Abstract
The thalamus and neocortex are two highly organized and complex brain structures that work in concert with each other. The largest synaptic input to the thalamus arrives from the neocortex via corticothalamic fibers. Using brain slices, we describe long-term potentiation (LTP) in corticothalamic fibers contacting the ventrobasal thalamus. Corticothalamic LTP is input-specific, NMDA receptor-independent, and reversible. The induction of corticothalamic LTP is entirely presynaptic and Ca2+-dependent. The expression of corticothalamic LTP is associated with a decrease in paired-pulse facilitation (PPF) and blocked by an inhibitor of the cAMP-dependent protein kinase A (PKA). Consistent with an involvement of cAMP and PKA, activation of adenylyl cyclase induced a synaptic enhancement that was associated with a decrease in PPF and occluded LTP. Corticothalamic LTP may serve to enhance the efficacy of cortico-cortical communication via the thalamus and/or to mediate experience-dependent long-term modifications of thalamocortical receptive fields.
Keywords: thalamus, neocortex, synaptic plasticity, cAMP, protein kinase A, long-term potentiation, long-term depression
Almost all of the information that reaches the neocortex arrives from the thalamus via thalamocortical fibers. In return, the thalamus receives a massive feedback from the neocortex via corticothalamic fibers. The number of corticothalamic fibers is one order of magnitude larger than the number of thalamocortical axons (Sherman and Guillery, 1996), and cortical afferents are the most abundant input to the thalamus (Guillery, 1969). In the somatosensory system, the most corticothalamic fibers originate in layer VI and send reciprocal connections to the ventrobasal thalamus (ventroposteromedial and lateral nuclei), leaving a fiber collateral in the reticular nucleus (Zhang and Deschenes, 1997). Corticothalamic fibers from layer VI act on distal dendrites of thalamic neurons, forming a fine plexus with simple branching and fine synaptic boutons (Robson, 1983). In addition, there is a group of corticothalamic fibers that originates in layer V and reaches the posterior nucleus of the thalamus, without innervating the ventrobasal thalamus or reticular nucleus (Hoogland, 1991; Ojima, 1994; Bourassa et al., 1995; Levesque et al., 1996; Vidnyansky et al., 1996). Corticothalamic synapses use glutamate as their neurotransmitter, which activates both non-NMDA and NMDA ionotropic glutamate receptors (Deschenes and Hu, 1990; Scharfman et al., 1990; Eaton and Salt, 1996; Kao and Coulter, 1997; Steriade et al., 1997; Turner and Salt, 1998). Metabotropic glutamate receptor-mediated responses have been described at these synapses in some studies (McCormick and von Krosigk, 1992; Eaton and Salt, 1996;Golshani et al., 1998), but not in others (Kao and Coulter, 1997;Turner and Salt, 1998). Repetitive stimulation of corticothalamic fibers produces short-term facilitation of EPSPs in thalamic neurons (Frigyesi, 1972; Steriade and Wyzinski, 1972; Tsumoto et al., 1978; Deschenes and Hu, 1990; Lindstrom and Wrobel, 1990; Scharfman et al., 1990; McCormick and von Krosigk, 1992; Steriade and Timofeev, 1997).
The functional role of corticothalamic pathways is unknown. They may play a role in modifying the size, strength, and selectivity of thalamocortical receptive fields (Yuan et al., 1985; Diamond, 1995;Weinberger, 1995; Ergenzinger et al., 1998) and/or serve to establish cortico-cortical communication via the thalamus (Guillery, 1995). In all these instances, a mechanism for bi-directional activity-dependent long-term synaptic plasticity in corticothalamic synapses would be advantageous. It would provide the capacity to modify the effectiveness with which the neocortex can influence the thalamus. The most characteristic forms of long-term synaptic plasticity are long-term potentiation (LTP) and long-term depression (LTD). Both LTP and LTD have been studied in numerous synapses (Bliss and Collingridge, 1993;Bear and Malenka, 1994; Nicoll and Malenka, 1995), but they have yet to be described in the thalamus. We therefore performed a series of experiments to explore long-term synaptic plasticity in corticothalamic fibers.
MATERIALS AND METHODS
Thalamocortical slices were prepared from adult (≥7 weeks) BALB/C mice according to the methods described by Agmon and Connors (1991). Slices were cut in ice-cold buffer using a vibratome and kept in a holding chamber for a least 1 hr. Experiments were performed in an interface chamber at 32°C. The slices were perfused constantly (1–1.5 ml/min) with artificial CSF (ACSF) containing (in mm): NaCl 126, KCl 3, NaH2Po4 1.25, NaHCO3 26, MgSO4 7H2O 1.3, dextrose 10, and CaCL22H2O 2.5. The ACSF was bubbled with 95% O2 and 5% CO2. Synaptic responses were induced using a concentric stimulating electrode (Frederick Haer Co.) placed in the thalamic radiation. The stimulus consisted of a 200 μsec pulse of <50 μA. The ventrobasal thalamus was easily and clearly identifiable with a dissecting microscope. Field recordings were made using a low-impedance pipette (∼0.5 MΩ) filled with ACSF or with ACSF containing 400 μm bicuculline methbromide (BMI). Intracellular recordings were performed using sharp electrodes (80–120 MΩ) filled with 3 mpotassium-acetate. The test stimulus was delivered at 0.05 Hz and was either single or a pair with a 50 msec interstimulus interval to evaluate paired-pulse facilitation (PPF). The data are expressed as mean ± SEM as a percentage of the baseline amplitude or slope. For single experiments, which represent typical examples, every response at 0.05 Hz is displayed.
