Abstract
ID is a slowly inactivating 4-aminopyridine (4-AP)-sensitive potassium current of hippocampal pyramidal neurons and other CNS neurons. AlthoughID exerts multifaceted influence on CNS excitability, whether ID is subject to modulation by neurotransmitters or neurohormones has not been clear.
We report here that one prominent effect of metabotropic glutamate receptor (mGluR) activation by short (3 min) exposure to 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD) (100 μm) is suppression of ID by acceleration of its inactivation. ID was identified as a target of mGluR-mediated modulation because inactivation of a component of outward current sensitive to 100–200 μm4-AP was accelerated by 1S,3R-ACPD, and because 4-AP occluded any further actions of 1S,3R-ACPD. Enhancement ofID inactivation was induced by the group I-preferring agonist RS-3,5-dihydroxyphenylglycine (3,5-DHPG) and the group II-preferring agonist 2S,2′R,3′R)-2-(2′,3′dicarboxycyclopropyl)-glycine (DCG-IV), but not by the group III-preferring agonistl(+)-2-amino-4-phosphonobutyric acid (L-AP4); it was blocked by the broadly acting mGluR antagonistS-α-methyl-4-carboxyphenylglycine (S-MCPG). Furthermore, inactivation of ID was enhanced by inclusion of GTPγS in the internal solution and blocked by inclusion of GDPβS.
Metabotropic GluR-induced suppression of IDwas manifest in three aspects of excitability previously linked toID by their sensitivity to 4-AP: reduction in input conductance and enhanced excitability at voltages just positive to the resting potential, reduced delay to action potential firing during depolarizing current injections, and delayed action potential repolarization. We suggest that mGluR-induced suppression ofID could contribute to enhancement of hippocampal neuron excitability and synaptic connections.
Keywords: metabotropic glutamate receptor, 4-aminopyridine, potassium current, ID, hippocampus, pyramidal neuron, regulation of excitability
Slowly inactivating potassium currents, such as ID, have been described in neurons from many regions of the CNS (McCormick, 1991; Surmeier et al., 1991; Ficker and Heinemann, 1992; Hammond and Crepel, 1992; Wu and Barish, 1992; Stefani et al., 1995; Bossu et al., 1996; Li and McArdle, 1997; Locke and Nerbonne, 1997a). As a class, these currents are often defined by their sensitivity to micromolar concentrations of 4-aminopyridine (4-AP), a characteristic that is striking in comparison with the millimolar concentrations required to block the more rapidly inactivating transient potassium currentIA, and in comparison with the 4-AP insensitivity of the relatively noninactivating potassium currentIK.
The higher sensitivity of ID to 4-AP is commonly used to separate it from other voltage-gated potassium currents [e.g., IA,IK, orIM (Rudy, 1988)] and has been used to identify three major influences of IDon the electrical behavior of neurons. First, application of micromolar 4-AP reduces the time between initiation of a long depolarizing current injection and onset of firing. For this reason, Storm (1988a) named this current the “delay” potassium current, and similar observations have been made in other investigations of CNS neurons (Locke and Nerbonne, 1997b) [also see Wu and Barish (1994) for a separation not using 4-AP]. This property ofID suggests a role in temporal integration of long epochs of excitatory input. Second, micromolar 4-AP reduces input conductance at voltages near the resting potential (Brown et al., 1990; Storm, 1990), suggesting thatID may influence the efficacy of synaptic input in eliciting postsynaptic action potentials. Third, micromolar 4-AP also delays action potential repolarization (Storm, 1987, 1988b; Wu and Barish, 1992; Bossu et al., 1996; Locke and Nerbonne, 1997b), suggesting that by influencing action potential duration ID may regulate Ca2+ entry and its sequelae.
The net effect of these influences ofID is a multifaceted regulation of excitability in CNS neurons. This is manifest as the ability of micromolar 4-AP to increase presynaptic fiber potentials, to enhance synaptic transmission, and to act as a potent convulsant (Llinás et al., 1976; Theslef, 1980; Buckle and Haas, 1982; Kuhnt and Grinvald, 1982; Haas et al., 1983; Rutecki et al., 1987; Szente and Baranyi, 1987; Perreault and Avoli, 1989, 1991, 1992; Barish et al., 1996; Obaid and Salzberg, 1996; Wheeler et al., 1996; M. E. Barish, R. Kajiwara, and T. Iijima, unpublished observations). Yet despite these demonstrations of the importance of IDin regulation of excitability, whetherID is subject to physiological modulation by neurotransmitters or neurohormones has not been clear.
We hypothesized that metabotropic glutamate receptors (mGluRs) might be linked to ID because several studies have implicated potassium currents in the pleiotropic effects of mGluR activation on hippocampal neuron excitability (Charpak et al., 1990;Lester and Jahr, 1990; Desai and Conn, 1991; Baskys, 1992; Swartz and Bean, 1992; Sahara and Westbrook, 1993). We report here that one prominent effect of mGluR activation by 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD) is suppression ofID by acceleration of its inactivation. Suppression of ID was sensitive to group I- and group II- but not group III-preferring agonists and antagonists. It was manifest in three aspects of excitability previously linked to IDby their sensitivity to 4-AP: reduction in input conductance and enhanced excitability at voltages just positive to the resting potential, reduced delay to action potential firing during depolarizing current injections, and delayed action potential repolarization. We suggest that mGluR-induced suppression ofID could contribute to persistent changes in hippocampal neuron excitability (Stratton et al., 1989;Gereau and Conn, 1994) and connectivity (Bortolotto and Collingridge, 1992, 1993; Bashir et al., 1993; Breakwell et al., 1996; Cohen and Abraham, 1996).
MATERIALS AND METHODS
Preparation of cultures. The procedures for preparation of these mixed neuron-glia cultures were identical to those used previously (Wu and Barish, 1992; Wu et al., 1998). Embryonic Swiss Webster mice were removed under sterile conditions from pregnant female mice, after anesthesia (by halothane inhalation) and cervical dislocation, using procedures meeting National Institutes of Health guidelines. Hippocampi were removed from fetuses and dissociated using papain (7.2 mg/10 ml, 35 min at 35°C; Worthington, Freehold, NJ) in Ca2+- and Mg2+-free HBSS. Dissociated cells were plated at ∼22,100 cells/cm2 (25,000 cells per coverslip) onto poly-d-lysine- and laminin-coated 12-mm-diameter glass coverslips (“Assistent”; Carolina Biological, Burlington, NC) in a 150 μl bubble of medium (described below) supplemented to 10% total serum. After cells were allowed to settle for 2 hr, each 35-mm-diameter Petri dish containing two coverslips was flooded with 1 ml of low-serum medium.
Low-serum medium, which facilitates growth of neurons on a sparse underlying layer of astroglial cells, consisted of an 8:2 mixture of MEM and F-12, supplemented with 2 mm glutamine, B-27 additive [1:50 (Brewer et al., 1993)], 2.5% fetal bovine serum, 2.5% horse serum, 0.5% antibiotic–antimycotic solution [(Sigma, St. Louis, MO) final concentrations: 50 U/ml penicillin, 0.05 mg/ml streptomycin, 0.13 μg/ml amphotericin B], and glucose to a total concentration of 25 mm. An antimitotic, ara-C (10 μm), was added after ∼48 hr to control astroglial proliferation. Except as noted, all components of tissue culture media, including sera and B-27, were purchased from Life Technologies (Gaithersburg, MD).
Procedures for electrophysiology.Procedures for whole-cell “tight seal” recordings were also standard, except that we have used an internal solution that maintains stable currents for up to 1 hr under favorable conditions. The solution consisted of the following (in mm): 125 potassium methylsulfate, 15 KCl, 2 MgCl2, 0.01 CaCl2, 0.11 BAPTA, 0.1 GTP (lithium salt), 4 ATP (magnesium salt), 20 HEPES, pH 7.30 (adjusted with Trizma base). Notable are the use of methylsulfate as an anion (Zhang et al., 1994;Velumian et al., 1997), low CaCl2 and BAPTA concentrations, and inclusion of GTP and ATP. The standard external solution contained (in mm): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 4.2 NaHCO3, 10 glucose, 15 HEPES, pH 7.30. Tetrodotoxin was added at 1 μm to block sodium currents. The bath chamber (volume 0.4 ml) was continuously perfused at a rate of 0.4 ml/min using a peristaltic pump. Channel blockers and other reagents were applied using a slightly pressurized large-bore (tip diameter ∼400 μm) puffer pipette.
Currents were recorded using an Axopatch 1B (modified for phase-lag series resistance compensation; Axon Instruments, Foster City, CA) and digitized and analyzed using a Digitata 1200a interface and pCLAMP v. 6 software (Axon Instruments). Series resistance was compensated to ∼80%, and currents were filtered using the amplifier’s Bessel filter at 1–2 kHz (−3 dB) and digitized at 2–5 kHz. Currents linear with membrane voltage (leak currents and residual capacity transients) were subtracted using a P/−4 voltage-step protocol. Voltages were corrected for junction potentials between electrode and bath solutions. Recordings were made at 28°C.
Statistical tests were made using Instat for Windows (Graph Pad, San Diego, CA). Data in the figures are presented as mean ± SD, with statistical significance evaluated by repeated-measures ANOVA with Dunnett’s multiple comparison post-test or by paired two-tailedt test, as appropriate.
All mGluR agonists and antagonists were purchased from Tocris Cookson (Ballwin, MO). Potassium methylsulfate was purchased from ICN (Aurora, OH). BAPTA was purchased from Molecular Probes (Eugene, OR). Other salts for physiological solutions were purchased from Sigma.
RESULTS
The data presented below were taken from pyramidal neurons in cultures of dissociated embryonic day 15–16 (E15–E16) hippocampal cells. Pyramidal neurons can be easily identified on the basis of the soma shape and the presence of a prominent apical dendrite. Neurons had been in culture for 4–12 d.
Under the conditions of relaxed intracellular Ca2+ chelation used here, both voltage-gated and Ca2+-dependent currents will be activated during voltage-clamp steps to voltages positive to approximately −20 mV (Storm, 1990). The Ca2+-dependent currents consist of both apamin- and charybdotoxin-sensitive components (Lancaster et al., 1991;Beck et al., 1997). These currents are considered only as they may relate to currents elicited as a consequence of mGluR activation.
