Abstract
The prelimbic/medial orbital areas (PL/MO) of the rat prefrontal cortex are connected to substantia nigra pars reticulata (SNR) through three main circuits: a direct nucleus accumbens (NAcc)–SNR pathway, an indirect NAcc–SNR pathway involving the ventral pallidum (VP) and the subthalamic nucleus (STN), and a disynaptic cortico-STN–SNR pathway. The present study was undertaken to characterize the effect of PL/MO stimulation on SNR cells and to determine the contribution of these different pathways. The major pattern of responses observed in the SNR was an inhibition preceded by an early excitation and followed or not by a late excitation. The inhibition resulted from the activation of the direct NAcc–SNR pathway because it disappeared after acute blockade of the glutamatergic cortico-striatal transmission by CNQX application into the NAcc. The late excitation resulted from the activation of the indirect NAcc–VP–STN–SNR pathway via a disinhibition of the STN because it disappeared after either CNQX application into the NAcc or blockade of the GABAergic striato-pallidal transmission by bicuculline application into the VP. The early excitation, which was markedly decreased after blockade of the cortico-STN transmission by CNQX application into the STN, resulted from the activation of the disynaptic cortico-STN–SNR pathway. Finally, the blockade of the cortico-STN–VP circuit by CNQX application into STN or VP modified the influence of the trans-striatal circuits on SNR cells. This study suggests that, in the prefrontal cortex–basal ganglia circuits, the trans-subthalamic pathways, by their excitatory effects, participate in the shaping of the inhibitory influence of the direct striato-nigral pathway on SNR neurons.
Keywords: basal ganglia circuits, prefrontal cortex, subthalamic nucleus, ventral striatum, nucleus accumbens, ventral pallidum, substantia nigra pars reticulata, in vivo single unit recordings, rat
The substantia nigra pars reticulata (SNR) and the internal pallidum (GPi), or entopeduncular nucleus in the rat, are the two main output stations of the basal ganglia. Through these output nuclei, information processed in the basal ganglia are directed to thalamic nuclei and pontomesencephalic structures. In current working models of basal ganglia (Alexander et al., 1986; Parent and Hazrati 1995a), the striatum is considered as a main input structure through which cortical information is transferred to the SNR and GPi. The striatum, which receives excitatory afferents from the entire cerebral cortex, projects to the SNR and GPi through a direct and an indirect pathway. The indirect pathway involves two tightly interconnected structures: the external segment of the globus pallidus and the subthalamic nucleus (STN). The STN also receives direct inputs from motor and prefrontal areas of the cerebral cortex and is thus considered as an input structure of the basal ganglia (Berendse and Groenewegen, 1991; Parent and Hazrati, 1995b). The neurons of this network are GABAergic, except those of the STN, which are glutamatergic.
The convergent nature of the cortico-striatal projections and the subsequent striato-nigral and striato-pallidal pathways has long been emphasized (Percheron and Filion, 1991). However, there is now growing evidence that signals originating from functionally distinct cortical areas are processed in separate striatal subterritories and remain segregated in the striato-pallido- and striato-nigro-thalamic pathways (Alexander et al., 1986; Groenewegen and Berendse, 1994; Deniau and Thierry, 1997). This led to the proposal cortico-basal ganglia circuits are essentially organized in parallel channels (Alexander et al., 1986).
We have recently described in the rat the anatomo-functional organization of the prefrontal channel originating from the prelimbic and medial orbital areas (PL/MO). PL/MO areas send an excitatory input to a restricted territory of the ventral striatum, the core of the nucleus accumbens (NAcc), which projects through a direct and an indirect pathway to the dorsomedial part of the SNR (Deniau et al., 1994; Montaron et al., 1996; Maurice et al., 1997, 1998b). The indirect pathway involves the region of the ventral pallidum (VP), denominated lateral ventral pallidum (VPl) by Zahm (1989), and the medial part of the STN. In addition, the medial STN receives a direct excitatory input from the prefrontal cortex and can thus be also viewed as an input structure in this prefrontal channel (Berendse and Groenewegen, 1991;Maurice et al., 1998a).
The present study was undertaken to determine the contribution of the trans-striatal and trans-subthalamic circuits in the influence exerted by PL/MO areas on the activity of the SNR. For this purpose, the responses evoked in SNR cells by PL/MO stimulation were characterized, and we investigated the effects of reversible blockade of the synaptic transmission in the NAcc, the VP, or the STN on these responses.
MATERIALS AND METHODS
Experiments were performed on 37 adult male Sprague Dawley rats (weighing 275–300 gm; Charles River, Saint-Aubin les Elbeuf, France). Animals were anesthetized with ketamine (100 mg/kg, i.p.; supplemented by 50 mg/kg, i.m., injections; Imalgène 500; Rhône-Mérieux, Courbevoie, France) and fixed in a conventional stereotaxic head frame (Horsley Clarke Apparatus; Unimécanique, Epinay-sur-Seine, France). Body temperature was monitored by a rectal thermometer and maintained at 37°C with a homeothermic warming blanket (Harvard Apparatus, Kent, UK).
Electrophysiological analysis. Single-unit activity of SNR cells was recorded extracellularly using glass micropipettes (6–10 MΩ) filled with a 0.6 m sodium chloride solution containing 4% Pontamine Sky Blue. Action potentials of single neurons were amplified with a World Precision Instruments (Hertfordshire, UK) DAM-5A differential preamplifier and displayed on a Tektronix (Marlow, UK) memory oscilloscope. Nigral neurons were identified as nondopaminergic on their classically defined electrophysiological characteristics: thin spikes (width, <2 msec) and ability to present high-frequency discharge (>10 Hz) without a decrease in the spike amplitude (Bunney et al., 1973; Deniau et al., 1978; Guyennet and Aghajanian, 1978). Spikes were separated from noise using a window discriminator and sampled on-line thanks to a computer connected to a CED 1401 interface (Cambridge Electronics Design, Cambridge, UK). Peristimulus time histograms were generated from 60 to 100 stimulation trials using 1 msec bins and plotted on a Hewlett-Packard plotter. The criterion used to establish the existence of an excitation was an increase greater than 50% in the number of spikes compared with the prestimulus frequency, for at least three consecutive bins. The duration of an inhibitory response corresponds to the time interval during which no spike was observed.
