is particularly important in the pro-epileptogenic effects of the neurotrophins.
Keywords: neurotrophins, BDNF, kindling, epilepsy, epileptogenesis, trk receptors
Elucidating the mechanisms of epileptogenesis in cellular and molecular terms may provide novel therapeutic approaches aimed at prevention of the disease. The distinguished French neurologist William Gowers noted, “The tendency of the disease (epilepsy) is to self-perpetuation; each attack facilitates the occurrence of another, by increasing the instability of the nerve elements” (Gowers, 1881). Direct support for Gowers’ idea that seizures beget seizures emerged from the discovery of the kindling model of epilepsy (Goddard et al., 1969); in this model, repeated focal application of initially subconvulsive electrical stimuli eventually results in intense focal and tonic–clonic seizures. Once established, this enhanced sensitivity to electrical stimulation persists for the life of the animal. Induction of repeated seizures by chemoconvulsants, including kainic acid, also can induce a kindling-like condition evident as an enhanced sensitivity to electrical stimulation-induced seizures (Vosu and Wise, 1975; Wasterlain and Jonec, 1983; Sutula et al., 1992; Croucher et al., 1995). The cellular and molecular events by which seizures beget more intense seizures are understood incompletely.
The discovery that limbic seizures increase the mRNA content of nerve growth factor (Gall and Isackson, 1989) led to the idea that seizure-induced expression of neurotrophic factors may contribute to the lasting structural and functional changes underlying epileptogenesis (Gall, 1993). Multiple investigators have found that the expression of genes encoding neurotrophic factors and their receptors is regulated prominently by seizure activity induced in diverse models. In particular, brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and trkB mRNA content are increased in kindling and other seizure models, whereas NT-3 mRNA content is decreased (Ernfors et al., 1991; Gall et al., 1991; Isackson et al., 1991; Dugich-Djordjevic et al., 1992a,b; Bengzon et al., 1993; Humpel et al., 1993; Merlio et al., 1993; Schmidt-Kastner and Olson, 1995;Mudo et al., 1996; Sato et al., 1996) (for review, see Gall, 1993). The magnitude of increase is greatest for BDNF mRNA and protein in the hippocampus, especially in the dentate gyrus (Lindvall et al., 1994;Nawa et al., 1995; Elmer et al., 1996b; Sato et al., 1996; Rudge et al., 1998).
A causal role for neurotrophins in epileptogenesis is supported by multiple studies of the kindling model. Funabashi et al. (1988) and Van der Zee et al. (1995) found that kindling development was delayed by intraventricular infusion of anti-NGF antisera. Kokaia et al. (1995)found a marked delay of kindling development in BDNF heterozygous mice (+/−) in which one BDNF allele had been inactivated by gene targeting. Recent work from this laboratory examined the effects of intraventricular administration of trk receptor “bodies” on kindling development; these receptor “bodies” contain the ligand-binding domain of distinct trk receptors fused to the Fc portion of human IgG1 and thus selectively bind distinct neurotrophins (Shelton et al., 1995). These studies demonstrated that infusion of trkB-Fc, but not trkA-Fc nor trkC-Fc, markedly inhibited kindling development; furthermore, the localization of infused trkB-Fc in the hippocampus, but not other regions, correlated with the anti-epileptogenic effects of trkB-Fc (Binder et al., 1999).
The anti-epileptogenic effects of trkB-Fc together with seizure-induced expression of BDNF suggested that trkB receptor activation may occur during epileptogenesis in the kindling model, but whether, when, or where trk receptors are activated in vivo is unknown. The development of phospho-specific trk antibodies that selectively detect phosphorylated trks provides the opportunity to assess directly the trk receptor activation ex vivo, using an immunohistochemical approach. Trk proteins are transmembrane receptor tyrosine kinases (RTKs) homologous to other RTKs such as the EGF receptor and insulin receptor family (Barbacid, 1994). Signaling by receptor tyrosine kinases involves ligand-induced receptor dimerization and dimerization-induced trans-autophosphorylation (Schlessinger and Ulrich, 1992; Guiton et al., 1994). Ligand-induced receptor tyrosine phosphorylation is required for neurotrophin-induced cellular responses (Barbacid, 1994). Tyrosine-490 is phosphorylated after neurotrophin application (Schlessinger and Ulrich, 1992); this allows specific intracellular target proteins to bind to the activated receptor via SH2 domains and leads to activation of the ras-MAP kinase cascade (Segal and Greenberg, 1996). The pY490 antibody detects phosphorylated trks on Western blots from cell lysates (Segal et al., 1996) and has been used in immunohistochemical assays to detect phosphorylated trks (Bhattacharyya et al., 1997; Schwartz et al., 1997). The goal of the present study was to determine whether, where, and when trk receptor activation occurred during epileptogenesis in the kindling and kainate models by using the pY490 antibody as an index of trk receptor activation.
MATERIALS AND METHODS
Antibodies. An affinity-purified phospho-specific trk antibody (pY490) directed against a synthetic phospho-tyr490 peptide corresponding to residues 485 to 493 (IENPQY*FSD) of human trkA was obtained commercially (New England Biolabs, Beverly, MA). This sequence is highly conserved among the three trk receptors and among rat, mouse, and human; the corresponding sequences of rat trks are trkA (MENPQYFSD), trkB (IENPQYFGI), and trkC (IENPQYFRQ). For peptide competition the pY490 phosphopeptide immunogen and cognate unphosphopeptide were used as described below; for an additional control, a dually tyrosine-phosphorylated trk phosphopeptide (STDY*Y*RVGG) (pY674/675) corresponding to residues 671–679 of human trkA was used.
In addition to the New England Biolabs pY490 antibody, a distinct affinity-purified polyclonal pY490 antibody directed against a synthetic phosphopeptide (VIENPQY*FGITNS) corresponding to residues 509–521 of rat trkB was used in the immunohistochemical assay (Segal et al., 1996). This peptide shares a seven-amino-acid sequence with that used for generation of the New England Biolabs antibody. Its specificity has been demonstrated previously in detecting phosphorylated trkA, trkB, and trkC on Western blots from cell lysates (Segal et al., 1996) and in immunohistochemical assays (Bhattacharyya et al., 1997; Schwartz et al., 1997).
Cell culture and Western blot analysis. To assess the specificity of the phosphotrk antibody, we treated cultured cells expressing trk receptors with neurotrophins and then subjected cell homogenates to Western blot analysis. PC12 cells expressing trkA were grown in six-well plates, using RPMI medium supplemented with 10% fetal bovine serum. Primary dissociated cortical cultures expressing trkB and trkC were prepared from E18 rat embryos and grown as previously described (Patel et al., 1996). Then 3 × 106 cortical cells were plated in six-well plates and treated after 5 d of growth in vitro. For treatment, dishes were washed gently with serum-free growth medium at 37°C for 15 min before the addition of reagents.