Of the voltage-gated potassium currents,IA is a rapidly activating and inactivating current sensitive to millimolar concentrations of 4-AP. The other voltage-gated currents can be separated into a slowly inactivating component and a relatively noninactivating or persistent component based on their differential sensitivity to micromolar concentrations of 4-AP (Brown et al., 1990; Storm, 1990; Ficker and Heinemann, 1992; Wu and Barish, 1992; Bossu et al., 1996; Li and McArdle, 1997). In studies of hippocampal neurons originating in various laboratories, the slowly inactivating and 4-AP-sensitive component has been termed ID (Storm, 1988a), IT,slow (Ficker and Heinemann, 1992), IK(AT) (Bossu et al., 1996), or IAs (Li and McArdle, 1997), and we have referred to it asID in previous studies (Wu and Barish, 1992, 1994; Wu et al., 1998). Similar 4-AP-sensitive currents have been termed IAs in neostriatal neurons (Surmeier et al., 1991; Gabel and Nisenbaum, 1998) and lateral geniculate relay neurons (McCormick, 1991), andID in visual cortical neurons (Albert and Nerbonne, 1995; Locke and Nerbonne, 1997a). Whether all such currents are equivalent is not clear, because differences have been noted in absolute sensitivities to 4-AP (block by tens vs hundreds of micromolar) activation voltage ranges (negative to vs positive toIA), sensitivities to TEA (block by low millimolar concentrations in some cases), and rates of removal of inactivation (hundreds of milliseconds vs seconds). Nevertheless, as a class they share certain functional roles, including control of the latency to first action potential generation during sustained depolarizing current injections, and control of action potential repolarization. In this study of cultured hippocampal neurons, the current sensitive to 100–200 μm 4-AP has been termed ID, and an example of its separation is presented in Figure 3 [also see Wu and Barish (1992); Wu et al. (1998)].
Fig. 3.
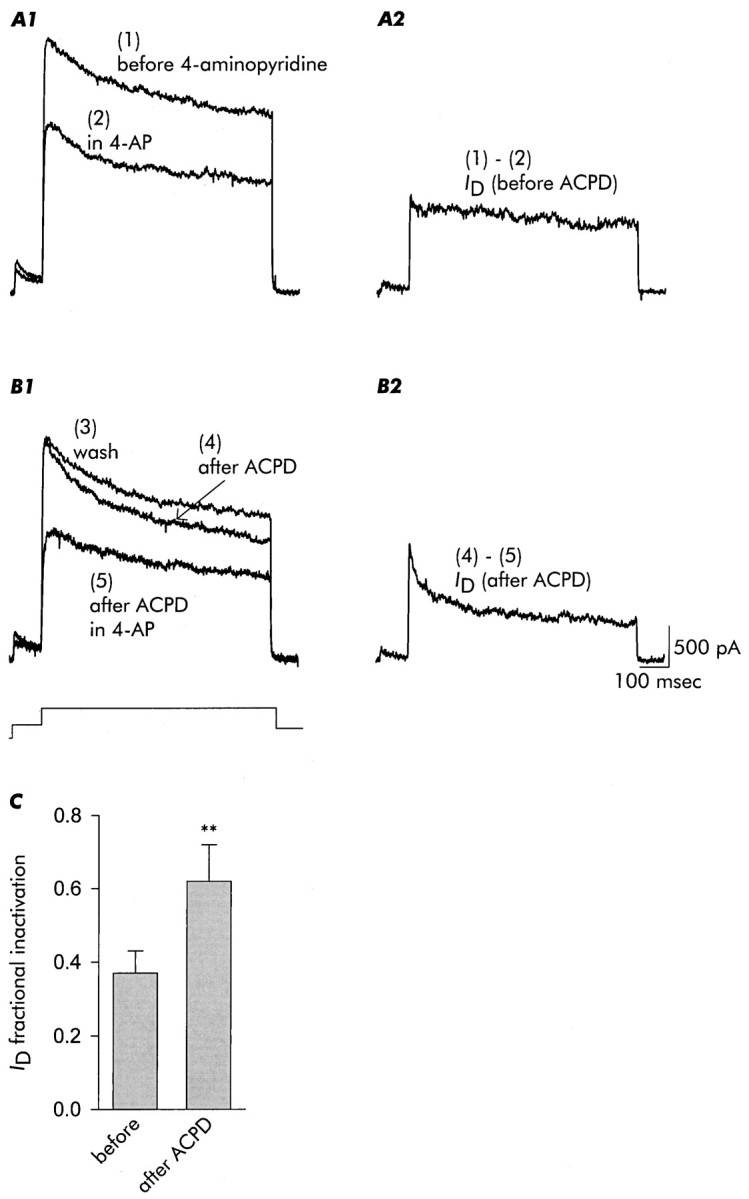
Inactivation ofID, as isolated by its sensitivity to 4-AP (100 μm), is accelerated after exposure to 1S,3R-ACPD. The five traces shown in this figure were acquired in the order indicated from the same neuron.A1, Currents recorded before (trace 1) and in the presence of 4-AP (trace 2).A2, ID isolated by subtraction as the difference between the two traces in A1. B1, After removal of 4-AP and recovery of outward current (trace 3), the neuron was exposed to 1S,3R-ACPD for 3 min as in the previous figure. After removal of 1S,3R-ACPD, inactivation of outward current was enhanced (trace 4). Outward current was then recorded in the presence of 4-AP (trace 5). B2,ID, again isolated as the 4-AP-sensitive current [trace (4) - (5)], inactivates more rapidly after exposure to 1S,3R-ACPD. C, Increase in fractional inactivation of ID, isolated by subtraction in each case, after exposure to 1S,3R-ACPD. Data are mean ± SD;n = 4.
In the experiments reported here, each test voltage-clamp step was preceded by a short prepulse to inactivateIA. Therefore the slowly relaxing potassium currents elicited by test depolarizations were composed ofID andIK and components ofIK(Ca), and we describe this mixture of currents as “delayed outward current” (delayed relative to initiation of the step depolarization) unlessID or other currents were specifically isolated.
Modulation of delayed potassium currents by 1S,3R-ACPD: enhancement and suppression of distinct components
Figure 1A1illustrates potassium currents recorded from a cultured hippocampal pyramidal neuron under control conditions. In the voltage protocol illustrated, the neuron was held at −70 mV. After a conditioning hyperpolarization to −120 mV, a prepulse to −40 mV eliminatedIA, and the currents remaining were recorded during 350-msec-long steps to voltages between −50 and +40 mV (in 10 mV increments). The pattern of currents shown is typical for cultured hippocampal pyramidal neurons.
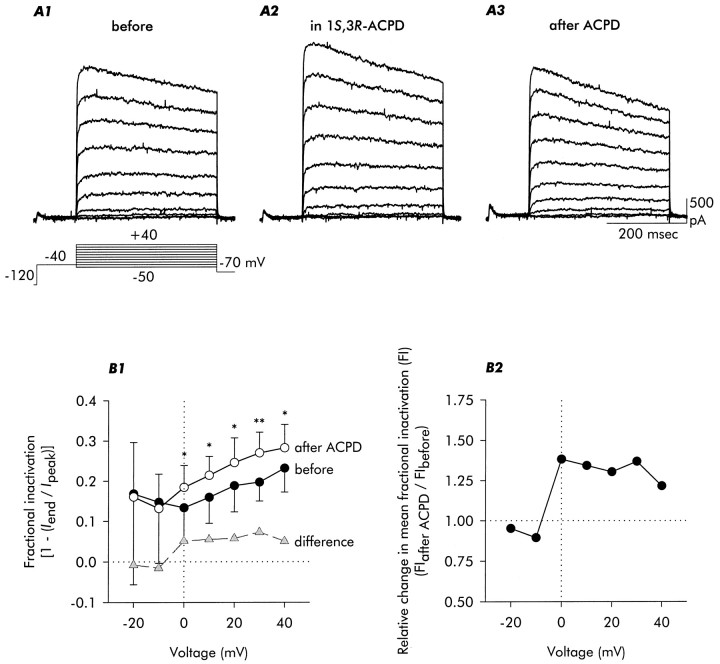
Fig. 1.
Delayed outward currents of mouse hippocampal neurons and patterns of changes observed during and after exposure to 100 μm1S,3R-ACPD. These currents demonstrate the immediate increase in current amplitude seen on application of 1S,3R-ACPD, and acceleration of delayed current inactivation observed after the exposure to agonist. Note that the changes in inactivation rate and steady-state amplitude were most evident at voltages positive to approximately −10 mV.A1, Control currents recorded under conditions that will maximize observation of delayed outward currents,ID,IK, and various forms ofIK(Ca), and minimize the contribution of IA (see Results). Currents were recorded at voltages between −50 and +40 mV (in 10 mV increments), as illustrated in the schematic. A2, Currents recorded 1 min after initiating exposure to 100 μm1S,3R-ACPD. A3, Currents recorded 10 min after termination of the 3-min-long exposure to 1S,3R-ACPD. B1, Voltage dependence of fractional inactivation of delayed outward currents [1 − (Iend/Ipeak)], illustrating the 1S,3R-ACPD-induced increase seen at voltages positive to −10 mV and the broad bell-shape of the change in fractional inactivation with voltage (difference). B2, Ratio of mean fractional inactivation for each test voltage; 1S,3R-ACPD increased mean fractional inactivation by ∼35% except at the most positive voltage. Data are mean ± SD; n = 7. Statistical significance is indicated in this and all subsequent figures: ns, not significant; *p < 0.05; **p < 0.01; and ***p < 0.001.
Application of the mGluR agonist 1S,3R-ACPD (100 μm) elicited an initial increase in outward current amplitude (Fig. 1A2) that was especially evident for peak current measured at voltages positive to approximately +20 mV. This amplitude increase was not considered in detail. After ∼1 min of exposure to the agonist, outward current began to decrease in amplitude and to inactivate more rapidly. On removal of 1S,3R-ACPD the amplitude increase reversed, whereas the increase in inactivation rate persisted (Fig.1A3). This change in inactivation rate was the major focus of this study.
We defined fractional inactivation, an index proportional to the extent of inactivation during a voltage step, as [1 − (Iend/Ipeak)], where Ipeak was the maximum current amplitude during the test depolarization andIend was the current amplitude at the end of the test pulse just before repolarization. Thus a fractional inactivation of 0 indicates no relaxation during the test depolarization, and a fractional inactivation of 1 indicates complete inactivation. As illustrated in Figure 1B1, exposure to 1S,3R-ACPD increased fractional inactivation at voltages positive to approximately −10 mV, and the magnitude of the increase (shown by the difference plot) had a broad bell-like shape. The relative increase in fractional inactivation, as defined by the ratio of fractional inactivations before and after exposure to 1S,3R-ACPD, was ∼35% at all but the most positive voltage examined (Fig.1B2).
