Abstract
Two experiments used functional magnetic resonance imaging (fMRI) to examine the cortical areas involved in establishing an expectation about the direction of motion of an upcoming object and applying that expectation to the analysis of the object. In Experiment 1, subjects saw a stationary cue that either indicated the direction of motion of a subsequent test stimulus (directional cue) or provided no directional information (neutral cue). Their task was to detect the presence of coherent motion in the test stimulus. The stationary directional cue produced larger modulations than the neutral cue, with respect to a passive viewing baseline, both in motion-sensitive areas such as left MT+ and the anterior intraparietal sulcus, as well as motion-insensitive areas such as the posterior intraparietal sulcus and the junction of the left medial precentral sulcus and superior frontal sulcus. Experiment 2 used an event-related fMRI technique to separate signals during the cue period, in which the expectation was encoded and maintained, from signals during the subsequent test period, in which the expectation was applied to the test object. Cue period activations from a stationary, directional cue included many of the same motion-sensitive and -insensitive areas from Experiment 1 that produced directionally specific modulations. Prefrontal activations were not observed during the cue period, even though the stationary cue information had to be translated into a format appropriate for influencing motion detection, and this format was maintained for the duration of the cue period (∼5 sec).
Keywords: attention, fMRI, motion perception, vision, event-related, cueing
People often form expectations about objects that are relevant to some behavioral goal. A real life example is a tennis player who is guessing the trajectory of the ball when preparing to return a serve. The return will be more accurate if the trajectory fits the player's expectation. This expectation is presumably represented as a pattern of neural activity in particular areas of the brain. When the expected object (e.g., the tennis ball) subsequently appears, the preexisting activity may modulate the neural activity produced by the object. This modulation enables the goal-relevant object to be attended and control the person's perception and responses. This framework suggests that when people are instructed to attend to an object, at least two types of signals can be measured: preparatory signals that represent the person's expectation of the goal-relevant object, which we call instruction signals, and signals that reflect the modulation of sensory activity by those instruction signals, which we call attentional modulations. Although there have been many demonstrations that attending to a visual stimulus affects neural activity in different areas of the human brain (Corbetta et al., 1991, 1993; Haxby et al., 1994; Heinze et al., 1994;Vandenberghe et al., 1996; Beauchamp et al., 1997; Mangun et al., 1997;O'Craven et al., 1997; Woldorff et al., 1997; Buchel et al., 1998;Cornette et al., 1998; Culham et al., 1998; Hillyard et al., 1998;Tootell et al., 1998; Wojciulik et al., 1998), it has been difficult in human neuroimaging studies to separate instruction signals from attentional modulations (but see Kastner et al., 1999). Because neuroimaging techniques have generally integrated brain activity over long periods, preexisting instruction signals have typically been integrated with subsequent attentional modulations. As a result, there is little information about which brain areas represent instruction signals.
Single-unit studies have separated these two signals, typically in match-to-sample paradigms in which the animal is shown a sample object (which generates the instruction signal) and has to determine whether it matches subsequent objects (which can result in attentional modulations) (Haenny and Schiller, 1988; Miller et al., 1993). Because most studies have recorded from a single visual cortical region (but see Ferrera et al., 1994; Miller et al., 1996), instruction signals and attentional modulations have not been characterized simultaneously over the entire brain. Several studies using this technique have found that sample-related signals during a delay period can be recorded in prefrontal regions (Funahashi et al., 1989), even in the presence of intervening nonsample items (Miller et al., 1996). These prefrontal areas have been interpreted as the source of the instruction signals that produce attentional modulations of subsequent sensory activity in posterior visual areas (Desimone and Duncan, 1995).
In the present paper, subjects were shown a stationary arrow cue that indicated the direction in which a subsequent test stimulus would move.Ball and Sekuler (1980) have previously shown that stationary direction cues improve motion detection. The directional cue should therefore establish instruction signals during the cue period that can modulate responses to the subsequent motion test stimulus. Experiment 1 measured the blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) response (Ogawa et al., 1990) during this task, using a blocked design that did not separate instruction signals during the cue period from attentional modulations during the test period. Experiment 2 introduced an event-related procedure (Buckner et al., 1996; Cohen et al., 1997; Courtney et al., 1997; Dale and Buckner, 1997; Zarahn et al., 1997; Friston et al., 1998) that separated these signals within the task explored in Experiment 1.
EXPERIMENT 1
Materials and Methods
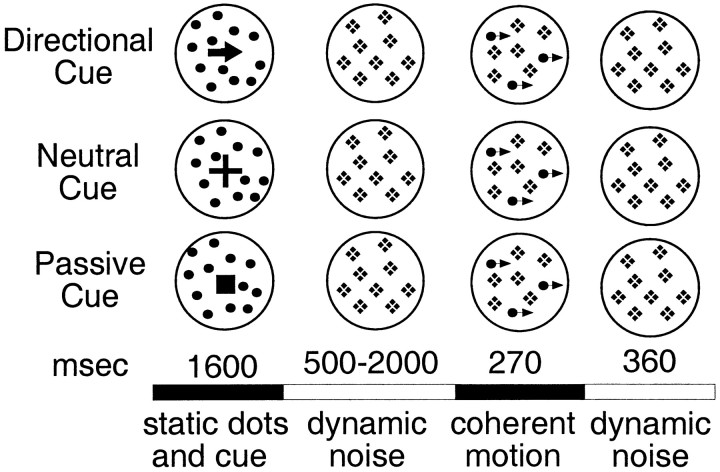
Stimuli and procedure. Seven subjects were tested with a blocked fMRI design in a cued motion coherence detection task. The stimulus consisted of a random array of 50 stationary dots positioned within an imaginary circular aperture of diameter 3.25°. The dots were white and presented on a dark screen. Each dot was a single pixel, 2 by 2 min. A central fixation cross was also present and remained throughout the trial. On each trial, subjects were shown a stationary cue, superimposed on a circular patch of random dots, consisting of either an arrow pointing in one of four directions (up, down, left, or right; directional cue), a plus sign (neutral cue), or a filled square (passive cue) (Fig. 1). The three cues were equated for area. The cue and static dots remained present for 1600 msec, at which point the cue was removed. For the next 500–2000 msec, in every display frame (30 msec/frame) each dot was displaced to a new location within the aperture, producing dynamic noise. After this initial period of dynamic noise, a percentage of the dots were replotted coherently in a single direction for 270 msec, yielding coherent motion at 4.2°/sec, whereas the remaining dots continued to be randomly replotted. The dots moving coherently were randomly chosen from the array each frame (e.g., the same dots were not moved coherently over the 270 msec interval but different dots were moved on different frames). Subjects were instructed to press an MR-compatible key with their right hand as quickly as possible if they detected motion. Catch trials, in which no coherent motion was presented, occurred on 17% of the trials and provided an estimate of the false alarm rate. After the 270 msec coherent motion, all dots were randomly replotted (dynamic noise) for an additional 360 msec. Two coherence percentages were used, randomly mixed over trials. These coherence percentages were determined for each subject in a behavioral presession. After short practice blocks in which subjects were familiarized with the task and display, performance was measured in two 80-trial blocks at four coherences (50, 40, 30, and 20). Two coherences were then selected so that the hit rate during the MR session would be ∼70–85% at one coherence level and 40–60% at the other level. The false alarm rate in the presession was low (∼6%), indicating that subjects used a conservative decision criterion. Radial motion scans involved random dot displays (consisting of 90 dots instead of 50) of the same speed (4.2°/sec) and spatial extent as in the coherence task. The speed was constant and did not vary with eccentricity.
Fig. 1.
Trial sequences for the three cueing conditions of Experiment 1. The solid circles were not present in the actual display but schematically indicate the extent of the stimulus. The crosses during the dynamic noise period schematically indicate that the dots, which were stationary during the static dots and cue period, were then randomly replotted to produce dynamic noise. Thearrowheads during the coherent motion period schematically indicate that some dots moved coherently. Dot density and size were the same throughout the trial.
In the directional cue condition, the signal dots always moved in the direction of the arrow. The cue therefore established a set to detect motion in a particular direction. In the neutral cue condition, the signal dots could move in any of the four cardinal directions. The cue therefore established a set to detect motion in any direction.
Five different scanning conditions were conducted in which different tasks were presented in alternating periods of 44 sec duration: (1) directional versus passive cue (four scans per subject), (2) neutral versus passive cue (four scans per subject), (3) directional versus neutral cue (four scans per subject), (4) passive cue versus fixation of a static dot array (two scans per subject), and (5) continuous motion versus fixation of a static dot array (two scans per subject). These scans were conducted to determine which areas were activated by sensory motion. The first three subjects received linear motion scans in which periods of continuous linear dot motion (left, right, up, and down) were alternated with control periods in which the dots were stationary. Because continuous linear motion might produce eye movements, the next four subjects received radial motion scans in which the dots moved inward and outward during the motion phase.
We use the term “motion-sensitive” in the text as an empirical label to refer to areas activated by the sensory motion condition. This term does not imply that these regions have the same sensitivity to motion or respond equally well to all types of motion. Because the areas activated by sensory motion were somewhat extensive, we considered an area activated during a condition as motion-sensitive if the Talairach coordinates of the peak activity in the condition (see Tables 4-6) were separated by a vector distance of <1 cm from the corresponding activation in the sensory motion condition.
Table 4.
Talairach coordinates of occipital from Experiment 2
| Region | Radial motion | Cue period | Test period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Zscore | x | y | z | Zscore | x | y | z | Zscore | |
| L calcarine | −7 | −97 | −8 | 5.6 | −17 | −89 | −8 | 8.7 | ||||
| R calcarine | 15 | −93 | −8 | 7.4 | ||||||||
| L ant coll | −25 | −67 | −12 | 8.5 | −27 | −65 | −14 | 6.3 | −27 | −65 | −14 | 9 |
| R ant coll | 29 | −63 | −12 | 10.1 | 29 | −63 | −14 | 6.9 | ||||
| R pos coll | 25 | −79 | −14 | 10.1 | 27 | −81 | −14 | 5.8 | 25 | −79 | −14 | 8.3 |
| L ant fusiform | −35 | −59 | −14 | 7.3 | ||||||||
| −41 | −65 | −16 | 8 | −41 | −63 | −12 | 6 | |||||
| R ant fusiform | 37 | −59 | −20 | 9.9 | ||||||||
| 45 | −61 | −12 | 5.7 | |||||||||
| L pos fusiform | −37 | −73 | −16 | 8.6 | −31 | −73 | −14 | 6.4 | ||||
| R pos fusiform | 37 | −75 | −16 | 5.6 | 33 | −75 | −6 | 6.1 | 33 | −71 | −6 | 6.4 |
| L MT+ | −39 | −73 | 2 | 19.8 | −39 | −75 | 0 | 7 | −45 | −69 | 4 | 14.7 |
| −43 | −71 | −6 | 8.5 | |||||||||
| −43 | −61 | −4 | 7.9 | |||||||||
| R MT+ | 41 | −65 | 4 | 19.6 | 41 | −63 | 4 | 7.2 | 41 | −65 | 4 | 12.6 |
| 41 | −77 | −6 | 6.8 | |||||||||
| L lateral occ | −27 | −89 | 0 | 14 | −33 | −85 | 2 | 8.7 | −33 | −85 | 2 | 6.7 |
| −27 | −87 | 12 | 7.6 | −25 | −87 | 2 | 8.1 | |||||
| R lateral occ | 27 | −85 | 4 | 18.3 | 31 | −85 | 2 | 9.9 | 29 | −83 | 8 | 8.7 |
| 37 | −81 | 2 | 14.5 | 23 | −87 | 2 | 8.5 | |||||
| 25 | −87 | −6 | 8.5 | |||||||||
Table 5.
