Abstract
The amyloid precursor protein (APP) of Alzheimer's disease can reduce copper (II) to copper (I) in a cell-free system potentially leading to increased oxidative stress in neurons. We used neuronal cultures derived from APP knock-out (APP−/−) and wild-type (WT) mice to examine the role of APP in copper neurotoxicity. WT cortical, cerebellar, and hippocampal neurons were significantly more susceptible than their respective APP−/−neurons to toxicity induced by physiological concentrations of copper but not by zinc or iron. There was no difference in copper toxicity between APLP2−/− and WT neurons, demonstrating specificity for APP-associated copper toxicity. Copper uptake was the same in WT and APP−/− neurons, suggesting APP may interact with copper to induce a localized increase in oxidative stress through copper (I) production. This was supported by significantly higher levels of copper-induced lipid peroxidation in WT neurons. Treatment of neuronal cultures with a peptide corresponding to the human APP copper-binding domain (APP142–166) potentiated copper but not iron or zinc toxicity. Incubation of APP142–166 with low-density lipoprotein (LDL) and copper resulted in significantly increased lipid peroxidation compared to copper and LDL alone. Substitution of the copper coordinating histidine residues with asparagines (APP142–166H147N, H149N, H151N) abrogated the toxic effects. A peptide corresponding to the zinc-binding domain (APP181–208) failed to induce copper or zinc toxicity in neuronal cultures. These data support a role for the APP copper-binding domain in APP-mediated copper (I) generation and toxicity in primary neurons, a process that has important implications for Alzheimer's disease and other neurodegenerative disorders.
Keywords: Alzheimer's, copper, free radicals, culture, knock-out, lipid peroxidation, neurons
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by amyloid plaques and neuronal cell loss or dysfunction. The major constituent of plaques is a 39–42 amino acid peptide, amyloid-β protein (Aβ) (Glenner and Wong, 1984; Masters et al., 1985) derived by proteolytic processing of full-length amyloid precursor protein (APP) (Kang et al., 1987). Aβ has an important role in neuronal dysfunction because the peptide is toxic to neurons (Koh et al., 1990; Yankner et al., 1990). APP belongs to a multigene family containing the amyloid precursor-like proteins 1 and 2 (APLP1 and APLP2) (Wasco et al., 1992; Slunt et al., 1994). APLPs share considerable homology with APP, including metal binding sites for zinc and copper (Bush et al., 1993; Hesse et al., 1994). The zinc-binding site may regulate homophilic binding (Beher et al., 1996), interact with other ligands such as heparan sulfate (Multhaup et al., 1994), or regulate coagulation factor inhibition (Van Nostrand, 1995) or protein folding. Copper binding to APP may be involved in electron transfer reactions as shown by the reduction of APP-bound copper (II) to copper (I) (Multhaup et al., 1996). This process was specific for copper with no reduction of iron (III), nickel (II), magnesium (II), or cobalt (II). It involved the interaction of cysteine residues at positions 144 and 158 and additional histidine residues on the same APP molecule. The APP-Cu (I) complex could reduce hydrogen peroxide to form an APP-Cu (II)-hydroxyl radical intermediate (Multhaup et al., 1998).
Although the normal function of copper reduction by APP is not known, excessive copper (I) and hydroxyl radical (OH·) formation can damage lipids and proteins (Gunther et al., 1995; Multhaup et al., 1996) and induce oxidative stress in neurons. Increased oxidative stress and altered copper homeostasis have been identified in AD (Deibel et al., 1996; Lovell et al., 1998) and other neurodegenerative diseases. Inherited forms of familial amyotrophic lateral sclerosis (FALS) can involve mutations in the cuproenzyme, Cu/Zn superoxide dismutase (Cu/ZnSOD), affecting copper metabolism and oxidative stress (Wiedaupazos et al., 1996; Yim et al., 1996). In Creutzfeldt-Jakob disease (CJD), the cellular prion protein (PrPc) binds copper (Brown et al., 1997a) and may be associated with lower Cu/ZnSOD activity in PrP-deficient neurons (Brown et al., 1997b) and increased susceptibility to copper toxicity and other forms of oxidative stress (Brown et al., 1998a).
To test the hypothesis that APP can interact with copper and mediate oxidative stress in neurons (Multhaup et al., 1996, 1998), we compared the effect of copper exposure on cultures of APP knock-out (APP−/−) and wild-type (WT) neurons. We found that WT neurons were more susceptible than APP−/− neurons to physiological concentrations of copper but not other metals. The WT neurons had increased levels of lipid peroxidation products consistent with copper-mediated oxidative stress. Similar effects were obtained with a peptide containing the APP copper-binding domain. These data demonstrate a copper–APP association that is relevant to AD.
MATERIALS AND METHODS
Materials. Poly-l-lysine, 3,[4,5 dimethylthiazol-2yl]-2,5 diphenyltetrazolium bromide (MTT), cytosine arabinofuranoside (Ara C), glycine, human low-density lipoprotein (LDL), bathocuproine disulphonate (BC), thiobarbituric acid (TBA), and butylated hydroxytoluene (BHT) were purchased from Sigma (St. Louis, MO). Metal salts were obtained from Ajax Chemicals. Glutamine, glutamate, glucose, B27 and N2 supplements, and gentamycin sulfate were obtained from Life Technologies (Gaithersburg, MD). Fetal calf serum (FCS) and horse serum (HS) were from the Commonwealth Serum Laboratories. The lipid peroxidation kit LPO 586 was obtained from Oxis International (Portland, OR).
Primary neuronal cultures. The generation of the APP−/− and APLP2−/− mice has been previously described (Zheng et al., 1995; von Koch et al., 1997). Control WT mice (C57BL6J × 129/Sv) correspond to genetically matched mice from which the knock-out mice were derived. Primary neuronal cultures of cerebral cortex, cerebellum, and hippocampus were established from knock-out and WT mice as previously described (White et al., 1998). Cortical astrocyte cultures were prepared in the same manner as neuronal cultures, however, cells were plated at a density of 100,000 cells/cm2 from embryonic day 16 (E16) mice, and the media were replaced with fresh serum-containing media after day 7. This method was found to produce confluent astrocyte cultures with few surviving neurons at day 10. MTT assays of astrocyte cultures revealed <10% difference in readings between wells.
Measurement of cell viability. Cell viability was determined using the MTT assay as previously described (White et al., 1998). Culture medium was replaced with 0.6 mg/ml MTT in control salt solution (CSS), pH 7.4, for 2 hr. The MTT solution was removed, and cells were solubilized with dimethylsulfoxide. Aliquots of 100 μl were measured with a spectrophotometer at 570 nm. Cell death was determined from culture supernatants using a lactate dehydrogenase (LDH) cytotoxicity detection kit (Boehringer Ingelheim).
Treatment of cultures with metals. Atomic absorption analysis of copper levels in serum-free medium before addition of CuCl2 revealed a background copper level of ∼0.2 μmol/l. Three- or 6-d-old neuronal cultures were washed twice with serum-free media, and copper, iron, and zinc were applied in fresh serum-free media for 16 hr or 3 d. For copper–glycine treatment (Brown et al., 1997a), a solution of 5 mmCuCl2 was incubated with a tenfold molar excess of glycine before treatment of cultures. Cell viability was determined using the MTT assay.
Treatment of cultures with peptides. Peptides of APP142–166 [corresponding to the human APP copper-binding domain and demonstrating copper reducing activity (Multhaup et al., 1998)], APP142–166H147N, H149N, H151N (APP142–166 with histidine to asparagine substitutions at positions 147, 149, and 151) and APP181–208 (the human APP zinc-binding domain) (Table1) were synthesized by manual 9-fluorenilmethoxy-carbonyl chemistry and purified by reverse phase-HPLC. Peptides were made as 2 mg/ml stock solutions in dH2O and added to APP−/− cortical cultures as indicated with or without metals (in MEM/N2 media) at day 2 in vitro. Cell survival was determined with the LDH assay.