The following drugs were stored as concentrated stock solutions and were diluted to the desired concentration using ACSF: 50 mmforskolin and 5 mm 1,9-dideoxyforskolin (dissolved in DMSO; Research Biochemicals, Natick, MA), 50 mm Rp-cAMPs (dissolved in water; Biomol, Plymouth Meeting, PA), 20 mm6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX) (dissolved in water; Research Biochemicals), 50 mm±-2-amino-5-phosphonovaleric acid (APV) (dissolved in water; Research Biochemicals), 40 mm BMI (dissolved in water; Sigma, St. Louis, MO), 50 mm cyclothiazide (dissolved in DMSO; Research Biochemicals), and α-methyl-4-carboxyphenylglycine (MCPG) (dissolved in the ACSF to 1 mm; research Biochemicals). All drugs were tested using bath application, although some drugs were also tested with local application. BMI was applied in either the bath (40 μm) or the extracellular recording pipette by filling the recording pipette with ACSF containing BMI (400 μm). CNQX and APV were applied in the bath (10–20 and 50–100 μm, respectively) or by using a low-impedance (∼0.5 MΩ) pipette filled with ACSF containing these drugs (100 and 250 μm, respectively). The drug-containing pipette was placed adjacent (∼500 μm) to the recording electrode, and the action of the drugs was monitored from the recording electrode.
RESULTS
LTP in corticothalamic fibers
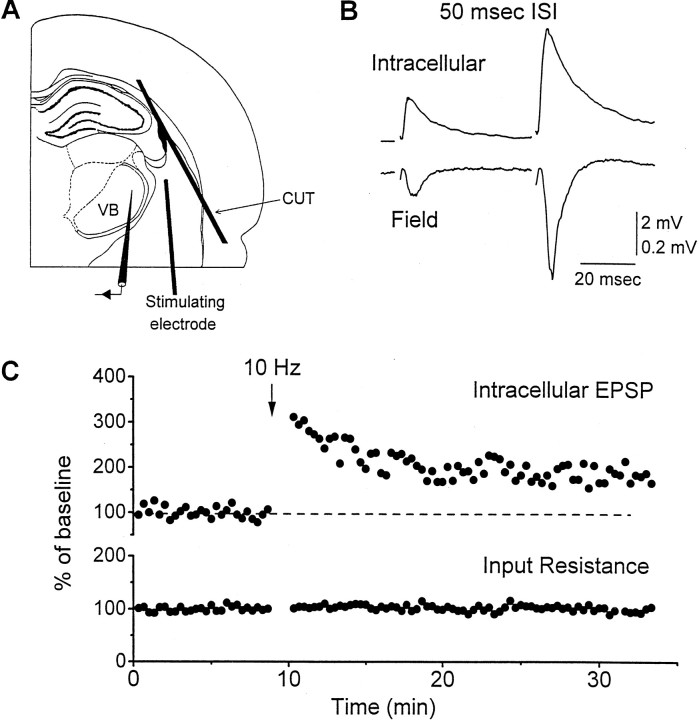
Field and intracellular potentials were recorded from neurons of the ventrobasal thalamus in brain slices of adult mice (Fig.1A). Orthodromic stimuli applied to the thalamic radiation evoked a negative potential in field recordings from populations of neurons and an EPSP in intracellular recordings from individual neurons of the ventrobasal thalamus (Fig. 1B). The field and intracellular EPSPs reflected a monosynaptic excitatory connection between corticothalamic fibers and neurons in the ventrobasal thalamus. This assertion is based on the following arguments. (1) The ventrobasal thalamus does not contain GABAergic interneurons, but it receives GABAergic input from the reticular nucleus (nRT), which is activated by corticothalamic fibers. To avoid the contribution of disynaptic IPSPs on corticothalamic responses, GABAA receptors were blocked locally by including BMI (400 μm) in the low-impedance (∼0.5 MΩ) extracellular recording electrode or in the bath (40 μm). (2) Stimulation of the thalamic radiation may activate thalamocortical fibers and thus the neocortex. To ensure that activity in cortical circuits did not feed back to the thalamus, we severed all connections between thalamus and neocortex with a cut just below the cortical white matter (Fig.1A). (3) Stimulation of the thalamic radiation could antidromically discharge neurons in the ventrobasal thalamus by directly activating their axons. However, antidromic activation cannot contribute to the recorded EPSPs because of the lack of recurrent connections between thalamic ventrobasal neurons. (4) Application of AMPA and NMDA receptor antagonists abolishes the field potential (see Fig. 5A). A short-latency (1–2 msec from the stimulus artifact) negativity resistant to glutamate receptor antagonists was observed in many extracellular recordings (see Fig. 5), and it was abolished by TTX (1 μm; data not shown). This short-latency non-synaptic component, the fiber volley, was monitored in many experiments as an index of fiber excitability. The field potential was also dependent on extracellular Ca+2 (see Fig. 5). (5) Consistent with a monosynaptic connection, both the intracellular and extracellular EPSPs have a constant and short latency of ∼3 msec, and both follow high-frequency (50 Hz) stimulation without failure. As for other monosynaptic excitatory connections, paired-pulse stimulation produces strong facilitation in both the field and intracellularly recorded EPSPs (Fig. 1B). Facilitation is a consistent finding among studies that test corticothalamic pathways (Frigyesi, 1972;Steriade and Wyzinski, 1972; Tsumoto et al., 1978; Deschenes and Hu, 1990; Lindstrom and Wrobel, 1990; Scharfman et al., 1990; McCormick and von Krosigk, 1992; Steriade and Timofeev, 1997). The only corticothalamic input to the ventrobasal thalamus arises from neocortical neurons in layer VI (Bourassa et al., 1995). Based on these arguments, we conclude that the synaptic responses we recorded in response to stimulation of the thalamic radiation result from the depolarization of ventrobasal neurons caused by the release of glutamate from corticothalamic fibers originating in layer VI neurons.
Fig. 1.
