Abstract
We tested the hypothesis that human CB1 cannabinoid receptors (hCB1) can sequester Gi/o-proteins from a common pool and prevent other receptors from signaling. Human CB1 cannabinoid receptors were expressed in superior cervical ganglion (SCG) neurons by microinjection of hCB1 cDNA. Expression of hCB1 cannabinoid receptors abolished the Ca2+ current inhibition by endogenous pertussis toxin-sensitive Gi/o-coupled receptors for norepinephrine (NE) and somatostatin (SOM) but not by endogenous pertussis toxin-insensitive Gs-coupled receptors for vasoactive intestinal polypeptide. Signaling by NE was rescued by expression of GαoB, Gβ1, and Gγ3. Expression of mGluR2 metabotropic glutamate receptors, another pertussis toxin-sensitive G-protein-coupled receptor, had no effect on the signaling by NE or SOM. Some hCB1 receptors were constitutively active because the cannabinoid receptor inverse agonist SR 141617A enhanced the Ca2+current. Some hCB1 receptors also appear to be precoupled to Gi/o-proteins because the cannabinoid agonist WIN 55,212–2 decreased the Ca2+ current at a time when no G-proteins were available to couple to α2-adrenergic and somatostatin receptors. In SCG neurons microinjected with a lower concentration of hCB1 cDNA, the effect of SR 141716A was reduced, and the response to NE and SOM was partially restored. Subsequent to the application of SR 141716A, the Ca2+ current inhibition by NE and SOM was abolished. These results suggest that both the active and inactive states of the hCB1 receptor can sequester Gi/o-proteins from a common pool. Cannabinoid receptors thus have the potential to prevent other Gi/o-coupled receptors from transducing their biological signals.
Keywords: calcium channels, G-proteins, cannabinoid receptor, G-protein-coupled receptors, patch clamp, ion channels, CB1, constitutively active receptors
The role of the cannabinoid receptor in brain function is thought to be related to the effects of marijuana (Cannabis sativa), which include euphoria, hypothermia, analgesia, appetite stimulation, and memory impairment. The brain CB1 cannabinoid receptor is a member of the G-protein-coupled receptor superfamily (Matsuda et al., 1990; Gérard et al., 1991). Heterologous expression studies have shown that the CB1 cannabinoid receptor inhibits adenylyl cyclase, activates an inwardly rectifying K+ channel, and inhibits N- and Q-type Ca2+ channels by coupling to pertussis toxin-sensitive G-proteins (Matsuda et al., 1990; Mackie et al., 1995;Pan et al., 1996). In hippocampal neurons, cannabinoid receptors inhibit glutamatergic synaptic transmission and Ca2+ currents and enhance the A-type K+ current (Deadwyler et al., 1993, 1995;Twitchell et al., 1997; Shen and Thayer, 1998). Acetylcholine release from the guinea pig myenteric plexus (Coutts and Pertwee, 1997) and hippocampal slice (Gifford and Ashby, 1996), GABA release from hippocampal slice (Katona et al., 1999), and norepinephrine release from sympathetic nerves has been shown to be inhibited by CB1 cannabinoid receptors (Ishac et al., 1996). Thus, the CB1 cannabinoid receptor functions to modulate neuronal excitability and neurotransmitter release.
The development of the CB1 receptor antagonist SR 141716A (Rinaldi-Carmona et al., 1994) led to the discovery that CB1 cannabinoid receptors can exist in a tonically active state. SR 141716A produced effects that were opposite those of cannabinoid agonists, indicating that SR 141716A was acting as an inverse agonist (Bouaboula et al., 1997; Landsman et al., 1997; MacLennan et al., 1998; Pan et al., 1998). We have shown that SR 141716A increased N-type Ca2+ currents in SCG neurons expressing CB1 receptors by inhibiting tonically active CB1 receptors (Pan et al., 1998). Bouaboula et al. (1997) reported that SR 141716A inhibited the activation of mitogen-activated protein kinase by the pertussis toxin-sensitive tyrosine kinase receptors insulin and insulin-like growth factor in cells transfected with hCB1 receptors. These authors hypothesized that SR 141716A converts a tonically active hCB1 receptor into an active negative state in which the receptor is coupled to a GDP-bound G-protein. Those G-proteins trapped by the inverse agonist would be unavailable to couple to other receptors.
The present study tested the hypothesis that hCB1 cannabinoid receptors can sequester G-proteins from a common pool and prevent other G-protein-coupled receptors from signaling. We predicted that hCB1 receptors would deplete a pool of pertussis toxin-sensitive G-proteins such that signaling by other pertussis toxin-sensitive G-protein-coupled receptors would be disrupted. Disruption of signaling would be confined to pertussis toxin-sensitive Gi/o-coupled receptors and not to Gs-coupled receptors. We also tested the hypothesis that the hCB1 cannabinoid receptors are capable of sequestering Gi/o-proteins in two naturally occurring receptor states, an active state coupled to Gi/o, and an inactive state precoupled to Gi/o-proteins in their inactive GDP-bound form. For precoupling to occur, the hCB1 receptor would be predicted to have a uniquely high affinity for Gi/o-proteins.
MATERIALS AND METHODS
Molecular biological procedures. The human brain cannabinoid receptor cDNA (from Dr. Tom I. Bonner, Laboratory of Cell Biology, National Institute of Mental Health, Bethesda, MD) was subcloned into the mammalian expression vector pCI (Promega, Madison, WI) as previously described (Pan et al., 1998). The metabotropic glutamate receptor mGluR2 cDNA (from Dr. Shigetada Nakanishi, Kyoto University, Kyoto, Japan) was subcloned into pCI using theSalI and NotI restriction sites. G-protein subunit cDNAs for GαoB, Gβ1, and Gγ3 (in pCIS, pCDM8.1, and pcDNA1, respectively) were obtained from Dr. Melvin Simon (Caltech, Pasadena, CA). Preparation of plasmid DNA was accomplished with a plasmid prep kit (Qiagen, Santa Clarita, CA).
Neuron preparation and microinjection. SCG neurons were enzymatically isolated from adult male Wistar rats (150–300 gm) following methods previously described (Ikeda et al., 1995) in accordance with the Committee on Animal Use for Research and Education. Neurons were allowed to attach to poly-d-lysine-coated 35 mm culture dishes for 4–5 hr before microinjection. The nuclei of single SCG neurons were microinjected with plasmids containing hCB1 cDNA, mGluR2 metabotropic glutamate receptor cDNA, or GαoB, Gβ1, and Gγ3 cDNA. Plasmids were diluted in TE buffer (10 mm Tris–HCl, pH 8, and 1 mm EDTA) to final injection concentrations of 10–200 ng/μl. The pEGFP-N1 plasmid (10 ng/μl) containing the coding sequence of the green fluorescent protein (Clontech, Palo Alto, CA) was used as a coinjection marker to identify neurons that were successfully injected. The plasmid solution was centrifuged (16,000 × g) in nonheparinized hematocrit tubes for 20 min to remove particulates. Approximately 1.5 μl of the plasmid solution was loaded into an injection pipette. Injection pipettes were pulled from fiber-filled capillary glass (1B120F-4; World Precision Instruments, Sarasota, FL) on a P-87 Flaming–Brown micropipette puller (Sutter Instrument Co., Novato, CA). The nucleus was microinjected with an Eppendorf (Madison, WI) 5242 microinjector and 5171 micromanipulator system with an injection pressure of 150–200 hPa and an injection time of 0.3–0.4 sec. Neurons that were successfully intranuclearly microinjected appeared green under fluorescent optics (Nikon Diaphot 300 and B2A filter cube; Southern Micro Instruments, Atlanta, GA) from expression of the green fluorescent protein. Approximately 30–40 neurons per dish were microinjected and, of those, <10% were successfully injected.
Electrophysiological recording and data analysis.Ca2+ currents from rat SCG neurons were recorded at room temperature (22–26°C) 16–20 hr after injection using the whole-cell variant of the patch-clamp technique (Hamill et al., 1981) with an Axopatch 200A patch-clamp amplifier (Axon Instruments, Foster City, CA). Patch electrode pipettes were pulled from borosilicate glass capillaries (Corning 7052; Garner Glass Co., Claremont, CA) on a P-87 Flaming–Brown micropipette puller (Sutter Instrument Co.). The patch electrodes were coated with Sylgard 184 (Dow Corning, Midland, MI) and fire-polished on a microforge (Narishige, Tokyo, Japan). Pipette resistances ranged from 1.8 to 3 MΩ when filled with the internal solution described below. The cell membrane capacitance and series resistance were electronically compensated to >80%. Whole-cell currents were low-pass filtered at 5 kHz using the Bessel filter of the clamp amplifier.
