Abstract
Astrocytes, oligodendrocytes, and oligodendrocyte/type 2 astrocyte progenitors (O2A cells) can all produce molecules that inhibit axon regeneration. We have shown previously that inhibition of axon growth by astrocytes involves proteoglycans. To identify inhibitory mechanisms, we created astrocyte cell lines that are permissive or nonpermissive and showed that nonpermissive cells produce inhibitory chondroitin sulfate proteoglycans (CS-PGs). We have now tested these cell lines for the production and inhibitory function of known large CS-PGs. The most inhibitory line, Neu7, produces three CS-PGs in much greater amounts than the other cell lines: NG2, versican, and the CS-56 antigen. The contribution of NG2 to inhibition by the cells was tested using a function-blocking antibody. This allowed increased growth of dorsal root ganglion (DRG) axons over Neu7 cells and matrix and greatly increased the proportion of cortical axons able to cross from permissive A7 cells onto inhibitory Neu7 cells; CS-56 antibody had a similar effect. Inhibitory fractions of conditioned medium contained NG2 coupled to CS glycosaminoglycan chains, whereas noninhibitory fractions contained NG2 without CS chains. Enzyme preparations that facilitated axon growth in Neu7 cultures were shown to either degrade the NG2 core protein or remove CS chains. Versican is present as patches on Neu7 monolayers, but DRG axons do not avoid these patches. Therefore, NG2 appears to be the major axon-inhibitory factor made by Neu7 astrocytes. In the CNS, NG2 is expressed by O2A cells, which react rapidly after injury to produce a dense NG2-rich network, and by some reactive astrocytes. Our results suggest that NG2 may be a major obstacle to axon regeneration.
Keywords: axon regeneration, chondroitin sulfate, NG2, extracellular matrix, proteoglycans, versican
Injury to the CNS generally results in the formation of a glial scar, a predominantly astrocytic structure that can constitute a no-go zone for regenerating axons. Reactive astrocytes upregulate their synthesis of many cell surface, secreted, and matrix molecules, but various lines of evidence point toward chondroitin sulfate proteoglycans (CS-PG) as being the major source of inhibitory molecules in the glial scar. For instance, Davies et al. (1997) showed that neurons grafted atraumatically into white matter tracts of adult rats were able to regenerate their axons. However, in some animals, axon growth failed, and in these animals the graft was enclosed by a ring of chondroitin sulfate. A different approach has been to remove reactive astrocytes from the brain by implanting a nitrocellulose filter. These cells express high levels of CS-PG, are inhibitory to axon growth, and can be made more permissive to growth by treatment with glycosaminoglycan (GAG)-degrading enzymes (McKeon et al., 1991). Many proteoglycans, particularly those carrying chondroitin sulfate, have been shown to be inhibitory to neurite outgrowth in vitro (Snow et al., 1990; Dou and Levine, 1994). Based on their distribution in the embryo, it has also been suggested that CS-PGs act as molecular barriers, preventing the growth of axons into inappropriate regions (Snow et al., 1990; Snow and Letourneau, 1992;Pindzola et al., 1993; Landolt et al., 1995).
As another approach toward determining which of the cell surface, diffusible, and matrix molecules present in glial scars might be critical to the inhibition of axon growth, we developed a panel of astrocyte cell lines displaying a range of abilities to promote growth (Geller and Dubois-Dalcq, 1988; Fok-Seang et al., 1995; Powell et al., 1997). The most inhibitory cell line, Neu7, was inhibitory to dorsal root ganglion (DRG), retinal, and cortical axons, whereas the A7 cell line was the most permissive. Analysis of the differences between the cell lines demonstrated that the inhibitory properties of the Neu7 cell line were primarily caused by their production of CS-PGs. Disruption of proteoglycan synthesis and enzymatic removal of the chondroitin sulfate made the cells and their extracellular matrix more permissive to axon growth, reduced the laminin-blocking activity of their conditioned medium (CM) (Smith-Thomas et al., 1994, 1995), and reduced their ability to form boundaries that repel axons (Powell et al., 1997). Importantly, it was also possible to increase axon growth through three-dimensional cultures of primary astrocytes by disrupting proteoglycan sulfation with chlorate (Smith-Thomas et al., 1995).
We have now screened these astrocyte cell lines for those large proteoglycans known to be expressed in the CNS to ascertain which are differentially expressed and might be responsible for the inhibition. We report that several CS-PGs are differentially expressed, but on the basis of functional assays, we believe that NG2 provides an important component of the inhibitory activity. This structurally unique CS-PG has been shown to be a potent inhibitor of neurite outgrowth in vitro (Dou and Levine, 1994) and is also highly expressed within the glial scar (Levine, 1994).
MATERIALS AND METHODS
Astrocyte cell lines
The A7 cell line was derived from cultured new-born rat optic nerve, immortalized with the SV40 large T antigen (Geller and Dubois-Dalcq, 1988). The remaining three GFAP-positive cell lines originated from cultures of neonatal rat cerebral cortex (Fok-Seang et al., 1995). Neu7 was immortalized by the neu oncogene, whereas T27A1 and T34.2 were immortalized by the temperature-sensitive form of the SV40 large T antigen. Cultures were maintained in DMEM containing 10% fetal calf serum (FCS) and 1% penicillin–streptomycin–fungizone (PSF). Where stated, Neu7 was grown in 10% horse serum, which made them even less permissive to neurite outgrowth (J. W. Fawcett, unpublished observation).
Sample preparation for SDS-PAGE
Supernatant. Cells in a 25 cm2 flask were grown to confluency in serum, rinsed three times in DMEM, and then incubated in exactly 2.5 ml of serum-free medium (DMEM containing 1% insulin–transferrin–selenium–BSA–linoleic acid (ITS+) (Collaborative Research, Paisley, Scotland). After 2 d, the serum-free CM was collected and concentrated 10-fold in a centricon-50 (Millipore, Bedford, MA) in the presence of a cocktail of protease inhibitors (Complete; Boehringer Mannheim). Each track received one-fifth of the concentrated CM (50 μl).
Metabolic labeling of cell lines. Cells (106) of each line were labeled overnight by addition of 250mCi of Na2-35SO4into the medium. The medium was taken off, the cells were lysed with 1% Triton X-100 buffer, and one-tenth of the supernatant or cell extract was loaded per lane after addition of reducing sample buffer. Proteins were separated in a 4–10% gel, and the dried gel was exposed to film.
Supernatant proteoglycan fractions. Confluent monolayers were incubated in serum-free medium. After 2 d, the conditioned media were collected, centrifuged at 100,000 × g for 1 hr, and applied to a Q-Sepharose anion exchange column (Amersham Pharmacia Biotech, Amersham, UK). The column was washed with sodium acetate buffer (containing 0.15 or 0.5 m NaCl), and a proteoglycan fraction was eluted with 1.0 mNaCl. This fraction was dialyzed against 50 mmammonium bicarbonate, lyophilized, and resuspended in PBS. The glycosaminoglycan content of the fractions was determined using the Blyscan assay (Biocolor, Belfast, UK). The inhibitory activity of each fraction was assayed by growing DRGs in 35 mm tissue culture dishes precoated overnight with 1.5 mg laminin and 50 gm of either the 0.5 or 1.0 m fraction. The DRGs were grown for 2 d in serum-free medium (DMEM, 1% ITS+, and 20 mm Ara-C) containing 50 mg of the appropriate fraction per dish. DRGs were scored to have grown axons if more than three axons had grown for one explant diameter or more.
Detergent lysates. Confluent cultures in 25 cm2 flasks were washed three times with 0.05 m Tris and 0.15 mNaCl, pH 7.4, and removed with a cell scraper. The cells were centrifuged, and the pellet was resuspended in 0.25 ml of the same buffer containing 1% Triton X-100 and the cocktail of protease inhibitors and incubated on ice for 1 hr. The suspension was centrifuged at 13,000 rpm for 10 min at 4°C. The protein content of the detergent lysate was determined using the Coomassie plus protein assay reagent (Pierce, Rockford, IL), and the volumes were adjusted to give equal loading to each lane.
Membrane fractions. Cells were lysed from the culture dish in a hypotonic buffer, homogenized using a 27 gauge needle, and layered on top of a sucrose step gradient (50 and 5% sucrose in PBS). After centrifugation for 10 min at 28,000 rpm, membranes were collected from the interface between the sucrose cushions and washed twice in PBS. Protein content was determined using the Bio-Rad (Hercules, CA) Protein Assay.
GAG lyase digestion
Samples of 0.05 ml were digested with 0.02 U of protease-free chondroitinase ABC (ChABC) (Boehringer Mannheim) for 2.5 hr at 37°C.
