Abstract
The accumulation of reactive oxygen species (ROS) in the brain is associated with several neurodegenerative conditions. ROS can affect ionic homeostasis leading to impaired neurotransmission. Here, we determined the ability of H2O2, a membrane permeant ROS, to alter intraneuronal Cl−, an important regulator of neuronal excitability. Real-time alterations in intracellular chloride, [Cl−]i, were measured with UV laser scanning confocal microscopy in hippocampal slices loaded with the cell-permeant form of 6-methoxy-N-ethylquinolium iodide (MEQ), a Cl−-sensitive fluorescent probe. In slices superfused with H2O2 for 10 min, there was a significant decrease in MEQ fluorescence (elevation in [Cl−]i) in area CA1 pyramidal cell soma but not in interneurons located in stratum radiatum. Alterations in [Cl−]i induced by H2O2were prevented by the iron chelator deferoxamine and the vitamin E analog Trolox, suggesting the involvement of free radicals. The influx of Cl− probably occurred through the GABA-gated Cl− channel because the effects of H2O2 were blocked by picrotoxin. In addition, HPLC analysis of the superfusates indicated that GABA and glutamate accumulated extracellularly after H2O2exposure. Excitatory amino acid receptor antagonists 2-amino-5-phoshopentanoic acid and 1,2,3,4-tetrahydro-6-nitro-2, 3-dioxo-benzo[f]quinoxaline-7-sulfonamide also attenuated the effect of H2O2 on MEQ fluorescence. The changes in [Cl−]i induced by H2O2were Ca2+-dependent and Na+-independent. After exposure of slices to H2O2, the ability of the GABA agonist muscimol to increase [Cl−]i was attenuated. Thus, ROS, like H2O2, may impair transmembrane Cl− gradients and reduce inhibitory neurotransmission, further promoting neuronal damage in oxidative stress-related disease and in aging.
Keywords: oxidative stress, intracellular chloride, hippocampal neurons, imaging, H2O2, GABA
Reactive oxygen species (ROS) are byproducts generated by cellular oxidative metabolism. However, enhanced production of ROS, exceeding the intrinsic antioxidant scavenging capacity, leads to the development of several pathophysiological conditions. Brain tissue may be especially vulnerable to ROS damage because of high oxygen consumption, moderate antioxidant capacity, and membranes rich in easily oxidized polyunsaturated lipids. Mounting evidence has implicated the role of ROS in the pathophysiology of neurodegenerative conditions such as Parkinson's disease, Huntington's disease, Alzheimer's disease, and in normal aging (for review, see Beal, 1995; Simonian and Coyle, 1996). An essential component of ROS-induced neurotoxicity may involve the modulation of various ion transport proteins and receptor-gated ion channels either directly via protein oxidation or indirectly via peroxidation of membrane phospholipids (Kourie, 1998).
The effect of ROS on synaptic transmission has been examined previously in the hippocampal slice. With exposure to oxygen free radicals, synaptic efficacy and the ability to generate spikes are impaired (Pellmar et al., 1989). EPSPs have been shown to be reduced (Pellmar, 1995) or enhanced (Frantseva et al., 1998) in neurons exposed to ROS. However, IPSPs were significantly reduced in both of these studies. Presynaptic and postsynaptic mechanisms have been implicated in the alteration of synaptic function by ROS (Colton et al., 1989; Pellmar, 1995), although specific targets have not been identified.
The sensitivity of inhibitory neurotransmission (i.e., GABAergic neurotransmission) to oxidative stress may be particularly important because a reduction in neuronal inhibition can lead to neuronal excitability. Previously, we reported that the generation of superoxide radicals in cerebral cortical synaptoneurosomes reduced GABAA receptor activity (Schwartz et al., 1988). In addition, we have demonstrated an increase in intracellular chloride ([Cl−]i) and a subsequent reduction in GABAA responses in hippocampal neurons after in vitro ischemia (oxygen–glucose deprivation) (Inglefield and Schwartz-Bloom, 1998). Previous reports indicate that alterations in GABAAreceptor-mediated inhibition may underlie hydrogen peroxide (H2O2)-induced changes in synaptic transmission (Katsuki et al., 1997). However, little is known about the effects of ROS on intracellular Cl− levels and GABAA receptor activity in an intact neuronal system.
Here, using optical imaging techniques and fluorescent dyes, we investigated the ability of H2O2, a membrane-permeant ROS, to affect intracellular Cl− in hippocampal area CA1 pyramidal neurons and interneurons. Our working hypothesis was that ROS can perturb transmembrane Cl− gradients within hippocampal neurons, resulting in reduced GABAA responses. We observed that exogenous H2O2 caused a Ca2+-dependent increase in intracellular Cl− in area CA1 pyramidal neurons of the hippocampus, followed by a reduction in GABAAresponses. We suggest that impairment of Cl− gradients by H2O2 could reduce GABAA receptor-mediated inhibitory transmission and promote neuronal damage in a variety of neurodegenerative conditions.
Part of this paper has been published previously in abstract form (Sah and Schwartz-Bloom, 1999).
MATERIALS AND METHODS
Materials. 6-methoxy-N-ethylquinolium iodide, (MEQ), carboxy-dichlorodihydrofluoresein diacetate (c-H2DCFDA), and propidium iodide were purchased from Molecular Probes (Eugene, OR). Trolox was obtained from Fluka BioChemika (Ronkonkoma, NY).d-2-amino-5-phosphopentanoic acid (d-AP-5) was obtained from Tocris Cookson (Ballwin, MO). Tiagabine and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX) were a gift from Novo Nordisk (MålØv, Denmark). All other drugs were purchased from Sigma (St. Louis, MO).
Preparation of dihydro-MEQ. For loading hippocampal slices, MEQ was reduced to its cell-permeable derivative, 6-methoxy-N-ethyl-1,2-dihydroquinoline (dihydro-MEQ) as described previously by our laboratory (Inglefield and Schwartz-Bloom, 1997). Briefly, reduction was performed by gradual addition of sodium borohydride (12% in H2O, 32 μm) to an aqueous solution of MEQ (16 μm) under nitrogen for 30 min. Formation of the dihydro derivative was accompanied by the appearance of a reddish-yellow oil that was extracted twice with ethyl acetate (0.5 ml). Organic extracts were combined and dried with anhydrous MgSO4, and the ethyl acetate was evaporated under N2 in a glass microvial. Reduced dye was stored at −80°C under N2 and used within 2–3 d for optimal loading of brain slices.
Hippocampal slice preparation and bath loading of dihydro-MEQ. Transverse hippocampal slices (300 μm) were prepared from 12- to 19-d-old Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) using a vibratome in ice-cold oxygenated (95% O2–5% CO2 mixture) physiological Ringer's buffer. Composition of the Ringer's buffer consisted of (in mm): 119 NaCl, 2.5 KCl, 1.0 NaH2PO4, 1.3 MgCl2, 2.5 CaCl2, 26 NaHCO3, and 11 glucose, pH 7.4. The osmolality of this buffer was 285–290 mOsm/l. For low Ca2+ or Na+-containing buffers, appropriate iso-osmotic ion equivalents were added to account for osmolality and pH changes caused by removal of ions. Slices were transferred to a nylon net submerged in oxygenated Ringer's buffer (room temperature) and allowed to equilibrate for at least 0.5–1 hr before dye loading. Bath loading of slices with resuspended dihydro-MEQ (final concentration of ∼400 μm) was carried out at room temperature for 30 min in Ringer's buffer. The slices were washed once in fresh oxygenated Ringer's to remove extracellular dye before transfer to the imaging chamber. Intracellularly trapped MEQ has high Cl− sensitivity, low toxicity, and a slow leakage rate (Biwersi and Verkman, 1991). Because the fluorescence of MEQ is quenched collisionally by Cl−, fluorescence intensity is inversely related to [Cl−]i.
