Abstract
Neurobiological studies of associative learning and memory have focused nearly exclusively on the analysis of neural plasticity resulting from paired stimuli. A second major category of associative-learning processes, one that has been conspicuously neglected in cellular studies, is that of conditioned inhibition (CI), learning that one stimulus signals the absence of another. The physiological bases of CI are obscure and unexplored. To study the behavioral and neural bases of CI, we exposed the nudibranch molluscHermissenda crassicornis to explicitly unpaired (EU) presentations of light and rotation. We report here thatHermissenda exhibited persistent increases in phototactic behavior after EU training. Retardation-of-learning test results provided further evidence that EU animals learned that light signaled the absence of rotation. The increased phototactic behavior of EU animals was paralleled by selective decreases in the magnitude of ocular type B cell photoresponses and the frequency of light-elicited action potentials: the first report of a neural correlate of noncoincidence learning. Plasticity arising from explicitly unpaired stimulus presentations raises provocative questions as to how noncoincidence is detected and represented within the nervous system.
Keywords: learning and memory, Hermissenda, conditioned inhibition, photoreceptors, rotation, noncoincidence
Neurobiological studies of associative learning and memory (Woody, 1986; Byrne, 1987; Krasne and Glanzmann, 1996) and presumptive neurophysiological models of these processes (Bliss and Collingridge, 1993; Linden and Connor, 1995) have focused nearly exclusively on the analysis of neural plasticity resulting from the presentation of temporally contiguous stimuli (paired-stimulation paradigms). In classical conditioning experiments, such procedures are exemplified by conditioning trials in which conditioned (CS) and unconditioned (US) stimuli are paired with one another, as in so-called conditioned excitation training paradigms.
A second major category of associative-learning processes, one that has been conspicuously neglected in cellular studies of learning and memory, is that of conditioned inhibitory learning (CI) (Rescorla, 1969; LoLordo and Fairless, 1985; Hearst, 1988), which occurs when an organism learns that one stimulus (CS) specifically signals the absence of another. A simple and reliable means of producing such noncoincidence learning with vertebrates is the explicitly unpaired procedure (Rescorla and Lolordo, 1965; Wasserman et al., 1974), in which CS and US are both presented during training but are separated by a fixed and relatively long temporal interval. Although a variety of cellular and molecular neural correlates have been reported for behavioral and/or neurophysiological paired-stimulation paradigms, the physiological bases of conditioned inhibitory learning are obscure and unexplored. This neglect of inhibitory learning is surprising, because of the importance of such learning for the behavioral adaptation of organisms. For example, it is just as important for the learning repertoire of animals that they be able to learn when and where food or a predator is not as it is to learn when and where they do occur.
To study the neural bases of this second fundamental category of associative-learning and memory processes, we exposed the nudibranch mollusc Hermissenda crassicornis to explicitly unpaired (EU) presentations of light and rotation and determined the effects of this training on phototactic behavior and the excitability of neurons that participate in its control. Because changes in ocular type B photoreceptor excitability have been implicated previously to play a causal role in phototactic suppression arising from pairings of light and rotation (Crow and Alkon, 1980; Farley and Alkon, 1982; Farley et al., 1983), we hypothesized that excitability changes in type B photoreceptors might also be produced by EU training.
We report here that Hermissenda exhibited reliable and persistent increases in phototactic behavior after exposure to EU training. Retardation-of-learning test results provided further evidence that EU animals learned that light signaled the absence of rotation. These behavioral changes were paralleled by selective decreases in the magnitude of type B cell photoresponses, as well as decreases in the frequency of light-elicited action potentials. Our results indicate that information concerning excitatory and inhibitory CS–US signal relations are represented and stored in a common class of neurons in the CNS of Hermissenda.
MATERIALS AND METHODS
Open-field phototactic behavior. Animals were tested in a Plexiglas box (40.5 × 35 × 7.5 cm) filled to a 2.5 cm level with refrigerated artificial seawater and surrounded on four sides by a black plastic shield. A 32 × 32 cm grid of black lines was placed underneath the open field, with the center of the field defined as the coordinate points (0,0). The grid coordinates were used to assign the location of the animal’s head. A Dyonics fiber optic light source (Andover, MA; model 375W) located 53 cm above the surface was adjusted to provide an ∼3-mm-diameter 100 μW·cm−2 cone of illumination, whose intensity decreased radially and uniformly as a function of distance from the center. A low-light Vicon CCD camera (Melville, NY; model VC275-24) was mounted above the fiber optic source and allowed continuous monitoring and videotape recording of an animal’s movement in the light gradient. During each open-field test, an individual 15 min dark-adapted animal was randomly started at either coordinate points (8,8) or (−8,8). Both placements represent a distance of ∼11.3 cm from the center of the gradient. There were no obvious differences in the behavior of animals treated similarly as a function of start position. The animal’s movements around the open field were then recorded over a 10 min period. All measurements were obtained from videotaped records, at 15 sec intervals, by an observer without knowledge of the animal’s training condition. After each test, the animal was returned to its home aquarium. All testing and training of animals were conducted during the intermediate 8 hr of a standard 12:12 hr light/dark cycle. Pre- and post-training tests of phototactic behavior were conducted 24 hr before and after the initiation and conclusion of training, respectively.
