Abstract
Patients sustaining lesions of the orbital prefrontal cortex (PFC) exhibit marked impairments in the performance of laboratory-based gambling, or risk-taking, tasks, suggesting that this part of the human PFC contributes to decision-making cognition. However, to date, little is known about the particular regions of the orbital cortex that participate in this function. In the present study, eight healthy volunteers were scanned, using H2150 PET technology, while performing a novel computerized risk-taking task. The task involved predicting which of two mutually exclusive outcomes would occur, but critically, the larger reward (and penalty) was associated with choice of the least likely outcome, whereas the smallest reward (and penalty) was associated with choice of the most likely outcome. Resolving these “conflicting” decisions was associated with three distinct foci of regional cerebral blood flow increase within the right inferior and orbital PFC: laterally, in the anterior part of the middle frontal gyrus [Brodmann area 10 (BA 10)], medially, in the orbital gyrus (BA 11), and posteriorly, in the anterior portion of the inferior frontal gyrus (BA 47). By contrast, increases in the degree of conflict inherent in these decisions was associated with only limited changes in activity within orbital PFC and the anterior cingulate cortex. These results suggest that decision making recruits neural activity from multiple regions of the inferior PFC that receive information from a diverse set of cortical and limbic inputs, and that the contribution of the orbitofrontal regions may involve processing changes in reward-related information.
Keywords: H2150 PET, decision making, orbitolateral PFC, orbitomedial PFC, frontal lobes, executive function
Human patients with damage to orbital regions of prefrontal cortex (PFC) are more likely to exhibit personality change and difficulties with social interactions than patients with damage to more dorsal regions of PFC (Stuss and Benson, 1986; Damasio, 1994; Rolls et al., 1994). However, understanding these behavioral changes in terms of compromised cognitive functions supported by orbital PFC has been complicated by clinical evidence that such difficulties in social cognition and real-life decision making are frequently not accompanied by marked changes in many important forms of cognitive function (Eslinger and Damasio, 1985; Saver and Damasio, 1991). Indeed, several of these other cognitive functions, different types of working memory (Goldman-Rakic, 1987, 1994, 1996; Petrides, 1994, 1995), the control of attention (Dias et al., 1996), and behavioral flexibility (Milner, 1964; Berman et al., 1995), have each been proposed to involve dorsolateral regions of PFC, highlighting the possibility that orbital sectors mediate distinctive mechanisms of particular importance to social cognition (Damasio et al., 1990).
Research into these issues has been advanced significantly by the demonstration that patients exhibiting such “acquired sociopathy” after orbital PFC damage also show consistent deficits on a gambling task involving choices between actions that differ in terms of the size and probabilities of their associated punishments and rewards (for review, see Bechara et al., 1994, 1996; Damasio, 1996a). Furthermore, work published recently in this journal (Bechara et al., 1998) found that orbital PFC patients exhibited difficulties with such decision making in the absence of consistent deficits on a modified delayed response task. Because dorsolateral PFC patients showed the opposite pattern of impairment, i.e., deficient delayed response performance but normal decision making, these data appear to confirm the relatively independent contributions made by the orbital and dorsolateral PFC to decision-making and working memory cognition, respectively. Overall, the trend of the clinical and experimental evidence suggests that the orbital PFC, presumably through its rich interconnections with limbic cortices and other neural stations deeply implicated in processes of incentive motivation and reinforcement (Damasio, 1994), represents an important site of contact between emotional or affective information and mechanisms of action selection (for review, see Rolls, 1996).
Although studies with neurological patients have highlighted the role of orbital PFC in decision-making cognition, functional imaging techniques offer the opportunity for specifying more closely which areas of the orbital PFC are particularly involved. The orbital cortex is relatively differentiated in terms of its cytoarchitecture and patterns of interconnectivity (Carmichael et al., 1994, 1995a,b). Moreover, it is likely to be functionally heterogeneous (Rolls, 1996). To address these issues, we used the slow bolus infusion method of water activation (H215O) to study a novel decision-making task in which subjects were asked to gamble accumulated reward on predictions about which of two mutually exclusive outcomes would occur. Critically, the largest reward was always associated with the least likely of the two outcomes, ensuring that the element of conflict inherent in risk taking was preserved.
MATERIALS AND METHODS
Subjects. Eight right-handed volunteers, all males, participated. None had a history of psychiatric or neurological illness. Their mean age was 31.9 ± 2.0 (SE) years, whereas their mean verbal IQ, estimated with the National Adult Reading Test (Nelson, 1982), was in the above average range at 120.9 ± 1. Each subject underwent 12 positron emission tomography (PET) scans and one magnetic resonance imaging (MRI) scan within a single session. All subjects gave informed, written consent for participation in the study after its nature and possible consequences had been explained to them. The study was approved by the Local Research and Ethics Committee.
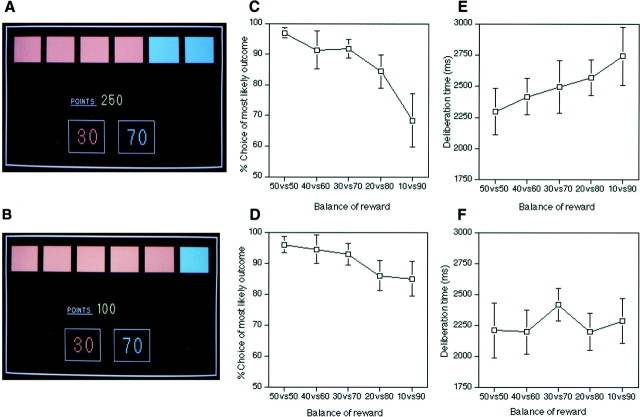
Task design. Two typical displays from the decision-making task are shown in Figure 1, Aand B. The subject was told that the computer had hidden a yellow token inside one of the red or blue boxes arrayed at the top of the screen and that he had to decide whether this token was hidden inside a red box or a blue box. However, this decision involved gambling a certain number of points associated with each choice. In these examples, if the subject chose red, then he gained 30 points if the yellow token was indeed hidden inside a red box, but lost 30 points if the token was hidden inside a blue box. On the other hand, if the subject chose blue, then he gained 70 points if the token was hidden inside a blue box, but lost 70 points if it was hidden inside a red box. The subject was told that there was an equal probability that the token would be hidden inside any of the six boxes. The subject indicated his decision by touching one of the two square response panels, located at the bottom of the display, containing the associated “stake” written in either red or blue ink. Immediately after a selection, one of the boxes opened to reveal the location of the token, accompanied by either a “You win!” or a “You lose!” message (written in large yellow helvetica font). If the subject chose the correct color, the stake associated with that color was added to the total points score; if the subject chose the wrong color, the same stake was subtracted. No monetary significance was attached to the points accumulated by the end of the task.
