Abstract
Considerable evidence supports a role for brainstem adrenergic and noradrenergic inputs to corticotropin-releasing hormone (CRH) cells of the hypothalamic paraventricular nucleus (PVN), in the control of hypothalamic–pituitary–adrenocortical (HPA) axis function. However, little is known about specific adrenoceptor (ADR) subtypes in CRH-containing cells of the PVN. Here we demonstrate, using dualin situ hybridization, that mRNA encoding α1b ADR is colocalized with CRH in the rat PVN. Furthermore, we confirm that these α1b ADR mRNA-containing cells are stress-responsive, by colocalization with c-fos mRNA after restraint, swim, or immune stress.
To determine whether expression of α1b ADR mRNA is influenced by circulating glucocorticoids, male rats underwent bilateral adrenalectomy (ADX) or sham surgery, and were killed after 1, 3, 7, or 14 d. In situ hybridization revealed levels of α1b ADR mRNA were increased in the PVN 7 and 14 d after ADX, but were not altered in the hippocampus, amygdala, or dorsal raphe. Additional rats underwent ADX or sham surgery and received a corticosterone pellet (10 or 50 mg) or placebo for 7 d. Corticosterone replacement (10 mg) reduced the ADX-induced increase in PVN α1b ADR mRNA to control levels, whereas 50 mg of corticosterone replacement resulted in a decrease in PVN α1b ADR mRNA as compared with all other groups. Furthermore, levels of plasma corticosterone were significantly correlated (inverse relationship) with α1b ADR mRNA in the PVN.
We conclude that α1b ADR mRNA is expressed in CRH-containing, stress-responsive cells of the PVN and is highly sensitive to circulating levels of corticosterone. Because activation of the α1B adrenoceptor is predominantly excitatory within the brain, we predict that this receptor plays an important role in facilitation of the HPA axis response.
Keywords: adrenergic, adrenoceptor, HPA axis, stress, CRH, hypothalamus, paraventricular nucleus, in situhybridization, adrenalectomy, glucocorticoid, corticosterone
The hypothalamic–pituitary–adrenocortical (HPA) axis is critical to an animal's response to stress (for review, see Akil et al., 1999). Parvocellular cells of the paraventricular nucleus of the hypothalamus (PVN) project to the external zone of the median eminence and release corticotropin-releasing hormone (CRH) and other corticotropin-releasing factors into the portal circulation to stimulate corticotrophs of the anterior pituitary. These cells subsequently release adrenocorticotropin hormone (ACTH), which in turn stimulates release of glucocorticoids (corticosterone in rat and cortisol in humans) from the adrenal cortex, a defining feature of a stress response. Considerable research has focused on elucidating the neural circuits that regulate the HPA axis at the level of the PVN, and a number of direct and indirect inputs have been demonstrated (Gaillet et al., 1991; Larsen and Mikkelsen, 1995; Cullinan et al., 1996; Li et al., 1996; Herman and Cullinan, 1997).
Direct adrenergic and noradrenergic inputs from the brainstem nucleus tractus solitarius and ventrolateral medulla to the parvocellular PVN have been described (Cunningham and Sawchenko, 1988; Cunningham et al., 1990), and the importance of these pathways in regulation of the HPA axis has been widely demonstrated (Gaillet et al., 1991; Itoi et al., 1994; Parsadaniantz et al., 1995; Smith et al., 1995). Evidence suggests that these inputs primarily have a facilitatory effect on HPA axis regulation (Guillaume et al., 1987; Plotsky, 1987; Plotsky et al., 1989). However, the adrenergic receptor (ADR) subtypes that may mediate the effects of epinephrine or norepinephrine at the level of the PVN have not been well characterized. Although considerable evidence supports an involvement of α1 adrenoceptors at the level of the PVN (Plotsky, 1987; Calogero et al., 1988; Kiss and Aguilera, 1992; Whitnall et al., 1993), the subtype or subtypes of α1 adrenoceptors have not been determined, in part because of the lack of selective ligands capable of discriminating between similar receptor subtypes. However, our laboratory and others have demonstrated by in situ hybridization that mRNA encoding the α1b ADR subtype is present at moderate levels in parvocellular cells of the rat PVN (McCune et al., 1993; Pieribone et al., 1994; Day et al., 1997). Stimulation of α1 adrenoceptors within the CNS is thought to be predominantly excitatory, via decreased potassium conductance and slow depolarization (Bevan et al., 1977; McCormick and Prince, 1988; Pan et al., 1994; Bergles et al., 1996), consistent with the hypothesis that activation of the α1B ADR subtype could be involved in stimulation of the HPA axis. To ascertain more directly whether the α1B ADR subtype might mediate excitation of CRH cells of the PVN, we determined, using dualin situ hybridization histochemistry, whether α1b ADR mRNA is expressed within CRH cells of the PVN. Furthermore, we investigated whether cells expressing this receptor are active after stress, by the colocalization of c-fos with α1b ADR mRNA in cells of the PVN after restraint, swim, or immune stress. In addition, because the gene for this receptor has been shown to contain a glucocorticoid response element (GRE) within its promoter region (Gao and Kunos, 1993), we went on to examine the effect of circulating glucocorticoids on expression of α1b ADR mRNA in different brain regions after bilateral adrenalectomy, with low or high dose corticosterone replacement. Our data suggest that α1b ADR mRNA is expressed in the majority of CRH-containing, stress-responsive cells of the PVN and that the levels of expression of this receptor within the PVN (but not other brain regions) are inversely related to circulating corticosterone levels.
MATERIALS AND METHODS
Animals. All procedures described were approved by the University of Michigan Committee on Use and Care of Animals. Adult male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were used throughout. All animals were allowed to habituate to the housing conditions for at least 10 d before any experimental manipulation. Rats were housed two or three per cage under conditions of constant temperature and humidity, on a 12 hr light/dark cycle (lights on at 7:00 A.M.), with ad libitum access to food and water.
