Abstract
GABA receptors (GABAA) are the major sites of fast synaptic inhibition in the brain and can be assembled from five subunit classes: α, β, γ, δ, and ε. Receptor function can be regulated by direct phosphorylation of β and γ2 subunits, but how kinases are targeted to GABAA receptors is unknown. Here we show that protein kinase C-βII (PKC-βII) is capable of directly binding to the intracellular domain of the receptor β1 and β3 subunits, but not to those of the α1 or γ2 subunits. Moreover, associating PKC-βII is capable of specifically phosphorylating serine 409 in β1 subunit and serines 408/409 within the β3 subunit, key residues for modulating GABAAreceptor function. The receptor for activated C kinase (RACK-1) was found also to bind to the β1 subunit intracellular domain, but PKC binding appeared to be independent of this protein. Using immunoprecipitation, the association of PKC isoforms and RACK-1 with neuronal GABAA receptors was seen. Furthermore, PKC isoforms associating with neuronal receptors were capable of phosphorylating the receptor β3 subunit.
Together, these observations suggest GABAA receptors are intimately associated with PKC isoforms via a direct interaction with receptor β subunits. This interaction may serve to localize PKC activity to GABAA receptors in neurons allowing the rapid regulation of receptor activity by cell-signaling pathways that modify PKC activity.
Keywords: GABAA receptor, β subunit, PKC, RACK-1, intracellular domain, protein kinase C
GABAAreceptors are the major sites of fast synaptic inhibition in the brain (Macdonald and Olsen, 1994; Rabow et al., 1995). GABAA receptors are part of a ligand-gated ion channel superfamily whose members include nicotinic acetylcholine, glycine, and 5HT3 receptors (Unwin, 1993). Members of this channel superfamily are believed to be heteropentamers, the subunits of which share a common transmembrane topology. This comprises a large N-terminal domain and four transmembrane domains (TMs) with a major intracellular domain between TMs 3 and 4 (Unwin, 1993). GABAA receptor subunits can be divided into five subunit classes with multiple members: α(1–6), β(1–3), γ(1–3), δ, and ε (Macdonald and Olsen, 1994; Rabow et al., 1995). Heterologous expression has revealed that the coexpression of receptor α, β, and γ subunits reproduces many of the physiological and pharmacological properties of neuronal GABAA receptors (Macdonald and Olsen, 1994; Rabow et al., 1995).
There is considerable interest in understanding the molecular mechanisms used by neurons to regulate GABAAreceptor function, with much emphasis at present focusing on the role of receptor phosphorylation. Studies on recombinant receptors have revealed that receptor β and γ subunits are the substrates of a range of protein kinases (Moss and Smart, 1996). Specifically, the β1–3 subunits are phosphorylated on a conserved serine residue (S409 or S410) by PKC, whereas PKA will differentially phosphorylate β subunits on S409 in vivo (Moss et al., 1992a,b; Krishek et al., 1994; McDonald and Moss, 1997; McDonald et al., 1998). There are additional phosphorylation sites for PKC, Ca2+–calmodulin type 2 dependent protein kinase (Cam KII) and cGMP-dependent protein kinase (PKG) within the β1, β3, and γ2 subunits (Moss et al., 1992a; McDonald and Moss 1994, 1997). The prototypic tyrosine kinase SRC will also phosphorylate specific sites within the γ2 and β1 subunits (Moss et al., 1995). In agreement with these observations, purified preparations of neuronal GABAA receptors are phosphorylated in vitro by PKA, PKC, and SRC (Kirkness et al., 1989; Browning et al., 1990; Valenzuela et al., 1995). GABAAreceptor phosphorylation can cause diverse functional effects, ranging from enhancements to inhibitions depending on the identity and location of the sites phosphorylated (Kapur and Macdonald, 1996; Lin et al., 1996; Moss and Smart, 1996; McDonald et al., 1998).
Although much progress has been made on identifying which receptor subunits are kinase substrates, little is presently understood regarding how specific kinases are targeted to GABAA receptors to ensure subunit-specific phosphorylation. To further investigate this, we have used subunit intracellular domains to look for interacting molecules that mediate GABAA receptor phosphorylation. Here we demonstrate that PKC-βII is targeted to GABAAreceptors via a direct interaction with receptor β subunits that is independent of the receptor for activated C kinase (RACK-1). Both PKC and RACK-1 immunoprecipitate with GABAAreceptors from cortical neurons. In addition, PKC isoforms associating with neuronal GABAA receptors are capable of phosphorylating the receptor β3 subunit. Together our observations suggest a critical role of receptor β subunits in targeting PKC activity to GABAA receptors.
MATERIALS AND METHODS
Production and purification of fusion proteins. The major intracellular loop between TM3 and TM4 of the GABAA receptors α1, β1, β2, β3, and the “short” form of the γ2 subunit (γ2s; Whiting et al., 1990;Kofuji et al., 1991) were cloned as BamHI–EcoRI fragments into pGex-4T3 (Pharmacia, Piscataway, NJ), for the production of glutathione-S-transferase (GST) fusion proteins. AnEcoRI–SmaI fragment comprising the entire coding sequence of RACK-1 was subcloned into pGEX-2TK. (Pharmacia). The RACK-1 cDNA was a kind gift of D. Mochly-Rosen, Stanford University (Ron et al., 1994). DNA constructs, the fidelity of which had been verified by DNA sequencing, were transformed intoEscherichia coli strain BL21 for protein expression. One liter cultures were grown, induced with isopropyl-B-d-thiogalactoylpyranoside (0.1 mm), sonicated, and the GST fusion proteins were then purified on glutathione agarose beads (Sigma, St. Louis, MO) as described previously (Smith and Johnson, 1988; Moss et al., 1992b).