Intracellular and field corticothalamic EPSPs and LTP. A, Diagram of the corticothalamic slice preparation. The typical positions of the stimulating and recording electrodes are shown. The stimulating electrode is placed in the thalamic radiation. The recording electrode is placed in the ventrobasal thalamus (VB). To ensure that activity in neocortical circuits did not feed back to the thalamus, all connections between thalamus and neocortex were severed with a cut below the cortical white matter. B, Paired-pulse facilitation of corticothalamic EPSPs. Intracellular and field EPSPs evoked in the ventrobasal thalamus in response to a pair of stimuli delivered in the thalamic radiation with a 50 msec interstimulus interval (ISI). C, LTP of corticothalamic EPSPs. Effect of 10 Hz stimulation (600 pulses in 6 trains of 100 pulses each delivered with a 10 sec interval between trains) on the amplitude of an intracellular EPSP recorded from the ventrobasal thalamus in response to stimulation of the thalamic radiation. The input resistance of the same neuron was also monitored with a 50 msec current pulse. Test stimuli were applied at 0.05 Hz, and all responses are displayed.
Fig. 5.
Corticothalamic LTP induction does not require postsynaptic activation but requires calcium. A, Field responses correspond to the experiment shown in C.B, Comparison of the effects of previous application of glutamate receptor antagonists (CNQX and APV) or 0 Ca2+ ACSF during which 10 Hz stimulation was applied (black) or was not applied (white). Shown are the amplitudes of the responses (mean ± SEM;n = 5–7) recorded 1 hr after recovery from complete field EPSP block (induced by CNQX plus APV or by 0 Ca2+ ACSF), represented as the percentage of the baseline amplitude (before field EPSP block). C, A typical experiment showing that, after a brief application of CNQX and APV, which completely blocks postsynaptic responses (compare1 and 2 in A), field EPSPs return to their baseline amplitudes. A subsequent application of CNQX and APV during which 10 Hz stimulation was applied produces LTP that is associated with a decrease in PPF. Subsequent application of CNQX and APV serves to demonstrate that the fiber volley did not change during LTP (compare 2 and 4 inA). D, Application of 10 Hz stimulation during complete block of synaptic transmission caused by 0 Ca2+ ACSF did not produce LTP. A subsequent application of 10 Hz stimulation in the presence of extracellular Ca+2 induces LTP. Data are presented as the percentage of the baseline field potential amplitude.
We first explored the competence of corticothalamic EPSPs to undergo LTP. To induce LTP, 600 pulses were delivered in six trains of 100 pulses each with an interval between trains of 10 sec. Trains consisted of 1 or 10 Hz. After 10 Hz stimulation, a lasting synaptic enhancement (LTP, >35 min) developed in both the field and the intracellular EPSP (Fig. 1C). Typically, 10 Hz stimulation produces a strong several-fold increase in the synaptic response, which decays within the next few minutes to a stable enhancement of ∼180% of the baseline. In a few experiments (n = 3), LTP was followed for 2 hr without decrement. LTP is demonstrated in both the field and intracellular EPSPs (Figs. 1C,2A). Intracellular recordings from ventrobasal thalamic neurons demonstrate that LTP is not associated with a change in input resistance (measured by applying a current pulse; 50 msec, 0.2 nA; n = 4) (Fig.1C). During intracellular recordings (n = 10), the amplitude of the evoked EPSP was set at an amplitude that did not reach threshold to trigger a low-threshold calcium spike, and none of the neurons included in the study were antidromically activated with the intensities used. Field recordings established that LTP is not associated with a change in fiber excitability, as determined by measuring the fiber volley (100 ± 3% of the baseline fiber volley amplitude measured 35 min after LTP induction; n= 4). After LTP has been induced, with 10 Hz stimulation, subsequent activation of the same corticothalamic fibers at 1 Hz (using the same number of stimuli) produces a lasting synaptic depression (LTD) or depotentiation that reverses LTP (Fig. 2A). For the remainder of the study, we investigated the properties of corticothalamic LTP induced by 10 Hz stimulation.
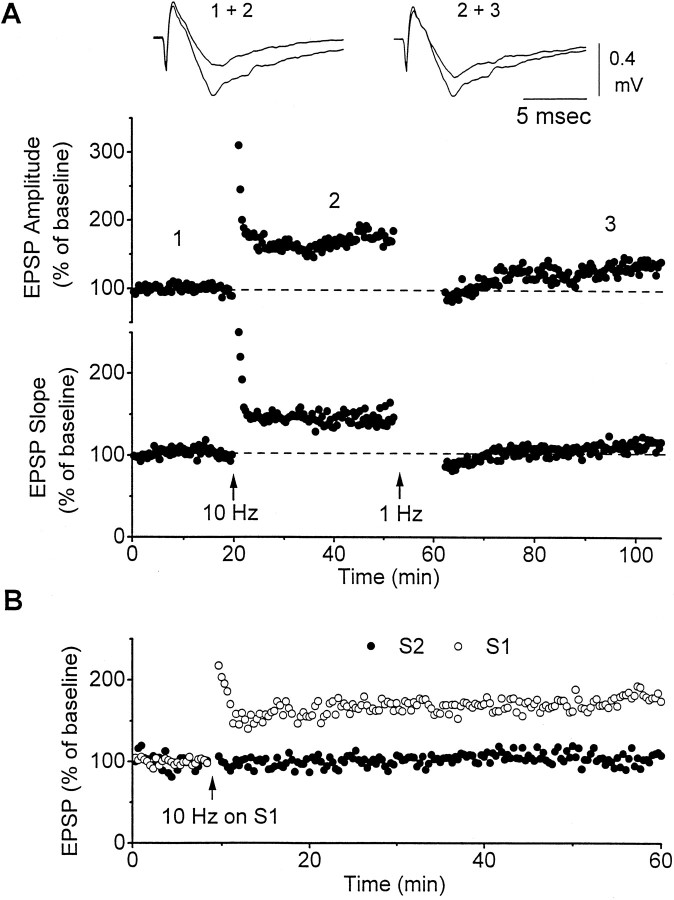
Fig. 2.
