Abstract
The question of whether recognition memory judgments with and without recollection reflect dissociable patterns of brain activity is unresolved. We used event-related, functional magnetic resonance imaging (fMRI) of 12 healthy volunteers to measure hemodynamic responses associated with both studying and recognizing words. Volunteers made one of three judgments to each word during recognition: whether they recollected seeing it during study (R judgments), whether they experienced a feeling of familiarity in the absence of recollection (K judgments), or whether they did not remember seeing it during study (N judgments). Both R and K judgments for studied words were associated with enhanced responses in left prefrontal and left parietal cortices relative to N judgments for unstudied words. The opposite pattern was observed in bilateral temporoccipital regions and amygdalae. R judgments for studied words were associated with enhanced responses in anterior left prefrontal, left parietal, and posterior cingulate regions relative to K judgments. At study, a posterior left prefrontal region exhibited an enhanced response to words subsequently given R versus K judgments, but the response of this region during recognition did not differentiate R and K judgments. K judgments for studied words were associated with enhanced responses in right lateral and medial prefrontal cortex relative to both R judgments for studied words and N judgments for unstudied words, a difference we attribute to greater monitoring demands when memory judgments are less certain. These results suggest that the responses of different brain regions do dissociate according to the phenomenology associated with memory retrieval.
Keywords: remember, know, recollection, familiarity, episodic memory retrieval, source, event-related fMRI
The “remember–know” procedure was introduced by Tulving (1985) to investigate the conscious experience accompanying memory retrieval. Participants in this procedure indicate with a remember (R) judgment those stimuli that evoke recollection of a specific episode in which the stimuli were experienced previously. For stimuli thought to have been experienced previously, but which do not evoke recollection of a specific episode, participants make a know (K) judgment. R judgments typically entail memory for the spatiotemporal context in which stimuli occurred or the mental associations triggered by their occurrence (“source” memory) (Johnson et al., 1993). K judgments typically entail a sense of familiarity, in the absence of information about the source of that familiarity (such as when one recognizes a face, but cannot remember to whom it belongs).
Behavioral evidence pertaining to the remember–know distinction includes claims that deeper encoding (Gardiner, 1988), lower normative frequency of stimuli (Gardiner and Java, 1990), and full versus divided attention (Gardiner and Parkin, 1990) increase R but not K judgments, whereas repeated shallow encoding (Gardiner et al., 1994) and subliminal priming (Rajaram, 1993) increase K but not R judgments. These claims, however, are based on the assumption that R and K judgments are exclusive, and different dissociations arise when R and K judgments are assumed to be independent or redundant (Yonelinas et al., 1996). Nonetheless, whether R and K judgments reflect qualitative or quantitative differences (Donaldson, 1996), they remain a useful means of operationalizing the subjective experience accompanying retrieval.
Little is known about the neural substrates that mediate R and K judgments. Knowlton and Squire (1995) found that amnesics with damage to the hippocampal formation or diencephalon showed reduced levels of both R and K judgments, whereas Schacter et al. (1997b) found that amnesics showed reduced levels of R but not K judgments. These results can be reconciled by scoring R and K judgments under an independence assumption, for which amnesics show reduced levels of both recollection and familiarity (Yonelinas et al., 1998). However, after reviewing the more general pattern of recognition and recall in amnesia, Aggleton and Brown (1998) argued that recollection depends on the hippocampus, whereas familiarity depends on the perirhinal cortex. An additional frontal role in recollection is suggested by studies showing that frontal patients are disproportionately impaired at retrieval of source information (Janowsky et al., 1989; Shimamura et al., 1990).
Distinct patterns of event-related potentials (ERPs) have been associated with R and K judgments. Smith (1993) found enhanced positive-going ERPs for R relative to K judgments 550–700 msec after stimulus, and Duzel et al. (1997) found that this difference was maximal over the left parietotemporal and bilateral frontal electrode sites. This pattern is consistent with the ERPs associated with correct and incorrect source judgments, which also diverge at these sites (Wilding and Rugg, 1996; Johnson et al., 1997). However, the anatomical generators of ERPs are notoriously difficult to localize. Here we capitalize on the high spatial resolution of event-related functional magnetic resonance imaging (fMRI) to localize differences in the hemodynamic response to individual R and K judgments.
MATERIALS AND METHODS
Participants. Twelve right-handed volunteers (six male), aged between 22 and 34 years (with a mean age of 26 years), gave informed consent to participate in the experiment.
Cognitive tasks. Participants were scanned during four sessions ordered as study–test–study–test conditions. Both conditions involved sequential, visual presentation of 90 stimuli, each stimulus prompting a manual response with the right hand. The stimuli were presented for 1 sec, followed by 7 sec of a central fixation cross.
In the study condition, the task was to press a key with either the index or middle finger to indicate whether the stimulus was a real word (a “lexical decision” task). Sixty stimuli were words; 30 were nonwords created by rearranging the letters of the remaining words assigned to that condition. A 1 min period of backward counting followed every study session to minimize any contribution of short-term memory to the subsequent test condition.
In the test condition, the 60 old words from the previous study condition were redisplayed, intermixed with 30 new words not seen before. Participants made one of three possible key presses with their index, middle, or ring fingers to indicate whether they consciously recollected seeing the word in the previous study episode (an R judgment), knew that the word was seen in the previous study episode but could not recollect any contextual information about its previous occurrence (a K judgment), or thought the word was new (an N judgment).
Participants were given brief practice on the study and test conditions before scanning, and the instructions for the R/K distinction [adapted from Rajaram (1993)] were clarified with examples. The instructions for responding emphasized accuracy over speed, and participants were reminded to focus on the fixation cross between stimuli.
Experimental materials and procedure. We obtained 240 five-letter nouns with a Kucera-Francis written frequency of 10–100 from the Medical Research Council Psycholinguistics database (http://www.psy.uwa.edu.au/uwa_mrc.htm) and assigned them randomly to each condition for each participant. The stimuli were presented in 24-point Helvetica font on a Macintosh computer and projected onto a screen ∼300 mm above the participant in the MRI scanner. The visual angle subtended by the stimuli was ∼2°.
