Abstract
Dendritogenesis, axonogenesis, pathfinding, and target recognition are all affected in distinct ways when Xenopus retinal ganglion cells (RGCs) are transfected with constitutively active (ca), wild-type (wt), and dominant negative (dn) Rho-family GTPases in vivo. Dendritogenesis required Rac1 and Cdc42 activity. Moreover, ca-Rac1 caused dendrite hyperproliferation. Axonogenesis, in contrast, was inhibited by ca-Rac1. This phenotype was partially rescued by the coexpression of dn cyclin-dependent kinase (Cdk5), a proposed effector of Rac1, suggesting that Rac1 activity must be regulated tightly for normal axonogenesis. Growth cone morphology was particularly sensitive to dn-RhoA and wt-Cdc42 constructs. These also caused targeting errors, such as tectal bypass, suggesting that cytoskeletal rearrangements are involved in target recognition and are transduced by these pathways.
Keywords: RhoA, Rac1, Cdc42, axonogenesis, dendritogenesis, GTPase
Studies in fibroblasts have demonstrated that Rho-family GTPases regulate the actin cytoskeleton. Changes in cell morphology occur after the microinjection of Cdc42, which induces filopodia; Rac1, which induces lamellipodia; and RhoA, which induces stress fibers (Hall, 1998). Recent studies have identified these GTPases as molecules involved in axon and dendrite formation during neuronal development (Luo et al., 1997; Tapon and Hall, 1997).
Work on neuritogenesis in vitro suggests that RhoA negatively influences growth cone and neurite formation. Wild-type (wt)-RhoA microinjection (Kozma et al., 1997) or exposure to lysophosphatidic acid (an activator of RhoA) (Jalink et al., 1994;Tigyi et al., 1996) causes growth cone collapse and neurite retraction in PC12 and N1E-115 cells. Also, the trituration of C3 exoenzyme (an inhibitor of RhoA activity) into dorsal root ganglion cells stimulates neurite outgrowth (Jin and Strittmatter, 1997), and both dominant negative (dn)-RhoA and C3 exoenzyme microinjection promote filopodia and lamellipodia formation in N1E-115 cell growth cones (Kozma et al., 1997). In contrast, the overexpression of wt-Cdc42 and Rac1 induces N1E-115 cell filopodia and lamellipodia formation (Kozma et al., 1997), and expression of constitutively active (ca)-Cdc42 and Rac1 promotes hippocampal neuron dendritic development (Threadgill et al., 1997). In addition, chicken Rac1B overexpression in retinal cells increases neurite number and branching, whereas dn-Rac1B inhibits neuritogenesis (Albertinazzi et al., 1998).
These GTPases also have been examined in vivo, where it is possible to look at axon and dendrite formation separately. ca- and dn-Cdc42 reduce sensory neuron dendrites in the fly, whereas mutant Rac1 proteins do not affect dendritogenesis (Luo et al., 1994). However, when expressed in mouse Purkinje cells, ca-Rac1 causes smaller but more numerous dendritic spines (Luo et al., 1996). Defects in axon initiation, extension, and pathfinding have been observed also. ca- and dn-Rac1 and Cdc42 all inhibit axonogenesis in fly sensory neurons (Luo et al., 1994), and ca-Rac1 greatly reduces the number of axon terminals in mouse Purkinje cells (Luo et al., 1996). In agreement with these observations, ca-Rac1 and Cdc42 inhibit axon extension in fly motor neurons (Kaufmann et al., 1998). However, dn-Rac1-expressing cells did extend axons, with a subset of neurons exhibiting pathfinding defects. Loss of Rac1 activity also has been implicated in pathfinding errors inCaenorhabditis elegans, because defects were observed after the loss of unc-73, an activator of Rac1 (Steven et al., 1998). In contrast, activated MIG-2, a novel Rac1/Cdc42-like molecule in C. elegans, causes axon pathfinding errors (Zipkin et al., 1997).
These findings highlight the importance of Rho-family GTPases in axonal and dendritic growth in vivo, but the findings are not always consistent among different systems. To try to develop a complete picture of the functions of these GTPases in a single cell type, we expressed wild-type and mutant RhoA, Rac1, and Cdc42 in developingXenopus retinal ganglion cells (RGCs). We report here that RhoA, Rac1, and Cdc42 have distinct effects on dendrite formation, axon initiation and extension, growth cone morphology, and target selection, which suggests specific roles for these small GTPases during RGC process development in vivo.
MATERIALS AND METHODS
Animals. Embryos were obtained by fertilizing eggs from adult female Xenopus laevis stimulated by injection of human chorionic gonadotropin (United States Biochemicals, Cleveland, OH). Embryos were raised in 10% Holtfreter’s (Holtfreter, 1943) at temperatures between 14 and 27°C. Embryos were staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967).
DNA constructs. Myc-tagged human wt-Rac1, Cdc42, and RhoA; ca-V12 Rac1 and Cdc42; ca-V14 RhoA; and dn-N17 Rac1 and Cdc42 cDNAs were obtained from Dr. Alan Hall (University College London, UK) and subcloned into pCS2, a eukaryotic expression vector designed by Dr. David Turner (University of Michigan, Ann Arbor, MI), dn-N19 Rho was obtained from Dr. Jerald Chun (University of California at San Diego) and subcloned into pCS2-myc. All constructs were designed with one myc-tag 5′ to the gene and were sequenced after being subcloned into CS2 to ensure that the genes were in-frame with the myc-tag. In vitro transcription–translation was performed by using the TNT Coupled Reticulocyte Lysate System kit (Promega, Madison, WI) to ensure that appropriately sized proteins were produced. Briefly, template cDNA was added to the cell-free transcription–translation lysate, and the reaction was allowed to proceed. The lysate was spiked with translation grade [35S]methionine (Amersham, Arlington Heights, IL), and the translation products were run on an SDS-PAGE gel. The proteins were visualized with autoradiography. The control luciferase reporter plasmid, RSVL, has been described previously (Holt et al., 1990). The green fluorescent protein (GFP)-myc fusion cDNA in CS2 was a gift of Dr. David Turner. Expression plasmids of Cdk5 and dn-Cdk5 were obtained from Dr. Anna Philpott (University of Cambridge, UK) and have been described previously (Philpott et al., 1997). Plasmid DNA was purified from E. coli by using Qiagen Maxiprep kits (Hilden, Germany).
DNA transfection. DNA lipofections were performed as described previously (Holt et al., 1990; Lilienbaum et al., 1995). Briefly, retinal cells were transfected by microinjecting a 1:3 mixture by weight of DNA and the transfectant DOTAP (Boehringer Mannheim) into the presumptive right eye of the anterior neural fold of stage 18–20 embryos. Embryos were cysteine-treated for 5 min to remove the jelly coat [2% l-cysteine (Sigma, St. Louis, MO) in 10% Holtfreter’s, pH 8] and placed in a dish containing 5% Ficol (Sigma) in 10% MMR [containing (in mm) 100 NaCl, 2 KCl, 1 MgSO4, 5 HEPES, 0.1 EDTA, and 2 CaCl2]. Borosilicate glass needles (FHC) were pulled on an electrode puller (Sutter Instruments, Novato, CA), filled with DNA/DOTAP, and mounted on a micromanipulator (Narishige, Tokyo, Japan) attached to a Picospritzer II (General Valve, Fairfield, NJ). Three to five extracellular injections of 5–10 nl each were made into the developing eye primordium. After injection the embryos were transferred to 10% Holtfreter’s.
