Abstract
GABAA receptors are believed to be heteropentamers that can be constructed from six subunit classes: α(1–6), β(1–4), γ(1–3), δ, ε, and π. Given that individual neurons often express multiple receptor subunits, it is important to understand how these receptors assemble. To determine which domains of receptor subunits control assembly, we have exploited the differing capabilities of the β2 and β3 subunits to form functional cell surface homomeric receptors. Using a chimeric approach, we have identified four amino acids in the N-terminal domain of the β3 subunit that mediate functional cell surface expression of this subunit compared with β2, which is retained within the endoplasmic reticulum. Substitution of these four amino acids—glycine 171, lysine 173, glutamate 179, and arginine 180—into the β2 subunit was sufficient to enable the β2 subunit to homo-oligomerize. The effect of this putative “assembly signal” on the production of heteromeric receptors composed of αβ and βγ subunits was also analyzed. This signal was not critical for the formation of receptors composed of either α1β2 or α1β3 subunits, suggesting that mutation of these residues did not disrupt subunit folding. However, this signal was important in the formation of βγ2 receptors. These residues did not seem to affect the initial association of β2 and γ2 subunits but appeared to be important for the subsequent production of functional receptors. Our studies identify, for the first time, key residues within the N-terminal domains of receptor β subunits that mediate the selective assembly of GABAA receptors.
Keywords: GABA receptor, homomeric, heteromeric, assembly, benzodiazepine, cell surface
GABAA receptors are the major sites of fast synaptic inhibition in the brain. Molecular cloning has revealed a multiplicity of GABAA receptor subunits that can be divided by sequence homology into six subunit classes: α(1–6), β(1–3), γ(1–4), δ, ε, and π. Alternative splicing further increases the repertoire of GABAAreceptors (Macdonald and Olsen, 1994; Rabow et al., 1995; Davies et al., 1997; Hedblom and Kirkness, 1997). Localization experiments have revealed a large spatial and temporal variation in subunit expression, with many individual neurons expressing multiple subunits (Laurie et al., 1992; Macdonald and Olsen, 1994; Rabow et al., 1995). Clearly, to understand the diversity of GABAA receptors expressed in neuronal membranes it is important to gain some insights into how these receptor subunits are assembled into functional hetero-oligomers.
To address this question, the assembly of recombinant receptors has been analyzed, focusing on receptors composed of α1, β2, and γ2 subunits, because this combination is believed to account for up to 50% of all benzodiazepine-sensitive receptors in the adult brain (Laurie et al., 1992; Benke et al., 1994; Macdonald and Olsen, 1994;Rabow et al., 1995). Collectively, it is apparent that GABAA receptors are assembled in the endoplasmic reticulum (ER), where access to the cell surface is limited to receptors composed of either α1β2 or α1β2γ2 subunits (Connolly et al., 1996a,b). The α1γ2 and β2γ2 combinations and homomeric subunits are retained within the ER (Connolly et al., 1996,a,b; Gorrie et al., 1997). ER-retained unassembled subunits are rapidly degraded (Gorrie et al., 1997). Recent studies focusing on the β3 subunit have shown that in contrast to homomeric α1, β2, or γ2L subunits, this protein has the capacity to access the cell surface on homomeric expression as determined by immunofluorescence (Connolly et al., 1996b). In addition, homomeric β3 subunits produce spontaneously gated ion channels on expression in either Xenopus oocytes or mammalian cells (Connolly et al., 1996b; Wooltorton et al., 1997).
Using subunit chimeras, we have exploited the differences in cell surface expression between the β2 and β3 subunits to identify key residues that are important in controlling receptor assembly. This approach has identified four amino acids in the N-terminal domain of the β3 subunit that mediate subunit homo-oligomerization and cell surface expression. These residues also selectively affected assembly with the γ2 subunit but not the α1 subunit. Together, these observations demonstrate that defined signals in the N-terminal domains of GABAA receptor subunits mediate selective subunit oligomerization and play a critical role in controlling receptor assembly.
MATERIALS AND METHODS
Cell culture and transfection. Human embryonic kidney 293 (A293) cells and African green monkey kidney (COS) cells were maintained in DMEM (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum, 100 U/ml streptomycin (Sigma, St. Louis, MO), and 100 U/ml penicillin (Sigma). Cells were electroporated (400 V, infinite resistance, 125 μF; Bio-Rad Gene Electroporator II) with 10 μg of DNA using equimolar ratios of expression constructs. For electrophysiology, the reporter plasmid for the S65T mutant jellyfish green fluorescent protein (Heim et al., 1995) was added to the transfection mixture. Transfected cells were maintained in culture for up to 70 hr before use.
DNA construction. The murine GABAA receptor cDNAs encoding the α1, γ2L, and γ2S (Whiting et al., 1990; Kofuji et al., 1991) subunits with the 9E10 epitope (between amino acids 4 and 5) and the β2 subunit cDNA with the FLAG epitope (between amino acids 4 and 5) in the cytomegalovirus-based pGW1 expression vector have been described previously (Connolly et al., 1996a). The β3 subunit cDNA in pGW1 was tagged with the FLAG epitope using the oligonucleotide CATGTTCCCGGGGTCCTTGTCATCGTCGTCCTTGTAGTCGTTTACGCTCTGby site-directed mutagenesis as described previously (Kunkel, 1985).
To generate the β2β3 chimera, a PstI/AvrII fragment encoding the C terminal of the β3 subunit was ligated into the(FLAG)β2, pGW1 AvrII/PstI vector using standard recombinant methods. To generate the β3β2 chimera, aPstI/HindIII fragment encoding the C terminal of β2 was ligated into the (FLAG)β3 pGW1HindIII/PstI vector. An XhoI site was introduced into both the β2 and β3β2 pGW1 constructs at a position corresponding to residue 154 of the mature proteins by site-directed mutagenesis using the oligonucleotides GCCATAGCTTTCAATCTCGAGTGTACAGTTTTGTTC (β2) and GCCATAGCTTTCAATCTCGAGAGTGCAGTTTTGCTC (β3) (Kunkel, 1985). ASacII/XhoI fragment encoding residues 1–153 of the β3 subunit was ligated into the (FLAG)β2 pGW1XhoI/SacII vector, and aXhoI/PstI fragment encoding residues 153–224 of the β3 subunit was ligated into the (FLAG)β2 pGW1XhoI/PstI vector to produce more refined chimeras. Further mutants were generated by site-directed mutagenesis using the oligonucleotides GGAGCTCGATCTTTGTCACGCCAGT and AGTGACAGCATTGTCATCGCCACGCC for the(FLAG)β3(DNTK) construct and GAAGCTCAATCCTTTCCACTCCTGTGA and CCTGTGACTGCCTTGTC ACCGCCGCGCCAG for the (FLAG)β2(GKER) construct.
Immunocytochemistry. Transfected cells plated on poly-l-lysine (10 μg/ml)-coated coverslips were fixed in 3% paraformaldehyde (in PBS) 15–18 hr after transfection, and immunofluorescence was performed as described previously (Connolly et al., 1996a). When cells were permeabilized, 0.05% vol/vol, NP40 was added to all solutions after fixation. The primary antibodies were applied for 1 hr at the following concentrations: anti-FLAG M2 mouse monoclonal antibody (IBI Ltd.), 9 μg/ml; 9E10 supernatant (Evan et al., 1985) diluted 1:2. Secondary antibodies, either fluoroscein-conjugated anti-mouse IgG (Pierce, Rockford, IL) or Alexa 488-conjugated anti-mouse IgG (Molecular Probes, Eugene, OR) at 1 μg/ml were applied for 45 min. Fluorescence images were analyzed by confocal microscopy (MRC 1000, Bio-Rad, Hercules, CA).
Quantitation of cell surface fluorescence by flow cytometrical sorting analysis. After transfection (15–18 hr), cells were blown gently into Ca2+/Mg2+-free PBS. Subsequent washes and antibody dilutions were performed in HBSS (Life Technologies) containing 2.5 mg/ml BSA and 2.5 mm EDTA at 4°C. Cells were incubated with primary antibody, purified 9E10, at 3 μg/ml or anti-FLAG antibody at 4 μg/ml, for 45 min, washed three times, and then incubated with Alexa 488-conjugated anti-mouse IgG at 1 μg/ml for a further 30 min, before they were washed twice and resuspended in Mg2+/Ca2+-free PBS. Cell fluorescence was measured using a Becton Dickinson FACS Calibur machine (Becton Dickinson, Mountain View, CA), and the percentage of transfected cells that were more fluorescent than mock-transfected cells was determined by calculating the number of cells above the boundary of fluorescence of mock-transfected cells on a fluorescence histogram. A statistical analysis of the apparent differences in cell-surface expression of different subunits or subunit combinations was performed using the Student’s t test.
