Abstract
GAL4-driven targeted expression of tetanus toxin light chain (UAS-TeTxLC) in a subset of chemosensory neurons of the larval antennomaxillary complex (AMC) and pharynx causes abnormal chemosensory behavior in Drosophila melanogaster. Consistent with strongest staining in the dorsal organ (DO), the presumed olfactory organ of the AMC, tetanus toxin-expressing larvae subjected to an olfactory preference assay show anosmic behavior to most volatile substances tested. Furthermore, we observed reduced responses to sodium chloride, fructose, and sucrose in gustatory plate assays. Surprisingly, the entire subset of labeled sensory neurons from the terminal (maxillary) organ (TO) of the AMC was found to project via the antennal nerve to the larval antennal lobe region. The maxillary nerve remained completely unstained. Hence, a subset of neurons from the TO builds an anatomical entity with projections from the DO. Our results suggest that the AMC contains both olfactory and gustatory sensilla, and that the DO is the main olfactory organ in larvae.
Keywords: Drosophila melanogaster, insect, larva, antennomaxillary complex, GAL4 enhancer trap line, tetanus toxin expression, smell, taste, behavior
A large number of substances can elicit specific olfactory and gustatory responses in larvae ofDrosophila melanogaster (Miyakawa, 1982; Monte et al., 1989;Ayyub et al., 1990; Jenkins and Tompkins, 1990; Cobb et al., 1992; Cobb and Dannet, 1994; Singh, 1997). Genetic and molecular studies describe several loci or genes involved in larval chemosensory perception (Rodrigues and Siddiqi, 1978; Monte et al., 1989; Siddiqi, 1991;Carlson, 1996; Cobb, 1996; Hekmat-Scafe and Carlson, 1996; Park et al., 1997). However, little is known about the receptor cells and brain centers required for larval chemosensation.
From anatomical studies we know that the peripheral larval chemosensory system consists mainly of three sensory organs, the dorsal organ (DO) and terminal organ (TO), also referred to as the antennomaxillary complex (AMC), and the ventral organ (Chu-Wang and Axtell, 1972;Stocker, 1994; Campos-Ortega and Hartenstein, 1997). The structural features of the perforated dome sensillum of the DO strongly imply a role in the perception of volatile substances. In contrast, sensilla surrounding the dome, as well as sensilla of the TO, have single pores and may therefore be contact chemoreceptors (Frederick and Denell, 1982; Singh and Singh, 1984; Stocker, 1994). Evidence for an olfactory function of the AMC has been provided through work on mutants of the gene ana, which is expressed in glial cells of the AMC (Park et al., 1997). Putative gustatory sensilla have also been described on the body wall and in internal sensory organs of the pharynx (Singh, 1997).
Development of the enhancer trap technology has provided us with a powerful tool to study chemosensory anatomy and perception (Riesgo-Escovar et al., 1992). The P[GAL4] system is extremely versatile to visualize and manipulate subsets of neurons in various ways (Fischer et al., 1988; Brand and Perrimon, 1993). The yeast transcription factor GAL4 directs expression of any gene fused to upstream activation sequence (UAS) elements and thus permits ectopic expression of different cell marker genes as well as toxin genes (Brand and Perrimon, 1993; Sweeney et al., 1995). Targeted expression of tetanus toxin light chain (UAS-TeTxLC) makes it possible to impair the function of neurons expressing GAL4 in enhancer trap lines. Tetanus toxin has been shown to specifically degrade synaptobrevin and thus to block evoked neurotransmitter release in Drosophila(Sweeney et al., 1995). We have created new P[GAL4] enhancer trap lines with expression patterns in the larval chemosensory system. In this study, we present a detailed anatomical and functional analysis of subsets of larval chemosensory neurons in P[GAL4] line GH86. Toxin expression in a number of cells of the AMC and the pharynx in line GH86, in combination with behavioral tests, confirms their expected function in olfaction and gustation. Furthermore, we provide evidence for chemical specificity of subsets of neurons.
MATERIALS AND METHODS
Strains. Line GH86 was isolated by remobilizing a lethal P element insertion on the X chromosome, P{GawB}l(1)BP1, as described by Brand and Perrimon (1993). Mapping of the P element insertion site of line GH86 was done by in situhybridization with biotin-labeled probes as described in Ashburner (1989). Wild-type lines Canton S (kindly provided by E. Buchner, Universität Würzburg, Germany) and Sevelen served as controls. Secondary reporter strains were UAS-lacZ (Brand and Perrimon, 1993), UAS-tau (Ito et al., 1997),UAS-GFP (Yeh et al., 1995), and UAS-TeTxLCtransformants (Sweeney et al., 1995). Line TNT-E shows the weakest TeTxLC expression of the three UAS-TeTxLC lines available, whereas line IMPT-TNT-Q4A contains an inactive UAS-TeTxLCconstruct (insertions on the second chromosome; both lines are a gift of S. Sweeney, Cambridge University, Cambridge, UK). Larvae and flies were raised on standard cornmeal food at 18° or 25°C.
Immunocytochemistry and microscopy. β-Galactosidase staining of embryos was modified from the method of Ashburner (1989), protocol 76. The formaldeyde fixation was replaced by 1% glutaraldehyde and was done for 45 min. β-Galactosidase staining of whole-mount larval brains and epidermis was done as previously described (Stocker et al., 1997). Ten micrometer cryosections of transheterozygotes p[GAL4]/UAS-tau and p[GAL4]/UAS-TeTxLC were stained using anti-TAU (1:2000; Sigma, St. Louis, MO) or anti-TeTxLC monoclonal antibodies (mAbs, 1:1000; kindly provided by H. Niemann, Universität Hannover, Hannover, Germany). Subsequently we applied the Vectastain ABC system (Vector Laboratories, Burlingame, CA) (Stocker et al., 1997). For whole-mount labeling of larval brains with anti-TeTxLC antibody, an HRP-conjugated secondary antibody from Bio-Rad (Hercules, CA) was used to reduce background staining (Sweeney et al., 1995). Staining of neuropil was done with mAb nc82 (a gift of A. Hofbauer, Universität Regensburg, Regensburg, Germany), and labeling of the DO and TO ganglia was achieved, using mAb 22C10 (kindly provided by S. Benzer, Caltech, Pasadena, CA). Whole mounts or 10 μm cryosections were fixed for 2 hr in 4% formaldehyde and PBS, pH 7.6, on ice, washed in 20% sucrose overnight, blocked in 3% normal serum and PBS/0.2% Triton X-100, and incubated with mAbs nc82 (1:10 dilution) and 22C10 (1:10) overnight at room temperature. Secondary antibody was anti-mouse F(ab′)2 coupled to indocarbocyanine fluorophore Cy3 (Jackson ImmunoResearch, West Grove, PA), diluted 1:100 in blocking solution. Preparations were mounted in Vectashield (Vector Laboratories). Confocal microscopy was performed with a BioRad MRC 1024 microscope equipped with a Kr/Ar laser. Pictures were taken as 0.7 μmZ series.
Olfactory tests. Larval plate assays for volatile substances were performed with modifications of the method of Aceves-Piña and Quinn (1979). Only feeding third instar larvae were used for the tests. The animals were washed out of the food with a 15% sucrose solution. After two rinses in water, 50 larvae were hand-picked and immediately tested. Tests were done on Petri dishes (diameter, 85 mm) covered with a layer of 1.2% agarose. To avoid diffusion of the test substance, plates were air-dried before use. Odor and control diluent (water or mineral oil) were placed on two small filter disks (Rufi 595, diameter, 10 mm; Schleicher & Schuell, Keene, NH) on opposite sides of the Petri dish (see Fig. 1). The filter disks can be placed on plastic supports (e.g., lids of 1.5 ml micro test tubes) to avoid diffusion through the agarose. For the small amounts of chemicals used in the assays (1 and 2 μl), no difference between responses was found for tests with or without plastic support. Concentrations and amounts were as follows: ethyl acetate (Merck, Darmstadt, Germany; 109623), propionic acid (Fluka, Buchs, Switzerland; 81910), and butanol (Fluka 19420), 1 μl undiluted; n-octyl acetate (Sigma O-0504), 2 μl undiluted; cyclohexanone (Fluka 29140), 1 μl undiluted; and 1 μl of a 1:10 dilution in mineral oil, respectively. Approximately 50 larvae were placed in the center of the plate before adding the test substances. The Petri dish was immediately covered with the lid. After 5 min, larvae were counted as shown in Figure1A. Only larvae on semicircular areas (radius, 30 mm) around the filter disks were included. Thus, the animals had to move at least one body length toward the source. We then calculated a response index (RI): Ns −Nc/Ns +Nc. Ns represents the number of animals at ≤30 mm from the odor source (inside areaaS in Fig. 1); Nc is the number of larvae found inside an identical surface on the opposite (control) side. Positive RIs indicate attraction; negative RIs indicate avoidance; and RI = 0 indicates indifferent behavior. Tests were done in artificial light, under a fume hood, and plates were turned occasionally during the test to compensate for visual cues.
