Abstract
Peripheral nerve injury in neonatal rats results in the death of the majority of the axotomized sensory neurons by 7 d after injury. In adult animals, however, all sensory neurons survive for at least 4 months after axotomy. How sensory neurons acquire the capacity to survive axonal injury is not known. Here we describe how the expression of the small heat shock protein 27 (HSP27) is correlated with neuronal survival after axotomy in vivo and after NGF withdrawal in vitro. The number of HSP27-immunoreactive neurons in the L4 DRG is low at birth and does not change significantly for 21 d after postnatal day 0 (P0) sciatic nerve axotomy. In contrast, in the adult all axotomized neurons begin to express HSP27. One week after P0 sciatic nerve section the total number of neurons in the L4 DRG is dramatically reduced, but all surviving axotomized neurons, as identified by c-jun immunoreactivity, are immunoreactive for HSP27. In addition, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling reveals that very few HSP27-expressing neurons are dying 48 hr after neonatal axotomy.In vitro, a similar correlation exists between HSP27 expression and survival; in P0 DRG cultures, neurons that express HSP27 preferentially survive NGF withdrawal. Finally, overexpression of human HSP27 in neonatal rat sensory and sympathetic neurons significantly increases survival after NGF withdrawal, with nearly twice as many neurons surviving at 48 hr. Together these results suggest that HSP27 in sensory neurons plays a role in promoting survival after axotomy or neurotrophin withdrawal.
Keywords: apoptosis, axotomy, nerve growth factor, heat shock protein, dorsal root ganglion, neonatal
Sensory neurons undergo substantial programmed cell death during early embryonic development (Oppenheim, 1991) and continue to be highly susceptible to cell death in the neonatal period. Peripheral nerve injury (Himes and Tessler, 1989), growth factor withdrawal (Eichler and Rich, 1989), ionizing radiation (Tong et al., 1997), capsaicin (Winter et al., 1993), and sindbis virus infection (Griffin et al., 1997) all induce cell death in neonatal DRG neurons. Adult sensory neurons, in contrast, are much more resistant to all of these insults. In particular, neither peripheral nerve injury (Swett et al., 1995; Lekan et al., 1997) nor NGF deprivation in vivo or in vitro (Johnson et al., 1980; Lindsay, 1988) results in significant early neuronal death in the adult. That adult sensory neurons become refractory to such varied death-inducing stimuli suggests that there is developmental regulation of genes or signal transduction pathways involved in neuronal death.
The pro-apoptotic protein Bax is downregulated while the anti-apoptotic protein Bcl-xL is upregulated in the neonate coincident with increased sensory neuronal ability to survive NGF withdrawal in vitro(Vogelbaum et al., 1998). However, levels of mRNAs encoding both Bcl-xL and another anti-apoptotic factor, Bcl-2, decrease in the DRG after adult sciatic nerve transection, whereas Bax mRNA levels do not change (Gillardon et al., 1994), suggesting that regulation of these factors alone cannot explain the increased ability of adult DRG neurons to survive axotomy. Bcl-xL and Bcl-2 mRNAs also decrease after neonatal axotomy; however, a small population of neonatal DRG neurons survives axotomy. The mechanism by which some neonatal neurons can survive axotomy or neurotrophic factor withdrawal whereas others do not is unknown.
We have found recently that the expression of heat shock protein 27 (HSP27) mRNA and protein in the DRG is dramatically upregulated after peripheral nerve injury (Costigan et al., 1998). HSP27 is constitutively expressed at low levels by a subpopulation of adult primary sensory neurons (Plumier et al., 1997; Costigan et al., 1998). After peripheral axotomy in the adult, however, virtually all injured sensory neurons express high levels of HSP27 (Costigan et al., 1998;Hopkins et al., 1998). Expression of HSP27 mRNA in the DRG is also developmentally regulated; low levels are detectable at embryonic day 15, and expression increases gradually, reaching adult levels by postnatal day 21 (P21) (Costigan et al., 1998).
HSP27 is induced by stressors in a variety of non-neuronal cell types and protects them from insults such as ischemia, heat shock, oxidative stress, and noxious chemicals (Huot et al., 1991; Lavoie et al., 1993;Wu and Welsh, 1996). HSP27 expression can protect against apoptotic as well as necrotic cell death in non-neuronal cells (Mehlen et al., 1995;Samali and Cotter, 1996), and exogenous HSP27 has been shown recently to decrease sensory neuron apoptosis after NGF withdrawal in vitro (Wagstaff et al., 1999). Here we investigate the role of HSP27 in neonatal sensory neurons after peripheral nerve injury and NGF withdrawal. Our findings suggest that HSP27 contributes significantly to the survival of sensory neurons under these conditions and is likely to be an important factor for survival of adult sensory neurons as well.
MATERIALS AND METHODS
Surgical procedures. Sprague Dawley rats were used for all procedures, following Massachusetts General Hospital and National Institutes of Health guidelines. Newborn pups no older than 12 hr were anesthetized by hypothermia until they were unresponsive to any form of mechanical stimuli. The left sciatic nerve was exposed at the midthigh level, ligated with 6.0 suture cotton, and transected. The wound was reclosed with cyanoacrylate, and the pup was returned to its mother. Sciatic nerve transection in adults was done as described previously (Costigan et al., 1998). For fluorogold double-labeling experiments, 3 μl of a 5% solution of fluorogold (Fluorochrome, Denver, CO) was injected into the nerve immediately after transection.
Tissue preparation. Animals were terminally anesthetized at various time points (24 hr to 21 d) after transection. L4/l5 DRGs ipsilateral to the injury were dissected and frozen immediately for immunohistochemistry. Cryosections (20–30 μm thick) of DRG were mounted on Silane-coated slides (Sigma, St. Louis, MO), air-dried, and fixed for 30 min in 4% paraformaldehyde at 4°C before preincubating in a dilution buffer (0.1 m PBS, 0.8% bovine serum albumin, 0.25% Triton X-100, and 5% normal goat serum) for 1 hr. After three rinses in PBS, sections were incubated in goat anti-HSP27 antibody (1:500–2000; Santa Cruz Biolabs, Santa Cruz, CA) in dilution buffer at 4°C for 48 hr. Sections were washed three times in PBS, incubated in secondary antibody (rabbit anti-goat; 1:200) for 2 hr at room temperature, and visualized using a standard Vectastain kit (Vector Laboratories, Burlingame, CA). Adult DRG tissue sections used in HSP27–c-jun double-labeling experiments were first incubated in goat anti-HSP27 antibody followed by an incubation in rabbit anti-c-jun (1:100; Oncogene Research Products, Cambridge, MA) antibody. Immunoreactive cells were visualized with Cy3-conjugated anti-goat and FITC-conjugated anti-rabbit secondary antibodies.
For fluorogold experiments, animals were perfused with 4% paraformaldehyde on postnatal day 7. DRGs were removed, post-fixed and cryoprotected, and sectioned on the cryostat (40 μm). Free-floating sections were then incubated with goat anti-HSP27 antibody as described above.