Sucrose density gradient fractionation. Receptor subunits were subjected to sucrose density gradient fractionation on 5–20% linear sucrose density gradients in lysis buffer (Gorrie et al., 1997). Before loading, solubilized cell extracts were clarified by centrifugation (100,000 × g for 10 min). Gradients were calibrated by loading parallel gradients with marker proteins (1 mg/ml) of known sedimentation coefficients: BSA, 4.3S; aldolase, 7.4S; catalase, 11.2S. Gradients were centrifuged in a Beckman SW55Ti rotor at 40,000 rpm for 14 hr at 4°C. The gradients were fractionated into fourteen 350 μl fractions, and receptor subunit sedimentation was analyzed by Western blotting. Alternatively, the (9E10)β2 subunit was immunoprecipitated from each fraction as described previously (Gorrie et al., 1997).
Western blotting. Receptor subunits were detected in gradient fractions using either anti-FLAG antibody or purified 9E10 antibody at 10 μg/ml. Western blotting was performed as described previously (Connolly et al., 1996a) using an enhanced chemiluminescent substrate (Pierce Supersignal substrate). The signals were quantitated using a Bio-Rad phosphorimager.
Immunoprecipitation. Cells werel-methionine-starved for 30 min before labeling with [35S]methionine (ICN/Flow) at 200 μCi/ml. Immunoprecipitation using FLAG or 9E10 antibodies was performed as described previously.
Electrophysiological analysis. Whole-cell recordings from transfected A293 cells were performed as described previously (Wooltorton et al., 1997) up to 70 hr after transfection. Drugs were rapidly applied via a modified U-tube. The expression of functional cell-surface homomeric β subunit receptors was assessed by their sensitivity to Zn2+ (10 μm), picrotoxin (10 μm), and pentabarbitone (1 mm). For αβ and βγ heteromers, GABA sensitivity was assessed. Control untransfected cells did not elicit membrane currents or change membrane conductances when exposed to these ligands.
RESULTS
GABAA receptor β2 and β3 subunits differ in their ability to access the cell surface
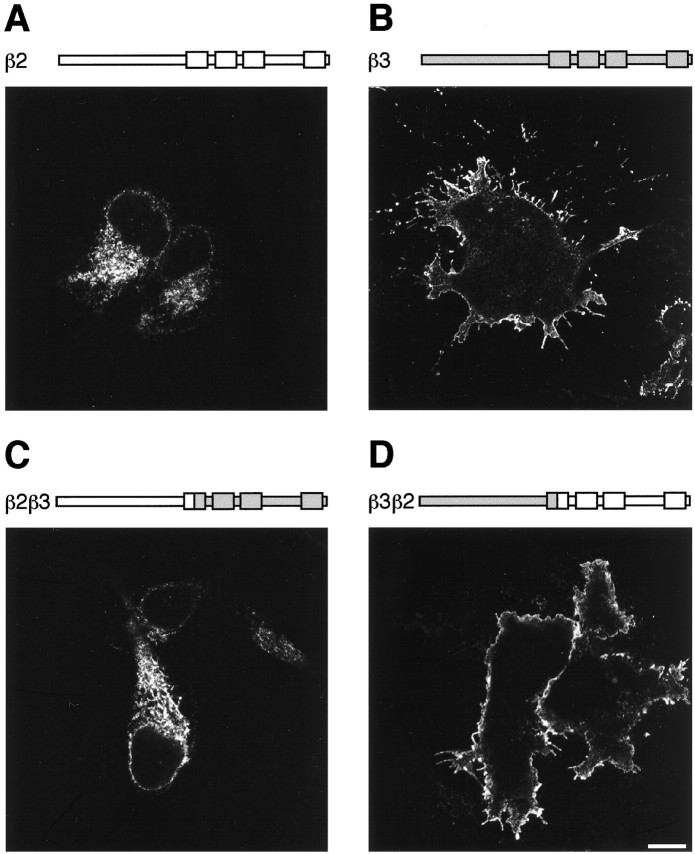
To examine the mechanisms underlying the assembly of GABAA receptors, receptor β subunits modified with reporter epitopes were expressed in A293 cells. Addition of reporter epitopes between residues 4 and 5 of selected GABAAreceptor subunits has been shown to be functionally silent (Connolly et al., 1996a,b). Receptor expression was analyzed by immunofluorescence with or without membrane permeabilization. Homomeric expression of (FLAG)β2 in A293 cells did not produce surface staining (Fig. 1). The staining pattern in permeabilized cells showed that this subunit is retained within the ER on homomeric expression (Connolly et al., 1996a,b; Gorrie et al., 1997). In contrast, homomeric expression of(FLAG)β3 produced robust surface expression in unpermeabilized cells (Fig. 1), as demonstrated previously in Madin–Darby canine kidney (MDCK) cells (Connolly et al., 1996b). Similar differences in surface expression of β2 and β3 were observed in both COS and baby hamster kidney cells, suggesting that this phenomena is not likely to be host cell specific (data not shown).
Fig. 1.
Surface expression of homomeric(FLAG)β subunits in A293 cells. Expression was determined by immunofluorescence using anti-FLAG M2 mouse monoclonal antibody and fluorescein-conjugated secondary antibodies with (+) or without (−) permeabilization 15–18 hr after transfection. Images were collected by confocal microscopy. The structure of each construct is indicated above the image, with the β2 sequence in white and β3 ingray. The four transmembrane domains in the C-terminal half of the subunits are represented by boxes.A, (FLAG)β2 (+); B,(FLAG)β3 (−); C,(FLAG)β2β3 (+); D,(FLAG)β3β2 (−). Scale bar, 10 μm.
Specific residues within the N-terminal domain of GABAAreceptor β subunits control cell surface expression
To determine the molecular basis of the differential ability of homomeric β subunits to access the cell surface, chimeras between(FLAG)β2 and (FLAG)β3 were produced. These constructs were produced at amino acid glutamine 224 within transmembrane domain 1 (TM1), which is identical in all β subunits (Yemer et al., 1989; Macdonald and Olsen, 1994; Rabow et al., 1995). Two chimeras were constructed in which the N-terminal and C-terminal portions of the (FLAG)β3 and(FLAG)β2 subunits were exchanged. These chimeras,(FLAG)β2/β3 and (FLAG)β3/β2, were expressed in A293 cells, and subunit localization was analyzed by immunofluorescence. The (FLAG)β3/β2 chimera, containing the N terminus of β3, was capable of robust cell surface expression as defined by staining in unpermeabilized cells, comparable to that seen with (FLAG)β3 (Fig. 1D). In contrast, the (FLAG)β2/β3 chimera containing the N terminus of β2 was not able to access the cell surface (Fig.1C). However, this protein could be seen in permeabilized cells where it appeared to be retained in the ER, like(FLAG)β2 (Connolly et al., 1996a). From this approach, it is clear that the N-terminal domain of (FLAG)β3 is important for determining cell surface expression.
To identify the regions of (FLAG)β3 responsible for mediating homomeric cell surface expression more precisely, further chimeras were produced. An alignment of the β3 and β2 subunit N-terminal domains is shown in Figure 2. There are 20 amino acid residues within the N terminus that differ between the β2 and β3 subunits. These differences are clustered in two distinct portions of the N-terminal domain (Fig. 2). Exchange of amino acids between isoleucine 154 and glutamine 224 from the(FLAG)β3 to the (FLAG)β2 subunit resulted in cell surface expression (Fig.3B). In contrast, substitution of residues 1–153 from (FLAG)β3 into(FLAG)β2 resulted in intracellular retention (Fig.3A). These studies clearly identify a role for amino acids between residues 154 and 224 within the β3 subunit in mediating cell surface homomeric expression. Using systematic site-directed mutagenesis, four amino acids were identified—G171, K173, E179, and R180 (single letter amino acid code)—within the(FLAG)β3 subunit that were critical in conferring cell surface expression on (FLAG)β2 (Fig. 3D). The individual mutation of D(171)G, N(173)K, T(179)K, or K(180)R in β2 did not promote cell surface homomeric expression (data not shown). As a control, the corresponding residues from (FLAG)β2, D171N173T179K180, were used to replace GKER in (FLAG)β3. Mutant(FLAG)β3(DNTK) was unable to access the cell surface and appeared to be ER-retained like(FLAG)β2 (Fig. 3C).