UV laser scanning confocal microscopy. The use of UV laser scanning confocal microscopy to measure Cl−-sensitive changes in MEQ fluorescence in hippocampal slices has been described previously by our laboratory (Inglefield and Schwartz-Bloom, 1997). After loading with MEQ, slices were submerged in a chamber that was superfused with oxygenated Ringer's buffer (flow rate of ∼1.5 ml/min) at room temperature. For all experiments, slices were allowed to equilibrate in the chamber for at least 10–15 min before the baseline recording period. The imaging chamber was positioned on the stage of an upright Nikon (Tokyo, Japan) Optiphot microscope. The laser scanning confocal microscope (Noran Odyssey; Noran Instruments, Middleton, WI) was equipped with an argon ion UV laser (50 mW output; Enterprise 653; Coherent-AMT, Kitchener, Ontario, Canada) and a digital imaging system using Image-1 software (Universal Imaging Corporation, West Chester, PA) for image and data acquisition. MEQ-loaded neurons were imaged by excitation with the 364 nm line of the UV laser (attenuated to 18% intensity using the acousto-optical modulator of this system). Fluorescent light was transmitted through a UV water-immersible objective (40× NA; Olympus Optical, Tokyo, Japan). Emission (Emmax of 440 nm) was imaged using a 400 nm barrier filter. Photomultipliers detected the fluorescent signal through a confocal slit at 1× electronic zoom (unless otherwise noted). The video frame rates (32 images per second) of the Noran Odyssey confocal microscope allowed rapid full-image (512 × 480 pixels) acquisition. To minimize photobleaching of the dye, repeated long-term illumination by the UV laser was limited. Previously, we have characterized the kinetics of photobleaching of MEQ to have a half-life (t1/2) of 173.8 sec (Inglefield and Schwartz-Bloom, 1997). Optical recordings totaling 1 sec of laser illumination were made every 2–5 min, ensuring total laser illumination of no more than 15 sec/slice. Images were 8-bit (256 intensity levels) and were recorded to a Panasonic (Secaucus, NJ) optical memory disk recorder (model LQ-3031) for off-line analysis. Before switching to H2O2 or drug-containing buffer, a minimum of 5 min of baseline was recorded to allow determination of the stable fluorescence of the cells. When inhibitors were used, they were superfused during the equilibration period (10–15 min) to ensure penetration within the slice.
Calibration of MEQ fluorescence sensitivity. Because MEQ is not a ratioable dye, absolute [Cl−]i concentrations were not measured. However, changes in MEQ fluorescence can be calibrated to estimate changes in intracellular [Cl−] using the Stern–Volmer relationship,F0/FCl= 1 + Kq[Cl], whereF0 is the total quenchable signal,FCl is the fluorescence in the presence of a given Cl− concentration, and Kq is the Stern–Volmer quench constant (inm−1) (Verkman, 1990). Previously, we have calibrated changes in MEQ fluorescence with intracellular Cl− under different experimental conditions (Inglefield and Schwartz-Bloom, 1997, 1998). For the imaging conditions described here, we determined the Stern–Volmer constant (Kq) to be 16 ± 1m−1. TheKq−1 equals 61 mm, the Cl−concentration to quench MEQ fluorescence by 50%.
Propidium iodide fluorescence imaging. In certain experiments, MEQ-loaded slices were superfused in the imaging chamber with propidium iodide (PI) (2 μg/ml) for 5 min before imaging. Propridium iodide was maintained in the buffer throughout the experiment to prevent washout from the extracellular space. Healthy cells are impermeable to PI. However, it enters neurons with damaged plasma membranes and fluoresces upon binding to nucleic acids. Detection of PI-positive neurons (indicating damaged plasma membranes) was achieved with a 488 nm excitation line and a 550 nm barrier filter.
Carboxy-dichlorofluorescein fluorescence imaging. To monitor the accumulation of intracellular peroxides, separate slices (non-MEQ-loaded) were loaded with the nonfluorescent dye c-H2DCFDA (10 μm) for 30 min at 30° C. This dye is freely permeable to cells, and it becomes trapped inside after hydrolysis to carboxy-dichlorodihydrofluorescein. Upon oxidation by intracellular oxidants, primarily peroxides, it forms fluorescent carboxy-dichlorofluorescein (c-DCF) (LeBel et al., 1992). Increases in c-DCF fluorescence dependent on intracellular oxidant status were measured using the 488 nm excitation line and a 515 nm barrier filter. Laser illumination was limited to 1 sec exposures at 5 min intervals for 15 min, and images were acquired using 16 frame averaging to minimize photo-oxidation of this dye. Control slices were exposed to H2O2-free Ringer's under identical conditions.
Data analysis. Only those cells that had <10% change in MEQ fluorescence during the initial baseline recording period and an initial fluorescence intensity between 90–200 optical density units were used for data analysis. Fluorescence intensity (optical density arbitrary units) was measured over a central area of 5–10 morphologically distinct somata per slice within the imaged field. Within each frame, the average fluorescence intensity, F, for either MEQ or c-DCF fluorescence was calculated and recorded. The percent change in fluorescence from baseline for each cell (ΔF) was calculated by the equation ΔF = (Fb −F/Fb) × 100, whereFb was basal fluorescence defined by the three frames preceding the experimental recordings. Thus, each cell served as its own control. There was a gradual rundown (baseline drift) of MEQ fluorescence (9.9 ± 2% over 20 min) in control slices primarily because of slow leakage of the dye. Thus, changes in MEQ fluorescence induced by H2O2 were always compared with the fluorescence in slices not exposed to H2O2 under similar conditions (controls). Typically, we obtained data from three to four stable cells per slice, from at least two slices per experiment, repeated at least three times.
Measurement of amino acid overflow. To quantify the amounts of GABA and glutamate released from the hippocampal slices, 100 μl of the superfusate was collected from slices exposed to H2O2 for 2 and 10 min. Superfusates were sampled from control slices at the same time points. Samples were stored at −20°C until analysis. Neurotransmitter amino acids in the superfusates were derivitized witho-phthalaldehyde and assayed by HPLC on a C18 reversed-phase column (4.6 × 80 mm; particle size, 3 μm; Zorbax; Hewlett-Packard Co., Newport, DE) using 700 nm cysteic acid as the internal standard (Lindroth and Mopper, 1979; Peterson et al., 1995). The limit of sensitivity was ∼50 fmol.