Neurotrophins (200 ng/ml; Promega, Madison, WI) were applied to PC12 or cortical cell cultures for 5 min at 37°C. After treatment, PC12 cells or cortical cultures were homogenized in 1:4 diluted Laemmli sample buffer with 1 mm sodium orthovanadate (0.0625 mTris-HCl, pH 6.8, 10% glycerol, 1.25% w/v sodium dodecyl sulfate, 5% βmercaptoethanol, 0.00125% bromphenol blue, and 1 mm sodium orthovanadate) by sonication for 15 sec; samples were boiled for 4 min, frozen, lyophilized, and resuspended in dH2O to one-fourth of the original volume.
For Western blots, samples were run on 6% SDS-PAGE gels and transferred to Immobilon-P membranes (Millipore, Bedford, MA). Membranes were fixed with 15 min immersion in 25% methanol/10% acetic acid, blocked for 1 hr in Blotto buffer (3% nonfat dry milk and 0.025% Tween-20 in TBS), and incubated overnight at 4°C in pY490 anti-phospho trk antibody (1:1000 in Blotto; New England Biolabs). Membranes subsequently were washed three times for 15 min in Blotto, incubated in peroxidase-conjugated goat anti-rabbit IgG (1:1000 in Blotto; New England Biolabs) for 1 hr at room temperature, washed three times for 15 min in Blotto, rinsed in TBS, incubated with a chemiluminescent detection reagent (Lumigen PS-3, Lumigen Technologies, Southfield, MI) for 1 min, and exposed to film.
After analysis of phosphotrk immunoblots, the membranes were incubated in stripping buffer (0.25 m glycine and 0.05% Tween 20, pH 2.5) at 80°C for 2 hr, reblocked with Blotto, and processed as described above, except that (1) primary antibody was a rabbit polyclonal antibody directed against the C terminus of all trks (Trk [C-14], 1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), and (2) a less sensitive chemiluminescence detection system was used (ECL, Amersham, Arlington Heights, IL).
Partial kindling by hippocampal stimulation. The 250–300 gm adult male Sprague Dawley rats (n = 38) were anesthetized with sodium pentobarbital (60 mg/kg) and placed in a stereotaxic frame. Bipolar electrodes made from Teflon-coated stainless steel wire were implanted into the right ventral hippocampus (n = 32; bregma as reference, coordinates: −4.8 mm anteroposterior, +5.2 mm lateral, 6.5 mm ventral to dura) or dorsal hippocampus (n = 9; coordinates: −3.3 mm anteroposterior, +2.0 mm lateral, 3.3 mm ventral to dura) (Paxinos and Watson, 1982). Electrodes were secured firmly to the skull with dental cement and anchor screws, and a ground wire was attached to one anchor screw. Animals were allowed to recover for 4 d after surgery before administration of the stimulations.
Each stimulation consisted of a 400 μA, 10 Hz, 10 sec train of 1 msec biphasic rectangular pulses with an interstimulus interval of 5 min, using a protocol for rapid hippocampal kindling adapted from previous studies (Lothman and Williamson, 1993; Elmer et al., 1996a). Behavioral (seizure class) and electrophysiological (electrographic seizure duration, ESD) parameters were recorded for each stimulation. Behavioral seizure class was scored according to Racine’s classification (Racine, 1972): Class 0, no behavioral change; Class 1, facial clonus; Class 2, head nodding; Class 3, unilateral forelimb clonus; Class 4, rearing with bilateral forelimb clonus; Class 5, rearing and falling (loss of postural control). Animals were stimulated until either one or seven hippocampal electrographic seizures (ESs) were elicited and then were killed at varying intervals (10 min, 3, 12, and 24 hr, and 1 week) thereafter. Sham-stimulated animals were treated identically, but no stimulation was given. This particular paradigm of partial kindling was selected because previous studies demonstrated that these are the minimal conditions required for seizure-induced increase of BDNF mRNA content (Bengzon et al., 1993; Elmer et al., 1996b); because ∼15 stimulations of ventral hippocampus are required to induce kindling as evident by Class 5 seizures, this paradigm is a form of partial kindling. These and other procedures involving animals followed National Institutes of Health guidelines for the care and use of experimental animals.
Kainic acid-induced status epilepticus. The 250–300 gm adult male Sprague Dawley rats (n = 23) were injected with kainic acid (15 mg/kg, i.p.) dissolved in saline or with saline alone. During the injection period the animals were observed continuously for tonic–clonic seizure activity. Animals were injected with 5 mg/kg kainic acid each half hour, starting 1 hr after the original 15 mg/kg injection until they exhibited continuous tonic–clonic seizure activity (status epilepticus). After at least 4 hr of continuous seizure activity, status epilepticus was terminated with pentobarbital (50 mg/kg, i.p.). Animals were killed immediately or at varying intervals (3, 12, 24, and 48 hr and 1 week) after pentobarbital treatment. This paradigm was selected because of previous studies establishing the conditions in which kainate-induced status epilepticus induced increased BDNF mRNA content (Dugich-Djordjevic et al., 1992a); this paradigm is an alternative method of inducing epileptogenesis, because many animals treated similarly exhibit spontaneous seizures when studied weeks to months later (Hellier et al., 1998).
Perfusion and histology. At various times after partial kindling or kainate status epilepticus, the animals were anesthetized (pentobarbital, 60 mg/kg, i.p.) and perfused intracardially with ice-cold 4% paraformaldehyde in 1× PBS containing 1 mmsodium orthovanadate (PBSV) for 5 min at 50 ml/min. Brains were dissected, post-fixed overnight at 4°C, cryoprotected in 20% sucrose and 1× PBV until they sank, and then frozen in isopentane in a dry ice/methanol bath. Coronal frozen sections (40 μm) were cut, and two sections per slide were wet-mounted in PBSV onto Superfrost (Corning, Corning, NY) slides, air-dried, and stored frozen at −70°C.
