Abstract
The Caenorhabditis elegans unc-13,unc-18, and unc-64 genes are required for normal synaptic transmission. The UNC-18 protein binds to theunc-64 gene product C. elegans syntaxin (Ce syntaxin). However, it is not clear how this protein complex is regulated. We show that UNC-13 transiently interacts with the UNC-18-Ce syntaxin complex, resulting in rapid displacement of UNC-18 from the complex. Genetic and biochemical evidence is presented that UNC-13 contributes to the modulation of the interaction between UNC-18 and Ce syntaxin.
Keywords: UNC-18, Ce syntaxin, UNC-13, SNARE complex, synaptic vesicle, exocytosis
At chemical synapses, neurotransmitter release is accomplished by a series of interactive steps between synaptic vesicles and plasma membrane, including targeting, docking, fusion, and exocytosis (Kelly, 1993; Bennett and Scheller, 1994; Südhof, 1995; Martin, 1997). The regulatory targeting of synaptic vesicles to the plasma membrane requires a core complex of neuronal synaptic proteins, solubleN-ethylmaleimide-sensitive factor attachment protein (SNAP) receptors, termed SNAREs (Söllner et al., 1993). These synaptic proteins include syntaxin, vesicle-associated membrane protein (VAMP) (also called synaptobrevin), and SNAP-25 (Calakos et al., 1994; Chaoman et al., 1994; Pevsner et al., 1994a; Hayashi et al., 1994).
In Caenorhabditis elegans, many potential presynaptic genes have been identified (Rand and Russell, 1984, 1985; Hosono et al., 1989; Hosono and Kamiya, 1991; Maruyama and Brenner, 1991; Nonet et al., 1993, 1998; Jorgensen et al., 1995; Miller et al., 1996).unc-18, one such candidate gene, is expressed in a neuron-specific manner, and its gene product (UNC-18) is present in presynaptic terminals (Hosono et al., 1992; Gengyo-Ando et al., 1993;Ogawa et al., 1998). The UNC-18 homolog n-Sec1 tightly associates with syntaxin (Hata et al., 1993; Pevsner et al., 1994b; Kee et al., 1995). n-Sec1 is not a component of either the 7S VAMP–SNAP-25 or 20S SNARE–SNAP–N-ethylmaleimide-sensitive factor protein complex, although syntaxin is present in both complexes (Pevsner et al., 1994b; Garcia et al., 1995). Binding of syntaxin to the component of the 7S complex is diminished in the presence of increasing concentrations of n-Sec1 (Pevsner et al., 1994a). From these results, it is hypothesized that n-Sec1 is associated with syntaxin before synaptic vesicle docking and may be a negative regulator of vesicle docking and/or release.
This hypothesis predicts that C. elegans null mutations of the unc-18 gene would lead to increased neurotransmitter release. However, unc-18 mutations have been shown to be associated with decreased transmitter release and with the accumulation of neurotransmitters at the presynaptic terminal (Hosono et al., 1987,1989). These results are also consistent with observations in yeast (Novick et al., 1980). To examine these apparently conflicting observations, we analyzed unc-18 mutants. We (Ogawa et al., 1998) and others (Saifee et al., 1998) found that unc-64encodes the mammalian syntaxin 1A homolog C. elegans(Ce syntaxin) and the product could bind to UNC-18. We further searched for factors interacting with UNC-18 and found theunc-13 gene product. UNC-13 has a potential phorbol ester binding domain (C1) and two probable calcium phospholipid-binding domains (C2) (Ahmed et al., 1992; Brose et al., 1995; Kazanietz et al., 1995). These structural features suggest that UNC-13 contributes to the calcium- and diacylglycerol-dependent regulation of transmitter release. We therefore performed detailed genetic and biochemical analyses of the three genes and their products. We report here UNC-13 dissociates UNC-18 from the UNC-18–Ce syntaxin complex. We propose that the three genes have a critical role in synaptic vesicle docking and subsequent processes. During the preparation of our manuscript, a factor from rat cerebral cytosol was found (tomosyn) that dissociates Munc-18 from the syntaxin-1a–Munc-18 complex (Fujita et al., 1998). Tomosyn differs from the mammalian UNC-13 homolog Munc-13 in protein structure.
