Abstract
The formation of neurocircuitry depends on the control of neurite outgrowth that, in turn, can be divided into two processes: nerve growth cone protrusion and neurite extension. It has long been known that the neural cell adhesion molecules L1 and NCAM-180 promote neurite outgrowth, but how they function in growth cones is unclear. We addressed the roles of L1 and NCAM-180 in neurite outgrowth by using microscale chromophore-assisted laser inactivation (micro-CALI) of these proteins to perturb their functions at precise times in single growth cones of embryonic chick dorsal root ganglion neurons grown in culture. Micro-CALI of L1 causes neurite retraction after a 10 min lag period but does not affect growth cone protrusion. In contrast, micro-CALI of NCAM-180 causes rapid growth cone retraction but does not affect neurite extension. The simultaneous inactivation of both these molecules resulted in both distinct effects that were segregated in time. The behavior of growth cones after these micro-CALI treatments resemble the drug-induced perturbation of microtubules for L1 and F-actin for NCAM-180. These findings suggest distinct roles in the growth cone for L1 and NCAM-180 in different steps of neurite outgrowth: L1 functions in neurite extension,whereas NCAM-180 functions in growth cone protrusion.
Keywords: CALI (chromophore-assisted laser inactivation), growth cone motility, neurite extension, chick dorsal root ganglion neurons, axon guidance, cell adhesion molecule
The neurites of embryonic neurons grow along specified routes to connect with their targets. Directed neurite outgrowth occurs by the coordination of two processes (Goldberg and Burmeister, 1986; Mitchison and Kirschner, 1988). The first process is the protrusion of the leading edge of the nerve growth cone, the sensory motile organelle at the tips of growing neurites. This step depends on the assembly of F-actin and its attachment to the membrane receptors that, in turn, bind to the substrate on which the growth cone moves (for review, see Letourneau, 1996). The second process is neurite extension, which occurs by the engorgement of microtubules into the periphery and the consolidation of these microtubules to form the nascent neurite (for review, see Sabry and Tanaka, 1995). How these processes occur remain unclear, but a family of cell adhesion molecules (CAMs) found on the growth cone membrane are likely to act during these steps (for review, see Bixby and Bookman, 1996). In our investigation of these interactions, we have focused on two CAMs, L1 (Rathjen and Schachner, 1984) and NCAM-180, a major NCAM isoform (Rutishauser et al., 1982) whose cytoplasmic domain is required to promote neurite outgrowth (Saffell et al., 1995).
L1 and NCAM-180 are members of the Ig superfamily (Rathjen and Schachner, 1984; Cunningham et al., 1987; Williams and Barclay, 1988). They are widely expressed on neural tissues during development (Rutishauser and Edelman, 1980; Lemmon and McLoon, 1986; Persohn and Schachner, 1987). L1 and NCAM-180 both act as substrates that promote neurite outgrowth in vitro (Rathjen et al., 1987; Doherty et al., 1989; Lemmon et al., 1989). L1 and NCAM-180 are both found on nerve growth cones (Letourneau and Shattuck, 1989), but it is unclear how they function there.
L1 and NCAM can both act by homophilic adhesion and were first identified by this function (Rutishauser et al., 1982; Rathjen and Schachner, 1984). They may also interact heterophilically with each other and with other molecules such as laminin (Grumet et al., 1993), signal transduction molecules (Williams et al., 1994; Wong et al., 1995), and cytoskeletal components (Pollerberg et al., 1987;Davis and Bennett, 1994). These interactions coupled with their expression patterns make L1 and NCAM-180 good candidates to function in growth cones. Functional perturbation (Chang et al., 1987; Bixby et al., 1987; Brittis et al., 1995) and genetic studies have implicated L1 (Dahme et al., 1997; Cohen et al., 1997) and NCAM-180 (Tomasiewicz et al., 1993) in neurite outgrowth, but how they function remains unclear.
To address their roles, we have generated the specific and localized inactivation of L1 and NCAM-180 in growth cones of dissociated chick dorsal root ganglia (DRG) neurons using micro-CALI. Micro-CALI generates a loss of function of specific proteins in living cells (Diamond et al., 1993). Inactivation occurs in the precise location of irradiation and only commences when irradiation is initiated such that the immediate consequences of acute and focal inactivation can be observed by video microscopy (for review, see Wang and Jay, 1996).
MATERIALS AND METHODS
Antibodies and reagents. Monoclonal antibody 8D9, which recognizes the extracellular domain of L1 (Lemmon and McLoon, 1986) and purified 8D9 antigen (chick L1) were gifts from Vance Lemmon (Case Western Reserve University, Cleveland, OH). Polyclonal antibody intra-L1, which recognizes the intracellular domain of mouse L1 but cross-reacts with chick L1 (Miura et al., 1992), was provided by Keiichi Uyemura, Masayuki Miura, and Yasuo Takeda (Keio University, Tokyo, Japan). Purified mouse L1 was a gift of Carl Lagenaur (University of Pittsburgh, Pittsburgh, PA). NCAM monoclonal antibodies that recognize the extracellular domain of all isoforms (5e) and the intracellular domain of NCAM-180 (4d) have been described previously (Frelinger and Rutishauser, 1986; Watanabe et al., 1986). Polyclonal anti-NCAM antibodies were the generous gift of Chi Hung Siu (University of Toronto, Toronto, Ontario, Canada). Purified bovine spectrin was provided by Vann Bennett (Duke University, Durham, NC). Anti-chicken spectrin (which cross-reacts with bovine spectrin), nonimmune IgG, and BSA were obtained from Sigma (St. Louis, MO). The intra-L1, 8D9, 4d, and 5e antibodies, BSA, and nonspecific IgG were labeled with malachite green (MG) isothiocyanate (Molecular Probes, Eugene, OR) to an average dye: protein molar ratio of 6–8 as described by Jay (1988).
Cell culture of DRG neurons. Dissected chick embryonic day 10–11 DRG were dissociated by incubation in 0.25% trypsin (Sigma) in HBSS at 37°C for 18 min followed by manual dissociation by pipetting as described by Bray (1991). The cells were then plated onto coverslips, coated with poly-l-lysine and laminin or L1. The neurons were cultured in Leibovitz L-15 media supplemented with nerve growth factor, gentamycin, glucose, and fetal bovine serum.
Immunocytochemistry. Chick DRG cultures were incubated at 37°C for 4 hr, by which time most neurons had produced neurites. The cells were then rinsed with warm HBSS and fixed in either freshly prepared 4% paraformaldehyde for 30 min at 37°C, or a solution of 95% ethanol and 5% acetic acid for 15 min at −20°C. After rinsing with PBS, the cells were blocked with 10% fetal calf serum in PBS, probed with primary antibodies against NCAM or L1, and incubated with fluorescence-conjugated secondary antibodies. Cells were observed by epifluorescence using a Zeiss confocal microscope. In all cases, matched controls were performed without primary antibody or with nonimmune IgG.
CALI of L1 in vitro. Purified 8D9 antigen (chick L1) or mouse L1 were coated at 100 μg/ml on a thin layer of nitrocellulose plated onto coverslips as previously described (Lagenaur and Lemmon, 1987). These samples were incubated with 15 μg/ml of MG-labeled 8D9 or MG-labeled intra-L1 antibodies in culture medium. Laser irradiation (620 nm) was performed for 2 min using a pulsed ND:YAG-driven dye laser (GCR-11; Spectra-Physics Corp.) with a spot size of 2 mm, a pulse width of 3 nsec, a pulse energy of 18 mJ at a frequency of 10 Hz. Dissociated chick DRG neurons were plated as described above and incubated for 2 hr at 37°C to permit neurite outgrowth. Neurons were fixed as described above. The percentage of cells bearing neurites were measured for neurons inside and outside of the laser spots.
CALI of NCAM-180 in vitro. Purified NCAM samples were incubated with MG-labeled antibodies (4d or 5e) on ice for 1 hr and transferred to a 96-prong plate (Nunc, Roskilde, Denmark) and subjected to 620 nm laser light as described above. Brain spectrin binding activity of the intracellular domain of NCAM-180 was assayed by coimmunoprecipitation. NCAM (1 μg) in PBS was incubated with 1 μg of brain spectrin for 2 hr on ice. Anti-NCAM rabbit polyclonal antibodies were then added to the mixture in tenfold excess along with immunoprecipitation buffer (1% Nonidet P-40 and 0.25% deoxycholate in PBS, pH 7.3), and the mixtures were incubated for 1 hr on ice. Samples were then mixed with killed Staphylococcus aureus cells (Calbiochem, La Jolla, CA) and incubated for 30 min on ice. S. aureus-bound proteins were sedimented by centrifugation and subsequently solubilized by boiling in 2× Laemmli sample buffer. Proteins were then fractionated by SDS-PAGE, and the gels were electroblotted. Blots were incubated with rabbit polyclonal antibodies against chicken spectrin, then incubated with HRP-conjugated protein A and visualized using an ECL kit (Amersham, Arlington Heights, IL).
Micro-CALI of L1 and NCAM in chick DRG growth cones. For micro-CALI directed to intracellular domains of L1 and NCAM-180, MG-labeled reagents were loaded into neurons by trituration as described in Sydor et al. (1996). To visualize protein loading, fluorescein-conjugated nonspecific IgG (1 mg/ml final concentration) was included with the MG-labeled reagent. When micro-CALI was performed against extracellular domains, MG-labeled reagents were added to the culture medium to a final concentration of 8 μg/ml and incubated for 30 min before to laser irradiation.