The stimuli were ordered such that every third stimulus in the study condition was a nonword, and every third stimulus in the test condition was a new word, to maximize blood oxygenation level-dependent (BOLD) signal contrast between words and nonwords and old and new words, respectively (R. Henson, O. Josephs, and K. Friston, unpublished observations). To reduce the risk of participants detecting this pattern, a random 10% of trial triplets were reordered. In fact, no participant reported detecting any pattern in the stimuli, and the behavioral data showed no evidence of this manipulation. The finger assignment of word–nonword and R–K–N judgments was counterbalanced across participants.
fMRI scanning technique. A 2T Siemens VISION system (Siemens, Erlangen, Germany) was used to acquire both T1 anatomical volume images (1 × 1 × 1.5 mm voxels) and T2*-weighted echoplanar (EPI) images (64 × 64 5 × 5 mm pixels; echo time = 40 msec) with BOLD contrast. Each echoplanar image comprised 34 2.5 mm axial slices taken every 3 mm, positioned to cover the cortex (the cerebellum was not imaged). Data were acquired during four 12 min sessions, separated by a 2 min rest period. A total of 245 volume images per session were taken continuously with an effective repetition time (TR) of 3 sec/vol, of which the first five volumes were discarded to allow for T1 equilibration effects. The ratio of interscan-to-interstimulus interval ensured an effective sampling rate of the hemodynamic response of 1 Hz.
Preprocessing. To correct for their different acquisition times, the signal measured in each slice was shifted relative to the acquisition of the middle slice using a sinc interpolation in time. All volumes were then realigned to the first volume and resliced using a sinc interpolation in space. Each volume was normalized to a standard EPI template volume (based on the Montreal Neurological Institute reference brain) (Cocosco et al., 1997) of 3 × 3 × 3 mm voxels in the space of Talairach and Tournoux (1988)using nonlinear basis functions. The T1 structural volume was coregistered with the mean realigned EPI volume and normalized with the same deformation parameters. Finally, the EPI volumes were smoothed with an 8 mm full-width half-maximum isotropic Gaussian kernel to accommodate further anatomical differences across participants and proportionally scaled to a global mean of 100.
Data analysis. Data were analyzed using Statistical Parametric Mapping (SPM97d, Wellcome Department of Cognitive Neurology, London, UK) (Friston et al., 1995). Stimuli in the test condition were classified according to seven event-types: correct R, K and N judgments, incorrect R, K and N judgments, and trial failures (when no judgment was made within 4 sec of the stimulus). Stimuli in the study condition were classified according to three basic event types: judgments to words, judgments to nonwords, and trial failures. Judgments to words in the study condition were further classified as words given an R judgment in the subsequent test condition, words given a K judgment in the subsequent test condition, words given an N judgment in the subsequent test condition, and trial failures in the subsequent test condition.
By treating the volumes acquired during each session as a time series, the hemodynamic responses to the stimulus onset for each event-type were modelled with a canonical, synthetic hemodynamic response function and its first-order derivative with respect to time (Josephs et al., 1997). The inclusion of the derivative accommodates for small deviations in the onset of the hemodynamic response (Friston et al., 1998). These functions were used as covariates in a general linear model, together with a constant term and a basis set of cosine functions with a cutoff period of 90 sec to remove low-frequency drifts in the BOLD signal (Holmes et al., 1997). The parameter estimates for the height of the canonical response for each event-type covariate resulting from the least mean squares fit of the model to the data were stored as separate images, and the estimates were averaged across the two sessions of each study and test condition.
Pairwise contrasts between the height parameter estimate for event types comprising at least 10 events were tested by voxel-specific, repeated-measures t tests across participants (effecting a random effects model), which were subsequently transformed to the unit normal Z-distribution to create a statistical parametric map for each contrast. Given that differential activity in several brain regions was predicted on the basis of previous studies of encoding and recognition of words, the regionally specific differences reported below consisted of four or more contiguous voxels surviving a threshold ofp < 0.001 (Z > 3.09). The maxima of these regions were localized on the normalized structural images and labeled using the nomenclature of Talairach and Tournoux (1988) andBrodmann (1909) for consistency with previous studies.
RESULTS
Behavioral data
Performance of the lexical decision task in the study condition was almost perfect, with 97% of words and 93% of nonwords classified correctly (the 2% of trials in study and test conditions in which participants failed to give a response within 4 sec of stimulus onset were removed from subsequent analyses). The mean reaction time for correct word classifications (M = 931 msec, SD = 145 msec) was shorter than for correct nonword classifications (M = 998 msec, SD = 187 msec), although this difference did not reach significance (t(12) = 1.49, p > 0.10). Mean reaction times for correct lexical decisions in the study condition did not differ significantly for words given an R (M = 931 msec, SD = 150 msec) or K (M = 970 msec, SD = 201 msec) judgment in the subsequent test condition (t(12) = 0.69).
The mean proportions and reaction times for each judgment type in the test condition are shown in Table 1. Collapsing across R and K judgments, overall memory performance was reasonable, as indexed by a hit–false alarm rate of 0.78 − 0.16 = 0.62. Although the difference was small (when scored under an exclusivity assumption), the hit rate (0.26) for K judgments was significantly greater than the false alarm rate (0.14),t(12) = 4.09, p < .01, confirming that K judgments were more than guesses. (When scored under an independence or redundancy assumption, the difference between hit, 0.26/(1 − 0.51) = 0.53, and false alarm, 0.14/(1 − 0.02) = 0.14, rates for K judgments was even more noticeable.)
Table 1.
Proportions of old and new words and reaction times (RT) for R, K, and N judgments in the test condition
| Judgment | Old words (n = 120) | New words (n = 60) | ||||
|---|---|---|---|---|---|---|
| R | K | N | R | K | N | |
| Number | ||||||
| Mean | 0.51 | 0.26 | 0.21 | 0.02 | 0.14 | 0.82 |
| SD | (0.31) | (0.15) | (0.18) | (0.04) | (0.14) | (0.16) |
| RT/msec | ||||||
| Mean | 1533 | 2361 | 2084 | 2280 | 2444 | 1698 |
| SD | (402) | (659) | (555) | (529) | (972) | (314) |
R judgments were almost twice as common as K judgments on average, although the range of R and K judgments was large, with one participant failing to give >10 R judgments and two participants failing to give >10 K judgments. These participants were removed from the relevant image contrasts below (their removal did not have any appreciable effect on the means and SDs reported in Table 1 or on the significance of tests performed on the behavioral data). The mean correct reaction times were longer for K judgments than R or N judgments;t(12) > 3.53, p < 0.01 in both cases. Mean correct reaction times did not differ significantly for R and N judgments (t(12) = 1.51, p> 0.10). The numbers of incorrect R, K, and N judgments were deemed insufficient to analyze these event-types further. Subsequent analyses are therefore restricted to correct judgments.
