Abstract
The present study expands the contemporary view of mitochondria as important participants in cellular Ca2+ dynamics and provides evidence that mitochondria regulate the supply of release-competent secretory granules. Using pharmacological probes to inhibit mitochondrial Ca2+ import, the ability of mitochondria to modulate secretory activity in single, patch-clamped bovine chromaffin cells was examined by simultaneously monitoring rapid changes in membrane surface area (ΔCm) and cytosolic Ca2+ levels ([Ca2+]c). Repetitive step depolarizations or action potential waveforms were found to raise the [Ca2+]c of chromaffin cells into the 1 μm to tens of micromolar range. Inhibiting mitochondria by treatment with carbonyl cyanidep-(trifuoro-methoxy)phenylhydrazone, antimycin–oligomycin, or ruthenium red revealed that mitochondria are a prominent component for the clearance of Ca2+ that entered via voltage-activated Ca2+ channels. Disruption of cellular Ca2+ homeostasis by poisoning mitochondria enhanced the secretory responsiveness of chromaffin cells by increasing the amplitude of the transient rise and the time course of recovery to baseline of the evoked Δ[Ca2+]c. The enhancement of the secretory response was represented by significant deviation of the Ca2+–exocytosis relationship from a standard relationship that equates Ca2+ influx and ΔCm. Thus, mitochondria would play a critical role in the control of secretory activity in chromaffin cells that undergo tonic or repetitive depolarizing activity, likely by limiting the Ca2+-dependent activation of specific proteins that recruit or prime secretory granules for exocytosis.
Keywords: membrane capacitance, exocytosis, FCCP, fura-2, furaptra, readily releasable pool
The immediate exocytotic release of neurotransmitters from synaptic vesicles at the active zone of a synaptic bouton is governed by Ca2+ influx and the rapid collapse of microdomains of high [Ca2+]c by diffusion (Neher, 1998). Colocalization of secretory granules and Ca2+ entry sites has also been proposed for adult bovine and calf chromaffin cells (Robinson et al., 1995;Elhamdani et al., 1998). In contrast, the exocytotic release of catecholamines from secretory granules is sensitive to changes in the exogenous Ca2+ buffering capacity. This observation may be explained by indications that only a small subset of granules colocalize with Ca2+ channels (Horrigan and Bookman, 1994; Klingauf and Neher, 1997). Although there is currently little direct evidence for mitochondrial Ca2+ dynamics regulating secretory responsiveness, the concept is reasonable because mitochondria have been postulated to function as the predominant Ca2+ clearance mechanism during prolonged or repetitive stimulus-activated Ca2+influx in sympathetic neurons (Thayer and Miller, 1990; Friel and Tsien, 1994; Werth and Thayer, 1994), adrenal chromaffin cells (Herrington et al., 1996; Park et al., 1996; Babcock et al., 1997; Xu et al., 1997), gonadotropes (Hehl et al., 1996), and neuroendocrine nerve endings (Stuenkel, 1994; Giovannucci and Stuenkel, 1997).
Mitochondria can sequester large amounts of calcium and function as a cytosolic Ca2+ buffer of low affinity and high capacity (Lehninger et al., 1967; Blaustein et al., 1977;Blaustein et al., 1978; Carafoli and Crompton, 1978; Carafoli, 1979;Nicholls and Akerman, 1982; Gunter et al., 1994). Pharmacologically induced or pathophysiologically mediated mitochondrial dysfunction leads to altered Ca2+ homeostasis in neurons (Thayer and Wang, 1995; Budd and Nicholls, 1996b;Schinder et al., 1996; Wang and Thayer, 1996; White and Reynolds, 1997;Nicholls and Budd, 1998). In addition, the notion that mitochondria participate in shaping changes in cytosolic calcium concentration ([Ca2+]c) during normal cellular functioning has recently been bolstered through the simultaneous monitoring of changes in [Ca2+]c and mitochondrial free Ca2+ levels ([Ca2+]m) (Sheu and Jou, 1994; Hajnoczky et al., 1995; Sparagna et al., 1995; Jou et al., 1996; Robb-Gaspers et al., 1998; Simpson and Russell, 1998). Despite the evidence, there remains a long-standing controversy as to the functional relevance of mitochondrial Ca2+ import during neuronal activity.
Modulation of the amplitude and kinetics of the evoked Δ[Ca2+]c exerts a regulatory influence on multiple steps that control the release of neurotransmitters (Herrington et al., 1996). For example, modest increases in [Ca2+]c augment the recruitment and passage of granules through the secretory pathway via the interaction of Ca2+ ions with distinct protein targets (Bittner and Holz, 1992; von Ruden and Neher, 1993; Neher and Zucker, 1993; Zucker, 1996; Bennett, 1997; Neher, 1998). In addition, it is generally thought that the efficient secretion of neuropeptide or catecholamine requires a level of stimulatory activity that is strong enough to evoke mitochondrial participation (Peng and Zucker, 1993; Nowycky et al., 1998). In the current study, the hypothesis that mitochondria regulate secretory activity by limiting rises in [Ca2+]c and the subsequent activation of specific proteins that recruit or prime secretory granules for exocytosis was tested by monitoring stimulus-evoked changes in [Ca2+]c and the secretory activity of single bovine chromaffin cells after selective pharmacological inhibition of mitochondrial Ca2+ transport.
MATERIALS AND METHODS
Preparation of bovine chromaffin cells. Primary dissociated cells from the medullas of fresh bovine adrenal glands obtained from a local commercial slaughterhouse (Murco, Plainwell, MI) were prepared by a collagenase digestion procedure (Bittner et al., 1986). Cultures were maintained in DMEM–F-12 (BioWhittaker, Walkersville, MD) containing 10% heat inactivated FCS. Cells were cultured as monolayers on collagen-coated glass coverslips (32 μg/ml in 0.01 N HCl), which formed the bottoms of 35 mm culture dishes (500,000–1,000,000 cells per dish). Before the start of an experiment, culture medium was replaced by superfusion with physiological saline for ∼20 min. Experiments were performed 1–8 d after the preparation of the cell cultures.
Electrophysiological recording ofIca and Cm.Standard whole-cell and perforated patch-clamp methods were used to evoke and record calcium currents and measure small, time-resolvable changes in membrane capacitance (ΔCm) from single chromaffin cells using a modified Axopatch 200A amplifier (Axon Instruments, Foster City, CA) and phase-tracking software (Pulse Control; Drs. Jack Herrington and Richard Bookman, University of Miami Medical School, Miami, FL). The ΔCm was monitored by applying a sine wave (60 mVp-p at 1201 Hz) to a holding potential of −90 mV. Sixteen samples per sinusoidal period were used to compute one Cmpoint each 6.6 msec, and calibration pulses (100 fF and 500 kΩ) were generated at the beginning of each trace. A train of 8 or 12 50–100 msec step depolarizations from −90 to 10 mV at 0.2 or 0.5 sec intervals was applied to evoke ICa, Δ[Ca2+]c, and ΔCm. For standard whole-cell patch recordings, pipettes were constructed out of 1.5 mm outer diameter (o.d.) capillary glass (Drummond Scientific, Broomall, PA) coated with Sylgard elastomer and fire polished to resistances of 2.5–7 MΩ. The standard intracellular recording solution contained (in mm):N-methyl-d-glucamine-Cl 128, HEPES 40, NaCl 10, Mg-ATP 4, GTP 0.2, Tris-EGTA 0.1, and fura-2, 0.15, pH adjusted to 7.1. For some experiments, 1 mmn-hydroxyethylethylenediaminetriacetic acid (HEDTA) or 10 μm ruthenium red (RR) was added to this solution. When necessary, osmolarity was maintained by ionic substitution. Conventional whole-cell recording was used for most experiments. For experiments in which cells were loaded with furaptra AM or stimulated by action potentials (see below), the perforated patch-clamp configuration was used. For these experiments, pipettes were constructed out of 1.5 mm o.d. borosilicate glass (catalog #TW150F-4; World Precision Instruments, Sarasota, FL). The pipette solution contained (in mm): cesium methanesulphonate 140, HEPES 10, MgCl2 1, EGTA 0.1, and amphotericin B 0.26, pH adjusted to 7.2 with CsOH. A concentrated stock solution of amphotericin B (30 μg/μl in methyl sulfoxide) was made fresh for each experiment and used within 1 hr. For recording of Ica, the superfusion solution was changed to a solution containing (in mm): tetraethylammonium chloride 137, CaCl2 10, MgCl2 2, HEPES 10, and glucose 19, pH adjusted to 7.15 with Tris. Test solutions containing mitochondrial inhibitors (0.5–1 μmcarbonyl cyanide p-(trifuoro-methoxy)phenylhydrazone (FCCP), 1 μm oligomycin, 10 μmantimycin and 10 μm oligomycin, or 100 μm CdCl2 were applied by local perifusion through a length of fused silica tubing (inner diameter of 300 μm) (PolyMicro Technologies, Inc., Phoenix, AZ) placed ∼50 μm from the cell. All compounds were purchased from Sigma (St. Louis, MO).