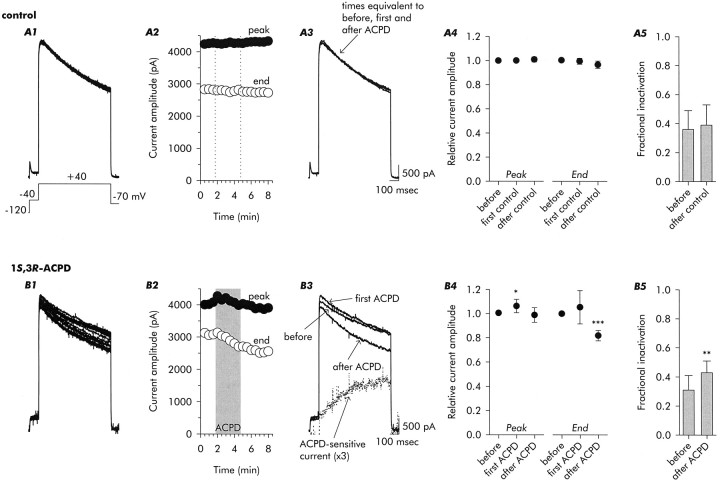
A more detailed analysis of changes in delayed outward currents is presented in Figure 2. Currents recorded under control conditions are shown in Figure 2A. In most of our analyses, measurements were made at a test voltage of +40 mV, after a conditioning hyperpolarization to −120 mV and a prepulse to −40 mV. The records of Figure 2A demonstrate stable recordings maintained during 16 test depolarizations applied at two per minute under control conditions for the 8-min-long standard test interval.
Fig. 2.
Time course of increase in delayed outward current amplitude followed by acceleration of inactivation, during and after 3-min-long exposures to 1S,3R-ACPD (100 μm). Control records in A, taken during test depolarizations to +40 mV, demonstrate the stability of currents when the standard internal solution was used (see Materials and Methods). Sixteen traces taken over an 8-min-long interval are shown inA1, and their peak and end amplitudes (end amplitude is the amplitude just before repolarization) are plotted inA2. The vertical dotted lines inA2 refer to the times at which mGluR agonist would be applied to and removed from experimental neurons, and the selected traces in A3, and the aggregate data presented inA4 and A5, are all from the times at which data were taken from experimental cells. Experimental records inB illustrate the increase in peak current amplitude seen immediately after application of 1S,3R-ACPD (compare beforeand first ACPD in B3) and the amplitude reduction and acceleration of inactivation that became evident after a few minutes and was maintained after removal of agonist (after ACPD in B3). Also shown in B3 is the waveform of the ACPD-sensitive current (computed by point-by-point subtraction as the difference between before andafter ACPD traces and multiplied by 3 for clarity), illustrating its progressive increase throughout the duration of the 800-msec-long test pulse. B4, Under normal recording conditions 1S,3R-ACPD increased peak current amplitude to ∼106% of control and reduced current amplitude at the time of repolarization to ∼83% of control. The decrease in current amplitude at the time of repolarization was reflected in an increase in fractional inactivation after exposure to 1S,3R-ACPD (B5). Data are mean ± SD; n = 9 for control,n = 6 for 1S,3R-ACPD.
The traces in Figure 2B1 illustrate the changes induced by exposure to 100 μm1S,3R-ACPD. In the standard protocol, agonist was applied for ∼3 min, added between the 3rd and 4th depolarizations and removed between the 9th and 10th. The typical pattern, which consisted of an initial increase in current amplitude followed by an acceleration of its inactivation, is evident in the traces and in the plots of peak current amplitude and current amplitude at the time of repolarization in Figure 2B2. In the selected traces shown in Figure2B3, the current to the first test depolarization (before) is compared with the first current recorded in the presence of agonist (first ACPD) and the final current recorded 3.5 min after removal of ACPD (after ACPD). Mean ± SD current amplitudes at the peak and at the time of repolarization are shown in Figure 2B4. In the aggregate, peak current amplitude was increased by ∼6% during the period that agonist was present in the external solution, and after exposure to 1S,3R-ACPD, current amplitude at the end of the test depolarization was decreased by ∼17%. We did not observe reversal of enhanced inactivation after removal of 1S,3R-ACPD during observations that lasted for up to ∼30 min.
The change in inactivation rate was manifest as an increase in fractional inactivation (Fig. 2B5). The inactivation time constant decreased from 850 msec before to 508 msec after exposure to ACPD in the example shown in Figure 2B3, which was typical.
Separation and identification of 1S,3R-ACPD-sensitive potassium currents
We did not study the conductances underlying the increase in current amplitude in detail; possible candidates include nonselective cation conductances (Crépel et al., 1994; Guérineau et al., 1995; Congar et al., 1997) and Ca2+-dependent potassium conductances (Shirasaki et al., 1994).
Our data indicate that mGluR activation accelerated inactivation of a 4-AP-sensitive current that is commonly noted asID (see introductory remarks). Of the various components of delayed outward current in hippocampal neurons,ID is differentially sensitive to micromolar concentrations of 4-AP. As illustrated in Figure3A1, subtraction of trace(2) in 4-AP from trace (1) before 4-AP yieldedID before exposure to 1S,3R-ACPD (Fig. 3A2). Then (Fig.3B1), after removal of 4-AP [trace (3) wash], application and removal of 1S,3R-ACPD resulted in accelerated inactivation of outward current [trace (4) after ACPD]. Subsequent addition of 4-AP [trace (5) after ACPD in 4-AP] and subtraction yieldedID after exposure to ACPD (Fig.3B2). At this time IDinactivation was substantially more rapid; in the aggregate, 1S,3R-ACPD increasedID fractional inactivation by ∼68%, from ∼0.37 to ∼0.62 (Fig. 3C).
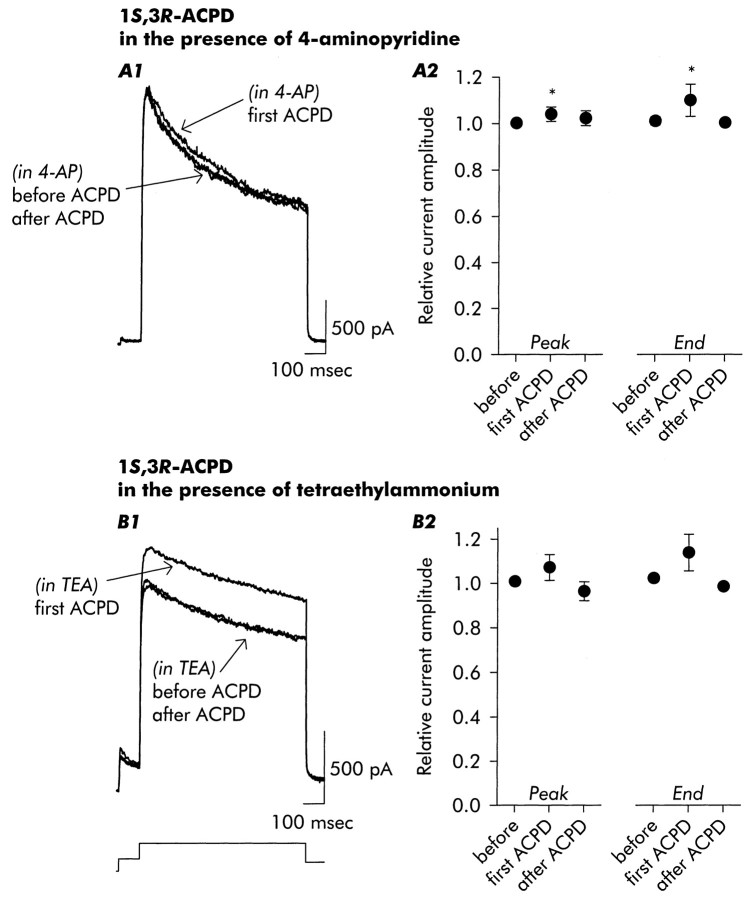
The increase in inactivation induced by mGluR activation was also analyzed using an occlusion paradigm in which we determined the ability of various agents to block effects of subsequent application of 1S,3R-ACPD. Most significantly, in the presence of 4-AP, the effects of 1S,3R-ACPD on inactivation observed after agonist removal were completely occluded (Fig. 4A), indicating that although ID was not the only current showing inactivation, acceleration of inactivation was restricted to 4-AP-sensitive current. At the same time, the increase in outward current seen in the presence of agonist was not affected (compare Fig. 4A2 with Fig.2B4).
Fig. 4.
After block ofID, 1S,3R-ACPD-induced changes in outward current inactivation were occluded. A1, A2, In the presence of 4-AP (200 μm), an increase in outward current was observed in the presence of 1S,3R-ACPD, but no acceleration of delayed current inactivation or reduction of current amplitude at the time of repolarization was observed. B1, B2, TEA (1.5 mm) also occluded 1S,3R-ACPD-induced changes in delayed current inactivation but spared the initial current increase. Data are mean ± SD; n = 5 for 4-AP,n = 3 for TEA.
Exposure to TEA (1.5 mm) also occluded the changes in inactivation rate elicited by 1S,3R-ACPD (Fig.4B). This observation is consistent with an action onID and other slowly inactivating potassium currents, which in some studies are reported to be sensitive to TEA (Ficker and Heinemann, 1992; Li and McArdle, 1997), although TEA does not preferentially block ID.
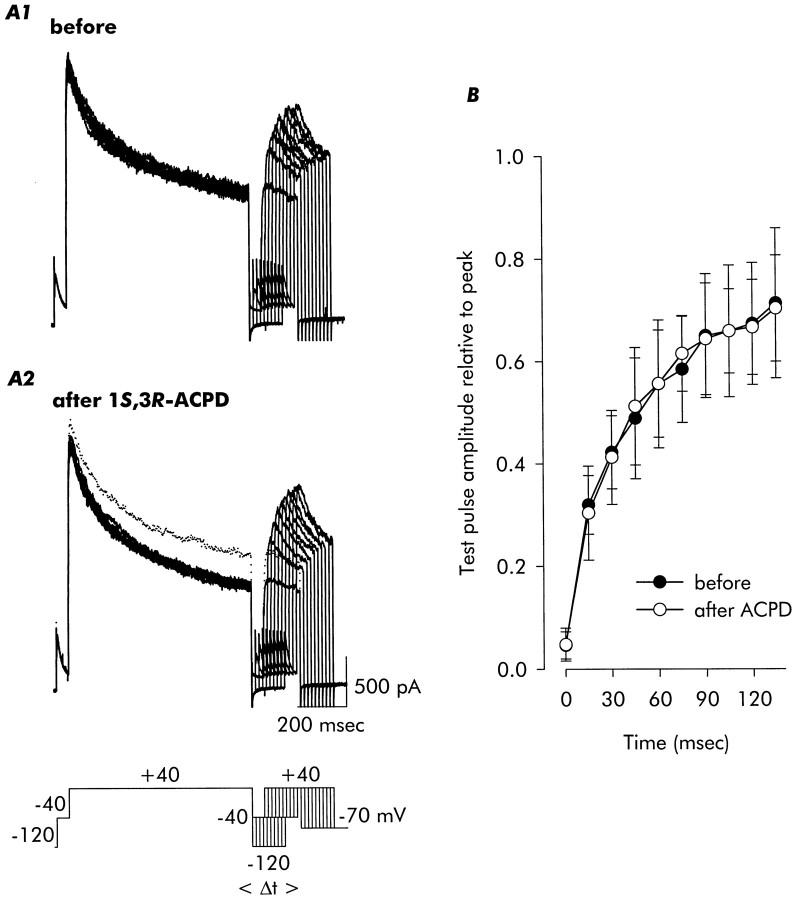
Recovery of ID from inactivation
Recovery of ID from inactivation was not affected by exposure to 1S,3R-ACPD. The records in Figure 5A show currents recorded using a two-pulse protocol, with a long depolarization to induce maximal inactivation followed by a shorter test pulse delivered at varying intervals (see legend). Despite the clear acceleration of inactivation induced by application of 1S,3R-ACPD (the dotted trace in the experimental records of Fig. 5A2 is the first control record, presented for reference), when recovery was assessed by normalizing test current amplitude to the initial peak of the initial current, its time course was not affected. As illustrated in Figure5B, the time constant of recovery was ∼82–83 msec in each case.
