Abstract
To examine the role of the intracellular N terminus in the G-protein modulation of the neuronal voltage-dependent calcium channel (VDCC) α1B, we have pursued two routes of investigation. First, we made chimeric channels between α1B and α1C, the latter not being modulated by Gβγ subunits. VDCC α1 subunit constructs were coexpressed with accessory α2δ and β2a subunits inXenopus oocytes and mammalian (COS-7) cells. G-protein modulation of expressed α1 subunits was induced by activation of coexpressed dopamine (D2) receptors with quinpirole in oocytes, or by cotransfection of Gβ1γ2 subunits in COS-7 cells. For the chimeric channels, only those with the N terminus of α1B showed any G-protein modulation; further addition of the first transmembrane domain and I-II intracellular linker of α1B increased the degree of modulation. To determine the amino acids within the α1B N terminus, essential for G-protein modulation, we made mutations of this sequence and identified three amino acids (S48, R52, and R54) within an 11 amino acid sequence as being critical for G-protein modulation, with I49 being involved to a lesser extent. This sequence may comprise an essential part of a complex Gβγ-binding site or be involved in its subsequent action.
Keywords: calcium channel, neuronal, G-protein, α1 subunit, Gβγ subunit, modulation
The inhibition of N- (α1B) and P/Q-type (α1A) calcium currents by receptors, usually acting through pertussis toxin-sensitive G-proteins, appears to be mediated by Gβγ subunits (Herlitze et al., 1996; Ikeda, 1996). There has been some controversy concerning whether the α1E calcium channel is G-protein-modulated (Page et al., 1998). We have now established that, whereas an N-terminally truncated isoform of rat α1E is not subject to modulation, an isoform with a full-length N terminus is G-protein-modulated, either by coexpression of Gβγ subunits or by activation of a G-protein-coupled receptor (Page et al., 1998), which would agree with results obtained previously for full-length human α1E (Qin et al., 1997).
A number of recent studies have established the importance of the intracellular loop that links transmembrane domains I and II, both in binding Gβγ and in mediating its effects to produce inhibition of the channel (Herlitze et al., 1997; Zamponi et al., 1997). However, this result is controversial, and several studies have suggested either that the I-II loop plays no role in G-protein modulation of α1B (Zhang et al., 1996) or α1E (Qin et al., 1997), or that alone it cannot mediate the effects of the Gβγ subunits (Page et al., 1997,1998; Simen and Miller, 1998). Nevertheless it is not disputed that the I-II loops of α1A, B, and E comprise a major binding site or sites for Gβγ and contain a QxxER amino acid consensus sequence common to many Gβγ-binding sites (De Waard et al., 1997; Herlitze et al., 1997; Zamponi et al., 1997; Dolphin et al., 1999). Secondly, a C-terminal Gβγ-binding site has recently been identified and proposed to be a region responsible for G-protein inhibition of human α1E (Qin et al., 1997). However, it is clear that there are also a number of other sites in the α1 subunit of G-protein-modulated calcium channels that are involved in expression of the inhibition by Gβγ. First, we have found that part of the intracellular N terminus of α1B and α1E is essential for their G-protein modulation (Page et al., 1998). Second, the transmembrane domain I has been found to have an important role (Zhang et al., 1996; Stephens et al., 1998b).
In the present study we have examined the critical nature of the intracellular N terminus of α1B, by making chimeric channels between α1B, which is strongly G-protein-modulated and α1C, which is not G-protein-modulated by this mechanism, and has a completely different N-terminal sequence. We have shown an absolute requirement for the α1B N terminus for observation of G-protein modulation in all the chimeric constructs. Second, we have made specific deletions and point mutations to identify the sequence in the N terminus of α1B that is responsible for conferring G-protein modulation.
MATERIALS AND METHODS
Materials
The following cDNAs were used: rat α1C (isoform CII, GenBank accession number M67515), rabbit α1B (D14157), rat β2a (M80545), rat α2δ-1 (neuronal splice variant, M86621), rat D2long receptor (X17458, N5→G), bovine Gβ1 (M13236), bovine Gγ2 (M37183), the C-terminal minigene of β-ARK (M34019), and mut-3 green fluorescent protein (GFP; U73901). All cDNAs were subcloned into the expression vector pMT2 (Swick et al., 1992).
Construction of chimeras
Chimeras were created using PCR following the methods described previously (Page et al., 1998; Stephens et al., 1998b). All constructs were subcloned into the pMT2 vector, and the sequences of the PCR products were confirmed using cycle-sequencing. The constructs were assembled as follows: bCCCC, amino acid residues 1–95 of α1B, 125–2143 of α1C; bBcCCC 1–359 α1B, 409–2143 α1C; bBbCCC 1–483 α1B, 525–2143 α1C; cBcCCC 1–124 α1C, 96–359 α1B, 409–2143 α1C; cBbCCC 1–124 α1C, 96–483 α1B, 525–2143 α1C; cCbCCC 1–408 α1C, 360–483 α1B, 525–2143 α1C; and bCbCCC 1–95 α1B, 125–408 α1C, 360–483 α1B, 525–2143 α1C. Chimeric primers were used with the reverse primer CCA CCA GCA GGT CCA GGA TAT TGA (R1). The resulting PCR product was extended against a template using a forward primer (pMT2F2) directed against the vector TCT CCA CAG GTG TCC ACT. The following chimeric primers were used: GTG CTG GGT GTG CTG AGC GGA GAG TTT for bBcCCC; CAG CCA GTA GAA GAC CTG TGC CTT CAC CAT (reverse primer R2) for bBbCCC; CAC CGA GTG GCC TCC ATT TGA AAT AAT T for bCCCC. These chimeras were used as templates to make others. The primers TTT GAG CGG AGA GTT TGC TAA GG and R2 were used to make the first PCR product, which was then extended on bCCCC to give bCbCCC. The chimeras cBbCCC and cBcCCC were made using bBbCCC and bBcCCC as templates. In each case, the PCR product made using the primers TGT TGA ATG GAA ACC GTT CGA GTA CAT G and R1 was extended on α1CpMT2 template to add the N terminus of α1C. For cCbCCC, restriction digestion of an MfeI site in domain I was used to substitute the N terminus of bCbCCC with that of α1C.
Construction of N-terminal deletion and point mutations
The α1B N terminus was truncated at the 5′ end by introducing a start codon before amino acid E7 to make α1B Δ2–6, Y45 (α1B Δ2–44), and Q51 (α1B Δ2–50). The following primers were used; CGC ACT AGT ATG GAG CTG GGC GGC CGC TAT (Δ2–6), CAG ACT AGT ATG TAC AAA CAG TCG ATC GCG (Δ2–44), and CAG ACT AGT ATG CAG CGC GCG CGG ACC AT (Δ2–50). The α1B Δ45–55 construct was made by using the primer GGC CAG CGG GTC CTC ATG GCG CTG TAC AAC to delete the 11 amino acids, YKQSIAQRART. For all of the α1B point mutations, primers were designed so that single residues were mutated to alanines or so that a number of residues were mutated within the same primer. The following primers were used; R52A-R54A, TCG ATC GCG CAG GCC GCG GCG ACC ATG GCG CT; Y45A, CAG CGG GTC CTC GCC AAA CAG TCG ATC; K46A, CGG GTC CTC TAC GCA CAG TCG ATC GCG; Q47A, GTC CTC TAC AAA GCG TCG ATC GCG CAG; S48A, CTC TAC AAA CAG GCG ATC GCG CAG C; I49A, TAC AAA CAG TCG GCC GCG CAG CGC GCG; Q51A, CAG TCG ATC GCG GCG CGC GCG CGG ACC; R52A, TCG ATC GCG CAG GCC GCG CGG ACC ATG; R54A, GCG CAG CGC GCG GCG ACC ATG GCG CTG; 45YKQSIA→AAAAA, GCC GCA GCA GCT GCC GCG CAG CGC GCG CGG (forward) and GGC AGC TGC TGC GGC GAG GAC CCG CTG (reverse); and 45YKQ→AAA, CGG GTC CTC GCC GCA GCG TCG ATC GCG CAG. The reverse primer used in each case was GTC GCT TCT GCT CTT CTT GG. For the PCR extension reactions, the forward primer used was AGC ACT AGT ATG GTC CGC TTC GGG GAC. The sequences of all constructs were verified.