Behavioral training. Behavioral training was conducted using methods and apparatus described in detail previously (Farley, 1987). Assignment of animals to treatment conditions was random in all experiments. In the first experiment reported (see Fig.2a), four training conditions were examined: EU, random control (RDM), light-alone (LT-A), and home cage (HC). On each of 3 consecutive training days, animals in the first three conditions experienced 30 trials of the indicated type (90 trials total). EU animals received presentations of light and rotation, separated by a constant 4.0 min interstimulus interval (ISI), with successive light presentations occurring every 9.0 min. RDM control animals received the same number of light and rotation presentations, but these were delivered randomly and independently of one another at an average ISI of 2.0 min. Some degree of overlap with rotation occurred by chance alone on ∼20% of the light presentations with this schedule. LT-A animals received 30 light presentations on each training day, separated by a constant 9.0 min ISI. All light (∼100 μW·cm−2) and rotation (100 rpm, 2.24 × g centrifugal force) presentations were 30 sec in duration. EU, RDM, and LT-A animals were all dark-adapted for 15 min before the first light presentation and were returned to the home aquarium immediately after training. HC control animals received no training on conditioning days and remained in their home aquaria. During each replication of the experiment, separate groups of four to seven animals were exposed to three of the four different training conditions described above. Two of these conditions were always the EU and HC control conditions, with the third condition tested being either the RDM or LT-A condition.
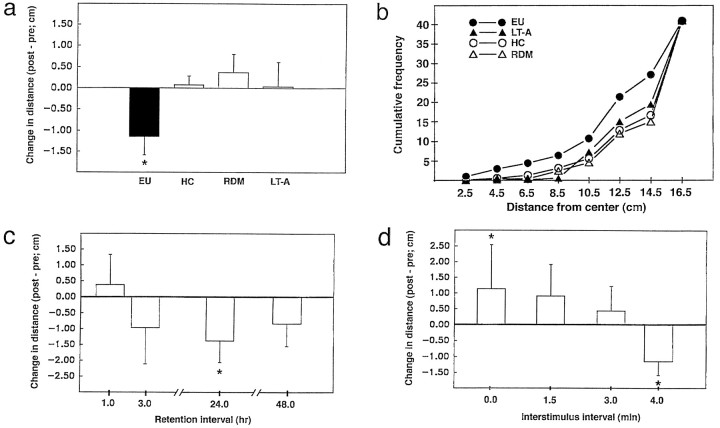
Fig. 2.
Associative characteristics, time course, and temporal specificity of enhanced phototaxis produced by explicitly unpaired training. a, Changes in average distance (post-training score minus pretraining score) from the center of illumination for EU (n = 57), HC (n = 64), LT-A (n = 23), and RDM (n = 31) animals. EU animals moved toward the center of the light gradient after training, whereas control animals’ phototactic behavior was either unchanged (LT-A, HC) or slightly suppressed (RDM). b, Cumulative frequency distributions of post-training distance-from-the-center scores for the different training conditions (SEMs have been omitted to avoid clutter). EU animals were significantly more likely to be found at distances closer to the center of the light gradient than were control animals.c, Time course of retained changes in phototaxis for EU animals. EU animals showed enhanced phototaxis at the 3, 24, and 48 hr retention intervals, with the greatest approach exhibited at 24 hr (*p < 0.05, paired-sample t test).d, Effects of varying the light–rotation ISI on open-field phototactic behavior. Animals exposed to a 4.0 min temporal separation between light and rotation (data from a have been replotted; n = 57) exhibited significantly enhanced phototaxis (approach), whereas shorter ISIs led to either no significant change in phototaxis [1.5 min (n = 8) and 3.0 min (n = 11) values] or suppressed phototactic behavior (withdrawal behavior for 0 min value;n = 6).
Retardation-of-learning experiments. Open-field phototactic behavior was measured (baseline tests), and animals were randomly assigned to one of three treatment conditions. Animals first received 3 d of standard EU, LT-A, or HC training (phase 1, days 1–3) as described above. All animals were subsequently exposed to two sessions of paired training (days 4–5) in the following manner. Twenty-four hours after the last training day in phase 1, animals were tested in the open field (post-test), returned to their home aquaria for 1–2 hr, and then exposed to 50 simultaneous light–rotation pairings (Farley, 1987, 1988). Animals were returned once again to their home aquaria for 1–2 hr after the first session of paired training and were then tested again in the open field (+50 pairings test). On the following day (day 5), animals received an additional 50 pairings and were then tested again (+100 pairings test) 1–2 hr after training. The change-in-distance scores reported (see Fig. 3) were measured relative to the initial (baseline) test of phototaxis.
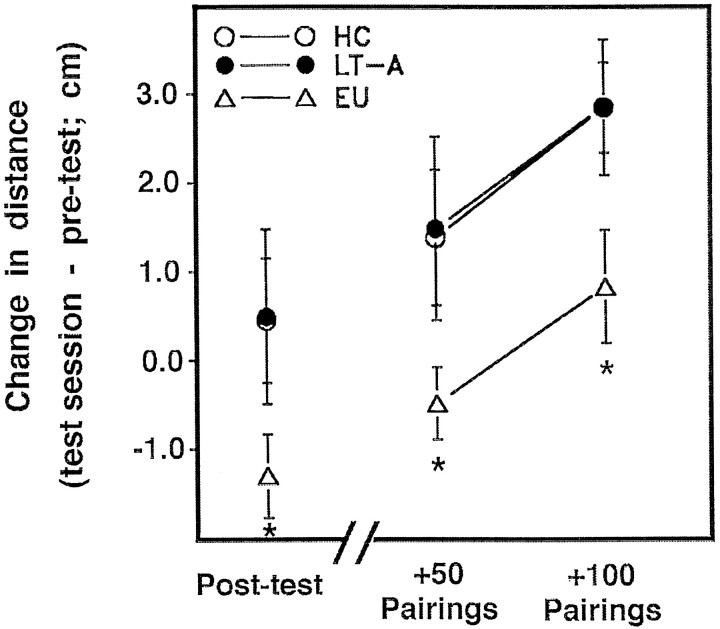
Fig. 3.