Experiment 1: expression of α1b ADR mRNA in CRH and stress-responsive cells of the PVN. Four animals, naive to any experimental manipulation were used to determine the extent of colocalization of α1b ADR mRNA in CRH-containing cells of the PVN. These data, combined with previous studies that have demonstrated that the majority of stress-induced c-fos mRNA or Fos protein-containing cells of the medial parvocellular PVN are CRH-positive (Ericsson et al., 1994; Rivest and Rivier, 1994;Day et al., 1999), suggested that α1b ADR mRNA-containing cells of the PVN are stress-responsive. To confirm this, five animals were handled daily for 1 week, then subjected to different stressors: 30 min restraint or swim stress (Cullinan et al., 1995), n = 2 per group, or intraperitoneal injection of human recombinant IL-1β, 5 μg/kg (Bachem), 30 min before killing (n = 1). Previous data suggested that the intraperitoneal injection procedure per se was not stressful, as indicated by a lack of corticosterone secretion and c-fos mRNA expression in the PVN after saline intraperitoneally (Day and Akil, 1996). Animals were killed by rapid decapitation 2–3 hr after lights on (9:00–10:00 A.M.). The brains were removed and frozen in isopentane cooled to −40 to −50°C, and stored at −80°C. Sections (10 μm) were cut on a cryostat (Bright) through the hypothalamus. Tissue was air-dried and stored at −80°C until processing for dual in situ hybridization, as described previously (Day et al., 1999). Briefly, cRNA probes complementary to CRH (courtesy of Dr. R. Thompson, University of Michigan, Ann Arbor, MI; 770 mer) or c-fos (Dr. T. Curran, St. Jude Children's Research Hospital, Memphis, TN; 680 mer) were generated and labeled with digoxigenin-UTP (dig-UTP; Boehringer Mannheim, Indianapolis, IN) using standard transcription methods. A cRNA probe complementary to α1b ADR mRNA (courtesy of Dr. R. Lefkowitz, Duke University Medical Center, Durham, NC; 766 mer) was generated and labeled with [35S]CTP and [35S]-UTP. Brain sections were hybridized overnight with both probes. The following day, sections were treated with RNase A (200 μg/ml) for 1 hr at 37°C and washed to a final stringency of 0.1× SSC at 65°C for 1 hr. Sections were then processed for visualization of the digoxigenin-labeled probe. Briefly, sections were incubated overnight with an antibody against digoxigenin, conjugated to alkaline phosphatase (sheep anti-dig-AP; Fab fragments; Boehringer Mannheim), diluted 1:20,000. After extensive washing, sections underwent a color reaction by addition of 0.45% nitro blue tetrazolium chloride (Boehringer Mannheim) and 0.35% 5-bromo-4-chloro-3-indoylphosphate, 4-toluidine salt (Boehringer Mannheim). After completion of the color reaction (∼18 hr) sections were rinsed and stripped of antibody by incubation with 0.1m glycine and 0.5% Triton-X 100, pH 2.2, for 10 min. Finally sections were fixed in 2.5% glutaraldehyde for 1 hr. These last steps were found to help prevent the increase in background after processing for radioactive signal. After exposure to x-ray film (5 d), sections were dipped in liquid emulsion (Ilford KD-5; Polysciences, Warrington, PA) and stored in light-tight boxes for 1 month. After this time sections were developed (Kodak D-19; Eastman Kodak, Rochester, NY), dehydrated, and coverslipped in a xylene-based mounting medium (Permount). The cellular distribution was determined using a Leica (Nussloch, Germany; Leitz DMR) microscope. Nonradioactive probes (CRH or c-fos) were visualized under bright field as a blue–purple precipitate, whereas the radioactive probe (α1b ADR) was visualized under dark field by silver grain distribution. It should be noted that the nonradioactivein situ hybridization technique is less sensitive than the radioactive technique, hence the digoxigenin-labeled cell population tends to be under-represented. Ideally, both combinations of radioactive and nonradioactive probes would be analyzed. However, the abundance of α1b ADR mRNA in the PVN was not sufficient to obtain reliable labeling with the nonradioactive in situ hybridization technique.
Sections at 50–80 μm intervals, three to five sections per animal, in the region of the medial parvocellular PVN were analyzed. Cell profile counts were determined at 40× magnification, with the aid of an eyepiece grid. No attempt was made to establish absolute numbers of cells within these structures. Rather, the numbers of cell profiles counted for each animal were used to approximate the relative percent colocalization of mRNA for that animal.
Experiment 2a: adrenalectomy time course. For the initial time course, 35 rats were used (mean weight, 313 ± 4 gm). Of these, 28 were anesthetized with pentobarbital and underwent bilateral adrenalectomy (ADX; four per group; n = 16) or sham surgery (SHAM; three per group; n = 12). The adrenalectomy was performed via a dorsolateral approach, and the incision was closed with wound clips. Animals were housed two or three per cage, and drinking water was replaced with a solution containing 0.9% saline and 5% dextrose. Animals were killed either 1 (22–24 hr), 3, 7, or 14 d after surgery. In addition, three unoperated (UNOP) animals were included that did not undergo any surgical manipulation, but were given the saline–dextrose solution to drink, and four naive animals (NAIVE) that were not subjected to any experimental manipulation. Animals were killed 2 hr before lights off (5:00 P.M.) so that there were detectable levels of plasma corticosterone in control animals against which to compare the effectiveness of the ADX surgery. Trunk blood was collected in chilled tubes containing EDTA, and plasma was separated and stored at −20°C for analysis of corticosterone. Brains were removed, frozen in isopentane cooled to −40 to −50°C, and stored at −80°C. Sections (10 μm) were cut on a cryostat through the hypothalamus, hippocampus, and brainstem, and stored at −80°C until processing for in situ hybridization (as described by Day et al., 1997).
Experiment 2b: adrenalectomy and corticosterone replacement.For the second part of the study, 30 animals were used, divided into the following groups: NAIVE (n = 6), SHAM (n = 6), ADX plus placebo (n = 6), ADX plus 10 mg of corticosterone (n = 6), and ADX plus 50 mg of corticosterone (n = 6). The mean start weight was 291 ± 3 gm. In addition to bilateral adrenalectomy, animals were implanted with a subcutaneous pellet (Innovative Research of America) within the dorsal neck region. Rats received either placebo, 10 mg, or 50 mg of corticosterone in the form of 21 d release pellets. Surgery was performed over a 2 d period,n = 3 per group per day. Drinking water for SHAM and all ADX groups was replaced with an isotonic solution containing 0.45% saline and 2.5% dextrose. Three days after surgery, a tail vein blood sample (75 μl) was taken from each animal 2 hr after lights on (9:00 A.M.). For this procedure, animals were restrained lightly, a lateral tail vein was punctured with the corner of a razor blade, and blood was collected in a heparinized capillary tube. Animals were killed 7 d after surgery, 1–2 hr before lights off (5:00–6:00 P.M.). Trunk blood and brains were collected as described in part 2a. Coronal brain sections (10 μm) were cut through the hypothalamus and hippocampus and stored at −80°C until processing for in situhybridization (as described by Day et al., 1997).
