Abstract
The present experiments used CGP 35348, a selective GABAB receptor antagonist with a significantly higher affinity for post- versus presynaptic receptors, to dissociate the role of antagonist concentration versus stimulation mode in determining whether GABAB receptor blockade facilitates or suppresses long-term potentiation (LTP). The antagonist was applied by pressure ejection to one of two recording sites in area CA1 of hippocampal slices before LTP was induced at both sites with either theta burst or high-frequency stimulation (TBS or HFS). TBS produced a dose-dependent facilitation of potentiation that turned into depression at the highest concentration tested, a result reflecting the dose-dependent balance between the drug’s postsynaptic disinhibitory effect and its action on presynaptic autoreceptors regulating the release of GABA. In contrast, HFS-induced LTP increased monotonically with drug concentration, suggesting that blockade of postsynaptic GABAB receptors is the only factor contributing to HFS-induced LTP. To test the relevance of the two sets of LTP results, we performed behavioral studies examining the effect of different dosages of antagonist on spatial retention and found that memory was enhanced at intermediate dosages but not at very low and high concentrations, reminiscent of the bell-shaped dose–response curve obtained for TBS-induced LTP. These findings are consistent with the notion that LTP induced by electrical stimulation modeled after endogenous theta-modulated activity patterns bears more relevance to behavior than does potentiation induced by arbitrary tetanic trains.
Keywords: LTP, memory, hippocampus, theta, GABAB, autoreceptors, postsynaptic, facilitation, impairment, dose–response curve
GABAergic interneurons are known to exert a powerful influence on the occurrence of long-term potentiation (LTP) by regulating the degree of local depolarization in the activity-receiving target areas. There are two main classes of GABA receptors, the GABAA and the GABAB type, each of which mediates inhibition via distinctly different cellular mechanisms. The present study focused on the GABAB receptor that modulates synaptic transmission by presynaptic inhibition of transmitter release via auto- and heteroreceptors and by increasing K+ conductance responsible for long-lasting (late) IPSPs. Our interest in the GABAB receptor emerged from previous findings that selective 5-HT3 receptor antagonism in behaving rats causes a reduction in the firing rate of a subset of hippocampal interneurons, thereby facilitating both the induction of LTP and the retention of memory (Stäubli and Xu, 1995; Reznic and Stäubli, 1997). The final trigger in this cascade of effects appeared to be a reduction in postsynaptic GABABreceptor–mediated currents. Thus, we sought to test this by selectively blocking postsynaptic GABAB receptors and determining whether such manipulation mimics the effect of the serotonergic antagonist on LTP and memory.
There is evidence that pre- and postsynaptic GABABreceptors exhibit differential responses with respect to time course and affinity to ligands (Olpe et al., 1990, 1993a,b; Davies et al., 1991; Isaacson et al., 1993). The complexity of mechanisms of GABAB action is further underlined by results that suggest both facilitation and suppression of potentiation, depending on the mode of LTP induction. Specifically, long tetanic trains enhanced LTP irrespective of antagonist concentration (Olpe and Karlsson, 1990; Olpe et al., 1993b), whereas brief theta pattern stimulation had no effect at low antagonist level (Davies et al., 1991) but suppressed LTP at high dosage (Davies et al., 1991; Mott and Lewis, 1991; Olpe et al., 1993b). Behavioral work on the effect of GABAB receptor blockade has produced results ranging from memory facilitation to impairment (e.g., Bianchi and Panerai, 1993; Carletti et al., 1993;Mondadori et al., 1993; Brucato et al., 1996; Getova et al., 1996). Possible reasons for these inconsistencies include the use of tasks with unknown relationships to hippocampal processes and the failure to establish dose–response relationships between the drug and the behavior tested.
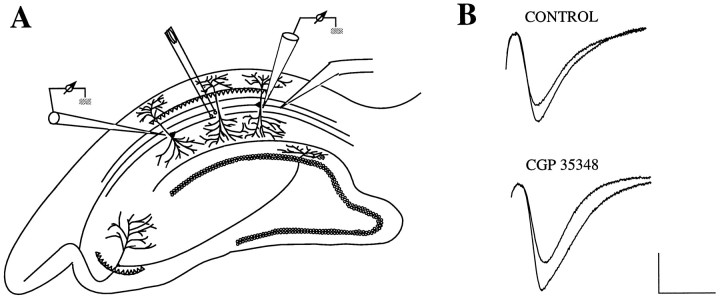
The goal of this study was (1) to disentangle the role of drug concentration versus LTP induction mode in determining whether GABAB antagonism facilitates or suppresses potentiation and (2) to assess the relevance of the observed physiological results to cognitive processes. All experiments involved CGP 35348, an antagonist with significantly higher affinity for post- versus presynaptic receptors (Olpe et al., 1990, 1993b; Davies et al., 1991) that crosses the blood–brain barrier (Olpe et al., 1993a). A technique was used in which potentiation could be simultaneously induced at two sites in the same hippocampal slice, only one of which was exposed to the antagonist, allowing for within-slice and same-time comparisons of test and control LTP (see Fig. 1). The drug was applied at increasing concentrations, and LTP was induced with either pairs of brief theta bursts or prolonged high-frequency trains. Behavioral testing involved increasing concentrations of CGP 35348 administered to rats before performing a radial maze task.