RESULTS
H2O2- induced changes in intracellular Cl−
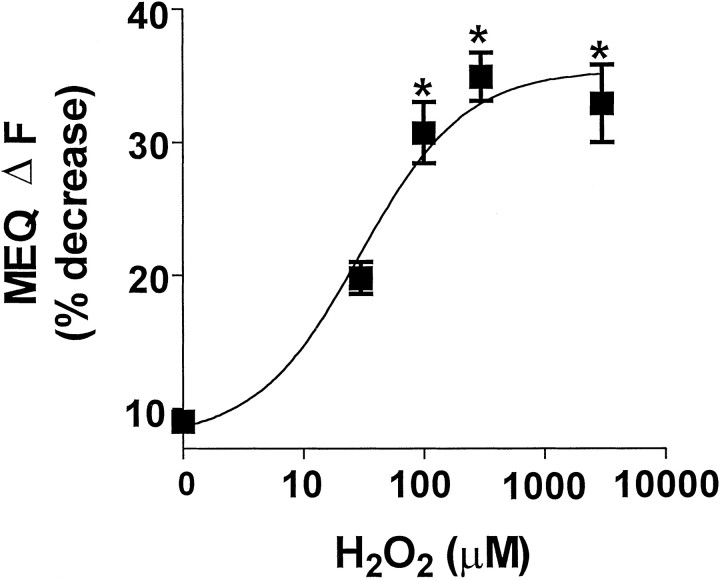
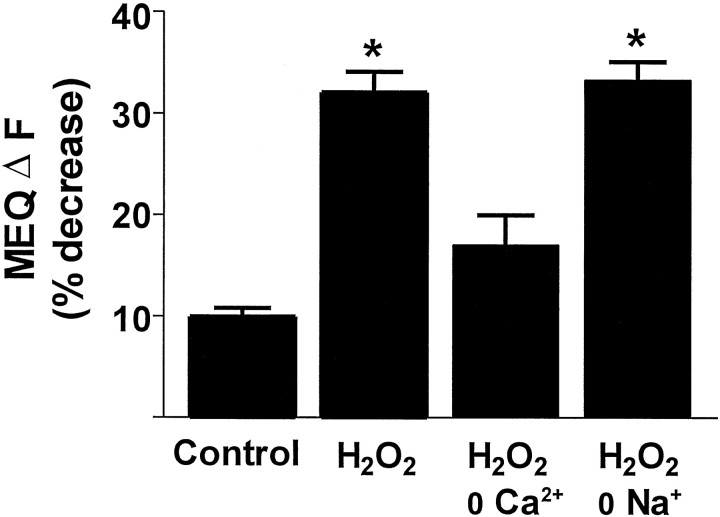
Freshly prepared hippocampal slices loaded with MEQ exhibited numerous fluorescent neurons with a uniform dye distribution in area CA1 stratum pyramidale (Fig.1A). Dendrites of several neurons were clearly visible depending on the optical plane of focus. In hippocampal slices exposed to H2O2 (300 μm; 0.001%), MEQ fluorescence decreased gradually in the cell soma of area CA1 stratum pyramidale (Fig. 1), indicating an accumulation of intracellular Cl−. When H2O2 was removed from the superfusion buffer after 10 min, no additional decrease in MEQ fluorescence was observed over the next 25 min (data not shown). Ten minutes after exposure to H2O2, MEQ fluorescence decreased by 32 ± 2% compared with a 9.9 ± 0.8% decrease in control slices over the same time period (also see Fig. 5). According to the Stern–Volmer relationship (see Materials and Methods), the decrease in MEQ fluorescence produced by 10 min H2O2 exposure (corrected for baseline drift) corresponds to an increase in intracellular Cl− of ∼27 mm(resting [Cl−]i typically ranges between 4 and 9 mm for neurons). The change in MEQ fluorescence produced by H2O2 in pyramidal neurons was concentration-dependent (Fig. 2). No change in MEQ fluorescence was observed below a concentration of 30 μmH2O2 compared with control cells. However, a significant reduction in MEQ fluorescence was observed at concentrations ≥100 μm of H2O2, and a maximal response was observed at 300 μmH2O2. Applying higher concentrations in the millimolar range produced no further decrease in MEQ fluorescence. We did not test concentrations above 10 mm because these concentrations have been reported to produce primary cytoplasmic and plasma membrane damage and rapid necrosis (Gardner et al., 1997). Unless noted otherwise, all experiments were performed with 300 μmH2O2.
Fig. 1.
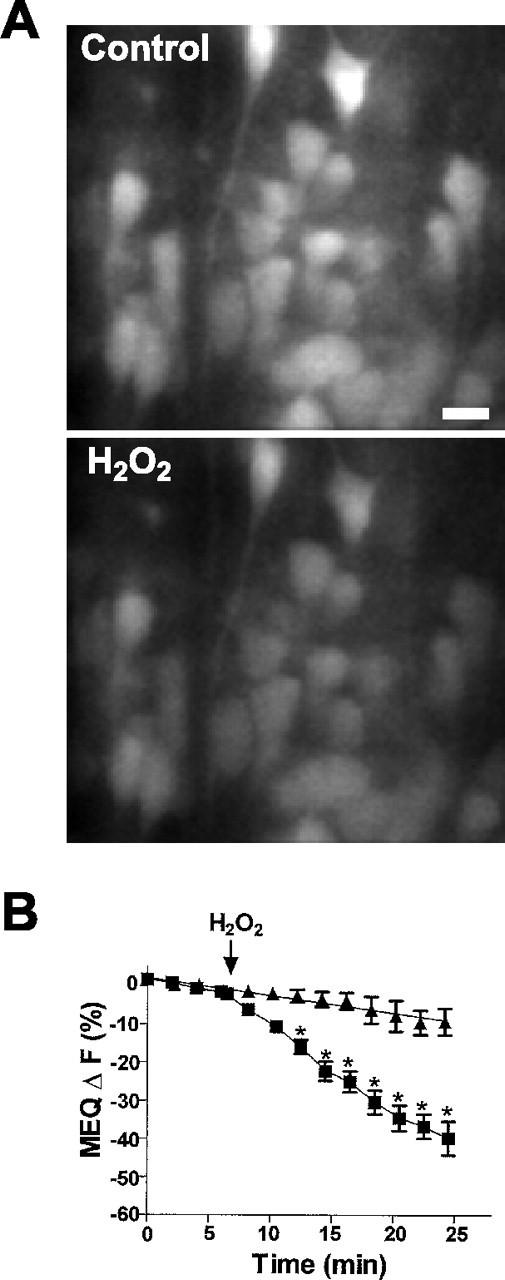
Effect of H2O2 on intracellular MEQ fluorescence in area CA1 pyramidal cells.A, Video confocal images of MEQ fluorescence before (Control) and 10 min after H2O2 (300 μm). The decreased fluorescence in the bottom indicates an increase in [Cl−]i. Scale bar, 20 μm. B, Time course for the H2O2-induced changes in MEQ fluorescence within individual pyramidal cells. After baseline recordings, hippocampal slices were superfused with 300 μm H2O2 (▪). The slow rundown (baseline drift) for control cells is also shown (▴). Data are mean ± SEM values of 8–14 cells for control and H2O2 (4 slices per condition) from three separate experiments. *p < 0.05 versus baseline fluorescence; repeated-measures ANOVA followed by Tukey's post hoc analysis.
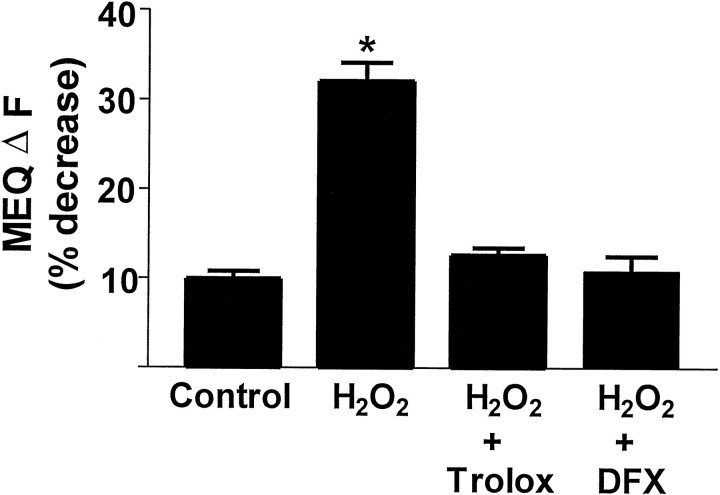
Fig. 5.
Effect of Trolox (50 μm) and deferoxamine mesylate (DFX; 100 μm) on H2O2-induced decreases in MEQ fluorescence. Hippocampal slices were superfused with 300 μmH2O2 for 10 min. The compounds were added 15 min before the addition of H2O2. Data are mean ± SEM values of 10–24 cells from at least three slices per condition. * p < 0.05 versus control; ANOVA followed by Tukey's test.
Fig. 2.
