Abstract
After cessation of repeated, intermittent amphetamine, we detected an emergent Ca2+-dependent component of amphetamine-induced dopamine release and an increase in calmodulin and Ca2+- and calmodulin-dependent protein kinase activity in rat striatum. This study examined the involvement of calmodulin-dependent protein kinase II (CaM kinase II) and synaptic vesicles in the enhanced Ca2+-dependent dopamine release in response to amphetamine or K+ in rat striatum. Rats were pretreated for 5 d with 2.5 mg/kg amphetamine or saline and withdrawn from drug for 10 d. The selective CaM kinase II inhibitor KN-93 (1 μm), but not the inactive analog KN-92, attenuated the Ca2+-dependent amphetamine-mediated dopamine release from amphetamine-pretreated rats but had no effect in saline-pretreated controls. [3H]Dopamine uptake was unaltered by repeated amphetamine or KN-93 and was Ca2+independent. Striatal dopamine release stimulated by 50 mmKCl was enhanced twofold after repeated amphetamine compared with that in saline controls but was unaffected by KN-93. To examine the requirement for dopaminergic vesicles in the Ca2+-dependent dopamine release, we administered reserpine to saline- and amphetamine-pretreated rats 1 d before killing. Reserpine pretreatment did not affect amphetamine-mediated dopamine release from either pretreatment group but completely ablated K+-mediated dopamine release. Reserpine did not disrupt the ability of 1 μm KN-93 to block the Ca2+-dependent amphetamine-mediated dopamine release from amphetamine-pretreated rats. The results indicate that the enhanced dopamine release elicited by amphetamine from chronically treated rats is dependent on Ca2+- and calmodulin-dependent phosphorylation and is independent of vesicular dopamine storage. On the contrary, the enhanced depolarization-mediated vesicular dopamine release is independent of Ca2+- and calmodulin-dependent phosphorylation.
Keywords: repeated amphetamine, dopamine transport, reserpine, CaM kinase II, depolarization, phosphorylation
The catecholamine dopamine (DA) can be released from terminals and dendrites by exocytosis, a Ca2+- and synaptic vesicle-dependent process, or by a Ca2+-independent, plasmalemma transporter-mediated mechanism that is used by the psychostimulant amphetamines (AMPHs) (Raiteri et al., 1979). Many behavioral effects elicited by AMPH are attributed to its ability to release DA from presynaptic nerve terminals and to inhibit the reuptake of DA from the synapse (Seiden et al., 1993). DA release after acute administration of AMPH is Ca2+ independent; it relies on cytosolic DA and vesicular DA that permeates into the neuronal cytoplasm from synaptic vesicles (Chiueh and Moore, 1975; Raiteri et al., 1979; Jones et al., 1998). Repeated, intermittent treatment of rats with AMPH or cocaine leads to an enhanced release of DA from areas such as the striatum and nucleus accumbens in response to stimuli such as AMPH or depolarization (Robinson and Becker, 1982; Robinson et al., 1985; Castañeda et al., 1988; Kolta et al., 1989; Wolf et al., 1993; Pierce and Kalivas, 1997a). The enhanced component of the AMPH-induced DA release after repeated AMPH or cocaine is distinguished from that released in nontreated rats by its Ca2+ dependency (Warburton et al., 1996; Iwata et al., 1997; Pierce and Kalivas, 1997a). We have identified additional Ca2+-related activities in rat striatum that develop after repeated, intermittent AMPH. After cessation of repeated AMPH, we found an increase in the Ca2+-binding protein calmodulin (CaM) and in the activity of Ca2+- and CaM-dependent protein kinase II (CaM kinase II) in striatal synaptosomes as well as an increase in the phosphorylated state of the vesicular protein synapsin I at the CaM kinase II substrate site (Iwata et al., 1996, 1997). Both CaM kinase II and synapsin I are involved in neurotransmitter release; CaM kinase II enhanced Ca2+-dependent glutamate release from nerve endings purportedly via an enhanced phosphorylation of synapsin I (Llinas et al., 1991; Nichols et al., 1992). Inhibitors of CaM kinase II blocked a Ca2+-dependent component of amphetamine-mediated DA release in the nucleus accumbens of rats treated with repeated cocaine (Pierce and Kalivas, 1997a) and the sensitized locomotor behavior (Pierce et al., 1998).
The goal of the present study was to examine the role of CaM kinase II in the Ca2+-dependent component of DA release in rat striatum after repeated AMPH in response to a challenge with AMPH. We also investigated whether the enhanced depolarization-mediated DA release after repeated, intermittent AMPH was dependent on CaM kinase II. The source of the releasable pool of DA affected by an AMPH challenge after a repeated AMPH treatment is unknown. Synaptic vesicles could be integral to DA release by both stimuli because DA release in response to both depolarization and AMPH is enhanced after repeated AMPH. To determine whether synaptic vesicles were a source of DA for the enhanced DA release after repeated AMPH, we examined whether reserpine treatment, which depletes vesicular DA, would abolish the amphetamine-mediated Ca2+-dependent DA release elicited by the repeated AMPH.
MATERIALS AND METHODS
Repeated AMPH regimen. Female Holtzman rats (179–190 gm; Harlan Sprague Dawley, Indianapolis, IN) were given intraperitoneal injections of AMPH (2.5 mg/kg) or saline once a day for 5 d. Saline- and AMPH-pretreated rats were killed 10 d after the last injection. This treatment was chosen because it produces a behavioral sensitization in response to a challenge dose of AMPH (Robinson and Becker, 1982).
Striatal slice preparation. Rats were killed by decapitation, and the striatum was removed and dissected on ice using a brain-cutting block as described (Heffner et al., 1980). The striatal tissue of each rat was divided into 1 mm3 pieces and placed immediately into ice-cold Krebs’ Ringer buffer (KRB) containing 125 mm NaCl, 2.7 mm KCl, 1 mmMgCl2, 1.2 mm CaCl2, 1.2 mm KH2PO4, 10 mm glucose, 24.9 mm NaHCO3, 0.25 mm ascorbic acid, and 10 μm pargyline. The buffer was oxygenated with 95% O2/5% CO2 for 1 hr, with pH at 7.4.