Fig. 5.
Recovery from inactivation, in contrast to onset of inactivation, was not affected by 1S,3R-ACPD. A two-pulse protocol was used in which an initial 750-msec-long conditioning depolarization to +40 mV (to induce inactivation) was followed, at intervals incremented by 15 msec during which the cell was held at −120 mV, by a 150-sec-long test depolarization (see pulse schematic). Although inactivation was clearly accelerated by exposure to 1S,3R-ACPD (the dotted trace in A2 is a control trace from A1 for reference), the time course of recovery from inactivation was not altered (B). Data are mean ± SD; n = 4 for control,n = 4 for 1S,3R-ACPD.
Involvement of GTP binding proteins
Metabotropic GluRs are coupled by GTP binding proteins to various second messengers, including phospholipases C and D, adenyl cyclase, and arachidonic acid. Experiments with GTP analogs indicated that acceleration of ID inactivation was a G-protein-mediated process. Inclusion of GTPγS, a nonhydrolyzable GTP analog (Gilman, 1984), in the internal solution resulted in maximal acceleration of ID inactivation (Fig.6A). Current amplitude at the time of repolarization was reduced by ∼23%, and the maximal effect was seen on the first test depolarization after application of 1S,3R-ACPD. Curiously, the increase in outward current in the presence of agonist was not evident. This could reflect masking by the large changes in ID, or maximal desensitization of the signaling pathway leading to outward current activation (Guérineau et al., 1997).
Fig. 6.

Evaluation of G-protein analogs on the actions of 1S,3R-ACPD. A1, A2, Inclusion of the nonhydrolyzable analog GTPγS (250 μm) in the internal solution resulted in almost maximal acceleration of delayed current inactivation immediately on application of 1S,3R-ACPD. B1, B2, In contrast, inclusion of the nondisplaceable analog GDPβS (500 μm) in the internal solution blocked any actions of 1S,3R-ACPD. Data are mean ± SD;n = 5 for GTPγS, n = 5 for GDPβS.
Conversely, inclusion of the nondisplaceable GDP analog GDPβS (Eckstein et al., 1979) blocked both the increase in outward current in the presence of agonist and the acceleration ofID inactivation (Fig.6B).
Effects of mGluR subtype-preferring antagonists and agonists
We performed a series of experiments comparing the actions of 1S,3R-ACPD with those of agonists preferring various mGluR subtypes. Metabotropic GluRs may be divided into groups I, II, or III on the basis of amino acid sequence and pharmacology, and additional mGluRs not conforming to this scheme have been proposed (Pin and Duvoisin, 1995; Conn and Pin, 1997; Albani-Torregrossa et al., 1998). 1S,3R-ACPD is an agonist preferring group I, group II, and a few group III mGluRs, as well as mGluRs coupled to phospholipase D (Pellegrini-Giampietro et al., 1996; Conn and Pin, 1997).
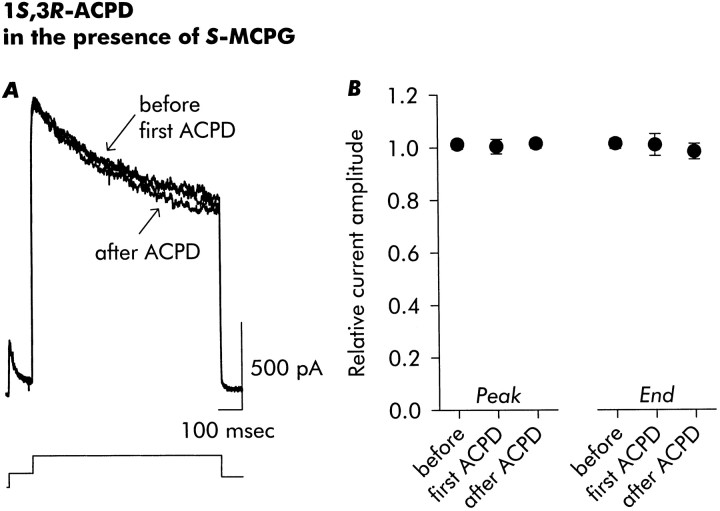
S-α-methyl-4-carboxyphenylglycine (S-MCPG) is a blocker preferring group I and group II mGluRs, is ineffective on most group III mGluRs or on ionotropic glutamate receptors, and is an agonist for phospholipase D-coupled mGluRs (Watkins and Collingridge, 1994; Pellegrini-Giampietro et al., 1996). As illustrated in Figure7, application of S-MCPG (1 mm) along with 1S,3R-ACPD virtually completely blocked both the increase in outward current in the presence of agonist and the subsequent acceleration ofID inactivation.
Fig. 7.
Effects of 1S,3R-ACPD were not observed when agonist was applied in the presence of the broadly acting mGluR antagonistS-MCPG (1 mm). The small reduction in steady-state current (after ACPD; A), although seen consistently (B), was not statistically significant. Data are mean ± SD;n = 4.
Exposure to RS-3,5-dihydroxyphenylglycine (3,5-DHPG; 100 μm), an agonist for group I mGluRs (Schoepp et al., 1994) and an antagonist for phospholipase D-coupled mGluRs (Pellegrini-Giampietro et al., 1996), failed to elicit the increase in outward current but did cause a significant decrease in steady-state current amplitude and increase in IDinactivation (Fig.8A).
Fig. 8.
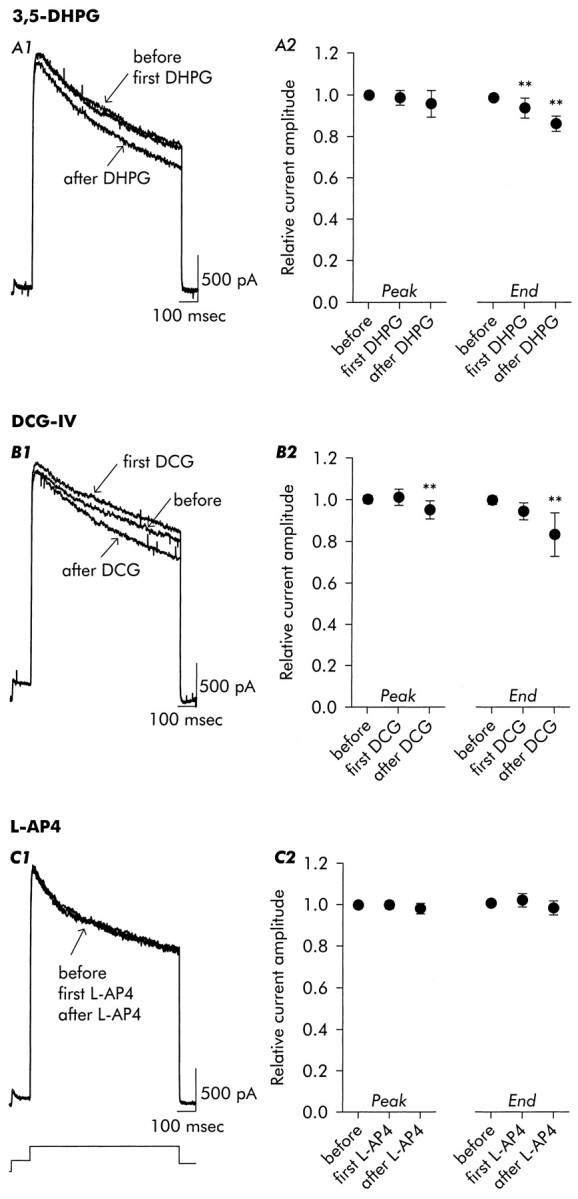
Evaluation of agonists acting preferentially on group I (3,5-DHPG), group II (DCG-IV), or group III (L-AP4) mGluRs.A1, A2, Application of 3,5-DHPG (100 μm) resulted in acceleration of inactivation in the presence of agonist and after its removal. B1, B2, Application of DCG-IV (100 μm) elicited a small increase in delayed current amplitude as well as acceleration of inactivation. In contrast, application of L-AP4 (100 μm) (C1, C2) failed to elicit change either in peak current amplitude or in inactivation rate and current amplitude at the time of repolarization. Data are mean ± SD; n = 9 for 3,5-DHPG, n = 11 for DCG-IV, n= 5 for L-AP4.
Application of the group II-preferring agonist (DCG-IV; 1–100 μm (Hayashi et al., 1993) (Fig. 8B) resulted in a small transient increase in outward current, followed by an enhancement of inactivation comparable to that seen with the group I agonist 3,5-DHPG (both showed a ∼14% reduction in steady state current amplitude).
Exposure to l(+)-2-amino-4-phosphonobutyric acid (L-AP4; 100 μm), an agonist preferring group III mGluRs and not active on phospholipase D-coupled mGluRs (Pellegrini-Giampietro et al., 1996), was without noticeable effect on delayed outward current (Fig. 8C).
1S,3R-ACPD-sensitive conductances active near the resting potential
In addition to modulation of currents activated at positive potentials, mGluR activation also reduces conductances active near the resting potential (Charpak et al., 1990; Guérineau et al., 1994). We assessed possible overlap between these conductances andID by examining the 4-AP sensitivity of currents suppressed by 1S,3R-ACPD during steps to voltages between −120 and −40 mV from the holding potential of −70 mV.
We determined slope conductance near the resting potential from the amplitudes of nonleak-subtracted steady-state currents measured between −60 and −40 mV. As shown in Figure9A, exposure to 1S,3R-ACPD reduced the slope conductance to ∼47% of control, and in most cells this decrease was reversible. 1S,3R-ACPD did not affect the slope conductance at voltages negative to −60 mV (data not shown).
Fig. 9.
