Abstract
This study investigates the mechanisms underlying death of cultured embryonic cortical neurons exposed to the DNA-damaging agent camptothecin and in particular the interdependence of the roles of cyclin-dependent kinases (Cdks), caspases, and mitochondrial function. Camptothecin evokes rapid neuronal death that exhibits nuclear features of apoptosis. This death is accompanied by loss of cytochromec and mitochondrial transmembrane potential as well as by induction of caspase-3-like activity and caspase-2 processing. The Cdk inhibitor flavopiridol provides long-term rescue from death and prevents loss of cytochrome c and mitochondrial transmembrane potential as well as caspase activation and processing. General caspase inhibitors rescue neurons from this rapid apoptotic death but do not prevent them from undergoing delayed death in which nuclear features of apoptosis are absent. Moreover, the caspase inhibitors do not affect early cytochrome c release and delay but do not prevent the loss of transmembrane potential. Agents that directly disrupt mitochondrial function without inducing cytochrome c release lead to a caspase-independent death. These observations favor a model in which (1) DNA damage leads to Cdk activation, which lies upstream of release of cytochromec and caspase activation; (2) cytochromec release is caspase-independent and may occur upstream of caspase activation; (3) early apoptotic death requires caspases; and (4) delayed nonapoptotic death that occurs in the presence of caspase inhibitors is a consequence of prolonged loss of mitochondrial function. These findings shed light on the mechanisms by which DNA damage kills neurons and raise questions regarding the general utility of caspase inhibitors as neurotherapeutic agents.
Keywords: apoptosis, mitochondria, cytochrome c, transmembrane potential, DNA damage, cell cycle
A detailed understanding of the cellular pathways that are elicited in response to various death-promoting stimuli can provide both fundamental insights and potential targets for therapeutic intervention. Toward this end, we have studied the role of elements of the cell cycle and of intracellular proteases in neuronal apoptosis elicited by withdrawal of trophic support (Park et al., 1996; Stefanis et al., 1996, 1997, 1998;Troy et al., 1997) and by exposure to DNA-damaging agents (Park et al., 1997, 1998a,b).
Neuronal death caused by DNA-damaging treatments such as chemotherapeutic agents and irradiation can be studied conveniently in cultured neurons (Morris and Geller, 1996; Gobbel et al., 1998). For instance, the topoisomerase-I inhibitor camptothecin induces rapid apoptotic death in CNS cultures (Morris and Geller, 1996). Studies of the mechanism of this death suggest roles for p53 and Bax (Xiang et al., 1997). Moreover, both pharmacological and molecular inhibitors of cyclin-dependent kinases (Cdks) protect cultured cortical neurons from camptothecin-induced apoptosis (Park et al., 1997, 1998a), thus supporting a role for aberrant activation of cell cycle components in this process.
In the current study we have further investigated the involvement of Cdks as well as the cysteine proteases known as caspases (Fraser and Evan, 1996; Salvesen and Dixit, 1997) and mitochondrial elements in camptothecin-induced death of cortical neurons. Our previous studies revealed that general inhibitors of caspases promote survival of DNA-damaged sympathetic neurons (Park et al., 1997, 1998b). Moreover, there is increasing recognition that mitochondria, and in particular loss of mitochondrial transmembrane potential and release of cytochromec, are key components in certain mammalian apoptotic pathways and may lie either upstream or downstream of caspases (for review, see Green, 1998; Green and Kroemer, 1998; Green and Reed, 1998).
Using cultures of embryonic cortical neurons, we have performed biochemical as well as biological studies to address various issues on the mechanisms by which camptothecin induces neuronal death. Our observations favor a model in which (1) DNA damage in cortical neurons causes activation of Cdks, which in turn lies upstream of loss of mitochondrial transmembrane potential, cytochrome c release, and caspase activation; (2) the activated caspases participate in rapid apoptotic death of neurons; (3) loss of mitochondrial cytochromec is not dependent on caspase activation; (4) caspases partially contribute to early loss of mitochondrial transmembrane potential; (5) if rapid apoptotic death is blocked by general caspase inhibitors, a delayed form of death occurs that does not demonstrate the classical nuclear manifestations of apoptosis; and (6) this delayed death may be attributable to the loss of mitochondrial function.
MATERIALS AND METHODS
Cell culture. Primary neuronal cortical cultures from embryonic day 18 (E18) rats were prepared as described previously (Friedman et al., 1993). After dissection, brain tissue was dissociated by mechanical trituration, and the cells were resuspended in medium consisting of Minimal Essential Medium/Ham’s F12 (1:1; both from Life Technologies, Gaithersburg, MD) supplemented with insulin (25 μg/ml), glucose (6 mg/ml), transferrin (100 μg/ml), progesterone (20 nm), putrescine (60 μm), and selenium (30 nm). This medium is referred to as complete serum-free medium (SFM) (Di Porzio et al., 1980). The cortical neurons were plated at a density of 100,000–200,000 cells/ml in 24 well or 35 mm poly-d-lysine-coated tissue culture dishes. Cultures were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Applications of reagents. One or 2 d after plating, the cortical neuron cultures were treated with 10 μmcamptothecin alone or in combination with 100 μmBoc-aspartyl(OMe)-fluoromethylketone (BAF), 100 μmN-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD-FMK), 1 μm flavopiridol, or 200 μmolomoucine. These reagents were added in a volume of SFM equal to one-fifth of the total volume in the well. In control cultures we added SFM without additives. In separate experiments, we treated the cultures with antimycin A (1 or 10 μm) or carbonyl cyanide-(trifluoromethoxy)-phenylydrazone (FCCP; 1 or 10 μm) with or without pretreatment for 2 hr with 100 μm BAF.
Assessment of survival. 12, 24, and 48 hr after application of the above reagents cortical neurons plated in 24-well dishes were lysed, and the number of intact nuclei was counted in a hemacytometer, as previously described (Rukenstein et al., 1991; Farinelli et al., 1998). Cell counts were performed in triplicate and are reported as mean ± SEM (n = 3). The data are expressed as a percentage of the number of neurons in the control cultures at each time point. All data shown are representative of at least two replicate experiments.
In a limited set of experiments we performed 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assays to ensure that the nuclear counts reflected functionally active neurons. We used the MTT cell proliferation kit by Boehringer Mannheim (Indianapolis, IN) and followed the manufacturer’s instructions. MTT reduction was assessed by determining absorbance values at 570 nm in a spectrophotometer. As a reference, we applied MTT to culture media without cells. MTT activity is reported relative to the activity present in control cultures and is the mean ± SEM (n = 3). Data are representative of two separate experiments.
Assays for nuclear apoptosis. We used the Hoechst dye 33342 (1 μg/ml; Sigma, St. Louis, MO) to stain nuclei of cortical neurons. Cortical neurons plated in 35 mm dishes were fixed with 4% paraformaldehyde, stained with the Hoechst dye, and visualized under a fluorescence microscope as previously described (Farinelli and Greene, 1996; Stefanis et al., 1998). Apoptotic nuclei were identified as nuclei with chromatin margination along the nuclear membrane or with chromatin condensation, with formation of discrete homogeneous chromatin clumps. The percentage of apoptotic nuclei was counted for each condition at 100× magnification in three separate fields of 100 cells each and is reported as the mean ± SEM (n = 3).
The terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay was performed using the Boehringer Mannheim kit. Briefly, neurons plated in 35 mm dishes were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 and 0.1% sodium citrate, incubated with the TUNEL reaction mixture, rinsed, and visualized under a fluorescent microscope. In certain cases, after the rinses we performed staining with propidium iodide (50 ng/ml) for 30 min at room temperature to counterstain neuronal nuclei. The percentage of TUNEL-positive cells was counted for each condition at 40× magnification in five separate fields of at least 100 cells each and is reported as the mean ± SEM (n = 5).
Preparation of cell lysates for Western blotting or assay of caspase activity. Cortical neurons plated in 35 mm dishes were harvested at the indicated time points after application of the reagents. Cells were rinsed three times in cold PBS and then collected in a buffer of 25 mm HEPES, pH 7.5, 5 mm EDTA, 1 mm EGTA, 5 mm MgCl2, 2 mm DTT, 10 μg/ml pepstatin and leupeptin, and 1 mm PMSF. The cellular material was left for 20 min on ice and then was sonicated on ice. The lysate was centrifuged for 20 min at 160,000 × g, and the supernatant was stored at −80°C to be used for the DEVD-amino-fluoro-coumarin (AFC)-cleaving assay and for Western immunoblotting (Stefanis et al., 1996, 1997, 1998). The pellet was used for poly-ADP-ribose polymerase (PARP) immunoblotting. The pellet was solubilized in 25 mm HEPES, pH 7.5, 5 mm EDTA, 2 mm DTT, 1% Triton X-100, 10 μg/ml pepstatin and leupeptin, and 1 mm PMSF, sonicated, and used for Western blotting with the PARP antibody. A signal corresponding to PARP was detected only in the pellet lysates and not in the supernatants. Protein concentrations were measured using the Bradford (1976)assay.
Western immunoblotting. Equal volumes of 2× sample buffer were added to soluble lysates of cortical neurons (50 μg of protein); the samples were boiled for 5 min and subsequently resolved by electrophoresis on SDS-polyacrylamide gels, transferred to nitrocellulose membranes, blocked in 5% nonfat milk in PBS, and incubated overnight at 4°C with anti-N-Nedd, an antibody directed against the N terminus of caspase-2 (1:250) (Stefanis et al., 1997;Troy et al., 1997) or with two anti-extracellular signal-regulated kinase (ERK) antibodies (1:2000 each; Santa Cruz Biotechnology, Santa Cruz, CA). Equal volumes of 2× sample buffer were added to lysates from cortical neuron pellets (50 μg of protein); the samples were boiled for 5 min and subsequently resolved on 10% SDS polyacrylamide gels, and similarly subjected to immunoblotting using the C2–10 PARP monoclonal antibody [Enzyme Systems Products (ESP), Dublin, Ca] (1:10,000). The blots were washed in washing buffer (PBS with 0.2% Tween 20), incubated for 1 hr at room temperature with anti-rabbit IgG antibody (Amersham, Arlington Heights, IL) (for anti-N-Nedd-2 and anti-ERK antibodies) or the anti-mouse IgG antibody (for the PARP antibody) (Amersham) at 1:1000 in blocking buffer, washed again in washing buffer, and then processed by ECL (Amersham) or with the Pierce (Rockford, IL) supersignal substrate system, according to the manufacturers’ instructions.