Fig. 2.
Sequence alignment of the N-terminal domains of the β2 and β3 subunits. Amino acids that differ between the β2 and β3 subunits are indicated (*). The joins between the two subunits in the β2β3 chimeras are shown by arrows. The four residues that affect cell surface expression are inbold. The presumed Cys–Cys loop is indicated. Theboxed region indicates the first presumed transmembrane domain.
Fig. 3.
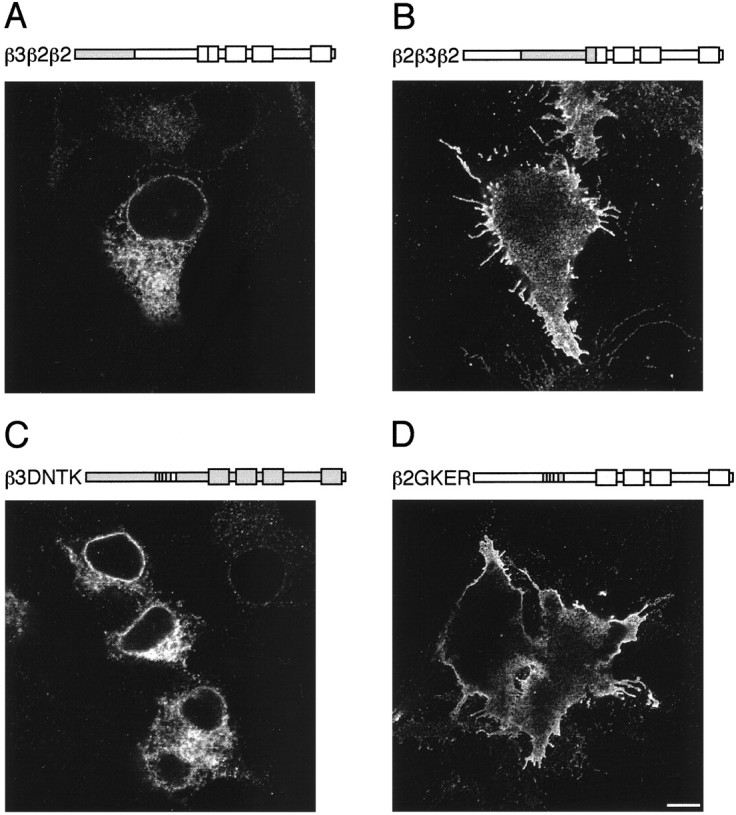
Surface expression of homomeric(FLAG)β2β3 chimeras in A293 cells. Expression was determined by immunofluorescence using anti-FLAG M2 mouse monoclonal antibody and fluorescein-conjugated secondary antibodies with (+) or without (−) permeabilization 15–18 hr after transfection, and images were collected by confocal microscopy. A,(FLAG)β3β2β2 (+); B,(FLAG) β2β3β2 (−); C,(FLAG)β3(DNTK) (+); D,(FLAG)β2(GKER) (−). Scale bar, 10 μm.
In addition to immunofluorescence studies, flow cytometrical sorting (FACS) was used to determine the levels of cell surface expression of homomeric β subunits. Live A293 cells were labeled by immunofluorescence using FLAG antibody followed by an Alexa 488-conjugated secondary antibody and analyzed by FACS. Figure4A shows typical results for mock-transfected A293 cells or cells expressing(FLAG)β2 or (FLAG)β3. Expression of(FLAG)β3 on the cell surface results in a clear shift of the histogram peak to higher fluorescence intensity. This shift in fluorescence was expressed as a percentage of cells expressing the FLAG epitope on the cell surface. Typically 30% of(FLAG)β3-transfected cells expressed the FLAG epitope. This value reflects transfection efficiency; therefore, when different subunits were compared, cell surface expression was calculated as a percentage of the cell surface expression seen for(FLAG)β3 in each experiment, which was normalized to 100%. Despite the fact that (FLAG)β2 cannot be detected on the cell surface by immunofluorescence microscopy, very low levels (∼2%) of (FLAG)β2 could sometimes be detected by FACS analysis. This is likely to represent cells that have become permeabilized during the staining procedure. The values obtained for(FLAG)β2 were not significantly different from mock-transfected cells (p > 0.05) (Fig.4B). The levels of cell surface expression for the(FLAG)β2(GKER) mutant were found to be variable but not significantly different from the(FLAG)β3 subunit (p > 0.05) (Fig.4B). Similarly, the number of(FLAG)β3(DNTK)-transfected cells in which the FLAG epitope was detected on the cell surface was not significantly different from that for(FLAG)β2-transfected cells (p > 0.05) (Fig. 4B) and is significantly less than for(FLAG)β3-transfected cells (p > 0.05).
Fig. 4.
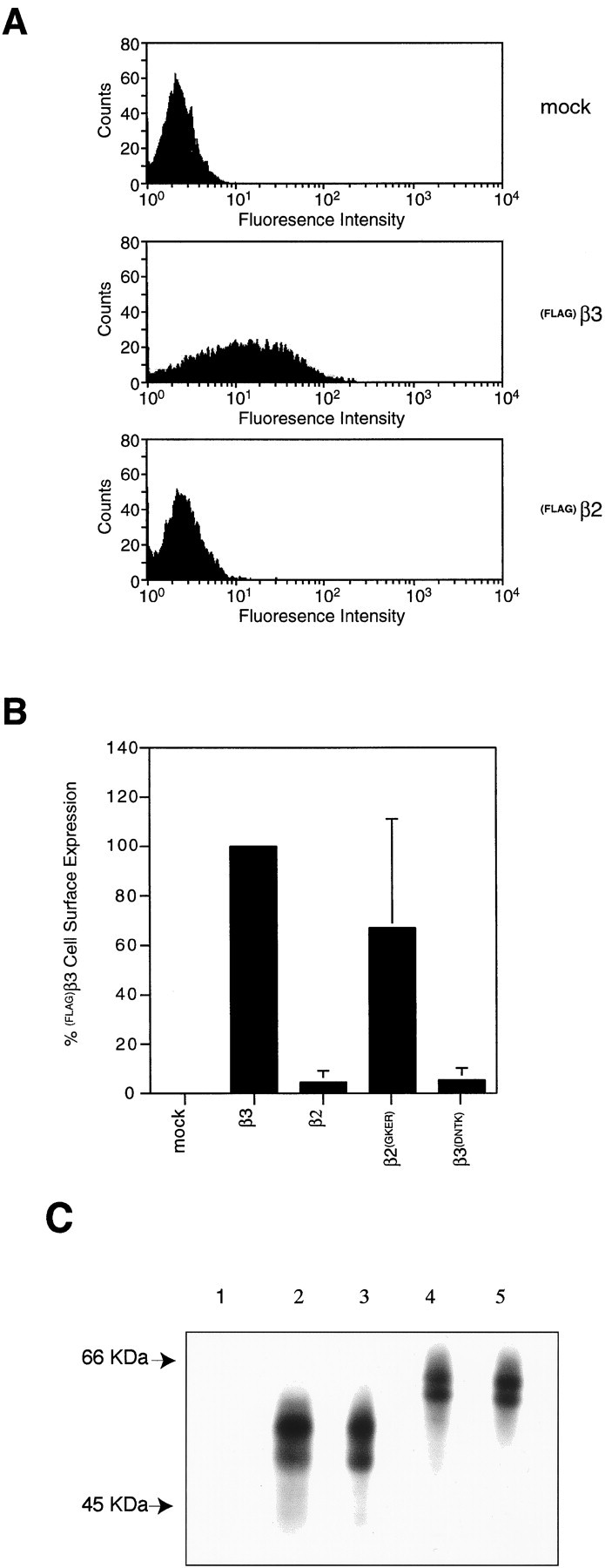
Quantitation of β subunit cell surface expression in A293 cells by FACS analysis. Cell surface β subunits were labeled by immunofluorescence on nonpermeabilized cells using anti-FLAG M2 mouse monoclonal antibody and an anti-mouse Alexa 488-conjugated secondary antibody. The cells were then subjected to flow cytometry analysis. A, Histograms showing the distribution of cells with different levels of cell surface fluorescence for mock-transfected cells (top panel) and cells transfected with either(FLAG)β3 (middle panel) or(FLAG)β2 (bottom panel) cDNAs.B, Relative levels of (FLAG)β subunit cell surface expression. The number of cells expressing the flag epitope on the cell surface was expressed as a percentage of the number of(FLAG)β3-transfected cells expressing the flag epitope on the cell surface (mock, n = 5;(FLAG)β3, n = 9;(FLAG)β2, n = 4;(FLAG)β2(GKER), n= 6; (FLAG)β3(DNTK),n = 5). C, Expression levels of(FLAG)β2 (lane 2),(FLAG)β2(GKER) (lane 3), (FLAG)β3 (lane 4),(FLAG)β3(DNTK) (lane 5), or control untransfected COS cells (lane 1) were assessed in COS cells labeled for 2 hr with 100 μCi/ml [35S]methionine. Expressing cells were then lysed, and receptor subunits were immunoprecipitated with FLAG antibody, resolved by SDS-PAGE, and visualized by autoradiography. The migration of molecular mass standards is indicated on theleft.
