Abstract
The suprachiasmatic nucleus (SCN) receives glutamatergic afferents from the retina and serotonergic afferents from the midbrain, and serotonin (5-HT) can modify the response of the SCN circadian oscillator to light. 5-HT1B receptor-mediated presynaptic inhibition has been proposed as one mechanism by which 5-HT modifies retinal input to the SCN (Pickard et al., 1996). This hypothesis was tested by examining the subcellular localization of 5-HT1Breceptors in the mouse SCN using electron microscopic immunocytochemical analysis with 5-HT1B receptor antibodies and whole-cell patch-clamp recordings from SCN neurons in hamster hypothalamic slices. 5-HT1B receptor immunostaining was observed associated with the plasma membrane of retinal terminals in the SCN. 1-[3-(Trifluoromethyl)phenyl]-piperazine HCl (TFMPP), a 5-HT1B receptor agonist, reduced in a dose-related manner the amplitude of glutamatergic EPSCs evoked by stimulating selectively the optic nerve. Selective 5-HT1A or 5-HT7receptor antagonists did not block this effect. Moreover, in cells demonstrating an evoked EPSC in response to optic nerve stimulation, TFMPP had no effect on the amplitude of inward currents generated by local application of glutamate. The effect of TFMPP on light-induced phase shifts was also examined using 5-HT1B receptor knock-out mice. TFMPP inhibited behavioral responses to light in wild-type mice but was ineffective in inhibiting light-induced phase shifts in 5-HT1B receptor knock-out mice. The results indicate that 5-HT can reduce retinal input to the circadian system by acting at presynaptic 5-HT1B receptors located on retinal axons in the SCN.
Keywords: circadian rhythms, serotonin, 5-HT1B receptor knock-out mice, retinal ganglion cells, presynaptic, hypothalamic slice
The hypothalamic suprachiasmatic nucleus (SCN) is a circadian oscillator and an important component of the mammalian circadian system responsible for the generation of behavioral and physiological circadian rhythms (see Klein et al., 1991;van den Pol and Dudek, 1993). The SCN receives a direct input from the retina via the retinohypothalamic tract (RHT) that arises from a small subset of retinal ganglion cells (Hendrickson et al., 1972; Moore and Lenn, 1972; Pickard, 1982; Moore et al., 1995). RHT afferents serve to entrain the endogenous SCN oscillator to the 24 hr environmental day–night cycle (Johnson et al., 1988). In addition, the SCN receives a dense serotonergic input from the midbrain raphe (Azmitia and Segal, 1978; Moore et al., 1978; Meyer-Bernstein and Morin, 1996). Although serotonergic input to the SCN is not required for the expression of circadian behavior (Block and Zucker, 1976; Morin and Blanchard, 1991), serotonin (5-HT) and 5-HT agonists can modify the response of the SCN to light (Miller and Fuller, 1990; Selim et al., 1993; Rea et al., 1994, 1995; Pickard et al., 1996; Pickard and Rea, 1997a,b; Ying and Rusak, 1997; Weber et al., 1998).
At present, 14 distinct 5-HT receptor subtypes are recognized (Saudou and Hen, 1994; Hoyer and Martin, 1997). Binding sites for several 5-HT receptor subtypes have been reported in the SCN, including the 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, and 5-HT7 receptors (Sumner et al., 1992; Manrique et al., 1993; Prosser et al., 1993; Miller et al., 1997). However, the functional organization and subcellular localization of 5-HT receptor subtypes in the SCN are not well understood.
5-HT1B receptors are located predominately on axon terminals in the CNS (Boschert et al., 1994). Pickard et al. (1996)hypothesized that 5-HT1B receptors in the SCN were located on RHT axon terminals and served to regulate retinohypothalamic input to the circadian system. Systemic administration of 5-HT1Breceptor agonists to rodents inhibits light-induced behavioral phase shifts and light-stimulated Fos expression in the SCN in a dose-dependent manner (Pickard et al., 1996; Pickard and Rea, 1997a). Although these data are consistent with the interpretation that 5-HT1B receptors are localized presynaptically on RHT terminals in the SCN, a direct demonstration is required.
To test the hypothesis directly, we (1) examined the subcellular localization of 5-HT1B receptors in the SCN using electron microscopic immunocytochemical analysis with 5-HT1Breceptor antibodies to determine whether 5-HT1B receptors were present in terminals of retinal origin, (2) conducted whole-cell patch-clamp recordings of SCN neurons in hypothalamic slices to determine whether the 5-HT1B receptor agonist 1-[3-(trifluoromethyl)phenyl]-piperazine HCl (TFMPP) could reduce the amplitude of glutamatergic EPSCs evoked by selective stimulation of the optic nerve and whether 5-HT1B receptor agonists could reduce the amplitude of inward currents generated by local application of glutamate to SCN cells receiving retinal afferents, and (3) determined the effect of TFMPP on light-induced behavioral responses in 5-HT1B receptor knock-out mice.
Parts of this paper have been published previously (Belenky et al., 1998; Pickard et al., 1998).
MATERIALS AND METHODS
Animals. Syrian hamsters (Mesocricetus auratus; male; Charles River Laboratories, Wilmington, MA) between 4 and 6 weeks of age were used for the in vitroelectrophysiological experiments. After arrival from the supplier, 28-d-old hamsters were housed in groups of four and maintained under a light/dark (LD) cycle of 14:10 hr (LD 14:10; lights on at 8 A.M.). Illuminance at cage level was ∼150 lux, and food and water were available ad libitum.
Mice (C57BL/6J; male; The Jackson Laboratory, Bar Harbor, ME) between 6 and 12 weeks of age were used for light microscopic analysis of RHT and 5-HT afferents in the SCN and for electron microscopic immunocytochemical analysis of 5-HT1B receptors in the SCN. In addition, C57BL/6J mice were used in behavioral experiments as wild-type controls along with 5-HT1B receptor knock-out mice. 5-HT1B receptor knock-out mice, generated originally on a 129/Sv-ter genetic background (Saudou et al., 1994), were outbred to the C57BL/6J background for 10 generations and generously supplied by Dr. René Hen (Columbia University, New York, NY). A breeding colony of 5-HT1B receptor knock-out animals was maintained in our laboratories, and these animals were used to examine the effect of a 5-HT1B receptor agonist on light-induced phase shifts of circadian wheel-running activity. All procedures used in the study adhered to guidelines approved by the Colorado State University Animal Care and Use Committee.
Electron microscopic immunocytochemistry. Mice were deeply anesthetized with pentobarbital (40 mg/kg). Before perfusion, ∼50 μl of heparin (5000 IU/ml; Choay, Paris, France) was injected into the left ventricle of the heart. Animals were perfused transcardially with PBS (0.1 m phosphate buffer with 0.9% saline, pH 7.3) followed by freshly prepared fixative containing 4% paraformaldehyde and 0.075% glutaraldehyde (Electron Microscopy Sciences, Fort Washington, PA) in 0.1 m phosphate buffer at pH 7.3. In some cases, 0.2% picric acid was added to the standard fixative. Brains were removed and immersed in the same fixative for an additional 2 hr at room temperature and were then stored in PBS overnight at 4°C. Brains were sectioned at 50 μm in the coronal plane using aVibratome (Oxford Instruments). Sections were collected into ice-cold PBS and then incubated sequentially in PBS containing 0.5% sodium borohydride (20 min), 15% sucrose and 5% glycerol (15 min), and 30% sucrose with 10% glycerol (30 min). Sections were then frozen in liquid nitrogen-cooled isopentane (2 methylbutane) and then in liquid nitrogen (30 sec each) and thawed in PBS.
Sections were immunostained as described previously (Belenky et al., 1996) using affinity-purified goat polyclonal antibodies raised against peptides corresponding to amino acids 371–390 or 365–383 mapping at the C terminal of the human or mouse 5-HT1B receptors, respectively (Santa Cruz Biotechnology, Tebu, France). Briefly, after thorough rinsing in PBS, the sections were incubated in a blocking solution containing 2% egg albumin, 0.5% glycine, and 0.5% lysine in PBS (1 hr; room temperature), followed by incubation in primary antiserum diluted 1:500 for anti-mouse 5-HT1B peptide or 1:1500–3000 for anti-human 5-HT1B peptide in PBS containing 1% egg albumin (24–48 hr at 4°C and then overnight at room temperature). Sections were then incubated in biotinylated anti-goat IgG (1:500) followed by avidin-biotinylated horseradish peroxidase (1:100) (ABC Elite kit; Vector Laboratories, Burlingame, CA). 3,3′-Diaminobenzidine tetrahydrochloride (DAB; 5 mg/20 ml with 4 μl of 30% H2O2) was used as the chromogen.
Sections containing the SCN were post-fixed in a mixture of 1% osmium tetroxide and 1.5% potassium ferricyanide in 0.1 mcacodylate buffer, dehydrated in ascending concentrations of ethanol, and flat-embedded between Permanox tissue culture slides (Nalge Nunc, Naperville, IL) in EM-BED812 (Electron Microscopy Sciences). Ultrathin sections prepared with a Reichert Ultracut and a diamond knife (Diatome) were lightly stained with lead citrate and viewed in JEOL-100CX or JEOL-2000 electron microscopes.