The electrical stimulation of the PL/MO areas, ipsilateral to the recording site in the SNR, was performed through a coaxial stainless steel electrode (diameter, 400 μm; tip-barrel distance, 300 μm) positioned stereotaxically [anterior (A), 12.7; lateral (L), 0.6; height (H), 5.5 mm from the interaural line] according to the atlas ofPaxinos and Watson (1986). Electrical stimuli consisted of monopolar pulses of 0.6 msec width and 200–600 μA intensity delivered at a frequency of 1.4 Hz.
At the end of each recording session, the tip of the stimulating electrode was marked by an electrical deposit of iron (15 μA anodal, 20 sec) and observed on histological sections after a ferri-ferrocyanide reaction. The tip of the recording electrode was marked by iontophoretic ejection of Pontamine Sky Blue (8 μA cathodal, 20 min), allowing the determination of the position of the recorded cells. Brains were removed and fixed in a 10% formalin solution, and the positions of electrodes were microscopically identified on serial frozen sections (100 μm) stained with safranin.
Drug applications. Pharmacological blockade of the glutamatergic transmission in the NAcc and the VP or of the GABAergic transmission in the VP was performed by local application of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and bicuculline, respectively. CNQX (500 μm; Research Biochemicals, Natwick, MA) and bicuculline (500 μm; Sigma, St Louis, MO) were applied through a microdialysis probe (CMA 102; Microdialysis AB, Stockholm, Sweden; membranes, 0.5 × 2 mm). The probe was positioned stereotaxically into the NAcc (A, 10.7; L, 1.7; H, 2.1) or the VP (A, 8.7; L, 2.5; H, 1.4) according to the atlas ofPaxinos and Watson (1986). At the beginning of each experiment, the probe was perfused with a Ringer’s phosphate solution (in mm: NaCl, 120; KCl, 4.8; CaCl2, 1.2; MgCl2, 1.2; NaH2PO4, 15.6) using a CMA 102 microinjection pump at a flow rate of 2 μl/min. When a SNR cell responding to PL/MO stimulation was recorded, a peristimulus time histogram (100 stimuli) corresponding to the control situation was generated. Then, the antagonist solution was perfused. The activity of the same cell was continuously recorded and, each fifth minute, its response to PL/MO stimulation was monitored, and a peristimulus time histogram was generated. The blockade of synaptic transmission was considered to be effective when nigral responses evoked by PL/MO stimulation were increased or decreased by at least 50%. The tested drug was then washed out by perfusion with the Ringer’s phosphate solution, and the same cell was recorded until the recovery of the control response. The blockade of the glutamatergic transmission in the STN was performed by a 1 min delivery of a saline solution containing CNQX (1 mm, pH 7.0; 0.3 μl) through a cannula (diameter, 400 μm) stereotaxically positioned into the STN (A, 5.2; L, 2.2; H, 1.7). Peristimulus time histograms of nigral responses evoked by PL/MO stimulation were generated before and after the receptor antagonist application, and the data were analyzed as mentioned above. When more than one cell was tested in the same animal, drug applications were separated by at least 2 hr after the recovery of the control response in the preceding cell.
Student’s t test (two-tailed) was used to compare excitatory and inhibitory responses observed before and after drug application.
RESULTS
Effects of PL/MO stimulation on the activity of SNR cells
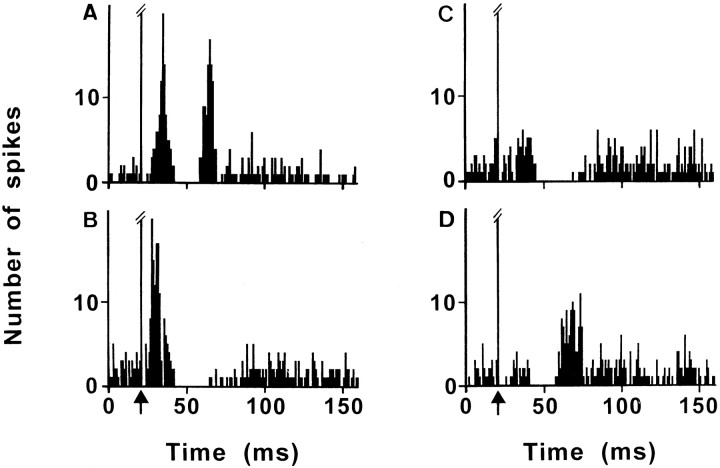
Responses evoked by electrical stimulation of PL/MO areas were investigated in 156 SNR cells recorded in 11 rats. As shown in Figure1 and Table1, PL/MO stimulation induced, in 80 cells, an inhibition preceded or not by an early excitation and followed or not by a late excitation. In 30 cells (37.5%), responses consisted of an inhibition [latency (L), 20.2 ± 0.5 msec; duration (D), 14.5 ± 1.0 msec] preceded by an early excitation (L, 8.6 ± 0.4 msec) and followed by a late excitation (L, 37.8 ± 1.1 msec). In eight cells (10%), the inhibition (L, 19.3 ± 2.0 msec; D, 16.1 ± 2.5 msec) was not preceded by an early excitation but was followed by a late excitation (L, 36.6 ± 2.2 msec). In 32 cells (40%), the inhibition (L, 23.0 ± 0.6 msec; D, 24.9 ± 2.0 msec) was preceded by an early excitation (L, 8.7 ± 0.4 msec) but was not followed by a late excitation. An inhibition without early or late excitations was observed in 10 cells (12.5%; L, 22.9 ± 1.1 msec; D, 23.0 ± 4.5 msec). Interestingly, the inhibition was significantly shorter (40.2%;p < 0.001) in cells with a late excitation (D, 14.6 ± 0.8 msec) than in cells without a late excitation (D, 24.4 ± 1.8 msec).
Fig. 1.
Patterns of responses evoked by PL/MO stimulation within SNR cells. The inhibition is preceded by an early excitation and is followed (A) or not (B) by a late excitation; the inhibition is not preceded by an early excitation and is followed (D) or not (C) by a late excitation.
Table 1.
Characteristics of the responses evoked by PL/MO stimulation within SNR cells
| Type of response | % of responding cells | Early excitation L (msec) | Inhibition | Late excitation L (msec) | |
|---|---|---|---|---|---|
| L (msec) | D (msec) | ||||
| Excitation–inhibition–excitation | 37.5% (n = 30) | 8.6 ± 0.4 | 20.2 ± 0.5 | 14.5 ± 1.0 | 37.8 ± 1.1 |
| Excitation–inhibition | 40.0% (n = 32) | 8.7 ± 0.4 | 23.0 ± 0.6 | 24.9 ± 2.0 | |
| Inhibition | 12.5% (n = 10) | 22.9 ± 1.1 | 23.0 ± 4.5 | ||
| Inhibition–excitation | 10.0% (n = 8) | 19.3 ± 2.0 | 16.1 ± 2.5 | 36.6 ± 2.2 | |
n, Number of cells.
