Abstract
Olfactory adaptation is shown to occur inDrosophila, at both behavioral and physiological levels. In a behavioral paradigm, the extent of adaptation is shown to depend on the dose and duration of the adapting stimulus. Half-maximal adaptation occurred after 15 sec of exposure to an odor, and recovery occurred with a half-time of 1.5 min, under a set of test conditions. Cross-adaptation was observed among all odor combinations tested, although to a lesser extent than when the same odor was used as both the adapting and the test stimulus. Mutants of the transient receptor potential (Trp) Ca2+ channel were normal in olfactory response, but defective in olfactory adaptation, when measured either behaviorally or in tests of antennal physiology. These results indicate that olfactory response and adaptation can be distinguished. Trp expression was detected in the developing antenna but, surprisingly, not in the mature antenna. These results, together with temperature-shift analysis of a temperature-sensitivetrp mutant, provide evidence of a role of Trp in olfactory system development.
Keywords: adaptation, olfaction, trp, channel, Drosophila, antenna, electrophysiology, behavior
A remarkable property of sensory systems is their ability to adapt to ambient conditions. Adaptation extends the operating range of sensory systems, in some cases over an enormous span of stimulus intensities (Torre et al., 1995). It may also play a role in complex functions of neuronal systems such as stimulus location (Kaissling et al., 1987).
There are multiple mechanisms through which sensory systems can be modified by experience, with various mechanisms operating over the course of milliseconds, seconds, minutes, or even weeks (Dethier, 1976;Wang et al., 1993; Colbert and Bargmann, 1995). Some changes occur during the course of a maintained stimulus; others occur as a result of repeated stimuli. Some occur within the receptor cell itself; others occur via central mechanisms.
In the vertebrate olfactory system, several mechanisms have been identified that play a role in the modification of signaling. In isolated olfactory cilia, the production of second messenger molecules is reduced after 100 msec of odor stimulation, and there is evidence of a role of protein kinases A and C, arrestin, and β-adrenergic receptor kinase family kinases in this process (Boekhoff and Breer, 1992; Dawson et al., 1993; Schleicher et al., 1993). Intact olfactory neurons show reduced electrical activity over the course of seconds after odor stimulation, and there is evidence that this reduction is mediated by an influx of Ca2+ through a cyclic nucleotide-gated channel; the Ca2+ influx apparently decreases the affinity of the channel for cAMP, thereby decreasing the amplitude and sensitivity of the response (Kurahashi and Menini, 1997). A form of odor adaptation that lasts over the course of minutes has been shown to be mediated by cGMP (Zufall and Leinders-Zufall, 1997). This kind of adaptation has been proposed to be mediated via cGMP activation of cyclic nucleotide-gated channels, with the resulting influx of Ca2+ then initiating a form of Ca2+-dependent feedback regulation.
In Caenorhabditis elegans, a form of olfactory adaptation is induced by 30 min of exposure to odors, with recovery then occurring over a period of hours (Colbert and Bargmann, 1995). This adaptation, documented in a series of elegant behavioral experiments, was affected by mutants of the adp-1 andosm-9 genes, with the adp-1 mutation affecting a Ca2+-dependent process and osm-9 encoding a protein with structural similarity to channels of the transient receptor potential (TRP) family (Colbert et al., 1997).
There has been little if any previous work on olfactory adaptation inDrosophila. Adaptation in the visual system ofDrosophila and other invertebrates has been found to depend on Ca2+ as an intracellular messenger, although the mechanism is not completely understood (Lisman and Brown, 1972; Hardie, 1991; Selinger et al., 1993; Ranganathan et al., 1994). InDrosophila, the trp mutant lacks almost all manifestations of visual adaptation (Cosens and Manning, 1969; Minke et al., 1975; Minke and Armon, 1980; Minke, 1982; Minke and Payne, 1991).trp was named on account of its decay to baseline during prolonged bright illumination, and it encodes a light-activated Ca2+ channel (Hardie and Minke, 1992; Peretz et al., 1994a,b; Niemeyer et al., 1996) whose sequence suggests that it is nonvoltage-gated (Montell and Rubin, 1989; Phillips et al., 1992).
Here we show that Drosophila exhibits olfactory adaptation. We demonstrate that the degree of adaptation depends on the dose and duration of the adapting stimulus, and we characterize the kinetics of recovery. Cross-adaptation is found to occur as well, although the response is reduced most to the odor used as the adapting stimulus.trp mutants show a strong defect in olfactory adaptation, as measured either behaviorally or physiologically. The olfactory responses of naive trp animals are normal, however, indicating that the processes of olfactory response and adaptation can be distinguished. Interestingly, we detect the trp gene product in the developing olfactory system but not in the mature antenna. This finding, along with temperature-shift analysis of a temperature-sensitive trp allele, indicates a role for the Trp Ca2+ channel in olfactory system development.
MATERIALS AND METHODS
Behavioral adaptation in the olfactory T maze. To test the ability of flies to adapt to an odor, we used an olfactory T maze in which flies choose between two airstreams, one containing odor and the other a control, as described elsewhere (Helfand and Carlson, 1989). Odors were dissolved in paraffin oil, and the airstreams were drawn at a rate of 1 l/min over the odor or paraffin diluent. A population of ∼25 flies was preexposed to odor (see below) for 1 min, unless otherwise specified, and then was tested for behavioral response in the T maze for 30 sec. We chose 30 sec to avoid adaptation that might occur during longer periods of exposure to the odorant; however, most flies appear to make a choice early in this 30 sec test period and retain their choice for the duration of the period (although we have not documented this observation rigorously). At the end of the period, flies were collected and counted. The response index (RI) was calculated by subtracting the number of flies in the tube containing the odor from the number of flies in the tube containing control air and dividing by the total. Thus in a test in which all flies are repelled by the odor, the RI would equal 1.0; if the flies were indifferent to the odor, the RI would equal 0. All behavioral tests were performed in the dark.
To preexpose the flies to odor before behavioral testing, we collected the flies in a plastic vial containing agarose. The vial was plugged with a plastic stopper through which two tubes passed, one for entry of the odor into the vial and one for exit of the odor. The airstream was drawn by a pump at a rate of 1 l/min through a bottle containing odorant dissolved in paraffin oil and then through the vial containing the flies. After the defined period of preexposure, ∼20 sec elapsed before the flies were transferred from the preexposure vial into the T maze.
Dosages, in both behavioral and physiological experiments, are indicated as dilution factors of the odorants in paraffin oil. The number of molecules that evaporated from the odorant solution, made contact with the olfactory organs, and entered the lumen of olfactory sensilla has not been determined.