Specificity of 5-HT1B receptor immunostaining was verified by increasing the dilutions of the primary antiserum, by omitting the primary antiserum, or by incubation in primary antiserum preabsorbed with an excess of the peptide used for raising the primary antibody (40 μg/ml; 48 hr at 4°C).
Light microscopic 5-HT immunocytochemistry. Serotonergic input to the mouse SCN was labeled with a rabbit polyclonal antibody generated against serotonin conjugated to BSA (Incstar, Stillwater, MN). Animals were perfused with PBS followed by 4% paraformaldehyde in PBS as described above. Sections were collected on a Vibratome as described above with an additional incubation in 1% H2O2 diluted with a PBS and 10% methanol solution. Primary antiserum (containing 0.3% Triton X-100) was used at a dilution of 1:25,000, followed by standard ABC Elite kit procedures with DAB as chromogen.
HRP-labeled RHT afferents to the SCN. RHT afferents to the SCN were examined in two mice. Animals were treated with atropine (0.6 mg/kg) and, under deep pentobarbital anesthesia (40 mg/kg), received binocular injections of 2 μl of a 30% HRP solution [horseradish peroxidase, type VI (Sigma, St. Louis, MO); 3 mg of HRP in 10 μl of 0.1 m phosphate buffer, pH 7.3] into the vitreous body using a glass micropipette. Twenty hours later, brains were removed and processed for the demonstration of anterogradely labeled retinal terminals in the SCN as described previously (Pickard and Silverman, 1981).
Hypothalamic slice preparation. Whole-cell recordings were conducted from SCN neurons from horizontal slices of the ventral hypothalamus. Animals were deeply anesthetized by halothane inhalation and killed by decapitation while anesthetized. Their brains were rapidly removed and immersed in ice-cold (0–4°C), oxygenated (95% O2/5% CO2) artificial CSF (ACSF) containing (in mm): 124 NaCl, 3 KCl, 26 NaHCO3, 1.4 NaH2PO4, 11 glucose, 1.3 CaCl2, and 1.3 MgCl2, pH 7.3–7.4, with osmolality of 290–315 mOsm/kg. Brains were blocked with a razor blade, and a horizontal slice (400–500 μm) containing the SCN and the proximal optic nerve was made from the ventral hypothalamus using a Vibratome. The slice was then transferred to an interface-type recording chamber, where it was perfused with warmed (32–35°C) and oxygenated ACSF. The ACSF used for recordings was identical to that used in the dissection. Added to the bath solution for some experiments were 5-HT (100 μm), the 5-HT1B receptor agonist TFMPP (10–300 μm) (Lucki et al., 1989), the 5-HT1Areceptor antagonist WAY-100635 maleate (2.5–5 μm) (Fletcher et al., 1994), and the 5-HT7 receptor antagonist ritanserin (5 μm) (Lovenberg et al., 1993). All serotonergic drugs were from Research Biochemicals (Natick, MA).
Patch-clamp recording. After an equilibration period of 1–2 hr, whole-cell current recordings were obtained in the SCN using patch pipettes with open resistances of 2–5 MΩ. Seal resistances were typically 1–4 GΩ, and series resistances were typically <20 MΩ, uncompensated. Patch pipettes were filled with (in mm): 130 K+-gluconate, 1 NaCl, 5 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 3 KOH, 2–4 ATP, and 0.2% biocytin (Sigma), pH 7.2–7.4. Pipettes were pulled from borosilicate glass capillaries of 1.65 mm outer diameter and 0.45 mm wall thickness (Garner Glass Company, Claremont, CA). Electrical stimulation of the optic nerve was performed using a stimulating electrode made from a twisted pair of teflon-coated platinum–iridium wires (75 μm diameter) inside a blunted glass micropipette placed over the nerve. Direct chemical stimulation of SCN neurons was made by pressure-applying glutamate (20 mm; 10–200 msec pulse) through a patch pipette positioned at the surface of the slice near the tip of the recording electrode (i.e., over the recorded neuron). Synaptic activity was recorded using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA), low-pass filtered at 5 kHz, digitized at 44 kHz (Neuro-corder; Neurodata), stored on videotape, and analyzed off-line on a desktop computer with pCLAMP programs (Axon Instruments). The criteria for detecting synaptic currents were fast rise times (<1 msec) and exponential decays. For each recording, the minimum stimulus intensity was determined, and then the intensity was increased until responses were obtained after at least five consecutive stimuli. More than five consecutive responses that varied by <0.5 msec from stimulus onset to the onset of the synaptic event within a given recording were considered to be of relatively constant latency. Measurements of 5–20 constant latency–evoked EPSCs were used to obtain mean amplitudes. After cells were in the whole-cell configuration, they were initially held near the resting membrane potential for 5–10 min to allow equilibration of the extracellular and recording electrode solutions. Evoked EPSCs were examined at rest and at more negative (−80 to −70 mV) holding potentials. Numbers are reported as the mean ± SEM. Effects of drugs were analyzed using an unpaired two-tailed Student’s t test.
Hypothalamic slice histology. Electrodes contained 0.2% biocytin to label recorded neurons and verify their location. After each recording, slices were fixed in 4% paraformaldehyde in 0.15m PB, pH 7.3, overnight at 4°C. After fixation, slices were rinsed three times for 5 min each in 0.01 m PBS, cryoprotected in PBS containing 30% sucrose, and sectioned in the horizontal plane at 50–60 μm on a sliding microtome. Reactions were also performed on whole-mount specimens. Biocytin-filled neurons were visualized by incubating the tissue with avidin–rhodamine (1:400) (Vector Laboratories) or with avidin–biotin–horseradish peroxidase complex (ABC kit; Vector Laboratories) in PBS (1:100), pH 7.3, containing 0.1–1% Triton X-100 (2–12 hr). For the ABC reaction, endogenous peroxidase was first removed (10% methanol and 3% H2O2 in PBS; 60–70 min), the labeled cell was visualized with DAB at a concentration of 0.06% with 0.003% H2O2 in PBS, pH 7.4, to confirm the location of the recorded neuron within the SCN, and the tissue was subsequently dehydrated through a graded series of ethanol and mounted in Permount. For the purposes of this study, recovered neurons were used only to verify their location within the SCN. Quantitative aspects of neuronal morphology will be addressed in a future study. In some slices, 5-HT–containing elements were also labeled using a rabbit polyclonal 5-HT antibody (see above) diluted 1:10,000 in PBS containing 1% Triton X-100 and 10% normal goat serum. The 5-HT–immunoreactive fibers were visualized with a fluorescein-conjugated secondary antibody (IgG; 1:400 in PBS; 4–12 hr).
Wheel-running activity rhythms. After at least 2 weeks in LD 12:12, wild-type and 5-HT1B receptor knock-out mice were transferred to individual cages equipped with activity wheels and were maintained in constant dark (DD) conditions until the experiment was terminated. Wheel-running activity was monitored continuously as described previously (Pickard et al., 1987; Rea et al., 1994) using a Zenith 248 computer running DATAQUEST III data acquisition software (Minimitter Company, Sunriver, OR). Activity records were generated in the standard manner, with each day’s activity presented beneath the previous day’s activity, and were analyzed using CIRCADIA software (Behavioral Cybernetics, Cambridge, MA) running on a Macintosh IIci computer.
The onset of wheel-running activity is designated as circadian time 12 (CT 12) and was used as a phase reference point for the timing of photic stimulation as described previously (Rea et al., 1994). The onset of wheel-running activity on the day of light stimulation was predicted by extrapolation of the least squares line through the activity onsets for at least 5 d preceding the day of stimulation.
Light-induced phase shifts. After 10 d in DD, mice received injections followed by light stimulation at CT 16 (4 circadian hours after predicted activity onset; 1 circadian hr = τ/24). Mice received intraperitoneal (1 ml/kg) injections of either vehicle (0.9% saline) or the 5-HT1B receptor agonist TFMPP (25 mg/kg) 30 min before light exposure. All injections were performed in the dark using infrared night vision goggles (ITT Night Vision, Roanoke, VA). Each animal received 10 min of white light (20 lux) at CT 16 using a light stimulation apparatus as described previously (Rea et al., 1994). After light stimulation, animals were returned to their wheel-running cages in DD.
Quantitation of phase shifts. Animals remained in DD for 10–14 d after photic stimulation. Phase shifts were calculated as the difference between the projected times of activity onset (CT 12) on the day after stimulation as determined by (1) extrapolation of the least squares line calculated from activity onset data collected during the 5 d before and including the day of stimulation and (2) back extrapolation of the least squares line through five activity onsets beginning as soon as a steady-state free run was resumed (2–4 d after photic stimulation) (Daan and Pittendrigh, 1976).
