Abstract
The neurochemical evidence of methamphetamine (MA)-induced toxicity to dopaminergic nerve terminals is well documented; however, the functional consequences are not clearly defined. The present study was designed to investigate whether MA-induced dopamine depletions affect locomotor activity, stereotypic behavior, and/or extracellular dopamine concentrations in the neostriatum. Male rats were treated with a neurotoxic regimen of MA (10 mg/kg, i.p., every 2 hr for four injections) or vehicle and tested for functional effects 1 week later. Animals that had received the neurotoxic regimen of MA showed a reduction in both caudate nucleus and nucleus accumbens dopamine contents of 56 and 30%, respectively. Furthermore, MA-treated rats exhibited a significant attenuation in spontaneous activity, as well as a significant diminution in MA (low dose)-stimulated locomotor activity as compared to vehicle-treated rats. However, there were no differences in the MA (low dose)-induced increases in extracellular dopamine concentrations in the caudate nucleus or the nucleus accumbens core of either group. Interestingly, the acute administration of higher doses of MA elicited a significantly augmented stereotypic response and a significantly attenuated increase in the extracellular concentration of dopamine in the caudate nucleus of rats treated with a neurotoxic regimen of MA as compared to vehicle-treated animals. These data indicate that MA-induced neurotoxicity results in abnormal dopamine-mediated behaviors, as well as a brain region-specific impairment in stimulated dopamine release.
Keywords: methamphetamine, neurotoxicity, dopamine, behavior, in vivo microdialysis, stereotypy, locomotor activity, sensitization, rat
Methamphetamine (MA) is a psychomotor stimulant that increases locomotor activity when administered at low doses and elicits stereotypic behavior when administered at higher doses (Kelly et al., 1975; Segal and Kuczenski, 1994). It is generally believed that dopaminergic transmission in the nucleus accumbens and the caudate nucleus mediates MA-induced hyperlocomotion and stereotypy, respectively (Creese and Iversen, 1974;Kelly et al., 1975; Kelly and Iversen, 1976; Lucot et al., 1980). Consistent with this idea, it is well documented that MA increases the extracellular concentration of dopamine in these brain regions, in part, by reversing the dopamine transporter and facilitating cytoplasmic dopamine release, as well as by releasing vesicular stores of dopamine (Liang and Rutledge, 1982; Schmidt and Gibb, 1985; O’Dell et al., 1991; Seiden et al., 1993; Cubells et al., 1994).
In addition to the acute neurochemical and behavioral effects of MA, repeated, high-dose administration of this stimulant produces long-term neurotoxicity to dopaminergic and serotonergic nerve terminals within the neostriata, as well as to serotonergic terminals in multiple forebrain regions, of rats, mice, monkeys, and guinea pigs (Seiden et al., 1975; Wagner et al., 1979; Morgan and Gibb, 1980; Ricaurte et al., 1980; O’Callaghan, 1991; O’Dell et al., 1991). The evidence for axon terminal damage includes long-term decreases in dopamine and serotonin contents, depletion of dopamine uptake sites, and decreases in both tyrosine and tryptophan hydroxylase activity (Hotchkiss and Gibb, 1980;Ricaurte et al., 1980; Wagner et al., 1980; Seiden et al., 1988). Furthermore, there is histochemical evidence of nerve terminal damage (i.e., reactive gliosis) (Pu and Vorhees, 1993; Broening et al., 1997).
Although the neurochemical consequences of MA-induced toxicity are well documented, less is known about whether functional effects accompany the long-term depletion of dopamine. Lucot et al. (1980) have reported that the administration of large doses (i.e., 100 mg/kg) of MA over several days results in an attenuation of subsequent MA-stimulated locomotor activity in rats (stereotypy was not assessed). Consistent with these results, Cass et al. (1997,1998) have reported deficits in evoked dopamine release in the caudate nucleus of MA-treated rats. However, reduced dopamine release is not a consistent finding (Robinson et al., 1990). More recently, Walsh and Wagner (1992) have reported impairments in active-avoidance, and Kita et al. (1998) have shown nocturnal hyperactivity in MA-treated rats.
It has been suggested that dopaminergic systems have substantial reserve capacity and that severe nigrostriatal dopamine reductions are required to reveal Parkinsonian-like symptoms (Stricker and Zigmond, 1976). However, the aforementioned studies (i.e., Lucot et al., 1980;Walsh and Wagner, 1992; Kita et al., 1998) suggest that functional deficits occur at more moderate levels of dopamine depletion. Therefore, the present study was designed to determine whether a neurotoxic dosing regimen of MA, followed by a 1 week recovery interval, results in changes in spontaneous locomotor activity, as well as in stimulated hyperlocomotion and stereotypic behavior. Furthermore, extracellular dopamine concentrations in the nucleus accumbens and the caudate nucleus were measured to determine whether MA-induced neurotoxicity results in neurochemical changes that parallel behavioral changes.
MATERIALS AND METHODS
Animals
Male Sprague Dawley CD rats (225–250 gm) were obtained from Charles Rivers Laboratories (Portage, MI) in groups of 64 and were housed two or three per cage with food and water available ad libitum. Animals were maintained on a 12 hr light/dark cycle for 1 week before experimental treatment began. Rats were housed in a vivarium fully accredited by the Association for the Assessment and Accreditation for Laboratory Animal Care. The protocol for this research was approved by the Institutional Animal Care and Use Committee.
Treatment procedures
Rats were treated in their home cages and randomly assigned to receive injections of either (+)-methamphetamine hydrochloride (Sigma, St. Louis, MO) 10 mg/kg, intraperitoneally (expressed as the salt) every 2 hr for a total of four injections (i.e., neurotoxic regimen of MA) or 0.9% NaCl (vehicle). Body temperatures were monitored using a Thermistor thermometer (Cole-Parmer Instruments, Vernon Hills, IL) throughout the drug treatment regimen. Animals reaching a temperature of ≥41.5°C were wetted and placed in ventilated cages until temperatures dropped to ≤40.0°C. These procedures were based on the known role of MA-induced hyperthermia in the induction of neurotoxicity (Bowyer et al., 1992, 1994), and the cooling intervention on the known lethal effects of hyperthermic responses exceeding 42°C.