Fig. 1.
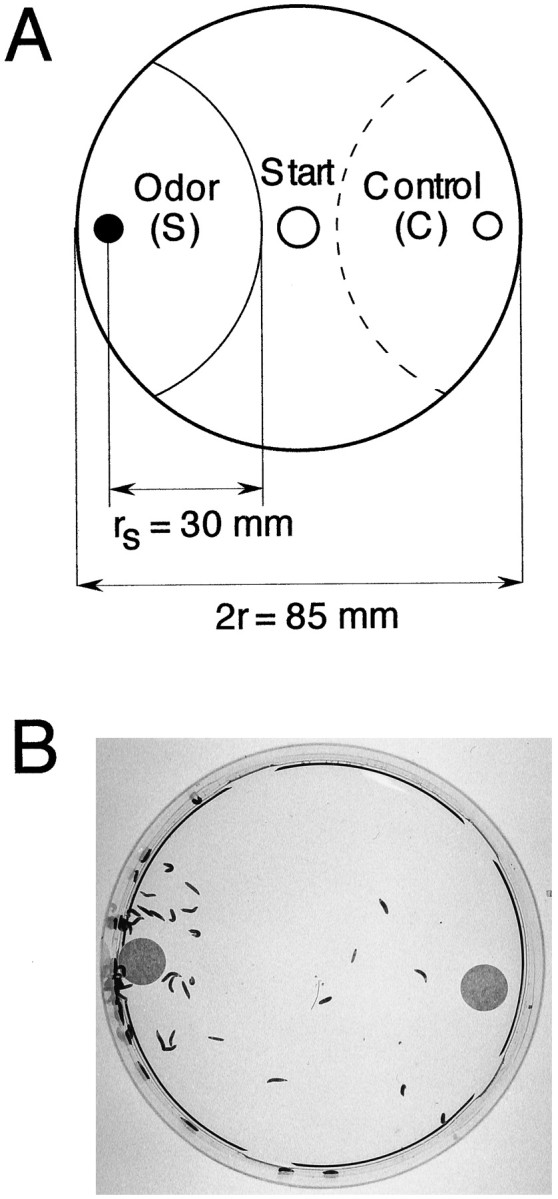
Olfactory larval plate assay. A, Schematic representation of the test setup. Small filter disks containing a test chemical (S) and control diluent (C) are placed on opposite sides of a Petri dish covered with a layer of agarose. Fifty animals are transferred to the start point and counted after 5 min in indicated semicircular areas. For calculation of response, see Materials and Methods. B, Larval plate assay of wild-type CS. The filter on the left contains 1 μl of undiluted propionic acid. The picture was taken 5 min after the test start.
Gustatory tests. For gustatory choice tests, I-plate Petri dishes (separated in two halves, Falcon 1003) were filled with 1% agarose and water (C) and agarose and test solution (S) on opposite halves (Lilly and Carlson, 1990). Chemicals tested were sucrose (Fluka 84100), fructose (Merck 5321), and NaCl (Fluka 71380). To avoid diffusion, plates were poured immediately before testing. Fifty larvae were placed on top of the separating plastic bridge and allowed to freely move on the entire plate. They were counted after 5, 15, and 30 min. An RI was calculated for each time point (RI =Ns −Nc/Ns +Nc, Ns andNc referring to the numbers present on test and control areas, respectively). Animals found at a distance of 0.5 cm from each side of the plastic bridge were not counted.
Statistics. Statistical analysis was done with StatView software (Abacus Concepts, Inc., Berkeley, CA). Because we cannot exclude a non-normal distribution of the relatively small number of observations, significance of behavioral differences was assessed with nonparametric tests (Mann–Whitney U test); significance level, p < 0.05 (Sokal and Rohlf, 1995). Only statistical analysis pertinent to the discussion of the results is presented in this work. Further statistical data are available on request.
RESULTS
Targeted expression of TeTxLC in line GH86 strongly impairs odor-driven behavior
P[GAL4] enhancer trap line GH86 was isolated in a screen for specific expression in the larval and adult chemosensory system. Chromosomal in situ hybridization revealed a single P[GAL4] insertion at 7C8/9 on the X chromosome (data not shown). Because of its very specific expression pattern in few neurons of the larval chemosensory system (see Fig. 7), we chose this line for functional studies of these particular neurons. We expected to find distinct behavioral defects when abolishing neuronal function by expressing tetanus toxin light chain (TeTxLC). The progeny from a cross of GH86 × TNT-E (UAS-TeTxLC transformant line) is fully viable and develops normally. No behavioral changes in feeding or locomotion could be detected during casual observations. These larvae are therefore well suited for functional analysis of the chemosensory system. The specific expression pattern in a subset of cells of the AMC and pharyngeal sensilla prompted us to test for changes both in olfactory and gustatory behavior. We used simple olfactory and gustatory paradigms, using a relatively small number of previously tested chemicals, most of which are food components ofDrosophila larvae, e.g., acetates, acids, ketones, and alcohols (Aceves-Piña and Quinn, 1979; Miyakawa, 1982; Monte et al., 1989; Ayyub et al., 1990; Jenkins and Tompkins, 1990; Cobb et al., 1992; Cobb and Dannet, 1994). In contrast to adult flies, larvae are generally attracted by most volatile substances when presented at high concentrations.
Fig. 7.