DA release assay. Striatal slices were weighed and placed into a designated chamber of a Brandel SF-12 superfusion apparatus (Gaithersburg, MD) onto a Whatman GF/B glass filter (Maidstone, UK). Superfusion chambers were maintained at 37°C, and medium was perfused through the chambers at 100 μl/min. Samples were collected at 5 min intervals. All chambers were perfused with KRB or drug for 30 min, followed by a 2.5 min bolus of the following drug combinations: 1 μm AMPH, 1 μm AMPH + 10 μmKN-93, 10 μm KN-93 alone, and KRB as control. The bolus of AMPH was given at fraction 7. Analysis of the time to travel through the tubing demonstrated that the bolus would arrive at the tissue at fraction 9. In some experiments, KN-92 was substituted for KN-93. Experiments measuring depolarization contained 50 mm KCl or 50 mm KCl + 1 μm KN-93. In these experiments, Na+ was commensurately lowered in the KRB to maintain osmolarity. The bolus drug combinations were replaced with fresh KRB or with KN-93 and/or KN-92, and sample collection continued for 40 more minutes. Samples were collected into vials containing 25 μl of internal standard solution [0.05N HClO4, 4.55 mm dihydroxybenzylamine (DHBA), 1 m metabisulfate, and 0.1 m EDTA]. Samples were stored at −70°C until analysis. The DA content of the samples was analyzed by HPLC with electrochemical detection using DHBA as an internal standard (Becker et al., 1984). Statistical significance was determined using one-way ANOVA with a post-test Tukey–Kramer multiple comparison analysis or by Student’s t test.
Reserpine treatment and total DA measurement. Female Holtzman rats were pretreated with repeated AMPH or saline as discussed above. On day 9 after the last dose of drug, rats were injected subcutaneously with 5 mg/kg reserpine or vehicle (1.5% glacial acetic acid) and killed 18 hr later. DA was measured in each striatum to determine the degree of depletion and was, as we found previously (Kantor and Gnegy, 1998), depleted 95% in both saline- and AMPH-pretreated rats.
[3H]DA uptake. Striatal slices from AMPH- and saline-pretreated rats were weighed, placed into vials containing KRB with and without 1.2 mm CaCl2 at 37°C for 5 min, and incubated for 20 more minutes in the presence or absence of 10 μm KN-93, 10 μm nomifensine, or 5 μm GBR-12935. After preincubation, [3H]DA (18.3 Ci/mmol) was added to a concentration of 33 nm, and the incubation proceeded for an additional 5 min. The reaction was terminated by the addition of 10 ml of ice-cold saline followed by filtration on GF/C filters (Whatman) and two more saline washes. Filters were counted in ScintiVerse BD in a Beckman LS 5800 scintillation counter. Data were adjusted for the weight of the slices.
Drugs. AMPH was obtained from the University of Michigan Laboratory of Animal Medicine (Ann Arbor, MI). HPLC-grade chemicals were purchased from American Bioanalytical (Natick, MA). KN-93, KN-92, KN-62, and GBR-12935 were purchased from Calbiochem (La Jolla, CA), and reserpine was obtained from Research Biochemicals (Natick, MA). Nomifensine was the generous gift of Dr. James Woods (Department of Pharmacology, University of Michigan, Ann Arbor, MI).
RESULTS
Effect of AMPH challenge on DA release from pretreated rats
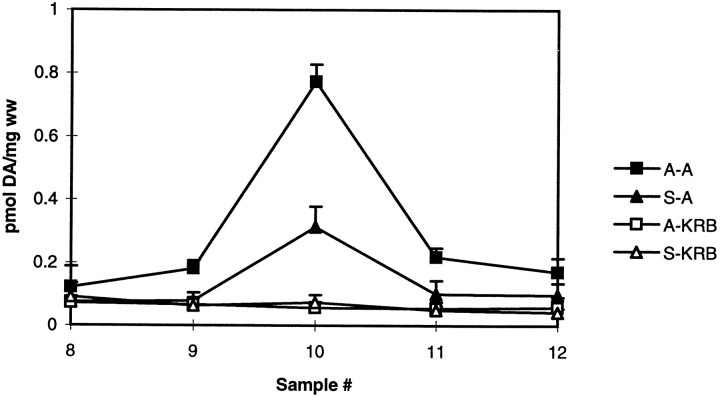
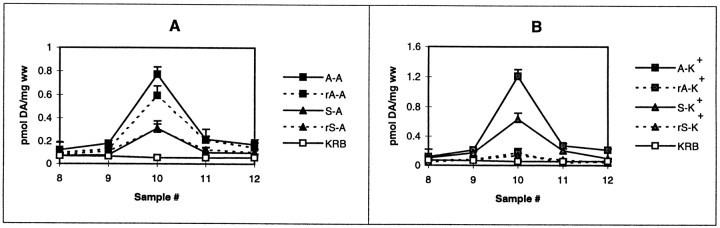
The endogenous DA released in response to 1 μm AMPH was measured in striatal slices 10 d after the last pretreatment dose of saline or AMPH (2.5 mg/kg per day for 5 d). As shown in Figure 1, AMPH-mediated DA release from striatal slices was twofold greater from AMPH-pretreated rats than from saline-pretreated rats. Baseline values did not differ.
Fig. 1.
AMPH-mediated DA release in AMPH- and saline-pretreated rats. Female Holtzman rats were pretreated with saline (triangles) or AMPH (squares) as described in Materials and Methods. DA release from the striatal slices was measured in response to AMPH (closed symbols) or KRB (open symbols) as described in Materials and Methods and reported as picomoles of DA per milligram wet weight (ww) ± SEM. Drugs given as a 2.5 min bolus at fraction 7 will reach the slices at fraction 9 (n = 3).A–A and S–A represent DA release from AMPH (A)- and saline (S)-pretreated rats after perfusion with 1 μm AMPH. A–KRB and S–KRBindicate basal DA levels from striatal slices from A- and S-pretreated rats. By the use of ANOVA to compare values for fraction 10, the peak DA responses differ (p < 0.0001). In post hocTukey–Kramer tests, S–KRB and A–KRBdiffer from S–A at p < 0.05 andA–A at p < 0.001.S–A differs from A–A atp < 0.001.