Concentration-dependent decrease in MEQ fluorescence by H2O2. MEQ-loaded slices were exposed to increasing concentrations of H2O2. The change in MEQ fluorescence (ΔF) was calculated as percent of baseline fluorescence for individual cells after 10 min of H2O2. Data are from 6–25 cells from at least three slices per condition. *p < 0.05 versus control; ANOVA and Tukey's post hocanalysis.
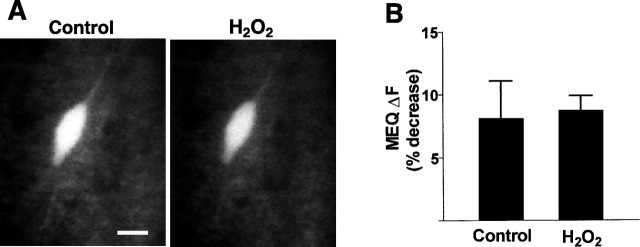
In contrast to pyramidal neurons, interneurons in area CA1 stratum radiatum were resistant to H2O2-induced changes in MEQ fluorescence (Fig. 3A). Ten minutes after H2O2exposure, there was no decrease in MEQ fluorescence compared with controls (Fig. 3B). These findings are consistent with the reported differences in vulnerability of area CA1 interneurons versus pyramidal neurons after ischemic insults in vivo (Crain et al., 1988; Inglefield et al., 1997).
Fig. 3.
Effect of H2O2 on intracellular MEQ fluorescence in interneurons located in area CA1 stratum radiatum. A, Video confocal images of MEQ fluorescence in an area CA1 interneuron before and 10 min after 300 μm H2O2 superfusion. Scale bar, 20 μm. B, H2O2 (10 min superfusion) causes no significant change in MEQ fluorescence in interneurons. Control bar represents the baseline drift (over 10 min) of MEQ fluorescence in cells exposed to Ringer's buffer without H2O2, under identical experimental conditions. Data are mean ± SEM of four to nine cells from four to six slices.
c-DCF fluorescence in neurons after H2O2
To verify that 10 min superfusion lead to adequate penetration of H2O2 within neurons, we assessed intracellular accumulation of H2O2 and other ROS in neuronal cell soma of CA1 pyramidal neurons using the oxidation-sensitive dye c-DCF. This dye has been used as a probe for evaluating H2O2 and other ROS, and therefore, it is an indicator of oxidative stress in biological systems (LeBel et al., 1992; Crow, 1997). There was a significant increase (53 ± 6.6%) in c-DCF fluorescence in area CA1 pyramidal neurons exposed to 300 μmH2O2 for 10 min (Fig.4). In addition, intense c-DCF fluorescence, which was punctate in distribution, was observed in the neuropil area in most slices. The punctate staining in the neuropil most likely reflected ROS accumulation in both terminals and dendrites, and it precluded observation of individual c-DCF-loaded interneurons. Control slices loaded with c-DCF and exposed to similar periods of laser illumination elicited minimal changes in c-DCF fluorescence during the experimental period (6.3 ± 2.2%), indicating negligible laser-induced auto-oxidation and photoconversion of the dye (Fig. 4B). The increase in c-DCF fluorescence was more rapid in some neurons, which showed significant increases in c-DCF fluorescence by 5 min of H2O2 superfusion (data not shown). The variability could be related to the intracellular H2O2 antioxidant status of individual neurons.
Fig. 4.
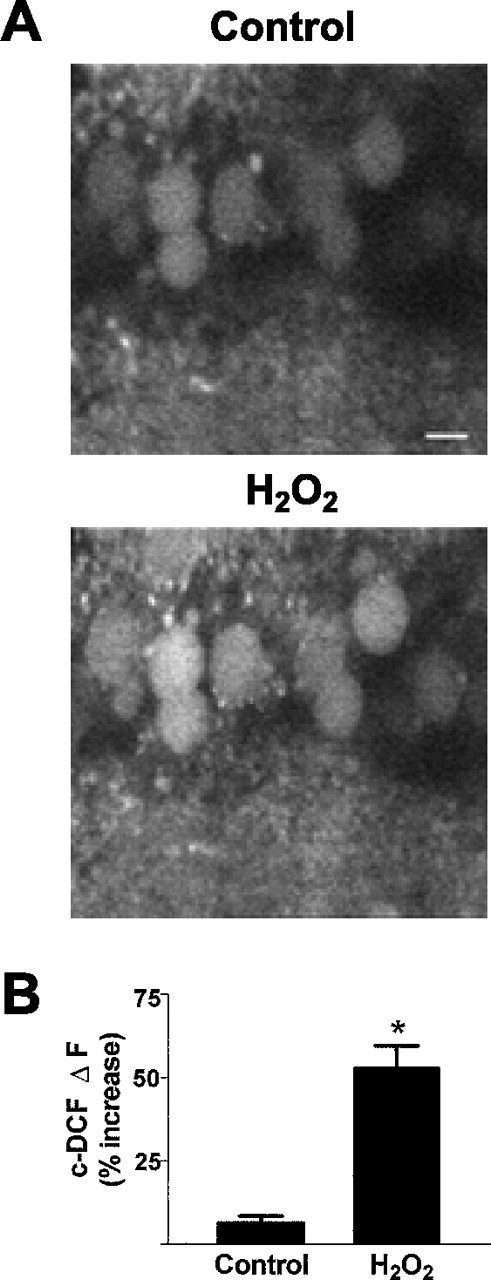
Increased c-DCF fluorescence in cells after 10 min H2O2, indicating elevation of ROS.A, Confocal video images of c-DCF fluorescence in area CA1 pyramidal cell soma before (Control) and after H2O2 superfusion. Scale bar, 20 μm.B, Changes in c-DCF fluorescence after 10 min H2O2. Control bar represents the baseline drift of c-DCF fluorescence in cells not exposed to H2O2 under identical experimental conditions. Data are mean ± SEM values of 12–22 cells from three to five slices. *p < 0.05 versus control; Student'st test.
H2O2 effects are mediated via free radicals
Normally, H2O2 is metabolized by glutathione peroxidase or catalase to water and a disulfide, or water and O2, respectively. If the antioxidant capacity is exceeded, H2O2, along with free Fe2+, promotes the production of hydroxyl radicals (OH·) via the Fenton reaction (Haber and Weiss, 1934), which peroxidizes lipids and proteins. To determine whether free radical generation was involved in the effect of H2O2 on MEQ fluorescence, slices were superfused with the iron chelator deferoxamine mesylate (100 μm). Deferoxamine completely prevented the H2O2-induced decrease in MEQ fluorescence (Fig. 5). In addition, superfusion of slices with Trolox (50 μm), a water-soluble form of α-tocopherol (vitamin E), prevented the ability of H2O2 to decrease MEQ fluorescence (Fig. 5). Thus, the H2O2-induced effects on [Cl−]i were probably a result of free radical generation, most likely hydroxyl radicals rather than H2O2 itself.