Corticosterone analysis. Levels of plasma corticosterone were analyzed as previously described (Day and Akil, 1996). Briefly, 10 μl duplicate samples of plasma were incubated overnight with an antibody against corticosterone (raised in our laboratory) and [3H]corticosterone (Amersham, Arlington Heights, IL). Bound versus free corticosterone was separated by charcoal extraction, and levels were calculated by comparison with a standard curve.
Semiquantitative mRNA analysis. Levels of α1b ADR mRNA were analyzed by computer-assisted optical densitometry. Brain section images from in situhybridization experiments were captured digitally (CCDcamera, model XC-77; Sony, Tokyo, Japan), and the relative optical density of the x-ray film was determined for each brain region using NIH Image 1.61 for Macintosh computer. A macro was written (Dr. Serge Campeau, University of Michigan) that enabled signal above background to be automatically determined. For each section, a background sample was taken over an area of white matter, and the signal threshold was calculated as mean gray value of background plus 3.5 × SD. The section was automatically density-sliced at this value, so that only pixels with gray values exceeding these criteria were included in the analysis. Results are either expressed as mean signal above background, which reflects mean gray values above background only, or as mean integrated density, which reflects the number of pixels above background (number of pixels above background × mean signal). In cases in which the number of pixels was included in the measurement, considerable care was taken to ensure equivalent areas were analyzed between animals.
Photography and image processing. Figures1, 2, and 4 were generated digitally. Bright-field and dark-field images were captured with a Sony CCD video camera (model DXC-970MD) attached to a Leica (Leitz DMR) microscope, or from the x-ray film on a Northern Lights light-box (Imaging Research), using MicroComputer Imaging Device (Ontario, Canada) image analysis system. Composites were formed within Adobe Photoshop and Adobe Illustrator. Brightness and contrast were altered to generate photographic quality prints.
Fig. 1.
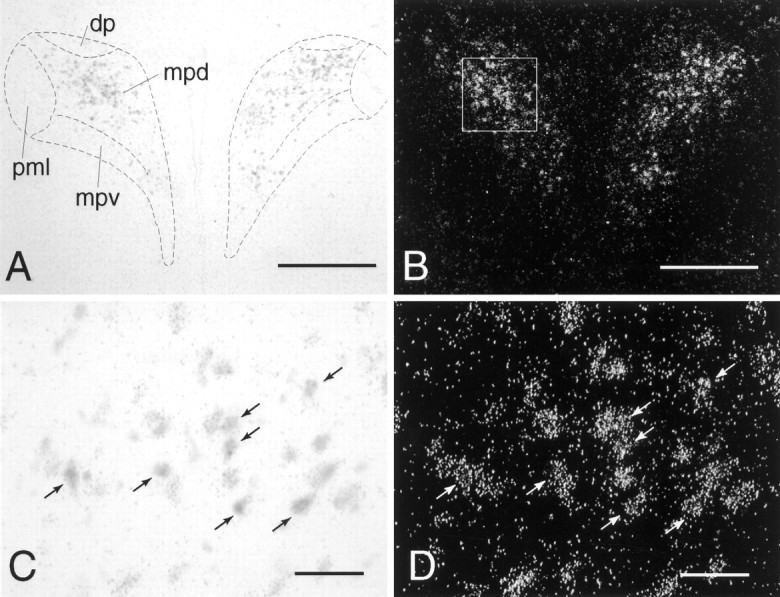
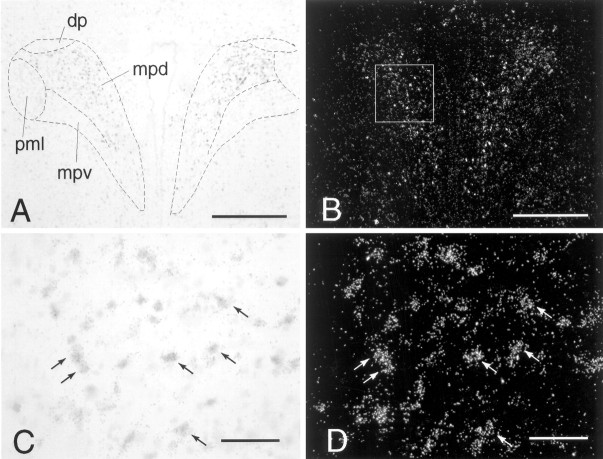
Dual in situ hybridization to show coexpression of α1b ADR mRNA in CRH mRNA-containing cells of the PVN. A, C, CRH mRNA in the PVN, labeled with a nonradioactive (digoxigenin) probe, viewed under bright-field illumination. B, D, α1b ADR mRNA in the PVN, labeled with a radioactive (35S) probe, viewed under dark-field illumination. Thebox in B represents the approximate area over which cells were counted on each side of the PVN.Arrows in C and D indicate examples of double-labeled cells. Scale bars: A, B, 400 μm; C, D, 50 μm. Subdivisions of the PVN:dp, dorsal parvocellular part; mpd,medial parvocellular part, dorsal zone; mpv, medial parvocellular part, ventral zone; pml, posterior magnocellular part, lateral zone.
Fig. 2.
Dual in situ hybridization to show coexpression of α1b ADR mRNA in c-fos mRNA-containing cells of the PVN 30 min after IL-1β (5 μg/kg, i.p.). A, C, c-fos mRNA in the PVN, labeled with a nonradioactive (digoxigenin) probe, viewed under bright-field illumination. B, D, α1b ADR mRNA in the PVN, labeled with a radioactive (35S) probe, viewed under dark-field illumination. The box in B represents the approximate area over which cells were counted on each side of the PVN.Arrows in C and D indicate examples of double-labeled cells. Scale bars: A, B, 400 μm; C, D, 50 μm.
Fig. 4.