RESULTS
5-HT immunoreactivity in the mouse SCN
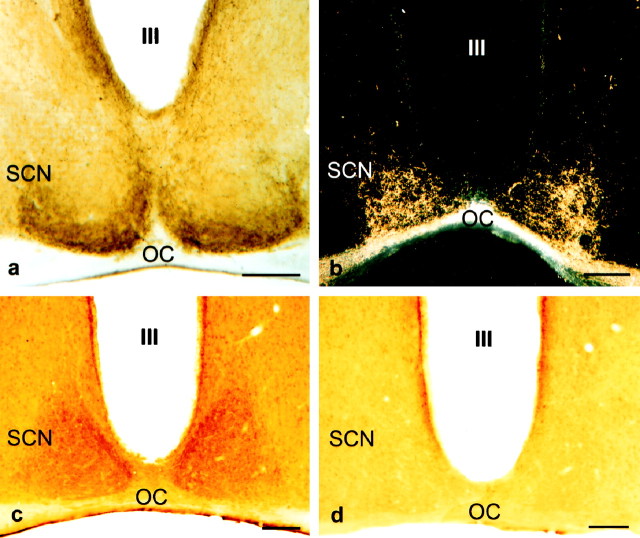
To facilitate comparison of the distribution of 5-HT processes, 5-HT1B receptors, and RHT afferents in the mouse SCN at the light microscopic level, we first examined the distribution of 5-HT–immunoreactive processes in coronal sections through the anterior hypothalamus of the mouse. 5-HT immunoreactivity was appreciably more dense in the SCN itself than in the hypothalamic neuropil surrounding the nucleus. Most 5-HT–labeled structures were thin fibers with irregularly spaced varicosities. At the rostral level of the SCN, 5-HT–immunoreactive elements were more numerous ventrally, forming a dense plexus just above the optic chiasm. Throughout the mid and caudal portions of the nucleus, many 5-HT–immunoreactive fibers were also observed in the lateral and dorsolateral regions of the nucleus (Fig.1a). At the most caudal level of the SCN, 5-HT fibers formed a dense plexus dorsally and medially, in the latter case parallel to the ependymal lining of the third ventricle. In general, the central region of the SCN (as viewed in the coronal plane) contained relatively few fine-caliber 5-HT–immunoreactive fibers. No immunoreactive perikarya were noted in the mouse SCN. The organization of 5-HT afferents described above is in general agreement with previous descriptions of 5-HT in the C57BL/6J mouse SCN (Cassone et al., 1988; Marchant et al., 1997).
Fig. 1.
Light micrographs of coronal sections through the mouse anterior hypothalamus illustrating the 5-HT-immunoreactive process in the mid-caudal SCN (a), the HRP-labeled retinal processes in the mid-caudal SCN viewed using dark-field optics (b), 5-HT1Breceptor immunoreactivity in the mid-caudal SCN (c), and the absence of 5-HT1Breceptor immunoreactivity in the SCN treated with 5-HT1Breceptor antiserum preabsorbed with the peptide used for raising the antiserum (d). OC, Optic chiasm;III, third ventricle. Scale bars, 100 μm.
RHT afferents to the mouse SCN
After bilateral injection of HRP into the vitreous body, labeled retinal fibers and preterminal axons were evident throughout the rostrocaudal extent of the SCN. In the very rostral SCN, labeled processes were predominately located ventrally in the nucleus (data not shown). More caudally, HRP-labeled fibers were more widely distributed over the SCN. In the middle and particularly the caudal aspects of the nucleus, retinal fibers were distributed throughout the SCN although they were more concentrated in the lateral and dorsolateral regions of the nucleus, overlapping the same region receiving 5-HT afferents (Fig.1b). The distribution of HRP-labeled RHT process in the mouse SCN described above is similar to that described previously using the autoradiographic method (Cassone et al., 1988) and a cholera toxin–HRP tracing procedure (Castel et al., 1993).
5-HT1B receptor immunoreactivity in the mouse SCN
5-HT1B receptor immunoreactivity was observed as a moderately intense, mainly diffuse immunostaining throughout the rostrocaudal extent of the SCN. The immunolabeling was slightly stronger in the ventromedial and much stronger in the dorsomedial regions of the SCN close to the ependymal layer lining the bottom of the third ventricle (Fig. 1c). Preabsorption of the primary antiserum with the peptide used for generating the antiserum completely eliminated specific 5-HT1B receptor immunoreactivity in the SCN (Fig. 1d).
Subcellular localization of 5-HT1B receptors in the SCN
Electron microscopic observations based on the preembedding immunoperoxidase technique demonstrated that in the mouse SCN, 5-HT1B receptor immunoreactivity was frequently associated with fine-caliber nonmyelinated axons that appeared to be filled with immunoperoxidase reaction end product. These immunopositive fibers were more frequently observed in the ventral region of the SCN close to myelinated fibers of the optic chiasm (Fig.2a). Clear immunostaining was also observed in the preterminal portions of the axons (Fig.2b) as well as in axonal terminals (Fig.3a,b). In the latter case, immunoreaction product was associated with the plasma membrane and, if intracellularly located, in close proximity to the plasma membrane. Immunoreactivity was never seen within the region of synaptic specializations. Many axonal preterminal and terminal profiles immunoreactive for the 5-HT1B peptide contained large, pale mitochondria with swollen cristae indicating that they were retinal in origin; not all retinal afferents in the SCN were 5-HT1Breceptor immunopositive (Figs. 2b, 3b). Incubation of sections containing the SCN in the absence of the primary antibody or with antibody preabsorbed with an excess of the specific peptide used for raising the antibodies completely abolished immunostaining (data not shown).
Fig. 2.
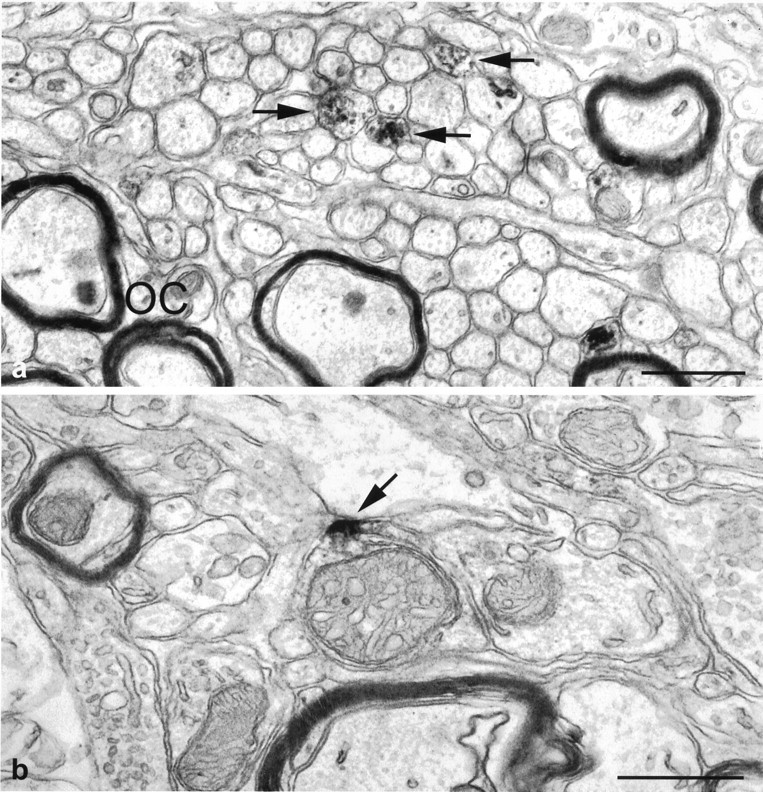
Electron micrographs of the mouse SCN showing immunoperoxidase-labeled 5-HT1B receptors in fibers (arrows) in the ventral region of the SCN close to myelinated fibers of the optic chiasm (OC) (a) and a 5-HT1B-immunopositive axonal profile judged to be optic in origin based on the ultrastructure of the mitochondria (b). Note that the immunoperoxidase reaction product (arrow inb) is associated with the plasma membrane. Scale bars, 0.5 μm.
Fig. 3.
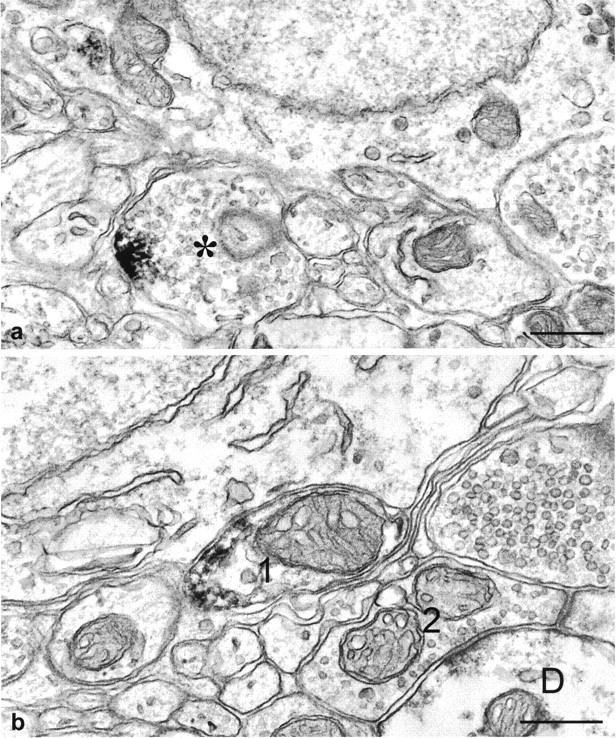
Electron micrographs illustrating immunoperoxidase-labeled 5-HT1B receptors in nerve terminals in the mouse SCN. a, In a nerve terminal containing numerous electron lucent synaptic vesicles and a few dense core vesicles, the immunoreaction product is located on the plasma membrane. b, Two optic profiles containing large pale mitochondria are shown. One of them (1), presumably a preterminal, is immunopositive for 5-HT1Breceptor peptide, whereas the other (2), making synaptic contact with a dendrite (D), is unlabeled. Scale bars, 0.5 μm.
