Abstract
Previous studies showed that sensory feedback from the body wall is important and sometimes critical for generating normal, robust swimming activity in leeches. In this paper, we evaluate the role of sensory feedback in intersegmental coordination using both behavioral and physiological measurements. We severed the ventral nerve cord of leeches in midbody and then made video and in situextracellular recordings from swimming animals. Our electrophysiological recordings unequivocally demonstrate that active intersegmental coordination occurs in leeches with severed nerve cords, refuting Schülter’s (1933) earlier conclusions that sensory feedback cannot coordinate swimming activity. Intersegmental coordination can in fact be achieved by sensory feedback alone, without the intersegmental interactions conveyed by the nerve cord.
Keywords: leech, swimming, sensory feedback, intersegmental coordination, CPG, oscillator, locomotion
The rhythmic motor patterns observed in animal locomotion, such as walking, swimming, and flying, are typically produced by neural oscillators. Because nearly all such motor patterns examined so far can be generated without sensory inputs (Grillner, 1975; Delcomyn, 1980), it is believed that these oscillators, or central pattern generators, are located within the CNS. In segmented animals, such as the leech, crayfish, and lamprey, functional individual oscillators have been found in most or all segments (Ikeda and Wiersma, 1964; Stent et al., 1978; Cohen and Wallen, 1980; Murchison et al., 1993; Hocker and Friesen, 1997). To produce effective movements along the whole body, however, segmental oscillators must be properly coupled and coordinated. For the expression of leech swimming movements, for example, the swim oscillator must generate an accurate intersegmental timing pattern to command phase-delayed, alternating contractions of dorsal and ventral muscles in consecutive body segments. This coordinated contraction generates a one wavelength sinusoidal-like undulation that is a highly effective waveform used by many elongated aquatic animals (Kristan et al., 1974).
The neuronal basis for intersegmental interaction has been studied in several animal systems. In the CNS, coordinating neurons found in crayfish (Stein, 1971; Paul and Mulloney, 1986) and synaptic connections found in the leech (Friesen et al., 1978; Weeks, 1981;Friesen, 1989) and the lamprey (Grillner et al., 1989) are thought to account for coordinating activity between different segments. Combined with studies at the systems level (Pearce and Friesen, 1985; Friesen and Pearce, 1993), these circuit-level data show that intersegmental coordination can be, and actually is, achieved by neuronal connections within the CNS. However, abundant evidence exists that sensory feedback from peripheral receptors is necessary for generating correct timing and motor patterns (Wilson, 1961; Kristan and Calabrese, 1976; Bassler, 1993; Pearson and Ramirez, 1997). For leech swimming, although an isolated nerve cord can generate coordinated swimming activity, intersegmental phase lags when sensory feedback is removed are significantly shorter than those observed in intact preparations (Kristan and Calabrese, 1976; Pearce and Friesen, 1984). These findings suggest that intersegmental coordination is not determined solely by interactions within the nerve cord.
We evaluated the role of sensory feedback in intersegmental coordination through systematic behavioral studies combined with physiological measurements. We severed the nerve cord of leeches and made video and in situ extracellular recordings from swimming animals. Video recordings of preparations with severed nerve cords (SNCs) were examined frame by frame and compared with control preparations. In situ extracellular recordings were made simultaneously from two sites on the nerve cord so that intersegmental phase relationships could be described more precisely. Our video records and electrophysiological recordings unequivocally demonstrate that active intersegmental coordination continues to occur in leeches with severed nerve cords after intersegmental interactions conveyed by the ventral nerve cord are removed.
A preliminary report of these results was presented earlier in an abstract (Yu and Friesen, 1997).
MATERIALS AND METHODS
Animals
Experiments were performed on adult medicinal leeches,Hirudo medicinalis, obtained from Leeches USA (Westbury, NY). The leeches were maintained in small aquaria in a light- and temperature-controlled room on a 12 hr light/dark daily cycle at 18–20°C. Before implanting recording wires and severing the nerve cord, we anesthetized leeches with cold (4°C) saline.
The leech CNS consists of large head and tail ganglia and a chain of 21 midbody ganglia (M1–M21) linked by three intersegmental connectives. Each midbody ganglion innervates the dorsal side of a body segment via a pair of dorsal posterior (DP) nerves. Extracellular recordings from DP nerves allow us to monitor swimming activity through the activity cycles of the swim dorsal excitor motoneuron DE-3.
Behavioral studies on freely swimming leeches
Experiments
We examined the swimming movements of twelve medium-sized adult leeches in an elongated Plexiglas tank (80 cm long, 15 cm wide, filled to a depth of 15 cm with deionized water at 18–20°C). We supplemented visual observations with videotape records made from the side to view the leeches in profile. A ruler in the background permitted us to measure distances traveled by the leeches and therefore calculate swim velocities (Fig. 1). For many experiments, we sutured small silver beads bilaterally to the body wall at midbody segments M7 and M14. These beads served as reference markers for measuring swim cycle period and intersegmental phase lag in behavioral experiments.
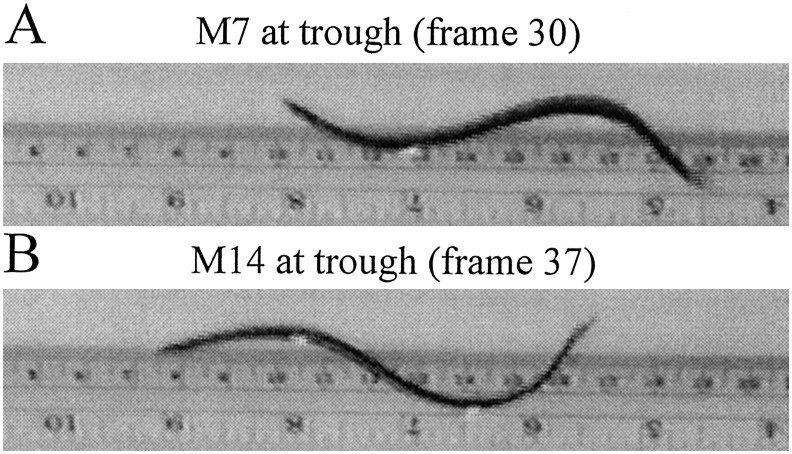
Fig. 1.
Measurement of intersegmental phase lags by video recording. The two video frames show side views of a swimming leech from a continuous video recording. Arrival of a trough at midbody segment M7 (A, frame 30) and then midbody segment M14 (B, frame 37). Because the video is recorded at 30 frames/sec, the time interval between the two frames is 7/30 or 0.23 sec. Given that the swim cycle period is 0.50 sec in this swimming episode, the phase lag between M7 and M14 is 166°. [The leech is swimming to the left, and the ruler in the background shows the distance traveled. Pictures here and in other similar figures were captured by a Matrox (Boca Raton, FL) Rainbow Runner video-capturing card and were enhanced with Corel (Ottowa, Canada) Photo-Paint.]
We examined four types of leech preparations: (1) intact control leeches without beads; (2) intact leeches with beads sewn to the body wall; (3) SNC leeches with the nerve cord severed between midbody ganglia M10 and M11 and with beads attached; and (4) half-leeches consisting of either the anterior or posterior halves of leeches, obtained by cutting previously tested animals in half at the M10 and M11 boundary. To obtain SNC leeches, we severed the nerve cord of experimental preparations with iridectomy scissors by first anesthetizing them with ice-cold (0–4°C) saline and then approaching the nerve cord through a small slit in the ventral aspect of the body wall. Leeches were allowed at least 15 min to recover from this surgery before they were videotaped. We tested the ability of these preparations to swim in a coordinated manner in response to tactile (using a wooden rod) or external electrical (3 V, 20 Hz) stimulation of the body wall.
Data analysis
We analyzed the videotaped records of leech swimming movements to obtain values for (1) cycle period, (2) intersegmental phase lag between segment M7 and M14, and (3) swim velocity.
Cycle period. To determine the cycle period, we counted the video frames required for successive crests and troughs to pass our position marker bead at body segment M7 and divided this number by 30 (for a frame rate of 30 frames/sec) to obtain the cycle period in seconds. To ensure maximum accuracy, we repeated these measurements for the progression of crests and troughs past M14, as well. All reported measurements are averages of at least three period measurements in five swim episodes (10 episodes for intact preparations). Similarly, we determined the cycle periods of half-leeches by counting the number of frames required for successive troughs to arrive at M7 for anterior halves and at M14 for posterior halves. For those preparations, we calculated the average period of several swim-like cycles in each of three swim episodes.
