Abstract
Rodents use two distinct navigation strategies that are based on environmental cues (landmark navigation) or internal cues (path integration). Head direction (HD) cells are neurons that discharge when the animal points its head in a particular direction and are responsive to the same cues that support path integration and landmark navigation. Experiment 1 examined whether HD cells in rats with lesions to the hippocampus plus the overlying neocortex or to just the overlying neocortex could maintain a stable preferred firing direction when the rats locomoted from a familiar to a novel environment, a process thought to require path integration. HD cells from both lesion groups were unable to maintain a similar preferred direction between environments, with cells from hippocampal rats showing larger shifts than cells from rats sustaining only cortical damage. When the rats first explored the novel environment, the preferred directions of the cells drifted for up to 4 min before establishing a consistent firing orientation. The preferred direction was usually maintained during subsequent visits to the novel environment but not across longer time periods (days to weeks). Experiment 2 demonstrated that a novel landmark cue was able to establish control over HD cell preferred directions in rats from both lesion groups, showing that the impairment observed in experiment 1 cannot be attributed to an impairment in establishing cue control. Experiment 3 showed that the preferred direction drifted when HD cells in lesioned animals were recorded in the dark. It was also shown that the anticipatory property of anterodorsal thalamic nucleus HD cells was still present in lesioned animals; thus, this property cannot be attributed to an intact hippocampus. These findings suggest that the hippocampus and the overlying neocortex are involved in path integration mechanisms, which enable an animal to maintain an accurate representation of its directional heading when exploring a novel environment.
Keywords: head direction, hippocampus, path integration, navigation, neocortex, spatial cognition
Rodents are believed to use two primary strategies in tandem when navigating: path integration and landmark navigation (Gallistel, 1989; Etienne et al., 1996). Path integration relies on the continuous monitoring of internally generated idiothetic cues, such as vestibular, proprioceptive, and motor efferent copy. The information provided by idiothetic cues is used to determine the animal’s current location relative to a fixed departure point as the animal moves through the environment (Mittelstaedt and Mittelstaedt, 1980; Wehner and Srinivasan, 1981). Under normal conditions, errors accumulate over time during the path integration process and are corrected by the second navigational strategy of referring to landmarks (Gallistel, 1990).
Two categories of neurons that are likely involved in navigation have been identified: place cells and head direction (HD) cells (O’Keefe and Dostrovsky, 1971; Taube et al., 1990a). HD cells preferentially discharge when the animal points its head in a particular direction in the horizontal plane, independent of location. The direction at which an HD cell fires maximally is known as the preferred direction of a cell. In contrast, place cells fire when the animal is at a particular location in an open arena, independent of its directional heading. HD and place cells are found throughout the limbic system and are responsive to sensory cues originating from external, as well as idiothetic, cue sources (O’Mara, 1995; Taube et al., 1996a). Moreover, the neural firing patterns of place and HD cells are believed to reflect the interplay between extrinsic and idiothetic cue sources in much the same way that path integration and landmark navigation strategies are thought to operate (Knierim et al., 1996; Taube et al., 1996a).
The hippocampus has been strongly implicated in the ability of rodents to navigate effectively. Lesions of the hippocampus impair performance on a variety of spatial learning tasks (O’Keefe and Nadel, 1978;Morris et al., 1982; Jarrard, 1993). These results have often been interpreted to indicate a deficit in cognitive mapping (O’Keefe and Nadel, 1978). Recent findings, however, offer an alternative interpretation. These studies suggest that hippocampal-lesioned animals are able to demonstrate place learning but instead may be impaired at path integration (Eichenbaum et al., 1990; Whishaw et al., 1995). Consistent with this notion are physiological data showing that hippocampal activity is influenced by a variety of factors associated with movement (Green and Arduini, 1954; Vanderwolf, 1969; Gavrilov et al., 1995; Sharp et al., 1995). Together, these results have led researchers to postulate that the rodent hippocampus is critically involved in path integration (McNaughton et al., 1996; Whishaw et al., 1997).
Previous experiments in control animals have shown that, when a rat moves into a novel environment, HD cells usually maintain their preferred direction within ±30° of the orientation of the cell in the familiar environment (Taube and Burton, 1995). Accurate maintenance of the preferred direction is thought to be accomplished via path integration mechanisms because initially there are no familiar landmark cues for orientation in a novel environment. Thus, if the preferred direction of a cell in an animal with a hippocampal lesion was to change substantially when it enters a novel environment, this result would suggest that the hippocampus is involved in path integration. Experiment 1 tested this hypothesis by measuring the response of HD cells in animals with hippocampal or overlying neocortical lesions as they entered a novel environment. We report that HD cells from both groups of animals are unable to accurately maintain their orientation in the novel environment compared with nonlesioned control animals, with the hippocampal group experiencing the greatest deficit. Experiment 2 demonstrated that the response of HD cells in Experiment 1 is not attributable to a general inability to incorporate novel landmark cues into the firing pattern of HD cells. Finally, in Experiment 3, we recorded HD cells in lesioned animals in the dark. The results provide further support for an impairment in path integration.
MATERIALS AND METHODS
Animals and training procedures
Female Long–Evans rats weighing ∼250 gm and between 90 and 180 d old at the start of training were used in the experiments. Animals were maintained on a 12 hr light/dark cycle. Each rat received one of two possible training schedules before surgery, depending on whether it was to be included in experiment 2. One group of animals that were later given either hippocampal lesions (n = 7) or neocortical lesions (n = 3) were trained over several weeks to forage for food pellets thrown randomly inside a gray cylindrical apparatus (76 cm in diameter, 51 cm in height). This training procedure resulted in a near uniform sampling of locations and head directions within the cylinder by the rat. The cylinder contained a large white cue card (73 × 51 cm) occupying ∼100° of arc along the cylinder wall. This card served as the most prominent visual cue within the cylinder and remained in the same location throughout training. The apparatus was surrounded by floor-to-ceiling black curtains arranged in a 2 m diameter circle. Gray photographic backdrop paper served as the floor of the cylinder. This floor paper was changed before all recording sessions to prevent the use of olfactory cues across sessions. Four DC-powered lights were symmetrically arranged 3 m above the cylinder, and a Sony (Tokyo, Japan) XC-711 color video camera was vertically aligned above the center of the cylinder. A white noise generator, connected to a black speaker placed in the rafters of the ceiling above the center of the apparatus, was used to mask extraneous sounds. The animals were carried to the room inside their home cage and introduced to the arena from a random location outside the cylinder each day. No attempt was made to disorient the animals during the training sessions.
A second group of animals that were later given lesions to either the hippocampus (n = 4) or neocortex (n = 5) were naive to the pellet-chasing task and the experimental context before being lesioned. Thus, for these animals, all of their experience within the room where the training, cell screening, and experiments took place occurred after sustaining their lesions. Animals in this group were handled for several weeks by the experimenter and given food pellets in their home cage. After surgery, the rats were trained on the pellet-retrieval task inside the cylindrical arena, as described above, except that the cue card was not present inside the cylinder.
A group of nonlesioned control animals (n = 4) were trained to forage for food pellets on either the standard (n = 2) or no-cue (n = 2) procedures described above. The purpose of this group was to replicate previous findings using the same experimental procedure (Taube and Burton, 1995), thus serving as a control for possible inter-experimenter differences. For statistical comparisons, a larger data set of control animals (n = 20) trained using the standard procedure was also included (Taube and Burton, 1995).
Surgical procedures
The surgical procedure for these experiments have been described previously in greater detail (Golob and Taube, 1997). Briefly, the animals were anesthetized using pentobarbital (40–45 mg/kg) and given atropine sulfate (25 mg/ml; 0.10 ml). A 10 wire microelectrode recording array was implanted in either the postsubiculum (PoS) or anterodorsal thalamic nucleus (ADN) (Kubie, 1984). The electrodes were placed just dorsal to either the PoS (anteroposterior, −6.6 mm; mediolateral, +2.2; dorsoventral, −1.6 relative to bregma) or the ADN (anteroposterior, −1.4; mediolateral, +1.3; dorsoventral, −4.0) based on a stereotaxic atlas (Paxinos and Watson, 1986). The electrode array was fixed to the skull using grip cement (Densply International) and was adjustable in the dorsoventral plane. Neurotoxic lesions of the entire hippocampus were made with ibotenic acid (Biosearch Technologies, Novato, CA) dissolved in sodium PBS (10 mg/ml). The ibotenic acid was injected via a glass micropipette at a total of 18 or 22 injection sites across both hemispheres with a volume of either 0.05 or 0.10 μl. Stereotaxic coordinates for the injection sites were identical to those described by Golob and Taube (1997). The neurotoxin was injected over ∼20–30 sec, and the pipette was left in place for at least 1 min after the injection. Bone wax was placed over the bore holes in an attempt to prevent the grip cement (which was used to attach the electrode array to the skull) from damaging the cortex. The lesioning procedure was a modification of the protocol originally described by Jarrard (1989).
Although we attempted to limit ancillary damage to structures adjacent to the hippocampus, all of the hippocampal animals also had some damage to the neocortex that overlies the hippocampus. The resulting cortical damage was likely caused by the use of grip cement to connect the recording electrode to the skull of the animal, because rats that were not implanted with electrodes exhibited much less cortical damage (our unpublished observations). To control for this cortical damage, another group of animals (n = 8) was given neocortical lesions using the same methods described above for the hippocampal animals, except the glass pipette was inserted only through the neocortex and ibotenic acid was not loaded into the pipette. Because these animals were also implanted with recording electrodes using grip cement, the overlying neocortex also sustained damage in these animals.
Cell screening and data acquisition
After surgery, the animals were allowed to recover for at least 7 d. After recovery, the activity on each of the 10 electrode wires was monitored daily as the animal foraged for food pellets inside the circular arena. If an HD cell was not identified on any of the electrode wires, the electrode array was advanced between 30 and 120 μm. Each screening session was separated by a minimum of 4 hr.
When a waveform from an HD cell was identified that was sufficiently isolated above background noise, its activity was recorded while the animal’s directional heading was simultaneously monitored using a two-spot video tracking system. The electrode signal was preamplified through a field–effect transistor in a source–follower configuration and then amplified and bandpass filtered (300–10,000 Hz). Cell spikes were isolated using a series of time–amplitude window discriminators (Bak Electronics), displayed on an oscilloscope, and saved onto a computer using a National Instruments (Austin, TX) data acquisition board with LabView software. Two light-emitting diodes (LEDs), separated by ∼10 cm, were used to indicate the animal’s directional heading. A red LED was positioned above the animal’s snout, and a green LED was located over the midline of the animal’s back when its head was facing forward. Neuronal spike activity and the location of the two LEDs were sampled at 60 Hz.