Reduction of whole-cell conductance near the resting potential by 1S,3R-ACPD, and occlusion by 4-AP. A, Slope conductance, measured between −60 and −40 mV, before, during, and (3 min) after exposure to 1S,3R-ACPD. B, Occlusion of 1S,3R-ACPD-induced suppression of resting conductance by addition of 4-AP to the external solution. 4-AP reduced whole-cell conductance at voltages positive to approximately −60 mV, and no further change was seen on application of 1S,3R-ACPD. Note that neither 4-AP nor 1S,3R-ACPD induced changes in conductance at voltages negative to −60 mV. Data are mean ± SD;n = 8 for 1S,3R-ACPD,n = 3 for 1S,3R-ACPD in 4-AP.
This reduction in slope conductance was occluded by 4-AP. As illustrated in Figure 9B, 4-AP caused a clear reduction in slope conductance near the resting potential [see also Brown et al. (1990); Storm (1990)]. When 1S,3R-ACPD was applied in the presence of 4-AP, there was no further change in slope conductance, indicating that mGluR activation was affecting a 4-AP-sensitive conductance. Identification of this current asID is consistent with its properties in cultured pyramidal neurons, because the foot of theID activation curve is near −60 mV and a window of current showing partial inactivation is seen between approximately −60 and −20 mV (Ficker and Heinemann, 1992; Wu and Barish, 1992). However, these observations may also reflect modulation of a different 4-AP-sensitive current.
Effects on electrogenesis
We specifically examined three aspects of excitability linked by other investigations to ID by the actions of micromolar concentrations of 4-AP: excitability near the resting potential, latency to action potential generation during sustained depolarizations, and waveform of action potential repolarization. In these current-clamp experiments, changes in excitability reflected the pleiotropic actions of mGluR activation, and the effects of 1S,3R-ACPD on excitability followed the biphasic pattern predicted from the voltage-clamp data (initial increase of outward current followed by enhancement ofID inactivation).
Excitability
The changes in excitability elicited by 1S,3R-ACPD are illustrated in Figure10. The initial resting potential, −62 mV, is indicated by the dotted line. At each time relative to application of 1S,3R-ACPD, a series of short current injections of increasing intensity were delivered through the patch pipette. 1S,3R-ACPD caused an immediate hyperpolarization and reduction in excitability (1.5 min in ACPD), which was followed by a return to the original resting potential and an enduring reduction in excitability (2 min in ACPD). Subsequently, after removal of 1S,3R-ACPD, persistent enhancement of excitability (5.5 min after ACPD) was observed.
Fig. 10.
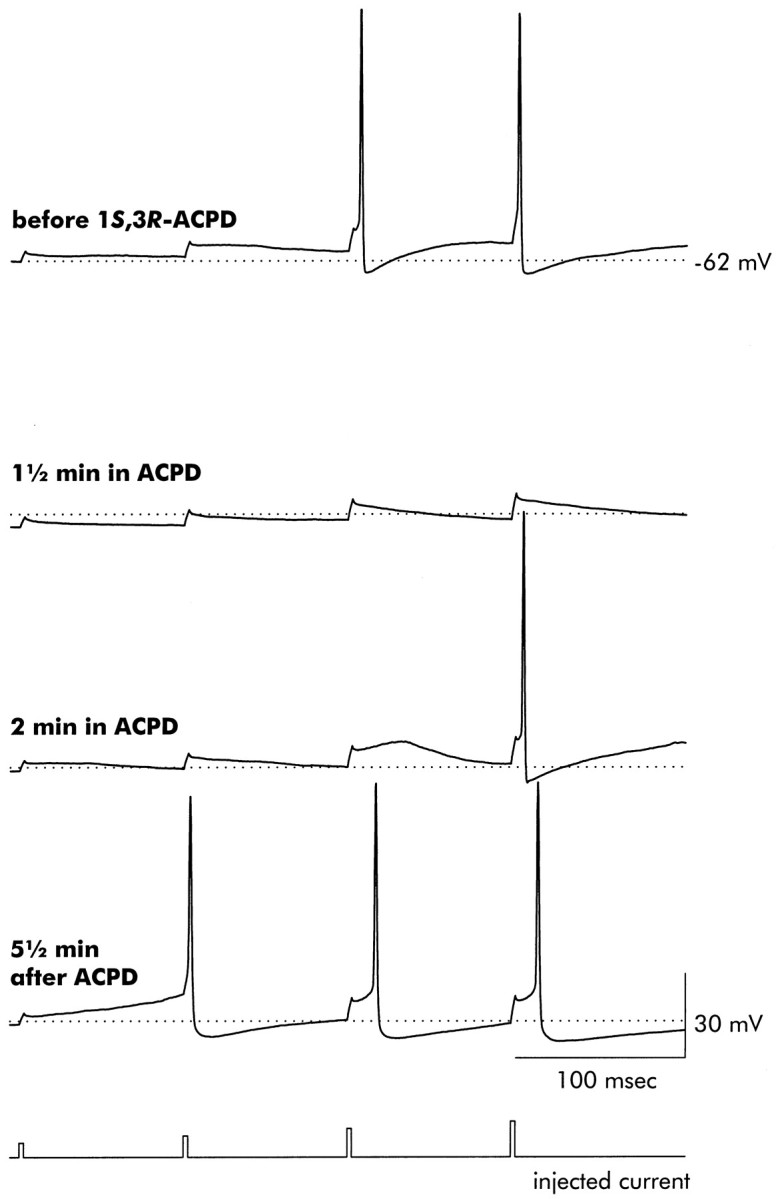
Exposure to 1S,3R-ACPD ultimately increases pyramidal neuron excitability. A series of short (duration 2.5 msec) depolarizing current injections of increasing amplitude (2, 4, 6, and 8 nA) were delivered, separated by 100 msec. The initial resting potential, −62 mV, is indicated by the dotted line. Before application of 1S,3R-ACPD, only the two largest current injections were sufficient to elicit action potentials. Exposure to 1S,3R-ACPD resulted in a transient hyperpolarization (1.5 min in ACPD), but excitability was reduced even after the return of the resting potential to near its initial value (2 min in ACPD). Note also the enhanced repolarization seen in the action potential recorded after 2 min in ACPD. In thebottom trace, recorded 5.5 min after washing off 1S,3R-ACPD, excitability was enhanced, as judged by the ability of the second current injection to elicit an action potential. The durations of these action potentials were also affected, as illustrated in Figure 12. Records are representative of six neurons that were similarly examined.
Response to sustained depolarization
A reduction in first action potential latency induced by 1S,3R-ACPD is illustrated in Figure11. In the control case, injection of positive-going current resulted in a rapid depolarization followed by a slower positive-going shift in membrane voltage that eventually reached threshold. During application of 1S,3R-ACPD, the resting potential shifted slightly positive (the original −74 mV resting potential is indicated), and the current injection evoked a single action potential followed by another after a long delay. Most significantly, after exposure to 1S,3R-ACPD was terminated, the cell returned to the original resting potential, the slow rise toward threshold was eliminated, and repetitive action potentials were generated immediately as the rapidly depolarizing membrane voltage reached threshold [also see Bashir et al. (1993)]. Note that at all three time points only minimal alterations were observed in the response to hyperpolarization (the dotted line at −80 mV marks the control response). Effects similar to those of 1S,3R-ACPD were also seen with 3,5-DHPG (100 μm; n = 3) and DCG-IV (100 μm; n = 3).
Fig. 11.
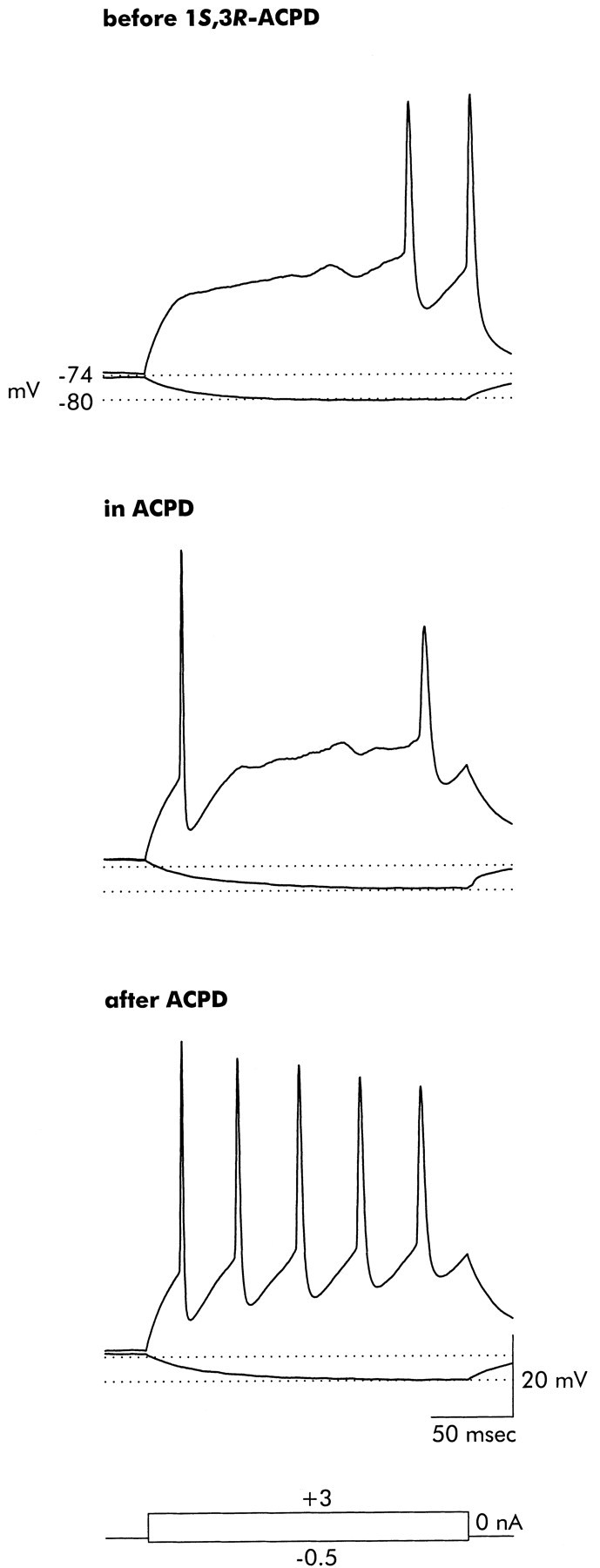
Exposure to 1S,3R-ACPD ultimately reduces the delay to onset of repetitive firing in response to sustained depolarizing current injection. Before application of 1S,3R-ACPD, a depolarizing current injection rapidly shifted voltage from the resting potential (−74 mV, indicated by the dotted line) to a slowly rising plateau from which an action potential was eventually generated. In the presence of 1S,3R-ACPD, a small positive shift in the resting potential resulted in initial generation of an action potential during the rapid depolarizing phase, but there was a long delay until the next action potential. After removal of ACPD (2 min wash) the delay to firing was completely eliminated, and the neuron fired steadily during the depolarizing current injection. Note that the response to the hyperpolarizing current injection (−80 mV, indicated by the second dotted line) was not altered during or after exposure to 1S,3R-ACPD. Records are representative of seven neurons that were similarly examined.