Intersegmental phase lag. We determined the rate of progression of the swimming wave (intersegmental phase lag) by first measuring the time interval between the arrival of a crest at M7 and its arrival at M14 and then repeated this measurement for the progression of troughs (Fig. 1). To convert these time delays to phase values, we divided the delays by the cycle period and multiplied by 360 to obtain the phase delays in degrees. For these and other measurements that depend on counting video frames, we estimated the arrival times of crests and troughs at the position markers to within 1/5 of a frame. To ensure accuracy of these measurements, we determined these intervals for six cycles in each of five swim episodes for leeches with severed nerve cords (10 episodes in the intact leeches).
Swim velocity. To calculate swim velocities, we noted the positions of the leeches at the beginning and end of a series of swim cycles using the ruler taped to the back of the tank. We then counted the number of elapsed video frames, calculated the time interval, and divided the distance by the time.
Finally, we determined the statistical significance of differences between values of cycle period, intersegmental phase delays, and swim velocity for the various preparations (e.g., intact vs severed nerve cords) with a Student’s two-tailed t test.
In situ extracellular recording from intact and SNC preparations
Experiments
We used two types of preparations in these experiments (Fig.2A): a control with the nerve cord intact and the SNC preparation with the nerve cord connectives severed between M10 and M11. Extracellular recordings were obtained from the DP nerves of ganglion M7 and ganglion M14 withen passant hook electrodes, which do not interrupt sensory and motoneuron traffic. These electrodes were fabricated from Teflon-coated silver wire that was insulated, except at the tip (W. B. Kristan, Jr., personal communication; Murray et al., 1996) (Fig. 2B), which was formed into a hook. To implant the hook electrodes, small slits were made from the leech ventral side to expose ganglia M7 and M14 and their associated DP nerves. A DP nerve was then drawn into a fine plastic tube with the exposed silver wire making direct contact with the nerve. The nerve and hook electrode were then insulated by injecting a 1:2 petroleum jelly/oil mixture into the tube. A length of wire close to the recording end was stitched into the skin and secured with a knot to keep the electrode stable and reduce artifacts caused by swim movements. In some preparations, the head ganglion was detached to facilitate swimming.
Fig. 2.
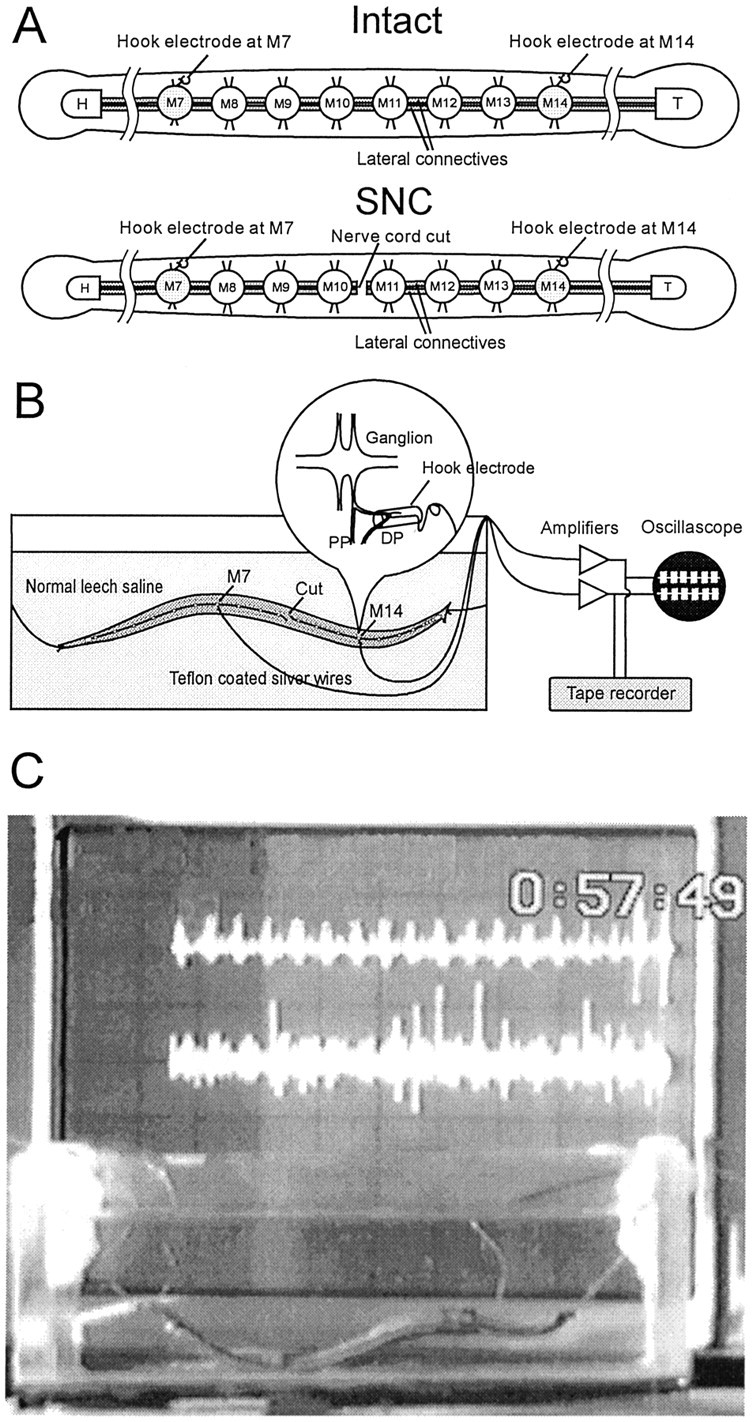
In situ recording.A, Two types of preparations used in the experiments. The leech ventral nerve cord is composed of a head ganglion (H), 21 midbody ganglia (M1–M21), and a tail ganglion (T). The median Faivre’s nerve and two lateral connective nerves link the ganglia. Interactions between the nerve cord and the body wall occur via the paired nerve roots that project from each midbody ganglion. The DP nerve, which branches from the posterior nerve root and innervates the dorsal side of the body wall, contains the large axon of the swim motoneuron DE-3.Top, Intact preparation with both the ventral nerve cord and the body wall intact. Bottom, SNC preparation with the ventral nerve cord severed between M10 and M11. B, Recording setup. Leeches were tethered by threads attached to head and tail suckers and suspended in a deep glass dish for physiological recording and videotaping. The lengths of threads tethering the leech were adjusted so that a full body wave could be developed. DP nerve activity was recorded in situ via fine silver hook electrodes. The inset illustrates the detail of a hook electrode. C, Snapshot of an experiment using the setup described above. The oscilloscope in the background displays signals recorded from the implanted electrodes.
To obtain physiological recordings of leech nerve activity in swimming animals, we suspended electrode-implanted leeches from suture thread into a glass dish (11.5 cm long, 6.5 cm wide, filled to a depth of 4 cm with cold saline). After the preparation was in place, the cold saline, which retards leech movements, was replaced with saline at room temperature. Electrical signals from the hook electrodes were amplified and filtered (P-15 preamplifiers set to pass signals in the range of 30 Hz to 1 kHz; Grass Instruments, Quincy, MA). Signals were further amplified for display on an oscilloscope and stored on magnetic tape (Vetter) for later analysis.
Data analysis
Records from the video tapes were digitized using a 12 bit analog-to-digital board (CFO-DAS16/TR; Computer Boards, Inc.) and the Leech Analysis Software (Computer Technology Center, University of Virginia, Charlottesville, VA). The digitized data were then exported to MATLAB (MathWorks, Inc., Natick, MA), where analyses were performed using our customized software, Rhythm Analysis System (programmed by Dr. Craig Hocker, University of Virginia, Charlottesville, VA).
