Abstract
The present study demonstrates that the regulator of G-protein–signaling protein type 4 (RGS4) is differentially regulated in the locus coeruleus (LC) and the paraventricular nucleus (PVN) of the hypothalamus by chronic stress and glucocorticoid treatments. Acute or chronic administration of corticosterone to adult rats decreased RGS4 mRNA levels in the PVN but increased these levels in the LC. Similarly, chronic unpredictable stress decreased RGS4 mRNA levels in the PVN but had a strong trend to increase these levels in the LC. Chronic stress also decreased RGS4 mRNA levels in the pituitary. The molecular mechanisms of RGS4 mRNA regulation were further investigated in vitro in the LC-like CATH.a cell line and the neuroendocrine AtT20 cell line using the synthetic corticosterone analog dexamethasone. Consistent with the findingsin vivo, dexamethasone treatment caused a dose- and time-dependent decrease in RGS4 mRNA levels in AtT20 cells but a dose- and time-dependent increase in CATH.a cells. RGS4 mRNA regulation seen in these two cell lines seems to be attributable, at least in part, to opposite changes in mRNA stability. The differential regulation of RGS4 expression in the LC and in key relays of the hypothalamic–pituitary–adrenal axis could contribute to the brain’s region-specific and long-term adaptations to stress.
Keywords: RGS proteins, glucocorticoids, chronic stress, locus coeruleus, paraventricular nucleus of the hypothalamus, HPA (hypothalamic–pituitary–adrenal) axis, CATH.a cells, AtT20 cells, cAMP pathway
The recently discovered regulators of G-protein–signaling (RGS) proteins negatively modulate G-protein function by accelerating the GTPase activity of G-protein α subunits. There has been intense interest in the function of RGS proteins, in particular, in their interaction with G-proteins. Several members of the RGS family inhibit Gαi, Gαo, Gαq, Gαz, or transducin (Gαt) subunits but not Gαs or Gα12 (Berman et al., 1996; Hunt et al., 1996; Watson et al., 1996; Hepler et al., 1997; Wieland et al., 1997). Because of its apparently exclusive expression in brain, RGS protein type 4 (RGS4) has received a wealth of attention. Experiments with purified recombinant proteins in vitro and with stably transfected mammalian cells show that RGS4 attenuates Gαi- and Gαq-mediated signaling by acting as a GTPase-activating protein (Hepler et al., 1997; Huang et al., 1997). On the basis of the crystal structure of RGS4 bound to AlF4−-activated Gαi, it has been suggested that RGS4 accelerates the GTPase activity of Gαi and Gαq by stabilizing the α subunit’s transition state for GTP hydrolysis (Tesmer et al., 1997).
We have characterized previously the distribution of mRNAs encoding RGS3–RGS11 in rat brain (Gold et al., 1997). Of these RGS proteins, RGS4 mRNA is relatively abundant in many brain regions, including several structures of the stress response circuitry, such as the cerebral cortex, amygdala, thalamus, paraventricular nucleus (PVN) of the hypothalamus, and locus coeruleus (LC). Although a recent study has shown changes in several RGS mRNAs after acute amphetamine treatment (Burchett et al., 1998), overall, little is known regarding the physiological consequences of altered RGS protein expression.
One functional consequence of RGS4 regulation could be modulation of signaling via the cAMP pathway. Thus, a decrease in RGS4 expression, by enhancing Gαi-mediated signaling, could result in diminished adenylyl cyclase activity. There is strong evidence that regulation of the cAMP pathway plays an important role in stress responses. For instance, corticotropin-releasing factor (CRF), the primary neurotransmitter controlling activity of the hypothalamic–pituitary–adrenal (HPA) axis, acts by stimulating adenylyl cyclase (Labrie et al., 1982; Litvin et al., 1984; Battaglia et al., 1987). Adrenal glucocorticoid, a key hormone of the stress response, exerts negative feedback control over the HPA axis by inhibiting CRF secretion by the PVN and adrenocorticotropic hormone (ACTH) secretion by the pituitary. These actions oppose the cAMP pathway, which is known to promote the synthesis and release of CRF and of ACTH in these tissues (Giguere et al., 1982; Labrie et al., 1982; Keller and Dallman, 1984; Litvin et al., 1984; Dorin et al., 1993). In contrast, chronic stress induces upregulation of several components of the cAMP pathway, including protein kinase A and adenylyl cyclase, in the LC (Melia et al., 1992), a brain region thought to mediate certain attentional and autonomic features of the stress response.
The goal of the present study was to investigate directly the effect of chronic stress and corticosterone treatments on RGS4 expression in brain regions associated with the stress response and to gain insight into the possible mechanisms involved by analyzing RGS4 expression in two cell lines in vitro. We show that chronic stress or glucocorticoid treatment decreases RGS4 mRNA levels in the PVN and pituitary but increases RGS4 mRNA levels in the LC. Decreased RGS4 expression in the PVN and pituitary, by potentiating Gαi function, could contribute to stress- and glucocorticoid-induced negative feedback of these brain regions. Conversely, increased RGS4 expression in the LC, by diminishing Gαi function, is consistent with an upregulation of the cAMP pathway known to occur in this brain region after chronic stress.
MATERIALS AND METHODS
Animal treatments. Adult male Sprague Dawley rats (initial weight, 190–240 gm; Charles River Laboratories, Wilmington, MA) were used in this study. Rats were caged in groups of two with food and water available ad libitum in a 12 hr light/dark cycle (lights off at 7 P.M.). All rats were received from the vendor several days before initiating various treatments to habituate them to our vivarium. In the acute corticosterone treatment, rats received a single injection of either corticosterone (40 mg/kg in sesame oil, s.c.; Sigma, St. Louis, MO) or vehicle and were used 6 hr later, at which time elevated plasma corticosterone levels have been documented (Pavlides et al., 1993). In the chronic corticosterone treatment, rats were implanted with sustained-release pellets (100 mg; 7 d release; Innovative Research of America, Toledo, OH) and used 7 d later as described (Ortiz et al., 1995). The control group received sham surgery. Chronic unpredictable stress, which involves animals being exposed to two of eight different stressors per day for 10 d, was administered exactly according to published procedures (Ortiz et al., 1996).
