Abstract
The ovarian hormones estradiol (E2) and progesterone (P) facilitate rat lordosis behavior in part by regulating the expression of and signal transduction by adrenoceptors in the hypothalamus (HYP) and preoptic area (POA). The major adrenoceptor subtype mediating E2 and P facilitation of lordosis is the α1-adrenoceptor. In the present studies, we tested the hypotheses that (1) α1-adrenoceptors in the HYP enhance lordosis responses by activating the nitric oxide (NO)–cGMP signaling pathway, and (2) coupling of α1-adrenoceptors to this signal transduction pathway is hormone-dependent. Basal levels of cGMP were significantly higher in HYP and POA slices from animals treated with E2 and P when compared with slices from ovariectomized controls or females treated with only E2 or P. When slices of HYP and POA from ovariectomized female rats were incubated with norepinephrine or the selective α1-adrenoceptor agonist phenylephrine, cGMP accumulation was observed only if slices had been derived from females treated with both E2 and P before experimentation. Moreover, α1-adrenoceptor stimulation of cGMP synthesis was blocked by an inhibitor of NO synthase, confirming that these receptors act by NO-mediated stimulation of soluble guanylyl cyclase. Behavioral studies demonstrated further that the cell-permeable cGMP analog 8-bromoadenosine-cGMP reverses the inhibitory effects of the α1-adrenoceptor antagonist prazosin on lordosis behavior in E2- and P-treated female rats. Thus, the NO–cGMP pathway mediates the facilitatory effects of α1-adrenoceptors on lordosis behavior in female rats, and previous exposure of the HYP and POA to both E2 and P are required to link α1-adrenoceptors to this pathway.
Keywords: estradiol, progesterone, α1-adrenoceptors, hypothalamus, lordosis, cGMP
Norepinephrine (NE) neurotransmission in the hypothalamus (HYP) and preoptic area (POA) is a key player in estradiol (E2) and progesterone (P) coordination of preovulatory gonadotropin secretion and reproductive behavior of female rodents (Kalra and Kalra, 1983; Etgen et al., 1992; Pfaff et al., 1994). Ample evidence implicates α1-adrenoceptors in these brain regions as the mediators of NE facilitation of gonadotropin secretion (Ramirez et al., 1984) and of lordosis, a major component of female reproductive behavior (Etgen et al., 1992). Moreover, E2priming elevates ligand binding and mRNA levels of the α1B-adrenoceptor subtype in HYP–POA (Petitti et al., 1992; Karkanias et al., 1996). The α1-adrenoceptors activate phospholipase C to produce inositol-1,4,5-triphosphate (IP3), which mobilizes intracellular calcium, and diacylglycerol, which activates protein kinase C (Johnson and Minneman, 1986, 1987). The α1-adrenoceptors also increase influx of extracellular calcium through calcium channels (Minneman, 1988). In HYP and POA slices from E2-primed female rats, P attenuates α1-adrenoceptor stimulation of IP3 formation and abolishes α1-adrenoceptor augmentation of adenylyl cyclase, a protein kinase C-mediated response (Petitti and Etgen, 1989,1990, 1992; Karkanias et al., 1995). However, the signal transduction mechanisms mediating α1-adrenoceptor facilitation of lordosis behavior are still unknown.
Nitric oxide (NO) is a diffusible messenger synthesized by NO synthase, a calcium–calmodulin-activated enzyme (Snyder and Bredt, 1991). Because NO synthase is activated by elevation of intracellular calcium, the effects of α1-adrenoceptors on reproductive function may be mediated by NO. Recently, NO was found to regulate the secretion of gonadotropin-releasing hormone (GnRH) both in vivo and in vitro (Bonavera et al., 1993; Moretto et al., 1993; Rettori et al., 1993, 1994; Mahachoklertwattana et al., 1994; Lopez et al., 1997). NO appears to act in the POA to stimulate GnRH release by activating soluble guanylyl cyclase to synthesize cGMP (Brann et al., 1997; Pu et al., 1997, 1998). Manipulations of brain NO and cGMP levels also modulate lordosis behavior (Fernandez-Guasti et al., 1983; Mani et al., 1994; Chu et al., 1999). Indeed, NO facilitates lordosis in hormone-treated females through activation of soluble guanylyl cyclase (Chu and Etgen, 1997; Chu et al., 1999).
Interestingly, brain NO synthase expression and mRNA levels increase in POA and HYP after estrogen priming (Okamura et al., 1994a,b; Ceccatelli et al., 1996; Pu et al., 1998). In addition, P increases guanylyl cyclase activity in some peripheral tissues (Vesely and Hill, 1980). HYP levels of cGMP increase significantly on the evening of proestrus, a time characterized by high levels of P and behavioral receptivity in gonadally intact rats (Kimura et al., 1980). Therefore, the present study tested the hypothesis that E2 and P promote the linkage of HYP–POA α1-adrenoceptors to the NO–cGMP pathway and that this signaling system mediates α1-adrenoceptor facilitation of lordosis behavior. We measured the cGMP response to NE and an α1-adrenergic agonist in HYP–POA slices of hormone-treated female rats. NO synthase inhibitors were used to examine the role of NO in NE-dependent cGMP accumulation. The ability of a cGMP analog to reverse the inhibition of lordosis behavior produced by α1-adrenergic antagonists was also evaluated.
MATERIALS AND METHODS
Determination of cGMP levels in vitro. All animal experimentation was performed in accordance with National Institutes of Health guidelines and was approved by the Institutional Animal Care and Use Committee. Female Sprague Dawley rats weighing 150–175 gm were purchased from Taconic Farms (Germantown, NY) and maintained with food and water ad libitum. Animals were ovariectomized under Metofane (Pitman-Moore, Mundelein, IL) anesthesia 1 d after arrival. Five or 6 d later, animals received the first of two daily injections of 2 μg E2 benzoate (EB) in 0.1 ml of peanut oil vehicle or 0.1 ml of oil (control) subcutaneously. P (200 μg) in 0.1 ml of oil or oil vehicle was injected subcutaneously 42–44 hr after the first EB or vehicle injection. Four hours after P or vehicle administration, animals were killed by decapitation. This time course of hormone treatment was chosen because ovariectomized animals reliably exhibit lordosis behavior under this regimen of hormone injections (Etgen et al., 1992; Chu et al., 1999). All animals were killed at the same time of day (10:00 A.M. to 11:00 A.M.) to control for reported diurnal rhythms in cGMP in the female rat POA (Pu et al., 1998).