Data processing of extracellular electrical recordings included three steps. First, we passed the records through a third-order Chebyshev high-pass digital filter to further reduce the low-frequency components caused by leech movements and to eliminate 60 Hz noise. Second, nerve impulses were extracted from the records by setting an appropriate threshold. Third, individual swim bursts were identified by a computer routine that identifies grouped impulses as discrete bursts. As in our previous studies (Pearce and Friesen, 1984; Friesen, 1989), the reference point (0°) for each swim cycle was assigned to the median impulse of each DP nerve burst. The cycle period was determined from the average time interval between the median impulses of consecutive swim bursts. The intersegmental phase lag between M7 and M14 (in degrees) was calculated by dividing the time delay between the midpoints of M7 and M14 swim bursts by the cycle period and then multiplying this quotient by 360°. Because phases are distributed around a 360° circle, circular statistics (Fisher, 1995) were used to rigorously analyze and display our results.
Extracellular recordings from isolated nerve cord preparations
Preparations and experiments
For these experiments, we used isolated leech nerve cords extending from midbody ganglia M2 to the tail brain held by pins in a glass-bottom dish. Extracellular recordings via suction electrodes were obtained from DP nerves emanating from M7 and M14 to obtain intersegmental phase relationship in the absence of sensory feedback (Kristan and Calabrese, 1976).
Data analysis
Similar to in situ recordings, records obtained in these experiments were digitized and then exported to MATLAB for data analysis. Cycle periods and intersegmental phase lags between M7 and M14 were calculated as described above.
RESULTS
Behavioral experiments in freely swimming leeches
Effects of beads on cycle periods
We sewed beads to the leech body wall to serve as reliable position reference markers at midbody segments M7 and M14. To determine whether this procedure influenced the expression of swimming activity, we examined swim movements in six leeches before and after attaching the beads. We observed almost no differences in the swim body wave expressed under the two conditions. Moreover, we found no significant differences in the cycle periods before (0.40 ± 0.01 sec; mean ± SEM; n = 10) and after (0.38 ± 0.01 sec; n = 10) sewing the beads to the body wall. The beads caused a 24% decrease in the leeches’ mean swim velocity (14.6 cm/sec before vs 11.1 cm/sec after). Although the reduction in swim velocity caused by the beads is statistically significant, the interpretation of our data were based on phase relationships and hence was not affected by swim velocity.
Intersegmental coordination and intersegmental phase lags
Our primary aim in these experiments was to determine by close observation of swimming leeches whether cutting the ventral nerve cord destroys intersegmental coordination between the anterior and posterior ends of a leech. Remarkably, we found that leeches with all neuronal connections severed in the ventral nerve cord can continue to generate well coordinated swim movements (Fig. 3). At first glance, these swim undulations appear to be identical in period and waveform to the movements of intact leeches. On closer examination, however, it appears that severing the nerve cord does have an effect; the leech then expresses a body waveform that is more than one full cycle. All eight preparations with the nerve cord severed between M10 and M11 generated swim movements, but in three preparations, well coordinated swimming occurred only infrequently, and hence quantitative analyses were not appropriate. Nevertheless, five leeches moved through the water essentially normally, and our analyses in this section and below are based on these five preparations.
Fig. 3.
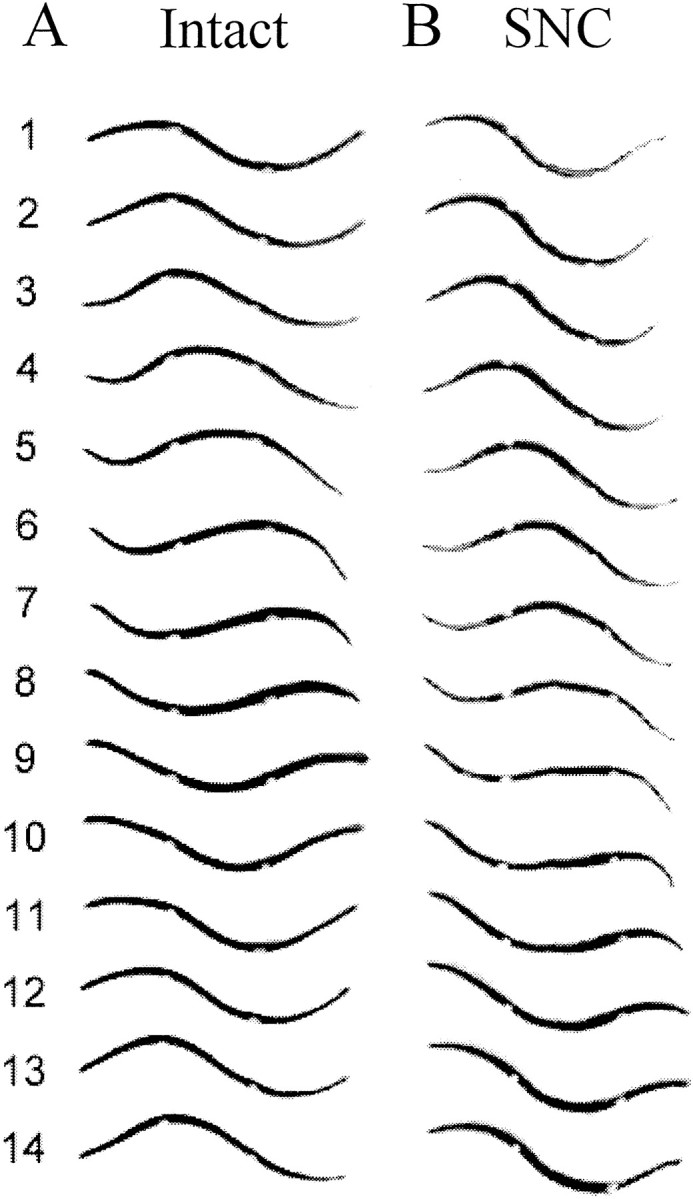
Swim body waves of a freely swimming leech before and after its nerve cord was severed. Each column shows sequential video frames of the leech in side view. Beads were sewn to the body wall at midbody segments M7 and M14 as markers to facilitate the determination of intersegmental phase relationships. A, Nerve cord intact. A full sinusoid-like swim wave was developed along the leech body, with a crest and a trough passing backward while the leech was swimming forward (anterior is to the left).Frames 1 and 12 show identical profiles, with a cycle period of 11/30 or 0.37 sec. The crest arrives at M7 and M14 in frames 2 and 6, so the crest-to-crest phase lag was [(6.0 − 2.0)/11] * 360° = 131°. Similarly, trough-to-trough phase lag was [(11.0 − 7.6)/11] * 360° = 111° (the trough arrives at M14 betweenframes 7 and 8 and was interpolated asframe 7.6). On average, the phase lag from M7 to M14 was 121°. B, The same leech after its nerve cord was severed. The entire length of the preparation maintains a smooth and strong swim body wave, indicating that the posterior end is active during swimming. More than a full sinusoid-like wave is developed, especially evident in frames 6 and 13 in which two troughs or crests can be observed. Frames 1and 14 are at the same phase angle, so the cycle period is 13/30 or 0.43 sec. Crest-to-crest phase lag is [(8.8 − 4.2)/13] * 360° = 127°, and trough-to-trough phase lag is [(14.8 − 9.4)/13] * 360° = 150°. The average phase lag from M7 to M14 is 139°.
We performed a quantitative examination of body wall dynamics in these five SNC preparations to understand the source of their altered swimming profile. Our approach was to determine the intersegmental phase lags in body movements in these animals by measuring intersegmental delays of the body wave between body segments M7 and M14. Two methods for determining these phase lags (examination of the progression of crests and of troughs) yielded nearly identical results. First, in our comparisons of phase lags before and after nerve cord transection (i.e., phase lags between M7 and M14), we discovered that, although the leeches still could maintain their coordinated swimming, phase lags increased after being cut (Table1). On average, the phase lag was 147° before the cut and 170° after the cut: a 23° or 16% increase in phase lag between M7 and M14. This difference is highly significant (p < 0.002; Student’s t test) for all five animals. These larger phase lags explain the expression of more than one cycle in the body wave of leeches with severed nerve cords.
Table 1.