MATERIALS AND METHODS
General handling. Culture, maintenance, and genetic manipulation were essentially as described previously (Brenner, 1974).
C. elegans strains. The wild-type Bristol strain N2 and the following mutations were used: LGI, unc-13(n2823, e51) and dpy-5(e61); LGIII, unc-64 (e246) anddpy-18 (e364); LGX, unc-18(cn347, md118, md183,md193, b403, md1054,md1412, md1417, e234,md120, md1307, md1401, e81,md1264, md426, md1094) andlon-2 (e678).
Construction of unc double mutants. Double mutants ofunc-13, unc-18, and unc-64 were tested by making hermaphrodites that are homozygous in one uncmutation (u1) and heterozygous in another (u2). The u2 mutation is linked to a Dpy or a Lon morphological marker (a). Self-progeny from these hermaphrodites (u1/u1; + +/u2 a) were inspected to determine survival.
Analyses of mutant phenotypes and ACh assay. Locomotion, trichlorfon resistance, and assays of ACh level of mutant alleles were performed as described previously (Harada et al., 1994).
Sequence determination. To determine the mutation sites, total RNA from mutant alleles was isolated by the CsCl gradient method (Ogawa et al., 1998). Poly(A+) RNAs were purified by Oligotex-dT30 (TaKaRa, Tokyo, Japan). The cDNAs amplified by reverse transcription-PCR were cloned into the pBluescript SK(+) vector and sequenced by the dye primer method or by the dideoxy chain termination method. The following oligonucleotides were used to amplify cDNAs and to determine their sequences: CE1845, GAAAGCTTATGTCACTCAAACAAATCGTTGGGCA (+1 to +26); CE1846, TCTCTAGATCATATGTCACGCGGTTTGTTC (+1755 to +1776); CE18SEQ2, AGCGTCGAGTTTTTGCTCAA (+608 to +627) ; CE18SEQ3, TGAGAGAAATGTTGAGCTCG (+579 to +598) ; CE18SEQ4, AACAGAATCAATCTGAGGCG (+1207 to +1226); and CE18SEQ5, GTTGATGGTGCCACTTTTGA (+1161 to +1180). Bold indicates aHindIII site, and italics indicates a XbaI site.
Preparation of recombinant proteins. pGEX vectors (Pharmacia Biotech, Uppsala, Sweden) for the bacterial expression of glutathione S-transferase (GST) fusion proteins containing the cytoplasmic domain of Ce syntaxin (amino acids 1–267) and the N-terminal domain of UNC-13 (UNC-13N) (amino acids 1–266) were constructed using PCR procedures. Expression of GST fusion proteins and subsequent purification using glutathione–Sepharose 4B beads were performed as described previously (Ogawa et al., 1996). Soluble recombinant syntaxin or UNC-13N was purified from the GST fusion protein by cleavage with Factor Xa or thrombin, respectively, and these digestions were stopped by the addition of 1 mm phenylmethylsulfonyl fluoride (PMSF). UNC-18 was prepared using a baculovirus expression system as described previously (Ogawa et al., 1996). Protein concentrations were estimated by Coomassie blue staining of protein bands after SDS-PAGE with bovine serum albumin as a standard.
In vitro binding assay. UNC-18 and GST fusion protein (either Ce syntaxin or UNC-13N) were incubated in 50 mm HEPES, pH 7.4, 150 mm NaCl, 0.5 mm PMSF, 10 μg/ml leupeptin, and 0.1% NP-40 for 1 hr at 4°C, and then glutathione–Sepharose 4B beads were added. After a 1 hr incubation, the beads were washed three times with 1 ml of 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.5% NP-40, and 0.5 mm PMSF and then washed once with 50 mmTris-HCl, pH 8.0. Proteins were eluted with 10 mm reduced glutathione and then analyzed by Western blotting using antibodies to UNC-18 after SDS-PAGE. The immunoreactive bands were visualized by enhanced chemiluminescence (ECL system; Amersham, Arlington Heights, IL). The binding ability was quantitated by using NIH Image software. Proteins were also visualized by autoradiography of125I-labeled sheep anti-rabbit secondary antisera (Amersham) and by phosphorimaging.