Micro-CALI on DRG growth cones was performed as previously described byChang et al. (1995). Cultures were maintained at 37°C with a stage incubator during the experiments. Micro-CALI was performed from 1 to 4 hr after plating. In a typical micro-CALI experiment, neurons were briefly observed by fluorescence microscopy to verify protein loading. Only loaded cells that were healthy and that produced growth cones with active motility were subjected to micro-CALI. Time-lapse video microscopy [phase contrast or differential interference contrast (DIC)] was used to record the behavior of growth cones. Images were taken every 15 sec using a computer-controlled shutter (1 sec exposure). Image enhancement was performed using custom-written software (Jay and Keshishian, 1990). Time-lapse observations were conducted for 40 min after laser irradiation for micro-CALI of L1 experiments and for only 10 min for micro-CALI of NCAM because neurons subjected to this treatment recovered by this time.
Measurement and quantitation of growth cone motility and neurite extension. Growth cone parameters measured were the rates of neurite extension, filopodial motility, and change in filopodial length. Filopodial and neurite lengths were measured every 1 min before and after CALI in all samples tested. Measurements were made in each frame of the time-lapse period before and after CALI as follows: 5 min before and after laser irradiation for CALI of NCAM-180 experiments using 4d; 10 min before and 20 min after laser irradiation for CALI of L1 experiments using 8D9 or intra-L1, CALI of NCAM and L1 experiments using 4d and 8D9, and other control CALI experiments. Quantitation was done using NIH Scion Imaging System software (Scion, Frederick, MD) and analyzed using Cricket Graph software (Malvern, PA). Data shown in Figure 8 are the averages (± SEM) of neurite extension rate (change in length per minute), filopodial length (change in length for 5 min), and filopodial motility (change in length per minute). For Table1, neurite retraction is defined as a neurite decreasing its length by continuous retraction for >10 min; growth cone collapse is defined as filopodia within the irradiated area shown decreasing its length by continuous retraction.
Fig. 8.
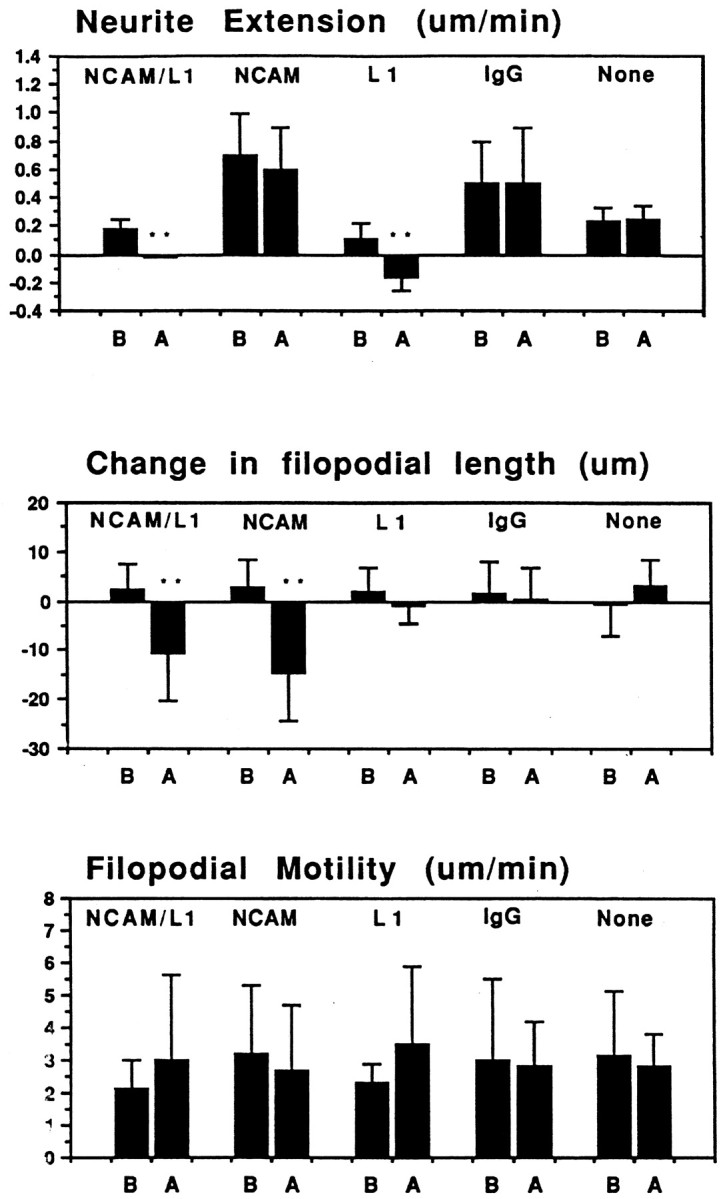
Quantitative analysis of micro-CALI of NCAM and L1. Average values for neurite extension, changes in filopodial length, and absolute rates of filopodial motility were determined for each experimental treatment, before (B) and after (A) laser irradiation.NCAM/L1, Simultaneous micro-CALI of NCAM and L1 using MG-labeled 4d and 8D9; NCAM, micro-CALI using MG-labeled 4d; L1, micro-CALI of L1 using MG-labeled 8D9;IgG, laser irradiation with MG-labeled nonimmune IgG;None, laser irradiation in the absence of MG-labeled reagents. Values that are significantly different (p < 0.01) than control values (None) are marked with double asterisks.
Table 1.
Summary of micro-CALI of L1 and NCAM effects
| Treatment | Neurite retraction | Local growth cone collapse |
|---|---|---|
| CALI of L1 (8D9) | 10 /12 | 1 /12 |
| CALI of L1 (intra L1) | 8 /12 | 0 /12 |
| CALI of NCAM-180 (4d) | 0 /25 | 21 /25 |
| CALI of NCAM (5e) | 0 /20 | 3 /20 |
| CALI of L1 & NCAM-180 (8D9 & 4d) | 12 /13 | 10 /13 |
| CALI using IgG | 0 /20 | 2 /20 |
| CALI using BSA | 0 /12 | 1 /12 |
| No treatment | 0 /13 | 0 /13 |
DRG neurites were treated with micro-CALI of L1, NCAM-180, both together, or a variety of controls. Growth cone motility and neurite outgrowth were assessed, and neurons were deemed to undergo neurite retraction if the neurite decreased in length after laser irradiation and increased in length before irradiation. Neurons were deemed to show local growth cone collapse when a net decrease in filopodial length was observed within the laser spot, and there was a net increase in filopodial length in the rest of the growth cone and within the chosen laser spot before irradiation.
RESULTS
To apply micro-CALI to address the function of a protein in growth cones, it is important to show that: (1) the antibody used for targeting is specific and recognizes the protein of interest in the growth cones; (2) the antibody can be loaded efficiently into growth cones; and (3) that CALI using this antibody disrupts the function of the protein in vitro. To target L1, we used the following antibodies: 8D9, which binds to the extracellular domain of L1 (Lemmon and McLoon, 1986), and intra-L1, which binds to the intracellular domain of chick and mouse L1 (Miura et al., 1992). To target NCAM, we used two anti-NCAM monoclonal antibodies: 4d, which recognizes the cytoplasmic domain of NCAM-180, and 5e, which recognizes the extracellular binding domain of all isoforms of NCAM (Frelinger and Rutishauser, 1986; Watanabe et al., 1986).
Specificity of L1 and NCAM antibodies
We confirmed that these antibodies recognize the appropriate L1 and NCAM isoforms in chick DRG neurons by Western blot analysis (Fig.1A). 8D9 recognizes two bands at 200 and 140 kDa (Fig. 1A, lane 1). The lower band likely represents a proteolytic product of L1 cleaved in the third fibronectin-III-like domain (Burgoon et al., 1991). 4d binds to purified NCAM-180 (Fig. 1A,lane 3), and this antibody recognizes only NCAM-180 in DRG lysates (lane 2) and whole brain lysates (lane 4). In contrast, 5e recognizes all three isoforms of NCAM in DRG lysates (Fig. 1A, lane 5).
Fig. 1.