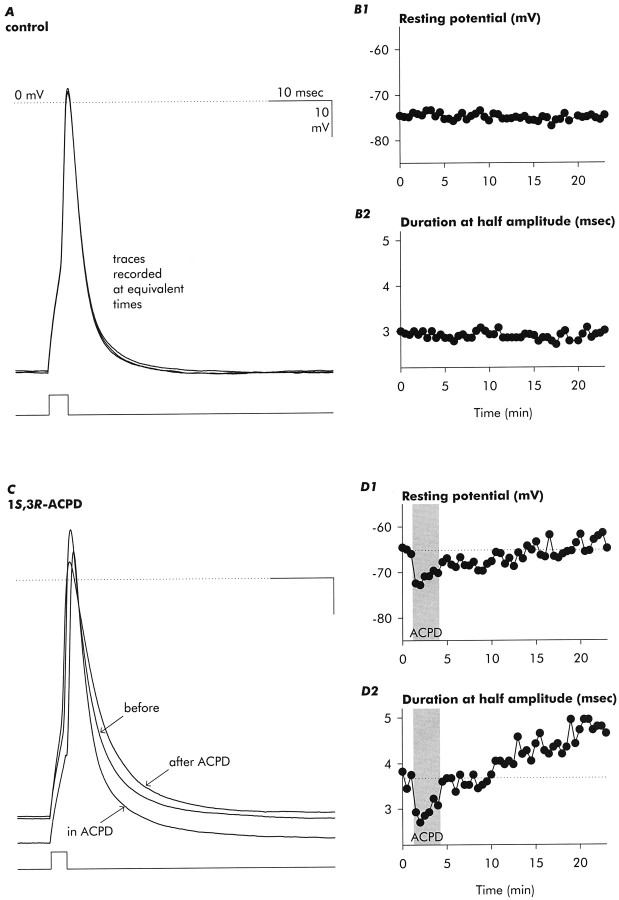
Action potential waveform
Individual action potentials showed the pattern of change illustrated in Figure 12: initial acceleration of repolarization followed by a sustained increase in duration. Shown in Figure 12C are superimposed action potentials by elicited current injections before, during, and after exposure to 1S,3R-ACPD. These action potentials illustrate that in the presence of ACPD the resting potential shifted negatively (a more typical finding than the positive shift shown in the previous figure) and that action potential repolarization was accelerated. Shortly after removal of 1S,3R-ACPD, the resting potential returned to control levels, and yet action potential repolarization was prolonged [also see Hu and Storm (1991)]. The progressive increase in action potential duration after removal of 1S,3R-ACPD, despite stabilization of the resting potential near its initial level, is shown graphically in Figure 12D. After ∼20 min, action potential duration (measured at half amplitude) increased by ∼27%, from ∼3.75 to ∼4.75 msec. In the aggregate, 1S,3R-ACPD increased action potential duration at half amplitude by 31.0 ± 8.4%, from 2.3 ± 1.0 msec to 3.1 ± 1.4 msec (mean ± SD, p = < 0.05;n = 4). No changes in these parameters were observed in control recordings (Fig. 12A,B).
Fig. 12.
Exposure to 1S,3R-ACPD ultimately increases action potential duration. A, Action potentials recorded in response to short (duration 2.5 msec) depolarizing current injections at times equivalent to those presented for the experimental neuron inC. In control neurons action potential waveforms remained stable, and neither resting potential (B1) nor action potential duration (B2) showed spontaneous changes. C, Action potentials recorded before, during, and after exposure to 1S,3R-ACPD, illustrating initial enhancement of repolarization, followed by increase in action potential duration (the trace shown was recorded 18 min after removal of agonist). A transient negative shift in the resting potential was followed by eventual recovery (D1), whereas action potential duration (measured at half amplitude) showed an initial decrease followed by progressive and sustained increase after wash off of 1S,3R-ACPD (D2). Records are representative of three control and four 1S,3R-ACPD-exposed neurons.
Applications of 4-AP elicited the responses expected for block ofID (increased excitability, reduction of latency to first action potential, delayed action potential repolarization). However, parameters of interest changed continuously and progressively over the duration of our measurements and did not reach a stable steady state from which to test occlusion of the actions of 1S,3R-ACPD.
DISCUSSION
In the experiments presented here, we have shown that activation of mGluR in cultured hippocampal pyramidal neurons by 1S,3R-ACPD results in a suppression ofID that persists after agonist removal. ID was isolated on the basis of its sensitivity to 100–200 μm 4-AP and in isolation showed accelerated inactivation after exposure to 1S,3R-ACPD. Furthermore, previous block ofID by 4-AP precluded subsequent effects of mGluR activation, indicating that additional 4-AP-insensitive conductances were not involved.
Application of 4-AP also occluded a reduction in resting conductance induced by 1S,3R-ACPD. This component of whole-cell resting conductance may be a manifestation of tonicID activation in a voltage region in which its activation and inactivation curves overlap (a “window current”), or an indication of another 4-AP-sensitive current, or both.
We further demonstrated that effects on excitability attributed toID by their sensitivity to 4-AP—increased excitability to short depolarizations, reduced latency to first action potential during sustained depolarizations, and increased action potential duration—are all also produced by exposure to 1S,3R-ACPD.
ID is a novel target for mGluR-initiated signaling
Although there are a number of other reports of potassium current modulation in hippocampal neurons by mGluR activation (Gerber and Gähwiler, 1994), in most cases the currents affected,IAHP,IM, andIK(slow), do not appear to overlap with ID as described here. An inhibition of IAHP andIM after mGluR activation by quisqualate was described by Charpak et al. (1990), but neitherIAHP norIM is blocked by 4-AP or TEA (Brown and Adams, 1980; Lancaster and Adams, 1986), both of which affectID, and the kinetics ofIM is quite different from that of ID (Brown and Adams, 1980). Somewhat closer is the reduction inIK(slow) by 1S,3R-ACPD described by Lüthi et al. (1996), but this study separated slow outward currents in rat hippocampal neurons into a 4-AP-sensitive but 1S,3R-ACPD-resistant ID(with inactivation kinetics similar to theID considered here), and a much more slowly inactivating 4-AP-resistantIK(slow) that was sensitive to 1S,3R-ACPD. These observations are reminiscent of those presented here, and the source of the differences is not obvious.Lüthi et al. (1996) studied rat hippocampal neurons in organotypic slice cultures, but differences between rat and mouse are not likely to be relevant because the properties ofID of pyramidal neurons from the two species grown in dissociated cell cultures are similar [compare Ficker and Heinemann (1992) for rat with Wu and Barish (1992) for mouse].
A possibly related observation from a study on neostriatal neurons was reported by Nisenbaum et al. (1996), who observed enhanced inactivation of outward current when GTP and KF were present in the internal solution. This effect was not seen when GTPβS was substituted for GTP, and these authors concluded that inactivation was accelerated as a consequence of G-protein stimulation. One component of outward current in neostriatal neurons isIAs, a slowly inactivating potassium current very similar to ID(Surmeier et al., 1991, 1994; Gabel and Nisenbaum, 1998). This current could be subject to G-protein-mediated regulation.
1S,3R-ACPD-induced reduction in conductance at voltages near or just positive to the resting potential, as described by Guérineau et al. (1994), who termed the current affectedIK,leak, and by Lüthi et al. (1997), does appear similar to the inhibition of resting conductance seen here. Shared properties include induction by 1S,3R-ACPD, block by S-MCPG, enhancement by GTPγS, and block by GDPβS. Neither Guérineau et al. (1994) nor Lüthi et al. (1997) examined the 4-AP sensitivity of their currents.
Mechanisms linking specific mGluR to particular potassium channel subunits
There is no clear answer to the question of which mGluR subtypes, and by implication second messenger systems, may be linked to modulation of ID. The group I-preferring agonist 3,5-DHPG (Schoepp et al., 1994) and the group II-preferring agonist DCG-IV (Hayashi et al., 1993) both enhancedID inactivation. This pharmacological profile was unexpected because of the divergent signaling characteristics of group I and group II mGluRs, but interactions between phosphoinositide- and cAMP-linked intracellular signaling pathways have been described (Gereau and Conn, 1994; Gereau et al., 1995; Nouranifar et al., 1998), and delayed potassium currents may be regulated by cAMP-sensitive phosphorylation reactions (Mu et al., 1997). An additional possibility is mGluR activation of phospholipase D in hippocampal neurons (Boss and Conn, 1992; Holler et al., 1993), which may be stimulated by both 3,5-DHPG and DCG-IV but is mediated by group I mGluRs (Klein et al., 1997) or, alternatively, by a novel mGluR subtype (Pellegrini-Giampietro et al., 1996).
A related issue is the molecular identities of the targets of mGluR modulation. Multiple cloned potassium channel subunits are sensitive to low concentrations of 4-AP (Grissmer et al., 1994), and one or more of these subunits may contribute to the channels carryingID in pyramidal neurons and be a target of the second messenger system(s) stimulated by mGluR activation. However, it is not clear at this time which of these subunits are actually involved.
Both of these issues will be investigated further.
Significance of ID modulation
The distinguishing property of IDis high sensitivity to 4-AP (see references herein). In his initial description of ID in CA1 pyramidal neurons, Storm (1988a) used 4-AP to perturbID and determined that the current influenced the time to first action potential generation during long depolarizing current injections (see introductory remarks). Subsequent investigations, also using 4-AP, have assigned it an additional role in repolarization of pyramidal neuron action potentials (see introductory remarks). Thus activation of ID will tend to both retard approach of the action potential to threshold and hasten repolarization of the action potential once it has been generated. Its importance in regulating excitability is demonstrated by the potent convulsant activity of 4-AP when used at concentrations selective for inhibition of ID (see introductory remarks). Because mGluR activation and 4-AP appear to target the same potassium current, one may predict that the more moderate inhibition of ID seen with mGluR activation could affect, in a less catastrophic manner, the same processes altered by 4-AP.
What might these changes be? First, reduction inID at voltages just positive to the resting potential will amplify any excitatory input, as has been seen after mGluR activation (Desai and Conn, 1991), and this inhibition could contribute to EPSP-spike potentiation (Breakwell et al., 1996). Reduction in ID near the resting potential could also contribute to the slow conductance decrease EPSP elicited by mGluR activation in hippocampal neurons (Charpak and Gähwiler, 1991; Gerber et al., 1993). Second, because suppression of ID reduces the delay to action potential firing, excitability during long trains of excitatory input may be increased. Third, because changes in the action potential waveform will affect Ca2+ entry (McCobb and Beam, 1991; Scroggs and Fox, 1992; Wheeler et al., 1996; Sabatini and Regehr, 1997), modulation of IDmay influence Ca2+-dependent neurotransmitter release (see introductory remarks). All of these actions of mGluR on ID could contribute to, among other things, the facilitating actions of mGluR activation on long-term potentiation (LTP) induction (Bashir et al., 1993; Cohen and Abraham, 1996), EPSP-spike potentiation associated with mGluR activation (Breakwell et al., 1996), and mGluR-induced slow-onset LTP (Bortolotto and Collingridge, 1992, 1993; Chinestra et al., 1994; Manahan-Vaughan and Reymann, 1995).