Talairach coordinates of parietal foci from Experiment 2
| Region | Radial motion | Cue period | Test period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Zscore | x | y | z | Zscore | x | y | z | Zscore | |
| L STg/SMg | −49 | −39 | 24 | 8.4 | −47 | −39 | 24 | 7.4 | ||||
| R STg/SMg | 59 | −35 | 18 | 7.4 | 49 | −39 | 18 | 6.4 | ||||
| L precuneus | −13 | −71 | 38 | 7.2 | ||||||||
| R precuneus | 13 | −67 | 36 | 7.7 | ||||||||
| L vIPs | −25 | −73 | 20 | 10.9 | −25 | −73 | 22 | 11.1 | −25 | −73 | 20 | 10.5 |
| R vIPs | 25 | −71 | 30 | 11.2 | 29 | −75 | 28 | 8.1 | 27 | −75 | 28 | 7.5 |
| 31 | −69 | 22 | 7.8 | 31 | −71 | 20 | 7.8 | |||||
| L ant IPs | −29 | −57 | 56 | 9.3 | ||||||||
| −31 | −53 | 50 | 4.9 | −31 | −53 | 48 | 8.8 | −43 | −45 | 56 | 8.4 | |
| −25 | −51 | 42 | 5.1 | −29 | −49 | 40 | 8.7 | −29 | −51 | 42 | 7.3 | |
| R ant IPs | 23 | −61 | 36 | 7.3 | 23 | −61 | 32 | 6.7 | ||||
| 23 | −57 | 48 | 8.5 | 25 | −57 | 48 | 8.7 | 25 | −57 | 46 | 9.5 | |
| 29 | −49 | 42 | 6.8 | 35 | −45 | 40 | 7 | |||||
| L pos IPs | −13 | −67 | 56 | 7 | ||||||||
| −23 | −71 | 46 | 9.7 | |||||||||
| −13 | −77 | 46 | 6.7 | |||||||||
| R pos IPs | 17 | −67 | 54 | 7.8 | ||||||||
| 21 | −65 | 46 | 7.8 | |||||||||
| 25 | −71 | 40 | 8.2 | |||||||||
Table 6.
Talairach coordinates of frontal and subcortical foci from Experiment 2
| Region | Radial motion | Cue period | Test period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Zscore | x | y | z | Zscore | x | y | z | Zscore | |
| L precentral/SFs | −27 | −9 | 52 | 7.2 | −29 | −11 | 52 | 7.8 | ||||
| L precentral | −45 | −9 | 52 | 6.2 | −41 | −9 | 48 | 5.3 | −41 | −11 | 44 | 8.3 |
| −47 | −1 | 42 | 8.2 | −49 | −3 | 42 | 9 | |||||
| −53 | 3 | 28 | 8.6 | |||||||||
| −45 | 1 | 20 | 8.6 | |||||||||
| −51 | 3 | 10 | 10 | |||||||||
| R precentral | 49 | 1 | 46 | 6.1 | ||||||||
| 39 | −9 | 56 | 6.7 | 37 | −9 | 52 | 6.4 | 37 | −7 | 46 | 9.6 | |
| 43 | 3 | 36 | 5.2 | 45 | 1 | 36 | 9.5 | |||||
| 33 | 7 | 28 | 8.8 | |||||||||
| 47 | 3 | 20 | 7.6 | |||||||||
| 49 | 9 | 12 | 7.8 | |||||||||
| SMA | 1 | −3 | 54 | 4.9 | −1 | −5 | 52 | 16.7 | ||||
| pre-SMA/ant cingulate | 3 | 7 | 46 | 4.3 | 1 | 7 | 46 | 15.7 | ||||
| ant cingulate | −9 | 9 | 36 | 11.2 | ||||||||
| 3 | 23 | 32 | 11.8 | |||||||||
| L DLPFC | −35 | 47 | 20 | 5.1 | ||||||||
| R DLPFC | 33 | 37 | 30 | 5.9 | ||||||||
| 39 | 31 | 20 | 4.8 | |||||||||
| 35 | 43 | 22 | 4.6 | |||||||||
| L insula/frontal operculum | −31 | 15 | 4 | 15.1 | ||||||||
| −35 | −5 | 12 | 11.6 | |||||||||
| R insula/frontal operculum | 31 | 17 | 4 | 17.5 | ||||||||
| 43 | 11 | 2 | 11.1 | |||||||||
| 35 | −5 | 10 | 7.5 | |||||||||
| L thalamus | −9 | −21 | 8 | 14 | ||||||||
| −9 | −3 | 8 | 9.8 | |||||||||
| R thalamus | 7 | −19 | 8 | 12 | ||||||||
| L putamen | −19 | 3 | 2 | 9.5 | ||||||||
| R putamen | 19 | 5 | 2 | 9 | ||||||||
Apparatus. Stimuli were displayed using an Apple (Cupertino, CA) Power Macintosh computer and projected to subjects with a Sharp (Mahwah, NJ) liquid crystal display projector (with a screen resolution of 640 × 480 pixels) onto a screen positioned at the head of the bore. Subjects viewed the screen through a mirror. A fiber-optic light-sensitive key press was used to record behavioral responses.
Scan acquisition. fMRI scans were collected on a Siemens AG (Erlangen, Germany) 1.5 tesla Vision system, using an asymmetric spin-echo echo-planar sequence sensitive to BOLD contrast (T2*; frame duration, 2.36 sec; T2* evolution time, 50 msec; flip angle, 90°) (Ogawa et al., 1990). During each scan, 128 frames of 16 contiguous 8 mm axial slices were acquired (3.75 × 3.75 mm in-plane resolution), allowing complete brain coverage at a high signal-to-noise ratio (Conturo et al., 1996). Functional images were acquired parallel to the anterior commissure–posterior commissure plane in each subject after prescribing slice position based on automatic measurements of rotation, translation, and tilt of the initial images to an average (n = 12) MP-RAGE anatomical image (target) representation of the atlas of Talairach and Tournoux (1988). Structural images were acquired using a sagittal MP-RAGE sequence, optimized for contrast-to-noise ratio and resolution (Epstein et al., 1994) (repetition time, 97 msec; echo time, 4 msec; flip angle, 12°; inversion time, 300 msec).
Analysis of BOLD responses. Functional data were realigned within and across runs to correct for head movement and coregistered with the anatomical data. A whole brain spatial normalization was applied to equate the overall signal intensity on each MR frame. For each subject, the scans for each condition were concatenated, and the linear trend at each voxel over each scan was removed.z-maps for each condition were then computed by comparing the MR signal during the experimental and control periods with a Wilcoxon summed ranks test and converting the test statistic to aZ score. The z-images were summed across subjects in atlas space (2 × 2 × 2 mm voxels) and divided by the square root of the sample size to yield a group z-image that was then corrected for multiple comparisons (Ollinger, 1997). An automatic search routine was used to determine the voxels yielding local maxima in the group z-image (Mintun et al., 1989). The time course of the BOLD response was computed for regions of interest (ROIs) formed from a 3 × 3 matrix of voxels (each voxel for the time course analysis was 3 × 3 × 3 mm), centered on these local maxima.
RESULTS
Behavior
ANOVAs were conducted on the behavioral data collected during the MR session to determine whether subjects were using the directional cue to improve their performance. After a directional cue, reaction time (RT) to detect motion was significantly faster (directional RT, 556 msec; neutral RT, 596 msec; F(1,6) = 40.7; p < 0.0005), and more motion targets were detected (directional hit rate, 68.3%; neutral hit rate, 58%;F(1,6) = 62.5; p < 0.0005), with no significant difference in false alarm rate (directional false alarm rate, 13.4%: neutral false alarm rate, 10.3%; F(1,6) = 0.82;p > 0.2). These effects did not interact with coherence level, indicating that the effects of the cue were similar for both coherence percentages. These data show that subjects were using the directional cue to improve their performance.
There may be several psychophysical mechanisms that underlie the effect of the cue. For example, the instruction signals from the cue could enhance the output of directionally selective mechanisms coding the cued direction or they could suppress the outputs of directionally selective mechanisms coding noncued directions (preventing false alarms from those channels). Recent analyses of the effects of a spatial attention cue (Lu and Dosher, 1998) have favored signal enhancement theories, but noise suppression or distractor exclusion theories may still apply in the present situation.a
Sensory motion
This condition was used to define motion-sensitive areas. Both linear and radial motion activated a large set of occipital areas that have previously been described, including human MT+b (Corbetta et al., 1991; Zeki et al., 1991;Watson et al., 1993; Tootell et al., 1995), a lateral occipital region, and a region near the junction of the superior temporal and supramarginal gyri (STg/SMg) (Corbetta et al., 1991; Dupont et al., 1994). Activations in the anterior intraparietal sulcus (ant IPs), with the most anterior part extending into the gyral surface posterior to the postcentral sulcus, and the ventral extension of the intraparietal sulcus into the occipital lobe (vIPs), just anterior and dorsal to its intersection with the transverse occipital sulcus, were also observed (Beauchamp et al., 1997; Culham et al., 1998).
Overall, the strongest activations were observed in MT+, which is thought to contain a homolog of MT in the macaque (Tootell et al., 1995), and the lateral occipital area, which probably corresponds to area KO of Dupont et al. (1997). Studies of motion processing that have involved large stimulus fields have produced little or no activation in the lateral occipital region (Reppas et al., 1997; Shulman et al., 1998), but studies using small fields (Dupont et al., 1997), such as the present work, have observed robust effects. Dupont et al. (1997)have suggested that this region is involved in the analysis of motion-defined contours, although large-field stimuli filled with motion-defined contours do not activate this region (Shulman et al., 1998).
Because continuous linear motion might produce tracking eye movements, identification of motion-sensitive areas is best addressed using the radial motion scans. Because a far more extensive set of data (n = 14 subjects) was collected with the radial motion condition in Experiment 2, detailed analysis of the radial motion data are deferred until the presentation of that experiment.
Directional and neutral cues
Neutral cues should activate regions reflecting a general set for motion, whereas directional cues should additionally activate regions involved in directionally specific sets. Both kinds of cues produced significant modulations, with respect to the passive cue baseline, in all of the areas activated by radial motion (Tables1, 2). Significant foci were also found in areas not activated in the radial motion condition, including the posterior intraparietal sulcus (pos IPs) and the junction of the medial precentral sulcus with the superior frontal sulcus.
Table 1.
Talairach coordinates of occipital foci from Experiment 1
| Region | Directional vs passive cue | Neutral vs passive cue | Directional vs neutral cue | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Zscore | x | y | z | Zscore | x | y | z | Zscore | |
| L calcarine | −11 | −97 | −8 | 11.3 | −17 | −99 | −6 | 13.2 | ||||
| −1 | −93 | −6 | 13.2 | |||||||||
| R calcarine | 19 | −91 | −4 | 9.4 | 19 | −91 | −6 | 11.2 | ||||
| R ant coll | 21 | −63 | −14 | 6.1 | ||||||||
| L pos coll | −17 | −85 | −16 | 9.2 | −17 | −85 | −16 | 11.9 | ||||
| R pos coll | 23 | −79 | −16 | 8.2 | 23 | −79 | −16 | 8.8 | ||||
| L pos fusiform | −43 | −75 | −16 | 5.2 | ||||||||
| R pos fusiform | 37 | −75 | −14 | 7.4 | 33 | −75 | −20 | 8.1 | ||||
| L MT+ | −49 | −63 | 4 | 9.6 | −49 | −63 | 4 | 8.9 | −49 | −61 | 4 | 4.3 |
| −43 | −69 | −6 | 11.1 | −43 | −69 | −6 | 10.1 | −43 | −69 | −6 | 6.2 | |
| R MT+ | 41 | −63 | 4 | 7.1 | 41 | −67 | 4 | 6.4 | 41 | −61 | 12 | 4.1 |
| L lateral occ | −29 | −89 | 2 | 11.7 | −27 | −89 | 0 | 11.7 | −35 | −83 | 2 | 4.6 |
| R lateral occ | 31 | −87 | 2 | 15.9 | 31 | −87 | 2 | 16.7 | ||||
| 29 | −81 | 10 | 10.7 | 29 | −83 | 10 | 5.2 | |||||
Table 2.
