Abstract
Sex-related differences in behavior are extensive, but their neuroanatomic substrate is unclear. Indirect perfusion data have suggested a higher percentage of gray matter (GM) in left hemisphere cortex and in women, but differences in volumes of the major cranial compartments have not been examined for the entire brain in association with cognitive performance. We used volumetric segmentation of dual echo (proton density and T2-weighted) magnetic resonance imaging (MRI) scans in healthy volunteers (40 men, 40 women) age 18–45. Supertentorial volume was segmented into GM, white matter (WM), and CSF. We confirmed that women have a higher percentage of GM, whereas men have a higher percentage of WM and of CSF. These differences sustained a correction for total intracranial volume. In men the slope of the relation between cranial volume and GM paralleled that for WM, whereas in women the increase in WM as a function of cranial volume was at a lower rate. In men the percentage of GM was higher in the left hemisphere, the percentage of WM was symmetric, and the percentage of CSF was higher in the right. Women showed no asymmetries. Both GM and WM volumes correlated moderately with global, verbal, and spatial performance across groups. However, the regression of cognitive performance and WM volume was significantly steeper in women. Because GM consists of the somatodendritic tissue of neurons whereas WM comprises myelinated connecting axons, the higher percentage of GM makes more tissue available for computation relative to transfer across distant regions. This could compensate for smaller intracranial space in women. Sex difference in the percentage and asymmetry of the principal cranial tissue volumes may contribute to differences in cognitive functioning.
Keywords: sex differences, neuropsychology, brain volume, magnetic resonance imaging, gray matter, white matter, cerebrospinal fluid, cognitive performance, segmentation, neuroanatomy
Sex differences in brain anatomy may explain some documented differences in behavior. Women perform better than men on verbal and memory tasks, whereas men excel in spatial tasks (Maccoby and Jacklin, 1974; Delgado and Prieto, 1996; Caplan et al., 1997; Collins and Kimura, 1997; McGivern et al., 1997). These differences were attributed to variation in hemispheric specialization of cortical function. Although the left hemisphere is generally dominant in verbal and the right in spatial processing (for review, seeSpringer and Deutsch, 1998), some neuropsychological studies have suggested less hemispheric specialization in women as compared with men (Witelson, 1976) (for review, see Hiscock et al., 1995).
Neuroanatomic substrates for functional asymmetry were suggested by larger volume of left cortical language regions (Geschwind and Levitsky, 1968), and sex differences were observed in such regions (Schlaepfer et al., 1995; Witelson et al., 1995; Harasty et al., 1997). Sex differences also were reported in corpus callosum morphometry (Witelson, 1989; Steinmetz et al., 1995). Because the callosum consists of myelinated connecting fibers, larger callosal volumes in women were interpreted as providing for better interhemispheric communication, hence less need for functional specialization of the two hemispheres (Witelson, 1989). These findings have been challenged as a byproduct of cranial volume (Jäncke et al., 1997), and studies correlating volume of the callosum and cognitive performance yielded mixed results (Hines et al., 1992; Clarke and Zaidel, 1994).
These results underscore the need to consider cranial volume when searching for regional anatomic differences. Perfusion data indicated a higher percentage of fast-clearing tissue, presumably gray matter, in the left hemisphere (Gur et al., 1980) and in women (Gur et al., 1982). However, the method was limited to measuring superficial cortex and only the percentage relative to a combined compartment of white matter and extracerebral tissue. More optimal neuroanatomic measures are feasible with quantitative MRI, using algorithms for tissue segmentation (Kohn et al., 1991; Filipek et al., 1994; Pfefferbaum et al., 1994; Blatter et al., 1995; Passe et al., 1997; Coffey et al., 1998). Such methods report results consistent with a proportionately higher percentage of gray matter in women. Filipek et al. (1994)studied 20 young adults and reported that, whereas men had larger brain volumes than women, the difference reached significance for WM, but not for GM. Similarly, Passe et al. (1997) reported that brain-size sex differences were primarily attributable to white matter volume. Sex differences in compartmental proportions for the entire supertentorial space have not been examined.
Establishing that anatomic findings provide substrates for sex differences in performance requires an association between tissue volume and performance on verbal and spatial tasks. Correlations between volume and performance measures generally have been small but consistent (Andreasen et al., 1993; Kareken et al., 1995; Reiss et al., 1996). However, no studies have addressed sex differences integrating neuroanatomic with cognitive measures.
We have described an automated procedure for tissue segmentation of intracranial compartments related to cytoarchitecture and connectivity: GM—the somatodendritic tissue of neurons (cortical and deep), WM—the axonal compartment of myelinated connecting fibers, and CSF (Kohn et al., 1991; Yan and Karp, 1994a). The present study applied this algorithm to examine sex differences in the composition of supertentorial brain for a prospective sample of young healthy adults.
SUBJECTS AND METHODS
Study design and population
The sample was recruited to study brain function in healthy people and to serve as normative comparison subjects for clinical studies. The 80 right-handed adults, 40 men and 40 women, age 18–45, were consecutive admissions to the protocols, recruited by advertisement in community newspapers. They underwent detailed medical, neurological, psychiatric, and neurocognitive evaluations to exclude for history of illness affecting brain function as well as major psychiatric illness in first-degree relatives (Shtasel et al., 1991;Kareken et al., 1995). Women were premenopausal. Groups did not differ on major sociodemographic characteristics (age: mean ± SD, men 27.0 ± 5.7, women 25.0 ± 5.3; education: men 14.8 ± 2.2, women 14.8 ± 1.7; parental education: men 12.4 ± 2.2, women 12.3 ± 1.9; IQ: men 109.9 ± 13.5, women 106.9 ± 12.9; all t < 1). Informed consent was obtained after the nature and possible consequences of the study were explained.
MRI measurement
Axial spin echo MRIs were acquired on a General Electric 1.5 tesla scanner with a repetition time of 3000 msec and echo times of 30 and 80 msec in planes parallel to the canthomeatal axis with in-plane resolution of 0.859 × 0.859 mm, 5 mm slice thickness, and no gaps. Images were resliced along the anterior-to-posterior commissural axis to standardize for head tilt and were imported electronically into the segmentation software package.