Affinity purification “pull-down” assays. Brains from adult Sprague Dawley rats were homogenized in buffer containing 1% Nonidet P-40, 0.5% deoxycholate, and (in mm) 150 NaCl, 10 triethanolamine, pH 7.6, 5 EGTA, 5 EDTA, 50 NaF, 1 Na orthovanadate, 100 PMSF, and 10 μg/ml leupeptin, pepstatin, antipain, and aprotinin. Insoluble material was removed by centrifugation at 50,000 ×gK for 30 min. Extracts (5 mg of protein) were then exposed to receptor fusion proteins (20 μg) at 4°C for 2 hr. Beads were washed twice in buffer 1 consisting of 0.4% Nonidet P-40 and (in mm) 500 NaCl, 10 triethanolamine, pH 7.6, 5 EGTA, 5 EDTA, 1 Na orthovanadate, 1 phenylmethylsulfonyl fluoride (PMSF), and then twice in buffer 1 supplemented with 50 mm NaCl. At this stage, the beads were either used in kinase assays or subjected to SDS-PAGE. Proteins binding to the fusions or GST alone were then detected by Western blotting. The antibodies used were as follows: anti-RACK-1 (mouse monoclonal; Transduction Laboratories, Lexington, KY) and anti-pan-PKC (rabbit polyclonal; Upstate Biotechnology, Lake Placid, NY). PKC-specific isoform antisera against the: α, βII, γ, δ, ε, ζ, η, and θ isoforms have been described previously (Kiley and Parker, 1995). Blots were visualized using ECL (Pierce, Rockford, IL).
In vitro phosphorylation. To analyze the capability of associating kinases to phosphorylate bound GABAAreceptor subunit intracellular domains, adult rat brain lysate was adsorbed with fusion proteins and washed as above. Beads were then washed in kinase buffer (in mm: 20 Tris, pH 7.4, 20 MgCl2, 1 EDTA, 1 EGTA, 1 ouabain, 1 Na orthovanadate, 0.1 DTT, and 2 MnCl2) and then incubated at 30°C for up to 30 min in kinase buffer containing 3–30 μCi γ32P-ATP at a final concentration of 20 μm (Amersham, Arlington Heights, IL). Beads were then pelleted, and bound material was separated by SDS-PAGE followed by autoradiography. To characterize the copurifying kinase activity, assays were performed in the presence of various kinase inhibitors; 0.1 μm PKA inhibitor/Walsh peptide (Promega, Madison, WI), 1 μm Cam KII inhibitor W7, (Calbiochem, La Jolla, CA) 0.1–0.5 μm PKC inhibitor peptide (19–36; Calbiochem).
Quantification of kinase activity interacting with GABAA receptor subunits. To analyze the level of kinase activity copurifying with each subunit, kinase assays were performed as above but in the presence of a core substrate peptide derived from neurogranin residues 28–43 (NG 28–43; Promega), a well characterized PKC substrate (Chen et al., 1993). The peptide was added to the reaction at a concentration of 50 μm with or without PKC(18–36) inhibitor peptide (10 μm) under the conditions described above. The reaction was stopped by adding an equal volume of ice-cold 150 mmH3PO4. The beads were then pelleted, and triplicate aliquots of the supernatant were spotted onto Whatman P-81 phosphocellulose filter papers. Papers were then washed with three 10 min changes of 150 mmH3PO4. The papers were then dried and subjected to Cherenkov scintillation counting. For all experiments, values were for control reactions lacking substrate, or beads not exposed to lysate were subtracted as blanks. Under the conditions used, the rate of phosphorylation was linear with respect to time.
Phosphoamino acid analysis. Phosphoamino acid analysis was performed on excised gel slices as described previously (Moss et al., 1992a; McDonald and Moss, 1994). Phosphoprotein gel slices were rehydrated, washed, and digested with trypsin (0.1 mg/ml, Sigma) for 24 hr. Digested samples were then hydrolyzed with 6N HCl for 1 hr at 100°C. The resulting phosphoamino acids were then subjected to thin layer electrophoresis and subjected to autoradiography.
Filter overlay binding. Filter overlay assays were performed as described by Li et al. (1992). Filters containing GST-GABAA receptor fusion proteins encoding the intracellular domains of the α1, β1, γ2s, and GST alone were probed with a GST-RACK-1 fusion protein produced in pGEX-2TK (Pharmacia), labeled via phosphorylation with the catalytic subunit of cAMP-dependent protein kinase (Promega) to a specific activity in excess of 106 cpm/μg. The PKC overlay assays were performed essentially as described by Ron et al. (1994)using PKC purified from rat brain, a generous gift from Rick Huganir (Johns Hopkins School of Medicine, Baltimore, MD). PKC-βII was detected using an antisera specific for this PKC isoform (Marais and Parker, 1989; Kiley and Parker, 1995).
Preparation and labeling of cortical neurons. Cortices were dissected from embryonic day 19 rats, and the tissue was incubated in 0.25% trypsin in HEPES-buffered saline (HBSS; Life Technologies, Gaithersburg, MD) for 15 min followed by three 5 min washes in HBSS. The tissue was then dissociated by tituration with a fire-polished glass pipette. Cells were then plated on 0.1 mg/ml poly-l-lysine-treated 10 cm tissue culture dishes at a density of 105cells/cm−2 and grown for 7 d before use. For metabolic labeling, the cultures were starved in methionine-free media for 30 min and then labeled with [35S]methionine (0.25 mCi/ml; ICN Biochemicals, Costa Mesa, CA) for 12 hr, supplemented with 5% normal media. For phorbol ester treatment, cells were exposed to 0.1 μm PDBu at 37°C for 20 min before lysis.
Immunoprecipitation. Cortical neurons were solubilized in a buffer containing 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid (CHAPS) and (in mm) 150 NaCl, 10 triethanolamine, pH 7.6, 5 EGTA, 5 EDTA, 50 NaF, 10 Na pyrophosphate, 1 Na orthovanadate, 100 PMSF, and 10 μg/ml leupeptin, pepstatin, antipain, and aprotinin. Solubilized receptors were immunoprecipitated using a rabbit polyclonal antisera specific for the β1 and β3 subunits (Moss et al., 1992b;McDonald et al., 1998) coupled to protein A Sepharose. Precipitated material was then separated by SDS-PAGE followed by autoradiography or Western blotting using antibodies against PKC isoforms, RACK-1 or BD17, an antibody that recognizes the GABAA receptor β2 and β3 subunits. Alternatively, precipitated material was subject to in vitro kinase assays. Briefly, beads were washed extensively in kinase buffer before the addition of γ32P-ATP to a final concentration of 1.0 μm and incubated at 30°C for 20 min. Reaction products were then separated by SDS-PAGE and visualized by autoradiography.