Fig. 1.
Typical displays from the decision-making task, and associated behavioral data across the present study.A, C,E, Example decision from the 4:2 condition, percentage of choice of the most likely outcome and mean deliberation times as a function of the balance of reward associated with the two outcomes. B, D,F, Example decision from the 5:1 condition, percentage of choice of the most likely outcome and mean deliberation times as a function of the balance of reward associated with the two outcomes.
At the start of each sequence, the subject was given 100 points and instructed to make whatever choices thought necessary to increase this score by as much as possible. It was emphasized that these choices might involve either conservative or risk-taking behavior. The ratio of colored boxes (5:1, 4:2, and 3:3) and the balance between the associated rewards (10 vs 90, 20 vs 80, 30 vs 70, 40 vs 60, and 50 vs 50) varied independently from trial to trial according to a fixed pseudorandom sequence. This sequence ensured that each balance of reward and each ratio of colored boxes co-occurred an equal number of times, with the restriction that on all trials with an unequal ratio of red and blue boxes (i.e., 5:1 or 4:2), the larger reward was always associated with the least likely outcome (i.e., the color with the fewest number of boxes; see Fig.1A,B), thus capturing the conflict inherent in risk-taking situations.
The data analyses centered around two main measures: (1) speed of decision making, i.e., how long it took the subject to decide which color of box was hiding the yellow token as measured by the mean deliberation time (measured in milliseconds), and (2) choice of the most likely outcome (associated with the smaller reward).
Design. For 8 of the 12 scans, the subject began working through sequences of decisions 1 min before the scan commenced. However, at the start of the scan window, i.e., when the “head count” began to rise, the experimenter advanced the subject to one of two conditions involving concealed runs of particular ratios of red and blue boxes (see below). After completing this concealed run, the subject was returned to his or her original place in the entire sequence that was then completed. Preliminary pilot tests had shown that each of these hidden runs occupied the typical subject for ∼1 min. Because most of the regional cerebral blood flow (rCBF) arising from the cognitive activity associated with any scan window coincides with the steepest increase in head counts (≈30 sec;Silbersweig et al., 1993), hidden runs of 1 min were sufficient to ensure that the rCBF data reflected the mental activity associated with the different conditions. On the remaining four scans, the subjects performed a purpose-designed visuomotor control task (see below).
Earlier work had shown that subjects appear to be more sensitive to the balance of reward associated with the two outcomes when the ratio of the colored boxes was 4:2 compared to when it was 5:1. For this reason, our design involved two conditions that allowed us to assess decision making with these different ratios. Thus, in the 4:2 choice conditions, the subject was scanned while making decisions that involved ratios of either 4 red:2 blue or 2 red:4 blue (e.g., Fig. 1A), and in which the reward associated with the two outcomes was always one of 30 vs 70, 20 vs 80, and 10 vs 90. As noted above, these choices tend to be particularly associated with reduced choice of the most likely outcome, as well as increased deliberation times, as a function of the balance of reward associated with the two possible outcomes.
In the 5:1 choice conditions, the subject was scanned while making decisions involving ratios of only 5 red:1 blue or 1 red:5 blue (e.g., Fig. 1B). Although the rewards associated with the two outcomes were the same as in the 4:2 choice conditions, i.e., 30 vs 70, 20 vs 80, and 10 vs 90, these decisions tend to be associated with more consistent choice of the most likely outcome, as well as relatively constant deliberation times. To control for possible differences in the amount of visual and motor processing associated with the 4:2 and 5:1 choice conditions, the presentation rate of trials in each of the 5:1 choice conditions was “yoked” to the latencies of choices in an earlier 4:2 condition. Additionally, because recent evidence has suggested that rCBF changes within orbital PFC and associated limbic circuitry can be seen with changes in reinforcement rate (Elliott et al., 1999), the frequency of reward within the scan windows of the 5:1 choice conditions was also yoked to earlier 4:2 choice conditions. This was achieved by having the computer select the location of the yellow token after the subject had made a response in the 5:1 conditions and thereby permitting the number of wins and losses to be balanced with the 4:2 conditions. In this way, differences in the rCBF in the 4:2 and 5:1 conditions cannot be attributed to gross differences in motor activity or rate of positive or negative feedback across conditions. The subject was not informed about this feature of the study design.
In the control condition, alternative displays showed only all red or all blue boxes with the yellow token already revealed at onset, thus ensuring that subjects were not able to covertly predict which color of box was hiding the yellow token. Moreover, all features of the displays that had previously indicated reward-based information, i.e., the total points score and the size of the rewards associated with two outcomes, were now marked with Xs. The subject was required to monitor the displays until one of the response panels brightened with a white border before touching that panel, the precise delay corresponding to the time required to make earlier decisions in a yoked 4:2 choice condition.
The twelve scans were divided into four runs of three scans each. The first scan in each run was always a 4:2 choice condition, whereas the second and third scans were always either a 5:1 choice condition or a control condition; the order of these two conditions was counterbalanced across scans within and between subjects. To remove linear time effects associated with earlier versus later scans, scan order was entered as a covariate (of no interest) in all analyses of the rCBF data. Before the first scan, but after the subject had been positioned in the scanner, the nature of the task and the task displays were explained to the subject, who was allowed to complete just one sample decision as training.
Scanning procedure and statistical analysis. Each subject was scanned in the presence of low background noise and dimmed ambient lighting. The task displays were presented on a MicroTouch 20C touch-sensitive screen controlled by a Pentium microcomputer. The screen was mounted at a viewing distance of ∼50 cm so that the subject could touch all areas of the screen with the index finger of the dominant hand, which was rested on the chest between responses.