Table 1.
Amino acid sequences of APP-derived peptides
| Peptide | Amino acid sequence | Metal binding | |
|---|---|---|---|
| APP142–166 | DVCETHLHWHTVAKETCSEKSTNLH | Cu | Wild-type |
| APP142–166 | DVCET NL NW NTVAKETCSEKSTNLH | — | H147N, H149N, H151N |
| APP181–208 | GVEFVCCPLAEESDNVDSADAEEDDSDV | Zn | Wild-type |
Peptides were synthesized as described in Materials and Methods. Peptide APP142–166 corresponds to the copper-binding domain of human APP (Hesse et al., 1994; Multhaup et al., 1996, 1998). The copper-binding sequence containing Cys144, His147, His149, His151, and Cys158 is conserved in mouse APP (De Strooper et al., 1991). APP142–166H147N,H149N,H151N corresponds to the copper-binding domain with substitution of the coordinating histidine residues to asparagines (underlined). Previous studies have shown these changes prevent copper binding (Hesse et al., 1994). APP181–208 corresponds to the human APP zinc-binding domain (Bush et al., 1993).
Measurement of 64Cu uptake and accumulation. Six-day-old primary cortical neuron cultures grown in MEM/N2 were used for 64Cu uptake assays. The growth media was replaced with fresh MEM/N2 containing 5–10 μCi/ml 64Cu (Australian Radioisotopes, Lucas Heights, New South Wales, Australia) and “no added copper” (trace) or medium with added “cold” CuCl2 to give a total copper concentration of 50 μm. After incubation at 37°C for 0.5, 4, 16, and 24 hr, cells were lysed in 0.1% SDS, 2 mm EDTA and collected in sterile 10 ml plastic tubes. 64Cu was measured in cell pellets using an LKB-Wallac (Gaithersburg, MD) Ultragamma counter and expressed as picomoles of Cu per microgram of protein.
Determination of lipid peroxidation in cultures. Lipid peroxidation was determined in cultures using the LPO 586 lipid peroxidation kit. CuCl2 or FeCl2 was added to 6-d-old cultures as for MTT assays, however, after 16 hr exposure to metals, the cells were extracted and processed as described in the kit instructions. A malondialdehyde (MDA) standard curve was established from 1,1,3,3 tetramethoxypropane supplied in the kit. The protein concentration of cell extracts was determined using a BCA protein assay kit (Pierce, Rockford, IL), and lipid peroxidation was calculated as nanomoles of MDA per milligram of protein and converted to percentage of untreated controls. As an additional measure of oxidative stress, the level of thiobarbituric acid-reactive substances (TBARS) was determined in metal-treated cultures. After exposure to CuCl2or FeCl2 for 16 hr, 400 μl of TBA solution (15% trichloroacetic acid, 1.25% TBA, and 5.5% HCl) was added to each culture well containing 600 μl medium (in 24 well plates). The supernatant from each well was transferred to a fresh 10 ml tube and heated at 95°C for 20 min, cooled to RT, and spun at 3000 ×g for 5 min to pellet precipitated protein. The clarified supernatant was read on a Bio-Rad (Hercules, CA) plate reader at 532 nm. Cell-free medium alone was incubated with the TBA solution as above and subtracted from test readings. The TBARS values are given as optical density (OD) units ( × 10−3)/well. Cell numbers were determined by cell viability and total protein (BCA) assays. To prevent oxidation during the extraction or incubation processes, 0.01% BHT dissolved in 100% ethanol was added to buffers.
Determination of LDL peroxidation by APP peptides. To measure the ability of APP peptides to induce lipid peroxidation, 0.5 mg/ml human LDL was incubated with CuCl2 (20 μm) or ZnCl2 (50 μm) and APP peptides (140 μm) in PBS (pH 7.4) for 16 hr at 37°C. The level of lipid oxidation was measured using the LPO 586 lipid peroxidation kit as described above. Lipid peroxidation was determined as nanomoles of MDA per milligram of LDL and converted to percentage of control (LDL and CuCl2).
Statistical analysis. Data represents the mean and SEM of experiments performed in at least three or four cultures measured in triplicate. In all cases, comparison of data was performed with ANOVA and Newman–Keuls tests.
RESULTS
Wild-type primary cortical neurons are more susceptible to copper toxicity than APP−/− neurons
There were no differences in cell viability between the WT, APP−/−, or APLP2−/− neurons in untreated cortical, cerebellar, or hippocampal cultures (data not shown). This indicates that under the basal culture conditions used, endogenous APP or APLP2 expression does not affect neuronal survival.
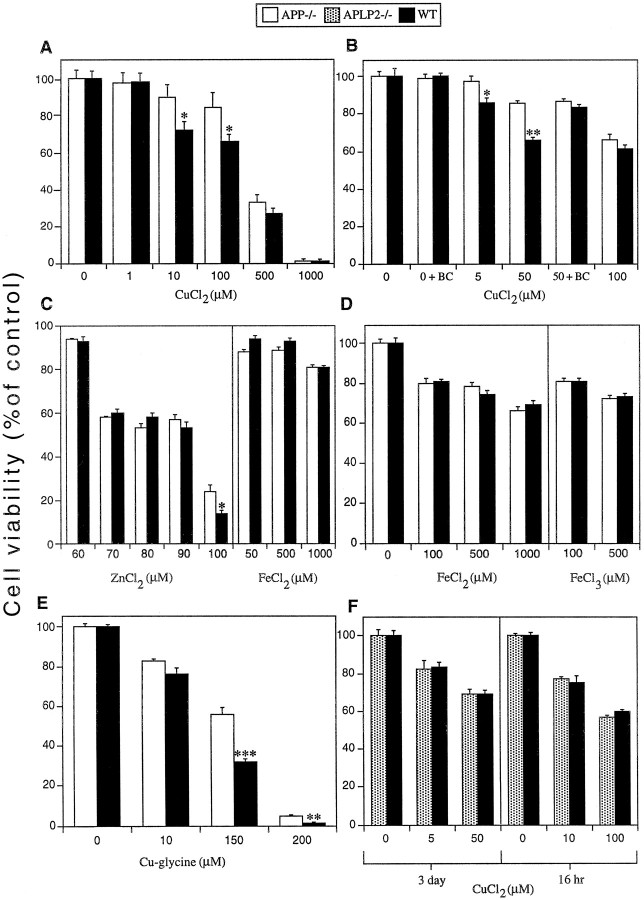
The reduction of copper (II) to copper (I) by APP has the potential to generate reactive oxygen species (ROS), which can induce oxidative stress (Multhaup et al., 1996, 1998). To determine if this can occur in a cellular environment, 6-d-old WT and APP−/− primary cortical neuronal cultures were exposed to CuCl2 for 16 hr. As shown in Figure 1A, WT neurons revealed significantly lower viability (∼20% lower) than APP−/− neurons exposed to 10 and 100 μm copper (*p < 0.01). Higher concentrations of copper (500 and 1000 μm) resulted in matching cell loss in both WT and APP−/− cultures. These findings were confirmed with the LDH cell survival assay. Exposure to 10 μm CuCl2 for 16 hr resulted in 83 ± 1.2% cell survival in WT neurons compared to 98 ± 1.6% in APP−/− neurons (p < 0.05). Similar levels of toxicity were obtained with CuSO4 (data not shown) indicating that the toxic effect is not dependent on the type of copper salt. A longer exposure to CuCl2 for 3 d resulted in significantly increased toxicity in WT compared to APP−/− neurons at 5 and 50 μm copper (*p < 0.05; **p < 0.01) but not at 100 μmcopper (Fig. 1B).
Fig. 1.