Talairach coordinates of parietal foci from Experiment 1
| Region | Directional vs passive cue | Neutral vs passive cue | Directional vs neutral cue | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Zscore | x | y | z | Zscore | x | y | z | Zscore | |
| R STg/SMg | 59 | −43 | 24 | 7.5 | 59 | −41 | 24 | 6.1 | ||||
| L vIPs | −27 | −77 | 26 | 7 | −23 | −73 | 30 | 4.9 | ||||
| −29 | −81 | 18 | 10.7 | −31 | −77 | 18 | 8.7 | |||||
| L ant IPs | −39 | −51 | 54 | 9.3 | 39 | −55 | 58 | 6.7 | −31 | −57 | 56 | 4 |
| −35 | −47 | 48 | 11.1 | −31 | −51 | 40 | 6.3 | −35 | −47 | 48 | 6.4 | |
| −31 | −43 | 38 | 8.7 | −35 | −45 | 46 | 6.1 | −33 | −43 | 38 | 5.8 | |
| −27 | −55 | 48 | 4.6 | |||||||||
| R ant IPs | 27 | −57 | 50 | 8.8 | 31 | −51 | 48 | 5 | ||||
| 29 | −47 | 42 | 9 | 31 | −47 | 53 | 6.4 | 31 | −45 | 40 | 5.8 | |
| L pos IPs | −13 | −69 | 54 | 12 | −15 | −71 | 48 | 8 | −15 | −67 | 52 | 8.9 |
| −23 | −71 | 46 | 13.2 | −23 | −71 | 46 | 9.1 | −23 | −71 | 46 | 8.5 | |
| −13 | −75 | 46 | 8.8 | |||||||||
| R pos IPs | 23 | −69 | 54 | 12.2 | 23 | −69 | 50 | 9.4 | 15 | −67 | 42 | 7.2 |
| 15 | −73 | 46 | 10.5 | 21 | −71 | 46 | 6.4 | |||||
Directional versus neutral cues
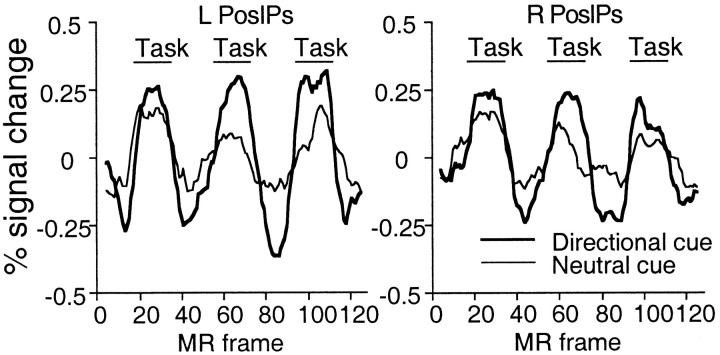
The activations specific to the use of directional cue information were isolated in the directional versus neutral scans (Tables 1, 2; Fig. 2A). This comparison yielded significant activations in motion-sensitive areas such as ant IPs and left MT+. Activations were also observed in motion-insensitive areas, particularly pos IPs (Fig.3), with a smaller activation at the junction of the left medial precentral and superior frontal sulci. Therefore, a directional cue increased the modulations observed after a neutral cue, in both motion-sensitive (e.g., ant IPs and left MT+) and motion-insensitive (e.g., pos IPs and left medial precentral sulcus) regions.
Fig. 2.
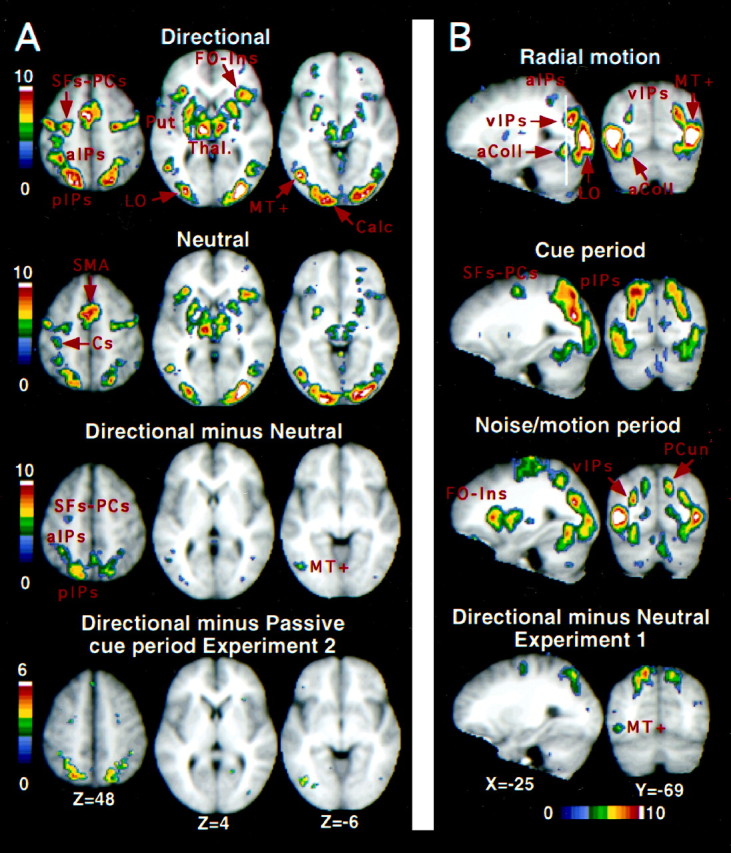
A, Group z-maps showing statistically significant activations for the conditions, directional cue versus passive cue (top row), neutral cue versus passive cue (second row), and directional cue versus neutral cue (third row). The bottom row shows the group z-map from Experiment 2 for a contrast comparing the directional cue and passive cue conditions during the cue period. The color scale represents the Z score of the activation, and all displayed pixels have passed a multiple-comparison procedure. SFs–PCs, Superior frontal sulcus–precentral sulcus; Cs, central sulcus;aIPs, anterior intraparietal sulcus;pIPs, posterior intraparietal sulcus;FO-Ins, frontal operculum/insula; Put, putamen; Thal, thalamus; LO, lateral occipital area; Calc, calcarine sulcus.B, Group z-maps of significant voxels activated in Experiment 2 during the radial motion condition (top row) and during the cue period (second row, within-trial model) and noise/motion period (third row, within-trial model) in the directional cue condition. The bottom row displays the group z-map from the directional cue versus neutral cue condition of Experiment 1. The color scale represents the Z score of the activation, and all displayed pixels have passed a multiple-comparison procedure. The white line through the top sagittal slice indicates the position of the coronal slice in the right column. Activation in the anterior intraparietal sulcus (aIPs), which courses laterally as it moves anterior, is only partly visible in the displayed sagittal slice (left column).aColl, Anterior collateral sulcus; PCun, precuneus.
Fig. 3.
Group time courses for the conditions, directional cue versus passive cue, and neutral cue versus passive cue.Task refers to time points during which the subject performed the directional cue or neutral cue task, with an additional two-frame delay to reflect the delayed hemodynamic response. The intervening time points involved the passive cue condition. The time courses have been smoothed by computing a nine-point running average; the linear trend has been removed; and the 0 point of the scale reflects the mean of the time courses. Time courses were derived from a 3 × 3 voxel ROI centered on the voxel yielding a local maximum peak Z score in the contrast, directional versus neutral cue, within the left (Talairach coordinate = −15, −67, 52) and right (Talairach coordinate = 15, −67, 52) posterior intraparietal sulcus. BOLD responses are significantly larger after directional than neutral cues.
Both the directional and neutral cue conditions also activated a large set of regions outside the visual system (Table3, Fig. 2A), including both dorsal and ventral sections of the precentral sulcus, the left central sulcus, supplementary motor area (SMA), anterior cingulate, insula/frontal operculum, thalamus, basal ganglia, and right cerebellum. A relatively weak but significant activation was also observed in dorsolateral prefrontal cortex (DLPFC) for both directional and neutral cues. None of these regions was significantly active in the direct comparison of directional and neutral cues (Fig.2A).
Table 3.
Talairach coordinates of frontal and subcortical foci from Experiment 1
| Region | Directional vs passive cue | Neutral vs passive cue | Directional vs neutral cue | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Zscore | x | y | z | Zscore | x | y | z | Zscore | |
| L precentral/SFs | −27 | −15 | 54 | 10.8 | −31 | −11 | 52 | 7.8 | −25 | −13 | 56 | 4.9 |
| −35 | −11 | 58 | 9.2 | −31 | −15 | 64 | 4.5 | |||||
| L precentral | −51 | −3 | 42 | 10.8 | −45 | −7 | 48 | 8 | −57 | −5 | 36 | 4.3 |
| −51 | 3 | 28 | 8.1 | −47 | −3 | 36 | 8.1 | |||||
| −53 | 1 | 18 | 6.8 | −51 | 3 | 26 | 6.7 | −59 | 7 | 18 | 4.1 | |
| R precentral/SFs | 25 | −11 | 50 | 10.3 | 23 | −7 | 56 | 8.5 | ||||
| 31 | −11 | 56 | 7.4 | |||||||||
| R precentral | 41 | −9 | 54 | 7.6 | 43 | −7 | 54 | 8.4 | ||||
| 51 | −1 | 46 | 8.3 | 51 | −3 | 46 | 6 | |||||
| 53 | 7 | 38 | 6.8 | 53 | 9 | 36 | 6.2 | |||||
| SMA | −3 | −1 | 52 | 12.8 | −3 | −1 | 52 | 11.5 | ||||
| −3 | −11 | 58 | 10.9 | −3 | −9 | 58 | 10.2 | |||||
| pre-SMA/ant cingulate | −9 | 3 | 46 | 11.8 | −9 | 3 | 44 | 10.2 | ||||
| 7 | 9 | 42 | 9.1 | 5 | 7 | 46 | 9.2 | |||||
| −3 | 11 | 42 | 9 | |||||||||
| ant cingulate | −9 | 11 | 36 | 8.8 | −9 | 13 | 36 | 7.7 | ||||
| 7 | 21 | 30 | 8.3 | 7 | 21 | 30 | 9.3 | |||||
| L DLPFC | −29 | 41 | 22 | 5.3 | ||||||||
| R DLPFC | 35 | 45 | 24 | 5.7 | 31 | 43 | 22 | 7.1 | ||||
| L insula/frontal operculum | −31 | 23 | 4 | 9.8 | −31 | 32 | 4 | 8.7 | ||||
| −31 | 15 | 10 | 8.8 | −31 | 17 | 10 | 7.6 | |||||
| R insula/frontal operculum | 31 | 21 | 12 | 11.1 | 31 | 21 | 12 | 9.1 | ||||
| 35 | 19 | 4 | 8.5 | |||||||||
| L thalamus | −11 | −21 | 8 | 15.1 | −9 | −23 | 8 | 13.2 | ||||
| −11 | −5 | 10 | 8.7 | −11 | −5 | 10 | 8.4 | |||||
| R thalamus | 7 | −21 | 8 | 13 | 7 | −19 | 8 | 11.4 | ||||
| 9 | −7 | 10 | 9.8 | 9 | −7 | 10 | 7.3 | |||||
| L putamen | 23 | −3 | 10 | 9.3 | 23 | −3 | 10 | 7.1 | ||||
| R putamen | −23 | −1 | 8 | 9.3 | −23 | −3 | 10 | 8 | ||||
Discussion
Both directional and neutral cues produced significant modulations with respect to a passive baseline in areas sensitive to radial motion, as well as areas not sensitive to motion. Directional cues, however, produced larger modulations in both motion-sensitive regions such as ant IPs and left MT+ and motion-insensitive regions such as pos IPs and the left medial precentral sulcus.
Although many studies have found that activity in human extrastriate cortex is modulated by attention to a dimension such as color or motion (Corbetta et al., 1991, 1993; Haxby et al., 1994;Heinze et al., 1994; Vandenberghe et al., 1996; Beauchamp et al., 1997;Mangun et al., 1997; O'Craven et al., 1997; Woldorff et al., 1997;Buchel et al., 1998; Cornette et al., 1998; Hillyard et al., 1998;Wojciulik et al., 1998), these studies have not shown that modulations can be specific to particular features of those dimensions such as red or motion to the left. The present study demonstrates modulations contingent on the use of specific feature information concerning direction of motion.
As noted in the introductory remarks, blocked fMRI paradigms, including the present experiment, have been unable to separate instruction signals from attentional modulations. The direction-specific modulations (i.e., modulations that were greater after a directional than neutral cue) in the intraparietal sulcus, for example, could reflect the encoding of the directional cue before the onset of the dynamic noise or the effects of that cue on the sensory activity subsequently evoked by the noise and/or coherent motion. The same ambiguity remains for the more general motion set modulations produced in the neutral cue condition. They could reflect tonic changes in the activation of motion areas that precede the sensory test stimulus or modulations of the activity evoked by that stimulus. Moreover, the present experiment indicated that a range of areas outside the visual system, such as thalamus, basal ganglia, insula/frontal operculum, and DLPFC, were also modulated by the active task. These modulations could reflect processes during the cue period or the subsequent test period. If prefrontal regions maintain instruction signals in posterior areas, for example, then some of these regions might be active during the cue period.
In Experiment 2, we separate instruction signals and attentional modulations using an event-related fMRI paradigm.