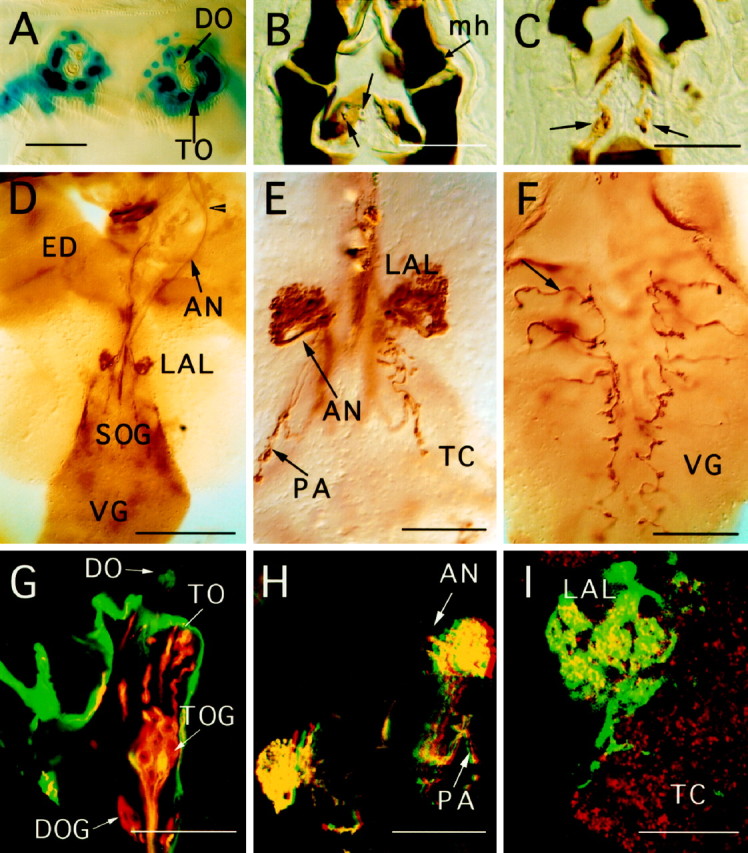
GAL4-driven expression pattern of line GH86 in third larval instar, using different UAS reporter genes. For tetanus toxin expression, the active UAS-TeTxLC was used in all preparations. All photographs are oriented with anterior ontop. A, Nuclear lacZ staining of chemosensory neurons of the DO and TO in a whole-mount preparation, showing 30–35 nuclei per side. B, C, Consecutive 10 μm cryosections stained with anti-tetanus antibody.Arrows indicate cuticular structures of the internal mouth organ and the corresponding chemosensory neurons.mh, Mouth hook. D, Overview of projection patterns visualized by anti-tetanus staining of a larval brain whole-mount preparation. The arrowhead marks the fusion point of projections from the TO with the AN. Afferent fibers arborize inside the LAL. ED, Eye-antennal imaginal disk;VG, ventral ganglion. E, Arborizations of AMC projections and fibers from pharyngeal sensilla (PA) at a higher magnification. F, Afferents (arrow) from the thoracicoabdominal peripheral nervous system shown in a whole-mount preparation of the VG. Irregularities of the arborization pattern can be seen along the midline. G, Confocal image of a section of the AMC. A UAS-GFP reporter construct was used to show expression of line GH86 in the AMC in greater detail (green). Counterstaining of chemosensory neurons was done with mAb 22C10 (red). Overlapping staining of GFP and mAb 22C10 is seen in yellow. Note that only two of eight neurons of the TO ganglion (TOG) show GFP expression in this focal plane. Intensely labeled GFP expression is seen in the dome region of the DO, and dendrites of the TO are strongly stained in red and yellow. Non-neuronal cells of the epidermis express large amounts of GFP. H,Red–green stereo image of chemosensory arborizations in the larval brain (UAS-GFP). Arborizations of the PA are detected in a different focal plane. I, Higher magnification of the GFP pattern in LAL shows local concentration of arborizations in a grape-like manner. A general neuropil staining was achieved with mAb nc82 in red. Scale bars: A–C, E, F, H, 50 μm; G, I, 25 μm; D, 200 μm.
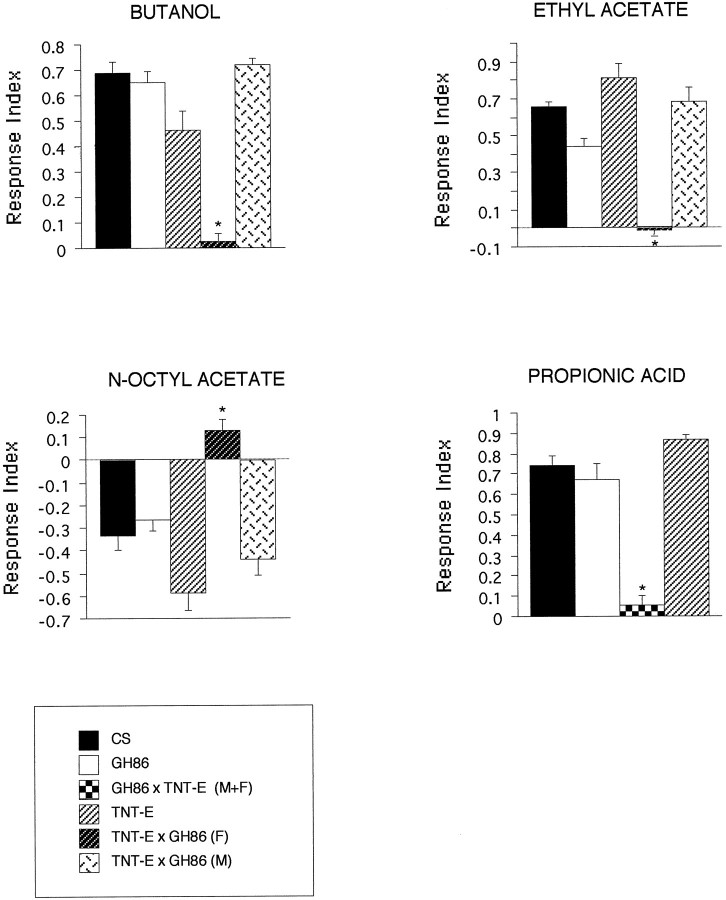
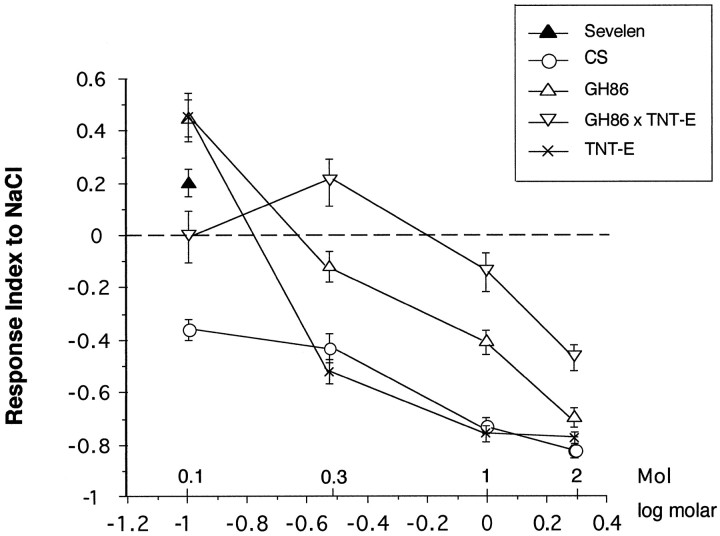
To test for odor-induced behavior, larvae were subjected to a choice assay on agarose plates (Fig. 1). Ethyl acetate, propionic acid, and cyclohexanone elicit strong positive responses in wild type. Butanol was previously shown to act as a weaker attractant (Cobb et al., 1992). N-Octyl acetate, a long-chain acetate, is one of the few larval repellent chemicals described (Cobb and Dannet, 1994). The results of the behavioral tests are depicted as RIs in Figures 2 and3. Because the P element insertion of line GH86 is localized on the X chromosome, male (M) heterozygotes +/Y; TNT-E/+ [Figs. 2, 3, TNT-E × GH86 (M)], which lack the P[GAL4] element, were compared with their female (F) siblings, GH86/+; TNT-E/+ [Figs. 2, 3, TNT-E × GH86 (F)] as an additional control in most tests. Only for tests with propionic acid, we used F1 larvae from the reciprocal cross, both males and females expressing GAL4 (GH86 × TNT-E in Fig. 2).
Fig. 2.
Odor-controlled behavior is severely impaired in larvae expressing TeTxLC driven by P[GAL4] in line GH86. Control lines CS, GH86, and TNT-E are homozygous. To indicate the genetic background of the tested animals, the genotypes of F1 larvae are always described by the parental cross; mothers are written on the left and fathers on theright of ×; e.g., a cross TNT-E × GH86 results in female (F) larvae, which contain the P[GAL4] element on the X chromosome and the UAS-TeTxLC construct on the second chromosome and males (M) lacking the P[GAL4] element. Consequently, only the females (F) are subject to TeTxLC expression. The reciprocal cross (GH86 × TNT-E) results in both males and females expressing TeTxLC. One microliter of undiluted butanol, ethyl acetate, and propionic acid and 2 μl of n-octyl acetate were used for the tests. Each bar consists of 5–10 independent tests. Error bars indicate SEM. For calculation of the RI, refer to Materials and Methods. Positive RIs indicate attraction; RI = 0 indicates indifferent behavior; and negative RI indicates aversion. Asterisks denote animals that express TeTxLC. Differences betweenTeTxLC-expressing animals and control lines are statistically highly significant (p < 0.005).
Fig. 3.