Antibodies. All embryos transfected with Rho-family GTPase cDNA or with GFP-myc cDNA were stained with the anti-myc monoclonal antibody 9E10 (Santa Cruz Biotechnology, Santa Cruz, CA) diluted to 1:500. Embryos transfected with luciferase were stained with an affinity-purified guinea pig anti-luciferase antibody used at 1:100. Donkey anti-mouse CY3-conjugated secondary was used at 1:100. Horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-guinea pig secondary antibodies from Jackson Laboratories (Bar Harbor, ME) were used at 1:500.
Immunocytochemistry and histology for vibratome-sectioned embryos. In general, the embryos were allowed to develop to stage 40 (∼45 hr at room temperature), fixed overnight at 4°C in 4% paraformaldehyde in 0.1 m PO4 buffer, rinsed several times with PBS [containing (in mm) 136.9 NaCl, 2.7 KCl, 81 Na2HPO4, and 1.5 KH2PO4, pH 7.4], and pigment-bleached overnight on a light box in methanol and 10% hydrogen peroxide. After several rinses in PBS and blocking solution [PBS with 5% normal goat serum (Core Cell Culture Facility, University of California at San Diego), 0.2% Fraction V bovine serum albumin (Sigma), and 0.5% Triton X-100 (Sigma)] the embryos were incubated overnight at 4°C in primary antibodies diluted in block, rinsed in block, incubated overnight in secondary antibodies diluted in block, and rinsed again. Then the embryos were incubated in 0.5% diaminobenzidine (Sigma) for 30 min, after which 1% hydrogen peroxide was added to catalyze the peroxidase reaction. Embryos were post-fixed in 1% glutaraldehyde for 30 min, embedded in gelatin/albumin [1.5% gelatin (Fisher, Pittsburgh, PA), 45% albumin (Sigma) in 0.9% saline], hardened with glutaraldehyde, and sectioned at 50 μm on an Oxford vibratome. Sections were collected in series on glass slides (Fisher), allowed to dry until the edges adhered, and then placed in 0.1 m PO4 buffer. The slides were dehydrated through a graded ethanol series followed by two butanol rinses, were cleared in xylenes, and were mounted in Permount (Fisher).
Immunocytochemistry and histology for cryostat-sectioned embryos.Stage 40–41 embryos were fixed in 4% paraformaldehyde, rinsed in PBS, saturated in 30% sucrose, transferred to OCT embedding medium (Tissue Tek, Miles, Elkhart, IN), and sectioned at 15 μm on a cryostat (Zeiss, Oberkochen, Germany). Immunocytochemistry to visualize myc-fusion proteins was performed on sectioned tissues as described above. Sections also were stained for condensed nuclei by being soaked in a 1:10,000 solution of Hoechst nuclear stain for 10 min, followed by PBS rinses. Apoptotic nuclei were visualized with an Apoptag kit (Oncor, Gaithersburg, MD). Briefly, sections were incubated in a terminal deoxynucleotidyl transferase solution along with digoxigenin-labeled nucleotides, rinsed with PBS, incubated with a fluorescein-conjugated anti-digoxigenin antibody, and viewed with epifluorescence.
Imaging and analysis. Immunopositive RGCs were drawn at 100× magnification with Nomarski optics, using a camera lucida attachment on a Nikon Optiphot-2; the drawings were scanned on a Color Onescanner (Apple), and parameters of cell morphology were measured by using National Institutes of Health Image software. RGCs were identified by their location in the ganglion cell layer directly abutting the lens. RGC processes were identified as axons if they grew along the vitreal surface toward the optic nerve head and as dendrites if they were oriented toward the inner plexiform layer. Cell body area was measured by tracing the main body of the outline of a cell, not including fine processes that could not be distinguished or drawn individually. Dendrite length was measured by tracing the length of the longest primary dendrite, and dendrite number was determined by counting the number of dendrite tips. Only cells that did not overlap significantly with other transfected cells and that were located in the flat, middle sections of the retina (generally including the lens) were used, because their laminar position could be classified with certainty. To avoid the inclusion of immature cells in the ciliary marginal zone, a zone of mitotically active cells that remains on the edges of the Xenopus retina into adulthood, we analyzed only cells in the inner two-thirds of the retinal semicircle. Growth cone area was measured from the widening of the axon at the base of the growth cone to the edges of the lamellipodia. Filopodial number and length were determined by counting the number of filopodia and measuring the longest filopodia. Axonal backbranches were counted two growth-cone lengths behind the base of the growth cone. All errors were calculated as SEM, and p values were determined by using Mann–Whitney nonparametric statistical analysis. Color slides were taken on a Zeiss Axiophot with Nomarski optics, scanned with a Nikon L5-1000 film scanner, and processed with Adobe Photoshop software.
Whole-mount embryo processing, imaging, and analysis.Embryos were transfected at stage 18 and allowed to develop to stage 41, at which point they were fixed and processed immunocytochemically as described above. Both HRP-conjugated and CY-3-conjugated secondary antibodies (Jackson Laboratories) were used. The brains were dissected out and mounted on slides; the transfected axons were viewed on a Nikon Optiphot-2. Axons visualized by using HRP-conjugated secondary antibodies were drawn at 20× magnification, using Nomarski illumination and a camera lucida; those visualized with cy-3 secary antibodies were drawn from a fluorescence image captured with a CCD camera (SpectraSource Instruments, Westlake Village, CA). The percentage of labeled axons in each brain that were in the diencephalon and in the tectum, that grew toward the basal optic nucleus (BON), or that made targeting errors was calculated. Targeting error percentages do not include axons that grew only as far as the diencephalon.
RESULTS
Overexpression of Rho-family GTPases in Xenopusretinal cells
Xenopus cells begin to express protein ∼8 hr after transfection, at stage 24 (Holt et al., 1990), and RGCs extend their first axons 6 hr after this, at stage 27 (Holt, 1989). Dendritogenesis usually begins 5 hr after axon initiation (Sakaguchi et al., 1984;Holt, 1989). Thus, the expressed proteins should be present at the time of process initiation and elaboration. Transfected cells were labeled immunocytochemically with an anti-myc primary and an HRP-conjugated secondary. Protein levels in expressing cells were not quantified; however, only cells that were stained darkly, and therefore most likely were expressing high levels of protein, were analyzed. A brown DAB reaction product was present throughout the cells from dendrite to growth cone tip. Potentially, the anti-myc staining may not be a precise reflection of cell morphology because of uneven protein distribution. However, the fact that the mutant proteins differ from wild type by a single amino acid substitution on the N terminus of the protein, which is not predicted to have an effect on intracellular localization (Adamson et al., 1992) and yet differential effects on cell morphology and process extension were observed, argues against this possibility. Labeled cells were present in all retinal laminae for all constructs, suggesting that neuronal migration within the retina was not perturbed (data not shown). Although a careful analysis of cell fate was not performed, all transfected cells were in distinct retinal laminae. Moreover, appropriate differentiated cell morphologies were observed for most constructs. In addition, transfected cells in stage 40 retina did not appear to be undergoing cell death, as revealed by Hoechst and Apoptag stain of their nuclei (data not shown), although we did not examine the possibility of cell death occurring at earlier stages.