To determine whether the differing wild-type and mutant subunits were expressed at similar levels, cells expressing FLAG-tagged versions of these constructs were metabolically labeled with [35S]methionine. Receptor β subunits were then immunoprecipitated and separated by SDS-PAGE (Fig. 4C).(FLAG)β3 migrated with a molecular mass of between 57 and 59 kDa, and (FLAG)β2 migrated as bands of 54 and 50 kDa, as determined previously (Connolly et al., 1996a; McDonald et al., 1998). This approach determined that β2, β2(GKER), β3, and β3(DNTK)were all expressed to similar levels (Fig. 4C).
Functional properties of β subunit chimeras
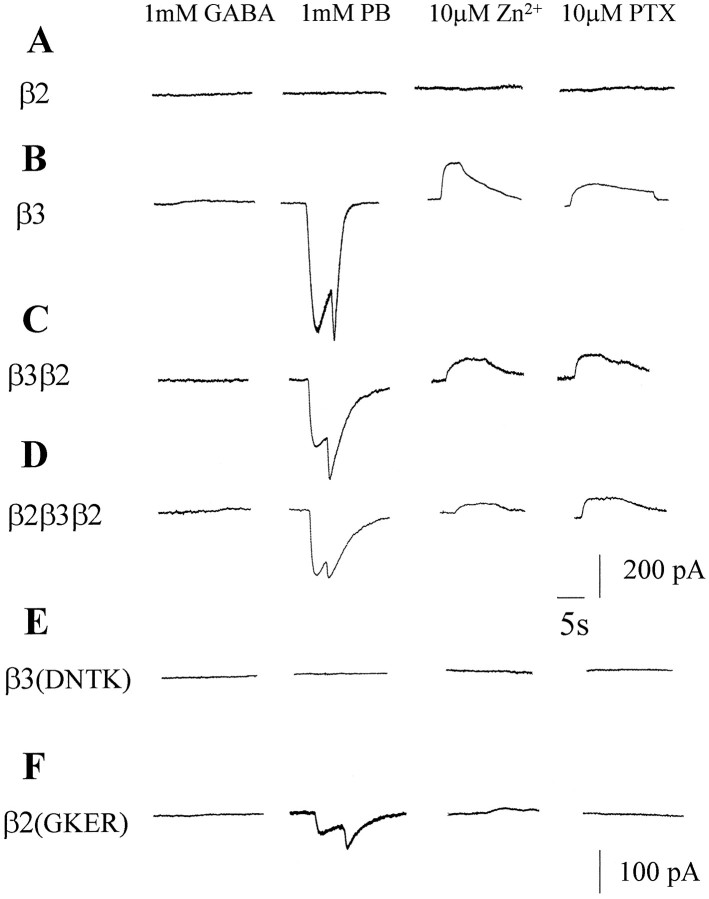
The ability of different β subunits to form functional homo-oligomeric receptors was also measured. Whole-cell currents generated in response to the application of various ligands from transfected A293 cells were recorded at a holding potential of −40 mV. A293 cells expressing β2 show no response to the application of GABA, pentobarbital, Zn2+, or picrotoxin (Fig.5A). In contrast, cells expressing β3 subunits are insensitive to the application of up to 1 mm GABA but display large inward currents with associated rebound currents in response to pentobarbital (Fig. 5B) (Wooltorton et al., 1997). The characteristic spontaneous gating of homo-oligomeric β3 receptors can be demonstrated by the generation of outward currents in response to the GABAA receptor inhibitors Zn2+ and picrotoxin (Fig. 5B). This is because the pipette electrolyte and external Krebs’ composition caused ECl to approximate 0 mV. Thus, the spontaneous gating of β3 homomers was manifest by a persistent inward current at the −40 mV holding potential. In agreement with the immunofluorescence studies, recordings made from cells expressing either the β3β2 or β2β3β2 chimeras resulted in functional cell surface receptors because 1 mmpentobarbital activated inward currents. These chimeras also exhibited spontaneous gating as the addition of 10 μmZn2+ or 10 μm picrotoxin elicited outward membrane currents (Fig. 5C,D). In contrast, β2β3 or β3β2β3 chimeras exhibited no sensitivity to pentobarbital (1 mm), Zn+2 (10 μm), or picrotoxin (10 μm) (n = 3; data not shown).
Fig. 5.
Functional analysis of β subunit homomers. Whole-cell currents were recorded from transfected A293 cells after the application of 1 mm GABA, 1 mm pentobarbital (PB), 10 μm Zn2+, or 10 μm picrotoxin (PTX) from A27.1p93 cells expressing A, β2; B, β3;C, β3β2; D, β2β3β2;E, β3(DNTK); and F, β2(GKER) constructs. Calibration bar:A–D, 200 pA; E, F, 100 pA. All of the cells were voltage-clamped at −40 mV, and each trace is representative of observations made from four to five determinations.
Whole-cell recordings were also made from cells expressing β subunit point mutants. Cells transfected with β3(DNTK) are insensitive to the application of GABA, pentobarbital, Zn2+, and picrotoxin (Fig. 5E), confirming that β3(DNTK) does not assemble into functional homomeric receptors. However, A293 cells expressing β2(GKER) exhibited a weak response to 1 mm pentobarbital (Fig. 5F), indicating that the four substitutions enable the β2 subunit to form functional homo-oligomeric receptors. In contrast, cells expressing β2(GKER) do not clearly display outward currents in response to Zn2+ or picrotoxin, suggesting that these subunits do not appear to form spontaneously open Cl− channels.
Therefore, the data derived from the immunofluoresence, FACS, and electrophysiological studies clearly identify four N-terminal amino acid residues, GKER, within (FLAG)β3 that are necessary for homomeric cell surface expression and are also sufficient to confer homomeric cell surface expression on the (FLAG)β2 subunit after mutation. Given that similar surface levels of β2(GKER) and β3 are seen (Fig. 4), the differences in the physiological properties of these homomeric receptors are of interest. These observations suggest that although the residues G171, K173, E179, and R180 are sufficient to mediate cell surface expression, other distinct residues are responsible for the unique pharmacological and physiological properties of β3 homomers.
Sucrose density gradient fractionation of receptor β subunits
The ER retention of (FLAG)β2 and the cell surface expression of (FLAG)β3 may reflect differences between the abilities of these two proteins to homo-oligomerize, because oligomerization is a prerequisite for ER exit (Hammond and Helenius, 1995). To analyze the oligomerization of receptor β subunits, detergent-solubilized cell extracts were fractionated on 5–20% linear sucrose density gradients. For these studies, expression in COS cells was used because they gave higher expression levels than A293 cells, facilitating biochemical analysis. Gradient fractions were subjected to Western blotting or immunoprecipitation with 9E10 antibody. The behavior of the β3(DNTK), β2(GKER), β2, and β3 subunits was identical in both COS and A293 cells (Fig. 1-3) with regard to cell surface expression (data not shown).
The (FLAG)β3 subunit exhibited a sedimentation coefficient of 9S as determined by reference to standards (Fig.6A,B). The sedimentation coefficient of (FLAG)β3 is distinct from(FLAG)β2, which exhibits a 5S coefficient (Fig.6A–C) (Gorrie et al., 1997). In contrast, β2 exhibits a coefficient of 9S when coexpressed with the α1 or the α1 and γ2 subunits to form functional cell surface receptors (Gorrie et al., 1997; Tretter et al., 1997). The distinct sedimentation coefficients of (FLAG)β2 and (FLAG)β3, combined with differential ER retention, suggested that these subunits differ in their abilities to homo-oligomerize. This issue was explored further by determining the sedimentation coefficients of(FLAG)β2(GKER) and(FLAG)β3(DNTK), which differ in their ability to access the cell surface (Figs. 3, 4).(FLAG)β3(DTNK), which is ER-retained, exhibited a sedimentation coefficient of approximately 5S (Fig.6A,B) like (FLAG)β2 (Fig.6A,C) (Gorrie et al., 1997). In contrast,(FLAG)β2(GKER), which like(FLAG)β3 can access the cell surface, had a sedimentation coefficient of 9S (Fig. 6A,C). Given that the(FLAG)β2,(FLAG)β2(GKER),(FLAG)β3, and(FLAG)β3(DNTK) proteins are all expressed to similar overall levels (Fig. 4C), these observations strongly suggest that the amino acids GKER in(FLAG)β3 mediate cell surface expression by facilitating subunit homo-oligomerization.