Retardation test of conditioned inhibitory properties of light. Changes in average distance scores for EU, LT-A, and HC animals at each of three test intervals (post-original training, post-50 pairings, post-100 pairings). EU animals showed significantly less withdrawal from light (phototactic suppression) than did control animals (repeated measures ANOVA, main effect of training condition,F(2, 24) = 3.42) after exposure to equivalent numbers of paired light–rotation presentations (+50 and +100 pairings), indicating that EU animals were retarded in the acquisition of excitatory learning relative to controls.
Electrophysiology. Intracellular recordings from synaptically intact type B photoreceptors were obtained 24–48 hr after the conclusion of training, using standard sharp-electrode recording methods (Farley and Auerbach, 1986; Farley, 1988; Farley and Schuman, 1991). One neuron per preparation was tested. After impalement of a B cell and stabilization of recording conditions, each preparation was dark-adapted for 15 min and subsequently exposed to three successive 30 sec light steps (6.0 × 10−4W·cm−2), separated by 2.5 min intervals (onset to onset). Steady-state light responses were measured during seconds 25–30, relative to the prelight resting membrane potential. Action potential frequencies were measured during seconds 5–30 of each light step. Input resistances were measured for dark-adapted cells, just before the first light step, from the asymptotic voltage drops produced by step injections (∼400 msec) of current (−0.5 to +0.5 nA, in 0.1 nA increments) through a balanced bridge circuit. Those scoring the records had no knowledge of the specific conditioning history of the preparation.
Data analysis. Data are expressed as means ± SEM. Statistical analyses of distance scores, photoreceptor light responses, action potential frequencies, resting input resistances, and membrane potentials were conducted by appropriate ANOVAs or Student’st tests. Cumulative frequency distributions of distance scores and dichotomous approach-withdrawal frequencies for open-field phototactic behavior were compared using χ2 tests. Correlation analyses used Pearson’s product-moment correlation coefficient. A pvalue < 0.05 was considered statistically significant.
RESULTS
EU training enhances open-field phototaxis
Phototactic behavior of adult Hermissenda was assessed in a center-illuminated open field (see Fig.1) by measuring the distance of the animal from the center (brightest portion) of the light gradient at consecutive 15 sec intervals, over a 10 min observation period. This measure, like those used for other bidirectional response systems (Wesierska and Zielinski, 1980; Best et al., 1985; Janssen et al., 1995), affords a simple and direct measure of conditioned inhibitory learning. Animals exposed to explicitly unpaired (4 min ISI) presentations of light and rotation moved toward the center of the light gradient when tested ∼24 hr after training (Fig.2a). In contrast, separate groups of animals exposed to equivalent numbers of random presentations of light and rotation (RDM), LT-A presentations, or no training at all (HC) failed to exhibit systematic changes in open-field phototactic behavior (Fig. 2a), although animals in all conditions locomoted throughout the post-training tests. The change-in-distance scores differed significantly among treatment conditions [F(3,171) = 3.73]. Planned comparisons indicated that the distance scores for EU animals were significantly different from each of the other three control conditions (Fig. 2a, *p < 0.05 for all Student’s t tests). There were no significant differences among any of the control groups (all t test scores < 1.0). There were no significant differences in baseline distance scores among groups [F(3,171) = 0.38, NS]. The average (mean ± SEM) baseline scores for EU, LT-A, RDM, and HC animals were 13.5 ± 0.31, 13.3 ± 0.53, 13.7 ± 0.37, and 13.8 ± 0.24 cm, respectively. The data summarized here represent the compilation of results from 30 separate replications, conducted over the course of several years. The enhancement of phototaxis by EU training was very reliable, with a statistically significant proportion of EU animals exhibiting approach toward light in each and every replication.
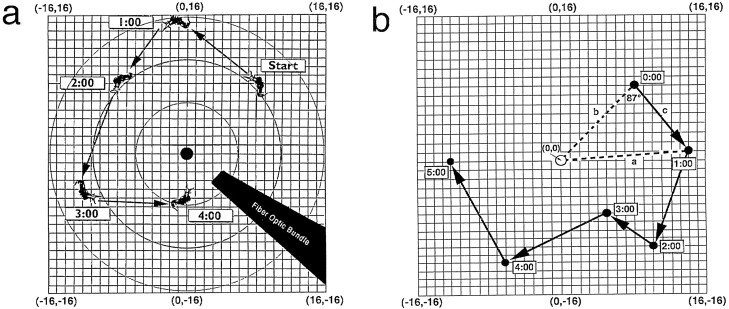
Fig. 1.
Open-field measurements of phototaxis.a, Measurement of distance scores. Phototactic behavior was scored from videotape by locating the animal’s position on thegridsheet underneath the open field. Each gridcoordinateposition (x,y) defined a distance-from-the-center score (x2 +y2)0.5 for each 15 sec time sample. The arithmetic average of these scores (n = 40 per animal per 10 min test) defined the average distance-from-the-center score for an animal during a given test. Boxednumbers indicate time in minutes. b, Illustration of measurement of trajectory angle scores. The schematic diagram illustrates a representative example of an animal’s movement in the open field over the course of 5 min. For each successive 1 min time sample (except the first), two straight (dashed)lines were drawn from the center of the light gradient (0,0) to the animal’s current (t =x;linesegmenta) and previous (t =x − 1 min; linesegmentb) gridlocations. A third linesegment (solidlinec) was then drawn connecting the two successivegridlocations. The angle formed bysegmentsb and c defined the direction of the animal’s movement, from one minute to the next, with respect to the center of the light gradient. Angles <90° indicate movement toward the center of illumination, angles >90° (maximum of 180°) indicate movement away from the center, and an angle of 90° indicates movement orthogonal to the center. The animal shown here moved from the starting position (8,8; 11.3 cm from the center) at t = 0 to point (14,1; 14.0 cm) att = 1 min, a directional angle of 87° relative to the center. The animal then moved to points (10,−10), (5,−6), (−6,−12), and (−12,0) during the following four successive 1 min intervals. The computed directional angles of movement were 62, 8, 74, and 58°, respectively. The corresponding distance-from-the-center scores for these time samples were 14.1, 7.8, 13.4, and 12.0 cm, respectively.