Behavioral measurements
Behavioral testing. One week after the initial treatment, rats were transported to the activity testing room in sets of 12 and tested in sets of four. Animals were placed in one of the four monitors for a 1 hr habituation period (activity was recorded every 10 min). After this period, each rat was removed and administered either vehicle or a dose of MA (0.5, 1.0, 2.0, 4.0, or 7.5 mg/kg, i.p.) and placed back in the monitors for another 2 hr (i.e., rats treated previously with a neurotoxic regimen of MA were administered a subsequent injection of MA or vehicle, similarly, rats treated previously with the vehicle were administered a subsequent injection of the vehicle or MA; n = 16/group). Each set of four animals tested had one animal from each treatment and each challenge condition represented (i.e., MA/MA, MA/vehicle, vehicle/MA, and vehicle/vehicle), such that groups were balanced by group for time of day. For the higher challenge dose experiments (i.e., 4.0 and 7.5 mg/kg MA) only the vehicle/MA and MA/MA groups were included, because data on 48 MA/vehicle and 48 vehicle/vehicle animals from the 0.5, 1.0, and 2.0 mg/kg MA experiments had already shown no differential response of MA treatment to a subsequent vehicle injection.
Locomotor activity. Activity chambers (40.6 × 40.6 cm) (model rxy2z; Accuscan, Columbus, OH) were equipped with 16-photodetector-LED pairs in each dimension (i.e., x,y, and two z planes) spaced 2.5 cm apart and located 2.2 cm above the floor. In addition, the floor contained four holes (3.2 cm in diameter) located close to each corner (i.e., 6 cm from each side wall). A set of photodetectors located 1.7 cm below the floor and another set located 15.9 cm above the floor measured hole pokes and rearings, respectively. Total distance was measured in centimeters traveled and is defined as sequential photobeam interruptions.
Stereotypic behavior. Video cameras were mounted above each chamber for scoring of stereotypic behavior. After review of the videotape, behavior was considered stereotypic if the animal remained in a stationary position and exhibited repetitive movements, such as sniffing, headweaving, licking, or biting. The duration of stereotypic behavior in a 30 sec period was timed at 10 min intervals for 2 hr after the administration of the subsequent injection of MA or vehicle. Results were reported based on the mean percent of time each treatment group (i.e., MA or vehicle) spent during each 10 min interval exhibiting stereotypic behavior, as described by Segal and Kuczenski (1987).
Biochemical measurements
Tissue analysis. Eight days after receiving the initial drug regimen (i.e., 1 d after behavioral assessment), rats were killed by decapitation, and the brains were rapidly removed. The nucleus accumbens and the caudate nucleus were dissected from 1.0 mm coronal sections, frozen rapidly on dry ice, and stored at −70°C until assayed. Tissue samples were homogenized in 0.2 N perchloric acid. After centrifugation (16,000 × g for 7 min.), the supernatant was injected onto a C18 reverse-phase column (Phenomenex, Torrance, CA) connected to a Coulochem II detector (ESA, Bedford, MA) or an LC-4B detector (BAS, West Lafayette, IN). The mobile phase used for the analysis of dopamine and serotonin consisted of 35 mm citric acid; 54 mmsodium acetate; 50 mg/l disodium ethylenediamine tetraacetate, 70 mg/l octanesulfonic acid sodium salt, 100 μl/l triethylamine, 6% acetonitrile, and 3% methanol, pH 4.2, set at a flow rate of 0.4 ml/min. Peak heights were quantified using a Hewlett-Packard integrator.
In vivo microdialysis. Three to five days after receiving a neurotoxic regimen of MA or vehicle, rats (separate groups of rats than were used for behavioral measurements) were anesthetized with an injection of a ketamine and xylazine (87/13 mg/kg, i.m.), and a guide cannula was implanted on the cortical surface above the nucleus accumbens core or the caudate nucleus for in vivomicrodialysis. These regions were chosen specifically because they demonstrate the greatest MA-induced depletion of dopamine (Morgan and Gibb, 1980; Ricaurte et al., 1980; Broening et al., 1997). On the morning of the experiment, a concentric style dialysis probe was inserted through the guide cannula into the nucleus accumbens core (anterior (A), 1.7 mm; lateral (L), −1.4 mm; ventral (V), −7.5 mm) or the caudate nucleus (A, 1.2 mm; L, 3.0 mm; V, −7.0 mm) according to the atlas of Paxinos and Watson (1986). The active portion of the membrane was 2.0 mm for the nucleus accumbens core and 4.5 mm for the caudate nucleus. Dulbecco’s PBS containing 1.2 mm CaCl2 and 10 mm glucose was perfused through the probe at a constant rate of 2.2 μl/min via an infusion pump. After an equilibration period of 1.5 hr, dialysis samples were collected every 30 min. At least three baseline samples were obtained before subsequent MA administration.
HPLC analysis of dopamine. The concentration of dopamine in the dialysate samples was determined via HPLC with electrochemical detection using the same procedure used for the analysis of tissue dopamine.
Statistics
For dialysis experiments, a two-way repeated measure ANOVA was used followed by post hoc analysis with Duncan’s test. For spontaneous locomotor activity, a two-treatment × six-interval (repeated measure) split-plot ANOVA was used to analyze the habituation phase, whereas a two-treatment × three-challenge × twelve-interval (repeated measure) split-plot ANOVA was used to analyze MA-stimulated activity. For stereotypy, a two-treatment × two-challenge × twelve-interval (repeated measure) split-plot ANOVA was used. Interactions were further analyzed by simple-effect analysis of variance if significant, after correction for nonsphericity using the Greenhouse-Geisser epsilon factors. Individual post hocgroup comparisons were made using Duncan’s multiple range test.
RESULTS
MA-induced dopamine and serotonin neurotoxicity
Rats that received the neurotoxic regimen of MA (i.e., 10 mg/kg every 2 hr for a total of four injections) showed a 56 and 30% reduction in dopamine concentrations in the caudate nucleus and the nucleus accumbens, respectively, 8 d after initial treatment (Table 1). In addition, the serotonin concentrations in the caudate nucleus and the nucleus accumbens of MA-treated rats were reduced by 50 and 63%, respectively, as compared to vehicle-treated rats.
Table 1.
MA-induced reduction of dopamine and serotonin contents in the nucleus accumbens and caudate nucleus
| Dopamine | Serotonin | |
|---|---|---|
| Nucleus accumbens | (ng/mg protein) | (ng/mg protein) |
| Vehicle-treated (n = 16) | 74.5 ± 4.0 | 9.1 ± 0.4 |
| MA-treated (n = 12) | 52.3 ± 6.3* | 3.4 ± 0.3* |
| Caudate nucleus | (ng/mg tissue) | (ng/mg tissue) |
| Vehicle-treated (n = 14) | 10.3 ± 0.4 | 0.44 ± 0.03 |
| MA-treated (n= 9) | 4.5 ± 0.9* | 0.22 ± 0.04* |
Rats were killed 8 d after treatment with MA (10 mg/kg every 2 hr for four injections) or with the vehicle. Tissue was homogenized in 0.2 N PCA and analyzed by HPLC-EC. Data are expressed as mean ± SEM.