Action potential clamp. Action potentials were evoked by brief current injection or by application of the nicotinic agonist DMPP (2 μm), and membrane voltage changes were recorded in the standard whole-cell configuration under the current-clamp mode of an Axopatch 200A amplifier with a sampling rate of 10 kHz. The pipette solution contained (in mm): KCl 135, HEPES 10, glucose 10, MgCl2 2, and EGTA 0.250, and pH was adjusted to 7.2. Action potentials from four cells were digitally recorded and averaged to produce a stimulus waveform used for subsequent patch-clamp experiments and were applied as a single stimulus or in trains of 144 action potentials at 5 Hz. For these experiments, the sampling rate was adjusted to match that of the stimulus waveform (100 μsec/Cm point). FifteenCm points were determined every 1.76 sec [after every 12th action potential (AP)]. The current output of the amplifier was transformed by a digital pulse code audio processor (PCM-701ES; Sony, Tokyo, Japan) and stored for playback on a video cassette recorder (Betamax SL-2700; Sony).
Epifluorescence measurement of [Ca2+]c. To determine Δ[Ca2+]c, 150 μm fura-2 or furaptra was included in the intracellular recording solution, and the fluorescence was monitored using dual wavelength microspectrofluorometry (SPEX Industries, Edison, NJ). Individual chromaffin cells were optically isolated using a 10 μm pinhole stop and then illuminated by epifluorescence through a 40× oil immersion objective (NA of 1.30) with alternating excitation wavelengths of 340 and 380 nm. The emission at 510 nm was measured by photomultiplier (15–100 msec/point), and the [Ca2+]c was obtained using the ratiometric method (Grynkiewicz et al., 1985):
Fura-2 and furaptra signals were calibrated using a solution similar to the intracellular patch recording solution and containing either nominal (10 mm EGTA, no added Ca2+) or saturating (2.9 mm) free [Ca2+] and constant free [Mg2+] of 0.74 mm(determined using Patcher's Power Tools XOP; Dr. Francisco Mendez, Department of Membrane Biophysics, Max-Planck-Institute for Biophysical Chemistry, Gottingen, Germany). After subtraction of background autofluorescence measured before rupture of the cell membrane patch,Rmin,Rmax, and β were determined to be 0.35, 11.4, and 9.6 for fura-2, and 0.47, 6, and 8.3 for furaptra, respectively. A KD value for fura-2 of 224 nm was taken from the literature (Grynkiewicz et al., 1985), and β was determined by multiplyingKD by the ratioF0/Fs. In experiments in which the perforated patch-clamp configuration was used, cells were loaded by perifusion with a physiological saline solution containing 1 μm fura-2 AM or furaptra AM. In these cells, background autofluorescence was determined after attainment of whole-cell configuration and washout of the dye. The dissociation constant (KD) of furaptra has been estimated to range between 20 and 53 μm (Raju et al., 1989; Hurley et al., 1992;Naraghi, 1997; Xu et al., 1997). Under our experimental conditions, theKD of furaptra was estimated to be 20 μm by matching the Δ[Ca2+]c for a specific Ca2+ influx as determined by fura-2 to that evoked by the same influx in furaptra loaded cells, and substituting R, Rmin,Rmax, and β into the equation above to solve for KD.
RESULTS
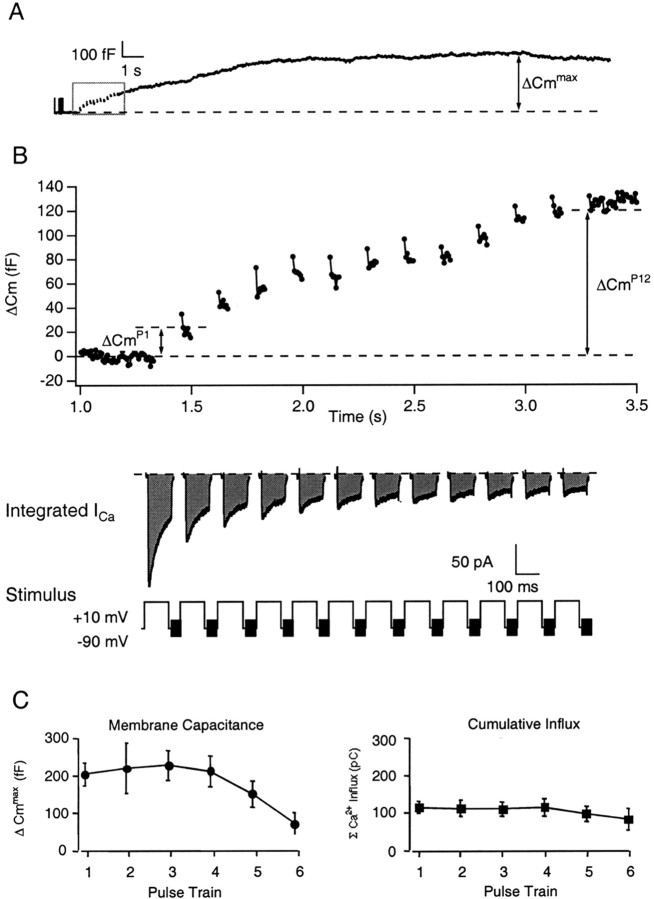
Unless otherwise indicated, experiments were performed in 10 mm external [Ca2+] using conventional whole-cell patch-clamp configuration to evoke and monitor both ΔCm andICa. The general experimental paradigm and nomenclature used is illustrated in Figure1, A and B, in which both the cumulative change in membrane capacitance after each step depolarization (ΔCmPn, where n indicates the position of a particular step depolarization within a pulse train) and the maximal ΔCm(ΔCmmax) were determined before and after drug application. The value of the ΔCmPn, which represents the Cm change with respect to the basal Cm value, was measured ∼20 msec after cessation of the step depolarization and includes any exocytotic activity that occurs during the interpulse intervals. The ΔCmmaxreflects the largest value achieved within 30 sec after initiation of the stimulus train. The ICacorresponding to each step depolarization was integrated, and the cumulative charge of entering Ca2+ ions (ΣQCa) was related to the ΣΔCm to investigate modulation of the Ca2+–exocytosis relationship. The bovine chromaffin cells used in the present study had a mean diameter of 15.2 μm and a resting whole-cellCm of 6.5 ± 0.6 pF (n = 36). Under conventional whole-cell patch-clamp conditions, application of an initial train of repetitive depolarizations induced an averaged cumulative, time-integrated Ca2+ influx of 161 ± 46 pC and a ΔCmmax of 248 ± 49 fF (n = 14). This increase corresponded to the exocytotic fusion of ∼65 secretory granules (3.8 fF/granule), assuming the average diameter of a single chromaffin granule is 0.356 μm with a specific membrane capacitance of 9 fF/μm2 (Albillos et al., 1997; Plattner et al., 1997). In control records, diminishment of theCm step amplitude evoked by each pulse during the train was observed in 57% of the cells. In these cells, the cumulative ΔCm evoked by the final step depolarization (ΔCmP8 or ΔCmP12) and the ΔCmmaxgave comparable values. This diminishment in the amplitude of theCm steps has been postulated to reflect the activity-dependent depletion of a pool of release-ready granules or a short-term change in the Ca2+–exocytosis relationship (Horrigan and Bookman, 1994; Engisch and Nowycky, 1996; Engisch et al., 1997). The remaining cells were found to exhibit a further average increase inCm (83 ± 25 fF) that persisted for 3.4 ± 1.9 sec after termination of the stimulus train (n = 6). This persistence of secretion may represent the exocytosis of release-ready granules that require the diffusional overlap of multiple Ca2+ domains or granules that require Ca2+-dependent recruitment and/or priming steps before fusion. Both types of responses were included in the averaged data relating Ca2+ influx andCmPn andCmmaxincreases.
Fig. 1.
Repetitive step depolarizations induce increases in membrane capacitance. A, Under standard whole-cell patch-clamp configuration, a train of depolarizing pulses was used to evoke both stepwise (inset) and maximal increases (ΔCmmax) in chromaffin cell surface area. B, The stepwise increases in ΔCm evoked during the pulse train (ΔCmPn) are shown on an expanded scale. Calcium currents were evoked by step depolarizations from a holding potential of −90 mV to a test potential of +10 mV. A 1200 Hz sine wave (60 mVp-p) was applied to the holding potential to monitor changes in Cm.C, Effect of repetitive pulse trains on ΔCmmax and the time-integrated Ca2+ influx. The ΔCmmax and the Ca2+ influx–train measured to six successive pulse trains applied under standard whole-cell patch-clamp conditions (n = 5). Each train consisted of 8–20 step depolarizations of 50 or 100 msec duration at 5 Hz. External [Ca2+]c was set at 10 mm. Two minutes of recovery time were allowed between each train.