Intersegmental phase lags between M7 and M14 before and after transection of the nerve cord in freely swimming leeches determined from video analysis (n = 5 swim episodes for each preparation)
| Preparation | 1 | 2 | 3 | 4 | 5 | Pooled data |
|---|---|---|---|---|---|---|
| Intact (mean ± SD) | 134 ± 10.0° | 142 ± 2.7° | 138 ± 11.0° | 152 ± 9.7° | 167 ± 9.1° | 147 ± 13.2° |
| SNC (mean ± SD) | 154 ± 5.2° | 149 ± 3.9° | 174 ± 4.0° | 185 ± 19.7° | 190 ± 8.3° | 170 ± 18.2° |
| Difference | 20° | 7° | 36° | 33° | 23° | 23° |
| Probability | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 |
Cycle periods of SNC preparations
Another potential difference between SNC and intact leeches is the cycle period. With the nerve cord severed, one might expect cycle period to be reduced because of the blocking of the projections of swim-initiating neurons, cells 204 and 205, and of the excitatory oscillator neuron, cell 208, which all have extensive intersegmental projections (Weeks, 1981, 1982a,b). Our results here are mixed. Two of the five leeches showed no significant difference in cycle period before and after the nerve cord was severed (p> 0.10). Among the three animals that did show a significant difference (p < 0.005), one showed a shorter cycle period, whereas the other two had longer cycle periods as the result of severing the nerve cord. Thus, cycle periods did not change significantly overall when the ventral nerve cord was severed. This result is unexpected because of the very large reduction in intersegmental excitation after transection of the nerve cord.
Swim velocity
In addition to increasing intersegmental phase lags, severing the ventral nerve cord also reduced swimming velocity. We measured the swim velocity of three animals both before and after lesioning the nerve cord and found that, although swim cycle periods were unchanged, the lesions reduced the average swim velocity from 11.1 to 6.3 cm/sec (p < 0.001). Obviously, the SNC leeches swam much less effectively than the intact ones.
Swimming behavior of the half-leeches
We subsequently cut all eight leeches described above in half between segments M10 and M11 to observe the swim-like movements generated independently by the anterior and posterior ends. Although swim cycle periods of half-leeches fall into the same range as those of intact leeches (0.28–0.64 sec), all preparations displayed significantly different cycle periods between the anterior and posterior halves (p < 0.005). In six preparations, the cycle periods of anterior halves were on average 20% longer than those of the posterior halves, whereas in the other two preparations the anterior halves exhibited a 30% shorter period on average. At first glance, the expression of swim-like movements in the anterior half resembles the flexions that characterize swimming inTritonia or the bending movements of larvalXenopus (Fig.4A, left column), and the amplitude is similar to that of the intact leech. Closer examination of the video tapes revealed that some anterior ends did in fact generate traveling swim waves. The posterior half-leech, on the other hand, developed nearly a full wavelength, with substantially reduced amplitude, almost as though it were a shortened but intact leech (Fig. 4B, right column). In summary, posterior, and sometimes anterior, halves generated traveling waves that in a few instances were robust enough to move them out of the field of the video camera.
Fig. 4.
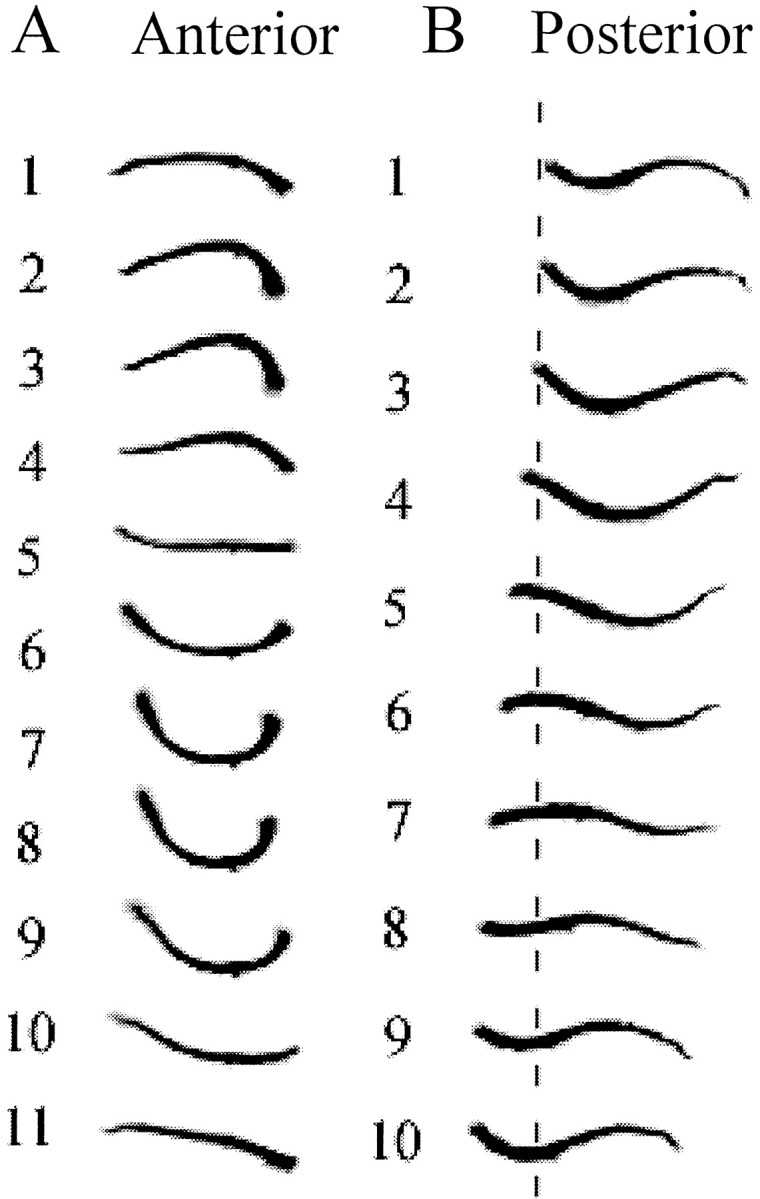
Swim body waves of anterior and posterior half-leeches. A, Eleven continuous frames captured from an anterior half-leech. Although the anterior half-leech seemed to be simply flexing its body up and down, a traveling wave might still be present (frames 6–10). Because the shape was only slightly nonuniform along the half-leech, we infer that phase lags between segments were very small. The camera was fixed during filming; hence, the vertical alignment of the leech silhouettes demonstrates that the anterior half did not progress forward. B, Ten consecutive frames captured from a posterior half-leech. A traveling wave is obvious, and sometimes a crest and a trough can be simultaneously observed in the same profiles (frames 1, 2, 6, 7,9, and 10), as in an intact leech. Although the amplitude of its swim wave is less than that of the anterior half, the posterior half travels forward approximately one-third of its body length in this swim cycle (indicated by thebroken line). In both columns, anterior is to the left.
In situ recording in intact, SNC, and isolated nerve cord preparations
Swimming behavior of the tethered leeches
To make low-noise in situ recording of long swim episodes, leeches were suspended in a glass dish from sutures attached to the head and tail suckers. After mechanical stimulation, these tethered leeches usually swam continuously for up to several minutes, or several hundred swim cycles. The swimming waveform was only altered slightly by the movement restrictions caused by the suspending threads. Although the leech was held up at both ends, giving the impression that the swim amplitude was larger than in freely swimming leeches, the sinusoid-like body wave nevertheless was well preserved and closely approximated swimming movements in freely swimming animals.
Comparison of swimming movements in a tethered intact leech (Fig.5A) and a tethered SNC leech (Fig. 5B) demonstrates that tethered leeches, like unrestrained ones, can swim in a coordinated manner even when the intersegmental connectives are severed in midbody. In approximately half of the preparations, the anterior end failed to generate swimming activity, whereas the posterior end swam vigorously. The anterior end, in contrast, swam by itself only rarely. However, when swimming undulations involved the entire leech, both ends appeared to be actively involved in generating the swimming wave. As Figure5A shows, tethered intact leeches exhibit more than one full wavelength, the result of constriction on the ends of the leech. In the tethered SNC preparations, a further increase in intersegmental phase lag occurs and is expressed as ∼1.5 cycles of body wave. Thus, as in freely swimming leeches, tethered SNC preparations have larger intersegmental phase lags than tethered intact animals. We present quantitative analyses below.
Fig. 5.