The brain was removed, a block containing the entire HYP-POA was dissected, and eight 400 μm slices were made with a McIlwain tissue chopper. Individual slices were collected in culture wells and preincubated for 90 min at 33–35°C in buffer containing (in mm): 26 NaHCO3, 11 glucose, 120 NaCl, 2 KCl, 1.18 KH2PO4, 1.19 MgSO4, and 2 CaCl2. The 90 min preincubation period allows cGMP levels in the slices to return to baseline from the elevated state caused by decapitation. An inhibitor of phosphodiesterase, 1 mm 1,3-isobutyl-1-methylxanthine, was also included in the medium. At the end of the 90 min preincubation, NE (100 μm) or the α1-adrenergic agonist phenylephrine (10 μm) was applied for 20 min. In some experiments, the NO synthase inhibitorsNG-nitro-l-arginine methyl ester (NAME) (100 μm) orNG-nitro-arginine (NOARG) (300 μm) or the α1-adrenergic antagonist prazosin (10 μm) was applied for 20 min before NE or phenylephrine.
Tissue slices were then placed in 5% trichloroacetic acid for deproteinization. Cyclic nucleotides and protein were separated by homogenization and centrifugation. Trichloroacetic acid was later removed by ether extraction. Samples containing cyclic nucleotides were then lyophilized and assayed for cGMP content by radioimmunoassay (Amersham, Arlington Heights, IL). This assay system provides a sensitive and specific assay for cGMP (detection limit, 0.04 pmol). Protein contents of the tissue slices were determined by the Lowry method (Lowry et al., 1951), and data are expressed as picomoles of cGMP per milligram of protein.
Distribution of basal cGMP levels along the anteroposterior axis of the POA–HYP. We consistently observe an uneven distribution of cAMP along the anteroposterior axis of POA–HYP (N. Petitti and A. M. Etgen, unpublished observations). Therefore, we analyzed the basal content of cGMP according to localization along the anteroposterior axis of POA–HYP. A significant effect of location was found (F(3,42) = 4.99;p < 0.05; n = 15). The most posterior quarter of the POA–HYP block had significantly lower cGMP levels (1.42 ± 0.26 pmol/mg protein) than the most anterior quarter (2.02 ± 0.29 pmol/mg protein). To avoid any possible masking effect caused by this uneven distribution, every two consecutive slices were used as a pair; one slice was treated with drug or vehicle, and one slice was untreated (basal cGMP). The order of treatment in a pair alternated from day to day of the same experiment. Likewise, drug treatment was randomly assigned to the four pairs of slices along the anteroposterior axis. We observed no effects of anteroposterior location on responses to any drugs. The ratio of cGMP levels from the two slices in a pair (treated/untreated) is usually presented in Results to provide a more informative within-subject comparison of drug effects.
Stereotaxic surgery and behavior testing. Female Sprague Dawley rats (175–200 gm) were maintained on a reverse14/10 hr light/dark cycle, with lights off at 11:00 A.M. The day after arrival, animals were anesthetized with xylazine (4 mg/kg) and ketamine (80 mg/kg), placed into a stereotaxic apparatus, and secured with ear bars and a nose piece set at +5.0 mm. A 26 gauge guide cannula (Plastics One, Roanoke, VA) was implanted into the third ventricle using coordinates from the atlas of Pellegrino et al. (1979): + 0.2 mm posterior, 0 mm lateral, and + 9 mm ventral with respect to bregma. Bilateral ovariectomy was done at the same time as stereotaxic surgery. Reproductive behavior testing was performed 1 week after surgery. Rats received two subcutaneous injections of 2 μg of EB 24 and 48 hr before behavioral testing. Some animals also received 200 μg of P 4 hr before testing. The hydrolysis-resistant cGMP analog, 8-bromoadenosine-cGMP (8-br-cGMP) (1 μm), was infused into the third ventricle in a volume of 2 μl 4 hr before behavior testing. The dose and timing of 8-br-cGMP infusion was based on previous experiments (Chu et al., 1999) showing that 8-br-cGMP can stimulate lordosis responding when infused into the third ventricle of EB-primed rats.
For reproductive behavior testing, experienced stimulus male rats were placed in 20 gallon glass arenas and allowed to adapt for 10 min. Females were then placed in the arenas with a male until they received 10 mounts with pelvic thrusting. A lordosis quotient (LQ = number of lordosis responses/number of mounts × 100) was used as a measure of behavioral receptivity. The quality of each lordosis was also scored on a scale of 0–3 (0, no lordosis; 1, shallow lordosis; 2, definite dorsiflexion of the spine; and 3, exaggerated lordosis). In addition, the presence or absence of proceptive (soliciting) behaviors was recorded. Animals were tested three times at hourly intervals, with the first test at 4 hr after drug or saline infusion into the third ventricle. To determine whether any treatments altered the general activity level of animals, two components of open field activity were measured in a 5 min test immediately after the first lordosis test. The number of lines crossed on a grid composed of 10 5 × 5 inch squares, and the number of rearing events, defined as the number of times the rat lifted both forepaws off the cage floor, were recorded.
Animals were anesthetized with 40 mg of ketamine and decapitated immediately after the final test for lordosis. The brain was removed, frozen in isopentane for 1 min at approximately −35°C, and stored at −70°C. Frozen brains were sectioned (40 μm) in the transverse plane on a cryostat microtome. Every fourth section was saved throughout the extent of the cannula tracks. Anatomical verification of the cannula placement was made according to the atlas of Pellegrino et al. (1979).
Materials and drug preparation. NAME and NOARG were purchased from Research Biochemicals (Natick, MA). EB and P were purchased from Steraloids, Inc. (Wilton, NH). Prazosin, NE, and phenylephrine were purchased from Sigma (St. Louis, MO), and 8-br-cGMP was purchased from Calbiochem (La Jolla, CA). Phenylephrine, NAME, and NOARG were prepared in distilled water. Prazosin was prepared in saline containing 25% propylene glycol. NE was prepared in 0.01 N HCl, and 8-br-cGMP was prepared in sterile saline.
Statistical analysis. Levels of cGMP or ratios of cGMP levels of slices in a pair were analyzed with either one-way or two-way ANOVA as appropriate, with hormone as the between-subject factor and drug as the within-subject factor, followed by Tukey post hoc tests. Behavioral data were analyzed using two-way ANOVA with drug as the between-subject factor and the test number as the within-subject factor, followed by Tukey post hoc tests. Differences were considered significant if p < 0.05.