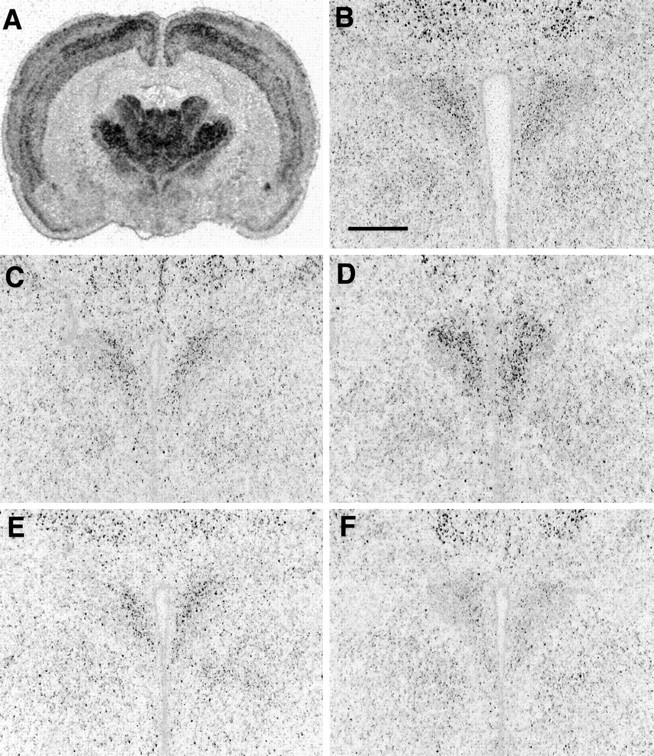
In situ hybridization to show expression of α1b ADR mRNA after bilateral ADX with low (10 mg) or high (50 mg) corticosterone replacement. A,Coronal brain section from a naive rat at the level of the PVN to show general distribution of α1b ADR mRNA.B–F, Expression of α1b ADR mRNA in the PVN of naive (B), SHAM (C), ADX (D), ADX plus 10 mg of corticosterone (E), or ADX plus 50 mg of corticosterone (F). Scale bar, 500 μm.
Statistical analysis. Data were analyzed by one-way ANOVA followed by Tukey–Kramer or Student–Newman–Keulspost hoc multiple comparisons test, as indicated in Results. Significance was set at p < 0.05.
RESULTS
Experiment 1: dual in situ hybridization
Expression of α1b ADR mRNA within the PVN was highest in the parvocellular region. Dual in situhybridization revealed that virtually all CRH cells (97.7 ± 0.6%) expressed α1b ADR mRNA (Fig. 1; mean number of double-labeled cells counted per animal = 554 ± 25; mean number of single-labeled CRH cells counted per animal = 13 ± 3; n = 4). In addition, the majority of α1b ADR mRNA cells (79.8 ± 0.5%), were double-labeled for CRH (mean number of double labeled cells counted per animal = 554 ± 25; mean number of single labeled α1b ADR cells counted per animal = 140 ± 5; n = 4). It should be noted that this is likely an underestimation of the true degree of colocalization, because of the relative insensitivity of the nonradioactive (CRH) compared with radioactive (α1b ADR) in situhybridization protocols.
Stress-induced c-fos mRNA expression in the PVN was found to be highly colocalized with α1b ADR mRNA. For restraint stress (n = 2), ∼94 and 96% of c-fos-positive cells were also labeled for α1b ADR mRNA, whereas 77 and 74% of α1b ADR-positive cells were also labeled for c-fos mRNA (mean number of double labeled cells counted per animal = 318; mean number of single labeled c-fos cells = 18; mean number of single-labeled α1b ADR = 103). For swim stress (n = 2), ∼83 and 94% of c-fos-positive cells were also labeled for α1bADR mRNA, whereas 84 and 64% of α1bADR-positive cells were also labeled for c-fos mRNA (mean number of double-labeled cells counted per animal = 149; mean number of single-labeled c-fos cells = 26, mean number of single-labeled α1b ADR = 43). For intraperitoneal IL-1β (n = 1; Fig. 2), ∼94% of c-fos positive cells were also labeled for α1b ADR mRNA, whereas 77% of α1b ADR-positive cells were also labeled for c-fos mRNA (number of double-labeled cells counted = 358; number of single-labeled c-fos cells = 16; number of single-labeled α1b ADR = 123).
Experiment 2a: ADX time course
Levels of corticosterone for all ADX animals were undetectable, whereas naive, unoperated, and sham controls had levels between 4 and 6 μg/dl at the time of killing (Table 1). Despite the addition of dextrose in the water, prolonged ADX resulted in significantly lower weight gain as compared with sham controls. At 7 d the percentage of weight gain was not significantly different between sham and ADX animals (sham, 14.0 ± 0.6%; ADX, 8.9 ± 1.1%). In contrast, after 14 d, the percentage of weight gain of ADX animals was significantly lower (p< 0.01; Tukey post hoc comparison test) than sham controls (sham, 18.9 ± 4.2%; ADX, 0.2 ± 2.7%).
Table 1.
Plasma levels of corticosterone at time of killing (2 hr before lights off) in animals subjected to bilateral ADX or SHAM surgery
| Group | Corticosterone (μg/dl) | n |
|---|---|---|
| Naive | 5.53 ± 0.98 | 4 |
| UNOP | 5.94 ± 0.13 | 3 |
| SHAM, 1 d | 4.40 ± 0.95 | 3 |
| SHAM, 3 d | 4.35 ± 0.43 | 3 |
| SHAM, 7 d | 5.86 ± 1.79 | 3 |
| SHAM, 14 d | 5.95 ± 0.78 | 3 |
| ADX, all groups | ND | 4/group |
Animals were killed 1, 3, 7, or 14 d after surgery. In addition, naive animals that had not experienced any experimental manipulation and UNOP animals, which did not undergo surgery, but were given saline–dextrose solution to drink, were included. Data are expressed as mean ± SEM. Levels of plasma corticosterone were not detectable (ND) in any of the ADX animals.
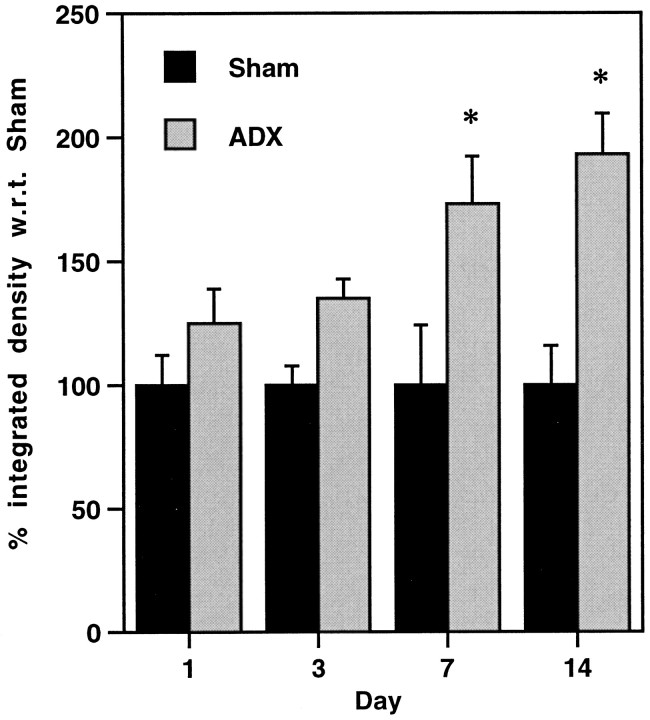
The effect of bilateral adrenalectomy on the expression of α1b ADR mRNA over time was studied. No significant differences between naive, unoperated, and sham controls were found. In contrast, levels of α1b ADR mRNA were significantly increased in the PVN of ADX animals relative to sham controls after 7 or 14 d (173 ± 19% and 193 ± 16%, respectively; p < 0.05, Tukey post hocmultiple comparison test; Fig. 3). This increase appears to be specific to the PVN, because no significant changes in expression of α1b ADR mRNA were observed for the hippocampus, lateral amygdala, or dorsal raphe (Table2).