Cell culture. AtT20 cells (purchased from American Type Culture Collection, Rockville, MD) were cultured in DMEM containing 10% fetal bovine serum. CATH.a cells were obtained from Dr. D. M. Chikaraishi (Duke University, Durham, NC) and were cultured in RPMI 1640 medium containing 4% fetal bovine serum and 8% horse serum. Initial experiments were also performed on SH-SY5Y neuroblastoma cells (a generous gift of Dr. S. Brene, Karolinska Institute) and C6 glioma cells (purchased from American Type Culture Collection), which were cultured similarly as the AtT20 cells. Cells were split at a ratio of 1:5 or 1:10 every 4–5 d. Cells were treated with dexamethasone (in ethanol; Sigma) at the indicated concentrations for 4 hr unless otherwise stated. mRNA stability was assayed using the transcription inhibitor actinomycin D (2 μg/ml; Calbiochem, La Jolla, CA). The effect of CRF or forskolin was tested by incubating the cells with CRF (100 nm; a generous gift of Dr. J. River, Salk Institute, La Jolla, CA) or forskolin (5 μm, in ethanol; Sigma) for 4 hr. Treatments were terminated at the indicated times by addition of an ice-cold guanidinium thiocyanate lysis buffer from the RNAqueous kit (Ambion, Austin, TX). Cell lysates were harvested for RNA extraction (see below).
Riboprobes. RGS4 templates were generated byHindIII digestion of pMK152 (obtained from Dr. M. Koelle, Yale University, New Haven, CT) (Koelle and Horvitz, 1996). Cyclophilin templates (pTRI-cyclophilin-mouse) were purchased from Ambion. Antisense riboprobes for RGS4 or cyclophilin were transcribed with T3 RNA polymerase (Boehringer Mannheim, Indianapolis, IN) in the presence of [32P]CTP (Northern blot analysis) or35S-UTP (in situ hybridization) (Dupont NEN, Boston, MA). Riboprobes for Northern analysis were purified with Nuc Trap minicolumns (Stratagene, La Jolla, CA). Riboprobes for in situ hybridization were purified by phenol–chloroform extraction followed by ethanol precipitation on dry ice with 2.5 mammonium acetate.
Northern blot analysis. RNA was prepared using RNAqueous kits (Ambion) and following the manufacturer’s protocol. The concentration of RNA was determined by spectrophotometry. Samples of 25 μg of RNA were electrophoresed through a formaldehyde–1.2% agarose gel containing ethidium bromide, transferred to Nitropure-supported nitrocellulose (MSI, Westboro, MA) by capillary blotting, and UV cross-linked to the membrane (Stratalinker; Stratagene). Northern blots were hybridized for 18 hr at 65°C in a roller tube oven in hybridization buffer containing 50% deionized formamide, 4× SSC, 20 mm Tris-HCl, pH 7.5, 0.1% SDS, 1× Denhardt’s solution, 10% dextran sulfate, 100 μg/ml denatured salmon sperm DNA, and 2 × 106 cpm/ml (RGS4) or 2 × 105 cpm/ml (cyclophilin) 32P-labeled riboprobes. Blots were washed at 65°C for 20 min in the following buffers: twice in 2× SSC and 0.1% SDS, once in 0.5× SSC and 0.1% SDS, and once in 0.1× SSC and 0.1% SDS. The blots were visualized and quantified by image analysis (Molecular Imager System GS-363; Bio-Rad, Hercules, CA). RGS4 mRNA levels were normalized to cyclophilin levels to control for variations in gel loading and RNA transfer.
In situ hybridization. In situhybridization of brain sections was conducted either on free-floating sections (Gall et al., 1995) or on slide-mounted sections (Vaidya et al., 1997). The two methods yielded equivalent results. In the free-floating hybridizations, rats were perfused transcardially with 50 ml of saline followed by 400 ml of 4% paraformaldehyde in 0.1m sodium phosphate buffer, pH 7.4. Brains were post-fixed, cryoprotected, and then sectioned at 30 μm in the coronal plane with a microtome. Serial sections between −0.68 and −1.04 mm bregma, which includes the large majority of the locus coeruleus (Paxinos and Watson, 1982), were analyzed for this nucleus. In the slide hybridizations, brains were fresh frozen and then sectioned at 14 μm in the coronal plane with a cryostat. The pituitary was analyzed with the slide hybridization method. Transverse pituitary sections (14 μm) were mounted on UltraStick slides (Becton Dickinson, Rutherford, NJ). RNA-labeling densities were determined by densitizing autoradiographic film using Image analysis software (NIH Image) as described previously (Gold et al., 1997). For high resolution analysis, sections were dipped in autoradiographic emulsion (NTB2; Eastman Kodak, Rochester, NY), exposed for 8 weeks, developed with D19 (Eastman Kodak), fixed with Kodak fixer, counterstained with cresyl violet, and coverslipped with DPX (Aldrich, Milwaukee, WI).
RESULTS
Regulation of RGS4 mRNA by chronic unpredictable stress
In initial studies, we examined the effect of chronic (10 d) unpredictable stress on the levels of RGS4 mRNA in rat brain byin situ hybridization. Of the many brain regions that express high levels of RGS4 (see Gold et al., 1997), the PVN and LC showed the most apparent effects of stress (Figs.1, 2). In the PVN, chronic stress caused a 28% decrease in RGS4 mRNA levels (p < 0.05, two-tailed Student’s ttest) (Fig.1A,B,G–I). In contrast, in the LC, there was a strong trend for increased (21%) RGS4 mRNA levels after chronic stress (p < 0.079) (Fig. 2A,B,G). Chronic stress had no marked effect in the thalamus or cerebral cortex, brain regions that also express high levels of RGS4 mRNA, or in the hippocampus, which expresses RGS4 mRNA at a very low level.
Fig. 1.