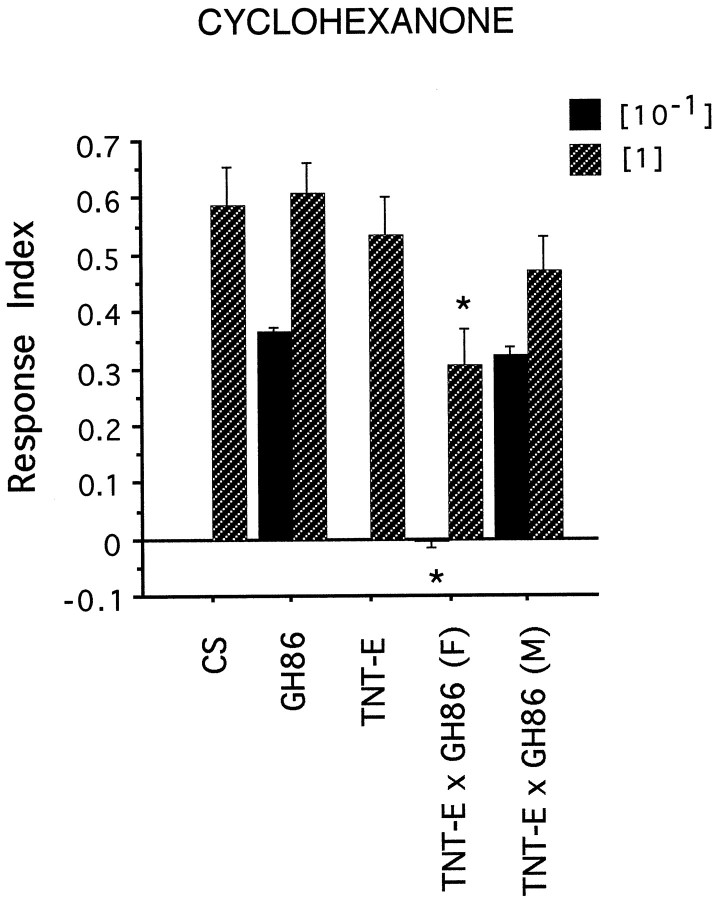
Undiluted cyclohexanone (1 μl) elicits a positive reaction in TNT-E × GH86 (F) larvae (concentration [1]). However, the response is significantly reduced, when compared with control lines GH86 (p = 0.007), wild-type CS (p = 0.01), and TNT-E (p = 0.03). No significant difference can be found from their male siblings GH86 × TNT-E (M) (p = 0.07). A dilution of [10−1] (1 μl) is sufficient to cause an indifferent behavior, which is statistically different from controls, male siblings, and GH86 homozygous larvae (p≤ 0.002). Abbrevations for larval genotypes are the same as in Figure2.
The P element insertion causes no behavioral defects. However, significant differences were found between TeTxLC-expressing larvae and the control lines wild-type Canton S (CS), GH86, and TNT-E. Butanol, ethyl acetate, and propionic acid did not seem to elicit any response, whereas all the control lines showed the expected preferences (Fig. 2). N-Octyl acetate, which elicited an avoidance behavior in control lines, seemed to be perceived as a weak attractant in our tests. However, because of the small number of tests, this may reflect high variability of undirected movement on the plates as indicated by the SD (RI = 0.13 ± 0.15 SD). The differences between control lines and TeTxLC-expressing animals were highly significant for all tests (p < 0.005). We therefore conclude that TeTxLC expression leads to anosmic behavior for these four chemicals. The only chemical that clearly elicited a behavioral response of GH86 × TNT-E (F) larvae was undiluted cyclohexanone (see Fig. 3). However, the response is significantly reduced when compared with control lines CS, GH86, and TNT-E. No significant difference was found for male siblings. Dilution of cyclohexanone by a factor of 10 clearly abolished the response ofTeTxLC-expressing larvae. Differences to control line GH86 and male siblings are highly significant (p ≤ 0.002).
For some odors we found significant differences in performance among control lines CS, GH86, and TNT-E (statistical analysis not shown). Similar variability was reported previously between different wild-type strains (Monte et al., 1989). It has to be stressed though that none of the control lines showed an indifferent behavior toward the odors, as is the case for tetanus toxin-impaired animals. In summary, we conclude that TeTxLC expression in the AMC efficiently blocks chemosensory afferents required for normal olfactory behavior and thus leads to a very strong anosmic behavioral phenotype.
TeTxLC expression in line GH86 reduces gustatory responses to sodium chloride and sugars
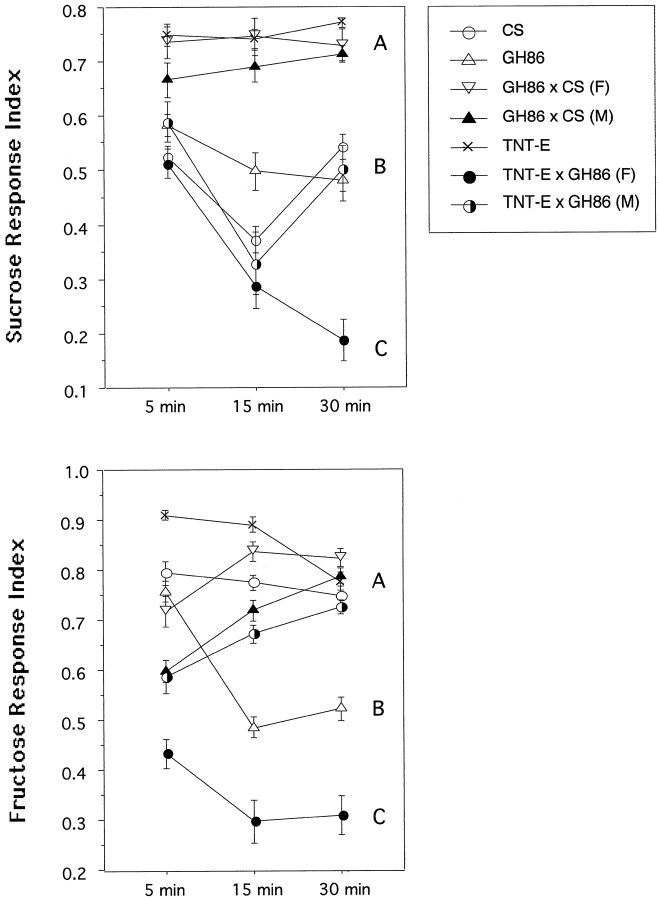
Insects can distinguish between the principal tastants sweet, sour, salty, and bitter (Singh, 1997). Behavioral responses to NaCl vary from positive to negative, depending on the concentration, whereas sugars are always attractive (Miyakawa, 1982; Jenkins and Tompkins, 1990). To assess larval behavior, we used a simple choice test on agarose Petri dishes. Control lines were the same as for the olfactory tests, namely wild-type CS, parental lines GH86, and TNT-E. Because line GH86 was found to behave differently in some tests, we included F1 larvae from a cross GH86 (F) × CS (M), to see whether this phenotype was caused by the insertion of the P element (see Figs.4, 6). The responses of TNT-E × GH86 (F) tetanus toxin-expressing females are significantly reduced with respect to all controls included in the test. For both sugars we find three levels of performance, A–C (Fig. 4). Female larvae (TNT-E × GH86), expressing TeTxLC, always show the weakest attraction to both sugars (level C). Their male siblings reach levels comparable with control lines. The smaller RI of homozygous GH86 larvae for fructose (level B) does not seem to be caused by the P element insertion, because the heterozgote F1 females and hemizgyote F1 males from the back-cross to wild-type CS (Fig. 4, GH86 × CS) behave like CS controls. Furthermore, male and female larvae did not respond differently to fructose. It is noteworthy that the same heterozygotes outperform both parent lines, CS and GH86, in the sucrose test. Genetic background and new combinations of the parental genomes seem to account for these behavioral differences. Taken together, our results suggest a significantly reduced response ofTeTxLC-expressing animals to fructose and sucrose. The time course of the response for different lines indicates that some of them show no significantly different RIs between 5 and 30 min, e.g., lines of level A on sucrose plates. For most lines, however, stable RIs are achieved between 15 and 30 min, e.g., lines of group A in the fructose assay. For some genotypes we still get different responses between 15 and 30 min, e.g., groups B and C in the sucrose test.