Fig. 6.
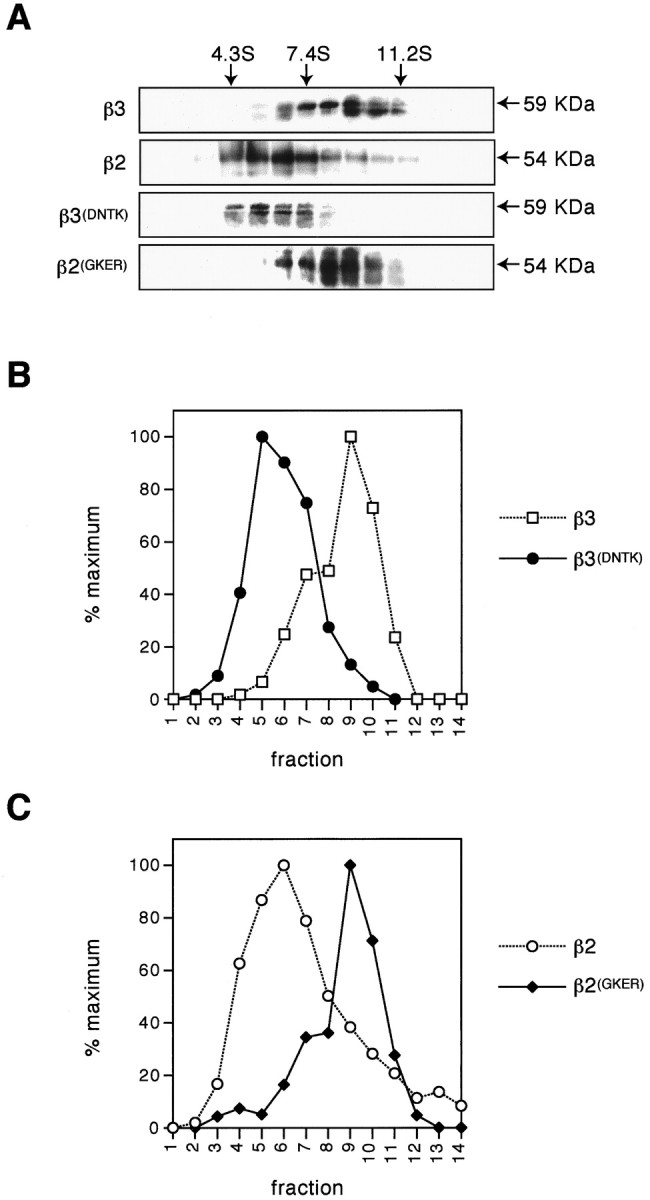
Differential sedimentation of(FLAG)β subunits on sucrose density gradients. COS cells transfected with (FLAG)β3,(FLAG)β3(DNTK), or(FLAG)β2(GKER) were subjected to sucrose density gradient fractionation 16 hr after transfection. Gradient fractions were separated by SDS-PAGE; the(FLAG)β subunits were detected by Western blotting using anti-FLAG M2 monoclonal antibody (A), and the signals were quantified using a Bio-Rad phoshorimager (B, ■, (FLAG)β3, ●,(FLAG)β3(DNTK); C, ○,(FLAG)β2, ♦,(FLAG)β2(GKER)). The data for(FLAG)β2 are taken from Gorrie et al. (1997) and represent immunoprecipitation of this protein from expressing cells after metabolic labeling with [35S]methionine. The level of β2 in each fraction was quantified using a Bio-Rad phosphorimager. Sedimentation coefficients of receptor subunits were determined by reference to the standards BSA (4.3S), aldolase (7.4S), and catalase (11.2S).
The amino acids responsible for mediating β3 subunit homo-oligomerization mediate cell surface expression with the γ2 subunit but not the α1 subunit
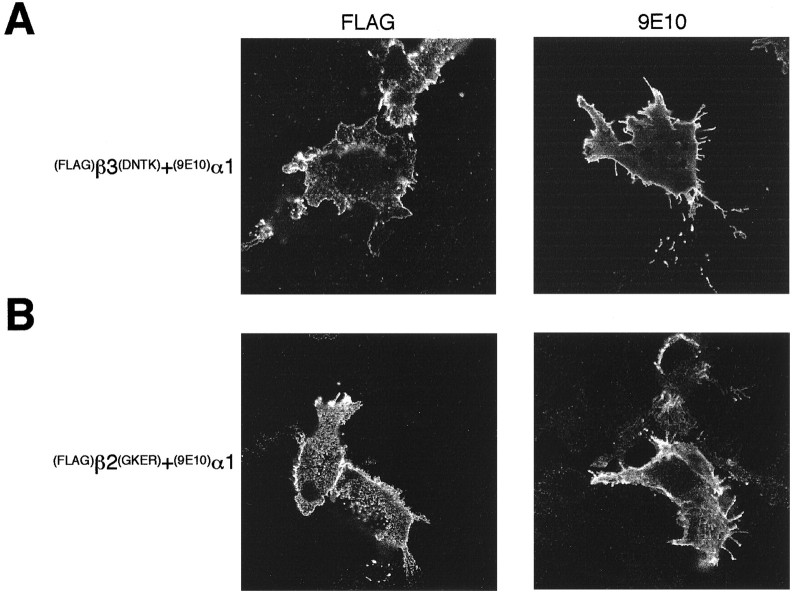
To determine whether the amino acids that control β3 subunit homo-oligomerization influence hetero-oligomerization, various(FLAG)β2 and (FLAG)β3 constructs were coexpressed with (9E10)γ2L or (9E10)α1 subunits. Both (9E10)α1 and (9E10)γ2L are ER-retained on homomeric expression (Connolly et al., 1996a,b), so expression was monitored by detecting the 9E10 reporter epitope at the cell surface. Coexpression of (9E10)α1 with either the (FLAG)β2(GKER) or(FLAG)β3(DNTK) constructs resulted in robust expression of both reporter epitopes on the cell surface (Fig.7). Likewise, both β2(GKER) and β3(DNTK) were able to assemble with the α1 and γ2 subunits to produce functional α1βγ2 receptors (data not shown). Because both the β2 and β3 subunits can produce functional receptors on coexpression with α1 and γ2 subunits, this result is not unexpected (Macdonald and Olsen, 1994; Rabow et al., 1995). However, coassembly with the α1 subunit indicates that the four mutations do not disturb the folding of β subunit polypeptides.
Fig. 7.
Surface expression of heteromeric(FLAG)β(9E10)α1 receptors in A293 cells. Expression was determined by immunofluorescence on nonpermeabilized cells 15–18 hr after transfection using anti-FLAG M2 mouse monoclonal antibody to detect (FLAG)β subunits (left panel) and 9E10 antibody to detect(9E10)α1 (right panel) followed by Alexa 488-conjugated secondary antibodies. Images were collected by confocal microscopy. A,(FLAG)β3(DNTK)(9E10)α1;B, (FLAG)β2(GKER) (9E10)α1. Scale bar, 10 μm.
Coexpression of (FLAG)β2 with (9E10)γ2L resulted in ER retention of both subunits, in agreement with earlier observations (Connolly et al., 1996a,b) (Fig.8A). However, coexpression of (FLAG)β3 and (9E10)γ2L resulted in robust cell surface expression of (9E10)γ2L (Fig. 8B). These results suggest clear differences in the ability of (FLAG)β2 and (FLAG)β3 to assemble with (9E10)γ2L. To determine whether the amino acids that mediate homomeric expression of β3 influence heteromeric expression, selected β subunit mutants were coexpressed with(9E10)γ2L. In contrast to the lack of surface expression of wild-type (FLAG)β2 with (9E10)γ2L, coexpression of (FLAG)β2(GKER) with(9E10)γ2L produced robust cell surface expression of both subunits (Fig. 8C). When (9E10)γ2L was expressed with (FLAG)β3(DNTK), cell surface expression of both subunits was also observed, although at reduced levels compared with cells coexpressing(FLAG)β3 and (9E10)γ2L (Fig.8D). Identical assembly behavior was seen with both the γ2L and γ2S splice variants. These observations indicate that the amino acid residues that mediate β3 subunit homo-oligomerization and cell surface expression also mediate hetero-oligomeric interactions between β3 and γ2 subunits.