Fig. 3.
Expression of α1b ADR mRNA in the PVN 1, 3, 7, or 14 d after bilateral adrenalectomy (ADX) or sham surgery. Data are expressed as percentage of integrated density with respect to (w.r.t.) sham. *p < 0.05; compared with appropriate sham group, Tukey post hoc comparison test.
Table 2.
Levels of α1b ADR mRNA in the hippocampus, dorsal raphe, and lateral amygdala after bilateral ADX or SHAM surgery
| Group | α1b ADR mRNA: % signal w.r.t. SHAM | n | ||
|---|---|---|---|---|
| Hippocampus | Dorsal raphe | Lateral amygdala | ||
| Naive | 103 ± 8 | 99 ± 5 | 96 ± 10 | 4 |
| UNOP | 102 ± 5 | 98 ± 21 | 91 ± 7 | 3 |
| SHAM, 1 d | 100 ± 7 | 100 ± 8 | 100 ± 2 | 3 |
| SHAM, 3 d | 100 ± 13 | 100 ± 9 | 100 ± 8 | 3 |
| SHAM, 7 d | 100 ± 5 | 100 ± 7 | 100 ± 3 | 3 |
| SHAM, 14 d | 100 ± 10 | 100 ± 5 | 100 ± 5 | 3 |
| ADX, 1 d | 96 ± 9 | 109 ± 8 | 99 ± 7 | 4 |
| ADX, 3 d | 108 ± 12 | 96 ± 15 | 97 ± 2 | 4 |
| ADX, 7 d | 113 ± 7 | 104 ± 10 | 94 ± 3 | 4 |
| ADX, 14 d | 96 ± 2 | 117 ± 10 | 94 ± 3 | 4 |
Animals were killed 1, 3, 7, or 14 d after surgery. In addition, naive animals that had not experienced any experimental manipulation and UNOP animals, which did not undergo surgery, but were given saline–dextrose solution to drink, were included. Levels of mRNA were set at 100% for each SHAM group, and data are expressed as mean percentage of signal (relative optical density above background; see Materials and Methods for more detail) ± SEM with respect to (w.r.t.) appropriate SHAM group. Naive and UNOP groups are expressed w.r.t. the mean of all SHAM animals. There were no significant differences in expression levels between groups as determined by one-way ANOVA.
Experiment 2b: ADX and corticosterone replacement
Levels of corticosterone (Table 3) were undetectable in five of the six animals in the ADX plus placebo group at either 3 d (A.M. sample) or 7 d (P.M. sample). The sixth animal had low but detectable levels of corticosterone (0.02 μg/dl A.M.; 0.2 μg/dl P.M.). On this basis, the animal was excluded from the study. The naive and sham groups had comparable levels of corticosterone for both A.M. and P.M. samples. For the ADX plus 10 mg of corticosterone group, levels of corticosterone were 2.42 ± 0.2 μg/dl after 3 d and 1.19 ± 0.29 μg/dl after 7 d. This represented a significant decrease (p < 0.01; Student's t test), possibly caused by a decreased secretion of corticosterone from the pellet over time. For the ADX plus 50 mg of corticosterone group, levels were 7.47 ± 0.88 μg/dl after 3 d and 6.83 ± 0.82 μg/dl after 7 d.
Table 3.
Plasma levels of corticosterone after bilateral ADX with low (10 mg) or high (50 mg) corticosterone replacement or SHAM surgery
| Group | Corticosterone (μg/dl) | % Weight gain | n | |
|---|---|---|---|---|
| A.M. | P.M. | |||
| Naive | 0.11 ± 0.04 | 5.74 ± 1.19 | 14.1 ± 0.8 | 6 |
| SHAM | 0.27 ± 0.14 | 7.30 ± 1.11 | 12.2 ± 1.3 | 6 |
| ADX | ND | ND | 3.0 ± 2.3* | 5 |
| ADX + 10 mg | 2.42 ± 0.20 | 1.19 ± 0.29** | 11.5 ± 1.2 | 6 |
| ADX + 50 mg | 7.47 ± 0.88 | 6.83 ± 0.82 | 2.5 ± 1.9* | 6 |
A naive group was also included that did not undergo any experimental manipulation. Blood was taken by tail vein sample ∼2 hr after lights on (A.M.), 3 d after surgery, and at the time of killing (trunk blood), 1–2 hr before lights off (P.M.), 7 d after surgery. Levels of plasma corticosterone were not detectable (ND) in any of the ADX animals. The percentage of weight gain over the 7 d period after surgery is also indicated. Data are expressed as mean ± SEM.
*p < 0.01, Tukey post hoc multiple comparisons test, with respect to naive, sham, and ADX + 10 mg groups. A significant decrease in corticosterone release was noted in the ADX + 10 mg group between days 3 and 7 (**p < 0.01; Student's t test).
Weight gain for the animals varied across groups (Table 3). Naive and sham groups exhibited a similar percentage of weight gain. ADX plus placebo gained significantly less weight than sham or naive animals (p < 0.01; Tukey post hoc multiple comparisons test). In contrast, a low dose of corticosterone reversed this effect, so that ADX plus 10 mg group exhibited a similar weight gain as the naive and sham animals. Animals treated with a high dose of corticosterone also demonstrated significantly lower weight gain than naive, sham, or ADX plus 10 mg groups (p < 0.01; Tukey post hoc multiple comparisons test).