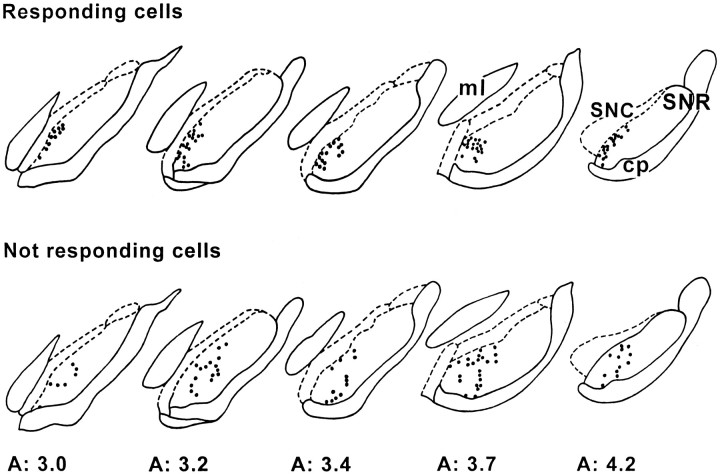
All these responding cells were located in the dorsomedial part of the SNR (Fig. 2). Within this SNR region, no obvious topographical distribution of the cells with distinct types of responses could be observed. In addition, it should be noted that in 6 of the 156 cells tested, an excitation with a mean latency of 25.4 ± 1.7 msec was observed instead of an inhibition. Finally, cells with no response to PL/MO stimulation (70 of the 156 cells tested) were mainly located more laterally and ventrally in the SNR.
Fig. 2.
Localization of SNR cells that responded to PL/MO stimulation. Note that responding cells are located in the medial part of the SNR, whereas more laterally located cells do not respond to PL/MO stimulation (Not responding cells). Eachdot represents a tested cell. Numbersindicate the distance, in millimeters, from the interaural line.cp, Cerebral peduncle; ml, medial lemniscus; SNC, substantia nigra pars compacta.
Effect of CNQX application into the NAcc on nigral responses evoked by PL/MO stimulation
The effect of a blockade of the glutamatergic cortico-striatal transmission by application of CNQX into the NAcc was examined in 13 SNR cells (eight rats) exhibiting a response to PL/MO stimulation. In control conditions, these cells presented the following patterns of responses: excitation–inhibition–excitation (four cells), excitation–inhibition (six cells), inhibition only (two cells), and inhibition–excitation (one cell).
In all these cells, the inhibition disappeared 8–25 min after the beginning of CNQX application (Fig. 3). In eight cells held long enough, the recovery of the inhibition occurred 25–65 min after the cessation of CNQX application. In addition, the late excitatory response observed in five cells in control conditions was markedly reduced under CNQX, with the maximal effect (63–97% decrease; p < 0.001) being observed 10–25 min after the beginning of the drug application (Fig. 3). In the three cells held long enough, the recovery of the late excitatory response was observed 40–65 min after the cessation of drug application. In contrast (Fig. 3), the CNQX treatment did not modify the early excitation that preceded the inhibition (10 cells).
Fig. 3.
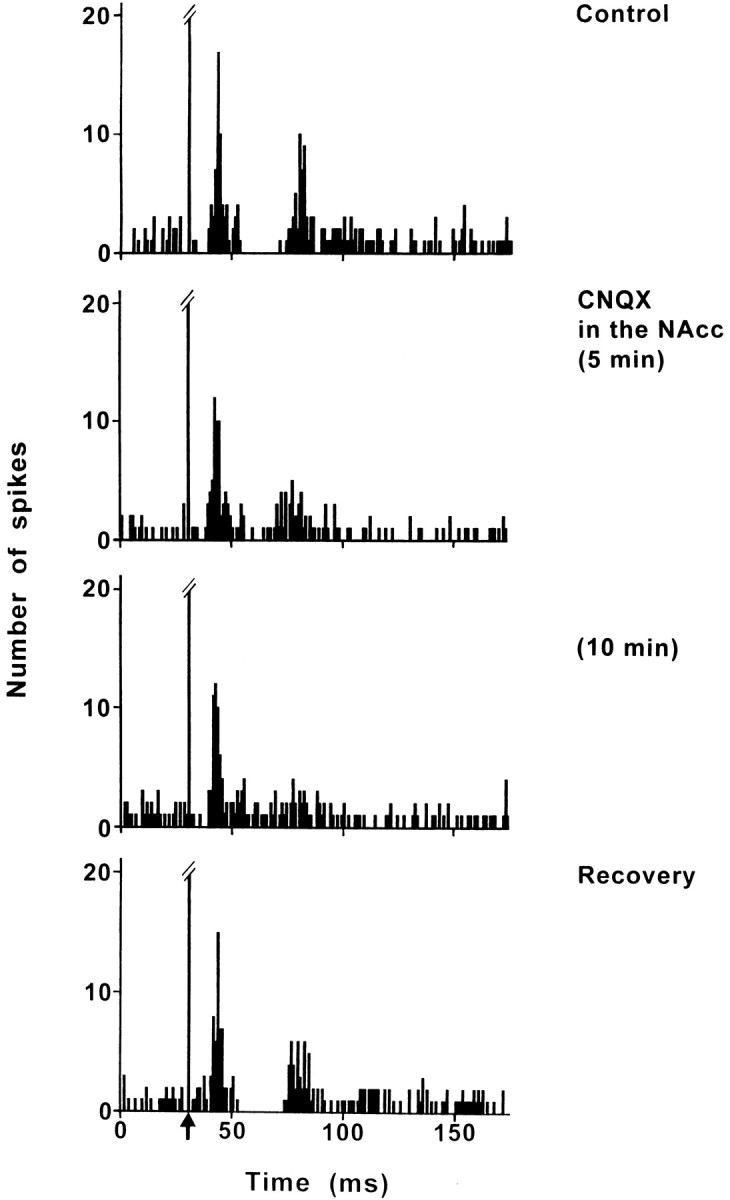
Effect of CNQX application into the NAcc on the response evoked by PL/MO stimulation in an SNR cell. Fromtop to bottom, The response exhibited a triphasic excitation–inhibition–excitation sequence in control conditions; under CNQX application into the NAcc, the early excitation was not affected, the inhibition disappeared, and the late excitation was markedly decreased. The maximal effect was observed 10 min after the beginning of CNQX application, and the recovery of the inhibition and of the late excitation occurred 40 min after the cessation of CNQX application. Each poststimulus time histogram represents 100 superimposed sweeps. Arrow indicates the stimulation artifact.