Whole-cell patch-clamp recordings from SCN neurons
Whole-cell patch-clamp recordings were obtained from 23 SCN neurons in horizontal hypothalamic slices from hamsters. Resting membrane potential was −48 ± 2 mV (mean ± SEM); input resistance ranged from 392 to over 1200 MΩ with a mean of 672 ± 61 MΩ. All neurons were located within the SCN, as determined by recording electrode placement, intracellular staining, and/or response to selective stimulation of the optic nerve.
Primary responses to optic nerve stimulation
To establish that the SCN-recorded neurons received retinal input, we monitored EPSCs while electrically stimulating the optic nerve rostral to the optic chiasm (i.e., in isolation from possible direct activation of local neurons). Relatively constant latency (0.5 msec variability) responses to stimulation of the optic nerve consisted of fast EPSCs and were observed in 19 neurons. All of the optic nerve–evoked constant latency EPSCs were inward at rest (Fig.4). The mean latency for the primary response to optic nerve stimulation was 4.4 ± 0.2 msec. With an estimated distance of ∼1.5 mm between recording and stimulating electrodes, this value indicated a conduction velocity for RHT fibers on the order of ∼0.3 m/sec, characteristic of thin, unmyelinated axons.
Fig. 4.
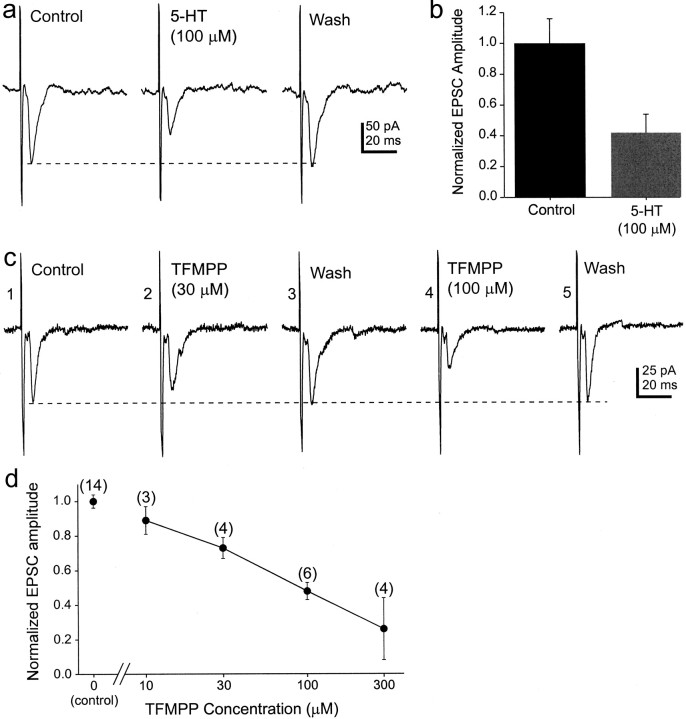
The effect of 5-HT and TFMPP on optic nerve-evoked EPSCs in the hamster SCN. a, 5-HT reduced the amplitude of optic nerve–evoked EPSCs. The average of 5–10 consecutive responses is shown for each condition. b, Cumulative responses from four neurons are graphed relative to the control response. At a concentration of 100 μm, 5-HT reduced the amplitude of the evoked EPSC by ∼60%. c,Trace 1, Electrical stimulation of the optic nerve resulted in an EPSC of relatively constant latency in this SCN neuron.Trace 2, Bath application of the 5-HT1Breceptor agonist TFMPP (30 μm) reduced the EPSC amplitude by ∼20%. Trace 3, The EPSC amplitude recovered after an ∼15 min wash to control recording conditions. Trace 4, A higher concentration of TFMPP (100 μm) further reduced the EPSC amplitude. Trace 5, The EPSC amplitude recovered after an ∼20 min wash. The average of five to eight consecutive responses, not including failures, is shown.d, Dose-dependent effect of TFMPP (10–300 μm) on optic nerve-evoked EPSC amplitude is shown. Data represent the mean ± SEM of three to six cells per dose. Thenumber of cells is indicated in parentheses above each concentration.
Effect of serotonin agonists on the evoked response
Serotonin (n = 4) or the 5-HT1Breceptor agonist TFMPP (n = 15) was bath applied to SCN neurons that displayed constant latency EPSCs after optic nerve stimulation. The primary effect of both receptor agonists was to decrease reversibly the amplitude of the optic nerve–evoked EPSC (Fig.4). Input resistance, determined by the whole-cell current induced by 10–20 mV voltage steps, was unaffected by TFMPP (649 ± 80 MΩ control vs 665 ± 82 MΩ TFMPP; n = 15), even though the EPSC amplitude was consistently reduced. The reduction in EPSC amplitude by TFMPP was dose dependent, becoming significant at 30 μm (p < 0.05) and saturating at 300 μm bath concentration (Fig. 4). These results suggested that TFMPP acted at 5-HT receptors to decrease retinal input to SCN neurons with little effect on neuron input resistance.
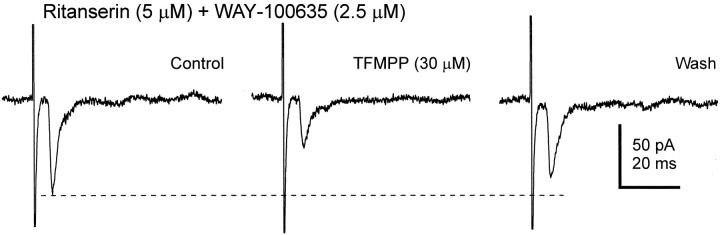
Although TFMPP is a 5-HT1B receptor agonist, it also has an affinity for the 5-HT1A receptor subtype (Hoyer et al., 1994). In addition, the presence of postsynaptic 5-HT7receptors has also been implicated in the SCN (Lovenberg et al., 1993;Kawahara et al., 1994). To determine whether the effect of TFMPP was caused by activation of 5-HT1A or 5-HT7receptors, we examined the effect of the agonist in four neurons in the presence of antagonists to those receptors. Neither WAY-100635 (5-HT1A receptor antagonist) nor ritanserin (5-HT7 receptor antagonist) inhibited the effect of TFMPP on the evoked response (Fig. 5). The effect of TFMPP on the evoked EPSC was therefore most likely mediated by activation of 5-HT1B receptors.
Fig. 5.
The effect of TFMPP in the presence of 5-HT1A and 5-HT7 receptor antagonists. The effect of TFMPP (a 5-HT1B receptor agonist) on the optic nerve-evoked EPSC was not blocked in the presence of the 5-HT1A and 5-HT7 receptor antagonists WAY-100635 and ritanserin, respectively. The average of five to eight responses is shown for each condition.
Effect of TFMPP on glutamate-evoked whole-cell currents
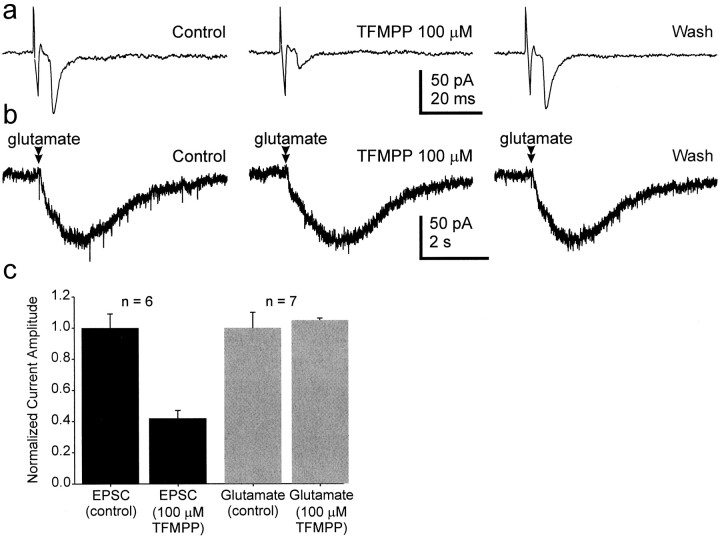
To determine whether TFMPP was acting at receptors located postsynaptically on the soma or dendrites of SCN neurons or presynaptically on retinal terminals, we examined the effect of TFMPP on the inward current evoked by application of glutamate directly to the recorded neuron. RHT input to the SCN is glutamatergic (Kim and Dudek, 1991; Castel et al., 1993; Rea et al., 1993; Mikkelsen et al., 1995; Ebling, 1996). If TFMPP were acting at postsynaptic 5-HT receptors that were closely associated with postsynaptic soma or dendritic glutamate receptors, then the effect of the agonist should also have been observed when postsynaptic glutamate receptors were activated directly by glutamate (Mooney et al., 1994). Whereas 100 μm TFMPP consistently reduced the amplitude of the optic nerve–evoked EPSC (six of six neurons; Fig. 4), the same concentration of TFMPP (100 μm) had no effect on the inward whole-cell current induced by direct glutamate application (Fig.6; n = 7). This was true for all seven neurons examined, including neurons in which the amplitude of the optic nerve–evoked EPSC was reduced by TFMPP (three of three cells). Therefore, the effect of TFMPP on the optic nerve–evoked EPSC was caused by activation of receptors located presynaptic to the recorded neuron, most likely on retinal terminals or preterminal axons.