Rho-family GTPases affect dendritogenesis
To determine whether Rho-family GTPases are involved in the formation of dendrites in Xenopus neurons in vivo, we introduced wt, ca, and dn versions of RhoA, Rac1, and Cdc42 or the control genes luciferase and GFP-myc via transient transfection into dividing primordial eye cells. Although all cell types in the retina were transfected, only RGCs were analyzed, because their identity can be determined by their laminar location and they allow for analysis of both axonal and dendritic structures. Normally, RGCs have an extensive dendritic tree that forms in the inner plexiform layer (Fig. 1A,B). The effect of expression of Rho-family GTPases on RGC morphology is illustrated in Figure 1C–K. Qualitatively, cells expressing activated RhoA and Rac1 displayed the most dramatic and consistent phenotypes. ca-RhoA expression resulted in the absence of nearly all dendrites (Fig. 1D), whereas ca-Rac1 caused a proliferation of processes, giving 70% of the cells expressing this construct a hairy appearance (Fig. 1G). The expression of other mutant and wt-GTPase proteins usually resulted in a general decrease in the number and length of dendrites.
Fig. 1.
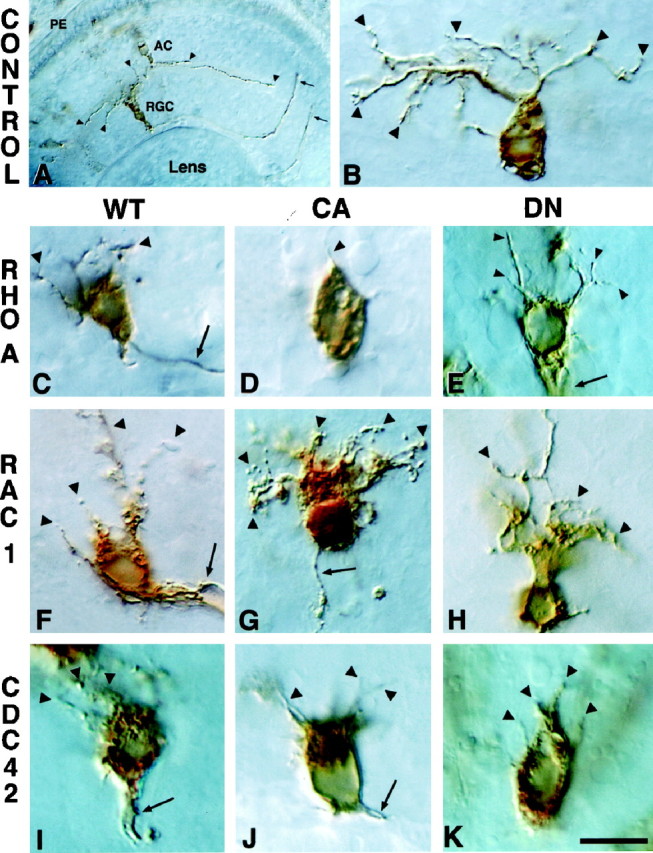
Expression of Rho-family GTPase mutants affects dendritogenesis. Shown are RGCs in sections ofXenopus eye immunolabeled with anti-luciferase (A, B) or anti-myc antibodies (C–K). All cells are visualized with HRP-conjugated secondary antibodies. Photomicrographs are oriented with the PE (pigment epithelium) at the top and the lens at the bottom. A, A low-power view of a retina with a luciferase-transfected RGC and an amacrine cell (AC). Arrowheads indicate RGC and AC dendrites; arrows indicate two RGC axons exiting the eye. B, A higher power view of a luciferase-expressing RGC. Arrowheads illustrate the extensive dendritic tree. The axon of this RGC is in the next section. C–K, RGCs expressing wt, ca, and dn RhoA, Rac1, and Cdc42.Arrowheads indicate dendrites; arrowsindicate axons. Scale bar, 15 μm.
The effect of different Rho-family GTPase transgenes was quantified by counting the number of dendrite tips and measuring the longest dendrite. Dendrites were defined as processes oriented toward the inner plexiform layer in the retina. Dendrite length and number could not be measured accurately for cells expressing ca-Rac1, because the processes could not be distinguished individually. Therefore, a third parameter, termed “extended cell area,” was examined also, in which the outline of the cell body, including processes that were not separable, was traced and measured. Most of the GTPase transgenes significantly decreased the average length of the longest dendrite, ranging from an ∼60% decrease caused by dn-Rac1 to an ∼90% decrease caused by ca-RhoA (Fig. 2A). Many constructs also significantly altered the number of dendrite tips (Fig.2B). Cells expressing wt- and ca-RhoA displayed the strongest phenotype, with an ∼70–90% decrease, and all forms of Cdc42 caused a significant decline in dendrite tip number. The dramatic effect of expressing ca-Rac1 on extended cell body area is illustrated in Figure 2C, in which the area is nearly double that of controls. A smaller, although significant, increase in extended cell area also was observed for wt-Rac1 and ca-Cdc42, which is probably attributable to some of these cells displaying a “hairy” phenotype. Thus, a loss of both Rac1 and Cdc42 activity negatively impacts dendrite formation in vivo, although an increase in Rac1 activity alone is enough to promote dendritogenesis.
Fig. 2.
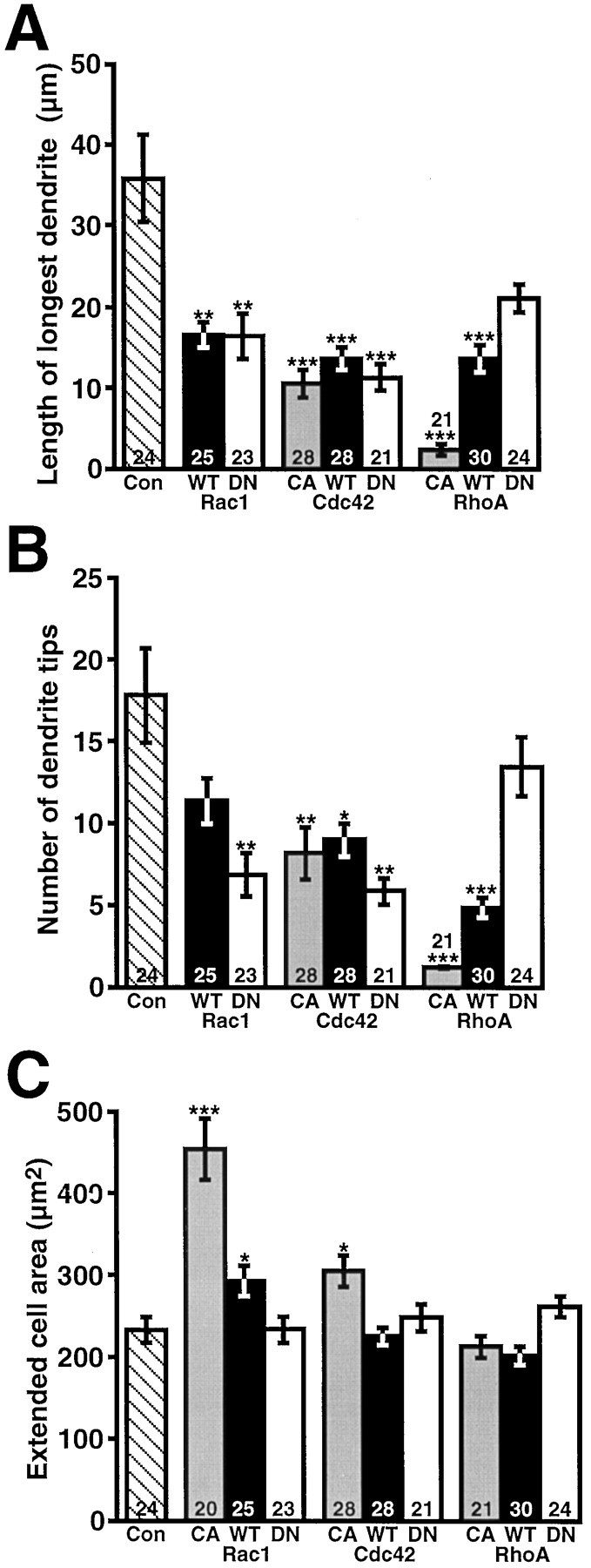
Most wt and mutant GTPases cause dendritic reduction, whereas ca-Rac causes process proliferation.A, Average length of the longest dendrite measured for RGCs expressing control proteins or ca-, wt-, or dn-GTPases.B, Average number of dendritic tips per RGC.C, Extended cell area of transfected RGCs (includes cell body plus closely growing, indistinguishable processes). Thenumbers in the bars indicate the RGCs that were analyzed. Error bars are SEM, and p values (*p < 0.05, **p < 0.01, ***p < 0.0001) indicate differences from control.