PET scans were obtained with the General Electrics Advance system, which produces 35 image slices at an intrinsic resolution of ∼5.0 × 5.0 × 5.0 mm. Using the bolus H215O methodology, rCBF was measured during four separate scans for each of the three experimental and control conditions (total = 12 scans). For each scan, subjects received a 20 sec intravenous bolus of H215O through a forearm cannula at a concentration of 300 MBq/ml−1 and a flow rate of 10 ml/min−1. With this method, each scan provided an image of rCBF integrated over a period of 90 sec from when the tracer first entered the cerebral circulation. The twelve PET scans were initially realigned using the first scan as a reference and then again using the mean of the scans as a reference, normalized to a standard brain template that forms part of the Statistical Parametric Mapping 98 (SPM98) software, corrected for global CBF value, and averaged across the eight subjects for each activation state. Then the images were smoothed using an isotropic Gaussian kernel at 16 mm full-width half-maximum (FWHM). Finally, blood flow changes between conditions were estimated for each voxel according to the general linear model, as implemented by Statistical Parametric Mapping (SPM 96; provided by the Wellcome Department of Cognitive Neurology, London, UK).
For each subject, a three-dimensional MRI volume (1.5 × 1.5 × 3.0 mm) was acquired using a 0.5 T system and Bruker console and resliced to be coregistered with the PET data. Composite stereotaxic MRI and PET volumes were merged to allow direct anatomical localization of regions with statistically significant rCBF change between conditions. Effects at each and every voxel were estimated according to the general linear model (Friston et al., 1995). Condition effects at each voxel were compared using linear contrasts. The resulting set of voxel t statistics constitute a statistical parametric map (SPM {t}). SPM {t} maps were transformed to the unit normal distribution SPM {Z} for display and thresholded at 3.09. The resulting foci were characterized in terms of spatial extent (k) and peak height (u). The significance of each region was estimated using distributional approximations from the theory of Gaussian fields. This characterization is in terms of the probability that the peak height observed (or higher) could occur by chance [PZmax > u] over the entire volume analyzed (i.e., a corrected p value).
In the case of comparisons between the decision-making and control scans, all predicted increases in rCBF were tested against a threshold of p < 0.05 corrected for multiple comparisons within a volume approximating the size of the orbital PFC. The technique for calculating such a threshold has been described elsewhere (Worsley et al., 1996). Predicted peaks were confined to orbital PFC in view of the considerable neuropsychological evidence that altered decision making is associated specifically with lesions in these cortical fields (Bechara et al., 1994, 1998; Rogers et al., 1999). To anticipate the results, decision making was exclusively associated with highly significant activations in the orbital PFC, with no evidence of increased activity in other parts of the PFC at either corrected or indeed uncorrected thresholds. Activations (and relative deactivations) beyond the frontal cortex (none of which were predicted a priori) are detailed in the tables and reported only briefly in the text if they survived the additional threshold of p < 0.05 corrected for multiple comparisons across the whole brain. As noted above, task-unrelated changes in rCBF associated with linear time effects associated with earlier versus later scans were removed by entering scan position as a covariate (of no interest) in all analyses.
RESULTS
Task performance
The behavioral data associated with each sequence of decisions (i.e., percentage of choice of the most likely outcome and mean deliberation times) were subject to multifactorial, repeated-measures ANOVA with the following within-subject factors: run (first, second, third, or fourth); ratio (4:2 or 5:1), and balance of reward (50 vs 50, 40 vs 60, 30 vs 70, 20 vs 80, or 10 vs 90). The proportions of trials on which subjects chose the most likely outcome were arcsine-transformed as is appropriate whenever variance is proportional to the mean (Howell, 1987). However, the data shown in the tables and figures represent untransformed values. In those instances in which the additional assumption of homogeneity of covariance in repeated-measures ANOVA was violated, as assessed using the Mauchly sphericity test, the degrees of freedom against which the F term was tested were reduced by the value of the Greenhouse-Geisser epsilon (Howell, 1987). Additional analyses were performed on the mean deliberation times and percentage of choice of the most likely outcome specifically associated with the concealed runs of decisions manipulated in the 4:2 and 5:1 conditions, to check that subjects’ behavior during the scan windows was similar to that seen over the entire set of decision-making sequences.
In general, subjects’ decision making was markedly influenced by the balance of rewards associated with the most and the least likely outcomes. Specifically, subjects’ choice of the most likely outcome was significantly reduced as the size of its reward was diminished in comparison with that of the least likely outcome (Table1;F(4,28) = 6.97; p = 0.001), whereas the time required to make these choices was significantly increased (F(4,28) = 3.05; p < 0.05). Moreover, as predicted, the extent to which the balance of rewards influenced subjects’ decisions tended to be greater when the ratio of red and blue boxes was 4:2 compared to when it was 5:1, both in terms of choice of the most likely outcome (F(4,28) = 3.43; p < 0.05) and time required to make decisions (F(4, 28) = 2.89; p < 0.05). Further analysis of simple effects demonstrated that deliberation times were significantly influenced by the balance of rewards with ratios of 4:2 (Fig.1E; F(4,28) = 3.73;p < 0.05) but not with ratios of 5:1 (Fig.1F; F(4,28) = 1.85). Choice of the most likely outcome was reduced by the changing balance of rewards with both ratios (Fig. 1C,D;F(4,28) = 7.96; p < 0.001; F(4,28) = 3.58;p < 0.05). Finally, subjects took significantly longer to make their choices with ratios of 4:2 compared to 5:1 (2505 ± 170 msec vs 2263 ± 164 msec;F(1,7) = 29.30; p = 0.001), especially in the earlier compared to later runs (F(3,21) = 3.99; p < 0.05). In general, deliberation times were increased in the earlier runs (F(3,21) = 30.81;p < 0.0001).
Table 1.
Decision-making performance (i.e. percentage of choice of the most likely outcome and mean deliberation times, plus SEs) as a function of the balance of rewards
| 50 vs 50 | 40 vs 60 | 30 vs 70 | 20 vs 80 | 10 vs 90 | |
|---|---|---|---|---|---|
| Percentage of choice of most likely outcome | 96.5 ± 2.1 | 93.0 ± 5.3 | 92.4 ± 3.0 | 85.2 ± 4.3 | 76.7 ± 7.0 |
| Scanned decisions | — | — | 91.6 ± 3.6 | 84.2 ± 5.2 | 73.5 ± 7.8 |
| Mean deliberation time (msec) | 2254 ± 198 | 2308 ± 160 | 2458 ± 162 | 2385 ± 141 | 2514 ± 202 |
| Scanned decisions | — | — | 2363 ± 199 | 2335 ± 105 | 2469 ± 171 |
Scanned decisions refer to those made during the scan windows of the 4∶2 and 5∶1 choice conditions.