Effect of KN-93 and KN-92 on AMPH-mediated DA release in pretreated rats
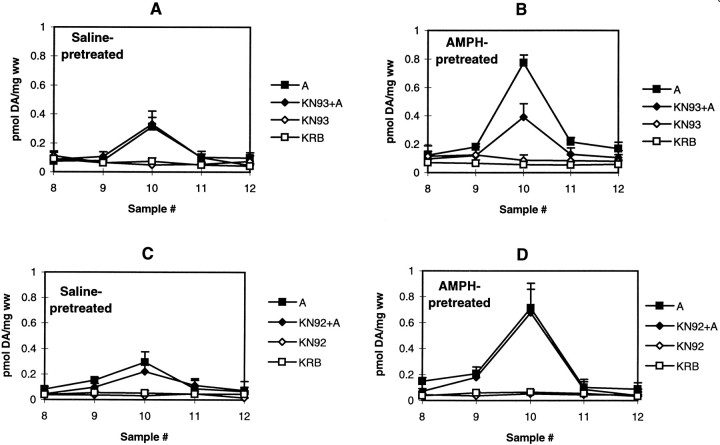
The effect of the selective CaM kinase II inhibitor KN-93 and its inactive analog KN-92 on AMPH-induced DA release in striatal slices from saline- and AMPH-pretreated rats was examined. At 10 μm, KN-93 had no effect on either basal DA levels or AMPH-mediated DA release in saline-pretreated rats (Fig.2A). KN-93, however, did significantly inhibit AMPH-induced DA release in AMPH-pretreated rats (Fig. 2B). The degree of inhibition was ∼50%; the amount of remaining DA released was equal to the level of AMPH-induced DA release in slices from saline-pretreated rats. At 10 μm, KN-92, the inactive analog of KN-93, had no effect on basal levels or DA released by 1 μm AMPH in either AMPH- or saline-pretreated rats (Fig. 2C,D). The same results were seen when striatal tissues were pretreated with a different inhibitor of CaM kinase II, KN-62. In striatal slices from saline-pretreated rats, values for peak DA released in response to 1 μm AMPH in the absence and presence of 5 μmKN-62 are 0.34 and 0.30 pmol/mg wet weight, respectively. In AMPH-pretreated rats, values for DA released in response to 1 μm AMPH in the absence and presence of 5 μmKN-62 are 0.93 and 0.44 pmol/mg wet weight, respectively. Values are the average from two experiments whose values did not differ >10%.
Fig. 2.
The effect of KN-93 and KN-92 on AMPH-mediated DA release in AMPH- and saline-pretreated rats. Rats were given repeated saline (A, C) or AMPH (B,D), and striatal slices were analyzed for DA release as described in Materials and Methods. DA release in striatal slices was measured in response to AMPH (closed symbols) or KRB (open symbols) in the absence (squares) or presence (diamonds) of 10 μm KN-93 or 10 μm KN-92. Results are reported as picomoles of DA per milligram wet weight (ww) ± SEM (n= 3). A, C, The effect of KN-93 or KN-92 on basal and AMPH-mediated DA release in saline-pretreated rats.B, D, The effect of KN-93 or KN-92 on AMPH-mediated DA release from AMPH-pretreated rats. Statistical analyses were performed using a one-way ANOVA with Tukey–Kramerpost hoc analysis on the peak AMPH response, fraction 10 (n = 3). A, C, ANOVA,p = 0.01; p < 0.05 for AMPH (A) compared with KN-93 (KN93) or KN-92 (KN92) alone and KRB.B, ANOVA, p < 0.0001;p < 0.001 for KRB andKN93 alone compared with A;p < 0.05 for KRB andKN93 alone compared with KN93+A; andp < 0.01 for A compared withKN93+A. D, ANOVA, p< 0.001; p < 0.01 for KRB andKN92 alone compared with A andKN92+A.
Depolarization-mediated DA release and the effect of KN-93 after repeated AMPH
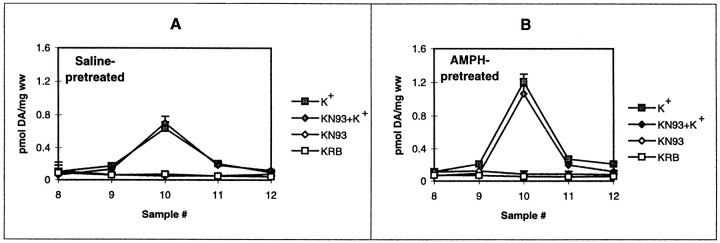
Castañeda et al. (1988) demonstrated that electrical- and depolarization-mediated DA release was enhanced in rats treated with a sensitizing regimen of repeated AMPH that differed from this regimen. To ensure that there was enhanced depolarization-mediated DA release in our regimen, we perfused striatal slices from saline- and AMPH-pretreated rats with 50 mm KCl. As shown in Figure3, depolarization-mediated DA release was increased twofold after withdrawal from the repeated AMPH regimen compared with that in saline controls. However, the K+-mediated DA release was not altered by KN-93 (Fig.4A,B) or KN-92 (data not shown) alone in striatal slices from either saline- or AMPH-pretreated rats.
Fig. 3.
K+-mediated DA release from AMPH- and saline-pretreated rats. Rats were given repeated saline (triangles) or AMPH (squares) as described in Materials and Methods. DA release from the striatal slices was measured in response to 50 mm KCl (closed symbols) or KRB (open symbols) as described in Materials and Methods and was reported as picomoles of DA per milligram wet weight (ww) ± SEM.A–K+ andS–K+ represent AMPH (A)- and saline (S)-pretreated rats given a bolus of 50 mm KCl, whereas A–KRB andS–KRB indicate basal DA levels from striatal slices from A- and S-pretreated rats. In comparing the peak K+ response,p < 0.03 forA–K+ compared withS–K+ (Student’s ttest; n = 6).
Fig. 4.
The effect of KN-93 on K+-mediated DA release from AMPH- and saline-pretreated rats. Rats were given repeated saline (A) or AMPH (B) as described in Materials and Methods. DA release from the striatal slices was measured in response to 50 mm KCl (closed symbols) or KRB (open symbols) in the absence (squares) or presence (diamonds) of 10 μm KN-93. Results are reported as picomoles of DA per milligram wet weight (ww) ± SEM (n= 3). Statistical analyses were performed using a one-way ANOVA with Tukey–Kramer post hoc analysis on the peak AMPH response, fraction 10. A, ANOVA, p< 0.03; all values for K+ differed from those for KRB at p < 0.05. B, ANOVA,p < 0.002; values for K+differed from those for KRB at p < 0.01. In neither A nor B did values with KN-93 (KN93) differ from their respective values without KN-93.