Dichotomous categorization of an individual animal’s post-training behavior as to whether it reflected approach toward the center (a decrease in the distance score ≥ 1.0 cm, relative to the preconditioning score), withdrawal (an increase ≥ 1.0 cm), or no change (a change score < 1.0 cm) revealed that a significantly greater percentage (61%) of EU animals moved toward the center after training, compared with HC (28%), RDM (29%), or LT-A (26%) animals [χ2(11, n = 175) = 30.74; p < 0.005]. Conversely, only 16% of EU animals withdrew from the center after training, whereas 20, 42, and 44% of HC, RDM, and LT-A animals, respectively, did so. Cumulative frequency analysis (Fig. 2b) of the distance scores indicated that EU training produced a clear and consistent shift in the animal’s preference for more highly illuminated areas of the open field (those closer to the center). A repeated measures ANOVA (training condition as the between-groups factor; distance from the center as the repeated measure) of the cumulative frequency distance scores revealed a significant interaction between training condition and frequency of position at various distances from the center of the light gradient [F(7,21) = 15.64]. The frequency scores for EU animals were significantly different from those of the three control conditions [χ2(7,n = 175) = 46.71]. None of the groups differed before training, nor did control groups differ after training (χ2 < 3.0 for all tests). Only EU animals increased the frequency with which they positioned themselves at distances closer to the center of the light gradient after training.
Analysis of animals’ movement trajectories in the open field (Fig.1b) confirmed the conclusions drawn from the distance scores. As a result of conditioning, EU animals’ movements were oriented toward the center (brightest area) of the light gradient. Before training, animals tended to move slightly away from their initial start position toward dimmer areas of the gradient, as evidenced by average trajectory angles of ∼99° [EU (98.1 ± 4.0°); HC (99.7 ± 4.0°); RDM (101.0 ± 6.1°); LT-A (99.2 ± 6.5°); no statistically significant differences between groups]. Average trajectory angles remained relatively constant for untrained [HC (100.3 ± 4.2°)] and random control [RDM (103.8 ± 6.0°)] animals but decreased for EU (94.3 ± 3.9°) and LT-A (92.0 ± 7.9°) animals after training. The failure of the LT-A treatment to produce consistent changes in the distance score (Fig. 2a,b) or results similar to that of EU animals in the retardation test (described below) suggest that the changes observed for LT-A animals in trajectory angle reflect a sampling error. Collectively, the distance score and trajectory angle results suggest that EU training resulted in a shift of the animals’ preferred level of illumination toward increased intensity values. The failure of control groups to exhibit consistent changes in both measures demonstrates the associative nature of the changes for EU animals. The fact that light signaled the absence of rotation for EU animals was critical for the enhanced phototaxis observed.
Temporal characteristics of inhibitory learning
To determine the persistence and time course of enhanced phototactic behavior produced by inhibitory conditioning, an additional group of EU-trained animals was tested 1, 3, 24, and 48 hr after the conclusion (day 3) of training. Shortly (1 hr) after the third training day, EU animals exhibited a slight, nonsignificant suppression of phototactic behavior that probably resulted from nonspecific effects of training and/or testing (Grover et al., 1987). Beginning 3 hr after training, EU animals exhibited enhanced phototactic behavior, with the greatest degree of approach behavior observed at the 24 hr retention interval (Fig. 2c). We cannot exclude the possibility that an additional factor contributing to the delayed appearance of approach was a delayed onset of the inhibitory learning process.
Pairings of light and rotation lead to suppressed phototactic behavior in Hermissenda (Crow and Alkon, 1980; Farley and Alkon, 1982; Farley et al., 1983), whereas explicitly unpaired presentations lead to enhanced phototaxis. How temporally separate must light and rotation be to produce enhanced phototaxis? Does excitatory learning give way to inhibitory learning in a gradual or abrupt manner as the light–rotation interstimulus interval (ISI) is increased? To address these issues, we systematically varied the ISI for separate groups of animals and measured open-field phototactic behavior 24 hr after the final training day. During each of 3 consecutive training days, animals received 50 presentations of light and rotation, as described previously. Animals that experienced EU training at a 4.0 min ISI exhibited enhanced phototaxis, whereas animals that experienced paired training (simultaneous and completely overlapping presentations of light and rotation; 0 min ISI) showed suppressed phototactic behavior (Fig. 2d). Animals exposed to EU training with a 1.5 or 3.0 min ISI showed no statistically significant changes in phototactic behavior, although there was a trend toward withdrawal. Between-group comparison of the distance change scores revealed that EU (4 min) and paired (0 min) groups differed significantly from the two intermediate ISI conditions and from each other (Fig. 2d, *p < 0.05). Thus, with the training procedures used here, light and rotation must be separated by an interval of at least ∼4.0 min to produce clear approach. The transition from excitatory conditioning (withdrawal) produced by simultaneous pairings (0 min ISI) to inhibitory learning (approach at the 4.0 min ISI) occurred over an interval of several minutes, and neither clear excitation nor inhibition was evidenced at intermediate ISI values. The temporal specificity of inhibition further underscores the associative character of the changes produced by EU training.