RESULTS
Phosphorylation of the β1 subunit intracellular domain by brain extracts
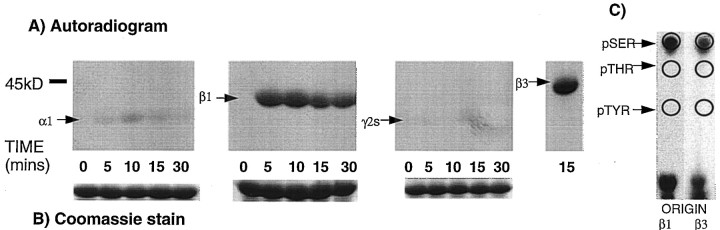
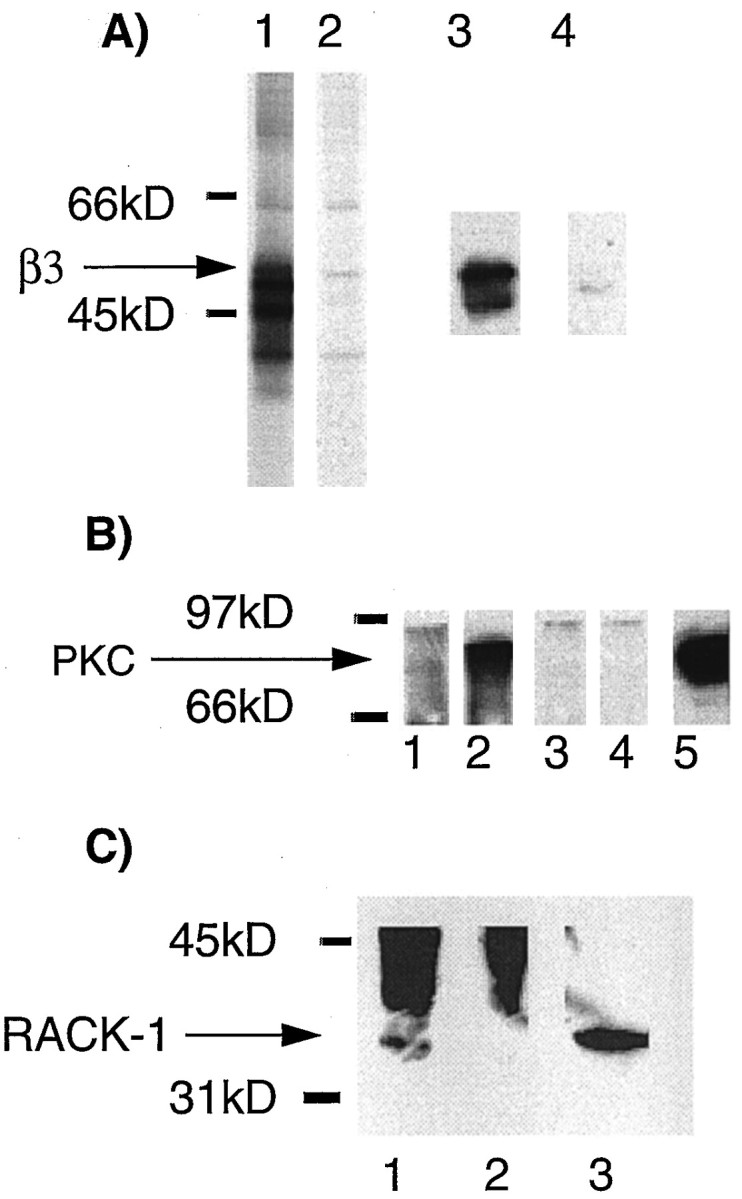
To identify molecules that interact with GABAA receptors and mediate phosphorylation, the intracellular domains of GABAA receptor subunits α1, β1, β3, and γ2S were expressed as GST fusion proteins (Moss et al., 1992; McDonald and Moss 1994). Purified fusion proteins immobilized on glutathione were then exposed to detergent-solubilized brain extracts. After extensive washing, bound material was then subjected to an in vitro kinase assay and phosphorylation was assessed by SDS-PAGE (Fig.1A). Using this regimen, β1-GST and β3-GST were found to be phosphorylated rapidly to high stoichiometry (∼0.2 mol/mol). However, α1-GST and γ2S-GST were found not to be significantly phosphorylated under identical conditions (Fig. 1). The GST backbone was also not phosphorylated in these assays (data not shown). Phosphorylated β1-GST and β3-GST were then subjected to phosphoamino acid analysis, which revealed that both subunit intracellular domains were phosphorylated on serine residues only (Fig. 1C).
Fig. 1.
Serine–threonine protein kinases from neuronal extracts phosphorylate the intracellular domain of the β subunits.A, α1-GST, β1-GST, β3-GST, or γ2-GST were exposed to solubilized neuronal extracts. After extensive washing, bound material was subjected to an in vitro kinase assay for various time periods as indicated, and the reaction products were subjected to SDS-PAGE followed by autoradiography. Similar results were seen in at least three separate experiments. B,Represents Coomassie staining of gels containing the α1-GST, β1-GST, and γ2S-GST fusion proteins demonstrating equivalence of loading. C, Gel slices containing the β1-GST and β3-GST phosphoproteins were subject to tryptic digestion followed by acid hydrolysis. The resulting phosphoamino acids were then separated by thin-layer chromatography and detected by autoradiography. The migration of phosphoserine (pSER), phosphothreonine (pTHR), and phosphotyrosine (pTYR) are indicated.
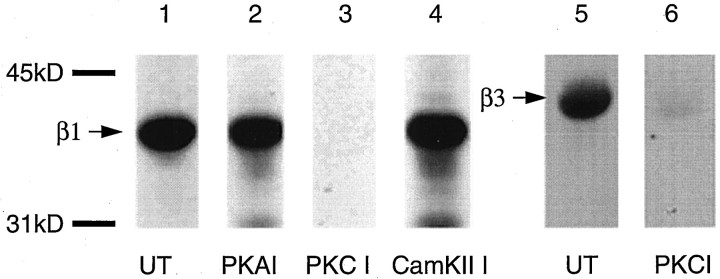
Previous studies have revealed that the β1 and β3 subunits can be phosphorylated by PKC, PKA, Cam KII, and PKG (Moss et al., 1992a,b;Krishek et al., 1994; McDonald and Moss, 1997; McDonald et al., 1998). To determine if any of these kinases were binding to, and phosphorylating, β1-GST, kinase inhibitors were used. Walsh peptide, a specific inhibitor of PKA and W7, an inhibitor of Cam KII, were without effect on β1-GST phosphorylation. However, phosphorylation of β1-GST was drastically reduced by the inclusion of a specific peptide inhibitor of PKC, PKCI(19–36), at a concentration of 100 nm (Fig. 2). Phosphorylation of β3-GST was also reduced by PKCI(19–36) (Fig. 2).
Fig. 2.