Effect of the CaM kinase II inhibitor on [3H]DA uptake
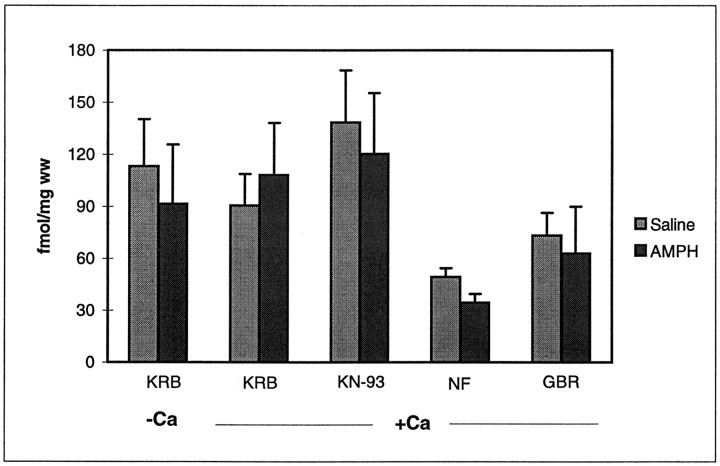
Because KN-93 could be inhibiting AMPH action by blocking its uptake, we assessed its effect on the uptake of [3H]DA in striatal slices from rats pretreated with saline or AMPH. Experiments were performed in the presence and absence of Ca2+ to determine whether there was a Ca2+-dependent component of [3H]DA uptake after repeated AMPH. In contrast to the Ca2+ dependency of transporter-mediated DA release after repeated AMPH, striatal DA uptake was Ca2+ independent in both AMPH- and saline-pretreated rats (Fig. 5). KN-93 did not significantly affect DA uptake in the striatum from either saline- or AMPH-pretreated rats. Nomifensine and GBR-12935, which are dopamine transporter blockers, inhibited [3H]DA uptake into striatal slices equally well from either AMPH- and saline-pretreated animals.
Fig. 5.
The effect of Ca2+ and KN-93 on [H3]DA uptake in AMPH- and saline-pretreated rats. Striatal slices from saline (light gray)- and AMPH (dark gray)-pretreated rats were incubated as indicated. Values in the left-hand set ofbars are those obtained when striata were incubated in KRB lacking CaCl2. All other samples were incubated in Ca2+-containing KRB. [3H]DA uptake was measured as described in Materials and Methods. In some experiments, slices were pretreated for 15 min with 10 μmKN-93, 10 μm nomifensine (NF), or 5 μm GBR-12935 (GBR). The results are expressed as femtomoles of DA per milligram wet weight (ww) of slices. Results represent the average ± SEM of three separate experiments. All values with nomifensine and GBR-12935 differed from values without these drugs atp < 0.05.
Effect of reserpine on AMPH-mediated DA release after repeated AMPH
Although KN-93 had no effect on depolarization-mediated DA release, the Ca2+ dependency of both the enhanced AMPH-mediated and the depolarization-mediated DA release suggested that synaptic vesicles could be involved in the increased response to both stimuli. To test for a requirement for synaptic vesicles in the enhanced stimulus-induced DA release after repeated AMPH, we pretreated rats with saline or AMPH and gave the rats reserpine (5 mg/kg, s.c.) or vehicle 9 d after the last treatment. There were thus four groups of rats differing in repeated treatment and injection type on day 9: saline-pretreated rats given vehicle on day 9 (Fig.6, S), saline-pretreated rats given reserpine on day 9 (rS), AMPH-pretreated rats given vehicle on day 9 (A), and AMPH-pretreated rats given reserpine on day 9 (rA). Rats were killed the next day, which was day 10 after the last drug injection, and DA release in response to either AMPH or K+ was measured in the striatal slices. The amount of DA released in response to 1 μm AMPH is shown in Figure 6A. Reserpine treatment on day 9 had no effect on endogenous DA release in response to 1 μm AMPH in saline-pretreated rats, as we have seen previously (Kantor and Gnegy, 1998). As shown in Figure6A, reserpine given on day 9 similarly had no significant effect on AMPH-mediated DA release in AMPH-pretreated rats. The controls (KRB alone in the perfusion) for all groups gave the same values and were treated as one line on Figure6A to avoid visual clutter. The effect of reserpine on K+-mediated DA release after repeated saline or AMPH is shown in Figure 6B. As expected, reserpine pretreatment abolished K+-mediated DA release in slices from rats repeatedly treated with either saline or AMPH.
Fig. 6.
The effect of reserpine on AMPH- and K+-mediated DA release in saline- and AMPH-pretreated rats. Rats received repeated saline or AMPH as described in Materials and Methods. On the ninth day after cessation of repeated saline or AMPH, rats were treated with either vehicle or reserpine (5 mg/kg, s.c.) such that four groups were formed: saline–vehicle (S), saline–reserpine (rS), AMPH–vehicle (A), and AMPH–reserpine (rA). Striatal slices were prepared from rats of each group, and DA release was measured in response to AMPH (A), 50 mm KCl (K+; B), or continued KRB (basal release; open square). KRB values in Aand B comprise the average of basal DA release data from saline, AMPH, and reserpine pretreatment that were all the same. Results are reported as picomoles of DA per milligram wet weight (ww) ± SEM (n = 6). Statistical analyses were performed using a one-way ANOVA with Tukey–Kramerpost hoc analysis on the peak AMPH response, fraction 10 (n = 3). A, DA release in response to AMPH (filled symbols) in saline- or AMPH-pretreated rats given vehicle (solid lines) or reserpine (dashed lines) the day before being killed (ANOVA, p < 0.0001). In post hocTukey–Kramer analysis, all values differed from KRB atp < 0.01, and all saline pretreatments (S–A and rS–A) differed from all AMPH pretreatments (A–A and rA–A) atp < 0.01. No group with reserpine pretreatment differed from the corresponding group without reserpine pretreatment (S–A vs rS–A) and (A–Avs rA–A). B, DA release in response to K+ (filled symbols) in saline- or AMPH-pretreated rats given vehicle (solid lines) or reserpine (dashed lines) the day before being killed (ANOVA, p < 0.0001). In post hocTukey–Kramer analysis, values from vehicle-treated rats (S–K+ andA–K+) differed from KRB values atp < 0.001 and from K+ values for reserpine-treated rats at p < 0.01. Values from reserpine-pretreated rats did not differ from KRB values (baseline).