RESULTS
Basal cGMP levels in HYP–POA slices of EB- and P-primed animals
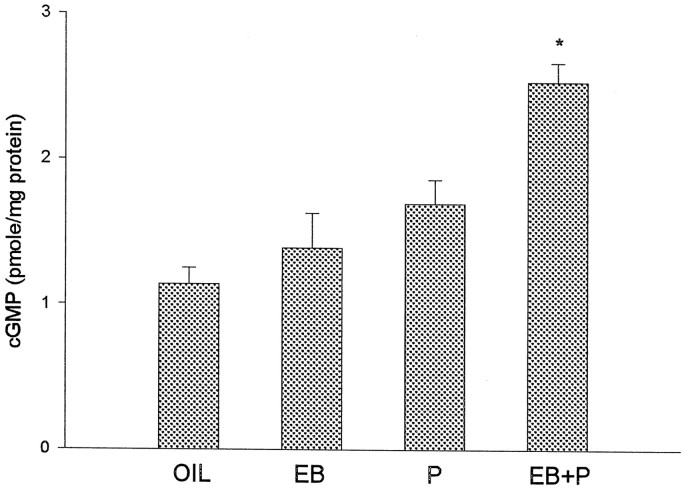
Because cGMP levels are reported to be highest on the afternoon of proestrus in gonadally intact female rats (Kimura et al., 1980) and because we hypothesize that E2 and P enhance the activity of NO–cGMP pathway, we first examined basal cGMP content in the POA–HYP slices obtained from ovariectomized animals treated with different hormones. The average cGMP content of all slices from the same rat was used to calculate the basal cGMP levels for each animal. There is a significant effect of hormone treatment on basal cGMP (F(3,18) = 14.50; p < 0.05; n = 5–7). Post hoc analysis indicated that slices from EB plus P-treated animals have significantly higher levels of cGMP than slices from control, EB- or P-treated animals (Fig. 1).
Fig. 1.
Priming with EB plus P increases basal cGMP levels in HYP–POA slices of ovariectomized female rats. Animals were injected subcutaneously with 2 μg of EB or 0.1 ml of vehicle (oil) at 24 and 48 hr before being killed. Some of the oil- and EB-treated rats were also injected subcutaneously with 200 μg of P 4 hr before being killed. The data are shown as mean ± SEM (n = 5–7). *p < 0.05 versus oil, EB, and P; Tukey test.
NE and phenylephrine stimulate cGMP accumulation only in EB plus P-treated animals
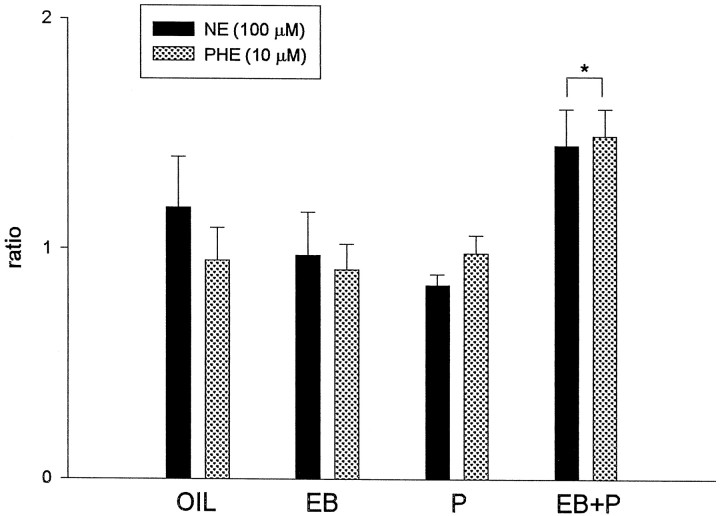
Because we hypothesize that NE, through α1-adrenoceptors, activates cGMP production in a hormone-dependent manner, we tested the effects of NE on cGMP accumulation in slices from rats killed after different hormone treatments. Phenylephrine, an α1-adrenergic agonist, was also used to determine the role of α1-adrenoceptors in regulation of cGMP synthesis. Ratios of cGMP levels from two consecutive slices (one treated with NE, phenylephrine, or vehicle; one untreated) in a pair are shown in Figure 2. ANOVA indicated that there was a significant main effect of hormone treatment (F(3, 36) = 4.39; p < 0.05; n = 5–7); the main effect of drug and the drug × hormone treatment interaction were not significant (p > 0.10). Figure 2 demonstrates that there is a significant increase of cGMP in response to 100 μm NE and 10 μmphenylephrine only in slices from EB plus P-treated females (Tukey test; p < 0.05). In the other hormone groups, neither NE nor phenylephrine significantly increased cGMP accumulation in POA–HYP slices. In addition, there was no difference between the magnitude of NE and phenylephrine stimulation, with both drugs increasing cGMP to ∼150% of basal levels, in the EB plus P slices. These results suggest that α1-adrenoceptors may mediate the effects of NE and phenylephrine on cGMP levels in EB plus P-primed animals.
Fig. 2.
NE and phenylephrine (PHE) stimulate cGMP production only in EB plus P-treated HYP–POA slices. Hormone treatments were the same as described in Figure 1. Either 100 μm NE or 10 μm phenylephrine was administered in vitro for 20 min. The ratio was calculated as the cGMP level in a drug-treated slice/the cGMP level in an adjacent, vehicle-treated slice. The data are shown as mean ± SEM (n = 5–7). *p < 0.05 versus oil, EB, and P; Tukey test.
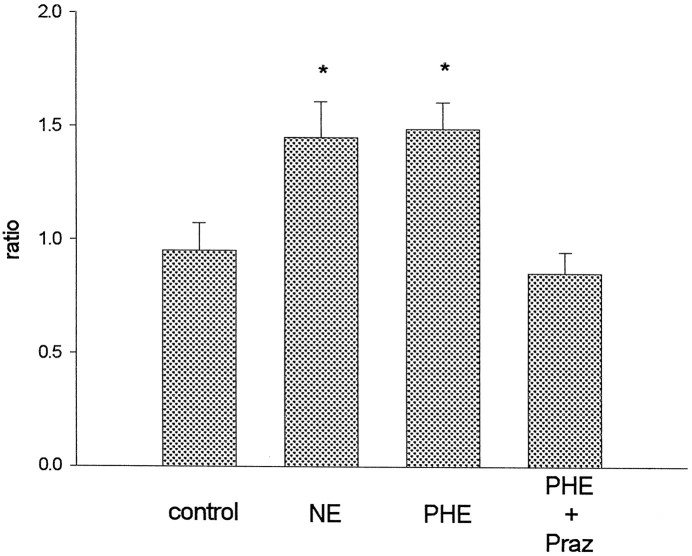
However, phenylephrine is known to act on the NE transporter to enhance NE release (Ari et al., 1989). Thus, phenylephrine effects might be mediated through NE acting at non-α1-adrenoceptors. Hence, the α1-adrenergic antagonist prazosin (10 μm) was used to examine the role of α1-adrenoceptors in phenylephrine-induced cGMP increases. The slices used in this experiment were from EB plus P-primed rats, because NE and phenylephrine stimulate cGMP production only in animals with EB plus P treatment. Figure3 demonstrates that prazosin blocks phenylephrine stimulation of cGMP synthesis (F(3,18) = 7.14; p < 0.05; n = 7). Again, responses to NE and phenylephrine are not different. Therefore, α1-adrenoceptors mediate the effects of phenylephrine on cGMP accumulation.