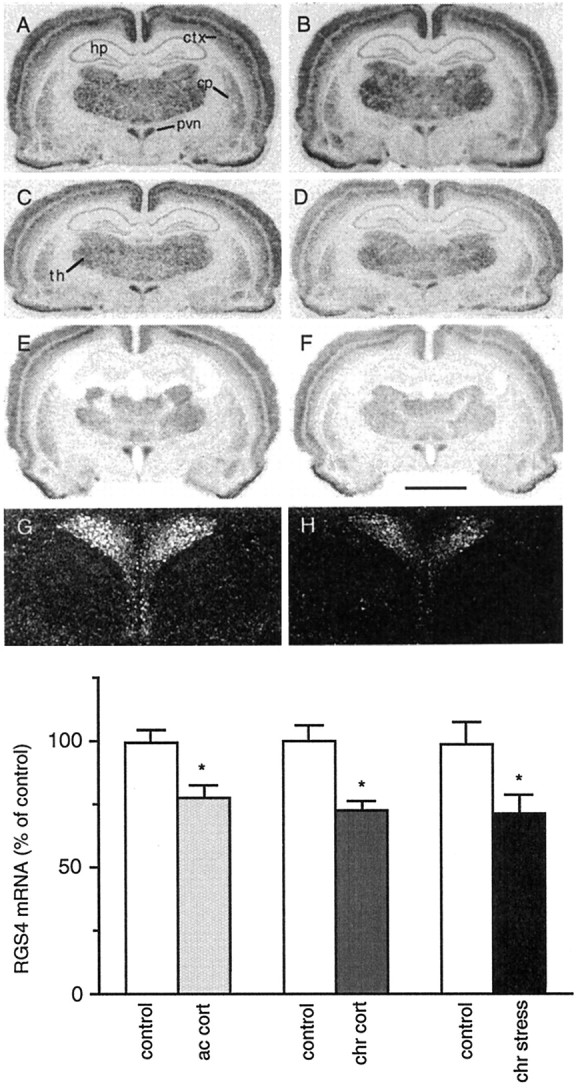
A–H, Bright-field film (A–F) and dark-field emulsion (G,H) autoradiograms of RGS4 expression in the PVN of control rats (A, C, E,G) or those treated with chronic stress (B, H; chr stress), acute corticosterone (D; ac cort), or chronic corticosterone (F; chr cort).cp, Striatum; ctx, cortex;hp, hippocampus; pvn, paraventricular nucleus of the hypothalamus; th, thalamus. Scale bar:A–F, 3.3 mm; G, H, 1.2 mm. I, Summary of results (mean ± SEM;n = 3–6; *p < 0.05, two-tailed t test).
Fig. 2.
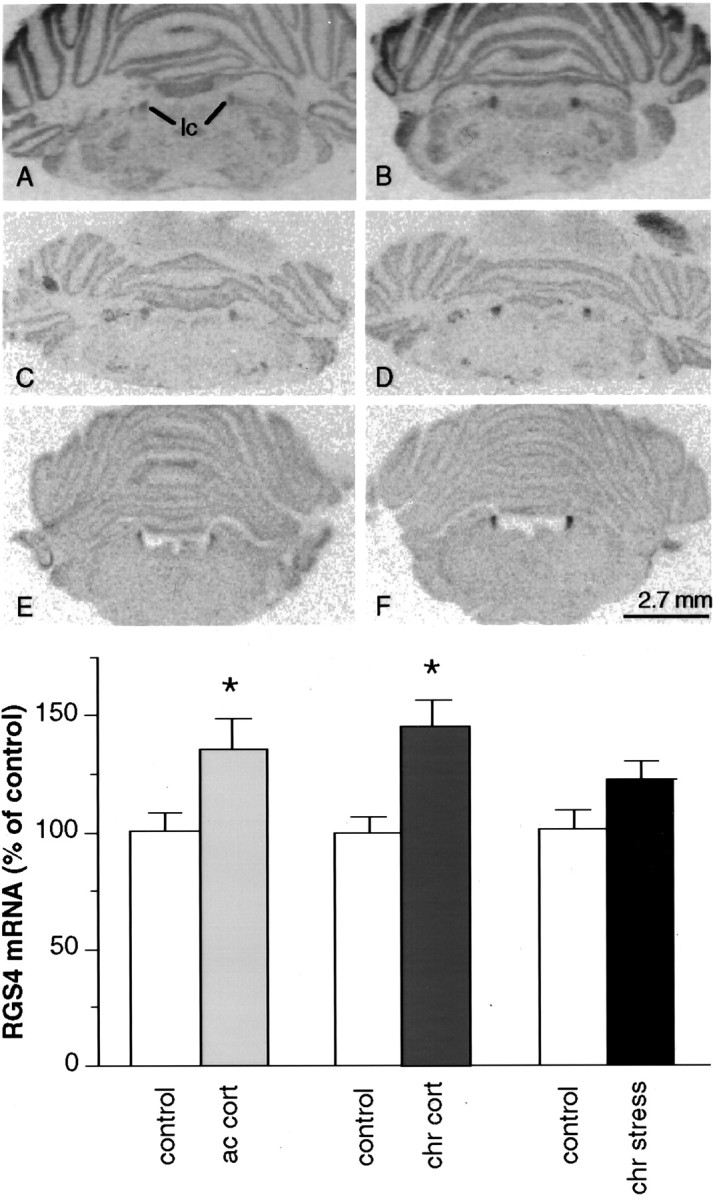
A–F, Film autoradiograms of RGS4 mRNA expression in the LC of control rats (A,C, E) or those treated with chronic stress (B; chr stress), acute corticosterone (D; ac cort), or chronic corticosterone (F; chr cort).lc, Locus coeruleus. G, Summary of results (mean ± SEM; n = 6; *p < 0.05, two-tailed ttest).
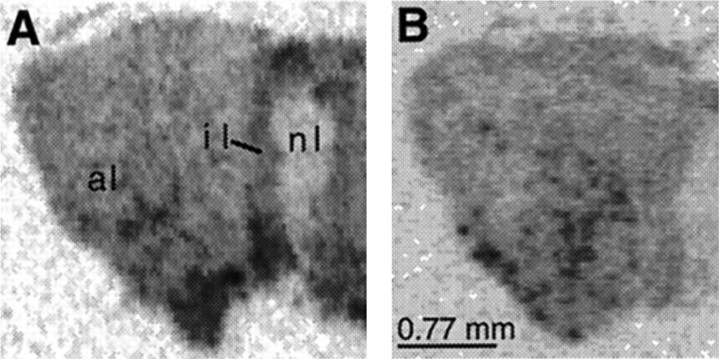
As shown in Figure 3, RGS4 mRNA was expressed at a relatively high level in the anterior and intermediate lobe of the pituitary. Interestingly, chronic stress decreased levels of RGS4 mRNA in both parts of the pituitary, with the most prominent effect in the intermediate lobe (49% decrease; p < 0.01) (Fig. 3).