Propidium iodide fluorescence in MEQ-loaded cells after H2O2
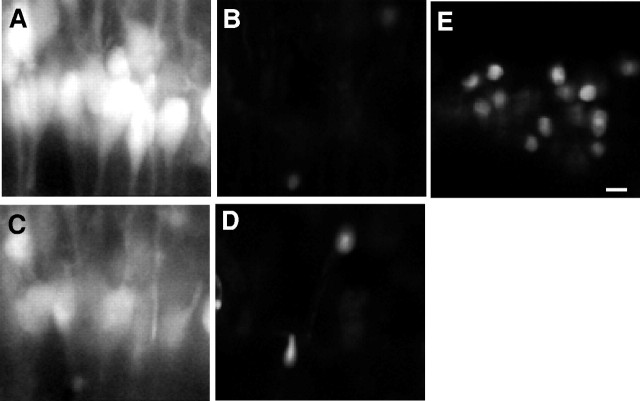
Free radicals, primarily OH·, can lead to peroxidation of membrane phospholipids, resulting in a loss of membrane integrity and cell death (Mattson, 1998). To determine whether neurons exposed to H2O2 maintained plasma membrane integrity, the MEQ-loaded slices were also loaded with PI, which is excluded from living cells with intact plasma membranes. As the plasma membrane becomes permeable, PI enters; it is relatively nonfluorescent until it binds to nuclear chromatin. Control cells loaded with MEQ showed no PI fluorescence (Fig.6A,B). Furthermore, cells continued to exclude PI after superfusion with H2O2 for 10 min (Fig.6D) and showed a significant reduction in MEQ fluorescence (Fig. 6C). Thus, the concentration of H2O2 used (300 μm) does not appear to damage plasma membranes. At the end of the experiment, the slices were incubated in Ringer's buffer containing 70% methanol as a positive control to permeabilize the cell membranes. Two minutes after exposure to methanol, there was intense nuclear staining of cells by PI (Fig. 6E), accompanied by a loss of MEQ fluorescence caused by damaged cell membranes (data not shown).
Fig. 6.
MEQ fluorescent cells within area CA1 pyramidal cell layer exclude PI 10 min after H2O2. An MEQ-loaded slice was constantly superfused with PI.A, MEQ fluorescent neurons in area CA1 before H2O2. B, Neurons inA imaged with the rhodamine filter for PI.C, MEQ fluorescent neurons 10 min after H2O2. D, Neurons inC imaged with the rhodamine filter for PI.E, As a positive control, the slice was exposed to methanol for 2 min to allow entry of PI into the same cells shown inA–D. All images were at a 1.7× electronic zoom to maintain registry between multiple laser lines. Scale bar, 20 μm.
H2O2-induced changes in [Cl−]i are secondary to release of GABA and activation of excitatory amino acid receptors by glutamate
Increases in intracellular Cl− in neurons may occur as a result of opening of ligand-gated Cl− channels, e.g., GABAA receptors, passive influx to accompany Na+, or an impairment of Cl− extrusion mechanisms (Alvarez-Leefmans, 1990). We performed specific antagonist and ion replacement experiments to determine potential mechanism(s) for the H2O2-induced accumulation of [Cl−]i. Superfusion of the slices with the noncompetitive GABAA receptor antagonist picrotoxin (100 μm) prevented the decrease in MEQ fluorescence of pyramidal cells produced by H2O2 (Table1), suggesting that Cl− influx was probably secondary to GABA release. Tetrodotoxin (2 μm) had no affect on the H2O2-induced change in MEQ fluorescence (Table 1), indicating a lack of involvement of voltage-dependent Na+ channels in the elevation of [Cl−]i by H2O2. To assess whether GABA was released by H2O2, we measured GABA levels in the superfusates after exposure of hippocampal slices to H2O2under identical conditions used for imaging (Fig.7). Within 2 min of H2O2 superfusion, there was a significant increase in the levels of GABA in the superfusate. In addition, glutamate levels increased by 2 min of H2O2 superfusion, but the increase was variable and did not reach statistical significance. To determine whether the H2O2-induced increase in [Cl− ]i involved the activation of excitatory amino acid (EAA) receptors, slices were incubated withd-AP-5 (50 μm) or NBQX (5 μm), selective antagonists of NMDA and AMPA receptors, respectively. Both compounds significantly attenuated the effect of H2O2 on MEQ fluorescence (Table 1), indicating that activation of EAA receptors by glutamate was also a component in the cascade of H2O2-mediated effects on intracellular Cl−.
Table 1.
H2O2-induced [Cl−]i elevation is prevented by the GABAA-Cl−channel blocker picrotoxin (PTX) and excitatory amino acid receptor antagonists (AP-5 and NBQX)
| Treatment | MEQ ΔF (% decrease) |
|---|---|
| Control | 9.9 ± 0.8 |
| H2O2 (300 μm) | 32.0 ± 2.0* |
| H2O2 + PTX (100 μm) | 10.0 ± 2.3 |
| H2O2 + AP-5 (50 μm) | 15.1 ± 1.0 |
| H2O2 + NBQX (5 μm) | 12.0 ± 1.6 |
| H2O2 + TTX (2 μm) | 32.5 ± 2.0* |
Slices were superfused with H2O2 for 10 min. Compounds were added 15 min before the addition of H2O2. Controls represent drift in MEQ fluorescence in the absence of compounds and H2O2. Data are mean ± SEM of 13–27 cells imaged from at least three individual slices in several experiments. *p < 0.05 versus control; ANOVA and Tukey'spost hoc test.
Fig. 7.
H2O2 causes an increase of amino acids (AA), glutamate (-▪-), and GABA (-▴-) in the superfusate of hippocampal slices. Superfusates were collected at 0 (basal), 2, and 10 min after 300 μmH2O2. Data are from a single experiment and represent three separate experiments. Accumulation of GABA was significantly above basal (p < 0.05) at 2 and 10 min (repeated measures ANOVA, followed by paired Student'st test).
Ca2+ ions mediate the H2O2-induced elevation in [Cl−]i
Oxidative species can cause the release of GABA via a Ca2+-dependent exocytotic process (Rego et al., 1996) and via a Ca2+-independent, Na+-dependent GABA carrier-mediated efflux (Oliveira et al., 1994). To investigate whether the H2O2-induced increase in intracellular Cl− required the presence of Ca2+, we superfused slices in a Ca2+-deficient Ringer's buffer containing 1 mm EGTA, before the addition of H2O2. The removal of extracellular Ca2 + markedly prevented the decrease in MEQ fluorescence by H2O2 (Fig. 8). Thus, the ability of H2O2 to elevate [Cl−]i appears to be dependent on the influx of Ca2+. To determine whether GABA carrier-mediated efflux (i.e., carrier reversal) contributed to the H2O2-induced increase in intracellular Cl−, we substituted Na+ (from NaCl) in the superfusion buffer with an equimolar concentration of choline chloride. The removal of Na+ had no effect on the ability of H2O2 to decrease MEQ fluorescence (Fig. 8).
Fig. 8.
H2O2-induced Cl− influx is Ca2+-dependent and Na+-independent. All ion-substituted buffers were iso-osmotic with the control Ringer's buffer. The Ca2+-deficient buffer also contained 1 mm EGTA. In the Na+-deficient buffer, choline chloride replaced NaCl. Data are mean ± SEM of 4–27 cells from at least three slices per condition. *p< 0.05 versus control; ANOVA followed by Tukey's post hoc test.
H2O2 decreases GABAA responses
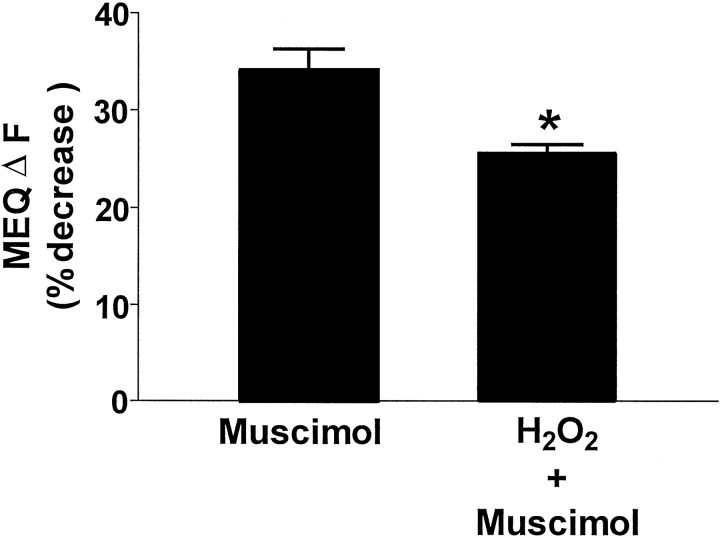
To determine whether the H2O2-induced elevation in intracellular Cl− could limit GABAA responses in the hippocampal slice, slices were superfused with H2O2for 10 min, and the H2O2was removed. The slices were superfused subsequently with the GABAA agonist muscimol (50 μm) for 10 min. At this time, the ability of muscimol to decrease MEQ fluorescence was significantly attenuated (by 25%; p< 0.05) compared with control slices not exposed to H2O2 (Fig.9).