Voltage-clamp protocols were generated with a Power Macintosh 8600/200 computer (Apple Computer, Cupertino, CA) equipped with a PCI-16 Host Interface card connected to an ITC-16 Data Acquisition Interface (Instrutech Corp., Port Washington, NY) using Pulse Control 5.0 XOPs (Richard J. Bookman, Jack D. Herrington, and Kenneth R. Newton, University of Miami, Miami, FL) with Igor software (WaveMetrics, Lake Oswego, OR). Ca2+ currents were elicited by voltage steps from a holding potential of −80 mV and digitized at 180 μsec per point. A double pulse protocol consisting of two 25 msec steps to +5 mV was used to elicit Ca2+ currents. The first step to +5 mV elicited the control Ca2+ current. The second step to +5 mV was preceded by a 50 msec step to +80 mV. The current elicited by the second voltage step to +5 mV is facilitated compared to the control current elicited by the first voltage step. Current amplitudes were measured isochronally 10 msec after the voltage step. Figures were generated using Igor (WaveMetrics) and Excel (Microsoft, Redmond, WA) with final preparation in Canvas (Deneba Systems, Miami, FL). Results are presented as means ± SEM where appropriate. Statistical significance was determined by Student'st test. The differences were considered significant atp < 0.05.
Solutions. To isolate Ca2+ currents for whole-cell recording, cells were bathed in an external solution that contained (in mm): 140 tetraethylammonium methanesulfonate, 10 HEPES, 15 glucose, 10 CaCl2, and 0.0001 tetrodotoxin (Calbiochem, La Jolla, CA), pH 7.4 (adjusted with methanesulfonic acid). The intracellular solution consisted of (in mm): 120N-methyl-d-glucamine, 20 tetraethylammonium chloride, 10 HEPES, 11 EGTA, 1 CaCl2, 4 MgATP, 0.1 Na2GTP, and 14 phosphocreatine, pH 7.2 (adjusted with methanesulfonic acid).
Two different techniques were used to apply drugs to the patched neurons during the course of this study. Drug solutions were applied from a macropipette (20–30 μm tip diameter; type N51A glass; Garner Glass Co.) lowered into the bath. To terminate drug application, the macropipette was removed from the bath, which was superfused at 1 ml/min. To apply multiple drugs, the SF-77B Perfusion Fast-Step device (Warner Instrument Corporation, Hamden, CT) was used. This device allowed for fast switching between control or drug-containing solutions. All compounds were diluted into the external solution from concentrated stock solutions on the day of the experiment. Stock solutions of 10 mm WIN 55,212–2 mesylate (Research Biochemicals International, Natick, MA) and SR 141716A (Sanofi Recherche, Montpellier, France) were prepared in dimethylsulfoxide. WIN 55,212–2 and SR 141716A were diluted into the external solution and briefly sonicated to facilitate dispersion. The final concentration of dimethylsulfoxide was <0.01%, which had no effect on the Ca2+ current. Stock solutions of 10 mm norepinephrine (Research Biochemicals International), 1 mm(d-Trp8)-somatostatin-14 (Bachem California Inc., Torrance, CA), 1 mm vasoactive intestinal polypeptide (VIP) (Bachem California Inc.), and 10 mml-glutamate (Sigma, St. Louis, MO) were made in water. All stock solutions were stored at −20 or −50°C.
RESULTS
CB1 cannabinoid receptor expression abolished the ability of norepinephrine and somatostatin to inhibit voltage-dependent Ca2+ currents in SCG neurons
If expression of hCB1 receptors leads to sequestration of G-proteins from a common pool, then other G-protein-coupled receptors would be unable to signal because of a lack of G-proteins with which to couple. Both α2-adrenergic and somatostatin receptors have been shown to couple to pertussis toxin-sensitive G-proteins and to inhibit voltage-dependent Ca2+ currents in SCG neurons (Ikeda and Schofield, 1989; Schofield, 1990, 1991). We predicted that expression of hCB1 receptors in SCG neurons would abolish the ability of norepinephrine and somatostatin to inhibit Ca2+ currents.
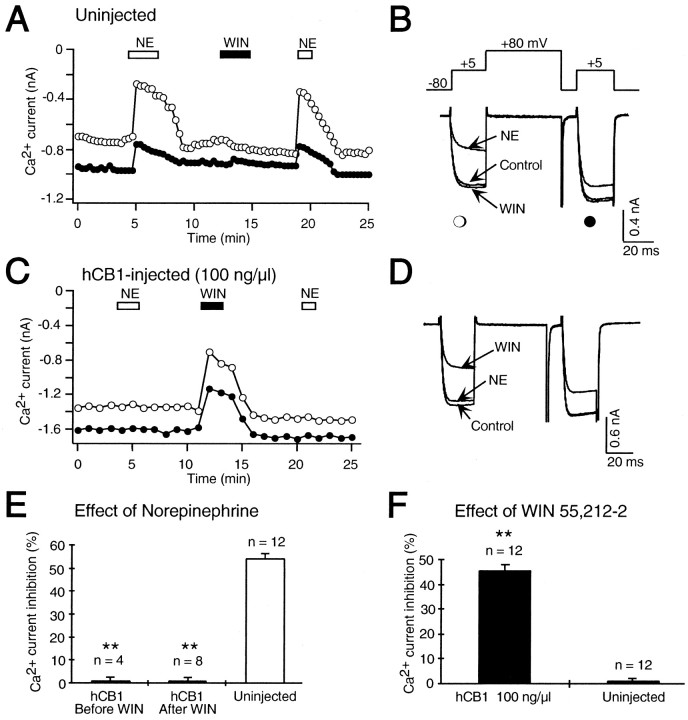
Whole-cell Ca2+ currents were recorded from control, uninjected SCG neurons. Figure1A illustrates the time course of the effect of the cannabinoid receptor agonist WIN 55,212–2 and NE on both control and facilitated Ca2+ currents elicited by a double pulse protocol (Fig. 1B). Application of NE (10 μm) inhibited both control and facilitated Ca2+ currents (Fig.1A,B). Application of WIN 55,212–2 had no effect on the Ca2+ current in this uninjected SCG neuron. Rat SCG neurons have been shown to contain CB1 mRNA as detected by the reverse transcription-PCR (Ishac et al., 1996). However, our experiments could not detect cannabinoid receptor modulation of Ca2+ currents from the soma of rat SCG neurons in culture. Because cannabinoid agonists inhibited the release of noradrenaline from sympathetic nerves innervating isolated rat atria (Ishac et al., 1996), CB1 receptors may be specifically localized in SCG neuronal terminals. Terminal localization of CB1 receptors has recently been shown in GABAergic hippocampal interneurons (Katona et al., 1999).
Fig. 1.
Expression of hCB1 cannabinoid receptors abolished inhibition of Ca2+ currents by norepinephrine (NE) in SCG neurons. A, A double pulse protocol (B, inset) was used to elicit control (open circle) and facilitated (filled circle) Ca2+ currents in a control, uninjected SCG neuron. The double pulse protocol was repeated every 10 sec, and the current amplitudes were plotted over the time course of the experiment. Application of 10 μmnorepinephrine (NE, open bar) reversibly decreased the Ca2+ current. A subsequent application of 1 μm WIN 55,212–2, the cannabinoid receptor agonist (WIN, filled bar), had no effect. A second application of norepinephrine again decreased the Ca2+ current.B, Superimposed current traces elicited by the double pulse protocol (top) from the same cell as shown inA in the absence (Control) and presence of norepinephrine (NE) and WIN 55,212–2 (WIN). The first voltage step to +5 mV elicited the control Ca2+ current (open circle), and the second step to +5 mV, which was preceded by a +80 mV step, elicited the facilitated (filled circle) Ca2+ current. C, In an SCG neuron previously microinjected with 100 ng/μl hCB1, cannabinoid receptor cDNA application of 10 μmnorepinephrine (NE, open bar) had no effect on the Ca2+ current. A subsequent application of 1 μm WIN 55,212–2 (WIN, filled bar) inhibited the Ca2+ current, which slowly recovered after washout. A second application of norepinephrine also had no effect on the Ca2+ current. D,Superimposed current traces from the same cell as shown inC in the absence (Control) and presence of WIN 55,212–2 (WIN) and norepinephrine (NE). E, Bar graph of control Ca2+ current inhibition by 10 μm norepinephrine in uninjected SCG neurons (Uninjected) and in SCG neurons microinjected with 100 ng/μl hCB1 cDNA either before (hCB1 Before WIN) or after (hCB1 After WIN) the application of 1 μm WIN 55,212–2. The effect of norepinephrine was significantly decreased (**p < 0.001) in SCG neurons microinjected with hCB1 cDNA. The number of neurons tested is indicated. F, Bar graph of control Ca2+ current inhibition by 1 μm WIN 55,212–2 in uninjected SCG neurons (Uninjected) and in SCG neurons microinjected with 100 ng/μl hCB1 cDNA (hCB1 100 ng/μl). WIN 55,212–2 significantly inhibited (**p < 0.001) the Ca2+ current in neurons microinjected with hCB1 cDNA compared to uninjected neurons. The number of neurons tested is indicated.