Phosphotrk immunohistochemistry. Slides (two sections per slide) were thawed in room temperature PBSV (10 min), endogenous peroxidase activity was quenched with 0.3% H2O2/MeOH (30 min), slides were washed in PBSV (10 min), blocked and permeabilized in PBSV, 5% normal goat serum, and 0.5% NP-40 (1 hr), and then washed in PBSV (10 min). Twenty microliters of 1° antibody (Ab) (1:10 NEB anti-pY490 diluted in PBSV and 5%NGS) were applied to each slide, and the slides were coverslipped and stored in a humidified chamber at 4°C overnight. For peptide competitions, phosphopeptide immunogen, cognate unphosphopeptide, or unrelated phosphopeptide was incubated at room temperature with the 1° antibody solution at indicated concentrations for at least 30 min before application to slides. The next day the coverslips were removed; the slides were washed in PBSV and 5% NGS (two times for 10 min), exposed to 2° Ab [1:200 biotinylated anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) diluted in PBSV and 5% NGS] (1 hr), washed in PBSV and 5% NGS (two times for 10 min), exposed to ABC reagent (Vectastain Elite, Vector Laboratories, Burlingame, CA) (30 min), washed in PBSV and 5% NGS (two times for 10 min), exposed to biotinyl tyramide solution (1:100 BT stock solution, Bio-Rad, Richmond, CA) (30 min), washed in PBSV and 5% NGS (two times for 10 min), exposed again to ABC reagent (30 min), washed in PBSV and 5% NGS (two times for 10 min), and developed for 10–30 min in DAB solution containing 0.03% H2O2 and 0.04% nickel ammonium sulfate. Then the slides were rinsed in PBS, dehydrated in ethanols, cleared in xylene, and coverslipped with Permount.
Quantification of staining intensity. Sections at equivalent coronal levels (−3.60 mm from bregma) (Paxinos and Watson, 1982) were analyzed, and Nissl-stained alternate sections were used to verify the identity of structures. For quantitative analysis of staining intensity, sections from each animal from the partial kindling protocol were analyzed by densitometry. Four hippocampi per animal (one slide per animal containing two adjacent sections, each with two hippocampi) were analyzed blinded to treatment. For densitometry, images of the immunoreactivity in the CA3 and dentate gyrus were captured with a high-resolution CCD camera interfaced with a light microscope (ZeissICM 405, Oberkochen, Germany) under a 10× objective and measured with a computer-assisted image analyzer (Image-1, Universal Imaging, West Chester, PA). For CA3 analysis, white and black reference images were obtained, and a square box the width of the pyramidal cell layer was placed in CA3a just proximal to the junction with CA2 to measure the average gray value for strata radiatum, lucidum, pyramidale, and oriens in individual hippocampi. Because the stratum pyramidale had the highest gray value (least immunoreactive), the results are presented as a percentage of reduction in gray value compared with stratum pyramidale for strata oriens, lucidum, and radiatum. For densitometry of the dentate hilus a similar procedure was used. A square box the width of the granule cell layer was placed to measure the average gray value in six different locations: outer molecular layer (OML), middle molecular layer (MML), inner molecular layer (IML), granule cell layer (GCL), hilar border with granule cell layer (hilus–GCL border), and deep hilus at the midpoint between blades of the granule cell layers (hilus). Results for OML, MML, IML, hilus-GCL border, and hilus are presented as a percentage of reduction in gray value compared with GCL.
RESULTS
Specificity of pY490 phosphotrk antibody
The specificity of the pY490 antibody from New England Biolabs was assessed in Western blot experiments in which PC12 cells were treated with vehicle or NGF, and E18 rat cortical cells were treated with vehicle, BDNF, or NT-3. In PC12 cells, treatment with NGF, but not vehicle, resulted in a strongly immunoreactive band at ∼140 kDa (Fig.1A1). Stripping the blot of antibody and reprobing with a pan-trk antibody that recognizes all trk receptors independent of phosphorylation state revealed a band of similar intensity in both lanes that comigrated with the phosphotrk-immunoreactive band (Fig.1A2). Similarly, in E18 cortical cells, treatment with BDNF and NT-3, but not vehicle, resulted in a strongly immunoreactive band at ∼140 kDa (Fig.1B1). Again, stripping the blot of antibody and reprobing with the pan-trk antibody revealed a band that comigrated with the phosphotrk-immunoreactive band in all lanes (Fig. 1B2). These results indicate that the pY490 antibody selectively recognizes phosphorylated trk proteins.
Fig. 1.
PY490 phosphotrk antibody recognizes phosphorylated trks. A1, Western blot of PC12 cultures treated with vehicle or NGF and probed with pY490 phosphotrk antibody. A2, Blot from A1 stripped of antibody and reprobed with pan-trk antibody.B1, Western blot of E18 cortical cultures treated with vehicle, BDNF, or NT-3 and probed with pY490 phosphotrk antibody. B2, Blot from B1 stripped of antibody and reprobed with pan-trk antibody.
Increased hippocampal phosphotrk immunoreactivity after partial kindling
Partial kindling induced by hippocampal stimulations produced a spatially selective increase of phosphotrk immunoreactivity in hippocampus. Phosphotrk immunoreactivity in the hippocampus of untreated or sham-stimulated controls was confined to the neuropil, particularly in the hilus of the dentate gyrus immediately beneath the granule cell layer; by contrast, there was no detectable immunoreactivity in the dentate granule cell or CA3 or CA1 pyramidal cell layers (Fig. 2B). In each of five animals killed 24 hr after partial hippocampal kindling, an increase of phosphotrk immunoreactivity was evident in the dentate hilus and in stratum lucidum of hippocampus (Fig.2C). The increased immunoreactivity was evident bilaterally in sections from dorsal hippocampus in those animals who had undergone stimulation of the right ventral hippocampus. In addition, increases of phosphotrk immunoreactivity may have been present in stratum oriens of CA3 and the molecular layer of the dentate gyrus of stimulated animals (Fig. 2C), but such findings were much less robust than stratum lucidum. No increase of immunoreactivity was observed in the dentate granule cell or pyramidal cell layers or in CA1 stratum lacunosum moleculare after partial kindling. Although phosphotrk immunoreactivity was evident in multiple areas of forebrain of the unstimulated controls, including neocortex, some thalamic nuclei, piriform cortex, and elsewhere, no obvious increases of immunoreactivity were evident in any of these regions after partial kindling. Importantly, the paucity of phosphotrk immunoreactivity in stratum lucidum and dentate hilus of unstimulated animals simplified detection of the increased immunoreactivity after partial kindling; by contrast, the abundant phosphotrk immunoreactivity in multiple areas of forebrain of unstimulated controls could obscure the detection of kindling-induced increases in some of these regions.
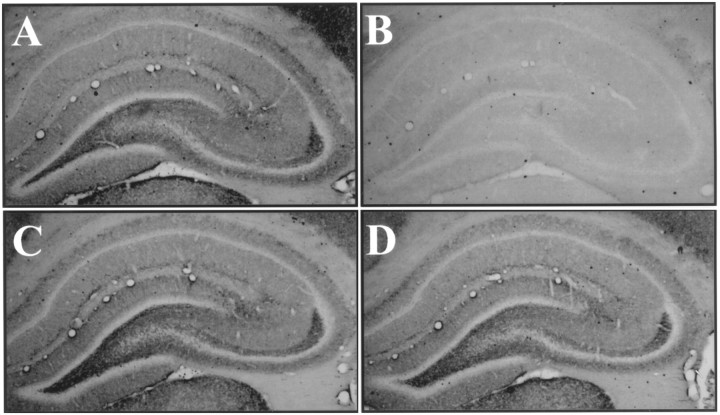
Fig. 2.