Expression of constructs and electrophysiological recording
Xenopus oocytes. Adult female Xenopus laevis were killed by anesthetic overdose in a 0.25% solution of tricaine, decapitated, and pithed. Oocytes were removed and defolliculated by treatment with 2 mg/ml collagenase type Ia in a Ca2+-free ND96 saline containing (in mm): NaCl, 96; KCl, 2; MgCl2, 1; and HEPES, 5, pH-adjusted to 7.4 with NaOH for 2 hr at 21°C. Plasmid cDNAs for the different α1 subunits, plus accessory β2a and α2δ subunits and rat D2 receptors, were mixed in a ratio of 3:4:1:3 (except where stated), and ∼10 nl was injected into the nuclei of stage V or VI oocytes. Injected oocytes were incubated at 18°C for 3–7 d in ND96 saline (as above plus 1.8 mm CaCl2) supplemented with 100 μg/ml penicillin, 100 IU/ml streptomycin (Life Technologies, Gaithersburg, MD), and 2.5 mm Na pyruvate. Whole-cell recordings from oocytes were made in the two-electrode voltage-clamp configuration with a chloride-free solution containing (in mm): Ba(OH)2, 5; TEA-OH, 80; NaOH, 25; CsOH, 2; and HEPES, 5 (pH 7.4 with methanesulfonic acid). In all experiments, oocytes were injected with 30–40 nl of a 100 mm solution of K3-1,2-bis (aminophenoxy) ethane-N,N,N′,N′-tetra-acetic acid (BAPTA) in order to suppress endogenous Ca2+-activated Cl− currents. Electrodes contained 3m KCl and had resistances of 0.3–2 MΩ. The holding potential (VH) was −100 mV, and the test potential (Vt) used for time course studies was 0 mV. All illustrated traces are at this potential, and the current amplitude was always measured 20 msec after the start of the test pulse. Membrane currents were recorded every 15 sec, amplified, and low-pass filtered at 1 KHz using a Geneclamp 500 amplifier and digitized through a Digidata 1200 interface (Axon Instruments, Foster City, CA). In all cases currents were leak subtracted on-line by a P/4 protocol.
COS-7 cells. Cells were cultured and transfected, using the electroporation technique, essentially as described previously (Campbell et al., 1995a). The α1, α2δ, β2a, and GFP cDNAs were used at 15, 5, 5, and 1 μg, respectively. When used, Gβ1 and Gγ2 were included at 2.5 μg each, or β-ARK was included at 5 μg. Blank pMT2 vector was included where necessary to maintain the total cDNA at 31 μg/transfection. Cells were replated using nonenzymatic cell dissociation medium (Sigma, St. Louis, MO), and then maintained at 25°C for between 1 and 16 hr before electrophysiological recording. Maximum GFP fluorescence and voltage-dependent calcium channel (VDCC) expression were observed between 2 and 4 d after transfection (Brice et al., 1997). Ca2+ channel currents were recorded using the whole-cell patch technique. Borosilicate glass 2–5 MΩ electrodes were used. The internal (electrode) and external solutions were similar to those described previously (Campbell et al., 1995b). The patch pipette solution contained in mm: Cs aspartate, 140; EGTA, 5; MgCl2, 2; CaCl2, 0.1; K2ATP, 2; and HEPES, 10; pH 7.2, 310 mOsm with sucrose. GDPβS (2 mm) was included where stated. The external solution contained in mm: tetraethylammonium (TEA) bromide, 160; KCl, 3; NaHCO3, 1.0; MgCl2, 1.0; HEPES, 10; glucose, 4; and BaCl2, 1 or 10, pH 7.4, 320 mOsm with sucrose. Whole-cell currents were elicited fromVH of −100 mV and recorded using an Axopatch 1D amplifier. Data were filtered at 2 kHz and digitized at 5–10 kHz. The junction potential between external and internal solutions was 6 mV, the values given in the figures and text have not been corrected for this. Current records are shown following leak and residual capacitance current subtraction (P/4 or P/8 protocol) and series resistance compensation up to 85%. Current amplitudes were measured 50 msec after the start of depolarization.
All experiments were performed at room temperature (18–20°C). Analysis was performed using pClamp6 and Origin software. Data are expressed as mean ± SEM. Statistical analysis was performed using paired or unpaired Student’s t test, as appropriate.
RESULTS
In a previous study we made chimeras between the rat brain α1E (rbEII) clone, which is not G-protein-modulated, and the strongly modulated α1B. The results of this study showed that rbEII was not modulated because it was N terminally truncated, and a full-length rat α1E isoform showed clear G-protein modulation, although not to such a great extent as α1B. We further showed the importance of the first domain of α1B in increasing the extent of G-protein modulation of α1B/α1E chimeras (Page et al., 1998; Stephens et al., 1998b), as has another recent study (Simen and Miller, 1998). In the present study, we wished to examine the distinct role of the N terminus of α1B in G-protein modulation. To do this we have taken two approaches. First, we have made chimeras between α1B and the α1C channel, which is not modulated by a Gβγ-mediated pathway under any conditions. Second, we have produced selective deletions and mutations of the α1B N-terminal sequence. With such constructs we can determine the domains necessary for the expression of G-protein modulation.
G-protein modulation of α1B/α1C chimeras by activation of the dopamine D2 receptor
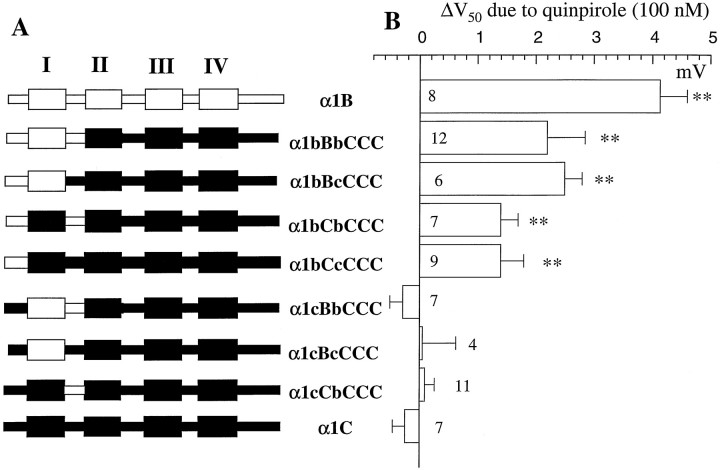
In this part of the study, all channels were expressed with the accessory subunits α2δ and β2a (unless stated) inXenopus oocytes, where they were coexpressed with the dopamine D2 receptor. A series of chimeras were made, in which the N terminus, first transmembrane domain, and I-II loop of α1B were systematically substituted for those in α1C, in different permutations. Figure 1Ashows the chimeras that were made, and the nomenclature employed, which uses capital letters for the transmembrane domains and small letters for the intracellular N-terminal and I-II loop. All chimeras contained the last three domains and C-terminal tail of α1C (denoted CCC), and all showed good expression levels with one exception (Table1). However, because α1B, α1C, and the chimeras between them showed differences in their voltage dependence of activation (Table 1), we could not compare G-protein modulation at a single step potential (Fig.2). Therefore, we have estimated the amount of G-protein modulation in two ways in Xenopusoocytes, first by determining the ability of the D2 agonist quinpirole to cause a depolarizing shift in the voltage dependence of activation, determined from current–voltage plots (Fig. 1B). Second, we have determined the percentage inhibition by quinpirole of the current activated at all potentials between −20 and +30 mV (Fig.2). In all cases, the modulation by quinpirole occurred within 30–60 sec of its application and was fully reversible.
Fig. 1.
G-protein modulation of chimeras between α1B and α1C. A, Chimeras made between α1B (white) and α1C (black), together with the nomenclature used. B, Chimeras and parental constructs were expressed in Xenopus oocytes together with α2δ and the dopamine D2 receptor. TheV50 in the absence and presence of the D2 agonist quinpirole (100 nm) was determined from current–voltage relationships performed before and during its application, as described in the legend to Table 1, and the ΔV50 was calculated (mean ± SEM). The number of experiments is given for each histogram bar. The statistical significance of ΔV50 was determined by paired t test; **p < 0.01.
Table 1.