Fig. 3.
Film autoradiograms of RGS4 mRNA expression in the pituitary of control (A) and chronic stress-treated (B) rats. Results shown are representative of the analysis of six rats in each treatment group.al, Anterior lobe; il, intermediate lobe;nl, neural lobe (posterior lobe).
Regulation of RGS4 mRNA by corticosterone treatments
We next studied the ability of glucocorticoids to mimic the effects of stress on RGS4 expression by examining the effect of acute and chronic corticosterone treatment on RGS4 mRNA levels in rat brain. In the PVN there was a 22% (p < 0.05) and 27% (p < 0.01) decrease in RGS4 mRNA levels after acute and chronic corticosterone treatment, respectively (Fig.1C–F,I). In contrast, in the LC there was a 35% (p < 0.05) and 46% (p < 0.05) increase in RGS4 mRNA levels after acute and chronic corticosterone treatment, respectively (Fig.2C–G). Acute and chronic corticosterone treatment had no discernible effect on the levels of RGS4 mRNA in other brain regions (Figs. 1, 2), except for the cingulate cortex where chronic treatment tended to decrease RGS4 expression (14%; p < 0.065).
Regulation of RGS4 mRNA by dexamethasone in vitro
To establish an in vitro model system with which to investigate further the regulation of RGS4 mRNA, we screened several cell lines for RGS4 mRNA expression. RGS4 mRNA was expressed in most of the cell lines examined, including CATH.a, AtT20, SH-SY5Y neuroblastoma, and U373 astrocytoma cells, but not in C6 glioma cells. Moreover, these initial studies showed that RGS4 mRNA levels were downregulated in CATH.a cells after a 4 hr CRF (100 nm) treatment, as well as in AtT20 cells after a 4 hr dexamethasone (0.2 μm) treatment (data not shown). CATH.a cells were derived from a brainstem tumor of a tyrosine hydroxylase–simian virus 40 T antigen transgenic mouse and resemble in many respects noradrenergic neurons of the LC (Suri et al., 1993; Widnell et al., 1994). AtT20 cells were derived from mouse pituitary and exhibit many properties of pituitary corticotrophs (ACTH-secreting cells). This cell line has long been used in studying stress mechanisms, particularly, the feedback inhibition by glucocorticoids on CRF and ACTH. Therefore, we decided to characterize further the regulation of RGS4 mRNA by the potent synthetic glucocorticoid dexamethasone in these two cell lines.
CATH.a cells
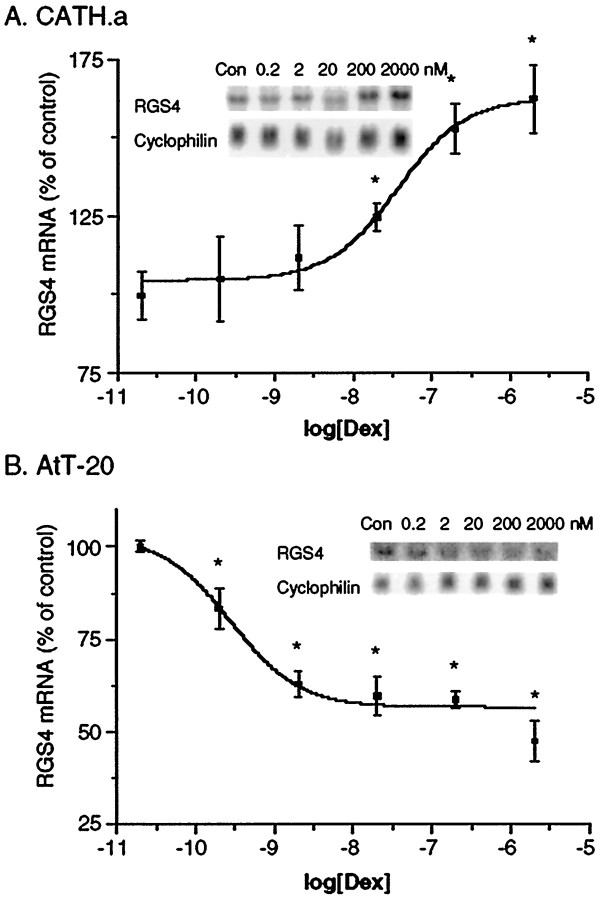
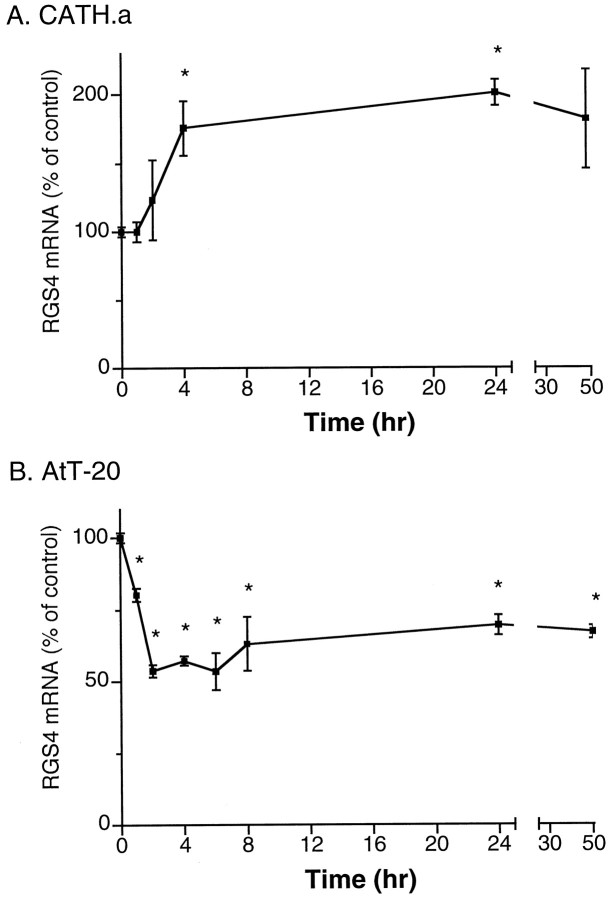
Dexamethasone induced a dose-dependent increase in RGS4 mRNA levels in CATH.a cells as determined by Northern blotting (Fig.4A). The effect of dexamethasone was detectable at subnanomolar concentrations, with an EC50 value of ∼37 nm. In addition, the upregulation of RGS4 mRNA levels occurred in a time-dependent manner (Fig. 5A). When 0.2 μm dexamethasone was used (Fig. 5A), the effect was significant within 4 hr. By 24 hr, levels of RGS4 mRNA were almost 200% that of controls. Longer incubations with dexamethasone (>24 hr) had diminished effects on RGS4 expression. However, even after 72 hr of dexamethasone exposure, increased levels of RGS4 mRNA persisted (∼50% increase; data not shown). Similar increases in RGS4 mRNA levels were seen with corticosterone itself (data not shown).