Footnotes
This work was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke Grant R01 NS23857. We thank M. Jill Flanagan for her assistance with preparation of this manuscript.
Correspondence should be addressed to Dr. Michael E. Barish, Division of Neurosciences, Beckman Research Institute of the City of Hope, Duarte, CA 91010.
REFERENCES
- 1.Albani-Torregrossa S, Pellicciari R, Moroni F, Pellegrini-Giampietro DE. Metabotropic glutamate receptors coupled to phospholipase D. In: Moroni F, Nicoletti F, Pellegrini-Giampietro DE, editors. Metabotropic glutamate receptors and brain function. Portland Press; London: 1998. pp. 251–260. [Google Scholar]
- 2.Albert JL, Nerbonne JM. Calcium-independent depolarization-activated potassium currents in superior colliculus-projecting rat visual cortical neurons. J Neurophysiol. 1995;73:2163–2178. doi: 10.1152/jn.1995.73.6.2163. [DOI] [PubMed] [Google Scholar]
- 3.Barish ME, Ichikawa M, Tominaga T, Matsumoto G, Iijima T. Enhanced fast synaptic transmission and a delayed depolarization induced by transient potassium current blockade in rat hippocampal slice as studied by optical recording. J Neurosci. 1996;16:5672–5687. doi: 10.1523/JNEUROSCI.16-18-05672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- 5.Baskys A. Metabotropic receptors and “slow” excitatory actions of glutamate agonists in the hippocampus. Trends Neurosci. 1992;15:92–96. doi: 10.1016/0166-2236(92)90018-4. [DOI] [PubMed] [Google Scholar]
- 6.Beck H, Clusmann H, Kral T, Schramm J, Heinemann U, Elger CE. Potassium currents in acutely isolated human hippocampal dentate granule cells. J Physiol (Lond) 1997;498:73–85. doi: 10.1113/jphysiol.1997.sp021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bortolotto ZA, Collingridge GL. Activation of glutamate metabotropic receptors induces long-term potentiation. Eur J Pharmacol. 1992;214:297–298. doi: 10.1016/0014-2999(92)90135-q. [DOI] [PubMed] [Google Scholar]
- 8.Bortolotto ZA, Collingridge GL. Characterisation of LTP induced by the activation of glutamate metabotropic receptors in area CA1 of the hippocampus. Neuropharmacology. 1993;32:1–9. doi: 10.1016/0028-3908(93)90123-k. [DOI] [PubMed] [Google Scholar]
- 9.Boss V, Conn PJ. Metabotropic excitatory amino acid receptor activation stimulates phospholipase D in hippocampal slices. J Neurochem. 1992;59:2340–2343. doi: 10.1111/j.1471-4159.1992.tb10131.x. [DOI] [PubMed] [Google Scholar]
- 10.Bossu JL, Capogna M, Debanne D, McKinney RA, Gähwiler BH. Somatic voltage-gated potassium currents of rat hippocampal pyramidal cells in organotypic slice cultures. J Physiol (Lond) 1996;495:367–381. doi: 10.1113/jphysiol.1996.sp021600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breakwell NA, Rowan MJ, Anwyl R. Metabotropic glutamate receptor dependent EPSP and EPSP-spike potentiation in area CA1 of the submerged rat hippocampal slice. J Neurophysiol. 1996;76:3126–3135. doi: 10.1152/jn.1996.76.5.3126. [DOI] [PubMed] [Google Scholar]
- 12.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal(TM), a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 13.Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- 14.Brown DA, Gähwiler BH, Griffith WH, Halliwell JV. Membrane currents in hippocampal neurons. Prog Brain Res. 1990;83:141–160. doi: 10.1016/s0079-6123(08)61247-9. [DOI] [PubMed] [Google Scholar]
- 15.Buckle PJ, Haas HL. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. J Physiol (Lond) 1982;326:101–122. doi: 10.1113/jphysiol.1982.sp014180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charpak S, Gähwiler BH. Glutamate mediates a slow synaptic response in hippocampal slice cultures. Proc R Soc Lond B Biol Sci. 1991;243:221–226. doi: 10.1098/rspb.1991.0035. [DOI] [PubMed] [Google Scholar]
- 17.Charpak S, Gähwiler BH, Do KQ, Knöpfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990;347:765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- 18.Chinestra P, Diabira D, Urban NN, Barrionuevo G, Ben-Ari Y. Major differences between long-term potentiation and ACPD-induced slow onset potentiation in hippocampus. Neurosci Lett. 1994;182:177–180. doi: 10.1016/0304-3940(94)90791-9. [DOI] [PubMed] [Google Scholar]
- 19.Cohen AS, Abraham WC. Facilitation of long-term potentiation by prior activation of metabotropic glutamate receptors. J Neurophysiol. 1996;76:953–962. doi: 10.1152/jn.1996.76.2.953. [DOI] [PubMed] [Google Scholar]
- 20.Congar P, Leinekugel X, Ben-Ari Y, Crépel V. A long-lasting calcium-activated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J Neurosci. 1997;17:5366–5379. doi: 10.1523/JNEUROSCI.17-14-05366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 22.Crépel V, Aniksztejn L, Ben-Ari Y, Hammond C. Glutamate metabotropic receptors increase a Ca2+-activated nonspecific cationic current in CA1 hippocampal neurons. J Neurophysiol. 1994;72:1561–1569. doi: 10.1152/jn.1994.72.4.1561. [DOI] [PubMed] [Google Scholar]
- 23.Desai MA, Conn PJ. Excitatory effects of ACPD receptor activation in the hippocampus are mediated by direct effects on pyramidal cells and blockade of synaptic inhibition. J Neurophysiol. 1991;66:40–52. doi: 10.1152/jn.1991.66.1.40. [DOI] [PubMed] [Google Scholar]
- 24.Eckstein F, Cassel D, Levkovitz H, Lowe M, Selinger Z. Guanosine 5′-O(2-thiodiphosphate): an inhibitor of adenylate cyclase stimulated by guanine nucleotide and fluoride ions. J Biol Chem. 1979;254:9829–9834. [PubMed] [Google Scholar]
- 25.Ficker E, Heinemann U. Slow and fast transient potassium currents in cultured rat hippocampal cells. J Physiol (Lond) 1992;445:431–455. doi: 10.1113/jphysiol.1992.sp018932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabel LA, Nisenbaum ES. Biophysical characterization and functional consequences of a slowly inactivating potassium current in neostriatal neurons. J Neurophysiol. 1998;79:1989–2002. doi: 10.1152/jn.1998.79.4.1989. [DOI] [PubMed] [Google Scholar]
- 27.Gerber U, Gähwiler BH. Modulation of ionic currents by metabotropic glutamate receptors in the CNS. In: Conn PJ, Patel J, editors. The metabotropic glutamate receptors. Humana; Totowa, NJ: 1994. pp. 125–146. [Google Scholar]
- 28.Gerber U, Lüthi A, Gähwiler BH. Inhibition of a slow response by a metabotropic glutamate receptor agonist in hippocampal CA3 pyramidal cells. Proc R Soc Lond B Biol Sci. 1993;254:169–172. doi: 10.1098/rspb.1993.0142. [DOI] [PubMed] [Google Scholar]
- 29.Gereau RW, IV, Conn PJ. A cyclic AMP-dependent form of associative synaptic plasticity induced by coactivation of β-adrenergic receptors and metabotropic glutamate receptors in rat hippocampus. J Neurosci. 1994;14:3310–3318. doi: 10.1523/JNEUROSCI.14-05-03310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gereau RW, IV, Winder DG, Conn PJ. Pharmacological differentiation of the effects of co-activation of β-adrenergic and metabotropic glutamate receptors in rat hippocampus. Neurosci Lett. 1995;186:119–122. doi: 10.1016/0304-3940(95)11300-l. [DOI] [PubMed] [Google Scholar]
- 31.Gilman AG. G-proteins and dual control of adenylate cyclase. Cell. 1984;36:577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- 32.Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 33.Guérineau NC, Gähwiler BH, Gerber U. Reduction of resting K+ current by metabotropic glutamate and muscarinic receptors in rat CA3 cells: mediation by G-proteins. J Physiol (Lond) 1994;474:27–33. doi: 10.1113/jphysiol.1994.sp019999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guérineau NC, Bossu JL, Gähwiler BH, Gerber U. Activation of a nonselective cationic conductance by metabotropic glutamatergic and muscarinic agonists in CA3 pyramidal neurons of the rat hippocampus. J Neurosci. 1995;15:4395–4407. doi: 10.1523/JNEUROSCI.15-06-04395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guérineau NC, Bossu JL, Gähwiler BH, Gerber U. G-protein-mediated desensitization of metabotropic glutamatergic and muscarinic responses in CA3 cells in rat hippocampus. J Physiol (Lond) 1997;500:487–496. doi: 10.1113/jphysiol.1997.sp022035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas HL, Wieser HG, Yasargil MG. 4-Aminopyridine and the fiber potentials in rat and human hippocampal slices. Experientia. 1983;39:114–115. doi: 10.1007/BF01960661. [DOI] [PubMed] [Google Scholar]
- 37.Hammond C, Crepel F. Evidence of a slowly inactivating K+ current in prefrontal cortical cells. Eur J Neurosci. 1992;4:1087–1092. doi: 10.1111/j.1460-9568.1992.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi Y, Momiyama A, Takahashi T, Ohishi H, Ogawa-Meguro R, Shigemoto R, Mizuno N, Nakanishi S. Role of a metabotropic glutamate receptor in synaptic modulation in the accessory olfactory bulb. Nature. 1993;366:687–690. doi: 10.1038/366687a0. [DOI] [PubMed] [Google Scholar]
- 39.Holler T, Cappel E, Klein J, Löffelholz K. Glutamate activates phospholipase D in hippocampal slices of newborn and adult rats. J Neurochem. 1993;61:1569–1572. doi: 10.1111/j.1471-4159.1993.tb13659.x. [DOI] [PubMed] [Google Scholar]
- 40.Hu G-Y, Storm JF. Excitatory amino acids acting on metabotropic glutamate receptors broaden the action potential in hippocampal neurons. Brain Res. 1991;568:339–344. doi: 10.1016/0006-8993(91)91423-x. [DOI] [PubMed] [Google Scholar]
- 41.Klein J, Iovino M, Vakil M, Shinozaki H, Löffelholz K. Ontogenetic and pharmacological studies on metabotropic glutamate receptors coupled to phospholipase D activation. Neuropharmacology. 1997;36:305–311. doi: 10.1016/s0028-3908(97)00024-5. [DOI] [PubMed] [Google Scholar]
- 42.Kuhnt U, Grinvald A. 4-AP induced presynaptic changes in the hippocampal slice as measured by optical recording and voltage sensitive optical probes. Pflügers Arch [Suppl] 1982;394:R45. [Google Scholar]