Double-labeling with terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and immunocytochemistry. Tissue was embedded in Tissue-Tek ornithine carbamyl transferase compound (Miles, Elkhart, IN) and sectioned at 20 μm on a cryostat. Sections were stained with the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method using the ApopTag Fluorescent detection kit (Oncor, Gaithersburg, MD). Sections for double-labeling were incubated in dilution buffer without goat serum, washed, and incubated in either goat anti-HSP27 (1:1000), mouse anti-glial fibrillary acidic protein (GFAP; 1:50; Boehringer Mannheim, Indianapolis, IN), or a mouse anti-neurofilament (200 kDa; 1:500; Sigma) at room temperature overnight. Secondary antibodies used were Cy3-conjugated rabbit anti-goat, Cy3-conjugated donkey anti-mouse, or Cy3-conjugated goat anti-rabbit (1:300; Jackson ImmunoResearch, West Grove, PA).
Cell counting. To estimate in vivo cell numbers and construct histograms free of size and shape biases, we counted total and HSP27-immunoreactive neurons in a double dissector paradigm (Pover et al., 1993). To do this, five pairs of sections (lookup and reference sections) were chosen in a systematic random manner for each animal. Those cells seen in each reference but not in the lookup section were identified and drawn, and their areas were calculated in square micrometers (n = 3–5 animals per condition).
Estimates of c-jun–HSP27 double-labeled cells that contributed axons to the sciatic nerve were made in the following way. Entire ganglia sweeps through every fourth section (15 sections total) were made using a 40× objective, and every c-jun-labeled, HSP27-positive, and double-labeled neuronal profile was counted (n = 3). For quantitative evaluation of fluorogold- or HSP27-positive ganglion cells, the numbers of fluorogold-labeled or HSP-immunoreactive sensory neuron profiles in every fourth section (10–15 sections per ganglion) were counted (n = 4). Cells undergoing apoptosis were recognized by the TUNEL method by an intensely fluorescent nucleus. Double-labeled cells exhibited both FITC (TUNEL positive) and Cy3 (HSP27, GFAP, or neurofilament). Profiles were counted in six to eight sections per ganglion from four animals.
Preparation of DRG neuronal cultures. Animals were decapitated, and ganglia were rapidly removed under aseptic conditions, placed in HBSS (Life Technologies, Gaithersburg, MD), and digested in 0.125% collagenase (Boehringer Mannheim) in DMEM for 20 min followed by 10 min in 0.25% trypsin (Sigma), all at 37°C. Ganglia were washed in DMEM, briefly centrifuged at 1000 rpm, and resuspended in F12 with 10% FBS (Life Technologies), N2 supplement (Life Technologies), 100 ng/ml 2.5 S NGF (Promega, Madison, WI), and penicillin and streptomycin (Sigma). Ganglia were then triturated through a flame-polished pipette ∼10 times, and the suspension was centrifuged through 15% BSA (fatty acid free; Sigma) in F12. The pellet was resuspended in F12 with the additives listed above, except that serum was reduced to 0.5%. In most experiments, cells were preplated onto uncoated tissue culture dishes for 1.5 hr at 37°C. Nonadherent cells were then recovered from the dishes by gentle pipetting and plated on polyornithine (Sigma)- and laminin (Sigma)-coated glass coverslips. Cells were grown at 37°C in 5% CO2 and fed every 48 hr. For counts of total neuronal survival, cells in a 50 mm2 area of each well were counted on the day of NGF withdrawal or refeeding and at 24 hr intervals thereafter. Cells with round, phase-bright bodies and intact neurites were counted as surviving neurons.
Preparation of SCG cultures. Cultures of SCG neurons were prepared essentially as described previously (Lewis et al., 1994). Briefly, SCG from P1 to P2 rat pups were dissected out and digested in collagenase P (5 mg/ml; Boehringer Mannheim) and dispase (1 mg/ml; Boehringer Mannheim) for 50 min at 37°C. DNase was added, and ganglia were triturated through a flame-polished Pasteur pipet to achieve a single-cell suspension. After washing, cells were plated onto poly-d-lysine- and laminin-coated dishes or slides in OPTI-MEM (Life Technologies) containing 10% fetal bovine serum (Life Technologies), penicillin, streptomycin, 2 mm glutamine, 4 mg/ml glucose, and 100 ng/ml NGF (Boehringer Mannheim). Cytosine arabinoside (ara-C; 10 μm) was included for the first 3 d of culture. On the fourth day, cells were refed with OPTI-MEM supplemented as above, except without serum and ara-C.
Immunocytochemistry of primary cultures. Cultures were fixed in 4% paraformaldehyde for 10 min, followed by three washes in PBS and a 30–60 min incubation at room temperature in PBS containing 0.25% Tween 20 and 3% of the appropriate serum (TPBS-3%), and then incubated overnight at 4°C in the same buffer containing appropriate dilutions of one of the antibodies listed below. After four washes in TPBS-3%, cultures were incubated for 40 min at room temperature with a 1:300 dilution of FITC- and/or Texas Red-conjugated secondary antibodies (Vector Laboratories) in PBS, followed by four 5 min washes in PBS, coverslipping with Vectashield (Vector Laboratories), and viewing on an epifluorescent microscope. Nuclei were visualized by including 10 μg/ml Hoechst 33342 (Boehringer Mannheim) in the first PBS wash after the secondary antibody incubation. Only protein gene product 9.5 (PGP9.5)-positive cells with clearly fragmented nuclei were scored as apoptotic neurons.
The following dilutions of primary antibodies were used: anti-HSP27 goat polyclonal antibody (Santa Cruz Biolabs) at 1:2000 to visualize endogenous HSP27 and anti-PGP9.5 rabbit polyclonal antibody (Accurate Biochemical) at 1:10,000 to identify neurons. To visualize human HSP27 in infected cells, a monoclonal antibody specific for human (not cross-reactive with rat) HSP27 (Stressgen) was used at 1:200 or 1:300. The anti-β-galactosidase monoclonal antibody (Promega) was used at a dilution of 1:500.
Virus infections. Adenovirus constructions have been described previously (Martin et al., 1997). All recombinant adenoviruses were purified on CsCl gradients (Graham and Prevec, 1995), plaque-titered on 293 cells, and checked for wild-type contamination by PCR for E1A. For infection, virus was added at a multiplicity of infection (m.o.i.) of 100 to P0 DRG cultures 15–18 hr after plating. At this m.o.i., 75–90% of neurons in neonatal DRG cultures were infected. NGF was withdrawn 36–48 hr later by removing virus-containing media completely and refeeding with medium containing a 1:300 dilution of an anti-NGF polyclonal antiserum or with NGF-containing medium as a control. Cultures were fixed with 4% paraformaldehyde 48 hr after NGF withdrawal. SCG cultures were infected on the fourth day after plating in OPTI-MEM without serum at m.o.i. values ranging from 20 to 100.
MTT assay for cell survival. For MTT assays, 3–5 × 103 cells were plated in each well of a 48-well plate. MTT assays were performed using the CellTiter 96Aq. kit (Promega).