Seizures increase phosphotrk immunoreactivity in hilus and CA3 stratum lucidum. A, Nissl-stained coronal section through hippocampus showing cell body layers (DG and CA1–CA3).B, Phosphotrk immunoreactivity in sham-stimulated animal. Note the presence of light immunoreactivity in neuropil but its absence in cell body layers. C, Phosphotrk immunoreactivity in an animal 24 hr after seven ventral hippocampal ESs. Note the marked increase in immunoreactivity in dentate hilus and stratum lucidum of CA3 (arrowheads); the remainder of hippocampal neuropil also appears slightly more immunoreactive, whereas the cell body layers still display an absence of immunoreactivity.
To assess the specificity of the phosphotrk immunoreactivity in unstimulated animals as well as after partial kindling, we performed the following experiments. Preincubation of the pY490 antibody with the pY490 phosphopeptide immunogen (300 nm) virtually abolished the immunoreactivity in sections from both unstimulated control (data not shown) and partially kindled animals (Fig.3B). The immunoreactivity is specific to the phosphorylated form of the protein sequence insofar as preincubation of the antibody with unphosphorylated peptide (300 nm) exerted no detectable effect on the immunoreactivity (Fig. 3C). Further evidence that the immunoreactivity is specific to the phosphotrk sequence derives from the observation that preincubation of the antibody with a hundred-fold greater concentration of an unrelated tyrosine phosphopeptide (30 μmphosphopeptide 674/675) produced no detectable attenuation of the phosphotrk immunoreactivity (Fig. 3D). Importantly, no immunoreactivity was detectable after omission of the primary antibody (NEB pY490) (data not shown). Additional immunohistochemical experiments were performed with an affinity-purified antibody raised against a distinct but overlapping phosphopeptide sequence that included the tyrosine phosphorylated form of 490 (Segal et al., 1996). Blinded analysis of sections from three pairs of control and partially kindled animals showed induction of phosphotrk immunoreactivity in hilus and stratum lucidum of CA3 in partially kindled animals similar to the NEB pY490 antibody, albeit with lower signal/noise ratio (data not shown).
Fig. 3.
Peptide competition of phosphotrk immunoreactivity. Shown is phosphotrk immunoreactivity in coronal sections of hippocampus from a single animal killed 24 hr after seven ventral hippocampal ESs. A, No peptide.B, Preincubation of pY490 antibody with 300 nm phosphopeptide 490 immunogen. C, Preincubation with 300 nm unphosphopeptide 490.D, Preincubation with 30 μm phosphopeptide 674/5.
Assessment of time course and quantitation of phosphotrk immunoreactivity after partial kindling
To assess the time course of the partial kindling-induced increase of phosphotrk immunoreactivity, we analyzed sections from animals killed at 3 hr (n = 5), 12 hr (n = 5), 24 hr (n = 5), or 1 week (n = 5) after hippocampal stimulation and compared them with control sham-stimulated animals (n = 5). Increases of phosphotrk immunoreactivity were evident in the dentate hilus and stratum lucidum of the hippocampus in each of the five animals killed at 24 hr but in none of the animals killed at the other time points (Fig.4, right column). Thus, partial kindling consistently led to a striking but transient increase in phosphotrk immunoreactivity in the hippocampus and in particular to the hilus and stratum lucidum of CA3.
Fig. 4.

Time course of phosphotrk immunoreactivity in hippocampus and CA3 after seven ventral hippocampal electrographic seizures. Shown is phosphotrk immunoreactivity in representative coronal sections of hippocampus from sham-stimulated animals and animals killed 3, 12, and 24 hr and 1 week after seven ventral hippocampal electrographic seizures. The whole hippocampus is shown on the left, and the CA3 region is shown on theright. Note the temporal (24 hr only) and spatial (hilus and stratum lucidum of CA3) pattern of the increase in phosphotrk immunoreactivity.
To assess quantitatively the anatomy and time course of partial kindling-induced changes in hippocampal phosphotrk immunoreactivity, we performed densitometric analysis of CA3 and the dentate hilus in sections from each animal. Analysis of the CA3 region disclosed increases of phosphotrk immunoreactivity in stratum lucidum in animals killed 24 hr after the last stimulation (one way ANOVA,p < 0.01); by contrast, no measurable increases were detectable in stratum lucidum at any of the other time points (Fig.5). In contrast to the increases evident in stratum lucidum, no significant increases were detected in either stratum radiatum or oriens, but a nonsignificant trend of an increase was in stratum oriens of CA3 (Fig. 5). Analysis of the strata of the dentate gyrus disclosed a similar time course in which increases were detected in the dentate hilus near the border of the GCL and also deep in the hilus in animals killed at 24 hr (p < 0.01), but not at other time points (p > 0.05), after hippocampal stimulation (Fig. 6). A nonsignificant trend to an increase of immunoreactivity was evident in the inner, middle, and outer molecular layers in animals killed at 24 hr after the last seizure (Fig. 6). In addition, the magnitude of partial kindling-induced phosphotrk immunoreactivity in hilus and CA3 stratum lucidum at 24 hr was not different between hippocampus ipsilateral versus contralateral to the stimulating electrode (data not shown), confirming that the effect is bilaterally symmetric. Together, these quantitative measures reinforced the impression obtained from visual analysis of the sections.
Fig. 5.

Time course of phosphotrk immunoreactivity in CA3 after seven ventral hippocampal kindling stimulations. The data are expressed as a percentage of reduction in gray value in the given stratum as compared with stratum pyramidale (see Materials and Methods); thus, higher values reflect more intense immunoreactivity. Each symbol corresponds to one animal. Horizontal lines denote mean values. **p < 0.01 compared with all of the other time points by ANOVA with post hoc Bonferroni’s test.
Fig. 6.

Time course of phosphotrk immunoreactivity in dentate gyrus after seven ventral hippocampal kindling stimulations. The data are expressed as a percentage of reduction in gray value in the given stratum as compared with the granule cell layer (see Materials and Methods); thus, higher values reflect more intense immunoreactivity. Each symbol corresponds to one animal.Horizontal lines denote mean values. **p < 0.01 compared with all of the other time points by ANOVA with post hoc Bonferroni’s test.OML, Outer molecular layer; MML, middle molecular layer; IML, inner molecular layer.