Biosynthetic labeling of cell cultures and immunoprecipitation
Cell lines were grown to confluency in 6-cm-diameter Petri culture dishes. For overall biosynthetic labeling, medium was changed to methionine–cysteine- and Na2SO4-free DMEM for 1 hr. Thereafter, 200 μCi/ml35S-methionine–cysteine (Amersham Pharmacia Biotech translabel) and 50 μCi/ml Na235SO4were added to the cultures for the times indicated. For biosynthetic labeling of sulfated carbohydrate groups, cultures were incubated overnight with 50 μCi/ml35SO4 in Na2SO4-free DMEM supplemented with 1% fetal calf serum. At the end of the labeling period, supernatants were collected and supplied with a mixture of protease inhibitors (EDTA and iodoacetamide at 5 mm, PMSF at 1 mm, soybean trypsin inhibitor at 10 μg/ml, and aprotinin, leupeptin and pepstatin, all at 5 μg/ml) and 0.1% BSA (w/v). Cell monolayers were washed twice with PBS (1×) and detergent solubilized in 1 ml of ice-cold solubilization buffer [0.15m NaCl, 20 mm Tris-HCl, 1 mm EGTA, 0.5% (v/v) Nonidet-40 (NP-40), 0.1% (w/v) BSA, and 0.05% (w/v) NaN3, pH 7.4] supplemented with protease inhibitors as indicated above. After 10 min on ice, cells were scraped off the culture dish, transferred to tubes, and kept on ice for another 30 min. Supernatants and detergent extracts were cleared by consecutive rounds of centrifugation at 800 × g, 4°C, for 10 min to remove large debris or cell nuclei, and subsequently at 100000 ×g, 4°C, for 45 min. For determination of incorporated activity, 50 μl aliquots from detergent extracts or culture supernatants were incubated with TCA (20% w/v) for 1 hr on ice. Precipitates were collected by centrifugation and washed twice with TCA (10% w/v), and the resulting pellets were dried, dissolved in preheated SDS sample buffer, boiled for 5 min, and finally quantified in a g-scintillation counter. For immunoprecipitation, 1 ml aliquots of biosynthetically labeled detergent-solubilized cell extracts or culture supernatants were precleared by incubation with 120 μl of preswollen Sepharose-protein A conjugate (10% v/v; Amersham Pharmacia Biotech) for 30 min to remove nonspecific binding to Sepharose-protein A beads. After removal of the Sepharose beads by centrifugation, the aliquots were mixed with 80 μg of IgG fraction of polyclonal anti-NG2 antibodies and incubated overnight at 4°C. The aliquots were subsequently incubated for 1 hr with 120 μl of preswollen Sepharose-protein A conjugate. Thereafter, the beads were washed by four cycles of centrifugation and resuspension in buffers of decreasing salt concentration. Finally, the beads were resuspended in 100 μl of SDS sample buffer and boiled for 5 min at 100°C (Faissner et al., 1994). A 50 μl aliquot of the resulting extract was counted in a g-scintillation counter, whereas the remaining 50 μl were resolved on a 4–10% gradient SDS-polyacrylamide gel. After the run, gels were fixed in 10% (v/v) acetate and 30% (v/v) methanol in water, vacuum-dried, and exposed to a phosphoimager (Fuji x-BAS 1000) plates at room temperature.
Western blot analysis
Polypeptides were separated in 5% SDS-polyacrylamide gels (without a reducing agent) and transferred to nitrocellulose (Hybond-C pure; Amersham) for ∼16 hr at 0.15 A. The blots were blocked for 1 hr in either 5% non-fat dried milk in TBS–0.05% Tween 20 or TBS–Tween 20 alone and then incubated for 2 hr in primary antibody. Various antibodies were used, and their concentrations and sources are in Table1. All subsequent washes were six times for 5 min, and incubations were performed in TBS–0.05% Tween 20. The blots were then incubated with biotinylated anti-mouse Ig or biotinylated anti-rabbit Ig (Amersham) for 1 hr, washed as before, incubated with alkaline phosphatase-avidin D (1:1000; Vector Laboratories, Burlingame, CA) for 1 hr, and washed, and reactive bands were visualized using a substrate of 5-bromo-4-chloro-3-indoyl-phosphate and nitroblue tetrazolium (Vector Laboratories). Alternatively, bands were visualized using an HRP-conjugated secondary antibody, followed by chemiluminescence used in accordance with the supplier’s instructions (ECL kit; Amersham).
Table 1.
Antibodies
| Antigen | Antibody | Host species | Source | Working dilution | ChABC | Reducing | Gel % |
|---|---|---|---|---|---|---|---|
| NG2 | a-NG2 | Rabbit | J. Levine | 1:1000 | Yes | No | 5% |
| Versican | 12C5 | Mouse IgG1 | R. A. Asher | 1:1000 | Yes | No | 5% |
| Neurocan | 1D1 | Mouse IgG1 | U. Rauch | 1:100 | Yes | ||
| Brevican | Pc | Rabbit | E. Gundelfinger | 1:1000 | Yes | No | 5% |
| CD44 | OX-50 | Mouse IgG1 | ECACC | 1:50 | Yes | No | 5% |
| CS/DS-PG | MO-225 | Mouse IgM | Seikagaku | 1:1000 | No | Yes | 5% |
| CS-PG | CS-56 | Mouse IgM | Sigma | 1:500 | No | No | 5% |
| KS | 5D4, 2D3 | Mouse | B. Caterson | 1:200 | No | ||
| CS-4 | 2B6 | Mouse | B. Caterson | 1:200 | Yes | ||
| CS-6 | 3B3 | Mouse | B. Caterson | 1:200 | Yes | ||
| HABR | 5C4 | Mouse | B. Caterson | 1:200 | No | ||
| APP | 22C11 | Mouse IgG | G. Multhaup |
ECACC, European Collection of Animal Cell Cultures; HABR, hyaluronic acid-binding region of aggrecan-like proteoglycans; KS, keratan sulphate; APP, Alzheimer precursor protein.
ELISA
The wells of a Microtest flexible assay plate (Falcon) were coated overnight with 0.2 ml of cell supernatant. The plates were washed three times with PBS and blocked for 1 hr with 4% non-fat dried milk in PBS–0.05% Tween 20. The primary antibody was applied in blocking buffer for 2 hr at 37°C and the HRP-conjugated secondary antibody for 1 hr at 37°C. Plates were developed with 2,2′-azino-di-[3-ethyl-benzthiazoline sulfonate], and the reaction was stopped by the addition of SDS to a final concentration of 0.2%. The reaction product was quantified with an ELISA reader at 405 nm.
Immunocytochemistry
Cells were seeded onto poly-l-lysine-coated glass coverslips and grown in DMEM containing 10% FCS. They were fixed in 4% paraformaldehyde and blocked in PBS containing 1% goat serum and 1% BSA, and the primary antibody was added for 1–2 hr. Generally, polyclonal antibodies were visualized using a fluorescent secondary antibody, whereas a biotinylated secondary antibody was used for monoclonal antibodies. For versican, it was necessary to fix in cold (−20°C) methanol. For double labeling, live cells were labeled for versican and CS-56, fixed in methanol, and then labeled with a rabbit anti-neurofilament antibody (Genosys, The Woodlands, TX).
Axon growth assays
Intact DRGs from neonatal rats were plated onto confluent monolayers of Neu7 cells, as described by Fok-Seang et al. (1995), in the presence of either the polyclonal anti-NG2 antibody (0.4 mg/ml) or the same concentration of a rabbit polyclonal antibody to laminin. The cultures were grown in media (DMEM containing 10% horse serum and 1% PSF) supplemented with 10 ng/ml nerve growth factor. Twenty-four hours later, the cultures were fixed in cold methanol, and the neurites were visualized using the anti-neurofilament monoclonal 3A10 (Developmental Studies Hybridoma Bank, Iowa City, IA). Neurite length was estimated as the mean of the 10 longest axons, measured from the edge of the explant to the growth tip. The total number of axons from each DRG was counted: when fasciculation was present, the number of axons in each fascicle was estimated by following the fascicle out to the margin of the halo of growing axons and counting the axons dissociating from the fascicle. Total outgrowth was the length multiplied by the number of axons. Results were from three separate assays. Neu7 matrix was made by water lysis of cell monolayers for 20 min, followed by washing in HBSS. DRGs were plated on the matrix in ITS+ serum-free medium with NGF, with and without 0.2 mg/ml anti-NG2, and growth was assayed 24 hr later. DRGs were counted as having grown axons if more than three axons had grown for more than one explant diameter.
Generation of cellular boundaries
Cellular boundaries were generated as we have described previously (Powell et al., 1997) by first plating A7 cells on poly-l-lysine-coated coverslips at a density of 3 × 104 cells per coverslip in DMEM–10% FCS. Twenty-four hours later, A7 or Neu7 cells were labeled with the vital dyes DiI or PKH26 and fast blue (0.1 μg/ml; Sigma, St. Louis, MO) and plated at a density of 3 × 103 cells per coverslip onto subconfluent A7 cells. Using this procedure, the cells formed a mixed monolayer within 24 hr. The monolayers are referred to as A7/A7 and A7/Neu7.
Cell culture and boundary assay
Cerebral cortical neuronal cultures were prepared as described by Morris and Geller (1996). Briefly, timed-pregnant Sprague Dawley rats were killed at embryonic day 17, and embryos were removed by cesarean section and placed in cold basal medium Eagle’s containing 0.02 m sodium-HEPES (BME-HEPES). The cortical hemispheres were dissected from each embryonic brain in cold BME-HEPES. Meningeal tissues were carefully removed from the tissue and incubated in BME-HEPES containing 0.025% trypsin (type-II) and 0.1% type-IV collagenase for 30 min at 37°C. The reaction was halted with 0.025% soybean trypsin inhibitor and 0.05% DNase I, and the tissue was gently triturated several times through a fire-polished Pasteur pipette. The cells were centrifuged at 1000 × g at 4°C for 5 min, filtered through sterile 40 μm mesh. Cortical neurons were resuspended in DMEM supplemented with 10% FCS, penicillin (50 μg/ml), and streptomycin (50 U/ml).