Effects of metals on cell viability in primary cultures of WT, APP−/−, and APLP2−/− cortical neurons. Three- or 6-d-old primary cortical neuronal cultures were exposed to copper, zinc, or iron salts for 3 d or 16 hr, respectively, and cell viability was determined using the MTT assay. A, WT neurons were significantly more susceptible to 10 and 100 μmCuCl2 toxicity than APP−/− neurons after 16 hr exposure (*p < 0.01).B, WT neurons were significantly more susceptible than APP−/− neurons to 5 and 50 μmCuCl2 toxicity after 3 d of exposure (from day 3in vitro) (*p < 0.05; **p < 0.01). The copper (I) chelator BC (50 μm) inhibited 50 μm CuCl2toxicity in WT cultures with no effect on copper toxicity in APP−/− cultures. BC (50 μm) (0 + BC) alone had no affect on cell viability.C, No significant difference in cell viability was observed between WT and APP−/− cortical neurons exposed to 60–90 μm ZnCl2 for 16 hr. However, WT neurons were significantly more susceptible than APP−/− neurons to ZnCl2 at 100 μm (*p < 0.05). No difference in cell viability was observed between WT and APP−/−neurons exposed to FeCl2 for 16 hr. D, No difference in cell viability was seen in WT and APP−/− neuron cultures exposed to FeCl2 or FeCl3 for 3 d (from day 3in vitro). E, WT neurons were significantly more susceptible than APP−/− neurons to copper toxicity induced by 150 and 200 μmcopper–glycine after 3 d of exposure (**p < 0.05; ***p < 0.01). Glycine alone at identical concentrations had no effect on neuronal viability. F,No difference in cell viability was seen in WT and APLP2−/− neuron cultures exposed to CuCl2 for 16 hr or 3 d.
To determine if the increased toxicity in WT cultures was specific for copper, we tested zinc [APP contains a zinc binding domain (Bush et al., 1993)] and iron (another redox reactive transition metal). There were no differences in viability between WT and APP−/− cortical neurons exposed to 60 and 90 μm ZnCl2 or with any concentration of FeCl2 or FeCl3 tested (Fig. 1C,D), whereas 100 μm ZnCl2induced significantly lower cell viability in WT neurons (*p < 0.05) (Fig. 1C). The large increases in toxicity between 60 and 70 μm and 90 and 100 μm ZnCl2 may indicate the ability of zinc to induce toxicity in neuronal cultures through increased activation of ionotropic glutamate receptors (Manev et al., 1997). Specific interaction of zinc with these receptors may have induced the threshold effect observed in our cultures. Significantly, there was no difference in the level of zinc toxicity between APP−/− and WT neurons except at the highest concentration (100 μm). The low level of cell viability observed at this concentration suggests that the difference is not physiologically relevant.
Because copper is normally complexed to other molecules such as amino acids in vivo (Linder, 1991; Brown et al., 1997a), we tested the neurotoxicity of copper chelated as a copper–glycine complex. This form of copper induced significantly greater toxicity in WT as compared to APP−/− neurons at 150 and 200 μm (**p < 0.05; ***p < 0.01) (∼50% lower viability in WT neurons exposed to 150 μm copper; Fig.1E). These data show that copper is toxic to primary neurons from WT and APP-deficient mice, however, both unbound and biologically chelated copper can induce greater toxicity in APP-expressing neurons at copper concentrations within the physiological range of 10–250 μm (Kardos et al., 1989; Linder, 1991), thus supporting the physiological relevance of these data.
If copper (I) generation by APP is responsible for increased copper toxicity in WT neurons, then chelation of copper (I) should abrogate toxicity. To test this, cultures were treated with the copper (I) chelator BC (50 μm) and 50 μmCuCl2. This resulted in the abolition of copper toxicity in WT neurons with no effect on APP−/− neurons (Fig.1B). A higher concentration of BC (80 μm) completely inhibited toxicity induced by 50 μm copper in both WT and APP−/− cultures (data not shown). These data support a role for copper (I) formation in mediating increased toxicity in WT compared to APP−/−neurons.
No alterations in copper toxicity in APLP2−/− neurons
APP and APLP2 are the most closely related members of the APP superfamily (Hesse et al., 1994), and APLP2 has the ability to reduce copper in vitro (Multhaup et al., 1996). To test whether APLP2 affects neuronal copper toxicity, we exposed APLP2 knock-out (APLP2−/−) and WT neurons to CuCl2 for 16 hr or 3 d. There was no difference in copper toxicity between WT and APLP2−/− neurons using the same concentrations of copper that induced a difference between WT and APP−/− neurons (Fig.1F). Therefore, basal levels of neuronal APLP2 may not mediate copper toxicity, or APLP2-associated toxicity could be masked by increased susceptibility to oxidative stress in APLP2−/− neurons. These findings demonstrate that decreased copper toxicity in APP−/− neurons is caused by a difference in APP expression and is not an artifact related to the gene knock-out procedure.
Copper toxicity is increased in WT compared to APP−/− primary neuronal cultures from cerebellumand hippocampus
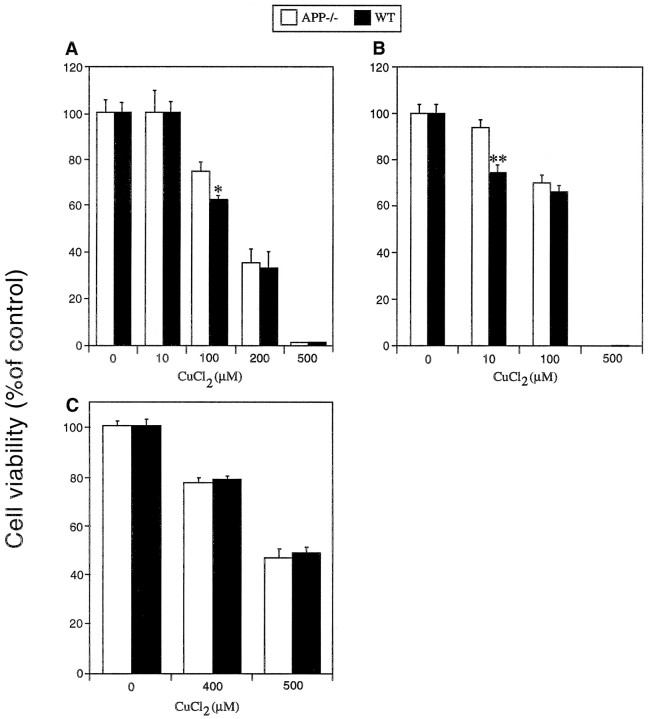
If the increased copper toxicity observed in WT cortical neurons is related to the copper-binding domain on APP, similar differences in toxicity should be seen in other APP-expressing neuronal populations. To test this, we exposed WT and APP−/−cerebellar granule neurons (CGNs) and hippocampal neurons to CuCl2 at day 6 in vitro (Fig.2A,B). WT CGNs and hippocampal neurons were significantly more susceptible to copper toxicity than their respective APP−/− cultures (*p < 0.01; **p < 0.05; Fig.2A,B) within the physiological range for copper. In contrast, astrocyte cultures revealed no significant difference in cell viability between WT and APP−/− cultures (Fig. 2C), suggesting that increased antioxidant levels in astrocytes may compensate for APP-associated copper toxicity.
Fig. 2.
Effects of copper on cell viability in WT and APP−/− CGNs, hippocampal neurons, and astrocytes. Six-day-old cultures of cerebellar or hippocampal neurons were exposed to CuCl2 for 16 hr, and cell viability was determined using the MTT assay. A, WT cerebellar neurons were significantly more susceptible to 100 μmCuCl2 than APP−/− neurons (*p < 0.05; see Materials and Methods).B, WT hippocampal neurons were significantly more susceptible to CuCl2 (10 μm) than APP−/− neurons (**p < 0.01; see Materials and Methods). C, Astrocyte cultures were established from WT and APP−/− mice cerebral cortices and exposed to CuCl2 at day 14 in vitro. No difference in cell viability was observed between WT and APP−/− astrocytes. Higher concentrations of CuCl2 were required to induce toxicity in astrocytes as compared to neurons.