Because secretory response characteristics of a single chromaffin cell may change in a time- or activity-dependent manner,Cm changes in response to successive pulse trains were also monitored. As shown in Figure 1C, there was a decline in both the amplitude of the depolarizing pulse-evoked Ca2+ currents and in the ΔCmmax with sustained dialysis (n = 5). Although the rate with which responsiveness declined was variable between cells, a significant enhancement of the ΔCmmaxbetween successive pulse trains applied at 2 min intervals under control conditions was rarely observed.
Effect of FCCP on the stimulus-evokedCm response
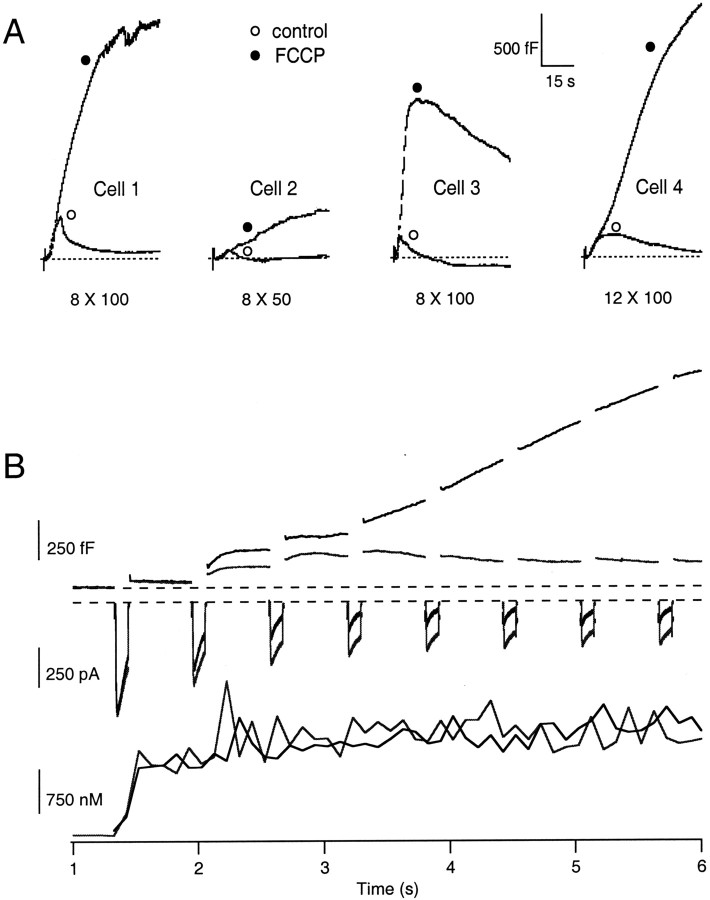
To determine the contribution of mitochondrial Ca2+ buffering to the control of catecholamine release, FCCP was used to dissipate the proton gradient across the inner mitochondrial membrane and reduce the electrochemical driving force (ψm + ΔpH) for mitochondrial Ca2+ import. The [Ca2+]c andCm changes evoked by repetitive stimuli before and during treatment with 0.5–1 μm FCCP were then compared. Neither FCCP (0.5–1 μm) nor oligomycin (1–10 μm) alone had any significant effect on basal levels of [Ca2+]cand Cm. However, as shown in Figure2A, the application of FCCP was found to potentiate the ΔCmmaxevoked by repetitive step depolarizations sevenfold over that of the control Cm response (n= 14; p < 0.001). Although FCCP treatment potentiated the evoked secretory response in nearly all cells tested, this enhancement varied from cell to cell in both magnitude and time course. The ΔCm for Cell 3 is shown on an expanded time scale in Figure 2B and includes the corresponding Δ[Ca2+]c and first and final ICa evoked by the stimulus trains. This type of response was observed in 43% of cells and demonstrated a moderate or profound increase in ΔCmPn during the pulse train, often despite decreased Ca2+ influx. By applying depolarizing stimuli to this cell at 1 Hz, it can be seen that, after FCCP treatment, the majority of the ΔCmPnincrease is not synchronized with Ca2+entry and occurs during the interpulse intervals. Because we are unable to isolate the Cm change evoked by active Ca2+ influx from that of the persistent Cm rise, we have focused on comparing the cumulative Cm changes evoked by Ca2+ influx (as a measure of the Ca2+–exocytosis relationship) between control and FCCP-treated cells. Despite decreased Ca2+ influx during FCCP treatment, there was little difference in the magnitude of the [Ca2+]c during the stimulus as reported by the fura-2 dye. This apparent discrepancy may be explained by an inability of the fura-2 dye to accurately report the large changes in [Ca2+]c induced by the strong stimuli used and further compounded by enhancement of the [Ca2+]c by FCCP (see next section). In the remaining cells, enhancement during the train was not readily evident and, in some cases, appeared to be diminished by FCCP treatment. However, when the ΔCmPn was normalized to account for diminished Ca2+influx, an enhanced Ca2+–exocytosis relationship was revealed (see below). It is important to note that, in most cells treated with FCCP, the majority of the ΔCm occurred after the stimulus train had ended. This persistent rise in the ΔCm often lasted for tens of seconds after voltage-dependent Ca2+ influx. It is unlikely that these effects resulted from depletion of cellular ATP levels or rundown of the plasma membrane Ca2+ pumps. Because FCCP treatment can elicit ATP consumption by reversal of theF0-F1ATP synthase, use of FCCP was always coupled with 1 μm oligomycin, a specific blocker of the mitochondrial ATP synthase (Budd and Nicholls, 1996a). Both the cytosolic ATP concentration (4 mm) and the pH (40 mm HEPES, pH. 7.1) were controlled by the use of the whole-cell recording configuration.
Fig. 2.
Effect of FCCP treatment on ΔCm and Δ[Ca2+]c evoked by repetitive step depolarizations under the whole-cell recording configuration with pipette solution containing 150 μm fura-2 (n = 14). A, Representative changes in ΔCm evoked by a train of 8 or 12, 50 or 100 msec depolarizations (stimulus noted below eachtrace) at 1 or 5 Hz before (open symbols) and during application of 0.5–1 μm FCCP (filled symbols). Dashed linesindicate prestimulus Cm baseline.B, The ΔCm replotted fromCell 3 on an expanded time scale and the corresponding Δ[Ca2+]c andICa evoked by stimulus train. Note that, despite a reduction in the ICa evoked during FCCP treatment, there was no effect on the Δ[Ca2+]c evoked during the stimulation.
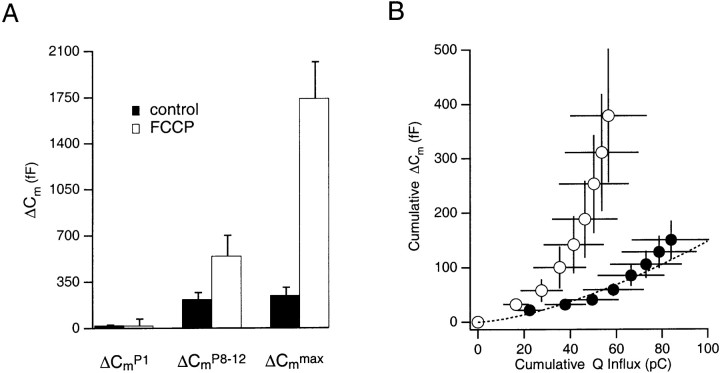
As shown in Figure 3A, the average ΔCmmaxevoked before FCCP treatment was 248 ± 49 fF, whereas that evoked during treatment with FCCP was 1743 ± 275 fF (n = 14). Unlike the modest increases inCmmax induced in control cells, the FCCP-treated cells demonstrated a considerable enhancement of the ΔCmmax. The persistent exocytotic response was maximal within tens of seconds after cessation of the stimulus train. On average, the ΔCmmax after FCCP treatment corresponded to the fusion of 1–2% of the estimated total granule content of a chromaffin cell (26,000–30,000 granules per cell) (Plattner et al., 1997) and an apparent increase in the number of granules available for release by this stimulus train from 65 to 457 granules. Because it is estimated that each bovine chromaffin cell contains 496 secretory granules that are either docked or in close proximity to the plasma membrane (Plattner et al., 1997), an interpretation is that the stimulus protocol induced the fusion of greater than 90% of this pool. Figure 3A shows that, in addition to the ΔCmmaxenhancement, the final ΔCmPn(ΔCmP8 or ΔCmP12) was also enhanced compared with control, indicating that increased secretory responsiveness developed during the stimulus train. As shown in Figure 3B, the FCCP-induced enhancement of the ΔCmmax was accompanied by a significant deviation from a standard relationship equating Ca2+ influx and the stepwise ΔCm in bovine chromaffin cells (Engisch and Nowycky, 1996; Engisch et al., 1997). The enhancement of the Ca2+–exocytosis relationship developed during the stimulus train, such that the latter ΔCmPn in the series, measured immediately after the termination of each depolarization, were enhanced significantly compared with control ΔCmPn steps. Figure 3B compares the Ca2+–exocytosis relationship evoked by eight step depolarizations in FCCP-treated cells with that of control cells and to a line fit to the standard Ca2+–exocytosis relationship (n = 8). This enhancement occurred despite rundown in the total amount of Ca2+ influx that accompanied FCCP treatment during the stimulus train (see below).