Additional analyses were performed on the decisions of the concealed runs constituting the 4:2 and 5:1 conditions (see above). These data were collected during a period beginning at the start of the scan windows and ending 60 sec later. The within-subject factors were unchanged except that the balance of rewards had only three levels instead of five (i.e., 30 vs 70, 20 vs 80, or 10 vs 90). Despite the reduced power available with this much restricted data set, decision-making performance within the scan windows of the 4:2 and 5:1 conditions was typical of the complete sequences. Thus, choice of the most likely outcome was significantly reduced as the size of its associated reward was diminished relative to that associated with the least likely outcome (Table 1;F(1.19,14) = 9.47; p< 0.01). The time required to make decisions also increased, although not significantly (F(2,14) = 1.3). Additionally, the balance of rewards influenced subjects’ choices more in the 4:2 condition than in the 5:1 condition (F(2,14) = 4.46; p < 0.05). Finally, as with the complete sequences, subjects took significantly longer to make their choices in the 4:2 compared to the 5:1 condition (2662 ± 200 msec vs 2373 ± 208 msec;F(1,7) = 20.53; p < 0.005), especially within the earlier runs of the study (F(3,20) = 4.94; p = 0.01). Deliberation times were significantly increased in the earlier compared to the later runs (F(3,21) = 18.25; p < 0.0001).
Regional cerebral blood flow changes
Decision-making versus control conditions
Subtraction of the rCBF associated with the visuomotor control conditions from that associated with the 4:2 and 5:1 conditions combined isolated significant and distinct activations in ventral, but not dorsolateral, sectors of the right PFC (Table2). Specifically, there was a highly significant peak positioned along the orbital frontal gyrus [Brodmann area 11 (BA 11); z score = 4.14; Fig.2A], another positioned more laterally along the most anterior and ventral portion of the middle frontal gyrus (BA 10/11; z score = 4.51; Fig. 2B), and a third significant peak positioned just anterior to the insular cortex, along the ventral part of the inferior frontal gyrus (BA 47; z score = 4.48; Fig.2C). There were no rCBF increases associated with decision making in other PFC areas.
Table 2.
Comparison of the combined rCBF from the 4∶2 and 5∶1 conditions with the rCBF associated with performance of the visuomotor control task
| BA | L/R | zscore | x | y | z | |
|---|---|---|---|---|---|---|
| (4∶2 + 5∶1)—control | ||||||
| Middle frontal gyrus | 10/11 | R | 4.51 | 40 | 54 | −8 |
| Inferior frontal gyrus | 47 | R | 4.48 | 34 | 20 | −4 |
| Orbital frontal gyrus | 11 | R | 4.14 | 20 | 42 | −28 |
| Fusiform gyrus | 18 | R | 5.21 | 32 | −86 | −12 |
| Superior parietal lobule | 7 | L | 5.48 | −32 | −54 | 52 |
| Superior parietal lobule | 7/40 | R | 5.78 | 30 | −66 | 40 |
| Superior parietal lobule | 7/40 | R | 5.41 | 46 | −52 | 52 |
| Cerebellum | Lateral | L | 4.66 | −42 | −68 | −20 |
| Cerebellum | Medial | R | 5.23 | 4 | −80 | −32 |
| Control—(4∶2 + 5∶1) | ||||||
| Medial frontal gyrus | 10 | L | 4.71 | −6 | 54 | −16 |
| Medial frontal gyrus | 10 | M | 4.12 | 0 | 60 | 0 |
| Precentral gyrus | 4 | L | 4.37 | −12 | −20 | 68 |
| Middle temporal gyrus | 39 | L | 5.22 | −46 | −72 | 24 |
| Uncus | 28/36 | L | 4.69 | −26 | 4 | −28 |
| Middle temporal gyrus | 21 | R | 4.70 | 60 | −48 | 8 |
| Superior temporal gyrus | 22 | R | 4.84 | 64 | −44 | 16 |
| Cerebellum | Lateral | R | 4.87 | 44 | −12 | −40 |
A threshold was set at p < 0.05 (zscore = 3.83) corrected for multiple comparisons within the orbital PFC (Worsley et al., 1996); all other reported peaks were significant at p < 0.05 corrected across the whole brain.
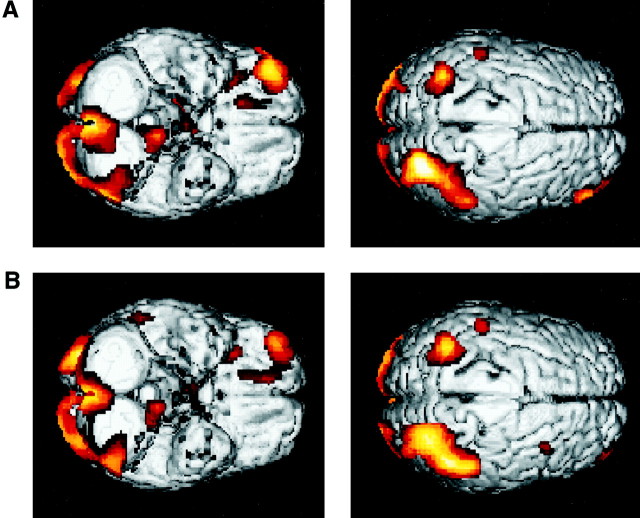
Fig. 2.
Peaks of activity-associated performance of the decision-making task compared to the visuomotor control task rendered onto the averaged MRI scans of the eight volunteer subjects used in the current study (threshold, p < 0.01).A, Peak of activation in orbitomedial PFC (BA 11);B, peak of activity within orbitolateral PFC (BA 10);C, activation within the inferior convexity (BA 47).
Additional activations not predicted a priori included a significant rCBF increase along the right fusiform gyrus (Table 2; BA 18;z score = 5.21). There was also a marked activation within the superior parietal lobule on the left (BA 7; zscore = 5.48), as well two distinct activations in the same area on the right (BA 7/40; z scores = 5.78 and 5.41). Finally, there were significant peaks within the lateral cerebellum on the left (z score = 4.66) and the medial cerebellum on the right (z score = 5.23).