Neuroradiological evaluation of the MRI. All MRI scans were evaluated neuroradiologically for technical quality and gross abnormalities; none was found. Only supertentorial tissue was included in the analyses, and thus the cerebellum and brainstem nuclei were excluded. This was done by using standard guidelines, as detailed in R. E. Gur et al. (1991). Several issues are addressed in this procedure. First, occipital lobes often project onto the same section as the cerebellar hemispheres and brainstem. Because the tentorium slopes, superiorly centrally, the margins had to be reconciled, and CSF in the superior cerebellar and quadrigeminal cisterns had to be subtracted. Second, the sella turcica was excluded because the enlargements of the CSF space in and around the pituitary gland depend on the intactness of the diaphragms sellae. However, CSF in the chiasmatic cistern was included. Third, other anatomic variables included the uppermost portion of the midbrain and the cisterns anterior to it. A line was drawn that connected the two cerebral peduncles with the basilar artery, and the brainstem posterior to that was excluded. The most superior portion of the midbrain and the CSF in the chiasmatic cistern anterior to this, along with structures of the hypothalamus (including the mamillary bodies, tuber cinereum and infundibular stalk, and optic chiasm), were included.
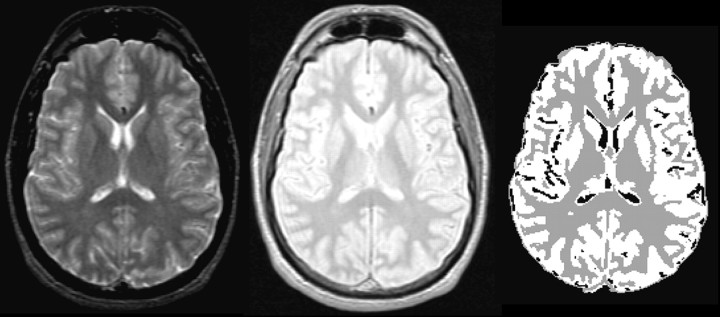
Neuroanatomical measures. The brain volume was extracted by automatically stripping scalp, skull, and meninges, using optimal thresholding and morphological operations on the image intensity and chamfer distance (Borgefors, 1986; Yan and Karp, 1994b). The chamfer distance is an easily computed approximation of the distance from any given point to the head surface. Some nonbrain regions such as bone marrow and the eyeballs could not be stripped reliably by this algorithm and were removed manually in an interactive program. The stripped MRI image was segmented into GM, WM, and CSF, using an adaptive Bayesian algorithm (Yan and Karp, 1994a, 1995), which models the image as a collection of tissue compartments with slowly varying mean intensity, plus white Gaussian noise. The mean intensity within each compartment was estimated by least-squares fitting to a cubic B-spline (Boor, 1978); this helps overcome “shading” effects and reduces partial voluming that can bias against small, isolated regions (e.g., sulcal CSF). Spatial interactions among adjacent voxel labels were modeled as a Markov random field with a three-dimensional second-order neighborhood system in which different potentials are used for the in-plane and axial directions to account for anisotropic voxel dimensions. The algorithm does an initial segmentation by using the K-means clustering on image intensity. Then the segmentation is improved iteratively by repeatedly estimating the (spatially varying) mean intensity of each compartment by fitting a B-spline over the entire image and resegmenting the image into compartments by maximizing the a posteriori (MAP) probability with the ICM algorithm (Besag, 1986). The number of spline control points is increased gradually. Combining spline representation and adaptation makes the segmentation more accurate and robust (Fig.1).
Fig. 1.
Illustration of the MRI segmentation process showing an acquired T2-weighted image (left), a proton density image (middle), and the segmented image (right) in which GM is depicted in white, WM in light gray, and CSF in black.
Neuropsychological measurement
As part of the neurocognitive evaluation, subjects were administered two tests of verbal abilities: the Vocabulary subscale of the Wechsler Adult Intelligence Scale–Revised (WAIS-R; Wechsler, 1981) and the California Verbal Learning Test (CVLT; Delis et al., 1987) plus two spatial tests: the Block Design subscale of the WAIS-R and the Judgment of Line Orientation test (Benton et al., 1983). The tests were administered within a week of the MRI study by trained neuropsychologists in adherence to standard procedures. For averaging and dimensional contrasts, the scores on individual tests were converted to standard equivalents (z-scores) and averaged to yield Global, Verbal, and Spatial performance indices. To test whether our sample replicates the reported finding of better verbal relative to spatial performance in women as compared with men, we calculated a “verbal superiority” index by subtracting Verbal minus Spatial.
RESULTS
To avoid inflating the probability of Type I (“experimenter-wise”) error, we limited data analysis to testing the specific hypotheses on a restricted number of dependent measures. We applied one-tailed tests when a prevailing hypothesis stipulates a specific direction to the finding (e.g., age is expected to correlate negatively with volume; volume is expected to correlate positively with performance) but did not correct the p values for multiple comparisons for such hypothesis-confirmatory analyses. For the main analysis of sex differences in cranial compartments we have applied a mixed-model multivariate analysis of variance (MANOVA), which is conservative but permits better generalization. We first will examine the effects of age, then present sex differences on the volumetric measures, and conclude by correlating the anatomic with cognitive performance measures.
Effects of age
An important factor to consider when evaluating both brain volume and neurocognitive measures is the effect of aging. Such effects have been observed throughout the lifespan, with increases from childhood to early adulthood, followed by decline in both parenchymal volume (Jernigan et al., 1990, 1991; Cowell et al., 1994; Blatter et al., 1995; Giedd et al., 1996a,b) and cognitive performance (Van Gorp et al., 1990) (for review, see Sternberg and Berg, 1992). Furthermore, such studies have suggested sex differences in the rate of age-associated decline, with men showing greater reduction in parenchymal volume (R. C. Gur et al., 1991; Cowell et al., 1994; Coffey et al., 1998). Therefore, evaluating sex differences in the relationships between volume and performance requires taking age into consideration.