Cleavage of fluorogenic substrate. Soluble lysates (10 μg of protein) were incubated at 37°C in a buffer of 25 mmHEPES, pH 7.5, 10% sucrose, 0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid, and 10 mm DTT with the fluorogenic substrate DEVD-AFC (15 μm; ESP), and the emitted fluorescence was measured in a fluorometer, as previously described (Stefanis et al., 1996).
Immunofluorescence. Cortical neurons plated in 35 mm dishes and treated as above for 10 or 20 hr were incubated with the mitochondrial dye Mitotracker (100 nm Mitotracker Orange CMTMRos; Molecular Probes, Eugene, OR) for 30 min at 37°C. This dye labels mitochondria with intact transmembrane potential and thus serves as an index of mitochondrial function. After this incubation, the cells were rinsed with PBS and fixed with 4% paraformaldehyde for 30 min at 4°C. After washing with PBS, the cells were incubated for 1 hr at room temperature with blocking solution [10% normal goat serum (NGS) in PBS with 0.4% Triton X-100]. The cells were then incubated for 1 hr at room temperature with a mouse monoclonal antibody against cytochrome c (clone 6H2.B4, dilution 1:500; PharMingen, San Diego, CA) in 2% NGS in PBS with 0.4% Triton X-100. After washing, the secondary antibody (goat anti-mouse FITC-conjugated, dilution 1:100; Molecular Probes) was applied for 45 min at room temperature in 2% NGS in PBS with 0.4% Triton X-100. After further washes coverslips were applied, and the cells were visualized under a fluorescent microscope. In certain cases, Hoechst dye 33342 was applied to the cells for 30 min after incubation with the secondary antibody.
To quantify the number of Mitotracker-positive cells that were cytochrome c-negative, we assessed in each condition 50 successive Mitotracker-positive cells for the presence of mitochondrial cytochrome c. Each assessment was repeated twice and is reported as the mean percentage of the two measurements. The percentage of cells that were Mitotracker-negative and the percentage of cells with nonapoptotic nuclei that were cytochrome c-negative or Mitotracker-negative were counted in a similar manner. Only cells with complete loss of staining or obvious diffuse cytoplasmic staining were counted as negative.
Materials. zVAD-FMK, BAF, and DEVD-AFC were obtained from ESP; flavopiridol was a generous gift from Dr. Peter Worland (National Institutes of Health, Bethesda, MD). Olomoucine was purchased from LC Laboratories. All other pharmacological and common tissue culture reagents were obtained from Sigma, except as indicated.
RESULTS
Cdk inhibitors and general caspase inhibitors protect E18 cortical neurons from cell death induced by camptothecin
We and others previously showed that the DNA-damaging agent camptothecin induces apoptotic death of PC12 cells, neonatal rat sympathetic neurons, and embryonic cortical neurons cultured in the presence of glia (Morris and Geller, 1996; Park et al., 1997). To study more closely the biochemical events that occur in cortical neurons after camptothecin application, we turned to a near-pure neuronal cell culture system. To this end, we prepared dissociated cortical neurons from E18 rat embryos and plated them in defined serum-free medium. Cultures maintained under these conditions have a very low percentage of glia (<2%; data not shown). One or 2 d after plating, we applied 10 μm camptothecin to the cultures. Visible neuronal degeneration started occurring ∼6 hr later. By 12 hr, the large majority of neurons were dead, as judged by counts of intact nuclei (Fig. 1A) or phase-contrast microscopy (Fig.2A,B). The rapid and synchronous nature of death of DNA-damaged cortical neurons has enabled us to perform a number of pharmacological and biochemical experiments.
Fig. 1.
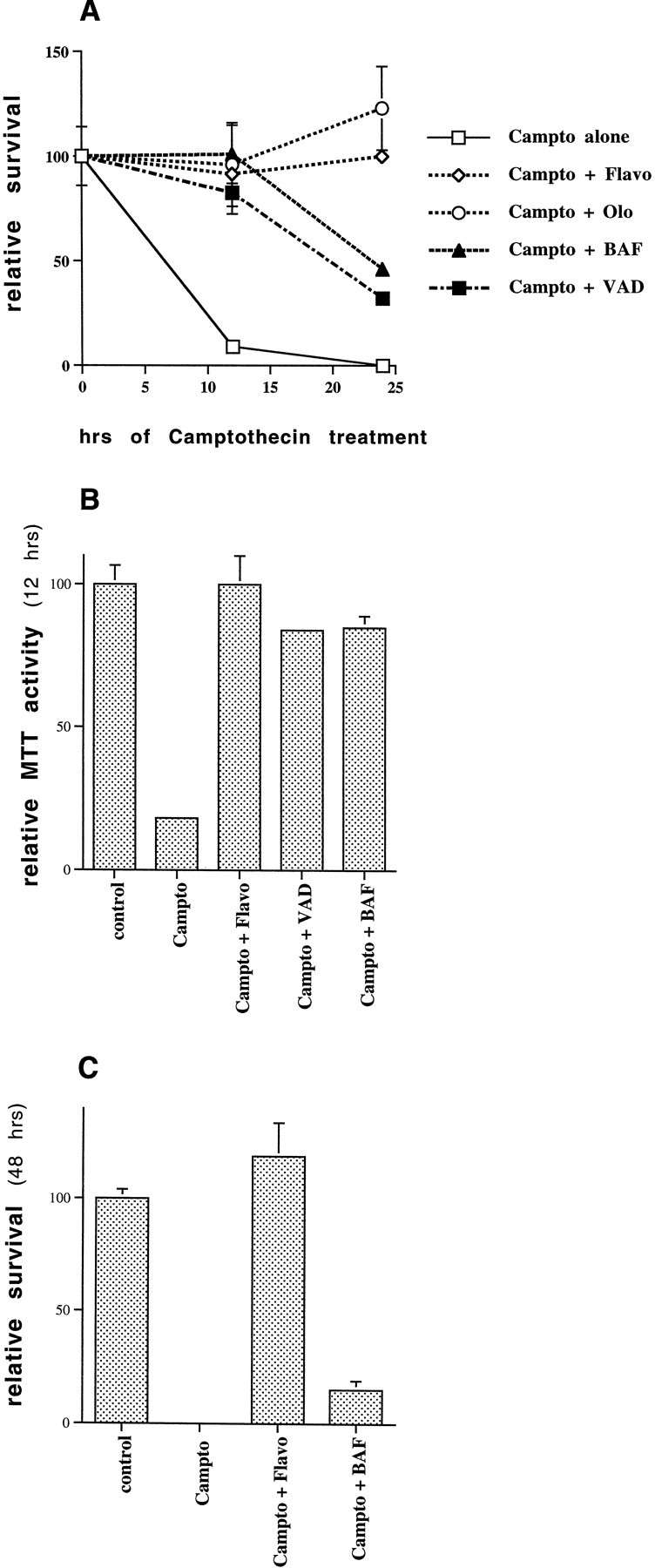
Effects of Cdk and caspase inhibitors on camptothecin-induced neuronal death. A, Rat E18 cortical neurons were plated in poly-d-lysine-coated 24 well dishes. The following day 10 μm camptothecin (Campto), with or without 1 μmflavopiridol (Flavo), 200 μm olomoucine (Olo), 100 μm BAF, or 100 μmzVAD-FMK (VAD), was applied to the cells. Survival was assessed 12 or 24 hr later by counting the number of intact nuclei, as described in Materials and Methods. Survival is reported relative to untreated control cultures and is the mean ± SEM (n = 3). B, Rat E18 cortical neurons were plated and treated with or without camptothecin and other additives as in A. Twelve hours later, MTT reducing activity was measured according to the manufacturer’s instructions (Boehringer Mannheim). MTT reducing activity is expressed relative to untreated controls and is reported as the mean ± SEM (n = 3). C, Rat E18 cortical neurons were treated with camptothecin alone or together with 1 μm flavopiridol or 100 μm BAF, and survival was assessed 48 hr later. Survival, assessed by counting the number of intact nuclei, is reported relative to untreated control cultures and is the mean ± SEM (n = 3).
Fig. 2.
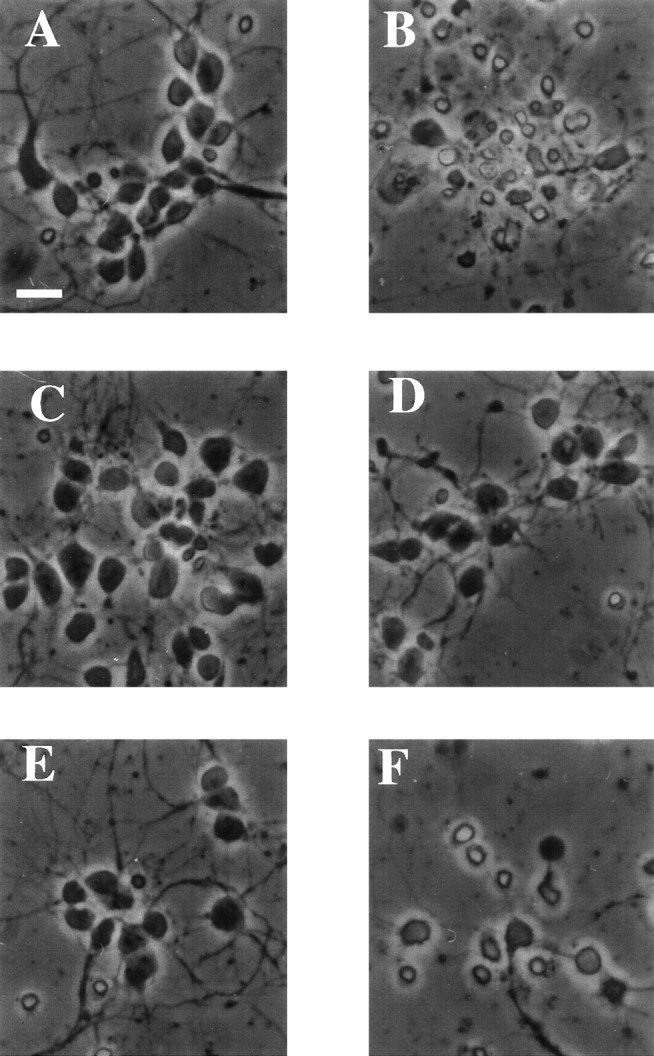
Photomicrographs of cultured E18 cortical neurons treated with camptothecin and Cdk and caspase inhibitors. Rat E18 cortical neurons were plated in poly-d-lysine-coated 24-well dishes. The following day 10 μm camptothecin, with or without 1 μm flavopiridol, or 100 μm BAF, was applied to the cells. Twelve hours later, photomicrographs were taken of cells that had been untreated (A) or treated with camptothecin alone (B) or in combination with flavopiridol (C) or BAF (D). Sister cultures were exposed for 24 hr to camptothecin and flavopiridol (E) or camptothecin and BAF (F) and photomicrographed. Scale bar, 25 μm.