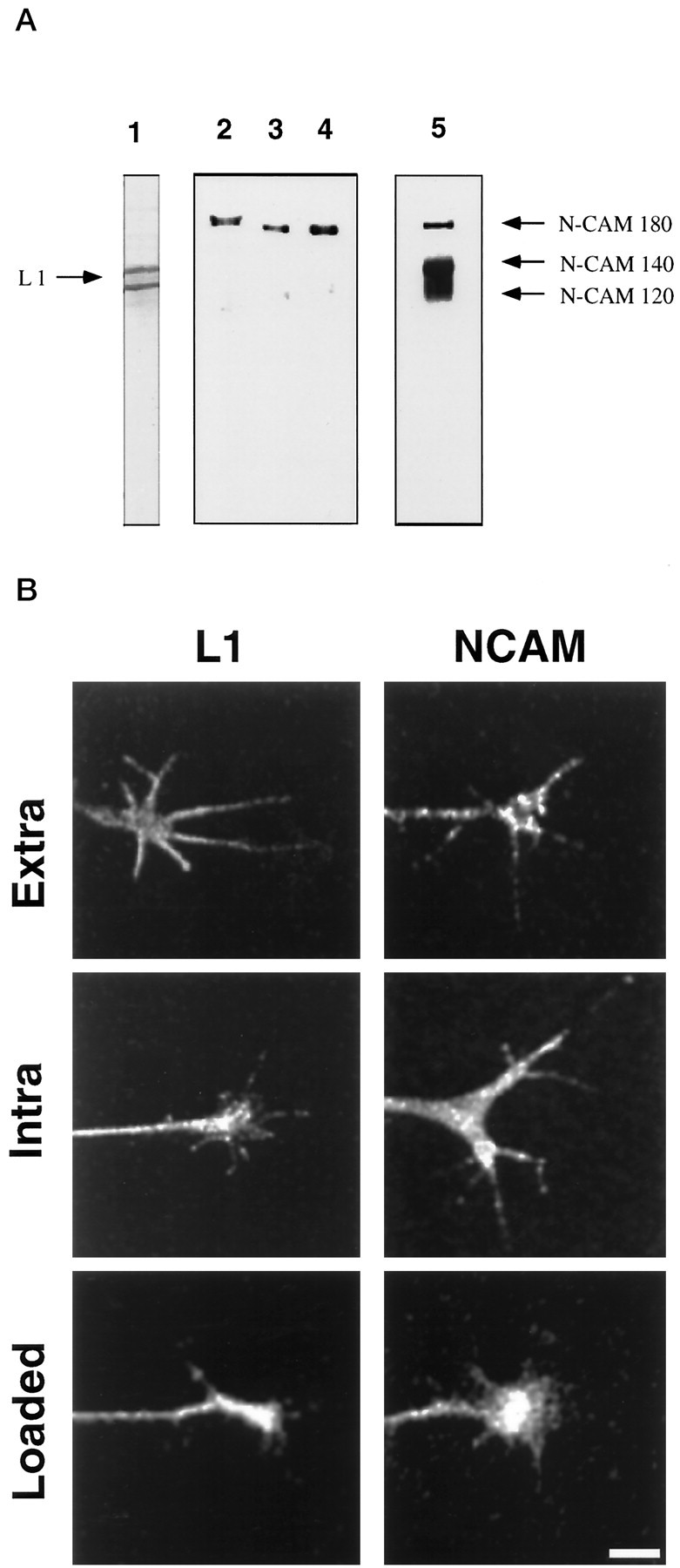
Immunocytochemistry, trituration loading, and specificity of L1 and NCAM antibodies in chick DRG neurons.A, Specificity of 8D9, 4d, and 5e is demonstrated by Western blot analysis. Lane 1, DRG lysate probed with 8D9. Two bands are observed, likely because of proteolysis. Lane 2, DRG lysate probed with 4d recognizes only NCAM-180;lane 3, purified NCAM-180 probed with 4d; lane 4, whole chick brain lysate probed with 4d; lane 5, DRG lysate probed with 5e, which recognizes all three NCAM isoforms. Note that different gels were used for lane 1 and for lanes 2–5 so that the migration distances do not correspond between these lanes. B,Trituration loading of intra-L1 followed by fixation and staining with secondary antibody (L1, Loaded) results in a similar pattern to that observed by immunocytochemistry with intra-L1 (L1, Intra) and with 8D9, which shows expression of L1 in growth cones (L1, Extra). Trituration loading of 4d followed by fixation and staining with secondary antibody (NCAM, Loaded) shows similar specific staining compared with indirect immunocytochemistry using 4d (NCAM, Intra) or with 5e (NCAM, Extra).
To show that these antibodies recognized their respective antigens in growth cones, we performed immunocytochemistry on chick DRG neuronal cultures using these reagents (Fig. 1B). Antibodies that recognize extracellular (Extra) or intracellular (Intra) domains of L1 and NCAM all stained DRG growth cones. These patterns are comparable to those previously reported for DRG neurons on laminin (Stoeckli et al., 1996) and for mouse cerebellar neurons (Persohn and Schachner, 1987). To perform micro-CALI of the intracellular domains of L1 and NCAM, MG-labeled antibodies (intra-L1 for L1 and 4d for NCAM-180) were loaded into DRG neurons by trituration (Sydor et al., 1996). We showed efficient loading of these antibodies by using fluorescent secondary antibody for immunocytochemistry (without additional primary antibody). This treatment resulted in immunostaining of the growth cone comparable to the indirect immunocytochemistry presented above (Fig. 1B, L1, Loaded;NCAM, Loaded). No staining was observed when primary antibodies were not loaded (data not shown). Generally, ∼80% of the neurons were loaded by trituration, and the antibodies were retained in growth cones with a 12 hr half-life (data not shown). We then used these antibodies to inactivate purified L1 and NCAM-180 using CALI to verify that micro-CALI would be useful for the selective perturbation of these molecules in neuronal growth cones.
CALI inactivates L1 and NCAM-180 in vitro
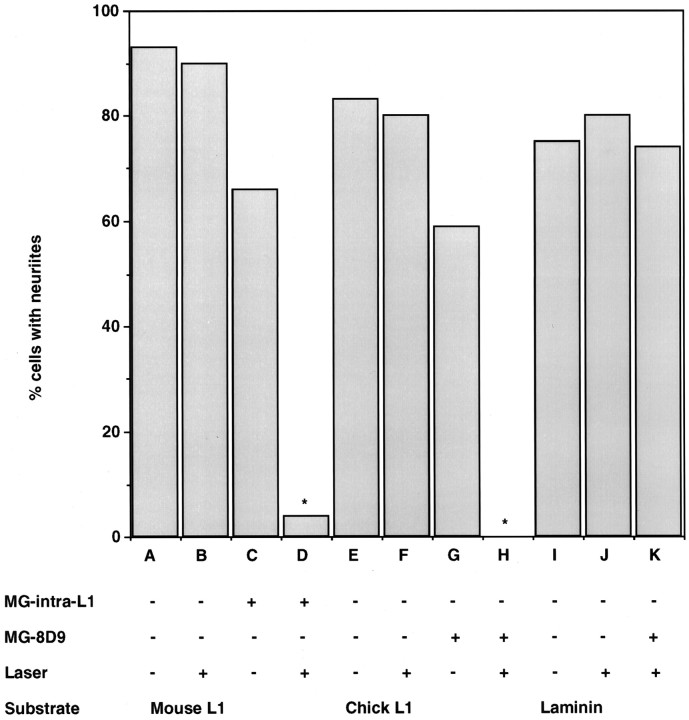
We performed CALI using MG-labeled 8D9 and intra-L1 incubated with samples of purified chick L1, mouse L1, or laminin. We used the resulting material as a substrate for chick DRG neuronal culture. We assayed the percentage of cells with neurites as a measure of the neurite-promoting activity of the CALI-treated samples. Figure2 shows that CALI of purified L1 in vitro inhibited its neurite-promoting activity when used as a substrate. The percentage of cells with neurites (>2 cell diameters) was assayed after 2 hr of culture in each dish, inside and outside the area of irradiation (∼2 mm spot). L1 substrate inactivated by CALI using either MG-labeled intra-L1 (lane D) or 8D9 (lane H) showed a large decrease in the percentage of cells with neurites. Antibody incubation alone for both MG-labeled intra-L1 or 8D9 had a slight effect on this parameter (lanes C, G). This may be explained by the high concentration of MG-labeled antibodies used for CALI of L1 in vitro. Experiments using micro-CALI of L1 on growth cones were designed using lower concentrations of MG-labeled antibodies. Laser irradiation without antibodies present had virtually no effect on neurite outgrowth on the L1 substrate (lanes B, F). CALI using MG-labeled 8D9 had no effect on the neurite-promoting activity of laminin (compare lane I). Similar results were obtained when neurite length was assayed except for MG-labeled 8D9 without laser light, the effect on neurite length was larger. These findings show that CALI of L1 in vitro affects its neurite-promoting activity. CALI of L1 directed to both intracellular and extracellular domains perturbs the ability of L1 to act as a permissive substrate.
Fig. 2.
CALI of purified L1 inhibits its neurite-promoting activity. CALI directed against L1 using intra-L1 or 8D9 resulted in marked inhibition of the percentage of cells with neurites.Lanes A–D show CALI of mouse L1 substrate using MG-labeled intra-L1: lane A, no treatment; lane B, laser irradiation without MG-labeled antibody; lane C, MG-labeled intra-L1 without laser irradiation; lane D, MG-labeled intra-L1 with laser irradiation. Lanes E–H show CALI of chick L1 substrate using MG-labeled 8D9:lane E, no treatment; lane F, laser irradiation without MG-labeled antibody; lane G, MG-labeled 8D9 without laser irradiation; lane H, MG-labeled 8D9 with laser irradiation. Lanes I–K show that laser irradiation using MG-8D9 did not affect neurite outgrowth-promoting activity of laminin. Lane I, No treatment; lane J, irradiation without MG-labeled 8D9;lane K, MG-labeled 8D9 with laser irradiation. The data are representative of three experiments with n > 100 neurons for each treatment.