Although the age range of the sample was restricted to young adulthood, examination of age effects on the volumetric and performance measures is nonetheless informative. Our sample can extend the characterization of this process to young adulthood, and if there are correlations with age, it is necessary to examine whether effects sustain the removal of age-related variance. The correlation between age and total intracranial volume was nil (r = 0.02), indicating no secular drift in head size. A small yet significant correlation was seen for GM volume (r = −0.26; df = 78;p < 0.01). This correlation was higher in men (r = −0.43; df = 38; p < 0.005) than in women (r = −0.29; df = 38;p < 0.05, one-tailed). Age did not correlate significantly with WM or CSF volumes. With regard to performance, the correlations between age and Global or Verbal performance were nonsignificant across or within groups. Spatial performance showed a small but significant correlation for the entire sample (r = −0.19; df = 73; p < 0.05, one-tailed). This correlation was larger in men (r = −0.35; df = 35; p < 0.025, one-tailed), and marginal in women (r = −0.19; df = 36;p = 0.114, one-tailed). Because of the significance of some correlations with age, all subsequent analyses also were performed on age-corrected values, using age as a covariate in the MANCOVAs and partialing it out in the correlational analyses. None of the effects that were significant using the raw values became nonsignificant with the age-corrected values, probably because there was no age difference between men and women. Therefore, the raw, uncorrected results will be presented.
Sex differences in compartmental volumes
As expected, intracranial volume in milliliters (ml), consisting of parenchyma, ventricles, and sulci (without subarachnoid space), was (mean ± SD) higher for men (1352.2 ± 104.9) than for women (1154.4 ± 85.1): t = 9.26; df = 78;p < 0.0001. The difference (14.6%) falls between the difference in height (8.2%) and weight (18.7%). Total parenchymal volume was 1229.6 ± 106.2 (range, 1033.9–1469.4) in men and 1072.3 ± 71.5 (range, 895.4–1196.0) in women: t= 7.77; df = 78; p < 0.0001. These compare well with estimates based on liquid displacement and with other MRI studies (Pakkenberg and Voight, 1964; Jernigan et al., 1990; Coffey et al., 1998). Hemispheric volumes are presented in Table1, and all are higher in men than in women.
Table 1.
Means and ranges (in ml) of hemispheric volumes for the three cranial compartments in men and women
| Men | Women | t(df = 78) | |||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | (allp < 0.001) | |
| GM | |||||
| L | 343.7 ± 34.3 | 255.9–413.3 | 319.3 ± 26.6 | 253.2–373.0 | 3.55 |
| R | 342.6 ± 34.3 | 252.8–409.1 | 319.7 ± 26.4 | 259.9–372.7 | 3.33 |
| WM | |||||
| L | 271.9 ± 33.4 | 216.0–374.7 | 216.4 ± 17.5 | 184.2–265.7 | 9.31 |
| R | 271.5 ± 33.7 | 213.1–373.1 | 217.0 ± 17.5 | 180.0–257.4 | 9.08 |
| CSF | |||||
| L | 60.5 ± 18.9 | 23.6–108.0 | 41.4 ± 17.8 | 14.4–79.8 | 4.66 |
| R | 62.1 ± 19.4 | 21.5–107.9 | 40.8 ± 17.2 | 15.8–71.2 | 5.21 |
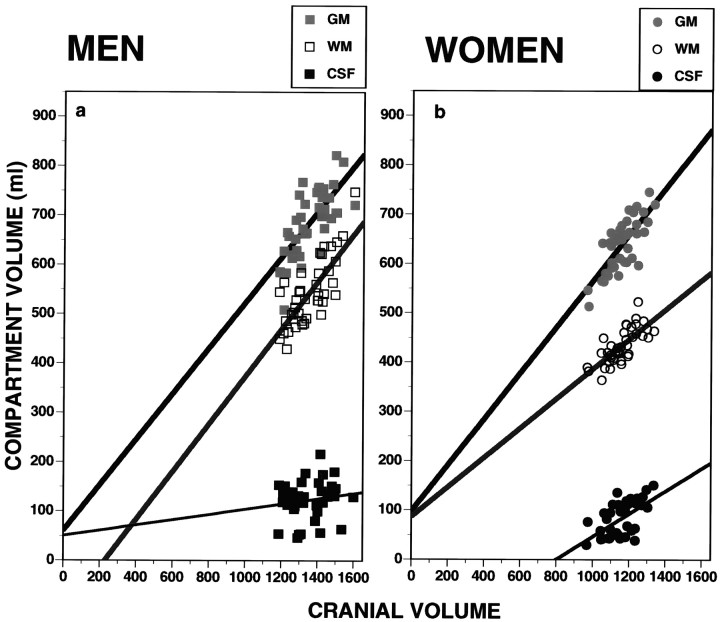
Examination of the relationship between total cranial volume and the volume of its three compartments (Fig. 2) indicates a divergence between men (Fig. 2a) and women (Fig.2b). Both in men and in women GM and WM are correlated with total cranial volume (men: r = 0.71 and 0.76 for GM and WM; women: r = 0.76 and 0.74, respectively; df = 38; p < 0.001). However, in testing for equality of slopes for the regression lines using Kleinbaum and Kupper’s (1978)method, we found that, whereas men have the same slope for GM (0.46 ± 0.07) and WM (0.48 ± 0.07, not significantly different), in women the slope for GM (0.47 ± 0.07) is identical to that of men, but the slope for WM (0.30 ± 0.04) is significantly shallower in comparison with GM in women (t = 2.11; df = 38; p < 0.05, two-tailed) and compared with WM in men (t = 2.23; df = 78; p < 0.025, two-tailed). Thus, in men increased cranial volume is associated with a proportional increase in GM and WM, whereas in women the increase in WM as a function of cranial volume is at a lower rate. The correlation between CSF volume and cranial volume was not significant in men (r = 0.15; df = 38) but was significant in women (r = 0.56; df = 38; p < 0.01). The sex difference in slope for this association was not significant: t = 1.73; df = 38; p = 0.08.
Fig. 2.
Scatterplots and regression lines for gray matter (GM), white matter (WM), and CSF against cranial volumes in men (left,squares) and women (right,circles).