RESULTS
HSP27 is constitutively expressed by a small subpopulation of sensory neurons throughout postnatal development
We analyzed the developmental regulation of HSP27 in the L4 DRG of rats by counting total numbers of HSP27-immunoreactive neurons at postnatal days 2, 7, and 21. A small percentage of DRG neurons is clearly positive for HSP27 at P2 (Fig.1A). HSP27 immunoreactivity is detectable in 1005 ± 211 L4 DRG neurons at P2, and the number of HSP27-immunoreactive neurons increases steadily postnatally (Fig. 1C). The number of cells immunoreactive for HSP27 at P21 is similar to that found in adults (Fig. 1C) (Costigan et al., 1998). Therefore, the proportion of L4 DRG neurons constitutively expressing HSP27 increases from a level of ∼7% at birth to ∼20% in adults.
Fig. 1.
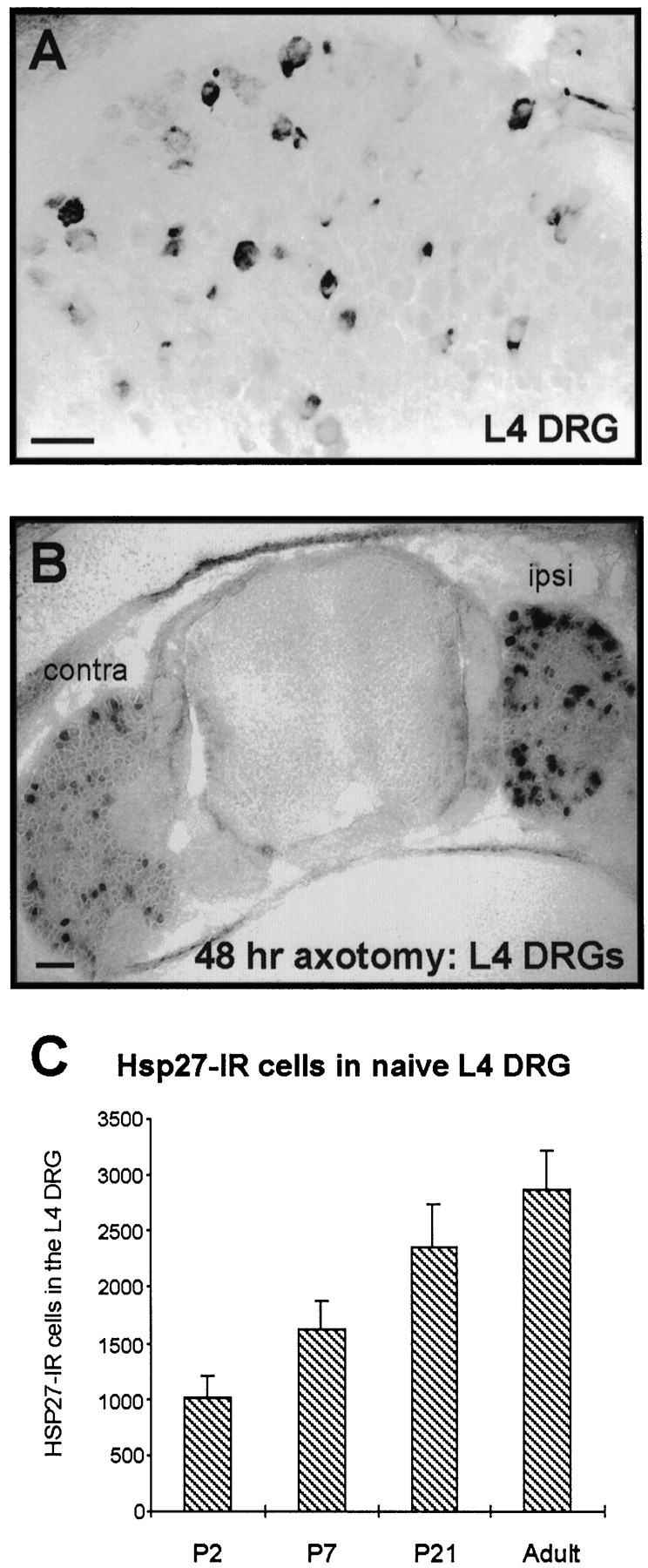
Postnatal expression of HSP27 protein in the DRG and expression after sciatic nerve injury. A, At P2, HSP27 immunoreactivity is detectable in a subpopulation of neurons in the L4 DRG of naive animals. B, Two days after nerve injury at P0, the intensity of HSP27 immunoreactivity increases dramatically in the ipsilateral (ipsi) DRG; however, the number of cells that are positive does not increase (see Results).C, The number of DRG neurons constitutively immunoreactive for HSP27 increases postnatally. Numbers are the mean ± SD from three or four animals per age group.contra, Contralateral. Scale bars, 50 μm.
HSP27 expression after neonatal nerve injury
Two days after a peripheral nerve section at P0, the total number of HSP27-immunoreactive cells is not significantly different from that in naive animals at this age (1005 ± 211 in naives; 1246 ± 130 after axotomy; p > 0.4). This is in marked contrast to the adult, in which essentially all injured neurons upregulate HSP27 within 48 hr after sciatic nerve transection (Costigan et al., 1998). After P0 axotomy, although total numbers do not change, HSP27-immunoreactive cells appear to express significantly greater amounts of the protein than do those in intact ganglia, as detected by a marked increase in the intensity of immunoreactivity (Fig. 1B).
Neurons that survive neonatal nerve injury express HSP27
To test whether those neurons that express HSP27 survive neonatal nerve injury, we assessed DRG neuronal loss after P0 axotomy, counting both total numbers of neurons and the numbers of HSP27-positive neurons 2 and 7 d after axotomy. Two days after axotomy at P0, the total number of L4 DRG neurons is reduced by 26% (p< 0.001). By 7 d, the number of neurons has fallen by >50% (p < 0.001; Fig.2A).
Fig. 2.
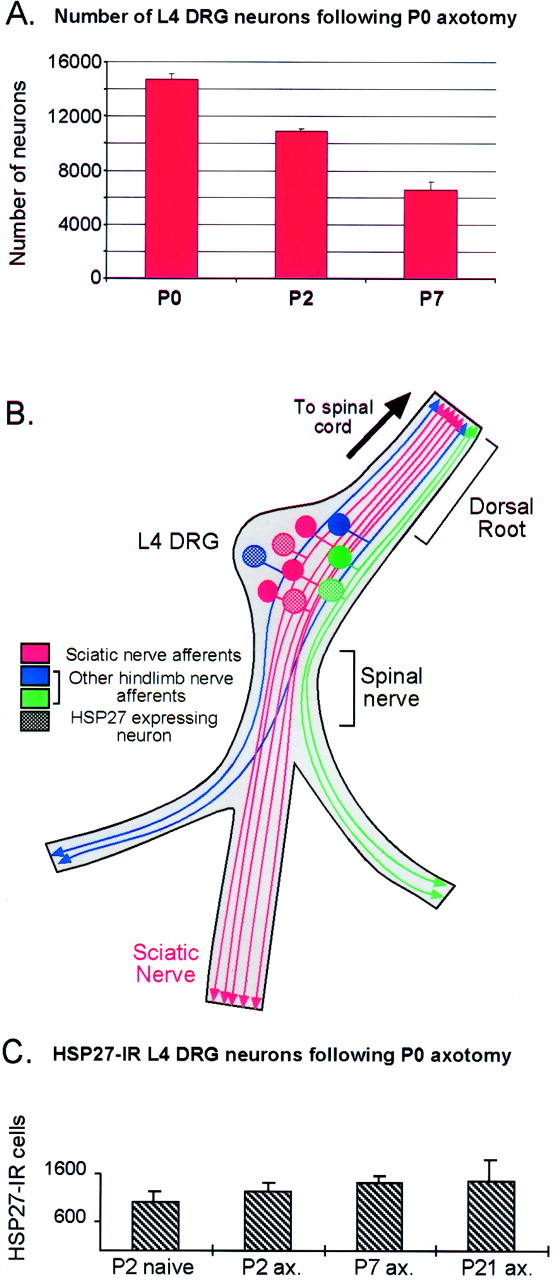
Most injured DRG neurons die after P0 axotomy, but the number of HSP27-immunoreactive neurons does not change.A, After nerve injury at P0, there is a dramatic loss of neurons in the L4 DRG by P7. C, However, the number of neurons expressing HSP27 does not decrease (n = 4–5 per group). B, Not all DRG neurons project into the sciatic nerve (schematic); therefore not all DRG neurons are axotomized by the sciatic nerve transection. ax., Axotomized.