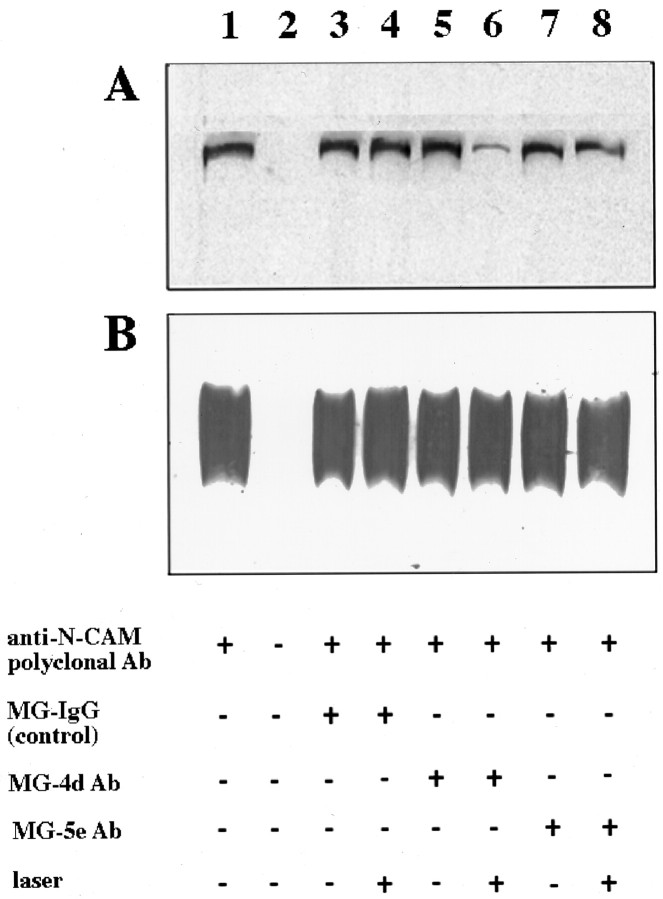
To test the efficacy of CALI of NCAM, we assayed NCAM binding to spectrin in vitro because purified NCAM is a poor substrate for DRG neurite outgrowth in our hands. NCAM-180 colocalizes with spectrin in growth cones, and these two proteins associate with each other in vitro (Pollerberg et al., 1987). CALI of the cytoplasmic domain of NCAM-180 (using antibody 4d) markedly reduced the amount of brain spectrin that bound to NCAM-180 (compare Fig.3A, lanes 5, 6). CALI of the extracellular portion of NCAM using MG-labeled 5e (Fig. 3A, lane 8) or irradiation in the presence of MG-labeled nonimmune IgG (Fig. 3A, lane 4) had little or no effect on spectrin binding. MG-labeled reagents without laser light also had little effect (compare Fig.3A, lane 1 with lanes 3, 5, 7). These data show that CALI of the cytoplasmic domain of NCAM-180 abolishes the spectrin-binding capacity of this domain, whereas CALI directed to the extracellular domain of all major NCAM isoforms (via MG-labeled 5e) does not. None of these treatments affect NCAM immunoprecipitation (Fig. 3B). These findings show that CALI of NCAM-180 in vitro disrupts its binding to brain spectrin.
Fig. 3.
CALI of NCAM-180 intracellular domain disrupts brain spectrin binding in vitro. Purified chick NCAM (all isoforms) was incubated with purified bovine brain spectrin after CALI using 4d, 5e, or nonspecific IgG. The complex was immunoprecipitated with anti-NCAM, dissociated in Laemmli sample buffer, fractionated by SDS-PAGE, and immunoblotted with anti-spectrin antibodies to assay NCAM-180/spectrin binding. A shows spectrin immunoblot, whereas B shows the same filter after being stripped and probed with anti-NCAM (all isoforms), showing that there were equivalent amounts of NCAM present in each lane.Lane 1, Anti-NCAM immunoprecipitation; lane 2, no anti-NCAM control; lane 3, preincubation of NCAM with MG-labeled nonspecific antibody (IgG); lane 4, the same conditions as lane 3 except subjected to laser irradiation; lane 5, preincubation with MG-labeled 4d (intracellular domain of NCAM-180); lane 6, same conditions as lane 5 except subjected to laser irradiation (CALI using 4d); lane 7, preincubation with MG-labeled 5e (extracellular domain of all NCAM isoforms); lane 8, same conditions as lane 7, except subjected to laser irradiation (CALI using 5e).
Together, these data show that CALI of L1 is effective when applied to both extracellular and intracellular regions, and CALI of NCAM-180 is effective only when applied to the intracellular domain. They suggest that micro-CALI of L1 and NCAM-180 in growth cones will affect their functions in situ.
Micro-CALI of L1 causes neurite retraction but does not affect growth cone protrusion
We applied micro-CALI using MG-labeled 8D9 to chick DRG neuronal growth cones in culture to address the specific role of L1 in neurite outgrowth. Figure 4 shows a typical experiment. Micro-CALI of L1 resulted in neurite retraction after a latency period of ∼10 min (Fig. 4D). Neurite extension resumed after ∼20 min and grew in a different direction (Fig.4F). In contrast, filopodial motility appeared to be unaffected by micro-CALI of L1. Long dynamic filopodia were observed throughout the time course (Fig. 4). Quantitation of the data from 12 micro-CALI of L1 experiments showed that retraction occurred for 10 of 12 neurons so treated (Table 1). The average onset time of retraction was 15.3 ± 6.6 min after the initiation of laser irradiation (n = 10). Neurite retraction also occurred for growth cones subjected to micro-CALI of the intracellular domain of L1 using MG-labeled intra-L1. This treatment caused neurite retraction in 8 of the 12 neurons tested (Table 1) and occurred with a similar latency (14.8 ± 4.1 min). In contrast, all control treatments, including irradiation of neurons loaded with MG-labeled nonimmune IgG or BSA did not affect neurite extension or filopodial motility (Table 1). Surprisingly, neurite retraction in response to micro-CALI of L1 was independent of substrate. Neurite retraction of similar magnitude and kinetics occurred for neurons plated on laminin or on L1 substrate (data not shown).
Fig. 4.
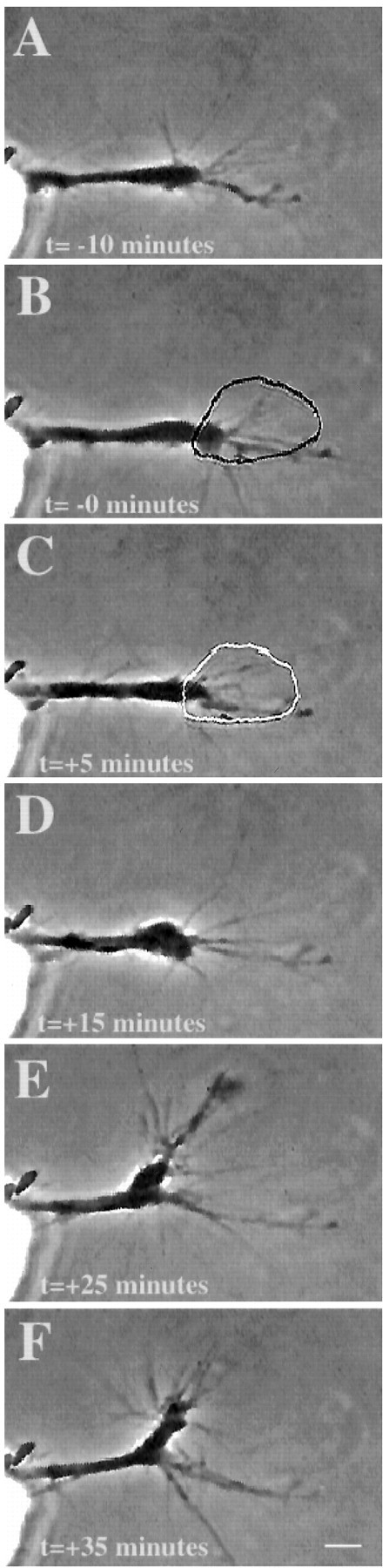
Micro-CALI of L1 causes neurite retraction. Micro-CALI of L1 causes neurite retraction but does not inhibit filopodial motility. A chick DRG growth cone was incubated with MG-labeled 8D9, and motility was observed by time-lapse video microscopy before laser irradiation (A). Laser irradiation of the growth cone (B, C) caused neurite retraction (D) after 10 min and subsequent recovery and growth along a different path (E, F) after 30 min. Filopodial motility is not affected. Scale bar, 10 μm.
Growth cone behavior in response to micro-CALI of L1 was also analyzed by quantitative morphometry measurements of video time-lapse images (see Fig. 8). The MG-labeled 8D9 antibody by itself caused a slight reduction in neurite extension rate (L1 before laser irradiation) compared to no treatment (None) but this reduction was significantly enhanced by laser irradiation (L1 after laser irradiation). Micro-CALI of L1 had no significant effect on the change in filopodial length or on the absolute rate of filopodial motility (regardless of retraction or extension). As such, the observed effects are not a general collapse of the growth cone and neurite as seen after addition of repulsive cues such as ephrin A5 (Drescher et al., 1995) or collapsin (Luo et al., 1993). Instead, micro-CALI of L1 causes neurite retraction and does not affect leading edge protrusion. From these findings, we suggest that L1 has a role in neurite extension but not leading edge protrusion.
Micro-CALI of NCAM-180 causes local growth cone collapse but does not affect neurite extension
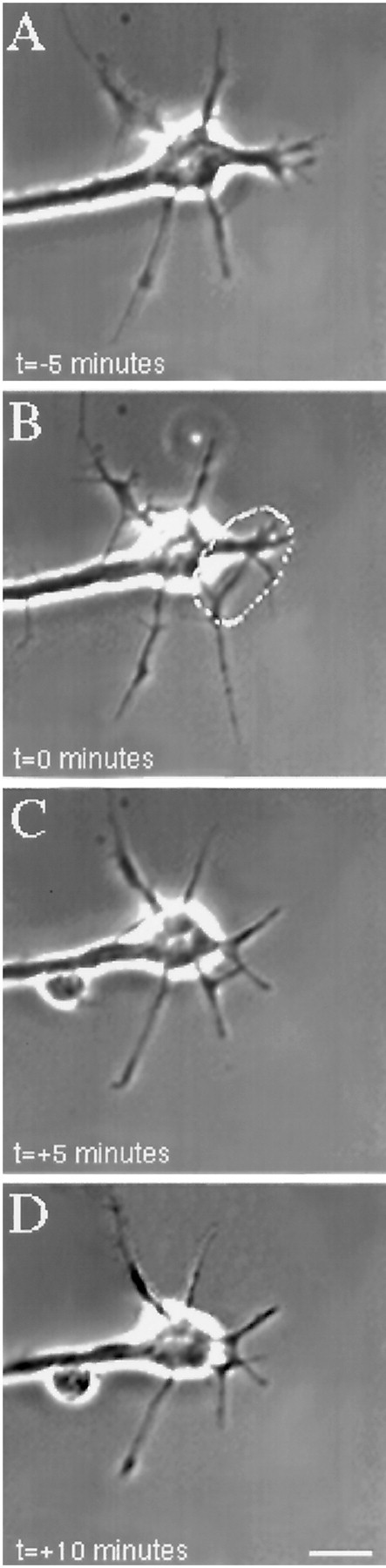
In contrast to the effects of micro-CALI of L1, micro-CALI with MG-labeled 4d resulted in a marked and localized retraction of the irradiated growth cone leading edge but did not cause neurite retraction (Fig. 5). When the laser spot was limited to one edge of the growth cone (Fig. 5B), localized filopodial and lamellipodial collapse was confined to the targeted region (Fig. 5, compare circles in B andC). The onset of growth cone collapse was rapid, occurring within 4 min after laser irradiation began (3.9 ± 2.5 min;n = 21). Neurons subjected to CALI of NCAM-180 (n = 25) showed local growth cone retraction in 84% of the cases examined, whereas none of these exhibited neurite retraction (Table 1). In contrast, 83% of the neurons treated with CALI of L1 (n = 12) showed neurite retraction, but only one of these showed observable filopodial collapse. Quantitative analysis of the time-lapse images revealed that micro-CALI of NCAM-180 using 4d caused significant (p < 0.01) filopodial retraction within the laser spot, but absolute rates of filopodial motility were not affected (see Fig. 8). MG-labeled 4d had no significant effect on neurite extension, regardless of laser light (Table 1). In fact, neurons loaded with MG-labeled 4d or nonimmune IgG showed a slight increase in neurite extension rates compared to untreated neurons (see Fig. 8).