EXPERIMENT 2
Materials and Methods
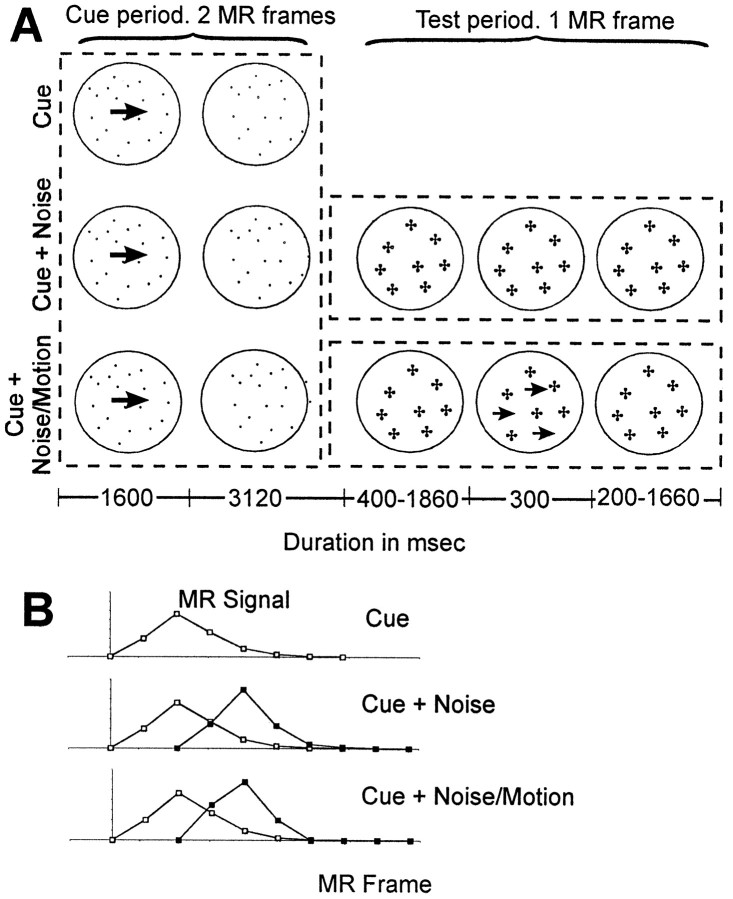
Procedure. Subjects were given a modified version of the task in Experiment 1. Only directional and passive cues were presented and eight directions were cued (in 45° increments from vertical) instead of four. As shown in Figure4A, the initial cue period was extended to two MR frames (4.72 sec). After the 1600 msec cue stimulus, only the static dots remained for the duration of the cue period. On 25% of the trials, the trial ended after the cue period (top row). As noted below, these cue trials were necessary for isolating the instruction signals initiated during the cue period. On the remaining trials, a test period of duration one MR frame (2360 msec) followed the cue period. On cue + noise trials (middle row), only dynamic noise was presented during the test period. On cue + noise/motion (bottom row) trials, coherent motion was presented for 300 msec at some point during the test period, with the remainder of the period filled with dynamic noise. A single coherence percentage was used for each subject. The initial level was determined in a behavioral presession so that the hit rate was between 70 and 85%. Subjects first received short blocks in which the coherence level was decreased until performance was approximately within the desired range. They then received two 30-trial blocks to confirm that the coherence level was appropriate. During the subsequent scanning session, adjustments in the coherence percentage were sometimes made between scans to ensure that performance remained within the desired range. Both neutral and directional cues were used in the behavioral presession so that the use of the directional cue information could be assessed for each subject.
Fig. 4.
A, Timelines for a cue, cue + noise, and cue + noise/motion trial in Experiment 2. The dotted boxes illustrate the three events (cue, noise, noise/motion) that initiate separate BOLD responses in the within-trial linear model (see B). The solid circles were not present in the actual display but schematically indicate the extent of the stimulus. The crosses in Test periodschematically indicate that the dots, which were stationary during the cue period, were randomly replotted during the test period, producing dynamic noise. The small arrows in Test period schematically indicate that some dots moved coherently. Dot density and size were the same for the cue and test periods.B, Illustration of hypothetical BOLD responses estimated by the within-trial model during the cue and test periods of the three trial types (cue, cue + noise, cue + noise/motion) for a voxel that is activated during both periods. During a cue trial, a single BOLD response related to the encoding of the cue is initiated. During both cue + noise and cue + noise/motion trials, two BOLD responses are initiated. The first is initiated during the cue period and is identical to that on cue trials. The second BOLD response is initiated two MR frames later at the beginning of the test period. The BOLD response during the noise period of cue + noise trials may be different from the BOLD response during the noise/motion period of cue + noise/motion trials. The within-trial linear model assumes that the observed signal on any MR frame is the sum of the signals from these ongoing BOLD responses. The goal of the model is to estimate separately at each voxel these hypothetical BOLD responses without making any assumption about their shape. The BOLD responses illustrated have a unimodal shape, but they would be estimated with equal precision by the model if they had multiple peaks or a single plateau that was sustained over an interval.
For all three trial types (cue, cue + noise, and cue + noise/motion), the end of the trial was signaled by a brief (500 msec) blanking of the random dots. The dots reappeared for the remainder of the intertrial interval. Fifty percent of the trials were cue + noise/motion, 25% were cue + noise, and 25% were cue, with the three types randomly mixed. Subjects also received separate radial motion scans identical to those in Experiment 1. Twelve subjects received seven scans with a directional cue, seven scans with a passive cue, and two radial motion scans. For these subjects, the intertrial interval lasted from two to four MR frames (4720–9440 msec). Two additional subjects received 14 directional cue scans and two radial motion scans. For these subjects, the intertrial interval lasted from one to three MR frames (2360–7080 msec).
Analysis of BOLD signal. fMRI data were initially analyzed as in Experiment 1, except that no spatial normalization was performed. The BOLD responses initiated during the cue and test periods were then estimated with linear regression using two models that assume that the MR signal on any frame is the sum of different components.
A between-trial model assumed that each component is the BOLD response initiated at the onset of a trial. Because trials are closely spaced, the MR signal on a trial will reflect the sum of the ongoing BOLD responses from the current and previous trials. The between-trial model estimated a separate time course for the BOLD response initiated by each trial type (cue, cue + noise, and cue + noise/motion), with the instruction signals from the cue given solely by the estimated time course for cue trials. This model ignores the fact that the cue period is common to all three trial types and treats each trial type as a separate unit.
The within-trial model takes advantage of this commonality by assuming that there can be multiple processes (e.g., a cue process and a test process) within a trial and that each process initiates a separate BOLD response (Fig. 4B). These responses, as well as ongoing responses from previous trials, are summed to yield the empirically measured fMRI response on a single frame. The within-trial model yields distinct estimates of the BOLD responses initiated during the cue, noise, and noise/motion periods and estimates the cue period response from all three trial types.
An important aspect of both the within and between trial models is that the time course of the BOLD response is estimated without making any assumption about its shape. The absence of shape assumptions distinguishes the present work from several other event-related methods (Courtney et al., 1997; Zarahn et al., 1997; Friston et al., 1998). Because it is difficult to predict the exact time course of different cognitive processes in different neural areas, it is useful to have methods that allow time courses to be recovered without assuming their shape. In the present work, both the within- and between-trial models estimated the BOLD response over an 18.8 sec (eight MR frames) interval. Both the within- and between-trial models require that the BOLD response is sufficiently linear (Boynton et al., 1996; Dale and Buckner, 1997) and that there are enough linearly independent equations to permit a unique estimate of the BOLD responses produced by each component in the model. When the components are different trial types, as in the between-trial model, the use of a random intertrial interval guarantees a sufficient number of independent equations to estimate the response to each trial type. When the components are two successive processes within a trial (e.g., the cue period and test period of the current experiment), as in the within-trial model, it is also necessary to present some trials (i.e., cue trials) in which only the first process is present.
The linear model yielded the time course of the BOLD response at each voxel for each condition. The estimates from the within-trial model yielded separate time courses for the cue and test periods, whereas the estimates from the between-trial model yielded a single time course over the entire trial. The within-trial time courses for each period were cross-correlated with three hemodynamic response functions (HRFs), each generated by convolving a γ function with a rectangular function specified by the period duration (Boynton et al., 1996), that were shifted by 1 sec intervals. A voxel-wise z-value was then computed based on the particular HRF yielding the largest cross-correlation. It is important to note that although the time course of the BOLD response was estimated without assuming a particular HRF, an HRF was assumed to generate a z-map. These voxel-wise z-maps were transformed to atlas space, summed across subjects, and divided by the square root of the sample size to yield a group z-image. This image was then corrected for multiple comparisons (Ollinger, 1997) and Bonferroni corrected for the number of HRFs (three) used in the cross-correlation. An automatic search routine was used to determine the voxels yielding local maxima in the group z-image (Mintun et al., 1989). The time course of the BOLD response was computed for ROIs formed from a 3 × 3 matrix of voxels (using 2 × 2 × 2 mm voxels) within a slice, centered on these local maxima.
The directional cue and passive cue conditions were compared by a within-subject ANOVA. First, the z-maps for the two cue conditions were computed for each individual within-atlas (Talairach) space, combined, and averaged across subjects to yield a group image that was not biased toward either condition. ROIs were defined on this combined image for all voxels passing the multiple comparison correction. These ROIs were applied to the data from each subject (in atlas space) separately for the passive and directional cue conditions, and the averaged time courses over these ROIs for each cue condition were computed. A within-subject ANOVA was then conducted with frame (i.e., time) and cue condition (directional or passive) as factors. Larger activations during the directional cue than passive cue condition were reflected in a frame by cue condition interaction.
An ANOVA was also used to determine whether activations during the noise/motion interval were modulated by whether the subject detected the motion stimulus and made a response. ROIs were defined from an image that combined the noise/motion–response and noise/motion–no response data. A within-subject ANOVA was then conducted on these regions with frame (i.e., time) and response (present or absent) as factors.
Results
Behavior
Data from a behavioral presession showed that subjects used the directional information provided by the cue to help detect the coherent motion. Subjects responded faster (509 vs 560 msec;F(1,12) = 26.3; p < 0.0005) and detected more motion targets (83.3 vs 73.8% hits;F(1,12) = 24.3; p < 0.0005) after directional than neutral cues, with no significant difference in false alarms (7.3 vs 5.3%;F(1,12) = 0.499). Some subjects tested during the behavioral session did not subsequently contribute MR data. This occurred because of technical problems during the scanning session (n = 3), scheduling, or other miscellaneous difficulties (n = 4) or because the effect of the cue on performance was ambiguous (n = 4). These latter subjects showed speed accuracy tradeoffs, with the directional cue producing opposite effects on reaction time and accuracy. Because the study was concerned with the effects of using a motion cue on the BOLD signal, these subjects were not tested in the MR scanner. However, when an analysis was conducted on all subjects who completed the behavioral presession, it was still the case that after a directional cue, reaction time was faster (F(1,23) = 33.6; p < 0.0001), and more motion targets were detected (F(1,23) = 18.0;p < 0.0005), with no significant difference in false alarms (F(1,23) = 2.09;p > 0.1). Group mean performance during the MR session was 80.1% hits and 12.8% false alarms, (average d′ = 2.10) with a mean reaction time of 570 msec.
Validation of the model
Restriction of central sulcus activations to the test period. Because motor responses were only made during the test period, the accuracy of the linear model in separating cue and test responses was evaluated by examining whether the linear model confined activations in the central sulcus to the test period. BOLD responses were estimated separately for trials (between-trial model) or periods (within-trial model) in which a response was made (hits or false alarms) or withheld (misses or correct rejections). Figure5, top row, shows the group time courses in left central sulcus from the between- and within-trial models. Time courses are also shown for SMA (middle row) and left parietal operculum (bottom row), which is thought to correspond to S-II (Burton et al., 1997), and presumably received feedback from the motor response and any preparatory motor adjustments.
Fig. 5.
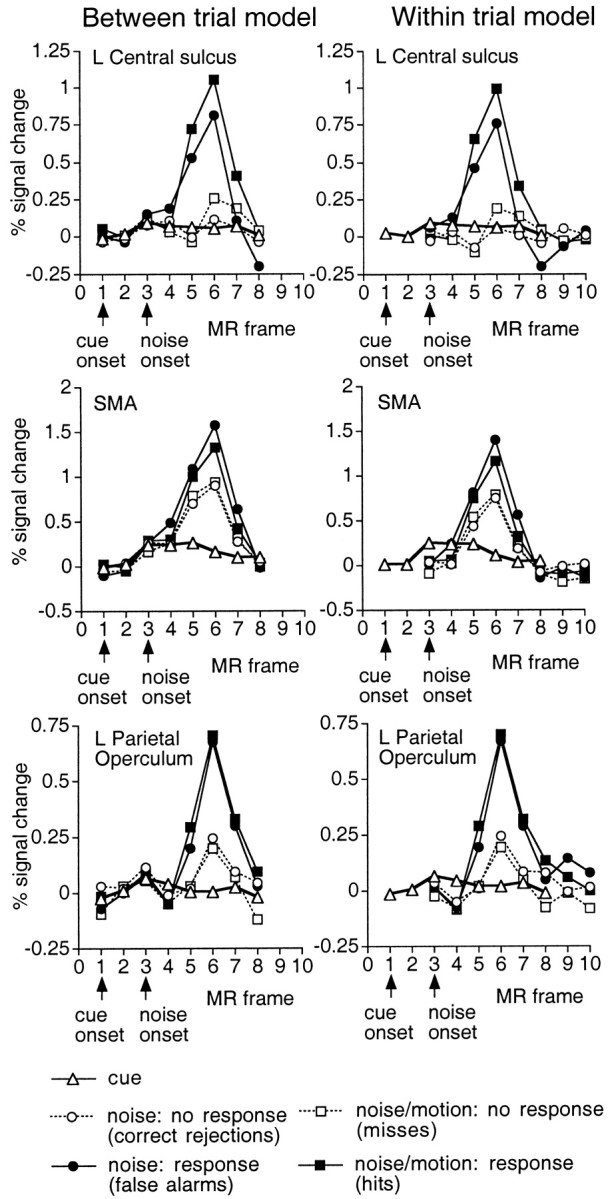
Group time course in the directional cue condition for activations in left central sulcus (top row), SMA (middle row) and left parietal operculum (bottom row) as a function of whether a motor response was executed (filled symbols, response; open symbols, no response) and the type of trial (left panel, between-trial model: cue only, cue + noise, cue + noise/motion) or the type of period within a trial (right panel, within-trial model: cue, noise, and noise/motion periods). Time courses are based on a 3 × 3 voxel ROI centered on the peak voxel in the z-map for noise/motion trials in which a response was made (hits) (central sulcus, Talairach coordinate = −39, −31, 58; SMA, −1, −5, 52; parietal operculum, −51, −21, 24). The small arrows on thex-axis indicate the MR frame for the onset of the cue and noise stimulus. For the within-trial model (right panel), the time course functions for the noise and noise/motion periods begin two MR frames after the functions for the cue, because the test period begins two frames after cue onset. No central sulcus activations are evident during a cue trial (left panel) or during the cue period of all trial types (right panel), whereas strong activations are observed during the test period (noise or noise/motion) of trials involving a response.