To express cannabinoid receptors we microinjected 100 ng/μl hCB1 cannabinoid receptor cDNA directly into the nucleus of SCG neurons. Application of the cannabinoid receptor agonist WIN 55,212–2 inhibited the Ca2+ current in this SCG neuron injected with hCB1 cDNA (Fig. 1C,D). WIN 55,212–2 inhibited the Ca2+ current 45.5 ± 2.4% (n = 12; p < 0.001) in SCG neurons microinjected with hCB1 receptor cDNA compared with 1.1 ± 0.6% (n = 12) in uninjected neurons (Fig.1F). Thus, microinjection of plasmids directly into the nucleus leads to expression of hCB1 receptors.
To determine whether expression of hCB1 receptors could abolish signaling by α2-adrenergic receptors, SCG neurons microinjected with hCB1 cDNA were challenged with NE. NE (10 μm) had no effect on the Ca2+ current in the SCG neuron microinjected with 100 ng/μl hCB1 cDNA (Fig.1C,D). In summary, NE (10 μm) decreased the Ca2+ current 54.7 ± 1.6% (n = 12) in uninjected neurons (Fig.1E). In contrast, NE had no effect on the Ca2+ current in neurons microinjected with 100 ng/μl hCB1 cDNA (Fig. 1E) either before (0.7 ± 1.2%; n = 4; p < 0.001) or after (0.8 ± 1.3%; n = 8; p< 0.001) the application of WIN 55,212–2.
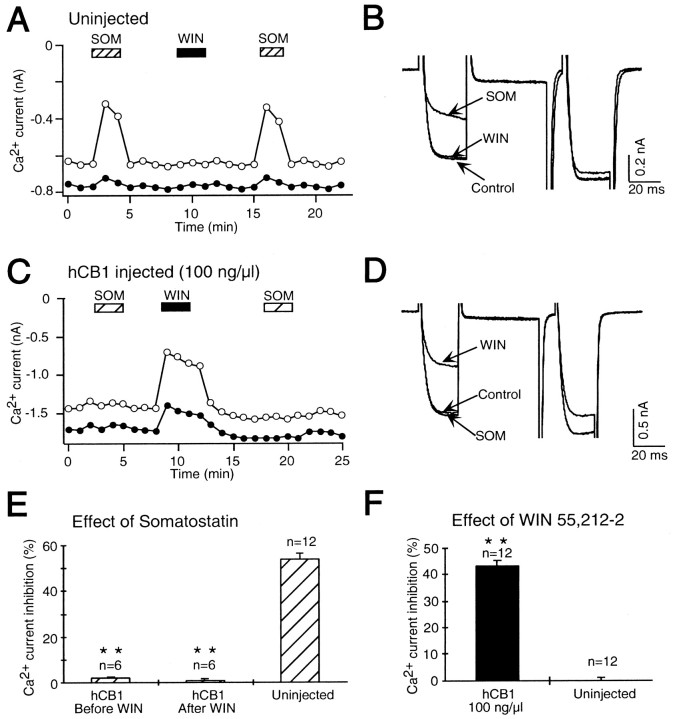
The effect of somatostatin was also inhibited by expression of hCB1 receptors. Figure 2Aillustrates the time course of the effect of SOM and WIN 55,212–2 on Ca2+ currents in a control, uninjected SCG neuron. Application of SOM (0.1 μm) inhibited the Ca2+ current, but WIN 55,212–2 (1 μm) had no effect (Fig.2A,B). In SCG neurons microinjected with 100 ng/μl hCB1 cDNA, WIN 55,212–2 inhibited the Ca2+ current, but SOM had no effect (Fig.2C,D). SOM (0.1 μm) inhibited the Ca2+ current in control, uninjected neurons 53.9 ± 1.3% (n = 12) (Fig.2E). In contrast, SOM had no significant effect in neurons microinjected with 100 ng/μl hCB1 cDNA either before (2.0 ± 0.4%; n = 6; p < 0.001) or after (0.9 ± 0.8%; n = 6; p< 0.001) the application of WIN 55,212–2. The hCB1 receptor was expressed in these neurons because WIN 55,212–2 inhibited the Ca2+ current 43.5 ± 2.0% (n = 12; p < 0.001) compared to uninjected neurons (0.1 ± 0.5%; n = 12) (Fig.2F). These results demonstrate that expression of the hCB1 receptor abolished the signaling by endogenous receptors for both NE and SOM in SCG neurons.
Fig. 2.
Expression of hCB1 cannabinoid receptors abolished inhibition of the Ca2+ current by somatostatin (SOM) in SCG neurons.A, Application of 0.1 μm somatostatin (SOM, hatched bar) reversibly decreased the Ca2+ current in an uninjected SCG neuron. A subsequent application of 1 μm WIN 55,212–2 (filled bar) had no effect. A second application of somatostatin again decreased the Ca2+ current.B, Superimposed current traces from the same cell as shown in A in the absence (Control) and presence of somatostatin (SOM) and WIN 55,212–2 (WIN). C, In an SCG neuron previously microinjected with 100 ng/μl hCB1 cannabinoid receptor cDNA, application of 0.1 μm somatostatin (hatched bar) had no effect on the Ca2+ current. A subsequent application of 1 μm WIN 55,212–2 (filled bar) inhibited the Ca2+ current. A second application of somatostatin also had no effect on the Ca2+ current.D, Superimposed current traces from the same cell as shown in C in the absence (Control) and presence of WIN 55,212–2 (WIN) and somatostatin (SOM). E, Bar graph of control Ca2+ current inhibition by somatostatin in uninjected SCG neurons (Uninjected) and in SCG neurons microinjected with 100 ng/μl hCB1 cDNA either before (hCB1 Before WIN) or after (hCB1 After WIN) the application of 1 μm WIN 55,212–2. The effect of somatostatin was significantly decreased (**p < 0.001) in SCG neurons microinjected with hCB1 cDNA. The number of neurons tested is indicated. F,Bar graph of control Ca2+ current inhibition by 1 μm WIN 55,212–2 in uninjected SCG neurons (Uninjected) and in SCG neurons microinjected with 100 ng/μl hCB1 cDNA (hCB1 100 ng/μl). The cannabinoid receptor agonist WIN 55,212–2 significantly inhibited (**p < 0.001) the Ca2+ current in neurons microinjected with hCB1 cDNA when compared to uninjected neurons. The number of neurons tested is indicated.