Effect of bicuculline application into the VP on nigral responses evoked by PL/MO stimulation
Bicuculline was applied into the VP to block the GABAergic transmission of the NAcc–VP pathway. The effect of bicuculline on the responses evoked by PL/MO stimulation was examined in 10 cells (eight rats). In control conditions, PL/MO stimulation induced an inhibition preceded by an early excitation and followed by a late excitation. In 9 of these 10 cells, bicuculline markedly reduced the late excitation, with the maximal effect (70–100% decrease; p < 0.001) being observed 8–35 min after the beginning of the drug application (Fig. 4). In eight cells held long enough, the recovery of the late excitation occurred 40–75 min after the cessation of bicuculline application. Interestingly, the disappearance of the late excitation was associated with a significant increase in the duration of the inhibition (control conditions: D, 15.8 ± 0.5 msec; bicuculline treatment: D, 24.4 ± 2.8 msec; mean increase, 54%; p < 0.01) without a significant change of the latency (control: L, 20.7 ± 0.6 msec; bicuculline: L, 21.3 ± 0.6 msec). Finally, bicuculline treatment did not affect the early excitatory response (Fig. 4).
Fig. 4.
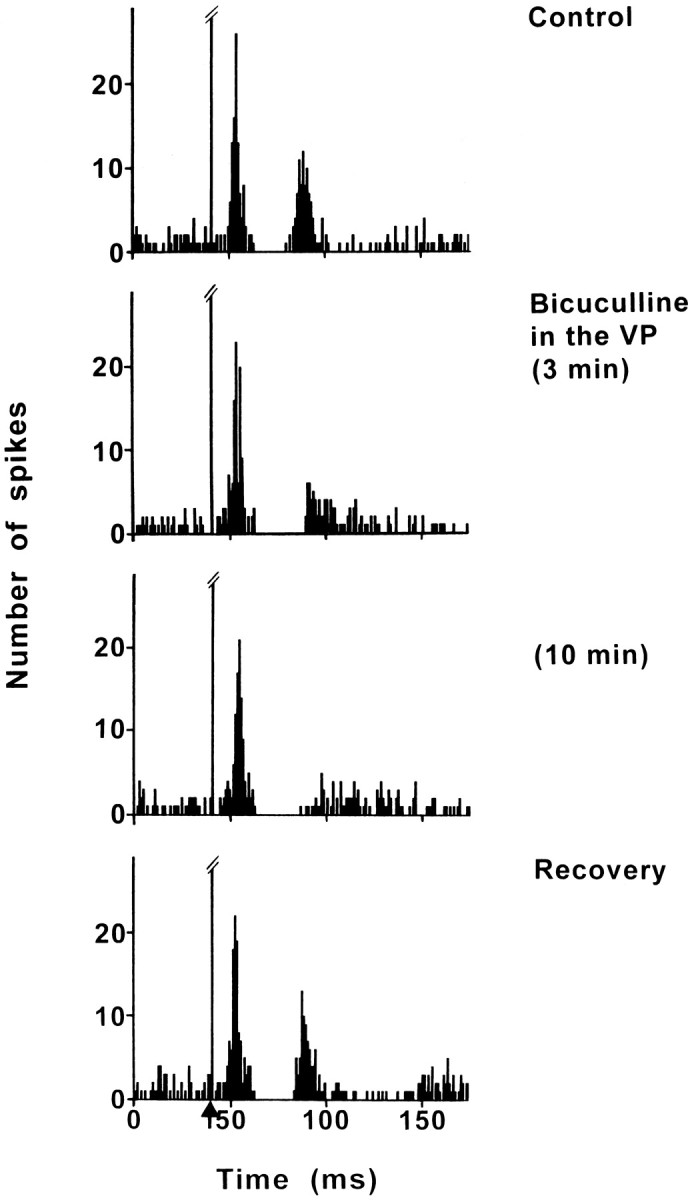
Effect of bicuculline application into the VP on the response evoked by PL/MO stimulation in an SNR cell. Fromtop to bottom, The response exhibited a triphasic excitation–inhibition–excitation sequence in control conditions; under bicuculline application into the VP, the early excitation was not modified, the duration of the inhibition was slightly increased, and the late excitation was markedly decreased. The maximal effect was observed 10 min after the beginning of bicuculline application, and the recovery of the late excitation occurred 60 min after the cessation of bicuculline application. Each poststimulus time histogram represents 100 superimposed sweeps. Arrowindicates the stimulation artifact.
Effect of CNQX application into the VP on nigral responses evoked by PL/MO stimulation
CNQX was applied into the VP to block the glutamatergic input from the STN and thus to investigate the possible influence of the subthalamo-pallidal loop on nigral responses evoked by PL/MO stimulation. In control conditions, the patterns of responses observed in the six SNR cells tested (four rats) were as follows: excitation–inhibition–excitation (five cells) and inhibition–excitation (one cell). In all cells, CNQX treatment markedly enhanced the late excitatory response (Fig.5). The duration of the late excitation increased from 13.0 ± 1.7 msec (control conditions) to 31.2 ± 2.8 msec (under CNQX; p < 0.001), although its latency was slightly decreased (control: L, 37.5 ± 1.1 msec; CNQX: L, 34.0 ± 1.0 msec; p < 0.05). The maximal effect (67–410% increase in the number of spikes during the excitatory period) occurred 15–35 min after the beginning of CNQX application, and a recovery of the response was observed 70–85 min after the cessation of the drug application in the three cells held long enough. Interestingly, this CNQX treatment slightly reduced the duration of the inhibition (control: D, 13.8 ± 1.2 msec; CNQX: D, 10.5 ± 1.0 msec; p < 0.05). Finally, the CNQX application did not significantly modify the early excitatory response recorded in control conditions (five cells).
Fig. 5.