Physiological adaptation in the antenna. To measure adaptation physiologically, we recorded electroantennograms (EAGs) using a modification of methods described elsewhere (Ayer and Carlson, 1992; Riesgo-Escovar et al., 1995). Briefly, a fly was mounted in a truncated micropipette tip with the anterior portion of the head protruding from the end of the tip. The micropipette tip was placed in wax on a microscope slide with the antennae facing upward. The indifferent electrode was inserted into the head capsule. The recording electrode was positioned along the dorsoventral axis on the surface of the third antennal segment in a region containing predominantly basiconic sensilla (Stocker, 1994). After a stable baseline was obtained, the EAG was initiated by a short pulse of odor applied through a syringe into an airstream (1 l/min) that was directed toward the antenna.
Flies were adapted while immobilized in the EAG apparatus by directing an airstream containing odor at the fly. The duration of this preexposure was 1 min and was controlled with a valve, which was used to switch the airstream from a bottle with paraffin oil to a bottle with odorant, either dissolved in paraffin oil or used undiluted. (The dead volume in this odor delivery system between the valve switch and the point of exit of the airstream was ∼15 ml.) Odor pulses were then administered by syringe (see above), immediately after this preexposure and at 0.5 or 1 min intervals thereafter. The recovery of the response was measured as the percentage of response amplitude of the naive fly as a function of time. The electrodes were not moved throughout the procedure.
Drosophila stocks and culture. trpP301,trpP313, and their parental control Oregon R were obtained from W. Pak, as wastrpCM. FortrpP313 andtrpCM, both of which were in aw− background, aw− Oregon R stock, also from W. Pak (Purdue University, West Lafayette, IN), was used as a control. Flies were cultured in a cornmeal–molasses–agar medium supplemented with dry active yeast and kept either at room temperature (∼22°C) or in 25°C incubators, except that the temperature-sensitive (ts) alleles were grown under permissive or restrictive temperatures for several experiments (see Figs. 7, 9), as described in Results.
Fig. 7.
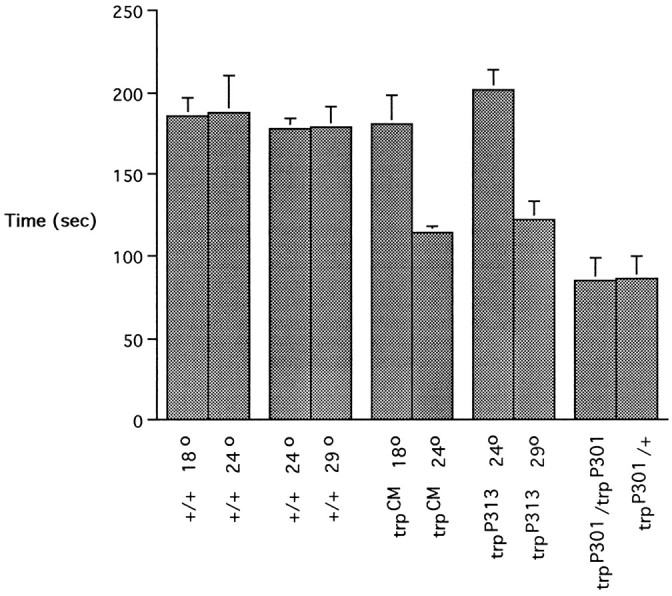
The physiological defect in olfactory adaptation maps to the trp locus. Half-recovery times are shown for two temperature-sensitive alleles,trpCM andtrpP313, cultured at both permissive and restrictive temperatures. Flies were preexposed to undiluted IAA for 1 min and then tested with a 10−1 dilution of IAA at subsequent intervals to determine the half-recovery time. Testing was at room temperature. n = 10 for all values. The data for the trpP301mutants were taken from the experiment shown in Figure5a and are included for comparison. Error bars indicate SEM.
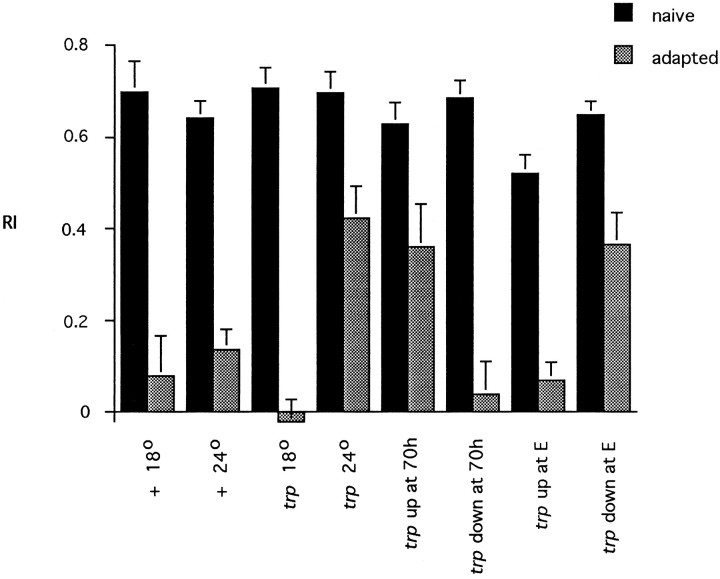
Fig. 9.
A developmental role for trp in olfactory adaptation. Flies (wild-type ortrpCM) either were grown continuously at 18 or 24°C or were shifted from 18 to 24°C (up) or from 24 to 18°C (down), either at 70 hr APF (at 70h) or at eclosion (at E; all flies were <8 hr old at the time of the shift). After the shift at eclosion, flies were cultured for 1–2 d at the temperature to which they had been shifted before being tested. For testing, flies were preexposed for 1 min with undiluted IAA and tested with a 10−1dilution of IAA in the T maze. The at E shifts were performed in a separate experiment. n = 10. Error bars indicate SEM.
Immunohistochemistry. Immunostaining was performed as described elsewhere (Störtkuhl et al., 1994). Flies were fixed for 3 hr in 4% paraformaldehyde at 4°C and subsequently incubated overnight in 25% sucrose in Drosophila Ringer’s solution. Cryosectioning was performed to produce 10 μm sections. After blocking with goat serum, the polyclonal anti-Trp antibody (diluted 1:500; kindly provided by B.-H. Shieh, Vanderbilt University, Nashville, TN) was applied to the sections overnight at 4°C. After washing, the biotinylated second-stage antibody was applied for 1 hr at 37°C, using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. Horseradish peroxidase staining was then performed.