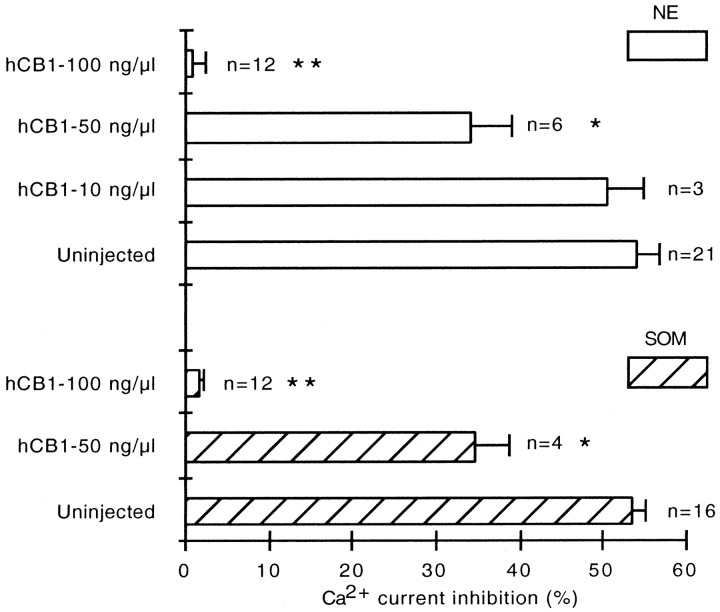
Inhibition of norepinephrine and somatostatin receptor signaling by expression of the hCB1 receptor depended on the concentration of hCB1 cDNA microinjected
To determine whether NE and SOM signal disruption depended on the abundance of hCB1 receptors, different concentrations of hCB1 cDNA were microinjected. In control, uninjected neurons, NE (10 μm) inhibited the Ca2+ current 54.0 ± 2.6% (n = 21) (Fig. 3). Microinjection of 10 ng/μl of hCB1 cDNA had no effect on the inhibition of the Ca2+ current by NE (50.4 ± 4.3%; n = 3). The effect of NE was significantly decreased (34.1 ± 4.9%; n = 6;p < 0.05) in SCG neurons microinjected with 50 ng/μl of hCB1 cDNA. The response to NE was completely abolished in neurons microinjected with 100 ng/μl hCB1 cDNA (0.8 ± 1.3%;n = 12; p < 0.001).
Fig. 3.
Dose-dependent effect of hCB1 cDNA on signaling by receptors for norepinephrine and somatostatin. Bar graph of control Ca2+ current inhibition by norepinephrine (NE, open bars) and somatostatin (SOM, diagonal bars) in uninjected SCG neurons (Uninjected) and in neurons microinjected with 10, 50, and 100 ng/μl hCB1 cDNA (hCB1). Microinjection of 50 ng/μl hCB1 cDNA significantly reduced (*p < 0.05) the inhibition of the Ca2+ current by both norepinephrine and somatostatin. Microinjection of 100 ng/μl hCB1 cDNA abolished (**p < 0.001) the inhibition of the Ca2+ current by both norepinephrine and somatostatin. The number of neurons tested is indicated.
SOM (0.1 μm) inhibited the Ca2+ current 53.5 ± 1.5% (n = 16) in uninjected neurons (Fig. 3). In SCG neurons microinjected with 50 ng/μl hCB1 cDNA, SOM inhibited the Ca2+ current 34.7 ± 3.9% (n = 4; p < 0.05). The response to SOM was abolished in neurons microinjected with 100 ng/μl hCB1 cDNA (1.5 ± 0.6%; n = 12; p < 0.001).
All neurons microinjected with hCB1 cDNA were checked for hCB1 receptor expression by measuring the effect of WIN 55,212–2 on the Ca2+ current. Receptor expression was low in SCG neurons microinjected with 10 ng/μl hCB1 cDNA, as indicated by a 12.9 ± 1.6% (n = 3) decrease in the Ca2+ current in response to 1 μm WIN 55,212–2. The effect of WIN 55,212–2 was similar in SCG neurons microinjected with 50 or 100 ng/μl hCB1 cDNA. WIN 55,212–2 decreased the Ca2+current 44.7 ± 1.3% (n = 10) in neurons microinjected with 50 ng/μl hCB1 cDNA and 44.5 ± 2.2% (n = 24) in neurons microinjected with 100 ng/μl hCB1 cDNA. WIN 55,212–2 had no effect (0.8 ± 0.6%; n= 34) in uninjected neurons.
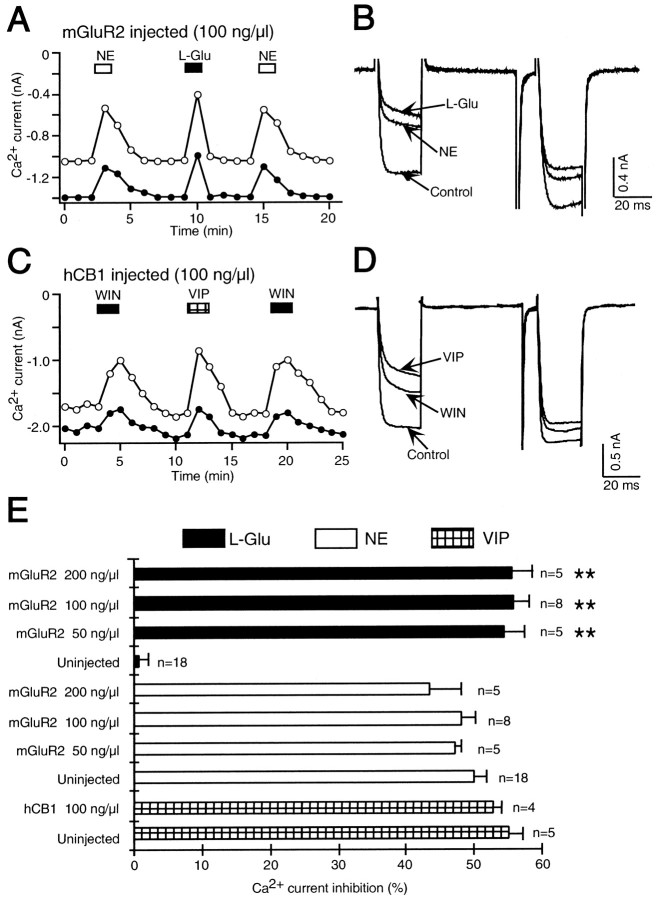
Heterologous expression of the mGluR2 metabotropic glutamate receptor had no effect on adrenergic and somatostatin signaling
To determine whether the block of adrenergic and somatostatin signaling is specific to expression of hCB1 receptors, SCG neurons were microinjected with mGluR2 metabotropic glutamate receptor cDNA. Heterologous expression of mGluR2 receptors has previously been shown to inhibit Ca2+ currents in SCG neurons by coupling to pertussis toxin-sensitive G-proteins (Ikeda et al., 1995).
In control, uninjected neurons l-glutamate had no effect on the Ca2+ current (1.6 ± 1.3%;n = 18), confirming the absence of endogenous metabotropic glutamate receptors (Fig.4E). In an SCG neuron microinjected with 100 ng/μl mGluR2 cDNA, NE (Fig.4A,B) reversibly reduced the Ca2+ current. A subsequent application ofl-glutamate (100 μm) also reversibly decreased the Ca2+ current demonstrating mGluR2 receptor expression. A second application of NE had an effect similar to the first application. In summary, NE (10 μm) reduced the Ca2+ current 48.0 ± 2.2% (n = 8) in neurons microinjected with 100 ng/μl mGluR2 cDNA (Fig. 4E), which was no different from the effect of NE (49.9 ± 1.9%; n = 18) in uninjected neurons. We also tested the effect of NE in neurons microinjected with 50 ng/μl mGluR2 cDNA. In these neurons NE inhibited the Ca2+ current 47.2 ± 0.9% (n = 5). l-glutamate decreased the Ca2+ current 55.7 ± 2.4% (n = 8) in neurons microinjected with 100 ng/μl mGluR2 cDNA and 54.5 ± 2.9% (n = 5) in neurons microinjected with 50 ng/μl mGluR2 cDNA, demonstrating mGluR2 receptor expression at both cDNA concentrations (Fig.4E).
Fig. 4.