Corticothalamic LTP is reversible and input-specific. A, Stimulation (10 Hz; 600 pulses) produces LTP that is expressed as a change in the slope and amplitude of the field potential. Application of 1 Hz stimulation (600 pulses) 30 min after LTP induction reverses LTP. Thenumbers in the graph correspond to the field recordings shown above. B, Responses were evoked by two stimulating electrodes placed in the thalamic radiation and recorded from a single electrode in the ventrobasal thalamus. Stimulation (10 Hz) delivered to one of the electrodes (S1; open circles) produced LTP but had no effect on the responses evoked from the other stimulating electrode (S2; closed circles). The amplitude of the field potential was measured.
The next experiment sought to investigate whether LTP was input-specific. Two stimulating electrodes were placed in the thalamic radiation on two independent sets of fibers. A population of thalamic neurons was monitored using a single recording electrode. To ensure that the electrodes activated independent sets of fibers, we tested for paired-pulse interactions between them. When two stimuli are delivered with a 50 msec interval through the same electrode, they activate the same set of fibers and produce robust synaptic facilitation. In contrast, if the first stimulus is delivered through one electrode and the second stimulus (50 msec latter) is delivered through another electrode, facilitation to the second stimulus would only occur if both electrodes activate the same set of fibers. Thus, by ensuring that facilitation to the second stimulus delivered through the second electrode does not occur, we can assume that the two electrodes activate different fibers. After determining that the two electrodes activated independent sets of fibers, we applied 10 Hz stimulation to one of them. LTP developed only in the pathway in which the 10 Hz stimulation was applied (n = 5) (Fig.2B). This indicates that corticothalamic LTP is input-specific.
Placing BMI locally in the recording pipette (400 μm) or in the bath (40 μm) tested the effect of blocking GABAA receptors on LTP induction. In other areas, such as the hippocampus (Schaffer collaterals) and the neocortex, the induction of LTP is eased by GABAA receptor block (Wigstrom and Gustafsson, 1983; Castro-Alamancos et al., 1995). However, we found no significant effect of BMI on the induction of LTP in corticothalamic fibers (Fig.3B). The average enhancement in the presence of BMI was 183% of the baseline, which is not significantly different from the average synaptic enhancement observed without BMI (n = 5; 35 min after LTP induction;t test; NS) (Fig. 3A). Despite this finding, we used local BMI application for the remainder of the experiments to be certain that our manipulations were not mediated through changes in disynaptic GABAA IPSPs.
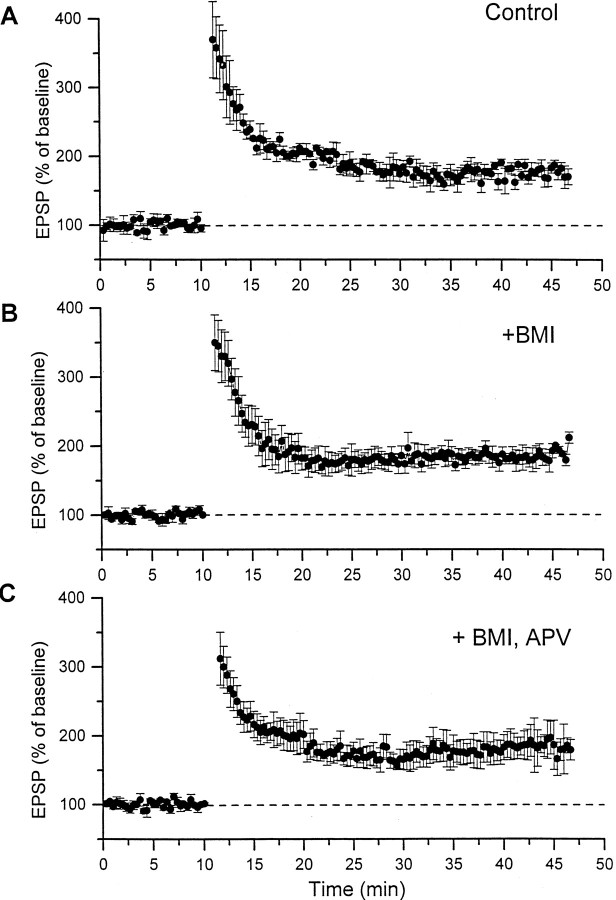
Fig. 3.
Corticothalamic LTP is not affected by NMDA receptor block or disinhibition. A, Summary of experiments (n = 5) showing the effects of 10 Hz stimulation on the amplitude of corticothalamic field potentials in normal ACSF (Control). B, Summary of experiments (n = 5) in which 10 Hz stimulation was applied in the presence of BMI (400 μm) in the field recording pipette. C, Summary of experiments (n = 5) in which 10 Hz stimulation was applied in the presence of APV (100 μm) in the bath. Data correspond to the amplitude of the field potential expressed as the mean ± SEM of every response at 0.05 Hz. Stimulation (10 Hz) was applied after a 10 min baseline.
We then examined whether the induction of LTP required the activation of NMDA receptors or metabotropic glutamate receptors. LTP induction was not significantly affected by bath application (100 μm) of the NMDA antagonist APV (Fig. 3C). Neither the initial large potentiation nor the persistent enhancement of the corticothalamic field potential were significantly affected in slices treated with APV compared with control slices (n= 5; 35 min after LTP induction; t test; NS). The effectiveness of APV was verified by demonstrating that Schaffer collateral LTP was blocked in the same slices (100 Hz tetanus for 1 sec repeated four times at 10 sec intervals). LTP induction was also not significantly affected by bath application (1 mm) of the metabotropic glutamate receptor antagonist MCPG (n = 4; 178 ± 9% of baseline responses; 35 min after LTP induction). Moreover, application of MCPG had no significant effect on the baseline field potential response (data not shown).