For the boundary assay, cerebral cortical neurons were plated on confluent A7/A7 or A7/Neu7 cellular substrates at a density of 3.5 × 104 cells per well and allowed to adhere for 3–4 hr. After adherence to the cellular substrates, 0.2 mg/ml rat anti-mouse immunoglobulin G1 (IgG1) (Cappel, West Chester, PA), rabbit anti-neurofilament 200 (Sigma) at 1:1000, 0.2 mg/ml of NG2, or 1.2 gm/ml CS-56 antibody were added. Sixteen hours after the addition of the IgG1 or NG-2 antibody, neurons on cellular substrates were labeled with the vital dye 5(6)-carboxyfluorescein diacetate, which intensely stains the soma and processes of living neurons (Petroski and Geller, 1994). One hundred neurites per coverslip were assessed as to their stopping, turning, or crossing upon encountering the A7/A7 or A7/Neu7 cellular boundaries, as described previously (Powell et al., 1997). Each experimental condition consisted of three coverslips, and the experiments were repeated three times.
RESULTS
Neu7 cells produce inhibitory proteoglycans
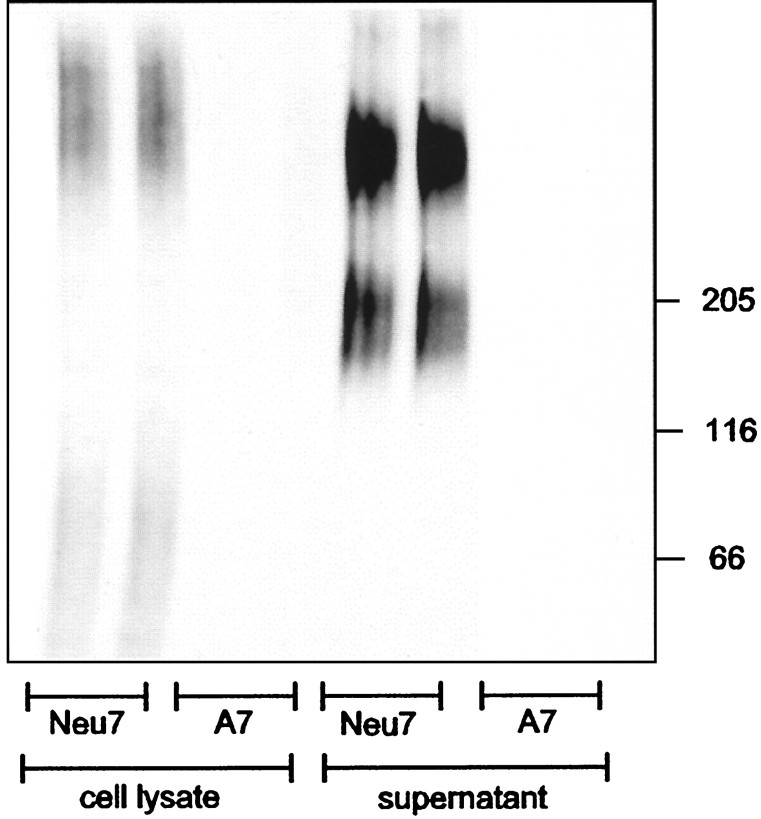
We first analyzed the proteoglycan synthesis by Neu 7 and A7 cell lines. The cells were grown for 12 hr in the presence of Na235SO4, and the cell lysate and CM were analyzed by SDS-PAGE. Because the majority of sulfate is incorporated into proteoglycans, this method provides a rough estimate of overall proteoglycan synthesis in the cell lines. Figure 1 shows that Neu7 incorporates much more sulfate than A7. The radiolabel was incorporated into species migrating at 80–90 kDa and greater than 350 kDa in the cell lysate, and into polydisperse material migrating at 140–180 and 350–500 kDa in the CM. Neu7 and A7 serum-free supernatants contain the same amount of total protein, measured by the Bio-Rad assay at 1.2 mg/ml.
Fig. 1.
Sulfate incorporation into cell lysate (left 4 tracks) and supernatant (right 4 tracks) from Neu7 and A7 cells after growing the cells in the presence of Na235SO4overnight. Neu7 cells incorporate much more sulfate than A7 into both types of sample.
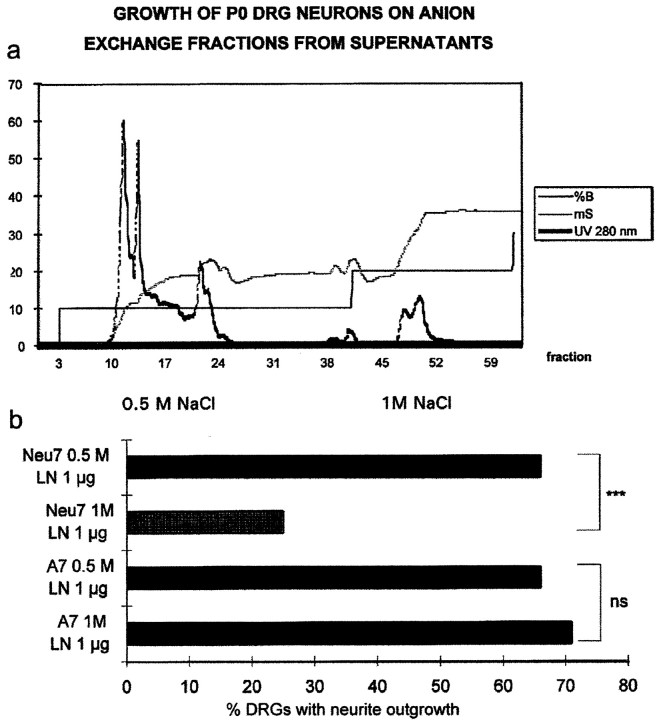
We have shown previously that the inhibitory activity in conditioned medium from the Neu7 line is found in fractions from an anion exchange column that contain sulfate (Smith-Thomas et al., 1994). In the present study, we fractionated CM from both Neu7 and A7 cells on an anion exchange column, and the fractions eluting with 0.5 m NaCl and 1.0 m NaCl were collected. We then tested the ability of the fractions to block the neurite outgrowth-promoting properties of laminin from explants of neonatal rat DRGs. The 0.5 mfractions from both A7 and Neu7 were not inhibitory to DRG outgrowth on laminin. However, the 1.0 m fraction from Neu7, but not from A7, was growth-inhibitory (Fig. 2). The glycosaminoglycan content of the 1 m fractions was tested by a modified dimethylene blue assay (Blyscan; Biocolor); the Neu7 1 m fraction contained 2.8 mg/ml, and the A7 1m fraction contained 0.9 mg/ml. Neu7 cells therefore produce more proteoglycan overall than A7, and the fraction of Neu7 supernatant eluting at 1.0 m NaCl contains the inhibitory activity.
Fig. 2.
Fractionation of Neu7 conditioned medium on an anion exchange column (a). %B is salt concentration, mS is the conductivity, andUV 280 nm is the UV absorption. The UV absorption curve shows that two absorption peaks come off the column, one at 0.5m NaCl and one at 1 m NaCl. These two fractions and equivalent fractions from the A7 cell line were tested for their ability to inhibit axon growth on a laminin substrate. Inb, the proportion of DRGs that grew axons on the four test substrates is shown. Only the 1 m fraction from Neu7 was inhibitory.
Expression of CNS proteoglycans by cell lines
We screened A7 and Neu7 cells for differential expression of a variety of individual proteoglycans and chondroitin sulfate epitopes that have been described in the CNS. These results are shown in Table 2(Table 2). The major differences we detected were much greater expression of NG2, versican, and the proteoglycans recognized by the CS-56 antibody in Neu7 cells.
Table 2.
Expression of proteoglycans
| Supernatant | Cells detergent extract | |||||||
|---|---|---|---|---|---|---|---|---|
| ELISA | Western blot | Immunofluorescence | Western blot | |||||
| A7 | Neu7 | A7 | Neu7 | A7 | Neu7 | A7 | Neu7 | |
| NG2 | − | +++ | − | +++ | − | +++ | − | ++ |
| Versican | − | +++ | +++ | − | − | |||
| Neurocan | − | − | − | − | − | − | − | − |
| Brevican | − | − | − | − | − | − | ||
| Perlecan | +++ | +++ | ++ | ++ | ++ | ++ | ||
| CS-56 mAb | + | + | − | + | + | +++ | ++ | ++ |
| 5D4, 2D3(a-KS) | + | ++ | + | + | + | + | ||
| 2B6 (a-C-4-S) | + | + | ++ | ++ | + | + | ||
| 3B3 (a-C-6-S) | + | + | + | + | + | ++ | ||
| MO-225 (a-CS) | − | − | ||||||
| 5C4 a-HABR | ++ | +++ | + | ++ | − | − | ||
−, Nondetectable; +, ++, or +++ indicates increasing levels of expression. HABR, Hyaluronic acid-binding region of aggrecan-like proteoglycans; KS, keratan sulphate.
Expression of the NG2 proteoglycan
Because our initial screen showed that NG2 was one of the proteoglycans that was made in large amounts by Neu7 but not by A7, we investigated its expression further.