Not all L4 DRG neurons project via the sciatic nerve; many contribute to more proximal nerves (Fig. 2B) and are uninjured after sciatic nerve section. The question then is how many axotomized neurons survive neonatal sciatic nerve section. Estimates of the percentage of L4 DRG neurons with sciatic projections vary between 50 and 70% (Himes and Tessler, 1989; Swett et al., 1991). These studies were done using fluorogold or HRP to label axons retrogradely in the sciatic nerve, and the variability may be caused in part by leakage of fluorogold into adjacent cells in the ganglion or lack of uptake of the dye by all axons in the nerve. Because c-jun has been shown to be upregulated by axotomized DRG neurons (Jenkins and Hunt, 1991; Jenkins et al., 1993), we counted the proportion of c-jun-positive neuronal profiles in adult L4 DRG 12 d after sciatic nerve section and found that 55.8 ± 3.8% express c-jun. This indicates that ∼55–60% of L4 DRG neurons are axotomized by sciatic transection, although this may be a slight underestimate because it has been suggested that a minority of injured sensory neurons do not upregulate c-jun (De Felipe and Belmonte, 1999). Assuming that only axotomized neurons die, this means that only 5–10% of the axotomized neurons survive 1 week after P0 sciatic nerve section (see Fig.2A). Despite this dramatic loss in injured neurons, there is no reduction in the total numbers of HSP27-positive cells in the L4 DRG at 2, 7, or 21 d after a P0 axotomy compared with that in the L4 DRG from a P2 naive rat pup (p values > 0.2; Fig. 2C). This suggests that cells expressing HSP27 preferentially survive axotomy. To confirm this we examined sections double-labeled for c-jun and HSP27 7 d after a P0 axotomy. At this time point, 100% of cells with clearly c-jun-positive nuclei also expressed HSP27 (Fig. 3). A few HSP27-positive cells were not c-jun positive; these are likely to reflect a small population of neurons without sciatic projections that constitutively express HSP27.
Fig. 3.
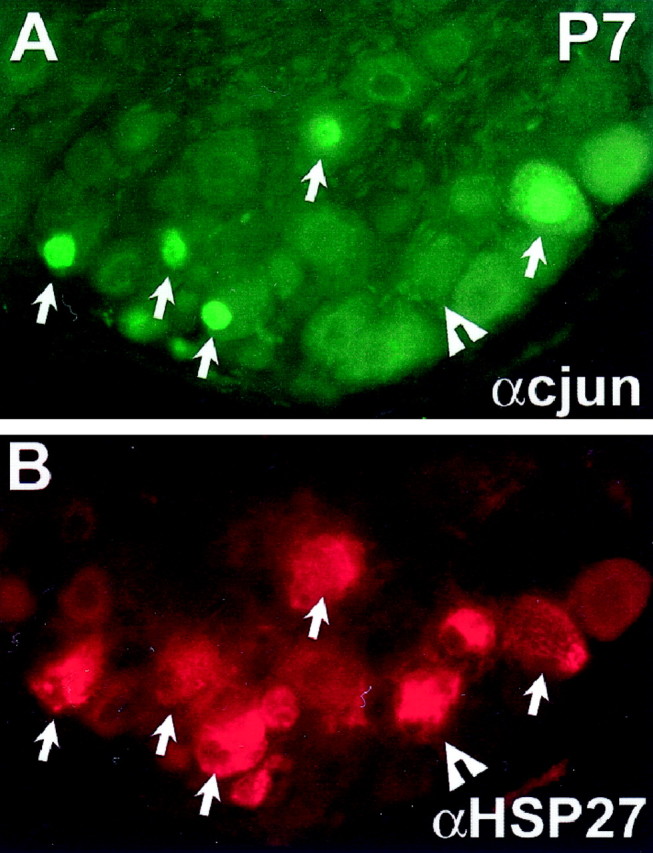
DRG neurons that survive P0 sciatic nerve section express HSP27. A, Seven days after P0 sciatic nerve section, axotomized neurons in the L4 DRG were identified by immunohistochemistry for c-jun. B, Double-labeling for HSP27 reveals that all DRG neurons with distinct nuclear c-jun labeling are also HSP27 immunoreactive.
To confirm these results with a second method, neurons projecting through the sciatic nerve were identified by retrograde labeling with fluorogold; 88 ± 0.9% of fluorogold-positive neuron profiles were HSP27 immunoreactive (data not shown). Although this is a lower proportion than that found with c-jun–HSP27 colocalization, collectively the data show that the vast majority of surviving axotomized neurons clearly express HSP27, in marked contrast to the naive state.
Most HSP27-expressing sensory neurons do not undergo DNA fragmentation after nerve injury
To exclude the possibility that all axotomized neonatal DRG neurons upregulate HSP27 but subsequently die, we double-labeled DRG cells with TUNEL (a marker of cells with fragmented DNA) and HSP27, neurofilament (to identify neurons), or GFAP (to identify Schwann and satellite cells) immunolabeling. As reported previously (Whiteside et al., 1998), there is a dramatic increase in TUNEL-positive profiles 48 hr after P0 axotomy (Fig.4A). The vast majority of these TUNEL-positive cells are neurons, as identified by neurofilament immunoreactivity (Fig. 4B,C), whereas very few TUNEL-positive cells were immunoreactive for GFAP (data not shown). At this time point, 9.5 ± 3.26% of neurofilament-immunoreactive profiles are TUNEL positive, whereas only 1.79 ± 2.65% of HSP27-immunoreactive profiles are positive for TUNEL (p < 0.005) (Fig. 4A). These data are likely to underestimate the difference in the percentage of dying cells in these two populations, because all L4 DRG neurons are neurofilament immunoreactive but only ∼60% are axotomized by sciatic nerve section and 26% of these are already lost at this time point. These results strongly support the hypothesis that HSP27-expressing sensory neurons are preferentially spared after nerve injury.
Fig. 4.