Fig. 4.
Dose–response analyses for dexamethasone (Dex) regulation of RGS4 mRNA expression in CATH.a (A) and AtT20 (B) cells. Data are expressed as the mean percent of control (± SEM;n = 3–6; *p < 0.05, two-tailed t test). Con, Control.
Fig. 5.
Time course study for dexamethasone regulation of RGS4 mRNA expression in CATH.a (A) and AtT20 (B) cells. Data are expressed as the mean percent of control (± SEM; n = 3–6; *p < 0.05, two-tailed ttest).
To characterize the molecular mechanism underlying the upregulation of RGS4 expression in CATH.a cells by dexamethasone, we examined the effect of dexamethasone on RGS4 mRNA stability. Cells were incubated with 200 nm dexamethasone for 1 hr, followed by addition of the transcription inhibitor actinomycin D. Exposure to dexamethasone increased the half-life of RGS4 mRNA from 2.6 ± 0.1 to 4.0 ± 0.03 hr (mean ± SEM; n = 4) (Fig.6A). This finding indicates that the dexamethasone-induced upregulation of RGS4 mRNA in CATH.a cells could be attributable, at least in part, to an increase in the stability of the mRNA.
Fig. 6.
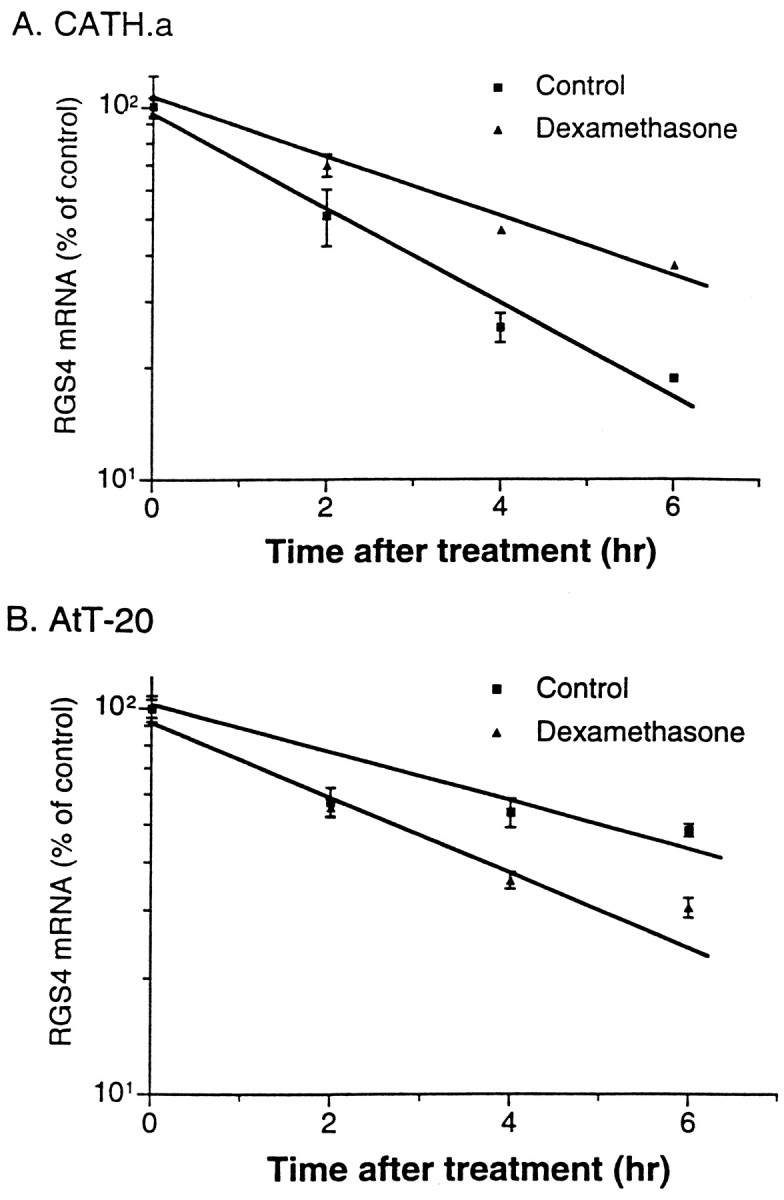
Effect of actinomycin D on dexamethasone regulation of RGS4 mRNA expression in CATH.a (A) and AtT20 (B) cells. Data are expressed as the mean percent of control (± SEM; n = 4).