Imaging data
The maxima of all brain regions showing differential event-related responses to correct R, K, and N judgments are shown in Tables 2-4. Below we discuss the responses of selected regions that were predicted on the basis of previous findings.
Table 2.
Maxima within regions showing significant BOLD signal changes in the comparisons between correct R and correct N judgments (excluding one participant who made insufficient numbers of R judgments)
| Region of activation | Left/right | Brodmann area (BA) | No. of voxels | Talairach coordinates | Z value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Increases for R judgments | |||||||
| Inferior frontal gyrus | L | 47 | 84 | −48 | 39 | −12 | 3.94 |
| Middle frontal gyrus | L | 9 | 19 | −54 | 24 | 33 | 3.34 |
| L | 46 | 37 | −60 | 27 | 21 | 3.99 | |
| Superior frontal gyrus | L | 10 | 17 | −12 | 63 | 18 | 4.11 |
| Medial frontal gyrus | L | 9 | 12 | −6 | 39 | 27 | 3.71 |
| Superior parietal gyrus | L | 7 | 173 | −33 | −60 | 45 | 4.18 |
| Inferior parietal gyrus | L | 40 | −51 | −45 | 39 | 3.67 | |
| Medial temporal gyrus | L | 30 | 12 | −12 | −36 | 3 | 3.62 |
| Posterior cingulate | L | 23 | 13 | −6 | −24 | 27 | 3.51 |
| L | 31 | 24 | −6 | −42 | 36 | 4.73 | |
| Precuneus | L | 7 | 11 | −6 | −75 | 42 | 3.42 |
| L | 7 | 6 | 0 | −66 | 33 | 3.71 | |
| Increases for N judgments | |||||||
| Middle frontal gyrus | R | 8 | 17 | 30 | 39 | 48 | 3.91 |
| Superior frontal gyrus | R | 6 | 15 | 18 | 6 | 54 | 4.18 |
| Insula | L | 21 | −51 | −6 | 3 | 3.59 | |
| R | 30 | 39 | −3 | −6 | 4.45 | ||
| Amygdala | L | 33 | −30 | 3 | −24 | 3.84 | |
| R | 10 | 33 | 3 | −24 | 4.22 | ||
| Precuneus | L | 7 | 47 | −12 | −45 | 57 | 4.51 |
| Inferior parietal gyrus | L | 40 | 16 | −51 | −21 | 30 | 3.90 |
| Precuneus | R | 7 | 224 | 21 | −45 | 51 | 4.73 |
| Inferior parietal gyrus | R | 40 | 42 | −30 | 45 | 4.27 | |
| Middle temporal gyrus | L | 37 | 122 | −33 | −75 | 12 | 3.98 |
| Middle temporal gyrus | R | 37 | 40 | 51 | −60 | 9 | 3.69 |
L, left; R, right; B, bilateral.
Table 3.
Maxima of regions showing significant BOLD signal changes in the comparisons between correct K and correct N judgments (excluding two participants who made insufficient numbers of K judgments)
| Region of activation | Left/right | Brodmann area (BA) | No. of voxels | Talairach coordinates | Z value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Increases for K judgments | |||||||
| Inferior frontal gyrus | L | 47 | 13 | −51 | 15 | −6 | 3.48 |
| R | 47 | 7 | 51 | 21 | −6 | 3.38 | |
| Middle frontal gyrus | L | 9 | 153 | −60 | 24 | 15 | 4.05 |
| R | 9 | 24 | 42 | 21 | 33 | 3.92 | |
| R | 46 | 34 | 51 | 39 | 21 | 4.21 | |
| Medial frontal gyrus | B | 9 | 202 | −9 | 42 | 24 | 5.63 |
| Precuneus | L | 19 | 6 | −24 | −63 | 42 | 3.79 |
| Increases for N judgments | |||||||
| Middle frontal gyrus | R | 8 | 11 | 18 | 54 | 42 | 3.72 |
| Insula | L | 4 | −36 | −6 | −3 | 3.57 | |
| R | 14 | 45 | −12 | −12 | 3.82 | ||
| Amygdala | L | 4 | −24 | −3 | −24 | 3.36 | |
| R | 9 | 24 | −9 | −18 | 3.39 | ||
| Precuneus | R | 31 | 17 | 12 | −51 | 33 | 4.01 |
| Inferior temporal gyrus | R | 20 | 34 | 63 | −15 | −18 | 4.29 |
| Middle temporal gyrus | R | 21 | 35 | 54 | −21 | −12 | 4.21 |
| Superior temporal gyrus | L | 22 | 14 | −60 | −33 | 15 | 3.76 |
| R | 42 | 14 | 66 | −21 | 15 | 3.77 | |
| Middle temporal gyrus | L | 37 | 7 | −51 | −51 | −9 | 3.59 |
| R | 39 | 17 | 57 | −63 | 15 | 3.63 | |
L, Left; R, right; B, bilateral.
Table 4.
Maxima of regions showing significant BOLD signal changes in the comparisons between correct R and correct K judgments at study and at test (excluding three participants who made insufficient numbers of R or K judgments)
| Region of activation | Left/right | Brodmann area (BA) | No. of voxels | Talairach coordinates | Z value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Test | |||||||
| Increases for R judgments | |||||||
| Superior frontal gyrus | L | 8 | 4 | −21 | 54 | 39 | 3.88 |
| Posterior cingulate | B | 24 | 13 | 0 | −30 | 36 | 3.66 |
| Inferior parietal gyrus | L | 40 | 7 | −57 | −51 | 39 | 3.89 |
| Superior parietal gyrus | L | 19 | 4 | −42 | −72 | 39 | 3.38 |
| Increases for K judgments | |||||||
| Middle frontal gyrus | R | 46 | 8 | 51 | 30 | 27 | 3.51 |
| Medial frontal gyrus | L | 9 | 8 | −12 | 39 | 27 | 3.55 |
| R | 9 | 14 | 9 | 39 | 30 | 3.64 | |
| Anterior cingulate | L | 24 | 4 | −12 | 9 | 36 | 3.51 |
| Superior temporal gyrus | L | 22 | 6 | −39 | −42 | 12 | 3.72 |
| Precuneus | L | 7 | 6 | −12 | −60 | 57 | 3.71 |
| Study | |||||||
| Increases for R judgments | |||||||
| Inferior frontal gyrus | L | 47 | 6 | −45 | 24 | −6 | 3.19 |
| Middle frontal gyrus | L | 9 | 25 | −57 | 18 | 27 | 3.98 |
| Brainstem | R | 17 | 12 | −12 | −9 | 3.80 | |
| Precuneus | L | 7 | 7 | −27 | −60 | 60 | 4.74 |
| Increases for K judgments | |||||||
| Parahippocampal gyrus | R | 35 | 6 | 18 | −15 | −24 | 3.77 |
| Precuneus | R | 7 | 5 | 3 | −69 | 33 | 3.83 |
| R | 7 | 6 | 18 | −54 | 48 | 3.67 | |
L, Left; R, right; B, bilateral.