Experimental design
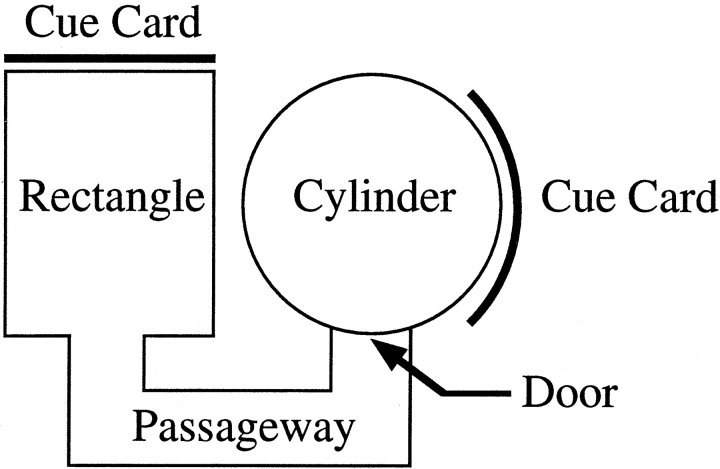
Experiment 1: behavioral testing in the dual-chamber apparatus. Experiment 1 was intended to provide the animal with a set of conditions in which it must path integrate to maintain its orientation while exploring a novel environment. The procedures closely followed those adopted by Taube and Burton (1995). Figure1 presents an overhead view of the dual-chamber apparatus. The dual-chamber apparatus is composed of two flat gray-colored open arenas, which are interconnected by a narrow passageway. The height of each section of the dual-chamber apparatus was 50 cm. The cylindrical arena was 76 cm in diameter, and the rectangle was 51 × 68.5 cm. The adjoining passageway was 15 cm wide and 40.5 cm long (as measured from the center). To prevent the walls from obscuring the LEDs from the view of the camera, the inner walls of the passageway and rectangle were slanted by 10–30°. A door was situated between the cylinder and the entrance to the alleyway to control access between the two sections of the apparatus. Both the cylinder and rectangle contained prominent white cue cards affixed to the wall. The card in the cylinder was centered at the 3:00 position and occupied ∼100° of arc, and the card in the rectangle was at 12:00 (see below). The cue card in the rectangle was not visible from the animal’s vantage point when initially entering the passageway. As with the cylinder used for cell screening, the floors of the cylinder and rectangle were covered by gray photographic backdrop paper. The passageway contained a gray painted wooden floor.
Fig. 1.
Overhead view of the dual-chamber apparatus. The novel portion of the apparatus includes the adjoining passageway and rectangular arena. Note that the cue card in the novel section of the apparatus is displaced 90° from the position of the card inside the cylinder.
The circular-shaped arena in the dual-chamber apparatus was similar to the cylinder the rat had been trained and screened in for several weeks. For this reason, the cylindrical section of the dual-chamber apparatus was considered to be a familiar environment to the animal. The passageway and rectangle together represented a novel environment to the animal because they had no previous exposure to them. HD cells in control animals maintain a similar preferred direction between both sections of the dual-chamber apparatus, and it is thought that idiothetic cues that are available during the animal’s journey through the passageway between the cylinder and rectangle are responsible for stabilizing the preferred direction (Taube and Burton, 1995).
The procedure for the dual-chamber apparatus experiment consisted of three phases. In the first recording session (cylinder session), the animal was placed in an opaque box and gently spun and translated for at least 1 min. The animal was then attached to the recording cable and placed in the cylindrical section of the dual-chamber apparatus. For the cylinder session, the rat was restricted to the cylindrical side of the apparatus for 8 min. After the cylinder session, the door between the cylinder and passageway was removed, permitting the animal to enter the novel section of the dual-chamber apparatus (novel session). Food pellets were scattered along the floor of the passageway and the rectangle but were no longer dropped into the cylinder. HD cell activity was recorded for 16 min in the novel session. If the animal failed to exit the cylinder after ∼4 min, it was gently guided into the passageway. After the rat entered the passageway, the door was replaced to obtain at least 6 min of uninterrupted sampling from within the novel environment. After 6 min, the door was again removed, allowing the rat to freely shuttle back and forth between the rectangle and cylinder for the remainder of the session.
To determine the stability of the preferred direction within the novel arena across sessions, HD cells were recorded ∼24 hr after the novel session. The animal was placed into an opaque box and gently spun (∼15 rpm) for ∼1 min. The rectangle and passageway were set up at the same locations they occupied within the circular curtain the previous day. Because the purpose of this phase of the experiment was to ascertain the consistency of the newly established preferred direction in the novel environment across days, the animals were not permitted to enter the cylinder; thus, their access was limited to the rectangle and passageway.
Experiment 2: novel-cue experiment. Experiment 2 was intended to assess the ability of a novel landmark to establish control over the preferred directions of HD cells in rats with hippocampus lesions. Cue control is demonstrated when angular rotation of the position of the cue leads to a similar angular shift in the preferred direction of HD cells. An earlier study showed that HD cells from hippocampal animals were able to establish a unique preferred direction in a novel environment and accurately maintain the preferred direction for at least several days to weeks (Golob and Taube, 1997). However, the animals were disoriented before entering the enclosures, and the presence of experience-dependent plasticity in the HD cell system in response to the novel arenas was inferred by the response of the cell to the shape of the environment. The novel-cue experiment was therefore conducted to facilitate a more valid comparison with the dual-chamber apparatus experiment described above and to determine whether a novel landmark could develop control over the preferred direction of the cell in hippocampal-lesioned animals. In the novel-cue experiment, the animals were not disoriented before being exposed to the novel cue, and evidence for experience-dependent change can be shown directly.
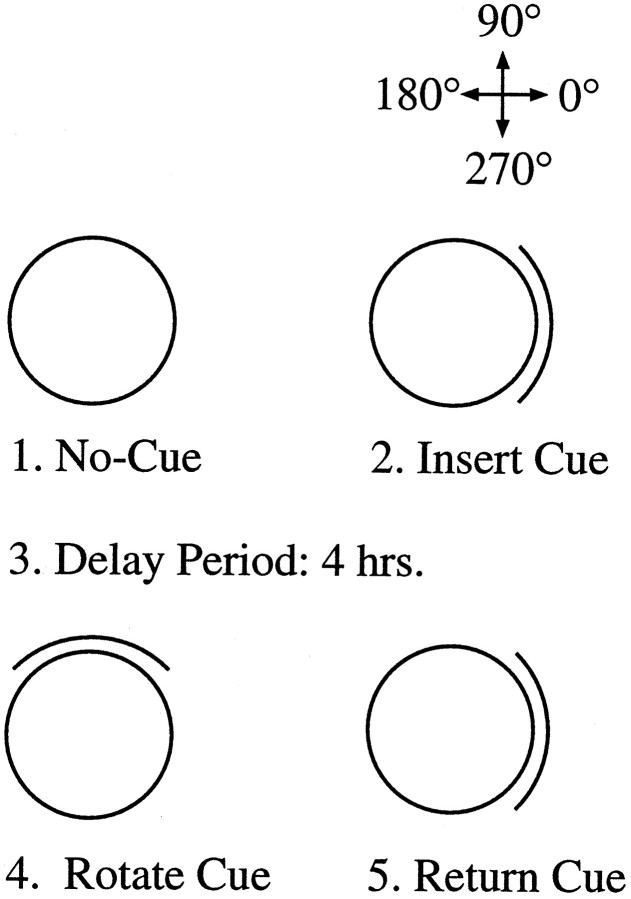
Figure 2 illustrates the procedure for the novel-cue experiment. For these animals, the novel-cue experiment preceded the dual-chamber apparatus experiment described above. All of the recording sessions in the novel-cue experiment lasted 8 min. The animal was initially recorded inside a cylinder that did not contain a cue card (no-cue session). Without removing the animal from the arena, a large, white cue card was placed against the wall of the cylinder, and another recording session was conducted (insert-cue session). After the insert-cue session, the animal was removed from the arena and returned to its cage for 4 hr. The 4 hr delay period was intended to prevent the use of a short-term strategy to maintain the preferred direction, which may be independent of the hippocampus. During the delay period, the floor paper was changed, and the cue card was rotated ± 90° (counterbalanced across animals) from its former position in the insert-cue session. The animal was returned to the room, and another 8 min session was recorded with the cue card in the rotated position (rotate-cue session). The rotate-cue session therefore probes the extent to which the cue card will influence the preferred direction of HD cells. If the cue card was able to establish control over the preferred direction of the cell during the insert-cue session, we would expect the preferred direction to also shift 90° in the correct direction during the rotate-cue session. After the rotate-cue session, a second session with the cue card returned to its initial position was then performed (return-cue session). For most animals, a second series of standard (cue at 0°)—rotate-cue—return-cue sessions were conducted the next day, without the delay period between the insert-cue and cue-rotation sessions.
Fig. 2.
Diagrammatic sequence for the procedure used in experiment 2. The overhead views of the cylindrical arena are shown during the four phases of the experiment. The surrounding environment for the no-cue session is identical to what the animal experienced during the daily screening sessions. After introduction of the white card during the insert-cue session, the animal is removed from the cylinder and returned to its home cage for at least 4 hr (Delay Period). The card is then rotated ±90°, and the animal is returned to the cylinder (Rotate Cue). Shortly afterward, a return-cue session is conducted in which the cue card is returned to the position it occupied in the insert-cue session. The floor paper was changed between all sessions after the insert-cue session to remove lingering olfactory cues. The designation of angular headings for experiments 1 and 2 are shown in the top right corner.
Experiment 3: HD cell recordings in the dark. Experiment 3 further assessed the ability of HD cells in lesioned animals to path integrate accurately. HD cells were recorded in a cylindrical chamber without a cue card while the rat foraged for food pellets in the dark. Illumination of the head stage-mounted LEDs was reduced to the minimum level needed for accurate tracking of the animal’s head direction. The amount the preferred direction of the cell shifted each minute was monitored over 20 min. Results were compared with sessions recorded in the light with and without the cue card present, and with sessions recorded from HD cells in intact blindfolded animals in the dark.
Data analysis
Graphs of firing rate versus HD were created by dividing the animal’s head direction into 60 6° bins and plotting them against the mean firing rate of each bin. The firing properties of a cell (such as peak firing rate, preferred direction, and firing range) were determined by fitting a triangular model to the firing rate versus HD graphs (Taube et al., 1990a). By mathematical convention, we assigned positive and negative signs for counterclockwise (CCW) and clockwise (CW) shifts, respectively. To quantify the difference in the preferred direction of an HD cell between two sessions or between two discrete portions within a single recording session, we used an algorithm that maximized the cross-correlation between the firing rate versus HD functions for the two sessions (Taube et al., 1990a). The function from one session was shifted in 6° increments relative to the function of the second session. The angular shift that yielded the maximum cross-correlation (Pearson’s r) was defined as the difference in preferred direction between sessions. If more than one cell was recorded in a session, the difference in preferred directions between sessions was calculated for each cell separately and then averaged to give a composite value. As in previous studies (Taube et al., 1990b; Taube, 1995; Golob and Taube, 1997), the HD cells shifted in register with one another, resulting in nearly equal shift values when cells were recorded simultaneously.