Fig. 3.
Prazosin (Praz) blocks the stimulatory effect of phenylephrine (PHE) on cGMP production in HYP–POA slices from EB plus P-treated animals. NE (100 μm), phenylephrine (10 μm), or vehicle (0.01N HCl) was administered in vitro for 20 min after 20 min preincubation with prazosin or vehicle (0.25% propylene glycol). Control slices were exposed to both vehicles. Ratios were calculated as described in Figure 2. The data are shown as mean ± SEM (n = 7). *p < 0.05 versus control; Tukey test.
NO mediates NE stimulation of cGMP formation in HYP–POA
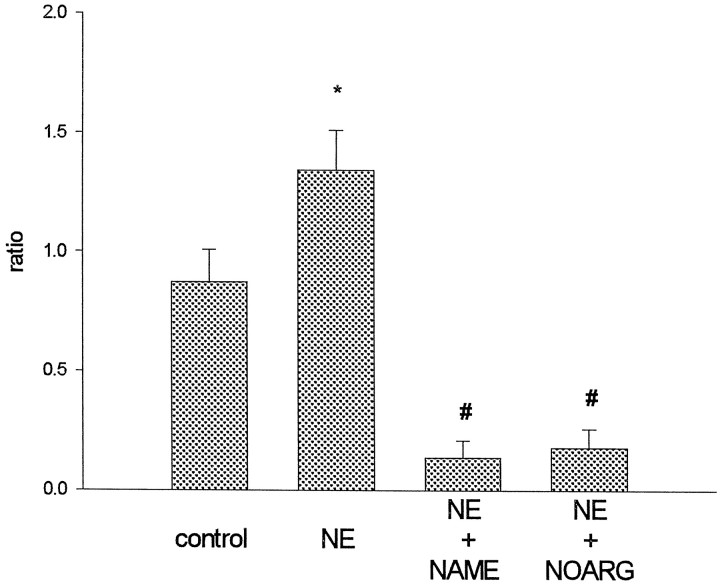
Next, we investigated the role of NO in α1-adrenoceptor activation of cGMP synthesis. We hypothesize that, in EB plus P-exposed slices, α1-adrenoceptor activation increases intracellular calcium via both the IP3 pathway and influx of extracellular calcium. NO synthase is thus activated to enhance the production of cGMP by soluble guanylyl cyclase. Two NO synthase inhibitors were used to test this hypothesis: NAME and NOARG. Both NAME and NOARG blocked NE-induced cGMP production (Fig.4) (F(3, 21) = 6.23; p < 0.05; n = 8). Furthermore, the two drugs markedly reduced the basal content of cGMP. In fact, the cGMP levels in some drug-treated slices were under the detection limit of the assay.
Fig. 4.
The NO synthase inhibitors NAME and NOARG block cGMP production in slices from EB plus P-treated animals. NAME (100 μm ), NOARG (300 μm), or vehicle (distilled water) was administered 20 min before stimulation with 100 μm NE or vehicle (0.01N HCl) for 20 min. Control slices were exposed to both vehicles. Ratios were calculated as described previously. The data are shown as mean ± SEM (n = 8). *p < 0.05 versus control; #p < 0.05 versus control and NE; Tukey test.
8-Br-cGMP reverses prazosin inhibition of lordosis behavior
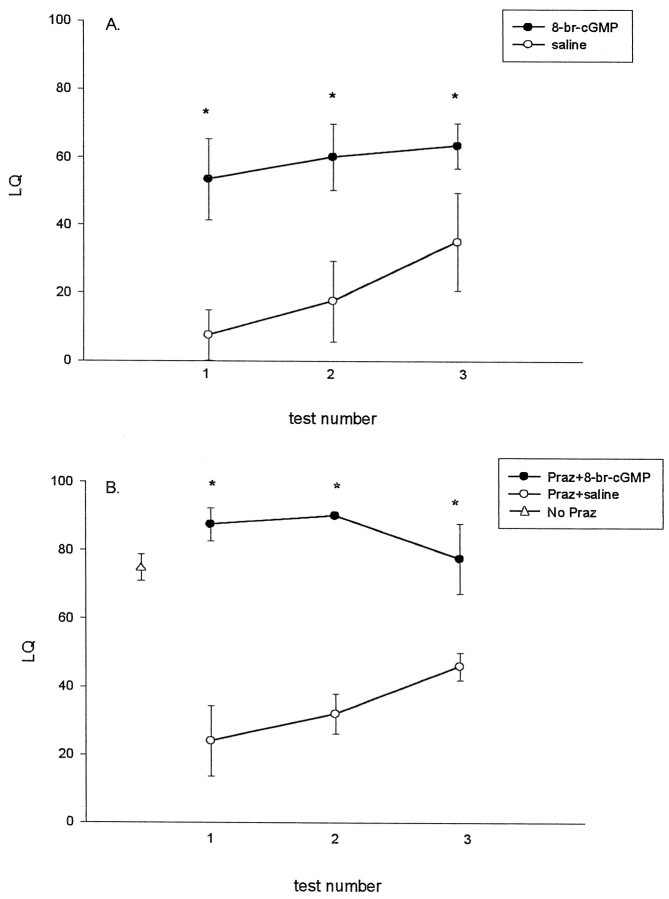
To test the hypothesis that α1-adrenoceptor activation of the NO–cGMP pathway is behaviorally relevant, we used a cell-permeable hydrolysis-resistant cGMP analog, 8-br-cGMP. It is well known that systemic (Vincent and Etgen, 1993) or intrahypothalamic (Etgen, 1990) administration of the α1-adrenergic antagonist prazosin inhibits lordosis behavior in E2 plus P-primed rats. Conversely, 8-br-cGMP and other cGMP derivatives potentiate lordosis behavior (Fernandez-Guasti et al., 1983; Chu et al., 1999). To reconfirm the facilitatory effects of 8-br-cGMP on lordosis in the present experiment, we first infused either 1 μm 8-br-cGMP or saline in a volume of 2 μl into the third ventricle of ovariectomized EB-primed females that did not receive P. It is easier to detect facilitatory effects of drugs such as 8-br-cGMP using low priming doses of EB, because female rats rarely exhibit lordosis behavior under these circumstances without addition of P. Four hours after 8-br-cGMP infusion, animals were tested for lordosis behavior and locomotor activities (rearing and grid crossing). Figure 5A shows that animals treated with 8-br-cGMP have threefold higher LQ scores than control animals (F(1,18) = 5.82;p < 0.05; n = 3–5). Infusion of 8-br-cGMP also significantly increases the quality of lordosis (data not shown).