We first ascertained whether the Cdk inhibitors flavopiridol and olomoucine would effectively promote survival in this model, as they had in the model of more protracted death of camptothecin-treated cortical neurons cultured in the presence of glia (Morris and Geller, 1996; Park et al., 1997). The Cdk inhibitors provided complete protection from camptothecin-stimulated cell death at 12 and 24 hr, as assessed by counts of intact nuclei or phase-contrast microscopy (Figs.1A, 2C). The survival effects were confirmed by measurement of MTT activity (Fig. 1B) and were maintained, at least in the case of flavopiridol, for up to 48 hr after camptothecin application (Figs. 1C,2E). Olomoucine alone (i.e., without camptothecin) tended to be toxic after 24 hr, so its survival potential in camptothecin-treated cultures was not assessed at 48 hr. The solvent (DMSO) alone did not promote survival.
We next tested the survival-promoting potential of two relatively general caspase inhibitors, zVAD-FMK and BAF (Deshmukh et al., 1996;Stefanis et al., 1996). In a previous study, BAF, but not zVAD-FMK, protected sympathetic neurons from DNA damage-induced cell death (Park et al., 1997, 1998b). In the case of E18 cortical neurons, however, both BAF and zVAD-FMK effectively promoted survival at 12 hr after camptothecin application, i.e., at a time when nearly all neurons treated with camptothecin alone were dead (Fig. 1A). At this time, neurons treated with camptothecin and the caspase inhibitors were indistinguishable from untreated controls, as judged by phase-contrast microscopy (Fig. 2D; data not shown). MTT assays further confirmed that the cells were metabolically intact (Fig. 1B). It should be stressed here that MTT reduction, although initially thought to reflect mitochondrial function, has recently been shown to be more closely related to mechanisms of endocytosis and thus is used here as a general index of metabolic function (Berridge and Tan, 1993; Hawtin et al., 1995; Liu et al., 1997). Although effective in the short term, the general caspase inhibitors did not maintain survival over a more prolonged period and thus delayed rather than prevented death. Despite the presence of the inhibitors, by 24 hr more than half of the neurons were dead, and by 48 hr only a few viable neurons remained (Fig. 1A,C). This delayed neuronal degeneration was evident by phase-contrast microscopy (Fig. 2F). BAF, applied together with 0.2% DMSO (equal to the DMSO concentration in cells treated with BAF and camptothecin), was not itself toxic under these circumstances.
Taken together these findings indicate that, in contrast to Cdk blockers, caspase inhibitors only delay camptothecin-evoked death. To explore this observation in further detail, we examined caspase activity in camptothecin-treated cultures.
Caspase activity is induced after application of camptothecin
We used several indices to measure caspase activity and activation in camptothecin-treated cultures. As one measure of caspase-3-like activity we assessed cleavage of the fluorogenic substrate DEVD-AFC by neuronal lysates prepared at successive time points after application of camptothecin. As shown in Figure3A, there was a marked induction of caspase-3-like activity, which was detectable by 4 hr after camptothecin application. At this time there was no visible neuronal degeneration.
Fig. 3.
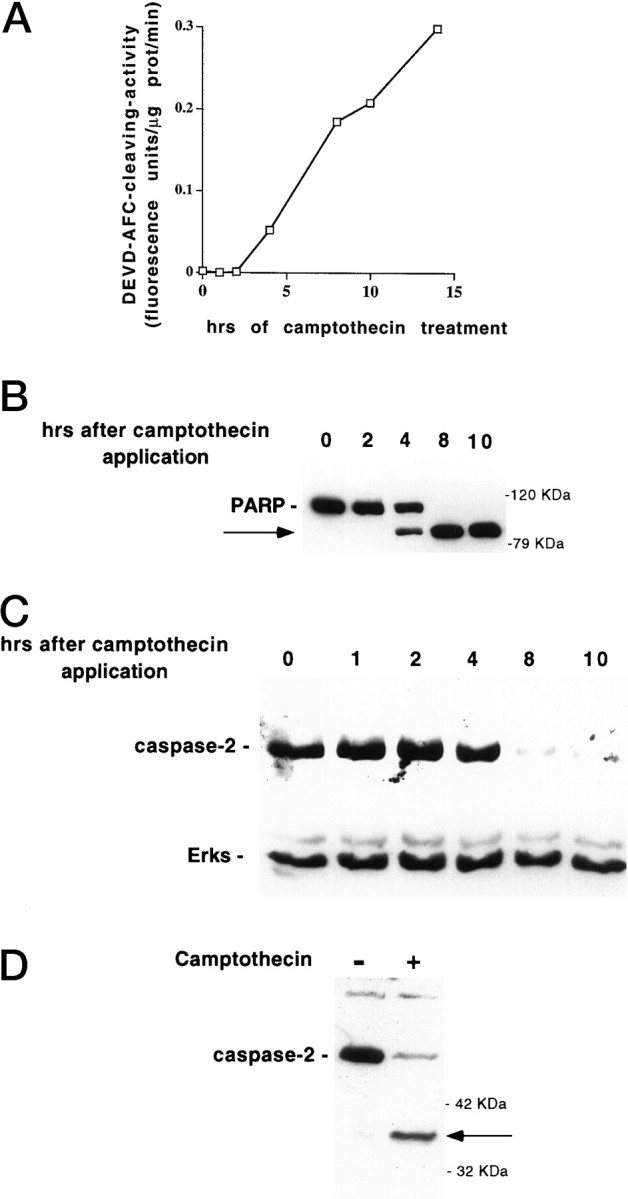
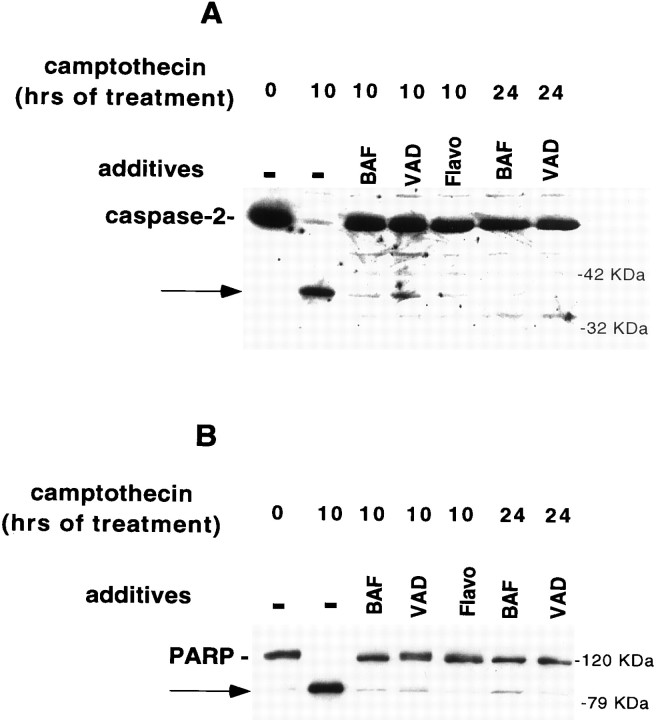
Induction of caspase activity in camptothecin-treated neurons. A, E18 cortical neurons were plated in poly-d-lysine-coated 35 mm dishes and the following day exposed to 10 μm camptothecin. At successive time points after camptothecin application, soluble neuronal lysates (10 μg) were generated and tested for their ability to cleave the fluorogenic substrate DEVD-AFC as described in Materials and Methods. Results are reported as fluorescence units per microgram of protein per minute. B, E18 cortical neurons were plated in poly-d-lysine-coated 35 mm dishes and the following day exposed to 10 μm camptothecin. At successive time points after camptothecin application, particulate neuronal lysates (50 μg) were generated as described in Materials and Methods and subjected to SDS-PAGE on a 10% gel. After Western immunoblotting with the C-2-10 monoclonal anti-PARP antibody (1:5000; (ESP), the bands were visualized by ECL (Amersham). The arrow indicates the 85 kDa cleavage product of PARP. C, E18 cortical neurons were plated in poly-d-lysine-coated 35 mm dishes and exposed the following day to 10 μm camptothecin. At successive time points after camptothecin application, soluble neuronal lysates (50 μg) were generated as described in Materials and Methods and subjected to SDS-PAGE on a 12% gel. After Western immunoblotting with the anti-N-Nedd (caspase-2) antibody (1:250) (top panel) or antibodies against ERK1 and ERK2 (1:2000 each, Santa Cruz) (bottom panel, after stripping the blot), the bands were visualized by ECL (Amersham). D, Lysates harvested from control E18 cortical neurons or from neurons treated with camptothecin for 8 hr were subjected to SDS-PAGE and assessed for caspase-2 processing, as in C. The arrowdenotes the 37 kDa caspase-2 cleavage product.
We also tested processing of the nuclear protein PARP. PARP represents a classic substrate for proteolytic cleavage by caspases including, but not limited to, the caspase-3-like subfamily. We found that PARP processing was induced by 4 hr after camptothecin application (Fig.3B).
Additional immunoblotting experiments assessed the induction of caspase-2 processing after camptothecin treatment. We previously showed that caspase-2 is processed after trophic deprivation in PC12 cells (Stefanis et al., 1997, 1998). In the present studies, we found a substantial reduction of the proform of caspase-2, which started being apparent by 8 hr after camptothecin treatment (Fig. 3C). That this reduction did not reflect a general protein degradation, but specific processing of caspase-2, is demonstrated by the observation that probing the same blot with an antibody against the ERKs showed no reduction in signal over the same period (Fig. 3C). In addition, a 36–37 kDa N-terminal cleavage product of caspase-2 was detected in lysates of cells treated with camptothecin for 8 hr (Fig.3D). This cleavage represents an intermediate step in the formation of activated caspase-2 (Xue et al., 1996, Stefanis et al., 1997, 1998). This cleavage product was seen as early as 4 hr after camptothecin application (data not shown).