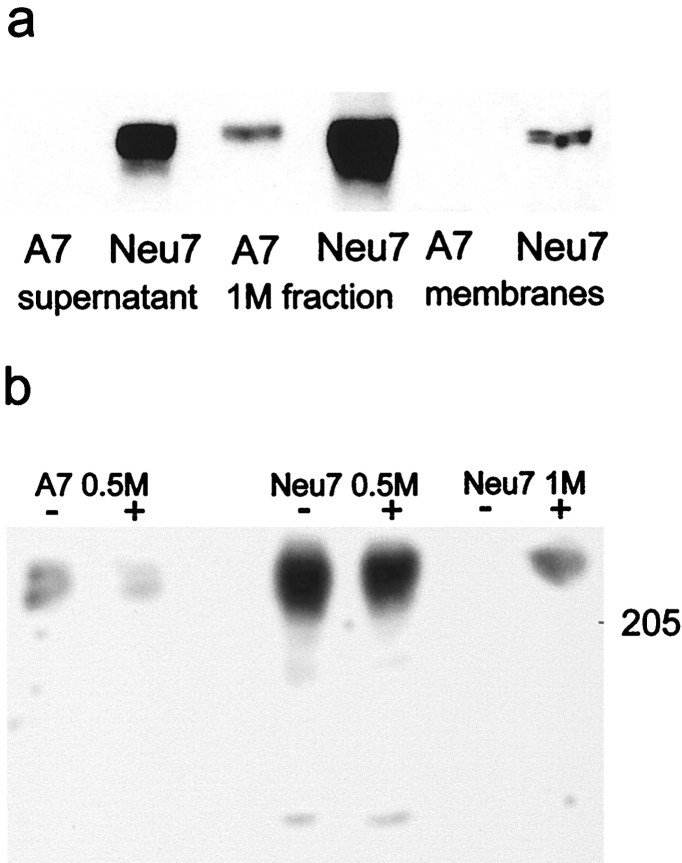
NG2 consists of a 300 kDa core protein bearing GAG chains composed entirely of chondroitin sulfate and is found in three forms: the full-length 300 kDa transmembrane form; a 290 kDa form released from the cell surface by proteolytic cleavage; and a 275 kDa membrane-associated form, which is also subject to proteolytic cleavage but which remains associated with the cell surface (Nishiyama et al., 1995). NG2 was detectable in detergent lysates and in cell culture supernatant only from Neu7 cells and not from any of the three more permissive cell lines A7, 27A1, and T34.2 (results for A7 and Neu7 are shown in Fig. 3a). The highly concentrated 1.0 and 0.5 m NaCl fractions of A7 and Neu7 supernatants were subjected to SDS-PAGE and examined using Western blots with anti-NG2 antibody. As shown in Figure 3a, there was a large amount of NG2 in the Neu7 1 mfraction and very little in the A7 1 m fraction, seen as a band of 270–300 kDa (our largest size marker was 205 kDa). Detection of NG2 in the 1.0 m fraction from A7, but not in the culture supernatant from which it was derived, is presumably a concentration effect, reflecting low levels of NG2 production by A7 cells, which is just detectable in highly enriched samples. As shown in Figure 3b, the NG2 band from the Neu7 1.0 m fraction could only be seen on blots in which the fraction had been digested with ChABC. In the 0.5m fractions, there was a large amount of NG2 in the Neu7 samples and a smaller amount in the A7 samples. These fractions produced bands at ∼300 kDa without previous digestion with ChABC, and chondroitinase digestion made no difference to the appearance of the bands. These results suggest that Neu7 makes large amounts of NG2, much of which is linked to CS GAG chains and therefore interacts strongly with the anion exchange column and comes off at 1.OM NaCl, and some of which is not linked to GAG chains and therefore interacts weakly with the column and comes off at 0.5m NaCl. The A7 line makes much smaller amounts of NG2, and the greater part of this is not linked to GAG chains and therefore comes off at 0.5 m NaCl. Immunocytochemistry revealed that most, but not all, Neu7 cells were NG2-positive in subconfluent cultures (Fig.4a,b); in fully confluent monolayers, staining was more uniform, with most cells expressing NG2. Axons will grow sparsely on Neu7 monolayers. In fully confluent monolayers that will support axon growth, the NG2 is fairly evenly distributed, so it is not possible to test whether axons preferentially avoid regions of higher NG2 expression (Fig.4c,d). A7 monolayers did not stain above background (data not shown).
Fig. 3.
a, Production of NG2 by A7 and Neu7 cells. In A7 cells, NG2 is not detectable in the supernatant or membrane fraction. It is only detectable in small amounts in the highly concentrated 1 m fraction from ion exchange fractionation. Neu7 produces large amounts of NG2 in cell surface and released forms, seen in the detergent lysate and culture supernatant samples. These samples were digested with chondroitinase ABC. B, NG2 in fractions from anion exchange. The fractions from both A7 and Neu7 coming off the column at 0.5 m NaCl contain NG2, which forms a discrete band in the absence of chondroitinase ABC digestion, and therefore has little or no GAG chain attached. There is a large amount of NG2 in the 1 m fraction from Neu7, but this cannot be seen as a discrete band unless it is digested with chondroitinase ABC, indicating that all the NG2 in this fraction carries GAG chains.
Fig. 4.
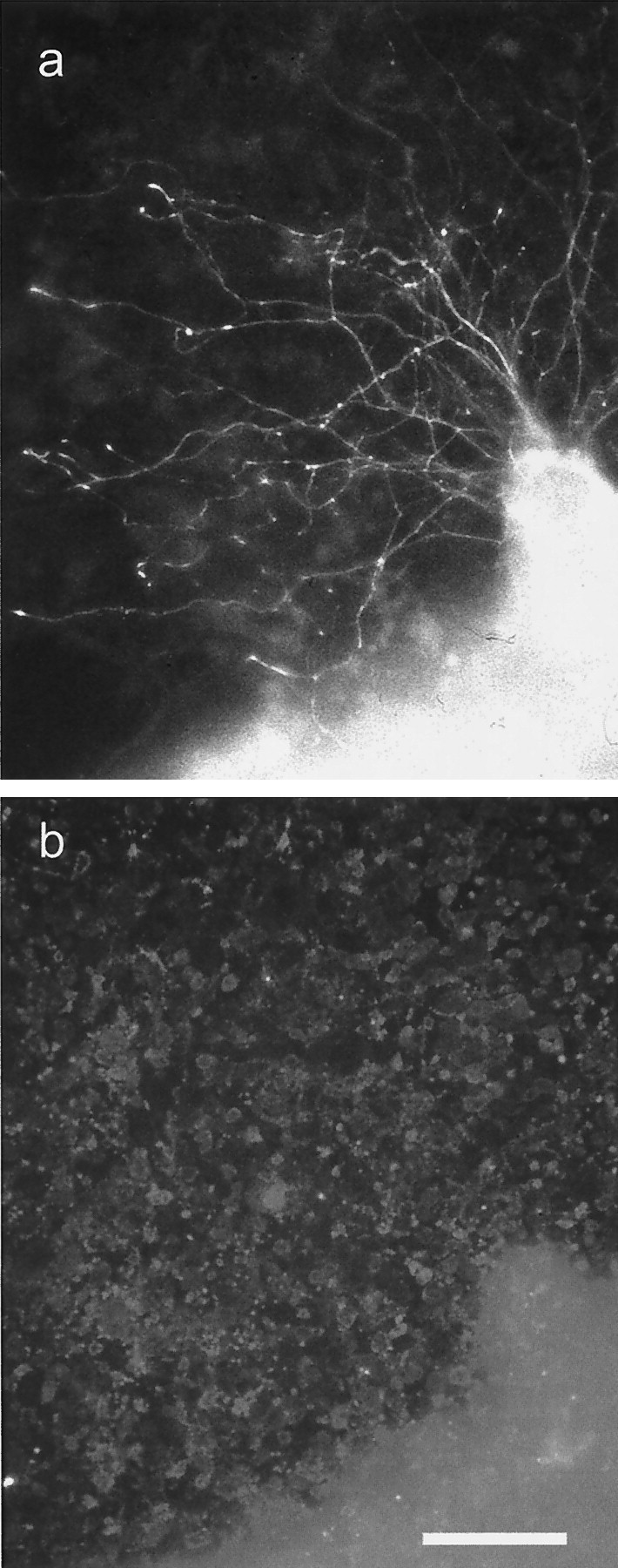
Axon growth from a postnatal DRG on an almost confluent culture of Neu7 cells. a is a neurofilament immunostain, and b is an NG2 immunostain. The axons had grown for 24 hr. Although most of the cells are stained for NG2, there is some unevenness in the intensity of NG2 staining on the Neu7 cells. However, during the 24 hr of the assay, the cells divide, migrate, and secrete NG2 into the medium, so correlations between axon path and NG2 staining are not meaningful.