RESULTS
Sequences and phenotypes of the unc-18 mutants
Mutation sites of the unc-18 alleles other thancn347 had not been determined (Hosono et al., 1992). We therefore analyzed all available unc-18 alleles by DNA sequencing and compared their phenotypes. Interestingly, mutations were all clustered in exons IV and IX (Fig.1A). Three mutations,md193, md426, and md1094, were found to result from Tcl insertions as observed in cn347. Four mutations, md118, md1264, md1307, andmd120, were small DNA rearrangements, including deletions and insertions. The remaining five mutations were single base changes; three mutations, e234, e81, andmd1412, had stop codons, and two, md1401 andb403, had amino acid substitutions.
Fig. 1.
Sequences of unc-18 mutant alleles.A, Map of sequenced mutations at theunc-18 locus. Boxes indicate coding regions. B, The altered codons and positions of amino acid substitutions are shown.
All unc-18 mutations had uncoordinated behaviors, abnormal ACh accumulation, resistance to AChE inhibitors, and a significantly slower growth rate than wild type. However, the severity of the behavioral impairment of mutants varied; that is, some of these mutants were quite uncoordinated (Table 1). Putative null alleles (stop codons or frameshifts) showed the strongest phenotype; that is, the animals were severely paralyzed, strongly resistant to trichlorfon, and accumulated ACh. We were especially interested in two missense mutations described in greater detail below;md1401 leads to mild behavioral defects, whereasb403 leads to a severe mutant phenotype.
Table 1.
Phenotypes of unc-18 mutant alleles
| Allele | Trichlorfon resistance1-a (μm) | Locomotion1-b | ACh levels (nmol/mg protein) | |
|---|---|---|---|---|
| forward(sec) | backward(sec) | |||
| +/+ | 20 | 3.3 ± 0.5 | 3.3 ± 0.7 | 0.45 ± 0.09 |
| md1401 | 20 | 7.6 ± 1.6 | 6.8 ± 1.3 | 0.67 ± 0.02 |
| md193 | 300 | >60 | >60 | 4.61 ± 0.02 |
| md118 | 300 | >60 | >60 | 2.62 ± 0.34 |
| e234 | >300 | >60 | >60 | 4.53 ± 0.07 |
| md426 | 100 | 8.4 ± 2.8 | 9.5 ± 2.3 | 4.74 ± 0.66 |
| md1094 | 50 | 8.0 ± 1.2 | 11.0 ± 2.4 | 1.27 ± 0.25 |
| e81 | 300 | >60 | >60 | 1.34 ± 0.07 |
| md1412 | >300 | >60 | >60 | 4.54 ± 0.21 |
| md1264 | 20 | 4.9 ± 0.9 | 5.2 ± 0.9 | 1.80 ± 0.16 |
| cn347 | 200 | >60 | >60 | 3.39 ± 0.59 |
| md1307 | 300 | >60 | >60 | 6.22 ± 0.15 |
| md120 | 250 | 53.0 ± 3.2 | 37.3 ± 4.3 | 3.09 ± 0.07 |
| b403 | 300 | >60 | >60 | 5.82 ± 0.75 |
Three larvae were grown on nematode growth medium-agar plates containing eight different concentrations of trichlorfon (10, 20, 50, 100, 150, 200, 250, and 300 μm). Shown are the highest concentrations of trichlorfon at which the animal in triplicate experiments was able to produce F2 progeny within 10 d.
The mean time for entire animals to cross a line was recorded when either the head or the tail regions were touched with toothpicks. Locomotion of 10 animals was measured.