Effect of reserpine treatment on KN-93 inhibition of AMPH-mediated DA release in pretreated rats
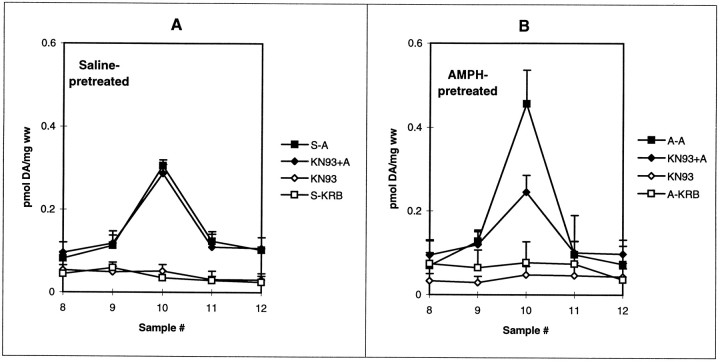
To help delineate the site of action of KN-93 in inhibiting striatal AMPH-mediated DA release in AMPH-pretreated rats, we examined the effect of reserpine on the KN-93 inhibitory action. Because the vesicles were obviously depleted by reserpine (Fig.6B), a lack of effect of KN-93 in reserpine-pretreated rats would suggest that the drug was acting on CaM kinase II on the synaptic vesicles. As shown in Figure2A, 10 μm KN-93 had no effect on AMPH-mediated DA release from saline-pretreated rats. Reserpine given on day 9 did not alter that result (Fig.7A). Similarly, the ability of 10 μm KN-93 to inhibit the enhanced component of AMPH-mediated DA release in AMPH-pretreated rats was unaltered by reserpine given on day 9 (Fig. 7B). KN-93 (10 μm) did not affect basal DA levels under any treatment condition.
Fig. 7.
The effect of KN-93 on AMPH-mediated DA release in rats treated with reserpine after previous saline and AMPH pretreatment. Rats received repeated injections of saline (A) or AMPH (B) and were treated with reserpine on withdrawal day 9 as described in Materials and Methods and the legend to Figure 6. DA release in striatal slices was measured in response to AMPH (closed symbols) or KRB (open symbols) in the absence (squares) or presence (diamonds) of 10 μm KN-93. Results are reported as picomoles of DA per milligram wet weight (ww) ± SEM (n = 3). Statistical analyses were performed using a one-way ANOVA with Tukey–Kramerpost hoc analysis on the peak AMPH response, fraction 10. A, The effect of KN-93 on basal and AMPH (A)-mediated DA release in saline (S)- and reserpine-pretreated rats. ANOVA,p < 0.0001; values with AMPH differed from all values without AMPH at p < 0.001; values with KN-93 (KN93) were no different from that of the comparable control. B, The effect of KN-93 on AMPH-mediated DA release from AMPH- and reserpine-pretreated rats. ANOVA, p < 0.0001; all values with amphetamine were different from those without AMPH at p < 0.01; value for AMPH + KN-93 differed from that for AMPH alone (KN93+A vs A) at p < 0.01.
Protein kinase C inhibitors attenuated AMPH- but not K+-stimulated DA release in saline- and AMPH-pretreated rats
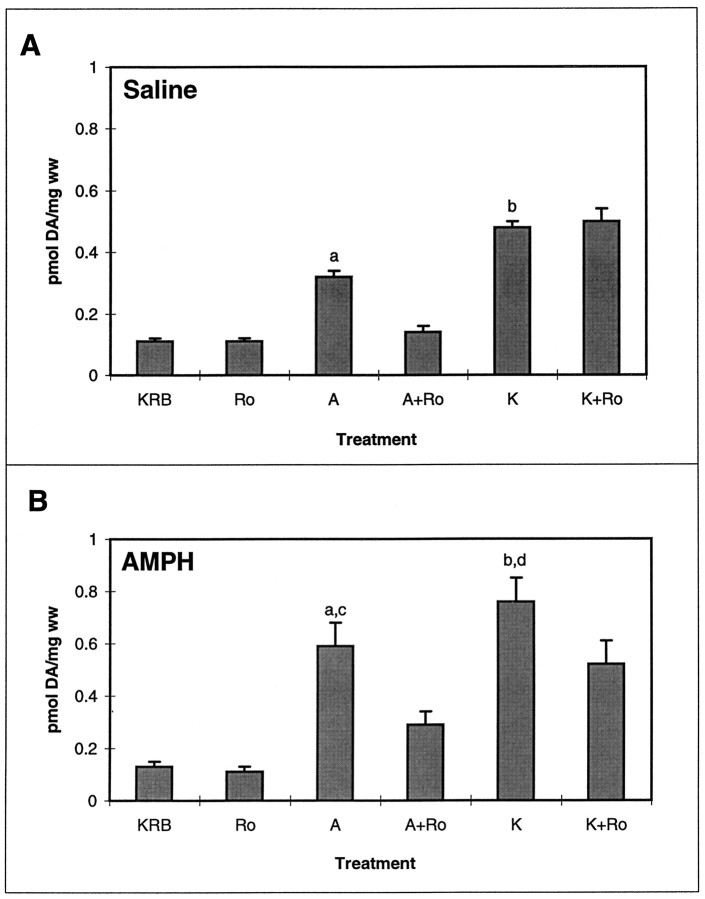
We have shown previously that inhibitors of protein kinase C (PKC) completely inhibit AMPH-mediated DA release in the rat striatum and nucleus accumbens independently of extracellular calcium (Kantor and Gnegy, 1998; Browman et al., 1999). The specificity of the CaM kinase II inhibition of AMPH-mediated DA release was examined by determining whether PKC inhibition attenuated DA release mediated by AMPH or K+ in AMPH-pretreated rats. Striatal slices from rats pretreated with saline or AMPH were perfused with 1 μm Ro31-8220 for 30 min before the bolus of AMPH or KCl. As shown in Figure8A, Ro31-8220 treatment completely blocked AMPH-mediated DA release in striatal slices from saline-pretreated rats but had only a partial effect in AMPH-pretreated rats (Fig. 8B). On the contrary, Ro31-8220 perfusion had no effect on K+-stimulated DA release in either saline- or AMPH-pretreated rats. There appeared to be a small degree of inhibition in AMPH-pretreated rats, but there was no significant difference between K+-stimulated values in the absence or presence of Ro31-8220. When calculated as fold stimulation over control, the values in AMPH-pretreated rats for K+ and K+ + Ro31-8220 samples were 5.9 ± 1.0 and 5.1 ± 1.2, respectively, again showing no significant difference.
Fig. 8.