For the dual-chamber apparatus experiment, the activity of the cell was sorted into individual data files, depending on whether the rat was located in the familiar or novel sections of the apparatus. An overall comparison using the cross-correlation program described above was then conducted to determine the differences in the preferred direction of the cell between each portion of the arena. For some analyses, the data were divided further into individual visits to either the familiar cylinder or the novel rectangle, as the animal repeatedly walked back and forth between the two sections of the dual-chamber apparatus. The first exposure to the novel environment, which lasted at least 6 min, was also divided into 1 min segments to examine the dynamics of cell firing with greater temporal resolution. The data were statistically analyzed using t tests and ANOVAs. The Rayleigh test was used to assess the angular distribution of preferred direction shifts (Batschelet, 1981). Comparisons between groups that contained a small n value used the Mann–WhitneyU test. For all statistical procedures, the significance level was p < 0.05.
Time shift analyses
Previous studies have shown that ADN HD cell activity anticipates the rat’s future directional heading by ∼25 msec (Blair and Sharp, 1995; Blair et al., 1997; Taube and Muller, 1998). The present experiments provided an opportunity to determine whether hippocampal lesions effected this temporal property. Two different types of analyses were performed. The first method examined the difference between the preferred firing directions of the cell when the animal was turning its head in the CW or CCW directions (Blair and Sharp, 1995). If the activity of the cell leads or lags the animal’s head direction, then the cell will reach its maximum firing rate at a different head direction for CW and CCW head turns. The difference in the preferred firing direction of the cell for CW and CCW turns is referred to as δ. The greater the degree to which the cell anticipates the animal’s current head direction, the larger the δ. Thus, if HD cells in lesioned animals lead or lag the animal’s head direction by different amounts than HD cells in control animals, then one would expect the two populations of cells to differ in the size of δ. We calculated δ by constructing two separate tuning curves, one for CW head turns >90°/sec and one for CCW head turns >90°/sec. Samples in which the animal’s angular velocity was <90°/sec were not included in the analyses.
The second method for determining the extent to which HD cell firing leads or lags the rat’s head direction involved shifting the spike series in relation to the head direction series and then comparing the tuning curves at various levels of alignment. The amount and direction the spike series has to be shifted to produce the strongest association between head direction and firing rate indicates whether the discharge of the cell most accurately predicts the animal’s past, current, or future head direction. The strength of the association between firing rate and head direction at different points in time was measured with three parameters: peak firing rate, range width, and information content. Range width is similar to directional firing range but measures the width of the tuning curve of the cell at 30% of the peak firing rate of the cell, rather than at its background levels. Information content represents how much information each spike conveys about the rat’s directional heading and was adapted to HD cells according to the model used by Skaggs et al. (1993) for place cells. The amount that the discharge of the cell leads or lags head direction was defined as the number of samples that the spike series needed to be shifted to attain the maximum peak firing rate, minimum range width, or maximum information content.
Once the optimal time shift values were calculated for each cell, we determined whether there was an overall difference in these values between lesioned and intact animals. We computed the mean of the optimal time shift values for cells in lesioned and intact animals and determined whether these means were statistically different.
Histology and lesion assessment
The electrodes were advanced between 2.0 and 2.5 mm before terminating the cell-screening procedures. Once the experiments were completed, the animals were deeply anesthetized with pentobarbital. An anodal current (15 μA) was passed through one of the electrode wires that recorded an HD cell to later determine the recording site using a Prussian blue reaction. The rats were perfused with saline, followed by a 10% formalin solution in saline. The brains were then removed and soaked in 10% formalin for at least 48 hr. Potassium ferrocyanide (∼2%) was added to the formalin solution for 24 hr, followed by a reimmersion of the brain in formalin for 24 hr. Finally, the brain was placed in a sucrose solution (20%) for 48 hr. The brains were then frozen and cut into 30 μm sections with a cryostat. Sections were stained with cresyl violet and examined microscopically to determine the amount of lesion damage and the recording sites. The amount of damage to the hippocampus (expressed as a percentage) was quantified in each animal by examining the slides, which corresponded to 11 sections illustrated in a stereotaxic atlas (Paxinos and Watson, 1986), and shading the damaged regions on paper. The sections ranged from −1.8 to −6.8 mm along the anteroposterior axis, with each section separated by 0.5 mm (e.g., −1.8, −2.3, −2.8, etc.). Using a square transparent grid overlay (representing 0.6 mm/side), the total area of the hippocampus was determined by summing the area of each section. For each animal, the area of the lesion in each section was calculated using the transparent grid overlay. Lesion percentages were then determined by dividing the total lesioned area by the total area of the hippocampus in the sections. In the animals given only neocortical lesions, the area of the lesion, in square millimeters, was calculated using the same 11 sections. Percentages are not given for this group because the lesion included several regions of neocortex.
RESULTS
Assessment of lesions
A total of 34 HD cells were recorded in 20 animals. Histological analysis showed that the electrodes were accurately positioned in either the ADN or PoS (data not shown). Figure3 shows coronal sections from animals in the control and hippocampus plus cortical lesion (HPC) groups. In the animals with hippocampal lesions, the overall amount of damage to the hippocampus varied from 70 to 100%. Six animals sustained a 90–100% complete lesion of the hippocampus, and six animals had lesions between 70 and 90%. Variability in the amount of damage to the ventral hippocampus accounted for the range of damage percentages, because the dorsal hippocampus was completely lesioned in all of the animals. In addition to the hippocampus, portions of the subiculum were lesioned in five of the six animals with >90% of the hippocampus lesioned. The subiculum was mostly spared in the five animals with less extensive hippocampal lesions. There was no difference in the results between animals with more complete lesions from animals with less complete lesions, and the animals from the two groups were therefore combined.
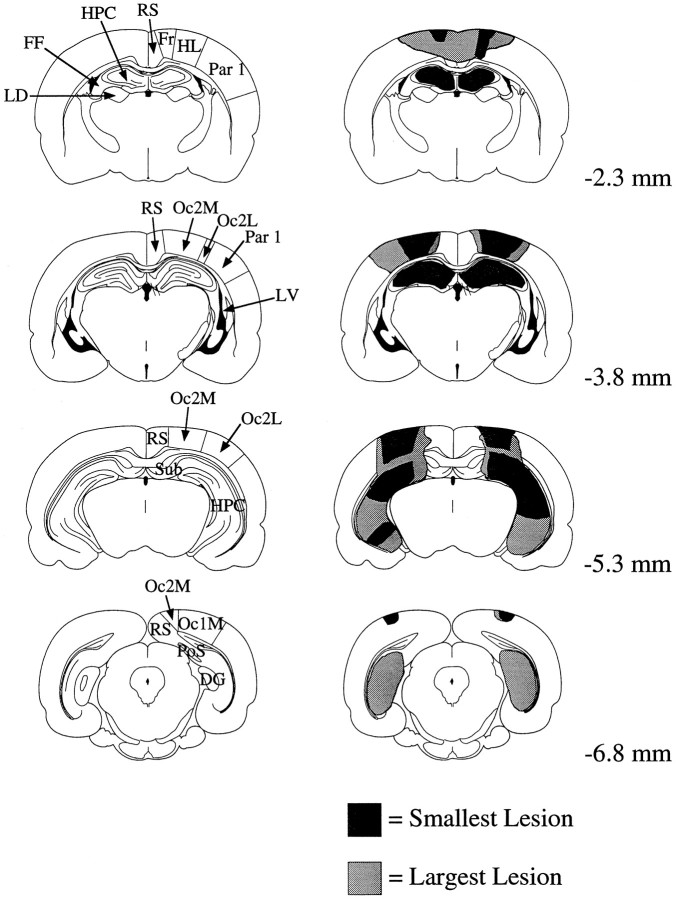
Fig. 3.
Schematic coronal sections comparing the largest and smallest lesions in the HPC group with a rat brain atlas (Paxinos and Watson, 1986). Each row illustrates a control and HPC section (left and right, respectively) at a particular distance relative to bregma (shown at thefar right). All shaded areas in the smallest lesion were also damaged by the largest lesion.DG, Dentate gyrus; FF, fimbria–fornix;FR, frontal area 1 and 2; HPC, hippocampus; HL, hindlimb area; LD, laterodorsal thalamic nucleus; LV, lateral ventricle;Oc1m, occipital 1 (medial); Oc2l,occipital 2, (lateral); Oc2m, occipital 2 (medial);Par 1, parietal 1; RS, retrosplenial cortex; Sub, subiculum.
In the cortical lesion (CTX) group, all animals sustained damage to the cortex overlying the hippocampus that was comparable with that found in the HPC group (Fig. 3). The mean area of the lesion was 57.0 mm2 (range, 31.0–90.4). The lesion area in two animals could not be accurately determined because of poor histology. There were no obvious differences in the results from animals having larger versus smaller total lesion areas. The cortical areas that usually sustained damage, as defined by Paxinos and Watson (1986), were frontal 1 and 2, somatosensory hindlimb area, parietal 1, and occipital 2 medial and lateral divisions. These damaged areas are primarily classified as motor and somatosensory regions, but the animals did not exhibit any noticeable motor or sensory impairments. The portions of the parietal cortex that were damaged may be homologous to the primate posterior parietal cortex and might involve the integration of sensory and motor functions. Figure4 illustrates the lesioned areas in the two animals with the greatest and least amount of cortical damage. The neocortical lesion group originally served as a control group for the unintentional damage to the cortex incurred by the group with hippocampal lesions; therefore, no particular region of cortex was purposefully lesioned.
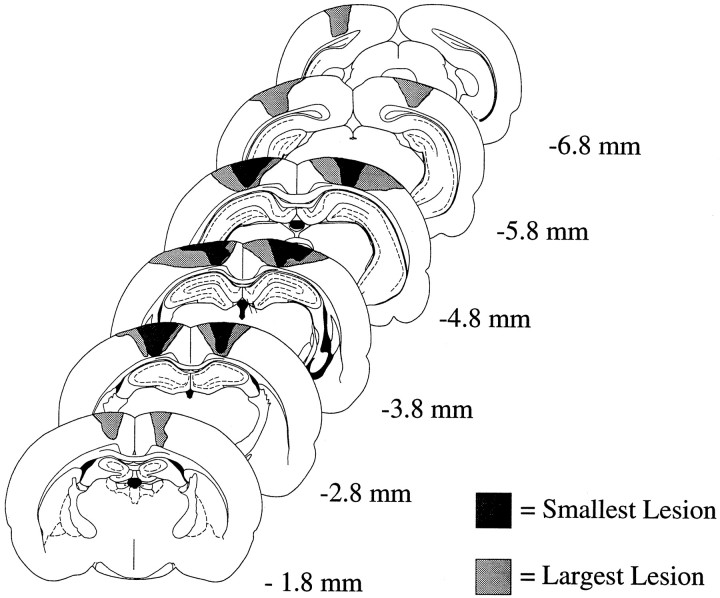
Fig. 4.
Schematic coronal sections showing cortical lesions from the CTX group. Examples from the two animals having the smallest and largest lesions are shown. The distance of each section from bregma is indicated at the far right. See Figure 3legend for classification of brain areas.