Protein kinase C inhibitors reduce neuronal extract serine–threonine-mediated phosphorylation of the intracellular domains of the GABAA receptor β subunits. The phosphorylation of β1-GST by neuronal extracts was analyzed with specific kinase inhibitors. Material associating with β1-GST from neuronal extracts was subjected to in vitro kinase assays alone (UT; lane 1) or in the presence of a specific PKA inhibitor peptide (Walsh peptide, 0.1 μm;lane 2), a specific inhibitory peptide of PKC (PKC(19–36), 0.1 μm; lane 3), or an inhibitor of Cam KII (W7, 1 μm; lane 4). Material associating with β3-GST from neuronal extracts was subjected to in vitro kinase assays alone (UT; lane 5) or the presence of a specific peptide inhibitor of PKC (PKC(19–36), 0.1 μm;lane 6). Phosphorylation was assessed by SDS-PAGE followed by autoradiography. Similar results were seen in at least three independent experiments.
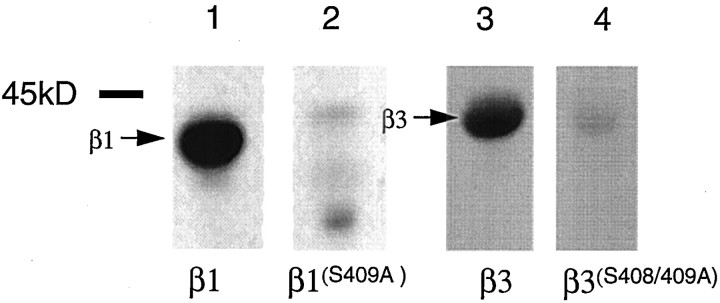
To further explore this observation, mutated versions of β1-GST and β3-GST were included in these kinase assays. These studies focused on mutant fusion proteins in which in vitro and in vivo PKC substrates within the intracellular domains of the β1 and β3 subunits had been mutated to alanine residues. Previous studies have demonstrated that S409 in the β1 subunit, and both S408 and S409 in β3 are PKC substrates (Moss et al., 1992a,b; Krishek et al., 1994). Accordingly β1(S409A)-GST and β3(S408/409A)-GST were exposed to neuronal extracts. Although there are additional serine residues in both the β subunit intracellular domains, mutation of S409 in β1 and S408/409 in β3 abolished phosphorylation of β1 and β3-GST, respectively (Fig. 3). Importantly, phosphorylation of these residues by PKC has been previously shown to regulate the function of heteromeric receptors containing the β1 or β3 subunits (Lin et al., 1996; Moss and Smart, 1996; McDonald et al., 1998). Together these observations suggest that PKC can interact with and phosphorylate defined serine residues within the β1 and β3 subunit intracellular domains.
Fig. 3.
Serine–threonine protein kinases from neuronal extracts do not phosphorylate the intracellular domains of serine-to-alanine-mutated GABAA β subunits. β1-GST, β1(S409A)-GST, β3-GST, and β3(S408/409A)-GST were exposed to neuronal extracts. Bound material was then subjected to an in vitro kinase assay. Phosphorylation was then assessed by SDS-PAGE followed by autoradiography. Similar results were seen in three independent experiments.
To control for possible differences in PKC substrate preferences between the various intracellular domains, material binding to the α1-GST, β1-GST, γ2-GST, and GST were all exposed to a PKC substrate peptide derived from neurogranin (Chen et al., 1993). The neurogranin peptide was phosphorylated by kinase activity binding to all three intracellular domains compared to GST alone (Fig.4). The highest level of neurogranin phosphorylation was seen with β1-GST. Importantly, 50% of the kinase activity associating with β1-GST could be specifically inhibited by PKCI(19–36) (>0.05; Fig. 4); in contrast, the kinase activity associating with α1-GST and γ2-GST was insensitive to PKCI(19–36). Together our results further demonstrate that PKC can interact with the intracellular domains of GABAA receptor β subunits. They also suggest another as yet unidentified serine–threonine kinase can also interact with the intracellular domains of the α1, β1, and γ2S subunits. However, none of these subunit intracellular domains appear to be phosphorylated by this kinase activity (Figs. 1-3).
Fig. 4.
Phosphorylation of Neurogranin peptide by PKC activity specifically associating with β1-GST. Material binding from adult rat brain extract to α1-GST (1), β1-GST (2), γ2-GST (3), or GST (4) alone was subject to an in vitro kinase assay using a substrate peptide derived from Neurogranin (Neurogranin 28–43, 50 μm) in the presence (black bars) and absence (white bars) of PKC19–36 inhibitor peptide (1 μm). Incorporation of 32P into this peptide was then measured and normalized to the protein input, n = 3 in each case. *Indicates significantly different from control (p > 0.05) as measured using the Student'st test.
PKC isoforms specifically interact with GABAA receptor β subunit intracellular domains
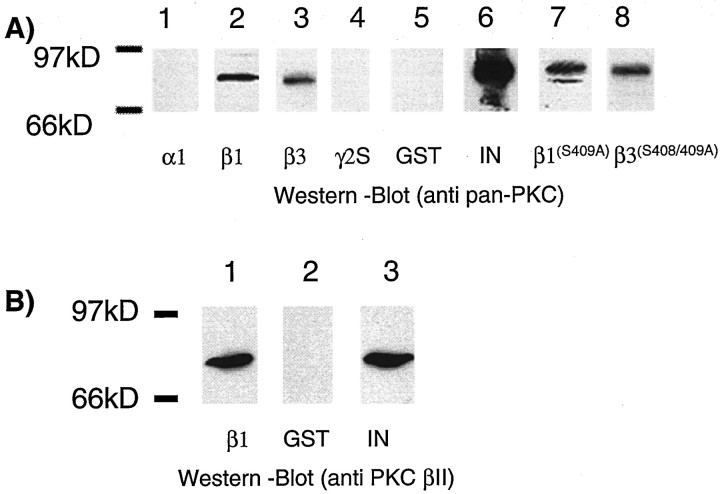
To further characterize the interaction of PKC with GABAA receptors, fusion proteins were exposed to neuronal extracts, and bound material was then subjected to Western blotting using a pan-PKC antisera. This antisera recognizes the α, βI, βII, and γ isoforms of PKC. Using this antisera, a band of 82 kDa was seen binding to β1-GST and β3-GST, not to α1-GST, γ2S-GST, or GST alone (Fig. 5). Importantly, PKC could also be detected binding to both β1(S409A)-GST and β3(S408/409A)-GST. This suggests that β1-GST and β3-GST are not simply acting as PKC substrate-binding proteins (Newton, 1997).