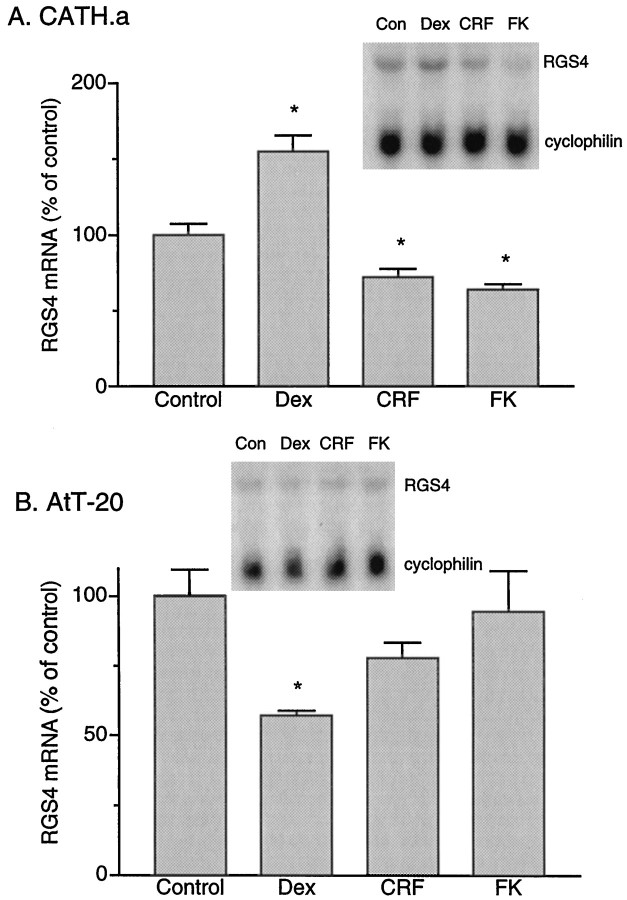
As mentioned above, in our initial experiments we detected a downregulation of RGS4 mRNA by CRF in CATH.a cells, an effect opposite to that seen with dexamethasone. Because CRF acts by stimulating adenylyl cyclase, it was of interest to determine whether forskolin, which directly activates the catalytic moiety of adenylyl cyclase, exerts a similar effect on RGS4 expression. Indeed, application of CRF (100 nm) or forskolin (5 μm) for 4 hr caused a 28 or 36% decrease in RGS4 mRNA levels, respectively (p < 0.05) (Fig.7A).
Fig. 7.
Comparison of the regulation of RGS4 mRNA expression in CATH.a (A) and AtT20 (B) cells by dexamethasone (Dex), CRF, and forskolin (FK). Data are expressed as the mean percent of control (± SEM; n = 4; *p < 0.05, two-tailed ttest).
AtT20 cells
In contrast to the upregulation of RGS4 mRNA expression seen in CATH.a cells, a dose-dependent downregulation of RGS4 mRNA was caused by dexamethasone treatment in AtT20 cells (Fig. 4B). The effect of dexamethasone was detectable at subnanomolar concentrations, with an EC50 value of ∼0.3 nm. Maximal effects of dexamethasone (close to a 50% inhibition) were seen at ∼20 nm. In addition, the downregulation of RGS4 mRNA levels in AtT20 cells was time-dependent. When 200 nm dexamethasone was applied, the effect was significant within 1 hr and peaked between 2 and 6 hr of treatment (Fig. 5B). At later time points, RGS4 mRNA levels partially recovered, even though dexamethasone was still present, but remained significantly suppressed after 50 hr.
A possible role for mRNA stability in dexamethasone regulation of RGS4 mRNA levels was also studied in AtT20 cells using actinomycin D. Exposure to dexamethasone significantly decreased the half-life of RGS4 mRNA from 6.2 ± 0.1 to 3.4 ± 0.1 hr (mean ± SEM;n = 3) (Fig. 6B). This finding is consistent with the possibility that the dexamethasone-induced downregulation of RGS4 mRNA in AtT20 cells may result, at least in part, from a decrease in mRNA stability.
Finally, we studied the effect of CRF and forskolin on RGS4 expression in AtT20 cells. In contrast to the results seen in CATH.a cells, application of CRF or forskolin did not produce any detectable change in RGS4 mRNA levels in AtT20 cells (Fig. 7B).
DISCUSSION
The present study demonstrates that expression of RGS4 mRNA is differentially regulated in the LC versus the PVN and pituitary by chronic stress and glucocorticoid treatments. In the LC, levels of RGS4 mRNA were significantly increased by acute and chronic corticosterone treatment, and there was a strong trend for an increase after chronic unpredictable stress. In contrast, in the PVN, levels of RGS4 mRNA were decreased by acute and chronic corticosterone treatment and by chronic stress. Similar decreases were seen in the anterior and intermediate lobe of the pituitary after chronic stress. To understand the molecular mechanisms underlying these changes, we studied the regulation of RGS4 mRNA by dexamethasone in the LC-like CATH.a cell line and the neuroendocrine AtT20 cell line. In CATH.a cells, levels of RGS4 mRNA were dose-dependently increased by dexamethasone, whereas, in AtT20 cells, levels of RGS4 mRNA were dose-dependently decreased by dexamethasone. Further studies showed that the opposite effects of dexamethasone on RGS4 expression in the two cell lines could be explained, at least in part, by opposite changes in the stability of RGS4 mRNA.
Previous studies have shown that the LC, which is the major noradrenergic nucleus in the brain, responds to stress with several adaptations. Acute or chronic stress increases the spontaneous firing rate of LC neurons (Abercrombie and Jacobs, 1987; Simson and Weiss, 1988; Pavcovich and Ramirez, 1991) and the expression of tyrosine hydroxylase, the rate-limiting enzyme for norepinephrine biosynthesis (Thoenen, 1970; Zigmond et al., 1974; Richard et al., 1988; Smith et al., 1991; Melia et al., 1992). In addition, it has been shown that chronic stress increases the levels of adenylyl cyclase and protein kinase A in this brain region, adaptations indicative of an upregulation of the cAMP pathway (Melia et al., 1992). Results from the current study demonstrate an additional adaptation to stress that could contribute further to the upregulated cAMP pathway in the LC. Thus, the chronic stress-induced increase in the levels of RGS4 expression in this brain region would be expected to promote cAMP formation by enhancing the inhibitory effect of RGS4 on Gαi and thereby reducing the inhibitory effect of this G-protein on adenylyl cyclase. It should also be mentioned that RGS4 seems to be expressed at particularly high levels in the LC relative to that of other RGS proteins examined to date (Gold et al., 1997).
Consistent with this hypothesis is the finding that RGS4 can exert a negative modulatory effect on Gαi function in vitro (Huang et al., 1997). However, the G-protein α subunit(s) regulated by this RGS protein in the brain in vivo remains unclear, becausein vitro studies indicate that RGS4 can also regulate Gαq (Hepler et al., 1997; Huang et al., 1997). This latter finding raises the interesting possibility that the stress- and glucocorticoid-induced upregulation of RGS4 in the LC might influence Gαq-mediated signaling cascades, in addition to the cAMP pathway, in this brain region.