Fig. 1.
Hippocampal setup for within-slice and same time–frame comparisons of control and test LTP.A, LTP is induced and recorded simultaneously in a control and test population of synapses at equidistant positions from the stimulating electrode in field CA1. Drug is applied using a pressure ejection system (Picospritzer) through a pipette placed within ∼150 μm of and at the same depth (∼50–100 μm) as the test recording electrode. B, Superimposed waveforms show representative baseline and potentiated responses recorded 45 min after LTP induction at the test and control site. The GABABreceptor antagonist CGP 35348 was ejected at the test site starting 15 min before LTP induction with two pairs of theta bursts separated by 30 sec. Calibration: 1 mV, 10 msec.
MATERIALS AND METHODS
In vitro hippocampal physiology
Rat hippocampal slices were prepared from 2- to 3-month-old Sprague Dawley rats and maintained in an interface chamber using standard conditions, as described in previous work (Stäubli et al., 1998). The rats were decapitated, and their brains were rapidly removed and placed in 0°C oxygenated (95% O2/5% CO2) artificial CSF (aCSF) of the following composition (in mm): NaCl 124, KCl 3, KH2PO4 1.25, MgSO4 2.5, CaCl2 3.4, NaHCO3 36, d-glucose 10, and l-ascorbate 2. The hippocampi were quickly dissected free in ice-cold aCSF, placed on a McIlwain tissue chopper, cut into 400 μm sections, and collected into a Petri dish containing ice-cold aCSF. The slices were then immediately placed on a nylon net in an interface chamber and maintained at a temperature of 31 ± 1°C. They were continuously perfused with preheated aCSF at a rate of 75 ml/hr while their upper surface was exposed to warm, humidified 95% O2/5% CO2.
Recording and stimulating began after an incubation time of at least 1 hr. All experiments involved local drug application via pressure ejection in combination with two extracellular recording sites (glass micropipettes filled with 2 mm NaCl) in the apical dendrites of fields CA1a and CA1c, with one of the two sites randomly selected for drug application and the other serving as control (Fig.1). Stimulation pulses were delivered to the Schaffer-commissural axons passing through the stratum radiatum using a bipolar-stimulating electrode (twisted nichrome wires, 65 μm) centered between the two recording electrodes, ∼500 μm from each. The stimulus strength was adjusted to produce two field EPSPs whose amplitudes were ∼60% of the maximum spike-free response. Experiments were initiated after establishing a stable baseline recording for at least 20 min.
CGP 35348 (a generous gift from Dr. W. Froestl, Novartis, Basel, Switzerland) is a selective antagonist for GABAB receptors with a half-maximal IC50 of 34 ± 5 μm(Bittiger et al., 1990). In slices, the drug has been shown to block postsynaptic GABAB receptor–mediated late IPSPs completely at a concentration of 100 μm (Davies et al., 1991; Olpe et al., 1990, 1993a). Presynaptic GABAB autoreceptors, on the other hand, were found to be fully antagonized at 1 mm, in accordance with the notion that CGP 35348 has a 10 times lower affinity for pre- compared with postsynaptic receptors (Davies et al., 1991). On the basis of the above information, the drug was tested at four concentrations: 100, 250, 500, and 1000 μm. It was diluted to its final concentration with aCSF and applied locally by pressure ejection (Picospritzer; General Valve, Fairfield, NJ) from a glass micropipette placed next to (within 150 μm) and at the same depth (∼50–100 μm) as the test recording electrode. Pipette ejection pressure was set at 8–12 psi (pulse duration, 10 msec) to supply ∼3 nl of drug every 5 sec. Drug application started 15 min before LTP induction and was terminated immediately thereafter. Control experiments involved local application of aCSF starting 15 min before LTP induction.
LTP was induced by either of two stimulation modes, both of which involved 100 Hz episodes but differed in duration and pattern of delivery. The first induction paradigm was called theta burst stimulation (TBS) and consisted of two four-pulse bursts at 100 Hz separated at theta frequency (i.e., 200 msec) and repeated once at 30 sec. This stimulation pattern mimics the typical firing mode of hippocampal pyramidal cells during learning, which consists of two to four sequential high-frequency bursts (100–400 Hz) paced at theta frequency intervals, with three to five spikes per burst (Otto et al., 1991; O’Keefe and Recce, 1993; Skaggs et al., 1996). The other induction paradigm, called high-frequency stimulation (HFS), consisted of a 200 msec long train of 21 pulses at 100 Hz that was repeated once at 30 sec. This second stimulation pattern, although not modeled after endogenous hippocampal activity, had been used by others testing the effect of GABAB receptor blockade on LTP (Olpe and Karlsson, 1990; Olpe et al., 1993b). The stimulation intensity was not increased during TBS or HFS. Within-slice comparisons between potentiation at the site receiving the injection of the antagonist (test response) versus potentiation at the site that did not (control response) were used to determine the effect of the drug on LTP.