Fig. 5.
Solubilized neuronal protein kinase C βII binds to the intracellular domain of the β1 and β3 subunits.A, α1-GST (lane 1), β1-GST (lane 2), β3-GST (lane 3), γ2S-GST (lane 4), GST (lane 5), β1(S409A)-GST (lane 7), and β3(S408/409A)-GST (lane 8) were exposed to neuronal extracts, and after extensive washing bound material was subjected to Western blotting with a pan-PKC antibody.Lane 6 (IN) represents 10% of the solubilized neuronal extract that was exposed to the respective fusion proteins. B, Material binding to β1-GST (lane 1) or GST (lane 2) was probed with antisera against the βII isoform of PKC via Western blotting. Lane 3 (IN) represents 10% of the solubilized neuronal extract that was exposed to the respective fusion proteins.
To identify the isoform of PKC interacting with β1-GST, bound material was probed with isoform-specific antibodies. Using an antibody directed against PKC-βII, a band of identical molecular mass was seen, as with the pan-PKC antisera (Fig. 5). In addition, small amounts of the α isoform of PKC were also detected binding to β1-GST and β3-GST (data not shown). In contrast, the β, γ, δ, ε, ζ, η, and θ PKC isoforms did not appear to interact with β1-GST as determined by Western blotting, using isoform-specific antibodies.
Together these results suggests that PKC isoforms are capable of interacting and phosphorylating the intracellular domain of receptor β subunits.
PKC-βII is capable of binding directly to GABAAreceptor intracellular domains
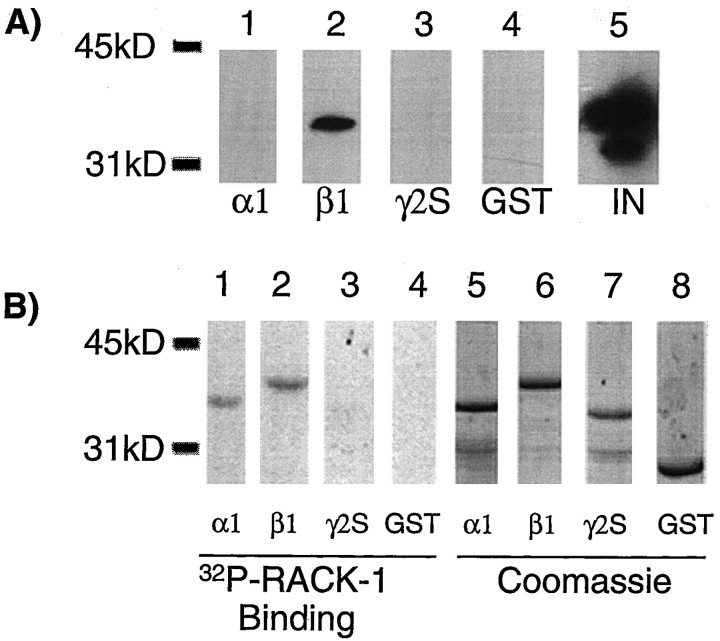
To test whether PKC-βII could interact directly with GABAA receptor subunits, gel overlay assays were used. A range of receptor intracellular domains were transferred to a membrane and then exposed to PKC purified from rat brain that had been activated in vitro. Material binding to the GABAA receptor intracellular domains was then visualized with antisera directed against PKC-βII. As a positive control, a GST fusion protein of the RACK-1 was included in this assay. PKC-βII could be seen binding directly to β1-GST and also to RACK-1, but not to α1-GST or GST alone (Fig.6). Importantly, Western blotting of the PKC preparation used for these experiments failed to detect RACK-1 (Fig. 6). Similar direct binding of PKC-βII was also seen with the intracellular domain of the β3 subunit (data not shown). An alternative, but less likely explanation is that PKC activity could be directed to GABAA receptor intracellular domains by another unidentified kinase anchoring protein within the PKC preparation used for the overlay assay.
Fig. 6.
The βII isoform of PKC can bind directly to the intracellular domain of the β1 subunit. α1-GST (lane 1), β1-GST (lane 2), RACK-1-GST (lane 3), or GST alone (lane 4) were transferred to a membrane and probed with PKC purified from rat brain. PKC binding to fusion proteins was then visualized with antisera against PKC βII by Western blotting. Lanes 5–8represent a Coomassie stain of an identical gel to show the equivalence in loading of the various proteins. C, The purified PKC preparation (50 ng; lane 1) used in the overlay assay and solubilized neuronal extract (100 μg; lane 2) were blotted with the pan-PKC antisera (top panel) and antibody specific for RACK-1.
RACK-1 associates with the β1 and α1 intracellular domains
Previous studies have shown that the PKC-β isoforms are targeted to substrates by anchoring proteins, such as RACK-1, a homolog of G-protein β subunits (Ron et al., 1994; Pawson and Scott, 1997;Mochly-Rosen and Gordon, 1998). To examine whether RACK-1 has a role in targeting PKC activity to GABAA receptors, material binding to receptor intracellular domains of GABAA receptors from brain extracts was blotted using antisera specific for RACK-1. Using this antibody, a major band of 36 kDa and a degradation product of 34 kDa were seen in brain extract (Fig. 7A). The 36 kDa band representing RACK-1 could be observed binding to β1-GST but not to either α1-GST, γ2S-GST, or GST alone (Fig. 7A).
Fig. 7.
RACK-1 can bind directly to GABAAreceptor subunit intracellular domains. A, α1-GST (lane 1), β1-GST (lane 2), γ2-GST (lane 3), or GST alone (lane 4) were exposed to neuronal extracts, and after extensive washing, bound material was probed with an antibody for RACK-1. Lane 5represents 10% of the input starting material. B,α1-GST (lane 1), β1-GST (lane 2), γ2-GST (lane 3), or GST (lane 4) fusion proteins were separated by SDS-PAGE and transferred to a membrane. Membrane was then probed with a radiolabeled GST-RACK-1 fusion protein. Bound RACK-1 was detected by autoradiography.Lanes 5–8 represent a Coomassie stain of an identical gel to show the equivalence in loading of the various proteins.
To determine if the interaction between RACK-1 and receptor intracellular domains was direct, gel overlay assays were used. Receptor GST fusions were transferred to a nitrocellulose membrane and probed with 32P-labeled RACK-1 expressed as a GST fusion protein. RACK-1 could be detected binding to β1-GST and also α1-GST, but not to γ2-GST or GST alone (Fig.7B). This suggests that RACK-1 is capable of binding directly to the intracellular domains of the α1 and β1 subunitsin vitro.