Biophysical properties and G-protein modulation of calcium channel α1 subunit chimeras in Xenopus oocytes
| α1BBBB | α1bBbCCC | α1bBcCCC | α1bCbCCC | α1bCcCCC | α1cBbCCC | α1cBcCCC | α1cCbCCC | α1CCCC | |
|---|---|---|---|---|---|---|---|---|---|
| With β2a | |||||||||
| IBa peak amplitude, nA (n) | 1195 ± 155 (8) | 1054 ± 130 (12) | 605 ± 90 (7) | 760 ± 103 (10) | 766 ± 267 (10) | 744 ± 139 (6) | 392 ± 82 (4) | 1355 ± 115 (11) | 657 ± 87 (10) |
| V50 for controlIBa (mV) | −12.0 ± 1.2 | −14.2 ± 0.8 | 3.7 ± 0.9 | −8.5 ± 1.3 | +0.4 ± 1.6 | −21.4 ± 1.3 | −8.3 ± 0.9 | −14.8 ± 1.3 | −8.7 ± 1.8 |
| V50 forIBa plus quinpirole (mV) | −7.8 ± 1.4 | −12.0 ± 0.9 | 6.2 ± 1.0 | −7.0 ± 1.3 | 1.8 ± 1.5 | −21.7 ± 1.2 | −8.2 ± 0.4 | −14.7 ± 1.4 | −8.9 ± 1.8 |
| Without β2a | |||||||||
| IBa peak amplitude, nA | 381 ± 46 (8) | 805 ± 212 (6) | 824 ± 161 (6) | 636 ± 138 (7) | No expression | 662 ± 199 (5) | 681 ± 104 (4) | 661 ± 104 (11) | 470 ± 82 (4) |
| V50 for controlIBa (mV) | −1.9 ± 0.1 | −9.1 ± 1.0 | 2.5 ± 1.7 | 3.5 ± 1.5 | −18.6 ± 2.0 | −7.0 ± 0.8 | −4.7 ± 0.8 | +9.9 ± 1.9 | |
| V50 for IBa plus quinpirole (mV) | 11.2 ± 1.6 | −5.4 ± 1.0 | 5.2 ± 1.6 | 5.4 ± 1.6 | −18.3 ± 2.4 | −6.7 ± 0.4 | −5.0 ± 0.7 | +9.2 ± 1.7 | |
| ΔV50 for IBa due to β2a (mV) | −10.1 | −5.1 | −3.7 | −12.0 | Not determined | −2.8 | −1.3 | −10.1 | −18.6 |
The parameters determined for the different α1 constructs (cotransfected with α2δ, and either with or without β2a) were measured as described in Materials and Methods and in the legend to Figure 1. Individual current density–voltage relationships were fitted with a Boltzmann equation I =Gmax·(V − Vrev)/(1 + exp[−(V − V50)/k]), whereGmax is the maximum conductance;Vrev is the reversal potential; k is the slope factor, and V50 is the voltage for 50% current activation. No systematic variation was seen ink, which was between 4.5 and 8 for all constructs, or inVrev, which was between +45 and +51 mV for all constructs. For simplicity, these values are not given. The current amplitude is given at the peak of the I–V relationship, which is between 0 and +10 mV.
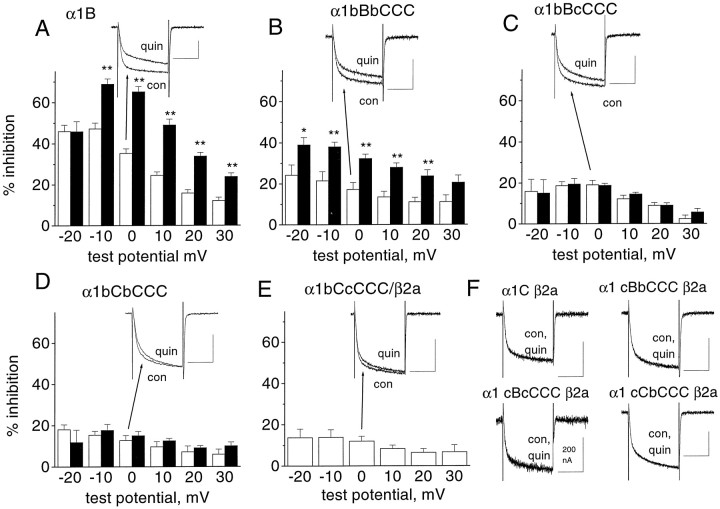
Fig. 2.
Voltage dependence of modulation of the chimeras between α1B and α1C by activation of the dopamine D2 receptor. The percentage inhibition by quinpirole (100 nm) was determined at voltages between −20 and +30 mV, from current–voltage relationships performed in the absence and presence of quinpirole. Measurements were made isochronally, 20 msec after the start of the voltage step. A, α1B; B, α1bBbCCC;C, α1bBcCCC; D, α1bCbCCC; andE, α1bCcCCC. Experiments were performed both in the presence (white bars) and the absence (black bars) of overexpressed β2a, except for α1bCcCCC, where no expression was seen in the absence of β2a. The numbers of experiments (with, without β2a) are 8, 6 (A); 12, 6 (B); 7, 6 (C); 10, 6 (D); and 9 (E). The statistical significance of the differences at each potential between inhibition in the presence and absence of β2a is indicated by *p < 0.05; **p < 0.01. Example currents in the presence of β2a are given as insets to parts A–Cfor α1B and for all the chimeras shown. They were expressed as described in the legend to Figure 1. These traces were evoked by a pulse from −100 to 0 mV, and therefore do not show the maximum inhibition. Traces are shown before (con) and during quinpirole (100 nm) application (quin).F, Example traces showing the lack of effect of quinpirole on α1C, α1cBbCCC, α1cBcCCC, and α1cCbCCC, all expressed with β2a. The calibration bars are all 50 msec and 500 nA, unless otherwise stated.
The modulation of α1B by activation of the dopamine D2 receptor with 100 nm quinpirole was voltage-dependent, as we have shown previously (Page et al., 1998; Stephens et al., 1998b). This is manifested by a depolarizing shift in the voltage for 50% activation of the current (V50) (Fig.1B), and also by a reduction in the percentage inhibition at increasing test potentials (Fig. 2A). Maximum inhibition was usually seen at a test potential ∼10 mV below the peak of the current–voltage relationship (47% at −10 mV for α1B; Fig. 2A). The transfer of the entire N terminus, first transmembrane domain, and I-II loop sequence of α1B into α1C gave a chimera showing G-protein modulation that was smaller at all potentials than the α1B parent (Figs. 1B,2B). The depolarizing shift in theV50 for α1bBbCCC was less than for α1B (Fig. 1B), and the maximum modulation was 24% at −20 mV (Fig. 2B). With respect to both measurements, a similar degree of modulation by quinpirole was seen for α1bBcCCC (Figs. 1B, 2C), providing strong evidence that the I-II linker from a modulatable channel such as α1B is not essential for exhibition of G-protein modulation. Modulation by quinpirole was also still present in the chimera α1bCbCCC (18% at −10 mV; Figs. 1B,2D). Furthermore, there was still a significant degree of modulation of the minimal chimera α1bCcCCC (13% at −10 mV; Figs. 1B, 2E), again indicating that the I-II linker from a modulatable channel is not essential for the observation of G-protein modulation.
In contrast, none of the chimeras containing the N terminus of α1C instead of α1B showed any inhibition by quinpirole at any potential from −30 to +40 mV under these conditions (inhibition by quinpirole at 0 mV: 0.66 ± 1.0% for α1cBbCCC, –0.4 ± 0.3% for α1cBcCCC, and –0.86 ± 0.92% for α1cCbCCC; nvalues given in Table 1) (Fig. 2F). There was also no quinpirole-induced depolarizing shift in theV50 for activation (Fig.1B). This was also the case for α1C (−0.25 ± 0.21% inhibition by quinpirole at 0 mV; Figs. 1B,2F). Thus, the N terminus of α1B is essential and sufficient for the expression of any G-protein modulation, whereas the first transmembrane domain and I-II linker of α1B can be substituted by that of α1C, and significant, although reduced, G-protein modulation is still observed.
Antagonism by β2a of G-protein modulation of the α1B/α1C chimeras
It has previously been shown that the G-protein modulation of α1E currents is antagonized by β2a (Qin et al., 1998). To study the interaction between the presence of overexpressed VDCC β2a and the extent of G-protein modulation, we also examined the degree of G-protein modulation by dopamine D2 receptor activation in the absence of exogenously coexpressed VDCC β subunit in Xenopusoocytes. Nevertheless, it should be stressed that Xenopusoocytes contain an endogenous β3-like subunit, and when this was depleted with an antisense construct, no functional currents were seen (Tareilus et al., 1997). The G-protein modulation of α1B and the chimera α1bBbCCC was found to be significantly greater in the absence of coexpressed β2a than in its presence (Fig.2A,B). In contrast, the extent of quinpirole-induced modulation of the α1bBcCCC and α1bCbCCC chimeras was not significantly increased in the absence of exogenous β2a (Fig.2C,D). Furthermore, the absence of β2a did not uncover G-protein modulation in any of the chimeras lacking the N terminus of α1B that were not modulated in the presence of β2a (results not shown). We were unable to examine α1bCcCCC currents in the absence of β2a, because no expression was observed (n = 3 experiments). These results suggest that the presence of the I-II linker and first transmembrane domain of α1B, although not being essential for G-protein modulation, are together required for the reduction of G-protein modulation in the presence of the exogenously expressed VDCC β2a subunit, seen under these conditions.