Intracranial volume consisted of 53.1 ± 4.0% (42.0–61.3) GM, 38.9 ± 3.0% (34.3–46.8) WM, and 8.0 ± 2.9% CSF. These values are comparable to estimates from other and similar methods (Risberg et al., 1975; Miller et al., 1980; Filipek et al., 1994;Coffey et al., 1998). The hypothesis of sex differences in the distribution and asymmetry of tissue was tested by using a mixed model MANOVA, with fixed effects for Compartment (GM, WM, CSF, repeated-measures factor) and Sex (grouping factor) and a random effect for subjects. The analysis of proportions of a whole, such as percentages of GM, WM, and CSF that make up the cranial volume, has great potential for erroneous results. This fact was recognized originally by Karl Pearson himself and was discussed extensively inAitchison (1986). The so-called “unit sum” constraint (the sum of these three proportions must add to one) results in spurious correlations when multiple proportions are included in a regression model and appropriate adjustments are not made. To address this problem, we included the intracranial volume as a covariate in the regression models for proportions.
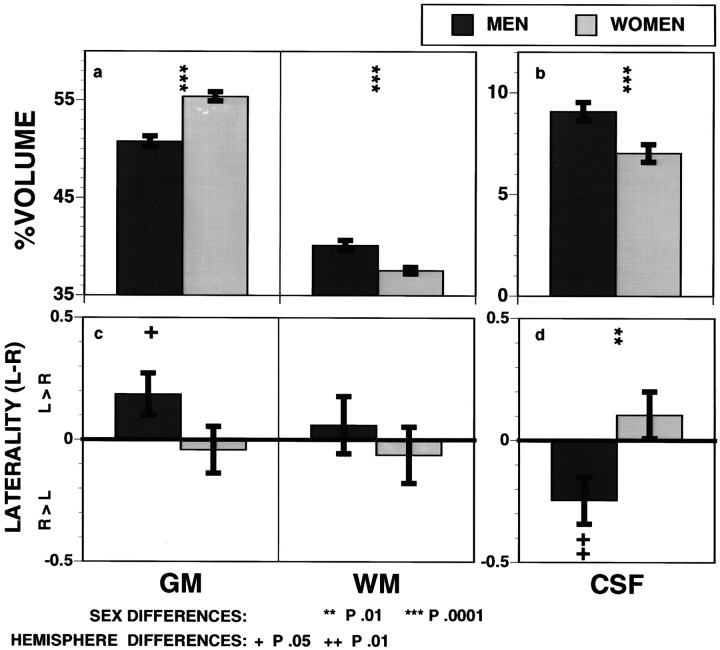
The hypothesized sex by compartment interaction was significant:F = 73.33; df = 2390; p < 0.0001. As seen in Figure 3, the interaction reflects a higher percentage of GM in women (55.4 ± 3.0%) than in men (50.8 ± 3.6%; t = 6.24, df = 78), extending to the whole brain our finding with 133xenon on superficial cortex (Gur et al., 1982). In contrast, men had a higher percentage of WM and a higher percentage of CSF (all p< 0.0001); for CSF the difference was significant only for sulcal measures (men, 7.8 ± 2.7; women, 6.0 ± 2.5;t = 3.10; df = 78; p = 0.001), not ventricular (or central) measures (men, 1.1 ± 0.4; women, 1.0 ± 0.4; t < 1).
Fig. 3.
Means ± SEM percentage of tissue and CSF averaged bilaterally (top) and examined as a laterality index (left minus right, bottom) in men (dark bars) and women (light bars).
Sex differences in hemispheric asymmetries were also significant, with greater asymmetries in the percentage of GM and the percentage of CSF in men as compared with women (Fig. 3c,d). As with133xenon, the percentage of GM was higher in the left for men: left–right difference = 0.19 ± 0.09% (t = 2.16; df = 38; p < 0.05, one-tailed). WM was symmetric (0.06 ± 0.12, t < 1), but the percentage of CSF was higher on the right (−0.25 ± 0.09; t = 2.56; df = 38; p < 0.01, two-tailed). This asymmetry of CSF in men was evident in sulcal (0.24 ± 0.09; t = 2.67; df = 38;p = 0.011, two-tailed), but not ventricular CSF (0.01 ± 0.03; t < 1). No asymmetries were significant in women, and the difference in laterality gradients between men and women was significant: more positive values for GM (t = 1.77; df = 78; p < 0.05, one-tailed) and more negative values for CSF (t = 2.59; df = 78; p < 0.01, two-tailed). Note that the hemispheric effects are quite small in absolute terms and do not mask the main sex differences in raw volumes. Thus, although men have a higher percentage of GM in the left relative to the right hemisphere whereas women have symmetric GM, women still have a higher percentage of GM than men in either hemisphere.
An alternative explanation for the higher percentage of GM in women is that the regression line relating GM and WM does not go through the origin; then the percentage of GM will decrease as a function of brain volume even if men and women have exactly the same relationship between tissue volumes. For example, Jäncke et al. (1997) reported that sex-related differences in the morphometry of the corpus callosum actually reflected a more general brain size effect; if female and male samples are matched on brain size, there should be no difference in the size of their corpus callosum. Furthermore, the slice thickness of 5 mm may introduce partial volume effects to which women will be more susceptible because of their smaller cranial volumes. We examined whether this could explain the difference in the percentage of GM by comparing the 21 men and 14 women with an overlapping range of intracranial volumes (1100–1350 ml). These groups did not differ in intracranial volume (men, 1265.9 ± 46.3; women, 1244.2 ± 45.7; t = 1.37; df = 33, not significant), yet women had a higher percentage of GM (54.5 ± 3.2) than men (50.9 ± 3.9): t = 2.86; df = 33;p = 0.007. This indicates a sex difference independent of head size. Finally, the difference remained when intracranial volume, height, and weight were entered as covariates in MANCOVAs, either on percentage or on raw volumetric values.
Correlations with performance measures
These anatomic findings may provide neural substrates for sex differences in cognition if volume correlates with performance on verbal and spatial tasks. We first examined whether this sample showed the reported sex difference of better verbal relative to spatial performance in women as compared with men. Men and women did not differ in the Global (mean of Verbal and Spatial) performance score (0.24 ± 0.65 and 0.06 ± 0.81, respectively; t = 1.08; df = 73; not significantly different). However, as expected, the “verbal superiority” index (Verbal minus Spatial) was positive in women (0.42 ± 0.84; paired t = 2.98; df = 38; p < 0.01) and negative in men (−0.35 ± 0.91; paired t = 2.37; p < 0.025), and the two groups differed (t = 3.76; df = 78;p < 0.001). This difference is attributable primarily to the spatial tasks in which men (0.42 ± 0.86) performed better than women (−0.14 ± 0.93; t = 2.71; df = 78; p < 0.01), whereas the sex difference in the opposite direction for the verbal tasks (men, 0.06 + 0.71; women, 0.26 + 0.88) was not significant (t = 1.06).