Effect of the PKC inhibitor Ro31-8220 on AMPH- and K+-mediated DA release in striatal slices from saline (A)- and AMPH (B)-pretreated rats. Striatal slices were incubated with 1 μm Ro31-8220 for 30 min before being given a 2.5 min bolus of KRB, 1 μm Ro31-8220 (Ro), 1 μm AMPH (A), 1 μm AMPH + 1 μm Ro31-8220 (A+Ro), 50 mm KCl (K), or 50 mm KCl + 1 μm Ro31-8220 (K+Ro). Results are presented as picomoles of DA per milligram wet weight (ww) ± SEM of the peak fraction containing maximal DA (n = 3). A, Effect of Ro31-8220 on DA release in saline-pretreated rats. ANOVA for AMPH-mediated DA release and controls, p < 0.0001.a, In post hoc Tukey–Kramer tests,A differs from KRB, Ro, and A+Ro samples at p < 0.01. ANOVA for K+-mediated DA release and controls,p < 0.0001. b, Kdiffers from KRB and Ro samples atp < 0.001. B, Effect of Ro31-8220 on DA release in AMPH-pretreated rats. ANOVA for AMPH-mediated DA release and controls, p < 0.0008.a, In post hoc Tukey–Kramer tests,A differs from KRB and Roat p < 0.01 and from A+Ro atp < 0.05. ANOVA for K+-mediated DA release and controls, p < 0.0003.b, K differs from KRB andRo at p < 0.001 and fromK+Ro at p < 0.01.KRB differs from K+Ro atp < 0.05. c, In comparison of saline pretreatment (A) versus AMPH pretreatment (B), saline–AMPH samples differ from AMPH–AMPH samples at p < 0.02 by Student’s ttest. d, Saline–K+ samples differ from AMPH–K+ samples at p < 0.01 by Student’s t test.
DISCUSSION
Our results demonstrate that, after reexposure to AMPH, the striatum from rats treated repeatedly with AMPH displayed a DA release that was twofold higher than that from saline-treated rats. The increase in released DA was Ca2+ dependent, and the involvement of a Ca2+- and CaM-dependent protein phosphorylation was demonstrated by the ability of KN-93 to inhibit the Ca2+-dependent component. Functioning synaptic vesicles seemed not to be integral to the enhanced AMPH-mediated DA release. The twofold enhancement in K+-induced DA release after repeated AMPH, although Ca2+ and vesicular dependent, differed from the AMPH-mediated release in that it was unaffected by KN-93. Similarly, there is a component of PKC dependence in the AMPH-mediated DA release that is not reflected in K+-stimulated DA release. The results concerning AMPH-mediated DA release correspond to those of Pierce and Kalivas (1997a) who, using microdialysis, demonstrated a Ca2+-dependent enhancement in AMPH-induced DA release in the nucleus accumbens of rats treated repeatedly with cocaine. Ca2+ entry into the terminal and activation of Ca2+- and CaM-dependent protein kinase activity were crucial to the enhanced AMPH-induced DA release, but exocytosis was not. Consequently, the enhancement in AMPH-induced DA release after repeated AMPH or cocaine may involve similar mechanisms.
Vesicular involvement in enhanced AMPH-mediated DA release
It has been postulated that the enhancement in AMPH-induced release in cocaine- or AMPH-pretreated rats could be caused by a redistribution of DA-containing synaptic vesicles that might position them more closely in apposition with plasmalemmal DA transporters (Robinson, 1991; Pierce and Kalivas, 1997b). “Leakage” or increased transport of DA from the vesicle could serve as the source of the enhanced DA. In support of that hypothesis, we found that repeated AMPH leads to a long-lasting elevation in site 3 phosphosynapsin I in rat striatum (Iwata et al., 1996, 1997). Dephosphosynapsin I mediates the formation of ternary complexes with synaptic vesicle and actin filaments. Phosphorylation of synapsin I at sites 2 and 3, substrate sites for CaM kinase II, abolishes those interactions. Because synapsin I is involved in maintaining a clustered, reserve pool of synaptic vesicles used during high synaptic activity (Pieribone et al., 1995), it is conceivable that disruption of this pool could lead to a wider distribution of vesicles that could enhance the supply of DA to be released in response to AMPH. Another role for vesicles could involve a direct interaction of AMPH with the vesicular monoamine transporter VMAT2 that could increase the availability of DA at the plasmalemma transporter. Because CaM kinase II is located on vesicles, as well as in the cytoplasm (Dunkley et al., 1988; Benfenati et al., 1992), vesicular CaM kinase II could be altering AMPH action at the vesicles.
Our studies, however, suggest that synaptic vesicles do not provide the source of DA for the enhanced AMPH response after repeated AMPH. Reserpine pretreatment completely abolished K+-stimulated DA release but did not affect AMPH-stimulated DA release in the striatum of rats pretreated with either saline or AMPH. In addition, reserpine pretreatment did not alter the ability of KN-93 to inhibit the Ca2+-dependent enhancement in AMPH-induced DA release. Although it is possible that the reserpine treatment did not deplete all of the vesicles, one might expect that the responses would have been diminished if vesicles were strongly involved in these effects. Reserpine completely ablated [3H]tetrabenazine binding to VMAT2 in rat striatum 1 d after injection, suggesting that vesicles are not functional (Naudon et al., 1995). Therefore, the synaptic vesicles do not seem to be the source of the increased DA released by AMPH through the transporter. The possibility that a vesicular CaM kinase II or a vesicular substrate for the enzyme is important for the process independent of active VMAT2 cannot be discounted, however. Our conclusion is predicated on the use of a relative low dose of AMPH, 1 μm, as our challenge dose. There is increasing vesicular involvement in AMPH-mediated DA release as the dose of AMPH is increased (Seiden et al., 1993). Had we used higher doses of AMPH, such as 100 μm, at which vesicles play a prominent role in the amount of DA released (Sulzer et al., 1995), no doubt a vesicular involvement would have been detected. However, we have demonstrated a sensitized DA release in response to 1 μmAMPH that remained unaltered by pretreatment with reserpine. Therefore the enhanced AMPH-mediated DA release after repeated AMPH is not dependent on functioning synaptic vesicles.