- 43.Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- 44.Lancaster B, Nicoll RA, Perkel DJ. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. J Neurosci. 1991;11:23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lester RAJ, Jahr CE. Quisqualate receptor-mediated depression of calcium currents in hippocampal neurons. Neuron. 1990;4:741–749. doi: 10.1016/0896-6273(90)90200-y. [DOI] [PubMed] [Google Scholar]
- 46.Li X-Y, McArdle JJ. Novel transient outward K+ current of mature murine hippocampal neurones. Pflügers Arch. 1997;434:195–202. doi: 10.1007/s004240050383. [DOI] [PubMed] [Google Scholar]
- 47.Llinás R, Walton K, Bohr V. Synaptic transmission in squid giant synapse after potassium conductance blockage with external 3- and 4-aminopyridine. Biophys J. 1976;16:83–86. doi: 10.1016/S0006-3495(76)85664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locke RE, Nerbonne JM. Three kinetically distinct Ca2+-independent depolarization-activated K+ currents in callosal-projecting rat visual cortical neurons. J Neurophysiol. 1997a;78:2309–2320. doi: 10.1152/jn.1997.78.5.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Locke RE, Nerbonne JM. Role of voltage-gated K+ currents in mediating the regular-spiking phenotype of callosal-projecting rat visual cortical neurons. J Neurophysiol. 1997b;78:2321–2335. doi: 10.1152/jn.1997.78.5.2321. [DOI] [PubMed] [Google Scholar]
- 50.Lüthi A, Gähwiler BH, Gerber U. A slowly inactivating potassium current in CA3 pyramidal cells of rat hippocampus in vitro. J Neurosci. 1996;16:586–594. doi: 10.1523/JNEUROSCI.16-02-00586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lüthi A, Gähwiler BH, Gerber U. 1S,3R-ACPD induces a region of negative slope conductance in the steady-state current-voltage relationship of hippocampal pyramidal cells. J Neurophysiol. 1997;77:221–228. doi: 10.1152/jn.1997.77.1.221. [DOI] [PubMed] [Google Scholar]
- 52.Manahan-Vaughan D, Reymann KG. 1S,3R-ACPD dose dependently induces a slow onset potentiation in the dentate gyrus in vivo. Eur J Pharmacol. 1995;294:497–503. doi: 10.1016/0014-2999(95)00579-x. [DOI] [PubMed] [Google Scholar]
- 53.McCobb DP, Beam KG. Action potential waveform voltage-clamp commands reveal striking differences in calcium entry via low and high voltage-activated calcium channels. Neuron. 1991;7:119–127. doi: 10.1016/0896-6273(91)90080-j. [DOI] [PubMed] [Google Scholar]
- 54.McCormick DA. Functional properties of a slowly inactivating potassium current in guinea pig dorsal lateral geniculate relay neurons. J Neurophysiol. 1991;66:1176–1189. doi: 10.1152/jn.1991.66.4.1176. [DOI] [PubMed] [Google Scholar]
- 55.Mu J, Zhuang S-Y, Kirby MT, Hampson RE, Deadwyler SA. Cannabinoid receptors have differential effects on potassium D-current and A-current channels, but are linked via the same cAMP-dependent processes. Soc Neurosci Abstr. 1997;23:1480. [Google Scholar]
- 56.Nisenbaum ES, Wilson CJ, Foehring RC, Surmeier DJ. Isolation and characterization of a persistent potassium current in neostriatal neurons. J Neurophysiol. 1996;76:1180–1194. doi: 10.1152/jn.1996.76.2.1180. [DOI] [PubMed] [Google Scholar]
- 57.Nouranifar R, Blitzer RD, Wong T, Landau E. Metabotropic glutamate receptors limit adenyl cyclase-mediated effects in rat hippocampus via protein kinase C. Neurosci Lett. 1998;244:101–105. doi: 10.1016/s0304-3940(98)00131-1. [DOI] [PubMed] [Google Scholar]
- 58.Obaid AL, Salzberg BM. Micromolar 4-aminopyridine enhances invasion of a vertebrate neurosecretory terminal arborization: optical recording of action potential propagation using an ultrafast photodiode-MOSFET camera and a photodiode array. J Gen Physiol. 1996;107:353–368. doi: 10.1085/jgp.107.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pellegrini-Giampietro DE, Torregrossa SA, Moroni F. Pharmacological characterization of metabotropic glutamate receptors coupled to phospholipase D in the rat hippocampus. Br J Pharmacol. 1996;118:1035–1043. doi: 10.1111/j.1476-5381.1996.tb15503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perreault P, Avoli M. Effects of low concentrations of 4-aminopyridine on CA1 pyramidal cells of the hippocampus. J Neurophysiol. 1989;61:953–970. doi: 10.1152/jn.1989.61.5.953. [DOI] [PubMed] [Google Scholar]
- 61.Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- 62.Perreault P, Avoli M. 4-aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci. 1992;12:104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pin J-P, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 64.Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 65.Rutecki PA, Lebeda FJ, Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 1987;57:1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- 66.Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci. 1997;17:3425–3435. doi: 10.1523/JNEUROSCI.17-10-03425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sahara Y, Westbrook GL. Modulation of calcium currents by a metabotropic glutamate receptor involves fast and slow kinetic components in cultured hippocampal neurons. J Neurosci. 1993;13:3041–3050. doi: 10.1523/JNEUROSCI.13-07-03041.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schoepp DD, Goldsworthy J, Johnson BG, Salhoff CR, Baker SR. 3,5-Dihydroxyphenylglycine is a highly selective agonist for phosphoinositide-linked metabotropic glutamate receptors in the rat hippocampus. J Neurochem. 1994;63:769–772. doi: 10.1046/j.1471-4159.1994.63020769.x. [DOI] [PubMed] [Google Scholar]
- 69.Scroggs RS, Fox AP. Multiple Ca2+ currents elicited by action potential waveforms in acutely isolated adult rat dorsal root ganglion neurons. J Neurosci. 1992;12:1789–1801. doi: 10.1523/JNEUROSCI.12-05-01789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shirasaki T, Harata N, Akaike N. Metabotropic glutamate response in acutely dissociated hippocampal CA1 pyramidal neurones of the rat. J Physiol (Lond) 1994;475:439–453. doi: 10.1113/jphysiol.1994.sp020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stefani A, Pisani A, Bonci A, Stratta F, Bernardi G. Outward potassium currents activated by depolarization in rat globus pallidus. Synapse. 1995;20:131–136. doi: 10.1002/syn.890200206. [DOI] [PubMed] [Google Scholar]
- 72.Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol (Lond) 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988a;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- 74.Storm JF. Evidence that two 4-aminopyridine (4-AP)-sensitive potassium currents, IA and ID, contribute to spike repolarization in rat hippocampal pyramidal neurons. Eur J Neurosci [Suppl] 1988b;1:42. [Google Scholar]
- 75.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- 76.Stratton KR, Worley PF, Baraban JM. Excitation of hippocampal neurons by stimulation of glutamate Qp receptors. Eur J Pharmacol. 1989;173:235–237. doi: 10.1016/0014-2999(89)90529-3. [DOI] [PubMed] [Google Scholar]
- 77.Surmeier DJ, Stefani A, Foehring RC, Kitai ST. Developmental regulation of a slowly-inactivating potassium conductance in rat neostriatal neurons. Neurosci Lett. 1991;122:41–46. doi: 10.1016/0304-3940(91)90188-y. [DOI] [PubMed] [Google Scholar]
- 78.Surmeier DJ, Wilson CJ, Eberwine J. Patch-clamp techniques for studying potassium currents in mammalian brain neurons. Methods Neurosci. 1994;19:39–67. [Google Scholar]
- 79.Swartz KJ, Bean BP. Inhibition of calcium channels in rat CA3 pyramidal neurons by a metabotropic glutamate receptor. J Neurosci. 1992;12:4358–4371. doi: 10.1523/JNEUROSCI.12-11-04358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szente M, Baranyi A. Mechanism of aminopyridine-induced ictal seizure activity in the cat neocortex. Brain Res. 1987;413:368–373. doi: 10.1016/0006-8993(87)91031-6. [DOI] [PubMed] [Google Scholar]
- 81.Thesleff S. Aminopyridines and synaptic transmission. Neuroscience. 1980;5:1413–1419. doi: 10.1016/0306-4522(80)90002-0. [DOI] [PubMed] [Google Scholar]
- 82.Velumian AA, Zhang L, Pennefather P, Carlen PL. Reversible inhibition of IK, IAHP, Ih and ICa currents by internally applied gluconate in rat hippocampal pyramidal neurones. Pflügers Arch. 1997;433:343–350. doi: 10.1007/s004240050286. [DOI] [PubMed] [Google Scholar]
- 83.Watkins J, Collingridge G. Phenylglycine derivatives as antagonists of metabotropic glutamate receptors. Trends Pharmacol Sci. 1994;15:333–342. doi: 10.1016/0165-6147(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 84.Wheeler DB, Randall A, Tsien RW. Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus. J Neurosci. 1996;16:2226–2237. doi: 10.1523/JNEUROSCI.16-07-02226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu R-L, Barish ME. Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. J Neurosci. 1992;12:2235–2246. doi: 10.1523/JNEUROSCI.12-06-02235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu R-L, Barish ME. Astroglial modulation of transient potassium current development in cultured mouse hippocampal neurons. J Neurosci. 1994;14:1677–1687. doi: 10.1523/JNEUROSCI.14-03-01677.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu R-L, Butler DM, Barish ME. Potassium current development and its linkage to membrane expansion during growth of cultured embryonic mouse hippocampal neurons: sensitivity to inhibitors of phosphatidylinositol 3-kinase and other protein kinases. J Neurosci. 1998;18:6261–6278. doi: 10.1523/JNEUROSCI.18-16-06261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, Weiner JL, Valiante TA, Velumian AA, Watson PL, Jahromi SS, Schertzer S, Pennefather P, Carlen PL. Whole cell recording of the Ca2+-dependent slow afterhyperpolarization in hippocampal neurones: effects of internally applied anions. Pflügers Arch. 1994;426:247–253. doi: 10.1007/BF00374778. [DOI] [PubMed] [Google Scholar]