It has been shown that during chronic stress the LC is exposed to increased levels of both CRF and glucocorticoids (Chappell et al., 1986). Although the effect of CRF on RGS4 expression in the LC in vivo remains unknown, our studies in the LC-like CATH.a cell line showed that CRF has an opposing effect on RGS4 mRNA levels compared with that of dexamethasone. If the same holds true in vivo, these opposing effects may account for the smaller change in RGS4 expression seen in the LC after chronic stress compared with that seen after acute or chronic corticosterone treatment alone.
In contrast to the upregulation of RGS4 expression observed in the LC, chronic stress and corticosterone treatments were found to downregulate levels of RGS4 mRNA in the PVN and pituitary, two components of the HPA axis. This downregulation of RGS4 expression could lead to a downregulation of the cAMP pathway in the PVN and pituitary, which could contribute to glucocorticoid-induced negative feedback on these tissues. It has been well documented that glucocorticoids exert a profound negative feedback on the PVN and pituitary. Part of this negative control is accomplished by inhibiting CRF and ACTH synthesis and release from the PVN and pituitary, respectively. The synthesis and release of CRF and ACTH are promoted by activation of the cAMP pathway (Giguere et al., 1982; Labrie et al., 1982; Keller and Dallman, 1984;Litvin et al., 1984; Dorin et al., 1993). Moreover, both CRF and ACTH act by stimulating adenylyl cyclase. Thus, the stress- and glucocorticoid-induced downregulation of RGS4 expression in the PVN and pituitary, via reduced inhibitory influence of Gαi function, would be expected to attenuate the formation of cAMP in these tissues and could be part of the mechanism underlying negative feedback in the HPA axis.
In addition to studying the LC and PVN, we also examined regulation of RGS4 mRNA levels in other brain regions, including the cerebral cortex and hippocampus. A previous study reported differential regulation of CRF receptor type 1 (CRFR1) mRNA in the frontal cortex and hippocampus by chronic stress or corticosterone treatments (Iredale et al., 1996). Expression of CRFR1 mRNA was decreased in the frontal cortex but increased in the hippocampus by chronic unpredictable stress. Chronic corticosterone administration did not affect receptor expression in either region. The results reported here in general show no dramatic effect of chronic stress or corticosterone treatments on RGS4 expression in the cortex and hippocampus, with the exception of a trend for a slight decrease in the cingulate cortex with chronic corticosterone exposure. However, it should be emphasized that these negative conclusions are based solely on the use of in situhybridization, which can lead to false-negative results particularly in the analysis of large brain structures (e.g., see Hayward et al., 1990).
Dexamethasone induced a dose-dependent increase in RGS4 mRNA levels in CATH.a cells and a dose-dependent decrease in AtT20 cells. The results in AtT20 cells are consistent with data from a previous study, in which dexamethasone treatment caused a dose- and time-dependent decrease in CRFR1 mRNA in these cells (Iredale and Duman, 1997). The decreases in RGS4 and CRFR1 expression could act in concert to produce a downregulation of the cAMP pathway. In both cell lines, the effect of dexamethasone took place between 1 and 2 hr; experiments with shorter time points are needed to determine whether the effects may occur even earlier. Results from the mRNA stability studies suggest that the regulation of RGS4 mRNA by dexamethasone in CATH.a and AtT20 cells occurs, at least in part, via changes in the stability of the mRNA. However, this finding does not eliminate the possibility that dexamethasone may also regulate RGS4 expression via additional mechanisms, for example, via altered rates of transcription of the RGS4 gene.
Results of the present study demonstrate changes in RGS4 mRNA levels in specific regions of the rat brain in response to chronic stress and glucocorticoid treatments. A critical question is whether equivalent changes occur in RGS4 protein levels; however this must await the availability of suitable antibodies directed at this protein. Nevertheless, the region-specific regulation of RGS4 expression seen in the LC and HPA axis could contribute to the complex types of adaptations (or maladaptations) that occur in the brain and could mediate long-term plasticity to prolonged periods of stress.
Footnotes
This work was supported by grants from the National Institute of Mental Health and the National Institute on Drug Abuse and by the Abraham Ribicoff Research Facilities of the Connecticut Mental Health Center, State of Connecticut Department of Mental Health and Addiction Services. We wish to thank Dr. J. P. Herman for suggestions on the pituitary in situ hybridization experiments, Dr. Y. Lee for helpful discussions throughout the course of this study, Dr. M. Charlton for providing U373 astrocytoma cell mRNA, and Ms. J. J. Spencer for technical help with the chronic unpredictable stress experiments.
Correspondence should be addressed to Dr. Eric J. Nestler, Department of Psychiatry, Yale University School of Medicine, 34 Park Street, New Haven, CT 06508.
Dr. Iredale’s present address: Central Research Division, Pfizer, Eastern Point Road, Groton, CT 06340.