Genetic interactions between unc-13,unc-18, and unc-64 mutations
Genetic, molecular, and biochemical analyses suggest thatunc-13, unc-18, and unc-64 gene products are all involved in neurotransmitter release (Miller et al., 1996). We therefore constructed double mutants to examine possible genetic interactions among these three genes. For these constructions, we used a mild (md1094) and a severe (cn347) allele of unc-18, a mild (n2823) and a severe (e51) allele ofunc-13, and the relatively mild (e246) allele of unc-64 (Table 2). The double mutants of unc-13 and unc-18 had phenotypes suggesting approximate additivity. Thus, the strain containing the two mild mutations was somewhat more severe than either of the single mutants, but not markedly so, and the strain containing the two severe mutations was lethal. In contrast, although theunc-64 (e246) allele is relatively mild, it greatly enhanced the phenotype of mutation with which it was combined, i.e., the unc-64 (e246) mutation in combination with either the mild unc-13 or the mildunc-18 mutations led to a severely paralyzed phenotype and, when combined with either the severe unc-13 allele or the severe unc-18 allele, led to a synthetic lethality.
Table 2.
Epistatic relationship between unc-13,unc-18, and unc-64 gene mutant alleles
| Allele | Trichlorfon resistancea (μm) | Locomotionb | ACh levels (nmol/mg protein) | |
|---|---|---|---|---|
| Forward (sec) | Backward (sec) | |||
| +/+ | 20 | 3.3 ± 0.5 | 3.3 ± 0.7 | 0.45 ± 0.09 |
| unc-13(e51) | 50 | >60 | >60 | 1.48 ± 0.07 |
| unc-13(n2823) | 50 | 5.2 ± 1.1 | 6.5 ± 2.4 | 0.69 ± 0.03 |
| unc-18(cn347) | 200 | >60 | >60 | 3.39 ± 0.59 |
| unc-18(md1094) | 100 | 8.0 ± 1.2 | 10.9 ± 2.4 | 1.27 ± 0.25 |
| unc-64(e246) | 200 | 11.7 ± 5.0 | 14.1 ± 4.0 | 2.61 ± 0.14 |
| unc-13(e51); unc-18(cn347) | lethal | |||
| unc-18(cn347); unc-64(e246) | lethal | |||
| uunc-13(e51); unc-64(e246) | lethal | |||
| unc-13(n2823); unc-18(md1094) | 150 | 13.1 ± 4.2 | 13.1 ± 2.7 | 6.47 ± 1.02 |
| unc-18(md1094); unc-64(e246) | 150 | >60 | >60 | 4.93 ± 0.46 |
| unc-13(n2823); unc-64(e246) | >300 | >60 | >60 | 6.69 ± 0.24 |
a, b See legend of Table1.
In addition, the extent of the elevation of ACh levels in double mutants is not comparable with the extent of the loss of coordination. Although the ACh level is only slightly elevated in unc-13(n2823) animals, in the double mutants withunc-18 (md1094) and unc-64(e246), it was quite high.
UNC-18 binds to an UNC-13 fragment in addition toCe syntaxin
We have shown previously that UNC-18 binds to theunc-64 gene product Ce syntaxin (Ogawa et al., 1996, 1998). We tested interaction between UNC-13, UNC-18, andCe syntaxin. In a preliminary work, proteins were visualized both by ECL and 125I-labeled secondary antisera (T. Sassa, unpublished results). Here, we present the former results because significant difference between the methods was not observed. A half-maximal binding (ED50) of UNC-18 toCe syntaxin occurred at 0.08 μm. Furthermore, we found that UNC-13N also bound to UNC-18 (Fig.2), with an ED50 of 0.04 μm. Parallel incubations with GST alone demonstrated that the binding was dependent on the fusion protein (data not shown).
Fig. 2.
Binding of UNC-18 to UNC-13 and toCe syntaxin. One micromolar GST alone or the GST fusion proteins consisting of the UNC-13N (top column,GST-UNC13N) or the cytoplasmic domain ofCe syntaxin (bottom column, GST-Ce syntaxin) was incubated with concentrations of UNC-18 ranging from 0.02 to 0.5 μm (A) and from 0.02 to 0.1 μm (B). Amounts of bound UNC-18 were determined by SDS-PAGE and immunoblotting. The ECL signal intensities were quantitated using NIH Image software, and the total value of each band was converted to picomoles on standard curves for UNC-18. The results are the mean of three experiments.Top, Immunoblot results from a single experiment.Ce syntaxin, ○; UNC-13N, ●.