EU training retards acquisition of phototactic suppression
We next determined whether EU training (days 1–3) would interfere with the acquisition of excitatory learning (via reduced phototaxis) when the light was subsequently paired with rotation [days 4–5; retardation test of CI (LoLordo and Fairless, 1985; Hearst, 1988; Williams et al., 1992)]. Animals that had received previous EU training showed significantly less phototactic suppression (Fig.3) than did controls tested 1–2 hr after each of 2 d of paired training [repeated measures ANOVA, main effect of training condition, F(2,24)= 3.42]. After 100 pairings of light and rotation, EU animals exhibited phototactic behavior similar to that of control animals before paired training (Fig. 3). The distance change scores for EU animals were significantly different from controls at all test intervals (Fig. 3, *p < 0.05 for all Student’st tests). There were no significant differences in baseline scores among the groups [F(2, 29) < 1.0]. For EU animals, the inhibitory-signaling relationship interfered with subsequent learning that light signaled the presence of rotation.
Type B photoreceptors encode inhibitory learning
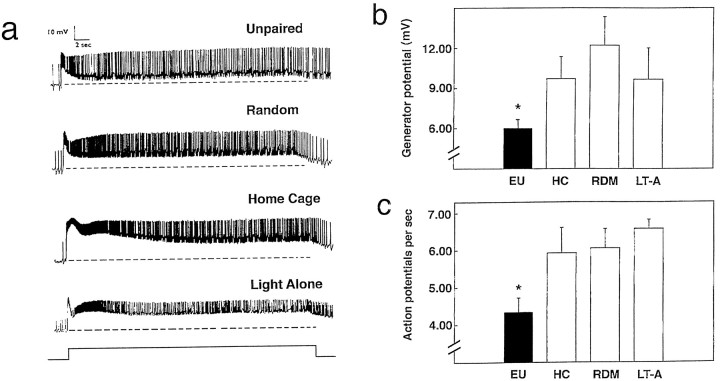
We next asked whether neurons known to encode an association between light and rotation resulting from pairings also encoded the inhibitory signal relation resulting from unpaired stimulation. Intracellular recordings were obtained from type B photoreceptors of randomly selected animals from each of the training conditions of the first experiment (results of Fig. 2a). Type B photoreceptors from EU animals responded to light with smaller steady-state depolarizing generator potentials, as well as fewer light-elicited action potentials (Fig. 4), than in cells from any of the other conditions. As observed previously for pairings of light and rotation (Farley, 1987), the training-associated differences were most pronounced for dark-adapted cells. There were no significant differences in light responses or action potential frequencies among the different control conditions. Repeated measures ANOVA (training condition as the between-groups factor; light step number as the repeated measure) of the steady-state light response revealed significant main effects of training condition [F(3,28) = 5.58] and light step [F(2,56) = 22.71] and a nonsignificant interaction [F(6,56) = 2.12, NS]. Light response amplitudes (average for three steps) were significantly smaller for cells from EU animals (n = 13), when compared with cells from RDM (n = 7) [t(18) = 3.63], HC (n = 7) [t(18) = 3.54], or LT-A (n = 5) [t(16) = 2.95] groups. There were no significant differences among the latter three groups [F(2,16) = 0.72, NS]. ANOVA of action potential frequency revealed nonsignificant main effects for training condition [F(3,28) = 2.16, NS] and light step [F(2,56) = 1.28, NS] but a significant interaction between the two [F(6,56) = 3.72]. Separate ANOVAs on light steps 1 [F(3,28) = 3.57] and 2 [F(3,28) = 3.12] revealed significant main effects of treatment, but not for light step 3 [F(3,28) = 1.95, NS]. Action potential frequencies (average of first two light steps) were smaller from EU animals, when compared with cells from RDM [t(18) = 2.17], HC [t(18) = 2.63], or LT-A [t(10) = 3.14] animals. There were no significant differences among the three latter groups [F(2,16) = 0.32, NS]. Resting membrane potentials [F(3,28) = 2.82, NS] and input resistances [F(3,23) = 0.79, NS] did not differ significantly as a function of training condition.
Fig. 4.
Neural correlates of EU training in type B photoreceptors. a, Representative recordings of light responses from dark-adapted Hermissenda type B photoreceptors from preparations exposed to the indicated training condition. Note the smaller steady-state response and reduced frequency of action potentials elicited by light in the photoreceptor from an EU animal relative to controls. All responses shown were obtained 24 hr after conditioning, for the first light step after 15 min of dark adaptation. The stimulusbar at thebottom of the figure illustrates the onset and offset of the light stimulus. b, c, Summary of the steady-state light response (average of 3 steps; b) and action potential frequency (average of light steps 1 and 2; c) data for type B photoreceptors. Note the significantly smaller light responses and fewer action potentials in cells from EU animals.
We also computed correlation coefficients between open-field phototactic behavior (change-in-distance scores) and both action potential frequency and steady-state light response (during the first light step) for individual animals, for each conditioning group, as well as when training history was ignored. In no case was a statistically significant correlation obtained, nor a suggestive trend observed. This represents a noteworthy departure from results obtained with light–rotation pairing paradigms, in which significant correlations between type B cell excitability/photoresponses and phototactic suppression for individual conditioned animals have been observed previously (Richards et al., 1984; Alkon et al., 1985; Goh et al., 1985; Farley and Jin, 1997).