Proportion of total protein and total proteoglycan represented by NG2
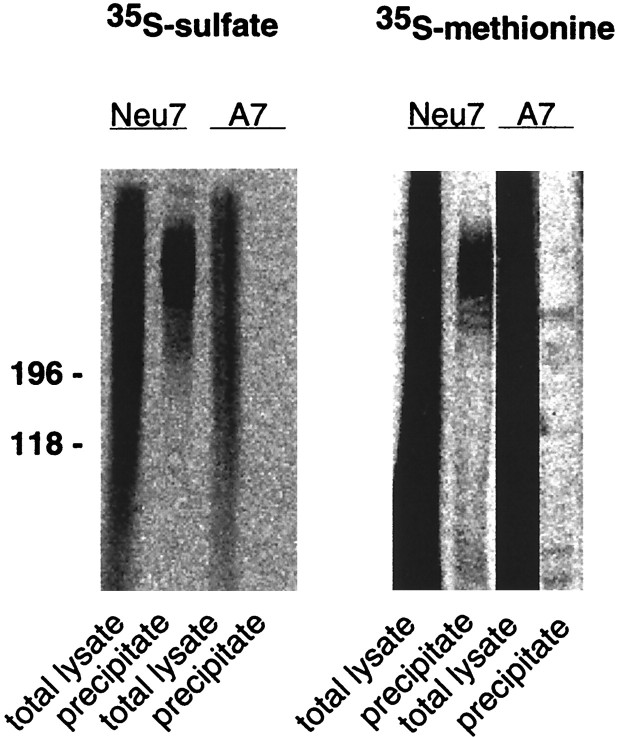
The Western blot analysis clearly suggested an enhanced expression of NG2 with attached GAG chains in the Neu7 compared with the A7 cell line. To obtain an estimate of the relative proportion of the proteoglycan in reference to the overall protein and proteoglycan synthesis in the respective cell lines, biosynthetic labeling and immunoprecipitation experiments were performed. When overall protein synthesis was evaluated using35S-methionine–cysteine, both Neu7 and A7 showed comparable anabolic activity with 153.4 × 106 cpm versus 192.7 × 106 cpm incorporated activity per dish in the detergent extracts of the monolayers, respectively (Figs.5,6). In contrast, there were marked differences in 35S-sulfate incorporation, with ∼2 × 106 cpm in the detergent extract and 8 × 106 cpm in the culture supernatant of Neu7, whereas A7 achieved only 0.5 × 106 cpm in the cell monolayer lysate and 2 × 106 cpm in the culture supernatant. Thus, because the incorporation of35S-SO4 primarily reflects glycosaminoglycan biosynthesis, the rate of GAG production seemed fourfold greater in Neu7 than in A7 under conditions of comparable protein synthesis. Furthermore, the major fraction of proteoglycans synthesized under these conditions accumulated in the culture supernatant. When immunoprecipitation experiments were performed with polyclonal anti-NG2 antibodies on35S-SO4-labeled cultures, no significant signal could be obtained from A7, whereas Neu7 produced a polydisperse smear in the expected molecular mass range (Fig. 5). Expressing immunoprecipitated NG2 as a percentage of overall incorporation, 0.24% of incorporated35S-SO4 was recovered from the detergent extract and 0.025% from the supernatant of Neu7. The values determined for the corresponding fractions of A7 were 0.012% and not measurable, respectively. A second cycle of immunoprecipitation yielded ∼10% of the signal intensity obtained in the first cycle, indicating nearly exhaustive precipitation. Thus, on the basis of 35S-SO4incorporation, A7 produced 20-fold less NG2 than Neu7. To examine whether this might reflect the overall lower rate of sulfate incorporation recorded for A7, an immunoprecipitation experiment was performed with detergent extracts of35S-methionine–cysteine-labeled cultures in which the major fraction of NG2 was expected. In this case, a faint band of NG2 could be detected in A7, which reflected ∼0.002% of incorporated label, whereas the Neu7-derived immunoprecipitate contained ∼0.005% of the labeled total protein. Again, polydisperse material could be revealed in addition to a faint band, which might reflect a higher degree of GAG attachment to NG2 in the Neu7 cell line (Fig. 5). Hence, on the level of35S-methionine–cysteine-labeling, the difference between the cell lines appeared less pronounced, although Neu7 still produced ∼2.5-fold more NG2 than A7. However, as far as the mature GAG-attached form of NG2 is concerned, Neu7 produced by far more than the A7 cell line.
Fig. 5.
Immunoprecipitation of NG2 from biosynthetically labeled Neu7 and A7 cultures. Cell lines were labeled with35S-SO4 or 35S-methionine–cysteine as indicated. An aliquot of cell monolayer detergent extracts and the corresponding immunoprecipitates were resolved on neighboring lanes of a 4–10% SDS-PAGE slab gel. Note that polydisperse NG2 can be recovered from the Neu7 lysates of both types of labeling, whereas A7 does not yield a detectable signal on 35S-SO4and only a faint band without smear after35S-methionine–cysteine labeling. A phosphoimager picture of the gel is shown.
Fig. 6.
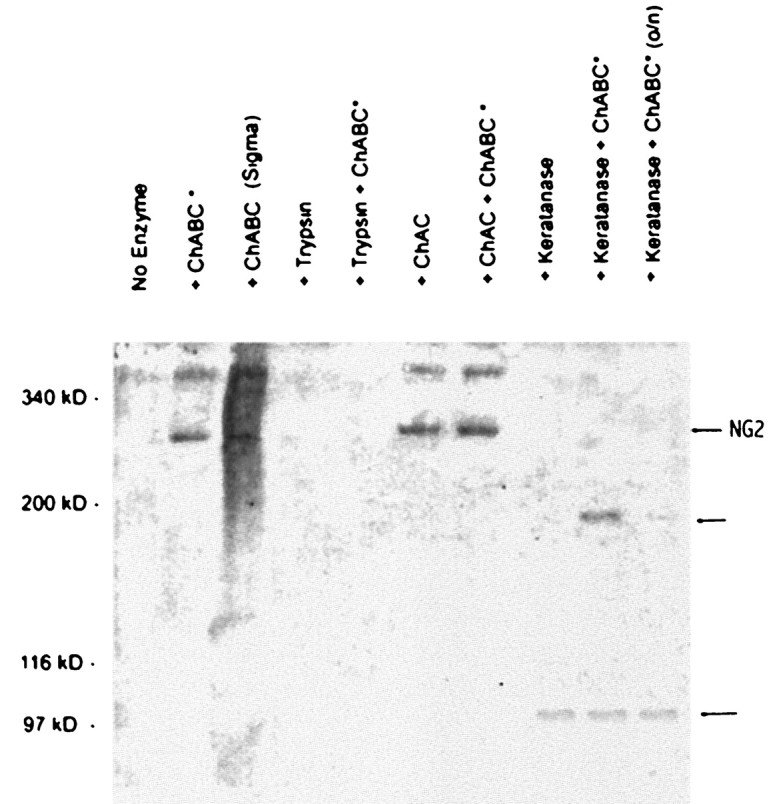
NG2 Western blot of Neu7 detergent lysate after digestion of samples with various enzymes. No NG2 band at ∼300 kDa is seen in the absence of digestion with chondroitinase ABC or AC, but after digestion with these enzymes, a sharp band is seen. Chondroitinase ABC and AC produce the same effect, and there is no additive effect. In Neu7 detergent lysate, a larger unidentified band at higher molecular weight is also seen. On digestion with keratanase, the 300 kDa band disappears, and a new band is seen at 100 kDa. When chondroitinase ABC is added to the keratanase, a 200 kDa band is seen in addition to the 100 kDa band. Digestion overnight with keratanase and chondroitinase (right lane) leads to almost complete disappearance of the 200 kDa band.
The NG2-blocking antibody increases axon growth on Neu7 cells and matrix
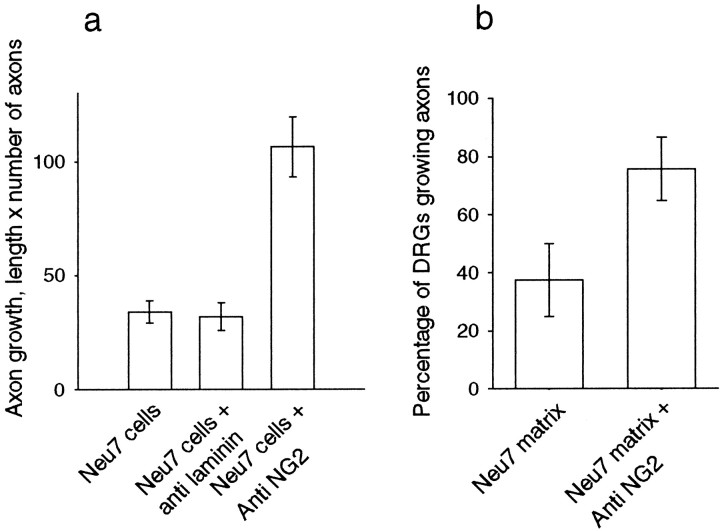
To ascertain whether NG2 is a functionally significant inhibitory molecule on Neu7 cells, we examined DRG axon growth on live Neu7 cells in the presence of an NG2 function-blocking antibody. We have shown previously (Dou and Levine, 1994) that axons will grow to some extent in the presence of NG2, but growth is increased by blocking NG2 with the antibody. This antibody recognizes both the released and transmembrane forms of NG2 (Fig. 3). As a control, we used a matrix-binding polyclonal antibody to laminin. Both antibodies were present at 0.4 mg/ml. Although the laminin antibody had no effect on the extent of axon growth, the presence of the anti NG2 antibody caused a large increase in axon growth (three separate assays, four wells with three DRGs per well for each condition in each assay; p< 0.001; t test) (Fig.7a). Growth was also assayed on Neu7 extracellular matrix produced by water lysis of cells. Only 38% of explants grew axons on this matrix, but in the presence of NG2 antibody, 75% grew axons (Fig. 7b).
Fig. 7.
a, Axon growth from DRGs plated on Neu7 cells in the absence of antibodies, in the presence of 0.4 mg/ml of polyclonal anti-laminin, or 0.4 mg/ml of polyclonal anti-NG2. The growth measurement is the number of axons growing from each DRG multiplied by the length in millimeters. NG2 antibody considerably increases axon growth, whereas the anti-laminin antibody is without effect. b, DRGs plated on Neu7 extracellular matrix. The proportion of DRGs producing three or more axons was counted.