REFERENCES
- 1.Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J Neurosci. 1987;7:2837–2843. doi: 10.1523/JNEUROSCI.07-09-02837.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battaglia G, Webster EL, De Souza EB. Characterization of corticotropin-releasing factor receptor-mediated adenylate cyclase activity in the rat central nervous system. Synapse. 1987;1:572–581. doi: 10.1002/syn.890010610. [DOI] [PubMed] [Google Scholar]
- 3.Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 4.Burchett SA, Volk ML, Bannon MJ, Granneman JG. Regulators of G protein signaling: rapid changes in mRNA abundance in response to amphetamine. J Neurochem. 1998;70:2216–2219. doi: 10.1046/j.1471-4159.1998.70052216.x. [DOI] [PubMed] [Google Scholar]
- 5.Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorin RI, Zlock DW, Kilpatrick K. Transcriptional regulation of human corticotropin releasing factor gene expression by cyclic adenosine 3′,5′-monophosphate: differential effects at proximal and distal promoter elements. Mol Cell Endocrinol. 1993;96:99–111. doi: 10.1016/0303-7207(93)90100-x. [DOI] [PubMed] [Google Scholar]
- 7.Gall C, Lauterborn J, Guthrie K. In situ hybridization: a sensitive measure of activity-dependent changes in neuronal gene expression. In: Stumpf WE, Solomon HF, editors. Autoradiography and correlative imaging. Academic; San Diego: 1995. pp. 379–399. [Google Scholar]
- 8.Giguere V, Labrie F, Cote J, Coy DH, Sueiras DJ, Schally AV. Stimulation of cyclic AMP accumulation and corticotropin release by synthetic ovine corticotropin-releasing factor in rat anterior pituitary cells: site of glucocorticoid action. Proc Natl Acad Sci USA. 1982;79:3466–3469. doi: 10.1073/pnas.79.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayward MD, Duman RS, Nestler EJ. Induction of the c-fos proto-oncogene during opiate withdrawal in the locus coeruleus and other regions of rat brain. Brain Res. 1990;525:256–266. doi: 10.1016/0006-8993(90)90872-9. [DOI] [PubMed] [Google Scholar]
- 11.Hepler JR, Berman DM, Gilman AG, Kozasa T. RGS4 and GAIP are GTPase-activating proteins for Gq alpha and block activation of phospholipase C beta by gamma-thio-GTP-Gq alpha. Proc Natl Acad Sci USA. 1997;94:428–432. doi: 10.1073/pnas.94.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Hepler JR, Gilman AG, Mumby SM. Attenuation of Gi- and Gq-mediated signaling by expression of RGS4 or GAIP in mammalian cells. Proc Natl Acad Sci USA. 1997;94:6159–6163. doi: 10.1073/pnas.94.12.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt TW, Fields TA, Casey PJ, Peralta EG. RGS10 is a selective activator of G alpha i GTPase activity. Nature. 1996;383:175–177. doi: 10.1038/383175a0. [DOI] [PubMed] [Google Scholar]
- 14.Iredale PA, Duman RS. Glucocorticoid regulation of corticotropin-releasing factor1 receptor expression in pituitary-derived AtT-20 cells. Mol Pharmacol. 1997;51:794–799. doi: 10.1124/mol.51.5.794. [DOI] [PubMed] [Google Scholar]
- 15.Iredale PA, Terwilliger R, Widnell KL, Nestler EJ, Duman RS. Differential regulation of corticotropin-releasing factor1 receptor expression by stress and agonist treatments in brain and cultured cells. Mol Pharmacol. 1996;50:1103–1110. [PubMed] [Google Scholar]
- 16.Keller WM, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 17.Koelle MR, Horvitz HR. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell. 1996;84:115–125. doi: 10.1016/s0092-8674(00)80998-8. [DOI] [PubMed] [Google Scholar]
- 18.Labrie F, Gagne B, Lefevre G. Corticotropin-releasing factor stimulates adenylate cyclase activity in the anterior pituitary gland. Life Sci. 1982;31:1117–1121. doi: 10.1016/0024-3205(82)90085-6. [DOI] [PubMed] [Google Scholar]
- 19.Litvin Y, PasMantier R, Fleischer N, Erlichman J. Hormonal activation of the cAMP-dependent protein kinases in AtT20 cells. Preferential activation of protein kinase I by corticotropin releasing factor, isoproterenol, and forskolin. J Biol Chem. 1984;259:10296–10302. [PubMed] [Google Scholar]
- 20.Melia KR, Rasmussen K, Terwilliger RZ, Haycock JW, Nestler EJ, Duman RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- 21.Ortiz J, DeCaprio JL, Kosten TA, Nestler EJ. Strain-selective effects of corticosterone on locomotor sensitization to cocaine and on levels of tyrosine hydroxylase and glucocorticoid receptor in the ventral tegmental area. Neuroscience. 1995;67:383–397. doi: 10.1016/0306-4522(95)00018-e. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- 23.Pavcovich LA, Ramirez OA. Time course effects of uncontrollable stress in locus coeruleus neuronal activity. Brain Res Bull. 1991;26:17–21. doi: 10.1016/0361-9230(91)90186-n. [DOI] [PubMed] [Google Scholar]
- 24.Pavlides C, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1982. [DOI] [PubMed] [Google Scholar]
- 26.Richard F, Faucon BN, Labatut R, Rollet D, Mallet J, Buda M. Modulation of tyrosine hydroxylase gene expression in rat brain and adrenals by exposure to cold. J Neurosci Res. 1988;20:32–37. doi: 10.1002/jnr.490200106. [DOI] [PubMed] [Google Scholar]
- 27.Simson PE, Weiss JM. Altered activity of the locus coeruleus in an animal model of depression. Neuropsychopharmacology. 1988;1:287–295. [PubMed] [Google Scholar]
- 28.Smith MA, Brady LS, Glowa J, Gold PW, Herkenham M. Effects of stress and adrenalectomy on tyrosine hydroxylase mRNA levels in the locus coeruleus by in situ hybridization. Brain Res. 1991;544:26–32. doi: 10.1016/0006-8993(91)90881-u. [DOI] [PubMed] [Google Scholar]
- 29.Suri C, Fung BP, Tischler AS, Chikaraishi DM. Catecholaminergic cell lines from the brain and adrenal glands of tyrosine hydroxylase-SV40 T antigen transgenic mice. J Neurosci. 1993;13:1280–1291. doi: 10.1523/JNEUROSCI.13-03-01280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4−-activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 31.Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 1970;228:861–862. doi: 10.1038/228861a0. [DOI] [PubMed] [Google Scholar]
- 32.Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. RGS family members: GTPase-activating proteins for heterotrimeric G-protein alpha-subunits. Nature. 1996;383:172–175. doi: 10.1038/383172a0. [DOI] [PubMed] [Google Scholar]
- 34.Widnell KL, Russell DS, Nestler EJ. Regulation of expression of cAMP response element-binding protein in the locus coeruleus in vivo and in a locus coeruleus-like cell line in vitro. Proc Natl Acad Sci USA. 1994;91:10947–10951. doi: 10.1073/pnas.91.23.10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wieland T, Chen CK, Simon MI. The retinal specific protein RGS-r competes with the gamma subunit of cGMP phosphodiesterase for the alpha subunit of transducin and facilitates signal termination. J Biol Chem. 1997;272:8853–8856. doi: 10.1074/jbc.272.14.8853. [DOI] [PubMed] [Google Scholar]
- 36.Zigmond RE, Schon F, Iversen LL. Increased tyrosine hydroxylase activity in the locus coeruleus of rat brain stem after reserpine treatment and cold stress. Brain Res. 1974;70:547–552. doi: 10.1016/0006-8993(74)90267-4. [DOI] [PubMed] [Google Scholar]