Fig. 8.
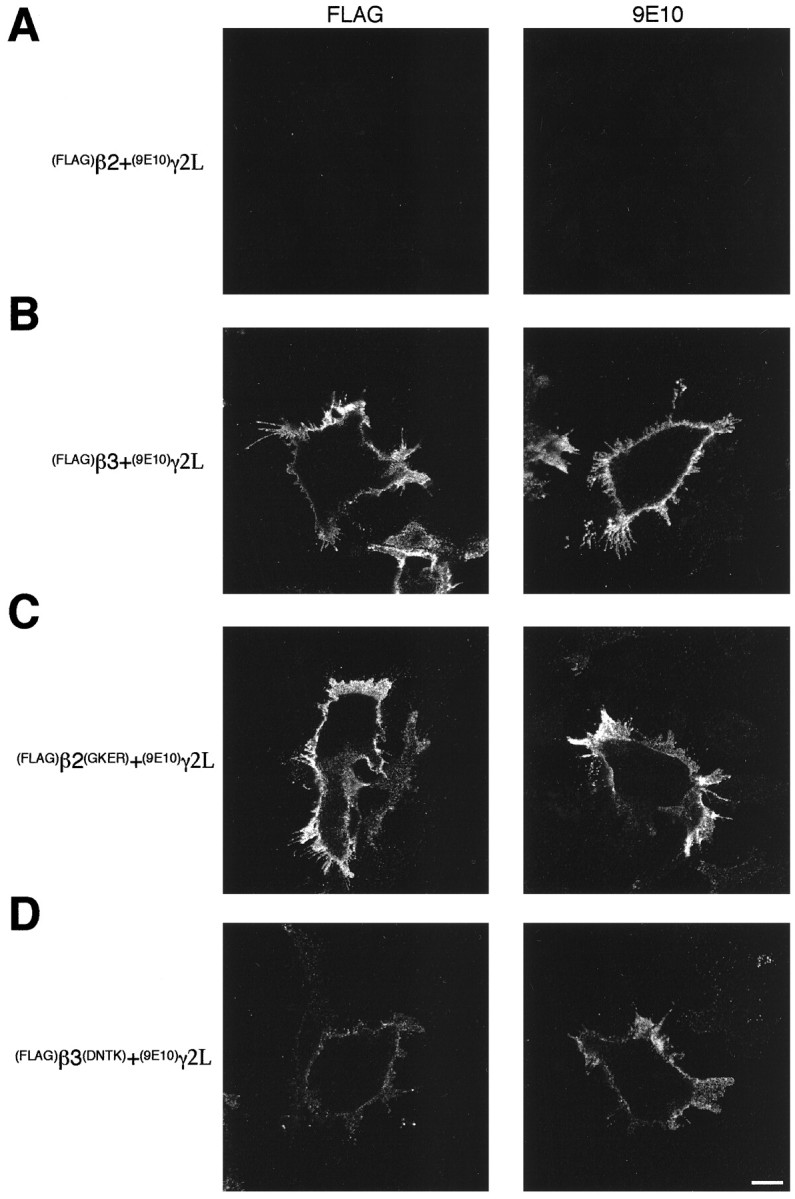
Surface expression of heteromeric(FLAG)β(9E10)γ2L receptors in A293 cells. Expression was determined by immunofluorescence on nonpermeabilized cells 15–18 hr after transfection using anti-FLAG M2 mouse monoclonal antibody to detect (FLAG)β subunits (left panel) and 9E10 antibody to detect(9E10)γ2L (right panel) followed by Alexa 488-conjugated secondary antibodies. Images were collected by confocal microscopy. Transfection and staining were performed simultaneously for each subunit combination, and the pictures were all taken using the same confocal microscope settings. A,(FLAG)β2 + (9E10)γ2L;B, (FLAG)β3 + (9E10)γ2L;C, (FLAG)β2(GKER)+ (9E10)γ2L; D,(FLAG)β3(DNTK) +(9E10)γ2L. Scale bar, 10 μm.
Cell surface βγ2 receptors also form functional ion channels
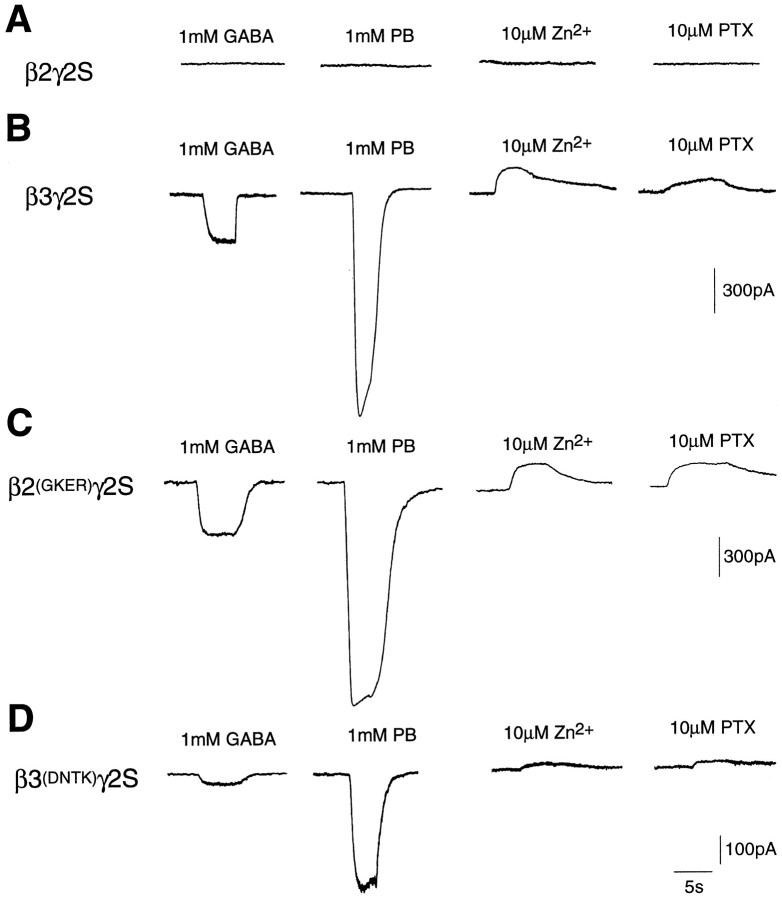
To investigate the abilities of the β subunits to form hetero-oligomeric receptors when coexpressed with the γ2S subunit, whole-cell currents generated in response to the application of various ligands in transfected A293 cells were recorded. Cells coexpressing β2 and γ2S subunits were insensitive to GABA, pentobarbital, Zn2+, and picrotoxin (Fig.9A), as described previously (Connolly et al., 1996a). In contrast, cells coexpressing β3 and γ2S exhibited both GABA- and pentobarbital-gated membrane currents (Fig. 9B). The pharmacology of these channels was distinct from that of β3 homomers, which are insensitive to GABA (Connolly et al., 1996a; Wooltorton et al., 1997). Application of the GABAA receptor inhibitors Zn2+ (10 μm) and picrotoxin (10 μm) to cells expressing β3 and γ2S resulted in the generation of outward currents (Fig. 9B), indicating that β3γ2S channels show a degree of spontaneous activity or that the cells express a mixed population of β3 homomers and β3γ2S receptors.
Fig. 9.
Functional analysis of βγ2S hetero-oligomers. Whole-cell currents were recorded in response to 1 mm GABA, 1 mm pentobarbital (PB), 10 μmZn2+, or 10 μm picrotoxin (PTX) from A293 cells expressingA, β2γ2S; B, β3γ2S;C, β2(GKER)γ2S; andD, β3(DNTK)γ2S. Cells were voltage-clamped at −40 mV holding potential, and each trace is representative of four to five determinations.