Subtraction of the combined rCBF of the 4:2 and 5:1 conditions from that of the control conditions also revealed evidence of relatively reduced activation associated with decision making within left anterior PFC; specifically, along the left medial frontal gyrus (Table 2; BA 10;z scores = 4.71 and 4.12). Additional unpredicted areas of reduced rCBF in the decision-making compared to control conditions were concentrated within predominantly temporal lobe areas (Table 2) and included the left middle temporal gyrus (BA 39; zscore = 5.22) and left uncus (BA 28/36; z score = 4.69), the right middle and superior temporal gyri (BA 21, zscore = 4.70; BA 22, z score = 4.84), as well as the left precentral gyrus (BA 4; z score = 4.37) and lateral cerebellum on the right (z score = 4.87).
In general, separate comparisons involving each of the decision-making conditions with the control condition reflected similar patterns of activation in the inferior and orbital PFC, as well as posterior temporal and parietal areas (Tables 3,4). In particular, decision making in the 4:2 condition (Fig. 3A) and the 5:1 condition (Fig. 3B) activated roughly the same three sites in right orbital PFC: laterally, along the anterior part of the middle frontal gyrus (BA 10/11; z scores = 4.24 in the 4:2 condition, 3.92 in the 5:1 condition); posteriorly, along the inferior frontal gyrus (BA 47; z scores = 4.07 in the 4:2 condition, 3.89 in the 5:1 condition); and, medially, in the region of the orbital frontal gyrus (BA 11; z score = 4.42 in the 4:2 condition; z score = 3.81 in the 5:1 condition).
Table 3.
Comparison of the rCBF associated with the 4∶2 conditions only with the rCBF associated with performance of the visuomotor control task
| BA | L/R | zscore | x | y | z | |
|---|---|---|---|---|---|---|
| 4∶2—control | ||||||
| Middle frontal gyrus | 10 | R | 4.24 | 42 | 50 | −8 |
| Inferior frontal gyrus | 47 | R | 4.07 | 34 | 20 | 0 |
| Orbital frontal gyrus | 11 | R | 4.42 | 22 | 40 | −32 |
| Fusiform gyrus | 18 | R | 4.06 | 30 | −84 | −12 |
| Superior parietal lobule | 7 | L | 4.70 | −28 | −58 | 48 |
| Superior parietal lobule | 7 | R | 5.25 | 30 | −68 | 40 |
| Cerebellum | Lateral | L | 4.50 | −42 | −68 | −20 |
| Cerebellum | Medial | R | 4.70 | 4 | −78 | −32 |
| Control—4∶2 | ||||||
| Orbital frontal gyrus | 10 | L | 4.54 | −4 | 54 | −20 |
| Precentral gyrus | 4/6 | L | 5.01 | −14 | −18 | 68 |
| Inferior temporal gyrus | 21 | R | 4.32 | 62 | −48 | 4 |
| Middle temporal gyrus | 20 | R | 4.35 | 46 | −10 | −36 |
A threshold was set at p < 0.05 (zscore = 3.83) corrected for multiple comparisons within the orbital PFC (Worsley et al., 1996); all other reported peaks were significant at p < 0.05 corrected across the whole brain.
Table 4.
Comparison of the rCBF associated with the 5∶1 conditions only with the rCBF associated with performance of the visuomotor control task
| BA | L/R | zscore | x | y | z | |
|---|---|---|---|---|---|---|
| 5∶1—control | ||||||
| Middle frontal gyrus | 10/11 | R | 3.92 | 36 | 56 | −12 |
| Inferior frontal gyrus | 47 | R | 3.89 | 32 | 20 | −4 |
| Orbital frontal gyrus | 11 | R | 3.81 | 18 | 48 | −28 |
| Fusiform gyrus | 18 | R | 5.04 | 32 | −86 | −12 |
| Superior parietal lobule | 7 | L | 5.14 | −32 | −54 | 52 |
| Superior parietal lobule | 40 | R | 5.04 | 32 | −66 | 44 |
| Inferior parietal lobule | 40 | R | 5.56 | 48 | −54 | 52 |
| Inferior parietal lobule | 40 | R | 5.00 | 50 | −42 | 48 |
| Cerebellum | Medial | R | 4.70 | 4 | −82 | −32 |
| Control—5∶1 | ||||||
| Middle temporal gyrus | 39 | L | 4.45 | −46 | −72 | 24 |
| Inferior temporal gyrus | 20 | L | 4.44 | −46 | −16 | −28 |
| Uncus | 28 | L | 4.65 | −26 | 4 | −28 |
| Superior temporal gyrus | 22 | R | 4.35 | 64 | −44 | 16 |
A threshold was set at p < 0.05 (zscore = 3.83) corrected for multiple comparisons within the orbital PFC (Worsley et al., 1996); all other reported peaks were significant at p < 0.05 corrected across the whole brain.
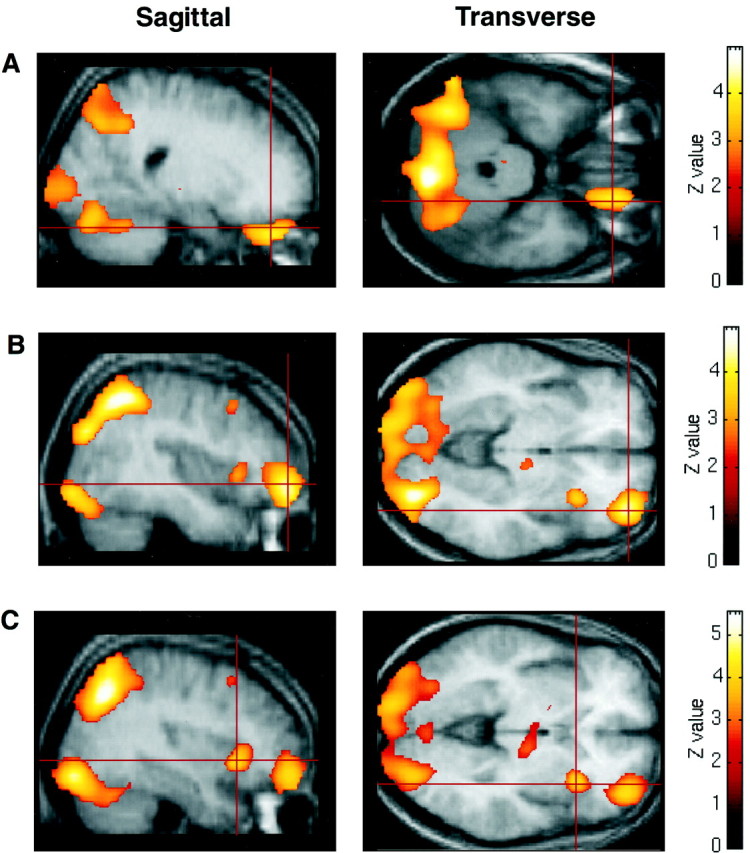
Fig. 3.