During noise or noise/motion trials in which a response was made (i.e., false alarms or hits), strong activations were observed in the left central sulcus (Fig. 5, top row). The latency of this activation was approximately two MR frames (4.72 sec) after the onset of the dynamic noise, indicating that it was related to the test period rather than the cue period. Conversely, no activation was seen during cue trials or noise and noise/motion trials in which a response was withheld (i.e., correct rejections or misses), confirming that activations from different trial types were properly segregated by the between trial model. Also, the within-trial model restricted central sulcus activations to test periods in which a motor response was made, with no activation evident during the cue period or the test periods of trials in which a response was withheld. This result confirms that signals during the test period were not inappropriately assigned to the cue period.
A strong SMA response was evident during test periods in which a response was made, but a somewhat smaller activation was also present during test periods in which a response was withheld (Fig. 5,middle row). Because subjects must be prepared to respond at any point during the presentation of the dynamic noise, this result is consistent with evidence that motor readiness activates SMA (Tanji et al., 1980; Alexander and Crutcher, 1990). Response preparation may also account for the very small SMA activation observed during the cue period. Parietal operculum (bottom row) showed a pattern midway between that in central sulcus and SMA, with strong activations during test periods involving a motor response, weak activation during test periods in which no response was made, and no activation during the cue period.
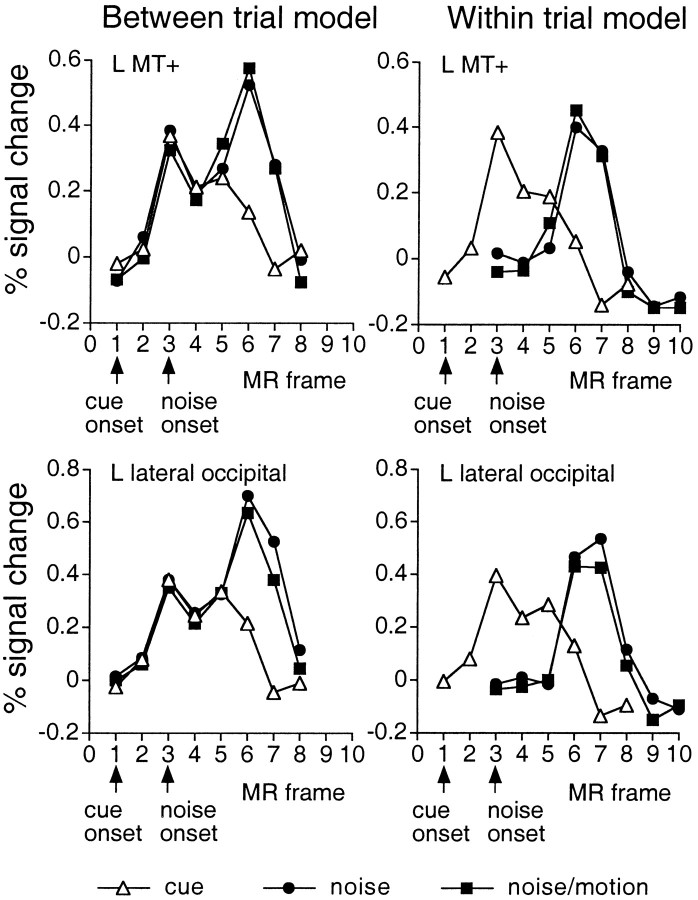
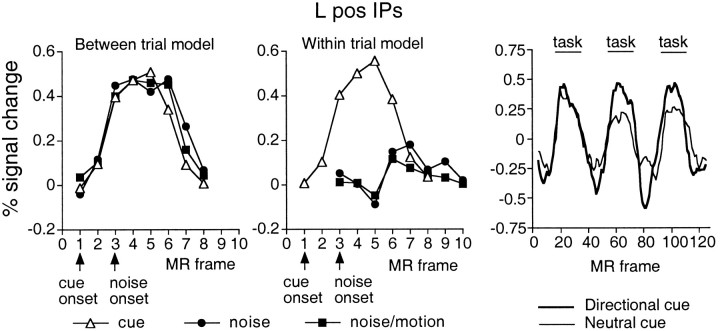
Independence of cue and test responses within the same voxel. Further confirmation of the success of the method in separating activations during the cue and test periods can be seen in the time courses of those voxels that were significantly activated during both periods (Fig. 6). The time course for left MT+ and the left lateral occipital area, for example, on cue + noise/motion trials (left panel) showed two peaks that followed the onset of the cue and test periods, respectively. The first peak matched identically with the time course on cue trials, indicating that the estimate of the signal during this initial peak, reflecting activity during the cue period, was not affected by the activity during the second peak, which presumably reflected the test period. The cue and test activations apparent from the between trial time courses were clearly separated by the within-trial model (right panel).
Fig. 6.
Group time courses in the directional cue condition for left MT+ (top row) and left lateral occipital area (bottom row) during cue, cue + noise, and cue + noise/motion trials (left panel, between-trial model) or during cue, noise, and noise/motion periods (right panel, within-trial model). Time courses are based on a 3 × 3 voxel ROI centered on the peak voxel in the z-map for the cue period (L MT+, Talairach coordinate = −43, −71, −6; L lateral occipital area, −33, −85, 2). Two responses are evident in the time courses for the between-trial model, corresponding to the separate activations during the cue and noise/motion period. The activation during the cue period on cue, cue + noise, and cue + noise/motion trials is the same, indicating that the estimation of the cue period was unaffected by the activations during the subsequent test period. The separate responses evident in the between-trial model time courses have been isolated by the within-trial model.
The time course on cue trials for both areas increased on frame 5 rather than decreasing. This “uptick” was probably caused by the brief offset of the random dots at the end of a trial to signal that the trial had ended. The cue trial, for example, ended at the onset of frame 3, and the uptick in the cue response was noticeable on frame 5.
Areas activated by radial motion
The radial motion scans were used to indicate which areas of the brain responded to sensory motion (Tables4-6). Radial motion activated several occipital regions (Fig.2B, top row), including MT+, lateral occipital area, a region near the junction of the STg/SMg, the calcarine sulcus, the posterior fusiform, and regions near the anterior and posterior collateral sulcus. Radial motion also activated the vIPs and a region buried within the ant IPs. Relatively weaker activations were also observed in the lateral precentral sulcus.
Areas activated during the cue period
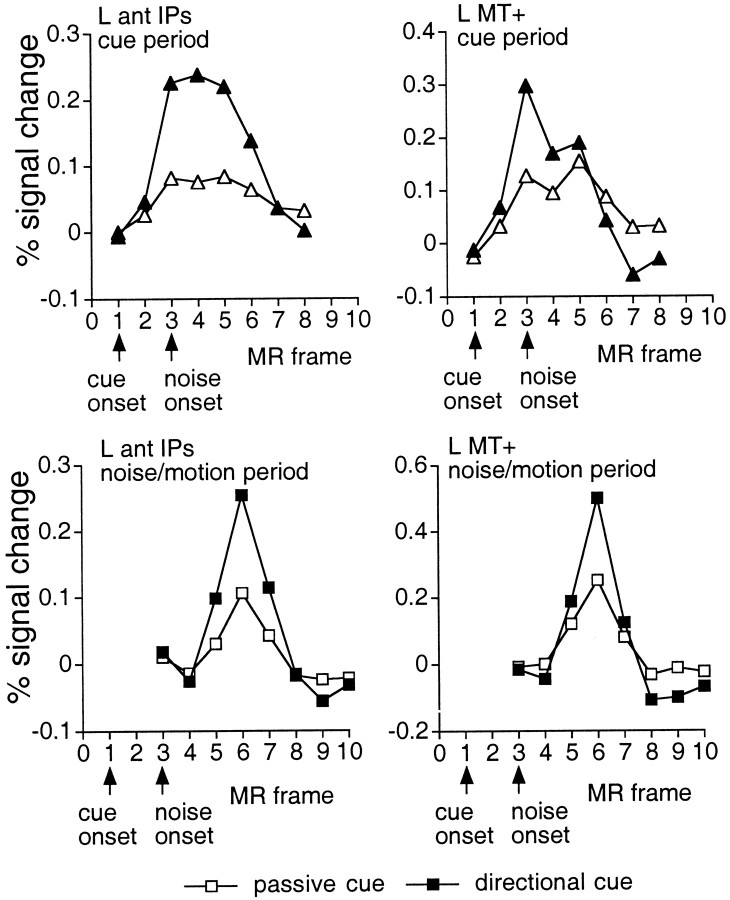
Motion-sensitive regions. We compared these motion-sensitive regions with the regions active during the cue period in which no motion was present. Activations were observed in ant IPs, vIPs, lateral occipital area, MT+, the anterior and posterior collateral sulcus, and the lateral precentral sulcus (Table 4, Fig.2B, middle row), with all areas showing greater activation after directional cues than passive cuesc [left (L) ant IPs,F(7,77) = 6.67; p < 0.0001; right (R) ant IPs, F(7,77) = 6.64; p < 0.0001; L vIPs,F(7,77) = 5.72; p < 0.0001; R vIPs, F(7,77) = 6.80;p < 0.0001; L lateral occipital area,F(7,77) = 7.09; p < 0.0001; R lateral occipital area,F(7,77) = 2.15; p < 0.05 L MT+, F(7,77) = 6.84;p < 0.0001; R MT+,F(7,77) = 3.45;p < 0.005; L precentral,F(7,77) = 3.51; p < 0.005; R precentral, F(7,77) = 4.55;p < 0.0005]. Figure 7,top row, shows the time course of activity during the cue period in L ant IPs and L MT+ after directional and passive cues. Both regions showed larger activations after directional cues.
Fig. 7.
Top row, Group time courses during the cue period (within-trial model) for two regions, L ant IPs and L MT+, which showed significantly larger activations in the directional cue than passive cue condition. Time courses are based on the ROIs used in the ANOVAs to compare directional and passive cues. Bottom row, Group time courses during the noise/motion period (within-trial model) for two regions that showed significantly larger activations in the directional cue than passive cue condition.
Motion-insensitive regions. Several motion-insensitive areas were also active during the cue period. The pos IPs was only activated during the cue period, with little or no activation observed during the test period (Table 5, Fig. 8; also compare the cue period activations in Fig. 2B, second row, with the activations during the noise/motion period in Fig.2B, third row). The between trial-model (Fig. 8,left panel) yielded very similar time courses for trials involving only a cue period and trials involving both cue and test periods. Correspondingly, the within-trial model (Fig. 8,middle panel) yielded significant activations during the cue period but only nonsignificant activity during the test period. As shown in Figure 8, right panel, this same region also showed greater activation during the directional cue than neutral cue condition in Experiment 1 (also see Fig. 2A, third row), as well as greater activation after directional cues than passive cues in Experiment 2 (L pos IPs,F(7,77) = 9.42; p < 0.0001; R pos IPs, F(7,77) = 12.2;p < 0.0001).
Fig. 8.
Left,middle panels, Group time courses in pos IPs from the directional cue condition during cue, noise, and noise/motion trials (between-trial model, left panel) or periods (within-trial model, middle panel). Time courses are based on an 3 × 3 voxel ROI centered on a voxel (Talairach coordinate = −23, −71, 46) yielding a peak Z score in the z-map for the cue period (Table 3). Significant activation is seen during the cue period but not during either the noise or noise/motion period.Right panel, Group time courses in Experiment 1 for the directional cue versus passive cue condition and neutral cue versus passive cue condition at the same voxel illustrated in the top row for Experiment 2. Activations are significantly larger for the directional than neutral cue condition.