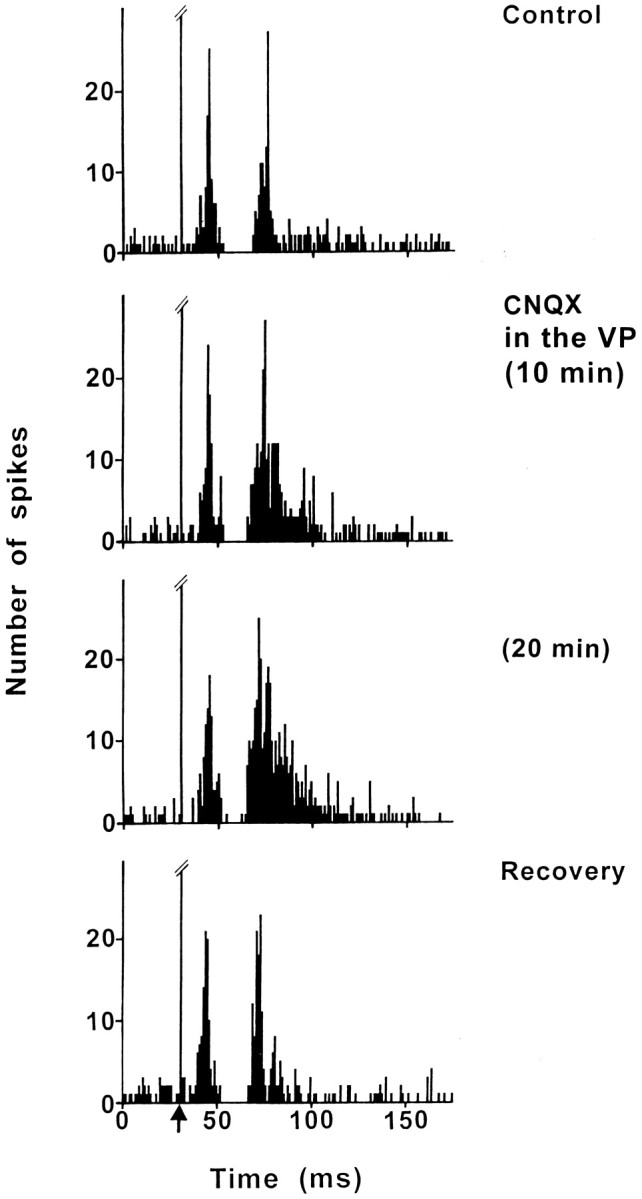
Effect of CNQX application into the VP on the response evoked by PL/MO stimulation in an SNR cell. Fromtop to bottom, The response exhibited a triphasic excitation–inhibition–excitation sequence in control conditions; under CNQX application into the VP, the early excitation was not significantly modified, the inhibition was still observed, and the late excitation was markedly increased. The maximal effect was observed 20 min after the beginning of CNQX application, and the recovery of the late excitation occurred 75 min after the cessation of CNQX application. Note that the duration of the inhibition and the latency of the late excitation were decreased under CNQX. Each poststimulus time histogram represents 100 superimposed sweeps.Arrow indicates the stimulation artifact.
Effect of CNQX application into the STN on nigral responses evoked by PL/MO stimulation
The effect of blockade of the glutamatergic cortico-subthalamic transmission by CNQX injection into the STN was examined in eight SNR cells (six rats) exhibiting a response to PL/MO stimulation. In control conditions, the responses evoked by PL/MO stimulation consisted of an inhibition preceded by an early excitation (eight cells) and followed (six cells) or not (two cells) by a late excitation. In all cases, the CNQX injection markedly decreased the early excitation (Fig.6). The maximal effect (52–91%) was observed 5–10 min after the drug application, and the recovery of the response occurred 35–50 min later in the five cells held long enough (Fig. 6). In addition, CNQX increased the duration of the inhibition (control: D, 20.0 ± 3.8 msec; CNQX: D, 44.5 ± 9.8;p < 0.05) and induced a disappearance of the late excitation (six cells).
Fig. 6.
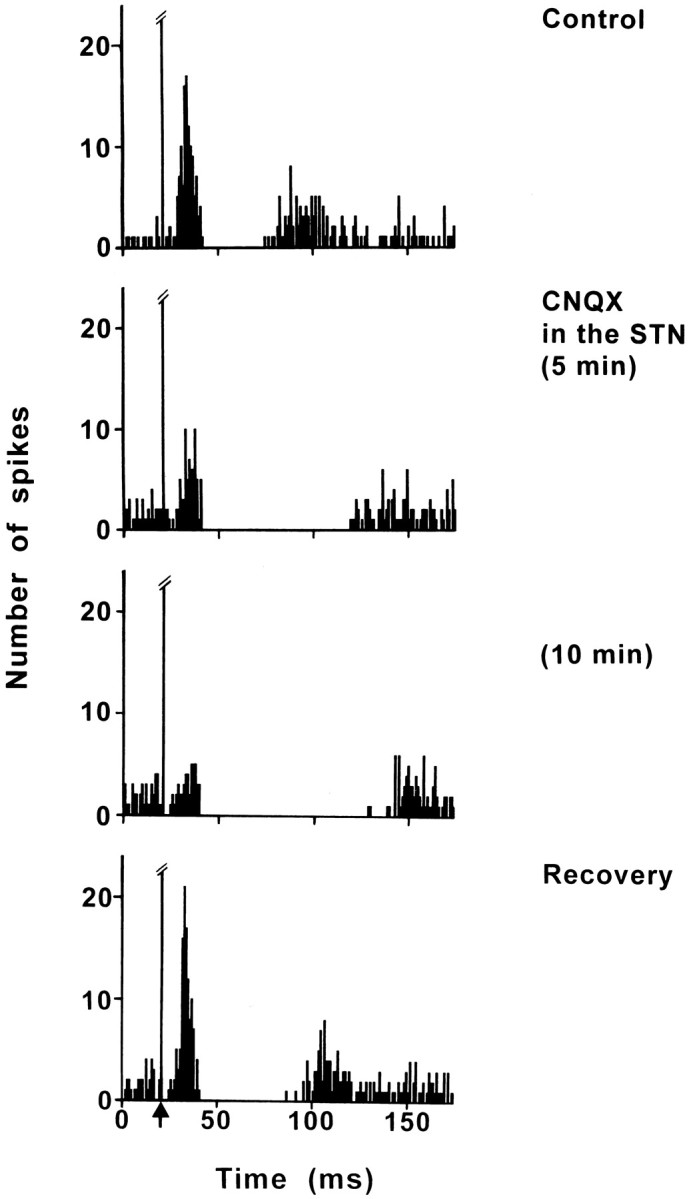
Effect of CNQX application into the STN on the response evoked by PL/MO stimulation in an SNR cell. Fromtop to bottom, The response exhibited a triphasic excitation–inhibition–excitation sequence in control conditions; under CNQX application into the STN, the early excitation was markedly decreased, the duration of the inhibition was increased, and the late excitation disappeared. The maximal effect was observed 10 min after the CNQX application, and the recovery occurred 50 min after CNQX application. Each poststimulus time histogram represents 100 superimposed sweeps. Arrow indicates the stimulation artifact.