Fig. 5.
Micro-CALI of the intracellular domain of NCAM 180 using MG-labeled 4d caused regional growth cone retraction.A, A growth cone was first observed for 5 min. A region of the growth cone was chosen for micro-CALI (white outline), laser irradiation was initiated att = 0 (B) and continued untilt = +5 min (C), and observed for 5 more minutes (D). In C, the filopodia and lamellipodia of the irradiated region retracted from the laser spot, whereas the rest of the growth cone did not appear to be affected. In D, the growth cone began to grow in another direction, causing a visible bend in the neurite. The time-lapse imaging was done using DIC microscopy instead of phase contrast as was used for Figures 4 and 6. Scale bar, 10 μm.
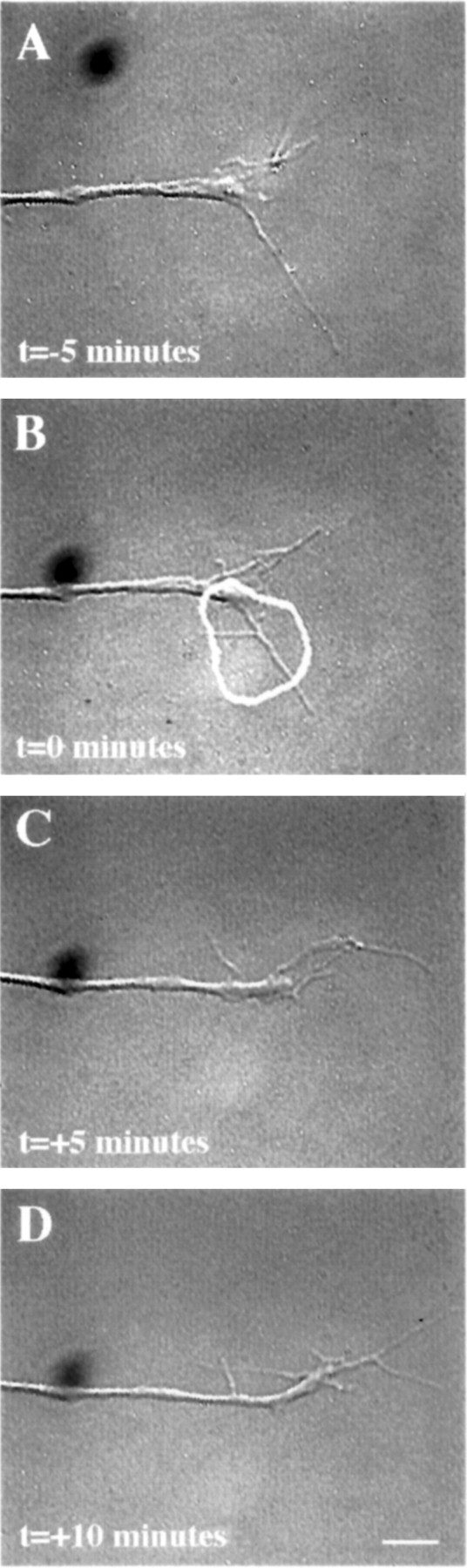
Micro-CALI using MG-labeled 5e to target the extracellular portion of NCAM isoforms had no observable effects (Fig.6). Filopodia in the irradiated region did not retract, and the growth cone often grew into the spot where the laser had been (Fig. 6B–D) nor was neurite extension affected (Fig. 6; see Fig. 8; Table 1). These experiments serve as negative controls for the previous experiments and show that laser irradiation of growth cones that are bound with MG-labeled antibody that binds to a growth cone membrane protein does not affect neurite outgrowth. These data suggest that the inactivation of the intracellular domain of NCAM-180 is specifically required for the leading edge retraction that we have observed. Additionally, laser irradiation of neurons loaded with MG-labeled nonimmune IgG or BSA did not affect growth cone behavior (see Fig. 8, Table 1).
Fig. 6.
Micro-CALI of the extracellular domain of all isoforms of NCAM using MG-labeled 5e does not affect growth cone behavior. A growth cone was incubated with MG-labeled 5e and observed by video time-lapse microscopy (A). A region of the growth cone is chosen for micro-CALI (white outline) and laser irradiated for 5 min (B, C). D,The growth cone is observed for an additional 5 min. Micro-CALI did not cause filopodial or lamellipodial retraction. Scale bar, 10 μm.
These findings suggest that NCAM-180 has an essential role in growth cone protrusion but not in neurite extension. Together with the experiments using micro-CALI of L1, our data suggest that L1 and NCAM-180 act on distinct processes during neurite outgrowth.
Asymmetric loss of NCAM-180 induces growth cone turning
Growth cone retraction caused by cytochalasin treatment can perturb growth cone guidance both in vitro and in vivo (Marsh and Letourneau, 1984; Bentley and Toroian-Raymond, 1986). We asked if the localized retraction of the leading edge caused by CALI of NCAM-180 could also alter the direction of subsequent growth cone movement. Generally growth cones in culture will continue to move straight on a uniform substrate and if they turn, there is a 50% probability of turning in each direction. CALI of the cytoplasmic domain of NCAM-180 caused a movement away from the laser spot and a visible change of angle of the nascent neurite (Fig. 5). In most cases, local filopodial collapse was followed by a change in lateral movement away from the laser spot during subsequent outgrowth (Table2). In our experiments, 16 of the growth cones treated asymmetrically with micro-CALI with 4d showed lateral movement and of these 14 moved away from the laser spot (odds ratio of 7). Both of these values were significantly different from all control treatments (p < 0.02). Thus micro-CALI of the cytoplasmic domain of NCAM-180 caused a significant increase in directed turning behavior away from the region of leading edge collapse. As both filopodia and lamellipodia retract, our experiments do not distinguish which component of the leading edge is responsible for directed motility.
Table 2.
Summary of turning behaviors resulting from micro-CALI of NCAM-180
| Behavior | Frequency observed for each treatment | ||
|---|---|---|---|
| Treatment | Micro-CALI using 4d | Micro-CALI using 5e | Micro-CALI Control IgG |
| Regional retraction | 21/25* | 3/20 | 2/20 |
| Lateral growth cone movement | 16/25 | 4/20 | 3/20 |
| Movement away | 14/16** | 2/4 | 1/3 |
| Movement toward | 2/16 | 2/4 | 2/3 |
| Odds ratio of moving away | 7.0*** | 1.0 | 0.5 |
Comparison of growth cone retraction and turning observed during micro-CALI of NCAM experiments. Regional retraction is defined as a decrease in length of the cord drawn from the neurite neck to the furthest extent of the leading edge within the area of irradiation during CALI. Lateral growth cone movement (defined operationally as components of leading edge extension that are perpendicular to the midline of the growth cone) was observed before and after micro-CALI, and changes in lateral movement were scored. “Moving away” means that the leading edge of the non-laser irradiated side is extending away from the laser spot and does not simply denote lamellipodial retraction. Of the growth cones that moved away from the laser spot, 93% also exhibited filopodial retraction for micro-CALI experiments targeting the intracellular domain of N-CAM 180 of cells growing on laminin. The odds ratio assumes a 50% probability for a change in lateral growth cone movement to be away from the laser spot (odds ratio = 1).
*p < 0.002 by Poisson's test.
**p < 0.02, significantly associated with filopodial retraction by McNemar's exact test.
***p < 0.02 by one-sample binomial test.
Simultaneous micro-CALI of L1 and NCAM-180
Neurons subjected to CALI of NCAM-180 showed growth cone collapse. In contrast, neurons treated with CALI of L1 showed neurite retraction but without observable growth cone collapse. These findings suggested that neurite extension and growth cone protrusion are distinct steps that may be segregated and are differentially regulated by L1 and NCAM-180, respectively. We addressed these hypotheses by performing micro-CALI of L1 and NCAM-180 together (which has not been tested before for any protein). We reasoned that if these processes are tightly linked, then the retraction of the leading edge might alter the kinetics of neurite retraction after micro-CALI of L1. Alternatively, if these processes are distinct, then CALI of both proteins should result in both growth cone retraction and neurite retraction with similar kinetics as seen when either L1 or NCAM-180 were inactivated separately.