The hCB1 cannabinoid receptor specifically disrupts signaling by Gi/o-coupled receptors and is not mimicked by expression of the mGluR2 metabotropic glutamate receptor. A, Expression of the mGluR2 metabotropic glutamate receptor, a pertussis toxin-sensitive G-protein-coupled receptor, did not alter the signaling of the receptor for norepinephrine. Application of 10 μm norepinephrine (NE, open bar) inhibited the Ca2+current in an SCG neuron microinjected with mGluR2 metabotropic glutamate receptor cDNA (100 ng/μl). A subsequent application of 100 μml-glutamate (L-Glu, filled bar) reversibly inhibited the Ca2+ current. A second application of norepinephrine also inhibited the Ca2+ current. B, Superimposed current traces from the same cell as shown in A in the absence (Control) and presence of l-glutamate (L-Glu) and norepinephrine (NE).C, Expression of the hCB1 cannabinoid receptor failed to affect signaling through the Gs-coupled receptor for vasoactive intestinal polypeptide (VIP). Application of 1 μm WIN 55,212–2 (WIN, filled bar) inhibited the Ca2+ current in an SCG neuron microinjected with hCB1 cDNA (100 ng/μl). A subsequent application of 10 μm VIP (hatched bar) reversibly inhibited the Ca2+ current. A second application of WIN 55,212–2 again inhibited the Ca2+ current.D, Superimposed current traces from the same cell as shown in C in the absence (Control) and presence of 10 μm VIP (VIP) and 1 μm WIN 55,212–2 (WIN). E, Bar graph of the inhibition of the control Ca2+ current byl-glutamate (filled bars), norepinephrine (open bars), and VIP (hatched bars) in uninjected SCG neurons (Uninjected), in neurons microinjected with 50, 100, or 200 ng/μl mGluR2 cDNA (mGluR2) and in neurons microinjected with hCB1 cDNA (hCB1). Uninjected neurons have no endogenous mGluR2 receptors and l-glutamate had no effect on the Ca2+ current (filled bar, Uninjected). In contrast, in neurons microinjected with 50, 100, or 200 ng/μl mGluR2 cDNA l-glutamate significantly inhibited the Ca2+ current (**p < 0.001, filled bars,mGluR2). The effect of norepinephrine on the Ca2+ current was no different in neurons microinjected with 50, 100, or 200 ng/μl mGluR2 cDNA (open bars, mGluR2) than in uninjected neurons (open bar, Uninjected). VIP inhibited the Ca2+ current in both uninjected neurons (hatched bar, Uninjected) and in neurons microinjected with 100 ng/μl hCB1 cDNA (hatched bar, hCB1 100 ng/μl). The number of neurons tested is indicated.
There was also no difference in the response to SOM in control, uninjected neurons compared to those microinjected with mGluR2 cDNA. In uninjected neurons, SOM (0.1 μm) reduced the Ca2+ current 51.4 ± 0.1% (n = 11). In neurons microinjected with 50 and 100 ng/μl mGluR2 cDNA, SOM inhibited the Ca2+ current 50.0 ± 1.4% (n = 5) and 50.5 ± 1.1% (n = 6), respectively (data not shown).
As a further control, mGluR2 cDNA was microinjected at twice the concentration of hCB1 receptor cDNA. In neurons microinjected with 200 ng/μl mGluR2 cDNA, NE decreased the Ca2+current 43.5 ± 4.6% (n = 5) (Fig.4E), which was no different from the NE-induced decrease of 48.8 ± 1.3% (n = 5) in uninjected neurons recorded on the same day. l-glutamate (100 μm) inhibited the Ca2+ current 55.6 ± 2.0% (n = 5) in the neurons microinjected with 200 ng/μl mGluR2 cDNA. Thus, heterologous expression of the mGluR2 metabotropic glutamate receptor had no effect on the signaling of endogenous NE and SOM receptors.
Heterologous expression of the hCB1 receptor had no effect on the signaling of a Gs-coupled receptor
To determine if expression of hCB1 receptors specifically disrupted signaling of pertussis toxin-sensitive G-protein-coupled receptors we looked for disruption of signaling by an endogenous Gs coupled receptor. VIP acts on endogenous receptors coupled to Gs in SCG neurons to inhibit the Ca2+ current (Zhu and Ikeda, 1994). In an SCG neuron microinjected with 100 ng/μl hCB1 cDNA, WIN 55,212–2 (Fig. 4C,D) reversibly reduced the Ca2+ current, demonstrating hCB1 receptor expression. A subsequent application of VIP (10 μm) also reversibly decreased the Ca2+ current. A second application of WIN 55,212–2 had an effect similar to the first application. In neurons microinjected with hCB1 cDNA, WIN 55,212–2 reduced the Ca2+ current 43.6 ± 1.7% (n = 4), and VIP decreased the Ca2+ current 53.0 ± 1.1% (n = 4) (Fig. 4E). In uninjected neurons, the effect of VIP was similar. VIP inhibited the Ca2+ current 55.2 ± 2.0% (n = 5). These results demonstrate that hCB1 receptor expression had no effect on signaling by an endogenous receptor coupled to the pertussis toxin-insensitive G-protein Gs.
The cannabinoid receptor inverse agonist SR 141716A trapped the hCB1 in an inactive state and sequestered G-proteins from a common pool
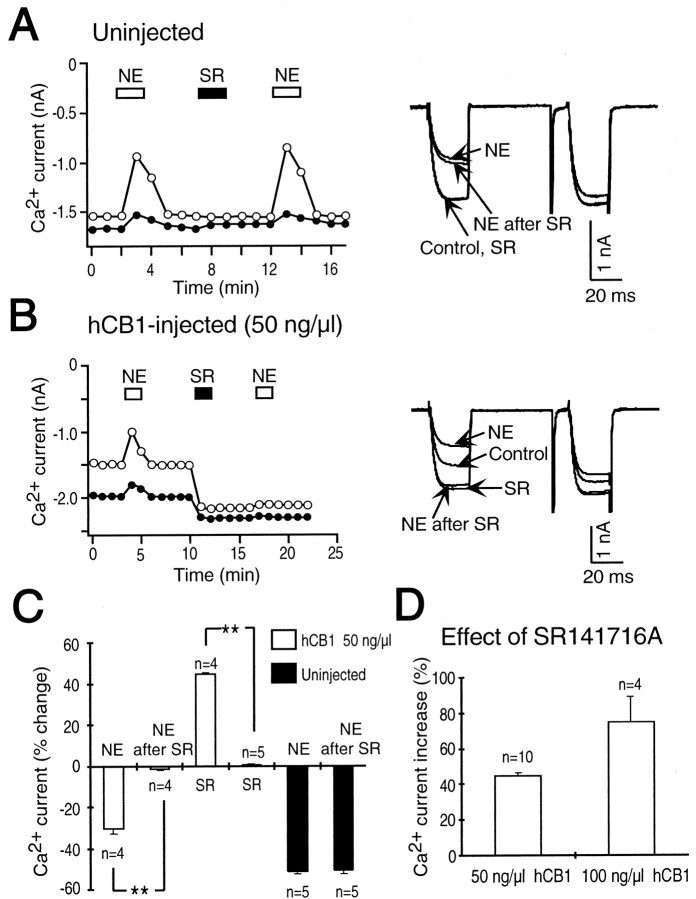
In SCG neurons microinjected with hCB1 cDNA, the cannabinoid receptor inverse agonist SR 141716A increased the Ca2+ current, an effect opposite that of the cannabinoid agonist WIN 55,212–2 (Pan et al., 1998). An increase in the Ca2+ current occurred as SR 141716A relieved a tonic inhibition of the Ca2+current caused by tonically active hCB1 receptors. Because an inverse agonist has higher affinity for the inactive state of the receptor, it will shift the balance to the inactive state and reverse the effects of a tonically active receptor. If SR 141716A can trap the hCB1 receptor in its inactive state together with its associated G-protein as proposed by Bouaboula et al. (1997), then this entrapment could sequester a common pool of G-proteins. To test whether G-proteins associated with receptors for NE or SOM could be sequestered by the hCB1 receptor trapped in its inactive state by the inverse agonist SR 141716A, experiments were performed to test NE and SOM both before and after application of SR 141716A. Figure5A shows the time course of the effect of NE and SR 141716A on the Ca2+ current in a control, uninjected neuron. NE (10 μm) reduced the Ca2+ current, but SR 141716A (1 μm) had no effect. A second application of NE inhibited the Ca2+ current. Neurons microinjected with 50 ng/μl hCB1 cDNA were tested with NE before and after application of the cannabinoid receptor inverse agonist SR 141716A. The first application of NE reversibly reduced the Ca2+ current. Application of SR 141716A increased the Ca2+ current, which remained elevated even after superfusion with external solution. A subsequent application of NE had no effect on the Ca2+ current. In SCG neurons microinjected with 50 ng/μl hCB1 cDNA, the first application of NE reduced the Ca2+ current 30.2 ± 2.6% (n = 4) (Fig. 5C), and SR 141716A increased the Ca2+ current 44.4 ± 0.9% (n = 4). In contrast, NE applied after SR 141716A had no significant effect on the Ca2+ current (1.8 ± 0.6%; n = 4; p < 0.001). In uninjected neurons, the first application of NE blocked the Ca2+ current 51.3 ± 1.3% (n = 5) (Fig. 5C), SR 141716A had no effect (0.4 ± 0.6%; n = 5), and the second application of NE inhibited the Ca2+ current 50.9 ± 1.7% (n = 5). Similar results were obtained for SOM in SCG neurons microinjected with 50 ng/μl hCB1 cDNA. The first application of SOM (0.1 μm) reduced the Ca2+ current 35.0 ± 2.5% (n = 6), SR 141716A increased the Ca2+ current 45.3 ± 0.7% (n = 6), and the second application of SOM had no effect on the Ca2+ current (1.7 ± 0.2%; n = 6; p < 0.001). In control, uninjected neurons the first application of SOM reduced the Ca2+ current 54.2 ± 1.0% (n = 6), SR 141716A had no effect on the Ca2+ current (0.7 ± 0.9%;n = 6), and the second application of SOM inhibited the Ca2+ current 51.2 ± 1.3% (n = 6) (data not shown). These results indicate that the inverse agonist-trapped inactive state of the hCB1 receptor could sequester G-proteins that were previously available to couple to α2-adrenergic and somatostatin receptors.