DISCUSSION
Our results indicate that the electrical stimulation of PL/MO areas of the rat prefrontal cortex induces in the SNR complex patterns of responses composed of an inhibition preceded or not by an early excitation and followed or not by a tardive excitation. These patterns of responses are similar to those described after stimulation of the sensorimotor frontal agranular cortex in the rat (Fujimoto and Kita, 1993; Ryan and Sanders, 1994). The present pharmacological data allowed the determination of the respective contribution of the trans-striatal and trans-subthalamic circuits in the nigral responses evoked by PL/MO stimulation (Fig. 7).
Fig. 7.
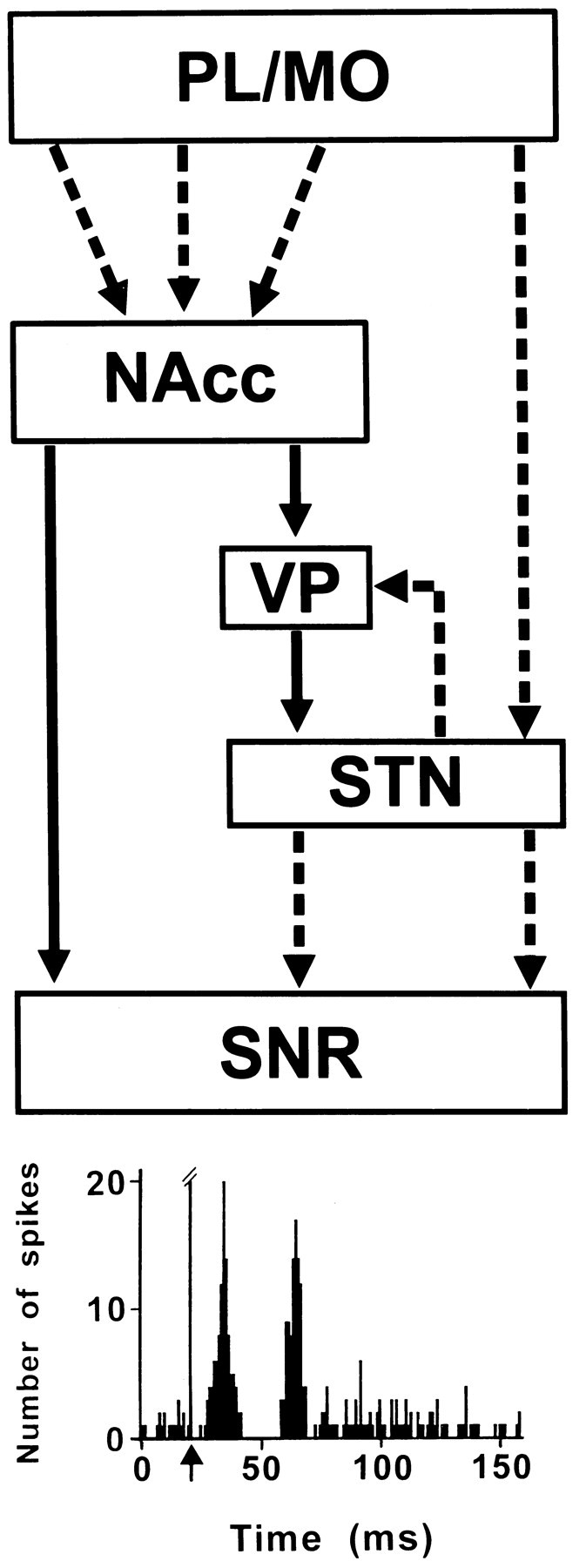
Schematic representation of the pathways involved in the SNR responses to prefrontal cortical stimulation.Broken and solid lines represent glutamatergic and GABAergic pathways, respectively.Bottom, Example of a complex response evoked in an SNR cell by stimulation of PL/MO areas of the prefrontal cortex. The early excitation is caused by the activation of the disynaptic cortico-STN–SNR pathway, the inhibition results from the activation of the direct NAcc–SNR pathway, and the late excitation involves the indirect NAcc–VP–STN–SNR pathway, which operates via disinhibition of the STN.
Role of the disynaptic cortico-subthalamo-nigral pathway
The cortico-subthalamic and subthalamo-nigral projections are glutamatergic and form asymmetrical synaptic contacts with their target neurons in the STN and the SNR (Smith et al., 1998). Based on the conduction time of these projections, it has been proposed that short latency excitations evoked in SNR cells by stimulation of the sensorimotor cortex are mediated through the disynaptic cortico-subthalamo-nigral pathway (Kita, 1994). Accordingly, the early excitations evoked in the SNR by frontal agranular cortex stimulation are no longer observed after excitotoxic lesion of the STN (Ryan and Sanders, 1994). Our data indicate the existence of a similar functional link between PL/MO areas and the medial SNR. Indeed, the latency of the early excitatory responses evoked by PL/MO stimulation in SNR cells is in the range of the conduction time of the disynaptic cortico-subthalamo-nigral pathway, and PL/MO stimulation induces a short latency excitation in STN cells projecting to the SNR (Maurice et al., 1998b). In addition, the early nigral excitatory responses evoked by PL/MO stimulation disappeared after CNQX application into the STN but persisted after the blockade of the cortico-NAcc transmission. This effect cannot be attributed to a diffusion of CNQX into the SNR because, in contrast to the reduced firing of nigral cells reported after local application of glutamatergic antagonists into the SNR (Robledo and Féger, 1990), the spontaneous activity of SNR cells was not modified (20.6 ± 3.4 Hz, before CNQX; 19.2 ± 2.2 Hz, under CNQX).