Fig. 4.
Effect of TeTxLC expression on responses to fructose and sucrose. The behavior of the same animals was monitored over 30 min. Animals were counted at the indicated time, and an RI was calculated as described in Materials and Methods. The concentration of both sugars was 1 m. Error bars indicate 0.5 × SEM. Each dot represents the mean of 10 independent tests. A–C, Significantly different response groups after 30 min assay time.
Fig. 6.
Test for a possible mutant effect attributable to the P element insertion. The reduced sensitivity of line GH86 to NaCl, as shown in the response curve in Figure 5, is not caused by the P element insertion. Heterozygotes GH86/CS from a cross GH86 (F) × CS (M) behave like the wild-type control CS in a test with 0.3m NaCl, despite the presence of the P element. Error bars indicate SEM. Each column includes 10 independent tests of 50 animals each.
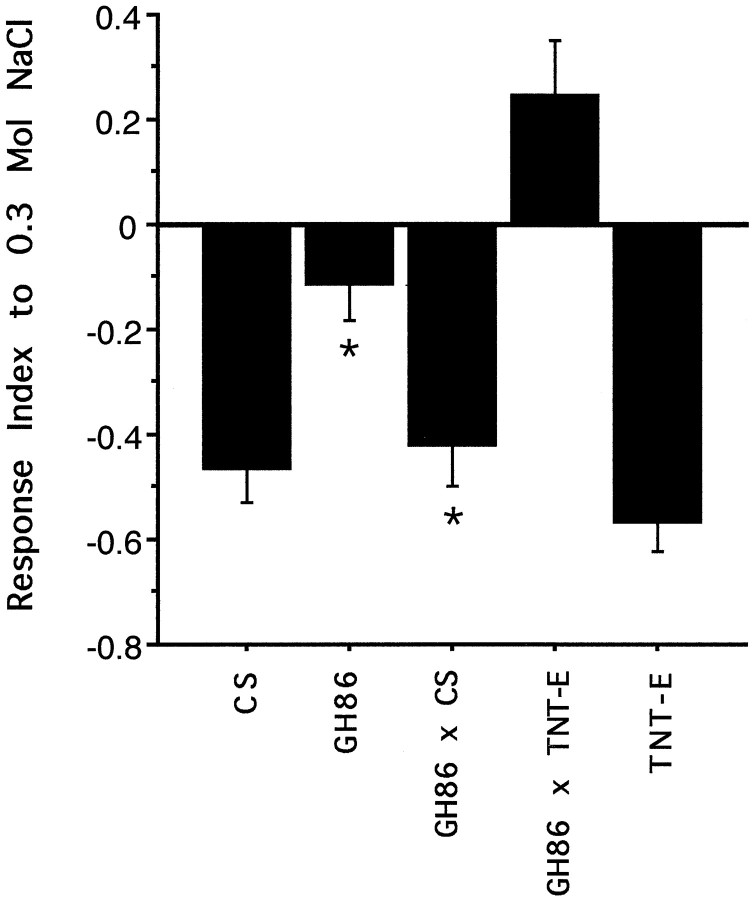
To assess the response to NaCl, tests were performed at different molarities (Fig. 5). To reduce the total number of tests, we expressed TeTxLC in both sexes, which were tested on the same plate (Fig. 5, GH86 × TNT-E). The response curve of these larvae is clearly different from those of the control lines. Larvae are repelled at high concentrations (2 and 1m) but are already clearly attracted at 0.3 mNaCl. The control lines GH86 and TNT-E show attraction only at 0.1 m. Wild-type CS is still repelled by 0.1 mNaCl. A second wild-type control line, Sevelen, however, was attracted, as reported previously for the Oregon R wild-type strain (Miyakawa, 1982). At 1 m NaCl the chemosensory system of lines CS and TNT-E seems to be saturated, or a maximal negative response is attained, whereas the RIs of homozygous GH86 and GH86 × TNT-E larvae still decline at the very high concentration of 2 mNaCl. Homozygous GH86 larvae show a significantly different response curve to NaCl than the other lines. To test for a possible mutative effect of the P element insertion, F1 from a cross GH86 (F) × CS (M) were tested. The results for these assays are shown in Figure6 for 0.3 m NaCl. Male and female larvae GH86 × CS were tested separately. Because their behavior was not different, data of both sexes were pooled. The behavior of wild-tpye line CS is not altered by introducing the P element-containing chromosome. Most importantly, males carrying the P element insertion on their single X chromosome show no defect. The reduced response of line GH86 cannot be attributed solely to the P element insertion and appears to result from differences in genetic background, acting either independently or in concert with alterations at the P element insertion site.
Fig. 5.
Response to NaCl. The response curve of animals subject to TeTxLC expression shows a reduced sensitivity at all tested concentrations compared with control lines. At 0.3m these larvae are clearly attracted by NaCl, whereas their parent lines GH86 and TNT-E are still repelled. Error bars indicate 0.5 × SEM. Each point represents the mean of 10 independent tests.
P[GAL4] insertion line GH86 shows specific expression in the chemosensory system
The specific expression pattern of P[GAL4] insertion line GH86 in a subset of the larval chemosensory neurons led us to investigate projection patterns of these neurons in more detail (Figs.7-9). This study focuses on the embryonic and larval expression patterns only. A detailed analysis was done in third instar larva using different UAS reporter genes. Strongest labeling in the nervous system was found in putative gustatory and olfactory sense organs of the larval head, in particular in the AMC (Fig. 7A), and internal chemosensory cells of the mouth parts (Fig. 7B,C). The ventral organ is devoid of expression. Only a subset of neurons in the ganglia of both the DO and TO can be visualized by reporter gene expression. By counting cell bodies in both ganglia of third instar larvae, using UAS-GFP and UAS-lacZ (nuclear) reporter constructs, we determined a total of 33–35 neurons on either side expressing GAL4 (n = 10). To determine the number of cells for each ganglion, we have used confocal microscopy of preparations, labeled with both the neuron-specific marker mAb 22C10 (Zipursky et al., 1984) and UAS-GFP (Fig. 7G). We find that approximately the same number of neurons, i.e., 16–18, express GFP in the DO and TO ganglia. Most or all of the dendrites from GFP-labeled neurons of the DO are found inside the central “dome” sensillum of the DO. Signal intensity between cells varies considerably.
Fig. 8.
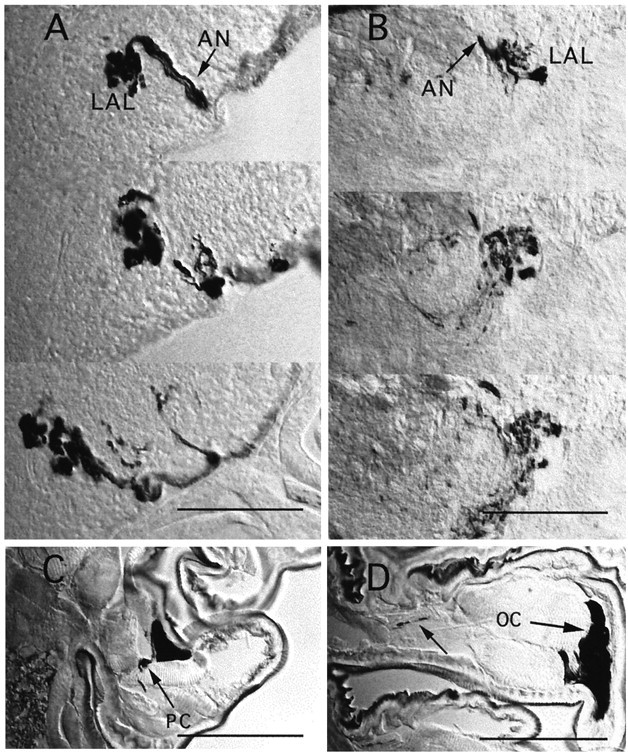
Anatomical differences betweenUAS-tau and UAS-TeTxLC expression.A, B, Two series of 10 μm cryosections through the LAL region of third instar larvae, showing TeTxLC staining (A) and TAU staining (B). The denser appearance of LAL projections in A, compared with B, suggests structural changes of presynaptic arborizations in afferents expressing active TeTxLC.C, Peripheral neuron of unknown identity (PC) stained in a thoracic segment. D, Strong oenocyte (OC) staining and faint axon staining (arrow) inside a peripheral nerve. Scale bars, 50 μm.