DISCUSSION
Our results indicate that Hermissenda can learn that light signals the absence of rotation. This learning persists for at least 2 d after training, is critically dependent on a relatively long (4 min) temporal gap separating the two stimuli, and retards subsequent learning that light signals the presence of rotation.
The requirement that light and rotation be separated by at least 4.0 min is presumably one reason why a previous study (Crow and Alkon, 1978) that included an explicitly unpaired control group failed to detect effects of this training condition on phototaxis. The ISIs used in that study were variable and ranged between 1.0 and 3.0 min. On the basis of analogous results from conditioning studies with vertebrates (for example, Janssen et al., 1995), we think it likely that the 4.0 min temporal separation requirement is not an absolute one but will instead depend on other temporal factors such as the overall cycle time, light and rotation durations, intensities, conditioning history, etc. A recent description of backward-conditioning–produced CI in honeybees (Hellstern et al., 1998) provides an interesting parallel to our finding that a relatively long ISI is required for CI inHermissenda. Conditioned inhibition of the honeybee’s proboscis extension reflex was maximal when a relatively long US–CS ISI (15 sec) was used. Shorter (6 sec) and longer (>120 sec) ISIs failed to produce significant CI (Hellstern et al., 1998).
Another factor that is likely to be involved in previous failures to detect inhibitory effects is the method of testing used. Our development and refinement of the open-field test (Lederhendler et al., 1980) was prompted by our inability to demonstrate enhanced phototaxis (decreased response latencies) for the EU-trained animals using the conventional straight-tube tests of phototaxis that have been used in the majority of studies with Hermissenda (Crow and Alkon, 1978, 1980; Farley and Alkon, 1982, 1987). During pretraining straight-tube tests of phototaxis, animals are already moving quite rapidly, making it difficult to observe further decreases in baseline latencies.
A result obtained from the retardation-of-excitatory-conditioning test (Fig. 2d) warrants comment. On the basis of conditioning studies with vertebrates, one might have expected that the CS preexposures received by the LT-A animals would also have retarded acquisition of phototactic suppression [i.e., “latent inhibition” (Lubow, 1989)]. But no such effect was observed, consistent with results from a previous study (Farley, 1987). We do not know for certain why Hermissenda do not exhibit latent inhibition. Two considerations seem relevant, however. First, latent inhibition effects are generally attributed to habituation and/or conditioned attentional processes that limit processing to stimuli that are inconsequential (Lubow, 1989; Hall, 1991). Second,Hermissenda fail to exhibit habituation-produced changes in phototaxis (Richards et al., 1984; Farley, 1987). The absence of both habituation and latent inhibition to light may reflect the lack of persistent decremental processes in the early stages of visual processing for Hermissenda. If such changes were to occur, they would be expected to compromise the efficiency of other neural interactions (e.g., sensory adaptation and lateral inhibition) that improve coding efficiency. The absence of habituation and/or latent inhibition can be further rationalized a posteriori on the grounds that light is certainly not an inconsequential or arbitrary stimulus forHermissenda.
Our results also demonstrate that like paired-stimulation training, unpaired light–rotation presentations produced persistent excitability changes in photoreceptors that contribute to learning-produced changes in phototactic behavior. Pairings of light and rotation increase the excitability of type B photoreceptor cells (Crow and Alkon, 1980;Farley and Alkon, 1982; Alkon et al., 1985; Farley, 1987, 1988), whereas unpaired presentations decrease it. Whether inhibitory conditioning affects other sites of plasticity within theHermissenda CNS in a manner opposite that of paired training (Farley et al., 1990; Frysztak and Crow, 1994; Schuman and Clark, 1994; Farley and Han, 1997) has not yet been determined. Similarly, we have not yet determined whether the same K+ conductance systems (Alkon et al., 1982; Farley, 1988) and signal transduction pathways (Farley and Auerbach, 1986; Farley and Schuman, 1991; Crow et al., 1998) that are affected by paired-stimulation training are also targeted by EU training.
Several factors may account for our failure to observe correlations between the phototactic behavior of individual CI-trained animals and the photoresponse amplitude or impulse activity of type B cells. First, the changes in B cells produced by EU training may be mere correlates of CI and not causally related to the conditioned changes in phototactic behavior. This seems unlikely because of the demonstrable causal role that B cells play in pairing-produced phototactic suppression (Farley et al., 1983) and the fact that for at least some motoneurons involved in phototaxis, B cells influence motoneuron activity rather directly through disynaptic pathways (Goh et al., 1985). Second, because we did not identify which B cell subtypes we recorded from in the present study, the lack of correlation may have arisen from the pooling of results for photoreceptor subtypes that change differentially. A third possibility is that the strength of the correlation between phototactic behavior and type B cell excitability may depend on the way in which phototactic behavior is tested (e.g., straight tube vs open field) and measured (latency vs spatial location). All studies that have reported significant B cell–phototaxis correlations have used the straight-tube test. This test restricts an animal’s trajectory of movement toward light to that of direct approach (i.e., a trajectory angle of ∼0°). Although convenient and easy to implement, the straight-tube test may artificially constrain and oversimplify phototactic behavior somewhat. Phototaxis in this test is typically quantified as the latency required to move to the light (or as a suppression ratio score that normalizes the post-training latency relative to the pretraining value). In contrast, we used open-field tests of phototaxis in the present study, which allows unrestricted locomotion throughout the light gradient. Furthermore, our quantification of phototaxis was based on the spatial location of the animal. Locomotion in an open-field light gradient and the spatial location measure may not be as directly relatable to the activity of individual photoreceptors as are linear movement and latency measures in the straight tube. A final possible reason for the lack of correlation in the present study should be mentioned. The extent to which phototactic movements are encoded by the aggregate activity of a population of photoreceptors, rather than by single cells, may (for unknown reasons) differ for animals exposed to CI versus paired training. At present, we have little reason to prefer any of the above possibilities over the others. A more thorough delineation and characterization of locomotor neural circuitry inHermissenda is undoubtedly crucial for a better understanding of how training-correlated changes in primary sensory neurons affect phototaxis.