Taken together, our data provide evidence for early activation of at least two caspase subclasses, the caspase-3-like caspases and caspase-2, before the death of embryonic cortical neurons after camptothecin treatment.
Cdk inhibitors block caspase activation
We and others have provided evidence for the role of elements of the cell cycle machinery, and in particular of the Cdks, in various paradigms of apoptotic cell death, including withdrawal of trophic support and application of DNA-damaging agents such as camptothecin to postmitotic neurons. It appears that in certain cell death models activation of Cdks occurs at a point downstream of caspase activation (Harvey et al., 1998), whereas in others Cdk inhibition protects neuronal cells by acting at a point upstream of caspase activation (Stefanis et al., 1996, 1998). To ascertain the relative positions of Cdks and caspase-2 processing and caspase-3-like activity in camptothecin-treated E18 cortical neurons, we treated cells with a combination of camptothecin and flavopiridol or olomoucine and 8–10 hr later assessed corresponding lysates for various indices of caspase activity and activation. As shown in Figure4A, both agents completely suppressed caspase-3-like activation, as judged by cleavage of the fluorogenic substrate DEVD-AFC. When these agents were applied directly to the assay, at concentrations that promote survival in culture, they showed no direct inhibition of caspase-3-like activity (data not shown). In addition, both agents completely suppressed the camptothecin-induced processing of caspase-2 (Fig.4B) and PARP (Fig. 4C) in intact cells. Therefore, Cdk inhibitors promote survival in this system at a point upstream of caspase-2- and caspase-3-like activation and activity.
Fig. 4.
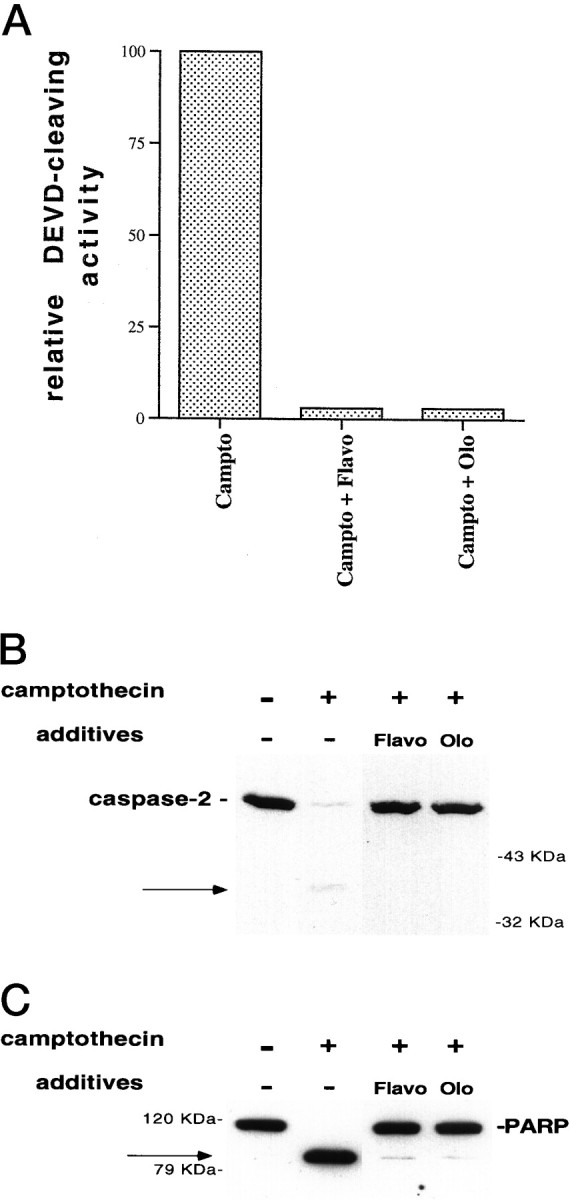
Cdk inhibitors block caspase activation in camptothecin-treated neurons. A, E18 cortical neurons were plated in poly-d-lysine-coated 35 mm dishes and the following day were treated with 10 μm camptothecin alone or together with 1 μm flavopiridol (Flavo) or 200 μm olomoucine (Olo). Ten hours later, soluble lysates were generated (10 μg) and assessed for their ability to cleave the fluorogenic substrate DEVD-AFC (see Materials and Methods). The results are representative of two separate experiments and are reported as percentage of activity of lysates treated with camptothecin alone. B, C, E18 cortical neurons were treated with camptothecin alone or in combination with flavopiridol (1 μm) or olomoucine (200 μm). Ten hours later, soluble (B) or particulate (C) neuronal lysates (50 μg) were generated as described in Materials and Methods and subjected to SDS-PAGE on a 12% (B) or 10% (C) gel. After Western immunoblotting with the anti-N-Nedd antibody (B) (1:250) or the C-2–10 monoclonal anti-PARP antibody (1:5000) (C), the bands were visualized by ECL (Amersham). The arrows indicate the 37 kDa cleavage product for caspase-2 (B) and the 85 kDa PARP cleavage product (C).
General caspase inhibitors completely block caspase-2 and PARP processing, even at 24 hr after camptothecin application, when cells are undergoing extensive neuronal degeneration
The most parsimonious explanation for the lack of prolonged protection from death in neurons treated with the combination of camptothecin and caspase inhibitors would be that the efficacy of the latter decreases with time. To test this possibility, we examined lysates of cells treated with these agents for 10 or 24 hr for the presence of caspase activity and activation. As shown in Figure5, A and B, both zVAD-FMK and BAF inhibited caspase-2 and PARP processing 10 hr after camptothecin application, when used at concentrations of 100 μm, which provide protection from cell death. These agents continued to inhibit caspase-2 and PARP processing even at 24 hr after camptothecin application; in contrast, at this time the survival efficacy of these agents had abated considerably (Figs.1A, 5A,B). At 24 hr, DEVD-AFC cleavage activity also remained completely inhibited by BAF and zVAD-FMK (data not shown).
Fig. 5.
BAF and zVAD-FMK block caspase activity, even after 24 hr of camptothecin exposure. E18 cortical neurons were treated with camptothecin alone or in combination with flavopiridol (Flavo; 1 μm), BAF (100 μm), or zVAD-FMK (VAD; 100 μm). Ten or 24 hr later, soluble (A) or particulate (B) neuronal lysates (50 μg) were generated as described in Materials and Methods and subjected to SDS-PAGE on a 12% (A) or 10% (B) gel. After Western immunoblotting with the anti-N-Nedd antibody (B) (1:250) or the C-2-10 monoclonal anti-PARP antibody (1:5000) (B), the bands were visualized by ECL (Amersham). The arrows indicate the 37 kDa cleavage product for caspase-2 (A) and the 85 kDa PARP cleavage product (B).
These data indicate that inhibition of all detectable caspase activity is achieved with the general caspase inhibitors BAF and zVAD-FMK even at a time (24 hr after camptothecin application) when there is >50% cell death. This supports the notion that there are two types of death promoted by camptothecin in cortical neurons, one that is dependent on caspases and one that is independent of caspase activity. The latter occurs in a delayed manner in neurons treated with the combination of camptothecin and general caspase inhibitors.
Delayed death in the presence of caspase inhibitors occurs in the absence of the classical nuclear manifestations of apoptosis
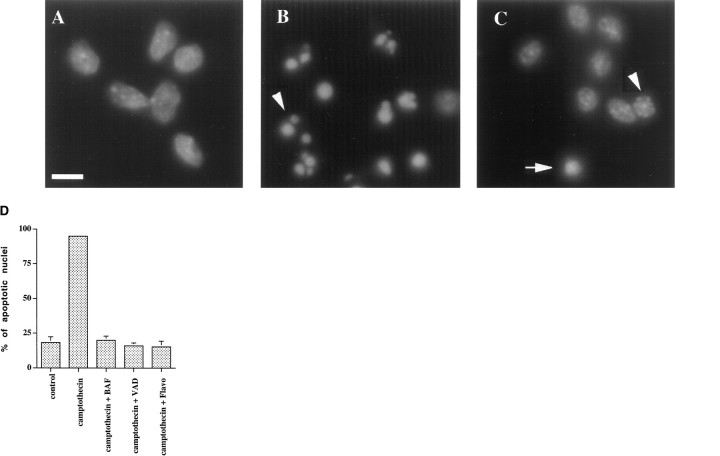
We next investigated more closely the delayed death that occurs at 22–24 hr after combined treatment with camptothecin and general caspase inhibitors. Nuclear counts and trypan blue uptake performed in parallel with these experiments confirmed that >50% of the neurons treated with the combination of camptothecin and the general caspase inhibitors were indeed dead by 24 hr (data not shown). We used Hoechst dye 33342 to stain neuronal nuclei and to characterize their morphology. Sixty-two percent of neurons treated with camptothecin alone for 10 hr showed the classical hallmarks of apoptosis, such as nuclear condensation into discrete dense chromatin clumps, and almost all neurons treated for 22 hr showed these apoptotic nuclear profiles (Fig. 6B,D). In contrast, cultures treated with the combination of camptothecin and caspase inhibitors or flavopiridol showed few apoptotic profiles, similar to control cultures (Fig. 6C,D). Some nuclei after combined treatment with camptothecin and BAF or zVAD-FMK were shrunken to variable degrees, but did not show chromatin condensation into discrete dense clumps (Fig. 6C, arrowhead).
Fig. 6.
Neurons treated with the combination of camptothecin and general caspase inhibitors die in a delayed manner in the absence of the classical nuclear manifestations of apoptosis. Rat E18 cortical neurons were plated in poly-d-lysine-coated 35 mm dishes and treated 2 d later with media alone (A) or 10 μm camptothecin alone (B) or together with 100 μmzVAD-FMK (C). Twenty hours later, the cells were fixed and stained with Hoechst dye 33342 (1 μg/ml; Sigma), and nuclei were visualized by fluorescence microscopy (magnification, 100×). Note the presence of multiple classical apoptotic profiles inB (one of which is highlighted by anarrowhead). An abnormally condensed nucleus is shown inC (arrow). Such nuclei were seen in similar numbers in control cultures. Some of the other nuclei inC are shrunken but do not demonstrate apoptotic morphology (an example of which is shown by thearrowhead). Scale bar, 10 μm. InD, the percentage of apoptotic nuclei for each condition was assessed and reported as mean ± SEM (three separate fields of 100 nuclei each). VAD, zVAD-FMK; Flavo, flavopiridol.