Micro-CALI of both L1 and NCAM-180 simultaneously resulted in both neurite retraction and leading edge collapse, and these distinct effects were segregated in time (Figs. 7,8). In the example shown, the irradiated growth cone lost most of its filopodia and lamellipodia after 5 min (Fig. 7C,D). After an additional 10 min, the neurite began retracting (Fig.7D,E). When CALI of L1 and NCAM-180 were done simultaneously, both filopodial collapse (77%) and neurite retraction (92%) were observed at high frequency (Table 1). The average times of onset of neurite retraction (22.5 ± 8.6 min) and filopodial retraction (3.9 ± 1.1 min) after the loss of both L1 and NCAM-180 were similar to those observed after the loss of either L1 or NCAM-180 alone. These findings show that CALI of two proteins together can be performed with easily interpretable results. Moreover, they support the hypotheses that growth cone protrusion and neurite extension can be segregated and are differentially regulated by L1 and NCAM-180, respectively.
Fig. 7.
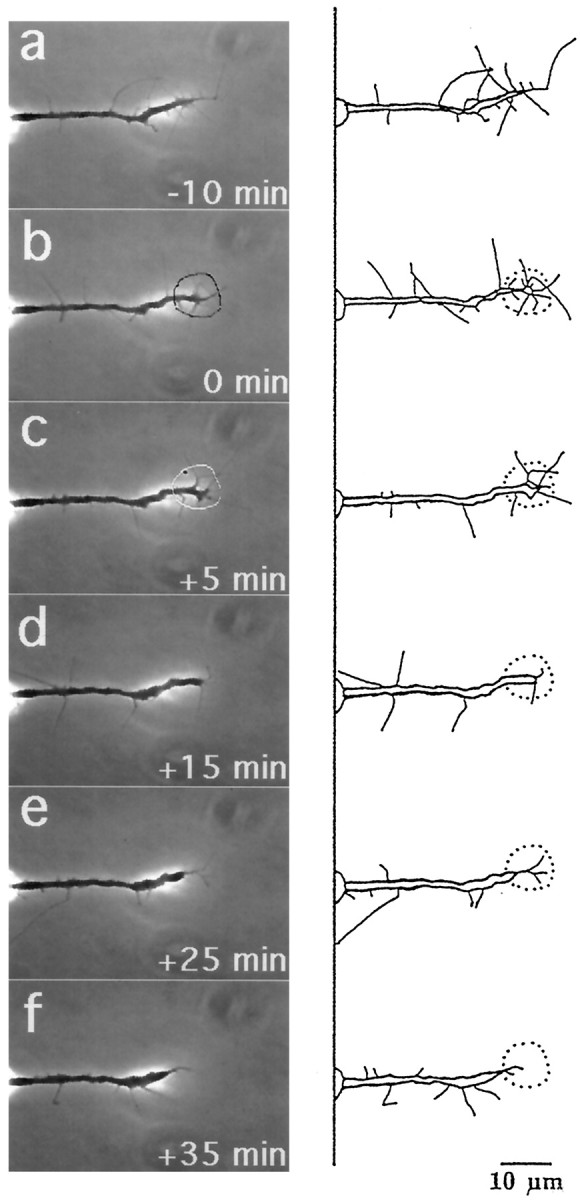
Micro-CALI of NCAM-180 and L1 simultaneously causes both neurite retraction and growth cone collapse. DRG neurons were loaded with MG-labeled 4d and incubated with MG-labeled 8D9. After observation for 10 min (a), the growth cone was irradiated for 5 min, starting at t = 0 (b, black outline in b) and ended at t = +5 min (c,white outline). Shortly thereafter, the filopodia and lamellipodia retract, and the growth cone continues to collapse, but the neurite does not decrease in length (d). Over the next 10 min the neurite retracts (e) and begins to recover after an additional 10 min (f). Scale bar, 10 μm.
DISCUSSION
We have shown that CALI of L1 and NCAM-180 causes their specific inactivation in vitro. In chick DRG growth cones, micro-CALI of L1 caused neurite retraction but had no effect on growth cone motility, and micro-CALI of the cytoplasmic domain of NCAM-180 caused local growth cone retraction and turning but had no effect on neurite extension. Because neurite extension and growth cone protrusion are differentially affected by the loss of L1 and NCAM-180, we suggest that these two adhesion molecules act on different pathways.
Inactivation by micro-CALI of L1 and NCAM-180 is specific. Laser irradiation of growth cones loaded with a variety of control reagents, including MG-labeled nonspecific IgG, BSA, and 5e, did not affect growth cone behavior. This last control is especially important because 5e binds to the growth cone membrane. CALI is spatially restricted to a half maximal radius of damage of ∼15 Å from each MG moeity (Liao et al., 1994). CALI-induced damage has been shown to be essentially limited to the polypeptide recognized by the MG-labeled antibody within multisubunit complexes (Liao et al., 1995) or to a single domain of a large protein such as myosin V (Wang et al., 1996). Thus, CALI-induced damage to the extracellular domain may not affect an intracellular domain that is separated by the thickness of the intervening plasma membrane (∼70 nm). Interestingly, inactivation of either the intracellular or extracellular domains of L1 caused neurite retraction, suggesting that there are critical domains of L1 required for neurite extension that are found on both sides of the membrane. The possibility that CALI-mediated damage could result in a constitutively active form of the targeted protein is unlikely here; the use of micro-CALI of L1 directed against two distinct domains had similar effects that were similar to the effects seen using function-blocking antibodies (Lemmon et al., 1989). Lastly, CALI of other growth cone proteins has caused a variety of different effects during neurite outgrowth (Sydor et al., 1996; Wang et al., 1996; Takei et al., 1998;Castelo and Jay, 1999).
Many studies have implicated L1 in neurite outgrowth. Previous studies using function-blocking antibodies have implicated L1 in neurite outgrowth and fasciculation (Chang et al., 1987; Rathjen et al., 1987; Lemmon et al., 1989; Miura et al., 1992). The use of genetic knock-outs have also implicated L1 in axon outgrowth or guidance. Transgenic mice lacking L1 have enlarged ventricles and dramatic hypoplasia of in the corticospinal tract (Cohen et al., 1997; Dahme et al., 1997). Kamiguchi et al. (1998) have suggested that both of these phenotypes may be caused by the absence of axons. For all of these studies the loss of L1 was chronic and global and could not show when or where L1 is required. Our findings support these studies and further show that L1 is required in the nerve growth cone for neurite extension. They suggest a cellular explanation for the defects observed in genetic knock-out mice; neurite extension is decreased by the absence of L1 in growth cones such that targets are not reached. Although some studies have implicated NCAM in neurite outgrowth (Silver and Rutishauser, 1984; Bixby et al., 1987; Doherty et al., 1989), others have suggested that L1 and not NCAM acts in neurite extension (Chang et al., 1987; Hankin and Lagenaur 1994). Genetic knock-outs of NCAM-180 (Tomasiewicz et al., 1993) or all NCAM isoforms (Cremer et al., 1994) showed primarily defects in embryonic neuronal migration and in spatial learning, but recent studies have suggested defects in axon growth and fasciculation in the hippocampus of older animals (Cremer et al., 1997; Treloar et al., 1997). Our findings suggest a role of NCAM-180 in the growth cone during leading edge protrusion that may be important either during outgrowth or plasticity.
How might L1 and NCAM-180 act in neurite outgrowth? Some studies have suggested distinct roles for L1 and NCAM during axon tract formation, fasciculation, and innervation of targets (Landmesser et al., 1988;Beggs et al., 1994; Hankin and Lagenaur, 1994; Ignelzi et al., 1994;Brittis et al., 1995). Others have suggested that these proteins may act together (Kadmon et al., 1990) to increase neurite outgrowth (Horstkorte et al., 1993). It has also been suggested that they function through a common pathway involving fibroblast growth factor receptor (Williams et al., 1994; for review, see Doherty et al., 1996). Our findings do not support a role for L1 and NCAM-180 interacting with each other or with a common pathway during neurite outgrowth because their inactivation affects different cellular processes.
Most studies have concentrated on L1 and NCAM acting via homophilic adhesion (Lemmon et al., 1989; Miura et al., 1992). However, there is a growing body of evidence that heterophilic associations of these proteins are also important. For example, L1 can interact with laminin (Grumet et al., 1993; Takeda et al., 1996) and integrin receptors (Yip et al., 1998). A variety of signal transduction molecules have been implicated in L1 and NCAM function that may be important for their cellular roles (Schuch et al., 1988; Beggs et al., 1994; Ignelzi et al., 1994; Wong et al., 1995; Doherty and Walsh, 1996). There is also evidence of interactions of L1 and NCAM-180 with cytoskeletal components (Pollerberg et al., 1987; Davis and Bennett, 1994). Our findings provide support for the importance of heterophilic interactions for L1 and NCAM-180 in the growth cone to extracellular components such as laminin (Grumet et al., 1993) or integrins (Yip et al., 1998). Consistent with this, transfection of full-length L1 increases cell migration on laminin (Takeda et al., 1996). These CAMs may also act via their interactions with the growth cone cytoskeleton because the processes affected by micro-CALI of L1 and NCAM-180 are dependent on microtubules and F-actin, respectively.