A peptide encoding the APP copper-binding domain potentiates copper toxicity in primary neuronal cultures
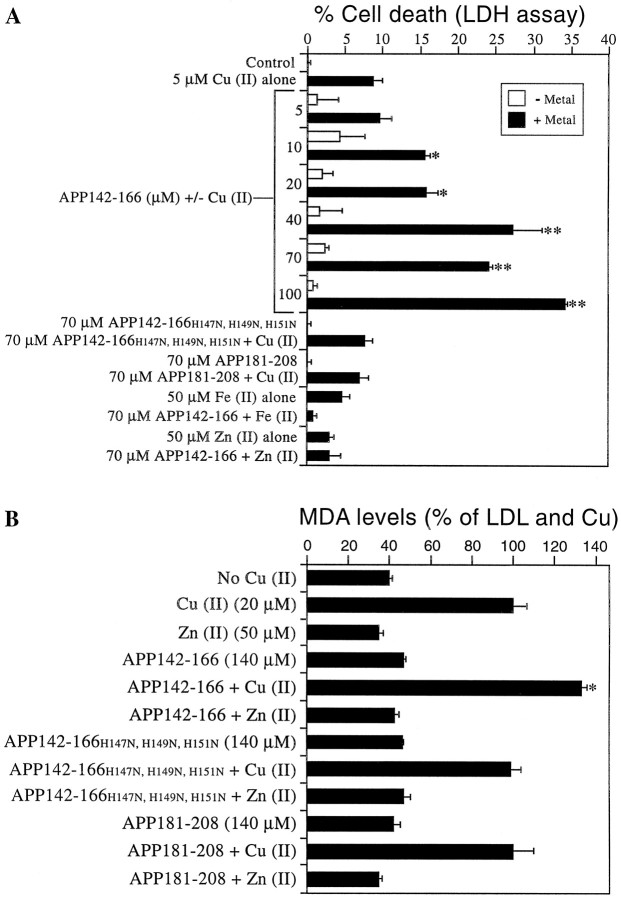
We have previously demonstrated that the copper-binding domain of APP induces copper (I) and hydroxyl radicals in a cell-free system (Multhaup et al., 1996, 1998). To determine if this region of APP is responsible for the increased copper toxicity observed in WT neurons, we exposed APP−/− cortical cultures to peptides containing the APP copper-binding domain (APP142–166) or the APP zinc-binding domain (APP181–208) (Table 1) and subtoxic levels of CuCl2. Cultures were exposed to 5–100 μm APP142–166 with and without 5 μmCuCl2 and assayed for release of LDH after 3 d (Fig. 3A). No significant increase in LDH release was observed in cultures treated with APP142–166 alone at any concentration. However, cultures exposed to 5 μm CuCl2 and 10 μm APP142–166 or greater produced a clear dose–response effect with a significant increase in LDH levels compared to copper alone (*p < 0.05; **p < 0.01; Fig. 3A). This was a specific effect because the CuBD mutant peptide, APP142–166H147N, H149N, H151N and the zinc-binding domain peptide, APP181–208 (70 μm) had no affect on LDH release alone or when applied with 5 μmCuCl2 (Fig. 3A). This activity is specific for copper because 70 μm APP142–166 did not potentiate either FeCl2- or ZnCl2-induced LDH release (Fig. 3A). These data indicate that the human APP copper-binding domain specifically potentiates cell death from low concentrations of copper.
Fig. 3.
Potentiation of copper-mediated cell death and lipid peroxidation by an APP-derived copper binding peptide.A, Two-day-old APP−/− neurons were exposed to APP142–166, APP142–166H147N, H149N, H151N, or APP181–208 with or without 5 μmCuCl2, 50 μm FeCl2, or 50 μm ZnCl2 for 3 d in serum-free medium. APP142–166 was added at concentrations of 5–100 μm at days 1 and 2. APP142–166H147N, H149N, H151N and APP181–208 were added at a concentration of 70 μm at days 1 and 2. CuCl2, FeCl2, or ZnCl2 were added at day 1 only. Cell survival was determined with the LDH assay. APP142–166 significantly increased cell death in the presence of 5 μm CuCl2 when added at concentrations of 10 μm or higher (*p < 0.05; **p < 0.01). APP142–166H147N, H149N, H151N and APP181–208 had no effect on cell death induced by CuCl2. APP142–166 had no effect on cell death induced by FeCl2 or ZnCl2. B, APP142–166, APP142–166H147N, H149N, H151N, and APP181–208 were incubated with 0.5 mg/ml LDL and 20 μm CuCl2or 50 μm ZnCl2 for 16 hr (37°C). MDA levels were measured with the LPO 586 assay kit and compared to LDL incubated with CuCl2 alone. A 140 μm concentration of APP142–166 (equivalent to the total concentration added to cultures) significantly increased lipid peroxidation in the presence of 20 μm CuCl2 (*p < 0.01). APP142–166H147N, H149N, H151N and APP181–208 had no effect on copper-induced lipid peroxidation. ZnCl2 did not increase lipid peroxidation induced by any peptide.
Free radical damage in neurons can be measured as lipid peroxidation products such as MDA. To determine that the toxicity induced by the APP copper-binding domain is related to copper-mediated free radical generation rather than alternative affects on cell metabolism, we added APP142–166 (140 μm, the same total concentration as added to cultures) and copper (20 μm) to LDL for 16 hr (37°C) and measured MDA accumulation. Addition of copper to LDL induced a significant increase in lipid peroxidation compared to LDL alone. Without added copper, APP142–166, APP142–166H147N, H149N, H151N, and APP181–208 did not significantly affect lipid peroxidation levels. In the presence of 20 μmcopper, APP142–166 increased lipid peroxidation by 33% compared to LDL and copper alone (*p < 0.01; Fig. 3B). APP H147N, H149N, H151N and APP181–208 had no effect on lipid peroxidation compared to LDL and copper (Fig.3B). Similarly, coincubation of (50 μm) ZnCl2 with the copper or zinc-binding peptides failed to induce toxicity compared to zinc alone (Fig. 3B). These data confirm that the APP copper-binding domain can induce oxidative stress (peroxidation) through a specific interaction with copper.
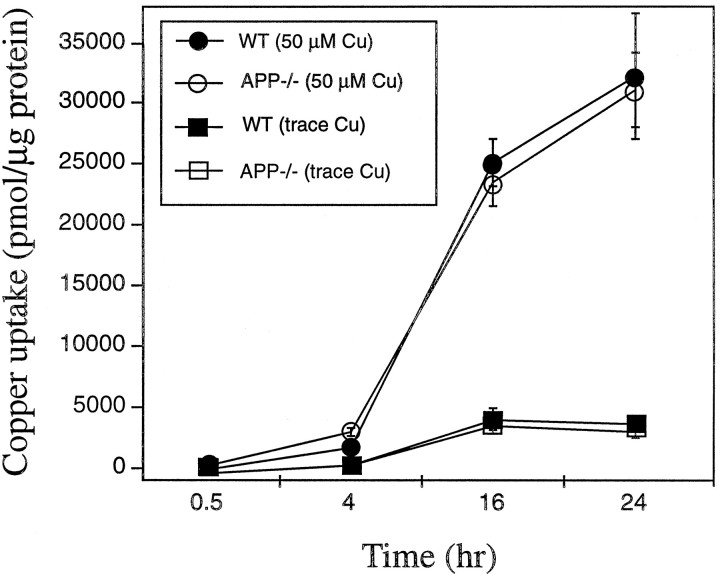
Wild-type and APP−/− neurons reveal similar levels of copper uptake
The increased copper toxicity in APP-expressing cultures may be caused by greater binding and uptake of copper than in APP−/− cultures. Alternatively, increased oxidative stress in WT cultures could result from localized generation of copper (I) by APP. 64Cu binding under the same conditions as were used to induce differential toxicity showed no significant differences in copper uptake between WT and APP−/− cortical neurons using either trace levels (∼1 μm) or 50 μm Cu (Fig.4). These data are consistent with APP inducing a localized increase in copper toxicity through copper (I) generation rather than increasing total copper uptake.