We also assessed the cells with TUNEL staining. TUNEL staining alone is not specific for apoptosis, but combined with evidence of apoptotic death by nuclear morphology, as in our case, it is a reliable indicator of nuclear apoptosis. Few neurons treated with camptothecin and the general caspase inhibitors for 24 hr demonstrated TUNEL staining compared with cells treated with camptothecin alone (Fig.7A–E). In fact, the numbers of TUNEL-stained cells in cultures exposed to camptothecin and caspase inhibitors were comparable with those in control cultures or in cultures treated with the combination of flavopiridol and camptothecin (Fig. 7E).
Fig. 7.
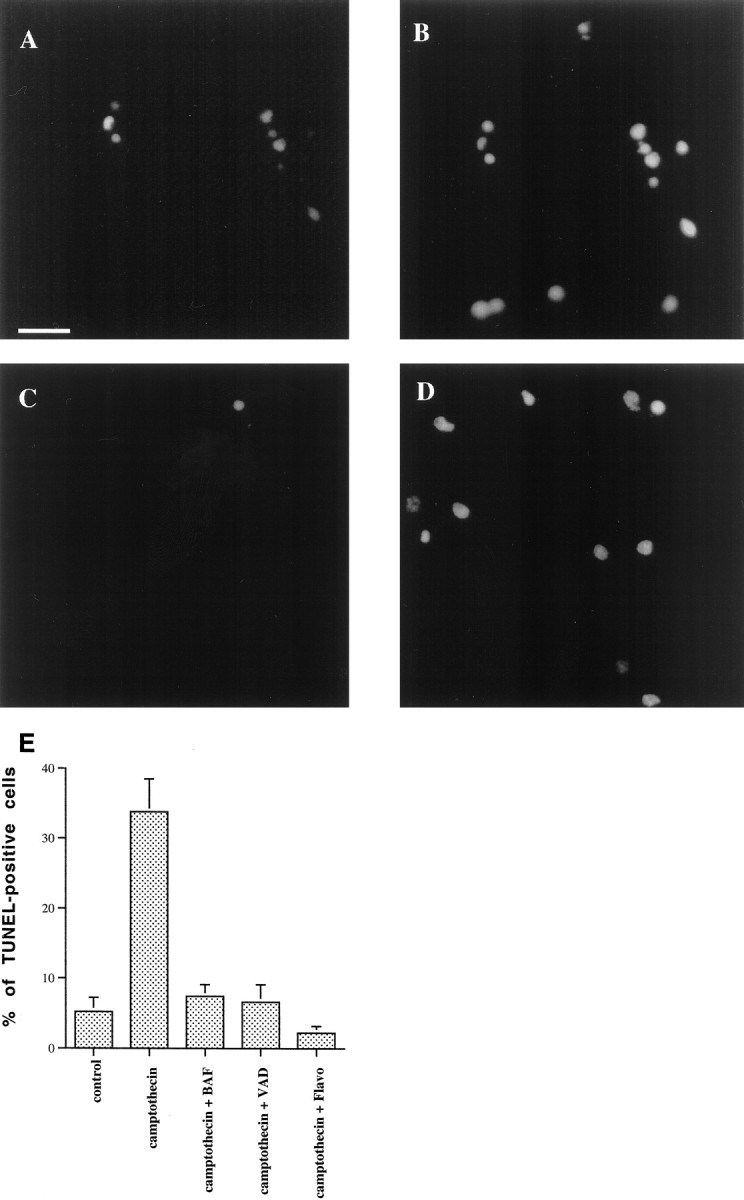
No induction of TUNEL staining in neurons treated with the combination of camptothecin and general caspase inhibitors. Rat E18 cortical neurons were plated in poly-d-lysine-coated 35 mm dishes and treated the following day with 10 μm camptothecin alone (A, B) or in combination with zVAD-FMK (100 μm) (C, D). Twenty-four hours later, the cells were fixed and stained for TUNEL analysis (A, C), according to the manufacturer’s instructions (Boerhinger Mannheim). Nuclei were counterstained with propropidium iodide (50 ng/ml; Sigma) (B, D). Propropidium iodide and TUNEL-positive nuclei were visualized under fluorescence microscopy (magnification, 40×). The percentage of TUNEL-positive nuclei for various conditions is reported in E as the mean ± SEM (5 separate fields of at least 100 nuclei each). Scale bar, 35 μm.VAD, zVAD-FMK; Flavo, flavopiridol.
These results indicate that the delayed death that occurred 24 hr after treatment with the combination of camptothecin and the general caspase inhibitors does not exhibit the nuclear manifestations typical of apoptosis.
Release of cytochrome c and delayed loss of mitochondrial transmembrane potential in camptothecin-treated cortical neurons occur in the presence of general caspase inhibitors but not flavopiridol
Translocation of cytochrome c from mitochondria to the cytoplasm has been shown to be an important feature of apoptotic cell death in a variety of models, although it does not appear to be a universal feature of apoptotic death (Liu et al., 1996; Li et al., 1997; Zhou et al., 1997; Green, 1998; Green and Reed, 1998). With the notable exception of Fas-mediated death (Krippner et al., 1996; Vander Heiden et al., 1997), in most instances cytochrome ctranslocation occurs upstream of initial caspase activation and is thought to play a causal role in this event (Bossy-Wetzel et al., 1998;Deshmukh and Johnson, 1998; Green, 1998; Green and Reed, 1998; Neame et al., 1998). Loss of mitochondrial transmembrane potential also occurs in apoptotic death, but its relationship to release of cytochromec and caspase activation has been less clear (Marchetti et al., 1996; Hirsch et al., 1997; Green and Kroemer, 1998; Neame et al., 1998).
We therefore investigated whether such release of mitochondrial cytochrome c and loss of mitochondrial transmembrane potential occur in our model and the relative position of these events within the cell death pathway. Cells were treated with camptothecin alone or in combination with either general caspase inhibitors or flavopiridol and 10 or 20 hr later incubated with the mitochondrial dye Mitotracker, which labels functional mitochondria. The cells were then fixed and stained with a cytochrome c antibody and the Hoechst dye. In control untreated cultures, neurons were stained with the cytochrome c antibody. This staining colocalized with Mitotracker staining, indicating that it was indeed mitochondrial and that the mitochondria had an intact transmembrane potential (Fig.8A, top row). All neurons with nonapoptotic nuclei showed Mitotracker and cytochromec staining, and all Mitotracker-positive cells were cytochrome c-positive (Table1).
Fig. 8.

A Loss of mitochondrial cytochromec staining in camptothecin-treated neurons occurs in the presence of caspase but not Cdk inhibitors. Rat E18 cortical neurons were plated in poly-d-lysine-coated 35 mm dishes and the following day were left untreated (top row) or treated with 10 μm camptothecin alone (campto,second row) or in combination with flavopiridol (1 μm) (Flavo, third row) or BAF (100 μm) (bottom row). Ten hours later, the cells were incubated with Mitotracker (100 nm; Molecular Probes) and then fixed and stained with a cytochromec antibody (PharMingen; 1:500) and Hoechst dye 33342 (Sigma). The cells were visualized under fluorescence microscopy (magnification, 100×). Exposure times were 25 sec for cytochromec (left column), 3.2 sec for Mitotracker (center column) and 1 sec for Hoechst dye staining (right column). The pictures across each row are from the same field. Note colocalization of cytochrome c and Mitotracker staining in controls and cells treated with camptothecin and flavopiridol. With camptothecin alone, cells with apoptotic nuclei (as shown by Hoechst staining) showed no cytochrome cstaining or low levels of diffuse staining (arrow). Some nonapoptotic cells in camptothecin-treated cultures retained Mitotracker and cytochrome c staining (arrowhead), whereas others still retained Mitotracker staining in the absence of cytochrome c staining (asterisk). Many cells treated with the combination of camptothecin and BAF retained Mitotracker staining but had lost cytochrome c staining (asterisk). Some cells retained both (arrowhead). Scale bar, 10 μm. B, Delayed loss of transmembrane potential in camptothecin-treated neurons occurs in the presence of caspase but not Cdk inhibitors. Rat E18 cortical neurons were treated with the combination of camptothecin and flavopiridol (top row) or BAF (bottom row) for 20 hr and then incubated with Mitotracker and fixed and stained with cytochromec antibody and the Hoechst dye, as in Figure8A. Neurons treated with flavopiridol retained Mitotracker and cytochrome c staining. In contrast, neurons treated with BAF lost both cytochrome c and Mitotracker staining. Scale bar, 10 μm.
Table 1.
Mitotracker and cytochrome cstaining
| Mitotracker+cells | Mitotracker− cells | ||
|---|---|---|---|
| Cytc+ | Cyt c− | ||
| Control | 84 | 0 | 16 |
| Campto | 31 | 4 | 65 |
| Campto + Flavo | 86 | 0 | 14 |
| Campto + BAF | 33 | 35 | 32 |
E18 cortical neurons were treated with medium alone (Control) or 10 μm camptothecin (Campto) alone or in combination with 1 μm flavopiridol (Flavo) or 100 μm BAF. Ten hours later, the cells were assessed for Mitotracker and cytochromec (Cyt c) staining, as described in Materials and Methods.
In cultures treated with camptothecin alone for 10 hr there was a marked reduction in Mitotracker and cytochrome c staining (Fig. 8A, second row). In most cells there was complete loss of cytochrome c staining. The loss of cytochrome c may reflect degradation soon after it is released into the cytoplasm, as reported elsewhere (Neame et al., 1998). In very few cells, a low level of diffuse cytochromec staining was detected, which presumably reflects translocation to the cytoplasm. All cells with apoptotic nuclei (as judged by staining pattern with the Hoechst dye) lost Mitotracker staining. Sixteen percent of cells with nonapoptotic nuclei lost cytochrome c staining, and 6% lost Mitotracker staining. Therefore, mitochondrial alterations appeared to precede the typical nuclear apoptotic changes. Overall, 65% of cells did not have Mitotracker staining. Of the Mitotracker-positive cells, 12% (or 4% of the total number of cells) were cytochrome c-negative (Table 1). The presence of such cells in the cultures, which had lost cytochrome c but not Mitotracker staining, suggests that in DNA-damaged neurons release of cytochrome c occurs before complete loss of transmembrane potential.