Correct R versus correct N judgments at test
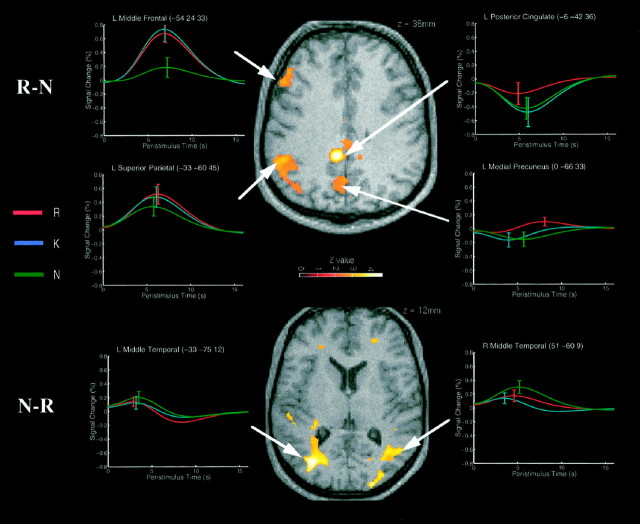
The regions exhibiting greater event-related responses to R than N judgments were strikingly left-lateralized, probably reflecting the verbal nature of the stimuli. The top panel of Figure1 shows a transverse slice through the left prefrontal, left parietal, posterior cingulate, and precuneus regions reported in Table 2. The left midlateral prefrontal region (BA 9/46) showed similar responses to R and K judgments, but less response to N judgments. Activation of nearby regions in comparison of old versus new stimuli was observed by Tulving et al. (1996). The left lateral superior parietal region (BA 7) showed a more graded pattern of responses, with the greatest to R judgments and the least to N judgments. Activity in this area may underlie the left parietal ERP difference observed between correct responses to old and new words (Rugg, 1995). The posterior cingulate region (BA 31) showed a decreased response relative to baseline fixation, with the least deactivation for R judgments. Given that deactivations may be a consequence of the global normalization of the BOLD signal (Aguirre et al., 1998), we emphasize only the differential nature of the response as a function of judgment type. The precuneus region (BA 7/31) showed a small activation for R judgments and small deactivations for K and N judgments. This region is consistently activated during episodic retrieval and may reflect reinstatement of visual images associated with words during study [Fletcher et al. (1996); although see Buckner and Peterson (1996)].
Fig. 1.
Regions showing enhanced event-related responses to correct R versus correct N judgments (top panel) and correct N versus correct R judgments (bottom panel). The anatomical slices are taken through a normalized T1 structural image of one participant’s brain. The activations reflect t tests on the height of the best-fitting canonical hemodynamic response function (HRF) across participants, thresholded at p < 0.01 for the purpose of illustration. The event-related plots are the sum of the best-fitting canonical HRF and its derivative (see Materials and Methods) from the voxel in the maxima of the activations, for the nine participants who made sufficient numbers of correct R, K, and N judgments. The error bars show the SE of the mean fitted HRF height across the nine participants (not the SE of the mean difference in fitted HRF heights for R and N judgments, which forms the error term in the repeated-measures t tests).
Other notable regions exhibiting greater responses to R than N judgments included a ventral region in left inferior frontal gyrus (BA 47), a more anterior region in left superior frontal gyrus (BA 10), and a posterior medial temporal region in the left hippocampus, close to the fornix. The left prefrontal regions have been associated with reflective processes by Nolde et al. (1999), and the posterior medial temporal region has been associated with episodic retrieval in a meta-analysis by LePage et al. (1998) (although see Schacter and Wagner, 1998) and in our own studies (Strange et al., 1999).
Several regions showed greater responses to N than R judgments. These included a large region in right parietal cortex, extending from precuneus to superior and inferior parietal gyri (BA 7/40), and large regions of temporoccipital cortex, particularly on the left and extending along the lingual, parahippocampal, and middle temporal gyri (BA 19/37). The reduced response elicited by R and K judgments relative to N judgments in the temporoccipital regions (Fig. 1, bottom panel) is consistent with the relative deactivations for familiar versus novel stimuli that have been attributed to perceptual priming (Blaxton et al., 1996; Buckner et al., 1996; Schacter and Buckner, 1998).
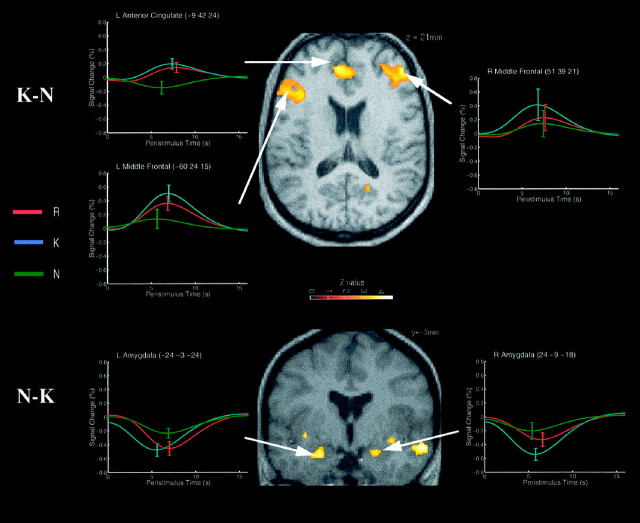
Correct K versus correct N judgments at test
The regions exhibiting greater responses to K than N judgments were confined mainly to left and right prefrontal cortices (Table3; Fig. 2,top panel) and included bilateral middle frontal gyri (BA 9), bilateral medial frontal gyri, centred in the cingulate sulci (BA 9/32), bilateral posterior inferior frontal gyri (BA 47), and a more anterior region of right midlateral prefrontal cortex (BA 46). The left prefrontal regions generally showed greater responses to R and K judgments than N judgments, whereas the right prefrontal regions generally showed greater responses to K judgments than R and N judgments. One or more of these regions are usually activated during episodic retrieval (for review, see Cabeza and Nyberg, 1997; Desgranges et al., 1998), particularly when retrieval is effortful (Schacter et al., 1996; Rugg et al., 1997; Buckner et al., 1998b). A small region of left lateral precuneus (BA 19) also exhibited greater responses to K than N judgments, but the spatial extent of left parietal activation was noticeably smaller than in the comparison of R and N judgments.