Role of the direct NAcc-SNR pathway
We have previously shown the existence of a functional link between the PL/MO areas and the NAcc neurons that innervate the SNR. Indeed, stimulation of PL/MO areas induces excitatory responses in NAcc neurons projecting to the SNR (Montaron et al., 1996), and stimulation of the NAcc inhibits the activity of SNR cells (Deniau et al., 1994). The present data demonstrate that the inhibitory responses observed in SNR cells after PL/MO stimulation result from the activation of the direct NAcc–SNR pathway: (1) the inhibitory responses have a latency compatible with the conduction time of the PL/MO–NAcc–SNR pathway and a duration similar to that observed after NAcc stimulation (Deniau et al., 1994); (2) the inhibitory responses were observed in cells located in the dorsomedial part of the SNR, in agreement with the distribution of NAcc projections (Montaron et al., 1996); and (3) finally, the inhibitory responses disappeared after acute blockade of the glutamatergic cortico-striatal neurotransmission by CNQX application into the NAcc, although they persisted after blockade of the striato-pallidal or cortico-subthalamic transmissions. It should be noted that the duration of CNQX application required to obtain a maximal effect in the SNR presented some variability. This is likely to be related to the diffusion time necessary for CNQX to produce a blockade of the synaptic transmission in the whole NAcc territory involved in the PL/MO–NAcc–SNR circuit.
Role of the indirect NAcc–SNR pathway
The indirect striato-nigral pathway, involving the external pallidum and the STN, has been proposed to exert an excitatory influence on the SNR (Alexander et al., 1986). Activation of striato-pallidal neurons inhibits the tonic discharge of the GABAergic pallidal neurons that project to the STN and consequently should lead to a disinhibition of STN. In turn, disinhibition of STN would result in an increased firing of SNR cells because STN–SNR projections are glutamatergic and excitatory.
The present data, along with our previous study (Maurice et al., 1998a), demonstrate that the late excitatory responses that follow the inhibition evoked in SNR cells by PL/MO stimulation are a result of the activation of the indirect NAcc–SNR pathway and result from the disinhibition of the STN. Indeed, the late nigral excitatory responses disappeared after blockade of either cortico-NAcc transmission by CNQX application into the NAcc or NAcc–VP transmission by bicuculline application into the VP. These treatments have also been shown to block the disinhibition of STN cells evoked by PL/MO stimulation (Maurice et al., 1998a). In addition, the latency of the late excitatory responses is in the range of the conduction time of the multisynaptic circuit linking the PL/MO areas to the SNR via the indirect basal ganglia pathway (Montaron et al., 1996; Maurice et al., 1997, 1998a,b).
Role of the STN–VP–STN loop
The STN and pallidum are two tightly interconnected structures, and pallido-subthalamic connections are topographically organized such that the GP and VP project to the STN region from which they receive an input (Groenewegen and Berendse, 1990; Smith et al., 1998). Electrophysiological data suggest that activation of STN cells projecting to the pallidum by cortical inputs leads to a feedback inhibition of the STN (Ryan and Clarke, 1991; Fujimoto and Kita, 1993). Confirming this hypothesis, we have recently shown that disinhibition of STN cells by PL/MO stimulation is markedly increased after blockade of the STN–VP glutamatergic transmission by CNQX application into the VP (Maurice et al., 1998a). The present data show that the late excitatory responses induced by PL/MO stimulation in SNR cells are also markedly increased after CNQX application into the VP. Together, these data indicate that the VP–STN feedback circuit modulates the late excitatory responses of SNR cells to PL/MO stimulation and further confirm that these late excitations result from the disinhibition of the STN.
Functional considerations
The terminals from the striatal and subthalamic afferent pathways form convergent synaptic contacts with individual neurons in the SNR (Smith et al., 1998). Accordingly, the present electrophysiological study shows that the trans-striatal and trans-subthalamic pathways related to PL/MO areas of the prefrontal cortex exert a converging synaptic influence on medial SNR cells. In addition, our data indicate that the inhibition exerted by the direct NAcc–SNR pathway on nigral cells is under the control of the trans-subthalamic pathways. The duration of this inhibition was decreased after CNQX application into the VP, a treatment that blocks the feedback STN–VP–STN inhibitory loop and thus enhances the disinhibition of the STN. In contrast, the duration of the inhibition was increased after bicuculline application into the VP, which blocks the disinhibition of the STN and consequently the late excitatory responses in the SNR. A more prolonged lengthening of the inhibition was observed after CNQX application into the STN, which blocks the cortico-subthalamic pathway. The marked lengthening of the inhibition cannot just be explained by the concomitant disappearance of the late excitatory responses but is likely to result from an increased inhibitory influence of the direct NAcc–SNR pathway. Because the VP receives excitatory inputs from the STN and sends GABAergic projections to the NAcc (Groenewegen et al., 1993), the blockade of the glutamatergic excitatory inputs to STN might lead to a disinhibition of the NAcc and thus would facilitate the activation of the NAcc–SNR pathway by PL/MO stimulation. On the other hand, the presence of metabotropic glutamate receptors of the group III (mGluR7) on GABAergic terminals in the SNR have recently been described (Kosinski et al., 1998). Assuming a presynaptic inhibitory influence of mGluR7 on transmitter release (Conn and Pin, 1997), it can be proposed that blockade of the activation of the glutamatergic subthalamo-nigral pathway by CNQX application into the STN could have reduced the inhibitory effect of mGluR7 receptors on GABA release, resulting in an increased inhibitory influence of the direct NAcc–SNR pathway.
In motor circuits of the basal ganglia, the activation of the direct striato-nigral GABAergic pathway, by inhibiting the tonically active GABAergic projection neurons of the SNR, leads to a disinhibition of their target nuclei in thalamus and brainstem (Chevalier and Deniau, 1990). This disinhibitory process very likely increases the excitability of the frontal cortex and is central to the physiology of the basal ganglia. It has been proposed that the trans-subthalamic pathways, by their excitatory influence on the SNR, participate in the spatio-temporal shaping of this disinhibitory process and thus contributes to the scaling of movements and inhibition of competing motor programs (Mink and Thach, 1993). An imbalance between the direct striato-nigral and the trans-subthalamic pathways is considered to be responsible for motor disorders, such as akinesia and dyskinesia (Albin et al., 1989; Chesselet and Delfs, 1996; Obeso et al., 1997). Similarly, in the PL/MO circuits of the basal ganglia, the present data show that SNR cells receive a direct inhibitory input from the NAcc and that the trans-subthalamic excitatory pathways participate in the shaping of the discharge of nigral output neurons. By analogy with motor circuits, it can be proposed that an imbalance between these pathways could be responsible for perturbation in prefrontal functions, such as perseveration and alterations in attentional and emotional processes.
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale. N.M. is a recipient of a fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche. We thank A. M. Godeheu and M. Saffroy for histological assistance and L. Darracq for his advice in the microdialysis technique.