Fig. 6.
TFMPP reduced the amplitude of evoked EPSCs but had no effect on the inward current evoked by direct application of glutamate. a, TFMPP reversibly reduced the amplitude of the optic nerve-evoked EPSC. The average of eight events is shown.b, In the same neuron, direct application of glutamate (20 mm) to the surface of the slice evoked an inward current that was not reduced by TFMPP. c, The effect of 100 μm TFMPP on the optic nerve-evoked EPSC and on the glutamate-evoked inward current in several neurons is shown. Thenumber of neurons examined is indicatedabove each column set.
Recorded neurons were located in the SCN
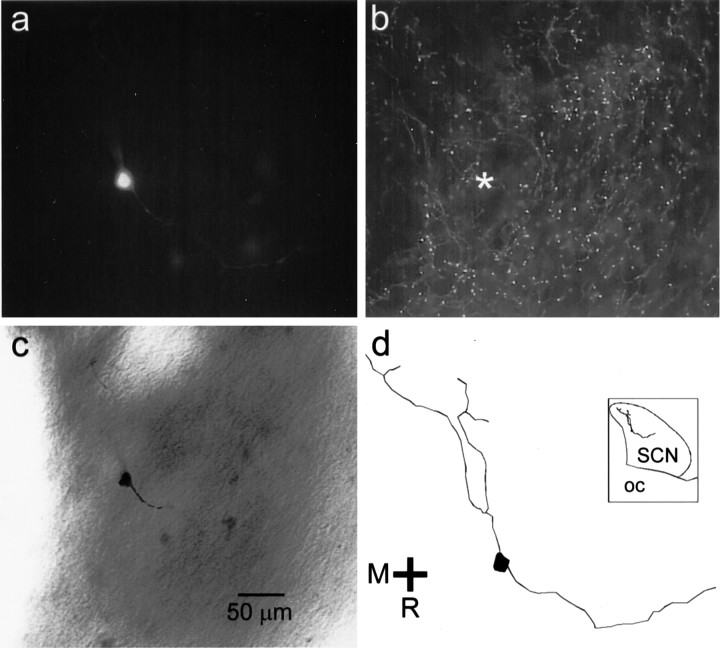
Neurons that responded to optic nerve stimulation were filled with biocytin during the whole-cell patch-clamp recording and were subsequently visualized with an avidin–rhodamine conjugate to confirm their location (Fig. 7a). Neurons generating optic nerve–evoked EPSCs were in a neuropil surrounded by 5-HT processes (Fig. 7b), and all neurons that responded to optic nerve stimulation were determined to be located within the boundaries of the SCN (Fig. 7c,d).
Fig. 7.
Location of an SCN neuron that responded to optic nerve stimulation and to TFMPP. a, This SCN neuron was filled with biocytin during a whole-cell patch-clamp recording and visualized with an avidin–rhodamine conjugate. b, The same tissue was labeled for 5-HT immunoreactivity. Immunopositive fibers are present near the position of the recorded neuron (asterisk). c, The same neuron was rereacted with avidin–biotin–horseradish peroxidase complex and visualized with DAB. d, The neuron was reconstructed digitally using Neurolucida (MicroBrightField). Inset, The position of the neuron relative to the SCN borders in thehorizontal plane of view is shown. OC, Optic chiasm.
Effects of systemic TFMPP on light-induced phase shifts
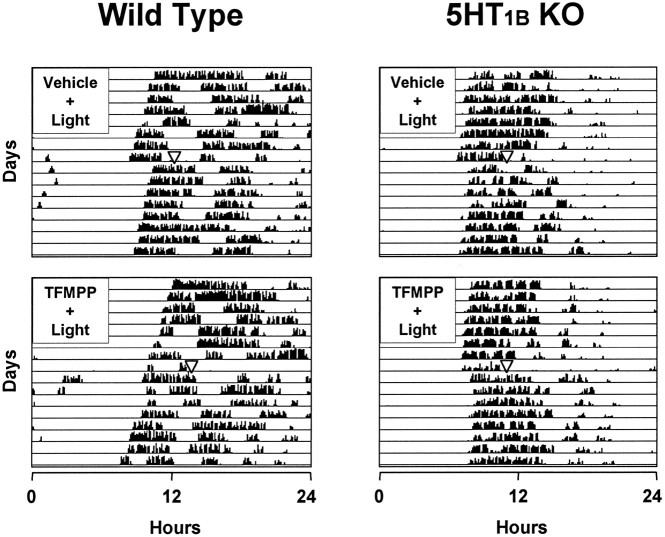
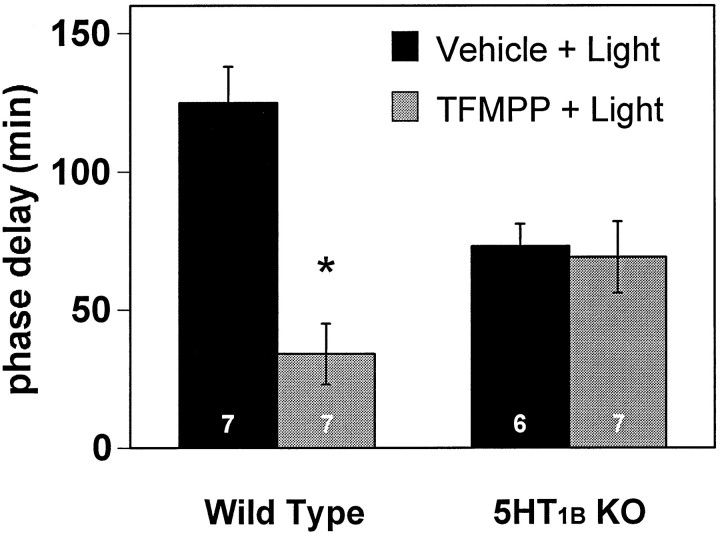
As an additional demonstration that the effect of TFMPP was caused by activation of the 5-HT1B receptor subtype, we examined the effect of TFMPP on light-induced phase shifts of the circadian rhythm of wheel-running activity in wild-type and 5-HT1Breceptor knock-out mice; we have shown previously that TFMPP inhibits light-induced phase shifts in hamsters and mice (Pickard et al., 1996;Pickard and Rea, 1997a). Wild-type mice that received intraperitoneal injections of vehicle 30 min before light stimulation at CT 16 exhibited large, stable phase delays of the free-running activity rhythm as expected. Injection of TFMPP (25 mg/kg of body weight) 30 min before light stimulation significantly reduced light-induced phase delays [−125 ± 12 min (vehicle + light; n = 7) vs −34 ± 10 min (TFMPP + light; n = 7);p < 0.001] (Figs. 8,9). 5-HT1B receptor knock-out mice injected with vehicle 30 min before light stimulation at CT 16 exhibited moderate phase delays. Injection of TFMPP (25 mg/kg of body weight) 30 min before light stimulation had no effect on light-induced phase delays in the 5-HT1B receptor knock-out mice [−73 ± 8 min (vehicle + light; n = 6) vs −69 ± 12 min (TFMPP + light; n = 7);p > 0.05] (Figs. 8, 9).
Fig. 8.
The effect of systemic administration of TFMPP on light-induced phase delays of the circadian rhythm of wheel-running activity in wild-type and 5-HT1B receptor knock-out (KO) mice is illustrated in representative actograms. Mice were maintained in DD throughout the experiment and received injections of vehicle (top) or TFMPP (25 mg/kg, i.p.) (bottom) at CT 15.5 followed by light exposure (10 min at 20 lux) at CT 16 to elicit phase delays. TFMPP reduced light-induced phase shifts in wild-type mice (left) but had little effect in the 5-HT1B receptor knock-out mouse (right). The approximate time of light stimulation is indicated by the inverted triangles.
Fig. 9.
Effect of systemic administration of TFMPP on light-induced phase delays of the free-running activity rhythm. Data represent the mean ± SEM of six to seven animals per group (numbers shown on each bar). Light-induced phase shifts were significantly smaller in TFMPP-treated wild-type mice compared with that in vehicle-injected animals (p < 0.001), whereas TFMPP produced no inhibition of light-induced phase shifts in 5-HT1B receptor knock-out (KO) mice (p > 0.05).