Experiment 1: dual-chamber apparatus
Novel sessions in the dual-chamber apparatus
During the 16 min novel session, the animals were provided access to both sections of the dual-chamber apparatus. When the nonlesioned control animals first entered the novel environment, the preferred direction remained approximately the same compared with the familiar section (i.e., the preferred direction was maintained between the familiar and novel environments). In contrast, for animals in the CTX and HPC groups, the preferred direction of each cell tended to drift for as long as 3–4 min during the initial visit to the novel environment. After this period, the cell established a particular preferred direction and maintained this orientation throughout the remainder of the first visit to the rectangle. The lesioned rats were then permitted to shuttle back and forth between the familiar cylinder and the novel rectangle throughout the remainder of the session. HD cell activity across multiple visits in the familiar and novel sections is analyzed separately below, but, in general, the preferred direction that was established during the first visit to the novel section was maintained during subsequent visits. To determine the overall firing characteristics of an HD cell in the familiar and novel sections, each sec data sample was sorted into two categories according to whether the animal was in the familiar or novel sections. The first 3 min of the initial visit were not included in the analysis, because this interval was the period when the preferred direction of most cells tended to vary (see below). The difference in preferred direction between the cylinder and passageway–rectangle were then computed for each animal.
The differences in preferred direction between the familiar and novel sections for the four nonlesioned animals were 0, 6, 6, and 30°. These values are well within the range of shifts reported in our previous study (the mean shift value was 18.0°; range, 0–30°;Taube and Burton, 1995). The results from these four animals were therefore combined with the results from our previous study, and together they represented the nonlesioned control group (n = 24). There was also no significant difference in the amount of shift in the preferred direction between the familiar and novel portions as a function of training procedure (no-cue vs standard training, Mann–Whitney U = 7.0, n = 4 and 7; p > 0.15) or recording site (ADN vs PoS, Mann–Whitney U = 11.0; n = 3 and 8;p > 0.80) in the HPC group. Similar results were also found for animals in the CTX group (training procedure, Mann–WhitneyU = 5.5; n = 5 and 3; p> 0.50; all animals, except for one, had electrodes implanted in the ADN). Therefore, for the purpose of statistical analysis, these animals were consolidated into a single HPC group and a single CTX group.
Figure 5A illustrates the firing rate versus HD functions for a typical HD cell from an animal from the HPC group when the animal was in either the familiar or novel sections of the dual-chamber apparatus. Note that the function maintains the same general shape in both environments. The primary difference in cell activity between the sections is the preferred direction, which shifted 102° CCW in the novel section relative to the familiar cylinder.
Fig. 5.
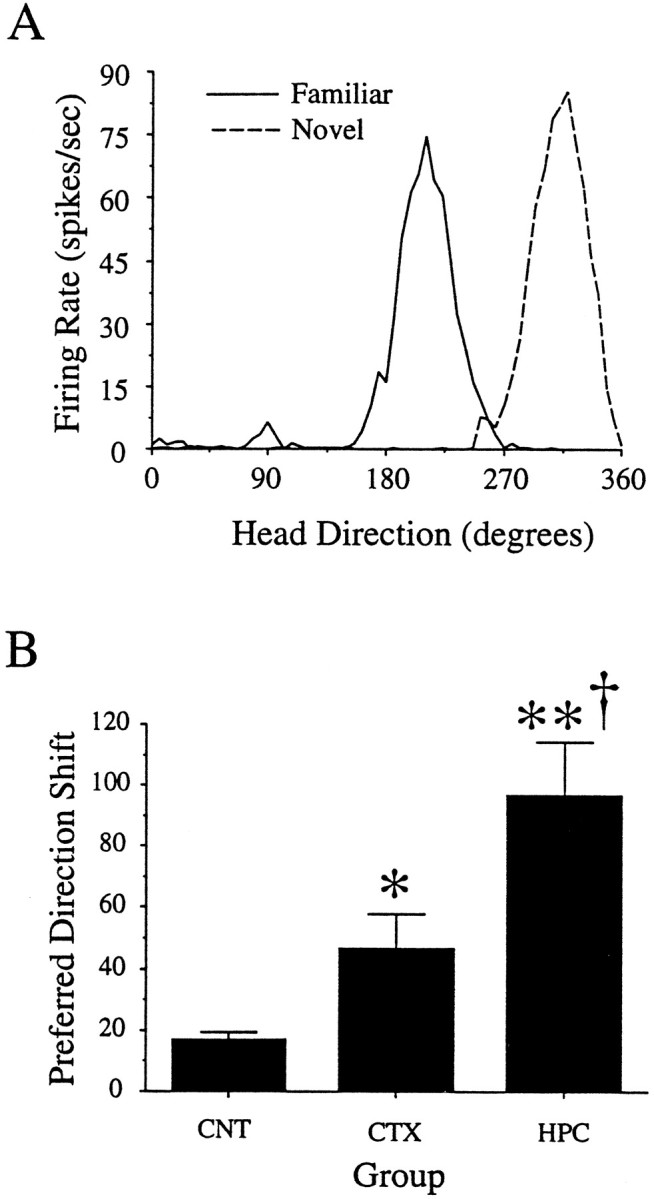
A, Firing rate versus HD plots for an HD cell from a hippocampal animal in the familiar and novel environments. This cell shifted its preferred direction 102° between the familiar (cylinder) and novel (rectangle–passageway) sections of the dual-chamber apparatus. The novel plot illustrates the firing activity of the cell after the initial 3 min of the rat’s first visit to the novel section. B, Group data showing the mean absolute values of directional shift between the familiar and novel sections for the three groups. Asterisks indicate levels of significance relative to controls (*p < 0.05; **p < 0.01). There was also a significant difference between the CTX and HPC groups (†p < 0.01).
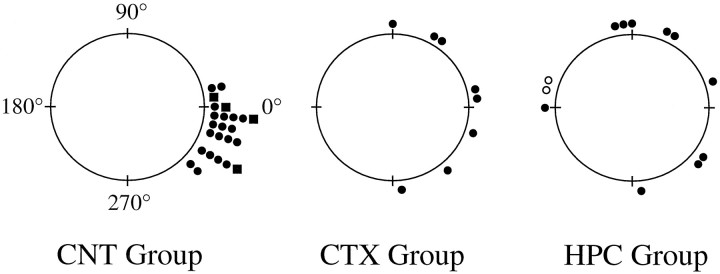
Overall, there were no significant differences in peak firing rate or firing range between the familiar and novel environments in either the HPC (peak firing rate, t = 0.28; df = 20;p > 0.70; firing range, t = 0.22; df = 20; p > 0.80) or CTX group (peak firing rate, t = 0.29; df = 14; p > 0.70; firing range, t = −0.29; df = 14;p > 0.78). Figure 5B shows the mean absolute shift in preferred direction between the familiar and novel environments for the HPC, CTX, and control groups. A one-way ANOVA comparing the directional shift values for the three groups showed a significant main effect for group (F = 20.3; df = 2,41; p < 0.0001). Post hoc Newman–Keuls tests revealed that the directional shift for the HPC group (96.5 ± 17.5) was significantly greater than the CTX (46.5 ± 11.3;p < 0.01) and control groups (17.0 ± 2.5;p < 0.01). In addition, the CTX group was significantly different from the control group (p < 0.05). Figure6 shows the group data in polar coordinates. Each mark indicates the amount of shift in the preferred direction of one cell per animal during the novel session. A Rayleigh test showed that the shifts in preferred direction were randomly distributed for the HPC group (r = 0.24;p > 0.50) but not for the CTX animals (r = 0.64; p < 0.05).
Fig. 6.
Polar plots showing the angular shifts in preferred direction during the first visit to the novel environment (novel session). Each dot on the periphery represents the magnitude of shift in the preferred direction for one HD cell. Each HD cell was recorded from a different animal. In general, HD cells in the control group maintained their preferred direction between the familiar and novel sections, with a small CW shift bias.Squares indicate the preferred direction shifts from the four control animals that were run in the present set of experiments. The CTX group had larger shifts than the controls, and these shifts were clustered around 0°. The HPC group had larger directional shifts than both the control and CTX groups, and the directional shifts were distributed randomly. The two open circles in the HPC group denote cells that drifted >180° in the CW direction.
In the earlier study using nonlesioned animals, it was reported that the small shift in preferred direction between sections of the dual-chamber apparatus was not randomly distributed. Rather, a bias toward CW shifts in preferred direction was noted (Taube and Burton, 1995). Of the 12 initial exposures to the novel environment in the HPC group, six drifted in the CCW direction, and six drifted in the CW direction. The shift directions were also corroborated by visual inspection at 1 min intervals, because it was possible for a cell to drift greater than 180°, a result that would be interpreted by the analysis program as a shift of lesser magnitude in the opposite direction. For the CTX group, the preferred direction of five cells changed in the CCW direction and three cells shifted their preferred direction in the CW direction. Grouping the results from both groups, a χ2 test indicated the absence of a bias in shift direction (χ2 = 0.20; df = 1; p > 0.60). Thus, in contrast to nonlesioned animals, when the rat entered the novel portion of the dual-chamber apparatus, HD cells in the HPC and CTX groups did not exhibit a tendency to drift in a CW direction.
In summary, HD cells from animals with hippocampal lesions were unable to maintain their preferred direction when the animal explored the unfamiliar section of the dual-chamber apparatus. The rats with cortical lesions exhibited a deficiency intermediate to the values found in the HPC and control animals. In addition, the HPC and CTX groups did not exhibit a bias in the direction of preferred direction shift as is typically observed in controls.
First visit to the novel environment
In the previous section, HD cell activity was averaged over the final 3–6 min of the first visit to the novel section. We were also interested in examining the HD cell responses throughout the entire first visit. In particular, we wanted to determine the preferred direction of the cell with greater temporal resolution to track the firing dynamics of the cell during this time period. After entering the novel environment, each rat was confined to this section of the dual-chamber apparatus for at least 6 min. Firing rate by HD plots in nonoverlapping 1 min increments were constructed for each rat during the first 6 min of the novel session and then compared with the preferred direction of the cell in the cylinder.
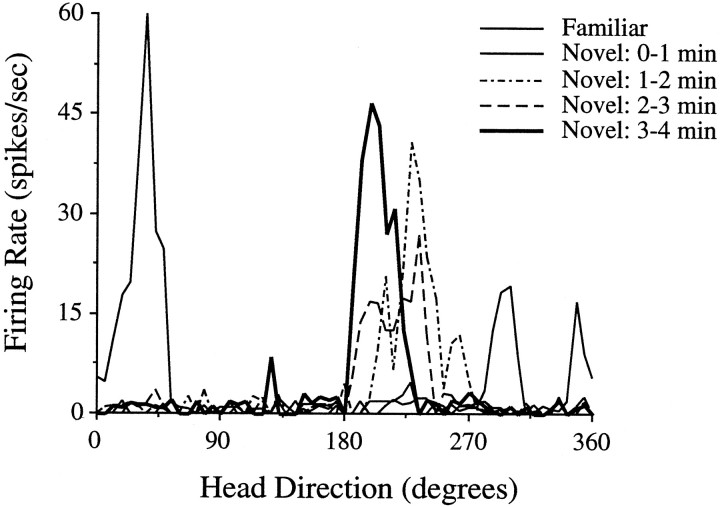
Figure 7 illustrates the successive drift in preferred direction for a hippocampal rat over the course of 4 min. After 3 min, the preferred direction stabilized at ∼205° and remained at that value for the rest of the visit. The preferred direction of most cells appeared to drift for 1–4 min before stabilizing. Figure 8 shows the change in preferred direction at 1 min intervals for all animals in the HPC group. Animals in the CTX group exhibited similar dynamics over time (data not shown). With one exception, the preferred directions of all the cells drifted during the first few minutes of the novel session, and, in almost every instance, the preferred direction drifted in a particular direction over time. Note that, after 1–3 min, most of the cells established a new preferred direction in the novel environment that was relatively stable for the remaining few minutes of the visit. If HD cells indeed rely on idiothetic cues to maintain their preferred direction during the interim period between first entering the environment and the establishment of cue control by landmark cues, the gradual drift we observed may indicate an impairment in the utilization of idiothetic cues as a consequence of the lesions.