LTP was further characterized by testing its effects on PPF. PPF is a well defined presynaptic process in which residual Ca2+ influx after the first of two stimuli results in an enhancement of transmitter release in response to the second stimulus (Zucker, 1989). In the mossy fibers of hippocampus and in the parallel fibers of cerebellum in which LTP is presynaptic and not dependent on NMDA receptor activation, LTP leads to a persistent depression of PPF (Zalutsky and Nicoll, 1990; Salin et al., 1996). We found that LTP in corticothalamic fibers is accompanied by a persistent reduction of PPF (Fig. 4). The reduction in PPF was very large during the initial minute of strong potentiation after the 10 Hz stimulation. During the stable synaptic enhancement, PPF was also significantly reduced. The average PPF 35 min after LTP induction was 70 ± 5% of the baseline (n = 5). Because PPF changes can be caused by changes in the desensitization of AMPA receptors (Wang and Kelly, 1996), we examined LTP and PPF in the presence of cyclothiazide (50 μm), which prevents the desensitization of AMPA receptors. In every case tested (n = 3), we observed that PPF was reduced during LTP in the presence of cyclothiazide (72 ± 7% of baseline) to a similar degree than control slices (70 ± 5% of baseline). These results suggest that the expression of LTP in corticothalamic synapses is presynaptic.
Fig. 4.
Corticothalamic LTP occludes PPF.A, Changes in paired-pulse facilitation during LTP. The field recordings show the effect of paired-pulse stimulation (50 msec interstimulus interval) before and 35 min after LTP induction. The field recordings correspond to the numbers shown inB. On the right, the second responses of the pair are scaled to reveal changes in PPF. Scaling is accomplished by changing the y-axis scale until the second responses of the pairs are equal in amplitude before and during LTP.B, Paired pulses (50 msec) were delivered at 0.05 Hz before and after the induction of LTP. Shown is the amplitude of the field potential to the first pulse (top) and the change in PPF associated with LTP displayed as a percentage of the baseline PPF (bottom).
Corticothalamic LTP induction does not require postsynaptic activation but requires calcium
The results show that corticothalamic LTP is input-specific, not dependent on the activation of NMDA receptors or disinhibition, and proceeds with a persistent occlusion of PPF. We next sought to test whether there is an actual postsynaptic involvement in the induction of corticothalamic LTP. To address this issue, we applied 10 Hz stimulation in the presence of NMDA and non-NMDA receptor antagonists (100 μm CNQX and 250 μm APV were applied using a pipette placed adjacent to the recording electrode). Figure5 shows such an experiment. An initial application of the glutamate receptor antagonists completely blocks evoked postsynaptic activity but leaves intact the fiber volley. Upon removal of the antagonists, the responses recover to their baseline amplitudes. The antagonists were applied again, but in this case, 10 Hz stimulation was delivered during the receptor blockade. Upon removal of the antagonists, the synaptic response did not return to control levels but increased to a stable enhancement (198 ± 9% of baseline after 1 hr; n = 5) (Fig. 5B). Moreover, LTP induced during postsynaptic block was accompanied by a significant reduction of PPF. A subsequent block of postsynaptic activity, which revealed again the fiber volley, demonstrates that LTP is not accompanied by a change in fiber excitability (Fig.5A,C). These results show that postsynaptic activity is not required for the induction of corticothalamic LTP.
The dependence of corticothalamic LTP on extracellular Ca2+ was also examined. Removal of Ca2+ from the ACSF (0 Ca2+ and 4 mmMg2+ ACSF) blocked corticothalamic synaptic transmission (Fig. 5D). Application of 10 Hz stimulation during complete block of synaptic transmission did not produce LTP; responses returned to baseline levels after restoration of Ca2+ (102 ± 6% of baseline;n = 7) (Fig. 5B). Subsequent application of 10 Hz stimulation in the presence of extracellular Ca2+ induces LTP (Fig. 5D). This finding, together with the lack of effect of blocking postsynaptic depolarization, indicates that a presynaptic rise in Ca2+ is necessary for the induction of LTP.
Expression of corticothalamic LTP involves cAMP and protein kinase A
LTP, which is NMDA receptor-independent and induced by a presynaptic increase in Ca2+, has been described in hippocampal mossy fibers and cerebellar parallel fibers (Zalutsy and Nicoll, 1990; Huang et al., 1994; Weisskopf et al., 1994; Salin et al., 1996). These forms of LTP and a similar type found in the lateral amygdala (Huang and Kandel, 1998) have been shown to depend on cAMP. To test the role of cAMP in corticothalamic LTP, we applied forskolin, which directly activates adenylyl cyclase and elevates cAMP levels. Forskolin enhanced corticothalamic synaptic responses, and this enhancement was long-lasting (at least 2 hr) despite the brief application (15 min; n = 5) (Fig.6). Similar to LTP induced with 10 Hz stimulation, forskolin-induced enhancement was associated with a decrease in PPF and with no change in the fiber volley (Fig. 6). The forskolin analog dideoxyforskolin (50 μm), which does not activate adenylyl cyclase but mimics other effects of forskolin, did not induce a lasting synaptic enhancement (103 ± 5% of baseline; 35 min after application; n = 3). Thus, direct activation of adenylyl cyclase mimics the effect of 10 Hz stimulation on corticothalamic fibers.
Fig. 6.