Role of CaM kinase II in enhanced AMPH-mediated DA release
The most parsimonious conclusion to explain the enhanced AMPH-induced DA release is that intraterminal CaM kinase II activity is maintaining phosphorylation of a substrate that enhances outward transport. We demonstrated that CaM kinase II activity is enhanced in striatal synaptosomes after repeated AMPH and that enhanced AMPH-induced DA release can be demonstrated in the synaptosomes (Iwata et al., 1997). The CaM kinase II activity is likely ongoing because the inhibitor KN-93 was readily effective. The nature of the substrate is unknown. It could be an ion channel or cytoskeletal protein affecting transporter function, or it could be the plasmalemmal DA transporter itself. The DA transporter from both rat and human contains a consensus sequence for CaM kinase II (Giros and Caron, 1993). Whatever the substrate, the modification is such that it affects primarily outward-directed transporter function. Because there is no change in basal DA release, the modification is unlikely to involve a global alteration in ion gradients such as that an inhibition of Na+,K+-ATPase would provide but could involve an AMPH-mediated effect on an ion gradient. Interestingly, ouabain-stimulated DA release from rat striatum is also enhanced after repeated AMPH (Kanzaki et al., 1992). The modification leading to the enhanced transporter-mediated DA release did not alter all transporter functions. We and others (Kuczenski and Segal, 1988) have found no change in DA uptake after repeated AMPH, showing that inward transport was unaltered. Ca2+ did not affect uptake in either saline- or AMPH-pretreated rats. This is in contrast to the report of Uchikawa et al. (1995) who found that Ca2+ and CaM increased [3H]DA uptake in naive rats. We also found that neither Ca2+ (data not shown) nor repeated AMPH altered the ability of maximal concentrations of the uptake blockers nomifensine and GBR-12935 to inhibit DA uptake. In microdialysis studies, Pierce and Kalivas (1997a) reported that levels of extraterminal DA in response to GBR-12909 were enhanced after repeated cocaine. This could be caused by the increase in exocytotic, impulse-dependent DA release occurring after repeated AMPH rather than by an altered effect of GBR-12909 on the DA transporter.
It is possible for a modification to affect outward transport through the plasmalemmal DA transporter without substantively altering inward transport. We found that selective inhibitors of protein kinase C blocked AMPH-mediated DA release in the striatum and nucleus accumbens from naive animals (Kantor and Gnegy, 1998; Browman et al., 1999) but slightly increased [3H]DA uptake (Kantor and Gnegy, 1998). Although the selective PKC inhibitor Ro31–8220 completely blocked AMPH-mediated DA release from naive (Kantor and Gnegy, 1998; Browman et al., 1999) and saline-pretreated rats (this study), this inhibitor only partially attenuated AMPH-mediated DA release from AMPH-pretreated rats. Inhibition of AMPH-induced DA release by the PKC inhibitors is entirely independent of extracellular calcium (Kantor and Gnegy, 1998). Thus the Ca2+-sensitive and -insensitive phases of AMPH-induced DA release in AMPH-pretreated rats seem to be separable and controlled by different protein kinase mechanisms.
Enhanced K+-mediated DA release
Repeated AMPH also led to an increase in depolarization-mediated, vesicular-dependent exocytotic release (this study) (Castañeda et al., 1988). Possible explanations for an increase in K+-evoked DA release are (1) movement of vesicles from a reserve to a more readily releasable pool, (2) an increase in the number of vesicles, (3) alteration in a plasmalemmal or vesicular membrane protein that heightens exocytosis, or (4) altered Ca2+ channel activity leading to increased intracellular Ca2+. The increase we reported in site 3 phosphosynapsin I (Iwata et al., 1996) could conceivably contribute to an increase in the readily releasable vesicular pool because it could decrease the attachment of vesicles to a confining cytoskeleton. On the other hand, KN-93 did not block K+-stimulated DA release, suggesting that ongoing Ca2+ and CaM protein phosphorylation does not modulate the depolarization-enhanced release. It is unlikely that the total number of vesicles increased because we found no change in total synapsin I (Iwata et al., 1996), which seems to be a component of every vesicle, including dopaminergic vesicles, in the striatum (Greengard et al., 1994). Although the increase in site 3 phosphosynapsin I could contribute to increased exocytotic release of DA, it is unclear whether the increase is in dopaminergic vesicles. A more probable option is that there is an increase in Ca2+ availability through Ca2+ channels that could lead to increased exocytotic release. Our results further suggest that ongoing PKC activity is not instrumental in mediating enhanced K+-induced DA release after repeated AMPH.
Conclusion
We have demonstrated that repeated AMPH treatment leads to an increase in DA released in response to AMPH and K+depolarization. Although the increased DA released in response to both stimuli was Ca2+ dependent, our experiments suggested that a Ca2+- and CaM-dependent phosphorylation was critical for the enhanced release by AMPH but not for that released by depolarization. On the contrary, the depolarization-mediated DA release was entirely vesicular, whereas vesicles were not required for the AMPH-mediated release. The results suggest that a CaM-dependent phosphorylation of a protein, perhaps the plasmalemma DA transporter itself or a membrane protein affecting the transporter function, could be responsible for the enhanced AMPH-induced release.
Footnotes
This work was supported by the National Institute on Drug Abuse (NIDA) Grant DA-05066 and NIDA Interdisciplinary Training Grant DA-07267 at the University of Michigan Substance Abuse Research Center (L.K.). We thank Terry Robinson for the use of his HPLC and for helpful conversations.
Correspondence should be addressed to Dr. Margaret E. Gnegy, Department of Pharmacology, 2220E MSRB III, University of Michigan Medical School, Ann Arbor, MI 48109-0634.