Fig. 7.
Drift in the preferred direction of a cell during the first exposure to the novel environment. The preferred direction of this cell was ∼40° in the familiar cylinder. When the animal entered the novel section, the preferred direction drifted in a CW direction during the first few minutes. After ∼3 min, the cell adopted, and subsequently maintained, a preferred direction of ∼205°. The reduced mean firing rates during the 1 min time periods are probably an artifact caused by averaging the changing preferred firing direction. The thick line from the 3–4 min epoch indicates the firing orientation that was eventually established and maintained across repeated visits to the novel environment.
Fig. 8.
Individual 1 min samples of directional shift across different HD cells in the HPC group. The shift in preferred direction for each 1 min time period was compared with the baseline measure when the animal was located inside the cylinder just before entering the rectangle–passageway. The graph indicates that the preferred directions (1) shifted during the first minute of exposure to the novel environment, (2) frequently continued to drift over the next 2 min, and (3) were relatively stable after ∼3 min.
Analysis of repeated visits to the cylinder and rectangle
After the initial ∼6 min within the novel environment, the door was removed, allowing the animal to freely shuttle back and forth between the cylinder and rectangle. We then examined the consistency of the preferred direction across multiple visits to the cylinder and the rectangle–passageway. For each animal, two pairwise matrices were assembled, comparing the preferred directions between all visits within the cylinder or the rectangle–passageway. For instance, in a rat that visited the cylinder three times, the difference in preferred direction for the first visit in the cylinder was compared with the preferred direction during the second and third visits. The difference in preferred direction was also calculated between the second and third visits. Thus, for an animal that had n visits to a particular chamber, there were Σ(n − 1) + (n − 2) +… (n − n) pairwise comparisons. The mean value of this matrix was computed for each animal. This procedure allows for each chamber visit to have an equal influence when determining the overall consistency of the preferred direction. The difference in preferred direction for each pairwise comparison was determined using the cross-correlation algorithm described in Materials and Methods. Comparisons that yielded an r value <0.55 were rejected from this analysis, and most visits were at least 30 sec in duration. The HPC and CTX groups visited the cylinder a mean of 2.9 ± 0.4 and 2.3 ± 0.2 times, respectively. The rectangle was visited 2.3 ± 0.5 and 2.5 ± 0.3 times by the HPC and CTX animals, respectively. The mean values for the consistency in the preferred direction across visits are illustrated in Table 1. The values forn were sometimes less than the total number of animals in each group because either some rats did not visit the rectangle more than once or the r value of the cross-correlation was <0.55. Overall, the HD cells accurately maintained their preferred direction across multiple visits to the familiar (cylinder–cylinder) and novel (rectangle–rectangle) sections of the arena. The mean values in the HPC group were a little larger than the CTX and control groups because of the seven occasions (of 32) in which the HD cell maintained its preferred direction between sections (discussed in greater detail below). Because of these outlier scores, the data were transformed by taking the square root of the average value for each animal to increase statistical power (Kirk, 1968). ANOVAs comparing the consistency of the preferred direction over multiple visits to the novel or familiar arenas revealed no significant differences between the consistency across visits to the cylinder (F = 2.75; df = 2,30; p > 0.08) or in the rectangle–passageway (F = 1.09; df = 2,26; p > 0.30). The consistency in the angular difference in preferred direction between the familiar and novel environments was also equivalent across the two groups (F = 0.78; df = 2,25;p > 0.40).
Table 1.
Consistency of preferred firing direction across visits
| Group | Cylinder–Cylinder | Rectangle–Rectangle | Cylinder–Rectangle |
|---|---|---|---|
| Control | 9.1 ± 1.6 (n = 14) | 10.9 ± 1.9 (n = 14) | 16.2 ± 2.4 (n = 13) |
| HPC | 32.5 ± 11.2 (n = 11) | 10.0 ± 7.6 (n = 7) | 29.0 ± 11.0 (n = 7) |
| CTX | 13.5 ± 4.4 (n = 8) | 7.9 ± 2.5 (n = 8) | 16.6 ± 5.9 (n = 8) |
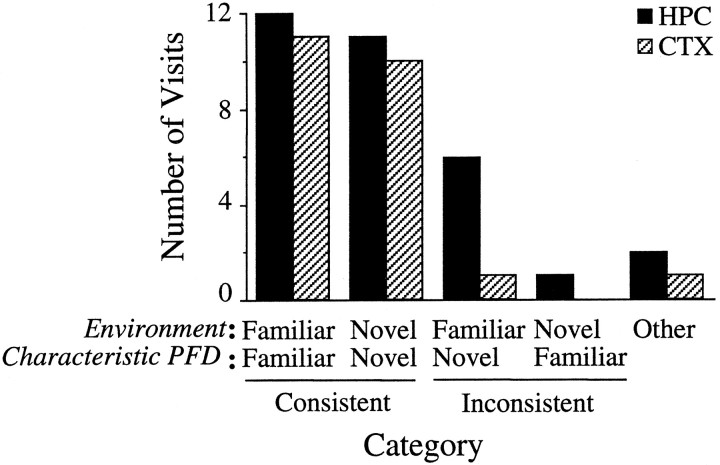
A more detailed analysis was undertaken by classifying the activity of the cell during each visit to the familiar and novel sections. For this analysis, the first visit to either side was used as the baseline for comparison with subsequent visits. Each visit to the familiar or novel section was categorized based on whether the preferred direction of the cell was within ±18° of the preferred direction in the previous visit to that chamber. Figure 9shows that, during the majority of return visits, the cell fired at the same preferred direction it had adopted during the animal’s initial visit (79.6% of 54 visits across both lesion groups). This consistency was found in both the familiar and the novel portions of the dual-chamber apparatus and across both the HPC and CTX groups. When the animal crossed between the familiar and novel sections, the cell would change its preferred direction, becoming congruent with the preferred direction established during the first visit. However, there were eight occasions (15.1%) when HD cells maintained the preferred direction characteristic of one section of the dual-chamber apparatus, even after the animal had entered the other section. On seven of these occasions, the cell maintained its preferred direction from the novel environment after the rat entered the familiar cylinder. On one occasion, the opposite pattern occurred in which the preferred direction of the cell in the cylinder was maintained after the rat had entered the novel environment. In the remaining three visits (5.7%), the preferred direction shifted more than ±18° away from the values established in the novel or familiar sections. Control animals have previously been shown to accurately maintain the preferred direction over repeated visits (Taube and Burton, 1995). These data indicate that HD cells in lesioned animals were capable of accurately maintaining their preferred direction, including the recently established orientation within the novel section, for short periods of time (<1 hr).
Fig. 9.
Frequency histogram categorizing the animal’s repeated visits to the familiar and novel sections.Environment indicates which part of the apparatus the animal was in for a given visit (familiar or novel).Characteristic PFD (preferred firing direction) indicates the typical preferred direction the cell adopted for an individual visit to one of the environments. The four pairs ofbars on the left represent all occasions when the preferred direction of a cell was within ±18° of its preferred direction in the animal’s first visit to that chamber. A cutoff value of 18° was chosen because it was at the upper range of values normally observed between sessions. A cell was consideredConsistent if its preferred direction was within ±18° of the direction established during the rat’s first visit to a given section (Familiar/Familiar orNovel/Novel). Inconsistent visits occurred when the preferred direction of a cell did not change after the animal crossed between sections, resulting in the cell firing within ±18° of the preferred direction typical of the other section of the dual-chamber apparatus (Familiar/Novel orNovel/Familiar). Other includes those visits in which the cell failed to fire within ±18° of its typical preferred direction in either section of the apparatus. In the vast majority of visits, the preferred direction of the cell appropriately matched the environment the animal currently occupied (Consistent). Occasionally, the firing of the cell was discordant with the environment (Inconsistent orOther). In seven of eight instances, the preferred direction characteristic of the rectangle–passageway was maintained when the animal returned to the cylinder.
Short-term stability of the preferred firing direction in the novel arena
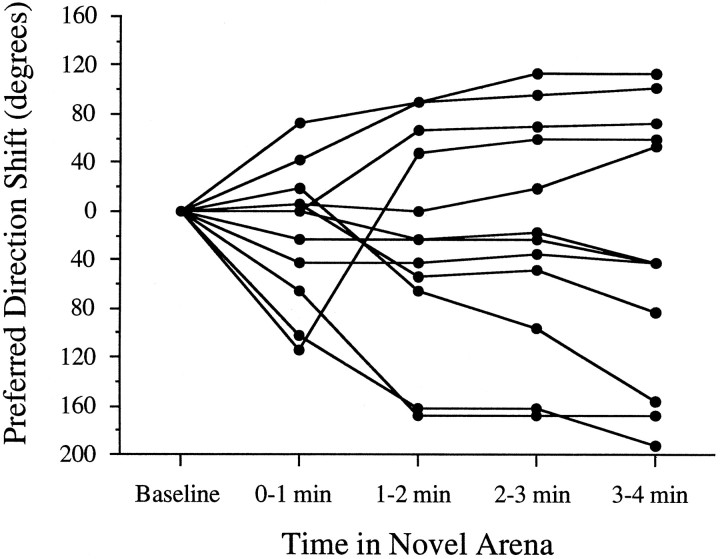
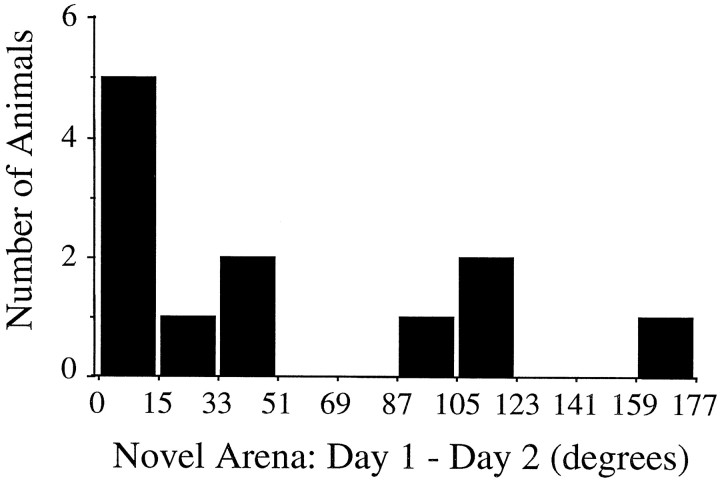
The consistency of the preferred direction in the novel arena across days was tested by reintroducing 12 rats (six HPC, six CTX; one cell per rat) to the rectangle–passageway ∼24 hr after the first novel session. Figure 10 illustrates the differences in preferred direction between days. Half of the cells (6 of 12) were consistent across days, with five cells varying by ≤12°. The remaining HD cells exhibited substantial disparities in preferred direction across days, differing by ≥90° in four animals (three HPC, one CTX). These findings demonstrate that, in some animals, the angular difference between the familiar and novel arenas established on the first day is not accurately maintained across days.