Forskolin enhances corticothalamic field potentials and occludes PPF and LTP. A, Change in paired-pulse facilitation during forskolin-induced enhancement. The field recordings represent the effect of two stimuli delivered with a 50 msec interval (ISI) before and 35 min after forskolin-induced enhancement. The field recordings correspond to thenumbers shown in B. On theright, the second responses of the pair are scaled to reveal changes in PPF. B, Paired-pulse stimulation (50 msec) was delivered at 0.05 Hz before, during, and after application of forskolin (50 μm). Plotted is the amplitude of the field potential to the first response (top) and the change in PPF (bottom), displayed as a percentage of the baseline PPF. During forskolin-induced enhancement, the evoked response was reduced to the baseline amplitude by decreasing the stimulus, and 10 Hz stimulation was applied. The stimulus amplitude was restored, and 10 Hz stimulation was delivered again. C, Summary of five experiments (mean ± SEM) in which forskolin enhanced corticothalamic field potentials.
If forskolin-induced LTP and 10 Hz-induced LTP share the same underlying mechanisms, elevation of cAMP by forskolin should occlude further potentiation by 10 Hz stimulation of corticothalamic fibers. To test this, we tried to induce LTP in corticothalamic fibers after forskolin had enhanced synaptic transmission. As shown in Figure 6, after application of forskolin, 10 Hz stimulation failed to induce LTP (106 ± 9% of baseline; 35 min after 10 Hz stimulation;n = 4). LTP was not observed either if the 10 Hz stimulation was applied after returning the field potential to the pre-forskolin baseline amplitude or if it was applied at the post-forskolin amplitude (Fig. 6). We were sure that these slices were capable of generating LTP because 10 Hz stimulation was tested before forskolin in a different set of corticothalamic fibers from the same slice or in other slices from the same animal, confirming that corticothalamic LTP could be generated.
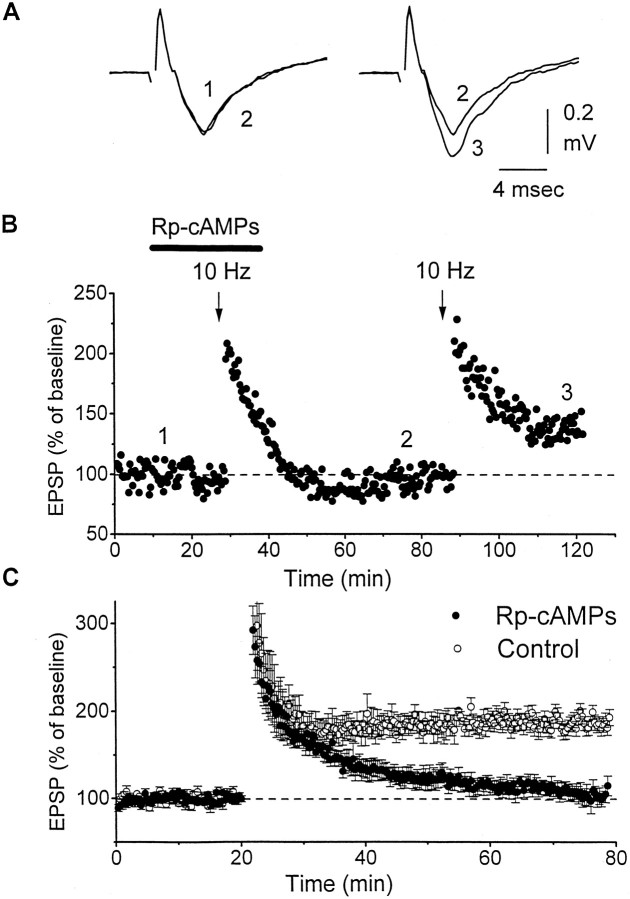
Because cAMP elevation is able to induce LTP in corticothalamic fibers, we tested whether an inhibitor of protein kinase A (PKA) blocked LTP. Bath application of Rp-cAMPs (50 μm), a potent and competitive PKA inhibitor, had no effect on baseline synaptic transmission; however, LTP was not generated by 10 Hz stimulation (n = 6) (Fig. 7). This effect was partially reversible in some experiments because application of 10 Hz stimulation after washout of the drug was able to induce LTP (130 ± 9% of baseline compared with 180% in control slices).
Fig. 7.
Corticothalamic LTP is blocked by a PKA inhibitor.A, The field recordings correspond to the experiment shown in B. B, Rp-cAMPs, an inhibitor of PKA, blocked corticothalamic LTP. Responses were evoked at 0.05 Hz, and Rp-cAMPs was included (50 μm) in the bath without any effect on the baseline after a 25 min application of Rp-cAMPs 10 Hz stimulation was applied, and LTP was not induced. In this experiment, a subsequent application of 10 Hz stimulation ∼40 min after Rp-cAMPs washout produced LTP. C, Summary of experiments in which 10 Hz stimulation was applied in control slices (n= 5) or in slices bathed in Rp-cAMPs (n = 6).
DISCUSSION
The present experiments reveal that corticothalamic fibers generate input-specific and reversible LTP in the ventrobasal thalamus. The results indicate that corticothalamic LTP is entirely presynaptic and appears to rely on the same mechanisms as LTP observed at hippocampal mossy fibers or cerebellar parallel fibers. The inability of glutamate receptor antagonists to impede the induction of corticothalamic LTP strongly suggests that this form of LTP is independent of postsynaptic depolarization or postsynaptic Ca2+ influx. However, extracellular Ca2+ is necessary for corticothalamic LTP, suggesting that a rise in presynaptic Ca2+is required. The results also show that LTP in corticothalamic fibers is not dependent on NMDA receptor activation or disinhibition. This reinforces the notion that postsynaptic mechanisms are not involved in the induction of corticothalamic LTP. In pathways in which postsynaptic depolarization and postsynaptic Ca2+ entry are important (e.g., neocortex, Schaffer collaterals), LTP induction is strongly facilitated by disinhibition and blocked by NMDA receptor antagonists. The decrease in PPF during corticothalamic LTP provides further evidence that not only the induction but also the expression of this form of LTP is presynaptic and therefore caused by an increase in neurotransmitter release. The results also show that activation of adenylyl cyclase by forskolin causes a long-lasting potentiation, which decreases PPF and occludes LTP induced by 10 Hz stimulation. Moreover, application of a PKA inhibitor blocks the generation of corticothalamic LTP. Together, the results can be interpreted by a model put forward to explain LTP in hippocampal mossy fibers (Huang et al., 1994; Weisskopf et al., 1994). In this model, presynaptic Ca2+ entry activates an adenylyl cyclase that, via cAMP and PKA, generates LTP through an increase in neurotransmitter release probability.