Fig. 9.
H2O2 attenuates the ability of muscimol to increase intracellular Cl−in area CA1 pyramidal cells. The GABAA agonist muscimol (50 μm) was applied for 10 min to the chamber containing control slices or slices previously exposed to 10 min H2O2. The ΔF is the change in MEQ fluorescence after 10 min of exposure of control or peroxidized slices to muscimol. The ΔF for each cell was calculated as described in Materials and Methods, except the fluorescence intensity values before muscimol addition were used for the Fb. Data are the mean ± SEM of 7–14 cells from at least three slices per condition. *p < 0.05 versus control; Student'st test.
DISCUSSION
In the present study, we used the Cl−-sensitive dye MEQ and laser scanning confocal microscopy to assess alterations in intraneuronal Cl− in the hippocampal slice after a mild oxidative insult induced by H2O2. The use of the brain slice preparation, which maintains a relatively intact circuitry, allowed us to image both pyramidal neurons and interneurons under identical conditions. A brief exposure of slices to H2O2 caused a significant increase in [Cl−]i in CA1 pyramidal cell soma. A comparison of the effects of H2O2 on MEQ and c-DCF fluorescence indicated that the increase in [Cl−]i occurred simultaneously with the accumulation of H2O2 and related ROS inside the cell soma. Normally, ambient levels of H2O2 in neurons are low because of its efficient destruction by glutathione peroxidase (Halliwell and Gutteridge, 1989). However, micromolar concentrations (∼100 μm) of H2O2 are found in brain after ischemia–reperfusion injury as measured by microdialysis (Hyslop et al., 1995). These micromolar concentrations can also be reached in aged brain tissue (Sohal et al., 1994; Auerbach and Segal, 1997), possibly because of an overproduction and/or reduced metabolism of H2O2.
In contrast to the acute sensitivity of pyramidal neurons to H2O2, interneurons located in area CA1 stratum radiatum did not demonstrate an increase in [Cl−]i in response to H2O2. Interestingly, differences in vulnerability of neuronal types to neurodegenerative processes have been observed in cerebral ischemia and in Huntington's disease (Crain et al., 1988; Inglefield et al., 1997; Calabresi et al., 1998). GABAergic interneurons in the hippocampus have been shown to survive long-term after an episode of cerebral ischemia, although they do suffer considerable damage (Nitsch et al., 1989; Inglefield et al., 1997). The exact reason(s) for the differing responses to H2O2 between these two cell types is not clear at present. However, some possible explanations may include the following: (1) different levels of intracellular antioxidants, (2) heterogeneity in GABAA-mediated Cl− currents, (3) different efficiencies of Cl− extrusion mechanisms, and (4) the neuronal circuitry. Interestingly, different levels of superoxide dismutase (SOD1 and SOD2 isoforms) have been reported in striatal cholinergic interneurons versus projection neurons, the latter being more susceptible to neurodegenerative processes (Yamada et al., 1995;Medina et al., 1996). Also, differential vulnerability of CA1 and CA3 subfields to superoxide and hydroxyl radicals has been reported in hippocampal slices (Wilde et al., 1997); this difference in vulnerability was attributed primarily to different levels of antioxidant enzymes and iron binding proteins between the two cell populations. Differences between pyramidal neurons and interneurons may also exist with respect to Cl− channel kinetics (Xiang et al., 1998) and GABAA receptor subunit composition (Sperk et al., 1997). In addition, differential responses to oxidative stress have been reported in thalamic and cortical neurons based on neuronal circuitary in which loss or preservation of certain pathways modified electrophysiological responses to H2O2(Frantseva et al., 1998). Future studies are required to fully understand the heterogeneity in responsiveness between hippocampal pyramidal neurons and interneurons to H2O2.
Alterations in [Cl−]i induced by H2O2 in pyramidal neurons were prevented in the presence of the iron chelator deferoxamine, suggesting that H2O2effects were probably mediated by hydroxyl radicals rather than H2O2 itself. This is consistent with previous reports in which the effects of H2O2 on synaptic efficacy were dependent on the presence of free iron in hippocampal slices (Pellmar et al., 1989). The ability of the antioxidant Trolox to prevent the increase in [Cl−]i by H2O2 also supports a role of free radicals in the actions of H2O2.
How does H2O2 produce elevated intraneuronal Cl− in pyramidal cell soma? Significant reduction of H2O2 action by the GABA-gated Cl− channel antagonist picrotoxin indicates that accumulation of Cl− probably occurred as a result of its influx through the GABAA ionophore, secondary to elevation of extracellular GABA levels. To support this, we found increased levels of GABA in superfusates collected from H2O2-exposed slices. Our studies agree with previous studies in which oxygen free radicals increased basal release of GABA in hippocampal slices and in cultured chick retinal neurons (Rego et al., 1996; Saransaari and Oja, 1998). However, the mechanism(s) by which H2O2 alters intraneuronal Cl− is probably more complex because it must also account for the dependence of H2O2 action on glutamate receptor activation and on extracellular Ca2+. The ability of H2O2 and other ROS to cause glutamate accumulation has also been documented. For example, elevated levels of excitatory amino acids have been reported in hippocampal slices and neuronal cultures on exposure to free radicals because of enhanced release (Pellegrini-Giampietro et al., 1988; Satoh et al., 1998) or impaired uptake (Volterra et al., 1994; Berman and Hastings, 1997). Free radicals can enhance basal release of excitatory amino acids in the presence or absence of extracellular Ca2+ (Gilman et al., 1994; Rego et al., 1996). In addition, enhancement of excitatory neurotransmission was observed after H2O2 in thalamocortical neurons, along with attenuated inhibitory transmission (Frantseva et al., 1998).
There is significant evidence implicating impaired Ca2+ homeostasis after oxidative insults (Mattson, 1998). Recently, Li et al. (1998) have reported that H2O2 increased the Ca2+ channel currents in cloned neuronal voltage-dependent Ca2+ channels expressed in Xenopus oocytes. Elevated [Ca2+]i after H2O2 has also been reported in synaptosomes (Tretter and Adam-Vizi, 1996). Interestingly, elevated [Ca2+]i in response to glutamate receptor activation has been shown to increase levels of ROS (Dugan et al., 1995; Reynolds and Hastings, 1995; Bindokas et al., 1996).
A model that includes the hippocampal circuit may be proposed to account for the mechanism(s) by which H2O2 increases intracellular Cl−. After exposure to H2O2, glutamate could accumulate (via Ca2+-dependent release and/or impaired uptake) at synapses with GABA interneurons, thereby activating glutamate receptors and causing GABA release. This process appears to be TTX-insensitive. Because the effects of H2O2 were blocked by glutamate receptor antagonists, Ca2+-dependent release or impaired uptake of GABA at interneuron terminals appear to be insignificant. Thus, glutamate terminals may be more sensitive to H2O2 than are GABA terminals. In addition to promoting Cl−influx, H2O2 may also affect Cl− extrusion mechanisms. Neurons contain ATP-dependent Cl− transporters, and these transporters may become impaired because H2O2 can decrease the ATP/ADP ratio (Tretter et al., 1997). Our model precludes any significant effects of H2O2directly on GABAA receptors, which have been reported to be sensitive to modulation by redox agents (Pan et al., 1995).