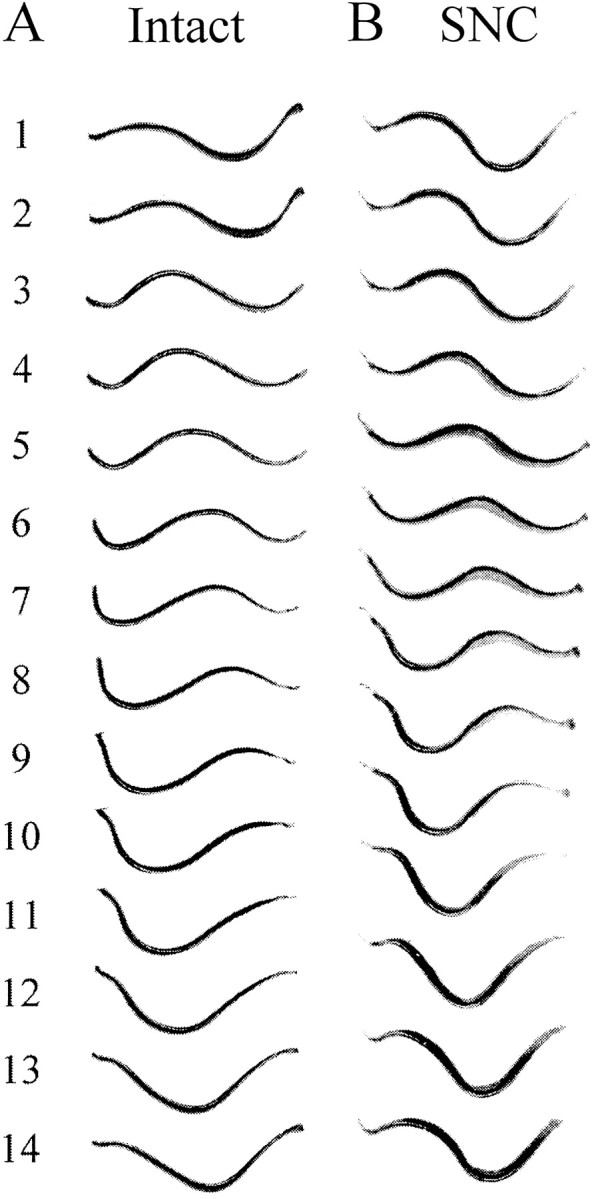
Swimming undulations in tethered leeches.A, Intact preparations. B, SNC preparations. Amplitudes are larger and appear somewhat distorted compared with freely swimming leeches (Fig. 3). More than one full wavelength is present, even in the intact preparations (anterior is to the left).
In situ extracellular recordings from swimming leeches
It appeared that, even with the nerve cord severed, both the anterior and posterior ends of the leech are active in generating coordinated swimming, contrary to the conclusions of Schülter (1933) earlier in this century. To verify our conclusion that both ends of the SNC leeches can generate coordinated, active contractions, we performed simultaneous in situ extracellular recordings of neuronal activity in DP nerves of tethered leeches.
As in the behavioral experiments on freely swimming leeches, SNC preparations were generated by severing the nerve cord between M10 and M11. Instead of sewing beads at M7 and M14, we implanted hook electrodes in these segments to record DP nerve activity from these two ganglia both before and after nerve cord transection. Although we were able to obtain such “before” and “after” data in two animals, in most preparations the technical difficulty of keeping in situ electrodes functioning while severing the nerve cord prevented us from achieving this aim. Thus, most of our results are ofin situ recordings obtained either from animals with intact nerve cords or after severing the ventral nerve cord connectives. To enhance the expression of swimming activity, we removed the head ganglion in some preparations (Brodfuehrer and Friesen, 1986).
As in similar earlier experiments on swimming leeches (Pearce and Friesen, 1984), bursting motoneuron activity (impulses from the dorsal excitor motoneuron DE-3) occurred at both recording sites in all intact preparations. Moreover, the bursts in M14 in the posterior third of the animal were phase-locked with those recorded from the more anteriorly located M7. In these intact preparations, the phase lag of M14 bursts with respect to M7 was nearly constant: less than one-third of a swim cycle (Fig. 6B). The new and interesting result is that, in SNC preparations, rhythmic bursting also occurs at both recording sites. Thus, the posterior end of the leech actively generates muscle rhythmic muscle contractions rather than following passively the movements generated by the anterior end, as suggested by Schülter. In addition, the bursts recorded in DP(14) were phase-locked to those of DP(7), demonstrating that swimming activity was coordinated even at the level of the CNS (Fig.6C; see below). Swim cycle periods were in the same range (0.4–0.7 sec) in both the intact and the SNC preparations, consistent with the behavioral experiments. There were also no significant differences in the number of impulses per burst (10–15 impulses per burst in both cases) or in impulse frequency (40–60 impulses/sec in both cases) between preparations with intact and transected nerve cords.
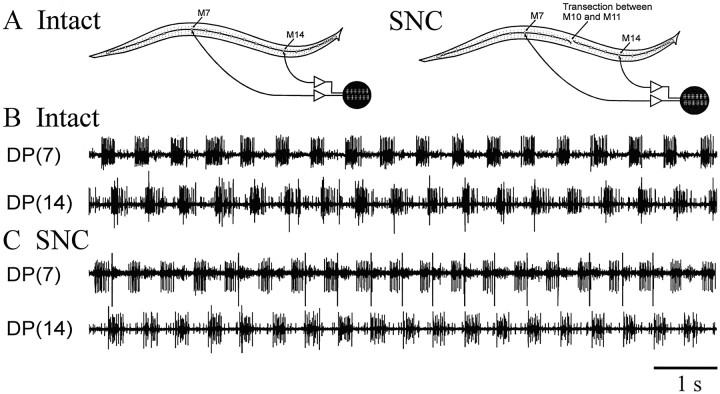
Fig. 6.
In situ extracellular recordings from tethered swimming leeches. Nerve impulses recorded from the DP nerves were generated by motoneurons DE-3, which command dorsal longitudinal muscle contraction during swimming. DE-3 neurons generate one burst of impulses per swim cycle; relative timing of DE-3 bursting in different segments was used to measure intersegmental phase relationship. A, An intact preparation (left) and an SNC preparation (right; different animal). B, A sample record from the intact leech. DE-3 activity in M7 and M14 was phase-locked, with a phase lag of less than one-third of a cycle. C, A sample record from the SNC leech. DE-3 activity in M7 and M14 was again phase-locked after nerve cord transection, but the phase lag between them is now approximately one-half of a swim cycle. [DP(7), DP nerve from M7; DP(14), DP nerve from M14. In theDP(14) trace in B and bothtraces in C, the largest spikes were identified as T-cell impulses by their large size and their activation by light touch.]
To facilitate the comparison of intersegmental phase lags in the two types of preparations, we summarize our data in polar plots (Fig.7). As depicted in Figure 6, the SNC preparations usually showed significantly larger intersegmental phase lags than did the intact preparations. The mean phase lag between M7 and M14 in the intact preparations was 96.5 or 13.8°/segment, a value very close to the 14.6°/segment found in the previous study on swimming activity of intact leeches (Pearce and Friesen, 1984). In contrast, the mean phase lag in the SNC preparations was 142.0 or 20.3°/segment, a highly significant difference (p < 0.001). Although one SNC preparation (Fig.7, #4) exhibited a 90.7° phase lag between the M7 and M14 recording sites, this number is still significantly larger than the phase lag recorded when the same animal had an intact nerve cord (78.8°).
Fig. 7.