The binding ability of mutant UNC-18 proteins derived fromb403 and md1401 was also tested. Themd1401 UNC-18 protein bound normally to both UNC-13N andCe syntaxin, whereas UNC-18 from the b403 mutant was defective in binding to Ce syntaxin and had greatly reduced ability to bind to UNC-13N (Fig.3).
Fig. 3.
Binding of md1401 andb403 mutant UNC-18 proteins to GST-Cesyntaxin (B) and GST-UNC-13N (C). A, The protein structure of UNC-18. The mutation sites of md1401 at 133 andb403 at 549 are indicated by arrows.B, C, Amounts of UNC-18 in bound forms (top) and used for the incubations (bottom) were determined by Western blotting.
An UNC-13 fragment can regulate the UNC-18–Cesyntaxin complex
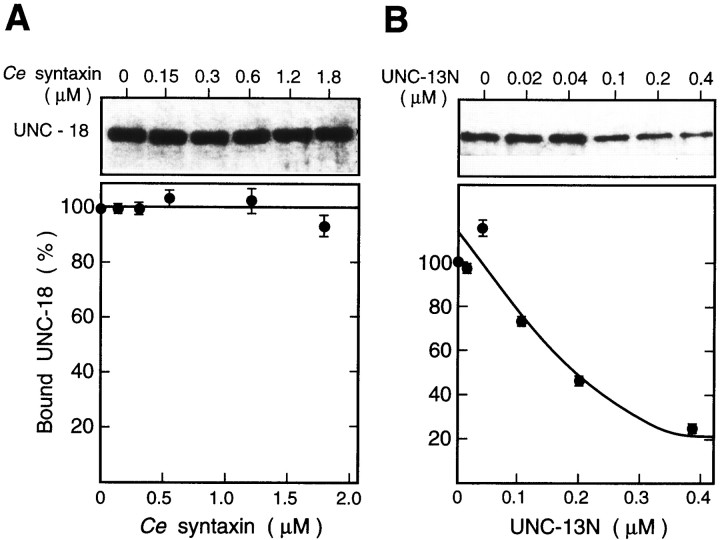
Ce syntaxin did not interfere with the formation of the UNC-13N–UNC-18 complex (Fig.4A). However, the amount of UNC-18–Ce syntaxin complex decreased when incubated with UNC-13N (Fig. 4B). These results suggest that UNC-13N either functions as an inhibitor of the complex formation or causes the displacement of UNC-18 from the complex.
Fig. 4.
Interaction of UNC-13N, UNC-18, andCe syntaxin. A, Binding of UNC-18 to GST-UNC-13N in the presence of Ce syntaxin. UNC-18 (0.2 μm) and GST-UNC-13N (1.2 μm) were incubated with the indicated concentrations of Ce syntaxin.B, UNC-18 (0.2 μm) and GST-Ce syntaxin (1.0 μm) were incubated with the indicated amount of UNC-13N. The value of each band of UNC-18 bound to immobilized GST-UNC-13N (A) or GST-Ce syntaxin (B) was expressed relative to that bound without Ce syntaxin (A) or UNC-13N (B).Top, Immunoblot results from a single experiment.
To know whether UNC-13N displaces UNC-18, we assayed the amount of UNC-18 released in the soluble fraction (Fig.5A). The amount of UNC-18 in the soluble fraction increased depending on the concentration of added UNC-13N. We then assayed the velocity of the release of UNC-18 from the UNC-18–Ce syntaxin complex. The release is very rapid, with most UNC-18 released within 1 min (Fig. 5B). The amount of UNC-18 released from the complex by UNC-13N (2 μm) is the same as that released by adding 10 mm reduced glutathione (data not shown).
Fig. 5.