Behavioral protocol
Animals. Behavioral training was conducted with a group of eight male Long–Evans rats that were 24–26 months of age at the time of testing. They were housed individually and kept on a 12:12 hr light/dark cycle, with the experiments being conducted during the light phase. The animals had restricted access to food to maintain their body weight at 90% of that of the same-age controls, while water was available ad libitum.
Spatial delayed nonmatch-to-sample task. An elevated eight-arm radial maze made of painted wood was used. It was kept in a well-lit room containing numerous distinct extramaze cues, such as posters, bookshelves, cabinets, and lamps, etc.. The center platform was 38 cm in diameter with eight arms radiating from it (12 cm wide, 56 cm long, and 3.5 cm high enclosing walls), separated from the central platform by Plexiglas guillotine-type doors that could be raised and lowered by the experimenter via a system of pulleys and strings.
Training involved one sample and one test trial per day. In the sample trial, three of the eight arms were blocked, and the animals were allowed to enter the open arms to retrieve a chocolate chip reward hidden in a recessed food well at the end of each arm. After a delay, they were returned to the maze for the test trial in which all eight arms were open, but rewards were contained only in the previously blocked arms (blocked arms were randomized across days). The number of incorrect entries (i.e., arms entered more than once) until all rewards were collected and the number of correct choices made before a reentry occurred were recorded. Rats trained extensively in this task generally perform accurately at delays up to 2 hr, but they begin to commit errors that increase in number the longer the intertrial interval. The present study involved rats with extensive previous experience in this task and used a delay of 8 hr between the sample and test trial.
CGP 35348 has been shown to penetrate the blood–brain barrier rapidly and to exhibit activity in vivo (Bittiger at al., 1990; Olpe et al., 1990; Froestl et al., 1996). In vivo experiments in rats have demonstrated that CGP 35348 given intraperitoneally has moderate effects on postsynaptic GABAB receptors at 30 mg/kg but causes an almost complete antagonism at 100 mg/kg. Side effects such as ataxia were observed at dosages >300 mg/kg (Olpe et al., 1990). The present study tested CGP 35348 at dosages between 12.5 and 300 mg/kg. Each concentration was administered over a period of 2 consecutive weeks in the following sequence: 50, 25, 12.5, 100, 200, and 300 mg/kg. The drug was dissolved in saline and administered intraperitoneally 30 min before the sample trial. On the first day of each week, all rats were tested with vehicle. To allow for within-animal comparisons of the drug’s effect on memory, we randomly divided the rats into two groups and administered either drug or vehicle in a counterbalanced manner on 4 consecutive days of the week, starting on the second of 5 weekly test days. Data analysis involved all drug and vehicle days except the first vehicle day of the week (i.e., 4 vehicle and 4 drug days per concentration and animal).
RESULTS
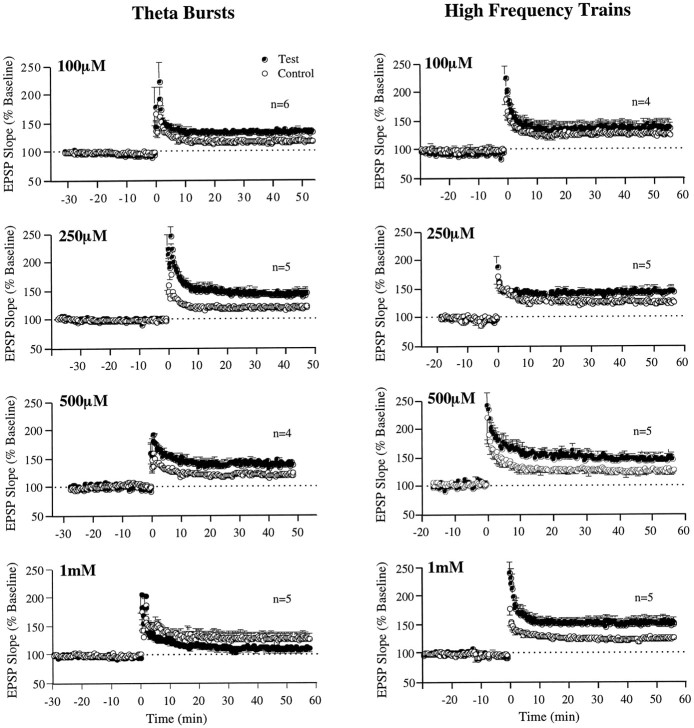
Local pressure ejection of the artificial CSF carrier vehicle used in these experiments had no detectable effect on slice physiology or potentiation. Figure 2 summarizes experiments in which the GABAB antagonist CGP 35348 was tested at four concentrations (100, 250, 500, and 1000 μm) for its effect on LTP induced by two different stimulation modes, TBS and HFS. Within-slice comparisons (pairedt test) between potentiation at the test site receiving the injection of GABAB antagonist versus potentiation at the control site were used to determine the effect of the drug on LTP. When TBS was used, the potentiation measured at the test site between 45 and 50 min after induction was significantly facilitated at 100 μm [t(5) = 5.1; p< 0.01] and 250 μm [t(4) = 4.53; p < 0.01] but was only marginally facilitated at 500 μm [t(3) = 1.79;p < 0.08] and was significantly suppressed at 1000 μm [t(4) = 3.01;p < 0.02]. The average within-slice difference (± SEM) between control and test sites during the last 5 min of recording was 16.5 ± 3.2 for 100 μm, 22.2 ± 5.5 for 250 μm, 12.5 ± 6.9 for 500 μm, and −14.4 ± 4.8 for 1000 μm. In brief, a bell-shaped dose–response curve characterized the interaction between the drug and TBS-induced LTP. This point is also illustrated in a later figure (see Fig. 4C), which displays the mean of the paired differences between the control and test site for each concentration.