Because the directional cue was a stationary arrow, one might also expect to see activations in ventral extrastriate regions that are involved in shape analysis and the determination of the direction of the arrow. Correspondingly, activations were found in a right hemisphere fusiform region, which was near a region reported by Haxby et al. (1994) as responding strongly to faces and was more active after a directional cue than passive cue (F(7,77) = 6.66; p < 0.0001). It may participate in the initial analysis of the shape of the arrow cue.
Interestingly, the directional cue did not significantly activate prefrontal regions. Although this is a null result and must be treated cautiously, the absence of prefrontal activations raises the possibility that instruction signals for visual tasks are not necessarily generated in prefrontal cortex. Frontal activations were confined to the junction of the left medial precentral sulcus and the left superior frontal sulcus. This activation was stronger after a directional than passive cue (F(7,77)= 7.64; p < 0.0001).
Correspondence of cue period activations and directionally specific activations. The regions from Experiment 1 that showed directionally specific activations were very similar to those active during the cue period. This result suggests that the activations during the cue period partly reflected the use of directionally specific cue information, although signals reflecting a general set for motion may also have been present. This argument seems particularly strong for pos IPs, because this region was not active during the test period. Figure2A compares the group image for the directional cue minus neutral cue contrast from Experiment 1 (third row) with the group image for the directional cue minus passive cue contrast from the cue period in Experiment 2 (bottom row). Both contrasts remove purely sensory effects. Very similar activations were observed in both ant and pos IPs, the left medial precentral sulcus, and left MT+. Table 7 lists the 15 foci with the largest Z scores from each contrast. All foci had passed the multiple correction procedure for statistical significance. Two foci from the directional cue minus neutral cue contrast were not included because they were actually produced by larger BOLDdecreases in the neutral than directional condition (i.e., neither the directional or neutral cue condition showed BOLD signal increases at that coordinate). Both contrasts showed the most robust activations in the same areas: ant and pos IPs, vIPs, the junction of the left medial precentral sulcus and the superior frontal sulcus, and left MT+. In many cases, coordinates were quite similar, even though the two experiments involved, among other things, different subjects, experimental design, and analysis techniques.
Table 7.
Comparison of directionally specific activations from Experiment 1 and cue period activations from Experiment 2
| Region | Cue period: directional minus passive (Exp 2) | Directional cue vs neutral cue (Exp 1) | ||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Zscore | x | y | z | Zscore | |
| L pos fusiform | −43 | −75 | −16 | 5.2 | ||||
| L MT+ | −41 | −75 | −6 | 4.4 | −43 | −69 | −6 | 6.2 |
| R lateral occ | 29 | −83 | 10 | 5.2 | ||||
| L vIPs | −25 | −71 | 22 | 4.7 | −23 | −73 | 30 | 4.9 |
| −29 | −83 | 24 | 4.6 | |||||
| R vIPs | 31 | −69 | 30 | 4.6 | ||||
| L ant IPs | −29 | −57 | 56 | 5.2 | ||||
| −35 | −47 | 48 | 6.4 | |||||
| −29 | −47 | 40 | 4.7 | −33 | −43 | 38 | 5.8 | |
| R ant IPs | 33 | −57 | 48 | 4.2 | 31 | −51 | 48 | 5 |
| 41 | −43 | 50 | 4.1 | |||||
| 31 | −45 | 40 | 5.8 | |||||
| L pos IPs | −9 | −67 | 54 | 5.4 | −15 | −67 | 52 | 8.9 |
| −23 | −69 | 48 | 5.3 | −23 | −71 | 46 | 8.5 | |
| −13 | −75 | 46 | 8.8 | |||||
| −15 | −61 | 68 | 5.5 | |||||
| R pos IPs | 17 | −65 | 50 | 5.4 | 15 | −67 | 42 | 7.2 |
| 25 | −71 | 40 | 4.3 | 21 | −71 | 46 | 6.4 | |
| L precuneus | −17 | −71 | 38 | 4.7 | ||||
| L precentral/SFs | −29 | −9 | 52 | 4.1 | −25 | −13 | 56 | 4.9 |
| R precentral/SFs | 25 | −11 | 52 | 4.1 | ||||
Areas activated during the test period
Motion-sensitive regions. The activations during the test period were more widespread than during the cue period and included most of the motion-sensitive areas defined by the radial motion condition (Fig. 2B, third row). Most areas were more active after a directional than passive cue (Fig. 7, bottom row) (L ant IPs, F(7,77) = 2.80;p < 0.05; R ant IPs,F(7,77) = 4.95; p < 0.0001; L vIPs, F(7,77) = 4.24;p = 0.0005; R vIPs,F(7,77) = 4.78; p < 0.0005; L MT+, F(7,77) = 9.96;p < 0.0001; R MT+,F(7,77) = 5.50; p < 0.0001; L lateral occipital area,F(7,77) = 2.28; p < 0.05; R lateral occipital area,F(7,77) = 4.85; p = 0.0001; L ant collateral sulcus,F(7,77) = 5.97; p < 0.0001; R ant collateral sulcus,F(7,77) = 6.72;p < 0.0001; R pos collateral sulcus,F(7,77) = 3.25; p < 0.005; R STg/SMg, F(7,77) = 4.23;p < 0.001). Previous studies have also reported modulations in motion-sensitive regions (Corbetta et al., 1991; Dupont et al., 1994; Beauchamp et al., 1997; O'Craven et al., 1997; Buchel et al., 1998; Cornette et al., 1998; Culham et al., 1998).
Motion-insensitive regions. During the test period (i.e., both for noise and noise/motion periods), many areas were observed that were not activated during radial motion or during the cue period (Fig.9, Table 6), including large sections of both dorsal and ventral precentral sulcus, SMA (Fig. 5) and pre-SMA, anterior cingulate, thalamus, basal ganglia, and the insula/frontal operculum (Fig. 9). Relatively weaker activations were also found in a region of DLPFC similar to that described in studies of working memory (Owen et al., 1996; Smith et al., 1996; Cohen et al., 1997). Modest but significant activation of DLPFC had also been observed in the neutral and directional cue conditions of Experi-ment 1. As shown in Figure 9,bottom row, DLPFC was not significantly active during the cue period of Experiment 2.d
Fig. 9.
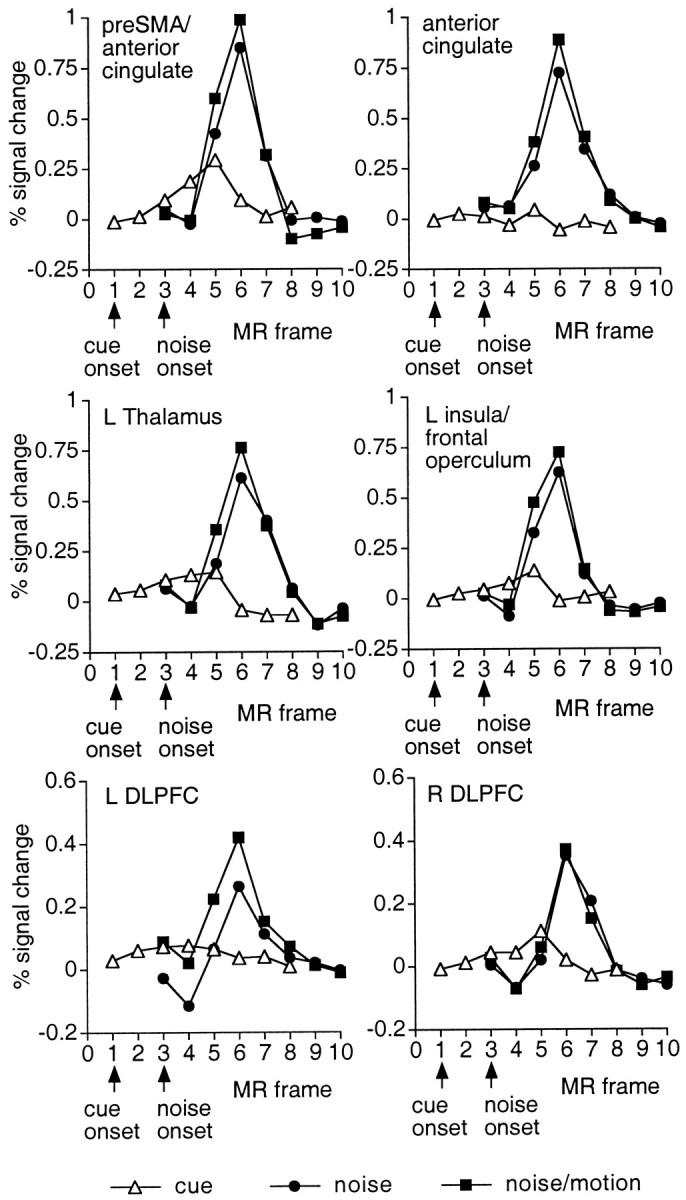
Group time courses during the cue, noise, and noise/motion periods. Time courses are based on a 3 × 3 voxel ROI centered on the voxel in the z-map for the noise/motion period that yielded a peak Z score (preSMA/anterior cingulate, Talairach coordinate = 1, 7, 46; anterior cingulate, 3, 23, 32; L insula/frontal operculum, −31, 15, 4; L thalamus, −9, 21, 8; L DLPFC, −35, 47, 20; R DLPFC, 33, 37, 30). Significant activation is evident during the test (noise, noise/motion) period but not the cue period.
Effects of detection and response. Most of the activations during the test period were present on trials in which a response was made or withheld, indicating that they did not require target detection or a motor response. However, some areas were modulated by this factor. A within-subject ANOVA compared the activations during the noise/motion period of trials in which motion was detected (i.e., a response was made) or not detected (i.e., no response). A similar analysis was not conducted on the data from the noise period (i.e., false alarms or correct detections), because fewer noise trials were presented, and the false alarm percentage for some subjects was quite low (<5%).
Significantly larger activations on noise/motion trials involving a response (see Fig. 5 for examples of time courses) were found in L central sulcus (F(7,77) = 10.7;p < 0.0001), L parietal operculum (F(7,77) = 5.71; p < 0.001), R cerebellum (F(7,77) = 4.59;p < 0.001), L thalamus (F(7,77) = 2.4; p < 0.05), R thalamus (F(7,77) = 3.22;p = 0.005), L putamen (F(7,77) = 3.71; p < 0.005), L lateral posterior frontal operculum (F(7,77) = 4.16; p = 0.001) (an area distinct from the region shown in Fig. 9), and a region in the anterior cingulate (F(7,77) = 2.56; p < 0.05). Although some of these effects were undoubtedly attributable to motor factors, it is possible that some reflected processes related to detection.
Larger activations on no-response trials were found in a number of motion-sensitive visual areas (Fig. 10,top two rows), including L IPs (F(7,77) = 2.3; p < 0.05), R IPs (F(7,77) = 4.61;p < 0.001), L vIPs (F(7,77) = 5.21; p < 0.001), R vIPs (F(7,77) = 3.69;p < 0.005), L MT+ (F(7,77) = 3.96; p = 0.001), and L lateral occipital area (F(7,77) = 4.47; p < 001), with marginal effects in the L pos collateral sulcus (F(7,77) = 2.08; p = 0.055) and R pos collateral sulcus (F(7,77) = 2.14; p = 0.049). Because subjects presumably stopped searching for a target once one had been detected, this effect probably reflects modulations produced by active processing of visual stimuli. In some of these areas, response and no-response time courses were initially similar, but the response time course fell off more rapidly than the no-response time course (Fig. 10, top two rows).
Fig. 10.