Fig. 9.
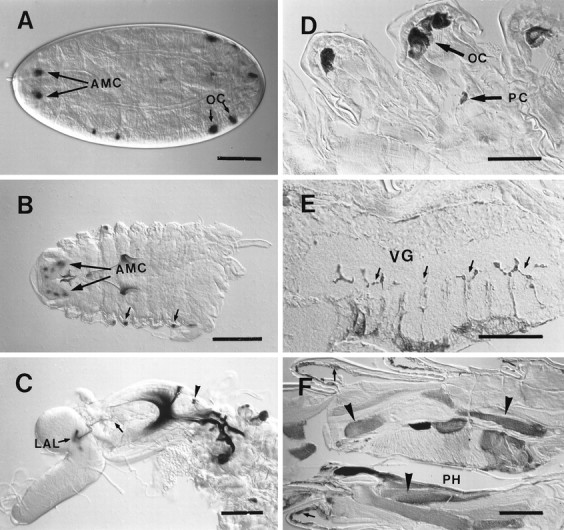
Expression pattern of line GH86 during development. A–C,UAS-lacZ; D–F,UAS-TeTxLC reporter genes. A, Expression is first detected in stage 17 embryos the AMC and oenocytes (OC); dorsal view on embryo, anterior is to theleft. B, First larval instar shows similar staining pattern as late embryos; small arrowspoint to oenocytes. C, Staining becomes stronger in second instar larva; the arborization pattern of AMC projections (arrow) inside the LAL is identical with third instar (see Fig. 7D,E). The arrowhead indicates cells of the pharyngeal mouth organ. D, E, Staining of cell bodies of peripheral neurons (PC), oenocyte staining (OC), and axons (E, arrows) entering the ventral ganglion (VG) are detected in second instar larvae using anti-TeTxLC antibodies. F, Expression in epidermis (arrows) and some muscles (arrowheads) of the pharynx (PH) is only found in third instar larva. Scale bars: A, C, F, 100 μm; B, D, E, 50 μm.
Afferent fibers leave both ganglia separately, forming thick axon bundles. However, after a short distance from the ganglion, the fibers of the TO fuse with those of the DO to form the antennal nerve. Surprisingly, the maxillary nerve remains completely unstained in line GH86. We conclude that a considerable number of afferents from the TO reach the brain via the antennal nerve. Fibers of the antennal nerve enter the brain near the antennal lobe region (LAL; Fig.7D). They arborize in a spherical neuropil inside the LAL, and some of them extend further ventrally toward the tritocerebrum (TC), forming a C-shaped band (Figs. 7E,H,8A,B). Counterstaining with mAb nc82, which labels the entire brain neuropil, shows that the LAL is connected via a very short stalk to the TC. The neuropil of the LAL seems to be divided into small subregions that correspond to a distinct staining pattern of arborizations in line GH86. Moreover, some regions of the LAL neuropil are devoid of GFP expression (see Fig.7I). This is reminiscent of the glomerular structure of the adult antennal lobe, although no clear subdivisions as in adult flies can be observed. Staining patterns with different UAS reporter constructs show the same overall morphology and projection pattern. Furthermore, in ∼200 lines examined in our screen, we have found similar projection patterns of peripheral nerves in two more P[GAL4] lines, GH327 and GH336.
In addition to the strong reporter gene expression in the AMC, we find labeling of three symmetrical pairs of chemosensory cells in internal pharyngeal sensilla (Fig. 7B,C). Two pairs are from the dorsal group, and one pair is localized on the ventral side (Singh and Singh, 1984; Singh, 1997). Axons from these sensilla enter the brain close to the entrance point of the antennal nerve (AN). Their fibers project ventrally toward the suboesophageal ganglion (SOG) and form small, bouton-like arborizations (see staining in Fig.7H).
In embryos, staining of the AMC begins at late stage 16 and stage 17, as shown by the UAS-lacZ reporter (Fig. 9A). In first and second instar larvae, AMC, pharyngeal sensilla, and chemosensory projections in the LAL are labeled (Fig.9B,C).
Additional expression in the peripheral nervous system (PNS) and in non-neuronal cells
In thoracic and abdominal segments, the only labeled peripheral neuronal cell bodies were localized close to the oenocytes (secretory cells of unknown function in larvae; Figs. 8C,D,9D). Afferents projecting to each neuromere of the ventral ganglion (Figs. 7F, 9E) seem to originate from these neurons of unknown function. Using tau,TeTxLC, lacZ, and nuclear lacZreporters, we never found staining in chemosensory cells of the body wall. Expression outside the nervous system was constant in oenocytes (Figs. 8C,D, 9A,B,D) and highly variable in the epidermis and some pharyngeal muscles (Fig. 9F). Expression in epidermal cells and muscles was only detected in the third larval instar (Figs. 7G, 9F). Strong reporter gene expression in salivary glands of GH86 larvae seems to be independent of the P element insertion site, because we observed similar staining in most of our enhancer trap lines.
In summary, enhancer trap line GH86 has a larval neuronal expression pattern that is restricted to the chemosensory system of the head region and a single peripheral neuron type found in each body segment.
TeTxLC expression leads to weak anatomical defects
Although no gross anatomical changes were detected when expressingTeTxLC in GH86 larvae, we noticed minor differences in sensory projection patterns. The bilateral symmetry of arborization patterns of the chemosensory afferents from the pharyngeal sensory neurons and the PNS seems to be disturbed in few cases (see Fig.7E,F). Afferents project into the correct target regions but exhibit local misrouting. Moreover, TeTxLCexpression clearly leads to a different morphology of arborizations inside the LAL and TC target regions, when compared with expression oftau, GFP (Figs. 7H,8A,B), or inactive TeTxLC (line IMPTNT-Q4A; data not shown). Arborizations of UAS-TeTxLCpreparations are swollen and seem to take up more space inside their target neuropil.
DISCUSSION
TeTxLC expression results in defects in olfactory and gustatory behavior
Using TeTxLC expression in line GH86, we were able to assign olfactory and gustatory functions to neurons of the larval AMC and pharynx of Drosophila melanogaster. Our results show that the sense of smell was almost completely blocked to the odorants tested. Tastants, on the other hand, still elicited responses, albeit at significantly reduced levels.
The results of our tests show that olfactory responses to butanol, ethyl acetate, n-octyl acetate, and propionic acid are completely abolished. Because expression of TeTxLC is limited to a subset of sensory neurons, and because no expression was found in central regions of the brain, we conclude that the animals were unable to smell the chemicals because their olfactory receptor cells were silenced. The most likely candidates are neurons of the AMC and, in particular, those sending dendrites into the dome of the DO. Reporter gene expression is predominant in the central region of the DO, which contains seven bundles of dendrite triplets below a single-walled, multiporous dome (Singh and Singh, 1984). Multiple pores have been found to be a typical feature of odorant sensilla in insects (Altner et al., 1977; Altner and Prillinger, 1980; Steinbrecht, 1996). We thus believe that most or all of the ∼18 labeled neurons of the DO are potential odorant receptors. This is supported by recordings from the DO, which was shown to respond to volatile components of banana (Oppliger et al., 1999). Considering the relatively small number of blocked neurons in line GH86, we hypothesize that odor detection in Drosophila larvae is solely mediated by neurons of the AMC.
Because expression does not include all sensory neurons of the DO, we expected that certain odors may still elicit a response, which is in fact the case for cyclohexanone. This demonstrates that larval olfactory neurons exhibit some odor specificity. The positive response to cyclohexanone may therefore be mediated by a neuron type devoid ofTeTxLC expression, which expresses a low-affinity receptor. Alternatively, a normal behavioral response may be achieved only via activation of several cyclohexanone-sensitive neurons acting in concert. Testing a larger number of odors may help us understand some of the mechanisms of olfactory detection and processing.