Fig. 5.
Facilitation of lordosis behavior by 8-br-cGMP and 8-br-cGMP reversal of prazosin inhibition of lordosis behavior. InA, 8-br-cGMP (1 μm) or saline was infused into the third ventricle of rats primed only with EB for 44 hr; hourly behavior testing began 4 hr later. In B, EB-primed rats were injected with 0.5 mg/kg prazosin 1 hr before infusion of 1 μm 8-br-cGMP or saline into the third ventricle. P (200 μg) was given subcutaneously to all the animals at the same time as 8-br-cGMP or saline infusion. (▵ indicates the mean LQ ± SEM from animals receiving the same hormone priming without prazosin or 8-br-cGMP; n = 13) (Chu and Etgen, 1997). The data are shown as mean ± SEM (n = 3–5).p < 0.05 versus saline; Tukey test.
A separate set of female rats implanted with a guide cannula into the third ventricle were primed with both EB and P to produce moderate-to-high levels of lordosis. The α1-adrenoceptor antagonist prazosin, in a dose known to reliably inhibit lordosis behavior (0.5 mg/kg, i.p.) (Vincent and Etgen, 1993), was given to all the animals 44 hr after the first EB injection. One hour later, 8-br-cGMP or saline was infused into the third ventricle concomitantly with subcutaneous injection of 200 μg of P. Female rats usually show LQ values of ∼75 with these doses of EB and P (Fig. 5B) (Chu and Etgen, 1997). Figure5B shows that prazosin reduces LQ scores to between 20 and 40 and that 8-br-cGMP reverses the inhibitory effects of prazosin on lordosis behavior (F(1,7) = 91.5;p < 0.05; n = 3). Similarly, 8-br-cGMP in the prazosin-injected animals also restores the quality of lordosis (data not shown). These data are consistent with the hypothesis that cGMP acts downstream from the α1-adrenoceptor to facilitate lordosis behavior in EB plus P-treated rats. Tests for locomotion for both sets of rats indicated that prazosin and 8-br-cGMP do not change locomotor activities (data not shown).
DISCUSSION
To our knowledge, this is the first evidence that ovarian steroids promote the linkage of a G-protein-coupled receptor, the α1-adrenoceptor, to a previously inactive signal transduction pathway. Moreover, the behavioral data demonstrate that the new signaling pathway (NO–cGMP) may mediate the facilitatory effects of NE on reproductive behavior. Reports from other laboratories suggest further that ovarian hormone-dependent, α1-adrenergic facilitation of GnRH release in female rats may also involve NO (Canteros et al., 1995, 1996; Kamat et al., 1995). Our past studies demonstrated that treatment with behaviorally relevant doses of estrogen alone enhances α1-adrenoceptor augmentation of cAMP formation, apparently by increased expression of α1B-adrenergic receptor binding sites and mRNA in the HYP–POA (Petitti and Etgen, 1990; Petitti et al., 1992;Karkanias et al., 1996). Interestingly, P treatment either in vivo (Petitti and Etgen, 1989, 1990) or in vitro(Petitti and Etgen, 1992) abolishes α1-adrenoceptor augmentation of cAMP accumulation in both HYP and POA slices, but only if animals were first exposed to E2. These changes in α1-adrenoceptor signal transduction are not correlated with changes in the affinity or density of α1-adrenoceptor binding sites (Petitti et al., 1992; Karkanias et al., 1995). P treatment also attenuates NE- and phenylephrine-stimulated IP3 formation in hypothalamic slices from E2-treated females (Karkanias et al., 1995). Thus, in female rats receiving hormone treatments that would result in high levels of lordosis behavior and in robust gonadotropin surges, we find marked changes in intracellular signaling by α1-adrenoceptors, which are known to mediate NE facilitation of both lordosis behavior and GnRH release.
In the present study, we demonstrate that combined treatment with E2 and P promotes α1-adrenoceptor activation of cGMP synthesis in HYP–POA slices. In fact, NE and phenylephrine do not activate cGMP production in these brain regions if the slices are not prepared from animals exposed to both EB and P. The ability of NO synthase inhibitors to block NE stimulation of brain slice cGMP accumulation indicates that NO-activated soluble guanylyl cyclase is the source of NE-induced cGMP synthesis. These data are consistent with the hypothesis that α1-adrenoceptors in the HYP–POA couple to the NO–cGMP second messenger system only after P administration to estrogen-primed animals. Moreover, when combined with our previous observations, the findings suggest that, after both E2 and P treatment, the ratio of cAMP/cGMP produced by activation of α1-adrenoceptors in the HYP–POA shifts in favor of cGMP. Hence, it is likely that activation of α1-adrenoceptors in the HYP–POA of E2 plus P-treated rats would result in greater activation of cGMP-dependent protein kinase and less activation of cAMP-dependent protein kinase. This change could have profound implications for the nature of cellular responses to endogenously released NE. For example, changes in the relative activities of cAMP-dependent protein kinase and cGMP-dependent protein kinase alter cellular responses to phospholipase A2 activation in smooth muscle (Murthy and Makhlouf, 1998), modulate olfactory signal transduction (Zufall and Leider-Zufall, 1998), and influence the expression of long-term depression in hippocampal slices (Santschi et al., 1999).
It is also interesting that combined treatment with EB and P increases basal cGMP levels in HYP–POA slices. This is a physiologically relevant finding because previous reports found that HYP levels of cGMP increase significantly on the evening of proestrus, a time characterized by high levels of E2 and P in gonadally intact rats (Kimura et al., 1980). NO synthase expression increases with estrogen priming in the POA and ventromedial HYP, measured by NADPH-diaphorase staining and by immunocytochemistry and immunoblotting for the brain isoform (Okamura et al., 1994a,b; Rachman et al., 1996; Pu et al., 1998). Thus, E2 may promote activation of NO generation (and therefore cGMP accumulation) in part by elevating levels of the synthetic enzyme NO synthase. Two early reports indicated that P alone increases guanylyl cyclase activity in skeletal, kidney, liver, and uterine tissues from guinea pigs and rats (Vesely, 1979; Vesely and Hill, 1980). Perhaps these two mechanisms together account for the observation that both basal and α1-adrenoceptor-stimulated cGMP levels are significantly elevated in the HYP–POA only when both hormones are administered. Recent studies of muscarinic receptor coupling to the NO–cGMP pathway suggest that prolonged, agonist-induced elevations in NO-stimulated cGMP levels may be attributable to influx of extracellular calcium rather than release of intracellular calcium from storage pools (Wotta et al., 1998). Therefore, it is interesting to speculate that the addition of P to E2-primed HYP–POA switches the preferred mode of α1-adrenoceptor signal transduction from mobilization of intracellular calcium to influx of extracellular calcium.