Fig. 2.
Regions showing enhanced event-related responses to correct K versus correct N judgments (top panel) and correct N versus correct K judgments (bottom panel). For details, see Figure 1.
Many regions that showed reduced responses for R relative to N judgments also showed reduced responses to K judgments, notably bilateral posterior middle temporal gyri (BA 37/39), bilateral insular cortex, and bilateral anterior medial temporal cortex. Although the spatial smoothing and averaging entailed by our statistical inference across participants makes precise localization difficult, the group maxima of the medial temporal regions was located just anterior to the temporal horn of the lateral ventricle in each participant’s normalized structural image, making the amygdala the most likely candidate. The response of these regions was a deactivation relative to baseline (Fig. 2, bottom panel), but with less deactivation for N than R or K judgments. This differential sensitivity to new versus old words is consistent with the proposal that anterior regions of the amygdala–hippocampal complex are sensitive to stimulus novelty (Tulving et al., 1996; Dolan and Fletcher, 1997; LePage et al., 1998; Strange et al., 1999), in this case the contextual novelty of the new words.
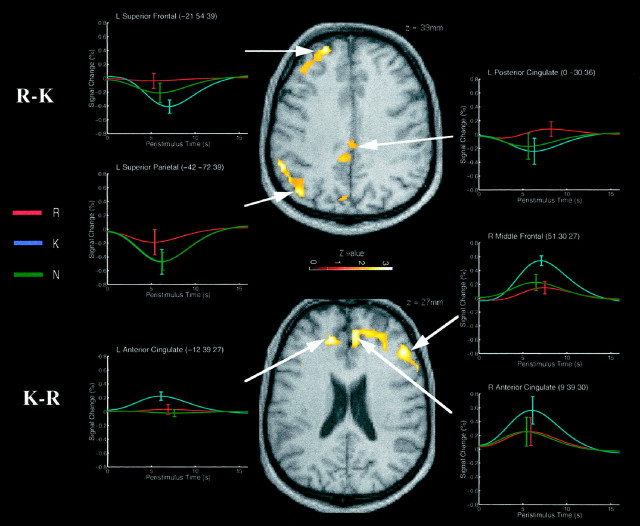
Correct R versus correct K judgments at test
The direct contrast of R against K judgments revealed a subset of the regions identified in the R versus N contrast, namely left inferior parietal (BA 40), left superior parietal (BA 7), and posterior cingulate (BA 24/31) regions, in addition to a region of left anterior superior frontal gyrus (BA 8/9; Table 4, Test). For the prefrontal and left parietal regions, this difference reflected greater deactivation for K (and N) judgments relative to R judgments (Fig. 3,top panel). For the posterior cingulate region, the difference reflected an activation for R judgments and deactivations for K (and N) judgments. The left superior parietal maximum is very close to that previously associated with retrieval of contextual information (Henson et al., 1999) and may underlie the left parietal ERP differences attributed to source retrieval (Wilding and Rugg, 1996;Allan et al., 1998). Anterior left prefrontal regions have also been associated with source retrieval by Nolde et al. (1998a), although the anterior region identified in the present study is more superior (BA 8/9 rather than BA 10).
Fig. 3.
Regions showing enhanced event-related responses to correct R versus correct K judgments (top panel) and correct K versus correct R judgments (bottom panel). For details, see Figure 1.
The reverse contrast of K against R judgments implicated three of the regions associated with the K versus N contrast, namely left and right cingulate sulci (BA 9/32) and right midlateral prefrontal cortex (BA 46). All three regions showed greater responses to K judgments than either R or N judgments (Fig. 3, bottom panel). These differences may reflect greater retrieval monitoring associated with K judgments (see Discussion), reflected by the longer reaction times for K than R or N judgments. The responses of the more posterior inferior and middle frontal regions did not appear to differentiate R and K judgments.
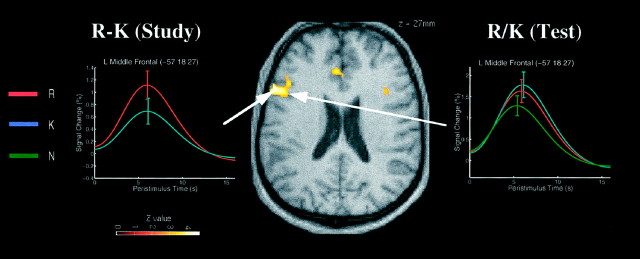
Correct R versus correct K judgments at study
Figure 4 shows a region in left posterior middle frontal gyrus (BA 9/44) that exhibited a greater response to words at study that were subsequently given an R judgment at test than words that were subsequently given a K judgment. Because every event in this comparison was associated with a correct lexical decision, the response times of which did not differ significantly as a function of the later recognition judgment, this subsequent memory effect is unlikely to reflect simple perceptual differences. Rather, it is likely to reflect differences in semantic elaborative or organizational processes that aid memory encoding (Fletcher et al., 1998). A similar finding was reported recently by Wagner et al. (1998b).
Fig. 4.
Regions showing enhanced event-related responses at study to words given correct R versus correct K judgments in the subsequent recognition test. The event-related plot on theleft shows the fitted response at study; the plot on theright shows the fitted response of the same voxel at test. For details, see Figure 1.
Responses measured at the same voxel, however, did not differentiate R and K judgments at test (although they did differentiate R and K judgments from N judgments). Similar response profiles, which distinguished R and K judgments at study but not at test, were also seen for a more ventral region of left inferior frontal gyrus (BA 47) and a lateral region of left precuneus (BA 7; Table 4, Study). Thus the brain regions in which activity during study predicted subsequent recollective experience did not necessarily reflect that experience during test. A right hippocampal–parahippocampal region showed greater response to words at study that were subsequently given K rather than R judgments, but given that this pattern was not parallelled at test, and that no predictions were made for this opposite pattern (cf. Brewer et al., 1998; Wagner et al., 1998b), we do not offer any explanation for responses in this or the precuneus regions in Table 4, Study.
DISCUSSION
The present study represents one of the first event-related fMRI experiments to identify brain regions that exhibit differential hemodynamic responses according to whether participants recognize stimuli from a previous study episode (cf. Schacter et al., 1997a;Buckner et al., 1998a). Moreover, it provides evidence that several brain regions exhibit differential responses as a function of whether correct recognition is associated with recollection, as operationalized by the remember–know procedure (Tulving, 1985). This combination of event-related fMRI with a subjective classification of events heralds an exciting future for the neuroimaging of human memory.