Fig. 3.
Effect of FCCP on ΔCmPn, ΔCmmax, and the Ca2+–exocytosis relationship under whole-cell recording conditions. A, The average ΔCmPn evoked by the first and last step depolarizations of a train and the ΔCmmax achieved within 30 sec after cessation of the stimulus, for control and FCCP-treated cells (n = 14). B, Comparison of the Ca2+–exocytosis relationship of chromaffin cells before (filled symbols) and during (open symbols) FCCP treatment (n = 8) to the standard Ca2+–exocytosis relationship (dashed line) described by Engisch et al. (1997).
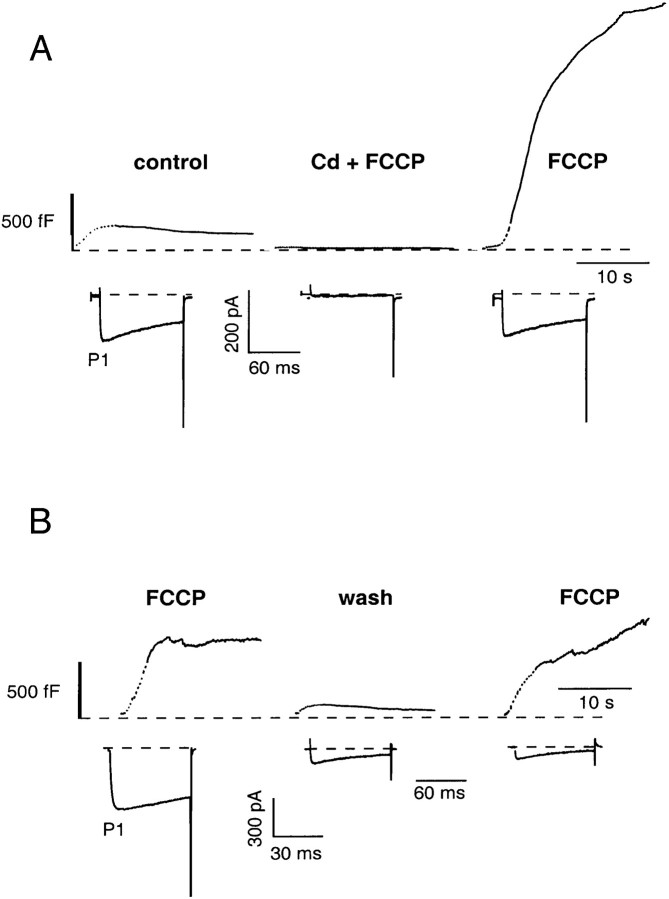
Additional experiments were performed to verify that the FCCP-induced enhancement of the secretory response was dependent on Ca2+ influx. As shown in Figure4A, application of 100 μm Cd2+ blocked Ca2+ influx through voltage-dependent Ca2+ channels during FCCP treatment and abolished the ΔCm andICa (Fig. 4A). A subsequent stimulus train after removal of Cd2+, but in the continued presence of FCCP, evoked an enhanced ΔCmmax(2233 ± 674 vs 292 ± 123 fF; n = 3). In addition, in a limited number of cells, removal of FCCP could restore the Cm response to control levels (Fig. 4B), consistent with the notion that the FCCP-induced increases in the evoked ΔCm were mediated by reduced mitochondrial Ca2+ import rather than collapse of cellular ATP levels. However, after the robust, FCCP-enhanced secretory response and prolonged elevation of [Ca2+]c, the majority of the cells treated did not respond to a subsequent stimulus train in the continued presence of FCCP.
Fig. 4.
Ca2+ dependence and reversibility of the FCCP-induced enhancement of the secretory response under whole-cell recording conditions. A,Cm traces comparing the ΔCm evoked before and during FCCP treatment in the presence of and after wash off of 100 μmCdCl2. Both the ΔCm and the effect of FCCP could be abolished by blocking Ca2+influx with CdCl2. Each of the ΔCm and Ca2+ current responses represent the averaged responses for three different cells.B, Representative ΔCmshowing that the enhanced secretory response could be reversed after wash off of FCCP.
FCCP-induced changes in [Ca2+]c dynamics
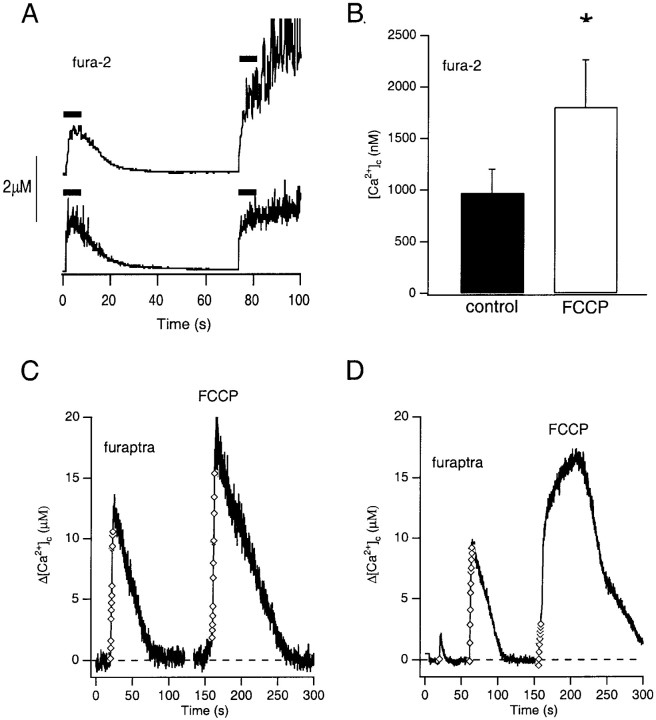
The FCCP-induced enhancement of the secretory response was accompanied by an alteration in the magnitude and time course of the [Ca2+]c response. To further establish that the increased secretory responsiveness during FCCP treatment resulted specifically from a derangement of Ca2+ homeostasis, the effect of FCCP on the Δ[Ca2+]cevoked by repetitive step depolarizations was more thoroughly examined using the Ca2+-sensitive fluorescent probes fura-2 and furaptra. The resting level of [Ca2+]c of chromaffin cells in standard or oligomycin-containing saline monitored with fura-2 was typically 125 ± 24 nm(n = 8). As shown in Figures 2B and5, A and B, [Ca2+]c increased in control cells to a plateau level by the third or fourth step depolarization in a train of depolarizing pulses. On average, the Δ[Ca2+]c was estimated by fura-2 to be 972 ± 228 nm and returned to a level just above that of prestimulus with an average time constant of 18 ± 3 sec after cessation of the stimulus (n = 8). After treatment with FCCP, the magnitude of the Δ[Ca2+]c was increased (1804 ± 457 nm; p< 0.017; n = 8) compared with control values, and the recovery of the [Ca2+]c was markedly slowed (t1/2 = 81 ± 29 sec; n = 5) or remained elevated. Moreover, the majority of the increase over control values occurred after the stimulus train and was represented by a slow upward drift of the [Ca2+]c level. Unexpectedly, the Δ[Ca2+]c in FCCP-treated cells also reached a plateau during the stimulus, indicating that the decrement of the Δ[Ca2+]c during influx was not solely a function of mitochondrial uptake.
Fig. 5.
Repetitive depolarizations evoke micromolar changes in [Ca2+]c of chromaffin cells. A, B, FCCP treatment significantly enhanced the maximum Δ[Ca2+]c evoked in chromaffin cells loaded with 150 μm fura-2 via patch pipette (n = 8). Bars indicate duration of the applied stimulus train. C, Comparison of evoked Δ[Ca2+]c before and during treatment with FCCP in a cell loaded with furaptra AM and stimulated by repetitive step depolarizations (12 pulses, 100 msec each) applied in the perforated patch-clamp configuration. D, Representative example of a delayed rise in [Ca2+]c in the presence of FCCP after the application of a train of 12 100 msec step depolarizations in the perforated patch-clamp configuration. Symbols denote application of a depolarizing pulse. The marked reduction of the Δ[Ca2+]c evoked during the stimulus train after FCCP treatment shown in D was not typical and was chosen to demonstrate the kinetics of the delayed rise in [Ca2+]c.