Fig. 5.
The cannabinoid receptor inverse agonist SR 141716A blocks the effect of norepinephrine on the Ca2+ current. A, Application of 10 μm norepinephrine (NE, open bar) inhibited the Ca2+ current in an uninjected SCG neuron. A subsequent application of 1 μm SR 141716A (SR, filled bar) had no effect on the Ca2+current. A second application of norepinephrine again inhibited the Ca2+ current. Right, Superimposed current traces in the absence (Control) and in the presence of norepinephrine (NE), SR 141716A (SR), and again in the presence of norepinephrine (NE after SR). B, In an SCG neuron microinjected with 50 ng/μl hCB1 cDNA, norepinephrine (NE, open bar) reversibly decreased the Ca2+current. A subsequent application of 1 μm SR 141716A (SR, filled bar) increased both the control (open circle) and facilitated (filled circle) Ca2+ current. The Ca2+ current remained enhanced after superfusion with external solution. A subsequent application of norepinephrine now failed to inhibit the Ca2+ current. Right, Superimposed current traces in the absence (Control) and presence of the first application of norepinephrine (NE), SR 141716A (SR), and the second application of norepinephrine (NE after SR).C, Summary of the changes in the control Ca2+ current amplitude in uninjected SCG neurons (filled bars) and in neurons microinjected with 50 ng/μl hCB1 cDNA (open bars). The effect of norepinephrine (NE, open bar) was abolished (**p < 0.001) after the application of SR 141716A (NE after SR, open bar). SR 141716A significantly increased (**p < 0.001) the Ca2+ current in neurons microinjected with 50 ng/μl hCB1 cDNA (SR, open bar) compared to uninjected SCG neurons (SR, filled bar). SR 141716A (NE after SR, filled bar) had no effect on the Ca2+ current inhibition by norepinephrine (NE, filled bar) in uninjected neurons. Number of neurons tested is indicated. D, SR 141716A increased the control Ca2+ current to a greater extent in SCG neurons microinjected with 100 ng/μl hCB1 cDNA than in neurons microinjected with 50 ng/μl hCB1 cDNA. The number of neurons tested is indicated.
The abundance of tonically active hCB1 receptors depended on the concentration of hCB1 cDNA microinjected
Disruption of NE and SOM signaling by expression of hCB1 receptors depended on the concentration of hCB1 cDNA microinjected. In neurons microinjected with 100 ng/μl hCB1 cDNA, signaling by NE and SOM was abolished, but in neurons microinjected with 50 ng/μl hCB1 cDNA, signaling by NE and SOM was reduced (Fig. 3). We hypothesized that the tonically active state of the hCB1 receptor was responsible for sequestering G-proteins and abolishing the effect of NE and SOM. If this hypothesis is correct, then there should be more tonically active receptors in neurons microinjected with 100 ng/μl hCB1 cDNA, and the effect of the inverse agonist SR 141716A should be greater than in neurons microinjected with 50 ng/μl hCB1 cDNA. In neurons microinjected with 50 ng/μl hCB1 cDNA, SR 141716A (1 μm) increased the Ca2+current 44.9 ± 0.8% (n = 10) (Fig.5D). In SCG neurons microinjected with 100 ng/μl hCB1 cDNA, SR 141716A increased the Ca2+current 75.1 ± 13.3% (n = 4) (Fig.5D). Thus, there are more tonically active hCB1 receptors in SCG neurons microinjected with 100 ng/μl hCB1 cDNA than in neurons microinejcted with 50 ng/μl hCB1 cDNA. These results lend support to the idea that the tonically active state of the hCB1 receptor can sequester G-proteins. However, not all hCB1 receptors are tonically active because the cannabinoid agonist WIN 55,212–2 can still decrease the Ca2+ current. These results suggest that hCB1 receptors have a high affinity for a limited pool of Gi/o-proteins in their GDP-bound form. The hCB1 receptor interconverts between an inactive state coupled to Gi/o in its GDP-bound form and an active state coupled to Gi/o in its GTP-bound form. The agonist stabilizes the receptor in its active state coupled to Gi/o in its GTP-bound form.
Rescue of NE effects by G-proteins
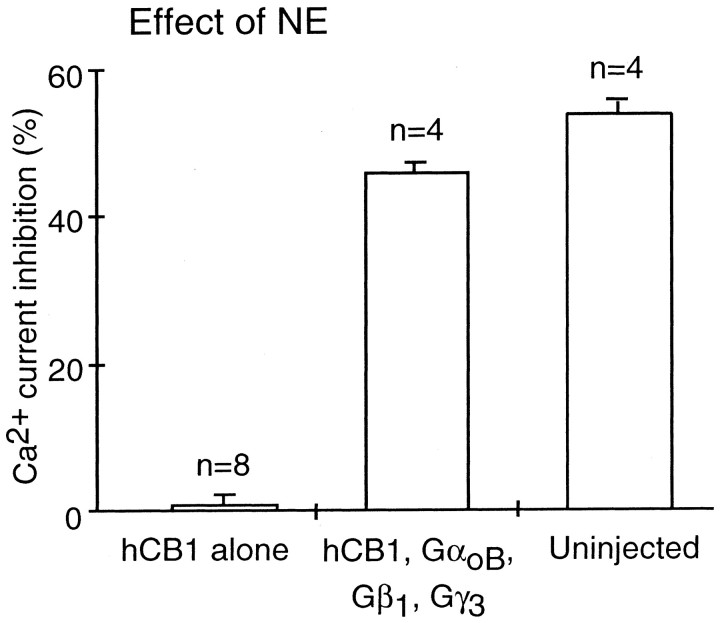
We hypothesized that we could rescue NE signaling in neurons expressing hCB1 receptors by expression of Gi/o-proteins. To test this hypothesis, nuclei of individual SCG neurons were coinjected with 100 ng/μl each of hCB1, GαoB, Gβ1, and Gγ3 cDNAs and 10 ng/μl green fluorescent protein cDNA. NE decreased the Ca2+current in control, uninjected neurons 55.4 ± 1.8% (n = 4). The effect of NE was abolished in neurons injected with hCB1 cDNA (0.8 ± 1.3%; n = 8). In neurons coinjected with hCB1, GαoB, Gβ1, and Gγ3 cDNAs WIN 55,212–2 (1 μm) inhibited the Ca2+ current 45.0 ± 2.0% (n = 4), and the effect of NE (10 μm) was restored to 46.0 ± 2.4% (n = 4) (Fig. 6).
Fig. 6.
Expression of G-protein subunits rescues the effect of NE in cells expressing hCB1 receptors. Bar graph of control Ca2+ current inhibition by 10 μmnorepinephrine in uninjected SCG neurons (Uninjected), in SCG neurons injected with hCB1 cDNA (hCB1), and in SCG neurons coinjected with hCB1, GαoB, Gβ1, and Gγ3 cDNA (hCB1, GαoB, Gβ1, Gγ3). The effect of norepinephrine was rescued in hCB1 expressing neurons after coexpression of GαoB, Gβ1, and Gγ3.