PKC and RACK-1 coimmunoprecipitate with neuronal GABAA receptors and phosphorylate receptor β subunits
Our in vitro binding studies suggest that PKC-βII and RACK-1 can bind to GABAA receptor β subunit intracellular domains and phosphorylate S409, a conserved phosphorylation site of critical importance for the regulation of GABAA receptor function (Moss et al., 1992b;Krishek et al., 1994; Lin et al., 1996; McDonald et al., 1998).
To examine the interaction of PKC with neuronal GABAA receptors, immunoprecipitation was used with an antisera specific for the β1 and β3 subunits (anti-β1/3;Moss et al., 1992a; McDonald et al., 1998). Detergent-solubilized extracts from cultured cortical neurons that express the GABAA receptor α1–5, β1, β3, and γ2 subunits (Benke et al., 1994; Macdonald and Olsen, 1994) were immunoprecipitated with anti-β1/3 or control non-immune antisera. Anti-β1/3 immunoprecipitated bands of 57, 55, 50, and 47 kDa from metabolically labeled cortical neurons (Fig.8A). Material precipitating with anti-β1/3 antisera was also Western-blotted with Bd 17, an antibody specific for the GABAAreceptor β2 and β3 subunits (Benke et al., 1994). Bd 17 recognized the 57 kDa band precipitated with anti-β1/3 (Fig. 8A, lanes 3, 4). Given that most GABAAreceptors only contain a single β subunit isoform (Benke et al., 1994; Li and De Blas, 1997), the 57 kDa band is therefore likely to represent the β3 subunit. To determine if PKC was capable of binding to neuronal GABAA receptors, cortical cultures were treated with phorbol esters and then immunoprecipitated with anti-β1/3 antisera. Precipitated material was then probed for PKC isoforms using pan-PKC antisera. A band of 82 kDa corresponding to PKC could be detected precipitating with anti-β1/3, but not with control antisera (Fig. 8B). Low levels of PKC immunoreactivity could be detected binding to GABAA receptors under basal conditions, (Fig.8B, lane 1) however, this interaction was dramatically increased by phorbol ester treatment, suggesting that activated PKC isoforms interact with GABAAreceptors in neurons (Fig. 8B, lane 2). Precipitated material was also probed for the presence of RACK-1. RACK-1 immunoreactivity could be detected coprecipitating with GABAA receptors (Fig. 8C).
Fig. 8.
Both RACK-1 and PKC isoforms immunoprecipitate with GABAA receptors containing the β3 subunit from cultured cortical neurons. A, Detergent-solubilized extracts from cortical neurons metabolically labeled with [35S]methionine were immunoprecipitated with anti-β1/3 (lane 1) or control nonimmune IgG (lane 2) and separated by SDS-PAGE. Receptor subunits were visualized by autoradiography. In addition, material precipitated with anti-β1/3 (lane 3) or control IgG (lane 4) was Western-blotted with a monoclonal antisera against the β2 and β3 subunits. B, Cortical cultures exposed to PDBu for 20 min (lanes 2, 4) or control cultures (lanes 1, 3) were precipitated with anti-β1/3 (lanes 1, 2) or control IgG (lanes 3, 4). Precipitated material was then Western-blotted with a pan-PKC antisera. Lane 5 represents 10% of the material used for the immunoprecipitation. C, Cortical cultures exposed to PDBu for 20 min and precipitated with anti-β1/3 (lane 1) or control IgG (lane 2) and Western-blotted with an antibody specific for RACK-1. Lane 3 represents 10% of the material used for the immunoprecipitation.
To determine if PKC associating with GABAAreceptors is catalytically active, precipitated material was subjected to an in vitro kinase assay using32γ-ATP; the reaction products were then separated by SDS-PAGE. Phosphorylation of a major band of 57 kDa representing the β3 subunit was observed using anti-β 1/3 antisera, but not control nonimmune sera (Fig. 9). In addition, a minor band of 55 kDa was also phosphorylated. Phosphorylation of these bands was evident under basal conditions, consistent with the interaction of PKC with GABAAreceptors seen in cortical neurons (Fig. 8). Phosphorylation of the 57 and 55 kDa bands was enhanced by phorbol ester treatment (Fig. 9). In contrast, the specific PKC inhibitor PKCI(19–36)completely abolished phosphorylation of the 57 and 55 kDa bands (Fig.9, lane 2).
Fig. 9.
PKC isoforms associating with GABAAreceptors in cortical neurons are capable of phosphorylating the receptor β3 subunit. Cortical cultures were treated with (+PdBu) or without (−PdBu) and immunoprecipitated with anti-β1/3 (lanes 1–3) or control IgG (lanes 4–6). Precipitated material was then subjected to an in vitro kinase assay in the presence (+PKCI) of absence of PKC(19–36) inhibitor (−PKCI) peptide, phosphorylation was assessed by SDS-PAGE and autoradiography.
Together, these results suggests that PKC isoforms and RACK-1 are closely associated with neuronal GABAA receptors. Furthermore, the PKC isoforms interacting with GABAA receptors are capable of phosphorylating receptor β subunits.
DISCUSSION
GABAA receptors are of central importance in mediating fast synaptic inhibition in the brain. Given the pivotal role these receptors play in synaptic transmission, it is of fundamental importance to understand how these ion channels are regulated. One mechanism that has received considerable attention is direct receptor phosphorylation.
Studies on recombinant and neuronal receptors have demonstrated that the receptor β and γ2 subunits are the substrates of a number of protein kinases (Moss and Smart, 1996). PKC for instance, has been shown to phosphorylate S409 in the β1 and S408/409 in β3 subunit (Moss and Smart, 1996; McDonald and Moss, 1997; McDonald et al., 1998). Likewise, PKC also phosphorylates residues in both the γ2L (S327/343) and γ2S (S327) subunits (Moss and Smart, 1996). Importantly, phosphorylation by PKC of these residues in the β1 and γ2 subunits can modulate the functional properties of recombinant receptors as demonstrated by site-directed mutagenesis (Moss and Smart, 1996). Phosphorylation produces diverse effects, from clear cut inhibitions using murine receptors (Kellenberger et al., 1992; Krishek et al., 1994) to enhancements with bovine receptors (Lin et al., 1994, 1996). These discrepancies may results from species of receptor expressed or differences in recording protocols and methodologies of kinase activation. Experiments using neuronal preparations have shown that PKC activity universally causes inhibition of GABAAreceptor function (Moss and Smart, 1996).