RESULTS
Drosophila adapt to olfactory stimuli
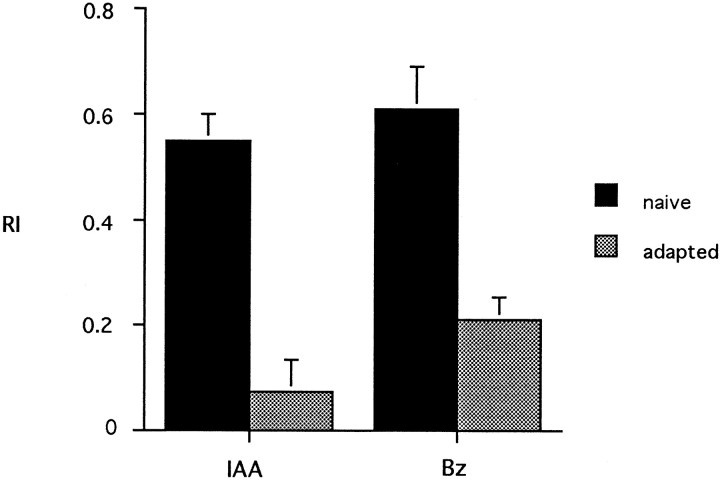
To determine whether Drosophila is capable of olfactory adaptation, we initially used a behavioral paradigm. Flies were exposed to an odor and then tested with the same odor in an olfactory T-maze choice test; their response was compared with that of naive flies. We found that flies preexposed to the odor of isoamyl acetate (IAA) showed a much lower response to a subsequent test stimulus of IAA than do naive flies (Fig. 1). Specifically, under the conditions used in this experiment, the RI of the preexposed flies was <15% that of naive controls. To determine whether this effect is restricted to IAA, we tested another odor, benzaldehyde (the odor of almond), and again found a reduction in response after preexposure (Fig. 1). We tentatively refer to this reduction in response as “adaptation,” a term that we use in a broad sense with no implication as to the mechanism or site of action.
Fig. 1.
Olfactory adaptation. Flies were preexposed to the odor of a compound (undiluted; see Materials and Methods for an explanation of dosage) and then tested with the odor of a 5 × 10−2 dilution of the same compound. For each value,n = 10 tests, with each test performed on a population of 25 flies. Bz, Benzaldehyde. Error bars indicate SEM.
Dose dependence and kinetics of adaptation
This form of adaptation shows dose dependence, in that a greater reduction in response is observed after preexposure to a greater dose of odor (Fig. 2a). The degree of adaptation also depends on the duration of the preexposure (Fig.2b). Full adaptation required 30 sec of preexposure, with no difference observed between 30 and 120 sec of preexposure, under the conditions used in Figure 2b. The preexposure time required for half-maximal adaptation was determined by interpolation to be 15 sec. Recovery occurred over the course of minutes (Fig. 2c), with a half-recovery time of 1.5 min under the test conditions we used.
Fig. 2.
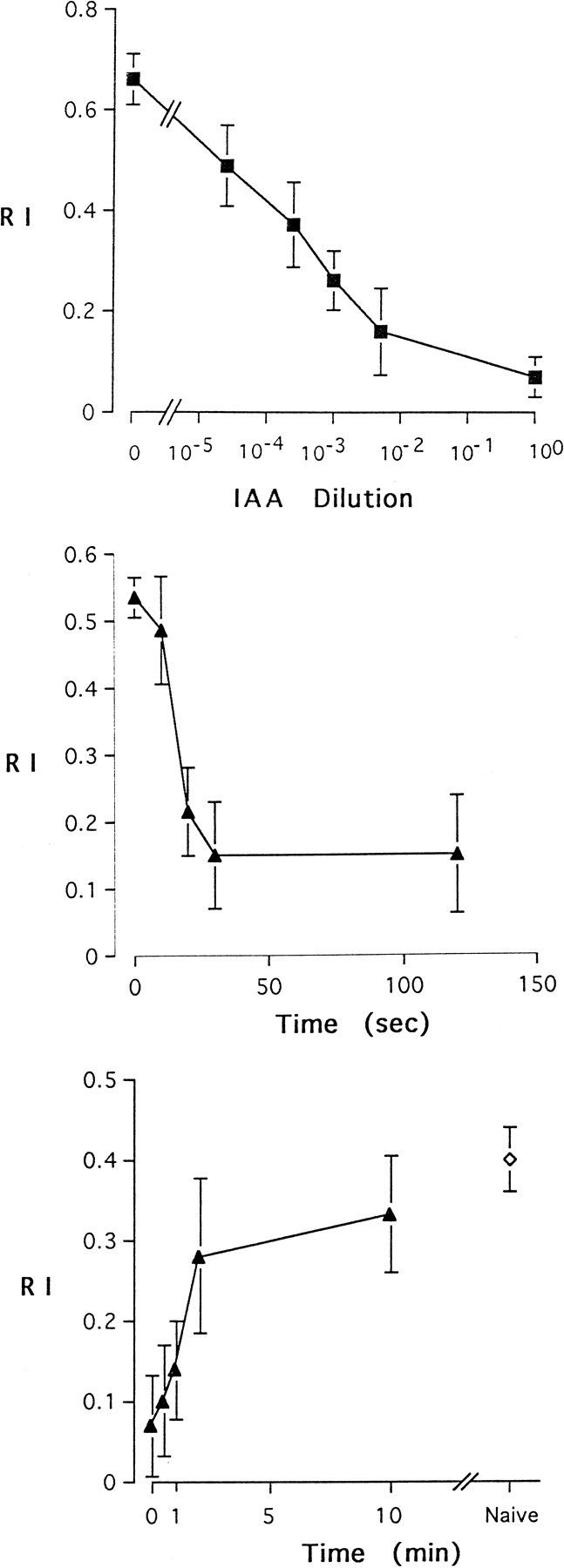
Parameters of adaptation. a, Dependence on concentration of the adapting stimulus. After exposure for 1 min to IAA of the indicated dilutions, flies were tested for response to a 5 × 10−2 dilution of IAA. The 0 dilution value indicates the paraffin oil diluent alone.n = 10 tests. b, Dependence on time of preexposure. Flies were preexposed to undiluted IAA for the indicated time and then tested with a 10−2 dilution of IAA. n = 10 tests. c, Recovery kinetics. Flies were preexposed for 1 min with undiluted IAA and then tested for response to a 10−3 dilution of IAA at the indicated times after the end of the preexposure period. Theopen diamond indicates the RI of naive animals and was positioned arbitrarily along the x-axis for clarity of presentation. n = 10 tests. Error bars indicate SEM in a–c.
Cross-adaptation
Does preexposure to one odor affect response to another? If adaptation occurs at the level of a signaling molecule that is shared by two odors, one might expect some degree of cross-adaptation. By contrast, if adaptation acts uniquely at the level of an odor-specific receptor molecule, for example, one would not expect to observe cross-adaptation.