Fig. 2.
Dose-dependent facilitatory and suppressive effects of the GABAB antagonist CGP 35348 on LTP induced by TBS versus HFS. Left, Comparisons of the amount of control and test LTP induced by TBS in the presence of increasing concentrations of CGP 35348, using the experimental setup illustrated in Figure 1. Right, Comparisons the same as those on theleft, except that LTP was induced by HFS. Then values indicate the number of slices (1 slice per animal) tested per concentration, and each data point represents the group mean of one response per animal (± SEM).
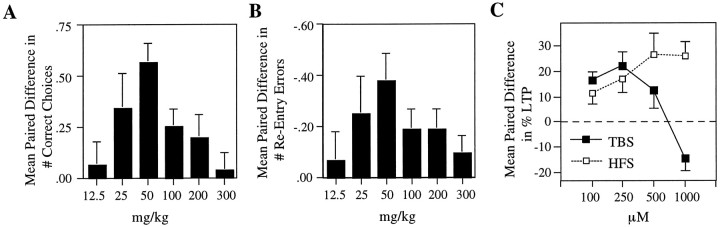
Fig. 4.
Comparison of mean within-rat differences in memory retention versus mean within-slice differences in TBS- and HFS-induced LTP as a function of drug dosage. A, Average within-rat improvement in the number of correct choices between the drug and vehicle condition. B, Average within-rat decrement in the number of reentry errors between the drug and vehicle condition. C, Average within-slice increment and decrement in the amount of potentiation between the test and control recording site for LTP induced by HFS versus TBS.
In contrast, when HFS was used to induce LTP, the potentiation measured at the test site between 45 and 50 min after induction was facilitated at all but the lowest drug concentrations [100 μm,t(3) = 1.49; p > 0.1; 250 μm, t(4) = 3.0; p< 0.05; 500 μm, t(4) = 2.93;p < 0.05; and 1000 μm,t(4) = 4.79; p < 0.01]. The average within-slice difference (± SEM) between the control and test sites during the last 5 min of recording grew steadily with increasing concentration and was 8.6 ± 5.8 at 100 μm, 17.3 ± 5.7 at 250 μm, 26.4 ± 9.0 at 500 μm, and 26.0 ± 6.2 at 1000 μm. Thus, a linear dose–response curve that leveled off at the high end characterized the interaction between the GABAB receptor blocker and LTP induced by HFS (see Fig. 4C). It may be speculated that concentrations >1 mm might have revealed inhibitory effects on HFS-induced LTP. However, as noted previously, CGP 35348 has an affinity of 34 μm for the postsynaptic GABAB receptor (Bittiger et al., 1990) and an ∼10 times lower affinity for the presynaptic site (Davies et al., 1991; Olpe et al., 1993b). According to standard calculations used in receptor-binding chemistry, a compound with these affinities can be expected to produce a virtually complete receptor–ligand saturation at 1 mm, both with respect to post- and presynaptic GABAB receptors. Thus, it is unlikely that testing concentrations >1 mm would have produced any additional changes other than side effects mediated by non-GABABreceptors.