Fig. 4.
Copper uptake in primary cultures of WT and APP−/− cortical neurons. Primary cortical cultures were exposed to 64Cu in MEM/N2 media for 0.5, 4, 16, and 24 hr. Cells were lysed in 0.1% SDS and counted in an LKB-Wallac Ultragamma counter. No differences were observed in the level of copper uptake over 24 hr between WT and APP−/−neurons.
Wild-type neurons reveal increased lipid peroxidation compared to APP−/− neurons
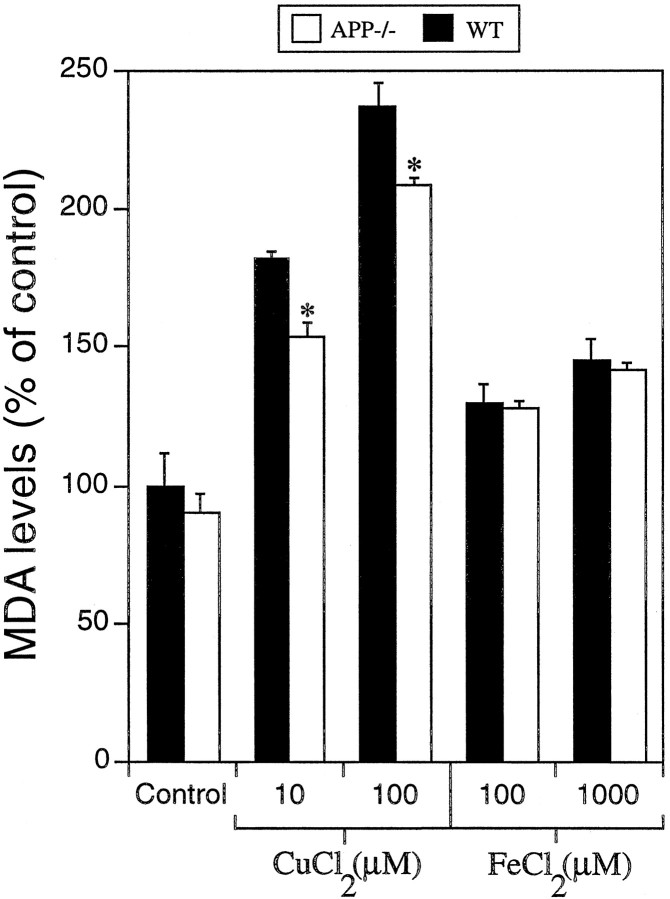
To determine if increased copper toxicity in WT neurons involves the generation of oxidative stress products by APP–copper interactions, we measured MDA levels and TBA-reactive aldehyde levels. Exposure of cortical neurons to 10 and 100 μmCuCl2 for 16 hr resulted in ∼18 and 13% higher levels of MDA, respectively, in WT as compared to APP−/− cultures (*p < 0.05; Fig. 5). Treatment of cultures with 100 and 1000 μm FeCl2resulted in no significant difference in MDA between WT and APP−/− neurons (Fig. 5). Analysis of TBARS levels in copper-treated cultures revealed similar results. There were no differences in the basal levels of aldehydic products between WT and APP−/− cultures. WT cortical neurons treated with 10 or 100 μmCuCl2 for 16 hr produced significantly greater levels (∼35 and 20%, respectively) of TBARS than APP−/− neurons (*p < 0.05; Table 2). In contrast, FeCl2 exposure resulted in no significant difference in TBARS levels between WT and APP−/− cultures (Table 2). The increased copper-induced lipid peroxidation in WT neurons correlates with the greater toxicity in WT neurons measured with the MTT or LDH assays and with the increased copper toxicity induced by APP142–166.
Fig. 5.
Malondialdehyde levels in neuronal cultures treated with copper or iron. WT and APP−/−cortical neuronal cultures were exposed to metals at indicated concentrations for 16 hr at day 6 in vitro. MDA levels were determined in cell extracts using the LPO 586 lipid peroxidation assay kit. Absorbance readings were compared to an MDA standard curve, adjusted for total protein concentration, and converted to percentage of control. One hundred percent MDA is equivalent to the reading for untreated WT cultures. Copper, but not iron, induced significantly greater levels of MDA (lipid peroxidation) in WT compared to APP−/− cultures (*p < 0.05).
Table 2.
TBARS levels in neuronal cultures exposed to copper or iron
| TBARS (OD Units × 10−3/well) | ||
|---|---|---|
| WT | APP−/− | |
| No treatment | 4 ± 3 | 6 ± 4 |
| CuCl2(10 μm) | 41 ± 7* | 27 ± 8 |
| CuCl2 (100 μm) | 50 ± 3* | 41 ± 5 |
| FeCl2 (100 μm) | 40 ± 8 | 37 ± 8 |
| FeCl2 (1000 μm) | 75 ± 4 | 77 ± 9 |
WT and APP−/− cortical neuronal cultures were exposed to metals at indicated concentrations for 16 hr at day 6in vitro. TBA solution was added to neurons and media and incubated at 95°C for 20 min. The values represent optical density (OD) units (× 10−3) per well (from 24 well plates) after correction for control media alone. Copper, but not iron, induced significantly greater levels of TBARS in WT compared to APP−/− cultures.
*p < 0.05.
DISCUSSION
Aβ deposition alone cannot explain the spatiotemporal pattern of cell loss characteristic of AD (Hardy et al., 1986). The possibility that copper may contribute to AD pathology is suggested by perturbed ceruloplasmin and copper levels in AD patients (Loeffler et al., 1996;Lovell et al., 1998) and the production of free radicals and increased Aβ aggregation in the presence of copper (Atwood et al., 1998, A. I. Bush, unpublished observations). There is increasing evidence that copper may also have an important role in other neurodegenerative disorders such as ALS (Andrus et al., 1998) and CJD (Viles et al., 1999; Wadsworth et al., 1999). The harmful effects of copper mis-metabolism are highlighted by illnesses such as Menkes and Wilson's diseases (Harris and Gitlin, 1996).
We have previously reported that the APP ectodomain can bind and reduce copper (II) to copper (I) (Multhaup et al., 1996, 1998). The present data demonstrate that this reaction also occurs in a cellular environment, resulting in generation of ROS and neurotoxicity from physiological concentrations of copper (Kardos et al., 1989). This was shown by the increased susceptibility of WT as compared to APP−/− primary neurons specifically to copper but not iron toxicity [another important mediator of oxidative stress (Xie et al., 1996)]. The increased toxicity observed in WT cortical, hippocampal, and CGN cultures with 5–150 μmcopper is clearly within the proposed physiological range of 10 μm for body fluid, 78 μm for CSF, and 250 μm for synaptic copper levels (Kardos et al., 1989;Linder, 1991). The same effect was observed when a copper–glycine complex was used in place of CuCl2, indicating that biologically bound copper can interact with APP and generate increased oxidative stress. Peptides corresponding to the APP copper-binding sequence have been shown to retain the copper reduction activity of full-length APP (Multhaup et al., 1996, 1998). The potentiation of toxicity by the APP142–166 peptide with a subtoxic level of copper strongly supports the hypothesis that the increased copper toxicity in WT neurons is mediated by this sequence. The specificity of this effect was shown by the lack of copper toxicity in cultures exposed to APP142–166H147N, H149N, H151N or APP181–208 and the inability of APP142–166 to potentiate toxicity from zinc or iron. The zinc-binding protein APP181–208 also failed to induce toxicity from ZnCl2 (data not shown). The APP-peptide concentrations used, although higher than predicted for endogenous APP levels [0.53–133 nm in CSF (Whyte et al., 1997)], are consistent with the dissociation constant (Kd) of APP142–166 for copper being 0.4 μm (our unpublished observation) as compared to 10 nm for full-length APP (Hesse et al., 1994). This 40-fold difference in theKd indicates the peptide would be less active, and therefore more peptide is required to compete with other soluble or cell-associated copper-binding molecules (Aschner, 1996;Loeffler et al., 1996; Brown et al., 1997a; Nishikawa et al., 1997) to induce the same level of copper reduction as full-length APP. Furthermore, the APP peptide remains soluble so a large proportion of the short-lived free radicals generated do not come into contact with the neuronal monolayer. In contrast, full-length APP is cell-associated or binds to cellular receptors and can generate a more specific and localized toxic effect. This is consistent with studies describing the need for a direct interaction between amyloidogenic peptides and neurons to induce toxicity even at peptide concentrations of 80 μm (Forloni et al., 1993; Brown et al., 1996;1998b; Ivins et al., 1998).