*p < 0.05 compared with appropriate vehicle-treated control (Student’s t test).
A neurotoxic regimen of MA reduces spontaneous locomotor activity during the habituation period
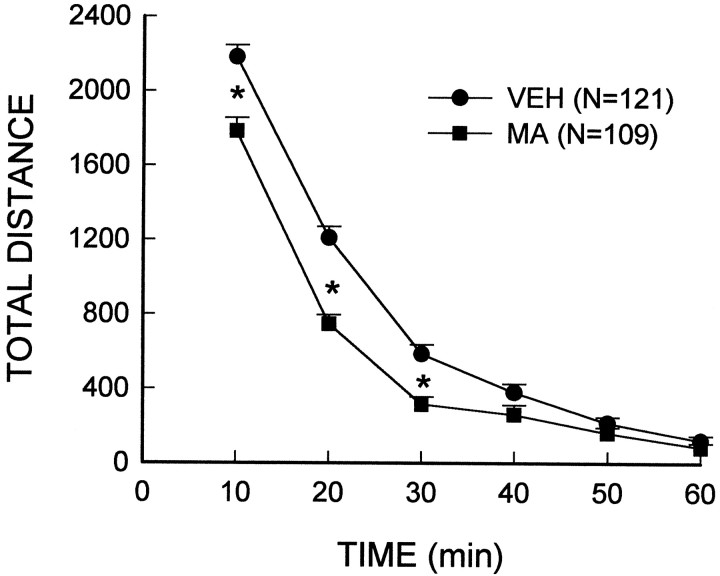
To determine whether the MA-induced loss of dopamine was accompanied by changes in dopamine-mediated behaviors, locomotor activity was monitored. Analysis of the total distance traveled during the habituation period included the rats that received subsequent low-dose (i.e., 0.5, 1.0, 2.0 mg/kg) injections of MA or vehicle after the habituation period. The main effect of the treatment (F(1,228) = 30.82; p< 0.00001) and the treatment × interval interaction (F(5,1140) = 9.44; p< 0.00001) were significant and indicated that the spontaneous activity of rats treated with a neurotoxic regimen of MA was significantly attenuated as compared to vehicle-treated controls (Fig.1). A posteriori group comparisons indicated that MA-treated animals exhibited lower activity during the initial exploratory phases (i.e., the first 30 min) of the habituation period than vehicle-treated rats; however, the locomotor activity of both groups reached comparable levels during the last 30 min of the habituation period. The fact that both MA- and vehicle-treated animals showed similar levels of activity at the end of the habituation period (i.e., before receiving a subsequent injection of MA) suggests that any subsequent MA-stimulated differences observed between the treatment groups cannot be attributed to pre-existing activity differences.
Fig. 1.
Reduced spontaneous activity in rats treated with a neurotoxic regimen of MA. MA (10 mg/kg, i.p.) or the vehicle (VEH) was administered every 2 hr for a total of four injections. After 7 d, rats were placed in activity chambers and monitored for a 1 hr habituation period. *Indicates values that differ significantly from those of the vehicle-treated controls (p < 0.05).
A neurotoxic regimen of MA results in the attenuation of subsequent MA (low dose)-induced locomotor activity
To determine whether receiving a neurotoxic regimen of MA produced any changes in stimulated locomotor activity, animals were administered a subsequent low dose of MA (i.e., 0.5, 1.0, or 2.0 mg/kg). The main effects of treatment (i.e., 10 mg/kg × 4 of MA or vehicle) (F(1,164) = 4.26; p < 0.05), challenge (i.e., MA or vehicle) (F(2,164) = 178.34; p< 0.00001), dose (i.e., 0.5, 1.0, or 2.0 mg/kg MA) (F(2,164) = 17.89; p< 0.00001), and interval (i.e., 10–120 min) (F(11,1804) = 49.24; p< 0.00001) were significant. In addition, the challenge × dose (F(2,164) = 18.37; p< 0.00001), interval × challenge (F(11,1804) = 44.45; p< 0.00001), interval × dose (F(22,1804) = 2.67; p< 0.05), and interval × challenge × dose (F(22,1804) = 3.32; p< 0.01) interactions also were significant. No differences were identified between MA- or vehicle-treated groups given a subsequent injection of the vehicle.
Further analyses of the individual low doses of MA indicated that the treatment and the treatment × interval interactions were significant for the 1.0 mg/kg (both p < 0.00001) and 2.0 mg/kg doses of MA (p < 0.0001 andp < 0.05, respectively) (Fig.2A,B). Neither factor was significant for the 0.5 mg/kg MA dose. Group comparisons demonstrated that the animals treated previously with a neurotoxic regimen of MA and administered a subsequent low dose injection of MA showed an increase in locomotor activity, however, the increase was significantly attenuated as compared to vehicle-treated animals administered the same low dose of MA.
Fig. 2.