Correlation between features of seizures and phosphotrk immunoreactivity
To determine the seizure parameters required for the induction of the phosphotrk immunoreactivity in this partial kindling paradigm, we correlated the immunohistochemical results with the behavioral and electrographic features of the seizures. Among the five animals killed at 24 hr, each of which exhibited the increased phosphotrk immunoreactivity, the total electrographic seizure duration was 280 ± 15 sec (range, 244–338 sec); the seizure duration of this subset was representative of the entire group (n = 20) of stimulated animals in the partial kindling experiments in which the mean duration was 265 ± 12 sec (range, 163–368 sec). The behavioral features of the seizures in this partial kindling paradigm consist of periodic wet dog shakes, a pattern typical of hippocampal seizures (Frush and McNamara, 1986). In some instances Class 1 and 2 seizures also were observed, with a return to overtly normal behavior typically occurring immediately on cessation of the brief seizures. No clonic or tonic seizures nor seizures of Class 3 or greater were observed. To determine whether a single electrographic seizure was sufficient to induce the increased phosphotrk immunoreactivity, we stimulated three additional animals once; they were killed 24 hr later. The increase of phosphotrk immunoreactivity in a pattern similar to that described in animals receiving seven stimulations was observed in one animal who exhibited the longest electrographic seizure (71 sec); no increase was evident in the other two animals who exhibited briefer seizures (39 and 30 sec, respectively). Taken together, these findings demonstrate that brief limbic seizures associated with the early stages of kindling development are sufficient to induce the increased phosphotrk immunoreactivity.
Anatomy and time course of phosphotrk immunoreactivity after kainate status epilepticus
The above findings demonstrated that brief hippocampal seizures with subtle behavioral correlates are sufficient to induce increased phosphotrk immunoreactivity in the hippocampal formation. To determine whether more intense seizures of a sustained nature might induce a distinct pattern of increased immunoreactivity, we induced hippocampal and tonic–clonic seizures persisting continuously for at least 4 hr by kainic acid; animals were killed at varying intervals thereafter. Although the typical pattern of phosphotrk immunoreactivity in hippocampal neuropil was evident in sections from vehicle-treated control animals, the pattern of increased phosphotrk immunoreactivity in dentate hilus and stratum lucidum was evident bilaterally in the hippocampus in sections from each animal killed either 24 (n = 5) or 48 (n = 4) hr after kainate-induced status epilepticus. No overt differences in immunoreactivity were evident in brain regions outside of the hippocampus. In contrast to the results from 24 or 48 hr, no increase of phosphotrk immunoreactivity was evident in sections from animals killed 3 hr (n = 4) after kainate; the characteristic pattern of increased phosphotrk immunoreactivity in hippocampus was detected in one of three animals killed 1 week after kainate-induced status epilepticus. Thus, kainate-induced status epilepticus leads to a dramatic increase in phosphotrk immunoreactivity, with a similar anatomic pattern and time course to that observed with partial kindling.
DISCUSSION
Our previous pharmacological studies led us to hypothesize that trkB receptors undergo activation during epileptogenesis but whether, where, or when this occurred was uncertain. The present work began to test this hypothesis by using an immunohistochemical measure of trk receptor activation. Two principal findings emerge. First, partial kindling induced by stimulation of the right ventral hippocampus evokes an increase of phosphotrk immunoreactivity with a highly specific anatomic and temporal pattern. The increased immunoreactivity is evident bilaterally in dentate hilus and CA3 stratum lucidum and is detectable at 24 hr, but not at 3 or 12 hr or 7 d after partial kindling. Second, more intense seizure activity evoked by kainate status epilepticus induces increased phosphotrk immunoreactivity with an anatomic distribution and time course similar to that induced by partial kindling.
Identity of the molecule reflected in increased phosphotrk immunoreactivity
Converging lines of evidence support the conclusion that a phosphorylated form of a trk receptor underlies the increased immunoreactivity induced by partial kindling or KA in these immunohistochemical experiments. Immunoblot experiments established that the antibody recognizes phosphorylated trk. That is, treatment of PC12 cells with NGF or cortical cells with BDNF or NT-3 induced a pY490-immunoreactive band that comigrates with trk (see Fig. 1). Furthermore, the specificity of the pY490 antibody in immunocytochemical studies was reinforced by preabsorption experiments; that is, partial kindling-induced phosphotrk immunoreactivity virtually was eliminated by preabsorption with the phosphotrk peptide, but not by the unphosphorylated trk peptide nor by a 100-fold greater concentration of an unrelated tyrosine phosphopeptide (see Fig. 3). These findings were reinforced by observations with a different polyclonal antibody raised against a distinct but overlapping pY490 phosphopeptide. Together, this evidence provides strong support that the immunoreactivity detected here reflects a phosphorylated form of a trk receptor.
Precisely which trk receptor is detected by the pY490 antibody in the immunohistochemical experiments is uncertain. The phosphopeptide used to raise the NEB antibody consists of nine amino acids present in human trkA; eight of these nine residues are conserved in rat trkA and seven in rat trkB and trkC. The induction of phosphotrk immunoreactivity on immunoblots after treatment with agonists of the trkA, trkB, and trkC receptors (NGF, BDNF, and NT-3, respectively) implies that the antibody can recognize each of these three receptors when phosphorylated at the site corresponding to pY490 (see Fig. 1). The abundance of mRNA of trkB and trkC, but not trkA, in dentate granule cell and CA3 pyramidal cell layers of rat hippocampus (Bengzon et al., 1993; Merlio et al., 1993;Cellerino, 1996) suggests that the phosphotrk immunoreactivity observed here is likely to be trkB or trkC. The increased phosphotrk immunoreactivity may reflect trkB or trkC that is expressed constitutively and simply post-translationally modified. Importantly, partial kindling evokes increased mRNA content of trkB and trkC in dentate granule cell and CA3 pyramidal cell layers within 2 hr after repeated seizures (Bengzon et al., 1993; Merlio et al., 1993); this raises the alternative possibility that the increased phosphotrk immunoreactivity reflects newly synthesized and post-translationally modified trkB or trkC.
Circumstantial evidence implicating seizure induction of BDNF expression as the cause of the increased phosphotrk immunoreactivity
What is likely responsible for the post-translational modification of trk that contributes to the increased phosphotrk immunoreactivity observed 1 d after the partial kindling paradigm or status epilepticus? The binding of neurotrophin to the trk receptor induces dimerization and trans-autophosphorylation of a subset of tyrosine residues (Schlessinger and Ulrich, 1992; Guiton et al., 1994). Phosphorylation of tyrosine 490 in particular, the molecular event presumably underlying the immunohistochemical change, is a valuable index of the ability of trk to serve as a scaffold for the assembly and activation of signaling molecules (Middlemas et al., 1994; Segal and Greenberg, 1996). Thus, it seems plausible that at least part of the mechanism underlying the increased phosphotrk immunoreactivity is the binding of neurotrophin to trk and its subsequent activation. The occurrence of the increased phosphotrk immunoreactivity after seizures implies that some consequence of the seizures is responsible; one possibility is that the seizure induced increased expression of a neurotrophin that is translated, transported and released, thereby activating trk.