Because the estimated KD for fura-2 is 224 nm, it is expected that when [Ca2+]c rises to ∼2 μm, ∼90% of fura-2 will become saturated (Augustine and Neher, 1992; Zhou and Neher, 1993). We, therefore, suspected that the loss of proportionality between Ca2+ influx evoked by repetitive depolarizations and dye fluorescence resulted from a loss of sensitivity because of dye saturation and that fura-2 measurements may underestimate the Δ[Ca2+]c in response to repetitive depolarizations. To address this concern, chromaffin cells were loaded with furaptra, which has a 100- to 250-fold lower affinity for Ca2+-binding. After loading with furaptra AM, the average increase in [Ca2+]c evoked by repetitive step depolarizations under perforated patch-clamp conditions was estimated in control cells at 7.2 ± 1.6 μm (n = 7). FCCP-treatment, however, significantly enhanced the evoked Δ[Ca2+]c, which averaged 12.9 ± 2.5 μm (n= 3). An example of the evoked changes in [Ca2+]c for a furaptra-loaded cell with a 15 μm diameter, before and during FCCP treatment, is shown in Figure 5C. The cytoplasmic Ca2+-binding ratio for bovine chromaffin cells is estimated to be near 100 (Neher and Zucker, 1993). Assuming that the estimated endogenous buffering capacity of a chromaffin cell does not change significantly above 2 μm, the depolarization-mediated Ca2+ influx of 580 pC for this cell should have raised the [Ca2+]c to ∼20 μm, assuming an accessible cell volume of 1450 μm3 [1767 μm3 × 0.85 (Xu et al., 1997)]. However, the [Ca2+]c increased to a value just over half that estimated (11.7 μm). After mitochondrial uncoupling by FCCP, however, a second train of depolarizations mediating a cumulative influx of 456 pC now raised the [Ca2+]c to 18.8 μm, indicating that under the control conditions mitochondria acted to rapidly buffer changes in [Ca2+]c in the micromolar range. Because experiments using furaptra were performed after loading of the indicator dye as the membrane-permeable form, no absolute estimate of the cytosolic concentration of the dye was made, making a quantitative estimate of the Ca2+-binding capacity of furaptra and of the contribution of mitochondria Ca2+buffering under control conditions equivocal. Interestingly, in four of seven cells, FCCP treatment revealed an additional increase in [Ca2+]c after cessation of the stimulus train (Fig. 5D). This delayed rise in [Ca2+]csuggested that mitochondria may also sequester Ca2+ released from an unidentified intracellular site or, when uncoupled, may themselves release stored Ca2+ in response to Ca2+ influx through voltage-activated Ca2+ channels, perhaps via permeability transition. However, the subpopulation of furaptra-loaded cells that exhibited the delayed [Ca2+]c rise after the stimulus train was not included in the estimates of [Ca2+]c rise in response to influx.
Other inhibitors of mitochondrial Ca2+ import augment the secretory response
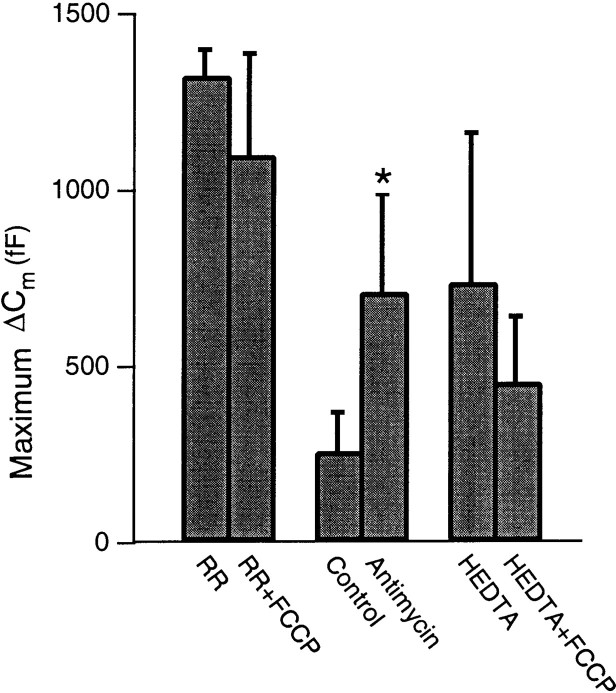
Under whole-cell patch-clamp configuration, enhanced secretory responsiveness, similar to that observed after uncoupling mitochondria with FCCP, could be induced by the inhibition of mitochondrial Ca2+ import using two other mitochondria-specific poisons, each with distinct mechanisms of action. The data from these experiments are summarized in Figure6. When 10 μm RR, a relatively specific blocker of the mitochondrial Ca2+ uniporter, was introduced through the patch pipette, the average evoked ΔCmmax was 1325 ± 75 (n = 4). This value was significantly larger than that evoked by the same stimulus under standard control conditions (248 ± 49 fF). When FCCP was applied in combination with RR, there was observed no further enhancement of the secretory response (1100 ± 289 fF; n = 4). The lack of an additive effect of these compounds on the secretory response suggests that these probes act at the same intracellular compartment.
Fig. 6.
Effects of additional inhibitors of mitochondrial Ca2+ import and reconstitution of Ca2+ buffering capacity by HEDTA under whole-cell recording conditions and after pharmacological dissipation of the ψm on the average evoked ΔCm. Cotreatment with 10 μmRR and 1 μm FCCP was not additive, suggesting that these compounds act at the same intracellular compartment (n = 4). Treatment with 10 μmantimycin also significantly enhanced the ΔCm (n = 6). FCCP treatment of cells dialyzed with pipette solution containing 1 mm the low-affinity Ca2+ buffer HEDTA and 220 μm free Mg2+ blocked the FCCP-induced enhancement of the secretory response (n = 3). All solutions contained 1–10 μm oligomycin.
In addition to RR, we used a combination of 10 μmantimycin A1 and 10 μm oligomycin to reduce the inner mitochondrial membrane potential (ψm) and, hence, the electromotive force for mitochondrial Ca2+ import. Antimycin A1 is an antibiotic substance that specifically inhibits electron flow between cytochrome b and c1 of the respiratory chain and blocks proton gradient generation at site 2. The ΔCmmaxevoked by repetitive step depolarizations before and during chemical hypoxia induced by 3–5 min of treatment with antimycin was 259 ± 111 and 711 ± 280 fF, respectively (n = 6;p < 0.04).
In addition to their effects on cellular Ca2+ homeostasis, inhibitors of mitochondrial function have been shown to alter the cellular levels of ATP–ADP, H+, Na+, and reactive oxygen species (ROS) in intact cells, each of which may affect the exocytotic response (Carriedo et al., 1998; Tenneti et al., 1998). Whereas the concentrations of ATP and ionic constituents can be maintained at relatively constant levels by the whole-cell patch-clamp configuration, significant amounts of ROS may be produced under our experimental conditions. To confirm the hypothesis that the increased secretory responsiveness after mitochondrial dysfunction resulted from a perturbation of [Ca2+]c dynamics, we attempted to “reconstitute” mitochondrial Ca2+ buffering capacity by including in the patch pipette solution a Ca2+ buffer with an affinity and capacity similar to that estimated for the mitochondrial component. It has been estimated that the mitochondria of bovine chromaffin cells have the capacity to sequester a total cytoplasmic Ca2+ load of 1 mm(Xu et al., 1997). Accordingly, 1 mm HEDTA was chosen to simulate the mitochondrial buffering capacity because it has an estimated buffering range of 1.3–8 μm under our experimental conditions. FCCP-induced uncoupling of mitochondrial Ca2+ import had no significant effect on either the ΔCm or the Δ[Ca2+]c when HEDTA was included in the patch pipette solution. As shown in Figure 6, the average peak values for these before and after FCCP-treatment were 738 ± 425 fF and 456 ± 186 nm, respectively (n = 4). The increased average ΔCmmax in HEDTA-loaded cells resulted from an enhancement of theICa, and inclusion of one cell in the data set that had uncommonly large ICa(>1 nA). Moreover, the Ca2+–exocytosis relationship between control and HEDTA-treated cells was not significantly different (data not shown). Thus, the enhanced secretory activity resulting from mitochondrial dysfunction was abolished by the intracellular application of an exogenous low-affinity Ca2+ buffer. Although the role of antioxidants was not directly tested, the above results suggest that the effect of mitochondrial inhibitors on secretory activity primarily results from a perturbation of Ca2+homeostasis rather than from ROS generation.