Coexpression of β subunits with α1 subunits in Xenopusoocytes and other systems results in a hyperpolarizing shift in current activation (for review, see Walker and De Waard, 1998), and it is of interest that this is greatest for α1B, α1C and those chimeras in which all the transmembrane domains are identical (α1bCbCCC and α1cCbCCC; Table 1). However, despite the reduced β2a-induced hyperpolarizing shift in the activation of the α1bBbCCC chimera, compared to α1B, there was still a clear β2a-induced reduction in the amount of G-protein inhibition at all potentials (Fig.2B), indicating that β2a was influencing this channel.
G-protein modulation of α1B/α1C chimeras by coexpression of Gβγ subunits
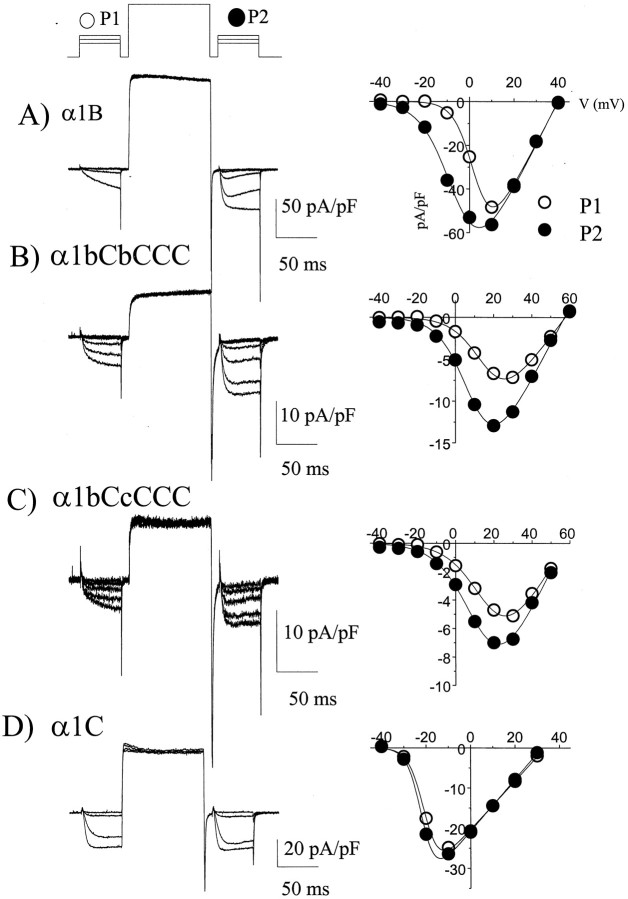
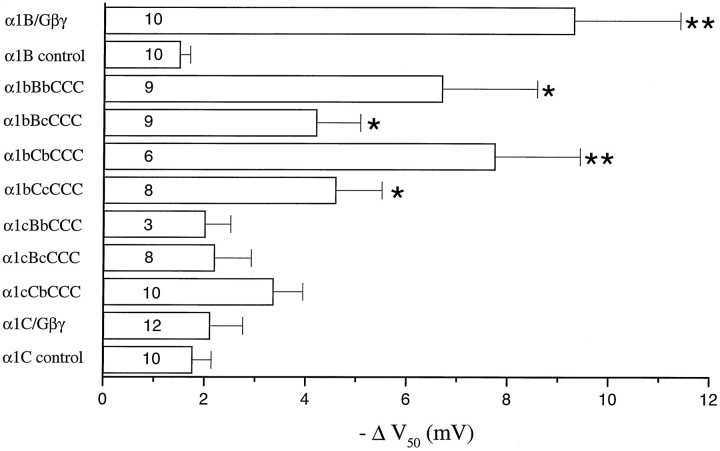
The role of Gβγ in mediating the inhibition observed was confirmed by coexpression of the chimeric α1 channels with α2δ, β2a, and Gβ1γ2 in COS-7 cells. A prepulse protocol was used (Fig.3, left panels), giving steps to potentials between −40 and +40 mV, before (P1) and 10 msec after (P2) a large depolarizing step to +120 mV (Page et al., 1998). The prepulse reverses Gβγ-mediated modulation, and hence P2 acts as an internal control. The Gβγ-mediated modulation was determined from the hyperpolarizing shift in the V50of the current–voltage relationship in P2 compared to that in P1 (Fig.3, right panels). For α1B, this shift was almost −10 mV (Figs. 3A, 4), and it was not significantly smaller for the chimeras α1bBbCCC and α1bCbCCC (Figs.3B, 4). It was reduced, but still significantly different from α1C for the α1bBcCCC and α1bCcCCC chimeras (Figs.3C, 4). Of the other chimeras, all of which had the N terminus of α1C, none showed any greater shift inV50 than α1C itself (Figs.3D, 4). In control experiments recorded in the absence of coexpressed Gβ1γ2 and in the presence of intracellular GDPβS, the shift in V50 caused by a depolarizing prepulse was approximately −1.8 mV for α1C (n = 10), very similar to the value for α1C coexpressed with Gβ1γ2 (−2.1 mV; Fig. 4). A similar level of control facilitation was observed for α1B (n = 10) (Fig. 4). Similar control results were obtained when the β-ARK1 Gβγ-binding domain was coexpressed, to act as a sink for endogenous Gβγ and prevent tonic modulation (Stephens et al., 1998a,b) (results not shown). This control prepulse potentiation is therefore likely to be caused by a mechanism other than G-protein modulation (Dolphin, 1996). The main discrepancy between the results examining direct Gβγ modulation and those examining receptor-mediated modulation involve the α1bCbCCC chimera, which is strongly modulated by overexpression of Gβ1γ2 (Figs.3B, 4), and more weakly modulated by receptor-mediated inhibition (Figs. 1B, 2D). The reason for this may relate to differences in Gβγ subtype or concentration between the two systems.
Fig. 3.
Examples of direct modulation by Gβ1γ2 of the chimeras between α1B and α1C. The α1 subunits shown were coexpressed with α2δ, β2a, Gβ1, and Gγ2 in COS-7 cells.Left panel, Traces obtained before and after a depolarizing prepulse (+120 mV, 100 msec). The prepulse protocol is above the top trace. Right panel, Current–voltage relationships (steps from −40 to +50 mV in 10 mV intervals, from a holding potential of −100 mV), measured 50 msec after the start of the step, for the currents in P1 (open circle) and P2 (filled circle). The current–voltage relationships were fitted (solid lines) with a modified Boltzmann equation as given in the legend to Table 1. The mean depolarizing shifts in V50 resulting from the depolarizing prepulse are given in Figure 4. A,Currents resulting from α1B expression (currents shown resulting from steps −40 to 0 mV, and recorded in 1 mmBa2+). B, Currents resulting from α1bCbCCC expression (steps −40 to +20 mV shown, recorded in 10 mm Ba2+). C, Currents resulting from α1bCcCCC expression (steps −40 to +20 mV shown, recorded in 10 mm Ba2+).D, Currents resulting from α1C expression (steps −40 to −10 mV shown, recorded in 1 mmBa2+). In this example the depolarizing prepulse was not preceded by a 10 msec step to the holding potential, but this had no effect on the subsequent results.
Fig. 4.
Modulation by Gβ1γ2 of the chimeras between α1B and α1C. Histogram giving the mean ± SEM of the hyperpolarizing shifts in V50 after a depolarizing prepulse for the same chimeras as in Figure 1. *p < 0.05; **p < 0.001 compared to α1C/Gβ1γ2. All α1B currents were recorded with 1 mm Ba2+ and all chimeras with 10 mm Ba2+ as charge carrier. It was checked for parental α1B that the use of 1 or 10 mmBa2+ did not affect the ΔV50 caused by a depolarizing prepulse. For the bars marked control, the parental constructs were expressed without Gβγ subunits, in the presence of GDPβS (1 mm), and a small prepulse-induced hyperpolarizing shift inV50 was observed for α1B and α1C. A similar control shift was also observed for all the chimeras tested [for example for α1bCbCCC the control ΔV50 was −2.7 ± 0.8 mV (n = 8)]. This shift was not significantly different from that for α1C coexpressed with Gβ1γ2. The number of experiments performed is given at the base of each bar.