Rac1 is required for axon initiation
The deregulation of Rho-family GTPases had a dramatic inhibitory effect on axon initiation. The expression of ca forms of RhoA, Rac1, and Cdc42 nearly eliminated RGC axons. Similarly, blocking Rac1 function with the dn version severely inhibited axonogenesis. Rarely, very short axons that failed to exit the eye were seen (arrows; see Fig. 1G,J), but labeled axons were never observed in the brain in vibratome sections for these constructs (data not shown). Several possibilities could explain the lack of axons. (1) Axons were never initiated. (2) Axons were formed initially but were retracted. (3) Axon initiation was delayed. To distinguish among these possibilities, we performed an in vivo time course. At no time were axons detected extending from cells expressing ca-GTPase mutants or dn-Rac1 (data not shown). Thus, an unregulated increase in the activity of these GTPases, or a loss of Rac1 activity, severely disrupts axon initiation. Cells expressing wt-RhoA, Rac1, and Cdc42 initiated and extended axons, indicating that the cells are able to compensate for the overexpression of wild-type proteins. Expression of dn-RhoA and Cdc42 did not block axon initiation, suggesting that these GTPases are not required for axon initiation.
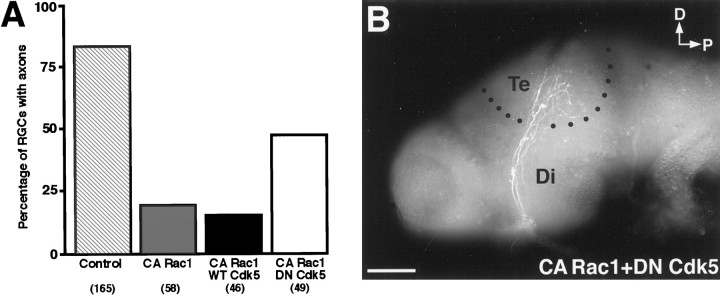
dn-Cdk5 coexpression with ca-Rac1 partially rescues axonogenesis
Cyclin-dependent kinase 5 (Cdk5) forms a complex with p35 and regulates neurite outgrowth in culture (Nikolic et al., 1996). A recent report shows that this complex colocalizes with Rac1 in neuronal growth cones and is a specific effector of Rac1 (Nikolic et al., 1998). We wondered whether the inhibition of axonogenesis observed after ca-Rac1 expression could be rescued by the coexpression of a dn version of Cdk5. Embryos were transfected with ca-Rac1 alone or along with wt- or dn-Cdk5. The coexpression rate for most constructs is generally >90% when the DNAs are mixed in the lipofection cocktail (Holt et al., 1990;Riehl et al., 1996). The number of transfected RGCs with axons in the retina was determined (Fig.3A). Nearly 85% of control GFP-expressing RGCs had axons. Using highly sensitive CY-3 secary antibodies on thin cryostat sections, we saw faintly labeled axons that were not visible with HRP-conjugated secondary antibodies on 19% of myc-tagged ca-Rac1-expressing cells. Cotransfection with wt-Cdk5 did not alter the number of ca-Rac1-expressing RGCs that formed axons, as observed by staining the myc-tag on the ca-Rac1 protein. However, cotransfection with dn-Cdk5 increased the percentage of ca-Rac1-expressing RGCs with axons to 48% (Fig. 3A). Because the cdk5 constructs were not myc-tagged, the rescue of axonogenesis in the case of dn-cdk5 must be attributable to coexpression with myc-tagged ca-Rac1. These axons projected normally to the optic tectum (Fig. 3B). This implies that, for normal axonogenesis to occur, the activity of Rac1 and its downstream effectors, such as p35/Cdk5, must be regulated tightly. The cotransfection of wt-Cdk5 in dn-Rac1-expressing RGCs was not able to rescue axonogenesis (data not shown). dn-Cdk5 cotransfection with GFP had no obvious effect on RGC axons as determined by GFP fluorescence; they projected normally to the tectum (data not shown). This finding contrasts with the results of Nikolic et al. (1996), who observed an inhibition of axon formation with the expression of dn-Cdk5 in neurons in culture. This difference may be attributable to compensatory mechanisms in vivo, or perhaps dn-Cdk5 was not expressed at high enough levels in our experiments to inhibit axon formation.
Fig. 3.
dn-Cdk5 rescues inhibition of axonogenesis caused by ca-Rac1. A, Percentage of transfected RGCs with axons exiting the eye in cryostat sections of stage 41 embryos. The numbers of axons that were examined are inparentheses. B, RGC axons coexpressing ca-Rac1 and dn-Cdk5 project dorsally up through the diencephalon (Di) to the optic tectum (Te) in a whole-mount brain. Black dots approximate the tectal borders. Scale bar, 150 μm.