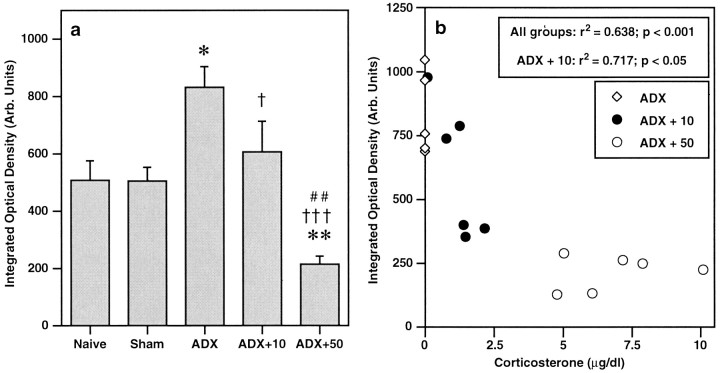
The effect of ADX with corticosterone replacement on expression of α1b ADR mRNA in the PVN is shown in Figures4 and 5. Expression was not significantly different in the PVN of sham rats, as compared to naive. As was observed in the initial time course experiment, levels of α1b ADR mRNA were increased in the PVN of ADX plus placebo animals (relative integrated optical density = 832 ± 73 arbitrary units; 164% of sham control), as compared with sham controls (relative integrated optical density = 506 ± 48 arbitrary units; p < 0.05, Student–Newman–Keuls). This effect was reversed by a low dose of corticosterone, so that there were no significant differences between levels of α1b ADR mRNA in the ADX plus 10 group compared with sham controls. In ADX animals replaced with a high dose of corticosterone (ADX plus 50), levels of α1b ADR mRNA were significantly decreased in the PVN compared with all other groups (relative integrated optical density = 215 ± 28 arbitrary units, 42% of sham control;p < 0.01, for each group comparison, Student–Newman–Keuls). It was determined that levels of α1b ADR mRNA in the PVN were significantly correlated (inverse relationship) with plasma corticosterone levels at the time of killing (Pearson analysis;r2 = 0.638, p< 0.001 for all groups combined;r2 = 0.717, p< 0.05 for ADX plus 10 mg of cortico-sterone group; Fig.5b). As was observed in the initial time course experiment, these changes appeared specific to the PVN. No changes in α1b ADR mRNA levels were observed in any hippocampal region in any of the treatment groups (Table4).
Fig. 5.
a, Expression of α1bADR mRNA in PVN after bilateral adrenalectomy (ADX) with low (10 mg) or high (50 mg) corticosterone replacement. Data are expressed as integrated optical density (arbitrary units). *p < 0.05, **p < 0.01 compared with sham group; †p < 0.05, †††p < 0.001 compared with ADX group; ##p < 0.01 compared with ADX plus 10 group; Student–Newman–Keuls post hocmultiple comparisons test. b, Correlation analysis (Pearson; all groups, r2 = 0.638; p < 0.001; ADX plus 10 mg group,r2 = 0.717;p < 0.05) between levels of α1b ADR mRNA in PVN and plasma corticosterone at time of killing, after bilateral adrenalectomy with corticosterone replacement (0, 10, or 50 mg, s.c.) for 7 d.
Table 4.
Levels of α1b ADR mRNA in hippocampal subfields after bilateral ADX with low (10 mg) or high (50 mg) corticosterone replacement or SHAM surgery
| Group | α1b ADR mRNA: % Integrated density w.r.t. SHAM | n | ||
|---|---|---|---|---|
| CA1 | CA3 | Dentate gyrus | ||
| Naive | 89 ± 11 | 91 ± 5 | 85 ± 8 | 6 |
| SHAM | 100 ± 11 | 100 ± 11 | 100 ± 8 | 6 |
| ADX | 102 ± 6 | 88 ± 8 | 97 ± 8 | 5 |
| ADX + 10 mg | 107 ± 9 | 104 ± 9 | 108 ± 5 | 6 |
| ADX + 50 mg | 109 ± 13 | 101 ± 11 | 95 ± 2 | 6 |
A naive group was also included that did not undergo any experimental manipulation. Data are expressed as mean percentage of integrated density ± SEM with respect to (w.r.t.) SHAM group. There were no significant differences between groups as determined by one-way ANOVA.
DISCUSSION
The data described in the present study demonstrate for the first time that mRNA encoding one subtype of adrenergic receptor, α1b ADR, is localized in virtually every CRH-containing parvocellular cell of the PVN. In addition, the majority of PVN cells found to be responsive to either restraint, swim, or immune stress, as indicated by expression of c-fos mRNA, were found tocontain α1b ADR mRNA. Within the PVN, α1b ADR mRNA appears extremely sensitive to levels of circulating glucocorticoids, because removal of the adrenal glands led to an upregulation of expression within 7 d, which was reversed by a low replacement dose of corticosterone. Exposure of adrenalectomized animals to a higher dose of corticosterone for 7 d led to a significant decrease in expression of α1b ADR mRNA in the PVN, as compared with sham-operated controls. Furthermore, in adrenalectomized rats with replaced corticosterone, levels of α1b ADR mRNA in the PVN were found to be correlated significantly (inverse relationship) with plasma levels of corticosterone.
Potential role of the α1B ADR in PVN activation or modulation
As outlined in the introductory remarks, many studies have indicated the importance of the brainstem ascending noradrenergic and adrenergic pathways in the control of the HPA axis. The data presented in the current study indicate that α1Badrenoceptors within CRH-containing cells of the PVN are potentially in an excellent position to elicit or modify an HPA axis response. Although α1b ADR mRNA appears highly colocalized with c-fos mRNA after stress (restraint, swim, or intraperitoneal IL-1β), we certainly cannot determine from these data whether this receptor subtype mediates excitation of CRH-containing PVN neurons, and ultimately the HPA axis, in response to these stimuli. Indeed, it seems most likely that a primarily systemic stress, such as IL-1β administration, uses catecholaminergic medullary pathways to a greater extent than a stressor with a significant neurogenic component, such as footshock (Li et al., 1996), and probably restraint. Hence, the function of PVN α1B adrenoceptors might be to mediate the activity of CRH-containing neurons in the case of an immune challenge, but may play a lessor role after other stressors. In keeping with this, a previous study has suggested that the PVN Fos response and corticosterone secretion after either restraint or hypertonic saline is not dependent on the α1B adrenoceptor (Williams and Morilak, 1997). However, it is of interest to note that in several brain regions excitation of α1 adrenoceptors has been shown to result in slow depolarization, such that the membrane potential is brought closer to the threshold for firing (Bevan et al., 1977; McCormick and Prince, 1988; Pan et al., 1994; Bergles et al., 1996). Hence, α1B adrenoceptors appear to be in an excellent position to modulate the activity of CRH-containing PVN neurons, and may well be involved in the integration of stress inputs at the level of the hypophysiotropic neurons of the PVN.