Effects of NG2-blocking antibody and CS-56 on axon crossing of A7–Neu7 boundaries
We have shown previously (Powell et al., 1997) that the axons growing from late embryonic cortical neurons seldom cross boundaries from A7 cells to Neu7 cells. Approximately 5% of axons will cross from the permissive to the inhibitory cell line, the rest either turning away from the boundary or stopping at the boundary. We have also shown that digesting proteoglycans with chondroitinase and keratanase, or disrupting their synthesis with xylosides or chlorate can increase the number of axons crossing these boundaries ∼10-fold to ∼40%. A major component of the inhibitory activity that prevents axons crossing onto the inhibitory Neu7 cell line is therefore attributable to proteoglycans. In the present study, we have repeated this assay in the presence of the function-blocking NG2 antibody or the CS-56 antibody. As observed previously, in untreated cultures or in cultures treated with a control antibody, the proportion of axons crossing from A7 to Neu7 was ∼10%. However, in the presence of anti-NG2 the proportion went up to 48% [p < 0.001; t test (Fig.8a), and in the presence of CS-56, the proportion went up from 16 to 42% (Fig. 8b); three independent estimations for each condition, each consisting of three coverslips with 100 or more axons counted on each coverslip); the same as we saw in previous experiments when we treated cultures with chondroitinase and keratanase. This and the result in the previous section together demonstrate that NG2 is a major component of the inhibitory proteoglycans produced by the Neu7 cell line.
Fig. 8.
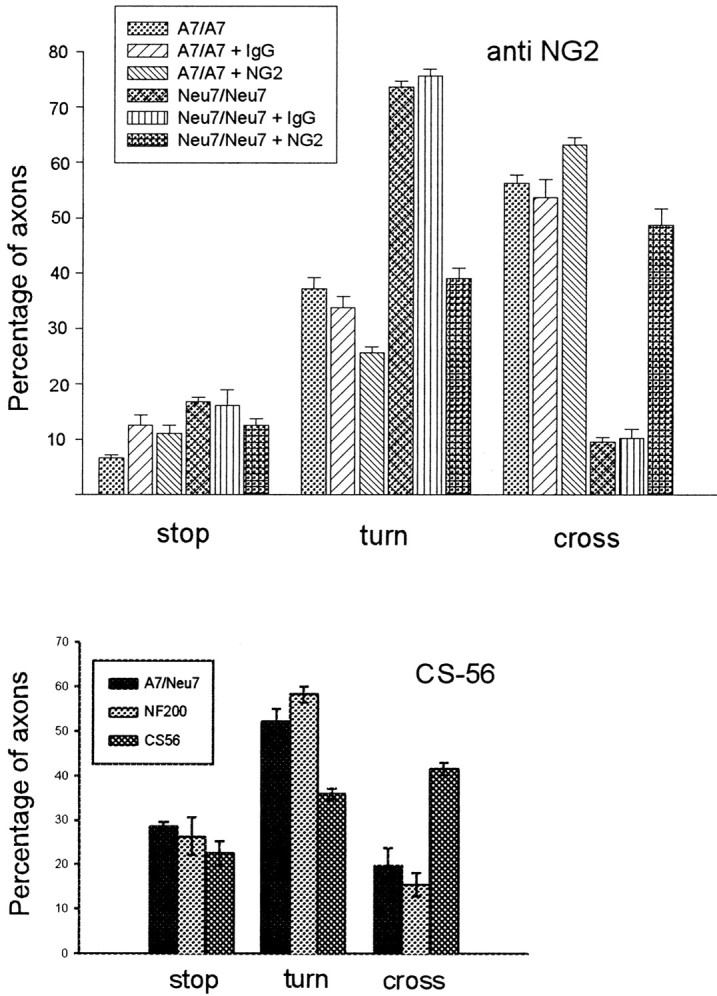
Anti-NG-2 significantly increases the ability of axons from cortical neurons to cross an A7–Neu7 cellular boundary. The behavior of 100 neurites per coverslip growing on A7 cells was assessed as they approached an Neu7 boundary, and classified as either stopped, turned, or crossed. In the presence of 0.2 mg/ml anti-NG2, the percentage of neurons that crossed onto Neu7 cells was increased from 10 to 48% (compare the two bars on the far right), whereas the number of neurites that turned was decreased from 75 to 39% (two bars on theright of the turn measurements). There was no change in the percentage of neurites that crossed or turned after the addition of a control IgG. The CS-56 monoclonal antibody, which binds to particular motifs in CS chains, was also tested for its ability to affect axonal boundary crossing. In the presence of CS-56, the proportion of axons able to cross from Neu7 to A7 approximately doubled.
We also investigated the effect of the CS-56 monoclonal antibody in this assay. This antibody binds to particular motifs in chondroitin sulfate chains (Sorrell et al., 1993). The presence of CS-56 also increased the ability of cortical axons to cross from A7 cells onto Neu7 (Fig. 8).
GAG lyases cleave the NG2 core protein
Smith-Thomas et al. (1994) concluded that the inhibitory nature of Neu7 was attributable in part to a dermatan sulfate and/or keratan sulfate proteoglycans. This conclusion was based on the results of bioassays after enzymatic digestion with various GAG lyases or disruption of proteoglycan synthesis with chlorate or xylosides. Cells or cell matrix pretreated with Sigma keratanase or ChABC (which cleaves chondroitin and dermatan sulfates) or grown in the presence of chlorate or xylosides (which affect GAG sulfation and attachment to core proteins, respectively) had increased permissiveness for axon growth, and conditioned medium treated in the same way or with trypsin had reduced ability to block the axon growth-promoting effects of laminin. However, because we now have evidence that NG2, a pure chondroitin sulfate proteoglycan, is primarily responsible for the proteoglycan-mediated inhibition associated with Neu7, we suspected that some of the GAG lyase enzymes might be cleaving the NG2 core protein. This is apparently so. Using Neu7 detergent lysates, we investigated the effects of the various enzymes used in the study bySmith-Thomas et al. (1994) on the intact proteoglycan and on the core protein after treatment with protease-free ChABC; the results are shown in Figure 6. In the absence of ChABC digestion, no NG2 band was seen, but after digestion, a band of ∼300 kDa was apparent, indicating that most of the NG2 in detergent lysates possesses GAG chains. As expected, trypsin cleaved the core into such small pieces that they are not resolved in a 5% gel. Treatment with chondroitinase AC led to the appearance of the core protein, and no further digestion occurred on the addition of ChABC. This confirms that the majority of the GAG on NG2 is chondroitin sulfate and not dermatan sulfate. After keratanase digestion, a single 100 kDa NG2-immunoreactive species was produced, and simultaneous removal of chondroitin sulfate with protease-free ChABC revealed that keratanase cleaved the intact 300 kDa core protein, producing two proteins of ∼200 and 100 kDa
Therefore, the enzyme preparations that removed the inhibition from Neu7 most effectively in our previous in vitro study are shown here to have either degraded the NG2 core or to have removed chondroitin sulfate chains.
Expression of versican
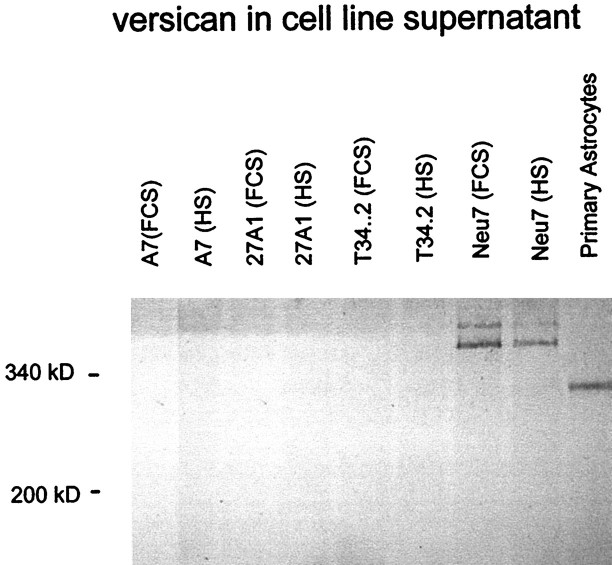
The other proteoglycan expressed at high level by Neu7 but not by A7 in our initial scan was versican. Using the 12C5 anti-versican monoclonal antibody (Asher et al., 1991), Western blotting showed there to be two forms of versican in the supernatant of Neu7 but not A7, 27A1, or T34.2 (Fig. 9). Versican was not detected in the detergent lysate of any of the cell lines. Although this antibody binds to the hyaluronic acid-binding region of versican and should therefore recognize all versican splice-variants, it has been shown not to cross-react with the related chondroitin sulfate proteoglycans aggrecan (Asher et al., 1995), neurocan, and brevican (R. A. Asher, unpublished observations). Interestingly, Neu7 cells produce different isoforms of versican from that seen in cultured rat cerebral cortical astrocytes (Fig. 9). Neu7 cells produce two species, both larger than the 340 kDa marker, that probably correspond to the V0 and V1 forms of the molecule, whereas the predominant form seen in cortical astrocytes is smaller and is probably V2. Immmunocytochemistry of Neu7 monolayers showed that versican is distributed in large blobs and patches rather than being evenly spread. This distribution is different from that seen with NG2 (Fig.10).
Fig. 9.
Production of versican by cell lines grown in FCS and horse serum (HS) and by primary astrocyte cultures purified by shaking off the top-dwelling cells. The Neu7 cell line produces two forms of versican, corresponding to the V0 and V1 variants, whereas the primary astrocyte cultures produce a smaller variant, corresponding to V2.
Fig. 10.
DRG axons growing over the surface of Neu7 cells, with the axons stained for neurofilament and the Neu7 cells stained for versican. The picture is a double exposure; axons and blobs are readily distinguishable. The versican is present in patches, with undetectable levels in between. The axons show no sign of avoiding the versican-rich areas.