Dissociation of UNC-18 from the UNC-18–Ce syntaxin complex by UNC-13N.A, UNC-18–Ce syntaxin complex immobilized to GST beads was prepared using UNC-18 (0.2 μm) and GST-Ce syntaxin (1.0 μm) as described in Materials and Methods. The GST beads were incubated with the indicated concentrations of UNC-13N at 30°C for 10 min. B, Time course of the displacement of UNC-18 by UNC-13N. The UNC-18–GST-Ce syntaxin complex was incubated with UNC-13N (2.0 μm) at 30°C and then centrifuged at the indicated times. The concentration of the released UNC-18 in the supernatant was expressed relative to the concentration of the released UNC-18 when the complex was incubated with 10 mm reduced glutathione.
DISCUSSION
Some synaptic genes, such as cha-1, snb-1,unc-17, and unc-64, are lethal if they are completely defective (Rand, 1989; Alfonso et al., 1993; Nonet et al., 1998; Saifee et al., 1998). In contrast, null mutations ofsnt-1 are viable but uncoordinated (Nonet et al., 1993). Theunc-18 gene appears to fall into the latter category because null mutations, although causing severe paralysis, were viable.
To explore the function of the unc-18 gene, we first analyzed the different mutations and their mutant phenotypes. Among them, we found two missense mutations, md1401 andb403. These mutation sites are located at the N and C terminal of UNC-18, respectively. However, although both of these mutations occur in predicted α-helices, they have very different amino acid substitutions, and they lead to quite different phenotypes. The N-terminal mutation md1401 is mildly defective, whereas the C-terminal mutation b403 shows a severely defective phenotype. Based on the computational three-dimensional structure, N. Hayashi (Fujita Health University, Aichi, Japan) predicted that the conformation is lost by the E-to-K substitution in theb403 mutation (personal communication). I-to-V substitution in the md1401 mutation may not bring out great conformational alteration, and the ability of the mutant UNC-18 to bindCe syntaxin and UNC-13 appears intact.
We have shown that UNC-18 has the ability to bind to UNC-13N, in addition to Ce syntaxin. A mammalian UNC-18 homolog, Munc-l8, can bind the Doc2 and Mint proteins, in addition to syntaxin (Okamoto and Südhof, 1997; Verhage et al., 1997). Therefore, UNC-18 also may bind to multiple proteins. The function of such interactions is not clear at present. It is noteworthy that UNC-18, once bound to Ce syntaxin, can lose its syntaxin-binding ability by interaction with UNC-13N. This step may be important for the late stage of synaptic transmission, including fusion and/or exocytosis. We suggest that UNC-18 induces a conformational change inCe syntaxin, which then acquires the ability to bind synaptic vesicles after the UNC-13N-dependent release of UNC-18 from the complex. The mammalian homolog Munc-13–1 has been shown to bind both syntaxin and Doc2 (Betz et al., 1997; Orita et al., 1997). Assays with full-length UNC-13 and UNC-18 were not done, however, because we have not yet established a method for preparation of the recombinant UNC-13 corresponding to the C-terminal region. Betz et al. (1997)performed coprecipitation and yeast two-hybrid experiments with Munc-13–1 and Munc-18–1. However, they did not observe the interaction.
We have found that UNC-13N, UNC-18, and Ce syntaxin form complexes with each other, although stable complex formation between UNC-13N and Ce syntaxin was not observed. We were unable to detect a ternary complex consisting of the three proteins. Instead, we observed that the UNC-18–Ce syntaxin complex is dissociated by UNC-13N. Recently, Betz et al. (1998) found that UNC-13 has binding ability to phorbor ester and diacylglycerol. Therefore, it is likely that UNC-13 activated by either Ca2+ or diacylglycerol dissociates UNC-18 from the UNC-18–Cesyntaxin complex.
In summary, we have demonstrated directly that the UNC-18–Ce syntaxin complex is dissociated by UNC-13N without forming a stable ternary complex. However, our findings do not exclude additional roles for the three proteins in synaptic transmission, given that the three proteins interact with numerous synaptic factors. For example, the syntaxin-1a–Munc-18 complex is dissociated by tomosyn (Fujita et al., 1998).