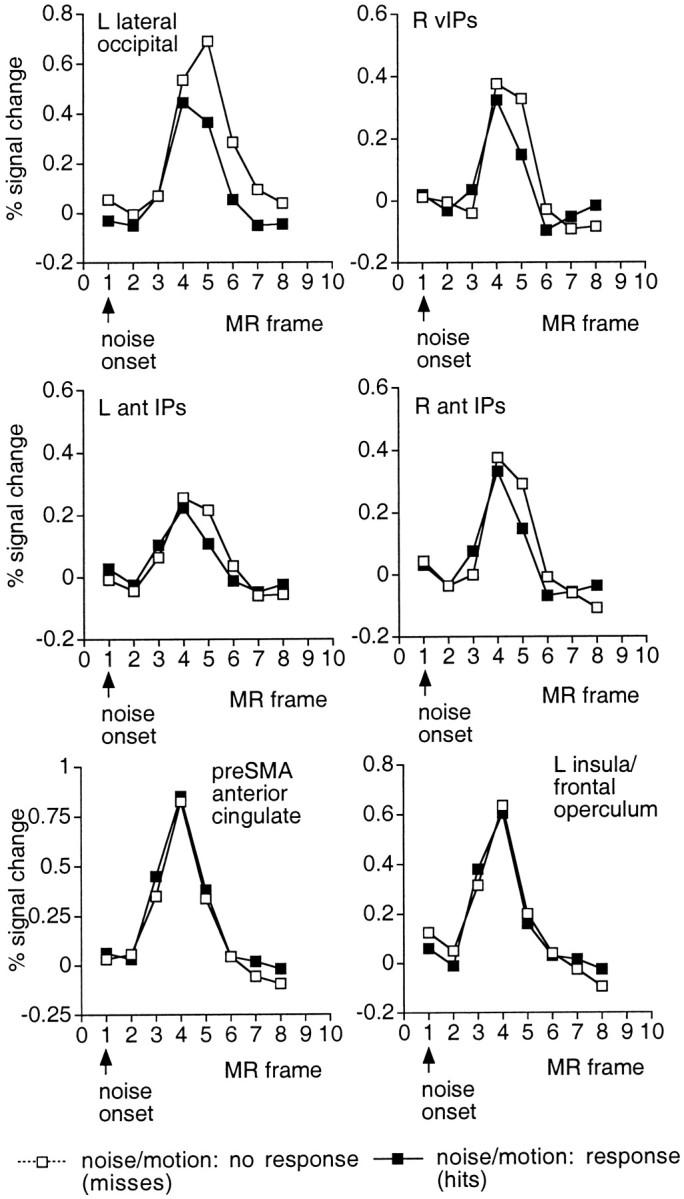
Group time courses during the noise/motion period as a function of whether a motor response was executed (filled symbols, hits, response; open symbols, misses, no response). Time courses are based on the ROIs used in the ANOVA to evaluate the effect of response/detection on activation. The top two rows show responses in motion-sensitive occipital and parietal areas. Activations were significantly larger when targets were missed and search could not be terminated. The bottom row shows activations in two frontal areas that were unaffected by response execution and target detection.
No significant effects of response and detection were found in the frontal operculum, SMA, and pre-SMA, more dorsal regions of the anterior cingulate, both dorsal and ventral precentral sulcus, and R DLPFC. Although null results must be treated cautiously,e in some areas the two functions were highly similar (Fig. 10, bottom row; a close correspondence was also found for activations in the dorsal anterior cingulate and precentral regions). Because processes sensitive to trial duration should produce larger activations in the no-response condition (Fig. 10, top two rows), these “null effect” areas presumably reflect processes independent of trial duration that occur once during the test period, such as the result of a decision process (respond or do not respond). A decision outcome would presumably occur earlier for response trials than no-response trials, because a target-absent decision could not be made until the trial was over. Time course functions for response trials should therefore be shifted about one-half an MR frame earlier than the functions for no-response trials, because a target appeared on average ∼1100 msec before the end of the trial. Although acknowledging the relatively coarse time sampling of the present experiment, there was little evidence for this shift in these null effect areas, suggesting that the activation was triggered at a similar time point (e.g., the beginning or end of the dynamic noise) for both response and no-response conditions.
GENERAL DISCUSSION
The results of Experiment 2 indicate that a stationary cue specifying a direction of motion activated a restricted set of regions. This set included areas sensitive to radial motion, such as MT+, the lateral occipital area, vIPs, ant IPs, and the lateral precentral sulcus, as well as regions not responding to radial motion, such as pos IPs, the junction of the left medial precentral sulcus and the superior frontal sulcus, and a right fusiform region. These areas define a circuit apparently responsible for encoding and maintaining instruction signals during the cue period. Frontal activations during the cue period were confined to precentral regions. We did not find evidence that prefrontal cortex maintained the instruction signals in posterior regions that were observed in this task.
Pathways for the encoding and maintenance of instruction signals
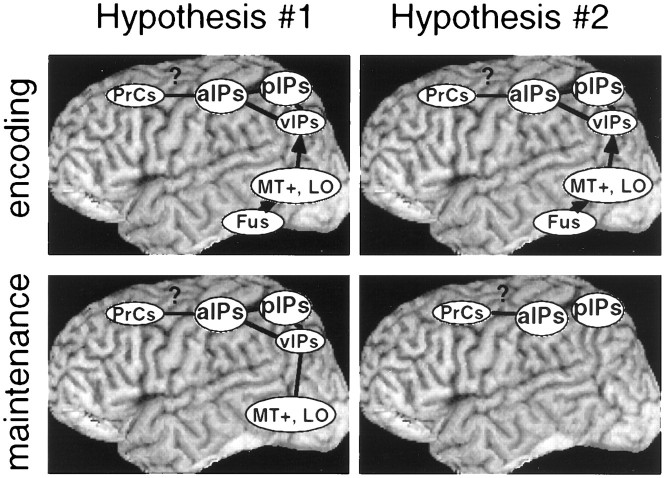
Although the present experiment cannot detail the exact function of the different parts of this circuit, it is worth noting two possibilities (Fig. 11). The first step in both is presumably to analyze the shape of the cue. This may partly occur in motion-insensitive fusiform areas, which have been implicated in neuroimaging studies of shape perception (Corbetta et al., 1991;Haxby et al., 1994; Wojciulik et al., 1998). The direction information extracted from the cue then needs to be recoded into a format that can influence the subsequent processing of the test stimulus. This transformation may involve motion-sensitive and motion-insensitive areas. The final encoded format, which can be maintained over the duration of the cue interval, may include some stages of the motion pathway defined by the radial motion condition (e.g., MT+ or the lateral occipital area), as well as motion-insensitive regions (Fig.11, Hypothesis #1). Alternatively, the final format may only involve later stages such as ant IPs, together with motion-insensitive regions (Fig. 11, Hypothesis#2). More ventral areas may only assist in the transformation of a fusiform shape code to a dorsal parietal motion code.
Fig. 11.
Two hypotheses concerning the areas that encode and maintain instruction signals. Hypothesis#1 asserts that both ventral and dorsal visual areas are used during both encoding and maintenance, whereasHypothesis #2 asserts that ventral areas assist during encoding but that the resulting instruction signals are maintained in parietal and possibly precentral regions.PrCs, Precentral sulcus; a, anterior;p, posterior; v, ventral;Fus, fusiform gyrus.
It may be possible to distinguish these two possibilities by extending the cue duration. The first hypothesis suggests that both ventral motion-sensitive areas and parietal areas should show sustained activity over the cue interval. The second hypothesis suggests that only parietal cortex should show sustained activity, whereas more ventral motion-sensitive areas should be transiently active during the transformation of the cue. The current experiment did not manipulate cue duration. Additionally, the uptick on frame 5 caused by the cue offset complicated any clear distinction between transient and sustained behavior. Ventral motion-sensitive areas, however, appeared to show a more transient time course, whereas dorsal areas, particularly pos IPs, showed a more sustained time course (e.g., compare the time course for MT+ in Fig. 7 with the time course for ant IPs in Fig. 7 or for pos IPs in Fig. 8). If confirmed, this result is consistent with hypothesis 2.
Experiment 2 did not involve a neutral cue condition. As a conservative approach with a new method, we collected more data with fewer conditions to increase the stability of the results. Although the correspondence of the cue period activations from Experiment 2 and the directionally specific activations from Experiment 1 suggest that the instruction signals observed during the cue period included directionally specific information, signals reflecting a general set for motion may also have been present.
Differences between the directional cue and passive cue conditions of Experiment 2 could reflect changes in arousal. Although a contribution of arousal cannot be ruled out with the present design, several points argue against this interpretation. First, the contrast between directional cues and neutral cues in Experiment 1 was controlled for arousal, yet the directional cue minus neutral cue activations in that experiment were very similar to the directional cue minus passive cue activations in Experiment 2. This argument seems particularly strong for the pos IPs activation, because it showed a robust directional minus neutral activation in Experiment 1 yet was only active during the cue period (not the test period) of Experiment 2 (Fig. 8). Second, we did not find significant modulations during the cue period in some visual regions that were significantly active during the test period, such as the calcarine sulcus and SMg/STg (Tables 4, 5). Only a subset of the visual regions that were active during the test period were also active during the cue period. Finally, responses in the parietal lobe appeared more sustained than those in the occipital lobe, suggesting that parietal regions may store the cue information. Time course differences between regions suggest that these regions play different roles in encoding and maintaining cue information and are not consistent with an arousal explanation.
Attentional modulations during the test period
During the test period, most motion-sensitive regions were modulated with respect to the passive condition. These attentional modulations have been demonstrated in many studies (Corbetta et al., 1991; Dupont et al., 1994; Beauchamp et al., 1997; O'Craven et al., 1997; Buchel et al., 1998; Cornette et al., 1998) and may reflect, in part, the interaction of instruction signals with the sensory signal during the test period. This interaction may occur because the instruction signals are either present throughout the motion pathway during both the cue and test periods (hypothesis 1) or are stored in parietal areas during the cue period and fed down during the test period (e.g., hypothesis 2). Single-unit studies of attentional modulations have shown that attention can increase the amplitude of the response of MT cells to moving stimuli (Treue and Maunsell, 1996). Moreover, this enhancement can be purely feature-based (i.e., nonspatial) (Treue and Trujillo, 1999). The attentional modulations in the present experiment may reflect these enhancements in single-unit activity.
Role of frontal cortex in the encoding, maintenance, and application of instruction signals
Prefrontal regions are thought to play important roles in working memory, which is typically defined as a limited capacity memory capable of transforming information in various ways. Anatomical work in nonhuman primates indicates that DLPFC and parietal cortex are richly interconnected (Goldman-Rakic, 1987), raising the possibility that instruction signals in parietal cortex may be maintained by signals from DLPFC.
It is therefore interesting that cue-related areas did not include prefrontal cortex, even though the symbolic cue information had to be translated into a format appropriate for influencing motion detection, and this format needed to be maintained for almost 5 sec. Frontal activations during the cue period were confined to precentral regions (i.e., the precentral sulcus and its intersection with the superior frontal sulcus).f Because parietal cortex was active during the cue period in the apparent absence of DLPFC activity, any linkage between parietal and frontal cortex during the cue period would have to be attributed to precentral regions.
Although the absence of activity in prefrontal regions during the cue interval is a null result and should be treated conservatively, prefrontal areas such as DLPFC and frontal operculum/insula, which are often found in imaging studies of working memory (Smith et al., 1995;Owen et al., 1996; Smith et al., 1996; Cohen et al., 1997; Courtney et al., 1997), were clearly activated during the test period. Working memory might be involved in applying the directional cue information to target detection during the test period. DLPFC and frontal operculum were activated equally well, however, in the directional and neutral cue conditions of Experiment 1, indicating that these areas were not involved in matching potential targets to the specified cue direction. DLPFC and frontal operculum were also activated both when subjects made or withheld a response, indicating that activation was not contingent on target detection or motor execution.
Some neuroimaging studies of working memory have concluded that prefrontal cortex is involved in maintaining information in working memory (Fiez et al., 1996; Cohen et al., 1997). Other studies have suggested that maintenance occurs in posterior association areas in the parietal and temporal lobe, and that prefrontal cortex is only active when information in storage must be transformed or processed in some fashion (Owen et al., 1996; Petrides, 1996; Smith et al., 1998). The results from the current study are more consistent with the latter view. It is possible, however, that prefrontal involvement during the cue period will be observed if the duration of the cue period is increased (Fiez et al., 1996), if the cue is presented in a more abstract format (e.g., linguistic), if the task involves match to sample (Miller et al., 1996) or recognition rather than simple detection, if the task requires a shift between rather than within perceptual dimensions (Owen et al., 1991), or if the task requires the storage of semantic (Fiez et al., 1996) or temporal order information (Smith et al., 1998), rather than the storage of simple visual attributes.
Methodological implications
The present results indicate that activations during the cue period can be isolated from those during the subsequent test period. This was most clearly shown by the restriction of central sulcus responses to the test period of trials in which a response was made (Fig. 5). Furthermore, separation of cue and test responses occurred even when a voxel was activated during both periods. The time course of the cue response was unaffected by the occurrence of a subsequent response during the test period (Fig. 6). Finally, the cue and test period activations of Experiment 2 largely summed to mirror the activations observed in the blocked design of Experiment 1, which combined both periods.
Critically, the separation of cue and test responses occurred in the absence of any assumption concerning the shape of the hemodynamic response. This is important, because it is difficult to predict the exact time course of different cognitive processes in different neural areas. The present techniques should be widely applicable to tasks in which different cognitive processes occur in different periods within a trial. They can be directly applied, for example, to any situation in which preparation for a task can be separated from task execution.