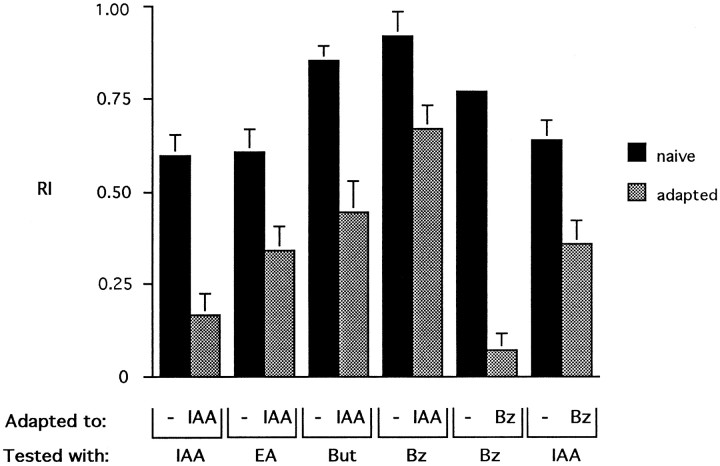
We found that animals adapted to IAA showed cross-adaptation to all other odors tested: another acetate ester (ethyl acetate), an alcohol (butanol), and an aldehyde (benzaldehyde) (Fig.3). In no case, however, was the reduction in response to these other odors as great as the reduction for IAA, measured in terms of either the absolute or fractional decline in mean RI. IAA is not the only odor that produces cross-adaptation; flies preexposed to benzaldehyde show a reduction in response to IAA, but again not as great as the reduction for benzaldehyde (Fig. 3).
Fig. 3.
Cross-adaptation. Flies were adapted with undiluted IAA or Bz for 1 min and then tested in the T maze with a 10−2 dilution of IAA, Bz, ethyl acetate (EA), or butanol (But). Flies indicated as being adapted to “−” are naive flies. n = 10 tests. Error bars indicate SEM.
Although several mechanisms of visual adaptation have been shown to be mediated via phosphorylation of opsin or by binding of other molecules to opsin (Scott and Zuker, 1997), our results support the notion that olfactory adaptation operates at least in part via steps downstream from the stimulated receptor molecule, which we assume to bind odors with a high degree of specificity.
Mutants of the Trp calcium channel are defective in adaptation
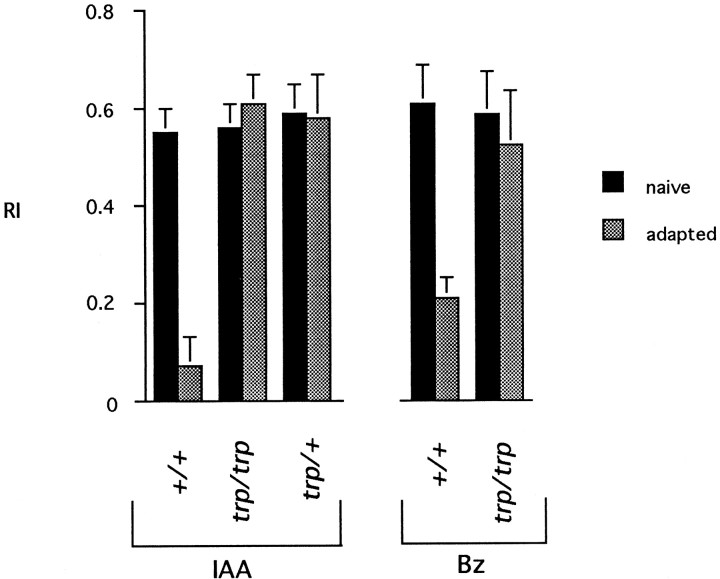
As a first step in investigating the genetic basis of olfactory adaptation, we tested a mutant of the trp gene, which encodes a light-activated Ca2+ channel (Hardie and Minke, 1992; Phillips et al., 1992). The olfactory response of naivetrpP301 flies was normal to each of two odors tested, IAA and benzaldehyde (Fig.4). However,trpP301 was severely defective in olfactory adaptation, when tested with either odor as the adapting stimulus in the T maze. Interestingly, the effect on adaptation was dominant, as if adaptation were very sensitive to the level of the Trp channel.
Fig. 4.
trpP301 is defective in olfactory adaptation as measured in a T maze. Naive flies and flies preexposed for 1 min to undiluted IAA or Bz were tested in the T maze with a 5 × 10−2 dilution of either IAA or Bz (i.e., preexposed flies were tested with the same odor to which they had been preexposed). n = 10 tests. Error bars indicate SEM.
The adaptation defect in trpP301 could lie at either a central or peripheral level. To test adaptation in the periphery, we used EAGs, extracellular recordings that are believed to measure the summed receptor potentials of olfactory neurons in the vicinity of the recording electrode (Ayer and Carlson, 1992). Initially we tested the EAG response of naive flies to a 10−1 dilution of IAA and found no difference between trpP301 and wild type; the EAG amplitudes were 9.2 ± 0.6 mV for wild type (± SEM;n = 30), 9.3 ± 0.9 mV fortrpP301 (n = 30), and 8.8 ± 0.5 mV fortrpP301/+ (n = 30). We then preexposed the animals to a dose of IAA and measured their EAG response at intervals thereafter.
We found that in wild-type flies, the EAG response was greatly reduced after the preexposure to IAA (Fig.5a). When tested immediately after preexposure and at 30 sec after preexposure, we detected no EAG response. EAG response began to recover thereafter; after 4 min, an odor stimulus evoked an amplitude one-half that of naive flies.
Fig. 5.