DISCUSSION
We found that expression of hCB1 cannabinoid receptors can sequester Gi/o-proteins and prevent signaling by α2-adrenergic and somatostatin receptors. Sequestration of G-proteins could be rescued by expression of G-protein subunits, GαoB, Gβ1, and Gγ3. G-protein sequestration is specific to hCB1 because expression of another Gi/o-coupled receptor, the mGluR2 metabotropic glutamate receptor, failed to have a similar effect. Expression of mGluR2 metabotropic glutamate receptors also indicates that it is unlikely that expression of hCB1 receptors alters the expression of α2-adrenergic or somatostatin receptors. Additionally, hCB1 receptors failed to alter the signaling of VIP receptors that couple to Gs-proteins (Zhu and Ikeda, 1994). Taken together, our results demonstrate that the ability to deplete a common pool of G-proteins is a property of hCB1 cannabinoid receptors and that sequestration of G-proteins is specific to the pertussis toxin-sensitive Gi/o-proteins.
Although it is possible that the complement of G-proteins expressed in SCG neurons may differ from central neurons, both SCG neurons expressing hCB1 receptors and hippocampal neurons with native CB1 receptors elicit a Ca2+ current inhibition that is pertussis toxin-sensitive (Pan et al., 1996; Twitchell et al., 1997). The precise complement, quantity, and localization of Gi/o-proteins in hippocampal neurons are unknown, whereas immunoblot analysis has indicated the presence of Gαo in SCG neurons (Caulfield et al., 1994). It remains possible that the quantity of Gi/o-proteins in central neurons might differ from those in SCG neurons to such an extent that sequestration of Gi/o-proteins by the CB1 cannabinoid receptor does not occur at all or to the same extent.
We have previously reported that SR 141716A acts as an inverse agonist in SCG neurons expressing hCB1 receptors (Pan et al., 1998). In the present study we found a greater effect of SR 141716A with increasing concentrations of hCB1 cDNA. SR 141716A increased the Ca2+ current 45% in neurons microinjected with 50 ng/μl hCB1 cDNA and 75% with 100 ng/μl hCB1 cDNA. Thus, the magnitude of the effect of SR 141716A depends on the abundance of tonically active hCB1 receptors, which is increased with increasing cDNA concentrations. If it is this tonically active state of the hCB1 receptor that can sequester G-proteins, then interference with norepinephrine and somatostatin signaling should depend on the concentration of hCB1 cDNA injected.
In SCG neurons microinjected with 100 ng/μl hCB1 cDNA, the inhibition of Ca2+ currents by norepinephrine and somatostatin was completely abolished; whereas, in neurons microinjected with 50 ng/μl hCB1 cDNA the response to norepinephrine and somatostatin was significantly reduced, but not abolished. These results indicate that hCB1 receptors can disrupt signaling by other pertussis toxin-sensitive G-protein-coupled receptors and that the magnitude of this disruption depends on the abundance of hCB1 receptors.
Felder et al. (1995) reported that the effects of oxotremorine-M and somatostatin on activation of an inwardly rectifying K+ current were abolished in cells expressing 7.6 pmol/mg protein of the CB2 cannabinoid receptor but were unaffected when the density was 189 fmol/mg protein. In cells expressing 800 fmol/mg protein of the CB1 receptor both WIN 55,212–2 and oxotremorine-M inhibited the Ca2+current. These results indicate that CB2 receptors at 7.6 pmol/mg protein can disrupt signaling by other G-protein-coupled receptors, but that CB1 receptors at 800 fmol/mg protein do not disrupt signaling.Bouaboula et al. (1997) found SR 141716A to be an inverse agonist that could sequester Gi/o-proteins in cells expressing hCB1 receptors at a density of 2 pmol/mg protein. Landsman et al. (1997) also found tonically active hCB1 receptors in cells expressing 2.6 pmol/mg protein hCB1 receptors. Although the density of hCB1 receptors expressed in SCG neurons in our study is unknown, hCB1 receptors were tonically active in all neurons that showed a significant inhibition of signaling by other Gi/o-coupled receptors. Thus, it is reasonable to expect that hCB1 receptor densities greater than or equal to 2 pmol/mg protein would disrupt signaling by Gi/o-coupled receptors. In vivo, CB1 cannabinoid receptors are predominantly expressed in the substantia nigra, globus pallidus, olfactory bulb, cerebellum, and hippocampus (Herkenham et al., 1990,1991a,b; Matsuda et al., 1993; Tsou et al., 1998). Cannabinoid receptor density in the substantia nigra of the rat brain was 6.3 pmol/mg protein, and in the hippocampal dentate gyrus molecular layer, the density was 4.1 pmol/mg (Herkenham et al., 1991a). Thus, neurons in the substantia nigra and hippocampus contain sufficiently high levels of CB1 receptors to sequester Gi/o and disrupt signaling by Gi/o-coupled receptors.
Bouaboula et al. (1997) reported that SR 141716A blocked the stimulation of mitogen-activated protein kinase by the pertussis toxin-sensitive receptor-tyrosine kinases insulin and insulin-like growth factor 1. They hypothesized that SR 141716A converted a tonically active hCB1 receptor to a suppressor receptor coupled to GGDP. To determine whether the inverse agonist-trapped inactive state could sequester Gi/o-proteins from a common pool, we tested SCG neurons microinjected with 50 ng/μl hCB1 cDNA. In neurons microinjected with this concentration of hCB1 cDNA, norepinephrine inhibited the Ca2+ current by 30%. The effect of norepinephrine was subsequently abolished after application of the CB1 cannabinoid receptor inverse agonist SR 141716A. The Gi/o-proteins initially available to couple to the α2-adrenergic receptor were, within minutes, no longer available. Similar results were obtained for somatostatin. Our results demonstrate that Gi/o-proteins are able to move freely between the α2-adrenergic or somatostatin receptors and the hCB1 cannabinoid receptors. SR 141716A stabilizes the inactive state, and the hCB1 receptors accumulate in this state. The successful competition of hCB1 receptors for Gi/o-proteins suggests that hCB1 receptors in their inactive state have a higher affinity for Gi/o-proteins than α2-adrenergic or somatotstatin receptors. Stabilization by SR 141716A of inactive hCB1 receptors precoupled to Gi/o blocked access to Gi/oby α2-adrenergic or somatostatin receptors.
In neurons microinjected with 100 ng/μl hCB1 cDNA the response to either norepinephrine or somatostatin was completely abolished, suggesting that Gi/o-proteins were unavailable to interact with these receptors. However, the cannabinoid agonist WIN 55,212–2 inhibited the Ca2+ current 45%, indicating that a population of hCB1 receptors were originally in an inactive state. Gi/o-proteins were available to couple to hCB1 receptors but not to α2-adrenergic or somatostatin receptors. These results indicate that hCB1 receptors also exist in an inactive state precoupled to Gi/o-proteins and that hCB1 receptors must have a greater affinity for Gi/o-proteins than α2-adrenergic and somatostatin receptors. Cannabinoid receptors also have a greater affinity for Gi/o-proteins than mGluR2 metabotropic glutamate receptors whose expression did not affect signaling by α2-adrenergic and somatotstatin receptors.