Isolation of the amino acid residues of the N terminus essential for G-protein modulation
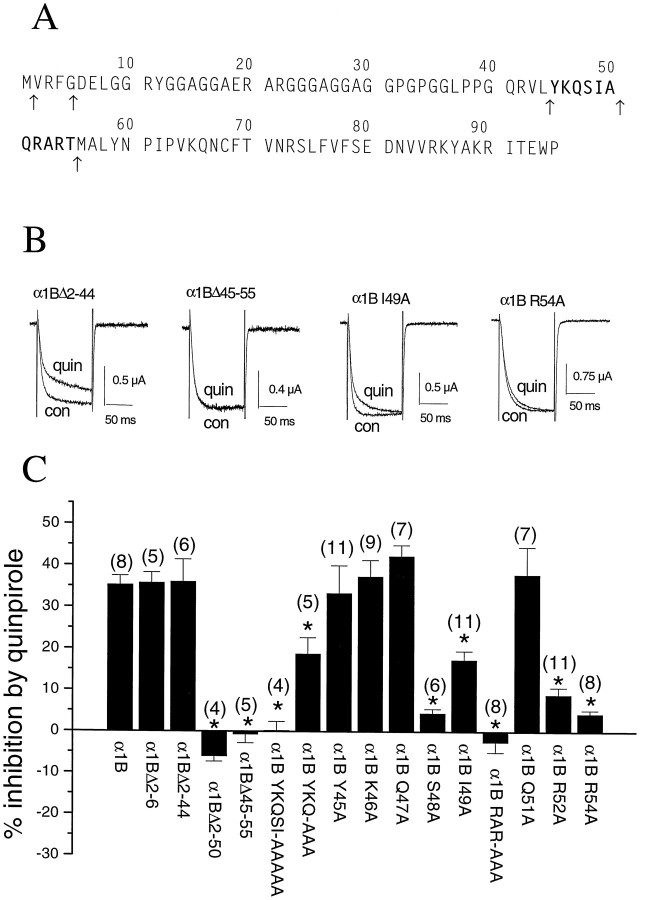
We have made a number of deletions to determine the amino acid sequences that are essential for G-protein modulation. From our previous study (Page et al., 1998), we found that the truncated Δ1–55 α1B construct was not G-protein modulated, in agreement with the N-terminally truncated α1E (rbEII) isoform, that is also not G-protein-modulated. In the present series of experiments, the effect of quinpirole (100 nm) was determined during steps to 0 mV, because none of the constructs showed major shifts in voltage dependence of current activation, compared to α1B. Inhibition of wild-type α1B was 35.3 ± 2.2% at 0 mV under these conditions (n = 8). We made a number of truncations: α1BΔ2–6 and Δ2–44, in line with regions of homology between the N-terminal sequences of all the G-protein modulated α1 subunits (Fig.5A). These two constructs were as strongly G-protein-modulated as α1B itself [respectively, 35.8 ± 2.5% (n = 5), and 36.1 ± 5.3% (n = 6) inhibition by quinpirole; Fig.5B,C]. This identifies the 11 amino acid sequence of α1B 45–55 (YKQSIAQRART) (Fig.5A) as being required for the G-protein modulation of α1B. To confirm this finding, deletion of only this sequence created a construct, α1B Δ45–55, in which G-protein modulation was completely abolished [−0.7 ± 2.1% inhibition by quinpirole (n = 5); Fig.5B,C].
Fig. 5.
The effect of various deletions and point mutations of the N terminus of α1B on inhibition ofIBa by the D2 agonist quinpirole. The sequence of the N terminus of α1B, with the 11 amino acid sequence identified as being involved in G-protein modulation inbold, and the points at which deletions were made shown by arrows beneath the sequence. Example traces, showing the effect of quinpirole (100 nm) onIBa in the α1B Δ2–44 mutant (left), the α1B Δ45–55 mutant (center left), the α1B I49A mutant (center right), and the α1B R54A mutant (right). Traces (100 msec duration) were obtained at a test potential of 0 mV, from a holding potential of −100 mV. Con, Control traces;quin, after perfusion of quinpirole. Histogram of the percentage inhibition by 100 nm quinpirole (mean ± SEM) of IBa in the various deletion and point mutants of the N terminus of α1B. The currents were activated at 0 mV, and the degree of inhibition was determined from the currents activated every 15 sec. The number of experiments for each condition is given in parentheses, and the significance of the differences compared to the inhibition of α1B are given by *p < 0.005.
Point mutations to alanine (A) were then carried out to identify the specific amino acids in this 11 amino acid sequence that are essential for G-protein modulation. Mutation of both arginines to alanine (R52A, R54A) produced a construct that showed no G-protein modulation (−2.5 ± 2.5% inhibition by quinpirole; n = 8). Point mutations of the individual amino acids in the QRART sequence (Q51A, R52A, and R54A) subsequently identified both arginines as being critical for G-protein modulation, because either mutation produced a construct that showed almost complete loss of inhibition by quinpirole (Fig. 5B,C).
The N-terminal part of this 11 amino acid sequence also contains residues that are critical for G-protein modulation. When YKQSI was mutated to AAAAA (Fig. 5A), the channel was not G-protein-modulated [0.3 ± 2.1% inhibition by quinpirole (n = 4); Fig. 5C]. To confirm the importance of the amino acids 45–50 (YKQSIA), an intermediate deletion α1B ΔN2–50 was made, to give a construct starting with methionine followed by Q51. This was also found not to be G-protein-modulated (Fig. 5C). Subsequent point mutations were made of the individual amino acids in the YKQSIA sequence to A (Y45A, K46A, Q47A, S48A, and I49A). This identified only the serine and, to a lesser extent, isoleucine in the sequence as being involved in G-protein modulation. These mutations resulted in reduced quinpirole-induced inhibition of IBa to 4.5 ± 1.0% (n = 6) for S48A and 17.4 ± 2.1% (n = 11) for I49A, respectively (Fig. 5C). Although the individual point mutants Y45A, K46A, and Q47A were all strongly G-protein-modulated by quinpirole, the modulation of the construct containing the triple mutation YKQ→AAA was reduced (18.8 ± 3.9% inhibition by quinpirole; n = 5; Fig. 5C), indicating an influence of these amino acids.
Modulation of N-terminal mutants of α1B by Gβγ
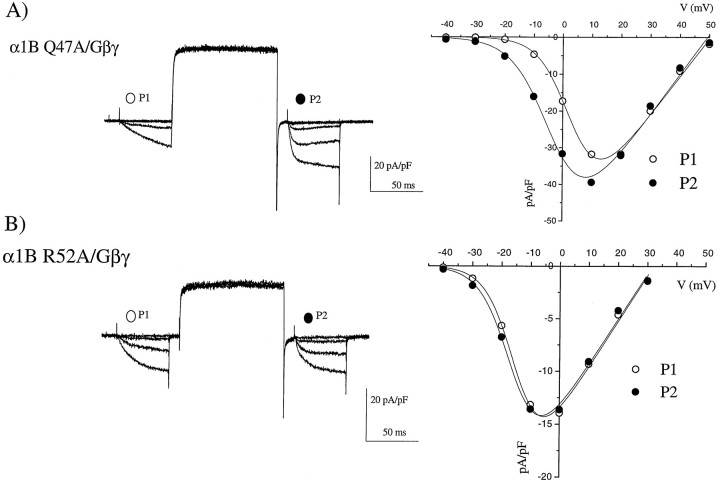
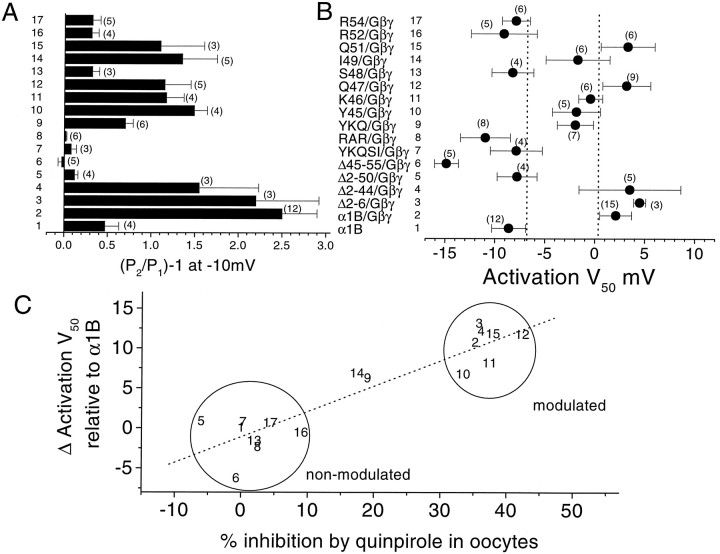
We have confirmed that the identified amino acids are similarly involved in direct Gβγ-induced modulation of α1B by performing experiments with coexpressed Gβ1γ2 in COS-7 cells. Examples of results obtained are shown in Figure 6. For the Q47A mutation, G-protein modulation was still observed, with slowly activating currents in P1 and a clear hyperpolarizing shift in the V50 for current activation resulting from the depolarizing prepulse (Fig. 6A). In contrast, for the R52A mutation, no G-protein modulation was observed (Fig. 6B). The mean results for all the constructs are given in Figure 7, expressed as P2/P1 facilitation ratio at −10 mV (Fig. 7A). The V50 for theIBa current–voltage relationship was also plotted, because this shows a depolarizing shift in G-protein-modulated channels, compared to the control α1B expressed in the absence of Gβγ (Fig. 7B). These two sets of measurements are strongly correlated (r = 0.76, data not shown), and the depolarizing shift in activationV50 is also highly correlated to the percentage inhibition by quinpirole observed for the same constructs in the Xenopus oocyte experiments (Fig.7C), suggesting that direct Gβγ modulation and quinpirole-induced modulation of these constructs are using the same mechanism. The I49A mutation stands out in both these systems as producing a reduction, but not a complete inhibition of modulation (Fig. 7C).