Presynaptic PKA-dependent mechanisms involved in the generation of LTP have been described in at least three different pathways: hippocampal mossy fibers (Huang et al., 1994; Weisskopf et al., 1994), parallel fibers (Salin et al., 1996), and lateral amygdala (Huang and Kandel, 1998). In all of these pathways, LTP is expressed presynaptically. Our results reinforce the notion that PKA-dependent LTP is presynaptic. It is reasonable to assume that a Ca2+-sensitive adenylyl cyclase should be present in the neurons that express this form of LTP. Indeed, mRNA for adenylyl cyclases are expressed at high levels in cerebellar granule cells, hippocampal dentate granule cells, and neocortical cells from which corticothalamic fibers originate (Glatt and Snyder, 1993). Presynaptic PKA-dependent LTD has also been recently described in mossy fibers (Kobayashi et al., 1996; Tzounopoulos et al., 1998). Further work will need to determine whether corticothalamic LTD is also mediated by similar mechanisms. Lack of postsynaptic involvement in LTP was also evidenced by the fact that LTP was triggered in neurons that did not produce either low-threshold calcium spikes or orthodromic and antidromic action potentials with the synaptic stimulation used. At the network level, activation of nRt neurons and the release of GABA in the ventrobasal thalamus seem to be unnecessary for LTP induction. This is suggested by the observation that glutamate receptor antagonists, which block corticothalamic fiber collaterals in nRt and thus GABA release, do not impede LTP induction.
The thalamus is the main relay station of information to the neocortex, which then feeds back to the thalamus through corticothalamic fibers. The existence of bi-directional long-term synaptic plasticity in this massive corticothalamic feedback provides an activity-dependent mechanism to enhance or depress the efficacy of communication between the neocortex and thalamus. Because the ventrobasal thalamus feeds back to the neocortex, LTP and LTD would provide the means to change the gain of information flow in the recurrent corticothalamic loop, thus modifying cortico-cortical communication via the thalamus.
When do corticothalamic neurons discharge at 10 Hz (which produces LTP) or at 1 Hz (which reverses LTP)? In whole animals, spindle oscillations occur at 10 Hz during drowsiness and the early stages of sleep. However, spindles are generated within the thalamus (for review, seeSteriade et al., 1993, 1997). Instead, during α-rhythms, 10 Hz activity is generated within the neocortex (Lopes da Silva et al., 1980). These cortical rhythms at 10 Hz occur during awake immobility just as an animal prepares for attentive processing but are abolished immediately after the animal begins active exploration (Rougeul-Buser and Buser, 1997). It is tempting to suggest that one of the functional roles of these cortical preparatory rhythms is to enhance the effectiveness of corticothalamic communication by potentiating corticothalamic synapses. Interestingly, 1 Hz slow oscillations, which would reverse LTP, are generated in the neocortex during slow-wave sleep (Steriade et al., 1993). One of the functions for cortical slow oscillations during sleep may be to reset (reduce) the efficacy of corticothalamic synapses.
Footnotes
This work was supported by the Medical Research Council of Canada, Fonds de la Recherche en Sante du Quebec, and the McGill University Research Development Fund. We thank Drs. Robert Malenka and John Robson for helpful comments on this manuscript.
Correspondence should be addressed to Dr. Manuel Castro-Alamancos, Montreal Neurological Institute, 3801 University Street, Room WB210, Montreal, Quebec H3A2B4, Canada.