Axon extension, target selection, and target recognition compromised by wt-Cdc42 and dn-RhoA
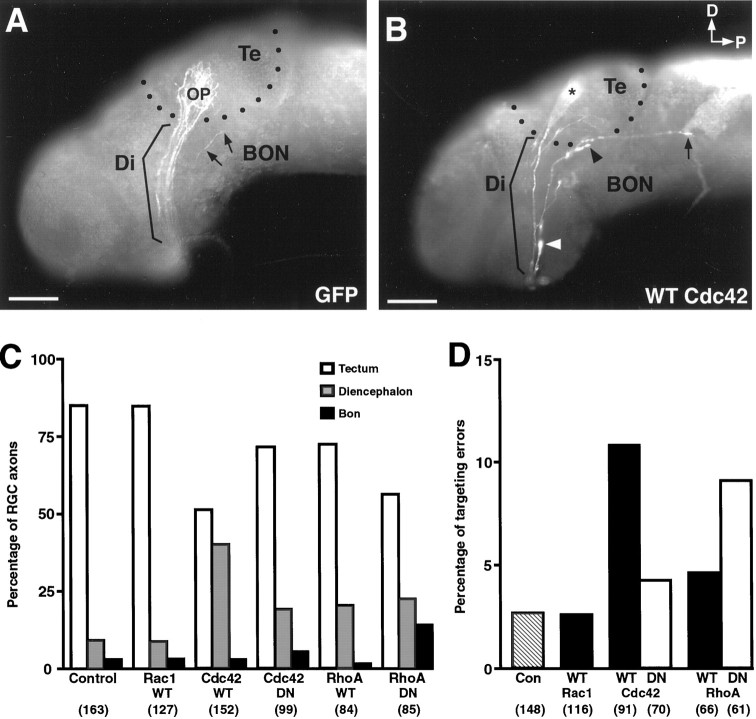
To determine whether Rho-family GTPases are important in pathfinding or target recognition, as has been shown in invertebrates (Zipkin et al., 1997; Kaufmann et al., 1998; Steven et al., 1998), we followed the trajectories of GTPase transgene-expressing axons in whole-mount brains. Although some axons expressing ca-Rac1 were observed by using CY-3 secary antibodies in whole-mount, this analysis could not be performed for this construct because there were too few axons observed and the staining was faint. Normally, the majority of RGC axons at stage 41 has grown dorsally through the contralateral diencephalon to innervate the optic tectum (Fig.4A). A small fraction of RGCs also normally innervates the basal optic nucleus (BON), located in the hindbrain. Axons were classified according to whether they were within the diencephalon (parentheses; Fig.4A,B), had innervated the optic tectum (dots; Fig. 4A,B), or were growing toward the basal optic nucleus (BON). Greater than 80% of control axons innervated the tectum, whereas ∼10% were still in the diencephalon, and <5% grew toward the BON (Fig. 4C). Just over 50% of wt-Cdc42-expressing cells had extended an axon to the tectum at the same stage, whereas 40% still had axons within the diencephalon. The growth cone of an axon can been seen just exiting the optic chiasm and entering the diencephalon in Figure 4B (white arrowhead). Thus, the overexpression of wt-Cdc42 seems either to delay axonogenesis developmentally or to cause growth cones to advance more slowly. A decrease in axons within the tectum also was observed for dn-RhoA-expressing cells. In this case, there was a fivefold increase in the percentage of cells that grew toward the BON as compared with the controls, indicating that the loss of RhoA function may impair the ability of RGC growth cones to make appropriate target choices.
Fig. 4.
Axon extension, target selection, and target recognition defects caused by Rho-family GTPases. A,GFP-transfected axons of the optic projection (OP) course dorsally through the diencephalon (Di) toward their target, the tectum (Te), in a control stage 41 brain. Black dots approximate the tectal borders. A single faintly labeled axon has left the main tract (arrows) to project toward the basal optic nucleus (BON). B, wt-Cdc42-expressing axons immunostained with an anti-myc antibody. Indicated are an axon just leaving the optic chiasm and entering the optic pathway in the diencephalon (white arrowhead), an out-of-focus axon that has terminated correctly within the tectum (asterisk), an axon on the ventral border of the tectum (blackarrowhead), and an axon that has extended past both the optic tectum and the BON (arrow). Scale bar, 150 μm. C, Percentage of RGC axons transfected with control (GFP), wt-, or dn-GTPase proteins that terminated within the optic tectum and were located in the diencephalic portion of the optic pathway or that projected toward the BON in stage 41 whole-mount brains. Axons that made target recognition errors were not included in this analysis. The numbers inparentheses indicate the axons that were examined.D, Percentage of RGC axons transfected with control (GFP), wt-, or dn-GTPase proteins that made target recognition errors in stage 41 whole-mount brains. Errors included axons that grew posteriorly past the tectum and axons that grew ventrally and dorsally along the tectal border but that did not innervate the tectum. Only axons that grew past the diencephalon were included in this analysis. The numbers in parentheses indicate the axons that were examined.
Retinal ganglion cells with axons that misexpressed GTPase transgenes did not display penetrant pathfinding phenotypes, because most of these exited the eye correctly, crossed the optic chiasm to enter the contralateral diencephalon, and then grew dorsally toward the tectum or the BON. However, many more axons expressing wt-Cdc42 and dn-RhoA made target recognition errors than did those expressing GFP or other GTPase constructs. A brain with several axons overexpressing wt-Cdc42 is shown in Figure 4B. One axon has grown past the tectum and the BON and is extending posteriorly into the hindbrain (arrow; Fig. 4B). Another axon (black arrowhead) also has turned at the ventral border of the tectum. Other errors that were observed include axons that grew along the border of the tectum dorsally but failed to innervate it. The percentage of axons making target recognition errors increased approximately threefold for both wt-Cdc42 and dn-RhoA as compared with the controls (Fig. 4D). No increased pathfinding or targeting errors were found with either wt-Rac1 overexpression (Fig.4D) or in RGCs expressing ca-Rac1 where axonogenesis was rescued with dn-Cdk5 (data not shown). These observations indicate a role for RhoA and Cdc42 in the transducing pathway or target recognition signals in this system.
Growth cone morphology altered by Cdc42 and RhoA
To examine why RhoA and Cdc42 affected target recognition, we looked at the morphology of growth cones, the structures responsible for sensing and responding to cues in the extracellular environment. Control RGC growth cones exhibited brush-like lamellipodia and finger-like filopodia (arrowheads; Fig.5A). wt-Cdc42-overexpressing growth cones were larger and more complex and commonly had an increased number of backbranches along their axons (arrows; Fig.5E). Of the growth cones expressing dn-RhoA, 58% had abnormal, thickened filopodia with a balled appearance (arrowheads; Fig. 5D). In both cases these growth cones had certain aspects of the transformed appearance of normal growth cones as they first entered the tectum, such as increased complexity, backbranching, and altered filopodial morphology (Harris et al., 1987). It is, therefore, interesting to speculate that these signaling pathways are involved in target recognition.
Fig. 5.