The striking left-lateralization of regions associated with recollection of old words (R judgments) relative to rejection of new words (N judgments) is surprising in light of numerous studies that have associated episodic retrieval with right prefrontal cortex (Shallice et al., 1994; Tulving et al., 1994; Buckner and Peterson, 1996; Nyberg et al., 1996; Fletcher et al., 1997). Most of these studies have used blocked rather than event-related designs and compared an episodic retrieval task with a comparable control task. One possibility is that the right prefrontal activations in these comparisons reflect the adoption of a retrieval mode (Kapur et al., 1995; Nyberg et al., 1995), the cognitive state arising whenever one attempts to refer to past experiences (Tulving, 1983). Because participants in the present study were attempting retrieval throughout the experiment, differential responses in right prefrontal cortex therefore might not be expected. Other studies (Rugg et al., 1996;Buckner et al., 1998b) have found right prefrontal activation when comparing blocks of a retrieval task in which only the ratio of old to new words varied. These activations tend to cluster in anterior regions of prefrontal cortex, and we may have failed to detect differential responses in these regions by virtue of diminished sensitivity owing to fMRI susceptibility artifacts in frontopolar regions.
Nonetheless, we did find differential responses in right posterior prefrontal cortex associated with old relative to new words when recognition was based on familiarity in the absence of recollection (i.e., K judgments). These regions included ventral and dorsal regions of posterior prefrontal cortex, medial frontal/anterior cingulate cortex, and dorsal midlateral prefrontal cortex. We attribute these differences to postretrieval processing (Shallice et al., 1994; Rugg et al., 1996). The right dorsolateral activation in particular we attribute to monitoring (cf. Nolde et al., 1999): processes that verify whether the products of retrieval attempts are sufficient for the current task (Burgess and Shallice, 1996). When these products include the spatiotemporal context of a word’s previous occurrence, an R judgment can follow immediately. When no such spatiotemporal information is forthcoming, yet the products of retrieval are associated with a feeling of familiarity, further retrieval attempts may ensue before a judgment is made (explaining why reaction times were longer for K judgments than R or N judgments). When repeated retrieval attempts fail to reinstate any contextual information, the decision remains whether to attribute the familiarity to a word’s previous occurrence (i.e., give a K or an N judgment). The relatively high proportion of misses in the present experiment suggests that many K judgments to old words were close to the K–N criterion (Donaldson, 1996; Yonelinas et al., 1996). In other words, we suggest that monitoring requirements during recognition are highest when familiarity levels are close to a response criterion. The right prefrontal activations associated with higher old/new word ratios in the studies of Rugg et al. (1996) and Buckner et al. (1998b) may therefore not reflect retrieval success per se but rather differing degrees of monitoring resulting from shifting response criteria, particularly given that these activations are sensitive to changes in task instructions (Wagner et al., 1998a).
Unlike their homologs in right prefrontal cortex, ventral and dorsal regions of left posterior prefrontal cortex exhibited greater responses to both R and K judgments relative to N judgments. Thus, although these regions indexed successful retrieval, they did not appear to distinguish recollection and familiarity or the degree of monitoring. They may subserve other postretrieval processes such as maintaining or manipulating retrieval products in working memory (Petrides et al., 1995). The left prefrontal region that did exhibit greater responses to R than K judgments was in anterior prefrontal cortex. Nolde et al. (1998) found that nearby, although generally inferior, regions of anterior left prefrontal cortex exhibited greater event-related responses to words in a source retrieval task than in a simple yes–no recognition task. Comparable results were observed in a recent blocked fMRI study (M. Rugg, P. Fletcher, P. Chua, and R. Dolan, unpublished observations). Anterior left prefrontal cortex therefore may be specialized for the reflective processes associated with source retrieval (Nolde et al., 1999), and damage to nearby regions may contribute to the source retrieval impairment observed in frontal patients (Janowsky et al., 1989; Shimamura et al., 1990).
A left posterior prefrontal region, close to that identified by Wagner et al. (1998b), exhibited a greater response to words at study that were subsequently given an R as opposed to a K judgment. However, no brain region in the present study showed a significantly greater response to R than K judgments at both study and test. This finding is troublesome for at least one interpretation of transfer-appropriate processing theory (Morris et al., 1977; Kolers and Roediger, 1984), which according to Blaxton et al. (1996) holds that the same brain regions differentiate memory performance at both study and test. An alternative proposal (Brewer et al., 1998; Wagner et al., 1998b) is that the results of processes performed in prefrontal cortex during encoding (such as semantic elaboration and organization) comprise the input to a medial temporal memory system. Such processes are less important when the words are seen again during test, when performance is assumed to be driven mainly by episodic retrieval from medial temporal structures.
Several regions within left parietal cortex also showed greater responses to R than K judgments. At least one of these regions, in left precuneus, also showed greater responses to K than N judgments, suggesting that some left parietal regions show a graded response to R, K, and N judgments. This accords with several studies in which the magnitude of the left parietal ERP old/new effect increases with the amount of contextual information retrieved (Allan et al., 1998), suggesting that the difference between R and K judgments may be quantitative rather than qualitative (Johnson et al., 1993; Donaldson, 1996). More generally, our findings of both left parietal and bilateral prefrontal differences between R and K judgments are highly consistent with ERP findings (Smith, 1993; Wilding and Rugg, 1996; Duzel et al., 1997; Johnson et al., 1997; Rugg et al., 1998). Furthermore, our data suggest a dissociation between the responses of parietal and prefrontal cortices, with the former showing a greater hemodynamic response to R judgments and the latter showing a greater hemodynamic response to K judgments.
Our findings are moot with respect to the neuropsychological findings of Knowlton and Squire (1995), who suggested that medial temporal structures are important for both R and K judgments. We identified a medial posterior region of left hippocampus that exhibited greater response to R than N judgments, but any difference between K and N judgments in this region failed to reach significance. This finding is more consistent with the suggestion of Aggleton and Brown (1998) that recollection requires hippocampal involvement. Nonetheless, no medial temporal structure showed a differential response in a direct comparison of R and K judgments. Indeed, whether one regards recollection as indexed by the R versus N comparison or by the R versus K comparison depends on whether one regards recollection and familiarity as independent, redundant, or exclusive (Knowlton and Squire, 1995; Yonelinas et al., 1996).