Physiological relevance
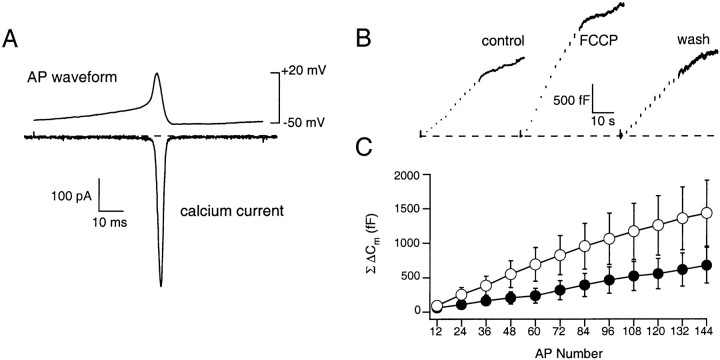
To determine whether mitochondrial Ca2+ import controls secretory activity under physiological conditions, experiments were performed using action potential waveforms as the depolarizing stimulus under perforated patch configuration and with the external Ca2+concentration reduced from 10 to 2.2 mm. In this manner, cytosolic proteins and the endogenous Ca2+buffer capacity of the cell are maintained and the influx driven by the depolarizing stimulus more closely approximates the physiological situation. Moreover, in rat and guinea pig preparations, splanchnic nerve or muscarinic stimulation has been shown to elicit bursts of action potentials (1–30 Hz) and the exocytotic release of catecholamine (Brandt et al., 1976; Kidokoro and Ritchie, 1980;Kajiwara et al., 1997; Inoue et al., 1998). In addition, acetylcholine-mediated depolarization of bovine chromaffin cells can induce trains of action potentials capable of inducing catecholamine secretion (Douglas et al., 1967). Accordingly, we recorded action potentials from chromaffin cells under current clamp and averaged them to use as a stimulus waveform (AP). This AP waveform was similar to those reported by others recorded from bovine and mouse chromaffin cells (Fenwick et al., 1982; Zhou and Misler, 1995; Moser, 1998). The prerecorded AP was then applied in a train at 5 Hz (144 APs) from a holding potential of −50 mV to evoke Ca2+influx, Δ[Ca2+]c, and ΔCm. An averagedICa activated during a single AP is shown in Figure 7A. The AP-evoked ICa had a peak amplitude and current integral of 450 ± 139 pA and 1.18 ± 0.41 pC (n = 6), respectively, and was completely blocked by the local application of 100 μmCd2+ (data not shown). TheICa activated at −16 mV reached a peak amplitude at −6 mV during the falling phase of the AP and had a half-width of 2.5 msec. The AP-evoked ΔCm did not exhibit activity-dependent depression of the ΔCm and was significantly enhanced from that predicted by the standard relationship determined using patterns of step depolarizations (see Discussion). For example, under control conditions, a train of 144 APs evoked a cumulative influx of ∼170 pC (530 × 106Ca2+ ions) and ΔCm and Δ[Ca2+]c of 673 ± 246 fF and 361 ± 67 nm(n = 6). This may indicate that, during AP-mediated secretory activity, granule recruitment is matched to support continued exocytosis or that trains of APs may activate a facilitationICa. The latter possibility was excluded because there was observed no facilitation of the Ca2+ currents evoked by repetitive application of APs, consistent with recent work that demonstrated theICa of adult bovine chromaffin cells do not facilitate (Engisch et al., 1997; Elhamdani et al., 1998). To estimate the contribution of mitochondrial Ca2+ import to the secretory response evoked by a physiological stimulation, we compared the AP-evoked ΔCm response before and during FCCP treatment. As shown in the representativeCm records in Figure 7B and averaged data in Figure 7C, a reduction of mitochondrial Ca2+ buffering capacity reversibly potentiated the ΔCm. The average ΔCm evoked during FCCP treatment was 1414 ± 466 fF (n = 6). After removal of FCCP, the ΔCm returned to 688 ± 324 fF, a value not significantly different from that evoked before FCCP treatment (n = 4). Although the increased responsiveness was less than that observed under stimulatory conditions that drive secretion maximally, these data indicate that mitochondrial import can contribute significantly to the control of secretory granule exocytosis during repetitive stimulatory activity.
Fig. 7.
Mitochondria regulate secretory activity under physiological conditions. APs were recorded under current clamp from bovine chromaffin cells and applied in trains at 5 Hz under the perforated patch-clamp configuration. External [Ca2+] was 2 mm. A, An averaged ICa evoked by an AP stimulus (n = 6). B, An averaged ΔCm evoked by 144 APs from three cells before, during, and after wash off of FCCP. C, Average data demonstrating that FCCP (open symbols) significantly enhanced the ΔCm induced by a train of APs (n = 6).
DISCUSSION
In response to multiple step depolarizations, the [Ca2+]c of bovine chromaffin cells was found to escalate from ∼0.1 to 1–20 μm, a range of [Ca2+]c in which mitochondria dominate Ca2+ clearance (Herrington et al., 1996; Xu et al., 1997). Pharmacological suppression of this low-affinity buffering mechanism by treatment with FCCP, RR, or antimycin–oligomycin rapidly potentiated the Δ[Ca2+]c and resulted in a threefold to sevenfold increase in the pool of secretory granules releasable by a standard stimulus pattern. Treatment with FCCP and RR in combination had no additive effect on secretion, suggesting that these compounds act on the same intracellular compartment. Simulating the endogenous buffering capacity of mitochondria by introduction of a low-affinity high-capacity Ca2+ buffer blocked the FCCP-induced enhancement of secretion. These findings indicate that mitochondria play an important role in the control of secretory activity in chromaffin cells.
Patterned activity has been shown to induce short-term changes in the secretory responsiveness of bovine chromaffin cells such that the rise in Cm evoked by repetitive stimulations can deviate from that predicted by a simple relationship describing Ca2+ influx and ΔCm (Engisch et al., 1997). To account for this deviation, it has been proposed that the efficacy with which Ca2+ can elicit a ΔCm may be enhanced by the activation of intracellular Ca2+ release or secretory granule mobilization, diminished by the recruitment of rapid Ca2+ clearance mechanisms (Hehl et al., 1996), or by desensitization of the secretory apparatus (Stuenkel and Nordmann, 1993; Hsu et al., 1996). In the present study, controlCm responses closely followed the standard Ca2+–exocytosis relationship described by Engisch and Nowycky (1996) and Engisch et al. (1997), but deviated from the predicted relationship when mitochondrial Ca2+ import was inhibited. The initial exocytotic steps, however, were not enhanced, suggesting that the exocytotic event per se was not directly modulated by mitochondrial inhibitors. In addition to the enhanced ΔCm that was directly coupled to Ca2+ influx, repetitive activity in the presence of mitochondrial inhibitors also produced a persistent, “asynchronous” rise in Cm that followed the cessation of the stimulus, but was, nevertheless, dependent on previous Ca2+ entry. This persistent rise in Cm likely resulted from global rises in [Ca2+]c and the recruitment for exocytosis of granules that are not located near the sites of Ca2+ influx.
The increased efficacy with which a train elicits secretion after FCCP treatment is represented by an increase in the number of granules recruited or available for release rather than a direct effect on the Ca2+ sensitivity of the release machinery. This interpretation is consistent with the observation that the ΔCmP1 was unaffected in both control and FCCP-treated cells, that significant enhancement of the Ca2+-exocytosis relationship developed during the stimulus train, and that most of the increase occurred during the interpulse intervals and after cessation of the stimulus train. Placed within the context of a two-step model for secretion in chromaffin cells (Heinemann et al., 1993, 1994; Smith et al., 1998), the enhanced magnitude and prolonged elevation of the [Ca2+]c associated with mitochondrial inhibition may act to drive the recruitment of granules into a releasable pool. Thus, long-term micromolar increases in [Ca2+]c evoked by strong repetitive stimuli in the presence of FCCP would act to either (1) increase the throughput of granules to the final exocytotic event in a stepwise enzymatic cascade in which granules exist in pools or states of varying releasability, or (2) increase the number of release sites that are activated by a given stimulus, in the way photolytic release of Ca2+ can evoke a massive secretory response. Implicit in the latter of these possibilities is the suggestion that global increases in [Ca2+]c must also act to drive persistent exocytosis from release sites distributed over the plasma membrane. These two scenarios are not necessarily exclusive, and experimental manipulations of mitochondrial Ca2+ uptake may provide useful tools for probing the recruitment and availability of secretory granules for exocytotic release.
A remarkable finding of the present study was that blocking mitochondrial Ca2+ import was found to enhance changes in Cm and [Ca2+]c when patterns of stimulation using natural (action potential) waveforms under conditions that preserved the physiological milieu were used to evoke secretion. For example, FCCP treatment potentiated the secretory response evoked by action potentials over twofold compared with control responses. Thus, even under conditions of moderate Ca2+ influx, mitochondria play a prominent role in limiting exocytotic activity in bovine chromaffin cells, primarily by rapidly clearing from the cytosol Ca2+ that accumulates during repetitive stimulations. Engisch et al. (1997) reported that enhancement is induced under conditions of minimal Ca2+entry. This is consistent with our observations that the total ΔCm evoked by trains of APs under control conditions was enhanced more than twofold from the predicted Ca2+–exocytosis relationship and, further, did not exhibit the use-dependent depression commonly observed when square-wave depolarizations were used to evoke secretion. Thus, it appears that natural waveforms or patterns of stimulation are more efficient at eliciting exocytotic fusion (Zhou and Misler, 1995;Engisch et al., 1997). Furthermore, after inhibition of mitochondrial Ca2+ import, the evoked ΔCm was enhanced more than fourfold from the standard Ca2+–exocytosis relationship, demonstrating that mitochondria normally limit secretory activity under physiologically relevant conditions.