DISCUSSION
The present study demonstrates that 5-HT1B receptors are localized to preterminal optic axons and retinal terminals in the SCN and that activation of these 5-HT1B receptors in vitro reduces retinohypothalamic glutamatergic input to SCN neurons. These results, taken together with the previous observations that bilateral enucleation reduces 5-HT1B receptor binding in the SCN and that 5-HT1B receptor agonists administered systemically or directly into the SCN inhibit light-induced behavioral phase shifts and light-induced Fos expression in the SCN of rodents (Pickard et al., 1996; Pickard and Rea, 1997a), strongly support the interpretation of a 5-HT1B receptor–mediated presynaptic inhibition of photic input to the SCN.
The electron microscopic observations provide a direct demonstration of 5-HT1B receptor immunoreactivity in unmyelinated retinal axons in the SCN. Optic axon terminals and preterminals immunoreactive for 5-HT1B receptors were positively identified in several instances by the presence of distinctive pale mitochondria with irregular cristae (Castel et al., 1993). Pale mitochondria, because of a swollen, electron lucent matrix, were initially suggested bySzentagothai et al. (1966) to be characteristic of optic boutons and are now believed to be common to all terminals of optic origin (Guillery, 1969; Montero and Wenthold, 1989; Morino et al., 1991). This simple diagnostic feature has been used by several investigators to identify RHT terminals and preterminals in the SCN (Guldner, 1978; Card and Moore, 1991; Guldner and Wolff, 1996). Moreover, it has been demonstrated previously that retinal terminals in the mouse SCN (identified after intraocular injection of anterograde tracers) virtually always contain swollen mitochondria with a pale matrix and irregular tubular cristae (Castel et al., 1993). It should also be noted that 5-HT1B receptor immunoreactivity was observed in nonretinal terminals in the SCN; many of these are most likely 5-HT terminals where the 5-HT1B receptor serves an autoreceptor function (Doucet et al., 1995). The fact that 5-HT1Breceptors may be heteroreceptors on RHT terminals and perhaps on other afferent terminals in the SCN (e.g., NPY terminals from the intergeniculate leaflet) and autoreceptors on 5-HT terminals may account for the apparent mismatch between 5-HT1B receptor immunoreactivity, 5-HT immunoreactivity, and the distribution of RHT labeling in the SCN. However, RHT labeling and 5-HT and 5-HT1B receptor immunoreactivity all overlap in the ventral SCN, the region in which we encountered the greatest number of optic terminals immunopositive for the 5-HT1B receptor. We conclude, based on these criteria, that 5-HT1B receptors are located on at least some RHT axon terminals.
5-HT1B receptor immunoreaction product was always associated with the plasma membrane in terminals and preterminal axons in the SCN but was never observed to be associated with synaptic specializations. These observations noted in the SCN are consistent with two brief reports describing 5-HT1B receptor immunoreactivity associated with the plasma membrane in terminals and preterminal axons, but not with synaptic specializations, in the substantia nigra and globus pallidus (Riad et al., 1996; Sari et al., 1997). The observations of 5-HT1B receptor immunoreactivity predominately in preterminal axons in the basal ganglia were made using antiserum different from the antibodies used in the present study (Langlois et al., 1995). Thus, the preterminal localization of 5-HT1B receptors does not seem to be related to the antibody used for immunocytochemical visualization and implies a potential site of action of 5-HT on transmitter release distant from the active zone of the bouton whose secretory activity is regulated.
The mechanism by which activation of 5-HT1B receptors in terminals (and preterminals) modulates transmitter release at the active zone is unknown. 5-HT1B receptors are negatively coupled to adenylyl cyclase (Hoyer et al., 1994). Thus, a decrease in the production of cAMP may be presumed to either regulate the availability of free Ca2+ at the site of neurotransmitter release or alter the sensitivity of the release machinery for Ca2+ by a variety of possible mechanisms. The ability of cAMP to serve as a long-range second messenger provides a mechanism by which activation of 5-HT1B receptors in the preterminal region might be capable of modulating neurotransmitter release at the active zone (Kasai and Petersen, 1994).
Serotonin and the 5-HT1B receptor agonist TFMPP were shown to strongly inhibit, via a presynaptic mechanism, EPSCs of SCN neurons evoked by optic nerve stimulation. This was similar to the effect of the GABAB receptor agonist baclofen acting at presynaptic GABAB receptors on RHT terminals in the SCN (Jiang et al., 1995). The interpretation that the TFMPP effect was attributable to presynaptic inhibition was indicated by the inability of the 5-HT1B receptor agonist to change the amplitude of the inward current evoked by direct application of glutamate that activates postsynaptic glutamate receptors. Had the effect of TFMPP been primarily at postsynaptic receptors, the agonist would have been expected to decrease the amplitude of the response to applied glutamate, which was not the case. The 5-HT1Breceptor–mediated presynaptic inhibition of transmitter release described in the present study in the SCN adds to a growing literature indicating that 5-HT has powerful presynaptic inhibitory effects, mediated via 5-HT1B receptors on axonal processes (Boschert et al., 1994), in several regions of the CNS including the cerebellum (Raiteri et al., 1986), spinal cord (Wu et al., 1991), midbrain (Johnson et al., 1992), cortex (Tanaka and North, 1993), tectum (Mooney et al., 1994), brainstem (Singer et al., 1996), and basal forebrain (Muramatsu et al., 1998).
The interpretation that TFMPP was exerting its inhibitory effect via activation of the 5-HT1B receptor is supported by the inability of 5-HT1A and 5-HT7 antagonists to alter the effect of TFMPP. Bath application of WAY-100635 and/or ritanserin had no effect on the ability of TFMPP to inhibit optic nerve–evoked EPSCs. Moreover, we have demonstrated previously that TFMPP and the more selective 5-HT1B receptor agonist CGS-12066B inhibit light-induced phase shifts of the circadian rhythm of wheel-running activity when administered systemically (Pickard et al., 1996; Pickard and Rea, 1997a). TFMPP and CGS-12066B also inhibit light-induced Fos expression in the rodent SCN (Pickard et al., 1996;Pickard and Rea, 1997a) (G. E. Pickard and M. A. Rea, unpublished observations); Fos expression is a cellular correlate of the behavioral response of the SCN circadian oscillator to light (Rea, 1989; Kornhauser et al., 1990). Finally, TFMPP was completely ineffective in inhibiting light-induced phase shifts in mice lacking 5-HT1B receptors. Thus, it would seem that TFMPP was exerting its inhibitory effect via activation of the 5-HT1Breceptor.
A role for serotonin in the photic regulation of circadian rhythms is clearly becoming established. Morin and colleagues first demonstrated a role for 5-HT in the regulation of photic input to the SCN by demonstrating changes in the phase angle of entrainment after depletion of brain 5-HT (Smale et al., 1990; Morin and Blanchard, 1991). More recently, 5-HT agonists have been shown to modify the response of the SCN to light via a presynaptic 5-HT1B receptor mechanism (Pickard et al., 1996; Pickard and Rea, 1997a,b) (this study) and a postsynaptic 5-HT1A or 5-HT7 receptor mechanism (Kawahara et al., 1994; Rea et al., 1994; Ying and Rusak, 1997; Weber et al., 1998). However, the functional significance of 5-HT inhibition and/or modulation of photic input to the SCN circadian system remains unclear.
To appreciate the role of 5-HT in the SCN, one might consider the effect of serotonin in a more general context. Jacobs and Fornal (1995)have suggested that the primary function of the brainstem serotonergic system is to modulate the actions of the fast neurotransmitter systems of the brain, such as glutamate and GABA, thereby facilitating motor output while concurrently inhibiting sensory information processing. Data relating 5-HT release in the SCN with locomotor activity are consistent with the general hypothesis of Jacobs and Fornal (1995) that the raphe-firing rate (and therefore 5-HT release) is increased during periods of increased motor activity: (1) 5-HT release in the SCN measured by in vivo microdialysis is significantly elevated during episodes of wheel-running activity in the hamster (Dudley et al., 1998), and (2) increased locomotor activity (increased 5-HT release?) during light exposure attenuates light-induced phase shifts of hamster circadian behavior (Ralph and Mrosovsky, 1992). The present data taken together with previous results indicating that activation of 5-HT1B presynaptic receptors inhibits light-induced phase shifts (Pickard et al., 1996; Pickard and Rea, 1997a) are consistent with the hypothesis that 5-HT modulates primary sensory afferents (Jacobs and Fornal, 1995).
Importantly, Jacobs and Fornal (1995) report that a dramatic decrease in raphe neuronal activity is observed under some specific conditions (e.g., orienting responses). In response to a novel stimulus, overt locomotor behavior is suppressed, and raphe firing ceases immediately. During an orientation response, motor activity is disfacilitated, and sensory afferent processing is disinhibited. In this context, disinhibition of 5-HT modulation of RHT input to the SCN during an orienting response could potentially increase the gain of these sensory afferents.