A common theme in accounts of neural plasticity that result from paired-stimulation paradigms is the involvement of various molecular coincidence detectors (e.g., NMDA receptors, IP3receptors, calcium-sensitive adenylate cyclase, PKC, etc.) (Bourne and Nicoll, 1995; Altman, 1996), which serve to integrate the effects of separate stimuli impinging on a cell within a brief time window. Such coincidence detection is thought to play an important role in the establishment of associative memories, perhaps in other forms of information processing in the adult, and also during development of the nervous system in the establishment of appropriate synaptic connectivity. Demonstrations of behavioral and neural changes arising from explicitly unpaired stimulus presentations thus raise provocative questions as to how such plasticity arises. Are there molecular “noncoincidence” detectors? Alternatively, perhaps familiar molecular coincidence detectors are involved, but the neural circuits and signal transduction pathways responsible for their activation transform unpaired behavioral stimulation into temporally overlapping signals at the subcellular and molecular level.
Footnotes
Correspondence should be addressed to Dr. Joseph Farley, Department of Psychology, Programs in Neural Science and Biochemistry, Indiana University, 1101 East 10th Street, Bloomington, IN 47405-7007.
This work was supported by grants from the National Institutes of Health (NS 30950) and the Indiana University Center for the Integrative Study of Animal Behavior. We thank Mary Janssen and Laura Friesen for their help in preliminary experiments.
REFERENCES
- 1.Alkon DL, Lederhendler I, Shoukimas JJ. Primary changes of membrane currents during retention of associative learning. Science. 1982;215:693–695. doi: 10.1126/science.7058334. [DOI] [PubMed] [Google Scholar]
- 2.Alkon DL, Sakakibara M, Forman R, Harrigan J, Lederhendler I, Farley J. Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behav Neural Biol. 1985;44:278–300. doi: 10.1016/s0163-1047(85)90296-1. [DOI] [PubMed] [Google Scholar]
- 3.Altman J. Coincidence detection in the nervous system: molecular and cellular mechanisms. Consequences for learning and memory. Human Frontier Science Program; Strasbourg, France: 1996. [Google Scholar]
- 4.Best MR, Dunn DP, Batson JD, Meachum CL, Nash SM. Extinguishing conditioned inhibition in flavor-aversion learning: effects of repeated testing and extinction of the excitatory element. Q J Exp Psychol. 1985;37:359–378. doi: 10.1080/14640748508401175. [DOI] [PubMed] [Google Scholar]
- 5.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 6.Bourne HR, Nicoll R. Molecular machines integrate coincident synaptic signals. Neuron. 1995;10:65–75. doi: 10.1016/s0092-8674(05)80029-7. [DOI] [PubMed] [Google Scholar]
- 7.Byrne JA. Cellular analysis of associative learning. Physiol Rev. 1987;67:329–439. doi: 10.1152/physrev.1987.67.2.329. [DOI] [PubMed] [Google Scholar]
- 8.Crow T, Alkon DL. Associative behavioral modification in Hermissenda: cellular correlates. Science. 1980;209:412–414. doi: 10.1126/science.209.4454.412. [DOI] [PubMed] [Google Scholar]
- 9.Crow T, Xue-Bian JJ, Siddiqi V, Kang Y, Neary JT. Phosphorylation of mitogen-activated protein kinase by one-trial and multi-trial classical conditioning. J Neurosci. 1998;18:3480–3487. doi: 10.1523/JNEUROSCI.18-09-03480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crow TJ, Alkon DL. Retention of an associative behavioral change in Hermissenda. Science. 1978;201:1239–1241. doi: 10.1126/science.694512. [DOI] [PubMed] [Google Scholar]
- 11.Farley J. Contingency learning and causal detection in Hermissenda. I. Behavior. Behav Neurosci. 1987;101:13–27. doi: 10.1037//0735-7044.101.1.13. [DOI] [PubMed] [Google Scholar]
- 12.Farley J. Associative training results in persistent reductions in a calcium-activated potassium current in Hermissenda type B photoreceptors. Behav Neurosci. 1988;102:784–802. [Google Scholar]
- 13.Farley J, Alkon DL. Associative neural and behavioral change in Hermissenda: consequences of nervous system orientation for light- and pairing-specificity. J Neurophysiol. 1982;48:785–807. doi: 10.1152/jn.1982.48.3.785. [DOI] [PubMed] [Google Scholar]
- 14.Farley J, Alkon DL. In vitro associative conditioning of Hermissenda: cumulative depolarization of type B photoreceptors and short-term associative behavioral changes. J Neurophysiol. 1987;57:1639–1668. doi: 10.1152/jn.1987.57.6.1639. [DOI] [PubMed] [Google Scholar]
- 15.Farley J, Auerbach S. Protein kinase C activation induces conductance changes like those seen in associative learning. Nature. 1986;319:220–222. doi: 10.1038/319220a0. [DOI] [PubMed] [Google Scholar]
- 16.Farley J, Han Y. Ionic basis of learning-correlated excitability changes in Hermissenda type A photoreceptors. J Neurophysiol. 1997;77:1861–1888. doi: 10.1152/jn.1997.77.4.1861. [DOI] [PubMed] [Google Scholar]