Neurite extension is highly dependent on microtubule dynamics (Sabry et al., 1991; Tanaka et al., 1995). The effects of micro-CALI of L1 resembles colchicine treatment (a microtubule inhibitor) both in the morphological changes observed and the time course of effect (Letourneau et al., 1987). As such, we suggest that L1 may function by regulating microtubules during neurite extension. Consistent with this notion, microtubules redistribute when growth cones encounter L1 in their environment (Burden-Gulley and Lemmon, 1996). Our findings are not consistent with a role for L1 in actin-mediated processes in the growth cone, although L1 does bind to ankyrin, an actin-associated protein (Davis and Bennett, 1994). Others have observed that cytochalasin B (an F-actin inhibitor) inhibits neurite outgrowth of neurons grown on laminin and NCAM but not on L1 (Abosch and Lagenaur, 1993). How L1 may act to regulate microtubules is not known, but the time delay that we see between CALI of L1 and neurite retraction is consistent with signal transduction instead of a direct physical interaction. One possible mechanism is the regulation of tyrosine phosphorylation. Atashi et al. (1992) showed that L1 activation in a growth cone-enriched membrane fraction specifically reduces the phosphorylation of membrane-associated tubulin at tyrosine residues through the modulation of protein tyrosine kinases. It has also been reported that tyrosine phosphorylation of tubulin decreased its polymerization rate (Matten et al., 1990).
The effects of micro-CALI of NCAM-180 resemble cytochalasin addition both in the morphological changes seen and the time course of effect (Letourneau et al., 1987). This suggests that NCAM-180 acts on F-actin-mediated processes at the leading edge. Actin-mediated processes are critical for axon guidance, as evidenced by the fact that depolymerization of F-actin by cytochalasin B inhibits pathfindingin vitro and in vivo (Marsh and Letourneau, 1984;Bentley and Toroian-Raymond, 1986). Indeed, the leading edge retraction by asymmetric loss of NCAM-180 by micro-CALI also caused growth cone turning. How NCAM-180 may act on actin-mediated processes is not known, but our findings suggest that NCAM-180 binding to brain spectrin, a major component of the F-actin cytoskeleton (Bennett et al., 1982), may be important. NCAM-180 binds brain spectrin (Pollerberg et al., 1987), and they colocalize in DRG growth cones (Letourneau and Shattuck, 1989).
How might L1 and NCAM-180 act in vivo? Our findings suggest that growth cone protrusion and neurite extension can be independently controlled by the action of these CAMs in the growth cone. Letourneau et al. (1987) postulated that neurite outgrowth is controlled by the “push” and “pull” exerted by anterograde movement of microtubules and F-actin-mediated growth cone protrusion, respectively. Either process may be rate-limiting for neurite outgrowth in vivo, depending on the cues in the environment near the growth cone. Thus, the local modulation of L1 and NCAM-180 function in the growth cone by environmental cues may differentially regulate the relative contribution of “push” and “pull” to neurite outgrowth of developing neurons.
Footnotes
This work was supported by the Ford Foundation (T.A.C.), a National Research Service Award fellowship (F.S.W.), National Institutes of Health Grants HL17411 (H.D.), HD18369 and EY06107 (U.R.), and NS34699 (D.G.J.), and the Esther A. and Joseph Klingenstein Fund (D.G.J.). We are indebted to Melitta Schachner, Chi Hung Siu, Vann Bennett, Keiichi Uyemura, Masayuki Miura, Yasuo Takeda, and Vance Lemmon for reagents. We are grateful to Daniel Branton, Tom Diefenbach, and Andrea Buchstaller for critical reading of this manuscript.
Correspondence should be addressed to Daniel Jay, Department of Physiology, Tufts University School of Medicine, Boston MA 02111. E-mail: djay01@emerald.tufts.edu.
Dr. Takei's present address: Calciosignal Net Project, Exploratory Research for Advanced Technology, Japan Science and Technology Corporation, Tokyo 113–0021, Japan.
REFERENCES
- 1.Abosch A, Lagenaur CF. Sensitivity of neurite outgrowth to microfilament disruption varies with adhesion molecule substrate. J Neurobiol. 1993;21:344–355. doi: 10.1002/neu.480240307. [DOI] [PubMed] [Google Scholar]
- 2.Atashi JR, Klinz SG, Ingraham CA, Matten WT, Schachner M, Maness PF. Neural cell adhesion molecules modulate tyrosine phosphorylation of tubulin in nerve growth cone membranes. Neuron. 1992;8:831–842. doi: 10.1016/0896-6273(92)90197-l. [DOI] [PubMed] [Google Scholar]
- 3.Beggs HE, Soriano P, Maness P. NCAM-dependent neurite outgrowth is inhibited in neurons derived from fyn-minus mice. J Cell Biol. 1994;127:825–833. doi: 10.1083/jcb.127.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett V, Davis J, Fowler VE. Brain spectrin, a membrane associated protein related in structure and function of erythrocyte spectrin. Nature. 1982;299:126–131. doi: 10.1038/299126a0. [DOI] [PubMed] [Google Scholar]
- 5.Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- 6.Bixby JL, Bookman RJ. Intracellular mechanisms of axon growth induction by CAMs and integrins: some unresolved issues. Perspect Dev Neurobiol. 1996;4:147–156. [PubMed] [Google Scholar]
- 7.Bixby JL, Pratt RS, Lilien J, Reichardt LF. Neurite outgrowth on muscle cell surfaces involves extracellular matrix receptors as well as Ca2+-dependent and -independent cell adhesion molecules. Proc Natl Acad Sci USA. 1987;84:2555–2559. doi: 10.1073/pnas.84.8.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray D. Isolated chick neurons for the study of axon growth. In: Banker G, Goslin K, editors. Culturing nerve cells. MIT; Cambridge, MA: 1991. pp. 104–120. [Google Scholar]
- 9.Brittis PA, Lemmon V, Rutishauser U, Silver J. Unique changes of ganglion cell growth cone behavior following cell adhesion molecule perturbations: a time-lapse study of the living retina. Mol Cell Neurosci. 1995;6:433–449. doi: 10.1006/mcne.1995.1032. [DOI] [PubMed] [Google Scholar]
- 10.Burden-Gulley SM, Lemmon V. L1, N-cadherin and laminin induce distinct distribution patterns of cytoskeletal elements in growth cones. Cell Motil Cytoskeleton. 1996;35:1–23. doi: 10.1002/(SICI)1097-0169(1996)35:1<1::AID-CM1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Burgoon MP, Grumet M, Mauro V, Edelman GM, Cunningham BA. Structure of the chicken neuron-glia cell-adhesion molecule, NG-CAM –origin of the polypeptides and relation to the Ig superfamily. J Cell Biol. 1991;112:1017–1029. doi: 10.1083/jcb.112.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castelo L, Jay DG. Radixin is involved in lamellipodial stability during nerve growth cone motility. Mol Biol Cell. 1999;10:1511–1520. doi: 10.1091/mbc.10.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang S, Rathjen FG, Raper JA. Extension of neurites on axons is impaired by antibodies against specific neural cell-surface glycoproteins. J Cell Biol. 1987;104:355–362. doi: 10.1083/jcb.104.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang HY, Takei K, Sydor AM, Born T, Rusnak F, Jay DG. Asymmetric retraction of growth cone filopodia following focal inactivation of calcineurin. Nature. 1995;376:686–690. doi: 10.1038/376686a0. [DOI] [PubMed] [Google Scholar]
- 15.Cohen NR, Taylor JSH, Scott LD, Guillery RW, Soriano P, Furley AJW. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol. 1997;8:26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- 16.Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, Barthels D, Rajewsky K, Wille W. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- 17.Cremer H, Chazal G, Goridis C, Represa A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol Cell Neurosci. 1997;8:323–335. doi: 10.1006/mcne.1996.0588. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236:799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- 19.Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17:346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- 20.Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/NrCAM/L1 family of nervous system cell adhesion molecules. J Biol Chem. 1994;269:27163–27166. [PubMed] [Google Scholar]
- 21.Diamond P, Mallavarapu A, Schnipper J, Booth J, Park L, O'Connor TP, Jay DG. Fasciclin I and II have distinct roles in the development of grasshopper pioneer neurons. Neuron. 1993;11:1–20. doi: 10.1016/0896-6273(93)90146-i. [DOI] [PubMed] [Google Scholar]
- 22.Doherty P, Barton CH, Dickson G, Seaton P, Rowett LH, Moore SE, Gower HJ, Walsh FS. Neuronal process outgrowth of human sensory neurons on monolayers of cells transfected with cDNAs for five human N-CAM isoforms. J Cell Biol. 1989;109:789–798. doi: 10.1083/jcb.109.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doherty P, Smith P, Walsh FS. Shared cell adhesion molecule (CAM) homology domains point to CAMs signalling via FGF receptors. Perspect Dev Neurobiol. 1996;4:157–168. [PubMed] [Google Scholar]
- 24.Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 25.Frelinger AL, III, Rutishauser U. Topography of NCAM structural and functional determinants II. Placement of monoclonal antibody epitopes. J Cell Biol. 1986;103:1729–1737. doi: 10.1083/jcb.103.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg DJ, Burmeister DW. Stages of axon formation: Observations of growth by Aplysia axons in culture using video-enhanced contrast-differential interference contrast microscopy. J Cell Biol. 1986;103:1921–1932. doi: 10.1083/jcb.103.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grumet M, Friedlander DR, Edelman GM. Evidence for the binding of Ng-CAM to laminin. Cell Adhes Commun. 1993;2:177–190. doi: 10.3109/15419069309095693. [DOI] [PubMed] [Google Scholar]