The enhanced secretory responsiveness may reflect the time- and activity-dependent activation of specific Ca2+-regulated proteins and their effectors whose function is to regulate the supply of release-competent secretory granules. For example, members of the protein kinase C family are one set of promising candidates for this regulatory control because they are activated by elevation of [Ca2+]c in chromaffin cells (TerBush et al., 1988), and phorbol ester treatment has been shown to induce a long-lasting enhancement of the secretory response (Bittner and Holz, 1993; Gillis et al., 1996; Billiard et al., 1997; Cox and Parsons, 1997; Misonou et al., 1998; Smith et al., 1998).
Presynaptic Ca2+ clearance by mitochondria may play a general role to regulate synaptic strength. This notion is based on long-standing information detailing the abundance of mitochondria at nerve endings (Fried and Blaustein, 1978), the multiple Ca2+ transport mechanisms associated with mitochondria (Sparagna et al., 1995; Gunter et al., 1998), and the ability to increase transmitter release when mitochondrial Ca2+ transport is inhibited (Alnaes and Rahamimoff, 1975; Melamed-Book and Rahamimoff, 1998). Recently,Peng (1998) demonstrated that mitochondria are an important, frequency-dependent mechanism for Ca2+removal after repetitive firing at peptidergic presynaptic terminals of bullfrog sympathetic ganglia. Also, a direct demonstration of activity-dependent mitochondrial Ca2+transport at the lizard neuromuscular junction was resolved by David et al. (1998). Using Oregon Green-5N and Rhod-2 dyes in combination to simultaneously monitor Δ[Ca2+]c and Δ[Ca2+]m, respectively, they found that repetitive stimulations (30–50 APs) raised the [Ca2+]mafter the onset of a rise in [Ca2+]c and demonstrated an enhancement of the Δ[Ca2+]c after interruption of Δ[Ca2+]m. Buffering of [Ca2+]c by mitochondria may also play an important role at some mammalian central synapses. For example, Borst and Sakmann (1996) estimated that, at a central fast synapse in the rat brain, ∼60 Ca2+ channel openings were required to evoke the release of neurotransmitter and demonstrated that the release event was subject to modulation by relatively slow-acting Ca2+ buffers. The sensitivity of the exocytotic response to changes in the Ca2+buffering capacity underscores the potential contribution of presynaptic Ca2+ clearance by mitochondria to modulate synaptic strength.
A key question to be addressed in future studies is whether mitochondrial Ca2+ import in neuroendocrine cells is a regulated process. Although this study has primarily focused on the effects of inhibition of Ca2+ import, Ca2+ efflux from the mitochondrion may function to produce a prolonged low-level elevation in [Ca2+] that could support recruitment and priming of fusion-competent secretory granules. For example, the use of inhibitors of mitochondrial Ca2+transport showed that, during tetanic stimulation of the crayfish neuromuscular junction, neurotransmitter release was enhanced and demonstrated that mitochondrial Ca2+efflux underlies the generation of post-tetanic potentiation (Kamiya and Zucker, 1994; Tang and Zucker, 1997). Accordingly, the notions that mitochondria normally can function to limit or sustain and augment secretory activity are not necessarily mutually exclusive concepts.
Footnotes
This work was supported by National Institutes of Health Grant NS36227 to E.L.S. We thank Drs. James Herrington, Ronald Holz, Mary Bittner, and Brandi Soldo for valuable discussion.
Correspondence should be addressed to David Giovannucci's present address: Department of Pharmacology and Physiology, School of Medicine and Dentistry, University of Rochester, 601 Elmwood Avenue, Rochester, NY 14642. E-mail: giovannucci@pharmacol.rochester.edu.
REFERENCES
- 1.Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- 2.Alnaes E, Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol (Lond) 1975;248:285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustine GJ, Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol (Lond) 1992;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett MK. Ca2+ and the regulation of neurotransmitter secretion. Curr Opin Neurobiol. 1997;7:316–322. doi: 10.1016/s0959-4388(97)80058-x. [DOI] [PubMed] [Google Scholar]
- 6.Billiard J, Koh DS, Babcock DF, Hille B. Protein kinase C as a signal for exocytosis. Proc Natl Acad Sci USA. 1997;94:12192–12197. doi: 10.1073/pnas.94.22.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittner MA, Holz RW. Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J Biol Chem. 1992;267:16219–162125. [PubMed] [Google Scholar]
- 8.Bittner MA, Holz RW. Protein kinase C and clostridial neurotoxins affect discrete and related steps in the secretory pathway. Cell Mol Neurobiol. 1993;13:649–664. doi: 10.1007/BF00711564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bittner MA, Holz RW, Neubig RR. Guanine nucleotide effects on catecholamine secretion from digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986;261:10182–10188. [PubMed] [Google Scholar]
- 10.Blaustein MP, Kendrick NC, Fried RC, Ratzlaff RW. Calcium metabolism at the mammalian presynaptic nerve terminal: lessons from the synaptosome. In: Cowan WM, Ferrendelli JA, Gurvitch G, editors. Approaches to the cell biology of neurons. Society for Neuroscience; Bethesda, MD: 1977. pp. 172–194. [Google Scholar]
- 11.Blaustein MP, Ratzlaff RW, Kendrick NK. The regulation of intracellular calcium in presynaptic nerve terminals. Ann NY Acad Sci. 1978;307:195–212. doi: 10.1111/j.1749-6632.1978.tb41943.x. [DOI] [PubMed] [Google Scholar]
- 12.Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 13.Brandt BL, Hagiwara S, Kidokoro Y, Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol (Lond) 1976;263:417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem. 1996a;66:403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- 15.Budd SL, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem. 1996b;67:2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- 16.Carafoli E. The calcium cycle of mitochondria. FEBS Lett. 1979;104:1–5. doi: 10.1016/0014-5793(79)81073-x. [DOI] [PubMed] [Google Scholar]
- 17.Carafoli E, Crompton M. The regulation of intracellular calcium by mitochondria. Ann NY Acad Sci. 1978;307:269–284. doi: 10.1111/j.1749-6632.1978.tb41957.x. [DOI] [PubMed] [Google Scholar]
- 18.Carriedo SG, Yin HZ, Sensi SL, Weiss JH. Rapid Ca2+ entry through Ca2+-permeable AMPA/kainate channels triggers marked intracellular Ca2+ rises and consequent oxygen radical production. J Neurosci. 1998;18:7727–7738. doi: 10.1523/JNEUROSCI.18-19-07727.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox ME, Parsons SJ. Roles for protein kinase C and mitogen-activated protein kinase in nicotine-induced secretion from bovine adrenal chromaffin cells. J Neurochem. 1997;69:1119–1130. doi: 10.1046/j.1471-4159.1997.69031119.x. [DOI] [PubMed] [Google Scholar]
- 20.David G, Barrett JN, Barrett EF. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J Physiol (Lond) 1998;509:59–65. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas WW, Kanno T, Sampson SR. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol (Lond) 1967;191:107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elhamdani A, Zhou Z, Artalejo CR. Timing of dense-core vesicle exocytosis depends on the facilitation L-type Ca channel in adrenal chromaffin cells. J Neurosci. 1998;18:6230–6240. doi: 10.1523/JNEUROSCI.18-16-06230.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engisch KL, Nowycky MC. Calcium dependence of large dense-cored vesicle exocytosis evoked by calcium influx in bovine adrenal chromaffin cells. J Neurosci. 1996;16:1359–1369. doi: 10.1523/JNEUROSCI.16-04-01359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engisch KL, Chernevskaya NI, Nowycky MC. Short-term changes in the Ca2+-exocytosis relationship during repetitive pulse protocols in bovine adrenal chromaffin cells. J Neurosci. 1997;17:9010–9025. doi: 10.1523/JNEUROSCI.17-23-09010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenwick EM, Marty A, Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol (Lond) 1982;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried RC, Blaustein MP. Retrieval and recycling of synaptic vesicle membrane in pinched-off nerve terminals (synaptosomes). J Cell Biol. 1978;78:685–700. doi: 10.1083/jcb.78.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci. 1994;14:4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillis KD, Mossner R, Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron. 1996;16:1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 29.Giovannucci DR, Stuenkel EL. Regulation of secretory granule recruitment and exocytosis at rat neurohypophysial nerve endings. J Physiol (Lond) 1997;498:735–751. doi: 10.1113/jphysiol.1997.sp021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 31.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 32.Gunter TE, Buntinas L, Sparagna GC, Gunter KK. The Ca2+ transport mechanisms of mitochondria and Ca2+ uptake from physiological-type Ca2+ transients. Biochim Biophys Acta. 1998;1366:5–15. doi: 10.1016/s0005-2728(98)00117-0. [DOI] [PubMed] [Google Scholar]