Cells coexpressing γ2S with β2(GKER) exhibit currents similar to those generated by cells expressing β3γ2S receptors. Application of GABA or pentobarbital generated inward currents; in contrast, both Zn2+ and picrotoxin blocked spontaneously open channels resulting in the generation of outward currents (Fig. 9C). Interestingly, cells coexpressing the β3(DNTK) mutant with γ2S also displayed inward currents in response to GABA or pentobarbital, but these currents were much smaller than those observed for β3γ2S or β2(GKER)γ2S receptors (Fig. 9D). This was a consistent feature that was independent of the transfection efficiency. Small outward currents were induced in response to the application of 10 μm Zn2+ or picrotoxin, indicating that some of the β3(DNTK)γ2S receptors gate spontaneously (Fig.9D). It is unlikely that mixed populations of β3(DNTK) homomers and β3γ2S receptors are expressed, because the former fail to form functional cell surface receptors. These electrophysiological observations correlate well with the immunofluorescence data, which suggested that β3(DNTK) formed cell surface receptors when coexpressed with γ2, but with a reduced efficiency compared with wild-type β3 subunits. Identical behavior was seen in these experiments with either splice variant of the γ2 subunit (data not shown).
Quantitation of βγ2 cell surface expression
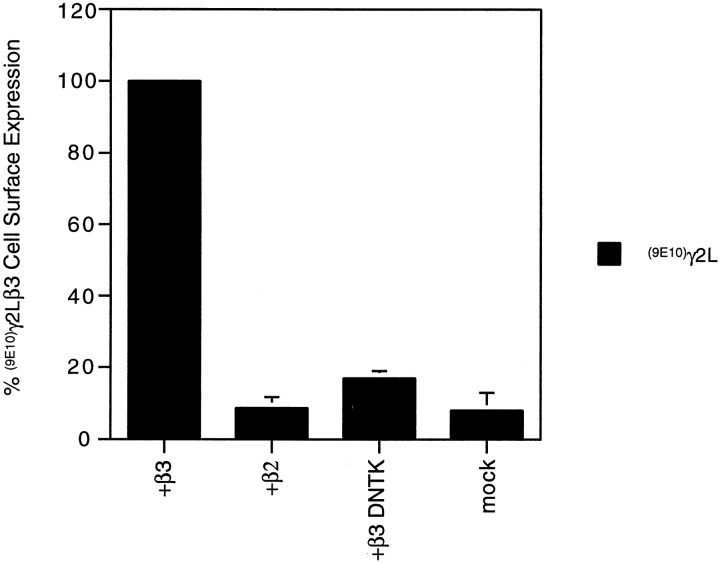
To confirm that the reduction in whole-cell currents produced on expression of β3(DNTK) and γ2 subunits compared with β3 and γ2 subunits was caused by a reduction in cell surface expression, coexpressing cells were subjected to flow cytometry to quantify heteromeric βγ2 receptor cell surface expression. This was achieved by monitoring the level of surface (9E10)γ2L using 9E10 antibody. In cells coexpressing β2 and(9E10)γ2L, the level of cell surface expression was low and similar to that observed for untransfected cells (Fig.10). For cells coexpressing β3 and(9E10)γ2L, (9E10)γ2L could be detected on the surface of ∼15% of cells, and this was used to normalize the cell surface expression of constructs in each experiment (Fig. 10). In(9E10)γ2Lβ3(DNTK)-transfected cells,(9E10)γ2L surface fluorescence was significantly decreased (p < 0.05) (Fig. 10) compared with(9E10)γ2Lβ3-expressing cells (Fig. 10) but was significantly greater than for both (9E10)γ2Lβ2 and mock-transfected cells (p < 0.05) (Fig. 10).
Fig. 10.
Relative levels of (9E10)γ2L subunit cell surface expression when expressed with different β subunits. Cell surface γ2L subunits were labeled by immunofluorescence on nonpermeabilized cells using 9E10 antibody and an Alexa-488 conjugated anti-mouse secondary antibody. The number of cells expressing the 9E10 epitope on the cell surface was measured by FACS analysis and expressed as a percentage of the number of(9E10)γ2Lβ3 transfected cells expressing the 9E10 epitope on the cell surface (n = 3 for each subunit combination).
Interaction of the γ2S subunit with β subunits
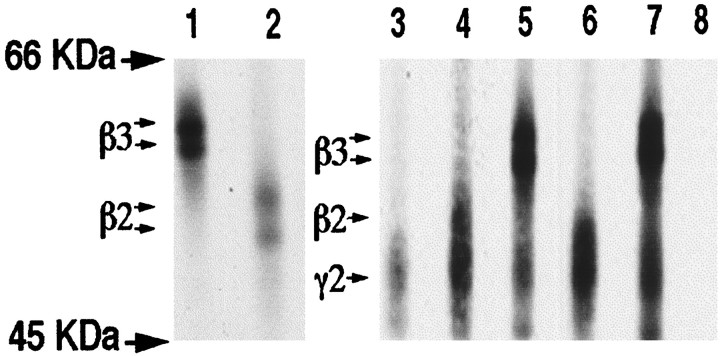
To determine whether changes in subunit oligomerization are responsible for the reduced cell surface expression of β3(DNTK)/γ2S constructs compared with β3/γ2S or β2(GKER)/γ2S, subunit oligomerization was analyzed by immunoprecipitation. For these experiments, a(9E10)γ2S construct was expressed with each of the β subunit constructs used in this study (Fig.11). The migration of homomeric β2, β3, and γ2S subunits is also shown for clarity in Figure 11. The β2 and β3 subunits migrated as bands of 50–54 and 57–59 kDa, respectively, whereas (9E10)γ2S migrated as a diffuse band of between 45 and 49 kDa. Association between the γ2 and β subunits was examined by immunoprecipitation using 9E10 antibody. Coimmunoprecipitation of β3 (57 and 59 kDa) and β3(DNTK) (57 and 59 kDa) with(9E10)γ2S was clearly observed because these proteins have distinct migrations on SDS-PAGE (Fig. 11). Because the close migration of β2 and γ2S, coimmunoprecipitation was more difficult to detect; however, the higher molecular mass species of β2 and β2(GKER) (54 kDa) (Fig. 11) coprecipitated with(9E10)γ2S. These results suggested that the reduced efficiency of surface expression of β3(DNTK)γ2S and the failure of β2γ2S to access the cell surface is not attributable to an inability to oligomerize, in agreement with earlier observations (Connolly et al., 1996a). Instead, the efficiency of assembly is likely to be affected at a later stage, possibly with the β2γ2 and β3(DNTK)γ2 combinations forming dimeric or trimeric complexes, which are processed inefficiently into functional receptors. Alternatively, these residues may affect the transport or targeting of assembled β/γ oligomers to the cell surface.
Fig. 11.
Coimmunoprecipitation of β subunits with the(9E10)γ2S subunit. COS cells or cells expressing β3 (lane 1), β2 (lane 2),(9E10)γ2S (lane 3), flag-tagged(FLAG)β2 + (9E10)γ2S (lane 4), (FLAG)β3 + (9E10)γ2S (lane 5),(FLAG)β2(GKER) +(9E10)γ2S (lane 6),(FLAG)β3(DNTK) +(9E10)γ2S (lane 7), or control cells were [35S]methionine-labeled and immunoprecipitated using 9E10 antibody coupled to protein A-Sepharose. Immune complexes were separated by SDS-PAGE using 8% gels. The migration of β3, β2, and γ2S is indicated as is the migration of molecular mass standards.
DISCUSSION
To identify amino acid residues that control GABAAreceptor assembly, we have used a chimeric approach to analyze the selective cell surface expression of the β3 subunit compared with the β2 subunit. This resulted in the identification of four amino acids—G171, K173, E179, and R180—within the N-terminal domain of β3 that are capable of conferring homomeric cell surface expression on β2. The sedimentation of β subunit constructs was compared by sucrose density gradient centrifugation. β2 migrated as a 5S complex; in contrast, β3 migrated as a 9S complex. Interconversion of the sedimentation coefficients of β2 and β3 could be achieved by replacing the amino acids GKER in β3 with DNTK from β2 and vice versa. Together, these observations suggest that these amino acids identified in our study mediate β3 subunit oligomerization. However, whether residues G171, K173, E179, and R180 all contribute equally to this process remains to be established.
The functional properties of the different β homomers were also examined. In agreement with earlier observations, β2 was unable to form functional channels. However, β3 produced pentobarbital-activated, Zn+2-sensitive responses (Connolly et al., 1996b). In agreement with its failure to homo-oligomerize and its resultant ER retention, expression of β3(DNTK) did not produce functional receptors. In contrast, pentobarbital-activated responses could be recorded from β2(GKER)-expressing cells. These pentobarbital-evoked responses were much smaller than those recorded from β3-expressing cells and unlike β3 homomers were not spontaneously gated (Wooltorton et al., 1997). Given that surface levels of β2(GKER) were similar to those of β3, these observations suggest that the residues GKER are sufficient to mediate β3 subunit homo-oligomerization, but other distinct N-terminal amino acid residues within this subunit are important for channel gating.