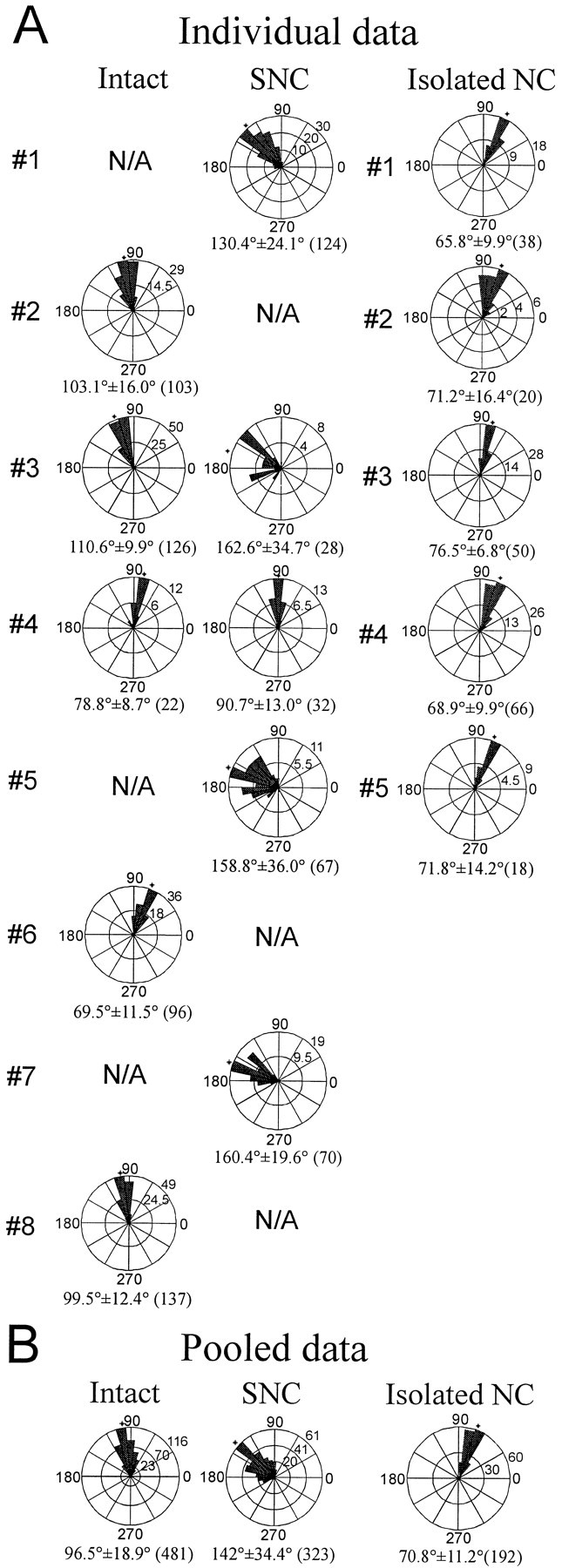
Intersegmental phase lags between M7 and M14 in intact, SNC, and isolated nerve cord preparations. In each plot, a counterclockwise 360° circle represents the swim cycle, 0/360° is the midpoint of DP(7) bursting, and the instantaneous phase lags between M7 and M14 are plotted as a circular histogram. The length of the filled wedgesrepresents
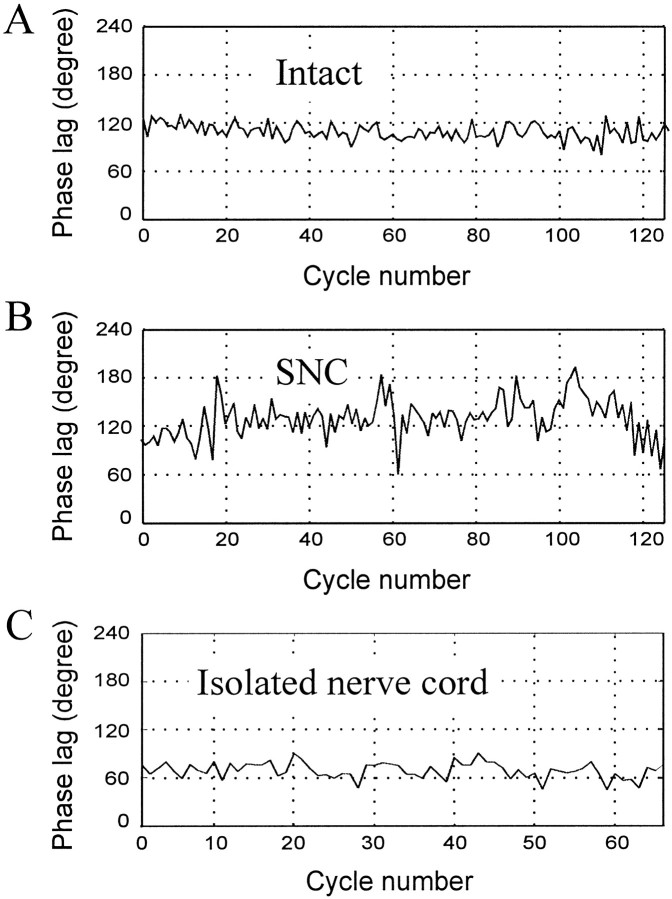
Another noticeable difference between the two types of preparations was that variability in the phase lags, measured as the SD of phase lags in M7 and M14 bursts, was much greater in SNC than in intact preparations. Variability in SNC preparations by this measure was twice that (mean SD = 25.5°) calculated for intact preparations (mean SD = 11.7°). The greater variability in intersegmental phase lags in SNC preparations is shown graphically in Figure8 in which instantaneous (cycle by cycle) phase lags between M7 and M14 bursting activity are plotted against the swim cycle number. The intact preparation (Fig. 8A) shows nearly constant phase relationships between these two recording sites, whereas the phase lag in the SNC preparation (B) fluctuates with large amplitude.
Fig. 8.
Instantaneous phase lags in intact, SNC, and isolated nerve cord preparations. Instantaneous phase lags between M7 and M14, measured for each individual swim cycle, are plotted against cycle number. A, An intact preparation (Fig.7A, #3). Although there is some fluctuation, the phase lags are relatively stable within the range of 80–130° during the whole swim episode. B, An SNC preparation (Fig. 7A, #1). Large fluctuations can be seen all through the swim episode, with phase lags as low as 60° and as high as 180°. C, An isolated nerve cord preparation (Fig. 7A,#4). Variance in cycle period is approximately the same as that of the intact preparation shown in A. Phase lags were obtained from DP records in tethered animals. the number of swim cycles that fall into the corresponding phase bin. An asterisk indicates the mean value of the histogram. Numbers under each plot are the mean ± SD; the total number of swim cycles included in the plots is inparentheses. A, Data from individual preparations. Each preparation is represented by its best swim episode(s). For two preparations (#3, #4of intact and SNC), DP nerve activity was recorded in both intact and SNC conditions. For others, different animals were used for these two conditions. Isolated nerve cord data are from five additional leech preparations. B, Pooled data. Data from all preparations of the same category are pooled in one plot. Phase lags were obtained from DP records in tethered animals.
Interestingly, some large T-cell (touch cell) spikes that are in phase with the swim rhythm can be observed in in situ DP recordings (Fig. 6B, bottom trace,C, top trace). These intermittent T-cell spikes are clearly not necessary for coordinated swimming but may have resulted from friction between the body wall and the implanted electrodes. Readjustment of the recording electrodes usually eliminated these T-cell impulses.
Extracellular recordings from isolated nerve cords
The nerve cord of the leech can generate fictive swimming activity even when completely isolated from the body wall and hence from any peripheral sensory inputs (Kristan and Calabrese, 1976). Previous experiments that used such isolated nerve cord preparations showed that the phase lag in the isolated nerve cord is ∼6–9°/segment for some middle ganglia (Kristan and Calabrese, 1976; Pearce and Friesen, 1984). This phase lag is not constant along the nerve cord but instead shows a monotonic gradient, with larger phase lags toward the posterior end. To make accurate comparisons of phase lags generated by the isolated nerve cord with those observed in the intact and SNC preparations, we measured the phase lag between M7 to M14 during the fictive swimming of the isolated nerve cord.
Our results (Fig. 7) show that the mean phase lag between M7 and M14 in isolated nerve cords is 70.8°, or 10.1°/segment, a value significantly less than that of intact or SNC preparations (p < 0.001; Student’s t test). Swimming in individual isolated nerve cord preparations is very stable (reflected by the small SD values in Fig. 7; see also Fig.8C), but not significantly more stable than in individual intact preparations. In examining variability between preparations of the same type, however, we found that phase lags within the set of isolated nerve cord preparations varied much less than within the sets of the intact or SNC preparations (Table2). The mean phase lags per segment between M7 and M14 range only from 9.4 ± 0.22 to 10.9 ± 0.16° (mean ± SEM) in the five isolated nerve cord preparations, but they are spread over a much larger range (9.9 ± 0.17 to 15.8 ± 0.16°) in the five intact preparations. The range of mean phase lags for the five SNC preparations is even larger (13.0 ± 0.33 to 23.2 ± 0.94°).