Glucocorticoid regulation of α1b ADR mRNA
Data from the present study demonstrate the regulation of α1b ADR mRNA in the PVN after removal of the adrenal glands. The reversal of this effect by replacement of corticosterone strongly suggests that expression of α1b ADR mRNA within the PVN is dependent on circulating glucocorticoids, consistent with the observation that the promoter region of the gene contains a GRE (Gao and Kunos, 1993). However, the data are in contrast to that predicted from in vitro studies, which demonstrated that dexamethasone and aldosterone increased α1b ADR mRNA abundance in DDT1 MF-2 smooth muscle cells because of increased transcriptional activation (Sakaue and Hoffman, 1991). However, it is well known that glucocorticoids regulate gene expression in a highly complex manner, interacting in different ways depending on a vast number of different factors, including cell type, glucocorticoid concentration, relative abundance of mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) within a cell, and relative expression of other transcription factors (Trapp and Holsboer, 1996; De Kloet et al., 1998;Gottlicher et al., 1998; Spencer et al., 1998). Thus, the cellular localization of a gene transcript will be important in determining the effects of circulating levels of glucocorticoids in vivo, above and beyond the presence or absence of a GRE. This may also explain the specificity of α1b ADR regulation to the PVN. In particular, despite the high expression of both MR and GR in the hippocampus (Herman et al., 1989), no regulation of α1b ADR mRNA was observed in any hippocampal subfield. However caution should be exercised in interpretation of this finding, because hippocampal expression of this receptor is extremely low under basal conditions, and hence changes may be hard to detect.
Is regulation of α1b ADR mRNA mediated by MR or GR?
In an effort to determine whether MR or GR are involved in the regulation of α1b ADR mRNA, low and moderate doses of corticosterone were used in the replacement study. MR has a 6- to 10-fold higher affinity for corticosterone than GR, and this receptor is thought to be more important than GR in situations when circulating levels of corticosterone are low, as occurs at the nadir of the diurnal rhythm (light phase for rats). In contrast, when circulating levels of corticosterone rise, either because of the normal circadian rhythm or after HPA activation, although the occupation of MR remains high, the relative importance of GR is increased (Reul and De Kloet, 1985; Dallman et al., 1991; De Kloet et al., 1998). The data presented here indicated that a relatively low dose of corticosterone (10 mg pellet) was able to reverse the increase in expression levels of α1b ADR mRNA obtained after bilateral adrenalectomy, which may indicate an involvement of MR. The demonstration that the higher dose of corticosterone (50 mg pellet) further depressed expression of α1b ADR mRNA in the PVN is consistent with the idea that this may be a GR-mediated effect. The relative paucity of MR in the PVN, compared with the abundance of GR (Reul and de Kloet, 1985), suggests that any role that MR plays in the regulation of expression of this receptor mRNA is probably indirect, whereas putative GR-mediated regulation may be direct. Although expression levels of α1b ADR mRNA in the PVN were significantly correlated (inverse relationship) with plasma levels of corticosterone, regulation of this receptor mRNA occurred over a relatively narrow range of corticosterone. It is conceivable therefore, that regulation of α1bADR mRNA in the PVN is highly sensitive to the balance of occupancy of MR and GR, and the use of compounds selective for these receptors will be useful in investigating this further.
Limits of interpretation
At this point, it is not clear whether regulation of mRNA encoding the α1b ADR is translated into functional protein. Autoradiography of α1 ADR in the PVN, using the nonspecific α1 ADR antagonist prazosin, has not demonstrated any changes in receptor level after ADX (Jhanwar-Uniyal and Leibowitz, 1986; Cummings and Seybold, 1988). However, we have shown previously that α1a ADR mRNA is expressed at relatively high levels in the PVN, primarily in magnocellular cells, but also at low levels in parvocellular cells (Day et al., 1997), and data from our laboratory suggest that this receptor mRNA is not regulated by glucocorticoids in vivo (our unpublished data). Hence, the effect of glucocorticoids on the α1B ADR may have been masked in receptor-binding studies. As antibodies against this receptor become available and as ligands with increased selectivity are generated that are able to distinguish between the different α1 receptor subtypes, semiquantitative immunoradiography and receptor autoradiography will be useful in determining if levels of α1B ADR protein are altered by glucocorticoids. In addition, the role of this receptor in an animal's response to chronic stress, in which the HPA axis is altered at many levels and often results in an increase in basal levels of corticosterone, will be of particular interest. Further studies will be needed to address these questions.
In conclusion, we have demonstrated the existence of a specific subtype of adrenergic receptor mRNA, α1b ADR, in CRH-containing, stress-responsive cells of the rat PVN. The levels of mRNA encoding this receptor in the PVN, but not in other brain regions, appear to be inversely related to circulating levels of corticosterone. Together these data indicate that the α1Badrenergic receptor is in a strong position to play a significant role in the complex and dynamic regulation of the HPA axis in response to stress.
Footnotes
This work was supported by National Institute on Drug Abuse Grant 5RO1 DA02265–18, National Institute of Mental Health Grant 2PO1 MH42251–11, and the Pritzker Network for the study of depression.
Correspondence should be addressed to Heidi E. W. Day, Mental Health Research Institute, University of Michigan, 205 Zina Pitcher Place, Ann Arbor, MI 48109-0720. E-mail: heididay@umich.edu.