Increased rCBF from the two decision-making conditions compared with the visuomotor control task rendered onto a representative brain (threshold, p < 0.01).A, 4:2 condition − control task; B, 5:1 condition − control task. Note the lack of activity within dorsolateral areas of the PFC.
The two decision-making conditions showed more limited distributions of reduced rCBF in comparison with the control conditions (Tables 3, 4). Specifically, in orbital PFC areas, only the 4:2 condition showed reduced significantly reduced activity within the left orbital gyrus (BA 11; z score = 4.54). However, both conditions were associated with marked deactivations in temporal areas: along the right inferior and middle temporal gyri in the 4:2 conditions (BA 21,z score = 4.32; BA 20, z score = 4.35), and along the left middle temporal gyrus (BA 39; zscore = 4.45), left inferior temporal gyrus (BA 20; zscore = 4.44) and left uncus (BA 28; z score = 4.65) in the 5:1 conditions. Additional rCBF deactivations were evident along the precentral gyrus on the left in the 4:2 condition (BA 4/6;z score = 5.01) and along the superior temporal gyrus on the right in the 5:1 condition (BA 22; z score = 4.35).
4:2 condition minus 5:1 condition
Direct subtraction of the rCBF associated with the 5:1 conditions from that associated with the 4:2 conditions isolated only modest changes in regional neural activity. Specifically, there was only a limited activation along the orbital frontal gyrus on the left (BA 11;z score = 3.28; Table 5), as well as a more extensive peak positioned along the anterior cingulate gyrus (BA 24; z score = 3.62). There was also some evidence of relatively increased rCBF in the area of the ventral striatum, just adjacent to the nucleus accumbens and putamen (z score = 3.92). However, none of these predicted or unpredicted rCBF changes survived correction for multiple comparisons. Subtraction of the rCBF in the 4:2 conditions from the rCBF in the 5:1 conditions revealed only a single area of changed rCBF along the left middle frontal gyrus (BA 6; z score = 4.26).
Table 5.
Direct comparison of the rCBF associated with the 4∶2 conditions with the rCBF associated with the 5∶1 conditions
| BA | L/R | zscore | x | y | z | |
|---|---|---|---|---|---|---|
| 4∶2—5∶1 | ||||||
| Orbital frontal gyrus | 11 | L | 3.28 | −14 | 34 | −32 |
| Anterior cingulate gyrus | 24 | L | 3.62 | −2 | 28 | 20 |
| Ventral putamen region | R | 3.92 | 28 | −8 | −8 | |
| 5∶1—4∶2 conditions | ||||||
| Middle frontal gyrus | 6 | L | 4.26 | −34 | 4 | 52 |
A threshold was set at p < 0.05 (zscore = 3.88) corrected for multiple comparisons within the orbital PFC (Worsley et al., 1996); all other reported peaks were significant at p < 0.05 corrected across the whole brain.
Covariates of interest
Further analyses, collapsed across the 4:2 and 5:1 conditions, failed to find any significant association between rCBF in any cortical area and the principal performance measures associated with the scanned sequences: percentage of choice of the most likely outcome, mean deliberation time, mean number of points earned during the scans, and total reward at the end of the scans. Activity within the anterior portion of the right orbital gyrus (BA 11; x = 14;y = 56; z = −20; zscore = 3.66) did show a positive relationship with the total change in points, i.e., summed losses or wins, from the start of the scan windows through to their completion. However, this increase did not survive correction the threshold set for multiple comparisons within the orbital PFC using the Worsley formula (see above).
DISCUSSION
The behavior of our subjects, across both the entire set of decision-making sequences completed during the study and the restricted sequences completed within the scan windows of the 5:1 and 4:2 conditions, indicated that the choice of the most likely outcome was significantly reduced when its associated reward was decreased in comparison with that associated with the least likely outcome. Deliberation times associated with these choices were also significantly increased. Thus, these behavioral data (Fig.1C–F) reflect the conflict inherent in “risky choices” in which the probability of relevant outcomes is pitted against the balance of their associated reinforcers. We have shown that, in a sample of healthy young adult males of relatively high intelligence, resolving this conflict in favor of one choice over another is associated with at least three distinct foci of rCBF increase within the inferior and orbital PFC: laterally, in the anterior part of the middle frontal gyrus (BA 10), medially, in the orbital gyrus (BA 11), and posteriorly, in the anterior portion of the inferior frontal gyrus (BA 47).
The multiple activations associated here with choices differing in the likelihood and size of their rewards help to explain the apparently greater incidence of deficient decision making in neurological patients sustaining damage to the orbital PFC compared to those sustaining damage in more dorsolateral and dorsomedial areas (Bechara et al., 1996, 1998, 1999; Rogers et al., 1999). In view of the current results, it seems that focal lesions of the orbital cortex, as the result of surgery or stroke (Damasio et al., 1996b), are likely to affect cortical areas encompassing the rCBF changes seen here, increasing the probability of deficits in resolving between competing actions on the basis of ambiguous or conflicting information (Bechara et al., 1994,1996, 1998, 1999; Eslinger and Damasio, 1985).
Choices in this study were not associated with any significant changes in neural activity within those dorsolateral prefrontal areas that have repeatedly been shown to mediate important aspects of the executive control of behavior such as working memory, planning, and attention (Goldman-Rakic, 1987, 1996; Petrides, 1994, 1995; Dias et al., 1996;Roberts et al., 1996). Thus, these results complement both experimental data indicating that impairments in decision making are dissociable from impairments in spatial memory (Bechara et al., 1998) and clinical assessments that ineffective decision making in real-life contexts can be accompanied by relatively normal performance on standard tests of frontal lobe function and measures of visuospatial performance, language, and memory (Eslinger and Damasio, 1985; Saver and Damasio, 1991; Rahman et al., 1999).