One medial temporal structure that did exhibit differential responses to both R and K judgments relative to N judgments was the amygdala, which showed less deactivation relative to baseline in response to N judgments than R or K judgments. The amygdala has previously been associated with novelty detection (Wilson and Rolls, 1993) and the encoding of emotionally salient stimuli (Babinsky et al., 1993; Cahill et al., 1996). The word stimuli used in the present study are unlikely to have much emotional significance, however, and other studies have shown that the amygdala is not necessary for episodic memory (Bechara et al., 1995; Parker et al., 1998). One possibility is that amygdala activity reflects an obligatory orienting or arousal response to novel stimuli, with the translation of novelty into effective memory encoding depending on other medial temporal structures.
Previous neuroimaging studies have provided good evidence for dissociable brain systems underlying, for example, episodic, semantic, and implicit memory (Gabrieli, 1998). In the present study, we have shown further that the subjective classification of stimuli afforded by event-related techniques allows neuroscientists to begin to addressTulving’s (1983) call for a scientific approach to the conscious experience accompanying memory retrieval.
Footnotes
This work was supported by Wellcome Trust Grant 051028/Z.
Correspondence should be addressed to Dr. Richard Henson, Wellcome Department of Cognitive Neurology, 12 Queen Square, London WC1N 3BG, UK.
REFERENCES
- 1.Aggleton JP, Brown MW (1999) Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci, in press. [PubMed]
- 2.Aguirre GK, Zarahn E, D’Esposito M. The inferential impact of global signal covariates in functional neuroimaging analyses. NeuroImage. 1998;8:302–306. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- 3.Allan K, Wilding EL, Rugg MD. Electrophysiological evidence for dissociable processes contributing to recollection. Acta Psychol. 1998;98:231–252. doi: 10.1016/s0001-6918(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 4.Babinsky R, Calabrese P, Durwen HF, Markowitsch HJ, Brechtelsbauer D, Heuser L, Gehlen W. The possible contributions of the amygdala to memory. Behav Neurobiol. 1993;6:167–170. doi: 10.3233/BEN-1993-6310. [DOI] [PubMed] [Google Scholar]
- 5.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 6.Blaxton TA, Bookheimer SY, Zeffiro TA, Figlozzi CM, Gaillard WD, Theodore WH. Functional mapping of human memory using PET: comparisons of conceptual and perceptual tasks. Can J Exp Psychol. 1996;50:42–56. doi: 10.1037/1196-1961.50.1.42. [DOI] [PubMed] [Google Scholar]
- 7.Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JDE. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:185–187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 8.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargelstellt auf Grund des Zellesbaues. Barth; Leipzig: 1909. [Google Scholar]
- 9.Buckner RL, Peterson SE. What does neuroimaging tell us about the role of prefrontal cortex in memory retrieval? Semin Neurosci. 1996;8:47–55. [Google Scholar]
- 10.Buckner RL, Raichle ME, Miezin FM, Petersen SE. Functional anatomic studies of memory retrieval for auditory words and visual pictures. J Neurosci. 1996;16:6219–6235. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckner RL, Koutstaal W, Schacter DL, Dale AM, Rotte M, Rosen BR. Functional-anatomic study of episodic retrieval. II. Selective averaging of event-related fMRI trials to test the retrieval success hypothesis. NeuroImage. 1998a;7:163–175. doi: 10.1006/nimg.1998.0328. [DOI] [PubMed] [Google Scholar]
- 12.Buckner RL, Koutstaal W, Schacter DL, Wagner AD, Rosen BR. Functional-anatomic study of episodic retrieval. I. Retrieval effort versus retrieval success. NeuroImage. 1998b;7:151–162. doi: 10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- 13.Burgess PW, Shallice T. Confabulation and the control of recollection. Memory. 1996;4:359–411. doi: 10.1080/096582196388906. [DOI] [PubMed] [Google Scholar]
- 14.Cabeza R, Nyberg L. Imaging cognition: an empirical review of PET studies with normal subjects. J Cognit Neurosci. 1997;9:1–26. doi: 10.1162/jocn.1997.9.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocosco CA, Kollokian V, Kwan RKS, Evans AC. Brainweb: online interface to a 3D MRI simulated brain database. NeuroImage. 1997;5:425. [Google Scholar]
- 17.Desgranges B, Baron J-C, Eustache F. The functional neuroanatomy of episodic memory: the role of the frontal lobes, the hippocampal formation, and other areas. NeuroImage. 1998;8:198–213. doi: 10.1006/nimg.1998.0359. [DOI] [PubMed] [Google Scholar]
- 18.Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson W. The role of decision processes in remembering and knowing. Mem Cognit. 1996;24:523–533. doi: 10.3758/bf03200940. [DOI] [PubMed] [Google Scholar]
- 20.Duzel E, Yonelinas AP, Mangun GR, Heinze HJ, Tulving E. Event-related brain potential correlates of two states of conscious awareness in memory. Proc Natl Acad Sci USA. 1997;94:5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fletcher PC, Shallice T, Frith CD, Frackowiak RSJ, Dolan RJ. Brain activity during memory retrieval: the influence of imagery and semantic cueing. Brain. 1996;119:1587–1596. doi: 10.1093/brain/119.5.1587. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher PC, Frith CD, Rugg MD. The functional neuroanatomy of episodic memory. Trends Neurosci. 1997;20:213–218. doi: 10.1016/s0166-2236(96)01013-2. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher PC, Shallice T, Dolan RJ. The functional roles of the prefrontal cortex in episodic memory. I. Encoding. Brain. 1998;121:1239–1248. doi: 10.1093/brain/121.7.1239. [DOI] [PubMed] [Google Scholar]
- 24.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 25.Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 26.Gabrieli JDE. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- 27.Gardiner JM. Functional aspects of recollective experience. Mem Cognit. 1988;16:309–313. doi: 10.3758/bf03197041. [DOI] [PubMed] [Google Scholar]
- 28.Gardiner JM, Java RI. Recollective experience in word and nonword recognition. Mem Cognit. 1990;18:23–30. doi: 10.3758/bf03202642. [DOI] [PubMed] [Google Scholar]
- 29.Gardiner JM, Parkin AJ. Attention and recollective experience in recognition memory. Mem Cognit. 1990;18:579–583. doi: 10.3758/bf03197100. [DOI] [PubMed] [Google Scholar]
- 30.Gardiner JM, Gawlik B, Richardson-Klavehn A. Maintenance rehearsal affects knowing not remembering: elaborative rehearsal affects remembering not knowing. Psychon Bull Rev. 1994;1:107–110. doi: 10.3758/BF03200764. [DOI] [PubMed] [Google Scholar]