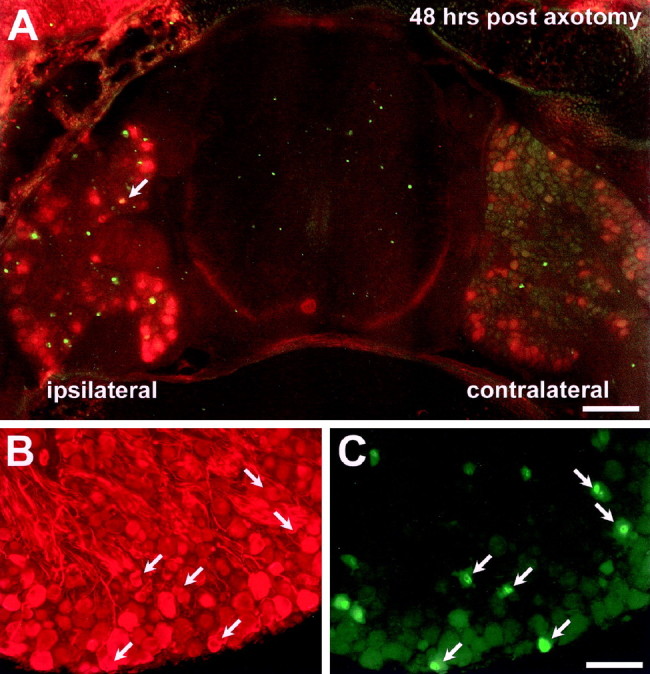
Most HSP27-immunoreactive neurons do not undergo apoptosis after P0 axotomy. A, A section of spinal cord with attached L4 DRG was double-labeled for HSP27 immunoreactivity (red/orange) and TUNEL (green) 48 hr after P0 sciatic nerve transection. The arrow indicates a single double-labeled cell that appears yellow. B, C, Sections of DRG were double-labeled for neurofilament (B) and TUNEL (C), demonstrating that most TUNEL-positive cells are also neurofilament immunoreactive (arrows).
Sensory neurons that express HSP27 preferentially survive NGF withdrawal in vitro
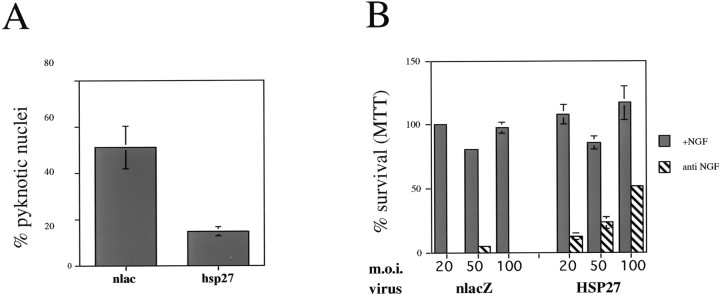
The survival of dissociated embryonic sensory neurons in vitro is dependent on the supply of exogenous neurotrophic factors, in particular NGF. At P0 only a minority of DRG neurons survive in dissociated culture without added growth factors (Eichler and Rich, 1989). HSP27 expression was examined in dissociated P0 neurons cultured in the presence or absence of NGF. After 48 hr of culture without NGF, we find that >60% of neurons are lost. The majority (80%) of the remaining neurons do not express HSP27, and 66.5 ± 2.3% of these HSP27-negative neurons have pyknotic nuclear profiles (Fig. 5a–c). In contrast, 20% of surviving neurons do exhibit HSP27 immunoreactivity, but only 8.7 ± 1.6% of these have pyknotic nuclei (p < 0.001; Fig. 5e). This demonstrates a strong correlation between HSP27 expression and neuronal survival in vitro after NGF withdrawal. However, HSP27 expression is not necessary for survival in the presence of NGF; the majority of sensory neurons cultured for 48 hr with NGF do not express HSP27 yet survive and extend long neurites (Fig. 5d).
Fig. 5.
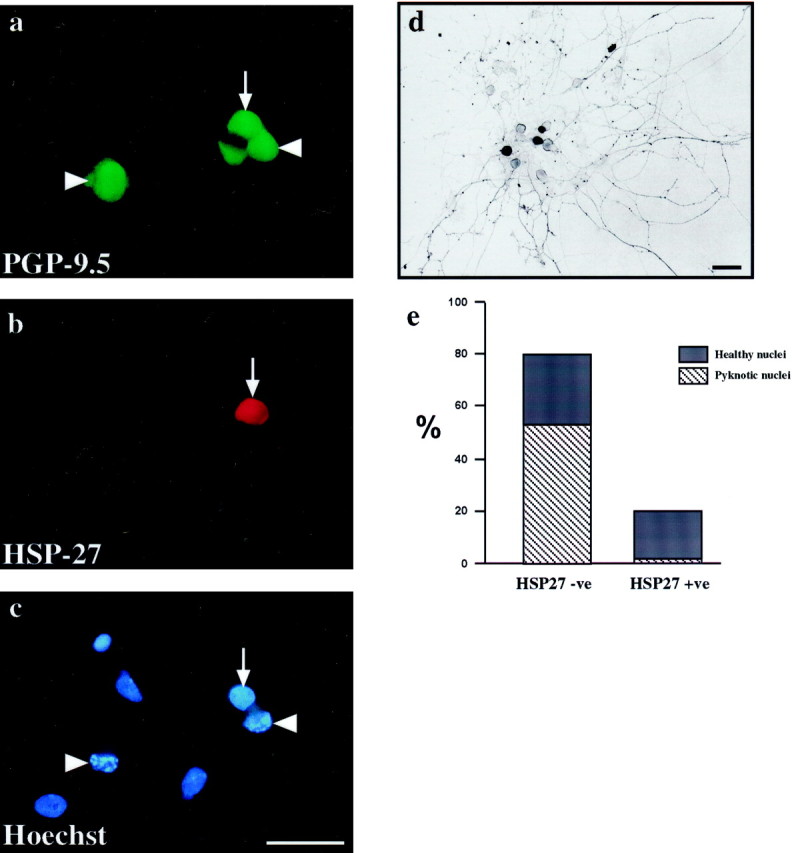
HSP27 expression correlates with survival after NGF withdrawal in vitro. Dissociated cultures from P0 lumbar DRG were cultured for 48 hr in the presence (d) or absence (a–c) of NGF.a–c, The same field is shown, immunostained for PGP9.5 to identify neurons (a), HSP27 (b), or Hoechst 33342 to visualize nuclear morphology (c). After 48 hr without NGF, neurons that expressed HSP27 had round healthy nuclei (arrows ina–c), whereas the majority of those that were negative for HSP27 immunoreactivity had pyknotic nuclei (arrowheads in a–c). e, The percentage of HSP27-immunopositive neurons with pyknotic nuclei was counted, and the same was done for HSP27-immunonegative neurons. Data shown are means ± SD from four independent experiments; a total of 5533 neurons were counted. d, In the presence of NGF, HSP27 was not necessary for either survival or neurite outgrowth (darkcells are HSP27 positive). Scale bars, 50 μm.