To gain further insights into how kinases are targeted to GABAA receptors, we have probed brain lysates with GST fusion proteins encoding subunit intracellular domains. Using the intracellular domain of the β1 and β3 subunits, a kinase activity could be detected specifically binding to and phosphorylating these proteins. The major substrate of this kinase within the β1 subunit intracellular domain was serine 409, and S408/S409 within the β3 subunit previously characterized functionally relevant phosphorylation sites for both PKA and PKC in receptor β subunits (Moss et al., 1992a,b; McDonald and Moss, 1994, 1997; Krishek et al., 1994; Lin et al., 1994, 1996; McDonald et al., 1998). The kinase activity in neurons phosphorylating the GABAAreceptor β1 and β3 subunit intracellular domains, was identified as being PKC because of its specific inhibition by PKC(19–36) inhibitor peptide. PKC-βII could be detected binding to β subunit intracellular domains but not to those of the α1 or γ2 subunits. The βII isoform of PKC has previously been shown to be the major PKC isoform associated with the cytoskeleton (Tanaka et al., 1991). In addition, within the hippocampus PKC βII is present in CA1 dendrites (Ase et al., 1988; Nicholls, 1997), consistent with a role for this PKC isoform in associating with and regulating the function of GABAA receptors. In addition to PKC, another as yet unidentified serine–threonine kinase activity could also be detected binding to the intracellular domains of the GABAA receptor α1, β1, and γ2S subunits. This kinase activity did not appear to phosphorylate any of these subunit intracellular domains but was able to phosphorylate a peptide substrate from neurogranin (Chen et al., 1993). Interestingly, there have been reports of a serine–threonine protein kinase that copurifies with GABAA receptors on benzodiazepine affinity chromatography. This activity was found to be independent of activators of PKC, PKA, or Cam KII (Sweetnam et al., 1988; Bureau and Laschet, 1995). Clearly, further studies will be needed to clarify the role of this kinase activity with regard to GABAAreceptor function.
The interaction of PKC with receptor β subunit intracellular domains may be direct or mediated by anchoring proteins such as RACK-1 and A kinase-anchoring proteins (AKAPs) (Pawson and Scott, 1997;Mochly-Rosen and Gordon, 1998). PKC-βII was able to bind directly to the GABAA receptor β1 subunit intracellular domain, but not to those of the α1 or γ2 subunits. Together, our observations suggest that interaction with β subunits could be a general mechanism for targeting PKC activity to GABAA receptors. Interestingly, RACK-1 was also able to bind directly to the intracellular domain of the α1 and β1 subunits. Previous studies have suggested that RACK-1 is of fundamental importance in mediating the binding of activated PKC-β isoforms with substrates in myocytes (Mochly-Rosen and Gordon, 1998). In the case of GABAA receptors however, PKC-βII can clearly bind to the intracellular domain of the GABAAreceptor β1 subunit independently of RACK-1. This may suggest that RACK-1 may play a differing role in the targeting of PKC activity to GABAA receptors. For instance, RACK-1 may increase the affinity of the interaction between GABAA receptors and PKC-βII, ensuring stoichiometrical phosphorylation (Ron et al., 1994; Mochly-Rosen and Gordon, 1998). By specifically blocking the binding of RACK-1 to GABAA receptors, it may be possible to address the role of RACK-1 in the regulation of receptor function by PKC phosphorylation.
To test the relevance of our observations using subunit intracellular domains, the interaction of PKC with neuronal GABAA receptors was analyzed using immunoprecipitation from extracts of cortical neurons. Using antisera against the β1 and β3 subunits, PKC and RACK-1 were detected coprecipitating with GABAA receptors from neuronal extracts. Furthermore, PKC activity associating with GABAA receptors was capable of phosphorylating a major protein of 57 kDa that was identified as the β3 subunit.
Together our observations suggest that in the brain GABAA receptors are intimately associated with PKC. This association in the case of PKC-βII is mediated via the direct interaction of this kinase with the receptor β subunits. This interaction may serve to localize PKC activity to GABAA receptors in the brain, allowing the rapid regulation of receptor activity by cell signaling pathways that modify PKC activity. Such rapid regulation may be a primary means of modifying the efficacy of synaptic inhibition and may therefore be an important mechanism in generating synaptic plasticity.
Footnotes
This work was supported by the Medical Research Council (UK) and the Wellcome trust.
Correspondence should be addressed to Dr. Stephen J. Moss, MRC-LMCB, University College, Gower Street, London WC1E 6BT. Email:steve.moss@UCL.ac.uk.
REFERENCES
- 1.Ase K, Saito N, Shearman MS, Kikkawa U, Ono Y, Igarashi K, Tanaka C, Nishizuka Y. Distinct cellular expression of βI- and βII-subspecies of protein kinase C in rat cerebellum. J Neurosci. 1988;8:3850–3856. doi: 10.1523/JNEUROSCI.08-10-03850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benke D, Fritschy JM, Trzeciak A, Bannwarth W, Mohler H. The distribution, prevalence and drug binding profile of GABAA receptors subtypes differing in the β subunit variant. J Biol Chem. 1994;269:27100–27107. [PubMed] [Google Scholar]
- 3.Browning MD, Bureau M, Dudek EM, Olsen RW. Protein kinase C and cAMP-dependent protein kinase phosphorylate the β subunit of the purified γ -aminobutyric acid A receptor. Proc Natl Acad Sci USA. 1990;87:1315–1318. doi: 10.1073/pnas.87.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bureau MH, Laschet JJ. Endogenous phosphorylation of distinct γ-aminobutyric acid type A receptor polypeptides by Ser/Thr and Tyr kinase activities associated with the purified receptor . J Biol Chem. 1995;270:26482–26487. doi: 10.1074/jbc.270.44.26482. [DOI] [PubMed] [Google Scholar]
- 5.Chen SJ, Klann E, Gower MC, Powell CM, Sessoms JS, Sweatt JD. Studies with synthetic peptide substrates derived from the neuronal protein neurogranin reveal structural determinants of potency and selectivity for protein kinase C. Biochemistry. 1993;32:1032–1039. doi: 10.1021/bi00055a006. [DOI] [PubMed] [Google Scholar]