Footnotes
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan, the Japan Society for the Promotion of Science (Research for the Future Grant 97L00401) to R.H., and National Institutes of Health Grant NS31439 to I.N.M. We thank S. Matsudaira and R. Kitamura for technical assistance.
Drs. Sassa and Harada contributed equally to this work.
Correspondence should be addressed to Ryuji Hosono, Department of Physical Information, Faculty of Medicine, Kanazawa University 5-11-80 Kodatsuno, Kanazawa, Ishikawa 920, Japan.
REFERENCES
- 1.Ahmed S, Maruyama IN, Kozma R, Lee J, Brenner S, Lim L. The Caenorhabditis elegans unc-13 gene product is a phospholipid-dependent high-affinity phorbol ester receptor. Biochem J. 1992;287:995–999. doi: 10.1042/bj2870995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993;261:617–619. doi: 10.1126/science.8342028. [DOI] [PubMed] [Google Scholar]
- 3.Bennett MK, Scheller RH. A molecular description of synaptic vesicle membrane trafficking. Annu Rev Biochem. 1994;63:63–100. doi: 10.1146/annurev.bi.63.070194.000431. [DOI] [PubMed] [Google Scholar]
- 4.Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc13–1 with the N terminus of syntaxin. J Biol Chem. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- 5.Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N. Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- 6.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–93. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brose N, Hofmann K, Hata Y, Südhof TC. Mammalian homo- logues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem. 1995;270:25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- 8.Calakos M, Bennett MK, Peterson KE, Scheller RH. Protein–protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 1994;263:1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- 9.Chaoman ER, An S, Barton N, Jahn R. SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem. 1994;269:27427–27432. [PubMed] [Google Scholar]
- 10.Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A, Scheller RH, Takai Y. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 11.Garcia E, McPherson PS, Chilcote TJ, Takei K, De Camilli P. rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J Cell Biol. 1995;129:105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gengyo-Ando K, Kamiya Y, Yamakawa A, Kodaira K, Nishiwaki K, Miwa J, Hori I, Hosono R. The C. elegans unc-18 gene encodes a protein expressed in motor neurons. Neuron. 1993;11:703–711. doi: 10.1016/0896-6273(93)90080-b. [DOI] [PubMed] [Google Scholar]
- 13.Harada S, Hori I, Yamamoto H, Hosono R. Mutations in the unc-41 gene cause elevation of acetylcholine levels. J Neurochem. 1994;63:439–446. doi: 10.1046/j.1471-4159.1994.63020439.x. [DOI] [PubMed] [Google Scholar]
- 14.Hata Y, Slaughter CA, Südhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–350. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof TC, Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosono R, Kamiya Y. Additional genes which result in an elevation of acetylcholine levels by mutations in Caenorhabditis elegans. Neurosci Lett. 1991;128:243–244. doi: 10.1016/0304-3940(91)90270-4. [DOI] [PubMed] [Google Scholar]
- 17.Hosono R, Sassa T, Kuno S. Mutations affecting acetylcholine levels in the nematode Caenorhabditis elegans. J Neurochem. 1987;49:1820–1823. doi: 10.1111/j.1471-4159.1987.tb02442.x. [DOI] [PubMed] [Google Scholar]
- 18.Hosono R, Sassa T, Kuno S. Spontaneous mutations of trichlorfon resistance in the nematode Caenorhabditis elegans. Zool Sci. 1989;6:697–708. [Google Scholar]
- 19.Hosono R, Hekimi S, Kamiya Y, Sassa T, Murakami S, Nishiwaki S, Miwa J, Taketo A, Kodaira K-I. The unc-18 gene encodes a novel protein affecting the kinetics of acetylcholine metabolism in the nematode Caenorhabditis elegans. J Neurochem. 1992;58:1517–1525. doi: 10.1111/j.1471-4159.1992.tb11373.x. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen EM, Hartwieg E, Schuske K, Nonet ML, Jin Y, Horvitz HR. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature. 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- 21.Kazanietz MG, Lewin NE, Bruns JD, Blumberg PM. Characterization of the cysteine-rich region of the Caenorhabditis elegans protein UNC-13 as a high affinity phorbol ester receptor. J Biol Chem. 1995;270:10777–10783. doi: 10.1074/jbc.270.18.10777. [DOI] [PubMed] [Google Scholar]