- 17.Farley J, Jin I. Potentiation of phototactic suppression in Hermissenda by compound conditioning results in potentiated excitability changes in type B and A photoreceptors. Behav Neurosci. 1997;111:309–319. doi: 10.1037//0735-7044.111.2.309. [DOI] [PubMed] [Google Scholar]
- 18.Farley J, Schuman E. Protein kinase C inhibitors prevent induction and continued expression of cellular memory in Hermissenda type B photoreceptors. Proc Natl Acad Sci USA. 1991;88:2016–2020. doi: 10.1073/pnas.88.5.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farley J, Richards WG, Ling L, Liman E, Alkon DL. Membrane changes in a single photoreceptor cause associative learning in Hermissenda. Science. 1983;221:1201–1203. doi: 10.1126/science.6612335. [DOI] [PubMed] [Google Scholar]
- 20.Farley J, Richards WG, Grover LM. Associative learning changes intrinsic to Hermissenda type A photoreceptors. Behav Neurosci. 1990;104:135–152. doi: 10.1037//0735-7044.104.1.135. [DOI] [PubMed] [Google Scholar]
- 21.Frysztak RJ, Crow TJ. Enhancement of type B and A photoreceptor inhibitory synaptic connections in conditioned Hermissenda. J Neurosci. 1994;14:1245–1250. doi: 10.1523/JNEUROSCI.14-03-01245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goh Y, Lederhendler, Alkon DL. Input and output changes of an identified neural pathway are correlated with associative learning in Hermissenda. J. Neurosci. 1985;5:536–543. doi: 10.1523/JNEUROSCI.05-02-00536.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grover LM, Farley J, Vold L. Training and testing determinants of phototaxic behavior in Hermissenda. Behav Neural Biol. 1987;47:275–306. doi: 10.1016/s0163-1047(87)90409-2. [DOI] [PubMed] [Google Scholar]
- 24.Hall G. Perceptual and associative learning. Clarendon; Oxford: 1991. [Google Scholar]
- 25.Hearst E. Learning and cognition. In: Atkinson RC, Herrnstein RJ, Lindzey G, Luce RD, editors. Stevens’ handbook of experimental psychology, 2nd Edition, Vol 2. Wiley; New York: 1988. pp. 3–109. [Google Scholar]
- 26.Hellstern F, Malaka R, Hammer M. Backward inhibitory learning in honeybees: a behavioral analysis of reinforcement processing. Learn Mem. 1998;4:429–444. doi: 10.1101/lm.4.5.429. [DOI] [PubMed] [Google Scholar]
- 27.Janssen M, Farley J, Hearst E. Temporal location of unsignaled food deliveries: effects on conditioned withdrawal (inhibition) in pigeon signtracking. J Exp Psychol Anim Behav Process. 1995;21:116–128. doi: 10.1037//0097-7403.21.2.116. [DOI] [PubMed] [Google Scholar]
- 28.Krasne F, Glanzmann D. What can we learn from invertebrate learning? Annu Rev Psychol. 1996;46:585–624. [Google Scholar]
- 29.Lederhendler, Barnes ES, Alkon DL. Complex responses to light of the nudibranch Hermissenda crassicornis (Gastrophoda: Ophistobranchia). Behav Neural Biol. 1980;28:218–230. [Google Scholar]
- 30.Linden DJ, Connor JA. Long-term synaptic depression. Annu Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- 31.LoLordo VM, Fairless JL. Pavlovian conditioned inhibition: the literature since 1969. In: Miller RR, Spear NE, editors. Information processing in animals: conditioned inhibition. Erlbaum; Hillsdale, NJ: 1985. pp. 1–49. [Google Scholar]
- 32.Lubow RE. Latent inhibition and conditioned attention theory. Cambridge UP; Cambridge, England: 1989. [Google Scholar]
- 33.Rescorla RA. Pavlovian conditioned inhibition. Psychol Bull. 1969;72:77–94. [Google Scholar]
- 34.Rescorla RA, Lolordo VM. Inhibition of avoidance behavior. J Comp Physiol Psychol. 1965;59:406–412. doi: 10.1037/h0022060. [DOI] [PubMed] [Google Scholar]
- 35.Richards WG, Farley J, Alkon DL. Extinction of associative learning in Hermissenda: behavior and neural correlates. Behav Brain Res. 1984;14:161–170. doi: 10.1016/0166-4328(84)90185-2. [DOI] [PubMed] [Google Scholar]
- 36.Schuman EM, Clark GA. Synaptic facilitation at connections of Hermissenda type B photoreceptors. J Neurosci. 1994;141:1613–1622. doi: 10.1523/JNEUROSCI.14-03-01613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wasserman EA, Franklin SR, Hearst E. Pavlovian appetitive contingencies and approach versus withdrawal to conditioned stimuli in pigeons. J Comp Physiol Psychol. 1974;86:616–627. doi: 10.1037/h0036171. [DOI] [PubMed] [Google Scholar]
- 38.Wesierska M, Zielinski K. Enhancement of bar-pressing rate in rats by the conditioned inhibitor of the CER. Acta Neurobiol Exp (Warsz) 1980;40:945. [PubMed] [Google Scholar]
- 39.Williams DA, Overmier JA, LoLordo V. A reevaluation of Rescorla’s early dictums about Pavlovian conditioned inhibition. Psychol Bull. 1992;111:275–290. [Google Scholar]
- 40.Woody CD. Understanding the cellular basis of learning and memory. Annu Rev Psychol. 1986;37:433–493. doi: 10.1146/annurev.ps.37.020186.002245. [DOI] [PubMed] [Google Scholar]