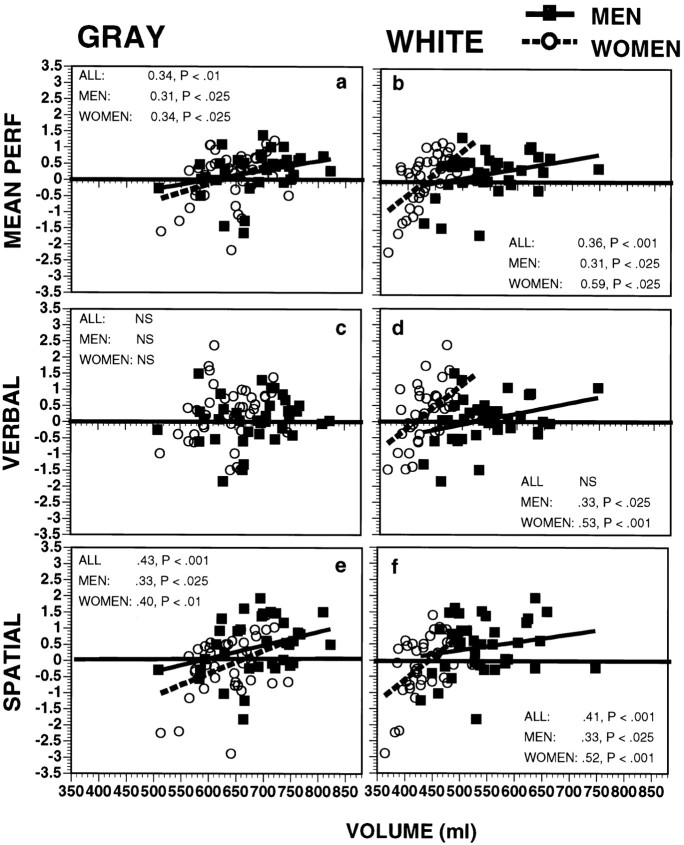
Further supporting the functional significance of the neuroanatomic findings, Global performance was correlated with intracranial volumes for the whole sample (r = 0.41; df = 78;p < 0.001, one-tailed), as well as for men (r = 0.39; df = 38; p < 0.01) and women (r = 0.40; df = 38; p < 0.001) considered separately. The correlation between intracranial volume and verbal performance was not significant for the entire sample or for men, but cranial volume did correlate with verbal performance in women (r = 0.40; df = 38; p < 0.01, one-tailed). Spatial performance was correlated with cranial volume for the entire sample (r = 0.51; df = 78;p < 0.001) as well as for men and women considered separately (r = 0.35; df = 38; p< 0.025; and r = 0.57; df = 38; p< 0.001, respectively). Although these correlations are moderate, scatterplots suggest that relationships were quite uniform across the range of volume and performance values for both GM and WM (Fig.4a,b), whereas the correlations with CSF volumes were nil. It is noteworthy that, whereas men and women had the same slope for the regression of global performance on volume (Fig. 4a), women showed steeper slopes of better performance associated with increased white matter volume (t = 3.13; p < 0.005). There was some divergence in this pattern of correlations between the verbal and the spatial performance scores (Fig. 4c–f). Verbal performance did not correlate with GM volume across the entire sample nor in men or women considered separately (Fig. 4c). WM was uncorrelated with verbal performance for the entire sample but showed significant correlations when men and women were considered separately (Fig. 4d). As with the Global score, the regression line for WM was steeper in women (t = 2.62;p < 0.01). Spatial performance correlated with both GM and WM volumes for the entire sample and for men and women separately (Fig. 4e,f). Again, the regression line for WM was steeper in women than in men (t = 2.56;p < 0.01). In contrast to correlations between absolute volumes and performance, none of the correlations with percentage values or laterality gradients (difference between hemispheres) was predictive of performance. It seems that sheer tissue volume, rather than proportion, is associated with better performance. Note that, despite the significant sex difference in spatial performance, most women performed comparably to men on the spatial tests. However, only one woman performed better than 1 SD above the mean, compared with nine men in this range, six of whom had WM volumes outside of the range of any of the women (Fig. 4d). Thus, larger volume is required for the highest levels of spatial performance than is permitted by the smaller cranial volume of women, despite their steeper regression of spatial performance on white matter volume. These conclusions should be considered tentatively because these correlations could be spurious, pending replication in other samples and across a wider range of cognitive measures.
Fig. 4.
Scatterplots and regression lines for gray matter (left column) and white matter (right column) against average cognitive performance (top row) and verbal and spatial performance (middleand bottom rows, respectively) in men (filled squares, solid regression line) and women (open circles, dashed regression line).
DISCUSSION
Before turning to the main focus of the study on sex differences in tissue volumes, it is noteworthy that the volume estimates with our automated segmentation approach compare well with earlier methods with postmortem brains (Pakkenberg and Voight, 1964; Miller et al., 1980) or with quantitative MRI (Jernigan et al., 1990; Coffey et al., 1998). Furthermore, the segmented volumes show similar association with age for this restricted age range, with GM showing reduced volume with age more than WM and the effect being stronger in men than in women (Raz et al., 1997; Coffey et al., 1998). This similarity adds confidence in the method, but apparently because the male and female samples did not differ in age, removing age effects by covariance analysis did not influence the findings.