Interestingly, the increased neuronal toxicity induced by the APP copper-binding peptide was found to be greater when assessed with the MTT assay than with the LDH assay. As MTT provides a measure of cell viability rather than actual cell death (as determined by the LDH assay), the data suggest that the APP copper-binding sequence can reduce neuronal viability and subsequently increase susceptibility to additional oxidative insults such as Aβ exposure, hypoglycemia, or GSH depletion. The significant and specific potentiation of copper toxicity by APP142–166 confirms the toxic potential of the APP copper-binding sequence. Together with the observation of increased copper-induced lipid peroxidation by the APP copper-binding peptide, our data supports the hypothesis that APP can generate copper (I) and ROS resulting in neuronal cell death. The inhibition of copper toxicity in WT cultures with the copper (I) chelator BC indicates that the increased toxicity in WT neurons is copper (I)-mediated. However, it is not known whether this effect is directly related to copper (I) production by APP or copper (I) oxidation of cysteine resulting in depletion of cellular glutathione.
Because WT and APP−/− neuronal cultures showed no difference in response to other inducers of oxidative stress, including Aβ and H2O2(Harper et al., 1998; White et al., 1998), the difference in copper toxicity in this study is not attributable to APP neuroprotective activity. In fact, a loss of APP expression should cause a decrease in cell survival, which is the opposite to that seen in the APP−/− neurons. The specificity of the effect with APP−/−, but not APLP2−/−, cultures is consistent with the observation that APLP2 reduces copper (II) less efficiently than APP in a cell-free system (Multhaup et al., 1996) and is expressed at lower levels than APP. In a cellular environment, variations in primary sequence or subcellular localization may be critical for determining the level of copper reduction and therefore toxicity induced by these proteins. This could explain the higher copper toxicity in WT neurons despite a lack of difference in total copper uptake between WT and APP−/− neurons. Although alternate copper transport systems (Harris et al., 1998;Nishihara et al., 1998) may compensate for the loss of APP in APP−/− neurons, the high efficiency of copper reduction and ROS generation by APP in WT neurons would result in increased copper toxicity without an overall increase in the cellular copper level.
Interestingly, Brown et al. (1998a) have demonstrated increased copper toxicity in PrP-deficient neurons (PrP is also a cuproprotein). This effect is the opposite to that shown with WT and APP−/− neurons and indicates different and specific mechanisms of copper metabolism and toxicity in each model. The increased toxicity in PrP−/−neurons may relate to their increased susceptibility to oxidative stress. Studies have shown that FALS-associated mutations to Cu/ZnSOD may induce interactions between copper and H2O2, resulting in neuronal toxicity without gross changes to copper levels or SOD activity (Yim et al., 1996, 1997; Liochev et al., 1997). Similar interactions between APP, copper, and ROS may occur with little change in total cellular copper binding. This provides further evidence for the importance of metals, and in particular copper, in neurodegenerative disorders and has important implications for metal chelation-based therapy.
The increased levels of lipid peroxidation in copper-treated WT cultures and in LDL coincubated with copper and APP142–166 supports our hypothesis that formation of APP-Cu (I) intermediates can result in the generation of toxic-free radicals and increased oxidative stress in neurons (Multhaup et al., 1996, 1998). This effect may be similar to the potent peroxidative activity of copper bound to human ceruloplasmin (Mukhopadhyay et al., 1997) and the demonstration of increased protein oxidation in G93A transgenic SOD1 mice (Andrus et al., 1998). Furthermore, other studies have highlighted the important role lipid peroxidation can have in neuronal oxidative stress and AD (Kruman et al., 1997; Montine et al., 1997; Sayre et al., 1997). Although increased lipid peroxidation could result from Aβ-mediated transition metal-induced ROS production (Bondy et al., 1998), it has been found that rodent Aβ does not generate ROS through interactions with copper (Bush, unpublished observations). This precludes mouse Aβ as the source of increased copper-associated oxidative stress in our cultures. Our findings indicate that increased lipid peroxidation may be an important intermediary in APP-copper toxicity and support an earlier report of neurotoxicity from high concentrations of APP (Milward et al., 1992). These findings may be related to the accelerated degeneration of APP-transfected neurons that reveal increased APP accumulation but no change in Aβ levels (Nishimura et al., 1998). However, other factors may also be involved because neuronal cell loss can be achieved in APP-transfected mice lacking the copper-binding domain (Hsiao, 1998). In fact, because of the ubiquitous distribution of both copper and APP in the brain, the specific pattern of neurodegeneration in AD and in animal models of AD requires the involvement of other factors such as changes in ceruloplasmin and metalloprotein regulation, glutathione status, and Aβ aggregation state. Aβ can generate ROS from transition metals (Bondy et al., 1998) and deplete neuronal glutathione levels (an important intracellular copper detoxifying molecule) (Freedman et al., 1989;Müller et al., 1997), suggesting that Aβ and APP may have multiple effects on neuronal copper toxicity. These factors could potentiate toxicity from additional stresses, including glucose deprivation, mitochondrial dysfunction, or perturbations to other neuronal antioxidant activities, all of which have been reported in AD.
Our findings demonstrating that increased oxidative damage and cell death can occur after interaction of APP and copper in a cell-based system identifies a novel role for APP in AD pathogenesis. Altered copper homeostasis has been reported in AD (Deibel et al., 1996;Loeffler et al., 1996; Lovell et al., 1998). Lovell et al. (1998)observed an increase in copper in the rim of senile plaques in AD brain, whereas Deibel et al. (1996) reported a decrease in overall copper levels in AD plaques. The apparent discrepancy in these findings could be related to the techniques used for copper measurement (micro-PIXE vs instrumental neutron activation) or tissue preparation. However, the data clearly indicate that perturbations to copper metabolism occur in AD patients, and this may result in a toxic gain of function through interaction with APP in vivo. This toxic process could contribute to the neuronal cell loss and dysfunction characteristic of AD. Furthermore, the ability of Aβ to increase APP expression (Saporito-Irwin et al., 1997; White et al., 1998) could create a positive feedback loop increasing both Aβ production and potentially toxic APP and thus augment the role of APP in AD pathogenesis.
Footnotes
This work is supported in part by grants from the National Health and Medical Research Council of Australia to C.L.M. K.B. is supported by the Deutsche Forschungsgemeinschaft and the Bundesministerium fur Forschung und Technologie. J.C. is supported by a grant from the Australian Institute of Nuclear Science and Engineering. We thank Dr. Sam Sisodia and Dr. Connie von Koch for the APLP2−/− mice, and we thank Denise Galatis for reading of this manuscript.
Correspondence should be addressed to Dr. Roberto Cappai, Department of Pathology, The University of Melbourne, Parkville, 3052 Victoria, Australia.