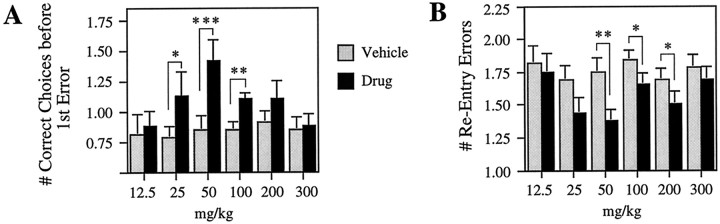
If the hypothesis that LTP plays a role in the encoding of information in tasks requiring the hippocampus is correct, then we would expect that one of the above sets of LTP data is predictive of the drug’s behavioral effects. To test this, we compared the ability to retain spatial memory in rats treated with increasing concentrations of GABAB receptor antagonist (12.5, 25, 50, 100, 200, and 300 mg/kg) versus performance on vehicle days. The drug caused no obvious behavioral side effects at any dosage, consistent with a study by Olpe et al. (1990) who found no measurable effects on motor performance at concentrations up to 1000 mg/kg. Average scores on vehicle versus drug days for each dosage are illustrated in Figure3, A and B. There was no overall significant difference in performance over time on vehicle days during the 12 weeks of behavioral testing, as shown by the results of a repeated measures ANOVA [F(5,35) = 0.152; p > 0.05]. Therefore, vehicle scores were pooled for further data analysis. A repeated measures ANOVA revealed a significant main effect for dosage on the number of correct choices before the first error [F(6,42) = 3.725;p < 0.01] and reentry errors [F(6,42) = 2.636; p < 0.05]. Subsequent within-animal comparisons (paired t test) for correct choices before the first error indicated significant improvements on drug versus vehicle days at 25 mg/kg [t(7) = 2.11; p < 0.05], 50 mg/kg [t(7) = 6.15; p < 0.001], and 100 mg/kg [t(7) = 3.06;p < 0.01]. However, memory facilitation was absent at the lowest and the two highest dosages tested (see Fig. 3A; i.e., at 12.5, 200, and 300 mg/kg [t(7) = 0.55, 1.66, and 0.36; p > 0.05, respectively]). The average within-animal increment in correct choices between drug and vehicle days was 0.06 ± 0.11 for 12.5 mg/kg, 0.34 ± 0.16 for 25 mg/kg, 0.56 ± 0.09 for 50 mg/kg, 0.25 ± 0.08 for 100 mg/kg, 0.19 ± 0.11 for 200 mg/kg, and 0.03 ± 0.09 for 300 mg/kg. Thus, as illustrated in Figure4A, a bell-shaped dose–response curve characterized the interaction between the drug and the improvement in correct choices before the first error.
Fig. 3.
Dose-dependent facilitatory effects of the GABAB antagonist CGP 35348 on retention in a spatial delayed nonmatch-to-sample task. A, Mean number of correct choices (± SEM) made by a group of eight rats before reentering any of the five arms visited in the sample trial 8 hr earlier. Each of the concentrations (12.5, 25, 50, 100, 200, and 300 mg/kg) was tested over a period of 2 weeks with drug and vehicle days counterbalanced, allowing us to use each rat as its own control.B, Experiment the same as that in A but for the number of reentries into arms visited previously (*p < 0.05; **p < 0.01; ***p < 0.001, paired t test).
Similarly, paired within-animal comparisons for the number of reentry errors showed a marginally significant reduction on drug versus vehicle days at 25 mg/kg [t(7) = 1.76;p = 0.06] and significant reductions at 50 mg/kg [t(7) = 3.55; p < 0.01], 100 mg/kg [t(7) = 2.39; p < 0.05], and 200 mg/kg [t(7) = 2.39;p < 0.05]. No facilitatory effects were apparent at the lowest and highest concentrations tested (see Fig. 3B; i.e., at 12.5 and 300 mg/kg [t(7) = 0.55 and 1.43; p > 0.05, respectively]). The average within-animal decrement in reentry errors between drug and vehicle days was −0.06 ± 0.11 for 12.5 mg/kg, −0.25 ± 0.14 for 25 mg/kg, −0.38 ± 0.11 for 50 mg/kg, −0.19 ± 0.08 for 100 mg/kg, −0.19 ± 0.08 for 200 mg/kg, and −0.09 ± 0.07 for 300 mg/kg. Thus, a bell-shaped dose–response curve characterized the interaction between the drug and the reduction in reentry errors, as illustrated in Figure 4B.
However, one caveat to keep in mind with respect to the above behavioral results is that, because the drug was administered systemically, we cannot be certain that the results are attributable to actions on hippocampal GABAB receptors only.
DISCUSSION
The present LTP experiments were prompted by previous work demonstrating that GABAB receptor blockade suppresses LTP in response to brief high-frequency bursts patterned at theta frequency (i.e., TBS) but facilitates LTP in response to long trains of high-frequency stimulation (i.e., HFS) (Olpe and Karlsson, 1990; Davies et al., 1991; Mott and Lewis, 1991; Olpe et al., 1993b). In an attempt to reconcile these apparently contradictory results, we conducted a parametrical study comparing the effects of TBS versus HFS on LTP induced under variable levels of GABAB receptor blockade. Our results indicate that the mode of induction is not the sole factor in determining whether GABAB receptor blockade suppresses or facilitates LTP but that the antagonist concentration is equally crucial. Thus, although HFS consistently enhanced LTP in a manner approximately proportional to the antagonist concentration, we found that a bell-shaped dose–response curve characterized the interaction between the drug and TBS-induced LTP. That is, TBS suppressed LTP at the highest dosage, in accordance with the results of others (Davies et al., 1991; Olpe et al., 1993b), but enhanced it at low and intermediate concentrations. These results provide the first demonstration that GABAB receptor antagonism is capable of facilitating TBS-induced LTP under certain conditions.