REFERENCES
- 1.Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- 2.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 3.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 4.Bourassa J, Pinault D, Deschenes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deschenes M, Hu B. Electrophysiology and pharmacology of the corticothalamic input to lateral thalamic nuclei: an intracellular study in the cat. Eur J Neurosci. 1990;2:140–152. doi: 10.1111/j.1460-9568.1990.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 7.Diamond ME. Cerebral cortex, Vol 11(Jones EG, Diamond IT, eds), pp189–219. Plenum; New York: 1995. Somatosensory thalamus of the rat. [Google Scholar]
- 8.Eaton SA, Salt TE. Role of N-methyl-d-aspartate and metabotropic glutamate receptors in corticothalamic excitatory postsynaptic potentials in vivo. Neuroscience. 1996;73:1–5. doi: 10.1016/0306-4522(96)00123-6. [DOI] [PubMed] [Google Scholar]
- 9.Ergenzinger ER, Glasier MM, Hahm JO, Pons TP. Cortically induced thalamic plasticity in the primate somatosensory system. Nat Neurosci. 1998;1:226–229. doi: 10.1038/673. [DOI] [PubMed] [Google Scholar]
- 10.Frigyesi TL. Intracellular recordings from neurons in dorsolateral thalamic reticular nucleus during capsular, basal ganglia and midline thalamic stimulation. Brain Res. 1972;48:157–172. doi: 10.1016/0006-8993(72)90176-x. [DOI] [PubMed] [Google Scholar]
- 11.Glatt CE, Snyder SH. Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature. 1993;361:536–538. doi: 10.1038/361536a0. [DOI] [PubMed] [Google Scholar]
- 12.Golshani P, Warren RA, Jones EG. Progression of change in NMDA, non-NMDA, and metabotropic glutamate receptor function at the developing corticothalamic synapse. J Neurophysiol. 1998;80:143–154. doi: 10.1152/jn.1998.80.1.143. [DOI] [PubMed] [Google Scholar]
- 13.Guillery RW. A quantitative study of synaptic interconnections in the dorsal lateral geniculate nucleus of the cat. Z Zellforsch Mikrosk Anat. 1969;96:39–48. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- 14.Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication. A brief review. J Anat. 1995;187:583–592. [PMC free article] [PubMed] [Google Scholar]
- 15.Hoogland PV, Wouterlood FG, Welker E, Van der Loos H. Ultrastructure of giant and small thalamic terminals of cortical origin: a study of the projections from the barrel cortex in mice using Phaseolus vulgaris leuco-agglutinin (PHA-L). Exp Brain Res. 1991;87:159–172. doi: 10.1007/BF00228517. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y-Y, Kandel ER. Postsynaptic induction and PKA dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y-Y, Li X-C, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 18.Kao C-Q, Coulter DA. Physiology and pharmacology of corticothalamic stimulation-evoked responses in rat somatosensory thalamic neurons in vitro. J Neurophysiol. 1997;77:1661–1676. doi: 10.1152/jn.1997.77.5.2661. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi K, Manabe T, Takahashi T. Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- 20.Levesque M, Gagnon S, Parent A, Deschenes M. Axonal arborizations of corticostriatal and corticothalamic fibers arising from the second somatosensory area in the rat. Cereb Cortex. 1996;6:759–770. doi: 10.1093/cercor/6.6.759. [DOI] [PubMed] [Google Scholar]
- 21.Lindstrom S, Wrobel A. Frequency dependent corticofugal excitation of principal cells in the cat’s dorsal lateral geniculate nucleus. Exp Brain Res. 1990;79:313–318. doi: 10.1007/BF00608240. [DOI] [PubMed] [Google Scholar]
- 22.Lopes da Silva FH, Vos JE, Mooibroek J, Van-Rotterdam A. Relative contributions of intracortical and thalamocortical processes in the generation of alpha rhythms, revealed by partial coherence analysis. Electroencephalogr Clin Neurophysiol. 1980;50:449–456. doi: 10.1016/0013-4694(80)90011-5. [DOI] [PubMed] [Google Scholar]
- 23.McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci USA. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 25.Ojima H. Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cereb Cortex. 1994;4:646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- 26.Robson JA. The morphology of corticofugal axons to the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1983;216:89–103. doi: 10.1002/cne.902160108. [DOI] [PubMed] [Google Scholar]
- 27.Rougeul-Buser A, Buser P. Rhythms in the alpha band in cats and their behavioural correlates. Int J Psychophysiol. 1997;26:191–203. doi: 10.1016/s0167-8760(97)00764-2. [DOI] [PubMed] [Google Scholar]
- 28.Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharfman HE, Lu SM, Guido W, Adams PR, Sherman SM. N-methyl-d-aspartate receptors contribute to excitatory postsynaptic potentials of cat lateral geniculate neurons recorded in thalamic slices. Proc Natl Acad Sci USA. 1990;7:4548–4552. doi: 10.1073/pnas.87.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- 31.Steriade M, Timofeev I. Short-term plasticity during intrathalamic augmenting responses in decorticated cats. J Neurosci. 1997;17:3778–3795. doi: 10.1523/JNEUROSCI.17-10-03778.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steriade M, Wyzinski P. Cortically elicited activities in thalamic reticularis neurons. Brain Res. 1972;42:514–520. doi: 10.1016/0006-8993(72)90552-5. [DOI] [PubMed] [Google Scholar]
- 33.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 34.Steriade M, Jones EG, McCormick DA. Thalamus, Vol 1. Elsevier; New York: 1997. [Google Scholar]
- 35.Tsumoto T, Creutzfeldt OD, Legendy CR. Functional organization of the corticofugal system from visual cortex to lateral geniculate nucleus in the cat (with an appendix on geniculo-cortical mono-synaptic connections). Exp Brain Res. 1978;32:345–364. doi: 10.1007/BF00238707. [DOI] [PubMed] [Google Scholar]
- 36.Turner JP, Salt TE. Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurons in vitro. J Physiol (Lond) 1998;510:829–843. doi: 10.1111/j.1469-7793.1998.829bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzounopoulos T, Janz R, Sudhof TC, Nicoll RA, Malenka RC. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron. 1998;21:837–845. doi: 10.1016/s0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- 38.Vidnyanszky Z, Gorcs TJ, Negyessy L, Borostyankoi Z, Kuhn R, Knopfel T, Hamori J. Immunocytochemical visualization of the mGluR1a metabotropic glutamate receptor at synapses of corticothalamic terminals originating from area 17 of the rat. Eur J Neurosci. 1996;8:1061–1071. doi: 10.1111/j.1460-9568.1996.tb01273.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang JH, Kelly PT. Regulation of synaptic facilitation by postsynaptic Ca2+/CaM pathways in hippocampal CA1 neurons. J Neurophysiol. 1996;76:276–286. doi: 10.1152/jn.1996.76.1.276. [DOI] [PubMed] [Google Scholar]
- 40.Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 42.Wigstrom H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- 43.Yuan B, Morrow TJ, Casey KL. Responsiveness of ventrobasal thalamic neurons after suppression of S1 cortex in the anesthetized rat. J Neurosci. 1985;5:2971–2978. doi: 10.1523/JNEUROSCI.05-11-02971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- 45.Zhang ZW, Deschenes M. Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: a single-cell labeling study. J Neurosci. 1997;17:6365–6379. doi: 10.1523/JNEUROSCI.17-16-06365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]