REFERENCES
- 1.Akil H, Campeau S, Cullinan WE, Lechan RM, Toni R, Watson SJ, Moore RY. Neuroendocrine systems I: overview–thyroid and adrenal axes. In: Zigmond M, Bloom F, Landis S, Roberts J, Squire L, editors. Fundamental neuroscience. Academic; San Diego: 1999. pp. 1127–1150. [Google Scholar]
- 2.Bergles DE, Doze VA, Madison DV, Smith SJ. Excitatory actions of norepinephrine on multiple classes of hippocampal CA1 interneurons. J Neurosci. 1996;16:572–585. doi: 10.1523/JNEUROSCI.16-02-00572.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevan P, Bradshaw CM, Szabadi E. The pharmacology of adrenergic neuronal responses in the cerebral cortex: evidence for excitatory α- and inhibitory β-receptors. Br J Pharmacol. 1977;59:635–641. doi: 10.1111/j.1476-5381.1977.tb07732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calogero AE, Galluchi WT, Chrousos GP, Gold PW. Catecholamine effects upon rat hypothalamic corticotropin-releasing hormone secretion in vitro. J Clin Invest. 1988;82:839–846. doi: 10.1172/JCI113687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 6.Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J Comp Neurol. 1996;368:88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Cummings S, Seybold V. Relationship of α-1 and α-2 adrenergic-binding sites to regions of the paraventricular nucleus of the hypothalamus containing corticotropin-releasing factor and vasopressin neurons. Neuroendocrinology. 1988;47:523–532. doi: 10.1159/000124965. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham ET, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham ET, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- 10.Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker C-D, Strack AM, Cascio CS. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol. 1991;4:517–526. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 11.Day HEW, Akil H. Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-β: implications for mechanism of action. Neuroendocrinology. 1996;63:207–218. doi: 10.1159/000126959. [DOI] [PubMed] [Google Scholar]
- 12.Day HEW, Campeau S, Watson SJ, Akil H. Distribution of α-1a, α-1b and α-1d adrenergic receptor mRNAs in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 13.Day HEW, Curran EJ, Watson SJ, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1β. J Comp Neurol. 1999;413:113–128. [PubMed] [Google Scholar]
- 14.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 15.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaillet S, Lachuer J, Malaval F, Assenmacher I, Szafarczyk A. The involvement of noradrenergic ascending pathways in the stress-induced activation of ACTH and corticosterone secretions is dependent on the nature of stressors. Exp Brain Res. 1991;87:173–180. doi: 10.1007/BF00228518. [DOI] [PubMed] [Google Scholar]
- 17.Gao B, Kunos G. Isolation and characterization of the gene encoding the rat α1B adrenergic receptor. Gene. 1993;131:243–247. doi: 10.1016/0378-1119(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 18.Gottlicher M, Heck S, Herrlich P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J Mol Med. 1998;76:480–489. doi: 10.1007/s001090050242. [DOI] [PubMed] [Google Scholar]
- 19.Guillaume V, Conte-Devolx B, Szafarczyk A, Malaval F, Pares-Herbute N, Grino M. The corticotropin-releasing factor release in rat hypophysial portal blood is mediated by brain catecholamines. Neuroendocrinology. 1987;46:143–146. doi: 10.1159/000124811. [DOI] [PubMed] [Google Scholar]
- 20.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 21.Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- 22.Itoi K, Suda T, Tozawa F, Dobashi I, Ohmori N, Sakai Y, Abe K, Demura H. Microinjection of norepinephrine into the paraventricular nucleus of the hypothalamus stimulates corticotropin-releasing factor gene expression in conscious rats. Endocrinology. 1994;135:2177–2182. doi: 10.1210/endo.135.5.7956940. [DOI] [PubMed] [Google Scholar]
- 23.Jhanwar-Uniyal M, Leibowitz SF. Impact of circulating corticosterone on α1- and α2-noradrenergic receptors in discrete brain areas. Brain Res. 1986;368:404–408. doi: 10.1016/0006-8993(86)90591-3. [DOI] [PubMed] [Google Scholar]
- 24.Kiss A, Aguilera G. Participation of α1-adrenergic receptors in the secretion of hypothalamic corticotropin-releasing hormone during stress. Neuroendocrinology. 1992;56:153–160. doi: 10.1159/000126223. [DOI] [PubMed] [Google Scholar]
- 25.Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute “stress” to the hypothalamic paraventricular nucleus. J Neurosci. 1995;15:2609–2627. doi: 10.1523/JNEUROSCI.15-04-02609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H-Y, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci USA. 1996;93:2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick DA, Prince DA. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons in vitro. J Neurophysiol. 1988;59:978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- 28.McCune SK, Voigt MM, Hill JM. Expression of multiple α adrenergic receptor subtype mRNAs in the adult rat brain. Neuroscience. 1993;57:143–151. doi: 10.1016/0306-4522(93)90116-w. [DOI] [PubMed] [Google Scholar]
- 29.Pan ZZ, Grudt TJ, Williams JT. α1-adrenoceptors in rat dorsal raphe neurons: regulation of two potassium conductances. J Physiol (Lond) 1994;478:437–447. doi: 10.1113/jphysiol.1994.sp020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsadaniantz SM, Gaillet S, Malaval F, Lenoir V, Batsche E, Barbanel G, Gardier A, Terlain B, Jacquot C, Szafarczyk A, Assenmacher I, Kerdelhue B. Lesions of the afferent catecholaminergic pathways inhibit the temporal activation of the CRH and POMC gene expression and ACTH release induced by human interleukin-1β in the male rat. Neuroendocrinology. 1995;62:586–595. doi: 10.1159/000127054. [DOI] [PubMed] [Google Scholar]
- 31.Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of α-1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plotsky PM. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic-pathways or central norepinephrine injection. Endocrinology. 1987;121:924–930. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- 33.Plotsky PM, Cunningham ET, Widmar EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev. 1989;10:437–458. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- 34.Reul JM, De Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 35.Rivest S, Rivier C. Stress and interleukin-1b-induced activation of c-fos, NGFI-B and CRF gene expression in the hypothalamic PVN: comparison between Sprague-Dawley, Fisher-344 and Lewis rats. J Neuroendocrinol. 1994;6:101–117. doi: 10.1111/j.1365-2826.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 36.Sakaue M, Hoffman BB. Glucocorticoids induce transcription and expression of the α1B adrenergic receptor gene in DTT1 MF-2 smooth muscle cells. J Clin Invest. 1991;88:385–389. doi: 10.1172/JCI115315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith DW, Buller KM, Day TA. Role of ventrolateral medulla catecholamine cells in hypothalamic neuroendocrine cell responses to systemic hypoxia. J Neurosci. 1995;15:7979–7988. doi: 10.1523/JNEUROSCI.15-12-07979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer RL, Kim PJ, Kalman BA, Cole MA. Evidence for mineralocorticoid receptor facilitation of glucocorticoid receptor-dependent regulation of hypothalamic-pituitary-adrenal axis activity. Endocrinology. 1998;139:2718–2126. doi: 10.1210/endo.139.6.6029. [DOI] [PubMed] [Google Scholar]
- 39.Trapp T, Holsboer F. Heterodimerization between mineralocorticoid and glucocorticoid receptors increases the functional diversity of corticosteroid action. Trends Pharmacol Sci. 1996;17:145–149. doi: 10.1016/0165-6147(96)81590-2. [DOI] [PubMed] [Google Scholar]
- 40.Whitnall MH, Kiss A, Aguilera G. Contrasting effects of central α-1-adrenoceptor activation on stress-responsive and stress-non-responsive subpopulations of corticotropin-releasing hormone neurosecretory cells in the rat. Neuroendocrinology. 1993;58:42–48. doi: 10.1159/000126510. [DOI] [PubMed] [Google Scholar]
- 41.Williams AM, Morilak DA. α-1B adrenoceptors in rat paraventricular nucleus overlap with, but do not mediate, the induction of c-fos expression by osmotic or restraint stress. Neuroscience. 1997;76:901–913. doi: 10.1016/s0306-4522(96)00351-x. [DOI] [PubMed] [Google Scholar]