REFERENCES
- 1.Andrus PK, Fleck TJ, Gurney ME, Hall ED. Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1998;71:2041–2048. doi: 10.1046/j.1471-4159.1998.71052041.x. [DOI] [PubMed] [Google Scholar]
- 2.Aschner M. The functional significance of brain metallothioneins. FASEB J. 1996;10:1129–1136. doi: 10.1096/fasebj.10.10.8751715. [DOI] [PubMed] [Google Scholar]
- 3.Atwood CS, Moir RD, Huang XD, Scarpa RC, Bacarra NME, Romano DM, Hartshorn MK, Tanzi RE, Bush AI. Dramatic aggregation of Alzheimer A-beta by Cu (II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273:12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- 4.Beher D, Hesse L, Masters CL, Multhaup G. Regulation of amyloid protein precursor (APP) binding to collagen and mapping of the binding sites on APP and collagen type I. J Biol Chem. 1996;271:1613–1620. doi: 10.1074/jbc.271.3.1613. [DOI] [PubMed] [Google Scholar]
- 5.Bondy SC, Guo-Ross SX, Truong AT. Promotion of transition metal-induced reactive oxygen species formation by β-amyloid. Brain Res. 1998;799:91–96. doi: 10.1016/s0006-8993(98)00461-2. [DOI] [PubMed] [Google Scholar]
- 6.Brown DR, Schmidt B, Kretzschmar HA. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature. 1996;380:345–347. doi: 10.1038/380345a0. [DOI] [PubMed] [Google Scholar]
- 7.Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, Fraser PE, Kruck T, Bohlens A, Schultz-Schaeffer W, Giese A, Westaway D, Kretzschmar HA. The cellular prion protein binds copper in vivo. Nature. 1997a;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- 8.Brown DR, Schulz-Schaeffer WJ, Schmidt B, Kretzschmar HA. Prion protein-deficient cells show altered response to oxidative stress due to decreased SOD-1 activity. Exp Neurol. 1997b;146:104–112. doi: 10.1006/exnr.1997.6505. [DOI] [PubMed] [Google Scholar]
- 9.Brown DR, Schmidt B, Kretzschmar HA. Effects of copper on survival of prion protein knockout neurons and glia. J Neurochem. 1998a;70:1686–1689. doi: 10.1046/j.1471-4159.1998.70041686.x. [DOI] [PubMed] [Google Scholar]
- 10.Brown DR, Schmidt B, Kretzschmar HA. Prion protein fragment interacts with PrP-deficient cells. J Neurosci Res. 1998b;52:260–267. doi: 10.1002/(SICI)1097-4547(19980501)52:3<260::AID-JNR2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Bush AI, Multhaup G, Moir RD, Williamson TG, Small DH, Rumble B, Pollwein P, Beyreuther K, Masters CL. A novel zinc(II) binding site modulates the function of the β A4 amyloid protein precursor of Alzheimer's disease. J Biol Chem. 1993;268:16109–16112. [PubMed] [Google Scholar]
- 12.Deibel MA, Ehmann WD, Markesbery WR. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer's disease: possible relation to oxidative stress. J Neurol Sci. 1996;143:137–142. doi: 10.1016/s0022-510x(96)00203-1. [DOI] [PubMed] [Google Scholar]
- 13.De Strooper B, Van Leuven F, Van Den Berghe H. The amyloid β protein precursor or proteinase nexin II from mouse is closer to its human homolog than previously reported. Biochim Biophys Acta. 1991;1129:141–143. doi: 10.1016/0167-4781(91)90231-a. [DOI] [PubMed] [Google Scholar]
- 14.Forloni G, Angeretti N, Chiesa R, Monzani E, Salmona M, Bugiani O, Tagliavini F. Neurotoxicity of a prion protein fragment. Nature. 1993;362:543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- 15.Freedman JH, Ciriolo MR, Peisach J. The role of glutathione in copper metabolism and toxicity. J Biol Chem. 1989;264:5598–5605. [PubMed] [Google Scholar]
- 16.Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 17.Gunther MR, Hanna PM, Mason RP, Cohen MS. Hydroxyl radical formation from cuprous ion and hydrogen peroxide: a spin-trapping study. Arch Biochem Biophys. 1995;316:515–522. doi: 10.1006/abbi.1995.1068. [DOI] [PubMed] [Google Scholar]
- 18.Hardy JA, Mann DM, Wester P, Winblad B. An integrative hypothesis concerning the pathogenesis and progression of Alzheimer's disease. Neurobiol Aging. 1986;7:489–502. doi: 10.1016/0197-4580(86)90086-2. [DOI] [PubMed] [Google Scholar]
- 19.Harper SJ, Bilsland JG, Shearman MS, Zheng H, Van der Ploeg L, Sirinathsinghji JS. Mouse cortical neurones lacking APP show normal neurite outgrowth and survival responses in vitro. NeuroReport. 1998;9:3053–3057. doi: 10.1097/00001756-199809140-00025. [DOI] [PubMed] [Google Scholar]
- 20.Harris ED, Qian Y, Tiffany-Castiglioni E, Lacy AR, Reddy MC. Functional analysis of copper homeostasis in cell culture models: a new perspective on internal copper transport. Am J Clin Nutr. 1998;67:988S–995S. doi: 10.1093/ajcn/67.5.988S. [DOI] [PubMed] [Google Scholar]
- 21.Harris ZH, Gitlin JD. Genetic and molecular basis for copper toxicity. Am J Clin Nutr. 1996;63:836S–841S. doi: 10.1093/ajcn/63.5.836. [DOI] [PubMed] [Google Scholar]
- 22.Hesse L, Beher D, Masters CL, Multhaup G. The βA4 amyloid precursor protein binding to copper. FEBS Lett. 1994;349:109–116. doi: 10.1016/0014-5793(94)00658-x. [DOI] [PubMed] [Google Scholar]
- 23.Hsiao K. Transgenic mice expressing Alzheimer amyloid precursor proteins. Exp Gerontol. 1998;33:883–889. doi: 10.1016/s0531-5565(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 24.Ivins KJ, Bui ETN, Cotman CW. β-amyloid induces local neurite degeneration in cultured hippocampal neurons: evidence for neuritic apoptosis. Neurobiol Dis. 1998;5:365–378. doi: 10.1006/nbdi.1998.0228. [DOI] [PubMed] [Google Scholar]
- 25.Kang J, Lemaire H, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K, Multhaup G, Beyreuther K, Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 26.Kardos J, Kovacs I, Hajos F, Kalman M, Simonyi M. Nerve endings from rat brain release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci Lett. 1989;103:139–144. doi: 10.1016/0304-3940(89)90565-x. [DOI] [PubMed] [Google Scholar]
- 27.Koh J, Yang LL, Cotman CW. β-Amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990;533:315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- 28.Kruman I, Bruce-Keller AJ, Bredesen D, Waeg G, Mattson MP. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci. 1997;17:5089–5100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linder MC. Biochemistry of copper. Plenum; New York: 1991. [Google Scholar]
- 30.Liochev SI, Chen LL, Hallewell RA, Fridovich I. Superoxide-dependent peroxidase activity of H48Q: a superoxide dismutase variant associated with familial amyotrophic lateral sclerosis. Arch Biochem Biophys. 1997;346:263–268. doi: 10.1006/abbi.1997.0298. [DOI] [PubMed] [Google Scholar]
- 31.Loeffler DA, LeWitt PA, Juneau PL, Sima AAF, Nguyen HU, DeMaggio AJ, Brickman CM, Brewer GJ, Dick RD, Troyer MD, Kanaley L. Increased regional brain concentrations of ceruloplasmin in neurodegenerative disorders. Brain Res. 1996;738:265–274. doi: 10.1016/s0006-8993(96)00782-2. [DOI] [PubMed] [Google Scholar]