Conclusions
(1) Linear models can be used to separate the BOLD signals from successive intervals within a task, without making any assumption about the shape of the hemodynamic response function.
(2) A stationary arrow cue that specified direction of motion enhanced the BOLD signal during a motion detection task, relative to a noninformative cue, in both dorsal (e.g., ant IPs) and ventral (e.g., left MT+) motion-sensitive regions (Experiment 1) as well as motion-insensitive regions (e.g., pos IPs).
(3) The arrow cue produced modulations in motion-sensitive regions before the onset of the test period in which moving stimuli were presented (Experiment 2), indicating that these regions were involved in the encoding and/or maintenance of instruction signals. The correspondence between these cue-related regions and the directionally specific regions from Experiment 1 suggests that the instruction signals in these regions included directionally specific information.
(4) The cue also produced modulations in motion-insensitive regions (Experiment 2), indicating a role for these regions in the encoding and/or maintenance of instruction signals. The most robust activation occurred in pos IPs. This region produced directionally specific activations in Experiment 1 but was not active during the test period (Experiment 2) and therefore contained instruction signals that included directionally specific information.
(5) Activations in precentral regions (Experiment 2) may maintain the instruction signals found in posterior regions, but we did not find evidence of instruction signals in prefrontal regions such as DLPFC or the insula/frontal operculum.
(6) In contrast, the DLPFC and frontal operculum were active during the test period, irrespective of whether stimuli were detected or motor responses were executed (Experiment 2). These regions were not sensitive to directionally specific cue information (Experiment 1) and therefore did not apply directional instruction signals to the detection of the test stimulus.
(7) Therefore, the cue and test intervals in a cued motion detection task activated overlapping sets of regions. Some regions were activated during both intervals; some were specific to the cue interval (e.g., pos IPs); and others were specific to the test interval (e.g., STg/SMg, thalamus, DLPFC, anterior cingulate, and frontal operculum/insula).
Footnotes
This research was supported by National Institutes of Health Grants EY00379A, EY12148, NS32979, and NS06833, the McDonnell Center for Higher Brain Function, and the Charles A. Dana Foundation.
Correspondence should be addressed to Gordon Shulman, Department of Neurology, Box 8111, Washington University, 660 South Euclid, St. Louis, MO 63110. E-mail: gordon@npg.wustl.edu.
aA reviewer also noted the possibility that subjects may use a different strategy in the neutral and directional cue conditions. Although subjects in the directional cue condition may monitor directionally specific motion channels, subjects in the neutral cue condition may look for changes in the overall speed distribution that are caused by the shift from dynamic noise to coherent motion. Even if this account is correct, subjects are using directional cue information in one condition and not the other.
bThe label MT+ (Beauchamp et al., 1997) indicates that this region may contain several areas, rather than just MT. Several motion-selective areas, for example, have been found in the superior temporal sulcus of the macaque (Desimone and Ungerleider, 1986).
cAs noted above, the cue time courses for most areas showed an uptick on frame 5. However, many of the active-passive differences during the cue period were also significant even when the contrast between the directional and passive conditions was restricted to frame 3, before the uptick. The exceptions were the left precentral gyrus, left lateral occipital area, and R MT+,although in all cases the means were in the expected direction. The image that combined the directional and passive cue conditions did not produce well defined activations in motion-sensitive fusiform regions. These regions were therefore not tested.
dThe DLPFC activation showed a slightly delayed time course, with no activation observed in the first three frames after test onset. This late time course reduced the observed Zscores, because the HRFs that were cross-correlated with the time courses obtained from the linear model were not sufficiently delayed. To check whether prefrontal or other activations might have been missed because of a late time course, HRFs with longer delays were used in the cross-correlation. Probing at longer delays did not uncover any new areas in the cue period image. For the test period image, the use of longer delays increased the Z scores in a number of regions, including visual regions, but most of these were already clearly present in the z-images determined with the shorter delays. Perhaps the most evident effect was in DLPFC, in which initially weak activations were raised to a modest level. In all cases, the coordinates of the voxel showing a peak Z score obtained with the earlier delays were very similar to those obtained from probing at slightly longer delays. Correspondingly, similar time courses were obtained with ROIs defined from z-maps derived with HRFs of slightly different delays.
eSMA, for example, showed a nonsignificant trend toward larger activations in the response condition. It is possible that greater activation on response trials in SMA attributable to motor execution was offset by the larger activation on no-response trials from motor readiness processes that were maintained for a longer duration.
fCourtney et al. (1998) have suggested that spatial working memory tasks, in which spatial locations must be maintained in memory for short periods, involve a region in the superior frontal sulcus just anterior to the frontal eye fields in the precentral region. Analysis of individual data suggested that the present medial precentral activation was related more to the precentral than superior frontal sulcus, but because the activations reported by Courtney et al. were extremely close in location, the correspondence between the present focus and their foci is unclear.
REFERENCES
- 1.Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. doi: 10.1152/jn.1990.64.1.133. [DOI] [PubMed] [Google Scholar]
- 2.Ball K, Sekuler R. Models of stimulus uncertainty in motion perception. Psychol Rev. 1980;87:435–469. [PubMed] [Google Scholar]
- 3.Beauchamp MS, Cox R, DeYoe EA. Graded effects of spatial and featural attention on human area MT and associated motion processing regions. J Neurophysiol. 1997;78:516–520. doi: 10.1152/jn.1997.78.1.516. [DOI] [PubMed] [Google Scholar]
- 4.Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchel C, Josephs O, Rees G, Turner R, Frith C, Friston K. The functional anatomy of attention to visual motion. Brain. 1998;121:1281–1294. doi: 10.1093/brain/121.7.1281. [DOI] [PubMed] [Google Scholar]
- 6.Buckner RL, Bandettini PA, O'Craven KM, Savoy RL, Petersen SE, Raichle ME, Rosen BR. Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:14878–14883. doi: 10.1073/pnas.93.25.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton H, MacLeod A, Videen TO, Raichle ME. Multiple foci in frontal and parietal cortex activated by rubbing embossed grating pattern across fingerpads: a positron emission tomography study in human. Cereb Cortex. 1997;7:3–17. doi: 10.1093/cercor/7.1.3. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–607. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 9.Conturo TE, RCM, Akbudak E, Snyder AZ, Yang T, Raichle ME. Sensitivity optimization and experimental design in functional magnetic resonance imaging. Soc Neurosci Abstr. 1996;22:7. [Google Scholar]
- 10.Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornette L, Dupont P, Rosier A, Sunaert S, Hecke P, Hichiels J, Mortelmans L, Orban GA. Human brain regions involved in direction discrimination. J Neurophysiol. 1998;79:2749–2765. doi: 10.1152/jn.1998.79.5.2749. [DOI] [PubMed] [Google Scholar]
- 13.Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- 14.Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- 15.Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RBH. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- 16.Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 18.Desimone R, Ungerleider LG. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J Comp Neurol. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupont P, Orban GA, De Bruyn B, Verbruggen A, Mortelmans L. Many areas in the human brain respond to visual motion. J Neurophysiol. 1994;72:1420–1424. doi: 10.1152/jn.1994.72.3.1420. [DOI] [PubMed] [Google Scholar]
- 20.Dupont P, De Bruyn B, Vandenberghe R, Rosier A, Michiels J, Marchal G, Mortelmans L, Orban G. The kinetic occipital region in human visual cortex. Cereb Cortex. 1997;7:283–292. doi: 10.1093/cercor/7.3.283. [DOI] [PubMed] [Google Scholar]
- 21.Epstein FH, Mugler JPI, Brookeman JR. Optimization of parameter values for complex pulse sequences by simulated annealing: application to 3D MP-RAGE imaging of the brain. Magn Reson Med. 1994;31:164–177. doi: 10.1002/mrm.1910310210. [DOI] [PubMed] [Google Scholar]
- 22.Ferrera VP, Rudolph KK, Maunsell JHR. Responses of neurons in the parietal and temporal visual pathways during a motion task. J Neurosci. 1994;14:6171–6186. doi: 10.1523/JNEUROSCI.14-10-06171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiez JA, Raife EA, Balota D, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friston KJ, Josephs O, Rees G, Turner R. Nonlinear event-related responses in fMRI. Magn Reson Med. 1998;39:41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- 25.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 26.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representation memory. In: Plum F, Mountcastle V, editors. The handbook of physiology: section 1. The nervous system, Vol V. Higher functions of the brain, Pt 1. American Physiological Society; Bethesda, MD: 1987. pp. 373–417. [Google Scholar]
- 27.Haenny PE, Schiller PH. State dependent activity in visual cortex. I. Single cell activity in V1 and V4 on visual tasks. Exp Brain Res. 1988;69:225–244. doi: 10.1007/BF00247569. [DOI] [PubMed] [Google Scholar]
- 28.Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Munte TF, Gos A, Scherg M, Johannes S, Hundeshagen H, Gazzaniga MS, Hillyard SA. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- 30.Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos Trans R Soc Lond B Biol Sci. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 32.Lu ZL, Dosher BA. External noise distinguishes attention mechanisms. Vision Res. 1998;38:1183–1198. doi: 10.1016/s0042-6989(97)00273-3. [DOI] [PubMed] [Google Scholar]
- 33.Mangun GR, Hopfinger JB, Kussmaul CL, Fletcher EM, Heinze H. Covariation in ERP and PET measures of spatial selective attention in human extrastriate cortex. Hum Brain Mapp. 1997;5:273–279. doi: 10.1002/(SICI)1097-0193(1997)5:4<273::AID-HBM12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 34.Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mintun MA, Fox PT, Raichle ME. A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography. J Cereb Blood Flow Metab. 1989;9:96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- 37.O'Craven KM, Rosen BR, Kwong KK, Treisman A, Savoy RL. Voluntary attention modulates fMRI activity in human MT-MST. Neuron. 1997;18:591–598. doi: 10.1016/s0896-6273(00)80300-1. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast depedent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ollinger JM. Correcting for multiple comparisons in fMRI activation studies with region-size dependent thresholds. Paper presented at the 5th Scientific Meeting of the International Society for Magnetic Resonance in Medicine 1997. Vancouver, April, 2:1672.
- 40.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions, or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- 41.Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: A positron emission tomography study. Cereb Cortex. 1996;6:31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- 42.Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1455–1461. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- 43.Reppas JB, Niyogi S, Dale AM, Sereno MI, Tootell RBH. Representation of motion boundaries in retinotopic human visual cortical areas. Nature. 1997;388:175–179. doi: 10.1038/40633. [DOI] [PubMed] [Google Scholar]
- 44.Shulman GL, Schwarz J, Miezin FM, Petersen SE. The effect of motion contrast on human cortical responses to moving stimuli. J Neurophysiol. 1998;79:2794–2803. doi: 10.1152/jn.1998.79.5.2794. [DOI] [PubMed] [Google Scholar]
- 45.Smith EE, Jonides J, Koeppe RA, Awh E, Schumacher EH, Minoshima S. Spatial versus object working memory: PET investigations. J Cogn Neurosci. 1995;7:337–356. doi: 10.1162/jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- 46.Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- 47.Smith EE, Jonides J, Marshuetz C, Koeppe R. Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci USA. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain (Rayport M, translator). Thieme; New York: 1988. [Google Scholar]
- 49.Tanji J, Taniguchi K, Saga T. Supplementary motor area: neuronal response to motor instructions. J Neurophysiol. 1980;43:60–68. doi: 10.1152/jn.1980.43.1.60. [DOI] [PubMed] [Google Scholar]
- 50.Tootell RBH, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tootell RBH, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- 52.Treue S, Maunsell JHR. attention modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 53.Treue S, Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- 54.Vandenberghe R, Dupont P, Debruyn B, Bormans G, Michiels J, Mortelmans L, Orban GA. The influence of stimulus location on the brain activation pattern in detection and orientation discrimination-a PET study of visual attention. Brain. 1996;119:1263–1276. doi: 10.1093/brain/119.4.1263. [DOI] [PubMed] [Google Scholar]
- 55.Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- 56.Wojciulik E, Kanwisher N, Driver J. Covert visual attention modulates face-specific activity in the human fusiform gyrus: fMRI study. J Neurophysiol. 1998;79:1574–1578. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- 57.Woldorff MG, Fox PT, Matzke M, Lancaster JL, Veeraswamy S, Zamarripa F, Seabolt M, Glass T, Gao JH, Martin CC, Jerabek P. Retinotopic organization of early visual spatial attention effects as revealed by PET and ERPs. Hum Brain Mapp. 1997;5:280–286. doi: 10.1002/(SICI)1097-0193(1997)5:4<280::AID-HBM13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 58.Zarahn E, Aguirre G, D'Esposito M. A trial-based experimental design for fMRI. NeuroImage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- 59.Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]