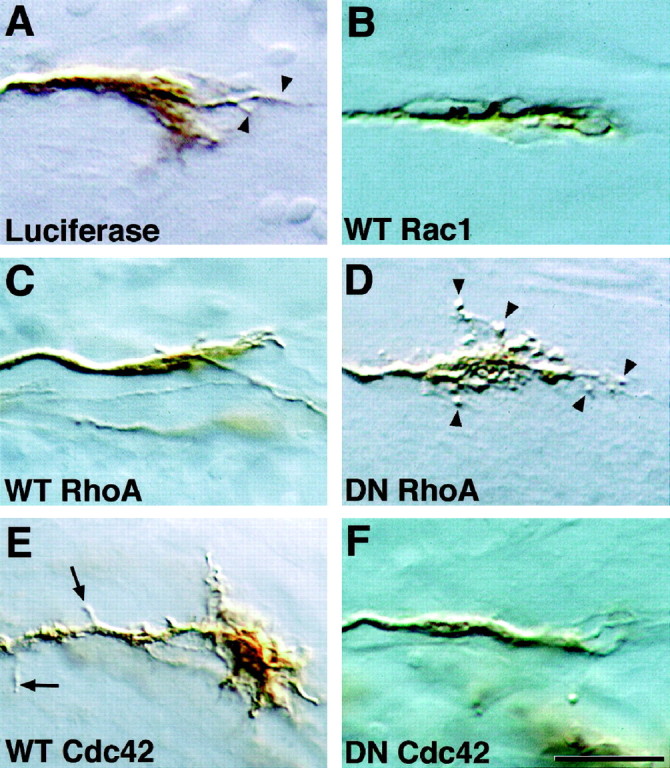
Rho-family GTPase mutants influence growth cone morphology. Shown are RGC growth cones in sections ofXenopus brain immunostained with anti-luciferase (A) or anti-myc antibodies (B–F). A, A growth cone transfected with the control protein luciferase;arrowheads indicate filopodia. B–F, Growth cones transfected with wt or dn mutant GTPases.Arrowheads in D indicate the abnormal, balled filopodia observed on dn-RhoA-expressing growth cones.Arrows in E indicate backbranches observed on axons of wt-Cdc42-expressing cells. Notice also the unusually large growth cone. Scale bar, 15 μm.
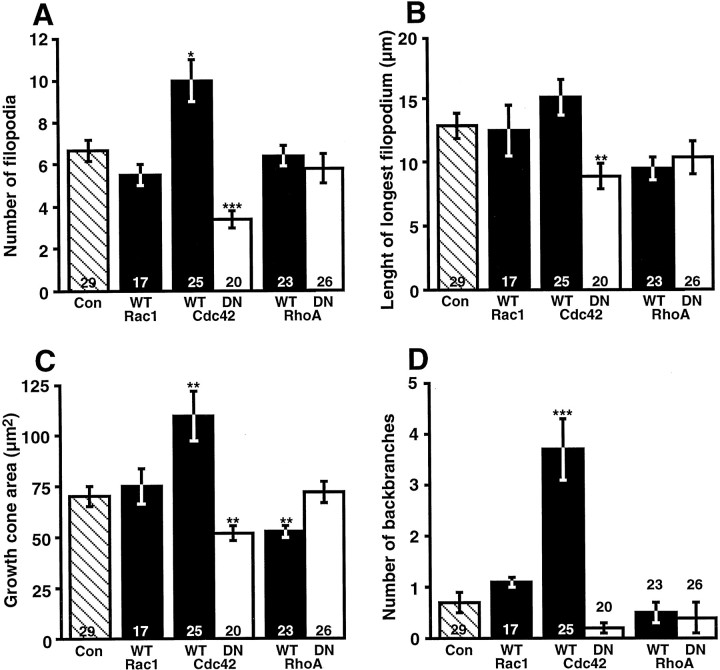
Growth cone morphology was analyzed by measuring the area, the number of filopodia, and length of the longest filopodium (Fig.6). For each construct the growth cones were examined at many different points in the pathway. However, the differences observed in growth cone morphology were not correlated with a preponderance of growth cones in a particular brain region. Growth cones overexpressing wt-Cdc42 had 50% more filopodia, were 40% larger, and had 80% more axonal backbranches than controls. Expressing dn-Cdc42 had the opposite effect: decreasing the number of filopodia on average by ∼50%, length of the longest filopodium by ∼30%, and growth cone area by ∼25%. Overexpressing wt-RhoA caused a similar decrease in growth cone area but did not affect significantly the filopodia number or length of the longest filopodium. Clearly, Cdc42 plays a major role in promoting growth cone structures in vivo. Interestingly, increasing the activity of this GTPase, and thus growth cone complexity, seems detrimental to either axon initiation or extension and to target recognition. Although dn-RhoA did not change significantly any of the parameters that were measured, it did alter filopodial morphology in many growth cones. This altered morphology potentially could explain the differences observed in target choice and recognition in growth cones in which RhoA function was inhibited.
Fig. 6.
Cdc42 regulates RGC growth cone complexity.A, Average number of filopodia per growth cone for RGC growth cones expressing control, wt-, or dn-GTPase proteins.B, Average length of the longest filopodium on RGC growth cones. C, Average growth cone area.D, Average number of backbranches found on the axon two growth-cone lengths behind the initial swelling of the growth cone. Thenumbers in the bars indicate the growth cones that were examined. Error bars are SEM, and pvalues (*p < 0.05, **p < 0.01, ***p < 0.0001) indicate differences from control.
DISCUSSION
This study demonstrates that Rho-family GTPases regulate the initiation, extension, and elaboration of dendrites, axons, and growth cones in vertebrate neurons in vivo. In addition, by expressing wt, ca, and dn forms of Rac1, RhoA, and Cdc42 in RGCs and examining multiple aspects of neuronal process development, we have illustrated differences in the functional roles for these GTPases in a single cell type. Several findings have emerged from this study, many of which are consistent with previous studies. (1) Axonogenesis is inhibited by the unregulated increase in activity of all three Rho-family GTPase members, but only Rac1 activity is required for axonogenesis. The partial rescue of the ca-Rac1 phenotype with dn-Cdk5 suggests that Rac1 activity must be maintained within certain levels for normal axonogenesis. (2) Dendritogenesis requires the function of both Rac1 and Cdc42, whereas the expression of ca-Rac1 results in a dramatic proliferation of dendrites. (3) Target recognition relies on a normal balance of Cdc42 and RhoA activity; Cdc42 and RhoA also have striking effects on growth cone morphology. Table1 summarizes these results.
Table 1.
Summary of effects of wt-, ca-, and dn-Rho-family GTPases on RGC axons, growth cones, and dendrites
| Construct | Axons | Growth cones | Dendrites | |
|---|---|---|---|---|
| Rac1 | CA | Absent | Absent | Proliferation |
| WT | Normal | Normal | Fewer | |
| Shorter | ||||
| Some proliferation | ||||
| DN | Absent | None | Fewer | |
| Shorter | ||||
| Cdc42 | CA | Absent | Absent | Fewer |
| Shorter | ||||
| WT | Fewer in tectum/shorter | More complex | Fewer | |
| Increased errors | Shorter | |||
| Some proliferation | ||||
| DN | Normal | Less complex | Fewer | |
| Shorter | ||||
| RhoA | CA | Absent | Absent | Fewer |
| Shorter | ||||
| WT | Normal | Smaller area | Fewer | |
| Shorter | ||||
| DN | Fewer in tectum | Abnormal filopodia morphology | Normal | |
| More toward BON | ||||
| Increased errors | ||||
Specificity of mutant Rho-family GTPases
The dn-GTPases used in this study function by competitive inhibition; they bind irreversibly to guanine nucleotide exchange factors (GEFs), molecules that regulate the exchange of GDP for GTP, thus preventing the activation of endogenous GTPases (Diekmann et al., 1991). Imperative to the interpretation of results obtained with these dns is the assumption that each affects only one GTPase and does not inhibit other family members. Two lines of evidence argue that these dns act specifically. First, differential effects were observed in this study after the expression of dn-Rac1, RhoA, or Cdc42. Second, specific GEFs for RhoA (Gebbink et al., 1997), Rac1 (Michiels et al., 1995), and Cdc42 (Zheng et al., 1996) have been identified. The ca mutants are unresponsive to GTPase-activating protein (GAP) stimulation of their intrinsic GTPase (Ridley et al., 1992) and thus exert their influence without interacting directly with endogenous regulatory proteins as the dn mutants do. Although these GTPases have not been demonstrated in theXenopus nervous system, it is likely that they are present, because Rho-family GTPases and their regulatory proteins are highly conserved and have been localized to nervous tissues in a variety of vertebrate and invertebrate species (Olofsson et al., 1988; Didsbury et al., 1989; Luo et al., 1994; Zipkin et al., 1997; Albertinazzi et al., 1998; Kuhn et al., 1998; Steven et al., 1998).