With regard to sex differences, these were found in all three principal supertentorial compartments. The finding that women have a higher percentage of GM than men replicates earlier studies with the133xenon clearance method (Gur et al., 1982). Because the methods rely on very different principles and assumptions, this replication adds confidence in the phenomenon. The newer methodology further enabled generalization of the effects beyond the cortical surface layers of brain that are sampled by the 133xenon clearance method. It also established effects related to sex differences and hemispheric asymmetry in WM and CSF, two compartments that could not be resolved with the 133xenon clearance method. We found that the higher percentage of GM in women is complemented by a globally higher percentage of WM and percentage of CSF in men. Although earlier studies with MRI have not evaluated comparable sex differences in tissue percentages, our raw volumes seem consistent with their reports of lower WM and CSF volume in women as compared with men (Filipek et al., 1994; Passe et al., 1997). On the other hand, we found that the absolute volume of GM was also lower in women, whereas the effect of Filipek and colleagues in this direction did not reach statistical significance. This, however, most likely reflects statistical power in view of our larger sample size.
Examination of the volumes in the context of total cranial volume indicated that, whereas in men there was a proportionate increase of GM and WM as a function of cranial volume, in women the slope of increase of WM was significantly shallower than that for GM. This sex difference in intracranial tissue composition may reflect adaptation to the smaller cranial volumes of women. Sexual anatomic dimorphism has been comparable at least since the Middle Pleistocene hominids (Arsuaga et al., 1997). Because GM is the somatodendritic tissue where computation is done whereas WM is the myelinated connective tissue needed for information transfer across distant regions, a higher percentage of GM in women increases the proportion of tissue available for computational processes. This is a reasonable evolutionary strategy because smaller crania require shorter distances for information transfer; hence there could be relatively less need for WM. The higher percentage of GM is bilateral in women, with laterality effects—higher left hemispheric percentage of GM and right hemispheric percentage of CSF—evident only in men. This is consistent with some behavioral and neurobiological data suggesting less hemispheric asymmetry in women (Hiscock et al., 1995). In a functional MRI study Shaywitz et al. (1995) reported that for phonological tasks men showed left lateralized inferior frontal gyrus activation, whereas women showed more bilateral activation in this region. The results were considered consistent with the hypothesis that men are more highly lateralized for language functions. On the other hand, it is noteworthy that the hemispheric asymmetries for our global measures were small relative to the sex differences in percentages. Thus, whereas men have a relatively higher percentage of GM in the left, they still have a lower percentage of GM than women in either hemisphere. This may differ for smaller structures.
The anatomic results suggest some parallels between sex differences in cognition and differences in GM because both women and the left language hemisphere have a higher percentage of GM, and women outperform men on language tasks. A direct examination of the functional significance of these anatomic findings was feasible via correlations between volume and cognitive performance measures. Considered separately, the performance data replicated earlier reports of better verbal relative to spatial performance in women as compared with men, against overall similar levels of average performance (Saykin et al., 1995; Delgado and Prieto, 1996; Caplan et al., 1997; Collins and Kimura, 1997; McGivern et al., 1997). Our finding of small but significant correlations between parenchymal volume and global measures of cognitive performance likewise replicates earlier reports (Andreasen et al., 1993; Kareken et al., 1995; Reiss et al., 1996; Raz et al., 1998). Examination of our segmented GM and WM volumes indicated a sex difference in this relationship. Although the slope of the relationship between GM and performance was identical for men and women, the slope for WM was significantly steeper in women than in men. The effect was seen for the global performance measure as well as for verbal and spatial performance considered separately. This supports the notion that the smaller crania of women enable a more efficient use of the available WM. For the verbal task, in which the overall correlation between parenchymal volume and performance is low, the higher percentage of GM in women and the steeper slope of improved performance with increased WM combine to confer on women a performance advantage. However, as seen from the association between WM volume and performance on the spatial tasks, men may perform better on tasks in which a high level of performance requires large volumes of WM. This suggests that verbal tasks require less intrahemispheric transfer than spatial tasks and that sex differences in performance would depend on the relative requirements for GM and WM. However, these correlations could be spurious and should be interpreted with extreme caution. Testing this hypothesis would require a wider range of tasks showing sex differences in performance and perhaps constructing new tasks designed to require either highly focal processing or transfer of information across distant cortical regions.
Our finding of a lower overall proportion of WM in women seems to contrast with reports of higher volumes of corpus callosum (Witelson, 1989; Steinmetz et al., 1995), which is a white matter structure. Although these findings have been challenged recently as an artifact of smaller cranial volume (Jäncke et al., 1997), several investigators have reported that sex differences in callosum have sustained corrections for cranial volume (Steinmetz et al., 1995;Davatzikos and Resnick, 1998). Conceivably, men and women differ in the relative amount of inter- and intrahemispheric communication. This possibility can be tested more specifically by using neurobehavioral and functional imaging methods (Clarke and Zaidel, 1994).
The present report is limited to gross tissue distribution, and we have examined only supertentorial tissue. More rigorous testing of hypotheses linking anatomy to behavior may entail correlating substructures with specific neurocognitive parameters. Higher resolution MRI enables the parceling of structures, but interpretation of regional differences is predicated on reference to values for the whole brain and to the entire intracranial volume. Thus, Giedd et al. (1996a) found that the sex difference in cerebellar volume was comparable to that in cerebral volume. However, examination of the cerebellum and brainstem can be performed more optimally with specialized procedures (Luft et al., 1998) and by using higher resolution T1-weighted sequences. This could shed more light on the involvement of infratentorial regions in motor and perhaps more complex cognitive processes (Malm et al., 1998). These anatomic differences may have implications to brain disorders, in which sex differences have been noted in frequency and severity (Weissman et al., 1993; Drislane et al., 1994; Gur et al., 1996; Payami et al., 1996). This methodology also can be extended to the question of sexual orientation (Wegesin, 1998). To understand further the effects of these neuroanatomic differences on behavior, we believe it also would be helpful to obtain functional and reproductive hormone measures. Normative parameters are prerequisite for depicting effects of brain dysfunction and their differential impact in men and women. It would be of particular relevance for evolutionary hypotheses to determine whether this sex-related divergence in brain tissue composition is uniquely human.
Footnotes
This work was supported by National Institutes of Health Grants MH-43380, MH-42191, MH-01336, MH-19112, and MO1RR0040. We thank Veda Maany, Oren Marom, and Daniel Widyono for assistance in image processing; Steven Arnold, Robert Grossman, Alan Rosenquist, Steven Siegel, and John Q. Trojanowski for their comments; and Oren Marom and Stephen Moelter for their help in manuscript preparation.