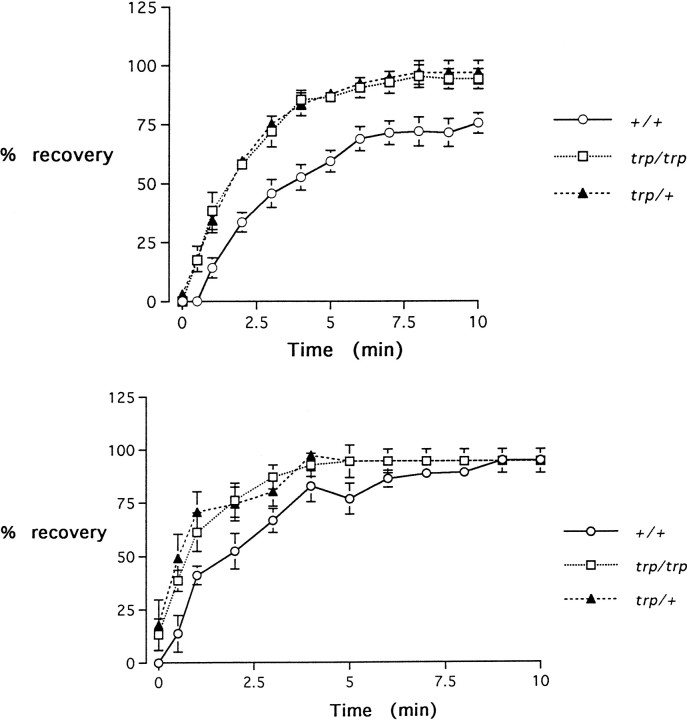
trpP301 is defective in olfactory adaptation as measured physiologically in EAGs.n = 10 tests. Error bars indicate SEM.a, Flies were preexposed to undiluted IAA for 1 min and then tested with a 10−1 dilution of IAA at the indicated times. The amplitudes were measured at each time point and plotted as a percentage of the amplitude observed for naive flies.b, Flies were preexposed to undiluted IAA and tested with a 10−1 dilution of Bz at the indicated times.
In trpP301, preexposure also caused a severe reduction in subsequent response (Fig. 5a). However, recovery occurred faster in trpP301, with a half-recovery observed after only ∼1.5 min. Interestingly, this quick recovery of normal physiology is dominant, like the behavioral defect in adaptation.
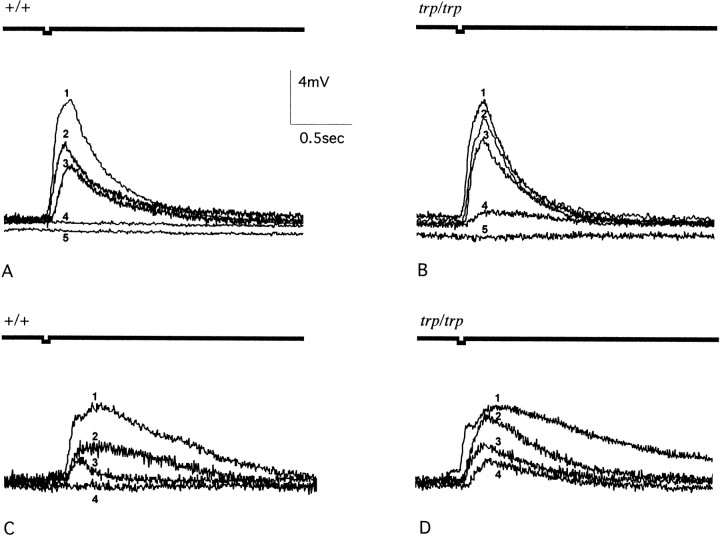
We also observed cross-adaptation in the antenna, in both wild type andtrpP301 (Fig. 5b). Preexposure to IAA caused a major decline in the response to benzaldehyde. At the earliest time point examined, the response oftrpP301 was greater than that of wild type, as if adaptation were less complete intrpP301 or as if some degree of recovery had already occurred. At the next time point examined, 30 sec, the difference in amplitude between trpP301and wild type is greater than that at the first time point, consistent with an abnormally quick recovery of IAA-adapted antennae to benzaldehyde in trpP301. Again, the effect of trpP301 is dominant. Four series of EAG traces showing adaptation and cross-adaptation in wild-type and trpP301 antennae are shown in Figure 6.
Fig. 6.
EAG traces from adapted flies. A,B, Wild-type (A) andtrpP301/trpP301(B) flies were preexposed to undiluted IAA for 1 min and then tested with a 10−1 dilution of IAA after 0 min (trace 5), 0.5 min (trace 4), 3 min (trace 3), or 6 min (trace 2). In both A andB, trace 1 shows the response of naive flies to the 10−1 dilution of IAA.C, D, Wild-type (C) andtrpP301/trpP301(D) flies were preexposed to undiluted IAA for 1 min and then tested with a 10−1 dilution of Bz after 0 min (trace 4), 0.5 min (trace 3), or 3 min (trace 2). In both Cand D, responses of naive flies to the 10−1 dilution of Bz are shown in trace 1. The test odor was delivered at the time indicated by the square wave in the thick horizontal line above each trace inA–D.
To determine whether the abnormal adaptation in the antenna results from the trpP301 mutation as opposed to an unidentified mutation in the stock, we tested two other alleles oftrp, trpCM andtrpP313, both shown to be ts with respect to their electroretinogram phenotypes (Minke, 1983; Wong et al., 1989;Lindsley and Zimm, 1992). When we tested mutants that had been cultured at their restrictive temperatures (24°C fortrpCM and 29°C fortrpP313), both showed strong abnormalities (Fig. 7), requiring shorter times for half-recovery than when cultured at the permissive temperatures (18 and 24°C, respectively). Mutants grown at their permissive temperatures were indistinguishable from wild-type controls raised at the same temperatures. The wild type showed no temperature sensitivity in either of two pairwise comparisons (18 vs 24°C and 24 vs 29°C). The simplest interpretation of these results is that the adaptation defect maps to the trp gene and that thetrp product is essential for normal olfactory adaptation.
Evidence of a role for Trp in the developing antenna
To investigate further the role of trp in olfactory adaptation, we first used an anti-Trp antibody to characterize its expression in the olfactory system. We had expected to detect Trp in the antenna, because trp mutants are defective in adaptation as measured in tests of antennal physiology. However, we were unable to detect Trp in the adult antenna using a polyclonal anti-Trp antibody that clearly stained the adult retina, nor was label observed in the antennal lobes of the adult brain. It seemed plausible that our inability to detect Trp in the adult antenna might result from low abundance of the protein, limited accessibility of the antigen, or differences in processing of the trp gene between the antenna and eye. We therefore analyzed antennal RNA for the presence oftrp RNA by reverse transcription (RT)-PCR, using nine different combinations of six primers. No PCR products were amplified from antennal RNA, although all primer pairs gave amplification products from head RNA. The antennal RNA was, however, able to support amplification of a product when primers were used for an antennal gene, OS9 (Raha and Carlson, 1994), indicating that the lack of a product from the antenna with trp primers was unlikely to be attributable to low quality of the antennal RNA preparation.
Trp was detectable in the developing antenna, however (Fig.8). Immunostaining with anti-Trp antibody showed label in the third antennal segment, the olfactory segment, at 75 hr after puparium formation (APF) (Fig. 8b). The label was not apparent at 66 hr APF (Fig. 8a) but became visible at ∼70 hr APF. To confirm that the label in fact represented the presence of Trp, we stained a trpP301mutant and found no labeling in developing antennae (Fig.8c). The absence of staining in the trp mutant strongly supports the notion that the staining observed in wild type (Fig. 8b) represents Trp; if the antibody were recognizing a cross-reacting species, we would have expected to observe staining in the mutant. At 75 hr APF, the antibody also detected expression in the wild-type antennal nerve and eye but not in the antennal lobe.