A role for RhoA in regulating target recognition and target choice
Our results confirm in vitro observations that increased RhoA activity inhibits process formation (Jalink et al., 1994; Gebbink et al., 1997; Kozma et al., 1997). The expression of ca-RhoA in vivo dramatically reduced the average length of the longest dendrite plus the number of dendrite tips and prevented axonogenesis. In addition, we have demonstrated novel requirements for RhoA activity in generating normal filopodial morphology and in target selection and recognition. It is of interest that growth cones in culture treated with C3-transferase, an inhibitor of RhoA, also display an abnormal morphology in that they exhibit little or no lamellipodial spreading (Jin and Strittmatter, 1997). The alterations in target selection and recognition observed in this study after dn-RhoA expression could be attributable to several factors. One may be improper myosin function in the growth cones. We have shown that myosin is required for normal Xenopus RGC growth cone motilityin vivo (Ruchhoeft and Harris, 1997), and it has been demonstrated that RhoA regulates myosin II activity (Amano et al., 1996; Kimura et al., 1996). It is also possible that these defects arise via RhoA effectors that interact with actin cytoskeleton, such as p140mDia, a protein that regulates the activity of profilin, an actin monomer-sequestering protein (Watanabe et al., 1997). Alternatively, other RhoA effectors that have been identified but not yet functionally characterized, such as PKN (Amano et al., 1996; Watanabe et al., 1996) and rhotekin (Watanabe et al., 1996), may be involved.
Rac1 is required for axon initiation and promotes dendritogenesis
The absence of axons after dn-Rac1 expression indicates that Rac1 activity is required for Xenopus RGC axon initiation, although Cdc42 activity is not. The expression of ca-Rac1 also resulted in the absence of axons, suggesting that either an unregulated increase or decrease in the activity of Rac1 is detrimental. This result agrees with a study by Luo et al. (1994) in Drosophila, in which the early expression of either dn- or ca-Rac1 eliminated axons and later expression caused growth cone stalling, and with a recent study in chick motor neurons in vitro in which both ca- and dn-Rac1 drastically reduced neurite length (Kuhn et al., 1998). Visualization of the actin cytoskeleton of growth cones in both of these systems revealed an increase in F-actin in ca-expressing growth cones and a decrease in dn-expressing growth cones, suggesting that Rac1 controls the cycling of actin polymerization and depolymerization. Too much or too little polymerization may upset the delicate balance required to generate an axon. That a dn version of Cdk5, an effector of Rac1 signaling, can partially rescue the inhibition of axonogenesis caused by ca-Rac1 in Xenopus RGCs supports this argument. The overexpression of wt-Rac1 did not prevent axonogenesis or affect growth cone morphology in this system. These cells may be able to compensate via regulatory mechanisms for the increased presence of a wt protein, whereas the activity of a nonregulatable ca form may be more difficult to overcome.
The expression of Rac1 mutants in this study also had a dramatic effect on dendritogenesis. dn-Rac1 caused a decrease in the length of the longest dendrite and the number of dendritic tips, whereas the introduction of a ca form resulted in a proliferation of dendrites. Other studies have shown that ca-Rac1 expression in mouse Purkinje cells in vivo increases the number of dendritic spines (Luo et al., 1996), ca-Rac1 expression in rat cortical neurons in culture increases dendrite number (Threadgill et al., 1997), and the overexpression of chicken Rac1B enhances neurite number and branching in retinal cells in culture (Albertinazzi et al., 1998). These results, as well as the data from this study, all point to a major role for Rac1 in dendrite formation. The influence of Rac1 on dendritic proliferation could be mediated via downstream effectors of Rac1, such as gelsolin (Arcaro, 1998; Azuma et al., 1998) and cofilin (Arber et al., 1998;Yang et al., 1998), which regulate actin polymerization cycling. Whatever the mechanism of dendritic proliferation, the differential effects of ca-Rac1 on dendrites versus axons in Xenopus RGCs suggest intriguing differences in the development of these structures. Growth cones have been observed on the tips of developing dendrites (Maslim et al., 1986), and the regulation of actin dynamics could vary from that found in axonal growth cones.
Cdc42 regulates growth cone morphology in vivo
Unlike Rac1, Cdc42 activity was not required for axon initiation, because dn-Cdc42-expressing RGCs initiated and extended axons normally. Manipulating Cdc42 function did impact growth cone morphology. Overexpression of the wt protein increased growth cone size and filopodia number, whereas dn-Cdc42 expression decreased growth cone size, filopodial number, and filopodial length. Intriguingly, overexpressing wt-Cdc42 caused both an increase in growth cone complexity and in the percentage of axons that made target recognition errors. Similar to what was observed with dn-Rho, only a small percentage (∼10%) of RGCs made target recognition errors. This may be attributable to variability among the mixed population of RGCs, some of which may be more sensitive to perturbation. In fly and worm, in which mutant GTPases have been found to cause pathfinding errors, only an identified subset of cells exhibits aberrant pathfinding (Zipkin et al., 1997; Kaufmann et al., 1998). wt-Cdc42-expressing axons also were significantly shorter than controls, although it is unclear whether this is attributable to slower axon extension or delayed axon initiation. Because of their increased size and complexity, these growth cones may have advanced more slowly or may have been hindered by abnormal filopodial and lamellipodial motility.
Interestingly, an unregulated increase or decrease of Cdc42 inhibited dendritogenesis, which is similar to the effect mutant Rac1 expression had on axon initiation in this system. Potentially, aspects of actin dynamics are controlled by Cdc42 as well as Rac1 during dendritogenesis. Cdc42 has been shown to induce actin polymerization in a cell-free system via a pathway involving p21-activating kinase (Pak1) (Zigmond et al., 1997).
Previous work on Rho-family GTPases has illustrated that the roles these molecules play can be extremely context-dependent, which could make the development of a generalized, comprehensive picture of their function impossible. In this study we have shown distinct roles for RhoA, Rac1, and Cdc42 in the formation of neuronal processes in a single cell type in a developing, in vivo, vertebrate preparation. The thorough nature of the present study may provide insight into the collaborative nature of these three proteins in the formation of axons, dendrites, and growth cones.
Footnotes
This work was supported by National Institutes of Health and Medical Research Council grants (to W.A.H. and C.E.H.) and a Lucille P. Markey Fellowship (to M.L.R.). We thank Alan Hall and Jerold Chun for providing various GTPase cDNA constructs and Anna Philpott for advice and for providing Cdk5 cDNA constructs. We also thank Sarah McFarlane, Barbara Lom, Emilie Marcus, Anne Vincent, and Nick Spitzer for helpful comments on this manuscript; Anna Marnick for library work; and Darwin Berg, Jim Posokony, David Rapaport, and Don Cleveland for allowing the use of their equipment.