Fig. 6.
Examples of the effect of α1B N-terminal mutations on Gβγ modulation in COS-7 cells. Coexpression of two α1B N-terminal mutations with α2δ, β2a, and Gβ1γ2, recorded with 1 mm Ba2+ charge carrier.Left panel, Current traces are shown, evoked by the same protocol given in Figure 3. Right panel,Current–voltage relationships are given, from −40 to +50 mV, in 10 mV intervals, before (open circles) and after (filled circles) the depolarizing prepulse, fitted (solid lines) with the modified Boltzmann equation given in the legend to Table 1. A, The α1B Q47A mutation (traces from −40 to 0 mV are shown). B,The α1B R52A mutation (traces from −40 to +10 mV are shown).
Fig. 7.
Mean effect of α1B deletions and mutations on Gβγ modulation in COS-7 cells. A, The P2/P1 ratio was determined in COS-7 cells overexpressing Gβ1γ2, from current amplitudes during steps to −10 mV before and after a depolarizing prepulse (+120 mV, 100 msec), for the same N-terminal deletions and point mutations shown in Figure 5. Comparison is made with α1B in the absence of Gβγ, recorded with 1 mm GDPβS in the patch pipette (1). The value [(P2/P1) − 1] is plotted, which will be 0 if there is no facilitation. B, The activation V50 was determined for the same constructs coexpressed with Gβ1γ2, and compared to the value for α1B in the absence of Gβγ, recorded with 1 mm GDPβS in the patch pipette (1). The dashed lines are 1 SEM more positive than the mean value for α1B (1), and 1 SEM more negative than the mean value for α1B/Gβγ (2). C,Correlation between Δ activation V50 (the data given in B, after subtraction of theV50 for α1B) on the y-axis, and the data from Figure 5C (percentage inhibition ofIBa by 100 nm quinpirole), on the x-axis. The numbers identifying the constructs refer to the bars in A and B. Regression analysis (dotted line) gives a coefficient,r of 0.92 (p < 0.001). The data divide into a group of modulated and a group of nonmodulated constructs, as identified, except for constructs 14 (I49A) and 9 (YKQ→AAA).
Basis for the reduction in receptor-mediated modulation of the N-terminal point mutation I49A
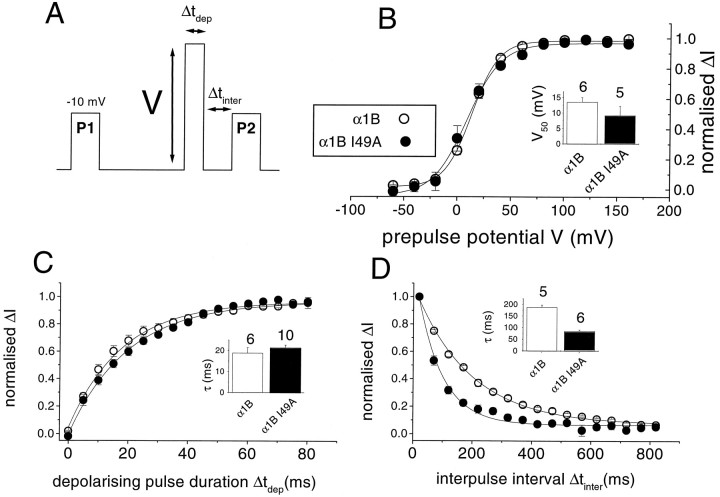
G-protein modulation of calcium channels is strongly voltage-dependent, in that more inhibition is observed at low than at high depolarizations (Bean, 1989). To examine the basis for the reduced modulation of the partially modulated mutant (α1B I49A) compared to α1B, we first examined, in Xenopus oocytes, the voltage dependence of the removal of inhibition by quinpirole, during a depolarizing prepulse (see Fig.8A for voltage protocol). There was no significant effect of the I49A mutation on the voltage dependence of the prepulse-induced facilitation in the presence of quinpirole (Fig. 8B), or on the time course of removal of quinpirole-induced inhibition during a 100 mV depolarizing prepulse. Single exponential fits gave τ values for removal of inhibition (possibly representing dissociation of Gβγ at this depolarized potential) of ∼20 msec for both constructs (Fig.8C). The only clear difference between I49A α1B and wild-type α1B was in the more rapid time course of reinstatement of G-protein modulation after its removal by a 100 msec prepulse to +100 mV (Fig. 8D). This could be fit to a single exponential with τreinhibition of 187 msec for α1B and 85 msec for the I49A mutant (Fig. 8D, inset).
Fig. 8.
Voltage dependence of inhibition, rate of loss of inhibition, and reinhibition rate for α1B and α1B I49A inXenopus oocytes. A, Voltage protocol, showing variation of the prepulse voltage (V), the prepulse duration (Δtdep), and the interpulse interval (Δtinter) between the prepulse and the test pulse. The prepulse potential was 100 mV and 50 msec duration, and the interpulse interval was 20 msec, unless these parameters were varied.B, Effect of increasing the 50 msec prepulse voltage (V) on prepulse facilitation in the presence of quinpirole. Facilitation was measured as (IBa in P2) − (IBa in P1) and normalized to the maximum facilitation observed (normalized ΔI). α1B (open circles), α1B I49A (filled circles). The inset histogram gives theV50 values (mean ± SEM, determined by fitting Boltzmann functions to the data from the number of individual experiments given above each bar) for α1B (white bar) and α1B I49A (black bar). C, Effect of increasing the duration of the 100 mV prepulse (Δtdep) on prepulse facilitation in the presence of quinpirole. Facilitation was measured as described inB. α1B (open circles), α1B I49A (filled circles). The insethistogram gives the τdissociation values (mean ± SEM, determined by fitting a single exponential to the data from the number of experiments given above each bar) for α1B (white bar) and α1B I49A (black bar).D, Effect of increasing the interval between the 100 mV, 50 msec prepulse and the subsequent test pulse P2 on the facilitation in the presence of quinpirole. Facilitation was measured as described in B: α1B (open circles), α1B I49A (filled circles). The insethistogram gives the τreinhibition values (mean ± SEM, determined by fitting a single exponential to the data from the number of experiments given above each bar) for α1B (white bar) and α1B I49A (black bar).
If we consider G-protein modulation as a simple bimolecular reaction, as has been done previously (Zhang et al., 1996; Stephens et al., 1998a):
where C is one of the closed states of the calcium channel α1B subunit, k1 is the association rate constant, and k−1is the dissociation rate constant for Gβγ. At equilibrium, from the law of mass action:
at the holding potential, 1/τreinhibition=k1[Gβγ]+k−1 (1)
| Equation 2 |
From our previous study (Stephens et al., 1998a), we estimated the Gβγ concentration to reach ∼300 nm, when Gβγ was overexpressed. Taking an approximate value of 100 nm for the concentration of Gβγ resulting from quinpirole-induced receptor activation in the present study [a value of 130 nm can be calculated making the assumptions described in Stephens et al. (1998a)], we can obtain estimates by substitution of steady-state inhibition and τreinhibition values into Equations 1 and 2, of k1 andk−1. For α1B,k1 is 25.2 μm−1sec−1, and k−1 is 2.8 sec−1, whereas for the I49A mutantk1 is 21.0 μm−1sec−1, and k−1 is 9.6 sec−1. Clearly, the major difference is an apparent 3.4-fold increase in the off-rate for Gβγ in the I49A mutant. However, assuming that reassociation of Gβγ is very slow at +100 mV, the dissociation of Gβγ during the prepulse to +100 mV, found from Figure 8C, is 53.2 sec−1 for α1B and 46.7 sec−1 for α1B I49A, indicating that the apparent off-rate is more rapid for both constructs at this depolarized potential [as previously observed in Stephens et al. (1998a)], and the difference between the parental α1B and the I49A mutant is lost.