Correspondence should be addressed to Dr. Anne-Marie Thierry, Chaire de Neuropharmacologie, Institut National de la Santé et de la Recherche Médicale U114, Collège de France, 11 place Marcelin Berthelot, 75231 Paris Cedex 05, France.
REFERENCES
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 3.Berendse HW, Groenewegen HJ. The connections of the medial part of the subthalamic nucleus in the rat: evidence for a parallel organization. In: Bernardi G, Carpenter MB, Di Chiara G, Morelli M, Stanzione P, editors. The basal ganglia III. Plenum; New York: 1991. pp. 89–98. [Google Scholar]
- 4.Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- 5.Chesselet MF, Delfs JM. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996;10:417–422. doi: 10.1016/0166-2236(96)10052-7. [DOI] [PubMed] [Google Scholar]
- 6.Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- 7.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 8.Deniau JM, Thierry AM. Anatomical segregation of information processing in the rat substantia nigra pars reticulata. In: Obeso JA, DeLong MR, Ohye C, Marsden CD, editors. The basal ganglia and new surgical approaches for Parkinson’s disease, Vol 74, Advances in Neurology. Lippincott-Raven; Philadelphia: 1997. pp. 83–96. [PubMed] [Google Scholar]
- 9.Deniau JM, Hammond C, Risk A, Féger J. Electrophysiological properties of identified output neurons of the rat subtantia nigra (pars compacta and pars reticulata): evidences for the existence of branched neurons. Exp Brain Res. 1978;32:409–422. doi: 10.1007/BF00238711. [DOI] [PubMed] [Google Scholar]
- 10.Deniau JM, Menetrey A, Thierry AM. Indirect nucleus accumbens input to the prefrontal cortex via the substantia nigra pars reticulata: a combined anatomical and electrophysiological study in the rat. Neuroscience. 1994;61:533–545. doi: 10.1016/0306-4522(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto K, Kita H. Response characteristics of subthalamic neurons to the stimulation of the sensorimotor cortex in the rat. Brain Res. 1993;609:185–192. doi: 10.1016/0006-8993(93)90872-k. [DOI] [PubMed] [Google Scholar]
- 12.Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. J Comp Neurol. 1990;294:607–622. doi: 10.1002/cne.902940408. [DOI] [PubMed] [Google Scholar]
- 13.Groenewegen HJ, Berendse HW. Motor and cognitive functions of the prefrontal cortex (Thierry AM, Glowinski J, Goldman-Rakic P, Christen Y), pp 51–77. Springer; Berlin: 1994. Anatomical relationships between the prefrontal cortex and the basal ganglia in the rat. [Google Scholar]
- 14.Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- 15.Guyenet PG, Aghajanian GK. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 1978;150:69–84. doi: 10.1016/0006-8993(78)90654-6. [DOI] [PubMed] [Google Scholar]
- 16.Kita H. Physiology of two disynaptic pathways from the sensorimotor cortex to the basal ganglia output nuclei. In: Percheron G, McKenzie JS, Féger J, editors. The basal ganglia IV. Plenum; New York: 1994. pp. 263–276. [Google Scholar]
- 17.Kosinski CM, Bradley SR, Conn PJ, Levey AI, Landwehrmeyer GB, Penney JB, Young AS, Standaert DG. Metabotropic glutamate receptor (mGluR7) mRNA and mGluR7a protein in the basal ganglia. Soc Neurosci Abstr. 1998;648:6. [Google Scholar]
- 18.Maurice N, Deniau JM, Ménétrey A, Glowinski J, Thierry AM. Position of the ventral pallidum in the rat prefrontal cortex-basal ganglia circuit. Neuroscience. 1997;80:523–534. doi: 10.1016/s0306-4522(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 19.Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the cortico-subthalamic circuits. J Neurosci. 1998a;18:9539–9546. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurice N, Deniau JM, Ménétrey A, Glowinski J, Thierry AM. Prefrontal cortex-basal ganglia circuits in the rat: involvement of the ventral pallidum and the subthalamic nucleus. Synapse. 1998b;29:363–370. doi: 10.1002/(SICI)1098-2396(199808)29:4<363::AID-SYN8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Mink JW, Thach WT. Basal ganglia intrisic circuits and their role in behavior. Curr Opin Neurobiol. 1993;3:950–957. doi: 10.1016/0959-4388(93)90167-w. [DOI] [PubMed] [Google Scholar]
- 22.Montaron MF, Deniau JM, Ménétrey A, Glowinski J, Thierry AM. Prefrontal cortex inputs of the nucleus accumbens-nigro-thalamic circuit. Neuroscience. 1996;71:371–382. doi: 10.1016/0306-4522(95)00455-6. [DOI] [PubMed] [Google Scholar]
- 23.Obeso JA, Rodriguez MC, DeLong MR. Basal ganglia pathophysiology. A critical review. In: Obeso JA, DeLong MR, Marsden CD, editors. The basal ganglia and new surgical approaches for Parkinson’s disease. Lippincott-Raven; Philadelphia: 1997. pp. 3–18. [PubMed] [Google Scholar]
- 24.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995a;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 25.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev. 1995b;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 2. Academic; New York: 1986. [DOI] [PubMed] [Google Scholar]
- 27.Percheron G, Filion M. Parallel processing in the basal ganglia: up to a point. Trends Neurosci [Letter] 1991;14:55–56. doi: 10.1016/0166-2236(91)90020-u. [DOI] [PubMed] [Google Scholar]
- 28.Robledo P, Féger J. Excitatory influence of rat subthalamic nucleus to substantia nigra pars reticulata and the pallidal complex: electrophysiological data. Brain Res. 1990;518:47–54. doi: 10.1016/0006-8993(90)90952-8. [DOI] [PubMed] [Google Scholar]
- 29.Ryan LJ, Clark KB. The role of the subthalamic nucleus in the response of globus pallidus neurons to stimulation of the prelimbic and agranular frontal cortices in rats. Exp Brain Res. 1991;86:641–651. doi: 10.1007/BF00230538. [DOI] [PubMed] [Google Scholar]
- 30.Ryan LJ, Sanders DJ. Subthalamic nucleus and globus pallidus lesions alter activity in nigrothalamic neurons in rats. Brain Res Bull. 1994;34:19–26. doi: 10.1016/0361-9230(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 31.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 32.Zahm DS. The ventral striatopallidal parts of the basal ganglia in the rat. II. Compartmentation of ventral pallidal efferents. Neuroscience. 1989;30:33–50. doi: 10.1016/0306-4522(89)90351-5. [DOI] [PubMed] [Google Scholar]