Correspondence should be addressed to Dr. Ruben C. Gur, Neuropsychiatry, 10th Floor, Gates Building, University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA 19104-4283.
REFERENCES
- 1.Aitchison J. The statistical analysis of compositional data. Chapman and Hall; New York: 1986. [Google Scholar]
- 2.Andreasen NC, Flaum M, Swayze V, O’Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WT. Intelligence and brain structure in normal individuals. Am J Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- 3.Arsuaga JL, Carretero JM, Lorenzo C, Gracia A, Martinez I, Bermudez de Castro JM, Carbonell E. Size variations in Middle Pleistocene humans. Science. 1997;277:1086–1088. doi: 10.1126/science.277.5329.1086. [DOI] [PubMed] [Google Scholar]
- 4.Benton AL, Hamsher KdeS, Varney NR, Spreen O. Contributions to neuropsychological assessment. Oxford UP; New York: 1983. [Google Scholar]
- 5.Besag J. On the statistical analysis of dirty pictures. J Royal Stat Soc. 1986;48:259–302. [Google Scholar]
- 6.Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Parker N, Kurth S, Horn SD. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. Am J Neuroradiol. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- 7.Boor CD. A practical guide to splines. Springer; New York: 1978. [Google Scholar]
- 8.Borgefors G. Distance transformations in digital images. Comput Vis Graph Image Process. 1986;34:344–371. [Google Scholar]
- 9.Caplan PJ, Crawford M, Hyde JS, Richardson JTE. Gender differences in human cognition. Oxford UP; New York: 1997. [Google Scholar]
- 10.Clarke JM, Zaidel E. Anatomical–behavioral relationships: corpus callosum morphometry and hemispheric specialization. Behav Brain Res. 1994;64:185–202. doi: 10.1016/0166-4328(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 11.Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, Bryan RN. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55:169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- 12.Collins DW, Kimura D. A large sex difference on a two-dimensional mental rotation task. Behav Neurosci. 1997;111:845–849. doi: 10.1037//0735-7044.111.4.845. [DOI] [PubMed] [Google Scholar]
- 13.Cowell PE, Turetsky BT, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobe. J Neurosci. 1994;14:4748–4755. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davatzikos C, Resnick SM. Sex differences in anatomic measures of interhemispheric connectivity: correlations with cognition in women but not men. Cereb Cortex. 1998;8:635–640. doi: 10.1093/cercor/8.7.635. [DOI] [PubMed] [Google Scholar]
- 15.Delgado AR, Prieto G. Sex differences in visuospatial ability: do performance factors play such an important role? Mem Cognit. 1996;24:504–510. doi: 10.3758/bf03200938. [DOI] [PubMed] [Google Scholar]
- 16.Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test: adult version. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 17.Drislane FW, Coleman AE, Schomer DL, Ives J, Levesque LA, Seibel MM, Herzog AG. Altered pulsatile secretion of luteinizing hormone in women with epilepsy. Neurology. 1994;44:306–310. doi: 10.1212/wnl.44.2.306. [DOI] [PubMed] [Google Scholar]
- 18.Filipek PA, Richelme C, Kennedy DN, Caviness VS. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- 19.Geschwind N, Levitsky W. Human brain: left–right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 20.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996a;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 21.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996b;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Gur RC, Packer IK, Hungerbuhler JP, Reivich M, Obrist WD, Amarnek WS, Sackeim HA. Differences in the distribution of gray and white matter in human cerebral hemispheres. Science. 1980;207:1226–1228. doi: 10.1126/science.7355287. [DOI] [PubMed] [Google Scholar]
- 23.Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217:659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- 24.Gur RC, Mozley PD, Resnick SM, Gottlieb GE, Kohn M, Zimmerman R, Herman G, Atlas S, Grossman R, Berretta D, Erwin R, Gur RE. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci USA. 1991;88:2845–2849. doi: 10.1073/pnas.88.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gur RE, Mozley PD, Resnick SM, Shtasel D, Kohn M, Zimmerman R, Herman G, Atlas S, Grossman R, Erwin R, Gur RC. Magnetic resonance imaging in schizophrenia. I. Volumetric analysis of brain and cerebrospinal fluid. Arch Gen Psychiatry. 1991;48:407–412. doi: 10.1001/archpsyc.1991.01810290019002. [DOI] [PubMed] [Google Scholar]
- 26.Gur RE, Petty RG, Turetsky BI, Gur RC. Schizophrenia throughout life: sex differences in severity and profile of symptoms. Schizophr Res. 1996;21:1–12. doi: 10.1016/0920-9964(96)00023-0. [DOI] [PubMed] [Google Scholar]
- 27.Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA. Language-associated cortical regions are proportionally larger in the female brain. Arch Neurol. 1997;54:171–176. doi: 10.1001/archneur.1997.00550140045011. [DOI] [PubMed] [Google Scholar]
- 28.Hines M, Chiu L, McAdams LA, Bentler PM, Lipcamon J. Cognition and the corpus callosum: verbal fluency, visuospatial ability, and language lateralization related to midsagittal surface areas of callosal subregions. Behav Neurosci. 1992;106:3–14. doi: 10.1037//0735-7044.106.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Hiscock M, Israelian M, Inch R, Jacek C, Hiscock-Kalil C. Is there a sex difference in human laterality? II. An exhaustive survey of visual laterality studies from six neuropsychology journals. J Clin Exp Neuropsychol. 1995;17:590–610. doi: 10.1080/01688639508405148. [DOI] [PubMed] [Google Scholar]
- 30.Jäncke L, Staiger J, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cereb Cortex. 1997;7:48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- 31.Jernigan TL, Press GA, Hesselink JR. Methods for measuring brain morphologic features on magnetic resonance images. Validation and normal aging. Arch Neurol. 1990;47:27–32. doi: 10.1001/archneur.1990.00530010035015. [DOI] [PubMed] [Google Scholar]