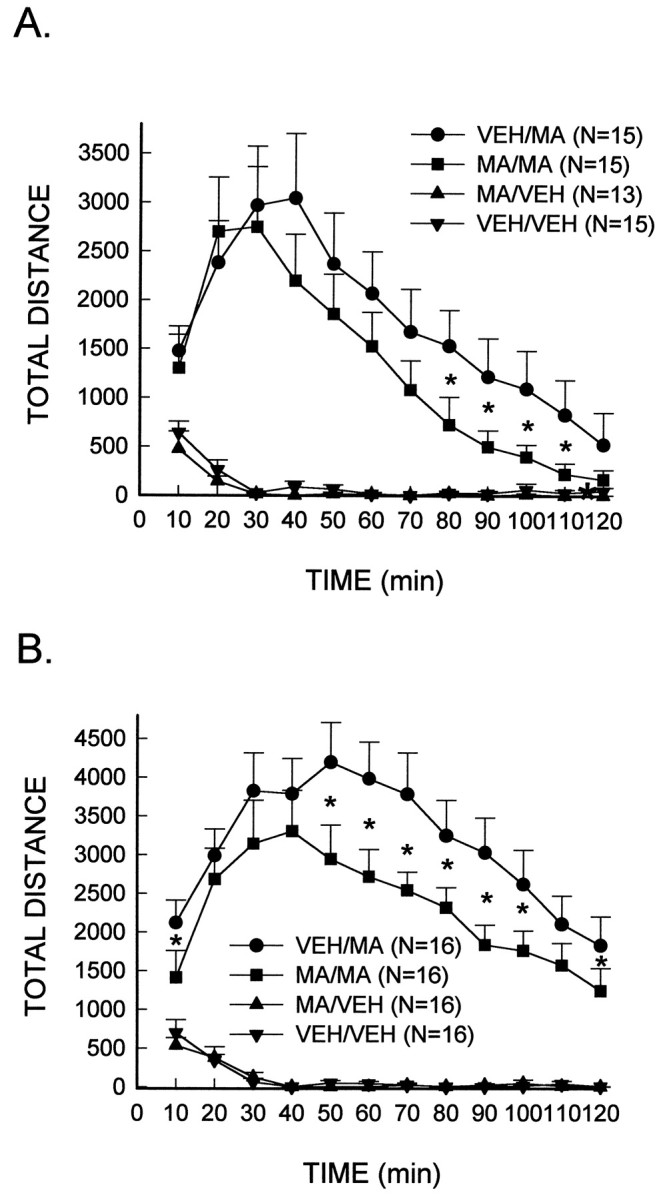
Reduced stimulated locomotor activity in MA-treated rats in response to a subsequent low-dose injection of MA. MA (10 mg/kg, i.p.) or the vehicle (VEH) was administered every 2 hr for a total of four injections. After 7 d, rats were placed in activity chambers and monitored for a 1 hr habituation period, after which they received an injection of MA, 1.0 mg/kg (A) or 2.0 mg/kg (B), or the vehicle. *Significantly different from VEH/MA-treated rats (p < 0.05).
In addition to locomotor activity, horizontal activity, rearing, and hole-poking measures also were analyzed. MA-treated animals administered a subsequent low-dose injection of MA exhibited a suppressed response for all three behaviors (i.e., horizontal activity, rearing, and hole-poking) as compared to control animals (data not shown).
A neurotoxic regimen of MA results in the augmentation of subsequent MA (high dose)-induced stereotypic behavior
To determine whether the MA-induced depletion of dopamine produced changes in stereotypic behavior, animals were administered a subsequent dose of MA (i.e., 4.0 or 7.5 mg/kg) that was known to elicit stereotypy as the dominant behavior (i.e., locomotor activity is suppressed). In contrast to the previous results indicating locomotor activity induced by lower doses of MA was attenuated in rats treated with a neurotoxic regimen of MA, stereotyped behavior elicited by higher doses of MA was significantly enhanced in these rats. At the 4.0 mg/kg dose of MA, treatment (F(1,28) = 6.71;p < 0.05) and treatment × interval (F(11,308) = 4.49; p< 0.001) effects were significant (Fig.3A). The same factors were significant at the 7.5 mg/kg dose, i.e., treatment (F(1,28) = 15.11; p < 0.001) and treatment × interval (F(11,308) = 3.27; p< 0.01) (Fig. 3B). Group comparisons performed at each interval to further analyze the interactions showed that at both the 4.0 and 7.5 mg/kg MA doses, the rats treated previously with a neurotoxic regimen of MA exhibited significantly augmented stereotypy at multiple early and middle test intervals as compared to their respective vehicle-treated controls.
Fig. 3.
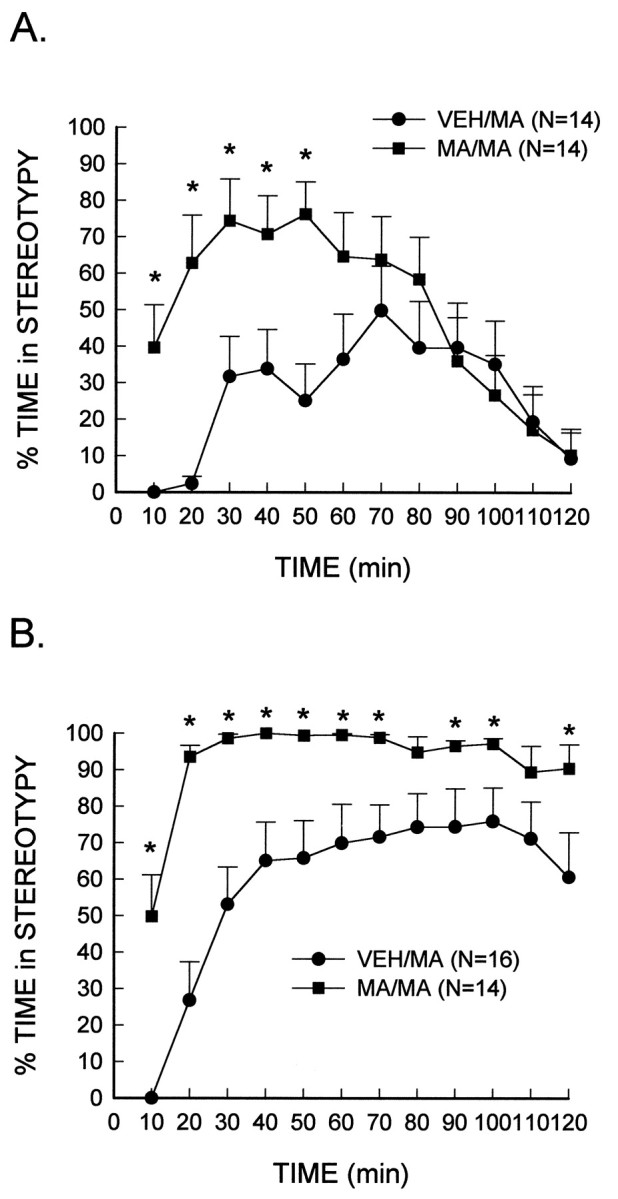
Augmented stereotypic behavior in MA-treated rats in response to a subsequent high-dose injection of MA. MA (10 mg/kg, i.p.) or the vehicle (VEH) was administered every 2 hr for a total of four injections. After 7 d, rats were placed in activity chambers and monitored for a 1 hr habituation period, after which they received an injection of MA, 4.0 mg/kg (A) or 7.5 mg/kg (B), or the vehicle. *Significantly different from VEH/MA-treated rats (p < 0.05).