- 32.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 33.Manev H, Kharlamov E, Uz T, Mason RP, Cagnoli CM. Characterization of zinc-induced neuronal death in primary cultures of rat cerebellar granule cells. Exp Neurol. 1997;146:171–178. doi: 10.1006/exnr.1997.6510. [DOI] [PubMed] [Google Scholar]
- 34.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milward E, Papadopoulos R, Fuller SJ, Moir RD, Small D, Beyreuther K, Masters CL. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- 36.Montine KS, Kim PJ, Olson SJ, Markesbery WR, Montine TJ. 4-hydroxy-2-nonenal pyrrole adducts in human neurodegenerative disease. J Neuropathol Exp Neurol. 1997;56:866–871. doi: 10.1097/00005072-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Mukhopadhyay CK, Mazumder B, Lindley PF, Fox PL. Identification of the prooxidant site of human ceruloplasmin: a model for oxidative damage by copper bound to protein surfaces. Proc Natl Acad Sci USA. 1997;94:11546–11551. doi: 10.1073/pnas.94.21.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller WE, Romero FJ, Perovic S, Pergande G, Pialoglou P. Protection of flupirtine on β-amyloid-induced apoptosis in neuronal cells in vitro: prevention of amyloid-induced glutathione depletion. J Neurochem. 1997;68:2371–2377. doi: 10.1046/j.1471-4159.1997.68062371.x. [DOI] [PubMed] [Google Scholar]
- 39.Multhaup G, Bush AI, Pollwein P, Masters CL. Interaction between zinc (II) and the heparin binding site of the Alzheimer's disease bA4 amyloid precursor protein (APP). FEBS Lett. 1994;355:151–154. doi: 10.1016/0014-5793(94)01176-1. [DOI] [PubMed] [Google Scholar]
- 40.Multhaup G, Schlicksupp A, Hesse L, Beher D, Ruppert T, Masters CL, Beyreuther K. The amyloid precursor protein of Alzheimer's disease in the reduction of copper (II) to copper (I). Science. 1996;271:1406–1409. doi: 10.1126/science.271.5254.1406. [DOI] [PubMed] [Google Scholar]
- 41.Multhaup G, Ruppert T, Schlicksupp A, Hesse L, Bill E, Pipkorn R, Masters CL, Beyreuther K. Copper-binding amyloid precursor protein undergoes a site-specific fragmentation in the reduction of hydrogen peroxide. Biochemistry. 1998;37:7224–7230. doi: 10.1021/bi980022m. [DOI] [PubMed] [Google Scholar]
- 42.Nishihara E, Furuyama T, Yamashita S, Mori N. Expression of copper trafficking genes in the mouse brain. NeuroReport. 1998;9:3259–3263. doi: 10.1097/00001756-199810050-00023. [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa T, Lee IS, Shiraishi N, Ishikawa T, Ohta Y, Nishikimi M. Identification of S100β protein as a copper-binding protein and its suppression of copper-induced cell damage. J Biol Chem. 1997;272:23037–23041. doi: 10.1074/jbc.272.37.23037. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura I, Uetsuki T, Dani SU, Ohsawa Y, Saito I, Okamura H, Uchiyama Y, Yoshikawa K. Degeneration in vivo of rat hippocampal neurons by wild-type Alzheimer amyloid precursor protein overexpressed by adenovirus-mediated gene transfer. J Neurosci. 1998;18:2387–2398. doi: 10.1523/JNEUROSCI.18-07-02387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saporito-Irwin SM, Thinskaran G, Ruffini L, Sisodia SS, Van Nostrand WE. Amyloid β-protein stimulates parallel increases in cellular levels of its precursor and amyloid precursor-like protein 2 (APLP2) in human smooth muscle cultures. Amyloid Int J Exp Clin Invest. 1997;4:54–60. [Google Scholar]
- 46.Sayre LM, Zelasko DA, Harris PLR, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 47.Slunt HH, Thinakaran G, von Koch C, Lo ACY, Tanzi RE, Sisodia SS. Expression of a ubiquitous, cross-reactive homologue of the mouse β-amyloid precursor protein (APP). J Biol Chem. 1994;269:2637–2644. [PubMed] [Google Scholar]
- 48.Van Nostrand WE. Zinc (II) selectively enhances the inhibition of coagulation factor XIa by protease nexin-2/amyloid β-protein precursor. Thromb Res. 1995;78:43–55. doi: 10.1016/0049-3848(95)00033-x. [DOI] [PubMed] [Google Scholar]
- 49.Viles JH, Cohen FE, Prusiner SB, Goodin DB, Wright PE, Dyson HJ. Copper binding to the prion protein: structural implications of four identical cooperative binding sites. Proc Natl Acad Sci USA. 1999;96:2042–2047. doi: 10.1073/pnas.96.5.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Koch CS, Zheng H, Chen H, Trumbauer M, Thinakaran G, van der Ploeg LHT, Price DL, Sisodia SS. Generation of APLP2 KO mice and early postnatal lethality in APLP2/APP double KO mice. Neurobiol Aging. 1997;18:661–669. doi: 10.1016/s0197-4580(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 51.Wadsworth JDF, Hill AF, Joiner S, Jackson GS, Clarke AR, Collinge J. Strain-specific prion-protein conformation determined by metal ions. Nat Cell Biol. 1999;1:55–59. doi: 10.1038/9030. [DOI] [PubMed] [Google Scholar]
- 52.Wasco W, Bupp K, Magendantz M, Gusella JF, Tanzi RE, Solomon F. Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease-associated amyloid β protein precursor. Proc Natl Acad Sci USA. 1992;89:10758–10762. doi: 10.1073/pnas.89.22.10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White AR, Zheng H, Galatis D, Maher F, Hesse L, Multhaup G, Beyreuther K, Masters CL, Cappai R. Survival of cultured neurons from APP knockout mice against Alzheimer's amyloid Aβ toxicity and oxidative stress. J Neurosci. 1998;18:6207–6217. doi: 10.1523/JNEUROSCI.18-16-06207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whyte S, Wilson N, Currie J, Maruff P, Malone V, Shafiq-Antonacci R, Tyler P, Derry KL, Underwood J, Li Q-X, Beyruther K, Masters CL. Collection and normal levels of the amyloid precursor protein in plasma. Ann Neurol. 1997;41:121–124. doi: 10.1002/ana.410410122. [DOI] [PubMed] [Google Scholar]
- 55.Wiedaupazos M, Goto JJ, Rabizadeh S, Gralla EB, Roe JA, Lee MK, Valentine JS, Bredesen DE. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 1996;271:515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- 56.Xie CX, Mattson MP, Lovell MA, Yokel RA. Intraneuronal aluminum potentiates iron-induced oxidative stress in cultured rat hippocampal neurons. Brain Res. 1996;743:271–277. doi: 10.1016/s0006-8993(96)01055-4. [DOI] [PubMed] [Google Scholar]
- 57.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 58.Yim HS, Kang JH, Chock PB, Stadtman ER, Yim MB. A familial amyotrophic lateral sclerosis-associated A4V Cu,Zn superoxide dismutase mutant has lower Km for hydrogen peroxide. J Biol Chem. 1997;272:8861–8863. doi: 10.1074/jbc.272.14.8861. [DOI] [PubMed] [Google Scholar]
- 59.Yim MB, Kang JH, Yim HS, Kwak HS, Chock PB, Stadtman ER. A gain of function of an amyotrophic lateral sclerosis-associated Cu,Zn superoxide dismutase mutant: an enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proc Natl Acad Sci USA. 1996;93:5709–5714. doi: 10.1073/pnas.93.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng H, Jiang MH, Trumbauer ME, Sirinathsinghji DJS, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, Stevens KA, Slunt HH, Sisodia SS, Chen HY, Van der Ploeg LHT. β-amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]