Table 2.
Phase lags and swim cycle periods in three different types of preparations (n = 5 individuals for each type)
| Isolated nerve cords | Intact animals | SNC preparations | |
|---|---|---|---|
| Phase lag per segment (mean ± SD) | 10.1 ± 0.56° | 13.8 ± 2.48° | 20.3 ± 4.40° |
| Range of phase lags | 9.4–10.9° | 9.9–15.8° | 13.0–23.2° |
| Swim cycle periods | 0.7–0.9 sec | 0.4–0.7 sec | 0.4–0.7 sec |
DISCUSSION
In these experiments, we systematically studied the swim behavior of the leeches after nerve cord transection. Video recordings of the SNC preparations strongly indicated that coordinated swim activity can be generated in leeches without an intact nerve cord, and our direct physiological recordings unambiguously demonstrated active, coordinated swimming. Quantitative analysis of both video and electrophysiological recordings consistently revealed that phase lags in SNC leeches are significantly larger than those of intact leeches. To further investigate the role of sensory inputs in intersegmental coordination, we also measured intersegmental phase lags in the isolated nerve cord and found smaller and more constant phase lags in these preparations.
Sensory feedback alone is capable of generating intersegmental coordination in leeches
The ability of the leech nerve cord to provide intersegmental coordination was demonstrated more than 60 years ago. Anterior and posterior half-leeches connected only by the nerve cord generate coordinated swimming movements (Schülter, 1933; Gray et al., 1938), but Schülter observed only a few cases of coordinated swimming after he severed the nerve cord. In these few cases, Schülter inferred from visual inspection that the posterior end did not generate muscle contraction but rather expressed undulations via traveling waves that progressed passively from the anterior end. He concluded that intersegmental coordinating information is conveyed exclusively through the nerve cord.
Here, we proved for the first time that an intact nerve cord is not necessary for intersegmental coordination in leeches. In eels, spinal cord transection in midbody results in irregular although coordinated swimming, as confirmed by EMG recording (Wallen, 1982). Similarly,McClellan (1990) reported coupled undulation above and below the transection site after acute transection of the middle of a lamprey spinal cord. The results obtained from these vertebrates, however, were not as clear and conclusive as ours obtained from the leech for the following reasons. (1) Correct anterior-to-posterior progression of swimming activity was well maintained in the SNC leeches, i.e., the anterior end always led the posterior end. In both lamprey and eel, however, such progression was severely disrupted by spinal transection to the extent that reversed posterior-to-anterior progressions were often recorded. (2) The swim cycle periods of the SNC leeches were in the range of intact leeches and nearly as stable. Eels swam erratically and much more slowly after spinal cord transection (without constant cycle period); in the transected lamprey, swimming degraded to many discontinuous episodes of erratic, albeit coupled, activity interspersed with uncoupled activity. (3) No drugs or special stimulation were required to elicit swimming in SNC leeches, but spinal cord-transected lamprey swam only after injection with NMDA. (4) Our recording technique enabled us to monitor neuronal activity directly and to make precise measurements of swim parameters, such as phase lags and cycle periods. In both eel and lamprey, only EMGs, yielding indirect measures of neuronal activity, were recorded from swimming animals.
Our results show that sensory feedback plays a greater role in coordinating locomotion in the leech than in lampreys and eels. More than that, we propose that sensory feedback alone is capable of generating intersegmental coordination in leeches. Although we cannot conclusively exclude a role for peripheral cross-branches of unidentified neurons between segments, no identified neurons can provide such peripheral intersegmental coordination.
What sensory receptors might provide coordinating sensory feedback, and how do they work? The T, P, and N cells are the most prominent mechanosensory neurons in the leech. However, they are not good candidates because they are usually silent during swimming (Kristan et al., 1974) (Fig. 6). Similarly, another class of mechanosensory neurons, the sensillar movement receptors, which are activated by water currents, are unlikely candidates because they are not specialized for either tension or length reception (Friesen, 1981). The best candidates for mediating the observed intersegmental coordination in our SNC preparations are six pairs of segmental stretch receptors in the body wall described by Blackshaw and Thompson (1988). The best studied of these neurons, the ventral stretch receptor, has a nonspiking axon and is hyperpolarized when the body wall is stretched. Our preliminary experiments show that this receptor neuron exhibits rhythmicity during swimming activity and interacts with swim-related neurons (X. Yu, J. Cang, and W. O. Friesen, unpublished observations).
Sensory feedback has an important and specific role in intersegmental coordination
Intersegmental interactions within the nerve cord and sensory feedback are each capable of generating intersegmental phase lags, but there are differences between the coordination generated from these two different sources. In isolated nerve cord preparations, phase lags per segment are small and very stable (10.1 ± 1.6° for M7 to M14; mean ± SD; Table 2). When only sensory feedback is present, intersegmental phase lags are large and much more variable (20.3 ± 4.9° for M7 to M14; mean ± SD). Compared with isolated nerve cord and SNC preparations, intact leeches exhibit phase lags that are relatively stable and have intermediate values, as observed in our in situ recordings (13.8 ± 2.7°; mean ± SD). An increase in the variability of intersegmental phase lags was also found in the spinal cord-transected lamprey (McClellan, 1990); indeed, the SD of intersegmental phase lag tripled in the lamprey after the spinal cord transection.
In comparing the phase lags observed in different leeches, it is interesting that little individual difference existed among the isolated nerve cord preparations used in our experiments. Indeed, the mean phase lags of these five preparations fell within a narrow range of 9.4–10.9°. In contrast, mean phase lags in the five intact preparations were spread over a much larger range of 9.9–15.8°, although swimming in these intact preparations individually was almost as stable as in isolated nerve cord preparations (Fig. 7).
These results demonstrate that intersegmental coordination generated by sensory feedback is not identical to that generated by the nerve cord and furthermore suggest that the CNS and sensory feedback have separate, specific roles in coordinating animal behavior. The swimming pattern observed in the isolated nerve cord preparations is generated by interactions within the nerve cord and is the prototype for the intact swimming leech. The pattern generated by the nerve cord is well coupled among different segments and varies little from individual to individual (indicated by the small individual variance among the isolated nerve cord preparations). This prototypical pattern is insufficient for generating normal swimming movements, however, and requires sensory feedback for the following reasons. (1) The intersegmental phase lag observed in the isolated nerve cord is too small to produce a full wavelength sinusoidal body wave. Sensory feedback is required to increase intersegmental phase lags enough to develop one full wavelength sinusoidal body wave. (2) The leech must adjust various swimming factors, such as body balance, swimming strength, intersegmental activity delay, swim period, and swim direction, in response to environmental changes, such as turbulence, currents, or obstacles. The prototype pattern generated by the CNS must rely on sensory inputs to make such adjustments. Sensory feedback allows the pattern to be fine-tuned, segment by segment and cycle by cycle, according to changes in the environment. (3) Body size and shape of leeches vary among individuals and change greatly with age and feeding condition, but the prototypical swimming pattern generated by the nerve cord is very similar among different preparations. Therefore, the swimming pattern must be modified to accommodate individual body characteristics and developmental changes to achieve optimal swimming mechanics. We propose that differences in the strengths of sensory feedback among animals gives rise to the large differences in intersegmental phase lags among individuals.
Phase lags generated during swimming movements by anterior and posterior half-leeches
The differences between the body waves generated by the anterior and posterior half-leeches is striking. The anterior half produces flailing motions (almost “C-shaped”), indicating that intersegmental phase lags are very small. In contrast, the posterior half-leech generates waves that resemble the full wavelength observed in the intact animal. The huge difference in body shapes indicates a fundamental difference in the phase lags generated by the half-systems. This discrepancy may simply be an enhancement of an effect already observed in the intact preparation in which close examination (Kristan et al., 1974; Friesen and Pearce, 1993) reveals that the radius of curvature is not constant along the animal but is greater in the anterior than the posterior, and thus intersegmental phase lags are also smaller in the anterior than posterior portions of intact swimming leeches. We demonstrated earlier that these differences are inherent in the central swim oscillator, because intersegmental phase lags in isolated nerve cord preparations are likewise smaller in the anterior than in the posterior nerve cord (Friesen and Pearce, 1993).