Fig. 10.
Consistency of the newly established preferred direction in the novel environment. The histogram shows the frequency in the magnitude the preferred direction shifted between days 1 and 2 in the novel section. The numbers along the abscissa indicate the absolute value of the angular shift. Approximately half the animals (6/12) maintained the same preferred direction (±12°) in the novel environment over 24 hr. Many of the remaining animals exhibited large differences in preferred direction between the two days, indicating that the preferred direction established the previous day, although stable within the same recording session, was unstable over 24 hr.
Subsequent sessions recorded from other HD cells
For some animals, we were able to repeat the dual-chamber apparatus experiment while recording a different HD cell. Other studies have reported that, whenever two cells were monitored simultaneously, their preferred directions were always separated by a fixed angle; thus, the effects of an environmental manipulation on the preferred direction for one cell were similar to the effects observed in other recorded cells (Taube et al., 1990a; Goodridge and Taube, 1995; Taube, 1995). This finding has also been observed for HD cells in rats with hippocampal lesions (Golob and Taube, 1997). Thus, it is unlikely that the above results are idiosyncratic to particular cells because other HD cells in the network presumably responded in a similar manner. Rather, the main purpose for conducting the dual-chamber apparatus experiment again was to determine whether the HD cell network is able to compensate for the lesion-induced effects by lessening the disparity between the preferred directions of the cell in the cylinder and rectangle–passageway on later tests.
Typically, several weeks separated the first (novel) and second (novel-repeat) experiments (mean number of days between the two sessions, 39.1 ± 9.7). HD cells were recorded from four rats in the HPC group and five rats in the CTX group. The mean absolute shift in the preferred direction between the familiar and novel portions of the apparatus was 100.5 ± 15.2° for the HPC group and 44.4 ± 8.2° for the CTX group. A repeated measures ANOVA showed a significant main effect for group (F = 52.49; df = 1,7; p < 0.001) but not for session day (novel vs novel-repeat, F = 0.64; df = 1,7;p > 0.40). The interaction between the two variables was also not significant (F = 0.91; df = 1,7;p > 0.30). These results argue against the development of an experience-dependent compensatory mechanism for reducing the disparity in preferred direction between sections of the dual-chamber apparatus. When examining the preferred direction shifts in the second novel session, we also noticed a tendency for the cells (eight of nine) to shift in the same direction as the HD cells recorded in the first novel session.
In contrast to the moderate predictability of shift direction, there was no relationship between the amount of angular shift between the first and second exposures to the rectangle–passageway (r = 0.35; n = 9; p > 0.40 from an expected population value of 0). In general, the scores tended to regress toward the mean for each group. Thus, animals with low preferred direction shifts during their first exposure to the dual-chamber apparatus often increased in the novel-repeat session, whereas animals with large directional shifts usually had a reduced shift magnitude in the novel-repeat session. Consistent with the variable short-term stability in the novel environment described above, these findings imply that the within session stability of the difference in preferred direction between the two chambers degrades over time. Apparently, the reestablishment of a new preferred direction difference value during the novel-repeat session is independent of the animals previous experience in the apparatus. Similar temporal instabilities have been observed in control animals (Taube et al., 1990b, their Table 2; our unpublished observations) and in animals with hippocampal lesions (Golob and Taube, 1997).
Experiment 2: novel cue
Comparison of the preferred direction between the no-cue and insert-cue sessions
The introduction of the cue card appeared to have only a small influence on the preferred direction of each recorded cell. A comparison between the no-cue and insert-cue sessions yielded a mean absolute change in preferred direction of 14.2 ± 2.8° in the HPC group and 13.2 ± 7.7° for the CTX group. These values are comparable with, although somewhat less than, the mean shift found in nonlesioned controls of 32.4 ± 17.9° (Goodridge et al., 1998).
Comparison of the preferred direction between the insert-cue session and the rotation test session
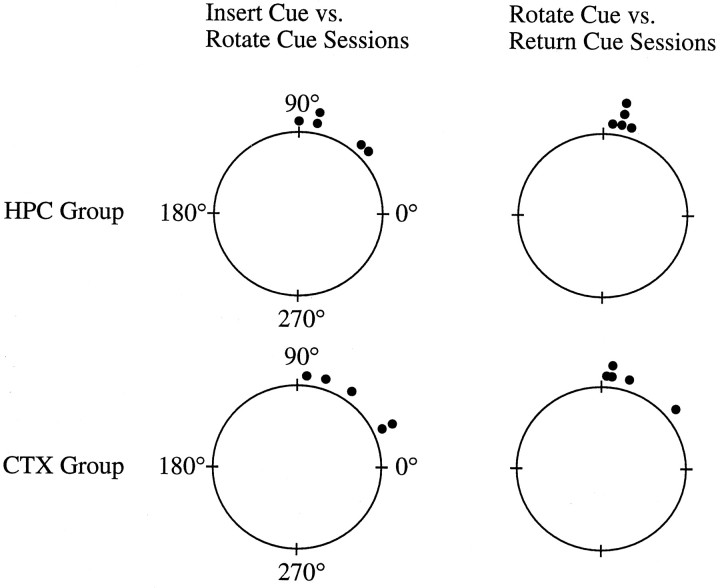
Polar plots illustrating the amount of shift in the preferred direction of the cell when the cue card was rotated ±90° are shown in Figure 11. Two sets of sequential comparisons are shown in Figure 11. The set on the leftillustrates the results from the rotation sessions when the cue card was rotated ±90°, and the set on the right shows the results from the return-cue sessions when the cue card was returned to its original position. The mean difference in preferred direction between the insert-cue and rotate-cue sessions was 72.0 ± 10.4° for the HPC animals (n = 5) and 51.6 ± 12.2° for the CTX group (n = 5). All of the cells shifted their preferred direction in the direction that the cue was rotated. For comparison, the mean shift in a group of nonlesioned animals was 70.8 ± 9.5° without the 4 hr delay (Goodridge et al., 1998). At test showed that the shift values for the lesioned animals did not differ from controls (t = −1.19; df = 8;p > 0.25), even though the controls experienced only a short (∼5 min) delay between the insert-cue and rotate-cue sessions. Cue control was further strengthened in some animals, because several cells updated their preferred directions more accurately when the cue card was returned to its former position in the return-cue session. The greater cue control was reflected by larger mean absolute changes in preferred direction between the rotate-cue and return-cue sessions (76.0 ± 2.0 and 73.2 ± 8.9° for the HPC and CTX groups, respectively). A repeated measures ANOVA comparing the change in preferred direction after the first and second cue rotation sessions among the HPC and CTX groups showed a nonsignificant effect for group (F = 1.16; df = 1,7; p > 0.30) and was also below the level of significance between the first and second rotation sessions (F = 2.987; df = 1,7;p > 0.10). The interaction was also nonsignificant (F = 1.06; df = 1,7; p > 0.30). In sum, the cue card was able to establish control over the preferred firing direction for all cells. These findings indicate that, even when the animals have extensive lesions to the hippocampus and overlying neocortex novel landmark cues are still capable of establishing control over the preferred directions of HD cells.
Fig. 11.
Polar plots illustrating the shift in preferred directions for HD cells in the HPC and CTX groups during the 90° cue card rotation sessions. A value of 90° corresponds to an equivalent shift of the preferred direction of a cell, and a 0° shift indicates an absence of cue control by the white card. In both groups of animals, most HD cells exhibited a change in preferred direction that followed the angular rotation of the novel cue. This result occurred in the first set of cue-rotation sessions after the 4 hr delay (insert-cue vs rotate-cue) and when the cue card was returned to its initial position a few minutes later (rotate-cue vs return-cue sessions). These data indicate that the novel environmental cue was able to rapidly establish control over the preferred directions of HD cells in the lesioned animals.
Experiment 3: dark sessions
If, as suggested in experiment 1, the lesions interfered with path integration, we would predict that the firing direction of HD cells would also drift under conditions in which access to extrinsic landmark cues is restricted and the animal’s navigation is confined to path integration mechanisms. To test this hypothesis, HD cells from two animals in the HPC group were recorded in a cylinder with the room lights either on or off. The cue card was removed during the dark sessions because it could potentially serve as a tactile and/or olfactory landmark cue. In addition, sessions were also recorded with the lights on but without the cue card. HD cells recorded from intact animals that were blindfolded during dark sessions in a previous study were used as a comparison group for the dark sessions in the lesioned animals (Goodridge et al., 1998).
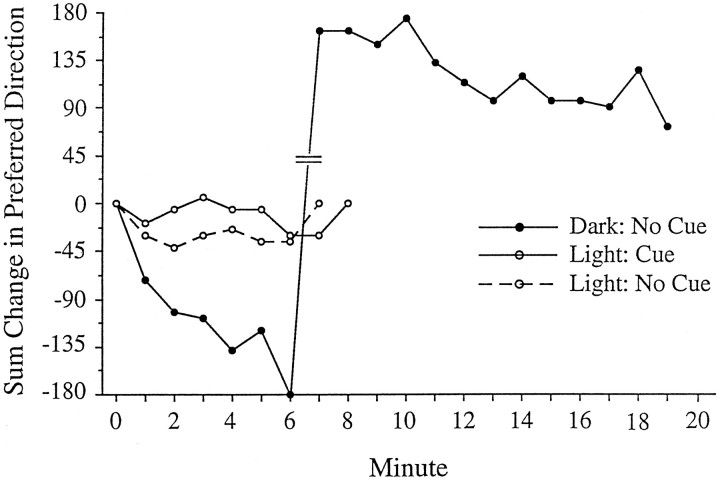
To track changes in preferred direction, each session was subdivided into nonoverlapping 1 min epochs. In general, there was substantial drift in the preferred direction recorded from HD cells in the HPC group when compared with the drift observed in either the light or dark sessions recorded from intact animals. Figure12 illustrates the drift in preferred direction across a 20 min dark session compared with the previous sessions with the lights on in a cell recorded from an HPC animal. We recorded seven HD cells from two lesioned animals (n = 1 and 6 per animal). Mean 1 min angular drift values were calculated by averaging the amount of preferred direction drift between adjacent 1 min epochs across a recording session (8 or 10 min) (e.g., change in preferred direction between 0–1 and 1–2 min, between 1–2 and 2–3 min, etc.). For the following values, the absolute value of the angular drifts was used. Two dark sessions were always recorded together in pairs without an intervening lights-on session. The same pattern of results was observed in both animals, although one rat contributed only a single light session and two dark sessions. For cells recorded from lesioned animals, the mean 1 min angular drift values for each session were as follows: light with cue card, 13.1 ± 1.2° (n = 5); light without cue card, 13.4 ± 3.3° (n = 4); and dark without cue card, 29.2 ± 3.1° (n = 12). The amount of drift in the dark sessions was significantly greater than the pooled value for all light sessions (t = −4.17; df = 19; p < 0.001). In the lights-on conditions, small CW and CCW drifts in preferred direction tended to cancel each other out, such that the change in preferred direction between the beginning and the end of the session was minimal. Although there were no apparent landmark cues for orientation in the session without the cue card, the preferred directions of the cells remained relatively stable for unknown reasons. It is possible that the rats may have used uncontrolled external cues that were visible in the lights-on session but not in the dark session (e.g., cues near the ceiling, small markings on the wall or floor). Finally, it was noted that when the cue card was returned and the lights were turned on after a dark session, the preferred direction of the cell drifted back to its original orientation over ∼1–3 min. This observation is consistent with previous reports showing that HD cells will orient by landmark cues when available (Goodridge and Taube, 1995).