Under seminatural conditions, nocturnal rodents typically spend the day in dark burrows. When they approach the entrance of their shelter before dusk, they have been observed to remain completely immobile in the entrance for up to several minutes before returning to their light-excluding shelter. Later, during the dark, they emerge fully for the start of their main activity period (DeCoursey, 1986a,b). This behavior at the entrance apparently not only informs the animal of conspecifics or predators but also serves a light-sampling function as well. Dawn and dusk are critical for entrainment of circadian rhythms, because it is at these times of day that exposure to light produces the corrective daily phase shifts necessary for maintaining circadian entrainment (see Boulos et al., 1996). Therefore, when nocturnal rodents approach the entrance of their burrow before dark, they are exposed to daylight for only brief durations during the light-sampling intervals. A decrease in 5-HT tone in the SCN (disinhibition) while immobile during these light-sampling intervals would increase the responsiveness of the SCN circadian oscillator to light, minimizing the time required to reset the circadian clock. Conversely, during active foraging at night, motor output is facilitated, and sensory input is inhibited via the serotonergic system, potentially lowering the sensitivity of the circadian system to light and thus decreasing the likelihood that inappropriate photic signals (e.g., lightning or moonlight) might phase shift the circadian clock. Therefore, we suggest that in a broad sense, serotonergic input to the SCN may function to adjust the gain of the response of the SCN circadian system to light. On the basis of the numerous 5-HT receptor subtypes found in the SCN, it seems likely that modulating photic input to the SCN is only one of many roles 5-HT may play in SCN circadian function.
Footnotes
This work was supported by National Institutes of Health Grants NS 35366 and NS 35615 and by a grant from the Air Force Office of Scientific Research (92-AL-004). We thank Dr. René Hen for supplying 5-HT1B receptor knock-out mice and Traci Sampson for excellent technical assistance.
Correspondence should be addressed to Dr. Gary E. Pickard, Department of Anatomy and Neurobiology, Colorado State University, Fort Collins, CO 80523-1670.
REFERENCES
- 1.Azmitia EC, Segal M. An autoradiographic analysis of differential ascending projections of the dorsal and median raphe nuclei of the rat. J Comp Neurol. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 2.Belenky M, Wagner S, Yarom Y, Matzner H, Cohen S, Castel M. The suprachiasmatic nucleus in stationary organotypic culture. Neuroscience. 1996;70:127–143. doi: 10.1016/0306-4522(95)00327-f. [DOI] [PubMed] [Google Scholar]
- 3.Belenky M, Sollars PJ, Pickard GE. Electron microscopic immunocytochemical localization of 5-HT1B receptors in the mouse suprachiasmatic nucleus. Soc Neurosci Abstr. 1998;28:1919. [Google Scholar]
- 4.Block M, Zucker I. Circadian rhythms of rat locomotor activity after lesions of the midbrain raphe nuclei. J Comp Physiol [A] 1976;109:235–247. [Google Scholar]
- 5.Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominately on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 6.Boulos Z, Macchi M, Houpt TA, Terman M. Photic entrainment in hamsters: effects of simulated twilights and nest box availability. J Biol Rhythms. 1996;11:216–233. doi: 10.1177/074873049601100304. [DOI] [PubMed] [Google Scholar]
- 7.Card JP, Moore RY. The organization of visual circuits influencing circadian activity of the suprachiasmatic nucleus. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic nucleus. The mind’s clock. Oxford UP; New York: 1991. pp. 51–76. [Google Scholar]
- 8.Cassone VM, Speh JC, Card JP, Moore RY. Comparative anatomy of the mammalian hypothalamic suprachiasmatic nucleus. J Biol Rhythms. 1988;3:71–79. doi: 10.1177/074873048800300106. [DOI] [PubMed] [Google Scholar]
- 9.Castel M, Belenky MA, Cohen S, Ottersen OP, Storm-Mathisen J. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. Eur J Neurosci. 1993;5:368–381. doi: 10.1111/j.1460-9568.1993.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 10.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol [A] 1976;106:253–266. [Google Scholar]
- 11.DeCoursey PJ. Light-sampling behavior in photoentrainment of a rodent circadian rhythm. J Comp Physiol [A] 1986a;159:161–169. doi: 10.1007/BF00612299. [DOI] [PubMed] [Google Scholar]
- 12.DeCoursey PJ. Circadian photoentrainment: parameters of phase delaying. J Biol Rhythms. 1986b;1:171–186. doi: 10.1177/074873048600100301. [DOI] [PubMed] [Google Scholar]
- 13.Doucet E, Pohl M, Fattaccini C-M, Adrien J, Mestikawy SE, Hamon M. In situ hybridization evidence for the synthesis of 5-HT1B receptor in serotonergic neurons of anterior raphe nuclei in the rat brain. Synapse. 1995;19:18–28. doi: 10.1002/syn.890190104. [DOI] [PubMed] [Google Scholar]
- 14.Dudley TE, DiNardo LA, Glass JD. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurosci. 1998;18:5045–5052. doi: 10.1523/JNEUROSCI.18-13-05045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebling FJP. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher A, Bill DJ, Cliffe IA, Forster EA, Jones D, Reilly Y. A pharmacological profile for WAY-100635, a potent and selective 5-HT1A receptor antagonist. Br J Pharmacol. 1994;112:91P. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- 17.Guillery RW. The organization of synaptic interconnections in the laminae of the dorsal lateral geniculate nucleus of the cat. Z Zellforsch. 1969;96:1–38. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- 18.Guldner FH. Synapses of optic nerve afferents in the rat suprachiasmatic nucleus. I. Identification, qualitative description, development and distribution. Cell Tissue Res. 1978;194:17–35. doi: 10.1007/BF00209231. [DOI] [PubMed] [Google Scholar]
- 19.Guldner FH, Wolff JR. Complex synaptic arrangements in the rat suprachiasmatic nucleus: a possible basis for the “Zeitgeber” and non-synaptic synchronization of neuronal activity. Cell Tissue Res. 1996;284:203–214. doi: 10.1007/s004410050580. [DOI] [PubMed] [Google Scholar]
- 20.Hendrickson AE, Wagoner N, Cowan WM. An autoradiographic and electron microscope study of retinohypothalamic connections. Z Zellforsch. 1972;135:1–26. doi: 10.1007/BF00307084. [DOI] [PubMed] [Google Scholar]
- 21.Hoyer D, Martin GR. 5-HT receptor classification and nomenclature: towards a harmonization with the human genome. Neuropharmacology. 1997;36:419–428. doi: 10.1016/s0028-3908(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. VII. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 23.Jacobs BL, Fornal CA. Serotonin and behavior: a general hypothesis. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven; New York: 1995. pp. 461–469. [Google Scholar]
- 24.Jiang ZG, Allen CN, North RA. Presynaptic inhibition by baclofen of retinohypothalamic excitatory synaptic transmission in rat suprachiasmatic nucleus. Neuroscience. 1995;64:813–819. doi: 10.1016/0306-4522(94)00429-9. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RF, Morin LP, Moore RY. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- 26.Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasai H, Petersen OH. Spatial dynamics of second messengers: IP3 and cAMP as long-range and associative messengers. Trends Neurosci. 1994;17:95–101. doi: 10.1016/0166-2236(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 28.Kawahara F, Saito H, Katsuki H. Inhibition by 5-HT7 receptor stimulation of GABAA receptor-activated current in cultured rat suprachiasmatic neurones. J Neurophysiol. 1994;478:67–73. doi: 10.1113/jphysiol.1994.sp020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: excitatory synaptic mechanisms. J Physiol (Lond) 1991;44:269–287. doi: 10.1113/jphysiol.1991.sp018877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus. The mind’s clock. Oxford UP; New York: 1991. [Google Scholar]
- 31.Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- 32.Langlois X, Gérard C, Darmon M, Chauveay J, Hamon M, El Mestikawy S. Immunolabeling of central serotonin 5-HT1Dβ receptors in the rat, mouse, and guinea pig with a specific anti-peptide antiserum. J Neurochem. 1995;65:2671–2681. doi: 10.1046/j.1471-4159.1995.65062671.x. [DOI] [PubMed] [Google Scholar]
- 33.Lovenberg TW, Baron BM, deLecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 34.Lucki I, Ward HR, Frazer A. Effect of 1-(m-chlorophenyl)piperazine and 1-(m-trifluoromethylphenyl)piperazine on locomotor activity. J Pharmacol Exp Ther. 1989;249:155–164. [PubMed] [Google Scholar]
- 35.Manrique C, Segu L, Hery F, Hery M, Faudon M, Francois-Bellan AM. Increase of central 5HT1B binding sites following 5,7-dihydroxytryptamine axotomy in the adult rat. Brain Res. 1993;623:345–348. doi: 10.1016/0006-8993(93)91452-x. [DOI] [PubMed] [Google Scholar]