Fig. 8.
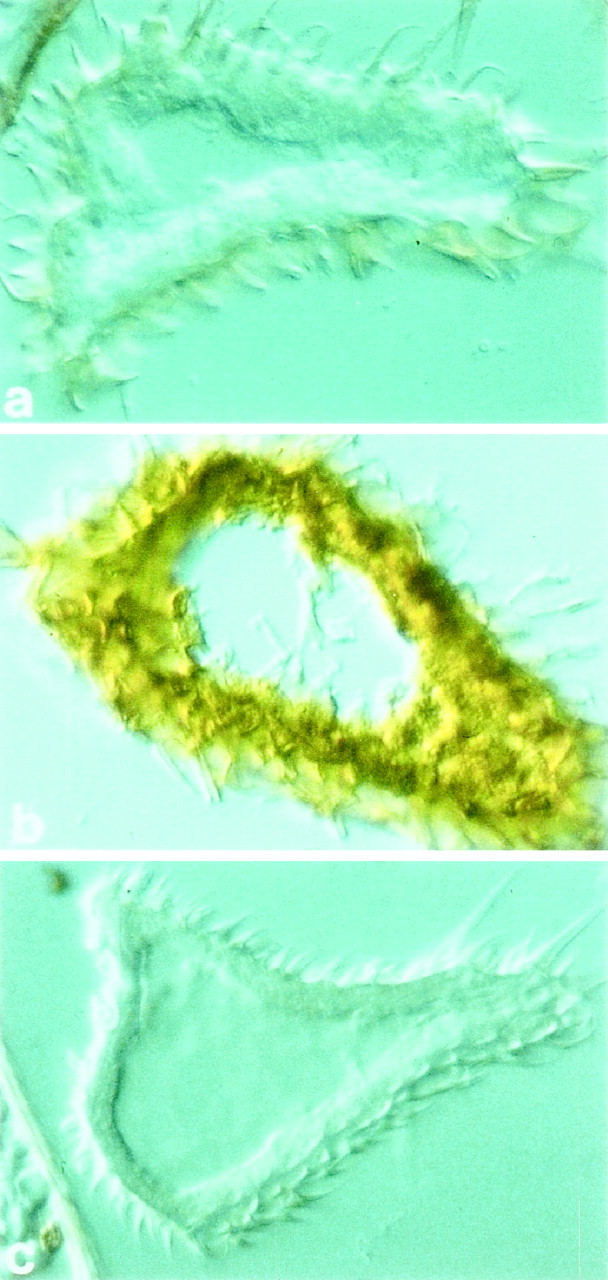
Immunostaining of the third antennal segment with anti-Trp antibody. a, Wild type at 66 hr APF.b, Wild type at 75 hr APF. c,trpP301 at 75 hr APF. As a means of verifying the difference between mutant and wild type, photographs of wild-type and mutant antennae (the eyes were not in the field of view) at 75 hr APF were scored in a blind test, and the genotypes were identified correctly for each of 20 photographs (10 of wild type and 10 of the mutant). The magnification is 2400×.
The detection of Trp in the developing, but not the mature, antenna suggests the possibility that olfactory adaptation is dependent on a role for Trp in the developing, but not the mature, antenna. We performed a temperature-shift experiment using the behavioral phenotype of the ts allele trpCM, which adapts normally if raised and maintained at 18°C but which shows a strong adaptation defect when raised and maintained at 24°C, the restrictive temperature (Fig. 9). Flies cultured at 18°C until 70 hr and then cultured at 24°C thereafter showed a defect in adaptation as severe as that in flies cultured at 24°C continuously. Flies cultured at 24°C until 70 hr and then cultured at 18°C continuously thereafter adapted normally as adults. These results are consistent with a requirement for Trp after, but not before, 70 hr, the time at which it first appears in the antenna. Most interesting, however, is that flies cultured at 18°C until eclosion and then shifted to 24°C adapted normally when tested 1–2 d later. By contrast, flies cultured at 24°C until eclosion and then shifted to 18°C showed a defect as strong as that in those cultured continuously at 24°C. These results support the hypothesis that normal olfactory adaptation depends on a role for Trp in development but not in the mature animal.
DISCUSSION
We have shown that Drosophila exhibits olfactory adaptation at both behavioral and physiological levels. Exposure to an odor stimulus reduces the behavioral response to a subsequent stimulus. The magnitude of this reduction depends on the concentration and on the duration of the preexposing stimulus, with half-maximal adaptation occurring after 15 sec of exposure under a set of test conditions. Recovery occurs over the course of minutes, with a half-recovery time of 1.5 min under a set of test conditions. Cross-adaptation occurs, but to a lesser extent than does adaptation to the test stimulus. At least some of the adaptation occurs in the peripheral olfactory system, because exposure to an odor stimulus reduces the electrical response of olfactory receptor neurons to a subsequent odor stimulus. Our characterization of olfactory adaptation in the wild type has laid a foundation for a genetic analysis of the process.
We have found evidence that mutants of the Trp Ca2+channel are defective in olfactory adaptation, as shown both in measurements of olfactory behavior and antennal physiology. An effect on adaptation was observed for three different alleles, including two ts alleles, which showed defects only at the restrictive temperature. Taken together, these results provide strong evidence that the adaptation defects in fact map to the trp locus and that Trp is required in some way for normal olfactory adaptation. A role for Trp in olfactory adaptation is reminiscent of the failure of trpmutants to show normal adaptation in the visual system (Minke et al., 1975; Minke and Armon, 1980; Minke, 1982; Minke and Payne, 1991), although the nature of the defect is different in the visual system, in which adaptation appears to occur to a greater extent in trpthan in wild type.