Conclusion
We have demonstrated that sensory feedback in the leech, even without an intact nerve cord, is capable of generating intersegmental coordination, albeit with greater than normal phase lags. Previous experiments on leech swimming movements focussed primarily on mechanisms within the nerve cord. Our new results show that the role of sensory feedback in generating animal swimming movements is more important than previously accepted and hence merits further investigation.
Footnotes
This research was supported by National Science Foundation Grants IBN 94–10779 and IBN 97–23320 (to W.O.F.). We thank Dr. Craig Hocker for his assistance in data analysis and Cameron McLaughlin for expert editorial assistance. We also thank Dr. Bill Kristan’s lab (University of California, San Diego, CA) for assistance with the in situ extracellular recording technique. We thank our colleagues Dr. Craig Hocker, Jianhua Cang, and Dr. Giselle Oda for their insightful comments. Finally, we thank two anonymous reviewers for their helpful critiques.
Correspondence should be addressed to W. Otto Friesen, Department of Biology, University of Virginia, Charlottesville, VA 22903-2477.
REFERENCES
- 1.Bassler U. The walking- (and searching-) pattern generator of stick insects, a modular system composed of reflex chains and endogenous oscillators. Biol Cybern. 1993;69:305–317. [Google Scholar]
- 2.Blackshaw SE, Thompson SWN. Hyperpolarizing response to stretch in neurons innervating leech body wall muscle. J Physiol (Lond) 1988;396:121–138. doi: 10.1113/jphysiol.1988.sp016954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodfuehrer PD, Friesen WO. Control of leech swimming activity by cephalic ganglia. J Neurobiol. 1986;17:697–705. doi: 10.1002/neu.480170612. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AH, Wallen P. The neuronal correlate of locomotion in fish: “fictive swimming” induced in an in vitro preparation of the lamprey spinal cord. Exp Brain Res. 1980;41:11–18. doi: 10.1007/BF00236674. [DOI] [PubMed] [Google Scholar]
- 5.Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980;210:492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- 6.Fisher NI. Statistical analysis of circular data. Cambridge UP; Victoria, Australia: 1995. [Google Scholar]
- 7.Friesen WO. Physiology of water motion detection in the medicinal leech. J Exp Biol. 1981;92:255–275. doi: 10.1242/jeb.92.1.255. [DOI] [PubMed] [Google Scholar]
- 8.Friesen WO. Neuronal control of leech swimming movements. In: Jacklet JW, editor. Cellular and neuronal oscillators. Dekker; New York: 1989. pp. 269–316. [Google Scholar]
- 9.Friesen WO, Pearce Mechanisms of intersegmental coordination in leech locomotion. Semin Neurosci. 1993;5:41–47. [Google Scholar]
- 10.Friesen WO, Poon M, Stent GS. Neuronal control of swimming in the medicinal leech. IV. Identification of a network of oscillatory interneurones. J Exp Biol. 1978;75:25–43. doi: 10.1242/jeb.75.1.25. [DOI] [PubMed] [Google Scholar]
- 11.Gray J, Lissmann HW, Pumphrey RJ. The mechanism of locomotion in the leech (Hirudo Medicinalis Ray). J Exp Biol. 1938;15:408–430. [Google Scholar]
- 12.Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol Rev. 1975;55:247–303. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- 13.Grillner S, Christenson J, Brodin L, Wallen P, Hill RH. Locomotor system in lamprey: neuronal mechanisms controlling spinal rhythm generation. In: Jacklet JW, editor. Cellular and neuronal oscillators. Dekker; New York: 1989. pp. 407–434. [Google Scholar]
- 14.Hocker CG, Friesen WO. Sensory feedback strongly affects swimming activity in the medicinal leech. Soc Neurosci Abstr. 1997;23:1048. [Google Scholar]
- 15.Ikeda K, Wiersma CAG. Autogenic rhythmicity in the abdominal ganglia of the crayfish: the control of the swimmeret movements. Comp Biochem Physiol. 1964;12:107–115. doi: 10.1016/0010-406x(64)90053-2. [DOI] [PubMed] [Google Scholar]
- 16.Kristan WB, Calabrese RL. Rhythmic swimming activity in neurons of the isolated nerve cord of the leech. J Exp Biol. 1976;65:643–668. doi: 10.1242/jeb.65.3.643. [DOI] [PubMed] [Google Scholar]
- 17.Kristan WB, Stent GS, Ort CA. Neuronal control of swimming in the medicinal leech. J Comp Physiol. 1974;94:97–119. [Google Scholar]
- 18.McClellan AD. Locomotor recovery in spinal-transected lamprey: regenerated spinal coordinating neurons and mechanosensory inputs couple locomotor activity across a spinal lesion. Neuroscience. 1990;35:675–685. doi: 10.1016/0306-4522(90)90338-5. [DOI] [PubMed] [Google Scholar]
- 19.Murchison D, Chrachri A, Mulloney B. A separate local pattern-generating circuit controls the movement of each swimmeret in crayfish. J Neurophysiol. 1993;70:2620–2631. doi: 10.1152/jn.1993.70.6.2620. [DOI] [PubMed] [Google Scholar]
- 20.Murray JA, Wilson RJA, Kristan WB. Motoneuron activity in freely swimming medicinal leeches. Soc Neurosci Abstr. 1996;22:1079. [Google Scholar]
- 21.Paul DH, Mulloney B. Intersegmental coordination of swimmeret rhythms in isolated nerve cords of crayfish. J Comp Physiol [A] 1986;158:215–224. [Google Scholar]
- 22.Pearce RA, Friesen WO. Intersegmental coordination of leech swimming: comparison of in situ and isolated nerve cord activity with body wall movement. Brain Res. 1984;299:363–366. doi: 10.1016/0006-8993(84)90720-0. [DOI] [PubMed] [Google Scholar]
- 23.Pearce RA, Friesen WO. Intersegmental coordination of the leech swimming rhythm. II. Comparison of long and short chains of ganglia. J Neurophysiol. 1985;54:1460–1472. doi: 10.1152/jn.1985.54.6.1460. [DOI] [PubMed] [Google Scholar]
- 24.Pearson KG, Ramirez JM. Sensory modulation of pattern-generating circuits. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, networks, and motor behavior. MIT; Cambridge, MA: 1997. pp. 225–235. [Google Scholar]
- 25.Stein PSG. Intersegmental coordination of swimmeret motoneuron activity in crayfish. J Neurophysiol. 1971;34:310–318. doi: 10.1152/jn.1971.34.2.310. [DOI] [PubMed] [Google Scholar]
- 26.Schülter E. Die Bedeutung des Centralnervensystems von Hirudo medicinalis für Locomotion and Raumorientierung. Z Wiss Zool. 1933;143:538–593. [Google Scholar]
- 27.Stent GS, Kristan WB, Jr, Friesen WO, Ort CA, Poon M, Calabrese RL. Neuronal generation of the leech swimming movement. Science. 1978;200:1348–1357. doi: 10.1126/science.663615. [DOI] [PubMed] [Google Scholar]
- 28.Wallen P. Spinal mechanisms controlling locomotion in dogfish and lamprey. Acta Physiol Scand Suppl. 1982;503:3–45. [PubMed] [Google Scholar]
- 29.Weeks JC. Neuronal basis of leech swimming: separation of swim initiation, pattern generation and intersegmental coordination by selective lesions. J Neurophysiol. 1981;45:698–723. doi: 10.1152/jn.1981.45.4.698. [DOI] [PubMed] [Google Scholar]
- 30.Weeks JC. Synaptic basis of swim initiation in the leech. I. Connections of a swim-initiating neuron (cell 204) with motor neurons and pattern-generating “oscillator” neurons. J Comp Physiol. 1982a;148:253–263. [Google Scholar]
- 31.Weeks JC. Synaptic basis of leech swimming. II. A pattern-generating neuron (cell 208) which mediates motor effects of swim-initiating neurons. J Comp Physiol. 1982b;148:265–276. [Google Scholar]
- 32.Wilson DM. The central nervous control of the flight in a locust. J Exp Biol. 1961;38:471–490. [Google Scholar]
- 33.Yu X, Friesen WO. Role of sensory feedback in generating coordinated swimming movement in leeches (Hirudo Medicinalis). Soc Neurosci Abstr. 1997;23:1048. [Google Scholar]