- 6.Kapur J, Macdonald RL. Cyclic AMP-dependent protein kinase enhances hippocampal dentate granule cell GABAA receptor currents. J Neurophysiol. 1996;76:2626–2634. doi: 10.1152/jn.1996.76.4.2626. [DOI] [PubMed] [Google Scholar]
- 7.Kellenberger S, Malherbe P, Sigel E. Function of the α1 β2 γ2S γ-aminobutyric acid type A receptor is modulated by protein kinase C via multiple phosphorylation sites. J Biol Chem. 1992;267:25660–25663. [PubMed] [Google Scholar]
- 8.Kiley SC, Parker PJ. Differential localization of protein kinase C isozymes in U937 cells: evidence for distinct isozyme functions during monocyte differentiation. J Cell Sci. 1995;108:1003–1016. doi: 10.1242/jcs.108.3.1003. [DOI] [PubMed] [Google Scholar]
- 9.Kirkness EF, Bovenkerk CF, Ueda T, Turner AJ. Phosphorylation of γ-aminobutyric acid (GABA)/benzodiazepine receptors by cyclic AMP-dependent protein kinase. Biochem J. 1989;259:613–616. doi: 10.1042/bj2590613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kofuji P, Wang JB, Moss SJ, Huganir RL, Burt DR. Generation of two forms of the γ-aminobutyric acid A receptor γ-2 subunit in mice by alternative splicing. J Neurochem. 1991;56:713–715. doi: 10.1111/j.1471-4159.1991.tb08209.x. [DOI] [PubMed] [Google Scholar]
- 11.Krishek BJ, Xie X, Blackstone CD, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 12.Li M, De Blas A. Co-existance of two β subunit isoforms in the same GABAA receptor. J Biol Chem. 1997;272:16564–16569. doi: 10.1074/jbc.272.26.16564. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Jan Y, Jan L. Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science. 1992;257:1225–1229. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- 14.Lin YF, Browning MD, Dudek EM, Macdonald RL. Protein kinase C enhances recombinant bovine α1 β1 γ2L GABAA receptor whole-cell currents expressed in L929 fibroblasts. Neuron. 1994;13:1421–1431. doi: 10.1016/0896-6273(94)90427-8. [DOI] [PubMed] [Google Scholar]
- 15.Lin YF, Angelotti TP, Dudek EM, Browning MD, Macdonald RL. Enhancement of recombinant α1 β1 γ2L γ-aminobutyric acid A receptor whole-cell currents by protein kinase C is mediated through phosphorylation of both β1 and γ2L subunits. Mol Pharmacol. 1996;50:185–195. [PubMed] [Google Scholar]
- 16.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 17.McDonald BJ, Moss SJ. Differential phosphorylation of intracellular domains of γ-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. J Biol Chem. 1994;269:18111–18117. [PubMed] [Google Scholar]
- 18.McDonald BJ, Moss SJ. Conserved phosphorylation of intracellular domains of γ-aminobutyric acid type A receptor β2 and β3 subunits by cAMP dependent protein kinase, protein kinase C, calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. Neuropharmacology. 1997;36:13377–1385. doi: 10.1016/s0028-3908(97)00111-1. [DOI] [PubMed] [Google Scholar]
- 19.McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. Adjacent phosphorylation of GABAA receptor β subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- 20.Mochly-Rosen D, Gordon AS. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 1998;12:35–42. [PubMed] [Google Scholar]
- 21.Moss SJ, Doherty CA, Huganir RL. Identification of the protein kinase A and protein kinase C phosphorylation sites within the intracellular domains of the β1, γ2S and γ2L GABAA receptor subunits. J Biol Chem. 1992a;267:14470–14476. [PubMed] [Google Scholar]
- 22.Moss SJ, Smart TG, Blackstone CD, Huganir RL. Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science. 1992b;257:661–665. doi: 10.1126/science.1323140. [DOI] [PubMed] [Google Scholar]
- 23.Moss SJ, Gorrie G, Amato A, Smart TG. Modulation of GABAA receptors by tyrosine phosphorylation. Nature. 1995;377:344–348. doi: 10.1038/377344a0. [DOI] [PubMed] [Google Scholar]
- 24.Moss SJ, Smart TG. Modulation of amino acid-gated ion channels by protein phosphorylation. In: Bradley RJ, Harris RA, Jenner P, editors. International review of neurobiology, Vol 39. Academic; San Diego: 1996. pp. 1–52. [DOI] [PubMed] [Google Scholar]
- 25.Nicholls DG. Protein kinase C and synaptic inhibition. In: Parker P, Dekker L, editors. Protein kinase C. Springer; Berlin: 1997. pp. 167–178. [Google Scholar]
- 26.Newton CA. Regulation of protein kinase C by cofactors. In: Parker P, Dekker L, editors. Protein kinase C. Springer; Berlin: 1997. pp. 25–39. [Google Scholar]
- 27.Pawson T, Scott J. Signaling through scaffold anchoring and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 28.Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1995;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- 29.Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the β subunit of G-proteins. Proc Natl Acad Sci USA. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith DB, Johnson KS. Single step purification of polypeptides expressed in E. coli as fusions with glutathione-S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 31.Sweetnam PM, Lloyd J, Gallombardo P, Malison RT, Gallager DW, Tallman JF, Nestler EJ. Phosphorylation of the GABAa/benzodiazepine receptor α subunit by a receptor-associated protein kinase. J Neurochem. 1988;4:1274–1284. doi: 10.1111/j.1471-4159.1988.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S, Tominaga M, Yasuda I, Kishimoto A, Nishizuka Y. Protein kinase C in rat brain synaptosomes. βII-subspecies as a major isoform associated with membrane-skeleton elements. FEBS Lett. 1991;294:267–270. doi: 10.1016/0014-5793(91)81445-e. [DOI] [PubMed] [Google Scholar]
- 33.Unwin N. Neurotransmitter action: opening of ligand-gated ion channels. Cell [Suppl] 1993;72:31–41. doi: 10.1016/s0092-8674(05)80026-1. [DOI] [PubMed] [Google Scholar]
- 34.Valenzuela CF, Machu TK, McKernan RM, Whiting P, VanRenterghem BB, McManaman JL, Brozowski SJ, Smith GB, Olsen RW, Harris RA. Tyrosine kinase phosphorylation of GABAA receptors. Brain Res Mol Brain Res. 1995;1–2:165–172. doi: 10.1016/0169-328x(95)00048-w. [DOI] [PubMed] [Google Scholar]
- 35.Whiting P, McKernan RM, Iversen L. Another mechanism for creating diversity in γ-aminobutyric acid type A receptors: RNA splicing directs expression of two forms of γ2 subunit, one of which contains a protein kinase C phosphorylation site. Proc Natl Acad Sci USA. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]