A neurotoxic regimen of MA produces alterations in the subsequent MA-induced increase of extracellular dopamine that are dose-dependent and brain region-specific
In vivo microdialysis was used to determine whether the MA-induced depletion of dopamine content in both the caudate nucleus and the nucleus accumbens would result in deficits in the extracellular concentrations of dopamine. There was no significant difference in the basal extracellular concentration of dopamine in rats treated previously with the neurotoxic regimen of MA or vehicle in either the caudate nucleus (MA, 6.0 ± 0.8 pg/20 μl; vehicle, 6.8 ± 0.4 pg/20 μl) or the nucleus accumbens core (MA, 1.9 ± 0.3 pg/20 μl; vehicle, 1.3 ± 0.2 pg/20 μl). Extracellular concentrations of dopamine increased ∼400% in both the caudate nucleus and the nucleus accumbens core after the acute injection of a low dose of MA (1.0 mg/kg) (Fig.4A,B). However, there was no significant difference in the magnitude of the increase in the extracellular concentration of dopamine in either brain region of MA- or vehicle-treated rats.
Fig. 4.
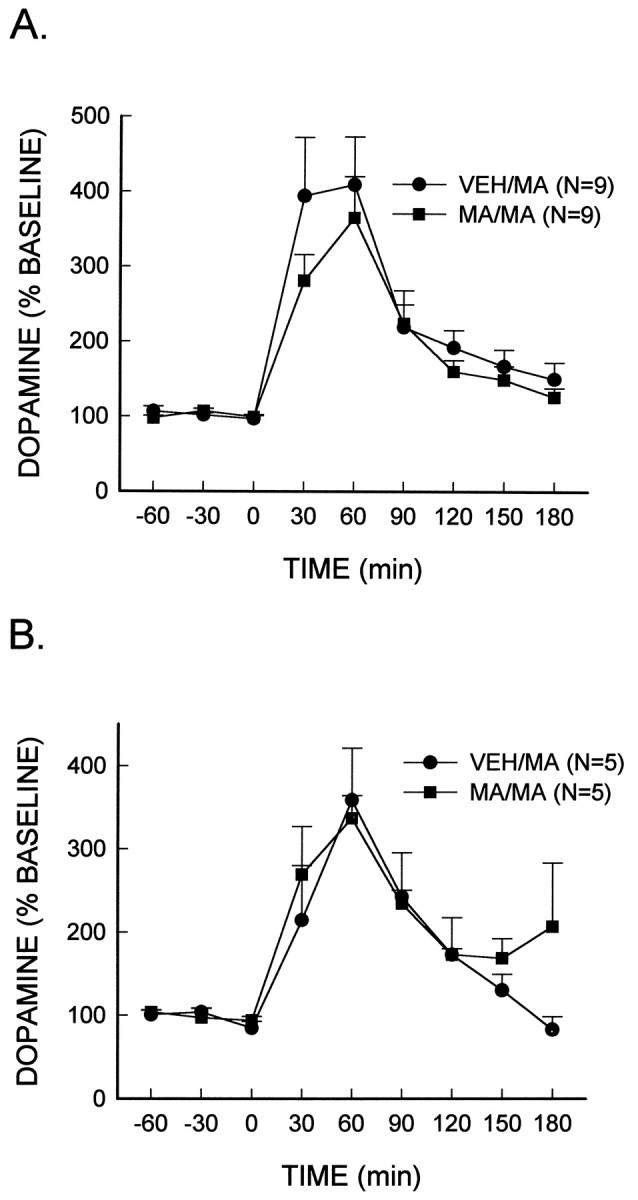
MA (low dose)-stimulated dopamine release. MA (10 mg/kg, i.p.) or the vehicle (VEH) was administered every 2 hr for a total of four injections. Seven days later, rats were administered a subsequent injection of MA (1.0 mg/kg, i.p.) at time 0, and extracellular dopamine concentrations were measured in either the caudate nucleus (A) or the nucleus accumbens core (B).
In addition, the extracellular concentration of dopamine in both brain regions was determined after the administration of a higher dose of MA. In rats that had previously received vehicle injections, the administration of MA (7.5 mg/kg) produced an increase in extracellular dopamine to ∼2200% of baseline values in both the caudate nucleus and the nucleus accumbens core (Fig.5A,B). In rats previously treated with a neurotoxic regimen of MA, the magnitude of the increase of extracellular dopamine in the caudate nucleus elicited by this dose of MA was significantly diminished (∼45%) (treatment × interval, F(8, 128) = 4.33; p < 0.0001). However, there was no difference in the MA-induced increase of extracellular dopamine in the nucleus accumbens core of MA or vehicle-treated rats.
Fig. 5.
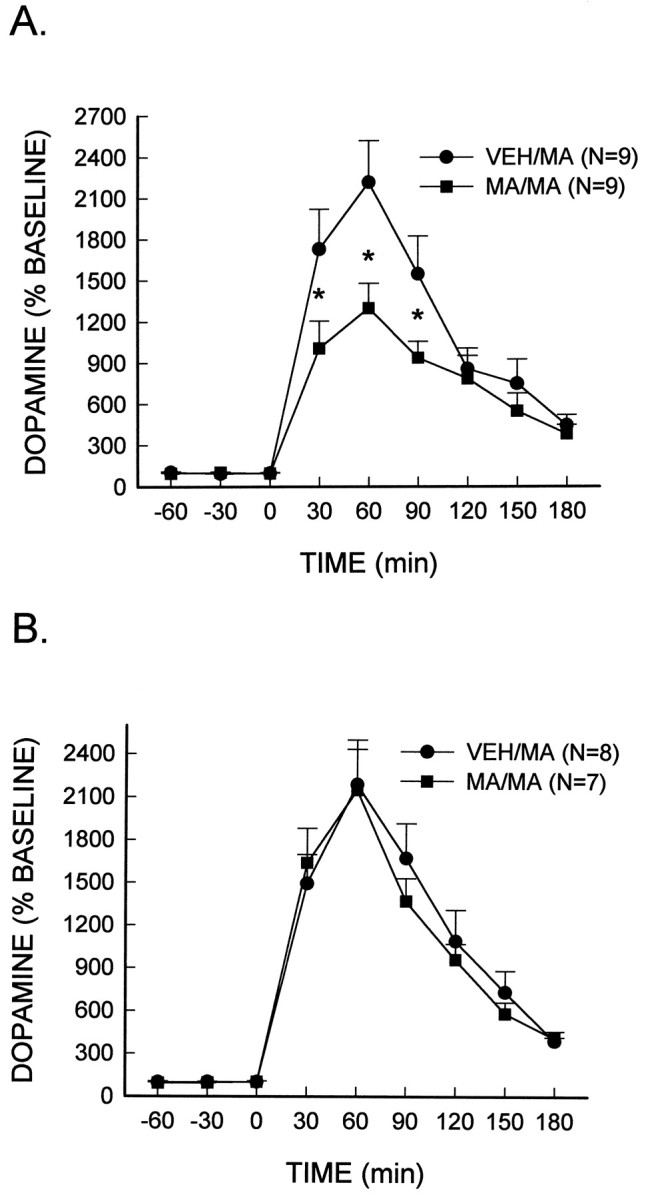
MA (high dose)-stimulated dopamine release. MA (10 mg/kg, i.p.) or the vehicle (VEH) was administered every 2 hr for a total of four injections. Seven days later, rats were administered a subsequent injection of MA (7.5 mg/kg, i.p.) at time 0, and extracellular dopamine concentrations were measured in either the caudate nucleus (A) or the nucleus accumbens core (B). *Significantly different from VEH/MA rats (p < 0.05).