Axon growth on Neu7 cells in relation to versican and CS-56
Because the distribution of versican on Neu7 monolayers is restricted to blobs with no versican in between (Fig. 10), it is possible to use this patterned surface to see whether growing axons avoid the versican-rich areas. DRGs were placed onto Neu7 monolayers, and 48–72 hr later, the cultures were double stained for versican and neurofilament. Staining for versican revealed an irregular and patchy distribution, but there was no evidence that the growing axons were avoiding these patches, nor was there any appearance of the axons having digested versican as they grew over the patches (Fig. 10). An almost identical picture was seen with the CS-56 epitope, whose distribution was patchy like that of versican, but again we saw no sign of axons avoiding these patches (data not shown).
DISCUSSION
Astrocytes are phenotypically diverse and vary in their ability to support neurite outgrowth (Grierson et al., 1990). This variability is reflected in our various cell lines (Fok-Seang et al., 1995) in which we have consistently observed that the Neu7 cell line is inhibitory to neuronal growth, whereas the A7 cell line is permissive; thus, differences between these cell lines provide an opportunity to determine which inhibitory molecules are functionally important in the context of the glial cell surface. In previous work, we have shown that much of the inhibitory activity of Neu7 cells can be ascribed to CS-PGs. Of the CS-PGs that were the subject of this study, three (NG2, versican, and the proteoglycan(s) recognized by the CS-56 antibody) were expressed at much higher levels in the inhibitory Neu7 cells than in the other cell lines.
The NG2 proteoglycan
NG2 consists of a 300 kDa core protein bearing GAG chains composed entirely of chondroitin sulfate and is found in three forms: the full-length 300 kDa transmembrane form, a 290 kDa form released from the cell surface by proteolytic cleavage, and a 275 kDa membrane-associated form, which is also produced by proteolytic cleavage but which remains associated with the cell surface (Nishiyama et al., 1995). Purified NG2 has been shown to be an inhibitor of axon growth from cerebellar granule neurons on both laminin and L1, whereas growth of sensory (DRG) axons is only inhibited on laminin. NG2 may therefore have more than one mode of action (Dou and Levine, 1994). A putative cell surface receptor has been identified on cerebellar granule neurons, although not on DRGs (Dou and Levine, 1997). NG2 has been shown to bind to collagens (types II, V, and VI), laminin, tenascin, and the PDGF receptor (Burg et al., 1996; Levine and Nishiyama, 1996; Nishiyama et al., 1996a,b). NG2 in the normal CNS is found on a population of cells thought to represent adult oligodendrocyte precursor cells (Reynolds and Hardy, 1997). After CNS damage, there is a massive recruitment of these cells to lesions within 48 hr. Numbers decline after 7 d but remain raised for several weeks (Levine, 1994). NG2 is also reported on astrocytes in the damaged spinal cord (Grill et al., 1998).
NG2 in cell lines
Neu7 cells produce far higher quantities of NG2 than A7, 27A1, or T34.2, producing both the 300 kDa intact transmembrane and the 290 kDa released forms of the molecule, both with and without CS GAG chains attached. To test whether a function-blocking NG2 antibody enhanced axon growth on Neu7 cells, we used three assays. In the first, DRG explants were grown on Neu7 cells, and the length and number of axons were measured 1 d later. In the second, DRG explants were cultured on Neu7 extracellular matrix. The third assay used a patterned substrate of permissive A7 and inhibitory Neu7 cells in which we have shown previously that only 5% of axons from cortical neurons cross from A7 to Neu7 but, after general disruption of all CS-PGs, this proportion goes up to 40% (Powell et al., 1997). Anti-NG2 increased growth in all assays; the addition of a blocking antibody led to a large increase in axon growth from DRG explants over the surface of Neu7 cells and matrix. In the boundary model, the proportion of cortical axons crossing from A7 cells to Neu7 was increased from ∼10 to ∼50% by the NG2-blocking antibody. These experiments demonstrate that NG2 functions in both the regulation of axon growth and axon guidance. By anion exchange fractionation, we produced a fraction that contained NG2 without GAG chains, which was not inhibitory to axon growth, and a fraction that contained NG2 with GAG chains, which was inhibitory. This and our previous results showing that the inhibitory activity of Neu7 cells is diminished by disruption of GAG chains suggests that, in these assays, NG2 requires its CS chains to be inhibitory. However, the fractions are not pure NG2 and contain versican and possibly other proteoglycans, as well as NG2, and the less inhibitory fraction contains laminin, so it is possible that inhibitory activity attributable to the protein core of NG2 alone would have been masked in the present assays. Our results therefore show that NG2 core protein and CS chains are both involved in the inhibitory activity of Neu7 cells but do not prove that they have to be attached to one another.
To estimate the amount of NG2 production as a proportion of total proteoglycan, we undertook metabolic labeling studies with35S-methionine and35S-sulfate, using immunopreciptitation with anti NG2 to determine the proportion of labeled material represented by NG2. This experiment confirmed the observation that only Neu7 makes the GAG-linked sulfated form of NG2, whereas Neu7 and to a lesser extent A7 make nonsulfate-labeled NG2. NG2 represents a minute component of the protein and a minor (<1%) variant of the total proteoglycan synthesis. In the latter case, however, the determination of the NG2 proportion is probably a slight underestimate, because some of the 35S-SO4 label could be linked to glycolipids, particular N-linked carbohydrates, or post-translational protein modifications. Because NG2 is a small fraction of total proteoglycan, it cannot be exerting its effect by the bulk carriage of CS chains. More probably, the effect of the CS chains is achieved by their being targeted to particular molecules by specific binding of the NG2 protein core to particular sites on matrix or axonal molecules.
Versican
Versican is a large secreted hyaluronate-binding proteoglycan of the aggrecan family found in many tissues, including the CNS. It carries large amounts of CS, with attachment sites for 12–15 GAG chains. Four isoforms, V0, V1, V2, and V3, have been identified and are generated from a single gene by alternative splicing (Zimmermann and Ruoslahti, 1989; Naso et al., 1994). Versican is inhibitory to neural crest migration in vitro and present in regions avoided by migrating neural crest cells in vivo, and its upregulation in the splotch mutant mouse is associated with defective neural crest cell migration (Landolt et al., 1995; Perris et al., 1996; Henderson et al., 1997).
Our results show that Neu7 cells, but not A7, T27A1, or T34.2, produce two forms of versican that are larger than the predominant form produced by primary astrocyte cultures. Staining Neu7 monolayers for versican revealed an irregular and patchy distribution, providing a patterned versican surface on which it was possible to look for avoidance behavior by growing axons. However, DRG axons showed no avoidance of versican-immunoreactive patches. It would appear therefore that, in this assay, either versican is either not inhibitory, a conclusion in agreement with that of Braunewell et al. (1995), or other molecules in the patches counteract its effects.
Other proteoglycans
The CS-56 antibody is reported to bind to CS chains on proteoglycans (Sorrell et al., 1993), which are strongly upregulated in the glial scar and the distribution of which correlates well with regions inhibitory to axon growth (Davies et al., 1997). Neu7 makes much more CS-56-immunoreactive material than the other cell lines. CS-56 antibody made Neu7 cells somewhat less inhibitory in our boundary crossing assay. However, because it binds to so many CS-PGs, this effect is difficult to interpret. It is possible that Neu7 makes CS-PGs that we have yet to identify, which bind CS-56.
Origin of the Neu7 cell line
In the CNS, NG2 is found on oligodendrocyte/type 2 astrocyte progenitors (O2A cells), endothelial cells, and meningeal cells but has only been reported on astrocytes in the injured spinal cord, not in cortical lesions (Levine, 1994; Grill et al., 1998). Cells of both O2A and astrocyte lineages can produce both GFAP and NG2 in vitro, as does Neu7. O2A cells can give rise to type 2 astrocytes, which are positive for NG2, GFAP, and versican-like Neu7; however, they also express A2B5 antigen, which Neu7 does not. Conversely, a subpopulation of type 1 astrocytes in culture can produce NG2, as well as GFAP, when stimulated with two cytokines that are present in injury sites: FGF-2 and TGFb (P. S. Fidler and J. W. Fawcett, unpublished observations; Hinkle et al., 1998). Therefore, Neu7 may be either a type 2 (O2A-derived) astrocyte or a type 1 astrocyte that has upregulated NG2 expression.
Inhibition in CNS injuries
Three glial cell types increase in numbers in CNS injuries: astrocytes, oligodendrocyte precursors, and microglia. Microglia are probably helpful to axon growth (Rabchevsky and Streit, 1997). However, reactive astrocytes can be inhibitory to axon growth (Fawcett et al., 1989; Fawcett, 1997), and oligodendrocytes (and myelin debris) contain yet further inhibitory activity (Pesheva et al., 1993; Schwab et al., 1993; McKerracher et al., 1994; Mukhopadhay et al., 1994; Faissner 1997).
Our demonstration that cell-associated NG2 is a highly potent inhibitory molecule also focuses attention on the oligodendrocyte precursors that produce it as a third major source of inhibitory activity in the damaged CNS. NG2 is highly expressed on the fine cellular processes of these cells in CNS injuries (Levine, 1994,Keirstead et al., 1998). This increase in immunoreactivity is rapid and declines after 7 d, so NG2 is positioned to block the earliest axon regeneration in the CNS. Neu7 cells provide an in vitromodel of this process, active against axons from diverse sources.
Three cell types are therefore involved in making the CNS inhibitory to axon regeneration. Myelin debris from oligodendrocytes and NG2 from oligodendrocyte precursors are present early, and later astrocytes produce a dense glial scar containing yet more inhibitory proteoglycans. To promote regeneration in vivo, it may be necessary to manipulate all three types of inhibitory glial cell or the molecules that they produce.