Hydrogen peroxide and other ROS alter synaptic inhibition, although the exact mechanisms are not known (Muller et al., 1993; Pellmar, 1995,Frantseva et al., 1998). A sustained increase in intracellular Cl− will decrease the Cl− gradient, and this should reduce subsequent GABAA-mediated hyperpolarization. Our findings are consistent with this process; the ability of muscimol to induce Cl− influx was reduced after slices were exposed to H2O2. Similarly, tonic activation of GABAA receptors by “ambient” GABA has been shown to elevate the resting Cl− permeability, and it causes a positive shift in the reversal potential for Cl− and a decrease in inhibitory transmission (Thompson and Gahwiler, 1989). Intense and repeated activation of GABAA receptors has also been reported to collapse the Cl−concentration gradient, leading to a positive shift inECl and a depolarizing response to GABA (Lambert and Grover 1995). This process may be important in conditions such as anoxia. In fact, Katchman et al. (1994) showed that the anoxia-induced suppression of monosynaptic GABAA receptor IPSCs in CA1 pyramidal neurons was caused by an altered IPSC reversal potential. In our previous studies, we also found that oxygen–glucose deprivation in the hippocampal slice decreased GABA responses caused by increased [Cl−]i (Inglefield and Schwartz-Bloom, 1998). Thus, when neurons are exposed to oxidative stress, increases in intraneuronal Cl− may explain the reduction in synaptic inhibition mediated by GABAA receptors.
Using optical imaging techniques within the hippocampal slice, we have demonstrated that exposure of neurons to mild oxidative stress produced by H2O2 increases Cl− levels within hippocampal pyramidal cell soma but not in interneurons. Impairment of Cl− gradients by H2O2 may reduce the efficacy of GABAA receptor-mediated inhibitory transmission and potentiate neurodegenerative damage in conditions such as Alzheimer's disease, Parkinson's disease, and aging.
Footnotes
This work was supported by National Institutes of Health Grants 5 T32 AG00029 (R.S.) and NS28791 (R.D.S.). We gratefully acknowledge the laboratory of Dr. J. Victor Nadler for analysis of HPLC samples and Dr. George Somjen for helpful comments on this manuscript.
Correspondence should be addressed to Dr. Rochelle D. Schwartz-Bloom, Department of Pharmacology and Cancer Biology, Box 3813, Duke University Medical Center, Durham, NC 27710. E-mail:schwa001@duke.edu.
REFERENCES
- 1.Alvarez-Leefmans FJ. Intracellular Cl− regulation and synaptic inhibition in vertebrate and invertebrate neurons. In: Alvarez-Leefmans FJ, Russell JM, editors. Chloride channels and carriers in nerve, muscle, and glial cells. Plenum; New York: 1990. pp. 109–158. [Google Scholar]
- 2.Auerbach JM, Segal M. Peroxide modulation of slow onset potentiation in rat hippocampus. J Neurosci. 1997;17:8695–8701. doi: 10.1523/JNEUROSCI.17-22-08695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beal MF. Aging, energy and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 4.Berman SB, Hastings TJ. Inhibition of glutamate transport in synaptosomes by dopamine oxidation and reactive oxygen species. J Neurochem. 1997;69:1185–1195. doi: 10.1046/j.1471-4159.1997.69031185.x. [DOI] [PubMed] [Google Scholar]
- 5.Bindokas VP, Jordán J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biwersi J, Verkman AS. Cell-permeable fluorescent indicator for cytosolic chloride. Biochemistry. 1991;30:7879–7883. doi: 10.1021/bi00246a001. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi P, Centonze D, Pisani A, Sancesario G, Gubellini P, Marfia GA, Bernadi G. Striatal spiny neurons and cholinergic interneurons express differential ionotropic glutamatergic responses and vulnerability: implications for ischemia and Huntington's disease. Ann Neurol. 1998;43:586–597. doi: 10.1002/ana.410430506. [DOI] [PubMed] [Google Scholar]
- 8.Colton CA, Fagni L, Gilbert D. The action of hydrogen peroxide on paired pulse and long-term potentiation in the hippocampus. Free Radic Biol Med. 1989;7:3–8. doi: 10.1016/0891-5849(89)90093-2. [DOI] [PubMed] [Google Scholar]
- 9.Crain BJ, Westerkam WD, Harrison AH, Nadler JV. Selective neuronal death after transient forebrain ischemia in the Mongolian gerbil: a silver impregnation study. Neuroscience. 1988;27:387–402. doi: 10.1016/0306-4522(88)90276-x. [DOI] [PubMed] [Google Scholar]
- 10.Crow JP. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for measurement of reactive nitrogen and oxygen species. Nitric Oxide. 1997;1:145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- 11.Dugan LL, Sensi SL, Canzoniero LMT, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frantseva MV, Jose L, Velazquez P, Carlan PL. Changes in membrane and synaptic properties of thalamocortical circuitary caused by hydrogen peroxide. J Neurophysiol. 1998;80:1317–1326. doi: 10.1152/jn.1998.80.3.1317. [DOI] [PubMed] [Google Scholar]
- 13.Gardner AM, Xu F-H, Fady C, Jacoby FJ, Duffey DC, Tu Y, Lichtenstein A. Apoptotic vs nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med. 1997;22:73–83. doi: 10.1016/s0891-5849(96)00235-3. [DOI] [PubMed] [Google Scholar]
- 14.Gilman SC, Bonner MJ, Pellmar TC. Free radicals enhance basal release of d-[3H]Aspartate from cerebral cortical synaptosomes. J Neurochem. 1994;62:1757–1763. doi: 10.1046/j.1471-4159.1994.62051757.x. [DOI] [PubMed] [Google Scholar]
- 15.Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc Lond B Biol Sci. 1934;147:332–351. [Google Scholar]
- 16.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford UP; New York: 1989. [Google Scholar]
- 17.Hyslop PA, Zhang Z, Pearson DV, Phebus LA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2in vitro. Brain Res. 1995;671:181–186. doi: 10.1016/0006-8993(94)01291-o. [DOI] [PubMed] [Google Scholar]
- 18.Inglefield JR, Schwartz-Bloom RD. Confocal imaging of intracellular chloride in living brain slices: measurement of GABAA receptor activity. J Neurosci Methods. 1997;75:127–135. doi: 10.1016/s0165-0270(97)00054-x. [DOI] [PubMed] [Google Scholar]
- 19.Inglefield JR, Schwartz-Bloom RD. Optical imaging of hippocampal neurons with a chloride-sensitive dye: early effects of in vitro ischemia. J Neurochem. 1998;70:2500–2509. doi: 10.1046/j.1471-4159.1998.70062500.x. [DOI] [PubMed] [Google Scholar]
- 20.Inglefield JR, Wilson CA, Schwartz-Bloom RD. Effect of transient cerebral ischemia on γ-aminobutyric acidA receptor α1-subunit-immunoreactive interneurons in the gerbil CA1 hippocampus. Hippocampus. 1997;7:511–523. doi: 10.1002/(SICI)1098-1063(1997)7:5<511::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Katchman AN, Vicini S, Hershkowitz N. Mechanism of early anoxia-induced suppression of the GABAA- mediated inhibitory postsynaptic current. J Neurophysiol. 1994;71:1128–1138. doi: 10.1152/jn.1994.71.3.1128. [DOI] [PubMed] [Google Scholar]
- 22.Katsuki H, Nakanishi C, Saito H, Matsuki N. Biphasic effect of hydrogen peroxide on field potentials in rat hippocampal slices. Eur J Pharmacol. 1997;337:213–218. doi: 10.1016/s0014-2999(97)01323-x. [DOI] [PubMed] [Google Scholar]