DISCUSSION
The molecular determinants for the inhibition of neuronal VDCC α1 subunits by Gβγ have been the subject of several studies. However, there remains no consensus of opinion concerning the functional importance of biochemically identified Gβγ-binding sites on the I-II loop and C terminus (De Waard et al., 1997; Page et al., 1997; Qin et al., 1997; Zamponi et al., 1997) (for review, see Dolphin, 1998). Furthermore, there has been little agreement on the extent of modulation of the E-type VDCCs (Bourinet et al., 1996; Toth et al., 1996; Yassin et al., 1996; Mehrke et al., 1997; Page et al., 1997; Qin et al., 1997). However, following our recent study (Page et al., 1998), it now seems clear that all α1E orthologues are G-protein-modulated when the long N terminus is present.
Requirement for the N terminus of α1B for G-protein modulation
The present study was performed to further our understanding of the involvement of the N terminus of the VDCC α1B in G-protein modulation, first identified by Page et al. (1998). We therefore made a series of chimeras between α1B, which is strongly G-protein-modulated, and α1C, which is not modulated by Gβγ, in the systems studied. Our conclusions are that the N terminus of α1B is absolutely essential for its G-protein modulation. No modulation was observed of any channel that contained the N terminus from α1C. The sequences of the intracellular N termini of α1B and α1C show little homology, and it is thus clear that the N terminus of α1B plays a role in G-protein modulation that cannot be substituted by that of α1C.
Role of the I-II linker and first transmembrane domain of α1B in G-protein modulation
In contrast to the results concerning the N terminus, the I-II linker of α1B was not completely essential; significant G-protein modulation was observed in the chimeras α1bBcCCC and α1bCcCCC, although the extent of modulation was less than for the control α1B. These results are of mechanistic interest because of the inability of the α1C I-II linker to bind Gβγ (De Waard et al., 1997; Qin et al., 1997; Dolphin et al., 1999), presumably because of the lack of the QxxER-binding motif. It is possible that Gβγ binding to the I-II linker of α1B increases its concentration close to its site of action, but is not directly involved in its functional effects.
Both the I-II linker and the first transmembrane domain of α1B are, however, essential for the observation of a reduction of G-protein modulation by overexpression of exogenous β2a subunit in theXenopus oocyte system. Whereas α1B itself and α1bBbCCC showed significantly greater modulation by quinpirole in the absence of coexpressed VDCC β2a subunit, the α1bBcCCC and α1bCbCCC chimeras exhibited a similar degree of inhibition by quinpirole in the presence and absence of coexpressed β2a. The mechanism of this partial antagonism by β2a remains unclear, but is not completely shared by other β subunits such as β1b (C. Canti and A. C. Dolphin, unpublished results).
The first transmembrane domain of α1B clearly has a role in G-protein modulation, as suggested previously (Zhang et al., 1996; Stephens et al., 1998b). We have found that, although it can be substituted by that of α1C, the α1bCbCCC chimera is less modulated than α1bBbCCC by quinpirole in the Xenopus oocyte system. It is possible that the first transmembrane domain mediates the effects of Gβγ subunits to slow current activation, via interference with the function of its voltage sensor. Evidence suggests that only one Gβγ subunit binds per α1 subunit, in a voltage-dependent manner (Stephens et al., 1998a; Zamponi and Snutch, 1998). We previously estimated the off-rate (k−1) of Gβγ subunits to be ∼1.3 sec−1 at −100 mV and 50 sec−1 at +120 mV (Stephens et al., 1998a). Thus, the binding of Gβγ is probably of higher affinity to the channel with the voltage sensors in their resting state. The action of Gβγ subunits is to delay channel opening and to produce a depolarizing shift in the voltage dependence of activation (Patil et al., 1996). Presumably, this is achieved either by slowing the movement of the voltage sensors (and the IS4 sensor in particular), in response to a change in transmembrane voltage, or reducing the efficiency of coupling of the voltage sensor to channel opening (Jones et al., 1997).
Some of our chimera results and conclusions do not agree with those of a previous work (Furukawa et al., 1998), which also made a chimera with the I-II linker of α1B in α1C and showed it to be G-protein-modulated, thus indicating that the I-II linker alone could mediate G-protein modulation. However, their chimera, together with other chimeras described in their paper, involved substitution of more than just the I-II linker of α1B. In the chimera in question, a region of α1B from part of IS5 through to IIS2 was substituted into α1C, with in addition several amino acid substitutions and deletions, and the results are thus not directly comparable. Furthermore, in their study the reciprocal chimera, made up of α1B with a region including the I-II linker of α1C, was also G-protein-modulated.
Our finding is that substitution into α1C of the region from the N terminus to the end of the I-II linker of α1B (α1bBbCCC) does not produce a channel that is as strongly modulated as α1B in theXenopus oocyte assay, although in the Gβγ overexpression assay, there was no significant difference between α1bBbCCC and α1B. Thus, it is likely that other regions in the rest of α1B may also contribute to the extent of G-protein modulation of α1B, possibly including the C terminus (Qin et al., 1997; Hamid et al., 1999), which may form part of a complex Gβγ-binding pocket.
Amino acids in the N terminus of α1B that are critical for G-protein modulation
From our mutational study of the N terminus, we identified the sequence between amino acids 45 and 55 (YKQSIAQRART) as being essential for G-protein modulation, because a deletion to amino acid 55 produced a construct that showed no G-protein modulation (Page et al., 1998), whereas a channel truncated to amino acid 44 was fully modulated, and a deletion of these 11 amino acids (45–55) resulted in a nonmodulated construct. Subsequently, we have identified three amino acids within this sequence, S48, R52, and R54, that when mutated to alanine, markedly reduce G-protein modulation of α1B, and a fourth amino acid (I49) that also shows an involvement. The substitution of just two amino acids (R52A, R54A) completely abolished G-protein modulation, whereas constructs containing the individual mutations still showed a small degree of modulation (4 and 9% inhibition by quinpirole, respectively). The R52A and R54A constructs also individually showed some slowing of current activation when coexpressed with Gβγ, whereas the double mutant did not (Fig.6B; results not shown). The RAR motif is reminiscent of the RAK motif found in one of the Gβγ-binding sites on GIRK4 (Krapivinsky et al., 1998).
The I49A mutation stands out as producing a reduction in G-protein modulation in both systems (Fig. 6C). It is of interest that the 11 amino acid motif we have identified is identical in rat α1E and α1A, except for I49, whose equivalent is lysine in α1E, and methionine in α1A. Furthermore, both α1A and α1Elong show less G-protein modulation than α1B in a number of systems (Zamponi et al., 1997; Page et al., 1998), possibly involving this amino acid substitution. In the present study we have observed that the τreinhibition after a depolarizing prepulse is more than twice as fast for α1B I49A (85 msec) than for α1B (187 msec; Fig. 8D). However, in our previous study we observed that the τreinhibition for both α1B and α1Elong was ∼95 msec (Page et al., 1998). We are currently re-examining the comparison between α1B and α1Elong under the present conditions (5 mm Ba2+, BAPTA-injected oocytes), to investigate whether our previous lack of observation of any difference in τreinhibitionbetween α1B and α1Elong was caused by an influence of niflumic acid, which we have subsequently found to affect G-protein modulation of α1B currents.
It is possible that the N terminus forms a Gβγ or VDCC β-binding site, or it may be involved in the downstream effects of Gβγ binding. We have observed that inactivation is increased in a number of the α1B mutants, suggesting an impairment of interaction with β2a (G. J. Stephens and A. C. Dolphin, unpublished results). However, one can consider that these mutations in the N terminus of α1B may alter the binding affinity for Gβγ (see Results for I49A). From this analysis the major difference is an apparent 3.4-fold increase in the off-rate for Gβγ in the I49A mutant. Thus, YKQSIAQRART may form part of a Gβγ-binding site, with I49 playing a modulatory role in binding affinity, or it may be involved in the interaction between Gβγ and VDCC β subunits.