Expression of human HSP27 in neonatal rat sensory neurons significantly increases survival after NGF withdrawal
To determine whether the correlation between HSP27 expression and neuronal survival reflects an anti-apoptotic action of the protein, we tested whether HSP27 expression could rescue dissociated P0 DRG neurons from NGF withdrawal. DRG cultures were infected with adenovirus expressing human HSP27 or control adenovirus expressing β-galactosidase and subsequently were cultured in the presence or absence of NGF. Infection with the control β-galactosidase adenovirus had no effect on the percentage of neurons with pyknotic nuclei in the presence or absence of NGF (data not shown). Two days after NGF withdrawal, 25 ± 3.5% of neurons infected with β-galactosidase control have pyknotic nuclei, whereas only 8.9 ± 0.75% of neurons expressing human HSP27 have pyknotic nuclei (p < 0.005; Fig.6a–e). To assess whether this decrease in pyknotic profiles was indicative of increased survival, total numbers of surviving neurons in a 50 mm2 section of each well were counted at 24, 48, and 72 hr after NGF withdrawal. Because 75–90% of neurons were routinely infected under the conditions used, we were able to use total counts without immunostaining for HSP27 or β-galactosidase. We found no significant difference between lacZ-infected and uninfected cultures at any time point in the presence or absence of NGF (data not shown). Comparison of the survival of lacZ-infected versus HSP27-infected neurons revealed significantly greater survival of HSP27-infected neurons at all time points examined (Fig.6f).
Fig. 6.
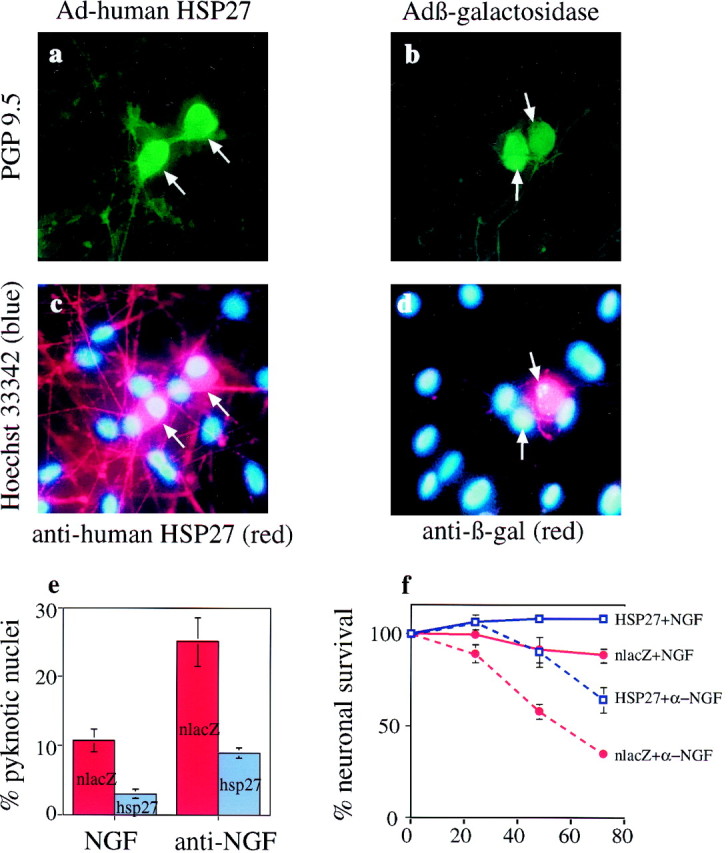
Expression of HSP27 in dissociated neonatal DRG neurons reduces nuclear pyknosis and increases survival after NGF withdrawal. a, b, P0 DRG cultures were infected with adenovirus (Ad) expressing human HSP27 (a) or β-galactosidase as a control (b). Forty hours later, NGF was withdrawn by refeeding with medium containing anti-NGF (1:250). Cultures were fixed 48 hr later and double immunostained with the neuron-specific antibody PGP9.5 and either anti-human HSP27 (a) or anti-β-galactosidase (-β-gal; b). The antibody to human HSP27 does not recognize rat HSP27, so it labels only infected cells. c, d, Nuclear morphology was visualized with Hoechst 33342. Arrows in a–dindicate neurons. e, The percentages of nlacZ- or HSP27-immunoreactive cells with pyknotic nuclei in the presence and absence of NGF were determined. Data shown are the means ± SEM of triplicates from a single experiment (200 cells were counted for each replicate). The experiment was repeated four times with similar results. f, To assess neuronal survival, total surviving neurons were counted in a 50 mm2 area of each well at the indicated times (in hours) after NGF withdrawal; typically, this area contained 150–200 neurons at the time of NGF withdrawal. Data shown are the means ± SEM of triplicates from a single experiment. The experiment was repeated three times with similar results.
Expression of HSP27 rescues neonatal sympathetic neurons from NGF withdrawal in vitro
To establish whether HSP27 can rescue other types of peripheral neurons from apoptosis, we infected dissociated cultures of neonatal rat SCG with the adenoviruses expressing human HSP27 or β-galactosidase and evaluated neuronal survival after NGF withdrawal. The control β-galactosidase adenovirus had no effect on cell survival at the m.o.i. values used in these experiments (data not shown). However, 24 hr after NGF withdrawal, human HSP27-expressing neurons had less than one-half as many pyknotic nuclei as did β-galactosidase-expressing controls (Fig.7A). As a measure of neuronal viability, mitochondrial function was assessed 48 hr after NGF withdrawal using the MTT metabolic assay, revealing that HSP27 expression rescues significant numbers of SCG neurons from NGF withdrawal (Fig. 7B). These results show that the survival-promoting effects of HSP27 are not specific to primary sensory neurons.
Fig. 7.
Expression of HSP27 in dissociated cultures of SCG neurons reduces nuclear pyknosis and increases survival after NGF withdrawal. Neuronal cultures of neonatal SCG were infected with adenovirus expressing human HSP27 or β-galactosidase as a control. Forty-eight hours later, NGF was withdrawn. A, After 24 hr, cells were fixed and immunostained for human HSP27 or β-galactosidase, and nuclear morphology was visualized with Hoechst 33342. HSP27-expressing neurons had far fewer pyknotic nuclei than did β-galactosidase-expressing control neurons. Data shown are means ± SEM of triplicates from a single experiment that was repeated twice with similar results. B, To assess neuronal survival, MTT assays were done 48 hr after NGF withdrawal from cultures infected with either human HSP27- or β-galactosidase-expressing adenoviruses at various m.o.i. values. Data shown are the means ± SEM of triplicates from a single experiment. Similar experiments were done three times with similar results.
DISCUSSION
Mature sensory neurons survive axotomy (Lekan et al., 1997) and NGF withdrawal (Lindsay, 1988), but most neonatal sensory neurons do not. One possible explanation for this is that injured adult DRG neurons themselves become the source of the survival-promoting growth factors normally supplied by the target tissue (Acheson et al., 1995). Alternatives are that adult sensory neurons decrease levels of molecules necessary for induction of cell death or induce or activate intrinsic survival factors. We have now shown that the regulation of the small heat shock protein HSP27 correlates strongly with sensory neuronal survival and that overexpression of human HSP27 in neonatal rat sensory neurons is sufficient to reduce apoptosis after NGF withdrawal in vitro. These data, together with the upregulation of HSP27 in primary sensory neurons after axotomy in the adult, suggest that HSP27 is an intrinsic survival factor that acts to reduce cell death in sensory neurons. The observation that HSP27 overexpression can also rescue sympathetic neurons from NGF withdrawalin vitro suggests that this role for HSP27 is not limited to sensory neurons.