- 23.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol. 1998;275:C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- 24.Lambert N, Grover L. The mechanism of biphasic GABA responses. Science. 1995;269:928–929. doi: 10.1126/science.7638614. [DOI] [PubMed] [Google Scholar]
- 25.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′, 7′-dichloro-fluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 26.Li A, Segui J, Heinemann SH, Hoshi T. Oxidation regulates neuronal voltage-dependent Ca2+ channels expressed in Xenopus oocytes. J Neurosci. 1998;18:6740–6747. doi: 10.1523/JNEUROSCI.18-17-06740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindroth P, Mopper K. High performance liquid chromatographic determination of picomole amounts of amino acids by precolumn derivatisation with o-phthaldialdehyde. Anal Chem. 1979;51:1667–1674. [Google Scholar]
- 28.Mattson MP. Modification of ion homeostasis by lipid peroxidation: roles in neuronal degeneration and adaptive plasticity. Trends Neurosci. 1998;20:53–57. doi: 10.1016/s0166-2236(97)01188-0. [DOI] [PubMed] [Google Scholar]
- 29.Medina L, Figueredo-Cardenas G, Reiner A. Differential abundance of superoxide dismutase in interneurons versus projection neurons and in matrix versus striosome neurons in monkey striatum. Brain Res. 1996;708:59–70. doi: 10.1016/0006-8993(95)01320-2. [DOI] [PubMed] [Google Scholar]
- 30.Muller M, Fontana A, Zbinden G, Gahwiler BH. Effects of interferons and hydrogen peroxide on CA3 pyramidal cells in rat hippocampal slice cultures. Brain Res. 1993;619:157–162. doi: 10.1016/0006-8993(93)91607-t. [DOI] [PubMed] [Google Scholar]
- 31.Nitsch C, Goping G, Klatzo I. Preservation of GABAergic perikarya and boutons after transient cerebral hippocampus in the gerbil CA1 hippocampal field. Neurosci Lett. 1989;495:243–252. doi: 10.1016/0006-8993(89)90218-7. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira CR, Agostinho P, Caseiro P, Duarte CB, Carvalho AP. Reactive oxygen species on GABA release. Ann NY Acad Sci. 1994;738:130–140. doi: 10.1111/j.1749-6632.1994.tb21798.x. [DOI] [PubMed] [Google Scholar]
- 33.Pan ZH, Bahring R, Grantyn R, Lipton S. Differential modulation by sulfhydral redox agents and glutathione of GABA- and glycine-evoked currents in rat retinal ganglion cells. J Neurosci. 1995;15:1384–1391. doi: 10.1523/JNEUROSCI.15-02-01384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellegrini-Giampietro DE, Cherici G, Alesiani M, Carla V, Moroni F. Excitatory amino acid release from rat hippocampal slices as a consequence of free-radical formation. J Neurochem. 1988;51:1960–1963. doi: 10.1111/j.1471-4159.1988.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 35.Pellmar TC. Use of brain slices in the study of free-radical actions. J Neurosci Methods. 1995;59:93–98. doi: 10.1016/0165-0270(94)00198-p. [DOI] [PubMed] [Google Scholar]
- 36.Pellmar TC, Neel KL, Lee KH. Free radicals mediate peroxidative damage in guinea pig hippocampus in vitro. J Neurosci Res. 1989;24:437–444. doi: 10.1002/jnr.490240314. [DOI] [PubMed] [Google Scholar]
- 37.Peterson CL, Thompson MA, Martin D, Nadler JV. Modulation of glutamate and aspartate release from slices of hippocampal area CA1 by inhibitors of arachidonic acid metabolism. J Neurochem. 1995;64:1152–1160. doi: 10.1046/j.1471-4159.1995.64031152.x. [DOI] [PubMed] [Google Scholar]
- 38.Rego AC, Santos MS, Oliveira CA. Oxidative stress, hypoxia and ischemia-like conditions increase the release of endogenous amino acids by distinct mechanisms in cultured retinal cells. J Neurochem. 1996;66:2506–2516. doi: 10.1046/j.1471-4159.1996.66062506.x. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3337. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sah R, Schwartz-Bloom RD. Imaging area CA1 hippocampal neurons in the brain slice after exposure to hydrogen peroxide: effects on intracellular chloride. J Neurochem [Suppl] 1999;72:S34A. [Google Scholar]
- 41.Saransaari P, Oja SS. Release of endogenous glutamate, aspartate, GABA, and taurine from hippocampal slices from adult and developing mice under cell-damaging conditions. Neurochem Res. 1998;23:563–570. doi: 10.1023/a:1022494921018. [DOI] [PubMed] [Google Scholar]
- 42.Satoh T, Tadahiro N, Yasuhiro A, Tomoko Y, Yasuyuki E, Hiroshi H. Production of reactive oxygen species and release of l-glutamate during superoxide anion-induced cell death of cerebellar granule neurons. J Neurochem. 1998;70:316–324. doi: 10.1046/j.1471-4159.1998.70010316.x. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz RD, Skolnick P, Paul SM. Regulation of γ-aminobutyric acid/barbiturate receptor-gated chloride ion flux in brain vesicles by phospholipase A2: possible role of oxygen radicals. J Neurochem. 1988;50:565–571. doi: 10.1111/j.1471-4159.1988.tb02948.x. [DOI] [PubMed] [Google Scholar]
- 44.Simonian NA, Coyle JT. Oxidative stress in neurodegenerative disease. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 45.Sohal RS, Ku H, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defences during aging and in food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 46.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABA(A) receptor subunits in the rat hippocampus. I. Immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 47.Thompson SM, Gahwiler BH. Activity-dependent disinhibition. II. Effects of extracellular potassium, furosemide, and membrane potential on ECl in hippocampal CA3 neurons. J Neurophysiol. 1989;61:512–523. doi: 10.1152/jn.1989.61.3.512. [DOI] [PubMed] [Google Scholar]
- 48.Tretter L, Adam-Vizi V. Early events in free radical-mediated damage of isolated nerve terminals: effects of peroxides on membrane potential and intracellular Na+ and Ca2+ concentrations. J Neurochem. 1996;66:2057–2066. doi: 10.1046/j.1471-4159.1996.66052057.x. [DOI] [PubMed] [Google Scholar]
- 49.Tretter L, Chinopoulos C, Adam-Vizi V. Enhanced depolarization-evoked calcium signal and reduced [ATP/ADP] ratio are unrelated events induced by oxidative stress in synaptosomes. J Neurochem. 1997;69:2529–2537. doi: 10.1046/j.1471-4159.1997.69062529.x. [DOI] [PubMed] [Google Scholar]
- 50.Verkman AS. Development and biological applications of chloride-sensitive fluorescent indicators. Am J Physiol. 1990;259:C375–C388. doi: 10.1152/ajpcell.1990.259.3.C375. [DOI] [PubMed] [Google Scholar]
- 51.Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in cortical astrocytes. J Neurosci. 1994;14:2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilde GJC, Pringle AK, Wright P, Iannotti F. Differential vulnerability of the CA1 and CA3 subfields of the hippocampus to superoxide and hydroxyl radicals in vitro. J Neurochem. 1997;69:883–886. doi: 10.1046/j.1471-4159.1997.69020883.x. [DOI] [PubMed] [Google Scholar]
- 53.Xiang Z, Huguenard JR, Prince DA. GABAA receptor-mediated currents in interneurons and pyramidal cells of rat visual cortex. J Physiol (Lond) 1998;506:713–730. doi: 10.1111/j.1469-7793.1998.715bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada K, Goto S, Oyama T, Yoshikawa M, Nagahiro S, Ushio Y. Striatal cells containing the Ca2+-binding protein calretinin (protein 10) in ischemia-induced neuronal injury. Acta Neuropathol (Berl) 1995;89:172–177. doi: 10.1007/BF00296362. [DOI] [PubMed] [Google Scholar]