The question arises as to how this complex interaction between drug level and LTP induction mode may be reconciled with our current understanding of the role of pre- and postsynaptic GABABreceptors in hippocampal physiology. It is well known that GABAB autoreceptors, located on interneuron terminals that synapse on pyramidal cells via GABAA receptors, mediate an IPSP refractory period that reaches its maximum at ∼200 msec (i.e., the theta period), leading to a prolongation of EPSPs occurring at this interval, thereby creating conditions needed for activation of the NMDA receptor currents that trigger LTP (Larson and Lynch, 1986). The exact mechanism underlying this feedforward IPSP suppression has been identified as transient hyperpolarization of the terminal, a process that peaks between 150 and 250 msec after autoreceptor activation, causing a suppression of GABA release to a subsequent input that is maximal if it occurs at the period of the theta rhythm (Larson and Lynch, 1986; Pacelli et al., 1989; Davies et al., 1991; Mott and Lewis, 1991). In contrast, activation of postsynaptic GABABreceptors on pyramidal cells triggers a slowly rising, long-lasting IPSP that has little inhibitory effect on fast AMPA receptor–mediated currents but is highly effective at counteracting responses mediated by NMDA receptors. From the above points it can be predicted that blockade of post- versus presynaptic GABAB receptors will have opposite effects on LTP. That is, a reduction in postsynaptic GABAB current is expected to increase the duration of dendritic NMDA receptor–mediated currents and thereby facilitate the induction of LTP, whereas blockade of presynaptic receptors is likely to counteract the autoinhibition of GABA release, thereby making it more difficult for LTP to occur.
As mentioned previously, pre- and postsynaptic GABABreceptors differ not only with respect to their physiological role but also in affinity for ligands; that is, postsynaptic receptors are completely blocked by 100 μm CGP 35348, whereas an ∼10-fold higher concentration is needed to antagonize presynaptic autoreceptor function fully (Davies et al., 1991; Olpe et al., 1993b).
How does the above set of information explain our finding that low drug levels facilitate LTP irrespective of stimulation protocol, whereas high levels have opposite effects depending on the induction mode? It seems reasonable to suggest that both TBS and HFS facilitated LTP at low to intermediate levels of CGP 35348 because postsynaptic receptors were the main target in this concentration range, producing an increase in the amount of dendritic depolarization during both induction paradigms. However, it is likely that TBS caused LTP suppression when the antagonist concentration was raised, because blockade of presynaptic GABAB receptors at high drug levels prevented the interneurons from entering their refractory period, thereby facilitating the release of GABA that is normally suppressed during TBS. HFS, on the other hand, still enhanced potentiation at high dosage because the physiological timing patterns and chemistries that regulate LTP induced with theta activity are likely to be of reduced importance when sustained pulse trains are used, as suggested by Arai and Lynch (1992); that is, LTP induced by HFS is less sensitive to the autoreceptor-mediated reduction in GABA release, leaving the disinhibitory effect of blocking postsynaptic GABABreceptors as the drug’s main contributing factor.