HSP27 as a survival factor for sensory neurons
HSP27 is expressed constitutively by 5–10% of DRG neurons at birth, and we find that a similar percentage preferentially survive both axotomy in vivo and NGF withdrawal in vitro. The fact that 88–100% of this small population of surviving, injured sciatic neurons express HSP27 1 week after a P0 axotomy, as opposed to only 5–10% in naive neonatal animals, suggests the selective survival of cells that express HSP27. Although the most parsimonious explanation for our results is that the cells that express HSP27 constitutively at birth are the same cells that upregulate its expression after axotomy and survive, this cannot be demonstrated directly. Two possible alternative explanations for our results are that a novel population of neurons upregulates HSP27, whereas the original population downregulates it or dies by P2, or that all injured DRG neurons upregulate HSP27 after P0 axotomy, but the majority die anyway. If either of these were the case, many TUNEL-labeled neurons at 48 or 72 hr after P0 axotomy should be HSP27 immunoreactive. Instead, we find that relatively few TUNEL-positive neurons express HSP27. In fact, our data support the hypothesis that HSP27-expressing neurons have a survival advantage after injury; <2% of HSP27-immunoreactive profiles are TUNEL positive 2 d after axotomy, whereas 9.5% of all neuronal profiles are TUNEL positive. The correlation between survival after axotomy and HSP27 expression also holds for adult animals; all axotomized adult DRG neurons upregulate HSP27 rapidly after injury, and all survive (Costigan et al., 1998).
Although we present strong evidence arguing that HSP27 can function as an intrinsic survival factor for sensory neurons, a small proportion of HSP27-expressing neurons die in all of the paradigms we examined. This suggests that HSP27 alone may not be sufficient for survival but requires the expression or activation of other factors not uniformly present in the mixed population of sensory neurons in the DRG.
Potential mechanisms for HSP27 survival activity
HSP27 stabilizes actin microfilaments (Lavoie et al., 1995), increases glutathione levels, and reduces generation of reactive oxygen species (Mehlen et al., 1996). It is also a molecular chaperone, enhancing protein refolding and associating with cytoplasmic protease complexes (Ciocca et al., 1993). Any of these roles may contribute to the rescue of neonatal sensory neurons from apoptosis. Actin proteolysis and depolymerization appear to play a role in neuronal apoptosis, including that induced by NGF withdrawal in embryonic chick sensory neurons (Villa et al., 1998). Inhibition of actin proteolysis by calpain inhibitors blocks nuclear fragmentation and increases survival of trophic factor-deprived ciliary neurons (Villa et al., 1998). As an actin-binding protein and molecular chaperone, HSP27 might act by protecting microfilaments from degradation and depolymerization. Because trophic factor withdrawal in pheochromocytoma 12 cells, sympathetic neurons, and embryonic motor neurons may also involve the generation of reactive oxygen species (Greenlund et al., 1995; Schulz et al., 1997; Estevez et al., 1998), the ability of HSP27 to elevate glutathione and reduce production of reactive oxygen species could also be important. Alternatively, the anti-apoptotic role of HSP27 may be the result of a novel function of this protein.
The function of HSP27 is apparently regulated by phosphorylation by MAP kinase activated protein kinase 2 (Ciocca et al., 1993). However, phosphorylation appears to have opposing effects on different actions of HSP27. Phosphorylation of HSP27 increases its ability to stabilize actin filaments and rescue cells from heat shock and other toxic stimuli (Lavoie et al., 1995; Huot et al., 1996; Guay et al., 1997), but it is the unphosphorylated form of HSP27 that appears to be important for rescuing NIH3T3 cells from apoptosis induced by tumor necrosis factor α (Mehlen et al., 1997). We are currently exploring the effects of HSP27 phosphorylation on its ability to promote survival of sensory neurons.
In conclusion, our data suggest that the expression of HSP27 confers a survival advantage to neonatal sensory neurons after injury or NGF deprivation. Similarly, the induction of HSP27 expression in all axotomized DRG neurons in the adult may contribute to their ability to survive axotomy in vivo and NGF withdrawal in vitro. Future characterization of the mechanisms by which HSP27 contributes to neuronal survival as well as the signals responsible for its regulation may identify new strategies for preventing neuronal death.
Footnotes
The work was funded by the Medical Research Council Grant G9431792 (C.J.W.) and National Institutes of Health Grant NS38253-01 (C.J.W.). We thank Jacqueta Meredith-Middleton for technical assistance and Drs. Anthony Rosenszweig and Ling Li for advice on adenoviral gene transfer.
Correspondence should be addressed to Dr. C. J. Woolf, Neural Plasticity Research Group, Department of Anesthesia and Critical Care, Massachusetts General Hospital East, 149 13th Street, Charlestown, MA 02129.
Drs. Lewis, Mannion, and White contributed equally to this work.