- 32.Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- 33.Kareken DA, Gur RC, Mozley PD, Mozley LH, Saykin AJ, Shtasel DL, Gur RE. Cognitive functioning and neuroanatomic volume measures in schizophrenia. Neuropsychology. 1995;9:211–219. [Google Scholar]
- 34.Kleinbaum DG, Kupper LL. Applied regression analysis and other multivariable methods. Wadsworth; Belmont, CA: 1978. [Google Scholar]
- 35.Kohn MI, Tanna NK, Herman GT, Resnick SM, Mozley PD, Gur RE, Alavi A, Zimmerman RA, Gur RC. Analysis of brain and cerebrospinal fluid volumes with MR imaging. I. Methods, reliability, and validation. Radiology. 1991;178:115–122. doi: 10.1148/radiology.178.1.1984289. [DOI] [PubMed] [Google Scholar]
- 36.Luft AR, Skalej M, Welte D, Kolb R, Burk K, Schulz JB, Klockgether T, Voigt K. A new semiautomated, three-dimensional technique allowing precise quantification of total and regional cerebellar volume using MRI. Magn Reson Med. 1998;40:143–151. doi: 10.1002/mrm.1910400119. [DOI] [PubMed] [Google Scholar]
- 37.Maccoby E, Jacklin C. The psychology of sex differences. Stanford UP; Stanford, CA: 1974. [Google Scholar]
- 38.Malm J, Kristensen B, Karlsson T, Carlberg B, Fagerlund M, Olsson T. Cognitive impairment in young adults with infratentorial infarcts. Neurology. 1998;51:433–440. doi: 10.1212/wnl.51.2.433. [DOI] [PubMed] [Google Scholar]
- 39.McGivern RF, Huston JP, Byrd D, King T, Siegle GJ, Reilly J. Sex differences in visual recognition memory: support for a sex-related difference in attention in adults and children. Brain Cogn. 1997;34:323–336. doi: 10.1006/brcg.1997.0872. [DOI] [PubMed] [Google Scholar]
- 40.Miller AK, Alston RL, Corsellis JA. Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: measurements with an image analyser. Neuropathol Appl Neurobiol. 1980;6:119–132. doi: 10.1111/j.1365-2990.1980.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 41.Pakkenberg H, Voight J. Brain weight of the Danes. Acta Anat (Basel) 1964;56:297–307. [Google Scholar]
- 42.Passe TJ, Rajagopalan P, Tupler LA, Byrum CE, MacFall JR, Krishnan KR. Age and sex effects on brain morphology. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1231–1237. doi: 10.1016/s0278-5846(97)00160-7. [DOI] [PubMed] [Google Scholar]
- 43.Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, Schellenberg GD. Gender difference in apolipoprotein E associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996;58:803–811. [PMC free article] [PubMed] [Google Scholar]
- 44.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 45.Raz N, Gunning FM, Head D, Dupuis JH, McQuain JM, Briggs SD, Thornton AE, Loken WJ, Acker JD. Selective aging of human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 46.Raz N, Gunning-Dixon FM, Head DP, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural MRI. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 47.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender, and IQ in children: a volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 48.Risberg J, Ali Z, Wilson EM, Wills EL, Halsey JH. Regional cerebral blood flow by 133xenon inhalation. Stroke. 1975;6:142–148. doi: 10.1161/01.str.6.2.142. [DOI] [PubMed] [Google Scholar]
- 49.Saykin AJ, Gur RC, Gur RE, Shtasel DL, Flannery KA, Mozley LH, Malamut BL, Watson B, Mozley PD. Normative neuropsychological test performance: effects of age, education, gender and ethnicity. Appl Neuropsychol. 1995;2:79–88. doi: 10.1207/s15324826an0202_5. [DOI] [PubMed] [Google Scholar]
- 50.Schlaepfer TE, Harris GJ, Tien AY, Peng L, Lee S, Pearlson GD. Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry Res. 1995;61:129–135. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- 51.Shaywitz B, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- 52.Shtasel DL, Gur RE, Mozley PD, Richards J, Taleff MM, Heimberg C, Gallacher F, Gur RC. Volunteers for biomedical research. Recruitment and screening of normal controls. Arch Gen Psychiatry. 1991;48:1022–1025. doi: 10.1001/archpsyc.1991.01810350062010. [DOI] [PubMed] [Google Scholar]
- 53.Springer SP, Deutsch G. Left brain, right brain: perspectives from cognitive neuroscience, 5th Ed. Freeman; New York: 1998. [Google Scholar]
- 54.Steinmetz H, Staiger JF, Schlaug G, Huang Y, Jäncke L. Corpus callosum and brain volume in women and men. NeuroReport. 1995;6:1002–1004. doi: 10.1097/00001756-199505090-00013. [DOI] [PubMed] [Google Scholar]
- 55.Sternberg R, Berg C, editors. Intellectual development. Cambridge UP; New York: 1992. [Google Scholar]
- 56.Van Gorp WG, Satz P, Mitrushina M. Neuropsychological processes associated with normal aging. Dev Neuropsychol. 1990;6:279–290. [Google Scholar]
- 57.Wechsler D. Wechsler adult intelligence scale–revised manual. Psychological Corporation; New York: 1981. [Google Scholar]
- 58.Wegesin DJ. Event-related potentials in homosexual and heterosexual men and women: sex-dimorphic patterns in verbal asymmetries and mental rotation. Brain Cogn. 1998;36:73–92. doi: 10.1006/brcg.1997.0964. [DOI] [PubMed] [Google Scholar]
- 59.Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- 60.Witelson DF. Sex and the single hemisphere: specialization of the right hemisphere for spatial processing. Science. 1976;193:425–427. doi: 10.1126/science.935879. [DOI] [PubMed] [Google Scholar]
- 61.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 62.Witelson SF, Glezer, Kigar DL. Women have greater density of neurons in posterior temporal cortex. J Neurosci. 1995;15:3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan MXH, Karp JS. Segmentation of 3D MR using an adaptive K-means clustering algorithm. Proc IEEE Med Imaging Conf. 1994a;4:1529–1533. [Google Scholar]
- 64.Yan MXH, Karp JS. Image registration of MR and PET based on surface matching and principal axes fitting. Proc IEEE Med Imaging Conf. 1994b;4:1677–1681. [Google Scholar]
- 65.Yan MXH, Karp JS. Information processing in medical imaging. In: Bizais Y, Barillot C, DiPaol R, editors. Information processing in medical imaging. Kluwer Academic; Dordrecht, The Netherlands: 1995. pp. 201–213. [Google Scholar]