The remaining sensilla of the TO and DO have terminal pores, suggesting that they might have a gustatory function (Singh, 1997). Reduced responses in gustatory choice assays may thus be attributable to the block of sensilla of the TO and/or pharynx. We cannot exclude, however, that gustatory responses are mediated by neurons from both the DO and TO. The well studied chemosensory system of Caenorhabditis elegans suggests a strong correlation between different morphological classes of sensory endings and the type of stimuli. However, two neurons were reported to react to both gustatory and olfactory stimuli (for review, see Mori and Ohshima, 1997). In contrast, the pharyngeal chemosensory neurons may be exclusively gustatory. It has to be stressed that the gustatory response is only impaired, not abolished, in animals expressing TeTxLC. Hence, chemosensory cells that do not express TeTxLC in line GH86 should account for the residual perception of tastants. Likely candidates are unlabeled cells of the AMC, the pharyngeal sensilla, and the putative chemoreceptors of the ventral organ, as well as epidermal sensilla of thoracic and abdominal segments. Similar slopes of the response curves to NaCl between experimental and control lines seem to indicate a quantitative element in the mechanisms of information processing. Disrupting the function of a subset of responsive cells does not lead to sudden behavioral changes for certain concentrations, but the information from the whole set of NaCl-responsive cells may be integrated by the CNS to determine the quality of the environment.
To refine and strengthen the functional analysis of subsets of the chemosensory system, independent GAL4 lines, showing overlapping expression patterns, should be studied. Such an extended analysis will also account for possible artifacts attributable to undetected reporter gene expression.
Structural changes in chemosensory afferents expressing TeTxLC
Surprisingly, expression of TeTxLC in line GH86 seems to cause slight morphological defects in sensory arborization patterns, i.e., a swelling of afferent terminals in their synaptic target regions. Using UAS-lacZ and UAS-GFP reporters in first and second instar larvae, we found no differences of the general projection patterns to third instar larvae. So far, we have not studiedTeTxLC expression in these earlier stages in detail. We therefore do not know whether the morphological abnormalities are already present at hatching or whether they are the consequence of blocked activity during larval life. Previous expression ofTeTxLC in the Drosophila neuromuscular junction abolished synaptic transmission without visible changes in synaptic morphology (Sweeney et al., 1995). Also, despite a feeding defect, no synaptic abnormalities were seen after blocking pharyngeal motor neurons in flies (Tissot et al., 1998). However, studies of the PNS and CNS of both vertebrates and invertebrates have shown that structural synaptic plasticity is regulated by presynaptic and postsynaptic activity (Zufall et al., 1997; Constantine and Cline, 1998; Davis and Goodman, 1998). Further detailed developmental and anatomical studies will be necessary to assess a possible role of neuronal activity in formation and/or maintenance of synaptic connections. Expression in muscles and epidermal cells in line GH86 does not cause a structural phenotype, which confirms the results ofSweeney et al. (1995). We are therefore convinced that the behavioral abnormalities are caused by expression of TeTxLC in the sensory neurons of the AMC and pharynx. From the previous work by Sweeney et al. (1995), it is likely that defects result from a functional block of neurotransmitter release. However, morphological defects attributable to TeTxLC expression during larval development may be the main cause of functional deficits. For lack of other markers labeling these particular neurons, we cannot currently exclude the possibility that the anatomical changes are a characteristic of line TNT-E itself. However, expression of inactive TeTxLC in line IMPTNT-Q4A does not change the appearance of synaptic regions when compared with nontoxic reporter genes. Because TNT-E and IMPTNT-Q4A are transformants with a similar genetic background (Sweeney et al., 1995), we believe the toxin to be responsible for these subtle changes in morphology.
Projection patterns of larval chemosensory neurons
Anatomical studies of enhancer trap line GH86 with different reporter genes enabled us to trace projection patterns of the larval AMC at high resolution. In a previous study, using Lucifer yellow and DiI backfills, we were unable to describe subpopulations of neurons and their projection patterns (Tissot et al., 1997). We had established the LAL as the main target of fibers from the DO and showed that the TO fibers reach the brain via the maxillary nerve and invade regions of the TC and SOG. The expression of line GH86 confirms the projection pattern of DO afferents. However, to our surprise, the labeled subset of afferents from the TO fuse with the antennal nerve and enter the brain together with the afferents from the DO. Thus we are unable to distinguish between projections from the DO versus TO inside the brain. They form spherical arborizations in the LAL and extend into a bent, C-shaped structure toward the TC. The SOG, another previously described target region of TO afferents (Tissot et al., 1997), is completely devoid of projections form the AMC. The SOG is therefore the target of another subset of fibers from the TO, which is not labeled in line GH86. This specific enhancer activity may well indicate a functional difference of subsets of TO neurons. Afferent projections of the pharyngeal sensory organs are not intermingled with the projections of the AMC but end in the TC–SOG region, which may thus be a purely gustatory target region.
Despite this seeming discrepancy of afferent pathways, we believe that the overall projection patterns found in line GH86 are not different from wild type. There are several lines of evidence for this. Reporter constructs such as UAS-GFP and UAS-lacZdo not interfere with the normal development of neurons and are thus excellent morphological markers. Although homozygotes of line GH86 show different RIs in some of our tests (e.g., NaCl), assays with GH86/CS heterozygotes have demonstrated that the P element insertion is not responsible for these differences. Furthermore, two independent P[GAL4] insertion lines from our screen show similar projection patterns (data not shown). Thus we conclude that the projection pattern of TO afferents reflects indeed a mixed nature of TO and DO projections. In agreement with this, a subset of afferents from the TO of Musca domestica larvae was reported to join the antennal nerve (Chu and Axtell, 1971; Chu-Wang and Axtell, 1972). Interestingly, in adult Drosophila the antennal lobe also receives afferents from antennal, maxillary, and pharyngeal sensilla (Singh and Nayak, 1985; Stocker et al., 1990).
The neuropil of the LAL is not composed of clearly separated glomerular subunits, as is the case for adult flies and other insects (for review, see Stocker, 1994). However, the staining pattern of line GH86 clearly indicates in some parts of the LAL a regionalization of arborizations. This organization may well reflect a functional specialization of subregions of the LAL as it was shown for adult glomeruli (Rodrigues, 1988).
Because reporter gene expression of P elements is dependent on neighboring, cell-specific enhancer elements, cloning of the underlying gene may provide us with important information about genetic specializations of neurons labeled in line GH86. Interestingly, the P element insert maps close to a region in 7D1, which contains several previously characterized olf genes (Ayyub et al., 1990).
Footnotes
This work was supported by Human Frontier Science Program Grant RG-93/94 B; 259-873-02 (to R.F.S.), by Swiss National Science Foundation Grants 31-42053.94 (to R.F.S.) and 31-52639.97 (to R.F.S. and G.H.), and by the Sandoz Foundation. We thank Sean Sweeney and Cahir O’Kane (University of Cambridge, Cambridge, UK) for theUAS-TeTxLC lines and helpful suggestions, Seymour Benzer (Caltech, Pasadena, CA) for the antibody 22C10, Klemens Störtkuhl (Ruhr-Universität, Bochum, Germany) for theUAS-GFP line, and Stephan Schneuwly (Universität Regensburg) for the UAS-tau line. Heiner Niemann (Universität Hannover) kindly provided us with the anti-TeTxLC antibody, and Alois Hofbauer (Universität Regensburg) provided us with antibody nc82. We thank Champakali Ayyub (Tata Institute of Fundamental Research, Bombay, India) for helpful comments on this manuscript and Adrian Aebischer and Louis-Félix Bersier (University of Fribourg) for help with the statistical analysis.
Correspondence should be addressed to Dr. Gertrud Heimbeck, Institute of Zoology and Program in Neuroscience, University of Fribourg, Perolles, CH-1700 Fribourg, Switzerland.