Neurons treated with the combination of camptothecin and flavopiridol for 10 hr (Fig. 8A, third row) or 20 hr (Fig.8B, top row) were indistinguishable from control untreated cells in terms of cytochrome c and Mitotracker staining. As in control cultures, all Mitotracker-positive cells were cytochrome c-positive. Therefore, flavopiridol prevents for a prolonged period the mitochondrial alterations induced by DNA damage.
In contrast, cultures treated with the combination of camptothecin and BAF showed substantial loss of mitochondrial cytochrome c at 10 hr, similar to the loss seen in cultures treated with camptothecin alone (Fig. 8A, bottom row; Table 1). Despite this loss of cytochrome c, almost all the neurons were alive at this point. Only 32% of the cells were Mitotracker-negative, compared with 65% in cultures treated with camptothecin alone (Table 1). Many more cells (51% of Mitotracker-positive or 35% of the total number of cells) were identified that were cytochrome c-negative but Mitotracker-positive (Fig. 8A, bottom row; Table 1). These data indicate that caspase inhibitors prevented to a certain extent the loss of mitochondrial transmembrane potential without affecting cytochrome c release. To ensure that Mitotracker-positive cells in this setting reflected functional mitochondria, we treated sister cultures with the mitochondrial uncoupler FCCP (10 μm) for 30 min before incubation with the Mitotracker. This resulted in a significant reduction of Mitotracker staining very close to background levels (data not shown).
Cells treated with the combination of BAF and camptothecin for 20 hr displayed no cytochrome c staining, and only an occasional cell demonstrated low levels of Mitotracker staining (Fig.8B, second row). Pretreatment with FCCP for 30 min before incubation with the Mitiotracker dye did not alter this staining to any appreciable degree (data not shown). Although >50% of the cells were dead at this time (Figs. 1, 2), Hoechst dye staining performed in parallel confirmed that the nuclei of these cells were nonapoptotic. Findings were similar for neurons treated with the combination of zVAD-FMK and camptothecin. Neurons treated with the caspase inhibitors alone showed no loss of mitochondrial staining (data not shown).
These results indicate that loss of mitochondrial cytochromec occurs downstream of Cdk activity and independently of the actions of caspases. In contrast, although loss of mitochondrial transmembrane potential also lies downstream of Cdk activity, it appears to be driven by both caspase-dependent and -independent mechanisms.
Direct inhibition of mitochondrial function leads to a nonapoptotic, caspase-independent death
The early release and loss of mitochondrial cytochromec that was observed in neurons treated with the combination of camptothecin and caspase inhibitors would be expected to lead to disruption of complexes III and IV of the respiratory chain (Mathews, 1985; Bossy-Wetzel et al., 1998) and eventually to inability to maintain mitochondrial transmembrane potential and to loss of mitochondrial function. The temporal correlation between the delayed loss of mitochondrial transmembrane potential and the delayed death in neurons treated with the combination of camptothecin and caspase inhibitors raised the possibility that mitochondrial dysfunction could be a contributing factor to this delayed death. To test whether inhibition of complex III and uncoupling of the mitochondrial transmembrane potential could lead to a death similar to the delayed death seen in cultures treated with camptothecin and caspase inhibitors, we treated the cultures with the complex III inhibitor antimycin A or the uncoupler of transmembrane potential, FCCP, for 12 hr in the presence or absence of BAF and assessed the number of surviving neurons by nuclear counts. We found that both these agents induced death that was not inhibited by BAF (Fig.9A,B). Assessment at 6 hr showed ongoing death that was not delayed in the presence of BAF (data not shown). Hoechst dye and TUNEL staining revealed no induction of apoptotic nuclear profiles in cultures treated for 10 hr with antimycin A or FCCP, whereas camptothecin-treated neurons, used as positive controls, demonstrated the expected induction of apoptotic nuclei (data not shown). Hoechst staining revealed some shrunken nuclei without the dense chromatin clumping characteristic of apoptotic nuclei (Fig.10, see nuclei of FCCP-treated neurons).
Fig. 9.
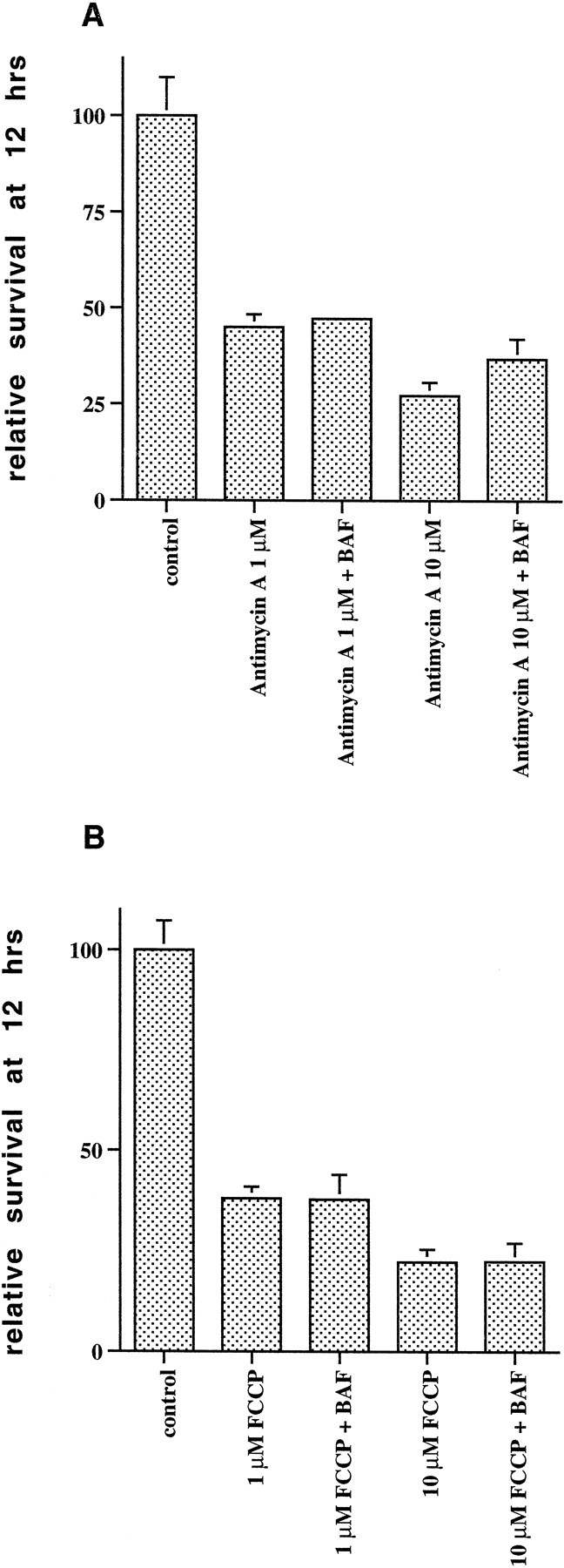
Inhibitors of mitochondrial function induce caspase-independent death of embryonic cortical neurons. Rat E18 cortical neurons were plated in poly-d-lysine-coated 35 mm dishes and 2 d later were treated with medium alone (control) or with 1 or 10 μmantimycin A (A) or 1 or 10 μm FCCP (B), with or without a 2 hr pretreatment with BAF (100 μm). Twelve hours later, the numbers of intact nuclei were assessed as described in Materials and Methods. Survival is reported relative to untreated control cultures and is the mean ± SEM (n = 3).
Fig. 10.
Death attributable to mitochondrial dysfunction occurs in the absence of cytochrome crelease. Rat E18 cortical neurons were plated in poly-d-lysine-coated 35 mm dishes and 2 d later were treated with media alone (control) or with 1 μm antimycin A or 1 μm FCCP. Ten hours later neurons were incubated with Mitotracker and stained with the anti-cytochrome c antibody and with the Hoechst dye, as described in Materials and Methods. There was colocalization of cytochrome c and Mitotracker staining in control cultures, a specific example of which is highlighted by anarrowhead. Neurons treated with antimycin A or FCCP retained cytochrome c staining. In contrast, Mitotracker staining was reduced to background levels. Note also shrunken, but not apoptotic, nuclei in FCCP-treated cultures.
These data indicate that antimycin A and FCCP induce a caspase-independent death without the classical nuclear features of apoptosis. They thus support the idea that loss of mitochondrial cytochrome c, dysfunction of complex III of the respiratory chain, and eventual loss of mitochondrial transmembrane potential and mitochondrial function could underlie the nonapoptotic delayed death seen in neurons treated with the combination of camptothecin and caspase inhibitors.
Death caused by mitochondrial dysfunction occurs without cytochromec release
To confirm that antimycin A and FCCP indeed lead to loss of mitochondrial transmembrane potential and to evaluate the possibility that cytochrome c is released from the mitochondria under these circumstances, we stained neurons treated for 10 hr with antimycin A (1 μm) or FCCP (1 μm) with Mitotracker and the cytochrome cantibody. We found that with these treatments, although there was loss of Mitotracker staining, mitochondrial cytochrome c staining was preserved (Fig. 10).
These data indicate that the nonapoptotic, caspase-independent death induced by mitochondrial dysfunction is not associated with release of cytochrome c from mitochondria. They further indicate that cytochrome c is not necessarily released when the mitochondrial transmembrane potential is lost.
DISCUSSION
Cdk activity is upstream of caspase activation and mitochondrial alterations in the death pathway triggered by camptothecin in E18 cortical neurons
The present findings confirm past reports that Cdk inhibitors protect neurons from death evoked by camptothecin and other DNA-damaging agents (Park et al., 1997, 1998b). Whereas flavopiridol is not known to affect kinases apart from Cdks, olomoucine may have additional effects, such as c-jun kinase inhibition, at least in vitro. However, neither agent prevented c-jun kinase activation when applied to trophic factor-deprived PC12 cells at concentrations that promote survival (Park et al., 1996). We have had similar findings with camptothecin-treated cortical neurons (D. S. Park, L. Stefanis, and L. A. Greene, unpublished data). Observations that protection is also provided by overexpression of the Cdk inhibitory proteins p16 and p27 or of dominant-negative forms of Cdks 4 and 6, and that camptothecin treatment evokes a large increase in neuronal cyclin-D-associated kinase activity (Park et al., 1998a) all support the requisite involvement of Cdk activity in this paradigm of death. In the current study, flavopiridol blocked both caspase activation and mitochondrial alterations, thus placing Cdk activity upstream of these responses to camptothecin.