Some of our results regarding the role of Trp in adaptation were unexpected. First, we found no evidence of trp expression in the mature antenna. We were, however, able to detect Trp expression in the developing antenna at 75 hr, a time at which antennal neurons are believed to be undergoing terminal differentiation (Dubin and Harris, 1997). Moreover, a temperature-shift experiment provided strong evidence that trp function is in fact required during development, but not in the mature antenna, for normal olfactory adaptation to occur. These data suggest the possibility that the Trp channel plays a role in the differentiation of antennal neurons, such that trp mutants when mature contain defects that make them unable to undergo normal adaptation. This interpretation is consistent with the demonstration of a role for Trp in the developing visual system; an extensive series of temperature-shift experiments withtrpP313 showed that function oftrp during the late pupal period is necessary for normal visual function in the adult (Wong et al., 1989). The critical period for trp expression during visual system development is consistent with the first appearance of trp mRNA in the eye (Montell et al., 1985). A variety of processes in retinal differentiation occur during the late pupal period (Cagan and Ready, 1989). These results led to the suggestion (Wong et al., 1989) that Trp might participate during development in the formation of a structure that is essential to visual function in the adult; if Trp is absent in the developing eye, the emerging adult suffers visual impairment. Interestingly, recent evidence supports the existence of a multicomponent structure including Trp, protein kinase C, thenorpA phospholipase C, InaD, and certain other phototransduction components (Huber et al., 1996; Shieh and Zhu, 1996;Chevesich et al., 1997; Tsunoda et al., 1997). We have detected Trp in the olfactory system during the period when its function is essential in the developing visual system. Perhaps Trp is required for the proper assembly of an analogous multicomponent structure during olfactory system development.
A role for Trp in olfactory system development therefore seems highly likely. However, the absence of detectable Trp in the mature olfactory system is surprising. It is formally possible that Trp protein is present but is in low abundance or is inaccessible to antibody, perhaps because it is located in dendritic membranes ensheathed by the cuticle of the olfactory sensory hairs. If Trp were present in the adult antenna, then the absence of trp RNA might be related to the lack of membrane turnover in olfactory neurons, in contrast to the high rate of membrane turnover in photoreceptor cells, which causes a continual need for new synthesis of phototransduction components. However, a more straightforward interpretation of the trpexpression data is that in the antenna, Trp has a role in development but not in olfactory transduction. Analysis performed with the temperature-sensitive trpCM mutant, which indicated a temperature-sensitive period during development but not during mature adulthood, strongly supports such a developmental role.
We were interested to find that the effects of trp were dominant, both in olfactory behavior and antennal physiology. There is ample precedent for dominant effects of channel mutations inDrosophila; there are dominant effects of Shakerpotassium channel mutations, and mutations of the parasodium channel are dominant in certain mutant backgrounds (Ganetzky, 1986; Lindsley and Zimm, 1992; Lilly et al., 1994). Moreover, there is precedent for a dominant mutation affecting olfactory adaptation inC. elegans, adp-1 (Colbert and Bargmann, 1995). One interpretation of the dominance of trp olfactory phenotypes is that the role of trp in the antenna is very sensitive to gene dosage, such that reducing the level of normal Trp by one-half is sufficient to engender a detectable adaptation phenotype. We note that the trpP301 mutant contained no detectable Trp protein in a Western blot, leading to the suggestion that it is a null allele (Montell and Rubin, 1989), consistent with a haplo-insufficient basis for its dominance and inconsistent with an explanation based on a gain of function. Why are the olfactory phenotypes dominant, whereas some or all of the visual phenotypes are recessive (Cosens and Manning, 1969)? One possibility is that there is some measure of genetic redundancy in the eye but not in the antenna. In this regard we note that the Trpl channel, which bears substantial sequence similarity to Trp, is present in the eye (Phillips et al., 1992) but was not detectable in the antenna by RT-PCR analysis (C. Warr and J. R. Carlson, unpublished observations).
The behavioral and physiological phenotypes oftrpP301 seem to differ in one sense: the behavioral studies reveal no evidence of any adaptation, whereas the physiological studies reveal a strong initial adaptation that decays more quickly than in the wild type. If there is strong adaptation in the antenna, why is there no adaptation as measured behaviorally? One explanation concerns methodological differences in the two paradigms. In the behavioral paradigm, more time (∼50 sec) elapses between the end of the defined preexposure period and the end of the assay, whereas EAG measurements are made over a much shorter interval. In Figure5a the trpP301 flies can be seen to have recovered some fraction of their normal antennal response within 30 sec, which may be sufficient to drive a full behavioral response. It is also possible that the behavior may be influenced by input from another olfactory organ, the maxillary palp (Ayer and Carlson, 1992), which may not adapt to the same extent as the antenna. We note that some degree of behavioral adaptation is observed intrpCM grown at the restrictive temperature (Fig. 9).
In summary, we have demonstrated that Drosophila exhibits olfactory adaptation, and we have characterized one form of adaptation both behaviorally and physiologically. Mutants of the Trp Ca2+ channel were shown to be defective in olfactory adaptation but not in olfactory sensation, demonstrating that the two processes can be distinguished. Analysis of trp expression in the olfactory system and temperature-shift analysis support a model in which Trp plays a role in olfactory system development. It is possible that Trp channel function is essential for a form of activity-dependent development; Trp may also be required for precise assembly of components essential for normal adaptation. In either case, a developmental role for Trp is of special interest in light of the accumulating body of evidence that olfactory transduction molecules, including receptors and a cyclic nucleotide-gated channel, are essential for development as well as for transduction (Coburn and Bargmann, 1996; Wang et al., 1998).
Footnotes
This work was supported by a grant from the Human Frontiers Science Program to J.R.C., the National Institutes of Health Grant R01 DC02174 to J.R.C., and the Deutsche Forschungsgemeinschaft Grants Sto 283/2-1 and Sto 283/2-2 to K.F.S. and H0 714/8-1 to B.T.H. We thank M. Freeman for performing the RT-PCR experiments, R. Mindnich for performing the immunocytochemistry and some of the temperature-shift experiments, and C. Warr, M. Freeman, M. de Bruyne, and L. Tompkins for helpful discussion.
Correspondence should be addressed to Dr. John R. Carlson, Department of Biology, Yale University, New Haven, Connecticut 06520-8103.