We showed previously that cGMP, produced by NO activation of soluble guanylyl cyclase, facilitates lordosis behavior in hormone-treated female rats (Chu and Etgen, 1997; Chu et al., 1999). Here, in the behavioral experiment, we report that 8-br-cGMP reverses the inhibitory effects of the α1-adrenergic antagonist prazosin on lordosis of EB plus P-primed female rats. Moreover, the ability of α1-adrenoceptors to activate soluble guanylyl cyclase in the HYP–POA appears to be hormone-dependent. Together, these results suggest that NO-stimulated cGMP production acts downstream of α1-adrenoceptor activation to enhance lordosis responsiveness. Our behavioral data also support the hypothesis that the NO–cGMP pathway mediates NE facilitation of hormone-dependent lordosis behavior. The lordosis-relevant molecular targets of cGMP action in the HYP–POA are not known. However, an inhibitor of cGMP-dependent protein kinase, a major mediator of NO-induced cGMP action (El-Husseini et al., 1998), and the P receptor antagonist RU 38486 both block cGMP facilitation of lordosis (Chu et al., 1999).
NO has also been proposed to mediate the estrogen-dependent effects of α1-adrenoceptors in the POA on the release of GnRH, the major neuropeptide controlling ovulation (Canteros et al., 1995, 1996; Kamat et al., 1995). In addition, NO mediates the stimulatory effects of glutamate (Mahachoklertwattana et al., 1994;Rettori et al., 1994), leptin (Yu et al., 1997), and oxytocin (Rettori et al., 1997) on GnRH secretion. These stimulatory effects of NO in the POA are believed to involve activation of soluble guanylyl cyclase, which leads to enhanced production of cGMP (Brann et al., 1997). We demonstrated in our previous studies (Chu and Etgen, 1997; Chu et al., 1999) that the NO–cGMP pathway, acting through cGMP-dependent protein kinase and the P receptor, is important in the regulation of lordosis behavior. Thus, it is possible that NO and cGMP, produced subsequent to activation of α1-adrenoceptors in the HYP and POA, are key players in the temporal coordination of ovulation with the period of behavioral sexual receptivity.
It is also possible that NO and cGMP facilitate lordosis behavior in part by enhancing GnRH release. GnRH facilitates the display of lordosis behavior in estrogen-primed female rats (for review, see Pfaff et al., 1994). GnRH neurons in the POA are surrounded by NO synthase-positive cells, although GnRH and NO synthase do not colocalize in the same cell (Bhat et al., 1995; Herbison et al., 1996). The P receptor, which mediates cGMP effects on lordosis (Chu et al., 1999), also mediates GnRH potentiation of lordosis (Beyer et al., 1997). Together, one might speculate that the NO–cGMP system is a convergent pathway for regulation of reproductive function by multiple neurotransmitters and hormones. NO, a small diffusible gas molecule and an important intercellular messenger, is especially suited to play such a coordinating role in the neuroendocrine control of reproduction.
The present study also demonstrates that both NE-stimulated cGMP production and the basal cGMP content in HYP–POA are derived predominantly from NO stimulation of soluble guanylyl cyclase. Cellular cGMP can come from two sources: the membrane-bound form of guanylyl cyclase and the soluble form of guanylyl cyclase. Guanylyl cyclase activity is considerably higher in the soluble than in the membrane fraction of most cell lysates (Garbers, 1989). The ability of NO synthase inhibitors to abolish NE activation of cGMP synthesis and to drastically reduce basal cGMP levels indicates that NO-stimulated soluble guanylyl cyclase is the main enzyme responsible for both basal and agonist-induced cGMP synthesis in HYP–POA.
In conclusion, present results indicate that treatment of female rats with behaviorally effective doses of E2 and P switches the preferred signaling pathways of α1-adrenoceptors in the HYP–POA away from IP3 formation and augmentation of adenylyl cyclase activity and to the production of NO–cGMP. Neither E2 nor P alone is able to effect this change in α1-adrenoceptor signal transduction. Moreover, combined administration of E2 and P raises the basal activity of the NO–cGMP pathway even before any receptor stimulation. The lordosis data demonstrate that the link between α1-adrenoceptors and the NO–cGMP pathway in the brain of EB plus P-treated animals is behaviorally relevant. Together, our data support the hypothesis that coupling of α1-adrenoceptors to the NO–cGMP pathway is hormone-dependent and that this pathway mediates NE (i.e., α1-adrenergic) facilitation of lordosis behavior in EB- and P-primed rats.
Footnotes
This research was supported by National Institutes of Health Grants HD29856 and MH41414 and by the Department of Neuroscience, Albert Einstein College of Medicine. We thank Jose Morales for his assistance in conducting parts of the experimental work and in figure preparation. The data in this paper are from a thesis, which was submitted in partial fulfillment of the requirements for the Degree of Doctor of Philosophy in the Sue Golding Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University. Portions of this work were presented in abstract form at the 1998 Annual Meeting of the Society for Neuroscience.
Correspondence should be addressed to Dr. Anne M. Etgen, Department of Neuroscience, F113, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461.