REFERENCES
- 1.Becker JB, Castaneda E, Robinson TE, Beer ME. A simple in vitro technique to measure the release of endogenous dopamine and dihydroxyphenylacetic acid from striatal tissue using high performance liquid chromatography with electrochemical detection. J Neurosci Methods. 1984;11:19–28. doi: 10.1016/0165-0270(84)90004-9. [DOI] [PubMed] [Google Scholar]
- 2.Benfenati F, Valtorta F, Rubenstein JL, Gorelick FS, Greengard P, Czernik AJ. Synaptic vesicle-associated Ca2+/calmodulin-dependent protein kinase II is a binding protein for synapsin I. Nature. 1992;359:417–420. doi: 10.1038/359417a0. [DOI] [PubMed] [Google Scholar]
- 3.Browman KE, Kantor L, Richardson S, Badiani A, Robinson TE, Gnegy ME. Injection of the protein kinase C inhibitor Ro31-8220 into the nucleus accumbens attenuates the acute response to amphetamine: tissue and behavioral studies. Brain Res. 1999;814:112–119. doi: 10.1016/s0006-8993(98)01040-3. [DOI] [PubMed] [Google Scholar]
- 4.Castañeda E, Becker JB, Robinson TE. The long-term effects of repeated amphetamine treatment in vivo on amphetamine, KCl and electrical stimulation evoked striatal dopamine release in vitro. Life Sci. 1988;42:2447–2456. doi: 10.1016/0024-3205(88)90343-8. [DOI] [PubMed] [Google Scholar]
- 5.Chiueh CC, Moore KE. d-Amphetamine-induced release of “newly synthesized” and “stored” dopamine from the caudate nucleus in vivo. J Pharmacol Exp Ther. 1975;192:642–653. [PubMed] [Google Scholar]
- 6.Dunkley PR, Jarvie PE, Rostas JAP. Distribution of calmodulin- and cyclic AMP-stimulated protein kinases in synaptosomes. J Neurochem. 1988;51:57–68. doi: 10.1111/j.1471-4159.1988.tb04835.x. [DOI] [PubMed] [Google Scholar]
- 7.Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 8.Greengard P, Benfenati F, Valtorta F. Synapsin I, an actin-binding protein regulating synaptic vesicle traffic in the nerve terminal. Adv Second Messenger Phosphoprotein Res. 1994;29:31–46. doi: 10.1016/s1040-7952(06)80005-4. [DOI] [PubMed] [Google Scholar]
- 9.Heffner TG, Hartman JA, Seiden LS. A rapid method for the regional dissection of the rat brain. Pharmacol Biochem Behav. 1980;13:453–456. doi: 10.1016/0091-3057(80)90254-3. [DOI] [PubMed] [Google Scholar]
- 10.Iwata S, Hewlett GHK, Ferrell ST, Czernik AJ, Meiri KF, Gnegy ME. Increased in vivo phosphorylation of neuromodulin and synapsin I in striatum from rats treated with repeated amphetamine. J Pharmacol Exp Ther. 1996;278:1428–1434. [PubMed] [Google Scholar]
- 11.Iwata S, Hewlett GHK, Ferrell ST, Kantor L, Gnegy ME. Enhanced dopamine release and phosphorylation of synapsin I and neuromodulin in striatal synaptosomes after repeated amphetamine. J Pharmacol Exp Ther. 1997;283:1445–1452. [PubMed] [Google Scholar]
- 12.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter [review]. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther. 1998;284:594–598. [PubMed] [Google Scholar]
- 14.Kanzaki A, Akiyama K, Otsuki S. Subchronic methamphetamine treatment enhances ouabain-induced striatal dopamine efflux in vivo. Brain Res. 1992;569:181–188. doi: 10.1016/0006-8993(92)90629-n. [DOI] [PubMed] [Google Scholar]
- 15.Kolta MG, Shreve P, Uretsky NJ. Effect of pretreatment with amphetamine on the interaction between amphetamine and dopamine neurons in the nucleus accumbens. Neuropharmacology. 1989;28:9–14. doi: 10.1016/0028-3908(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 16.Kuczenski R, Segal DS. Psychomotor stimulant-induced sensitization: behavioral and neurochemical correlates. In: Kalivas PW, Barnes CD, editors. Sensitization in the nervous system. Telford; Caldwell, NJ: 1988. pp. 175–205. [Google Scholar]
- 17.Llinas R, Gruner JA, Sugimori M, McGuinness TL, Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol (Lond) 1991;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naudon L, Leroux-Nicollet I, Raisman-Vozari R, Botton D, Costentin J. Time-course of modifications elicited by reserpine on the density and mRNA synthesis of the vesicular monoamine transporter, and on the density of the membrane dopamine uptake complex. Synapse. 1995;21:29–36. doi: 10.1002/syn.890210105. [DOI] [PubMed] [Google Scholar]
- 19.Nichols RA, Chilcote TJ, Czernik AJ, Greengard P. Synapsin I regulates glutamate release from rat brain synaptosomes. J Neurochem. 1992;58:783–785. doi: 10.1111/j.1471-4159.1992.tb09788.x. [DOI] [PubMed] [Google Scholar]
- 20.Pierce RC, Kalivas PW. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci. 1997a;17:3254–3261. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997b;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 22.Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- 23.Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P. Distinct pools of synaptic vesicles in neurotransmitter release. Nature. 1995;375:493–497. doi: 10.1038/375493a0. [DOI] [PubMed] [Google Scholar]
- 24.Raiteri M, Cerrito F, Cervoni AM, Levi G. Dopamine can be released by two mechanisms differentially affected by the dopamine transport inhibitor nomifensine. J Pharmacol Exp Ther. 1979;208:195–202. [PubMed] [Google Scholar]
- 25.Robinson TE. The neurobiology of amphetamine psychosis: evidence from studies with an animal model. In: Nakazawa T, editor. Taniguchi Symposia on Brain Sciences, Vol 14, Biological basis of schizophrenic disorders. Japan Scientific Societies; Tokyo: 1991. pp. 185–201. [Google Scholar]
- 26.Robinson TE, Becker JB. Behavioral sensitization is accompanied by an enhancement in amphetamine-stimulated dopamine release from striatal tissue in vitro. Eur J Pharmacol. 1982;85:253–254. doi: 10.1016/0014-2999(82)90478-2. [DOI] [PubMed] [Google Scholar]
- 27.Robinson TE, Becker JB, Moore CJ, Castañeda E, Mittleman G. Enduring enhancement in frontal cortex dopamine utilization in an animal model of amphetamine psychosis. Brain Res. 1985;343:374–377. doi: 10.1016/0006-8993(85)90760-7. [DOI] [PubMed] [Google Scholar]
- 28.Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 29.Sulzer D, Chen T-K, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchikawa T, Kiuchi Y, Yura A, Nakachi N, Yamazaki Y, Yokomizo C, Oguchi K. Ca2+-dependent enhancement of [3H]dopamine uptake in rat striatum: possible involvement of calmodulin-dependent kinases. J Neurochem. 1995;65:2065–2071. doi: 10.1046/j.1471-4159.1995.65052065.x. [DOI] [PubMed] [Google Scholar]
- 31.Warburton EC, Mitchell SN, Joseph MH. Calcium dependence of sensitised dopamine release in rat nucleus accumbens following amphetamine challenge: implications for the disruption of latent inhibition. Behav Pharmacol. 1996;7:119–129. [PubMed] [Google Scholar]
- 32.Wolf ME, White FJ, Nassar R, Brooderson RJ, Khansa MR. Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J Pharmacol Exp Ther. 1993;264:249–255. [PubMed] [Google Scholar]