Fig. 12.
HD cell responses in the dark recorded from a lesioned animal. The graph shows the running total drift in preferred direction over 20 min. In the sessions with the lights on, the preferred direction fluctuated around 0°. In contrast, in the dark session without the cue card, the preferred direction continuously drifted in the clockwise direction. The drift is not attributable to the absence of the cue card, because the drift during light sessions with and without the cue card was very small (see Experiment 3: dark sessions in Results). Hash marks between minutes 6 and 7 indicate the “wrap-around” point across 180°. The actual drift magnitude between minutes 6 and 7 was −18°.
In addition to the difference between the lights-on and dark sessions, the drift in preferred direction under darkened conditions was significantly greater in lesioned animals than in blindfolded control animals (mean angular drift, 10.5 ± 2.3°) (t = 4.66; df = 13; p < 0.001). For the lesioned animals, the mean 1 min angular drift values were not only greater than controls in the dark, but the drift in the preferred direction was usually in one direction (CW for both animals). To a first approximation, this resulted in a continuous drift away from the initial preferred direction over the course of a recording session. In contrast, the small drifts in preferred direction in blindfolded control animals in the dark were evenly distributed in the CW and CCW directions, resulting in only minor net changes in the preferred firing direction at the end of the session compared with the lesioned animals.
In sum, these findings provide further support for the notion that the lesions interfered with the rat’s ability to maintain a consistent preferred direction via path integration mechanisms.
Time shift analyses in lesioned animals
The time shift analyses were only performed on HD cells recorded in the ADN from the HPC group (n = 18), because the number of HD cells recorded in the PoS of lesioned animals was too small to yield reliable results. Control values were obtained from data reported in a previous study (Taube and Muller, 1998). The mean optimal time shifts for peak firing rate, range width, and information content were 0.28 ± 0.81, 0.92. ± 0.61, and 1.06 ± 0.47 samples, respectively. Because one sample is equivalent to 16.67 msec, these values correspond to 4.67, 15.33, and 17.67 msec, respectively. For comparison, the mean optimal time shift values for ADN cells in nonlesioned animals were (in samples) 1.85 ± 0.42, 1.27 ± 0.26, and 1.06 ± 0.18, respectively. t tests comparing HD cells from intact and lesioned animals showed no significant differences for the peak firing rate (t = −1.9; df = 17; p > 0.07), range width (t = −0.58; df = 17; p > 0.50), or information content measures (t = −0.01; df = 19; p > 0.90). Using the separation angle measure, the angular difference in cells from the lesioned animals was 4.61 ± 2.38°, a value that was also not significantly different from intact controls (6.03 ± 0.92°) (t = −0.60; df = 17; p > 0.50). These results suggest that the anticipatory properties of ADN HD cells are unlikely to be generated by hippocampal processing.
DISCUSSION
The results from experiment 1 indicate that lesions to either the hippocampus and overlying neocortex or only the overlying neocortex, prevented the accurate maintenance of the preferred direction of an HD cell when an animal locomoted from a familiar to novel environment. Furthermore, this impairment was significantly greater for rats with combined hippocampal and neocortical lesions than animals given only cortical lesions.
We favor the interpretation that the disparity in preferred direction values between the familiar and novel environments in lesioned animals is attributable to a disruption in the path integration process. Because there are no familiar landmarks to use for orientation in the novel environment, the task is thought to require accurate path integration to maintain the preferred direction of the cell (Taube and Burton, 1995). Furthermore, preliminary findings indicate that self-locomotion cues, and not vestibular or optic flow information, are critical for maintaining a consistent preferred direction between the two arenas (Taube et al., 1996b). The initial drift of the preferred direction is unlikely attributed to a lack of cue control from arena or uncontrolled room cues outside the curtain in the lesioned animals for three reasons. First, HD cells in the lesioned animals responded well to rotation of a novel cue in experiment 2 in which all HD cells shifted their preferred direction after one or two sessions when the novel cue card was placed in a rotated position. Second, previous cue rotation experiments with hippocampal animals in familiar environments also showed that the cue card exerted control over the preferred direction of the cell (Golob and Taube, 1997). Third, the finding in experiment 1 that the shift in preferred direction was maintained across multiple visits into the novel chamber suggests that cues within this chamber (e.g., the geometric shape of the passageway and rectangle, the cue card in the rectangle) are able to control the preferred direction of the cell.
Another explanation that could account for the shift in preferred direction in the lesioned animals is an attentional impairment. Previous theories of associational learning have proposed that animals with hippocampal lesions exhibit attentional disturbances (Douglas and Pribram, 1966; Schmajuk and Moore, 1989; Han et al., 1995). By extension, a lesioned animal would be unable to continuously attend to its internal, self-locomotion cues as it moved between environments. However, HD cells in hippocampal animals could respond to the introduction of a novel cue card in experiment 2 and, in previous experiments, showed evidence that they incorporated the geometric features of a novel environment (Golob and Taube, 1997). Thus, the attentional hypothesis has difficulty accounting for our results, unless the attentional deficit is confined solely to self-locomotion cues and does not effect landmark information. In addition, the areas of the overlying cortex that sustained damage were primarily sensory and motor areas, brain areas that are not typically implicated in attentional processes.
Together, the most parsimonious explanation to account for our results is that the lesioned animals were impaired at angular path integration. Data from lesioned animals recorded in the dark are also consistent with this explanation. McNaughton et al. (1996) postulated that one of the functions of the hippocampus is to serve as a path integrator. Consistent with this notion are recent experiments by Whishaw and Maaswinkel (1998), showing that rats with fimbria–fornix lesions are impaired at taking a direct route back to their burrow after they have searched for and retrieved a food reward. In contrast, Alyan and McNaughton (1999) recently demonstrated that rats with hippocampal lesions accurately performed a task thought to require path integration.
Path integration probably involves several processes, including (1) the establishment of an initial reference point, (2) monitoring the appropriate motion cues, (3) computation of the animal’s new position based on its initial position and subsequent movements, and (4) a mnemonic component that stores “on-line” the distance and direction of the initial reference point relative to the animal’s current position. Because the hippocampus has historically been associated with mnemonic processes, it is possible that the inability of hippocampal-lesioned animals to maintain a constant preferred direction when path integration processes are required is because of the animal’s poor ability to remember the initial reference point. Nonetheless, a disruption in any one of the above processes would interfere with accurate path integration. There is no evidence ora priori reason to suggest that the path integration processes are executed in a single brain area. Therefore, one might expect that damage to one of several brain areas will affect path integration abilities. Our findings that lesions to either the hippocampus or overlying neocortex prevent the maintenance of a stable preferred direction are consistent with the notion that multiple brain regions participate in path integration.
HD cell activity could be related to these path integration processes in several ways. First, a given landmark could simultaneously calibrate the firing orientation of HD cells and serve as a reference point for path integration. As a rat locomotes through territory devoid of familiar landmarks, it most likely relies on self-motion cues to maintain an accurate preferred direction (Taube and Burton, 1995). Consequently, the animal could in principle derive the correct direction for returning to its departure point based on self-motion information. The directional system may therefore be used to compute the directional component of path integration (Blair et al., 1999). The linear component of path integration is unlikely to be represented by HD cells, which are only mildly influenced by linear velocity (Taube et al., 1990a; Taube, 1995). If the computations involved in path integration are divided into separate linear and angular components that are implemented by distinct populations of cells, it will be important to identify where the linear component of path integration is computed and how the two types of information reconverge for the animal to navigate successfully.
Comparison of the CTX and HPC groups
The preferred direction instability in CTX animals is consistent with studies showing that parietal cortex ablations can lead to substantial (DiMattia and Kesner, 1988) or mild (Save and Moghaddam, 1996) deficits on an allocentric water maze task. Marked performance impairments on an egocentric water maze task, which probably required the use of idiothetic cues, were also reported (Save and Moghaddam, 1996). Neurons recorded from the cortex above the hippocampus exhibit a variety of movement-related correlates (McNaughton et al., 1994). These findings suggest that the functions performed by the lesioned neocortical areas in our study involve the use of idiothetic cues and are consistent with interpretations suggesting that the parietal cortex is involved in transformations of the location of stimuli from retinal to head- or body-centered coordinates (Andersen, 1997). A “mass action effect” provides the simplest explanation for the difference in the magnitude of directional shift between the HPC and CTX groups. The HPC group sustained additional tissue damage, causing a larger shift in preferred direction. Anatomical considerations suggest that there are functional differences between the neocortical areas we lesioned and the hippocampus that are relevant to path integration. The hippocampus, via the entorhinal and perirhinal cortices, receives input from all of the association cortices (Amaral and Witter, 1995). In contrast, the damaged neocortical areas in the CTX group were mostly primary and secondary sensorimotor cortex, areas that may be involved in processing idiothetic information. Because these lesions partially damaged several cortical areas, it is likely that they did not interfere completely with the transmission of idiothetic information. Consequently, only a moderate impairment was observed in the CTX group, and the preferred direction shifts were not random. Additional damage to the hippocampus could have affected the on-line storage of the animal’s current orientation or other aspects of the path integration process. The random distribution of preferred direction shifts in the HPC group, but not the CTX group, is consistent with this notion.
Establishment of cue control by novel landmark cues
In both experiments, landmark cues were able to establish control over the preferred directions of HD cells. When the animals were exposed to the novel section of the dual-chamber apparatus for the first time, the preferred direction eventually settled on a consistent value. For most cases in both groups of lesioned animals, this preferred direction was quite different from the preferred direction of the cell in the cylinder. The preferred direction of a cell, however, was usually consistent across multiple visits to either the familiar or novel section. If landmark cues within the novel environment were unable to establish control over the preferred direction of a cell, then the preferred direction presumably would have drifted as it did when the rat first entered the novel section. Because this result did not occur, it indicates that landmarks within the dual-chamber apparatus were able to calibrate the firing orientation of HD cells.