Involvement of caspase(s) in camptothecin-evoked rapid apoptotic death
We found that camptothecin triggers rapid death of cortical neurons by a pathway resulting in typical apoptotic nuclear changes. Both this rapidly occurring death and the nuclear changes were blocked by general caspase inhibitors. In addition, we detected activity and activation corresponding to caspases 2 and 3 before the onset of death in these cultures. Therefore, caspases appear essential for the execution of a rapid apoptotic death program induced by camptothecin.
Role of mitochondria in early apoptotic death
In at least some systems, cytochrome c translocation from mitochondria to cytoplasm triggers caspase activation and apoptotic death (Liu et al., 1996; Li et al., 1997; Zhou et al., 1997;Green, 1998; Green and Reed, 1998). Thus, the early cytochromec release in camptothecin-treated neurons, which was not blocked by caspase inhibition, may play a causal role in the type of death that is rapid, caspase-dependent, and apoptotic in nature. Loss of mitochondrial transmembrane potential, which leads to release of a variety of mitochondrial molecules, has also been implicated in apoptotic death (Marchetti et al., 1996; Susin et al., 1996, 1997;Hirsch et al., 1997; Green and Kroemer, 1998). We found that both release of mitochondrial cytochrome c and loss of transmembrane potential occur before nuclear apoptotic changes. Moreover, cytochrome c release occurred before complete loss of transmembrane potential. In the presence of caspase inhibitors the loss of transmembrane potential was delayed, and many neurons that had lost cytochrome c staining by 10 hr of camptothecin treatment retained Mitotracker staining. It is thus tempting to construct a model in which cytochrome c release leads to caspase activation, which in turn leads or contributes to loss of transmembrane potential. Consistent with this, caspases can induce permeability transition and loss of transmembrane potential in isolated mitochondria (Susin et al., 1997). However, we cannot rule out that low-level permeability transition that leads to only small decrements of mitochondrial transmembrane potential, without complete loss of Mitotracker staining, induces release of cytochrome c(and/or other mitochondrial factors) and consequent caspase activation, leading to a circular feedback loop, as proposed by Green and Kroemer (1998). These possibilities are summarized in a model for rapid camptothecin-induced apoptotic death (Fig.11A). Previous studies in non-neuronal and neuronal systems have situated caspases either downstream (Green and Kroemer, 1998; Neame et al., 1998) or upstream (Marchetti et al., 1996; Bossy-Wetzel et al., 1998) of disruption of mitochondrial transmembrane potential. The relative position of these events may be dependent on the cell death model and the participation of “initiator” and “effector” caspases.
Fig. 11.
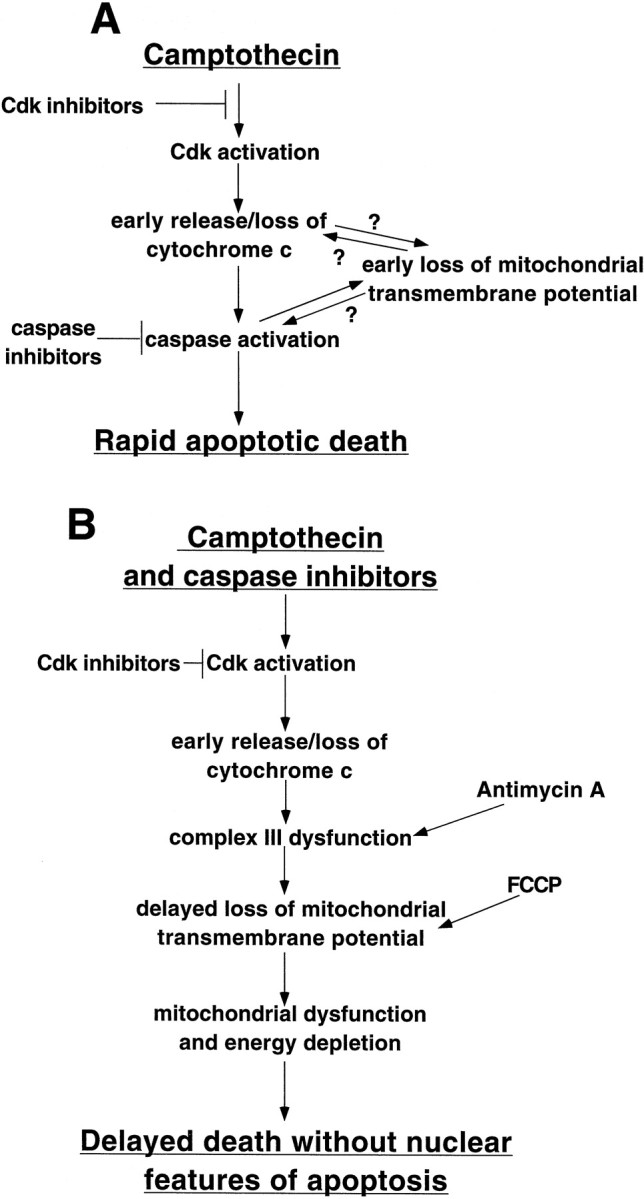
Provisional model of the death pathway(s) elicited by camptothecin treatment of E18 cortical neurons.A, Model of early apoptotic pathway elicited by camptothecin. B, Model of delayed death without classical nuclear features of apoptosis that occurs in the presence of caspase inhibitors and camptothecin or inhibitors of mitochondrial function
Delayed, caspase-independent death of camptothecin-treated cortical neurons
Although general caspase inhibitors blocked rapid apoptotic death elicited by camptothecin, they did not provide long-term protection. This did not appear to be caused by decreased caspase inhibition at later time points. In contrast to the rapid death that occurs with camptothecin alone, the delayed death that occurs with caspase inhibitors and camptothecin does not show the characteristic nuclear changes of apoptosis. Similar findings have been reported in other systems. For example, overexpression of Bax leads to caspase-independent death of Jurkat T cells, which does not demonstrate nuclear apoptosis (Xiang et al., 1996). Treatment of human fibroblasts with a variety of death-initiating stimuli in the presence of caspase inhibitors results in death, but nuclear and membrane manifestations of apoptosis are blocked (McCarthy et al., 1997). Nonapoptotic death of K+-deprived cerebellar granule cells occurs in the presence of caspase inhibitors, and these agents do not significantly alter the kinetics of death (Miller et al., 1997). This contrasts with the present system of DNA damage in which general caspase inhibitors delayed death and in which the neurons were morphologically and metabolically intact at a time (12 hr) when neurons treated with camptothecin alone were almost all dead. Recently, Johnson et al. (1999) reported a caspase-independent death in postnatal cortical cultures treated with camptothecin. It remains to be seen whether this death shares similar mechanisms with the delayed death in the embryonic cultures.
Apoptotic and nonapoptotic pathways to death in camptothecin-treated neurons
The current study has disclosed two distinct types of death in camptothecin-treated cortical neurons. One occurs rapidly (6–12 hr), has the nuclear morphological features of apoptosis, and is dependent on caspase activity. The other, which is observed only when caspase activity is blocked (and therefore caspase-independent), is delayed (12–48 hr) and does not exhibit classical nuclear manifestations of apoptosis.
There are two possible interpretations of the presence of caspase-dependent and -independent types of cortical neuron death triggered by camptothecin. One possibility is that both death programs are induced by camptothecin and propagate independently. If this is the case, then the nonapoptotic program must have a significant time lag compared with the rapid apoptotic program, and both must be blocked by flavopiridol. A potential mechanism here could be that neurons eventually succumb because of the accrued degree of DNA damage sustained by camptothecin. At odds with this, however, flavopiridol should not prevent DNA damage but does provide long-term protection.
The second possibility is that a single, flavopiridol-sensitive pathway is initiated that, if unhindered, leads to caspase activation and consequent rapid apoptotic death. If this program is blocked at the step of caspase activation, then other steps in the pathway continue that over time lead to delayed death. In this case, death occurs without manifesting nuclear apoptotic changes, because these changes require caspases.
Role of mitochondria in delayed death without nuclear features of apoptosis
Tentative support, and a conceptual framework, for the second alternative is provided by observations regarding the role of mitochondria in cell death. Our studies indicate early loss of mitochondrial cytochrome c and delayed loss of transmembrane potential in neurons simultaneously treated with camptothecin and caspase inhibitors. Disappearance of cytochrome c from the mitochondria should lead to impairment of complexes III and IV of the respiratory chain (Mathews, 1985; Bossy-Wetzel et al., 1998). Over time this should lead to energy depletion and inability to maintain mitochondrial transmembrane potential (Green, 1998; Green and Reed, 1998). Therefore, the delayed loss of transmembrane potential in the presence of caspase inhibitors could be attributable, at least in part, to loss of mitochondrial cytochrome c (Fig.11B). It is also possible that additional caspase-independent mechanisms contribute to the delayed loss of transmembrane potential. We propose that in either case the impairment of mitochondrial function that occurs in the presence of camptothecin and caspase inhibitors is responsible for the delayed cell death lacking the nuclear features of apoptosis (Fig. 11B). Consistent with this idea, we found that agents that inhibit complex III of the respiratory chain or that uncouple the mitochondrial transmembrane potential induce caspase-independent death without nuclear apoptosis. Interestingly, this death occurs without cytochromec release. Depending on the local milieu and their energy state, neurons may or may not be able to maintain energy sources adequate for long-term survival under such conditions. For instance, cultured NGF-deprived sympathetic neurons survive for long periods in the presence of general caspase inhibitors (Deshmukh et al., 1996), although cytochrome c and Mitotracker staining are lost from the mitochondria (Neame et al., 1998). In contrast, it may be that E18 cortical neurons cannot sustain viability in the face of such disruption of mitochondrial function. Nicotera and colleagues and others have proposed that cellular ATP levels are a determinant of the mode of death; if ATP levels are sufficient, death is apoptotic, and if not, death occurs by a nonapoptotic pathway (Eguchi et al., 1997; Leist and Nicotera, 1997; Leist et al., 1997). This idea is consistent with the loss of mitochondrial function and the nonapoptotic death that occurs in cortical neurons exposed to both camptothecin and caspase inhibitors.