Analysis of the temporal and anatomic patterns of seizure-mediated regulation of neurotrophins supports the candidacy of BDNF. The mRNA and protein content of both BDNF and NGF is increased after seizures (Ernfors et al., 1991; Gall et al., 1991; Isackson et al., 1991;Dugich-Djordjevic et al., 1992a; Bengzon et al., 1993; Humpel et al., 1993; Mudo et al., 1996; Sato et al., 1996); by contrast, the mRNA content of NT-3 is decreased after seizures (Bengzon et al., 1993;Schmidt-Kastner and Olson, 1995; Mudo et al., 1996), whereas NT-4 mRNA content in hippocampus is undetectable (Ernfors et al., 1991; Gall et al., 1991; Isackson et al., 1991; Dugich-Djordjevic et al., 1992a,b;Bengzon et al., 1993; Humpel et al., 1993; Merlio et al., 1993; Timmusk et al., 1993; Schmidt-Kastner and Olson, 1995; Mudo et al., 1996; Sato et al., 1996). The seizure-mediated regulation of BDNF protein peaks at 24 hr, the time point corresponding to increased phosphotrk immunoreactivity, whereas the content of NGF protein peaks at 1 week after seizures (Bengzon et al., 1992; Nawa et al., 1995;Elmer et al., 1996a; Rudge et al., 1998). The anatomic distribution of BDNF further supports its candidacy in that immunohistochemical studies have localized the basal and seizure-mediated increase of BDNF immunoreactivity to the dentate hilus and stratum lucidum of CA3 (Conner et al., 1997; Yan et al., 1997b; Rudge et al., 1998) (C. Gall, unpublished results), a pattern coinciding with that of the increased phosphotrk immunoreactivity.
The occurrence of increased NPY immunoreactivity after seizures provides additional circumstantial evidence supporting BDNF. Direct intracerebral infusion of BDNF, but not NGF, is sufficient to evoke increased amounts of NPY mRNA and peptide levels (Croll et al., 1994), implicating the activation of trkB receptors. Moreover, both kindling and kainate-induced seizures induce increased neuropeptide Y as detected immunohistochemically in the dentate hilus and CA3 stratum lucidum (Marksteiner et al., 1990; Tønder et al., 1994). The occurrence of the increased NPY immunoreactivity at the same time as the peak of the seizure-induced BDNF content (24 hr) and in the same anatomic distribution (dentate hilus and stratum lucidum) of the BDNF suggests that BDNF induced the increase of NPY, presumably by activating trkB. The identification of the increased phosphotrk immunoreactivity in the predicted anatomic pattern and at the predicted time point is consistent with this suggestion and thereby provides additional circumstantial evidence that the phosphotrk is phosphotrkB.
Cellular site of seizure-induced phosphotrk immunoreactivity
What is the likely cellular site of partial kindling-induced phosphotrk immunoreactivity? The light microscopic distribution of the increased phosphotrk immunoreactivity in the dentate hilus and stratum lucidum of CA3 corresponds to the mossy fiber axons of the dentate granule cells. One possibility is that the cellular site of phosphotrk immunoreactivity resides on postsynaptic targets of the mossy fibers, including dendrites of the CA3 pyramidal cells and potentially interneurons in stratum lucidum, together with a diversity of additional potential targets in the dentate hilus; the presence of >20 types of hilar neurons provides a large number of possible targets. By contrast, presynaptic localization of the immunoreactivity intrinsic to the mossy fiber axons would be sufficient to account for its presence throughout the dentate hilus and stratum lucidum. Although the “presynaptic” locale is the most parsimonious explanation, ultrastructural studies will be required to address this question. In either case the distribution of the phosphotrk immunoreactivity, both constitutively and after partial kindling, differs from that of trkB-like immunoreactivity as revealed by studies that used an affinity-purified antibody directed against an extracellular trkB peptide sequence (Fryer et al., 1996; Yan et al., 1997a). That is, the trkB-like immunoreactivity was distributed preferentially on cell bodies and dendrites of hippocampal pyramidal and granule cells (Fryer et al., 1996; Yan et al., 1997a), whereas mossy fiber axons do not display strong trkB immunoreactivity (Yan et al., 1997a). Importantly, this trkB antibody does not distinguish between full-length and truncated (Barbacid, 1994) forms of trkB receptors; because the truncated forms predominate in the mature rat brain (Knusel et al., 1994; Fryer et al., 1996), it seems plausible that the phosphotrk immunoreactivity may reflect a subset of the trk proteins recognized by the anti-trkB antibody.
trkB receptors and epileptogenesis: effects on synaptic transmission?
Elucidating the answers to the questions considered in the preceding paragraphs will be necessary to understand the significance of trk receptor activation in epileptogenesis in this model. If our suspicion that the increased phosphotrk immunoreactivity reflects the activation of trkB is correct, this finding—together with the finding that pharmacological interventions limiting trkB activation inhibit kindling development (Binder et al., 1999)—raises the following question: what consequences of trkB receptor activation contribute to the increased excitability of kindling? We favor the idea that BDNF-mediated activation of trkB enhances excitatory transmission at the mossy fiber→CA3 pyramidal cell synapse, either directly by enhancing the efficacy of the mossy fiber→CA3 excitatory synapse or indirectly by reducing the efficacy of the mossy fiber synapse onto inhibitory interneurons in stratum lucidum. Indeed, BDNF has been demonstrated to enhance excitatory synaptic transmission (Lohof et al., 1993; Kang and Schuman, 1995; Levine et al., 1995; Stoop and Poo, 1996) and reduce inhibitory synaptic transmission (Penschuck et al., 1997;Tanaka et al., 1997) in hippocampus. A critical level of BDNF/trkB activation appears to be vital for modulation of synaptic efficacy: hippocampal slices from BDNF knock-out animals exhibit impaired LTP induction (Korte et al., 1995, 1996; Patterson et al., 1996), and pretreatment of adult hippocampal slices with trkB-Fc reduces LTP (Figurov et al., 1996). Interestingly, acute application of exogenous BDNF to hippocampal slices preferentially enhances the efficacy of the excitatory mossy fiber synapse onto CA3 pyramidal cells (Scharfman, 1997). These results implicating BDNF in the modulation of synaptic transmission coincide with the observation of increased excitability of CA3 pyramidal cells in kindled animals as detected by increased epileptiform bursting induced by elevated K+ or lowered Mg2+ in isolated hippocampal slices (King et al., 1985; Behr et al., 1998). The pivotal role of the CA3 pyramidal cells in promoting epileptiform activity in the hippocampus and the role of BDNF in hippocampal synaptic transmission, together with the localization of seizure-induced trk receptor activation in CA3 stratum lucidum, suggest that enhancing the mossy fiber excitation of CA3 pyramidal cells (either directly or indirectly) may be a pivotal mechanism by which BDNF activation of trkB promotes epileptogenesis.