Footnotes
This work was supported by The Wellcome Trust and the European Community (Marie Curie Fellowship to C.C.). We thank the following for generous gifts of cDNAs: T. Snutch (University of British Colombia, Vancouver, Canada), rat α1C; H. Chin (National Institutes of Health, Bethesda, MD), rat α2δ-1; Y. Mori (Seriken, Okazaki, Japan), rabbit α1B; E. Perez-Reyes (Loyola University, Chicago, IL), rat β2a; P. G. Strange (Reading, UK), rat D2 receptor; M. Simon (CalTech, Pasadena, CA), bovine Gβ1 and Gγ2; R. Lefkowitz (Duke University, Durham, NC), β-ARK1; T. Hughes (Yale, New Haven, CT), mut-3 GFP; Genetics Institute (Cambridge, MA), pMT2. We also thank M. Li and J. Richards for technical assistance.
Correspondence should be addressed to Prof. A. C. Dolphin, Department of Pharmacology (Medawar Building), University College London, Gower Street, London WC1E 6BT, UK.
REFERENCES
- 1.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage-dependence. Nature. 1989;340:153–155. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 2.Bourinet E, Soong TW, Stea A, Snutch TP. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci USA. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different β subunits in the membrane expression of the α1A and α2 calcium channel subunits: studies using a depolarisation-sensitive α1A antibody. Eur J Neurosci. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- 4.Campbell V, Berrow N, Brickley K, Page K, Wade R, Dolphin AC. Voltage-dependent calcium channel β-subunits in combination with alpha1 subunits have a GTPase activating effect to promote hydrolysis of GTP by Gαo in rat frontal cortex. FEBS Lett. 1995a;370:135–140. doi: 10.1016/0014-5793(95)00813-o. [DOI] [PubMed] [Google Scholar]
- 5.Campbell V, Berrow NS, Fitzgerald EM, Brickley K, Dolphin AC. Inhibition of the interaction of G protein Go with calcium channels by the calcium channel β-subunit in rat neurones. J Physiol (Lond) 1995b;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Waard M, Liu HY, Walker D, Scott VES, Gurnett CA, Campbell KP. Direct binding of G protein βgamma complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 7.Dolphin AC. Facilitation of Ca2+ current in excitable cells. Trends Neurosci. 1996;19:35–43. doi: 10.1016/0166-2236(96)81865-0. [DOI] [PubMed] [Google Scholar]
- 8.Dolphin AC, Page KM, Berrow NS, Stephens GJ, Canti C. Dissection of the calcium channel domains responsible for modulation of neuronal voltage-dependent calcium channels by G proteins. Ann NY Acad Sci. 1999;868:160–174. doi: 10.1111/j.1749-6632.1999.tb11285.x. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa T, Nukada T, Mori Y, Wakamori M, Fujita Y, Ishida H, Fukuda K, Kato S, Yoshii M. Differential interactions of the C terminus and the cytoplasmic I-II loop of neuronal Ca2+ channels with G-protein alpha and beta gamma subunits. I Molecular determination. J Biol Chem. 1998;273:17585–17594. doi: 10.1074/jbc.273.28.17585. [DOI] [PubMed] [Google Scholar]
- 10.Hamid J, Nelson D, Spaetgens R, Dubel S, Snutch TP, Zamponi G. Identification of an integration center for cross-talk between protein kinase C and G protein modulation of N type calcium channels. J Biol Chem. 1999;274:6195–6202. doi: 10.1074/jbc.274.10.6195. [DOI] [PubMed] [Google Scholar]
- 11.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein βgamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 12.Herlitze S, Hockerman GH, Scheuer T, Catterall WA. Molecular determinants of inactivation and G protein modulation in the intracellular loop connecting domains I and II of the calcium channel α1A subunit. Proc Natl Acad Sci USA. 1997;94:1512–1516. doi: 10.1073/pnas.94.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 14.Jones LP, Patil PG, Snutch TP, Yue DT. G-protein modulation of N-type calcium channel gating current in human embryonic kidney cells (HEK 293). J Physiol (Lond) 1997;498:601–610. doi: 10.1113/jphysiol.1997.sp021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krapivinsky G, Kennedy ME, Nemec J, Medina I, Krapivinsky L, Clapham DE. Gβγ binding to GIRK4 subunit is critical for G protein-gated K+ channel activation. J Biol Chem. 1998;273:16946–16952. doi: 10.1074/jbc.273.27.16946. [DOI] [PubMed] [Google Scholar]
- 16.Mehrke G, Pereverzev A, Grabsch H, Hescheler J, Schneider T. Receptor-mediated modulation of recombinant neuronal class E calcium channels. FEBS Lett. 1997;408:261–270. doi: 10.1016/s0014-5793(97)00437-7. [DOI] [PubMed] [Google Scholar]
- 17.Page KM, Stephens GJ, Berrow NS, Dolphin AC. The intracellular loop between domains I and II of the B type calcium channel confers aspects of G protein sensitivity to the E type calcium channel. J Neurosci. 1997;17:1330–1338. doi: 10.1523/JNEUROSCI.17-04-01330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page KM, Canti C, Stephens GJ, Berrow NS, Dolphin AC. Identification of the amino terminus of neuronal Ca2+ channel α1 subunits α1B and α1E as an essential determinant of G protein modulation. J Neurosci. 1998;18:4815–4824. doi: 10.1523/JNEUROSCI.18-13-04815.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patil PG, De Leon M, Reed RR, Dubel S, Snutch TP, Yue DT. Elementary events underlying voltage-dependent G-protein inhibition of N-type calcium channels. Biophys J. 1996;71:2509–2521. doi: 10.1016/S0006-3495(96)79444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Direct interaction of Gβγ with a C terminal Gβγ binding domain of the calcium channel α1 subunit is responsible for channel inhibition by G protein coupled receptors. Proc Natl Acad Sci USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin N, Platano D, Olcese R, Costantin JL, Stefani E, Birnbaumer L. Unique regulatory properties of the type 2a Ca2+ channel β subunit caused by palmitoylation. Proc Natl Acad Sci USA. 1998;95:4690–4695. doi: 10.1073/pnas.95.8.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simen AA, Miller RJ. Structural features determining differential receptor regulation of neuronal Ca channels. J Neurosci. 1998;18:3689–3698. doi: 10.1523/JNEUROSCI.18-10-03689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephens GJ, Brice NL, Berrow NS, Dolphin AC. Facilitation of rabbit α1B calcium channels: involvement of endogenous Gβγ subunits. J Physiol (Lond) 1998a;509:15–27. doi: 10.1111/j.1469-7793.1998.015bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens GJ, Canti C, Page KM, Dolphin AC. Role of domain I of neuronal Ca2+ channel α1 subunits in G protein modulation. J Physiol (Lond) 1998b;509:163–169. doi: 10.1111/j.1469-7793.1998.163bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swick AG, Janicot M, Cheneval-Kastelic T, McLenithan JC, Lane DM. Promoter-cDNA-directed heterologous protein expression in Xenopus laevis oocytes. Proc Natl Acad Sci USA. 1992;89:1812–1816. doi: 10.1073/pnas.89.5.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tareilus E, Roux M, Qin N, Olcese R, Zhou JM, Stefani E, Birnbaumer L. A Xenopus oocyte β subunit: Evidence for a role in the assembly/expression of voltage-gated calcium channels that is separate from its role as a regulatory subunit. Proc Natl Acad Sci USA. 1997;94:1703–1708. doi: 10.1073/pnas.94.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toth PT, Shekter LR, Ma GH, Philipson LH, Miller RJ. Selective G-protein regulation of neuronal calcium channels. J Neurosci. 1996;16:4617–4624. doi: 10.1523/JNEUROSCI.16-15-04617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker D, De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels. Trends Neurosci. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- 29.Yassin M, Zong SQ, Tanabe T. G-protein modulation of neuronal class E (α1E) calcium channel expressed in GH3 cells. Biochem Biophys Res Commun. 1996;220:453–458. doi: 10.1006/bbrc.1996.0426. [DOI] [PubMed] [Google Scholar]
- 30.Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gβγ subunit. Proc Natl Acad Sci USA. 1998;95:4035–4039. doi: 10.1073/pnas.95.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JF, Ellinor PT, Aldrich RW, Tsien RW. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G proteins. Neuron. 1996;17:991–1003. doi: 10.1016/s0896-6273(00)80229-9. [DOI] [PubMed] [Google Scholar]