- 31.Henson RNA, Shallice T, Dolan RJ (1999) Right prefrontal cortex and memory retrieval: an fMRI test of the monitoring hypothesis. Brain, in press. [DOI] [PubMed]
- 32.Holmes AP, Josephs O, Büchel C, Friston KJ. Statistical modelling of low frequency confounds in fMRI. NeuroImage. 1997;5:S480. [Google Scholar]
- 33.Janowsky JS, Shimamura AP, Kritchevsky M, Squire LR. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav Neurosci. 1989;103:548–560. doi: 10.1037//0735-7044.103.3.548. [DOI] [PubMed] [Google Scholar]
- 34.Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Rev. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- 35.Johnson MK, Kounios J, Nolde SF. Electrophysiological brain activity and memory source monitoring. NeuroReport. 1997;8:1317–1320. doi: 10.1097/00001756-199703240-00051. [DOI] [PubMed] [Google Scholar]
- 36.Josephs O, Turner R, Friston K. Event-related fMRI. Hum Brain Mapp. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 37.Kapur S, Craik F, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in memory retrieval: a PET study. NeuroReport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 38.Knowlton BJ, Squire LR. Remembering and knowing: two different expressions of declarative memory. J Exp Psychol. 1995;21:699–710. doi: 10.1037//0278-7393.21.3.699. [DOI] [PubMed] [Google Scholar]
- 39.Kolers PA, Roediger HL. Procedures of mind. J Verb Learn Verb Behav. 1984;23:425–449. [Google Scholar]
- 40.LePage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 41.Morris CD, Bransford JD, Franks JJ. Levels of processing versus transfer appropriate processing. J Verb Learn Verb Behav. 1977;16:519–533. [Google Scholar]
- 42.Nolde SF, Johnson MK, D’Esposito M. Left prefrontal activation during episodic memory: an event-related study. NeuroReport. 1998;9:3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- 43.Nolde SF, Johnson MK, Raye CL (1999) The role of prefrontal cortex during tests of episodic memory. Trends Cognit Sci, in press. [DOI] [PubMed]
- 44.Nyberg L, Tulving E, Habib R, Nilsson LG, Kapur S, Cabeza R, McIntosh AR. Functional brain maps of retrieval mode and recovery of episodic information. NeuroReport. 1995;7:249–252. [PubMed] [Google Scholar]
- 45.Nyberg L, Cabeza R, Tulving E. PET studies of encoding and retrieval: the HERA model. Psychon Bull Rev. 1996;3:135–148. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- 46.Parker A, Wilding E, Akerman C. The von Restorff effect in visual object recognition in humans and monkeys: the role of frontal/perirhinal interaction. J Cognit Neurosci. 1998;10:691–703. doi: 10.1162/089892998563103. [DOI] [PubMed] [Google Scholar]
- 47.Petrides M, Alivisatos B, Evans AC. Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proc Natl Acad Sci USA. 1995;92:5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajaram S. Remembering and knowing: two means of access to the personal past. Mem Cognit. 1993;21:89–102. doi: 10.3758/bf03211168. [DOI] [PubMed] [Google Scholar]
- 49.Rugg MD. ERP studies of memory. In: Rugg MD, Coles MGH, editors. Electrophysiology of mind. Oxford UP; Oxford: 1995. pp. 132–170. [Google Scholar]
- 50.Rugg MD, Fletcher PC, Frith CD, Frackowiak RSJ, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- 51.Rugg MD, Fletcher PC, Frith CD, Frackowiak RSJ, Dolan RJ. Brain regions supporting intentional and incidental memory: a PET study. NeuroReport. 1997;8:1283–1287. doi: 10.1097/00001756-199703240-00045. [DOI] [PubMed] [Google Scholar]
- 52.Rugg MD, Schloerscheidt AM, Mark RE. An electrophysiological comparison of two indices of recollection. J Mem Lang. 1998;39:47–69. [Google Scholar]
- 53.Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 54.Schacter DL, Wagner AD (1999) Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus, in press. [DOI] [PubMed]
- 55.Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schacter DL, Buckner RL, Koutstaal W, Dale AM, Rosen BR. Late onset of anterior prefrontal activity during true and false recognition: an event-related fMRI study. NeuroImage. 1997a;6:259–269. doi: 10.1006/nimg.1997.0305. [DOI] [PubMed] [Google Scholar]
- 57.Schacter DL, Verfaellie M, Anes MD. Illusory memories in amnesic patients: conceptual and perceptual false recognition. Neuropsychology. 1997b;11:331–342. doi: 10.1037//0894-4105.11.3.331. [DOI] [PubMed] [Google Scholar]
- 58.Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RSJ, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- 59.Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–814. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- 60.Smith ME. Neurophysiological manifestations of recollective experience during recognition memory judgements. J Cognit Neurosci. 1993;5:1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- 61.Strange BA, Fletcher, PC, Henson, RNA, Friston, KJ, Dolan, RJ (1999) Segregating the functions of human hippocampus. Proc Nat Acad Sci USA, in press. [DOI] [PMC free article] [PubMed]
- 62.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. George Thieme Verlag; Stuttgart: 1988. [Google Scholar]
- 63.Tulving E. Elements of episodic memory. Oxford UP; Oxford: 1983. [Google Scholar]
- 64.Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- 65.Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tulving E, Markowitsch HJ, Craik FIM, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 67.Wagner AD, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal cortex and recognition memory: functional-MRI evidence for context-dependent retrieval processes. Brain. 1998a;121:1985–2002. doi: 10.1093/brain/121.10.1985. [DOI] [PubMed] [Google Scholar]
- 68.Wagner AD, Schacter DL, Rotte M, Koustaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998b;21:188–191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 69.Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- 70.Wilson FAW, Rolls ET. The effects of stimulus novelty and familiarity on neuronal activity in the amygdala of monkeys performing recognition memory tasks. Exp Brain Res. 1993;93:367–382. doi: 10.1007/BF00229353. [DOI] [PubMed] [Google Scholar]
- 71.Yonelinas AP, Dobbins I, Szymanski MD, Dhaliwal HS, King L. Signal detection, threshold, and dual-process models of recognition memory: ROCs and conscious recollection. Consciousness Cognit. 1996;5:418–441. doi: 10.1006/ccog.1996.0026. [DOI] [PubMed] [Google Scholar]
- 72.Yonelinas AP, Kroll NEA, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12:323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]