Footnotes
This work was supported by National Institutes of Health Grant NS-17771 (J.O.M.). We thank R. Segal for helpful comments and R. Segal and H. Ruan for their kind gifts of antibodies and peptides.
Correspondence should be addressed to Dr. James O. McNamara, 401 Bryan Research Building, Duke University Medical Center, Durham, NC 27705.
REFERENCES
- 1.Barbacid M. The trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 2.Behr J, Lyson KJ, Mody I. Enhanced propagation of epileptiform activity through the kindled dentate gyrus. J Neurophysiol. 1998;79:1726–1732. doi: 10.1152/jn.1998.79.4.1726. [DOI] [PubMed] [Google Scholar]
- 3.Bengzon J, Soderstrom S, Kokaia Z, Kokaia M, Ernfors P, Persson H, Ebendal T, Lindvall O. Widespread increase of nerve growth factor protein in the rat forebrain after kindling-induced seizures. Brain Res. 1992;587:338–342. doi: 10.1016/0006-8993(92)91016-8. [DOI] [PubMed] [Google Scholar]
- 4.Bengzon J, Kokaia Z, Ernfors P, Kokaia M, Leanza G, Nilsson OG, Persson H, Lindvall O. Regulation of neurotrophin and trkA, trkB, and trkC tyrosine kinase receptor messenger RNA expression in kindling. Neuroscience. 1993;53:433–446. doi: 10.1016/0306-4522(93)90207-v. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya A, Watson FL, Bradlee TA, Pomeroy SL, Stiles CD, Segal RA. Trk receptors function as rapid retrograde signal carriers in the adult nervous system. J Neurosci. 1997;17:7007–7016. doi: 10.1523/JNEUROSCI.17-18-07007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder DK, Routbort MJ, Ryan TE, Yancopoulos GD, McNamara JO. Selective inhibition of kindling development by intraventricular administration of trkB receptor body. J Neurosci. 1999;19:1424–1436. doi: 10.1523/JNEUROSCI.19-04-01424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cellerino A. Expression of messenger RNA coding for the nerve growth factor receptor trkA in the hippocampus of the adult rat. Neuroscience. 1996;70:613–616. doi: 10.1016/s0306-4522(96)83001-6. [DOI] [PubMed] [Google Scholar]
- 8.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS—evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croll SD, Wiegand SJ, Anderson KD, Lindsay RM, Nawa H. Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur J Neurosci. 1994;6:1343–1353. doi: 10.1111/j.1460-9568.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 10.Croucher MJ, Cotterell KL, Bradford HF. Amygdaloid kindling by repeated focal N-methyl-d-aspartate administration: comparison with electrical kindling. Eur J Pharmacol. 1995;286:265–271. doi: 10.1016/0014-2999(95)00459-6. [DOI] [PubMed] [Google Scholar]
- 11.Dugich-Djordjevic MM, Tocco G, Lapchak PA, Pasinetti GM, Najm I, Baudry M, Hefti F. Regionally specific and rapid increases in brain-derived neurotrophic factor messenger RNA in the adult rat brain following seizures induced by systemic administration of kainic acid. Neuroscience. 1992a;47:303–315. doi: 10.1016/0306-4522(92)90246-x. [DOI] [PubMed] [Google Scholar]
- 12.Dugich-Djordjevic MM, Tocco G, Willoughby DA, Najm I, Pasinetti G, Thompson RF, Baudry M, Lapchak PA, Hefti F. BDNF mRNA expression in the developing rat brain following kainic acid-induced seizure activity. Neuron. 1992b;8:1127–1138. doi: 10.1016/0896-6273(92)90133-x. [DOI] [PubMed] [Google Scholar]
- 13.Elmer E, Kokaia M, Kokaia Z, Ferencz I, Lindvall O. Delayed kindling development after rapidly recurring seizures: relation to mossy fiber sprouting and neurotrophin, GAP-43, and dynorphin gene expression. Brain Res. 1996a;712:19–34. doi: 10.1016/0006-8993(95)01424-1. [DOI] [PubMed] [Google Scholar]
- 14.Elmer E, Kokaia Z, Kokaia M, Carnahan J, Nawa H, Bengzon J, Lindvall O. Widespread increase of brain-derived neurotrophic factor protein in the rat forebrain after kindling-induced seizures. Soc Neurosci Abstr. 1996b;22:2089. [Google Scholar]
- 15.Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- 16.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 17.Frush DP, McNamara JO. Evidence implicating dentate granule cells in wet dog shakes produced by kindling stimulations of entorhinal cortex. Exp Neurol. 1986;92:102–113. doi: 10.1016/0014-4886(86)90128-7. [DOI] [PubMed] [Google Scholar]
- 18.Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, Kromer LF. Developmental and mature expression of full-length and truncated trkB receptors in the rat forebrain. J Comp Neurol. 1996;374:21–40. doi: 10.1002/(SICI)1096-9861(19961007)374:1<21::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Funabashi T, Sasaki H, Kimura F. Intraventricular injection of antiserum to nerve growth factor delays the development of amygdaloid kindling. Brain Res. 1988;458:132–136. doi: 10.1016/0006-8993(88)90504-5. [DOI] [PubMed] [Google Scholar]
- 20.Gall CM. Seizure-induced changes in neurotrophin expression: implications for epilepsy. Exp Neurol. 1993;124:150–166. doi: 10.1006/exnr.1993.1186. [DOI] [PubMed] [Google Scholar]
- 21.Gall CM, Isackson PJ. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989;245:758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- 22.Gall CM, Lauterborn J, Bundman M, Murray K, Isackson P. Seizures and the regulation of neurotrophic factor and neuropeptide gene expression in brain. Epilepsy Res Suppl. 1991;4:225–245. [PubMed] [Google Scholar]
- 23.Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- 24.Gowers WR. Epilepsy and other chronic convulsive diseases. Churchill; London: 1881. [Google Scholar]
- 25.Guiton M, Gunn-Moore FJ, Stitt TN, Yancopoulos GD, Tavare JM. Identification of in vivo brain-derived neurotrophic factor-stimulated autophosphorylation sites on the trkB receptor tyrosine kinase by site-directed mutagenesis. J Biol Chem. 1994;269:30370–30377. [PubMed] [Google Scholar]
- 26.Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 27.Humpel C, Wetmore C, Olson L. Regulation of brain-derived neurotrophic factor messenger RNA and protein at the cellular level in pentylenetetrazol-induced epileptic seizures. Neuroscience. 1993;53:909–918. doi: 10.1016/0306-4522(93)90476-v. [DOI] [PubMed] [Google Scholar]
- 28.Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- 29.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 30.King GL, Dingledine R, Giacchino JL, McNamara JO. Abnormal neuronal excitability in hippocampal slices from kindled rats. J Neurophysiol. 1985;54:1295–1304. doi: 10.1152/jn.1985.54.5.1295. [DOI] [PubMed] [Google Scholar]