- 33.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 34.Hehl S, Golard A, Hille B. Involvement of mitochondria in intracellular calcium sequestration by rat gonadotropes. Cell Calcium. 1996;20:515–524. doi: 10.1016/s0143-4160(96)90094-9. [DOI] [PubMed] [Google Scholar]
- 35.Heinemann C, von Ruden L, Chow RH, Neher E. A two-step model of secretion control in neuroendocrine cells. Pflügers Arch. 1993;424:105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- 36.Heinemann C, Chow RH, Neher E, Zucker RS. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994;67:2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 38.Horrigan FT, Bookman RJ. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994;13:1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 39.Hsu SF, Augustine GJ, Jackson MB. Adaptation of Ca(2+)-triggered exocytosis in presynaptic terminals. Neuron. 1996;17:501–512. doi: 10.1016/s0896-6273(00)80182-8. [DOI] [PubMed] [Google Scholar]
- 40.Hurley TW, Ryan MP, Brinck RW. Changes of cytosolic Ca2+ interfere with measurements of cytosolic Mg2+ using mag-fura-2. Am J Physiol. 1992;263:C300–C307. doi: 10.1152/ajpcell.1992.263.2.C300. [DOI] [PubMed] [Google Scholar]
- 41.Inoue M, Fujishiro N, Imanaga I. Hypoxia and cyanide induce depolarization and catecholamine release in dispersed guinea-pig chromaffin cells. J Physiol (Lond) 1998;507:807–818. doi: 10.1111/j.1469-7793.1998.807bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jou MJ, Peng TI, Sheu SS. Histamine induces oscillations of mitochondrial free Ca2+ concentration in single cultured rat brain astrocytes. J Physiol (Lond) 1996;497:299–308. doi: 10.1113/jphysiol.1996.sp021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kajiwara R, Sand O, Kidokoro Y, Barish ME, Iijima T. Functional organization of chromaffin cells and cholinergic synaptic transmission in rat adrenal medulla. Jpn J Physiol. 1997;47:449–464. doi: 10.2170/jjphysiol.47.449. [DOI] [PubMed] [Google Scholar]
- 44.Kamiya H, Zucker RS. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- 45.Kidokoro Y, Ritchie AK. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol (Lond) 1980;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klingauf J, Neher E. Modeling buffered Ca2+ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehninger AL, Carafoli E, Rossi CS. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- 48.Melamed-Book N, Rahamimoff R. The revival of the role of the mitochondrion in regulation of transmitter release. J Physiol (Lond) 1998;509:2. doi: 10.1111/j.1469-7793.1998.002bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misonou H, Ohara-Imaizumi M, Murakami T, Kawasaki M, Ikeda K, Wakai T, Kumakura K. Protein kinase C controls the priming step of regulated exocytosis in adrenal chromaffin cells. Cell Mol Neurobiol. 1998;18:379–390. doi: 10.1023/A:1022593330685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moser T. Low-conductance intercellular coupling between mouse chromaffin cells in situ. J Physiol (Lond) 1998;506:195–205. doi: 10.1111/j.1469-7793.1998.195bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naraghi M. T-jump study of calcium binding kinetics of calcium chelators. Cell Calcium. 1997;22:255–268. doi: 10.1016/s0143-4160(97)90064-6. [DOI] [PubMed] [Google Scholar]
- 52.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 53.Neher E, Zucker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- 54.Nicholls D, Akerman K. Mitochondrial calcium transport. Biochim Biophys Acta. 1982;683:57–88. doi: 10.1016/0304-4173(82)90013-1. [DOI] [PubMed] [Google Scholar]
- 55.Nicholls DG, Budd SL. Mitochondria and neuronal glutamate excitotoxicity. Biochim Biophys Acta. 1998;1366:97–112. doi: 10.1016/s0005-2728(98)00123-6. [DOI] [PubMed] [Google Scholar]
- 56.Nowycky MC, Seward EP, Chernevskaya NI. Excitation-secretion coupling in mammalian neurohypophysial nerve terminals. Cell Mol Neurobiol. 1998;18:65–80. doi: 10.1023/A:1022575126738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park YB, Herrington J, Babcock DF, Hille B. Ca2+ clearance mechanisms in isolated rat adrenal chromaffin cells. J Physiol (Lond) 1996;492:329–346. doi: 10.1113/jphysiol.1996.sp021312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng YY. Effects of mitochondrion on calcium transients at intact presynaptic terminals depend on frequency of nerve firing. J Neurophysiol. 1998;80:186–195. doi: 10.1152/jn.1998.80.1.186. [DOI] [PubMed] [Google Scholar]
- 59.Peng YY, Zucker RS. Release of LHRH is linearly related to the time integral of presynaptic Ca2+ elevation above a threshold level in bullfrog sympathetic ganglia. Neuron. 1993;10:465–473. doi: 10.1016/0896-6273(93)90334-n. [DOI] [PubMed] [Google Scholar]
- 60. Plattner H, Artalejo AR, Neher E. Ultrastructural organization of bovine chromaffin cell cortex-analysis by cryofixation and morphometry of aspects pertinent to exocytosis. J Cell Biol 139 1997. 1709 1717[Erratum (1998) 140:973]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raju B, Murphy E, Levy LA, Hall RD, London RE. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989;256:C540–C548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- 62.Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson IM, Finnegan JM, Monck JR, Wightman RM, Fernandez JM. Colocalization of calcium entry and exocytotic release sites in adrenal chromaffin cells. Proc Natl Acad Sci USA. 1995;92:2474–2478. doi: 10.1073/pnas.92.7.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheu SS, Jou MJ. Mitochondrial free Ca2+ concentration in living cells. J Bioenerg Biomembr. 1994;26:487–493. doi: 10.1007/BF00762733. [DOI] [PubMed] [Google Scholar]
- 66.Simpson PB, Russell JT. Role of mitochondrial Ca2+ regulation in neuronal and glial cell signalling. Brain Res Brain Res Rev. 1998;26:72–81. doi: 10.1016/s0165-0173(97)00056-8. [DOI] [PubMed] [Google Scholar]
- 67.Smith C, Moser T, Xu T, Neher E. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron. 1998;20:1243–1253. doi: 10.1016/s0896-6273(00)80504-8. [DOI] [PubMed] [Google Scholar]
- 68.Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- 69.Stuenkel EL. Regulation of intracellular calcium and calcium buffering properties of rat isolated neurohypophysial nerve endings. J Physiol (Lond) 1994;481:251–271. doi: 10.1113/jphysiol.1994.sp020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stuenkel EL, Nordmann JJ. Intracellular calcium and vasopressin release of rat isolated neurohypophysial nerve endings. J Physiol (Lond) 1993;468:335–355. doi: 10.1113/jphysiol.1993.sp019775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- 72.Tenneti L, D'Emilia DM, Troy CM, Lipton SA. Role of caspases in N-methyl-d-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem. 1998;71:946–959. doi: 10.1046/j.1471-4159.1998.71030946.x. [DOI] [PubMed] [Google Scholar]
- 73.TerBush DR, Bittner MA, Holz RW. Ca2+ influx causes rapid translocation of protein kinase C to membranes. Studies of the effects of secretagogues in adrenal chromaffin cells. J Biol Chem. 1988;263:18873–18879. [PubMed] [Google Scholar]
- 74.Thayer SA, Miller RJ. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol (Lond) 1990;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thayer SA, Wang GJ. Glutamate-induced calcium loads: effects on energy metabolism and neuronal viability. Clin Exp Pharmacol Physiol. 1995;22:303–304. doi: 10.1111/j.1440-1681.1995.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 76.von Ruden L, Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993;262:1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]
- 77.Wang GJ, Thayer SA. Sequestration of glutamate-induced Ca2+ loads by mitochondria in cultured rat hippocampal neurons. J Neurophysiol. 1996;76:1611–1621. doi: 10.1152/jn.1996.76.3.1611. [DOI] [PubMed] [Google Scholar]
- 78.Werth JL, Thayer SA. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994;14:348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.White RJ, Reynolds IJ. Mitochondria accumulate Ca2+ following intense glutamate stimulation of cultured rat forebrain neurones. J Physiol (Lond) 1997;498:31–47. doi: 10.1113/jphysiol.1997.sp021839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu T, Naraghi M, Kang H, Neher E. Kinetic studies of Ca2+ binding and Ca2+ clearance in the cytosol of adrenal chromaffin cells. Biophys J. 1997;73:532–545. doi: 10.1016/S0006-3495(97)78091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Z, Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J Biol Chem. 1995;270:3498–3505. [PubMed] [Google Scholar]
- 82.Zhou Z, Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol (Lond) 1993;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zucker RS. Exocytosis: a molecular and physiological perspective. Neuron. 1996;17:1049–1055. doi: 10.1016/s0896-6273(00)80238-x. [DOI] [PubMed] [Google Scholar]