REFERENCES
- 1.Ari IL, Schwarz L, Atlas D. Cholinergic-induced [3H] noradrenaline release in rat brain cortical slices is mediated via a pertussis toxin sensitive GTP binding protein and involves activation of protein kinase C. Cell Signal. 1989;1:461–470. doi: 10.1016/0898-6568(89)90031-4. [DOI] [PubMed] [Google Scholar]
- 2.Beyer C, Gonzalez-Flores O, Gonzalez-Mariscal G. Progesterone receptor participates in the stimulatory effect of LHRH, prostaglandin E2, and cyclic AMP on lordosis and proceptive behaviours in rats. J Neuroendocrinol. 1997;9:609–614. doi: 10.1046/j.1365-2826.1997.00617.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhat GK, Mahesh VB, Lamar CA, Ping L, Aguan K, Brann DW. Histochemical localization of nitric oxide neurons in the hypothalamus: association with gonadotropin-releasing hormone neurons and co-localization with N-methyl-d-aspartate receptors. Neuroendocrinology. 1995;62:187–197. doi: 10.1159/000127004. [DOI] [PubMed] [Google Scholar]
- 4.Bonavera JJ, Sahu A, Kalra PS, Kalra SP. Evidence that nitric oxide may mediate the ovarian steroid-induced luteinizing hormone surge: involvement of excitatory amino acids. Endocrinology. 1993;133:2481–2487. doi: 10.1210/endo.133.6.8243268. [DOI] [PubMed] [Google Scholar]
- 5.Brann DW, Bhat GK, Lamar CA, Mahesh VB. Gaseous transmitters and neuroendocrine regulation. Neuroendocrinology. 1997;65:385–395. doi: 10.1159/000127201. [DOI] [PubMed] [Google Scholar]
- 6.Canteros G, Rettori V, Franchi A, Genaro A, Cebral E, Faletti A, Gimeno M, McCann SM. Ethanol inhibits luteinizing hormone-releasing hormone (LHRH) secretion by blocking the response of LHRH neuronal terminals to nitric oxide. Proc Natl Acad Sci USA. 1995;92:3416–3420. doi: 10.1073/pnas.92.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canteros G, Rettori V, Genaro A, Suburo A, Gimeno M, McCann SM. Nitric oxide synthase content of hypothalamic explants: increase by norepinephrine and inactivated by NO and cGMP. Proc Natl Acad Sci USA. 1996;93:4246–4250. doi: 10.1073/pnas.93.9.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceccatelli S, Grandison L, Scott RE, Pfaff DW, Kow LM. Estradiol regulation of nitric oxide synthase mRNAs in rat hypothalamus. Neuroendocrinology. 1996;64:357–363. doi: 10.1159/000127139. [DOI] [PubMed] [Google Scholar]
- 9.Chu HP, Etgen AM. A potential role of cyclic GMP in the regulation of lordosis behavior of female rats. Horm Behav. 1997;32:125–132. doi: 10.1006/hbeh.1997.1413. [DOI] [PubMed] [Google Scholar]
- 10.Chu HP, Morales JC, Etgen AM. Cyclic GMP may potentiate lordosis behaviour by progesterone receptor activation. J Neuroendocrinol. 1999;11:107–113. doi: 10.1046/j.1365-2826.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 11.El-Husseini AED, Bladen C, Williams JA, Reiner PB, Vincent SR. Nitric oxide regulates cyclic GMP-dependent protein kinase phosphorylation in rat brain. J Neurochem. 1998;71:676–683. doi: 10.1046/j.1471-4159.1998.71020676.x. [DOI] [PubMed] [Google Scholar]
- 12.Etgen AM. Intrahypothalamic implants of noradrenergic antagonists disrupt lordosis behavior in female rats. Physiol Behav. 1990;48:31–36. doi: 10.1016/0031-9384(90)90256-4. [DOI] [PubMed] [Google Scholar]
- 13.Etgen AM, Ungar S, Petitti N. Estradiol and progesterone modulation of norepinephrine neurotransmission: implications for the regulation of female reproductive behaviour. J Neuroendocrinol. 1992;4:255–271. doi: 10.1111/j.1365-2826.1992.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Guasti A, Rodriguez-Manzo G, Beyer C. Effect of guanine derivatives on lordosis behavior in estrogen-primed rats. Physiol Behav. 1983;31:589–592. [PubMed] [Google Scholar]
- 15.Garbers DL. Cyclic GMP and the second messenger hypothesis. Trends Endocrinol Metab. 1989;1:64–67. doi: 10.1016/1043-2760(89)90004-0. [DOI] [PubMed] [Google Scholar]
- 16.Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendocrinol. 1996;8:73–82. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RD, Minneman KP. Characterization of α1-adrenoceptors which increase cyclic AMP accumulation in rat cerebral cortex. Eur J Pharmacol. 1986;129:293–305. doi: 10.1016/0014-2999(86)90439-5. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RD, Minneman KP. Differentiation of α1-adrenergic receptors linked to phosphatidylinositol turnover and cyclic AMP accumulation in rat brain. Mol Pharmacol. 1987;31:239–246. [PubMed] [Google Scholar]
- 19.Kalra SP, Kalra PS. Neural regulation of luteinizing hormone secretion in the rat. Endocr Rev. 1983;4:311–351. doi: 10.1210/edrv-4-4-311. [DOI] [PubMed] [Google Scholar]
- 20.Kamat A, Yu WH, Rettori V, McCann SM. Glutamic acid induces luteinizing hormone releasing hormone release via α receptors. Brain Res Bull. 1995;37:233–255. doi: 10.1016/0361-9230(94)00280-e. [DOI] [PubMed] [Google Scholar]
- 21.Karkanias GB, Petitti N, Etgen AM. Progesterone attenuation of α1-adrenergic receptor stimulation of phosphoinositol hydrolysis in hypothalamus of estrogen-primed female rats. Endocrinology. 1995;136:1993–1999. doi: 10.1210/endo.136.5.7720647. [DOI] [PubMed] [Google Scholar]
- 22.Karkanias GB, Ansonoff MA, Etgen AM. Estradiol regulation of α1b-adrenoceptor mRNA in female rat hypothalamus-preoptic area. J Neuroendocrinol. 1996;8:449–455. doi: 10.1046/j.1365-2826.1996.04716.x. [DOI] [PubMed] [Google Scholar]
- 23.Kimura F, Kawakami M, Nakano H, McCann SM. Changes in adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate concentrations in the anterior pituitary and hypothalamus during the rat estrous cycle and effects of administration of sodium pentobarbital in proestrus. Endocrinology. 1980;106:631–635. doi: 10.1210/endo-106-2-631. [DOI] [PubMed] [Google Scholar]
- 24.Lopez FJ, Moretto M, Merchenthaler I, Negro-Vilar A. Nitric oxide is involved in the genesis of pulsatile LHRH secretion from immortalized LHRH neurons. J Neuroendocrinol. 1997;9:647–654. doi: 10.1046/j.1365-2826.1997.t01-1-00618.x. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Mahachoklertwattana P, Black SM, Kaplan SL, Bristow JD, Grumbach MM. Nitric oxide synthesized by gonadotropin-releasing hormone neurons is a mediator of N-methyl-d-aspartate (NMDA)-induced GnRH secretion. Endocrinology. 1994;135:1709–1712. doi: 10.1210/endo.135.4.7523101. [DOI] [PubMed] [Google Scholar]