- 31.Knusel B, Rabin SJ, Hefti F, Kaplan DR. Regulated neurotrophin receptor responsiveness during neuronal migration and early differentiation. J Neurosci. 1994;14:1542–1554. doi: 10.1523/JNEUROSCI.14-03-01542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kokaia M, Ernfors P, Kokaia Z, Elmer E, Jaenisch R, Lindvall O. Suppressed epileptogenesis in BDNF mutant mice. Exp Neurol. 1995;133:215–224. doi: 10.1006/exnr.1995.1024. [DOI] [PubMed] [Google Scholar]
- 33.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindvall O, Kokaia Z, Bengzon J, Elmer E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 37.Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 38.Lothman EW, Williamson JM. Rapid kindling with recurrent hippocampal seizures. Epilepsy Res. 1993;14:209–220. doi: 10.1016/0920-1211(93)90045-9. [DOI] [PubMed] [Google Scholar]
- 39.Marksteiner J, Ortler M, Bellmann R, Sperk G. Neuropeptide Y biosynthesis is markedly induced in mossy fibers during temporal lobe epilepsy of the rat. Neurosci Lett. 1990;112:143–148. doi: 10.1016/0304-3940(90)90193-d. [DOI] [PubMed] [Google Scholar]
- 40.Merlio JP, Ernfors P, Kokaia Z, Middlemas DS, Bengzon J, Kokaia M, Smith ML, Siesjo BK, Hunter T, Lindvall O. Increased production of the TrkB protein tyrosine kinase receptor after brain insults. Neuron. 1993;10:151–164. doi: 10.1016/0896-6273(93)90307-d. [DOI] [PubMed] [Google Scholar]
- 41.Middlemas DS, Meisenhelder J, Hunter T. Identification of trkB autophosphorylation sites and evidence that phospholipase C-γ1 is a substrate of the trkB receptor. J Biol Chem. 1994;269:5458–5466. [PubMed] [Google Scholar]
- 42.Mudo G, Jiang XH, Timmusk T, Bindoni M, Belluardo N. Change in neurotrophins and their receptor mRNAs in the rat forebrain after status epilepticus induced by pilocarpine. Epilepsia. 1996;37:198–207. doi: 10.1111/j.1528-1157.1996.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 43.Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 44.Patel M, Day BJ, Crapo JD, Fridovich I, McNamara JO. Requirement for superoxide in excitotoxic cell death. Neuron. 1996;16:345–355. doi: 10.1016/s0896-6273(00)80052-5. [DOI] [PubMed] [Google Scholar]
- 45.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knock-out mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; Sydney: 1982. [DOI] [PubMed] [Google Scholar]
- 47.Penschuck S, Fritschy J-M, Thoenen H, Berninger B. Regulation of GABAA receptor expression by BDNF in hippocampal neurons in vitro. Soc Neurosci Abstr. 1997;23:45. [Google Scholar]
- 48.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 49.Rudge JS, Mather PE, Pasnikowski EM, Cai N, Corcoran T, Acheson A, Anderson K, Lindsay RM, Wiegand SJ. Endogenous BDNF protein is increased in adult rat hippocampus after a kainic acid induced excitotoxic insult, but exogenous BDNF is not neuroprotective. Exp Neurol. 1998;149:398–410. doi: 10.1006/exnr.1997.6737. [DOI] [PubMed] [Google Scholar]
- 50.Sato K, Kashihara K, Morimoto K, Hayabara T. Regional increases in brain-derived neurotrophic factor and nerve growth factor mRNAs during amygdaloid kindling, but not in acidic and basic fibroblast growth factor mRNAs. Epilepsia. 1996;37:6–14. doi: 10.1111/j.1528-1157.1996.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 51.Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol. 1997;78:1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- 52.Schlessinger J, Ulrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992;9:381–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt-Kastner R, Olson L. Decrease of neurotrophin-3 mRNA in adult rat hippocampus after pilocarpine seizures. Exp Neurol. 1995;136:199–204. doi: 10.1006/exnr.1995.1096. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA. Abnormal cerebellar development and foliation in BDNF −/− mice reveals a role for neurotrophins in CNS patterning. Neuron. 1997;19:269–281. doi: 10.1016/s0896-6273(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 55.Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 56.Segal RA, Bhattacharyya A, Rua LA, Alberta JA, Stephens RM, Kaplan DR, Stiles CD. Differential utilization of trk autophosphorylation sites. J Biol Chem. 1996;271:20175–20181. doi: 10.1074/jbc.271.33.20175. [DOI] [PubMed] [Google Scholar]
- 57.Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoop R, Poo MM. Synaptic modulation by neurotrophic factors: differential and synergistic effects of brain-derived neurotrophic factor and ciliary neurotrophic factor. J Neurosci. 1996;16:3256–3264. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sutula T, Cavazos J, Golarai G. Alteration of long-lasting structural and functional effects of kainic acid in the hippocampus by brief treatment with phenobarbital. J Neurosci. 1992;12:4173–4187. doi: 10.1523/JNEUROSCI.12-11-04173.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Timmusk T, Belluardo N, Metsis M, Persson H. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. Eur J Neurosci. 1993;5:605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 62.Tønder N, Kragh J, Finsen B, Bolwig TG, Zimmer J. Kindling induces transient changes in neuronal expression of somatostatin, neuropeptide Y, and calbindin in adult rat hippocampus and fascia dentata. Epilepsia. 1994;35:1299–1308. doi: 10.1111/j.1528-1157.1994.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 63.Van der Zee CE, Rashid K, Le K, Moore KA, Stanisz J, Diamond J, Racine RJ, Fahnestock M. Intraventricular administration of antibodies to nerve growth factor retards kindling and blocks mossy fiber sprouting in adult rats. J Neurosci. 1995;15:5316–5323. doi: 10.1523/JNEUROSCI.15-07-05316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vosu H, Wise RA. Cholinergic seizure kindling in the rat: comparison of caudate, amygdala, and hippocampus. Behav Biol. 1975;13:491–496. doi: 10.1016/s0091-6773(75)91109-8. [DOI] [PubMed] [Google Scholar]
- 65.Wasterlain CG, Jonec V. Chemical kindling by muscarinic amygdaloid stimulation in the rat. Brain Res. 1983;271:311–316. doi: 10.1016/0006-8993(83)90293-7. [DOI] [PubMed] [Google Scholar]
- 66.Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of trkB in the central nervous system of the adult rat. J Comp Neurol. 1997a;378:135–157. [PubMed] [Google Scholar]
- 67.Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997b;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]