A second goal of this project was to compare the drug’s effect on LTP and learning. Assuming that LTP-like mechanisms are engaged during learning, it seemed reasonable to expect that GABABblockers might be effective means to manipulate memory. Previous work by others examining the contribution of GABAB receptors to learning demonstrated a facilitatory action of an orally administered GABAB antagonist (CGP 36742) in various behavioral paradigms including shock avoidance tests, social learning, and operant conditioning (Bianchi and Panerai, 1993; Carletti et al., 1993;Mondadori et al., 1993; Getova et al., 1996). Although these studies suggested a role for GABAB receptors in memory formation, they failed to demonstrate that the compound promoted both the occurrence of synaptic plasticity in a given brain site and the formation of memory known to be subserved by that same site. Because of our LTP results, we tested CGP 35348 at increasing concentrations for its effect on retention in the radial maze, a task well known to involve hippocampal circuitries. Although low and intermediate dosages were expected to enhance memory, it was conceivable that higher dosages might produce either facilitation or impairment, depending on whether HFS- or TBS-induced LTP better mimics the process by which synapses normally change their strength during learning. We found that the drug enhanced hippocampal memory moderately at low dosage and substantially at intermediate dosages and had no effect at high concentration, reminiscent of the bell-shaped dose–response curve that describes the interaction between the drug and TBS-induced LTP. However, the question arises whether the drug concentrations used in the behavioral study reached the brain in the concentration range in which selective effects on pre- and postsynaptic receptors occur? As mentioned above, in vivo experiments by others have shown that the dosage range for modest to almost complete postsynaptic GABAB receptor blockade spans 30–100 mg/kg (Olpe et al., 1990). On the basis of these observations and given that the affinity of CGP 35348 for pre- versus postsynaptic receptors is ∼10 times lower, we can assume autoreceptor blockade to be absent or moderate at 100 mg/kg but substantial at 500 mg/kg and complete at 1000 mg/kg. Our memory performance results correspond well with the above notion. We found memory facilitation at dosages between 25 and 100–200 mg/kg, drug levels at which postsynaptic blockade is dominant. The peak of enhancement occurred at 50 mg/kg, with facilitation monotonically decreasing at increasingly higher and lower dosages. Thus, the effect of the drug on behavior best mimics the effect of the drug on TBS-induced LTP, because both facilitation of LTP and memory occur as long as the antagonist predominantly blocks postsynaptic receptors (between 100 and 500 μm in slices and 25 and 200 mg/kg in vivo) but diminishes progressively as the proportion of receptors that is presynaptic increases. This is not the case for HFS-induced LTP, which was increasingly facilitated the larger the dosage and, in fact, shows the largest enhancement at 1 mm at which both pre- and postsynaptic receptors are known to be blocked.
The implications of the above observation are two-fold. First, it underscores the notion that LTP induced by electrical stimulation that mimics endogenous theta-modulated neural activity patterns typically present when the rat is in learning mode bears more relevance to memory than does LTP induced with arbitrary tetanic trains. Second, it supports the hypothesis that both learning and LTP make use of the same underlying cellular mechanism and that LTP-like enhancements in synaptic efficacy might actually occur during learning. It is of interest in this regard that LTP in area CA1 of the awake animal is best elicited by a few brief theta bursts, whereas prolonged tetanic episodes frequently cause nonspecific neuronal depression and loss of response (Stäubli and Scafidi, 1997).
The highest behavioral dosage examined (300 mg/kg) did not produce memory impairment but simply lacked facilitatory effects, in contrast to our LTP study in which TBS suppressed potentiation at the highest dosage (1 mm) tested. This incongruity may, at least partly, be explained by the choice of our paradigm that involved highly trained rats committing an average of 1.5–2 errors on control days, thus leaving little room for further impairments. Another reason may be that 1 mm CGP 35348 in slices is a much higher dosage than the concentration that reaches the brain after systemic injection of 300 mg/kg (animals injected with dosages larger than 300 mg/kg manifested mild signs of physical discomfort, which led us to stop the experiment at 300 mg/kg). That both of these explanations may apply is suggested by results from another study (Brucato et al., 1996) that involved CGP 46381, an antagonist with a several times higher affinity for the GABAB site than has CGP 35348 [IC50 of CGP 46381 is 4.9 vs 34 μm for CGP 35348 (Olpe et al., 1993a)]. The drug was tested at 100 mg/kg, a dosage demonstrated to be sufficient for blockade of presynaptic autoreceptors in vivo, and, as expected, was found to suppress both TBS-induced LTP in anesthetized rats and acquisition in the Morris water maze, a task known to be relatively stressful and therefore prone to revealing impairments, especially in naive rats.
The present results would gain additional strength if LTP was recorded in the freely moving rat and it was demonstrated that the threshold dosage for memory enhancement and LTP facilitation in the awake brain is similar. In any event, the present study has benefited from the use of CGP 35348 in that it enabled us to distinguish between the usefulness of two distinct LTP induction paradigms for identifying the basic physiologies and chemistries active during learning. It is clear that further developments of GABAB antagonists that selectively interact with the postsynaptic site are likely to be of immense therapeutic value.
Footnotes
This work was supported in part by the Whitehall Foundation Grant M97R05 to U.S. and by the National Science Foundation Grant IBN-9726779 to U.S. We thank Dr. Wolfgang Froestl (Novartis, Basel, Switzerland) for the generous supply of CGP 35348 and Sherman Wiebe for critical comments on this manuscript.
Correspondence should be addressed to Dr. Ursula Stäubli, Cortex Pharmaceuticals, Inc., 15231 Barranca Parkway, Irvine, CA 92618.