- 27.Mani SK, Allen JM, Rettori V, McCann SM, O’Malley BW, Clark JH. Nitric oxide mediates sexual behavior in female rats. Proc Natl Acad Sci USA. 1994;91:6468–6472. doi: 10.1073/pnas.91.14.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minneman KP. α1-adrenergic receptor subtypes, inositol phosphates, and sources of cell Ca2+. Pharmacol Rev. 1988;40:87–119. [PubMed] [Google Scholar]
- 29.Moretto M, Lopez FJ, Negro-Vilar A. Nitric oxide regulates luteinizing hormone-releasing hormone secretion. Endocrinology. 1993;133:2399–2402. doi: 10.1210/endo.133.5.8104781. [DOI] [PubMed] [Google Scholar]
- 30.Murthy KS, Makhlouf GM. Differential regulation of phospholipase A2 (PLA2)-dependent Ca2+ signaling in smooth muscle by cAMP and cGMP-dependent kinases. Inhibitory phosphorylation of PLA2 by cyclic nucleotide-dependent kinases. J Biol Chem. 1998;273:34519–34526. doi: 10.1074/jbc.273.51.34519. [DOI] [PubMed] [Google Scholar]
- 31.Okamura H, Yokosuka M, Hayashi S. Estrogenic induction of NADPH-diaphorase activity in the preoptic neurons containing estrogen receptor immunoreactivity in the female rat. J Neuroendocrinol. 1994a;6:597–601. doi: 10.1111/j.1365-2826.1994.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 32.Okamura H, Yokosuka M, McEwen BS, Hayashi S. Colocalization of NADPH-diaphorase and estrogen receptor immunoreactivity in the rat ventromedial hypothalamic nucleus: stimulatory effect of estrogen on NADPH-diaphorase activity. Endocrinology. 1994b;135:1705–1708. doi: 10.1210/endo.135.4.7925135. [DOI] [PubMed] [Google Scholar]
- 33.Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. Plenum; New York: 1979. [Google Scholar]
- 34.Petitti N, Etgen AM. Progesterone depression of norepinephrine-stimulated cAMP accumulation in hypothalamic slices. Mol Brain Res. 1989;5:109–119. doi: 10.1016/0169-328x(89)90002-8. [DOI] [PubMed] [Google Scholar]
- 35.Petitti N, Etgen AM. α1-Adrenoceptor augmentation of β-stimulated cAMP formation is enhanced by estrogen and reduced by progesterone in rat hypothalamic slices. J Neurosci. 1990;10:2842–2849. doi: 10.1523/JNEUROSCI.10-08-02842.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petitti N, Etgen AM. Progesterone promotes rapid desensitization of α1-adrenergic receptor augmentation of cAMP formation in rat hypothalamic slices. Neuroendocrinology. 1992;55:1–8. doi: 10.1159/000126089. [DOI] [PubMed] [Google Scholar]
- 37.Petitti N, Karkanias GB, Etgen AM. Estradiol selectively regulates α1b-noradrenergic receptors in the hypothalamus and preoptic area. J Neurosci. 1992;12:3869–3876. doi: 10.1523/JNEUROSCI.12-10-03869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neil JD, editors. The physiology of reproduction, Vol 2. Raven; New York: 1994. pp. 107–197. [Google Scholar]
- 39.Pu S, Horvath TL, Diano S, Naftolin F, Kalra PS, Kalra SP. Evidence showing that β-endorphin regulates cyclic guanosine 3′,5′-monophosphate (cGMP) efflux: anatomical and functional support for an interaction between opiates and nitric oxide. Endocrinology. 1997;138:1537–1543. doi: 10.1210/endo.138.4.5086. [DOI] [PubMed] [Google Scholar]
- 40.Pu S, Kalra PS, Kalra SP. Ovarian steroid-independent diurnal rhythm in cyclic GMP/nitric oxide efflux in the medial preoptic area: possible role in preovulatory and ovarian steroid-induced LH surge. J Neuroendocrinol. 1998;10:617–625. doi: 10.1046/j.1365-2826.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- 41.Rachman IM, Pfaff DW, Cohen RS. NADPH diaphorase activity and nitric oxide synthase immunoreactivity in lordosis-relevant neurons of the ventromedial hypothalamus. Brain Res. 1996;740:291–306. doi: 10.1016/s0006-8993(96)00901-8. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez VD, Feder HH, Sawyer CH. The role of brain catecholamines in the regulation of LH secretion: a critical inquiry. In: Martini L, Ganong WF, editors. Frontiers in neuroendocrinology, Vol 8. Raven; New York: 1984. pp. 27–84. [Google Scholar]
- 43.Rettori V, Belova N, Dees WL, Nyberg CL, Gimeno M, McCann SM. Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc Natl Acad Sci USA. 1993;90:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rettori V, Kamat A, McCann SM. Nitric oxide mediates the stimulation of luteinizing-hormone releasing hormone release induced by glutamic acid in vitro. Brain Res Bull. 1994;33:501–503. doi: 10.1016/0361-9230(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 45.Rettori V, Canteros G, Renoso R, Gimeno M, McCann SM. Oxytocin stimulates the release of luteinizing hormone-releasing hormone from medial basal hypothalamic explants by releasing nitric oxide. Proc Natl Acad Sci USA. 1997;94:2741–2744. doi: 10.1073/pnas.94.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santschi L, Reyes-Harde M, Stanton PK (1999) Chemically-induced, activity-dependent LTD elicited by simultaneous activation of PKG and inhibition of PKA. J Neurophysiol, in press. [DOI] [PubMed]
- 47.Snyder SH, Bredt DS. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991;12:125–128. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]
- 48.Vesely DL. Testosterone and its precursors and metabolites enhance guanylate cyclase activity. Proc Natl Acad Sci USA. 1979;76:3491–3494. doi: 10.1073/pnas.76.7.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vesely DL, Hill DE. Estrogens and progesterone increase fetal and maternal guanylate cyclase activity. Endocrinology. 1980;107:2104–2109. doi: 10.1210/endo-107-6-2104. [DOI] [PubMed] [Google Scholar]
- 50.Vincent PA, Etgen AM. Steroid priming promotes oxytocin-induced norepinephrine release in the ventromedial hypothalamus of female rats. Brain Res. 1993;620:189–194. doi: 10.1016/0006-8993(93)90155-g. [DOI] [PubMed] [Google Scholar]
- 51.Wotta DR, Parsons AM, Hu J, Grande AW, El-Fakahany EE. M1 muscarinic receptors stimulate rapid and prolonged phases of neuronal nitric oxide synthase activity: involvement of different calcium pools. J Neurochem. 1998;71:487–497. doi: 10.1046/j.1471-4159.1998.71020487.x. [DOI] [PubMed] [Google Scholar]
- 52.Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology. 1997;138:5055–5058. doi: 10.1210/endo.138.11.5649. [DOI] [PubMed] [Google Scholar]
- 53.Zufall F, Leider-Zufall T. Role of cGMP in olfactory transduction and adaptation. Ann NY Acad Sci. 1998;855:199–204. doi: 10.1111/j.1749-6632.1998.tb10566.x. [DOI] [PubMed] [Google Scholar]