In conclusion, our findings shed light on the mechanisms by which the DNA-damaging agent camptothecin kills cortical neurons. Based on these findings as well as past work, we favor a provisional model in which (1) DNA damage leads to Cdk activation; (2) this in turn promotes and is required for loss of mitochondrial cytochrome c and early loss of transmembrane potential as well as (3) rapid activation of caspases (which may be dependent on cytochrome c release); (4) caspase activation leads to rapid apoptotic death; and (5) if caspase activity is blocked, loss of cytochrome c (perhaps in conjunction with additional mechanisms) results in delayed loss of transmembrane potential and mitochondrial dysfunction, which eventually leads to death by a nonapoptotic mechanism. These findings may be applicable to other models in which caspase inhibition does not significantly promote neuronal survival, such as K+- and serum-deprived cerebellar granule cells (Miller et al., 1997). They also suggest that there may be limitations to the extent to which caspase inhibitors may be effective as neurotherapeutic agents.
Footnotes
This work was supported in part by grants from National Institutes of Health–National Institute of Neurological Diseases and Stroke, the Blanchette Rockefeller Foundation, and the ALS Foundation. L.S. was supported in part by a grant from the Lucille P. Markey Trust and a Wellcome Burroughs career award in biomedical sciences. D.S.P. was supported in part by the Aaron Diamond Foundation and the Medical Research Council of Canada. We thank Carol M. Troy for the generous gift of the caspase-2 antibody, James E. Goldman for the use of his fluorescent microscope, and Giovanni Manfredi, Robert E. Burke, Toni Barrientos, and Andy Giovanni for helpful discussions.
Correspondence should be addressed to Leonidas Stefanis, Department of Pathology, Taub Center for Alzheimer’s Disease Research and Center for Neurobiology and Behavior, Columbia University College of Physicians and Surgeons, 630 West 168th street, P&S 15-401, New York, NY, 10032.
Dr. Park’s present address: Neuroscience Research Institute, University of Ottawa, 451 Smyth Road, Ottawa, Ontario KIH8M5, Canada.
REFERENCES
- 1.Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–482. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- 2.Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:39–47. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Deshmukh M, Johnson EM., Jr Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron. 1998;21:695–705. doi: 10.1016/s0896-6273(00)80587-5. [DOI] [PubMed] [Google Scholar]
- 5.Deshmukh M, Vasilakos J, Deckwerth TL, Lampe PA, Shivers BD, Johnson EM., Jr Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor of the ICE family. J Cell Biol. 1996;135:1341–1354. doi: 10.1083/jcb.135.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Porzio U, Daguet MC, Glowinski J, Prochiantz A. Effect of striatal cells on in vitro maturation of mesencephalic dopaminergic neurones grown in serum-free conditions. Nature. 1980;288:370–373. doi: 10.1038/288370a0. [DOI] [PubMed] [Google Scholar]
- 7.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 8.Farinelli SE, Greene LA. Cell cycle blockers mimosine, ciclopirox and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support. J Neurosci. 1996;16:1150–1162. doi: 10.1523/JNEUROSCI.16-03-01150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farinelli SE, Greene LA, Friedman WJ. Neuroprotective actions of dipyridamole on cultured CNS neurons. J Neurosci. 1998;18:5112–5123. doi: 10.1523/JNEUROSCI.18-14-05112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser A, Evan G. A license to kill. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 11.Friedman WJ, Ibanez CF, Hallbook F, Persson H, Cain LD, Dreyfus CF, Black IB. Differential actions of neurotrophins in the locus coeruleus and basal forebrain. Exp Neurol. 1993;119:71–78. doi: 10.1006/exnr.1993.1007. [DOI] [PubMed] [Google Scholar]
- 12.Gobbel GT, Bellinzona M, Vogt AR, Gupta N, Fike JR, Chan PH. Response of postmitotic neurons to X-irradiation: implications for the role of DNA damage in neuronal apoptosis. J Neurosci. 1998;18:147–155. doi: 10.1523/JNEUROSCI.18-01-00147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 14.Green DR, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- 15.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 16.Harvey KJ, Blomquist JF, Ucker DS. Commitment and effector phases of the physiological cell death pathway elucidated with respect to Bcl-2, caspase, and cyclin-dependent kinase activities. Mol Cell Biol. 1998;18:2912–2922. doi: 10.1128/mcb.18.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawtin SR, Dobbins AC, Vipula JT, Shearman MS. β-Amyloid inhibition of MTT reduction is not mimicked by inhibitors of mitochondrial respiration. Biochem Soc Trans. 1995;23:56S. doi: 10.1042/bst023056s. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch T, Marchetti P, Susin SA, Dallaporta B, Zamzami N, Marzo I, Geuskens M, Kroemer G. The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene. 1997;15:1573–1582. doi: 10.1038/sj.onc.1201324. [DOI] [PubMed] [Google Scholar]
- 19.Johnson MD, Kinoshita Y, Xiang H, Ghatan S, Morrison RS. Contribution of p53-dependent caspase activation to neuronal cell death declines with neuronal maturation. J Neurosci. 1999;19:2996–3006. doi: 10.1523/JNEUROSCI.19-08-02996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krippner A, Matsuno-Yagi A, Gottlieb RA, Babior BM. Loss of function of cytochrome c in Jurkat cells undergoing Fas-mediated apoptosis. J Biol Chem. 1996;271:21629–21636. doi: 10.1074/jbc.271.35.21629. [DOI] [PubMed] [Google Scholar]
- 21.Leist M, Nicotera P. The shape of cell death. Biochem Biophys Res Commun. 1997;236:1–9. doi: 10.1006/bbrc.1997.6890. [DOI] [PubMed] [Google Scholar]
- 22.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Kim CN, Pohl J, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69:581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- 26.Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184:1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathews FS. The structure, function and evolution of cytochromes. Prog Biophys Mol Biol. 1985;45:1–56. doi: 10.1016/0079-6107(85)90004-5. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy NJ, Whyte MKB, Gilbert CS, Evan GI. Inhibition of Ced3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller TM, Moulder KL, Knudson CM, Creedon DJ, Deshmukh M, Korsmeyer SJ, Johnson EM., Jr Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J Cell Biol. 1997;139:205–217. doi: 10.1083/jcb.139.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris EJ, Geller HM. Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell cycle-independent toxicity. J Cell Biol. 1996;134:757–770. doi: 10.1083/jcb.134.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neame SJ, Rubin LL, Philpott KL. Blocking cytochrome c activity within intact neurons inhibits apoptosis. J Cell Biol. 1998;142:1583–1593. doi: 10.1083/jcb.142.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park DS, Farinelli SE, Greene LA. Inhibitors of cyclin-dependent kinases promote survival of post-mitotic neuronally differentiated cells and sympathetic neurons. J Biol Chem. 1996;271:21898–21905. doi: 10.1074/jbc.271.14.8161. [DOI] [PubMed] [Google Scholar]
- 33.Park DS, Morris EJ, Greene LA, Geller HM. G1/S cell cycle blockers and inhibitors of cyclin-dependent kinases suppress camptothecin-induced apoptosis. J Neurosci. 1997;17:1256–1270. doi: 10.1523/JNEUROSCI.17-04-01256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park DS, Morris EJ, Padmanabhan J, Shelanski ML, Geller HM, Greene LA. Cyclin-dependent kinases participate in death of neurons evoked by DNA-damaging agents. J Cell Biol. 1998a;143:457–467. doi: 10.1083/jcb.143.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park DS, Morris EJ, Stefanis L, Troy CM, Shelanski ML, Geller HM, Greene LA. Multiple pathways of neuronal death induced by DNA-damaging agents, NGF deprivation, and oxidative stress. J Neurosci. 1998b;18:830–840. doi: 10.1523/JNEUROSCI.18-03-00830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rukenstein A, Rydel RE, Greene LA. Multiple agents rescue PC12 cells from serum-free cell death by translation- and transcription-independent mechanisms. J Neurosci. 1991;11:2552–2563. doi: 10.1523/JNEUROSCI.11-08-02552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 38.Stefanis L, Park DS, Yan CYI, Farinelli SE, Troy CM, Shelanski ML, Greene LA. Induction of CPP32-like activity in PC12 cells by withdrawal of trophic support. J Biol Chem. 1996;271:30663–30671. doi: 10.1074/jbc.271.48.30663. [DOI] [PubMed] [Google Scholar]
- 39.Stefanis L, Troy CM, Qi H, Greene LA. Inhibitors of trypsin-like serine proteases inhibit processing of the caspase Nedd-2 and protect PC12 cells and sympathetic neurons from death evoked by withdrawal of trophic support. J Neurochem. 1997;69:1425–1437. doi: 10.1046/j.1471-4159.1997.69041425.x. [DOI] [PubMed] [Google Scholar]
- 40.Stefanis L, Troy CM, Qi H, Shelanski ML, Greene LA. Caspase-2 (Nedd-2) processing and death of trophic factor-deprived PC12 cells and sympathetic neurons occur independently of caspase-3-like activity. J Neurosci. 1998;18:9204–9215. doi: 10.1523/JNEUROSCI.18-22-09204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Susin SA, Zamzami N, Castedo M, Daugas E, Wang H, Geley S, Fassy F, Reed JC, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troy CM, Stefanis L, Greene LA, Shelanski ML. Nedd2 is required for apoptosis after trophic factor withdrawal, but not superoxide dismutase (SOD1) down-regulation, in sympathetic neurons and PC12 cells. J Neurosci. 1997;17:1911–1918. doi: 10.1523/JNEUROSCI.17-06-01911.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 45.Xiang H, Kinoshita Y, Knudson CM, Korsmeyer SJ, Schwartzkroin PA, Morrison RS. Bax involvement in p53-mediated neuronal cell death. J Neurosci. 1997;18:1363–1373. doi: 10.1523/JNEUROSCI.18-04-01363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang J, Chao DT, Korsmeyer SJ. Bax-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue D, Shaham S, Horvitz HR. The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of human CPP32 protease. Genes Dev. 1996;10:1073–1083. doi: 10.1101/gad.10.9.1073. [DOI] [PubMed] [Google Scholar]
- 48.Zhou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4: participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]