REFERENCES
- 1.Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 2.Ciocca DR, Oesterreich S, Chamness GC, McGuire WL, Fuqua SA. Biological and clinical implications of heat shock protein 27,000 (Hsp27): a review. J Natl Cancer Inst. 1993;85:1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- 3.Costigan M, Mannion RJ, Kendall G, Lewis SE, Campagna JA, Coggeshall RE, Meredith-Middleton J, Tate S, Woolf CJ. Heat shock protein 27: developmental regulation and expression after peripheral nerve injury. J Neurosci. 1998;18:5891–5900. doi: 10.1523/JNEUROSCI.18-15-05891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Felipe C, Belmonte C. c-Jun expression after axotomy of corneal trigeminal ganglion neurons is dependent on the site of injury. Eur J Neurosci. 1999;11:899–906. doi: 10.1046/j.1460-9568.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- 5.Eichler ME, Rich KM. Death of sensory ganglion neurons after acute withdrawal of nerve growth factor in dissociated cell cultures. Brain Res. 1989;482:340–346. doi: 10.1016/0006-8993(89)91197-9. [DOI] [PubMed] [Google Scholar]
- 6.Estevez AG, Spear N, Manuel SM, Radi R, Henderson CE, Barbeito L, Beckman JS. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998;18:923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillardon F, Wickert H, Zimmerman M. Differential expression of bcl-2 and bax mRNA in axotomized dorsal root ganglia of young and adult rats. Eur J Neurosci. 1994;6:1641–1644. doi: 10.1111/j.1460-9568.1994.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 8.Graham FL, Prevec L. Methods for construction of adenovirus vectors. Mol Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 9.Greenlund LJ, Korsmeyer SJ, Johnson EM., Jr Role of BCL-2 in the survival and function of developing and mature sympathetic neurons. Neuron. 1995;15:649–661. doi: 10.1016/0896-6273(95)90153-1. [DOI] [PubMed] [Google Scholar]
- 10.Griffin DE, Levine B, Tyor WR, Tucker PC, Hardwick JM. Age-dependent susceptibility to fatal encephalitis: alpha virus infection of neurons. Arch Virol Suppl. 1997;9:31–39. doi: 10.1007/978-3-7091-9326-6_4. [DOI] [PubMed] [Google Scholar]
- 11.Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 12.Himes BT, Tessler A. Death of some DRG neurons and plasticity of others following sciatic nerve section in adult and neonatal rats. J Comp Neurol. 1989;284:215–230. doi: 10.1002/cne.902840206. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins DA, Plumier JC, Currie RW. Induction of the 27-kDa heat shock protein (Hsp27) in the rat medulla oblongata after vagus nerve injury. Exp Neurol. 1998;153:173–183. doi: 10.1006/exnr.1998.6870. [DOI] [PubMed] [Google Scholar]
- 14.Huot J, Roy G, Lambert H, Chretien P, Landry J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991;51:5245–5252. [PubMed] [Google Scholar]
- 15.Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- 16.Jenkins R, Hunt SP. Long-term increase in the levels of c-jun mRNA and jun protein-like immunoreactivity in motor and sensory neurons following axon damage. Neurosci Lett. 1991;129:107–110. doi: 10.1016/0304-3940(91)90731-8. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins R, McMahon SB, Bond AB, Hunt SP. Expression of c-Jun as a response to dorsal root and peripheral nerve section in damaged and adjacent intact primary sensory neurons in the rat. Eur J Neurosci. 1993;5:751–759. doi: 10.1111/j.1460-9568.1993.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson EM, Jr, Gorin PD, Brandeis LD, Pearson J. Dorsal root ganglion neurones are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980;210:916–918. doi: 10.1126/science.7192014. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- 20.Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lekan HA, Chung K, Yoon YW, Chung JM, Coggeshall RE. Loss of dorsal root ganglion cells concomitant with dorsal root axon sprouting following segmental nerve lesions. Neuroscience. 1997;81:527–534. doi: 10.1016/s0306-4522(97)00173-5. [DOI] [PubMed] [Google Scholar]
- 22.Lewis SE, Rao MS, Symes AJ, Dauer WT, Fink JS, Landis SC, Hyman SE. Coordinate regulation of choline acetyltransferase, tyrosine hydroxylase, and neuropeptide mRNAs by ciliary neurotrophic factor and leukemia inhibitory factor in cultured sympathetic neurons. J Neurochem. 1994;63:429–438. doi: 10.1046/j.1471-4159.1994.63020429.x. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurones. J Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin JL, Mestril R, Hilal-Dandan R, Brunton LL, Dillmann WH. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997;96:4343–4348. doi: 10.1161/01.cir.96.12.4343. [DOI] [PubMed] [Google Scholar]
- 25.Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo AP. Constitutive expression of human HSP27, Drosophila HSP27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:363–374. [PubMed] [Google Scholar]
- 26.Mehlen P, Kretz-Remy C, Preville X, Arrigo A-P. Human hsp27, Drosophila hsp27 and human αβ-crystallin expression-mediated increase in glutathaione is essential for the protective activity of these proteins against TNFα-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 27.Mehlen P, Hickey E, Weber LA, Arrigo A-P. Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFα in NIH-3T3-ras cells. Biochem Biophys Res Commun. 1997;241:187–192. doi: 10.1006/bbrc.1997.7635. [DOI] [PubMed] [Google Scholar]
- 28.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 29.Plumier J-CL, Hopkins DA, Robertson HA, Currie RW. Constitutive expression of the 27-kDa heat shock protein (Hsp27) in sensory and motor neurons of the rat nervous system. J Comp Neurol. 1997;384:409–428. [PubMed] [Google Scholar]
- 30.Pover CM, Orr MH, Coggeshall RE. A method for producing unbiased histograms of neuronal profile sizes. J Neurosci Methods. 1993;49:123–131. doi: 10.1016/0165-0270(93)90116-9. [DOI] [PubMed] [Google Scholar]
- 31.Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 32.Schulz JB, Bremen D, Reed JC, Lommatzsch J, Takayama S, Wullner U, Loschmann PA, Klockgether T, Weller M. Cooperative interception of neuronal apoptosis by BCL-2 and BAG-1 expression: prevention of caspase activation and reduced production of reactive oxygen species. J Neurochem. 1997;69:2075–2086. doi: 10.1046/j.1471-4159.1997.69052075.x. [DOI] [PubMed] [Google Scholar]
- 33.Swett JE, Torigoe Y, Elie VR, Bourassa CM, Miller PG. Sensory neurons of the rat sciatic nerve. Exp Neurol. 1991;114:82–103. doi: 10.1016/0014-4886(91)90087-s. [DOI] [PubMed] [Google Scholar]
- 34.Swett JE, Hong CZ, Miller PG. Most dorsal root ganglion neurons of the adult rat survive nerve crush injury. Somatosens Mot Res. 1995;12:177–189. doi: 10.3109/08990229509093656. [DOI] [PubMed] [Google Scholar]
- 35.Tong JX, Vogelbaum MA, Rich KM. Radiation-induced apoptosis in dorsal root ganglion neurons. J Neurocytol. 1997;26:771–777. doi: 10.1023/a:1018566431912. [DOI] [PubMed] [Google Scholar]
- 36.Villa PG, Henzel WJ, Sensenbrenner M, Henderson CE, Pettman B. Calpain inhibitors, but not caspase inhibitors, prevent actin proteolysis and DNA fragmentation during apoptosis. J Cell Sci. 1998;111:713–722. doi: 10.1242/jcs.111.6.713. [DOI] [PubMed] [Google Scholar]
- 37.Vogelbaum MA, Tong JX, Rich KM. Developmental regulation of apoptosis in dorsal root ganglion neurons. J Neurosci. 1998;18:8928–8935. doi: 10.1523/JNEUROSCI.18-21-08928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagstaff MJ, Collaco-Moraes Y, Smith J, de Belleroche JS, Coffin RS, Latchman DS. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem. 1999;274:5061–5069. doi: 10.1074/jbc.274.8.5061. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside G, Doyle CA, Hunt SP, Munro FE. Differential time course of neuronal and glial apoptosis in neonatal rat dorsal root ganglia after sciatic nerve axotomy. Eur J Neurosci. 1998;10:3400–3408. doi: 10.1046/j.1460-9568.1998.00346.x. [DOI] [PubMed] [Google Scholar]
- 40.Winter J, Woolf CJ, Lynn B. Degenerative and regenerative responses of sensory neurones to capsaicin-induced damage. In: Wood J, editor. Capsaicin in the study of pain. Academic; London: 1993. pp. 139–160. [Google Scholar]
- 41.Wu W, Welsh MJ. Expression of the 25-kDa heat-shock protein (HSP27) correlates with resistance to the toxicity of cadmium chloride, mercuric chloride, cis-platinum(II)-diammine dichloride, or sodium arsenite in mouse embryonic stem cells transfected with sense or antisense HSP27 cDNA. Toxicol Appl Pharmacol. 1996;141:330–339. doi: 10.1006/taap.1996.0290. [DOI] [PubMed] [Google Scholar]