REFERENCES
- 1.Arai A, Lynch G. Factors regulating the magnitude of LTP induced by theta pattern stimulation. Brain Res. 1992;598:173–184. doi: 10.1016/0006-8993(92)90181-8. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi M, Panerai AE. Reversal of scopolamine-induced amnesia by the GABAB receptor antagonist CGP 35348 in the mouse. Cognit Brain Res. 1993;1:135–136. doi: 10.1016/0926-6410(93)90018-z. [DOI] [PubMed] [Google Scholar]
- 3.Bittiger H, Froestl W, Hall R, Karlsson G, Klebs K, Olpe H-R, Pozza MF, Steinmann MW, Van Riezen H. Biochemistry, electrophysiology and pharmacology of a new GABAB antagonist: CGP 35348. In: Bowery NG, Bittiger H, Olpe H-R, editors. GABAB receptors in mammalian function. Wiley; Chichester, UK: 1990. pp. 47–60. [Google Scholar]
- 4.Brucato FH, Levin ED, Mott DD, Lewis DV, Wilson WA, Swartzwelder HS. Hippocampal long-term potentiation and spatial learning in the rat: effects of GABAB receptor blockade. Neuroscience. 1996;74:331–339. doi: 10.1016/0306-4522(96)00131-5. [DOI] [PubMed] [Google Scholar]
- 5.Carletti R, Libri V, Bowery NG. The GABAB antagonist CGP 36742 enhances spatial learning performance and antagonises baclofen-induced amnesia in mice. Br J Pharmacol [Suppl] 1993;109:74P. [Google Scholar]
- 6.Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABAB autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- 7.Froestl W, Mickel SJ, Mondadori C, Olpe H-R, Pozza MF, Waldmeier PC, Bittiger H. GABAB receptor antagonists: new tools and potential new drugs. In: Giardina D, Piergentili A, Pigini M, editors. Perspectives in receptor research, Vol 24. Elsevier; Amsterdam: 1996. pp. 253–270. [Google Scholar]
- 8.Getova D, Bowery NG, Spassov V. Effects of GABAB receptor antagonists on learning and memory retention in a rat model of absence epilepsy. Pharmacol Rev Commun. 1996;8:141–143. doi: 10.1016/s0014-2999(96)00877-1. [DOI] [PubMed] [Google Scholar]
- 9.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 10.Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- 11.Mondadori C, Jaekel J, Preiswerk G. CGP 36742: the first orally active GABAB blocker improves the cognitive performance of mice, rats, and rhesus monkeys. Behav Neural Biol. 1993;60:62–68. doi: 10.1016/0163-1047(93)90729-2. [DOI] [PubMed] [Google Scholar]
- 12.Mott DD, Lewis DV. Facilitation of the induction of long-term potentiation by GABAB receptors. Science. 1991;252:1718–1720. doi: 10.1126/science.1675489. [DOI] [PubMed] [Google Scholar]
- 13.O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 14.Olpe H-R, Karlsson G. The effects of baclofen and two GABAB-receptor antagonists on long-term potentiation. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:194–197. doi: 10.1007/BF00166964. [DOI] [PubMed] [Google Scholar]
- 15.Olpe H-R, Karlsson G, Pozza MF, Brugger F, Steinmann M, Van Riezen H, Fagg G, Hall RG, Froestl W, Bittiger H. CGP 35348: a centrally active blocker of GABAB receptors. Eur J Pharmacol. 1990;187:27–38. doi: 10.1016/0014-2999(90)90337-6. [DOI] [PubMed] [Google Scholar]
- 16.Olpe H-R, Steinmann MW, Ferrat T, Pozza MF, Greiner K, Brugger F, Froestl W, Mickel SJ, Bittiger H. The actions of orally active GABAB receptor antagonists on GABAergic transmission in vivo and in vitro. Eur J Pharmacol. 1993a;233:179–186. doi: 10.1016/0014-2999(93)90048-m. [DOI] [PubMed] [Google Scholar]
- 17.Olpe H-R, Wörner W, Ferrat T. Stimulation parameters determine role of GABAB receptors in long-term potentiation. Experientia. 1993b;49:542–546. doi: 10.1007/BF01955159. [DOI] [PubMed] [Google Scholar]
- 18.Otto T, Eichenbaum H, Wiener S, Wible C. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- 19.Pacelli GJ, Su W, Kelso SR. Activity-induced depression of synaptic inhibition during LTP-inducing patterned stimulation. Brain Res. 1989;486:26–32. doi: 10.1016/0006-8993(89)91273-0. [DOI] [PubMed] [Google Scholar]
- 20.Reznic J, Stäubli U. Effects of 5-HT3 receptor antagonism on hippocampal cellular activity in the freely moving rat. J Neurophysiol. 1997;77:517–521. doi: 10.1152/jn.1997.77.1.517. [DOI] [PubMed] [Google Scholar]
- 21.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 22.Stäubli U, Scafidi J. Studies on long-term depression in area CA1 of the anesthetized and freely moving rat. J Neurosci. 1997;17:4820–4828. doi: 10.1523/JNEUROSCI.17-12-04820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stäubli U, Xu F. Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J Neurosci. 1995;15:2445–2452. doi: 10.1523/JNEUROSCI.15-03-02445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stäubli U, Chun D, Lynch G. Time-dependent reversal of LTP by an integrin antagonist. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]