Correspondence should be addressed to Dr. Maureen Ruchhoeft, University of California at San Diego, 3115 Pacific Hall, Mail Code 0357, La Jolla, CA 92093.
REFERENCES
- 1.Adamson P, Paterson HF, Hall A. Intracellular localization of the p21 Rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albertinazzi C, Gilardelli D, Paris S, Longhi R, de Curtis I. Overexpression of a neural-specific Rho-family GTPase, cRac1B, selectively induces enhanced neuritogenesis and neurite branching in primary neurons. J Cell Biol. 1998;142:815–825. doi: 10.1083/jcb.142.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 4.Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 5.Arcaro A. The small GTP-binding protein Rac promotes the dissociation of gelsolin from actin filaments in neutrophils. J Biol Chem. 1998;273:805–813. doi: 10.1074/jbc.273.2.805. [DOI] [PubMed] [Google Scholar]
- 6.Azuma T, Witke W, Stossel TP, Hartwig JH, Kwiatkowski DJ. Gelsolin is a downstream effector of Rac for fibroblast motility. EMBO J. 1998;17:1362–1370. doi: 10.1093/emboj/17.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didsbury J, Weber R, Bokock G, Evans T, Snyderman R. Rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem. 1989;264:16378–16382. [PubMed] [Google Scholar]
- 8.Diekmann D, Brill S, Garrett MD, Totty N, Hsuan J, Monfries C, Hall C, Lim L, Hall A. Bcr encodes a GTPase-activating protein for p21rac. Nature. 1991;351:400–402. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- 9.Gebbink MF, Kranenburg O, Poland M, van Horck FP, Houssa B, Moolenaar WH. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–1613. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 11.Harris WA, Holt CE, Bonhoeffer F. Retinal axons with and without their soma, growing to and arborizing in the tectum of Xenopus embryos: a time-lapse study of single fibers in vivo. Development. 1987;101:123–133. doi: 10.1242/dev.101.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Holt CE. A single-cell analysis of early retinal ganglion cell differentiation in Xenopus: from soma to axon tip. J Neurosci. 1989;9:3123–3145. doi: 10.1523/JNEUROSCI.09-09-03123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt CE, Garlick N, Cornel E. Lipofection of cDNAs in the embryonic vertebrate central nervous system. Neuron. 1990;4:203–214. doi: 10.1016/0896-6273(90)90095-w. [DOI] [PubMed] [Google Scholar]
- 14.Holtfreter J. Properties and functions of the surface coat in amphibian embryos. J Exp Zool. 1943;93:251–323. [Google Scholar]
- 15.Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenar W. Inhibition of lysophosphatidic- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann N, Wills ZP, Van Vactor D. Drosophila Rac1 controls motor axon guidance. Development. 1998;125:453–461. doi: 10.1242/dev.125.3.453. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 19.Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodeling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn TB, Brown MD, Bamburg JR. Rac1-dependent actin filament organization in growth cones is necessary for β1-integrin-mediated advance but not for growth cone advance on poly-d-lysine. J Neurobiol. 1998;37:524–540. doi: 10.1002/(sici)1097-4695(199812)37:4<524::aid-neu3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Lilienbaum A, Reska AA, Horwitz AF, Holt CE. Chimeric integrins expressed in retinal ganglion cells impair process outgrowth in vivo. Mol Cell Neurosci. 1995;6:139–152. doi: 10.1006/mcne.1995.1013. [DOI] [PubMed] [Google Scholar]
- 22.Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 23.Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 24.Luo L, Jan LY, Jan YN. Rho family GTP-binding proteins in growth cone signaling. Curr Opin Neurobiol. 1997;7:81–86. doi: 10.1016/s0959-4388(97)80124-9. [DOI] [PubMed] [Google Scholar]
- 25.Maslim J, Webster M, Stone J. Stages in the structural differentiation of retinal ganglion cells. J Comp Neurol. 1986;254:382–402. doi: 10.1002/cne.902540310. [DOI] [PubMed] [Google Scholar]
- 26.Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- 27.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis. Daudin; Amsterdam: 1967. [Google Scholar]
- 28.Nikolic M, Dudek H, Kwon YT, Ramos YFM, Tsai L-H. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 29.Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai L-H. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- 30.Olofsson B, Chardin P, Touchot N, Zahraoui A, Tavitian A. Expression of the ras-related ralA, Rho12, and rab genes in adult mouse tissues. Oncogene. 1988;3:231–234. [PubMed] [Google Scholar]
- 31.Philpott A, Porro EB, Kirschner MW, Tsai LH. The role of cyclin-dependent kinase 5 and a novel regulatory subunit in regulating muscle differentiation and patterning. Genes Dev. 1997;11:1409–1421. doi: 10.1101/gad.11.11.1409. [DOI] [PubMed] [Google Scholar]
- 32.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 33.Riehl R, Johnson KJ, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt C. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 34.Ruchhoeft ML, Harris WA. Myosin functions in Xenopus retinal ganglion cell growth cone motility in vivo. J Neurobiol. 1997;32:567–578. [PubMed] [Google Scholar]
- 35.Sakaguchi DS, Murphey RK, Hunt RK, Tompkins R. The development of retinal ganglion cells in a tetraploid stain of Xenopus laevis: a morphological study utilizing intracellular dye injection. J Comp Neurol. 1984;224:231–251. doi: 10.1002/cne.902240205. [DOI] [PubMed] [Google Scholar]
- 36.Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Ruiz Morales A, Hogue CW, Pawson T, Culotti J. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92:785–795. doi: 10.1016/s0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- 37.Tapon N, Hall A. Rho, Rac, and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 38.Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 39.Tigyi G, Fischer DJ, Sebok A, Yang C, Dyer DL, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J Neurochem. 1996;66:537–548. doi: 10.1046/j.1471-4159.1996.66020537.x. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe G, Saito Y, Madaule P, Reid T, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka A, Narumiya S. Protein kinase N (PKN) and PKN-related rhophilin as targets of small GTPase Rho. Science. 1996;271:645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang N, Higuchi O, Mizuno K. Cytoplasmic localization of LIM-kinase 1 is directed by a short sequence within the PDZ domain. Exp Cell Res. 1998;241:242–252. doi: 10.1006/excr.1998.4053. [DOI] [PubMed] [Google Scholar]
- 43.Zheng Y, Fischer DJ, Santos MF, Tigyi G, Pasteris NG, Gorski JL, Xu Y. The faciogenital dysplasia gene product FGD1 functions as a Cdc42Hs-specific guanine-nucleotide exchange factor. J Biol Chem. 1996;271:33169–33172. doi: 10.1074/jbc.271.52.33169. [DOI] [PubMed] [Google Scholar]
- 44.Zigmond SH, Joyce M, Borleis J, Bokoch GM, Devreotes PN. Regulation of actin polymerization in cell-free systems by GTPγS and Cdc42. J Cell Biol. 1997;138:363–374. doi: 10.1083/jcb.138.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zipkin ID, Kindt RM, Kenyon CJ. Role of a new Rho family member in cell migration and axon guidance in C. elegans. Cell. 1997;90:883–894. doi: 10.1016/s0092-8674(00)80353-0. [DOI] [PubMed] [Google Scholar]