Given the intrinsic connectivity within orbital PFC (Barbas and Pandya, 1989; Carmichael and Price, 1995b), the activations of the present study are not likely to be functionally independent. Nevertheless, their distribution within the inferior and orbital cortex reflects the diversity of cell types and connectivity extrinsic to the PFC. Thus, the strong activations around the orbital frontal gyrus fell within an area that, in the primate brain, has a distinctive granular cytoarchitecture (Carmichael and Price, 1994) and receives rich innervation from all major stations of limbic–hippocampal circuitry (Morecroft et al., 1992; Carmichael and Price, 1995a). By contrast, the peaks around the inferior frontal and middle frontal gyri (BA 47 and 10/11) were located in areas that have a relatively agranular composition (Carmichael and Price, 1994) and receive more pronounced input from distinct sensory association cortices (Jones and Powell, 1970; Barbas, 1988; Morecroft et al., 1992; Carmichael and Price, 1995b). Thus, decision making in this study activated distinct areas of inferior and orbital PFC that have access to heteromodal sources of information and are ideally positioned to integrate sensory and object-based processing of exteroceptive stimuli with processing of their associated reward–punishment valence. Moreover, in addition to its reciprocal connections with medial temporal systems (Jones and Powell, 1970), the orbitomedial and orbitolateral PFC provide important output pathways into the ventral striatum (Haber et al., 1995) and are able to interface such “affective” information with mechanisms of action selection routed through corticostriatal loops (Rolls, 1996).
The orbital PFC is also a prominent target of the monoamine neuromodulatory projections (Thierry et al., 1973). Indeed, the orbital PFC is just one station in an extensive circuitry, incorporating the ventral striatum and amygdala, implicated in processes of reinforcement and incentive motivation and under strong influence from mesocorticolimbic dopamine input (DiChiara and Imperato, 1988;Koob and Bloom, 1988; Wise and Rompré, 1989). Consequently, recent findings that subjects with a history of chronic amphetamine abuse show a pattern of decision-making deficits that closely resembles that shown selectively by patients sustaining damage to orbital PFC suggests that decision-making cognition may be susceptible to altered neuromodulation, perhaps affecting orbital PFC function (Rogers et al., 1999). Converging evidence that this is the case can be seen in the demonstration of marked impairments in the decision making of normal volunteers after acute plasma tryptophan depletion (Rogers et al., 1999), raising the further possibility that reduction in central 5-hydroxytryptamine, itself strongly associated with disorganized, impulsive, and aggressive behavior (Linnoila et al., 1983), is associated with altered decision making in laboratory settings.
The contributions of the orbital PFC to decision making are poorly understood; resolving choices between small, likely rewards and larger, unlikely rewards must recruit several, as yet unspecified, cognitive operations (Bechara et al., 1997; Rogers et al., 1999). However, the proposal that the orbital PFC is involved in the representation of stimulus–reward relationships (for review, see Iversen and Mishkin, 1970; Jones and Mishkin, 1972; Dias et al., 1996; Rolls, 1996) seems especially pertinent because effective real-life decision making must require accurate information about the current reward valence of relevant exteroceptive stimuli. However, the nature of this information remains controversial. On the one hand, the orbital PFC may help to mediate decision making by providing action selection mechanisms with direct information about the reinforcing properties of all types of unconditioned and conditioned stimuli (Rolls, 1996); on the other hand, the orbital PFC may reactivate somatic states previously conditioned to salient features of the choice confronting the subject (Damasio, 1994).
In this context, it is notable that although the reward offered to our subjects, experimenter-defined “points” having no monetary significance, was rather abstract and arbitrary in character, it is clear that the decision making of our subjects was sensitive to the combination of size and probability of rewards associated with the two response options (Fig. 1C–F). Moreover, decision making per se over this kind of reward, although effective in activating extensive parts of orbital PFC, did not activate other stations in the circuitry associated with processes of reinforcement such as the ventral striatum and amygdala. Although the detection of rCBF changes in these smaller structures may have been hampered by the width of smoothing filter applied to our data (FWHM = 16 mm), the present results suggest that the orbital PFC is particularly implicated in mediating decision making over “secondary” reinforcement (i.e., reinforcement conditioned to stimuli associated with “primary” reward; see also Bechara et al., 1999). Exploring whether the orbital PFC participates in a wider network mediating primary reinforcement requires manipulating the type of reinforcement available to subjects in similar tasks.
The strong activations seen in the orbital PFC during the decision-making conditions compared to the control conditions contrasts with the more restricted activity apparent in the direct comparisons between the 4:2 and 5:1 conditions. In general, the decision of the 4:2 conditions were more affected by the balance of reinforcers than those of the 5:1 conditions and were associated with marked increases in deliberation times (Fig. 1). Although the limited increase in rCBF seen within the anterior cingulate gyrus is entirely consistent with its proposed role in response selection mechanisms in coordination with interconnected limbic circuitry and orbital PFC (Vogt et al., 1992), the absence of large activations in the orbital PFC itself suggests that this area makes a necessary contribution to decision making that does not depend to any great extent on the degree of conflict inherent in the choice. However, our design deliberately matched reinforcement density across the 4:2 and 5:1 conditions. Recent studies suggest that the activity of the orbital PFC is sensitive to changes in acquired reward (Elliott et al., 1999) and violations of expectations (Nobre et al., 1999). Thus, research into the relationship between decision making, orbital PFC activity, and magnitude of reward also seems warranted.
Finally, appropriate deliberation about the available options in our decision-making task may also have required the temporary suppression of activated or primed responses, for example, those directed toward actions associated with larger but less probable rewards, and this suppression may have been reflected in the activations seen in the inferior convexity during the 4:2 and 5:1 conditions (Kawashima et al., 1996; Konishi et al., 1998; Krams et al., 1998). However, our peaks within the inferior convexity are somewhat ventral to those most recently associated with this inhibitory function (cf. Konishi et al., 1998) and are closer to activations previously seen in working memory studies (Owen et al., 1996; Smith et al., 1996; Courtney et al., 1998). Because it has been proposed that the inferior convexity is involved in the retrieval of information from posterior cortical areas (Petrides, 1994, 1995, 1996; Owen et al., 1996), it is possible that this area contributes to decision making, not by mediating some generic inhibitory function, but by mediating retrieval and/or comparator operations, e.g., over recent reinforcing events, needed for effective choices. Converging evidence that this is the case can be seen in a significant association (n = 84;r = −0.39; p < 0.001) between deliberation times in a decision-making task similar to the one used here and performance on a spatial span task (E. Bazanis, R. D. Rogers, J. H. Dowson, T. W. Robbins, and B. J. Sahakian, unpublished observations) that has previously been shown to activate the same area of ventrolateral PFC as activated in the current study (Owen et al., 1996). Finally, the decision-making deficits of orbital PFC patients do not take the form of impulsive or disinhibited responding (Bechara et al., 1996), but rather slow and ineffective deliberation about the conflicting options for action (Rogers et al., 1999), again suggesting that the contribution of the orbital PFC to decision-making cognition is not the provision of a simple inhibitory mechanism.