Footnotes
This work was supported by The Wellcome Trust (to P.S.F. and R.A.A.); the International Spinal Research Trust, Action Research, and Association de Recherche sur le Cancer (to A.D.); Fondation de la Recherche Medicale, Deutsche Forschungsgemeinschaft (Grant Fa 159/11–1), and Centre National de la Recherche Scientifique (to A.F.); and National Institutes of Health Grant NS25168 (to H.M.G.).
Drs. Fidler and Schuette contributed equally to this work.
Correspondence should be addressed to James Fawcett, Department of Physiology, Downing Street, University of Cambridge, Cambridge CB2 3EG, United Kingdom.
REFERENCES
- 1.Asher R, Perides G, Vanderhaeghen J-J, Bignami A. Extracellular matrix of central nervous system white matter: demonstration of an hyaluronate–protein complex. J Neurosci Res. 1991;28:410–421. doi: 10.1002/jnr.490280314. [DOI] [PubMed] [Google Scholar]
- 2.Asher RA, Scheibe RJ, Keiser HD, Bignami A. On the existence of a cartilage-like proteoglycan and link proteins in the central nervous system. Glia. 1995;13:294–308. doi: 10.1002/glia.440130406. [DOI] [PubMed] [Google Scholar]
- 3.Braunewell KH, Pesheva P, McCarthy JB, Furcht LT, Schmitz B, Schachner M. Functional involvement of sciatic nerve-derived versican and decorin-like molecules and other chondroitin sulfate proteoglycans in ecm-mediated cell-adhesion and neurite outgrowth. Eur J Neurosci. 1995;7:805–814. doi: 10.1111/j.1460-9568.1995.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 4.Burg MA, Tillet E, Timpl R, Stallcup WB. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J Biol Chem. 1996;271:26110–26116. doi: 10.1074/jbc.271.42.26110. [DOI] [PubMed] [Google Scholar]
- 5.Davies SJA, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–684. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 6.Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dou CL, Levine JM. Identification of a neuronal cell surface receptor for a growth inhibitory chondroitin sulfate proteoglycan (NG2). J Neurochem. 1997;68:1021–1030. doi: 10.1046/j.1471-4159.1997.68031021.x. [DOI] [PubMed] [Google Scholar]
- 8.Faissner A. The tenascin gene family in axon growth and guidance. Cell Tissue Res. 1997;290:331–341. doi: 10.1007/s004410050938. [DOI] [PubMed] [Google Scholar]
- 9.Faissner A, Clement A, Lochter A, Streit A, Mandl C, Schachner M. Isolation of a neural chondroitin sulfate proteoglycan with neurite outgrowth promoting properties. J Cell Biol. 1994;126:783–799. doi: 10.1083/jcb.126.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fawcett JW. Astrocytic and neuronal factors affecting axon regeneration in the damaged central nervous system. Cell Tissue Res. 1997;290:371–377. doi: 10.1007/s004410050943. [DOI] [PubMed] [Google Scholar]
- 11.Fawcett JW, Housden E, Smith-Thomas L, Meyer RL. The growth of axons in three dimensional astrocyte cultures. Dev Biol. 1989;135:449–458. doi: 10.1016/0012-1606(89)90193-0. [DOI] [PubMed] [Google Scholar]
- 12.Fok-Seang J, Smith-Thomas L, Muir E, Du J-S, Housden E, Johnson AR, Faissner A, Geller HM, Keynes RJ, Rogers JH, Fawcett JW. An analysis of astrocytic cell lines with different abilities to promote axon growth. Brain Res. 1995;689:207–223. doi: 10.1016/0006-8993(95)00575-b. [DOI] [PubMed] [Google Scholar]
- 13.Geller HM, Dubois-Dalcq M. Antigenic and functional characterization of a rat central nervous system-derived cell line immortalized by a retroviral vector. J Cell Biol. 1988;107:1977–1986. doi: 10.1083/jcb.107.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grierson JP, Petroski RE, Ling DSF, Geller HM. Astrocyte topography and tenascin/cytotactin expression: correlation with the ability to support neuritic outgrowth. Dev Brain Res. 1990;55:11–19. doi: 10.1016/0165-3806(90)90100-d. [DOI] [PubMed] [Google Scholar]
- 15.Grill RJ, Stallcup WB, Tuszynski MH. Temporal upregulation and spatial distribution of putative inhibitory and growth permissive substrate molecules in the injured adult rat spinal cord. Soc Neurosci Abstr. 1998;24:1054. [Google Scholar]
- 16.Henderson DJ, Ybot-Gonzalez P, Copp AJ. Over-expression of the chondroitin sulfate proteoglycan versican is associated with defective neural crest migration in the pax3 mutant mouse (splotch). Mech Dev. 1997;69:39–51. doi: 10.1016/s0925-4773(97)00151-2. [DOI] [PubMed] [Google Scholar]
- 17.Hinkle DA, Harney JP, Cai A, Hilt DC, Yarowsky PJ, Wise PM. Basic fibroblast growth factor-2 and interleukin-1β regulate S100 expression in cultured astrocytes. Neuroscience. 1998;82:33–41. doi: 10.1016/s0306-4522(97)00223-6. [DOI] [PubMed] [Google Scholar]
- 18.Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- 19.Landolt RM, Vaughan L, Winterhalter KH, Zimmermann DR. Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development. 1995;121:2303–2312. doi: 10.1242/dev.121.8.2303. [DOI] [PubMed] [Google Scholar]
- 20.Levine JM. Increased expression of the ng2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine JM, Nishiyama A. The NG2 chondroitin sulfate proteoglycan: a multifunctional proteoglycan associated with immature cells. Perspect Dev Neurobiol. 1996;3:245–259. [PubMed] [Google Scholar]
- 22.McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 24.Morris EJ, Geller HM. Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell-cycle independent toxicity. J Cell Biol. 1996;134:757–770. doi: 10.1083/jcb.134.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muir D. Metalloproteinase-dependent neurite outgrowth within a synthetic extracellular-matrix is induced by nerve growth-factor. Exp Cell Res. 1994;210:243–252. doi: 10.1006/excr.1994.1036. [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 27.Naso MF, Zimmermann DR, Iozzo RV. Characterization of the complete genomic structure of the human versican gene and functional analysis of its promoter. J Biol Chem. 1994;269:32999–33008. [PubMed] [Google Scholar]
- 28.Nishiyama A, Lin XH, Stallcup WB. Generation of truncated forms of the ng2 proteoglycan by cell- surface proteolysis. Mol Biol Cell. 1995;6:1819–1832. doi: 10.1091/mbc.6.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF α-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996a;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Interaction between NG2 proteoglycan and PDGF α-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996b;43:315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 31.Perris R, Perissinotto D, Pettway Z, Bronner FM, Morgelin M, Kimata K. Inhibitory effects of PG-H/aggrecan and PG-M/versican on avian neural crest cell migration. FASEB J. 1996;10:293–301. doi: 10.1096/fasebj.10.2.8641562. [DOI] [PubMed] [Google Scholar]
- 32.Pesheva P, Gennarini G, Goridis C, Schachner M. The F3/11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1–160/180. Neuron. 1993;10:69–82. doi: 10.1016/0896-6273(93)90243-k. [DOI] [PubMed] [Google Scholar]
- 33.Petroski RE, Geller HM. Selective labeling of embryonic neurons cultured on astrocyte monolayers with 5(6)-carboxyfluorescein diacetate (CFDA). J Neurosci Methods. 1994;52:23–32. doi: 10.1016/0165-0270(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 34.Pindzola RR, Doller C, Silver J. Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions. Dev Biol. 1993;156:34–48. doi: 10.1006/dbio.1993.1057. [DOI] [PubMed] [Google Scholar]
- 35.Powell EM, Fawcett JW, Geller HM. Proteoglycans provide neurite guidance at an astrocyte boundary. Mol Cell Neurosci. 1997;10:27–42. doi: 10.1006/mcne.1997.0629. [DOI] [PubMed] [Google Scholar]
- 36.Rabchevsky AG, Streit WJ. Grafting of cultured microglial cells into the lesioned spinal cord of adult rats enhances neurite outgrowth. J Neurosci Res. 1997;47:34–48. [PubMed] [Google Scholar]
- 37.Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 38.Schwab ME, Kapfhammer JP, Bandtlow CE. Inhibitors of neurite growth. Annu Rev Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- 39.Smith-Thomas L, Fok-Seang J, Stevens J, Du J-S, Muir E, Faissner A, Geller HM, Rogers JH, Fawcett JW. An inhibitor of neurite outgrowth produced by astrocytes. J Cell Sci. 1994;107:1687–1695. doi: 10.1242/jcs.107.6.1687. [DOI] [PubMed] [Google Scholar]
- 40.Smith-Thomas L, Stevens J, Fok-Seang J, Muir E, Faissner A, Rogers JH, Fawcett JW. Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J Cell Sci. 1995;108:1307–1315. doi: 10.1242/jcs.108.3.1307. [DOI] [PubMed] [Google Scholar]
- 41.Snow DM, Letourneau PC. Neurite outgrowth on a step gradient of chondroitin sulfate proteoglycan (CS-PG). J Neurobiol. 1992;23:322–336. doi: 10.1002/neu.480230311. [DOI] [PubMed] [Google Scholar]
- 42.Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 43.Sorrell JM, Carrino DA, Caplan AI. Structural domains in chondroitin sulfate identified by anti-chondroitin sulfate monoclonal antibodies. Immunosequencing of chondroitin sulfates. Matrix. 1993;13:351–361. doi: 10.1016/s0934-8832(11)80040-5. [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8:2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]