The contribution of the amino acids within β3 controlling homo-oligomerization in mediating heteromeric receptor assembly was analyzed. Coexpression of α and β subunits in heterologous systems results in the production of GABA-gated channels (Macdonald and Olsen, 1994; Rabow et al., 1995). That the substitution of residues GKER and DNTK between β2 and β3 does not affect assembly with α1 is not unexpected but is of significance. This result suggests that the amino acids identified in our study are likely to constitute an assembly signal that mediates subunit oligomerization rather than having effects on gross subunit folding.
The production of functional receptors composed of β and γ subunits is less consistent. The formation of both β2γ2 (Draguhn et al., 1990; Sigel et al., 1990) and β3γ2 receptors (Zezula et al., 1996) has been reported. Other studies have reported that coexpression of β1 and γ2S (Angelotti et al., 1993) or β2 and γ2L (Connolly et al., 1996a) does not result in the formation of functional receptors. The observation that β2 and γ2 do not coassemble into cell surface receptors is consistent with earlier results (Connolly et al., 1996a,b). However, β3 was able to assemble with γ2 to form functional GABA-gated cell surface receptors. These results indicate clear differences in the abilities of the β2 and β3 subunits to assemble with the γ2 subunit. Substitution of G171, K173, E179, and R180, key residues in β3 subunit homo-oligomerization, into the β2 was sufficient to allow assembly with the γ2 subunit. However, substitution of these residues for the corresponding amino acids from β2, DNTK, reduced the efficiency of, but did not completely prevent, assembly with the γ2 subunit. Immunoprecipitation of γ2 from COS cell lysates results in the coimmunoprecipitation of coexpressed β2, β3, β2(GKER), or β3(DNTK), suggesting that the initial steps of βγ oligomerization are unaffected by these amino acids. However, the production of a 9S complex that presumably represents functional receptors (Gorrie et al., 1997; Tretter et al., 1997) appeared to be drastically reduced for the β3(DNTK) construct compared with either wild-type β3 or β2(GKER) constructs. Together, these observations suggest that the GKER residues within the β3 subunit are not essential for the initial steps in βγ subunit oligomerization but play a critical role in facilitating the production of functional tetrameric or pentameric subunit assemblies.
The role of the residues identified in our study in the production of receptors composed of αβγ subunits that are believed to account for most GABAA receptor subtypes in the brain remains to be determined (Macdonald and Olsen, 1994; Rabow et al., 1995). Although the production of receptors composed of α1β2γ3 and α1β2γ3 was unaffected by the amino acids mediating β3 subunit homo-oligomerization, they may play a role in the assembly of receptors containing other α subunit variants. Alternatively, they may also be of importance in the production of heteromeric receptors where the γ subunit is substituted for either the δ or ε subunits (MacDonald and Olsen, 1994; Rabow et al., 1995; Davies et al., 1997).
The four residues that control the interactions of the β3 subunit during assembly are not located in a region of the polypeptide sequence that is homologous to the assembly domains that have been identified for nicotinic ACh receptor subunits or glycine receptor subunits (Gu et al., 1991; Chavez et al., 1992; Kuhse et al., 1993; Kreienkamp et al., 1995). However, an asparagine residue in the N terminus of the ρ1 subunit that mediates cell surface homomeric expression is located in a region homologous to the β3 assembly signal defined here (Hackam et al., 1997). Presumably this assembly signal within the β3 subunit will interact in concert with other as yet undefined assembly signals to ensure the fidelity of GABAA receptor assembly.
In conclusion, the results of this study provide the first direct evidence of defined signals in the N-terminal domain of GABAA receptor subunits that are important in mediating subunit selective receptor assembly.
Footnotes
This work was supported by the Medical Research Council (United Kingdom) and the Wellcome Trust.
Correspondence should be addressed to Dr. S. J. Moss, The Medical Research Council Laboratory for Molecular Cell Biology, University College London, Gower Street, London WC1E 6BT, United Kingdom.
REFERENCES
- 1.Angelotti TP, Macdonald RL. Assembly of GABAA receptor subunits: α1β1 and α1β1γ2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993;13:1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benke D, Fritschy JM, Trzeciak A, Bannwarth W, Mohler H. Distribution, prevalence, and drug binding profile of gamma-aminobutyric acid type A receptor subtypes differing in the beta-subunit variant. J Biol Chem. 1994;269:27100–27107. [PubMed] [Google Scholar]
- 3.Chavez RA, Maloof J, Beeson D, Newsom-Davis J, Hall ZW. Subunit folding and alpha delta heterodimer formation in the assembly of the nicotinic acetylcholine receptor. Comparison of the mouse and human alpha subunits. J Biol Chem. 1992;267:23028–23034. [PubMed] [Google Scholar]
- 4.Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996a;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Connolly CN, Wooltorton JRA, Smart TG, Moss SJ. Subcellular localization of γ-aminobutyric acid type A receptors is determined by receptor β subunits. Proc Natl Acad Sci USA. 1996b;93:9899–9904. doi: 10.1073/pnas.93.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABA(A) receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 7.Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- 8.Evan GI, Lewis G, Ramsey G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorrie GH, Vallis Y, Stephenson A, Whitfield J, Browning B, Smart TG, Moss SJ. Assembly of GABAA receptors composed of α1 and β2 subunits in both cultured neurons and fibroblasts. J Neurosci. 1997;17:6587–6596. doi: 10.1523/JNEUROSCI.17-17-06587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Y, Camacho P, Gardner P, Hall ZW. Identification of two amino acid residues in the epsilon subunit that promote mammalian muscle acetylcholine receptor assembly in COS cells. Neuron. 1991;6:879–887. doi: 10.1016/0896-6273(91)90228-r. [DOI] [PubMed] [Google Scholar]
- 11.Hackam AS, Wang TL, Guggino WB, Cutting GR. The N-terminal domain of human GABA receptor ρ1 subunits contains signals for homooligomeric and heterooligomeric interaction. J Biol Chem. 1997;272:13750–13757. doi: 10.1074/jbc.272.21.13750. [DOI] [PubMed] [Google Scholar]
- 12.Hammond C, Helenius A. Quality control in the secretory pathway. Curr Opin Cell Biol. 1995;7:670–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 13.Hedblom E, Kirkness EF. A novel class of GABAA receptor subunit in tissues of the reproductive system. J Biol Chem. 1997;272:15346–15350. doi: 10.1074/jbc.272.24.15346. [DOI] [PubMed] [Google Scholar]
- 14.Heim R, Cubitt AB, Tsien RY. Improved green fluorescent proteins. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- 15.Kofuji P, Wang JB, Moss SJ, Huganir RL, Burt DR. Generation of two forms of the gamma-aminobutyric acid A receptor gamma 2-subunit in mice by alternative splicing. J Neurochem. 1991;56:713–715. doi: 10.1111/j.1471-4159.1991.tb08209.x. [DOI] [PubMed] [Google Scholar]
- 16.Kreienkamp HJ, Maeda RK, Sine SM, Taylor P. Intersubunit contacts governing assembly of the mammalian nicotinic acetylcholine receptor. Neuron. 1995;14:635–644. doi: 10.1016/0896-6273(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 17.Kuhse J, Laube B, Magalei D, Betz H. Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry. Neuron. 1993;6:1049–1056. doi: 10.1016/0896-6273(93)90218-g. [DOI] [PubMed] [Google Scholar]
- 18.Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurie DJ, Wisden W, Seeberg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 21.McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. Adjacent phosphorylation sites on GABAA receptor β subunits determine regulation by cAMP-dependent protein kinase. Nature Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- 22.Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1995;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- 23.Sigel E, Baur R, Trube G, Mohler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- 24.Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiting P, McKernan RM, Iversen LL. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc Natl Acad Sci USA. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wooltorton JA, McDonald BJ, Moss SJ, Smart TG. Identification of a Zn+2 binding site on the murine GABAA receptor complex: dependence on the second transmembrane domain of β subunits. J Physiol (Lond) 1997;505.3:633–640. doi: 10.1111/j.1469-7793.1997.633ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yemer S, Schofield PR, Draguhn A, Werner P, Kohler M, Seeberg PH. GABAA receptor β subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989;8:1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zezula J, Slany A, Sieghart W. Interaction of allosteric ligands with GABAg1 receptors containing one, two or three different subunits. Eur J Pharmacol. 1996;301:207–214. doi: 10.1016/0014-2999(96)00066-0. [DOI] [PubMed] [Google Scholar]