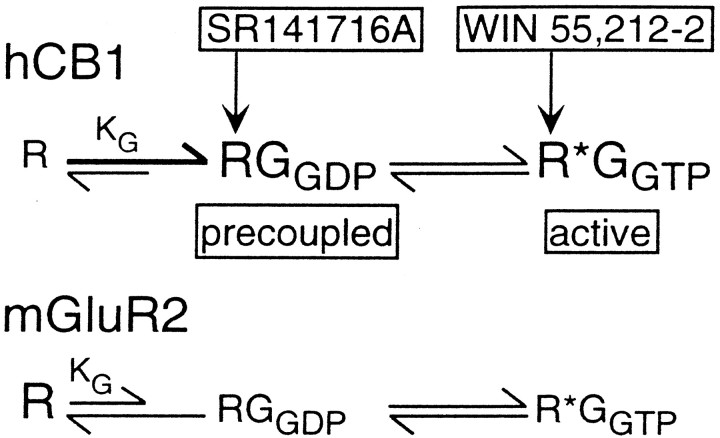
Our results are consistent with a model in which hCB1 cannabinoid receptors exist predominantly in two states, an inactive R state precoupled to Gi/o in its GDP-bound form and an active R* state coupled to Gi/o in its GTP-bound form (Fig. 7). The active R*-GGTP state would have a higher affinity for the cannabinoid agonist WIN 55,212–2. The inactive R-GGDP state would have a higher affinity for the cannabinoid inverse agonist SR 141716A. Both the active R*-GGTP state and the inactive R-GGDP state can sequester G-proteins. This model is not inconsistent with the cubic ternary complex model for G-protein-coupled receptors (Kenakin, 1996). The cubic ternary complex model includes a stable complex between an inactive receptor and a G-protein. Kenakin (1996) proposed an inactive receptor G-protein complex, even though there was no evidence for its existence. Consequently, Kenakin (1996) proposed that the association constant between the inactive receptor and G-protein, KG, must be very small. Our results provide evidence that the inactive hCB1 receptor can form a stable complex precoupled with Gi/o. For precoupling to occur, the hCB1 association constant, KG, must be large. The magnitude of KG between the receptor and G-protein is the major difference between cannabinoid receptors, including both CB1 and CB2 (Bouaboula et al., 1999) and other G-protein-coupled receptors such as the mGluR2 metabotropic glutamate receptor (Fig. 7). This means that cannabinoid receptors would exist predominantly in either a G-protein-precoupled inactive R-GGDP state or an active R*-GGTP state. This model differs from the three-state receptor model proposed by Bouaboula et al. (1997). According to their three-state receptor model, SR 141716A converts a tonically active hCB1 receptor into an active negative state in which the receptor is coupled to Gi/o in its GDP-bound form. We observed the existence of an inactive hCB1 receptor precoupled to GGDP. This naturally occurring inactive R-GGDP state of the receptor would have a high affinity for SR 141716A, and SR 141716A would act to stabilize the inactive R-GGDP state of the receptor.
Fig. 7.
Model of hCB1 cannabinoid and mGluR2 metabotropic glutamate receptor states. The hCB1 cannabinoid receptor has a high association constant, KG, between the inactive receptor R and Gi/o-proteins in their inactive GDP-bound state. Therefore, the hCB1 receptor has a high probability of being in an inactive state precoupled to G-proteins, the RGGDPstate. The inverse agonist SR 141716A has a high affinity for the inactive RGGDP state and acts to stabilize this state. The hCB1 receptor can also exist in a tonically active state coupled to active G-proteins in their GTP-bound state. This active R*GGTP state of the receptor has a high affinity for the cannabinoid agonist WIN 55,212–2. Therefore, hCB1 receptors exist predominantly in two states: the RGGDP state and the R*GGTP state. In contrast, the mGluR2 metabotropic glutamate receptor has a small association constant, KG, between the receptor and the Gi/o-proteins and thus has a lower probability of existing in the RGGDP state. The mGluR2 receptor also does not exhibit tonic receptor activity. Therefore, the mGluR2 receptor would exist predominantly in an inactive R state not coupled to a G-protein.
In summary, we have shown that the human CB1 cannabinoid receptor has the ability to block signaling by other pertussis toxin-sensitive Gi/o-coupled receptors by sequestering a common pool of Gi/o-proteins. Sequestration of G-proteins by CB1 cannabinoid receptors likely occurs in two receptor states, an inactive R-GGDP state and an active R*-GGTP state. Our study suggests that CB1 cannabinoid receptors may act as dominant receptors controlling the biological signals of other pertussis toxin-sensitive Gi/o-coupled receptors.
Footnotes
This work was supported by a grant from the National Institutes of Health, National Institute on Drug Abuse Grant DA10350 to D.L.L. We thank Dr. Tom I. Bonner (National Institute of Mental Health, Bethesda, MD) for the human CB1 cannabinoid receptor cDNA, Dr. Shigetada Nakanishi (Kyoto University, Kyoto, Japan) for the mGluR2 receptor cDNA, Dr. Melvin Simon (Caltech, Pasadena, CA) for GαoB, Gβ1, and Gγ3 cDNA, and Sanofi Recherche (Montpellier, France) for SR 141716A. We also thank Dr. Nevin Lambert and Dr. Clare Bergson for helpful discussions.
Correspondence should be addressed to Dr. Deborah L. Lewis, Department of Pharmacology and Toxicology, Medical College of Georgia, 1120 15th Street, Augusta, GA 30912-2300. E-mail: DLewis@mail.mcg.edu.
Dr. Vásquez's present address: Centro Universitario de Investigaciones Biomedicas, Universidad de Colima, 28000 Colima, Colima, Mexico.
REFERENCES
- 1.Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, Maffrand JP, Le Fur G, Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- 2.Bouaboula M, Desnoyer N, Carayon P, Combes T, Casellas P. Gi-protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implications for intracellular signalization cross-regulation. Mol Pharmacol. 1999;55:473–480. [PubMed] [Google Scholar]
- 3.Caulfield MP, Jones S, Vallis Y, Buckley NJ, Kim GD, Milligan G, Brown DA. Muscarinic M-current inhibition via Gα q/11 and α-adrenoceptor inhibition of Ca2+ current via Gαo in rat sympathetic neurones. J Physiol (Lond) 1994;15:415–422. doi: 10.1113/jphysiol.1994.sp020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutts AA, Pertwee RG. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br J Pharmacol. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deadwyler SA, Hampson RE, Bennett BA, Edwards TA, Mu J, Pacheco MA, Ward SJ, Childers SR. Cannabinoids modulate potassium current in cultured hippocampal neurons. Receptors Channels. 1993;1:121–134. [PubMed] [Google Scholar]
- 6.Deadwyler SA, Hampson RE, Mu J, Whyte A, Childers S. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neuron via a cAMP-dependent process. J Pharmacol Exp Ther. 1995;273:734–743. [PubMed] [Google Scholar]
- 7.Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- 8.Gérard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gifford AN, Ashby CR. Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212–2, and is potentiated by the cannabinoid antagonist, SR 141716A. J Pharmacol Exp Ther. 1996;277:1431–1436. [PubMed] [Google Scholar]
- 10.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 11.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991a;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991b;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda SR, Schofield GG. Somatostatin blocks a calcium current in rat sympathetic ganglion neurons. J Physiol (Lond) 1989;409:221–240. doi: 10.1113/jphysiol.1989.sp017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda SR, Lovinger DM, McCool BA, Lewis DL. Heterologous expression of metabotropic glutamate receptors in adult rat sympathetic neurons: subtype-specific coupling to ion channels. Neuron. 1995;14:1029–1038. doi: 10.1016/0896-6273(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 16.Ishac EJN, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenakin T. The classification of seven transmembrane receptors in recombinant expression systems. Pharmacol Rev. 1996;48:413–463. [PubMed] [Google Scholar]
- 19.Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR 141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- 20.Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLennan SJ, Reynen PH, Kwan J, Bonhaus DW. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br J Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature (Lond) 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J. Comp. Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- 24.Pan X, Ikeda SR, Lewis DL. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol Pharmacol. 1996;49:707–714. [PubMed] [Google Scholar]
- 25.Pan X, Ikeda SR, Lewis DL. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoic receptor activity. Mol Pharmacol. 1998;54:1064–1072. doi: 10.1124/mol.54.6.1064. [DOI] [PubMed] [Google Scholar]
- 26.Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara P, Soubrié P, Brelière JC, Le Fur G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 27.Schofield GG. Norepinephrine blocks a calcium current of adult rat sympathetic neurons via an α2-adrenoceptor. Eur. J. Pharmacol. 1990;180:37–47. doi: 10.1016/0014-2999(90)90590-3. [DOI] [PubMed] [Google Scholar]
- 28.Schofield GG. Norepinephrine inhibits a Ca2+ current in rat sympathetic neurons via a G-protein. Eur J Pharmacol. 1991;207:195–207. doi: 10.1016/0922-4106(91)90031-c. [DOI] [PubMed] [Google Scholar]
- 29.Shen M, Thayer SA. The cannabinoid agonist Win55,212–2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- 30.Tsou K, Brown S, Sanudo-Pena, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 31.Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Ikeda SR. VIP inhibits N-type Ca2+ channels of sympathetic neurons via a pertussis toxin-insensitive but cholera toxin-sensitive pathway. Neuron. 1994;13:657–669. doi: 10.1016/0896-6273(94)90033-7. [DOI] [PubMed] [Google Scholar]