DISCUSSION
In the present study, the repeated high dose administration of MA to rats resulted in alterations in both spontaneous and stimulated behavior, as well as in evoked dopamine release. The MA-induced depletion of dopamine content may be responsible, in part, for these observations. Joyce et al. (1983) have reported that bilateral injections of 6-hydroxydopamine into the nucleus accumbens result in a marked decrease in spontaneous activity. Although Joyce et al. (1983) obtained almost a 95% depletion of dopamine in the nucleus accumbens with the 6-hydroxydopamine-induced lesions, our results with a 30% decrease in dopamine content in this same brain region of MA-treated rats suggest that a nearly complete loss does not have to be obtained in order to affect the exploratory phase of spontaneous activity.
Alternatively, the suppressed spontaneous activity of MA-treated animals observed in the present study could be caused by the dosing regimen of MA (i.e., 10 mg/kg of MA administered every 2 hr for a total of four injections within a single day) that was used rather than the depletion of dopamine. Robinson and Camp (1987) have reported a decrease in basal locomotor activity in rats that have received repeated, daily doses of d-amphetamine. Whereas the dosing paradigm of Robinson and Camp (1987) was not neurotoxic to dopamine neurons, the deficit in basal activity that they reported for d-amphetamine-treated rats was similar to that reported in the present study for rats that received a neurotoxic regimen of MA. Thus, it is not clear whether the deficit in spontaneous activity of MA-treated rats observed presently is the result of dopamine depletion or is a compensatory response to repeated dopaminergic stimulation.
The administration of a low dose of MA (1.0 or 2.0 mg/kg, i.p.) resulted in significant deficits in stimulated locomotor activity in MA-treated rats as compared to vehicle-treated controls. The attenuated locomotor response seen in MA-treated rats was not caused by the emergence of stereotypic behavior as determined by videotape scoring. Our data are consistent with results obtained by Lucot et al. (1980)for the 1.0 mg/kg dose of MA, although these investigators reported no difference in the locomotor activity of MA- and vehicle-treated rats in response to a 2.0 mg/kg dose of MA. However, Lucot et al. (1980) did not monitor stereotypic behavior even though locomotion was highest at the 1.0 mg/kg dose, reduced at 2.0 mg/kg, and absent at 4.0 mg/kg. Thus, it is possible in the Lucot et al. (1980) study that the animals became less hyperactive and more stereotypic at the 2.0 and 4.0 mg/kg doses of MA.
In the present study, it was hypothesized that the observed deficits in stimulated locomotor activity of rats treated with a neurotoxic regimen of MA may be caused by the loss of dopamine. However, no significant differences in the extracellular concentration of dopamine were observed between MA- and vehicle-treated rats in either the caudate nucleus or the nucleus accumbens core before or after a subsequent injection of MA (1.0 mg/kg). Thus, there appeared to be no correlation between MA-induced dopamine release and the observed behavioral effects. Such a lack of correlation has been reported several times in animals repeatedly exposed to d-amphetamine and later tested for functional changes (Callaway et al., 1989; Kuczenski and Segal, 1989; Kuczenski et al., 1991; Segal and Kuczenski, 1992). One explanation for the lack of a deficit observed in the present study may be attributable to presynaptic compensatory changes that occur in the remaining dopamine neurons. For example, 6-hydroxydopamine-induced lesions in the striatum result in an increase in the amount of dopamine efflux per remaining nerve terminal (Snyder et al., 1986; Stachowiak et al., 1987; Zigmond et al., 1989). Therefore, residual dopamine nerve terminals may be able to compensate for the MA-induced loss of dopamine. Alternatively, the MA-induced decrease of high-affinity dopamine reuptake sites may allow dopamine to remain in the synapse longer and to diffuse to more distant sites, thereby maintaining neurochemical function (Wagner et al., 1980; Doucet et al., 1986; Kelly and Wightman, 1987).
Although the amount of extracellular dopamine measured may remain unchanged in MA- and vehicle-treated animals, the observed deficit in locomotor activity may still be evident. For example, a decrease in dopamine receptor binding has been demonstrated in the caudate putamen after repeated high-dose injections of MA without any alteration in receptor affinity (Schmidt et al., 1985; McCabe et al., 1987), although this is not a consistent finding (Robinson and Becker, 1986). Based on these data, a decrease in binding sites could result in deficits in dopamine-mediated functions, e.g., locomotor activity.
Although MA-treated rats demonstrated deficits in locomotor activity when injected subsequently with a low dose of MA (i.e., 1.0 or 2.0 mg/kg), these rats exhibited an augmentation in stereotypic behavior when given higher doses of MA (4.0 or 7.5 mg/kg). This illustrates the utility of pharmacological challenges in unmasking underlying functional changes. Moreover, the enhanced behavioral response (i.e., quicker onset and greater inten- sity of stereotypy) exhibited by rats treated with a neurotoxic regimen of MA is similar to results observed in animals that have become sensitized tod-amphetamine (Segal and Kuczenski, 1994) and is noteworthy in light of the concomitant attenuation in the MA-induced increase of the extracellular dopamine concentration. Although an increase in stimulated dopamine release has been reported in amphetamine-sensitized rats, there are several reports indicating that animals exhibiting sensitization have diminished dopamine release after stimulation (Kuczenski and Segal, 1988, 1989; Segal and Kuczenski, 1992). Therefore, these results suggest that the neurochemical and behavioral responses are not necessarily correlated.