Footnotes
This work was supported by a Programme Grant from the Wellcome Trust to T.W.R., B.J.E., A.C.R., and B.J.S., and by Technology Foresight (J.D.P.). This is a publication of the Medical Research Council Cooperative on Brain, Behavior, and Neuropsychiatry. We thank Matthew Brett for his advice and help with data analysis.
Correspondence should be addressed to Robert D. Rogers, Department of Psychiatry, University of Oxford, Warneford Hospital, Oxford, OX3 7JX, UK.
REFERENCES
- 1.Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- 2.Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 3.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 4.Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- 5.Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 6.Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision-making within human prefrontal cortex. J Neurosci. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman KF, Randolph C, Gold J, Goldberg TE, Coppola R, Ostrem JL, Carson RE, Herscovitch P, Weinberger DR. Physiological activation of a cortical network of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 10.Carmichael ST, Price JL. Limbic connections of the orbital cortex and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995a;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 11.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995b;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 12.Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- 13.Damasio AR. Descartes’ error. Grosset/Putnam; New York: 1994. [Google Scholar]
- 14.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996a;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 15.Damasio H. Human neuroanatomy relevant to decision-making. In: Damasio AR, editor. Neurobiology of decision-making. Springer; Berlin: 1996b. pp. 1–12. [Google Scholar]
- 16.Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;4:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- 17.Dias R, Robbins TW, Roberts AC. Disassociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 18.DiChiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott R, Friston KJ, Dolan RJ. Dissociable neural response associated with reward, punishment and risk-taking. NeuroReport. 1999;9:S355. [Google Scholar]
- 20.Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- 21.Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 22.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behaviour by representational knowledge. In: Plum F, Mountcastle VB, editors. Handbook of physiology, Vol 5. American Physiological Society; Bethesda, MD: 1987. pp. 373–417. [Google Scholar]
- 23.Goldman-Rakic PS. The issue of memory in the study of prefrontal functions. In: Thierry AM, Glowinski J, Goldman-Rakic PS, Christen Y, editors. Motor and cognitive functions of the prefrontal cortex. Springer; Berlin: 1994. pp. 112–122. [Google Scholar]
- 24.Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 25.Haber SN, Kunisho K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howell DC. Statistical methods for psychology, Ed 2. Duxbury; Boston: 1987. [Google Scholar]
- 27.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 28.Jones EG, Powell TPS. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- 29.Jones B, Mishkin M. Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurology. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- 30.Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, Yanagisawa T, Fukuda H. Functional anatomy of GO/NO-GO discrimination and response selection–a PET study in man. Brain Res. 1996;728:79–89. [PubMed] [Google Scholar]
- 31.Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- 32.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 33.Krams M, Rushworth MFS, Deiber MP, Frackowiak RSJ, Passingham RE. The preparation, execution and suppression of copied movements in the human brain. Exp Brain Res. 1998;120:386–398. doi: 10.1007/s002210050412. [DOI] [PubMed] [Google Scholar]
- 34.Linnoila M, Virkkunen M, Stein M, Nuptial A, Ripon R, Goodwill FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- 35.Milner B. Some effects of frontal lobectomy in man. In: Warren JM, Akert K, editors. The frontal granular cortex and behavior. McGraw-Hill; New York: 1964. [Google Scholar]
- 36.Morecroft RJ, Geula C, Mesulam M-M. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- 37.Nelson HE. National adult reading test (NART) test manual. NFER-Nelson; Windsor: 1982. [Google Scholar]
- 38.Nobre AC, Coull JT, Frith CD. Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nat Neurosci. 1999;2:11–12. doi: 10.1038/4513. [DOI] [PubMed] [Google Scholar]
- 39.Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex. 1996;6:31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- 40.Petrides M. Frontal lobes and working memory: evidence from investigations of the effects of cortical excision in human primates. In: Boller F, Grafman J, editors. Handbook of neuropsychology, Vol 9. Amsterdam; Elsevier: 1994. pp. 59–82. [Google Scholar]
- 41.Petrides M. Functional organisation of the human frontal cortex for mnemonic processing. Ann NY Acad Sci. 1995;76:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- 42.Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc B Biol Sci. 1996;351:1455–1461. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- 43.Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain. 1999;122:1469–1493. doi: 10.1093/brain/122.8.1469. [DOI] [PubMed] [Google Scholar]
- 44.Roberts AC, Robbins TW, Weiskrantz L. Executive and cognitive functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1387–1527. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- 45.Rogers RD, Everitt BJ, Baldacchino A, Blackmore AJ, Swainson R, London M, Deakin JWF, Sahakian BJ, Robbins TW. Dissociating deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–329. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 46.Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rolls ET. The orbitofrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1433–1444. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- 48.Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29:1241–1249. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- 49.Silbersweig DA, Stern E, Frith CD, Cahill C, Schnorr L, Grootonk S, Spinks T, Clark J, Frackowiak R, Jones T. Detection of thirty-second cognitive activations in single subjects with positron emission tomography: a new low-dose H215O regional cerebral blood flow three-dimensional imaging technique. J Cereb Blood Flow Metab. 1993;13:617–629. doi: 10.1038/jcbfm.1993.80. [DOI] [PubMed] [Google Scholar]
- 50.Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial and working memory using PET. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- 51.Stuss DT, Benson DF. The frontal lobes. Raven; New York: 1986. [Google Scholar]
- 52.Thierry AM, Blanc G, Sobel A, Stinus L, Glowinski J. Dopamine terminals in the rat cortex. Science. 1973;182:499–501. doi: 10.1126/science.182.4111.499. [DOI] [PubMed] [Google Scholar]
- 53.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 54.Wise RA, Rompré PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 55.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]