- 22.Kee Y, Lin RC, Hsu S-C, Scheller RH. Distinct domains of syntaxin are required for synaptic vesicle fusion complex formation and dissociation. Neuron. 1995;14:991–998. doi: 10.1016/0896-6273(95)90337-2. [DOI] [PubMed] [Google Scholar]
- 23.Kelly RB. Storage and release of neurotransmitters. Cell. 1993;72:43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- 24.Martin TFJ. Stages of regulated exocytosis. Trends Cell Biol. 1997;7:271–276. doi: 10.1016/S0962-8924(97)01060-X. [DOI] [PubMed] [Google Scholar]
- 25.Maruyama IN, Brenner S. A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc Natl Acad Sci USA. 1991;88:5729–5733. doi: 10.1073/pnas.88.13.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc Natl Acad Sci USA. 1996;93:12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nonet ML, Grundahl K, Meyer BJ, Rand JB. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- 28.Nonet ML, Saifee O, Zhao H, Rand JB, Wei L. Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J Neurosci. 1998;18:70–80. doi: 10.1523/JNEUROSCI.18-01-00070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novick PJ, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa H, Hayashi N, Hori I, Kobayashi T, Hosono R. Expression, purification and characterization of recombinant C. elegans UNC-18. Neurochem Int. 1996;29:553–563. doi: 10.1016/0197-0186(95)00012-7. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa H, Harada S, Sassa T, Yamamoto H, Hosono R. Functional properties of the unc-64 gene encoding a Caenorhabditis elegans syntaxin. J Biol Chem. 1998;273:2192–2198. doi: 10.1074/jbc.273.4.2192. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto M, Südhof TC. Mints, Munc18-interacting proteins in synaptic vesicle exocytosis. J Biol Chem. 1997;272:31459–31464. doi: 10.1074/jbc.272.50.31459. [DOI] [PubMed] [Google Scholar]
- 33.Orita S, Naito A, Sakaguchi G, Maeda M, Igarashi H, Sasaki T, Takai Y. Physical and functional interactions of DOC2 and Munc13 in Ca2+ dependent exocytotic machinery. J Biol Chem. 1997;272:16081–16084. doi: 10.1074/jbc.272.26.16081. [DOI] [PubMed] [Google Scholar]
- 34.Pevsner J, Hsu S-C, Braun JEA, Calakos N, Ting AE, Bennett MK, Scheller RH. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994a;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 35.Pevsner J, Hsu S-C, Scheller RH. n-Sec1: a neural-specific syntaxin-binding protein. Proc Natl Acad Sci USA. 1994b;91:1445–1449. doi: 10.1073/pnas.91.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rand JB. Genetic analysis of the cha-1-unc-17 gene complex in Caenorhabditis. Genetics. 1989;122:73–80. doi: 10.1093/genetics/122.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rand JB, Russell RL. Choline acetyltransferase-deficient mutants of the nematode Caenorhabditis elegans. Genetics. 1984;106:227–248. doi: 10.1093/genetics/106.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rand JB, Russell RL. Molecular basis of drug-resistance mutations in C. elegans. Psychopharmacol Bull. 1985;21:623–630. [PubMed] [Google Scholar]
- 39.Saifee O, Wei L, Nonet ML. The Caenorhabditis elegans unc-64 encodes a syntaxin that interacts genetically with synaptobrevin. Mol Biol Cell. 1998;9:1235–1252. doi: 10.1091/mbc.9.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 41.Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 42.Verhage MJ, de Vries KJ, Roshol H, Burbach PH, Gispen WH, Südhof TC. DOC2 proteins in rat brain: complementary distribution and proposed function as vesicular adaptor proteins in early stages of secretion. Neuron. 1997;18:453–461. doi: 10.1016/s0896-6273(00)81245-3. [DOI] [PubMed] [Google Scholar]