REFERENCES
- 1.Ayer RK, Carlson J. Olfactory physiology in the Drosophila antenna and maxillary palp: acj6 distinguishes two classes of odorant pathways. J Neurobiol. 1992;23:965–982. doi: 10.1002/neu.480230804. [DOI] [PubMed] [Google Scholar]
- 2.Boekhoff I, Breer H. Termination of second messenger signalling in olfaction. Proc Natl Acad Sci USA. 1992;89:471–474. doi: 10.1073/pnas.89.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cagan R, Ready D. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- 4.Chevesich J, Kreuz A, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]
- 5.Coburn C, Bargmann C. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 6.Colbert H, Bargmann C. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron. 1995;14:803–812. doi: 10.1016/0896-6273(95)90224-4. [DOI] [PubMed] [Google Scholar]
- 7.Colbert H, Smith T, Bargmann C. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosens D, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 9.Dawson T, Arriza J, Jaworsky D, Borisy F, Attramadal H, Lefkowitz R, Ronnett G. B-Adrenergic receptor kinase-2 and B-arrestin-2 as mediators of odorant-induced desensitization. Science. 1993;259:825–829. doi: 10.1126/science.8381559. [DOI] [PubMed] [Google Scholar]
- 10.Dethier V. The hungry fly. Harvard; Cambridge, MA: 1976. [Google Scholar]
- 11.Dubin A, Harris G. Voltage-activated and odor-modulated conductances in olfactory neurons of Drosophila melanogaster. J Neurobiol. 1997;32:123–137. doi: 10.1002/(sici)1097-4695(199701)32:1<123::aid-neu11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Ganetzky B. Neurogenetic analysis of Drosophila mutations affecting sodium channels: synergistic effects on viability and nerve conduction in double mutants involving tip-E. J Neurogenet. 1986;3:19–31. doi: 10.3109/01677068609106892. [DOI] [PubMed] [Google Scholar]
- 13.Hardie R. Whole-cell recordings of the light induced current in dissociated Drosophila photoreceptors: evidence for feedback by calcium permeating the light-sensitive channels. Proc R Soc Lond [Biol] 1991;245:203–210. [Google Scholar]
- 14.Hardie R, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 15.Helfand S, Carlson J. Isolation and characterization of an olfactory mutant in Drosophila with a chemically specific defect. Proc Natl Acad Sci USA. 1989;86:2908–2912. doi: 10.1073/pnas.86.8.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber A, Sander P, Gobert A, Bahner M, Hermann R, Paulsen R. The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J. 1996;15:7036–7045. [PMC free article] [PubMed] [Google Scholar]
- 17.Kaissling K, Strausfeld C, Rumbo E. Adaptation processes in insect olfactory receptors. Ann NY Acad Sci. 1987;510:104–112. doi: 10.1111/j.1749-6632.1987.tb43475.x. [DOI] [PubMed] [Google Scholar]
- 18.Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- 19.Lilly M, Kreber R, Ganetzky B, Carlson J. Evidence that the Drosophila olfactory mutant smellblind defines a novel class of sodium channel mutants. Genetics. 1994;136:1087–1096. doi: 10.1093/genetics/136.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsley D, Zimm GG. The genome of Drosophila melanogaster. Academic; San Diego: 1992. [Google Scholar]
- 21.Lisman J, Brown J. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972;59:701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minke B. Light-induced reduction in excitation efficiency in the trp mutant of Drosophila. J Gen Physiol. 1982;79:361–384. doi: 10.1085/jgp.79.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minke B. The trp is a Drosophila mutant sensitive to temperature. J Comp Physiol [A] 1983;151:283–286. [Google Scholar]
- 24.Minke B, Armon E. Intermediate processes in phototransduction: a study in Drosophila mutants. Photochem Photobiol. 1980;32:553–562. [Google Scholar]
- 25.Minke B, Payne R. Spatial restriction of light adaptation and mutation-induced inactivation in fly photoreceptors. J Neurosci. 1991;11:900–909. doi: 10.1523/JNEUROSCI.11-04-00900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minke B, Wu C-F, Pak W. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- 27.Montell C, Rubin G. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 28.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- 29.Niemeyer B, Suzuki E, Scott K, Jalink K, Zuker C. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 30.Peretz A, Sandler C, Kirschfeld K, Hardie R, Minke B. Genetic dissection of light-induced Ca2+ influx into Drosophila photoreceptors. J Gen Physiol. 1994a;104:1057–1077. doi: 10.1085/jgp.104.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peretz A, Suss-Toby E, Rom-Glas A, Arnon A, Payne R, Minke B. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 1994b;12:1257–1267. doi: 10.1016/0896-6273(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 32.Phillips A, Bull A, Kelly L. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- 33.Raha D, Carlson J. OS9: a novel olfactory gene of Drosophila expressed in two olfactory organs. J Neurobiol. 1994;25:169–184. doi: 10.1002/neu.480250208. [DOI] [PubMed] [Google Scholar]
- 34.Ranganathan R, Bacskai B, Tsien R, Zuker C. Cytosolic calcium transients: spatial localization and role in Drosophila photoreceptor cell function. Neuron. 1994;13:837–848. doi: 10.1016/0896-6273(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 35.Riesgo-Escovar J, Raha D, Carlson J. Requirement for a phospholipase C in odor response: overlap between olfaction and vision in Drosophila. Proc Natl Acad Sci USA. 1995;92:2864–2868. doi: 10.1073/pnas.92.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schleicher S, Boekhoff I, Arriza J, Lefkowitz R, Breer H. A B-adrenergic receptor kinase-like enzyme is involved in olfactory signal termination. Proc Natl Acad Sci USA. 1993;90:1420–1424. doi: 10.1073/pnas.90.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott K, Zuker C. Lights out: deactivation of the phototransduction cascade. Trends Biochem Sci. 1997;22:350–354. doi: 10.1016/s0968-0004(97)01100-6. [DOI] [PubMed] [Google Scholar]
- 38.Selinger Z, Doza Y, Minke B. Mechanisms and genetics of photoreceptors desensitization in Drosophila flies. Biochim Biophys Acta. 1993;1179:283–299. doi: 10.1016/0167-4889(93)90084-3. [DOI] [PubMed] [Google Scholar]
- 39.Shieh B, Zhu M. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron. 1996;16:991–998. doi: 10.1016/s0896-6273(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 40.Stocker R. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 41.Störtkuhl K, Hofbauer A, Keller V, Gendre N, Stocker R. Analysis of immunocytochemical staining patterns in the antennal system of Drosophila melanogaster. Cell Tissue Res. 1994;275:27–38. doi: 10.1007/BF00305373. [DOI] [PubMed] [Google Scholar]
- 42.Torre V, Ashmore J, Lamb T, Menini A. Transduction and adaptation in sensory receptor cells. J Neurosci. 1995;15:7757–7768. doi: 10.1523/JNEUROSCI.15-12-07757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker C. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Wysocki C, Gold G. Induction of olfactory receptor sensitivity in mice. Science. 1993;260:998–1000. doi: 10.1126/science.8493539. [DOI] [PubMed] [Google Scholar]
- 46.Wong F, Schaefer E, Roop B, LaMendola J, Johnson-Seaton D, Shao D. Proper function of the Drosophila trp gene product during development is important for normal visual transduction in the adult. Neuron. 1989;3:81–94. doi: 10.1016/0896-6273(89)90117-7. [DOI] [PubMed] [Google Scholar]
- 47.Zufall F, Leinders-Zufall T. Identification of a long-lasting form of odor adaptation that depends on the carbon monoxide-cGMP second-messenger system. J Neurosci. 1997;17:2703–2712. doi: 10.1523/JNEUROSCI.17-08-02703.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]