In addition, whereas MA-treated rats demonstrated a significant reduction in the MA (7.5 mg/kg)-induced increase of the extracellular concentration of dopamine in the caudate nucleus as compared to vehicle-treated rats, an attenuated response was not observed in the nucleus accumbens core. This result may be attributed, in part, to a greater loss of dopamine in the caudate nucleus that results in the activation of different compensatory mechanisms (e.g., upregulation of postsynaptic receptors), which does not occur in the nucleus accumbens. Moreover, the differing circuitry of the two brain regions also may contribute to the observed consequential differences of MA-induced neurotoxicity.
The seemingly paradoxical findings observed in MA-treated rats in the present study [i.e., augmented stereotypy with concomitant reduction in the MA (7.5 mg/kg)-stimulated increase in the extracellular concentration of dopamine in the caudate] suggests that presynaptic mechanisms do not account for the enhanced stereotypy. Alternatively, the augmented response may be caused by a postsynaptic upregulation of dopamine receptors in the caudate nucleus. Although, it has been suggested that for an upregulation of receptors to occur within the dopaminergic system, damage to caudate dopamine neurons has to be nearly complete, i.e., ≥90% (Mishra et al., 1974; Stricker and Zigmond, 1976; Creese et al., 1977; Graham et al., 1990; Schwarting and Huston, 1997). However, increases in dopamine receptor sensitivity have been shown to occur in response to the repeated exposure tod-amphetamine (Robinson and Becker, 1986).
In the present study, it is difficult to determine whether the augmented behavioral response demonstrated by MA-treated rats was caused by the repeated exposure to MA (i.e., sensitization) or by the depletion of dopamine from nerve terminals. Certainly the temporal pattern of stimulant administration is an important factor in the development of behavioral augmentation, although a great deal of variation occurs in the dosing regimens with little difference in the production of sensitization (Segal and Geyer, 1985; Paulson et al., 1991). For example, one or two daily injections ofd-amphetamine for 2 weeks or a single injection ofd-amphetamine have both been shown to elicit a sensitized behavioral response (Browne and Segal, 1977; Robinson and Becker, 1986).
In summary, the treatment of rats with a neurotoxic regimen of MA results in a depletion of dopamine and serotonin in the caudate nucleus and the nucleus accumbens that is accompanied by a reduction in spontaneous locomotor activity, low-dose MA-induced locomotion, and augmented high-dose MA-induced stereotypy. The MA-induced depletion of dopamine or the repeated administration of MA may be responsible for the decrease in stimulated dopamine release and the development of augmented stereotypic responses. The present results demonstrate that MA- induced neurotoxicity alters dopamine-mediated function and may serve as a useful model of functional consequences of moderate dopamine depletion.
Footnotes
This work was supported by National Institute on Drug Abuse Grants DA07427 (G.A.G.) and DA06733 (C.V.V).
Correspondence should be addressed to Dr. Charles V. Vorhees, Division of Developmental Biology, The Children’s Hospital Research Foundation, 3333 Burnet Avenue, Cincinnati, OH 45229-3039.
REFERENCES
- 1.Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260:817–824. [PubMed] [Google Scholar]
- 2.Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- 3.Broening HW, Pu C, Vorhees CV. Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens core while sparing the shell. Synapse. 1997;27:153–160. doi: 10.1002/(SICI)1098-2396(199710)27:2<153::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Browne RG, Segal DS. Metabolic and experiential factors in the behavioral response to repeated amphetamine. Pharmacol Biochem Behav. 1977;6:545–552. doi: 10.1016/0091-3057(77)90115-0. [DOI] [PubMed] [Google Scholar]
- 5.Callaway CW, Kuczenski R, Segal DS. Reserpine enhances amphetamine stereotypies without increasing amphetamine-induced changes in striatal dialysate dopamine. Brain Res. 1989;505:83–90. doi: 10.1016/0006-8993(89)90118-2. [DOI] [PubMed] [Google Scholar]
- 6.Cass WA. Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther. 1997;280:105–113. [PubMed] [Google Scholar]
- 7.Cass WA, Manning MW, Dugan MT. Effects of neurotoxic doses of methamphetamine on potassium and amphetamine evoked overflow of dopamine in the striatum of awake rats. Neurosci Lett. 1998;248:175–178. doi: 10.1016/s0304-3940(98)00374-7. [DOI] [PubMed] [Google Scholar]
- 8.Creese I, Iversen SD. A role of forebrain dopamine systems in amphetamine induced stereotyped behaviour in the rat. Psychopharmacology. 1974;39:345–357. doi: 10.1007/BF00422974. [DOI] [PubMed] [Google Scholar]
- 9.Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral sensitivity. Science. 1977;197:596–598. doi: 10.1126/science.877576. [DOI] [PubMed] [Google Scholar]
- 10.Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doucet G, Descarries L, Garcia S. Quantification of the dopamine innervation in adult rat neostriatum. Neuroscience. 1986;19:427–445. doi: 10.1016/0306-4522(86)90272-1. [DOI] [PubMed] [Google Scholar]
- 12.Graham WC, Crossman AR, Woodruff GN. Autoradiographic studies in animal models of hemi-parkinsonism reveal dopamine D2 but not D1 receptor supersensitivity I: 6-OHDA lesions of ascending mesencephalic dopaminergic pathways in the rat. Brain Res. 1990;514:93–102. doi: 10.1016/0006-8993(90)90439-i. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- 14.Joyce EM, Stinus L, Iversen SD. Effect of injections of 6-OHDA into either nucleus accumbens septi or frontal cortex on spontaneous and drug-induced activity. Neuropharmacology. 1983;22:1141–1145. doi: 10.1016/0028-3908(83)90051-5. [DOI] [PubMed] [Google Scholar]
- 15.Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- 16.Kelly RS, Wightman RM. Detection of dopamine overflow and diffusion with voltammetry in slices of rat brain. Brain Res. 1987;423:79–87. doi: 10.1016/0006-8993(87)90827-4. [DOI] [PubMed] [Google Scholar]
- 17.Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- 18.Kita T, Takahashi M, Wagner GC, Kubo K, Nakashima T. Methamphetamine-induced changes in activity and water intake during light and dark cycles in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1185–1196. doi: 10.1016/s0278-5846(98)00069-4. [DOI] [PubMed] [Google Scholar]