REFERENCES
- 1.Aceves-Piña EO, Quinn WG. Learning in normal and mutant Drosophila larvae. Science. 1979;206:93–95. doi: 10.1126/science.206.4414.93. [DOI] [PubMed] [Google Scholar]
- 2.Altner H, Prillinger L. Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int Rev Cytol. 1980;67:69–131. [Google Scholar]
- 3.Altner H, Sass H, Altner I. Relationship between structure and function of antennal chemo-, hygro-, and thermoreceptive sensilla in Periplaneta americana. Cell Tissue Res. 1977;176:389–405. doi: 10.1007/BF00221796. [DOI] [PubMed] [Google Scholar]
- 4.Ashburner M. Drosophila. A laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 5.Ayyub C, Paranjape J, Rodriques V, Siddiqi O. Genetics of the olfactory behaviour in Drosophila melanogaster. J Neurogenet. 1990;6:285–262. doi: 10.3109/01677069009107114. [DOI] [PubMed] [Google Scholar]
- 6.Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development [Suppl] 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 7.Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. Springer; Berlin: 1997. [Google Scholar]
- 8.Carlson JR. Olfaction in Drosophila: from odor to behavior. Trends Genet. 1996;12:175–180. doi: 10.1016/0168-9525(96)10015-9. [DOI] [PubMed] [Google Scholar]
- 9.Chu IW, Axtell RC. Fine structure of the terminal organ of the house fly larva, Musca domestica. Z Zellforsch. 1971;117:17–34. doi: 10.1007/BF00331098. [DOI] [PubMed] [Google Scholar]
- 10.Chu-Wang IW, Axtell RC. Fine structure of the terminal organ of house fly larva, Musca domestica. Z Zellforsch. 1972;127:287–305. doi: 10.1007/BF00306874. [DOI] [PubMed] [Google Scholar]
- 11.Cobb M. Genotypic and phenotypic characterization of the Drosophila melanogaster olfactory mutation Indifferent. Genetics. 1996;144:1577–1587. doi: 10.1093/genetics/144.4.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobb M, Dannet F. Multiple genetic control of acetate-induced olfactory responses in Drosophila melanogaster larvae. Heredity. 1994;73:444–455. doi: 10.1038/hdy.1994.192. [DOI] [PubMed] [Google Scholar]
- 13.Cobb M, Bruneau S, Jallon JM. Genetic and developmental factors in the olfactory response of Drosophila melanogaster larvae to alcohols. Proc R Soc Lond B Biol Sci. 1992;248:103–109. doi: 10.1098/rspb.1992.0048. [DOI] [PubMed] [Google Scholar]
- 14.Constantine PM, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 15.Davis GW, Goodman CS. Genetic analysis of synaptic development and plasticity: homeostatic regulation of synaptic efficacy. Curr Opin Neurobiol. 1998;8:149–156. doi: 10.1016/s0959-4388(98)80018-4. [DOI] [PubMed] [Google Scholar]
- 16.Fischer JA, Giniger E, Maniatis T, Ptashne M. GAL4 activates transcription in Drosophila. Nature. 1988;332:853–856. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]
- 17.Frederick RD, Denell RE. Embryological origin of the antenno-maxillary complex of the larva of Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int J Insect Morphol Embryol. 1982;11:227–233. [Google Scholar]
- 18.Hekmat-Scafe DS, Carlson JR. Genetic and molecular studies of olfaction in Drosophila. Ciba Found Symp. 1996;200:285–296. doi: 10.1002/9780470514948.ch20. [DOI] [PubMed] [Google Scholar]
- 19.Ito K, Sass H, Urban J, Hofbauer A, Schneuwly S. GAL4-responsive UAS-tau as a tool for studying the anatomy and development of the Drosophila central nervous system. Cell Tissue Res. 1997;290:1–10. doi: 10.1007/s004410050901. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins JB, Tompkins L. Effects of amiloride on taste responses of Drosophila melanogaster adults and larvae. J Insect Physiol. 1990;36:613–618. [Google Scholar]
- 21.Lilly M, Carlson J. smellblind: a gene required for Drosophila olfaction. Genetics. 1990;124:293–302. doi: 10.1093/genetics/124.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyakawa Y. Behavioural evidence for the existence of sugar, salt and amino acid taste receptor cells and some of their properties in Drosophila larvae. J Insect Physiol. 1982;28:405–410. [Google Scholar]
- 23.Monte P, Woodard C, Ayer R, Lilly M, Sun H, Carlson J. Characterization of the larval olfactory response in Drosophila and its genetic basis. Behav Genet. 1989;19:198–283. doi: 10.1007/BF01065910. [DOI] [PubMed] [Google Scholar]
- 24.Mori I, Ohshima Y. Molecular neurogenetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Bioessays. 1997;19:1055–1064. doi: 10.1002/bies.950191204. [DOI] [PubMed] [Google Scholar]
- 25.Oppliger FY, Guerin PM, Vlimant M (1999) Neurophysiological and behavioural evidence for an olfactory function for the dorsal organ and gustatory one for the terminal organ in Drosophila melanogaster larvae. J Insect Physiol, in press. [DOI] [PubMed]
- 26.Park Y, Caldwell MC, Datta S. Mutation of the central nervous system neuroblast proliferation repressor ana leads to defects in larval olfactory behavior. J Neurobiol. 1997;33:199–211. [PubMed] [Google Scholar]
- 27.Riesgo-Escovar J, Woodard C, Gaines P, Carlson J. Development and organization of the Drosophila olfactory system: an analysis using enhancer traps. J Neurobiol. 1992;23:947–964. doi: 10.1002/neu.480230803. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues V. Spatial coding of olfactory information in the antennal lobe of Drosophila melanogaster. Brain Res. 1988;453:299–307. doi: 10.1016/0006-8993(88)90170-9. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues V, Siddiqi O. Genetic analysis of chemosensory pathways. Proc Indian Acad Sci. 1978;87B:147–160. [Google Scholar]
- 30.Siddiqi O. Olfaction in Drosophila. Chem Senses. 1991;3:79–96. [Google Scholar]
- 31.Singh RN. Neurobiology of the gustatory systems of Drosophila and some terrestrial insects. Microsc Res Tech. 1997;39:547–563. doi: 10.1002/(SICI)1097-0029(19971215)39:6<547::AID-JEMT7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Singh RN, Nayak S. Fine structure and primary sensory projections of sensilla on the maxillary palp of Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int J Insect Morphol Embryol. 1985;14:291–306. [Google Scholar]
- 33.Singh RN, Singh K. Fine structure of the sensory organs of Drosophila melanogaster Meigen larva (Diptera: Drosophilidae). Int J Insect Morphol Embryol. 1984;13:255–273. [Google Scholar]
- 34.Sokal RR, Rohlf FJ. Biometry. Freeman; New York: 1995. [Google Scholar]
- 35.Steinbrecht RA. Structure and function of insect olfactory sensilla. Ciba Found Symp. 1996;200:158–174. doi: 10.1002/9780470514948.ch13. [DOI] [PubMed] [Google Scholar]
- 36.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 37.Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- 38.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 40.Tissot M, Gendre N, Hawken A, Störtkuhl KF, Stocker RF. Larval chemosensory projections and invasion of adult afferents in the antennal lobe of Drosophila. J Neurobiol. 1997;32:281–297. doi: 10.1002/(sici)1097-4695(199703)32:3<281::aid-neu3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Tissot M, Gendre N, Stocker RF. Drosophila P[Gal4] lines reveal that motor neurons involved in feeding persist through metamorphosis. J Neurobiol. 1998;37:237–250. [PubMed] [Google Scholar]
- 42.Yeh E, Gustafson K, Boulianne GL. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc Natl Acad Sci USA. 1995;92:7036–7040. doi: 10.1073/pnas.92.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zipursky SL, Venkatesh TR, Teplow DB, Benzer S. Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell. 1984;36:15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]
- 44.Zufall F, Shepherd GM, Barnstable CJ. Cyclic nucleotide gated channels as regulators of CNS development and plasticity. Curr Opin Neurobiol. 1997;7:404–412. doi: 10.1016/s0959-4388(97)80070-0. [DOI] [PubMed] [Google Scholar]