- 28.Hankin MH, Lagenaur CF. Cell adhesion molecules in the early developing mouse retina: Retinal neurons show preferential outgrowth in vitro on L1 bur not NCAM. J Neurobiol. 1994;25:472–487. doi: 10.1002/neu.480250503. [DOI] [PubMed] [Google Scholar]
- 29.Horstkorte R, Schachner M, Magyar JP, Vorherr T, Schmitz B. The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans implicated in association with L1 and neurite outgrowth. J Cell Biol. 1993;121:1409–1421. doi: 10.1083/jcb.121.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ignelzi MA, Miller DR, Soriano P, Maness PF. Impaired neurite outgrowth of src-minus cerebellar neurons on the cell adhesion molecule L1. Neuron. 1994;12:9473–9484. doi: 10.1016/0896-6273(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 31.Jay DG. Selective destruction of protein function by chromophore-assisted laser inactivation. Proc Natl Acad Sci USA. 1988;85:5454–5458. doi: 10.1073/pnas.85.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jay DG, Keshishian H. Laser inactivation of fasciclin I disrupts axon adhesion of grasshopper pioneer neurons. Nature. 1990;348:548–550. doi: 10.1038/348548a0. [DOI] [PubMed] [Google Scholar]
- 33.Kadmon G, Kowitz A, Altevogt P, Schachner M. The neural cell adhesion molecule N-CAM enhances L1-dependent cell-cell interactions. J Cell Biol. 1990;110:193–208. doi: 10.1083/jcb.110.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamiguchi H, Hlavin ML, Lemmon V. Role of L1 in neural development: what the knockouts tell us. Mol Cell Neurosci. 1998;12:48–55. doi: 10.1006/mcne.1998.0702. [DOI] [PubMed] [Google Scholar]
- 35.Lagenaur CF, Lemmon V. An L1-like molecule 8D9 antigen, is a potent substrate for neurite extension. Proc Natl Acad Sci USA. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landmesser L, Dahm L, Schultz K, Rutishauser U. Distinct roles for adhesion molecules during innervation of embryonic chick muscle. Dev Biol. 1988;130:645–670. doi: 10.1016/0012-1606(88)90358-2. [DOI] [PubMed] [Google Scholar]
- 37.Lemmon V, McLoon SC. The appearance of an L1-like molecule in the chick primary visual pathway. J Neurosci. 1986;6:2987–2994. doi: 10.1523/JNEUROSCI.06-10-02987.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemmon V, Farr KL, Lagenaur C. L1-mediated axon outgrowth via a homophilic binding mechanism. Neuron. 1989;2:1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- 39.Letourneau PC. The cytoskeleton in nerve growth cone motility and axonal pathfinding. Perspect Dev Neurobiol. 1996;4:111–124. [PubMed] [Google Scholar]
- 40.Letourneau PC, Shattuck TA. Distribution and possible interaction of actin-associated proteins and cell adhesion molecules of nerve growth cones. Development. 1989;105:505–519. doi: 10.1242/dev.105.3.505. [DOI] [PubMed] [Google Scholar]
- 41.Letourneau PC, Shattuck TA, Ressler AH. “Push” and “pull” in neurite elongation: observations of the effects of different concentrations of cytochalasin B and taxol. Cell Motil Cytoskel. 1987;8:193–209. doi: 10.1002/cm.970080302. [DOI] [PubMed] [Google Scholar]
- 42.Liao JC, Roider J, Jay DG. Chromophore-assisted laser inactivation of proteins is mediated by the photogeneration of free radicals. Proc Natl Acad Sci USA. 1994;91:2659–2663. doi: 10.1073/pnas.91.7.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao JC, Berg LJ, Jay DG. Chromophore-assisted laser inactivation of subunits of the T cell receptor in living cells is spatially restricted. Photochem Photobiol. 1995;62:923–929. doi: 10.1111/j.1751-1097.1995.tb09157.x. [DOI] [PubMed] [Google Scholar]
- 44.Luo YL, Raible D, Raper JA. Collapsin - a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 45.Marsh L, Letourneau P. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol. 1984;99:2041–2047. doi: 10.1083/jcb.99.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matten WT, Aubry M, West J, Maness PF. Tubulin is phosphorylated by pp60c-src in nerve growth cone membranes. J Cell Biol. 1990;111:1959–1970. doi: 10.1083/jcb.111.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 48.Miura M, Asou H, Kobayashi M, Uyemura K. Functional expression of a full length cDNA coding for rat neural cell adhesion molecule L1 mediates homophilic intercellular adhesion and migration of cerebellar neurons. J Biol Chem. 1992;267:10752–10758. [PubMed] [Google Scholar]
- 49.Persohn E, Schachner M. Immunoelectron microscopic localization of the neural cell adhesion molecules L1 and NCAM during postnatal development of the mouse cerebellum. J Cell Biol. 1987;105:569–576. doi: 10.1083/jcb.105.1.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollerberg E, Burridge K, Krebs KE, Goodman SR, Schachner M. The 180 kD component of the neural cell adhesion molecule NCAM is involved in cell-cell contacts and cytoskeleton-membrane interactions. Cell Tissue Res. 1987;250:227–236. doi: 10.1007/BF00214676. [DOI] [PubMed] [Google Scholar]
- 51.Rathjen FG, Schachner M. Immunological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984;3:1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rathjen FG, Wolff JM, Frank R, Bonhoeffer F, Rutishauser U. Membrane glycoproteins involved in neurite fasciculation. J Cell Biol. 1987;104:343–353. doi: 10.1083/jcb.104.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutishauser U, Edelman GM. Effects of fasciculation of the outgrowth of neurites from spinal ganglia in culture. J Cell Biol. 1980;87:370–378. doi: 10.1083/jcb.87.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutishauser U, Hoffman S, Edelman GM. Binding properties of a cell adhesion molecule from neural tissue. Proc Natl Acad Sci USA. 1982;79:685–689. doi: 10.1073/pnas.79.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabry JH, Tanaka E. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 56.Sabry JH, O'Connor TP, Evans L, Toroian-Raymond A, Kirschner M, Bentley D. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991;115:381–395. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saffell JL, Doherty P, Tiveron MC, Morris RJ, Walsh FS. NCAM requires a cytoplasmic domain to function as a neurite outgrowth-promoting neuronal receptor. Mol Cell Neurosci. 1995;6:521–531. doi: 10.1006/mcne.1995.0004. [DOI] [PubMed] [Google Scholar]
- 58.Schuch U, Lohse MJ, Schachner M. Neural cell adhesion molecules influence second messenger systems. Neuron. 1988;3:13–20. doi: 10.1016/0896-6273(89)90111-6. [DOI] [PubMed] [Google Scholar]
- 59.Silver J, Rutishauser U. Guidance of optic axons in vivo by a preformed adhesive pathway on neuroepithelial endfeet. Dev Biol. 1984;106:485–499. doi: 10.1016/0012-1606(84)90248-3. [DOI] [PubMed] [Google Scholar]
- 60.Stoeckli ET, Ziegler U, Bleiker AJ, Groscurth P, Sonderegger P. Clustering and functional cooperation of Ng-CAM and axonin-1 in the substratum-contact area of growth cones. Dev Biol. 1996;177:15–29. doi: 10.1006/dbio.1996.0141. [DOI] [PubMed] [Google Scholar]
- 61.Sydor AM, Su AL, Wang FS, Xu A, Jay DG. Talin and vinculin play distinct roles in filopodial motility in the neuronal growth cone. J Cell Biol. 1996;134:1197–1207. doi: 10.1083/jcb.134.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeda Y, Asou H, Murakami Y, Miura M, Kobayashi M, Uyemura K. A nonneuronal isoform of cell adhesion molecule L1: tissue-specific expression and functional analysis. J Neurochem. 1996;66:2338–2349. doi: 10.1046/j.1471-4159.1996.66062338.x. [DOI] [PubMed] [Google Scholar]
- 63.Takei K, Shin RM, Inoue T, Kato K, Mikoshiba K. Regulation of nerve growth mediated by inositol 1,4,5-trisphosphate receptors in growth cones. Science. 1998;282:1705–1708. doi: 10.1126/science.282.5394.1705. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka E, Ho T, Kirschner M. The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol. 1995;128:139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomasiewicz H, Ono K, Yee DL, Thompson C, Goridis C, Rutishauser U, Magnuson T. Genetic deletion of a neural cell-adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron. 1993;11:1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- 66.Treloar H, Tomasiewicz H, Magnuson T, Key B. The central pathway of primary olfactory axons is abnormal in mice lacking the N-CAM-180 isoform. J Neurobiol. 1997;32:643–658. doi: 10.1002/(sici)1097-4695(19970620)32:7<643::aid-neu1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 67.Wang FS, Jay DG. CALI and the dissection of growth cone motility. Trends Cell Biol. 1996;6:442–445. doi: 10.1016/s0962-8924(96)40005-8. [DOI] [PubMed] [Google Scholar]
- 68.Wang FS, Wolenski JS, Cheney RE, Mooseker MS, Jay DG. Requirement of myosin V in filopodial extension in neuronal growth cones. Science. 1996;273:660–663. doi: 10.1126/science.273.5275.660. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe M, Frelinger AL, III, Rutishauser U. Topography of NCAM structural and functional determinants I. Classification of monoclonal antibody epitopes. J Cell Biol. 1986;103:1721–1727. doi: 10.1083/jcb.103.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams AF, Barclay AN. The immunoglobulin superfamily-domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 71.Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, NCAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 72.Wong EV, Kenwrick S, Willems P, Lemmon V. Mutations in the cell adhesion molecule L1 cause mental retardation. Trends Neurosci. 1995;18:168–172. doi: 10.1016/0166-2236(95)93896-6. [DOI] [PubMed] [Google Scholar]
- 73.Yip PM, Zhao X, Montgomery AMP, Siu CH. The Arg-Gly-Asp motif of the cell adhesion molecule L1 promotes neurite outgrowth via interaction with the αVβ3 integrin. Mol Biol Cell. 1998;9:277–290. doi: 10.1091/mbc.9.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]