- 19.Kuczenski R, Segal DS. Psychomotor stimulant-induced sensitization: behavioral and neurochemical correlates. In: Kalivas P, Barnes T, editors. Sensitization in the nervous system. Telford; Caldwell, NJ: 1988. pp. 175–205. [Google Scholar]
- 20.Kuczenski R, Segal DS. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, cocaine and fencamfamine: relationship between locomotor and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci. 1991;11:2703–2712. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang N, Rutledge C. Evidence for carrier-mediated efflux of dopamine from corpus striatum. Biochem Pharmacol. 1982;302:479–484. doi: 10.1016/0006-2952(82)90057-0. [DOI] [PubMed] [Google Scholar]
- 23.Lucot JB, Wagner GC, Schuster CR, Seiden LS. The effects of dopaminergic agents on the locomotor activity of rats after high doses of methylamphetamine. Pharmacol Biochem Behav. 1980;13:409–413. doi: 10.1016/0091-3057(80)90247-6. [DOI] [PubMed] [Google Scholar]
- 24.McCabe RT, Hanson GR, Dawson TM, Gibb JW. Methamphetamine-induced reduction in D1 and D2 dopamine receptors as evidenced by autoradiography: comparison with tyrosine hydroxylase activity. Neuroscience. 1987;23:253–261. doi: 10.1016/0306-4522(87)90287-9. [DOI] [PubMed] [Google Scholar]
- 25.Mishra RK, Gardner EL, Katzman R, Makman MH. Enhancement of dopamine-stimulated adenylate cyclase activity in rat caudate after lesions in substantia nigra evidence for denervation supersensitivity. Proc Natl Acad Sci USA. 1974;71:2883–2887. doi: 10.1073/pnas.71.10.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan ME, Gibb JW. Short-term and long-term effects of methamphetamine on biogenic amine metabolism in extrastriatal dopaminergic nuclei. Neuropharmacology. 1980;19:989–995. doi: 10.1016/0028-3908(80)90010-6. [DOI] [PubMed] [Google Scholar]
- 27.O’Callaghan Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1991;270:197–206. [PubMed] [Google Scholar]
- 28.O’Dell SJ, Weihmuller FB, Marshall JF. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlates with subsequent neurotoxicity. Brain Res. 1991;564:256–260. doi: 10.1016/0006-8993(91)91461-9. [DOI] [PubMed] [Google Scholar]
- 29.Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- 31.Pu C, Vorhees CV. Developmental dissociation of methamphetamine-induced depletion of dopaminergic terminals and astrocyte reaction in rat striatum. Dev Brain Res. 1993;72:325–328. doi: 10.1016/0165-3806(93)90201-k. [DOI] [PubMed] [Google Scholar]
- 32.Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- 33.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 34.Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- 35.Robinson TE, Yew J, Paulson PE, Camp DM. The long-term effects of neurotoxic doses of methamphetamine on the extracellular concentration of dopamine measured with microdialysis in striatum. Neurosci Lett. 1990;110:193–198. doi: 10.1016/0304-3940(90)90810-v. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt CJ, Gibb JW. Role of dopamine uptake carrier in the neurochemical response to methamphetamine and effects of amfonelic acid. Eur J Pharmacol. 1985;109:73–80. doi: 10.1016/0014-2999(85)90541-2. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt CJ, Gehlert DR, Peat MA, Sonsalla PK, Hanson GR, Wamsley JK, Gibb JW. Studies on the mechanism of tolerance to methamphetamine. Brain Res. 1985;343:305–313. doi: 10.1016/0006-8993(85)90748-6. [DOI] [PubMed] [Google Scholar]
- 38.Schwarting RKW, Huston JP. Behavioral and neurochemical dynamics of neurotoxic meso-striatal dopamine lesions. Neurotoxicology. 1997;18:689–708. [PubMed] [Google Scholar]
- 39.Segal DS, Geyer MA. Animal models of psychopathology. In: Cavenar JO, editor. Psychiatry. Lippincott; Philadelphia: 1985. pp. 1–18. [Google Scholar]
- 40.Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- 41.Segal DS, Kuczenski R. In vivo microdialysis reveals a diminished amphetamine-induced dopamine response corresponding to behavioral sensitization produced by repeated amphetamine pretreatment. Brain Res. 1992;571:330–337. doi: 10.1016/0006-8993(92)90672-v. [DOI] [PubMed] [Google Scholar]
- 42.Segal DS, Kuczenski R. Behavioral Pharmacology of Amphetamine. In: Cho AK, Segal DS, editors. Amphetamine and its analogs: psychopharmacology, toxicology and abuse. Academic; San Diego: 1994. pp. 115–150. [Google Scholar]
- 43.Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1975;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- 44.Seiden LS, Commins D, Vosmer G, Axt L, Marek G. Neurotoxicity in dopamine and serotonin terminal fields: a regional analysis in nigrostriatal and mesolimbic projections. Ann NY Acad Sci. 1988;537:161–172. doi: 10.1111/j.1749-6632.1988.tb42104.x. [DOI] [PubMed] [Google Scholar]
- 45.Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;32:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 46.Snyder GL, Stachowiak M, Keller RW, Jr, Stricker EM, Zigmond MJ. Release of endogenous DA and DOPAC from striatal slices after DA-depleting lesions: effects of stimulation frequency and DA synthesis inhibition. Soc Neurosci Abstr. 1986;12:136. [Google Scholar]
- 47.Stachowiak MK, Keller RW, Stricker EM, Jr, Zigmond MJ. Increased dopamine efflux from striatal slices during development and after nigrostriatal bundle damage. J Neurosci. 1987;7:1648–1654. doi: 10.1523/JNEUROSCI.07-06-01648.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stricker ED, Zigmond MJ. Recovery of function following damage to central catecholamine containing neurons; a neurochemical model of the lateral hypothalamic syndrome. In: Sprague JM, Epstein AN, editors. Progress in psychobi- ology and physiological psychology. Academic; New York: 1976. pp. 121–189. [Google Scholar]
- 49.Wagner GC, Seiden LS, Schuster CR. Methamphetamine induced changes in brain catecholamines in rats and guinea pigs. Drug Alcohol Depend. 1979;4:435–438. doi: 10.1016/0376-8716(79)90076-0. [DOI] [PubMed] [Google Scholar]
- 50.Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- 51.Walsh SL, Wagner GC. Motor impairments after methamphetamine-induced neurotoxicity in the rat. J Pharmacol Exp Ther. 1992;263:617–626. [PubMed] [Google Scholar]
- 52.Zigmond MJ, Berger TW, Grace AA, Stricker ED. Compensatory responses to nigrostriatal bundle injury. Mol Chem Neuropathol. 1989;10:185–200. doi: 10.1007/BF03159728. [DOI] [PubMed] [Google Scholar]