Experiment 2 clearly demonstrated that HD cells are able to link their firing patterns to a novel spatial cue, even in the absence of the hippocampus. Introduction of the cue card had little influence on the preferred direction, indicating that the directional orientation of a cell relative to an environmental feature is not predetermined. Therefore, any relationship between the orientation of the preferred direction of a cell and the location of a cue must develop over time as a consequence of the animal’s experience. After rotating the cue card, the preferred direction in five of five cells in the HPC group and four of five cells in the CTX group also rotated a similar amount, thus demonstrating a strong-to-moderate degree of cue control. These results confirm earlier findings that the hippocampus is not required for this form of episodic spatial memory (Golob and Taube, 1997). Although the hippocampus is not necessary for cue control, it has recently been shown that the PoS is necessary for accurate cue control in ADN HD cells (Goodridge and Taube, 1997). The findings that the orientation of HD cells in the novel environment after the recording session on the first day were often inconsistent, suggest that cue control in the novel environment weakens, or disappears, over time. The preferred direction instability across 24 hr in particular is quite different from the consistency usually observed in hippocampal animals when placed into novel arenas after a disorientation procedure (Golob and Taube, 1997). Perhaps the interplay between path integration and extrinsic cues affects the process of establishing cue control, whereas in the earlier study, path integration mechanisms were presumably nullified by disorienting the animal before introducing it into the novel environment.
Time course for stabilizing the preferred firing direction and establishing cue control
In experiment 1 the preferred direction of a cell drifted for as long as 4 min before establishing a consistent orientation in the novel environment. This result may be related to the finding that it takes a few minutes to integrate novel cues into the firing patterns of HD cells (Goodridge et al., 1998). When a rat is exposed to a novel environment, it may be forced to rely on path integration-based information until the environmental landmark cues are able to be linked with the directional system. A similar interaction between the continuous monitoring of idiothetic cues and periodic reorientation by landmarks is a prominent theme in several theories of navigation (Gallistel, 1990; Etienne et al., 1996).
Conclusions
In summary, our findings suggest that both the hippocampus and overlying neocortex are important for angular path integration. Moreover, novel landmark cues are capable of gaining control over preferred directions in HD cells in animals with lesions of the hippocampus and/or overlying neocortex, indicating that episodic spatial information concerning landmark cues can be encoded and retrieved by brain regions other than the hippocampus and overlying cortex.
Footnotes
This work was supported by National Institute of Mental Health Grants MH48924 and MH01286 to J.S.T. Preliminary reports of this research were presented at the 26th and 27th Annual Society for Neuroscience meetings in Washington, DC and New Orleans, LA in 1996 and 1997. We thank Robert Stackman, Matthew Tullman, Joshua Bassett, and Andrew Wong for valuable discussions concerning these experiments.
Correspondence should be addressed to Dr. Jeffrey S. Taube, Dartmouth College Department of Psychological and Brain Sciences, 6207 Moore Hall, Hanover, NH 03755.
REFERENCES
- 1.Alyan SH, McNaughton BL. Hippocampectomized rats are capable of homing by path integration. Behav Neurosci. 1999;113:19–31. doi: 10.1037//0735-7044.113.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system, Ed 2. Academic; San Diego: 1995. pp. 443–493. [Google Scholar]
- 3.Andersen RA. Multimodal integration for the representation of space in the posterior parietal cortex. Phil Trans R Soc Lond B Biol Sci. 1997;352:1421–1428. doi: 10.1098/rstb.1997.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batschelet E. Circular statistics in biology. Academic; New York: 1981. [Google Scholar]
- 5.Blair HT, Sharp PE. Anticipatory head direction signals in anterior thalamus: evidence for a thalamocortical circuit that integrates angular head motion to compute head direction. J Neurosci. 1995;15:6260–6270. doi: 10.1523/JNEUROSCI.15-09-06260.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair HT, Lipscomb BW, Sharp PE. Anticipatory time intervals of head-direction cells in the anterior thalamus of the rat: implications for path integration in the head-direction circuit. J Neurophysiol. 1997;78:145–159. doi: 10.1152/jn.1997.78.1.145. [DOI] [PubMed] [Google Scholar]
- 7. Blair HT, Sharp PE, Cho J, Goodridge JP, Stackman RW, Golob EJ, Taube JS. Path integration in the rat head-direction circuit. Advances in neural information processing systems, Vol 10 Touretzky D, Mozer M, Hasselmo M. 1999. MIT; Cambridge, MA, in press. [Google Scholar]
- 8.DiMattia BD, Kesner RP. Spatial cognitive maps: differential role of parietal cortex and hippocampal formation. Behav Neurosci. 1988;102:471–480. doi: 10.1037//0735-7044.102.4.471. [DOI] [PubMed] [Google Scholar]
- 9.Douglas R, Pribram KH. Learning and limbic lesions. Neuropsychologia. 1966;4:197–220. [Google Scholar]
- 10.Eichenbaum H, Stewart C, Morris RG. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etienne AS, Maurer R, Seguinot V. Path integration in mammals and its interaction with visual landmarks. J Exp Biol. 1996;199:201–209. doi: 10.1242/jeb.199.1.201. [DOI] [PubMed] [Google Scholar]
- 12.Gallistel CR. Animal cognition: the representation of space, time and number. Annu Rev Psychol. 1989;40:155–189. doi: 10.1146/annurev.ps.40.020189.001103. [DOI] [PubMed] [Google Scholar]
- 13.Gallistel CR. The organization of learning. MIT; Cambridge, MA: 1990. [Google Scholar]
- 14.Gavrilov VV, Wiener SI, Berthoz A. Enhanced hippocampal theta EEG during whole body rotations in awake restrained rats. Neurosci Lett. 1995;197:239–241. doi: 10.1016/0304-3940(95)11918-m. [DOI] [PubMed] [Google Scholar]
- 15.Golob EJ, Taube JS. Head direction cells and episodic spatial information in rats without a hippocampus. Proc Natl Acad Sci USA. 1997;94:7645–7650. doi: 10.1073/pnas.94.14.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodridge JP, Taube JS. Preferential use of the landmark navigational system by head direction cells. Behav Neurosci. 1995;109:49–61. doi: 10.1037//0735-7044.109.1.49. [DOI] [PubMed] [Google Scholar]
- 17.Goodridge JP, Taube JS. Interaction between the postsubiculum and anterior thalamus in the generation of head direction cell activity. J Neurosci. 1997;17:9315–9330. doi: 10.1523/JNEUROSCI.17-23-09315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodridge JP, Dudchenko PA, Worboys KA, Golob EJ, Taube JS. Cue control and head direction cells. Beh Neurosci. 1998;112:749–761. doi: 10.1037//0735-7044.112.4.749. [DOI] [PubMed] [Google Scholar]
- 19.Green JD, Arduini A. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 20.Han JS, Gallagher M, Holland P. Hippocampal lesions disrupt decrements but not increments in conditioned stimulus processing. J Neurosci. 1995;15:7323–7329. doi: 10.1523/JNEUROSCI.15-11-07323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarrard LE. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. J Neurosci Methods. 1989;29:251–259. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- 22.Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 23.Kirk RE. Experimental design: procedures for the behavioral sciences. Brooks/Cole; Belmont, CA: 1968. [Google Scholar]
- 24.Knierim JJ, Kudrimoti HS, McNaughton BL. Neuronal mechanisms underlying the interaction between visual landmarks and path integration in the rat. Int J Neural Syst. 1996;7:213–218. doi: 10.1142/s012906579600018x. [DOI] [PubMed] [Google Scholar]
- 25.Kubie JL. A drivable bundle of microwires for collecting single-unit data from freely moving rats. Physiol Behav. 1984;32:115–118. doi: 10.1016/0031-9384(84)90080-5. [DOI] [PubMed] [Google Scholar]
- 26.McNaughton BL, Mizumori SJ, Barnes CA, Leonard BJ, Marquis M, Green EJ. Cortical representation of motion during unrestrained spatial navigation in the rat. Cereb Cortex. 1994;4:27–39. doi: 10.1093/cercor/4.1.27. [DOI] [PubMed] [Google Scholar]
- 27.McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- 28.Mittelstaedt ML, Mittelstaedt H. Homing by path integration in a mammal. Naturwissenschaften. 1980;67:566–567. [Google Scholar]
- 29.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 30.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 31.O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon; Oxford: 1978. [Google Scholar]
- 32.O’Mara SM. Spatially selective firing properties of hippocampal formation neurons in rodents and primates. Prog Neurobiol. 1995;45:253–274. doi: 10.1016/0301-0082(94)00050-r. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1986. [DOI] [PubMed] [Google Scholar]
- 34.Save E, Moghaddam M. Effects of lesions of the associative parietal cortex on the acquisition and use of spatial memory in egocentric and allocentric navigation tasks in the rat. Behav Neurosci. 1996;110:74–85. doi: 10.1037//0735-7044.110.1.74. [DOI] [PubMed] [Google Scholar]
- 35.Schmajuk NA, Moore JW. Effects of hippocampal manipulations on the classically conditioned nictitating membrane response: simulations by an attentional-associative model. Behav Brain Res. 1989;32:173–189. doi: 10.1016/s0166-4328(89)80083-x. [DOI] [PubMed] [Google Scholar]
- 36.Sharp PE, Blair HT, Etkin D, Tzanetos DB. Influences of vestibular and visual motion information on the spatial firing patterns of hippocampal place cells. J Neurosci. 1995;15:173–189. doi: 10.1523/JNEUROSCI.15-01-00173.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. An information - theoretic approach to deciphering the hippocampal code. In: Hanson SJ, Cowan JD, Giles CL, editors. Advances in neural information processing, Vol 5. Morgan Kaufman; San Mateo, CA: 1993. pp. 1030–1037. [Google Scholar]
- 38.Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taube JS, Burton HL. Head direction cell activity monitored in a novel environment and during a cue conflict situation. J Neurophysiol. 1995;74:1953–1971. doi: 10.1152/jn.1995.74.5.1953. [DOI] [PubMed] [Google Scholar]
- 40.Taube JS, Muller RU. Comparisons of head direction cell activity in the postsubiculum and anterior thalamus of freely moving rats. Hippocampus. 1998;8:87–108. doi: 10.1002/(SICI)1098-1063(1998)8:2<87::AID-HIPO1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990a;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990b;10:436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taube JS, Goodridge JP, Golob EJ, Dudchenko PA, Stackman RW. Processing the head direction cell signal: a review and commentary. Brain Res Bull. 1996a;40:477–484. doi: 10.1016/0361-9230(96)00145-1. [DOI] [PubMed] [Google Scholar]
- 44.Taube JS, Stackman RW, Dudchenko PA. Head direction cell activity monitored following passive transport into a novel environment. Soc Neurosci Abstr. 1996b;22:1873. [Google Scholar]
- 45.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 46.Wehner R, Srinivasan M. Searching behavior of desert ants, genus cataglyphis (formicidae, hymenoptera). J Comp Physiol. 1981;142:315–338. [Google Scholar]
- 47.Whishaw IQ, Maaswinkel H. Rats with fimbria–fornix lesions are impaired in path integration: a role for the hippocampus in “sense of direction.”. J Neurosci. 1998;18:3050–3058. doi: 10.1523/JNEUROSCI.18-08-03050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whishaw IQ, Cassel JC, Jarrard LE. Rats with fimbria–fornix lesions display a place response in a swimming pool: a dissociation between getting there and knowing where. J Neurosci. 1995;15:5779–5788. doi: 10.1523/JNEUROSCI.15-08-05779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whishaw IQ, McKenna JE, Maaswinkel H. Hippocampal lesions and path integration. Curr Opin Neurobiol. 1997;7:228–234. doi: 10.1016/s0959-4388(97)80011-6. [DOI] [PubMed] [Google Scholar]