- 36.Marchant EG, Watson NV, Mistlberger RE. Both neuropeptide Y and serotonin are necessary for entrainment of circadian rhythms in mice by daily treadmill running schedules. J Neurosci. 1997;17:7974–7987. doi: 10.1523/JNEUROSCI.17-20-07974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikkelsen JD, Larsen PJ, Mick G, Vrang N, Ebling FJP, Maywood ES, Hastings MH, Moller M. Gating of retinal inputs through the suprachiasmatic nucleus: role of excitatory neurotransmitters. Neurochem Int. 1995;27:263–272. doi: 10.1016/0197-0186(95)00039-b. [DOI] [PubMed] [Google Scholar]
- 39.Miller JD, Fuller CA. The response of suprachiasmatic neurons of the rat hypothalamus to photic and serotonergic stimulation. Brain Res. 1990;515:155–162. doi: 10.1016/0006-8993(90)90590-8. [DOI] [PubMed] [Google Scholar]
- 40.Miller JD, Edgar DM, Rea MA. 5HT receptors in the aged SCN. Soc Neurosci Abstr. 1997;23:241. [Google Scholar]
- 41.Montero VM, Wenthold RJ. Quantitative immunogold analysis reveals high glutamate levels in retinal and cortical synaptic terminals in the lateral geniculate nucleus of the macaque. Neuroscience. 1989;31:639–647. doi: 10.1016/0306-4522(89)90429-6. [DOI] [PubMed] [Google Scholar]
- 42.Mooney RD, Shi MY, Rhoades RW. Modulation of retinotectal transmission by presynaptic 5-HT1B receptors in the superior colliculus of the adult hamster. J Neurophysiol. 1994;72:3–13. doi: 10.1152/jn.1994.72.1.3. [DOI] [PubMed] [Google Scholar]
- 43.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 44.Moore RY, Halaris AE, Jones BA. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- 45.Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J Comp Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- 46.Morin LP, Blanchard J. Depletion of brain serotonin by 5,7-DHT modifies hamster circadian rhythm response to light. Brain Res. 1991;566:173–185. doi: 10.1016/0006-8993(91)91696-x. [DOI] [PubMed] [Google Scholar]
- 47.Morino P, Bahro M, Cuenod M, Streit P. Glutamate-like immunoreactivity in the pigeon optic tectum and effects of retinal ablation. Eur J Neurosci. 1991;3:366–378. doi: 10.1111/j.1460-9568.1991.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 48.Muramatsu M, Lapiz MDS, Tanaka E, Grenhoff J. Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur J Neurosci. 1998;10:2371–2379. doi: 10.1046/j.1460-9568.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- 49.Pickard GE. The afferent connections of the suprachiasmatic nucleus of the golden hamster with emphasis on retinohypothalamic projection. J Comp Neurol. 1982;211:65–83. doi: 10.1002/cne.902110107. [DOI] [PubMed] [Google Scholar]
- 50.Pickard GE, Rea MA. TFMPP, a 5HT1B receptor agonist, inhibits light-induced phase shifts of the circadian activity rhythm and c-fos expression in the mouse suprachiasmatic nucleus. Neurosci Lett. 1997a;231:95–98. doi: 10.1016/s0304-3940(97)00534-x. [DOI] [PubMed] [Google Scholar]
- 51.Pickard GE, Rea MA. Serotonergic innervation of the hypothalamic suprachiasmatic nucleus and photic regulation of circadian rhythms. Biol Cell. 1997b;89:513–523. doi: 10.1016/s0248-4900(98)80007-5. [DOI] [PubMed] [Google Scholar]
- 52.Pickard GE, Silverman AJ. Direct retinal projections to the hypothalamus, piriform cortex and accessory optic nuclei in the golden hamster as demonstrated by a sensitive anterograde horseradish peroxidase technique. J Comp Neurol. 1981;196:155–172. doi: 10.1002/cne.901960111. [DOI] [PubMed] [Google Scholar]
- 53.Pickard GE, Ralph MR, Menaker M. The intergeniculate leaflet partially mediates effects of light on circadian rhythms. J Biol Rhythms. 1987;2:35–56. doi: 10.1177/074873048700200104. [DOI] [PubMed] [Google Scholar]
- 54.Pickard GE, Weber ET, Scott PA, Riberdy AF, Rea MA. 5-HT1B receptor agonists inhibit light-induced phase shifts of behavioral circadian rhythms and expression of the immediate-early gene c-Fos in the suprachiasmatic nucleus. J Neurosci. 1996;16:8208–8220. doi: 10.1523/JNEUROSCI.16-24-08208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pickard GE, Smith BN, Mitchell TW, Dudek FE. 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the golden hamster suprachiasmatic nucleus in vitro. Soc Neurosci Abstr. 1998;28:1185. doi: 10.1523/JNEUROSCI.19-10-04034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prosser RA, Dean RR, Edgar DM, Heller HC, Miller JD. Serotonin and the mammalian circadian system. I. In vitro phase shifts by serotonergic agonists and antagonists. J Biol Rhythms. 1993;8:1–16. doi: 10.1177/074873049300800101. [DOI] [PubMed] [Google Scholar]
- 57.Raiteri M, Maura G, Bonanno G, Pittaluga A. Differential pharmacology and function of two 5-HT1 receptors modulating transmitter release in rat cerebellum. J Pharmacol Exp Ther. 1986;237:644–648. [PubMed] [Google Scholar]
- 58.Ralph MR, Mrosovsky N. Behavioral inhibition of circadian responses to light. J Biol Rhythms. 1992;7:353–359. doi: 10.1177/074873049200700408. [DOI] [PubMed] [Google Scholar]
- 59.Rea MA. Light increases Fos-related protein immunoreactivity in the rat suprachiasmatic nuclei. Brain Res Bull. 1989;23:577–581. doi: 10.1016/0361-9230(89)90204-9. [DOI] [PubMed] [Google Scholar]
- 60.Rea MA, Buckley B, Lutton LM. Local administration of EEA antagonists blocks light-induced phase shifts and c-Fos expression in hamster SCN. Am J Physiol. 1993;34:R1191–R1198. doi: 10.1152/ajpregu.1993.265.5.R1191. [DOI] [PubMed] [Google Scholar]
- 61.Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci. 1994;14:3635–3642. doi: 10.1523/JNEUROSCI.14-06-03635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rea MA, Barrera J, Glass JD, Gannon RL. Serotonergic potentiation of photic phase shifts of circadian activity rhythm. NeuroReport. 1995;6:1417–1420. doi: 10.1097/00001756-199507100-00014. [DOI] [PubMed] [Google Scholar]
- 63.Riad M, Jodoin N, Garcia S, Langlois X, Darmon M, Hamon M, Descarries L. Axonal localization of the serotonin 5-HT1B receptor in rat brain. Soc Neurosci Abstr. 1996;22:1329. [Google Scholar]
- 64.Sari Y, Lefevre K, Bancila M, Quignon M, Miquel M, Langlois X, Hamon M, Vergé D. Light and electron microscopic immunocytochemical visualization of 5-HT1B receptors in the rat brain. Brain Res. 1997;760:281–286. doi: 10.1016/s0006-8993(97)00400-9. [DOI] [PubMed] [Google Scholar]
- 65.Saudou F, Hen R. 5-Hydroxytryptamine receptor subtypes in vertebrates and invertebrates. Neurochem Int. 1994;25:503–532. doi: 10.1016/0197-0186(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 66.Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot M-C, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 67.Selim M, Glass JD, Hauser UE, Rea MA. Serotonergic inhibition of light-induced Fos protein expression and extracellular glutamate in the suprachiasmatic nuclei. Brain Res. 1993;621:181–188. doi: 10.1016/0006-8993(93)90105-v. [DOI] [PubMed] [Google Scholar]
- 68.Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- 69.Smale L, Michels KM, Moore RY, Morin LP. Destruction of the hamster serotonergic system by 5,7-DHT: effects on circadian rhythm phase, entrainment, and response to triazolam. Brain Res. 1990;515:9–19. doi: 10.1016/0006-8993(90)90570-2. [DOI] [PubMed] [Google Scholar]
- 70.Sumner BEH, Rosie R, Fink G. Relative density of 5-hydroxytryptamine receptor subtype mRNAs in female rat neuroendocrine brain determined by in situ hybridization histochemistry. Mol Cell Neurosci. 1992;3:215–223. doi: 10.1016/1044-7431(92)90041-y. [DOI] [PubMed] [Google Scholar]
- 71.Szentagothai J, Hamori J, Tombol T. Degeneration and electron microscope analysis of the synaptic glomeruli in the lateral geniculate body. Exp Brain Res. 1966;2:283–301. doi: 10.1007/BF00234775. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka E, North RA. Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol. 1993;69:1749–1757. doi: 10.1152/jn.1993.69.5.1749. [DOI] [PubMed] [Google Scholar]
- 73.van den Pol AN, Dudek FE. Cellular communication in the circadian clock, the suprachiasmatic nucleus. Neuroscience. 1993;56:793–811. doi: 10.1016/0306-4522(93)90128-3. [DOI] [PubMed] [Google Scholar]
- 74.Weber ET, Gannon RL, Rea MA. Local administration of serotonin agonists blocks light-induced phase advances of the circadian activity rhythm in the hamster. J Biol Rhythms. 1998;13:209–218. doi: 10.1177/074873098129000057. [DOI] [PubMed] [Google Scholar]
- 75.Wu SY, Wang MY, Dun NJ. Serotonin via presynaptic 5-HT1 receptors attenuates synaptic transmission to immature rat motoneurons in vitro. Brain Res. 1991;554:111–121. doi: 10.1016/0006-8993(91)90178-x. [DOI] [PubMed] [Google Scholar]
- 76.Ying S-W, Rusak B. 5-HT7 receptors mediate serotonergic effects on light-sensitive suprachiasmatic nucleus neurons. Brain Res. 1997;755:246–254. doi: 10.1016/s0006-8993(97)00102-9. [DOI] [PubMed] [Google Scholar]